Highlights
-
•
Gut microbiota are known to be associated with various metabolic syndromes.
-
•
Diversity of gut microbiota decreases in PLWH with prediabetes.
-
•
Streptococcus and Anaerostignum are more abundant in the prediabetes group.
-
•
Further study of alteration in gut microbiota on glucose metabolism is warranted.
Keywords: Gut microbiota, HIV, People living with HIV, Plasma glucose, Prediabetes
Abstract
The prevalence of prediabetes is rapidly increasing in general population and in people living with HIV (PLWH). Gut microbiota play an important role in human health, and dysbiosis is associated with metabolic disorders and HIV infection. Here, we aimed to evaluate the association between gut microbiota and prediabetes in PLWH. A cross-sectional study enrolled 40 PLWH who were receiving antiretroviral therapy and had an undetectable plasma viral load. Twenty participants had prediabetes, and 20 were normoglycemic. Fecal samples were collected from all participants. The gut microbiome profiles were analyzed using 16S rRNA sequencing. Alpha-diversity was significantly lower in PLWH with prediabetes than in those with normoglycemia (p<0.05). A significant difference in beta-diversity was observed between PLWH with prediabetes and PLWH with normoglycemia (p<0.05). Relative abundances of two genera in Firmicutes (Streptococcus and Anaerostignum) were significantly higher in the prediabetes group. In contrast, relative abundances of 13 genera (e.g., Akkermansia spp., Christensenellaceae R7 group) were significantly higher in the normoglycemic group. In conclusion, the diversity of gut microbiota composition decreased in PLWH with prediabetes. The abundances of 15 bacterial taxa in the genus level differed between PLWH with prediabetes and those with normoglycemia. Further studies on the effect of these taxa on glucose metabolism are warranted.
Graphical Abstract
1. Introduction
Type 2 diabetes mellitus (T2DM) prevalence is increasing and has led to higher rates of diabetes-related morbidity and mortality in adults worldwide. In Thailand, T2DM prevalence was 8.3% in 2020, increasing from 7.5% in 2009 (Aekplakorn et al., 2011). Prediabetes, a state of abnormal glucose homeostasis with blood glucose levels not yet reaching the diabetes diagnosis criteria, is associated with diabetes complications, including early nephropathy, sensory neuropathy, retinopathy, and cardiovascular diseases (Brunner et al., 2006; Nathan et al., 2007; Plantinga et al., 2010; Sumner et al., 2003; Xu et al., 2009). Prediabetes prevalence is also rapidly increasing worldwide, and up to 10% of people with prediabetes progress to T2DM yearly (Tabák et al., 2012).
Because of the increased access to antiretroviral therapies (ART) for people living with HIV (PLWH), a significant reduction in acquired immunodeficiency syndrome (AIDS)-associated morbidity and extension of the predicted lifespan have been observed (Palella et al., 1998). Nonetheless, the non-AIDS events have become an increasing burden. ART, HIV itself, and the aging process increase the risk of noncommunicable diseases, including insulin resistance, hypertension, metabolic disorders, and cardiovascular diseases (Aekplakorn et al., 2011; Chantrathamachart et al., 2006; Prioreschi et al., 2017). Compared to the general population, prediabetes and T2DM in PLWH are more prevalent (Brown et al., 2005; Phuphuakrat et al., 2020; Srivanich et al., 2010).
Gut microbiota, as intestinal microorganisms (bacteria, archaea, viruses, and eukaryotic microbes), play an essential role in human health by influencing cellular metabolism, immune regulation, and the inflammatory process (Feng et al., 2018; Valdes et al., 2018). Gut dysbiosis or adverse change in microbiome contributes to insulin resistance, T2DM, obesity, inflammatory bowel diseases, autoimmunity, and carcinogenesis (Sommer et al., 2017). The composition of the gut microbiota differs between people with and without T2DM (Sedighi et al., 2017). Changes in the composition of the gut microbiota play a role in glucose metabolism by alterations in systemic lipopolysaccharide concentrations, bile acid metabolism, short-chain fatty acid production, gut hormone secretion, and circulating branched-chain amino acids (Utzschneider et al., 2016). Likewise, the composition of the gut microbiota and metabolites is altered in PLWH with T2DM as compared to those without T2DM (Moon et al., 2018). Moreover, dietary intervention and physical exercise as well as HIV infection can change the composition of gut microbiota and their metabolism (Chen et al., 2018; Zhao et al., 2018).
A few studies (Hoel et al., 2018; Moon et al., 2018) have investigated the association between gut microbiota and T2DM in PLWH. The data on gut microbiota in PLWH with prediabetes are markedly limited, and the differences of gut microbiota in PLWH with normoglycemia and those with prediabetes are not well understood. The purpose of this study was to evaluate the association between gut microbiota and the prediabetes status in PLWH.
