Highlights
-
•
Nanomaterials have been shown to affect gut microbiota (GM) both in vivo and in vitro.
-
•
The effects have been shown to depend on size, dose, dose duration and functional groups.
-
•
In general, more studies seem to indicate dose-dependent adverse effects of NMs towards GM.
-
•
Standardized protocols are needed for characterization of NMs, dosing, and test systems (both in vitro and in vivo).
Keywords: Nanomaterials, Gut microbiota, Dysbiosis, Exposure, Metabolic disease
Abstract
Some nanomaterials (NMs) have been shown to possess antimicrobial activity and cause GM dysbiosis. Since NMs are being used widely, a systematic assessment of the effects of NMs on GM is warranted. In this systematic review, a total of 46 in vivo and 22 in vitro studies were retrieved from databases and search engines including Science-Direct, Pubmed and Google scholar. Criteria for assessment of studies included use of in vitro or in vivo studies, characterization of NMs, use of single or multiple doses as well as consistency of results. GM dysbiosis has been studied most widely on TiO2, Ag, Zn-based NMs. There was moderate evidence for GM dysbiosis caused by Zn- and Cu-based NMs, Cu-loaded chitosan NPs and Ag NMs, and anatase TiO2 NPs, as well as low evidence for SWCNTs, nanocellulose, SiO2, Se, nanoplastics, CeO2, MoO3 and graphene-based NMs. Most studies indicate adverse effects of NMs towards GM. However, more work is required to elucidate the differences on the reported effects of NM by type and sex of organisms, size, shape and surface properties of NMs as well as effects of exposure to mixtures of NMs. For consistency and better agreement among studies on GM dysbiosis, there is need for internationally agreed protocols on, inter alia, characterization of NMs, dosing (amounts, frequency and duration), use of sonication, test systems (both in vitro and in vivo), including oxygen levels for in vitro models.
Graphical Abstract
Graphical abstract
.
1. Introduction
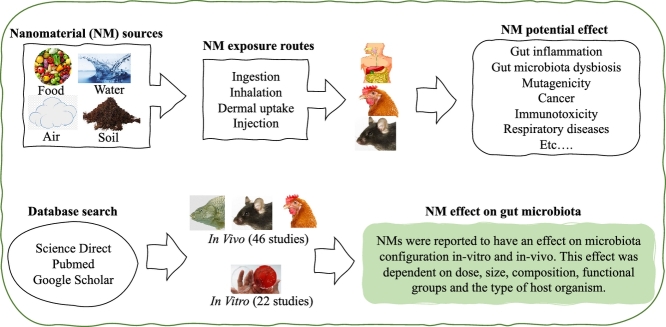
Nanomaterials (NMs) are defined as materials that have structural components smaller than 100 nanometers in at least one dimension (Buzea et al., 2007). Due to unique biological and physico-chemical properties that emerge at the nanoscale (RS and RAE, 2004), NMs have found many applications in medicines, food, pesticides electronics, textiles and many others. However, NMs can penetrate human skin, lungs and the gastro-intestinal tract and translocate into systemic circulation and eventually to tissues and organs (Nemmar et al., 2002; Bachler et al., 2015). Moreover, NMs may have indirect adverse effects on humans through modification of gut microbiota (GM), which play many indispensable roles in human health, including metabolism (Rowland et al., 2018), immunity (Geuking et al., 2014), neurobehavioral processes (MacFabe, 2012) and many others. The disturbance of normal GM configuration, often referred to as GM dysbiosis, has been linked with several human diseases and conditions, including, inter alia, asthma (Johnson and Ownby, 2017), diabetes and obesity (Moreno-Indias et al., 2014), colon cancer (Garrett, 2019), inflammatory bowel disease (IBD) (Garrett, 2019) as well as central nervous system disorders (Carabotti et al., 2015; Chin-Chan et al., 2015; Zhu et al., 2017).
Titanium dioxide (TiO2), starch and silicon dioxide (SiO2) NMs are often intentionally added in food (Dekkers et al., 2011; Weir et al., 2012b; Chen et al., 2017b), while silver NPs (AgNPs), nano-clay, zinc oxide (ZnO) NPs, TiO2 are often used in food packaging because of their antimicrobial properties (Bumbudsanpharoke et al., 2015; Radusin et al., 2016). For example, the estimated mean intake of TiO2 NPs for the Dutch population ranged from 0.19 mg/kg bw/day in elderly people, 0.55 mg/kg bw/day for 7–69-year olds, to 2.16 mg/kg bw/day in young children (Rompelberg et al., 2016). Furthermore, use of number of NMs in medical formulations, including liposomes, organic polymers, micelles, metals/metal oxides, carbon nanotubes (CNTs) and other inorganic materials, may result to significant exposure of NMs to the human gut.
The human gut can also be exposed to NMs from other routes other than the oral route. For example, negative effects on GM are expected to result from ingested NMs from mucociliary clearance of inhaled NMs (Pietroiusti et al., 2017) as well as intravenous injections. As an example of the latter, Lee et al. (2012) detected considerable fecal excretion of Ag NPs in rats following intravenous administration.
Various investigations are yielding results on the effects of NMs on GM. However, in certain cases, these studies, which usually employ a variety of methods and approaches, appear to yield contradictory results for the same type of NM. Despite the fact that a number of narrative reviews have been done on the subject, such as those by Bouwmeester et al. (2018), Lamas et al. (2020), and Zhang et al. (2020), a systematic assessment of the evidence of the impacts of NMs on GM in various species is required.
2. Methods
Scientific manuscripts published from 2010 to 2020 were identified from electronic search engines such as Pubmed, Google scholar, and ScienceDirect, using terms that included ‘nanomaterials’, ‘nanoparticles’, ‘ultrafine particles’, ‘gut microbiota’, ‘commensal gut microorganisms’, ‘commensal gut microbiota’. The electronic searches were complemented by snowballing. The PECO (Population, Exposure Comparator and Outcome) strategy was used (Hoffmann et al., 2017): Experimental studies using GM (P), NMs (E) and control groups (C) were evaluated to identify the presence or absence of negative effects (O).
2.1. Characteristics and quality of included studies
Risk of bias was reduced by involvement of three independent researchers to assess the eligibility of studies. All the included studies had to involve the assessment of the adverse effects of NMs on GM, in vivo or in an in vitro system. Failure to meet this requirement resulted in the automatic exclusion of the study. However, some methods and study designs were considered more relevant than others for evaluating the effects of NMs on GM. For example, studies conducted in vivo would be considered to provide more reliable evidence than those conducted in vitro (ex vivo) because of the inherent limitations associated with in vitro studies (Pearce et al., 2018). In addition, a study using one dose level would be considered less effective than those that use more than one dose levels, since a proof of a biological gradient or dose-response is among the Braford-Hill criteria of causality (Swaen and van Amelsvoort, 2009). This is useful in this assessment even though the study is not attempting to prove causality.
The use of negative controls would detect both suspected and unsuspected sources of spurious causal inferences (Lipsitch et al., 2010). While a negative control does not trigger a response, the evidence can be enhanced by the use of a positive control which triggers a response from the baseline in the expected direction and to a defined extent (Leist et al., 2010). Use of a positive control in the study assessing effects of NMs on GM would be used as an indicator for performance and sensitivity of the test system (Hothorn, 2014).
The toxicological properties of NMs are often affected by their physico-chemical properties. Therefore, characterization of these properties is of fundamental importance in nanotoxicology. However, since different techniques for characterizing physico-chemical properties of NMs have strengths and limitations, more than one method is required to characterize o nano-specific properties such as size and morphology. The criteria used in this study are presented in Table 1 below.
Table 1.
Criteria for the assessment of the strength of the included studies
| Score | Description |
| 1 | In vitro (ex vivo) studies using one dose level together with a negative control, without adequate characterization of NMs |
| 2 | In vitro (ex vivo) studies with one dose level together with a negative control and positive control as well as adequate characterization of NMs or in vivo studies using one dose level together with a positive and/or negative control without adequate characterization of NMs |
| 3 | In vitro (ex vivo) studies using multiple dose levels together with a negative and/or positive control as well as adequate characterization of NMs or in vivo studies using one dose levels together with a positive and/or negative control as well as adequate characterization of NMs |
| 4 | In vivo studies using multiple dose levels together with a negative and/or control with minimal characterization of NMs |
| 5 | In vivo studies using multiple dose levels together with a negative and/or positive control as well as adequate characterization of NMs |
As shown in Table 2, the quality of evidence for each conclusion statement was rated as low, moderate, or high, depending on the confidence of the results on the effects of specific NMs on GM. The quality rating was based on the strength of the outcome in the studies behind the conclusion as well as the consistency of the results across similar studies.
Table 2.
Significance of the quality of evidence for conclusion statements (adapted from Debia et al. (2016)
| Quality | Description |
| High | Strong evidence from numerous studies with consistent results. Further studies are unlikely to change the confidence in the conclusion statement |
| Moderate | Overall moderately to highly strong evidence and/or possible inconsistency in results. Further studies may change the confidence in the conclusion statement |
| Low | Overall weak evidence or inconsistency in results from a limited number of studies. Further research is very likely to change the confidence in the conclusion statement: based on |
3. Results
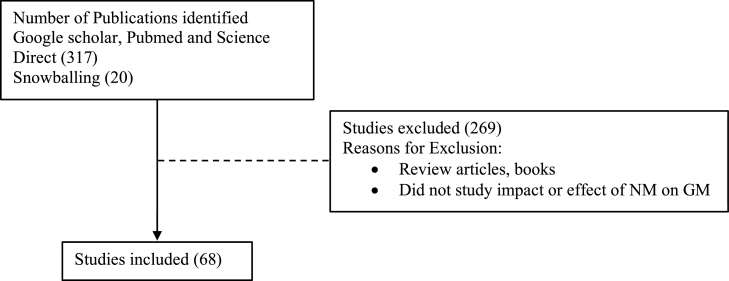
After excluding review articles, books and studies that did not assess impact or effect of NM on GM, a total of 68 journal articles were identified (46 from Google scholar, PubMed and Science Direct, as well as a further 20 articles from snowballing) (Figure 1).
Fig. 1.
Flow Diagram of Study Selection
3.1. Effect of nanomaterials on gut microbiota
3.1.1. In vivo studies
3.1.1.1. Effect of NMs on the gut microbiota of model organisms
Carbon-based NMs have been shown to have significant effects on GM in model animals such as mice and rats. As an example, oral administration of 0.05, 0.5, and 2.5 mg/kg bw day single-walled carbon nanotubes (SWCNTs) for 7 days caused a metabolic inflammation response together with an increase in the abundance of proinflammatory bacteria Alitipes_uncultured_bacterium and Lachnospiraceae bacterium A4 (Chen et al., 2018a). On the other hand, 10 weeks repeated oral exposure to multi-walled carbon nanotubes (MWCNTs; 4 or- 40 mg/week) did not cause changes in the composition of GM in mice (Christophersen et al., 2016). Other carbon-based NMs that have been studied include graphene, which had greater effects on Gram-positive bacteria than Gram-negative bacteria in mice (Xie et al., 2016), as well as fullerenol (Li et al., 2018a), fullerenes (Đurašević et al., 2020), nanofibrils (nanocellulose) (Khare et al., 2020), Chitin nanofibers (CNFs) (Azuma et al., 2015) and Cu-loaded chitosan NPs (Han et al., 2010). The results of these studies are summarized in Table 3.
Table 3.
Summary of effects of NMs on GM from in vivo tests
| Nanomaterial | Species | Exposure | Effects on GM | Evidence score | Reference |
|---|---|---|---|---|---|
| SWCNTs(1.04 – 1.17nm x 1-5 µm) | Mice | Oral administration of 0, 0.05, 0.5, and 2.5 mg/kg bwt/d for 7 days | Dose-dependent increases in the abundance of proinflammatory bacteria as well as significant shifts of Firmicutes | 5 | Chen et al. (2018a). |
| MWCNTs (49-74 nm x 3.86-5.7 µm) | Mice | Oral and pulmonary administration of 0, 4, 40 mg/week for 10 weeks | No changes in the composition of GM | 4 | Christophersen et al. (2016) |
| Fullerenol 100 nm and 90 nm | Mice | 0 and 20 mg/kg per day by gavage, for a month | A shift in the overall structure of GM; a marked increase in bacteria belonging to putative SCFAs-producing genera | 3 | Li et al. (2018a). |
| Fullerenes (size not stated) | Rats | Dietary 0 and 5 mg/kg b.w day, 12 weeks | Shift in GM structure towards the bacteria that ameliorate lipid homeostasis | 2 | Đurašević et al. (2020). |
| Nanocellulose (50 nm) | Rats | 0 or 10 ml/kg bw of 1% twice weekly for five weeks by gavage | Enrichment of specific species and reductions in populations of species that produce large amounts of SCFAs | 3 | Khare et al. (2020). |
| TiO2 (29 nm anatase) | Rats | Oral administration of 0, 2, 10, 50 mg/ kg daily for 30 days | Dose-dependent GM dysbiosis | 5 | Chen et al. (2019a). |
| TiO2 (29 nm anatase nm) | Rats | Oral administration of 0, 2, 10, 50 mg/ kg daily for 90 days | Dose-dependent increases in the abundance of Lactobacillus_reuteri and a decrease in the abundance of Romboutsia) | 5 | Chen et al. (2019b). |
| TiO2 (anatase 16.8 nm | Mice | 0, 2.5 mg/kg bw/day for 7 days by oral gavage | No obvious GM dysbiosis | 3 | Chen et al. (2017a) |
| TiO2 (220 nm and 255 nm, 85% anatase, 15% rutile) | Rats | 0, 10 mg/kg bw/day for 7 days by intragastric gavage | No obvious GM dysbiosis | 3 | Talbot et al. (2018) |
| Rutile (16, 148, and 361 nm) and anatase (20, 135 and 420 nm)TiO2 | Mice | 0, 100 mg/kg/day for 28 days by gavage | More pronounced shift in GM structures for rutile | 3 | Li et al. (2018b) |
| 30 nm TiO2 NPs (majority anatase) | Mice | 0, 40 mg/kg by oral gavage for 8 weeks | Changes in composition of GM | 3 | Zhu et al. (2020). |
| Food-grade TiO2 (28-1,158 nm) | Mice | 0, 2, 10 and 50 mg/kg/day in drinking water for 4 weeks | Minimal impact on the composition of GM. | 5 | Pinget et al. (2019). |
| TiO2 (10 nm primary size, undefined crystal structure) | Zebra fish | Three months exposure to aquatic 100 µg/L TiO2 NPs and BPA (0, 2, and 20 µg/L) | Antagonistic interaction at the lower BPA concentration but synergistic interaction at higher BPA concentrations. |
2 | Chen et al. (2018b) |
| TiO2 NPs (10-65 nm mixed anatase and rutile) | M. hemolymph | Aquatic 0, 100 μg/L for 4 days | Decreases in the abundance of some genera but increases in others | 3 | Auguste et al. (2019) |
| TiO2 (anatase, 10, 50, and 100 nm) | Mice | Dietary exposure of 0.1% for 3 months, ad libitum | Significant decrease in the abundance of several probiotic taxa | 3 | Mu et al. (2019) |
| TiO2 NPs (anatase <100 nm) | Mice | Oral 1 mg/kg bw /day for 7 days | Altered GM composition including a reduced richness of Bifidobacterium |
3 | Li et al. (2019b) |
| TiO2 NPs (food-grade from chocolates) | White albino mice | Ingestion of 0, 50 and 100 µg/day for 18 days | Inhibited growth and activity of probiotic formulation of B. coagulans, E. faecalis, and E. faecium | 5 | Khan et al. (2019) |
| TiO2 NPs (6–10 nm anatase) | Silkworm (Bombyx mori) | Ingestion of 0 and 5 mg/L | Changes in the abundance of individual bacterial species | 3 | Li et al. (2020) |
| Ag (12.2 nm) | Mice | Oral gavage of 0, 2.5 mg/kg bw/day for 7 days | Shifts in inter- and intra- phyla abundance of Bacteroidetes and Firmicutes | 3 | Chen et al. (2017a) |
| Ag (55 nm) | Mice | Dietary 0-4600 ppb (0-1140 µg/kg b.w./d) for 28 days | Disturbance in α-diversity) and β-diversity | 5 | Van Den Brûle et al. (2015) |
| Ag (50 nm nanosheres and 45 nm nanocubes) coated with PVP | Rats | Oral administration of 0, 3.6 mg/kg b.w./day for two weeks | Nanocubes caused decreases in Clostridium spp., B. uniformis, Christensenellaceae, and C. eutactus; nanospheres caused decreases in Oscillospira spp., Dehalobacterium spp., Peptococcaeceae, Corynebacterium spp., Aggregatibacter pneumotropica; | 3 | Javurek et al. (2017) |
| Ag NPs (10, 75 and 110 nm) | Rats | Oral gavage of 0, 9, 18 and 36 mg/kg bw/day for 13 weeks | Size- and dose-dependent changes to ileal mucosal microbial populations, as well as an apparent decrease in Firmicutes phyla | 5 | Williams et al. (2015) |
| Ag NPs (20 and 110 nm PVP and citrate-coated) | Mice | Oral gavage of 0, 10 mg/kg bw/day for 28 days | No effect on the membership, structure, or diversity of GM | 3 | Wilding et al. (2016) |
| Ag NPs (<100 nm) | Zebra fish | 0, 500 mg/kg in food for 14 days | Minor changes in community richness and diversity as the controls | 3 | Merrifield et al. (2013) |
| Ag NPs (14 nm, PVP stabilized) | Rats | Oral gavage of 0, 2.25, 4.5 or 9 mg/kg bw/day for 28 days | No effect on the balance between the two main phyla of gastrointestinal tract bacteria, Firmicutes and Bacteriodetes. | 5 | Hadrup et al. (2012) |
| Ag NPs (55 nm) | Zebra fish | Aquatic Ag NPs (0, 10, 33 or 100 μg L−1) for 35 days. | Significantly altered GM compositions in male zebrafish, but not in females |
5 | Ma et al. (2018) |
| Ag NPs | Drosophila melanogaster | Varying aquatic doses from 10 μg/ml to 9000 μg/mL | Reduction in the diversity of the GM of larvae with a rise in the predominance of Lactobacillus brevis and a decrease in Acetobacter compared to control | 5 | Han et al. (2014) |
| Ag NPs | Lepidopteran pest Spodoptera litura | Aquatic 1.0, 0.1, 0.01 and 0.001 g/mL for 21 days | Very significant reductions in Klebsiella pneumoniae, Bacillus licheniformis, Bacillus cereus and Citrobacter freundi, Enterobacter cloacae | 5 | Bharani and Namasivayam (2017). |
| Ag NPs | Earth worm E. fetida | 0, 10, 26, 64, 160 and 400 mg/kg dry soil | significant negative effect on the relative abundance of Firmicutes and Patescibacteria; Significant positive effect on the relative abundance of Bacteroidetes | 5 | Swart et al. (2020b) |
| Ag NMs (from a water filtration system) | Mice | 0.2 mL per day for 45 days by ingestion | Increase in the relative abundance of Bacteroidetes in the faeces of females and decrease in the relative abundance of Firmicutes | 3 | Wu et al. (2020) |
| ZnO NPs (<50 nm) | Mice | 0, 26 mg/kg inter-gastric administration for 30 days | Marked changes in GM composition that was closely associated with neurobehavioral impairments and dysfunctions. | 3 | Chen et al. (2020). |
| ZnO NPs (23.0) nm, | Pig | Dietary 0, 600, 2000 mg/kg for 14 days. | Increases in the bacterial richness and diversity in ileum, with decreased bacterial richness and diversity in cecum and colon; | 5 | Xia et al. (2017) |
| ZnO NPs (30 nm) | Chicken | Dietary 0, 25, 50 and 100 mg/kg for 9 weeks. | Dose-dependent changes in bacterial richness, metabolism of glucose and some amino acids as well as choline, lactate, and methionine | 5 | Feng et al. (2017) |
| ZnO NPs (30 nm | C carpio. | Dietary 0 and 500 mg kg−1 ZnO NPs for 6 weeks | No significant effects on the intestinal microbial community |
2 | Chupani et al. (2019) |
| HAHp (3.0) and ZnO NPs | Mice | Oral administration of 0 and 1.0 g/kg bw for 14 days |
Increases in the abundances of Firmicutes and reduced abundances of Bacteriodetes in female mice; enrichment of probiotic-type bacteria in the feces of female mice | 2 | Song et al. (2018) |
| Zn NPs (90 nm) | Chicken | Dietary 0, 5 mg/kg of feed for 28 days | Increase in Bacteroides and Faecalibacterium; decrease in Lactobacillus | 2 | Yausheva et al. (2018) |
| Zn NPs (30 nm) | Mice | 0, 0.5, 5, 50 mg/kg in feed for 5 days | Amelioration of GM composition responsible for in initiating and maintaining IBD | 3 | Li et al. (2017) |
| Zn/Cu alloy NPs (65 nm) | Chicken | 0, 2.84 mg/kg of feed for 42 days | A slight increase in the bacteria belonging to taxa Bacteroidetes and a decrease in Firmicutes |
2 | Yausheva et al. (2018) |
| SiO2 NPs (11 nm) | Mice | Oral gavage 0, 2.5 mg/kg bw/day for 7 days | Increased microbial species richness and diversity within the intestinal tract | 3 | Chen et al. (2017a). |
| SiO2 NPs (49 nm) | Mice | Oral administration 0, 5.0 mg/kg b.w. one every two days for five weeks | GM dysbiosis that reportedly promoted lung epithelial damage by triggering the Notch pathway | 3 | Ju et al. (2020) |
| Fe NPs (50 nm) | Chicken | 0, 8 mg/kg of feed for 28 days | No significant changes in GM | 2 | Yausheva et al. (2018) |
| Iron(III) oxo-hydroxidenano (10-nm) | Rats | 20 mg Fe/kg diet as Fe(II) sulfate or 20 mg Fe/kg diet as nano Fe(III). | Increase in the proportion of Lactobacillus spp. and a decrease in Bacteroides spp. | 2 | Pereira et al. (2014) |
| CuO NPs | Collembolans | 0, 100 mg Cu/kg dry soil | Reduction in both diversity and abundance of GM as well as gut-associated ARGs | 2 | Chen et al. (2018b). |
| CuO NPs (183 nm) | Earth worms E. fetida | 0, 160 mg/kg dw soil, 28 days | Negative effect on the relative abundance of C. Lumbricincola’ and positive effect on Aeromonas. | 3 | Swart et al. (2020b) |
| CuO NPs (20 and 50 nm) | Earth worm E. fetida | 0, 10, 26, 64, 160 and 400 mg/kg dry soil | The alpha-diversity of treated replicates was different from the controls | 5 | Swart et al. (2020a) |