2. Materials and methods
2.1. Study participants and design
This cross-sectional study was conducted at an infectious disease clinic in Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. We included PLWH from a previous cross-sectional study for the prevalence of prediabetes among PLWH (Phuphuakrat et al., 2020), aged 20 years or older, and willing to participate in the study. PLWH using glucose-lowering medications, having a history of diabetes, and those who were pregnant were excluded. Patients were screened for prediabetes and consecutively enrolled with a goal of 20 participants with prediabetes (prediabetes group) and 20 participants with normoglycemia (normoglycemia group). All participants had received ART for more than 6 months, had HIV viral loads less than 50 copies/mL, and had CD4 counts more than 200 cells/mm3. Their medical histories were retrieved. Anthropometric parameters were measured by physicians, and all clinical samples, including feces and blood, were collected under standard techniques. The protocol was approved by the Institutional Review Board, Faculty of Medicine, Ramathibodi Hospital, Mahidol University (COA. MURA2020/1203). Written informed consent was obtained from each participant. All methods were performed in accordance with the relevant guidelines and regulations.
2.2. Measurement and laboratory determinations
2.2.1. Prediabetes
According to the American Diabetes Association (ADA) Standards of Medical Care in Diabetes-2019, prediabetes was defined as fasting plasma glucose (FPG) levels of 100-125 mg/dL or 2-h plasma glucose (2h PG) levels 140-199 mg/dL during a 75-g oral glucose tolerance test (OGTT) or hemoglobin A1c (HbA1c) 5.7- <6.5% (American Diabetes Association, 2019). All participants had fasted at least 12 h before the test. At baseline, blood samples were collected for FPG and HbA1c. After a 75-g glucose solution was taken, blood samples were collected at 120 min for 2h PG.
2.2.2. Fecal sample collection
Fecal samples were self-collected by participants using a clean disposable spatula and a plastic container over the toilet seat. Participants washed their hands and cleaned the perianal area before sample collection. Fecal samples were immediately refrigerated at -80°C.
2.2.3. RNA extraction and high-throughput sequencing
Genome DNA was extracted with the QIAamp® Fast DNA Stool Mini Kit (QIAGEN, Hilden, Germany) following the manufacturers’ instructions. Polymerase chain reaction (PCR) amplification was performed on the 16S rRNA gene using 341F (TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG) and 805R (GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC) primers, targeting the V3-V4 variable regions and sparQ HiFi PCR master mix (Quanta bio, Beverly, MA, USA). The amplification condition consisted of an initial denaturation step at 94°C for 3 min, followed by 25 cycles of 98°C for 20 s, 55°C for 30 s, 72°C for 30 s, and a single final extension step at 72°C for 5 min. Additionally, an internal transcribed spacer of nuclear ribosomal DNA amplification was performed using the primers ITS-1F and ITS-2R (Gardes and Bruns, 1993) with PCR conditions as follows: an initial denaturation step at 94°C for 3 min, followed by 25 cycles of 98°C for 20 s, 60°C for 30 s, 72°C for 30 s, and a single final extension step at 72°C for 5 min. Subsequently, both metagenomic marker amplicons were purified using AMPure XP beads and indexed using 5 µl of each Nextera XT index primer (Illumina, San Diego, CA, USA) in a 50-µl PCR reaction, followed by 8-10 cycles of PCR conditions, as above. The final PCR products were cleaned, pooled, and diluted to the final loading concentration at 6 pM. Cluster generation and 250-bp paired-end read sequencing were performed on an Illumina MiSeq (Illumina).
2.2.4. Bioinformatics analysis
Microbiome bioinformatics was performed with QIIME 2 2019.10 (Bolyen and Rideout, 2019). Raw sequence data were demultiplexed and quality filtered using the q2-demux plugin followed by denoising with DADA2 (via q2-dada2) (Callahan et al., 2016). A phylogeny tree was constructed using the SEPP q2-plugin, placing short sequences into a sepp-refs-gg-13-8.qza phylogenetic reference tree for the 16S marker gene (Janssen et al., 2018). Alpha-diversity metrics [Shannon diversity, Faith's Phylogenetic Diversity (Faith, 1992), and observed operational taxonomic units (OTUs)], beta-diversity metrics (weighted UniFrac, unweighted UniFrac, Jaccard distance, and Bray-Curtis dissimilarity) (Lozupone and Knight, 2005; Lozupone et al., 2007), and Principle Coordinate Analysis (PCoA) were performed using q2-diversity after samples were rarefied (subsampled without replacement) to the minimum number of sequences. Taxonomy was assigned to ASVs using the q2-feature-classifier (Bokulich et al., 2018) to classify sklearn naïve Bayes taxonomy classifier against the SILVA database (Quast et al., 2013).