| CuO NPs (<50 nm) | Enchytraeus crypticus | 0 and 100 mg Cu/ kg soil (dry weight). For 21 days | Marked increases in the alpha-diversity as well as shifts in GM communities, | 2 | Ma et al. (2020) |
| Cu NPs (55 nm) | Chicken | 0, 1.7 mg/kg of feed for 28 days | A decrease in Blautia | 2 | Yausheva et al. (2018) |
| Cu NPs (87nm nm) | Zebra fish | 0, 500 mg/kg in food for 14 days | Suppression of beneficial bacterial strains such as C. somerae | 3 | Merrifield et al. (2013) |
| MoO3 NPs (92 nm) | Zebra fish | Aquatic 0.2 and 0.4 mg/dm3 for 7 days | Changes in intestinal GM diversity | 5 | Aleshina et al. (2020) |
| Se NPs (size not indicated) | Chicken | 0, 0.3, 0.9 and 1.5 mg/kg in feed | Increases in the abundance of beneficial bacteria, such as Lactobacillus and Faecalibacterium | 5 | Gangadoo et al. (2018) |
| GR (0.5 µm x 1.6 nm) GO nanosheet (0.3 µm x 1.76 nm) and rGO (42 nm x 13 nm) | Zebra fish. | 0, 1 µg /day for 21 days, dietary exposure | Increases in the relative abundance of Fusobacteria and the genus Cetobacterium and Lactobacillus, but decreases in Firmicutes and Pseudomonas | 3 | Zheng et al. (2019). |
| Graphene | Rats | Dietary 1, 10 and 100 μg/day | Changes in GM diversity and community structure, with greater effects at 1 μg/day than at 10 μg/day and 100 μg/day, | 5 | Xie et al. (2016) |
| GO (321.74 nm x 0-1.2 nm) | Zebra fish. | Aquatic 0.05, 0.5, and 5 mg L−1 for 25 days | Disruption of GM diversity at both phylum and genus levels, with notable increases in pathogenic bacteria, | 5 | Jia et al. (2019) |
| Nano-polystyrene (nanoplastic, 100 nm) | Marine fish Larimichthys crocea | Aquatic 14-day exposure (0, 5.50 × 10−12, 5.50 × 10−9, 5.50 × 10−7 mg/L) | Significant changes in the proportions of Bacteroidetes, Proteobacteria and Firmicutes, as well as significant increases in potentially pathogenic Parabacteroides and Alistipes | 4 | Gu et al. (2020). |
| Nano-polystyrene (50 – 100 nm) | Soil oligochaete Enchytraeus crypticus | 10% soil (dry weight basis) | Significant decreases in the relative abundance of Rhizobiaceae, Xanthobacteraceae and Isosphaeraceae but an increase in Amoebophilaceae | 2 | Zhu et al. (2018). |
| Nano-sized plastics (44 nm) | Shrimp | Aquatic 0, 50 μg/L for 21 days | Changes in GM, amino acids and fatty acids as well as microbial activities | 3 | Chae et al. (2019) |
| Lead-halide perovskite NPs (889 - 1206 nm) | Zebra fish | 24 hour aquatic exposure to 0, 5, 10, 50, 100 and 200 mg/L and dietary exposure (500 mg/kg) | No significant changes in GM | 5 | Patsiou et al. (2020) |
| CNFs and SDA CNFs | Mice | Oral administration 0.1% (v/v) in tap water for 28 days | Increases in Bacteroidales as well as changes in the metabolism of acyl-carnitines and fatty acids | 3 | Azuma et al. (2015). |
| Cu-loaded chitosan NPs | Rats | Dietary 80 and 160 mg/kg bw administered for 21 days | Increase of caecal Bifidobacteria and Lactobacillus but decrease of total aerobes, total anaerobes, Clostridium, Salmonella and coliforms | 4 | Han et al. (2010) |
| Cu-loaded chitosan NPs | Pigs | 100 mg/kg in feed | A significant decrease in the abundance of E. coli in duodenum, jejunal, and caecum as well as an increase in the abundance of lactobacillus and bifidobacterium | 3 | Wang et al. (2012) |
| Citral-loaded nanostructured systems | Silver catfish (Rhamdia quelen). | Dietary 0.25 g/kg for 21 days | Reduced total bacterial population in the fish intestine, | 3 | Sutili et al. (2019) |
Metal-based NMs have also been reported to cause GM dysbiosis. For example, daily oral administration of 0, 2, 10, 50 mg/kg anatase TiO2 NPs (29 nm) to rats for 30 days could induce GM dysbiosis such as increase in L. gasseri, Turicibacter, and L. NK4A136_group and a decrease in Veillonella (Chen et al., 2019a). An extension of the exposure period from 30 days to 90 days significantly affected the diversity of GM in a dose-dependent manner, by enriching Lactobacillus_reuteri and depleting Romboutsia in feces (Chen et al., 2019b). Similarly, three-month low-dose dietary exposure of 0.1% anatase TiO2 NPs to mice could interfere with the GM balance by significantly decreasing the abundance of several probiotic taxa including Bifidobacterium and Lactobacillus, even though there was no significant effect on the total abundance of GM (Mu et al., 2019). GM dysbiosis caused by TiO2 NPs was also reported by other authors (Li et al., 2018b; Khan et al., 2019; Li et al., 2019b; Zhu et al., 2020), which was in contrast to studies by Pinget et al. (2019), Chen et al. (2019a), Li et al. (2018b), where no obvious GM dysbiosis was observed.
Conflicting studies were also reported on the effects of Ag NPs on GM. For example, exposure to 2.5 mg/kg bw/day for 7 days caused shifts in inter- and intra- phyla abundance of Bacteroidetes and Firmicutes, reduction in the Firmicutes/Bacteroidetes ratio, increases in the lowly abundant families of bacteria, as well as decreases in the probiotic bacteria genus Lactobacillus (Chen et al., 2017a). Similarly, 28-day exposure of 1.18 to 36 mg/kg b.w./d Ag NPs to mice, caused dose-dependent disturbance in bacterial diversity as well as an increase in the ratio between Firmicutes and Bacteroidetes phyla (Van Den Brûle et al., 2015). However, Wilding et al. (2016) could show that a 28-day repeated oral administration of 10 mg/kg bw/day 20 and 110 nm polyvinylpyrrolidone (PVP) and citrate-coated Ag NPs could not affect the membership, structure, or diversity of GM in mice. Similarly, Hadrup et al. (2012) could show that Ag NPs do not affect the balance between the two main phyla of gastrointestinal tract bacteria, Firmicutes and Bacteriodetes. A summary of the effects of GM on model organisms is presented in Table 3.
3.1.1.2. Effects of NMs on the gut microbiota of domestic animals
Several studies have been conducted in chickens. For example, Feng et al. (2017) showed that the diversity of the bacterial community was negatively correlated with increasing amounts of ZnO NPs and was significantly decreased at 100 mg/kg bw. In addition, ZnO NPs changed metabolism of glucose and some amino acids, where choline, lactate, and methionine correlated positively with bacterial richness. Negative effects of GM in chickens were reported for dietary Zn and Cu/Zn alloy NPs Yausheva et al. (2018), intra-amniotic administered ZnO NPs, TiO2 NPs and SiO2 (Kolba et al., 2020). On the other hand, Gangadoo et al. (2018) reported positive effects in chickens for selenium NPs (Se NPs) including an increase in the abundance of beneficial bacteria, such as Lactobacillus and Faecalibacterium as well as changes in SFCAs , especially butyric acid.
Effects of NMs on GM have also been reported in pigs. For example, dietary administration of 150, 300, or 450 mg/kg, and 3000 mg/kg ZnO NPs for 21 days elicited beneficial effects on intestinal GM by reducing counts of E. coli in porcine cecum, colon, and rectum (Pei et al., 2019). Other NMs that caused GM dysbiosis in pigs include Cu-loaded chitosan (Wang et al., 2012).
3.1.1.3. Effects of NMs on the gut microbiota of aquatic organisms
NMs have also been shown to affect the configuration of GM in many aquatic species. For example, a 21-day dietary exposure to graphene materials, including monolayer graphene powder (GR), graphene oxide nanosheet (GO) and reduced graphene oxide powder (rGO), resulted in the increase of the abundance of Fusobacteria and the genus Cetobacterium and Lactobacillus in zebra fish (Danio rerio) (Zheng et al., 2019). Jia et al. (2019) also reported a disruption of GM configuration at both phylum and genus levels, with increases in pathogenic bacteria, caused by chronic exposure (25 days) to 0.05, 0.5, and 5 mg L−1 GO to zebrafish. Gut microbiota dysbiosis has also been reported for nano-polystyrene in Larimichthys crocea (Gu et al., 2020) as well as citral-loaded nanostructured systems comprising of nanoemulsions (NEs) and alginate NPs in silver catfish (Rhamdia quelen) (Sutili et al., 2019). A summary of all in vivo tests including effects on GM on insects and soil micrograms is presented in Table 3.
3.1.2. In vitro (ex vivo) studies
The toxic effects of NMs on GM have also been reported in vitro systems. For example, exposure of 10 nm ZnO, 10 nm CeO2 and 21 nm TiO2 NPs to a colon cell culture model at concentrations of 0.01 µg/L, 0.1 and 3 mg/L respectively was reported to cause non-lethal yet significant changes to the microbial community's phenotypic traits including SCFA production, sugar content of the extracellular polymeric substance, hydrophobicity and electrophoretic mobility (Taylor et al., 2015). Similarly, 25 nm food-grade TiO2 NPs tested in vitro at 100 and 250 mg/L caused minor effects on human GM, that were limited to a modest decrease in the relative abundance of the dominant Bacteroides ovatus in favor of Clostridium cocleatum (Dudefoi et al., 2017). Radziwill-Bienkowska et al. (2018) also noted that food-grade TiO2, which differs from the P25 Organization for Economic Co-operation and Development (OECD) reference TiO2, could induce some physiological alterations in the most sensitive species, and thus affecting GM composition and functioning. Moreover, using a laboratory-scale in vitro model human colon reactor, Waller et al. (2017) observed compositional, phenotypic, and biochemical changes in GM caused by both industrial and food-grade TiO2 NPs, with more pronounced effects elicited by the food-grade TiO2 NPs. More results of in vitro studies on GM are summarized in Table 4.
Table 4.
Summary of effects of NMs on GM from in vitro (ex vivo) tests
| Nanomaterial | Test system | Effects | Evidence score | Reference |
|---|---|---|---|---|
| ZnO NPs (10 nm) | A continuous replicated colon containing 0.01 µg/L of ZnO for 5 days | Nonlethal, significant changes to the microbial community's phenotype | 2 | Taylor et al. (2015) |
| CeO2 (10 nm) | A continuous replicated colon containing 0.01 µg/L of CeO2 for 5 days | Nonlethal, significant changes to the microbial community's phenotype | 2 | Taylor et al. (2015) |
| TiO2 (27 nm, 82% anatase and 18% rutile) | A continuous replicated colon containing 3 mg/L of TiO2 for 5 days | Nonlethal, significant changes to the microbial community's phenotype | 2 | Taylor et al. (2015) |
| TiO2 NPs (25 nm, undefined crystal structure) | A continuous Human Gut Simulator system, 100 mg/day dose for 7 days | Modest reduction in community density with no impact on community diversity and evenness. | 2 | Agans et al. (2019) |
| TiO2 NPs (food-grade, 25 nm) | In vitro static culture at a concentration of 100 and 250 mg/L | A modest decrease in the relative abundance of the dominant Bacteroides ovatus in favor of Clostridium cocleatum | 3 | Dudefoi et al. (2017) |
| TiO2 NPs (food-grade) | In vitro static culture at a concentration of 32, 62.5, 125, 320 mg/mL. | Induction of some physiological alterations in the most sensitive species, and thus affecting GM composition and functioning. | 3 | Radziwill-Bienkowska et al. (2018) |
| TiO2 NPs (Food-grade isolated from chocolates) (40 nm) | 125–250 μg/ml for 48 hours | Inhibited the growth and activity of probiotic formulation of Bacillus coagulans, Enterococcus faecalis, and Enterococcus faecium | 2 | Khan et al. (2019). |
| TiO2 NPs (industrial -252 nm, 75% anatase and 25% rutile-and food-grade- 212 nm, 98% anatase and 2% rutile) | In vitro model human colon reactor, 36 mg/.day | Compositional, phenotypic, and biochemical changes in GM caused by both industrial and food-grade TiO2 NPs, with more pronounced effects elicited by the food-grade TiO2 NPs. | 2 | Waller et al. (2017) |
| Se NPs (unknown size) | In vitro static culture at a concentration of 0.9 mg/kg | Significant reduction in the abundance of pathogenic E. cecorum without significant disturbance to the total GM community | 2 | Gangadoo et al. (2019) |
| Nanostructured lipid carriers (NLC, 211 nm) | In vitro static culture, 0, 1.25, 2.5 and 5% v/v | This selective eradication of H. pylori without affecting the other tested human GM | 3 | Seabra et al. (2018) |
| Ag NPs (11 nm), PVP-capped | Cultured GM from human stool exposed NPs at 0, 25, 100 and 200 mg/L | Reduction in gas production as well as changes in fatty acid methyl ester profiles | 2 | Das et al. (2014) |
| Ag NPs (49 nm), | Static GIS1 simulator, 200 mg /mL | A decrease in Bacteroides, Enterobacteriaceae, and Lactobacillus populations | 2 | Vamanu et al. (2018) |
| Ag NPs (10 nm), citrate capped | In vitro static culture, 25, 50 and 100 μg/mL for 24 hours | Concentration, temperature- and time-dependent inactivation of gastrointestinal phages/virus manner | 3 | Gokulan et al. (2018) |
| Ag NPs (30–50 nm) | Human Gut dynamic Simulator system, 100 mg/day dose for 7 days | Drastic reduction of GM population density. | 2 | Agans et al. (2019) |
| Ag NPs, polyethylene glycol (PEG) and glutathione (GSH) stabilized | Continuous SIMGI® simulator, 88 μg/ml of PEG-AgNPs, 20 and 61 μg/ml of GSH-Ag, for 8 days | No significant changes in the composition and metabolic activity of GM | 3 | Cueva et al. (2019). |
| Ag NPs (14 nm), citrate-capped | In vitro batch fermentation models, inoculated with human fecal matter and 1 μg/mL NPs for 24 hours | No effect on the composition and diversity of fecal microflora and their metabolic profiles | 2 | Cattò et al. (2019) |
| Nanocellulose fibres (4–5 nm) | In vitro static culture, 500, 250, 100 and 50 µg/mL | A bacteriostatic effect of on Escherichia coli and none on Lactobacillus reuter | 2 | Lopes et al. (2020) |
| SWCNTs (1–3μm) and MWCNTs (> 50 μm) | In vitro static culture (20, 50 100 µg/L | Broad-spectrum antimicrobial activity against L. acidophilus, B. adolescentis, E. coli, E. faecalis, and S. aureus; greater antimicrobial activity for thin and rigid SWCNTs than MWCNTs. | 1 | Chen et al. (2013). |
| Graphene oxide | in vitro static culture, 10 to 500 µg/ml for 24 hours | No adverse effect on human intestinal gram-negative E. coli K-12 and gram-positive L. acidophilus ADH, and Bifidobacterium animalis Bif-6 | 3 | Nguyen et al. (2015) |
4. Discussion
This study identified 46 studies conducted in vivo on a number of models such as mice, rats, terrestrial insects, aquatic organism, and soil organisms, as well as 22 in vitro studies including those that mimic the human digestive tract. The type and magnitude of effects of NMs on GM in various organisms generally depended on the type of NM and were likely to be affected by many experimental factors such as test organism or model, dose, route of exposure, type of adminstration (single or repeated), size of NMs, exposure duration, time points for assessment of effects, methods of characterization of physico-chemical properties as well as methods used to assess GM dysbiosis. Comparison of results from different studies was complicated by large variations in these conditions.
This review could show that different NMs elicited different effects in GMs. Furthermore, different forms of the same types of NM caused different effects in some cases. For example Chen et al. (2019a) and Li et al. (2018b) reported GM dysbiosis in mice caused by TiO2 NMs, in contrast to Pinget et al. (2019), Chen et al. (2017a) and Talbot et al. (2018) who reported no obvious GM dysbiosis in murine species. However, the differences in the results reported for TiO2 may be attributed to the variations in forms of TiO2, especially the differences between food-grade and non-food grade TiO2 NPs (EFSA, 2016). It is important to note that toxicity of food-grade TiO2 to GM differs from that of the P25 OECD reference or industrial TiO2 NPs (Waller et al., 2017; Radziwill-Bienkowska et al., 2018). The contradictions in the toxicity of the two forms of TiO2 on GM were also observed in vitro (Waller et al., 2017).
Contrasting results were also reported for Ag NPs both in vivo (Hadrup et al., 2012; Williams et al., 2015; Wilding et al., 2016; Javurek et al., 2017) and in vitro (Hadrup et al., 2012; Das et al., 2014; Vamanu et al., 2018; Agans et al., 2019; Cueva et al., 2019). The reasons for these differences are not clear. The mechanisms of action of Ag NPs against GM, which appear to be the release of Ag+ ions, oxidative stress, blockage of DNA replication as well as the destruction of bacterial cell membranes (Li et al., 2019a), may explain the shape-dependent differences in the effects caused by NCs and NPs as reported by Javurek et al. (2017). Nevertheless, there is need for more studies on the effects of shape of NMs on their toxicity towards GM. More studies are also required on the effects of functional groups since adverse effects of functionalized Ag NPs were reported in one in vivo (Javurek et al., 2017) and two in vitro studies (Das et al., 2014; Gokulan et al., 2018), but not in some in vivo (Hadrup et al., 2012; Wilding et al., 2016) and in vitro (Cattò et al., 2019; Cueva et al., 2019) studies. Although sex-dependent toxic effects on GM are reported for some conventional substances (Ba et al., 2017; Lozano et al., 2018), the sex-dependent effects of Ag NPs on zebrafish GM that were reported by Ma et al. (2018) and Wu et al. (2020 require further investigation. Similarly, as size-dependent effects were observed in one study only (Williams et al., 2015), there is need to assess the effects of size of NMs on toxicity towards GM.
In contrast to size-dependent effects that were observed in a few studies, dose dependency was observed in many studies (Van Den Brûle et al., 2015; Williams et al., 2015; Wilding et al., 2016; Chen et al., 2018a; Chen et al., 2019a; Chen et al., 2019b). Nevertheless, there were numerous studies that only used one dose, in addition to a naïve or negative control. As discussed earlier, these studies would be less effective since proof of a dose response is needed to establish causality (Swaen and van Amelsvoort, 2009). In addition, effects assessed at one-time point may be different from those observed over time.
As shown in Table 3, the majority of in vivo investigations involved NMs being administered orally (through diet or oral gavage), with fewer studies involving environmental exposure via water or soil. It is interesting to observe that environmental exposure to 10, 33 and100 μg L−1 Ag NPs (55 nm) for 35 days significantly altered GM compositions in male zebrafish (Ma et al., 2018), while dietary exposure of the same species to 500 mg/kg Ag NPs (<100 nm) for 14 days only caused minor changes in community richness and diversity (Merrifield et al., 2013). The differences in effects could not only be attributed to differences in the routes of exposure, but also differences in size of NMs and duration of exposure. Unfortunately, a comparison of the effects of route of administration could not be made in the study by Christophersen et al. (2016), which involved both oral and pulmonary exposure of MWCNTs to mice, since the authors only focused on the effects of on GM following oral exposure.
In nanotoxicology, inconsistencies have been reported among both in vitro and in vivo studies because of lack of standardized dispersion protocols and lack of proper characterization of NMs (Sayes et al., 2007b; Sayes et al., 2007a; Cohen et al., 2015). The method used to disperse the NMs affects the extent of agglomeration and surface properties, which would in turn affect their toxicity. Several protocols for preparing NP dispersions prescribe levels of delivered acoustic energy to the solution and sonication times to limit agglomeration (Pradhan et al., 2016). Many authors such as Chen et al. (2018a), Ju et al. (2020), Christophersen et al. (2016), (Zhu et al., 2020) and (Pinget et al., 2019) used various sonication procedures to prepare NMs for the study of GM dysbiosis, while other authors, including Khare et al. (2020), Li et al. (2018a), Li et al. (2018b), and Mu et al. (2019) did not report the use of any sonication procedures. Since variations in dispersion procedures could affect results among similar NMs, use of standard protocols, such as the one proposed by DeLoid et al. (2017), cannot be overemphasized.
Standard protocols are also required for assessing the physico-chemical properties that affect the toxicological properties of NMs, including inter alia size, shape, surface area, charge, chirality, functional groups. Indeed, different techniques for measuring each physico-chemical property have strengths and limitations that complicate the choice of the most suitable method. For the determination of size and morphology of NMs for example, widely used techniques include transmission electron microscopy (TEM), scanning transmission electron microscopy (SEM) and dynamic light scattering (DLS). Various combinations of these techniques were used in the majority of the studies, although a few studies such as those by Chupani et al. (2019), Song et al. (2018) and Yausheva et al. (2018), did not specify the methods for characterizing the pertinent nano-specific physico-chemical properties of NMs like size, shape and surface area. Differences in techniques and methods used for characterization could indeed lead to differences in results among similar NMs.
Differences in results between studies could also be attributed to differences in techniques or methods used to analyze the microbiota, especially differences in the type of samples and sites of collection, whether culture-dependent or culture-independent approaches were used, as well as differences in techniques used to analyze the microbiota (Goodrich et al., 2014; Van Den Brûle et al., 2015). As there are numerous factors involved, it is difficult to ascertain that methodological differences were responsible for differences in effects caused by a particular NM among a number of studies. Overall, the majority of the studies utilized PCR amplification of 16S rRNA for culture-independent characterization of GM configuration, with a very small number of studies using single colonies.
Very high doses were used in some studies where negative effects of NMs on GM were reported. For example, Li et al. (2018b) used a dose of 100 mg TiO2 /kg/day whereas human exposure to TiO2 in the USA has been estimated at 1-2 mg/kg day for children and 0.2 – 0.7 mg/kg day in adults (Weir et al., 2012a). Similarly, although migration from food packaging has been estimated to result to dietary Ag NPs exposure of between 5.89 × 10−5 and 8.9 × 10 −5 mg/kg.day (Cushen et al., 2014), doses as high as 500 mg/kg (equivalent to 7.1 mg/kg.day in humans) were used (Merrifield et al., 2013). On the other hand, Chen et al. (2018b) claims to have exposed zebra fish to an environmentally relevant aquatic concentration of 100 µg/L of TiO2 NPs. For effective and relevant results, studies are required to estimate real-life exposure levels of NMs for humans and other species and to ascertain if these doses are sufficiently large to alter GM in a manner that can result in toxicologically significant outcomes (Utembe and Kamng'ona, 2021). Admittedly, it will be difficult to determine the exposure levels of GM to NMs through other routes other the oral route. For example, in the studies of effects of CNTs on GM, doses ranged from 0.05 to 5 mg/kg bw/d, while only small fractions of inhalable CNTs doses (4.07 μg/day 4.07 in humans and 2 ng/day in mice) are expected to be transported from the lung to the GI tract through mucociliary transport (Erdely et al., 2013).
Table 3 shows large variations in the studies in terms of duration of exposure (and examination of effects) which ranged from a few days to 3 months (90 days). This important aspect makes the comparison GM dysbiosis studies very challenging. A very notable example includes studies conducted on 29 nm anatase TiO2 NMs for 30 days by Chen et al. (2019a) and the same NMs for 90 days by Chen et al. (2019b). While the TiO2 NMs are reported to have elicited dose-dependent changes on GM in both studies, the changes in specific species of GM appear to be different. Therefore, future studies should take dose frequency and duration as well as the period for the examination of the effects into consideration.
For the NMs that affect GM through disruption of the cell wall, the existence or nature of cell wall is expected to influence toxicity. In that regard, Gram-negative bacteria would be expected to have higher tolerance than Gram-positive bacteria because of differences in cell wall structures. While Gram-positive bacteria possess a cytoplasmic membrane and a thick peptidoglycan layer, gram-negative bacteria possess a thick cell wall that consists of two cell membranes, an outer membrane, and a plasma membrane, as well as one thin peptidoglycan layer (Fu et al., 2005). Indeed, Xie et al. (2016) reported greater tolerance of Gram-negative bacteria towards graphene than Gram-positive bacteria. Similarly, Ag NPs cause adverse effects on Gram-negative bacteria at lower concentrations than in Gram-positive bacteria (Fröhlich and Fröhlich, 2016; Zhang et al., 2020). Nevertheless, as ZnO NPs negatively affect both groups of bacteria at similar concentrations (Fröhlich and Fröhlich, 2016), there is need for more studies on factors that contribute to the differential effects of NMs on Gram-positive and Gram-negative bacteria
Some NMs affect GM through the generation of ROS which can directly damage biomolecules, including the cell wall and DNA. The generation of ROS in aerobic environments may not be similar to the generation of ROS at anaerobic conditions. Therefore, the rates and mechanisms of GM disruption at high oxygen levels may not be relevant in anaerobic or anoxic environments that prevail in some parts of the GM tracts (Zhang et al., 2020). However, the majority of in vitro studies have been conducted in aerobic conditions, and the continuous simulator cited in this paper such as those by Cueva et al. (2019), Taylor et al. (2015) and Agans et al. (2019) did not make any reference of the oxygen levels or aerobic/anaerobic conditions of the various compartments.
A very important issue in the study of effects of NMs on GM involves the choice of test systems or models among in vitro and in vivo systems. Although in vitro model systems offer relatively more realistic exposure environments, while at the same time enabling higher control over experimental conditions, it is only animal models that enable studies under realistic host environment, where several bacterial phyla interact and influence each other (Zhang et al., 2020). On the other hand, although animal models seem to be the most reliable, it is important to realize that the GM in animals may be very different from human GM. Among animal models, mice are the most widely used in GM studies because they possess similar structure of gastrointestinal tract to humans (Velmurugan, 2018). In Table 3, mice appear 20 times, compared to rats that appear 11 times. Eight (8) of the studies were performed on zebrafish which is an emerging model in GM studies. Although adult mammalian GM communities are characterized by high abundances of Bacteroidetes and Firmicutes as opposed to a high abundance of Proteobacteria, Fusobacteria, and Firmicutes in zebrafish (Catron et al., 2019), studies of GM dysbiosis in zebra fish can provide useful information for humans since greater than 70% of its genes are homologous to those in humans (Howe et al., 2013). Table 3 also shows the emerging use of chickens as an animal model in GM studies, owing to increasing use of NM formulations for growth promotion (Yausheva et al., 2018). Few studies were performed on pigs, which have been recognized as a superior model compared to other non-primate models because of their physiological, metabolic and nutritional similarities with humans pigs (Heinritz et al., 2013).
Indeed, the choice of test organism appear to be the source of variation among a number of studies. For example, exposure of C carpio to 500 mg kg−1 ZnO NPs (30 nm) through the diet for 6 weeks did not cause significant effects in intestinal microbial community (Chupani et al., 2019). On the other hand, exposure of chickens to a much lower dietary dose of 50 and 100 mg/kg (30 nm) ZnO NPs for 9 weeks caused dose-dependent changes in bacterial richness as well as metabolism of glucose and some amino acids (Feng et al., 2017).
In in vitro or ex vivo studies, many studies assessed the direct effects of NPs on the growth and metabolism of specific monocultures of bacteria isolated from gut, using incubation conditions that mimic in vivo conditions. However, the use of isolated bacteria assesses the effects of NMs on specific species or strains of bacteria. On the contrary, the most suitable in vitro models are those that use human faeces samples, which contain realistic and more representative GM diversity. The monoculture models are still useful for assessing specific effects of NMs or for elucidating mechanisms of NM interaction with microorganisms (Campos et al., 2022).
Most studies have evaluated the adverse effects of single NMs on GM under laboratory-controlled conditions, whereas real-life exposures involve mixtures of NMs. In that regard, TiO2 NPs and BPA elicited antagonistic effects at low BPA concentration and synergistic effects at high BPA concentrations (Chen et al., 2018b). Similarly, synergistic dysbiotic effects were also reported for SiO2 NPs and low dose radiation (Ju et al., 2020). Therefore, there is need to assess the combined effects of various mixtures of NMs as well as mixtures of GM and other substances on GM.
This systematic review used subjective, arbitrary and potentially disputable score criteria to assess the quality of the evidence of toxicity of NMs towards GM. These indicative criteria will hopefully initiate debate and discussions, and serve as a starting point for the development of universally agreed quality assessment criteria and indicators in GM dysbiosis studies. In the end, this study has shown that for consistency and better agreement among studies on GM dysbiosis, there is need for internationally agreed protocols on, inter alia, characterization of NMs, dosing (amounts, frequency and duration), use of sonication, test systems (both in vitro and in vivo), as well as the oxygen levels on in vitro models.
A summary of the quality of evidence by type of NM is presented in Table 5. While the effects of various NMs on GM can lead to many metabolic diseases, the effects offer opportunities of using these NMs as micronutrient preparations that can be used to enhance bacterial diversity and correct dysbiosis (Chen et al., 2017a; Li et al., 2017; Yausheva et al., 2018; Gangadoo et al., 2019).
Table 5.
Summary of quality of evidence by type of nanomaterial
| Nanomaterial | Total number of in vivo studies | Total number of ex vivo studies | General comment on GM dysbiosis | Quality of evidence rating |
|---|---|---|---|---|
| SWCNTs | 1 | 1 | Changes in GM, more studies required | Low |
| MWCNTs | 1 | 1 | No changes in GM, more studies required | Low |
| Fullerenes | 2 | 0 | Changes in GM, more studies required | Moderate |
| Nanocellulose | 1 | 1 | Changes in GM, more studies required | Low |
| TiO2 | ||||
| Anatase | 7 | 0 | Six studies showed dysbiosis, one study showed no obvious dysbiosis | Moderate |
| Rutile | 1 | 0 | More studies required | Low |
| Food-grade | 2 | 3 | Minimal or modest impact on the composition of GM. More studies required | Low |
| Mixed or undefined crystal structure | 4 | 3 | Modest GM dysbiosis | Low |
| Silver | 12 | 6 | Some studies indicate Ag NPs cause GM dysbiosis, while other indicate otherwise. Same results for functionalized NPs | Moderate |
| ZnO | 4 | 1 | Significant changes in GM | Moderate |
| Zn | 2 | 0 | Significant changes in GM | Moderate |
| Zn/Cu alloy | 1 | 0 | Slight changes in GM | Low |
| HAHp (3.0) and ZnO NPs | 1 | 0 | Significant changes in GM | Low |
| SiO2 | 2 | 0 | Changes in GM, more studies required | Low |
| Fe | 1 | 0 | No significant changes in GM | Low |
| Nanoparticulate Iron(III) oxo-hydroxidenano | 1 | 0 | Significant changes in GM | Low |
| CuO | 4 | 0 | Significant changes in GM | Moderate |
| Cu | 2 | 0 | Significant changes in GM | Moderate |
| Se | 1 | 1 | Significant changes in GM | Low |
| GR, GO nanosheet and rGO | 3 | 1 | Some studies indicate GM dysbiosis, others do not | Low |
| Nano-polystyrene | 2 | 0 | Significant changes in GM | Moderate |
| Nanoplastics | 1 | 0 | Changes in GM | Low |
| Lead-halide perovskite | 1 | 0 | No changes in GM | Low |
| CeO2 | 0 | 1 | Changes in GM | Low |
| MoO3 NPs | 1 | 0 | Significant changes in GM diversity | Low |
| CNFs and (SDA) and CNFs | 1 | 0 | Significant changes in GM diversity | Low |
| Cu-loaded chitosan NPs | 2 | 0 | Significant changes in GM | Moderate |
| Citral-loaded nanostructured systems comprising of nanoemulsions (NEs) and alginate NPs | 1 | 0 | Significant changes in GM | Low |
| Citral-loaded nanostructured systems | 1 | 0 | Significant changes in GM | Low |
5. Conclusion
This systematic review has shown the potential of NMs to cause GM dysbiosis in many species as well as in humans. Overall, there was moderate evidence for GM dysbiosis caused by Zn-based NMs, Ag NPs, Cu-based NMs, Cu-loaded chitosan NPs and anatase TiO2 NPs. Effects of various NMs on GM in humans and other animals present opportunities for using in the treatment of GM dysbiosis-related illnesses and conditions.
The effects of NMs on GM varied depending on the NM type and organism. Some NMs, particularly TiO2 and Ag, produced contradictory outcomes both in vivo and in vitro. As a result, more research into the effects of NM size on GM toxicity is required. More research into the effects of form, functional groups, and other physico-chemical properties is also needed. The effects of concurrent exposure to combinations of NMs on GM must also be evaluated. For consistency and better agreement among studies on GM dysbiosis, there is need for internationally agreed protocols on, inter alia, characterization of NMs, dosing (amounts, frequency and duration), use of sonication, test systems (both in vitro and in vivo), as well as the levels of oxygen for in vitro models.
Disclaimer
The article has been prepared in the authors’ own capacity. The opinions, findings and conclusions in this article are therefore the authors’ own and do not necessarily reflect or represent the views of the Kamuzu University of Health Sciences, the National Institute for Occupational Health (NIOH), the national Health Laboratory Service (NHLS) or the University of Johannesburg.
Author contributions
W.U, N.T. and A.W.K. were all involved in literature search, writing and editing of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are very grateful to Dr. N. Sanabria for her kind assistance in proofreading and editing the manuscript.
References
- Agans RT, Gordon A, Hussain S, Paliy O. Titanium dioxide nanoparticles elicit lower direct inhibitory effect on human gut microbiota than silver nanoparticles. Toxicol Sci. 2019;172(2):411–416. doi: 10.1093/toxsci/kfz183. [DOI] [PubMed] [Google Scholar]
- Aleshina E, Miroshnikova E, Sizova E. Transformation of microbiota of fish intestines and gills against the background of molybdenum oxide nanoparticles in environment. Int J Environ Sci Technol. 2020;17(2):721–732. [Google Scholar]
- Auguste M, Lasa A, Pallavicini A, Gualdi S, Vezzulli L, Canesi L. Exposure to TiO2 nanoparticles induces shifts in the microbiota composition of Mytilus galloprovincialis hemolymph. Sci Total Environ. 2019;670:129–137. doi: 10.1016/j.scitotenv.2019.03.133. [DOI] [PubMed] [Google Scholar]
- Azuma K, Izumi R, Kawata M, Nagae T, Osaki T, Murahata Y, Tsuka T, Imagawa T, Ito N, Okamoto Y. Effects of oral administration of chitin nanofiber on plasma metabolites and gut microorganisms. Int J Mol Sci. 2015;16(9):21931–21949. doi: 10.3390/ijms160921931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba Q, Li M, Chen P, Huang C, Duan X, Lu L, Li J, Chu R, Xie D, Song H. Sex-dependent effects of cadmium exposure in early life on gut microbiota and fat accumulation in mice. Environ Health Perspect. 2017;125(3):437–446. doi: 10.1289/EHP360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachler G, Losert S, Umehara Y, von Goetz N, Rodriguez-Lorenzo L, Petri-Fink A, Rothen-Rutishauser B, Hungerbuehler K. Translocation of gold nanoparticles across the lung epithelial tissue barrier: Combining in vitro and in silico methods to substitute in vivo experiments. Particle and fibre toxicology. 2015;12(1):1–18. doi: 10.1186/s12989-015-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharani RANamasivayam SKR. Biogenic silver nanoparticles mediated stress on developmental period and gut physiology of major lepidopteran pest Spodoptera litura (Fab.)(Lepidoptera: Noctuidae)—An eco-friendly approach of insect pest control. J Environ Chem Eng. 2017;5(1):453–467. [Google Scholar]
- Bouwmeester H, van der Zande M, Jepson MA, et al. Effects of food‐borne nanomaterials on gastrointestinal tissues and microbiota. Wiley Interdiscip Rev Nanomed. 2018;10(1):e1481. doi: 10.1002/wnan.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumbudsanpharoke N, Choi J, Ko S. Applications of nanomaterials in food packaging. J Nanosci Nanotechnol. 2015;15(9):6357–6372. doi: 10.1166/jnn.2015.10847. [DOI] [PubMed] [Google Scholar]
- Buzea C, Blandino P, II, Robbie K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases. 2007;2(4):MR17–MR172. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- Campos D, Goméz-García R, Oliveira D, Madureira AR. Intake of nanoparticles and impact on gut microbiota: in vitro and animal models available for testing. Gut Microbiome. 2022;3:1–16. doi: 10.1017/gmb.2021.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203–209. [PMC free article] [PubMed] [Google Scholar]
- Catron TR, Gaballah S, Tal T. Using zebrafish to investigate interactions between xenobiotics and microbiota. Current Pharmacology Reports. 2019;5(6):468–480. [Google Scholar]
- Cattò C, Garuglieri E, Borruso L, Erba D, Casiraghi MC, Cappitelli F, Villa F, Zecchin S, Zanchi R. Impacts of dietary silver nanoparticles and probiotic administration on the microbiota of an in-vitro gut model. Environ Pollut. 2019;245:754–763. doi: 10.1016/j.envpol.2018.11.019. [DOI] [PubMed] [Google Scholar]
- Chae Y, Kim D, Choi M-J, Cho Y, An Y-J. Impact of nano-sized plastic on the nutritional value and gut microbiota of whiteleg shrimp Litopenaeus vannamei via dietary exposure. Environ Int. 2019;130 doi: 10.1016/j.envint.2019.05.042. [DOI] [PubMed] [Google Scholar]
- Chen H, Wang B, Gao D, Guan M, Zheng L, Ouyang H, Chai Z, Zhao Y, Feng W. Broad-spectrum antibacterial activity of carbon nanotubes to human gut bacteria. Small. 2013;9(16):2735–2746. doi: 10.1002/smll.201202792. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhao R, Wang B, Cai C, Zheng L, Wang H, Wang M, Ouyang H, Zhou X, Chai Z. The effects of orally administered Ag, TiO2 and SiO2 nanoparticles on gut microbiota composition and colitis induction in mice. NanoImpact. 2017;8:80–88. [Google Scholar]
- Chen H, Zhao R, Wang B, Zheng L, Ouyang H, Wang H, Zhou X, Zhang D, Chai Z, Zhao Y. Acute Oral Administration of Single-Walled Carbon Nanotubes Increases Intestinal Permeability and Inflammatory Responses: Association with the Changes in Gut Microbiota in Mice. Adv Healthc Mater. 2018;7(13) doi: 10.1002/adhm.201701313. [DOI] [PubMed] [Google Scholar]
- Chen j, Zhang S, Chen C, Jiang X, Qiu J, Qiu Y, Zhang Y, Wang T, Qin X, Zou Z, Chen C. Crosstalk of gut microbiota and serum/hippocampus metabolites in neurobehavioral impairments induced by oral zinc oxide nanoparticles exposure. Nanoscale. 2020;12(41):21429–21439. doi: 10.1039/d0nr04563b. [DOI] [PubMed] [Google Scholar]
- Chen L, Guo Y, Hu C, Lam PK, Lam JC, Zhou B. Dysbiosis of gut microbiota by chronic coexposure to titanium dioxide nanoparticles and bisphenol A: Implications for host health in zebrafish. Environ pollut. 2018;234:307–317. doi: 10.1016/j.envpol.2017.11.074. [DOI] [PubMed] [Google Scholar]
- Chen Q-j, Zheng X-m, Zhou L-l, Dong X-f, Wang J-h. Study on the nano-starch and its application in food industry. Food & Machinery. 2017;7:47–60. [Google Scholar]
- Chen Z, Han S, Zhou D, Zhou S, Jia G. Effects of oral exposure to titanium dioxide nanoparticles on gut microbiota and gut-associated metabolism in vivo. Nanoscale. 2019;11(46):22398–22412. doi: 10.1039/c9nr07580a. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhou D, Han S, Zhou S, Jia G. Hepatotoxicity and the role of the gut-liver axis in rats after oral administration of titanium dioxide nanoparticles. Part Fibre Toxicol. 2019;16(1):1–17. doi: 10.1186/s12989-019-0332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Frontiers in cellular neuroscience. 2015;9:1–22. doi: 10.3389/fncel.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophersen DV, Jacobsen NR, Andersen MH, Connell SP, Barfod KK, Thomsen MB, Miller MR, Duffin R, Lykkesfeldt J, Vogel U. Cardiovascular health effects of oral and pulmonary exposure to multi-walled carbon nanotubes in ApoE-deficient mice. Toxicology. 2016;371:29–40. doi: 10.1016/j.tox.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Chupani L, Barta J, Zuskova E. Effects of food-borne ZnO nanoparticles on intestinal microbiota of common carp (Cyprinus carpio L.) Environ Sci Pollut Res. 2019;26(25):25869–25873. doi: 10.1007/s11356-019-05616-x. [DOI] [PubMed] [Google Scholar]
- Cohen JM, DeLoid GM, Demokritou P. A critical review of in vitro dosimetry for engineered nanomaterials. Nanomedicine. 2015;10(19):3015–3032. doi: 10.2217/nnm.15.129. [DOI] [PubMed] [Google Scholar]
- Cueva C, Gil-Sánchez I, Tamargo A, Miralles B, Crespo J, Bartolomé B, Moreno-Arribas MV. Gastrointestinal digestion of food-use silver nanoparticles in the dynamic SIMulator of the GastroIntestinal tract (simgi®). Impact on human gut microbiota. Food Chem Toxicol. 2019;132 doi: 10.1016/j.fct.2019.110657. [DOI] [PubMed] [Google Scholar]
- Cushen M, Kerry J, Morris M, Cruz-Romero M, Cummins E. Evaluation and Simulation of Silver and Copper Nanoparticle Migration from Polyethylene Nanocomposites to Food and an Associated Exposure Assessment. Journal of Agricultural and Food Chemistry. 2014;62(6):1403–1411. doi: 10.1021/jf404038y. [DOI] [PubMed] [Google Scholar]
- Das P, McDonald JA, Petrof EO, Allen-Vercoe E, Walker VK. Nanosilver-mediated change in human intestinal microbiota. J Nanomed Nanotechnol. 2014;5(5):1. [Google Scholar]
- Debia M, Bakhiyi B, Ostiguy C, Verbeek JH, Brouwer DH, Murashov V. A systematic review of reported exposure to engineered nanomaterials. Ann Occup Hyg. 2016;60(8):916–935. doi: 10.1093/annhyg/mew041. [DOI] [PubMed] [Google Scholar]
- Dekkers S, Krystek P, Peters RJ, Lankveld DP, Bokkers BG, van Hoeven-Arentzen PH, Bouwmeester H, Oomen AG. Presence and risks of nanosilica in food products. Nanotoxicology. 2011;5(3):393–405. doi: 10.3109/17435390.2010.519836. [DOI] [PubMed] [Google Scholar]
- DeLoid GM, Cohen JM, Pyrgiotakis G, Demokritou P. Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nature protocols. 2017;12(2):355–371. doi: 10.1038/nprot.2016.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudefoi W, Moniz K, Allen-Vercoe E, Ropers M-H, Walker VK. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem Toxicol. 2017;106:242–249. doi: 10.1016/j.fct.2017.05.050. [DOI] [PubMed] [Google Scholar]
- Đurašević S, Nikolić G, Todorović A, Drakulić D, Pejić S, Martinović V, Mitić-Ćulafić D, Milić D, Kop TJ, Jasnić N. Effects of fullerene C60 supplementation on gut microbiota and glucose and lipid homeostasis in rats. Food Chem Toxicol. 2020 doi: 10.1016/j.fct.2020.111302. [DOI] [PubMed] [Google Scholar]
- EFSA Re-evaluation of titanium dioxide (E 171) as a food additive. EFSA J. 2016;14(9):e04545. [Google Scholar]
- Erdely A, Dahm M, Chen BT, Zeidler-Erdely PC, Fernback JE, Birch ME, Evans DE, Kashon ML, Deddens JA, Hulderman T, Bilgesu SA, Battelli L, Schwegler-Berry D, Leonard HD, McKinney W, Frazer DG, Antonini JM, Porter DW, Castranova V, Schubauer-Berigan MK. Carbon nanotube dosimetry: from workplace exposure assessment to inhalation toxicology. Part Fibre Toxicol. 2013;10(1):1–14. doi: 10.1186/1743-8977-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Min L, Zhang W, Liu J, Hou Z, Chu M, Li L, Shen W, Zhao Y, Zhang H. Zinc oxide nanoparticles influence microflora in ileal digesta and correlate well with blood metabolites. Front Microbiol. 2017;8:992–1002. doi: 10.3389/fmicb.2017.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich EEFröhlich E. Cytotoxicity of nanoparticles contained in food on intestinal cells and the gut microbiota. Int J Mol Sci. 2016;17(4):509. doi: 10.3390/ijms17040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu G, Vary PS, Lin C-T. Anatase TiO2 nanocomposites for antimicrobial coatings. J Phys Chem B. 2005;109(18):8889–8898. doi: 10.1021/jp0502196. [DOI] [PubMed] [Google Scholar]
- Gangadoo S, Bauer BW, Bajagai YS, Van TTH, Moore RJ, Stanley D. In vitro growth of gut microbiota with selenium nanoparticles. Anim Nutr. 2019;5(4):424–431. doi: 10.1016/j.aninu.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadoo S, Dinev I, Chapman J, Hughes RJ, Van TTH, Moore RJ, Stanley D. Selenium nanoparticles in poultry feed modify gut microbiota and increase abundance of Faecalibacterium prausnitzii. Appl Microbiol Biotechnol. 2018;102(3):1455–1466. doi: 10.1007/s00253-017-8688-4. [DOI] [PubMed] [Google Scholar]
- Garrett WS. The gut microbiota and colon cancer. Science. 2019;364(6446):1133–1135. doi: 10.1126/science.aaw2367. [DOI] [PubMed] [Google Scholar]
- Geuking MB, Köller Y, Rupp S, McCoy KD. The interplay between the gut microbiota and the immune system. Gut microbes. 2014;5(3):411–418. doi: 10.4161/gmic.29330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokulan K, Bekele AZ, Drake KL, Khare S. Responses of intestinal virome to silver nanoparticles: safety assessment by classical virology, whole-genome sequencing and bioinformatics approaches. Int J Nanomedicine. 2018;13:2857–2867. doi: 10.2147/IJN.S161379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich Julia K, Di Rienzi Sara C, Poole Angela C, Koren O, Walters William A, Caporaso JG, Knight R, Ley Ruth E. Conducting a Microbiome Study. Cell. 2014;158(2):250–262. doi: 10.1016/j.cell.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Wang S, Wang X, Yu X, Hu M, Huang W, Wang Y. Nanoplastics impair the intestinal health of the juvenile large yellow croaker Larimichthys crocea. J Hazard Mater. 2020;397:1–8. doi: 10.1016/j.jhazmat.2020.122773. [DOI] [PubMed] [Google Scholar]
- Hadrup N, Loeschner K, Bergström A, Wilcks A, Gao X, Vogel U, Frandsen HL, Larsen EH, Lam HR, Mortensen A. Subacute oral toxicity investigation of nanoparticulate and ionic silver in rats. Arch Toxicol. 2012;86(4):543–551. doi: 10.1007/s00204-011-0759-1. [DOI] [PubMed] [Google Scholar]
- Han X, Geller B, Moniz K, Das P, Chippindale AK, Walker VK. Monitoring the developmental impact of copper and silver nanoparticle exposure in Drosophila and their microbiomes. Science of the total environment. 2014;487:822–829. doi: 10.1016/j.scitotenv.2013.12.129. [DOI] [PubMed] [Google Scholar]
- Han XY, Du WL, Fan CL, Xu ZR. Changes in composition a metabolism of caecal microbiota in rats fed diets supplemented with copper-loaded chitosan nanoparticles. J Anim Physiol Anim Nutr. 2010;94(5):e138–e144. doi: 10.1111/j.1439-0396.2010.00995.x. [DOI] [PubMed] [Google Scholar]
- Heinritz SN, Mosenthin R, Weiss E. Use of pigs as a potential model for research into dietary modulation of the human gut microbiota. Nutrition research reviews. 2013;26(2):191–209. doi: 10.1017/S0954422413000152. [DOI] [PubMed] [Google Scholar]
- Hoffmann S, de Vries RB, Stephens ML, Beck NB, Dirven HA, Fowle JR, Goodman JE, Hartung T, Kimber I, Lalu MM. A primer on systematic reviews in toxicology. Archives of toxicology. 2017;91(7):2551–2575. doi: 10.1007/s00204-017-1980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn LA. Statistical evaluation of toxicological bioassays–a review. Toxicology Research. 2014;3(6):418–432. [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javurek AB, Suresh D, Spollen WG, Hart ML, Hansen SA, Ellersieck MR, Bivens NJ, Givan SA, Upendran A, Kannan R. Gut dysbiosis and neurobehavioral alterations in rats exposed to silver nanoparticles. Sci Rep. 2017;7(1):1–15. doi: 10.1038/s41598-017-02880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia P-P, Sun T, Junaid M, Xiong Y-H, Wang Y-Q, Liu L, Pu S-Y, Pei D-S. Chronic exposure to graphene oxide (GO) induced inflammation and differentially disturbed the intestinal microbiota in zebrafish. Environ Sci Nano. 2019;6(8):2452–2469. [Google Scholar]
- Johnson CCOwnby DR. The infant gut bacterial microbiota and risk of pediatric asthma and allergic diseases. Translational Research. 2017;179:60–70. doi: 10.1016/j.trsl.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Z, Ren G, Zhou M, Jing J, Xiang J, Liu X, Huang R, Zhou P-K. Exposure to a combination of silica nanoparticles and low-dose radiation aggravates lung fibrosis in mice via gut microbiota modulation. Environ Sci Nano. 2020;7(12):3979–3998. [Google Scholar]
- Khan ST, Saleem S, Ahamed M, Ahmad J. Survival of probiotic bacteria in the presence of food grade nanoparticles from chocolates: an in vitro and in vivo study. Appl Microbiol Biotechnol. 2019;103(16):6689–6700. doi: 10.1007/s00253-019-09918-5. [DOI] [PubMed] [Google Scholar]
- Khare S, DeLoid GM, Molina RM, Gokulan K, Couvillion SP, Bloodsworth KJ, Eder EK, Wong AR, Hoyt DW, Bramer LM, Metz TO, Thrall BD, Brain JD, Demokritou P. Effects of ingested nanocellulose on intestinal microbiota and homeostasis in Wistar Han rats. NanoImpact. 2020;18 doi: 10.1016/j.impact.2020.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolba N, Guo Z, Olivas FM, Mahler GJ, Tako E. Intra-amniotic administration (Gallus gallus) of TiO2, SiO2, and ZnO nanoparticles affect brush border membrane functionality and alters gut microflora populations. Food Chem Toxicol. 2020;135:1–38. doi: 10.1016/j.fct.2019.110896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas B, Martins Breyner N, Houdeau E. Impacts of foodborne inorganic nanoparticles on the gut microbiota-immune axis: potential consequences for host health. Part Fibre Toxicol. 2020;17:1–22. doi: 10.1186/s12989-020-00349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim P, Yoon J, Lee B, Choi K, Kil K-H, Park K. Serum kinetics, distribution and excretion of silver in rabbits following 28 days after a single intravenous injection of silver nanoparticles. Nanotoxicology. 2012;7(6):1120–1130. doi: 10.3109/17435390.2012.710660. [DOI] [PubMed] [Google Scholar]
- Leist M, Efremova L, Karreman C. Food for thought... considerations and guidelines for basic test method descriptions in toxicology. Alternatives to animal experimentation. ALTEX. 2010;27(4):309–317. doi: 10.14573/altex.2010.4.309. [DOI] [PubMed] [Google Scholar]
- Li J, Tang M, Xue Y. Review of the effects of silver nanoparticle exposure on gut bacteria. J Appl Toxicol. 2019;39(1):27–37. doi: 10.1002/jat.3729. [DOI] [PubMed] [Google Scholar]
- Li J, Chen H, Wang B, Cai C, Yang X, Chai Z, Feng W. ZnO nanoparticles act as supportive therapy in DSS-induced ulcerative colitis in mice by maintaining gut homeostasis and activating Nrf2 signaling. Sci Rep. 2017;7(1):1–11. doi: 10.1038/srep43126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lei R, Li X, Xiong F, Zhang Q, Zhou Y, Yang S, Chang Y, Chen K, Gu W. The antihyperlipidemic effects of fullerenol nanoparticles via adjusting the gut microbiota in vivo. Part Fibre Toxicol. 2018;15(1):1–11. doi: 10.1186/s12989-018-0241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yang S, Lei R, Gu W, Qin Y, Ma S, Chen K, Chang Y, Bai X, Xia S. Oral administration of rutile and anatase TiO 2 nanoparticles shifts mouse gut microbiota structure. Nanoscale. 2018;10(16):7736–7745. doi: 10.1039/c8nr00386f. [DOI] [PubMed] [Google Scholar]
- Li M, Li F, Lu Z, Fang Y, Qu J, Mao T, Wang H, Chen J, Li B. Effects of TiO2 nanoparticles on intestinal microbial composition of silkworm, Bombyx mori. Sci Total Environ. 2020;704 doi: 10.1016/j.scitotenv.2019.135273. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Y, Li B, Cui J, Gao N, Sun H, Meng Q, Wu S, Bo J, Yan L, Wu J, Chen R. Prebiotic protects against anatase titanium dioxide nanoparticles-induced microbiota-mediated colonic barrier defects. NanoImpact. 2019;14 [Google Scholar]
- Lipsitch M, Tchetgen ET, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology (Cambridge, Mass) 2010;21(3):383–388. doi: 10.1097/EDE.0b013e3181d61eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes VR, Strømme M, Ferraz N. In Vitro Biological Impact of Nanocellulose Fibers on Human Gut Bacteria and Gastrointestinal Cells. Nanomaterials. 2020;10(6):1159–1174. doi: 10.3390/nano10061159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano VL, Defarge N, Rocque L-M, Mesnage R, Hennequin D, Cassier R, de Vendômois JS, Panoff J-M, Séralini G-E, Amiel C. Sex-dependent impact of Roundup on the rat gut microbiome. Toxicology Reports. 2018;5:96–107. doi: 10.1016/j.toxrep.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Chen Q-L, O'Connor P, Sheng GD. Does soil CuO nanoparticles pollution alter the gut microbiota and resistome of Enchytraeus crypticus? Environ Pollut. 2020;256 doi: 10.1016/j.envpol.2019.113463. [DOI] [PubMed] [Google Scholar]
- Ma Y, Song L, Lei Y, Jia P, Lu C, Wu J, Xi C, Strauss PR, Pei D-S. Sex dependent effects of silver nanoparticles on the zebrafish gut microbiota. Environ Sci Nano. 2018;5(3):740–751. [Google Scholar]
- MacFabe DF. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microbial ecology in health and disease. 2012;23(1):19260. doi: 10.3402/mehd.v23i0.19260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield DL, Shaw BJ, Harper GM, Saoud IP, Davies SJ, Handy RD, Henry TB. Ingestion of metal-nanoparticle contaminated food disrupts endogenous microbiota in zebrafish (Danio rerio) Environ pollut. 2013;174:157–163. doi: 10.1016/j.envpol.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Moreno-Indias I, Cardona F, Tinahones FJ, Queipo-Ortuño MI. Impact of the gut microbiota on the development of obesity and type 2 diabetes mellitus. Front Microbiol. 2014;5:190–200. doi: 10.3389/fmicb.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu W, Wang Y, Huang C, Fu Y, Li J, Wang H, Jia X, Ba Q. Effect of long-term intake of dietary titanium dioxide nanoparticles on intestine inflammation in mice. J Agric Food Chemy. 2019;67(33):9382–9389. doi: 10.1021/acs.jafc.9b02391. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoet PM, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts M, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105(4):411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Lin M, Mustapha A. Toxicity of graphene oxide on intestinal bacteria and Caco-2 cells. J Food Prot. 2015;78(5):996–1002. doi: 10.4315/0362-028X.JFP-14-463. [DOI] [PubMed] [Google Scholar]
- Patsiou D, del Rio-Cubilledo C, Catarino AI, Summers S, Fahmi AM, Boyle D, Fernandes TF, Henry TB. Exposure to Pb-halide perovskite nanoparticles can deliver bioavailable Pb but does not alter endogenous gut microbiota in zebrafish. Sci Total Environ. 2020;715:1–12. doi: 10.1016/j.scitotenv.2020.136941. [DOI] [PubMed] [Google Scholar]
- Pearce SC, Coia HG, Karl JP, Pantoja-Feliciano IG, Zachos NC, Racicot K. Intestinal in vitro and ex vivo models to study host-microbiome interactions and acute stressors. Front Physiol. 2018;9:1584. doi: 10.3389/fphys.2018.01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei X, Xiao Z, Liu L, Wang G, Tao W, Wang M, Zou J, Leng D. Effects of dietary zinc oxide nanoparticles supplementation on growth performance, zinc status, intestinal morphology, microflora population, and immune response in weaned pigs. J Sci Food Agric. 2019;99(3):1366–1374. doi: 10.1002/jsfa.9312. [DOI] [PubMed] [Google Scholar]
- Pereira DIA, Bruggraber SFA, Faria N, Poots LK, Tagmount MA, Aslam MF, Frazer DM, Vulpe CD, Anderson GJ, Powell JJ. Nanoparticulate iron(III) oxo-hydroxide delivers safe iron that is well absorbed and utilised in humans. Nanomed-Nanotechnol. 2014;10(8):1877–1886. doi: 10.1016/j.nano.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietroiusti A, Bergamaschi E, Campagna M, Campagnolo L, De Palma G, Iavicoli S, Leso V, Magrini A, Miragoli M, Pedata P. The unrecognized occupational relevance of the interaction between engineered nanomaterials and the gastro-intestinal tract: a consensus paper from a multidisciplinary working group. Particle and fibre toxicology. 2017;14(1):1–23. doi: 10.1186/s12989-017-0226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinget G, Tan J, Janac B, Kaakoush NO, Angelatos AS, O'Sullivan J, Koay YC, Sierro F, Davis J, Divakarla SK. Impact of the food additive titanium dioxide (E171) on gut microbiota-host interaction. Front Nutr. 2019;6:1–13. doi: 10.3389/fnut.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan S, Hedberg J, Blomberg E, Wold S, Odnevall Wallinder I. Effect of sonication on particle dispersion, administered dose and metal release of non-functionalized, non-inert metal nanoparticles. Journal of Nanoparticle Research. 2016;18(9):285. doi: 10.1007/s11051-016-3597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radusin TI, Ristić IS, Pilić BM, Novaković AR. Antimicrobial nanomaterials for food packaging applications. Food Res. 2016;43(2):119–126. [Google Scholar]
- Radziwill-Bienkowska JM, Talbot P, Kamphuis JB, Robert V, Cartier C, Fourquaux I, Lentzen E, Audinot J-N, Jamme F, Réfrégiers M. Toxicity of food-grade TiO2 to commensal intestinal and transient food-borne bacteria: new insights using nano-SIMS and synchrotron UV fluorescence imaging. Front Microbiol. 2018;9:1–14. doi: 10.3389/fmicb.2018.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompelberg C, Heringa MB, van Donkersgoed G, Drijvers J, Roos A, Westenbrink S, Peters R, van Bemmel G, Brand W, Oomen AG. Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population. Nanotoxicology. 2016;10(10):1404–1414. doi: 10.1080/17435390.2016.1222457. [DOI] [PubMed] [Google Scholar]
- Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. European Journal of Nutrition. 2018;57(1):1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RSRAE . The Royal Society & The Royal Academy of Engineering; London: 2004. Nanoscience and nanotechnologies: Opportunities and uncertainties. [Google Scholar]
- Sayes CM, Reed KL, Warheit DB. Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicological Sciences. 2007;97(1):163–180. doi: 10.1093/toxsci/kfm018. [DOI] [PubMed] [Google Scholar]
- Sayes CM, Marchione AA, Reed KL, Warheit DB. Comparative Pulmonary Toxicity Assessments of C60 Water Suspensions in Rats: Few Differences in Fullerene Toxicity in Vivo in Contrast to in Vitro Profiles. Nano Letters. 2007;7(8):2399–2406. doi: 10.1021/nl0710710. [DOI] [PubMed] [Google Scholar]
- Seabra CL, Nunes C, Brás M, Gomez-Lazaro M, Reis CA, Gonçalves IC, Reis S, Martins MCL. Lipid nanoparticles to counteract gastric infection without affecting gut microbiota. Eur J Pharm Biopharm. 2018;127:378–386. doi: 10.1016/j.ejpb.2018.02.030. [DOI] [PubMed] [Google Scholar]
- Song R, Yao J, Shi Q, Wei R. Nanocomposite of half-fin anchovy hydrolysates/zinc oxide nanoparticles exhibits actual non-toxicity and regulates intestinal microbiota, short-chain fatty acids production and oxidative status in mice. Marine drugs. 2018;16(1):23. doi: 10.3390/md16010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutili FJ, Kreutz LC, Flores FC, CdB da Silva, Kirsten KS, APdS Voloski, Frandoloso R, Pinheiro CG, Heinzmann BM, Baldisserotto B. Effect of dietary supplementation with citral-loaded nanostructured systems on innate immune responses and gut microbiota of silver catfish (Rhamdia quelen) J Funct Foods. 2019;60 [Google Scholar]
- Swaen Gvan Amelsvoort L. A weight of evidence approach to causal inference. Journal of clinical epidemiology. 2009;62(3):270–277. doi: 10.1016/j.jclinepi.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Swart E, Goodall T, Kille P, Spurgeon DJ, Svendsen C. The earthworm microbiome is resilient to exposure to biocidal metal nanoparticles. Environ Pollut. 2020;267:1–14. doi: 10.1016/j.envpol.2020.115633. [DOI] [PubMed] [Google Scholar]
- Swart E, Dvorak J, Hernádi S, Goodall T, Kille P, Spurgeon D, Svendsen C, Prochazkova P. The effects of in vivo exposure to copper oxide nanoparticles on the gut microbiome, host immunity, and susceptibility to a bacterial infection in earthworms. Nanomaterials. 2020;10(1337):1–21. doi: 10.3390/nano10071337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P, Radziwill-Bienkowska JM, Kamphuis JB, Steenkeste K, Bettini S, Robert V, Noordine M-L, Mayeur C, Gaultier E, Langella P. Food-grade TiO 2 is trapped by intestinal mucus in vitro but does not impair mucin O-glycosylation and short-chain fatty acid synthesis in vivo: implications for gut barrier protection. J Nanobiotechnology. 2018;16(1):1–14. doi: 10.1186/s12951-018-0379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AA, Marcus IM, Guysi RL, Walker SL. Metal oxide nanoparticles induce minimal phenotypic changes in a model colon gut microbiota. Environ Eng Sci. 2015;32(7):602–612. [Google Scholar]
- Utembe WKamng'ona AW. Gut microbiota-mediated pesticide toxicity in humans: methodological issues and challenges in risk assessment of pesticides. Chemosphere. 2021 doi: 10.1016/j.chemosphere.2021.129817. [DOI] [PubMed] [Google Scholar]
- Vamanu E, Ene M, Biță B, Ionescu C, Crăciun L, Sârbu I. In vitro human microbiota response to exposure to silver nanoparticles biosynthesized with mushroom extract. Nutrients. 2018;10(607):1–12. doi: 10.3390/nu10050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Brûle S, Ambroise J, Lecloux H, Levard C, Soulas R, De Temmerman P-J, Palmai-Pallag M, Marbaix E, Lison D. Dietary silver nanoparticles can disturb the gut microbiota in mice. Part Fibre Toxicol. 2015;13(1):1–16. doi: 10.1186/s12989-016-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velmurugan G. Gut microbiota in toxicological risk assessment of drugs and chemicals: The need of hour. Gut microbes. 2018;9(5):465–468. doi: 10.1080/19490976.2018.1445955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller T, Chen C, Walker SL. Food and industrial grade titanium dioxide impacts gut microbiota. Environ Eng Sci. 2017;34(8):537–550. [Google Scholar]
- Wang M-Q, Du Y-J, Wang C, Tao W-J, He Y-D, Li H. Effects of copper-loaded chitosan nanoparticles on intestinal microflora and morphology in weaned piglets. Biol Trace Elem Res. 2012;149(2):184–189. doi: 10.1007/s12011-012-9410-0. [DOI] [PubMed] [Google Scholar]
- Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environmental science & technology. 2012;46(4):2242–2250. doi: 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir A, Westerhoff P, Fabricius L, Hristovski K, Von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol. 2012;46(4):2242–2250. doi: 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding LA, Bassis CM, Walacavage K, Hashway S, Leroueil PR, Morishita M, Maynard AD, Philbert MA, Bergin IL. Repeated dose (28-day) administration of silver nanoparticles of varied size and coating does not significantly alter the indigenous murine gut microbiome. Nanotoxicology. 2016;10(5):513–520. doi: 10.3109/17435390.2015.1078854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Milner J, Boudreau MD, Gokulan K, Cerniglia CE, Khare S. Effects of subchronic exposure of silver nanoparticles on intestinal microbiota and gut-associated immune responses in the ileum of Sprague-Dawley rats. Nanotoxicology. 2015;9(3):279–289. doi: 10.3109/17435390.2014.921346. [DOI] [PubMed] [Google Scholar]
- Wu J, Li C, Zhang J, Menzies NW, Bertsch PM, Wang P, Kopittke PM. Release of silver from nanoparticle-based filter paper and the impacts to mouse gut microbiota. Environ Sci Nano. 2020;7(5):1554–1565. [Google Scholar]
- Xia T, Lai W, Han M, Han M, Ma X, Zhang L. Dietary ZnO nanoparticles alters intestinal microbiota and inflammation response in weaned piglets. Oncotarget. 2017;8(39):64878–64891. doi: 10.18632/oncotarget.17612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Wu B, Zhang X-X, Yin J, Mao L, Hu M. Influences of graphene on microbial community and antibiotic resistance genes in mouse gut as determined by high-throughput sequencing. Chemosphere. 2016;144:1306–1312. doi: 10.1016/j.chemosphere.2015.09.076. [DOI] [PubMed] [Google Scholar]
- Yausheva Е, Miroshnikov S, Sizova Е. Intestinal microbiome of broiler chickens after use of nanoparticles and metal salts. Environ Sci Pollut Res. 2018;25(18):18109–18120. doi: 10.1007/s11356-018-1991-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mortimer M, Guo L-H. Interplay between engineered nanomaterials and microbiota. Environ Sci Nano. 2020;7(9):2454–2485. [Google Scholar]
- Zheng M, Lu J, Lin G, Su H, Sun J, Luan T. Dysbiosis of gut microbiota by dietary exposure of three graphene-family materials in zebrafish (Danio rerio) Environ Pollut. 2019;254:1–10. doi: 10.1016/j.envpol.2019.112969. [DOI] [PubMed] [Google Scholar]
- Zhu B-K, Fang Y-M, Zhu D, Christie P, Ke X, Zhu Y-G. Exposure to nanoplastics disturbs the gut microbiome in the soil oligochaete Enchytraeus crypticus. Environ Pollut. 2018;239:408–415. doi: 10.1016/j.envpol.2018.04.017. [DOI] [PubMed] [Google Scholar]
- Zhu X, Han Y, Du J, Liu R, Jin K, Yi W. Microbiota-gut-brain axis and the central nervous system. Oncotarget. 2017;8(32):53829–53838. doi: 10.18632/oncotarget.17754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhao L, Liu Z, Zhou Q, Zhu Y, Zhao Y, Yang X. Long-term exposure to titanium dioxide nanoparticles promotes diet-induced obesity through exacerbating intestinal mucus layer damage and microbiota dysbiosis. Nano Res. 2020:1–11. [Google Scholar]