2.3. Statistical methods
Baseline demographics of the participants are presented as mean ± standard deviation (SD) or median [interquartile range (IQR)] for continuous variables and as frequency (%) for binary or categorical variables. Chi-square tests were used to analyze categorical variables. Student's t-test was used to compare means, and the Mann–Whitney U test was used to compare medians between the prediabetes and normoglycemic groups, depending on the data distribution. The alpha-diversity of microbiota in OTU levels between the prediabetes and normoglycemic groups was determined using four measures: Shannon diversity, Faith's phylogenetic diversity, observed OTUs richness, and Evenness and presented in box-and-whisker plots. The dissimilarity of microbial community compositions (beta-diversity) between participants in the prediabetes and normoglycemic groups were estimated using weighted UniFrac, unweighted UniFrac, Jaccard distance, and Bray-Curtis dissimilarity. Alpha and beta diversity were analyzed using Kruskal-Wallis and PerMANNOVA (number of permutations=999), respectively. Significantly differential taxa abundances between condition groups were tested using linear discriminant analysis (LDA) effect size (LEfSe). Statistical significance was considered as p-value <0.05, and all reported probability tests were two-sided. Statistical analysis was conducted using SPSS statistical software package, version 18.0 (SPSS, Chicago, IL, USA).
3. Results
Of the 40 PLWH, 20 participants were in the prediabetes group and 20 participants were in the normoglycemia group. The mean age was 51.3 ± 6.0 years, and 65% of the participants were male. The duration of HIV infection and ART was 14.8 ± 5.7 and 11.7 ± 5.5 years, respectively. As expected, FPG, 2h PG, and HbA1c levels were significantly higher in the prediabetes group than in the normoglycemia group. Demographic, anthropometric, HIV-related, and other biochemical parameters did not significantly differ between the two groups (Table 1).
Table 1.
Characteristics of participants with normoglycemia and prediabetes
| Characteristics | Normoglycemia (N=20) | Prediabetes (N=20) | P-value |
|---|---|---|---|
| Demographic and anthropometric parameters | |||
| Age (years) | 51.8±6.6 | 50.9±5.6 | 0.625 |
| Male, N (%) | 13 (65.0) | 13 (65.0) | >0.999 |
| History of smoking, N (%) | 10 (50.0) | 7 (35.0) | 0.337 |
| History of alcohol drinking, N (%) | 16 (80.0) | 12 (60.0) | 0.168 |
| Underlying diseases, N (%) | |||
| Hypertension | 4 (20.0) | 3 (15.0) | 0.677 |
| Dyslipidemia | 9 (45.0) | 7 (35.0) | 0.519 |
| NAFLD | 0 (0.0) | 1 (5.0) | 0.311 |
| Body weight (kg) | 62.7±12.8 | 63.7±12.4 | 0.814 |
| Body mass index (kg/m2) | 23.1±4.6 | 24.1±4.0 | 0.501 |
| SBP (mmHg) | 130.5±15.5 | 129.5±14.5 | 0.842 |
| DBP (mmHg) | 81.3±8.1 | 82.1±10.6 | 0.790 |
| Waist circumference (cm) | 84.8±10.8 | 85.4±10.7 | 0.878 |
| Waist hip circumference ratio | 0.91±0.07 | 0.91±0.06 | 0.825 |
| HIV-related parameters | |||
| Duration of HIV infection (years) | 15.2±6.1 | 14.4±5.4 | 0.682 |
| Type of ART regimen, N (%) | |||
| NNRTI-based | 13 (65.0) | 13 (65.0) | >0.999 |
| PI-based | 7 (35.0) | 7 (35.0) | >0.999 |
| Duration of ART (years) | 11.5±6.1 | 11.9±5.1 | 0.834 |
| CD4 cell counts (cells/mm3) | 559.8±264.0 | 475.0±204.5 | 0.263 |
| Biochemical parameters | |||
| FPG (mg/dL) | 91.8 ±7.9 | 101.4±13.6 | 0.009 |
| 2h PG (mg/dL) | 113.5±37.0 | 138.7±37.3 | 0.041 |
| HbA1c (%) | 5.32±0.21 | 5.99±0.22 | <0.001 |
| Triglycerides (mg/dL) | 130.5 (81.8 – 185.3) | 134.0 (99.5 – 198.5) | 0.379 |
| HDL cholesterol (mg/dL) | 51.2±11.6 | 46.2±11.4 | 0.181 |
| LDL cholesterol (mg/dL) | 141.3±36.0 | 130.0±33.4 | 0.308 |
| Total cholesterol (mg/dL) | 219.0±42.0 | 203.4±36.3 | 0.215 |
Data were presented as mean±SD or median (interquartile range).
2h PG = 2-h plasma glucose; ART = antiretroviral therapy; DBP = diastolic blood pressure; FPG = fasting plasma glucose; HbA1c = hemoglobin A1c; HDL = high-density lipoprotein; LDL= low-density lipoprotein; NAFLD = non-alcoholic fatty liver disease; NNRTI = non-nucleoside reverse transcriptase inhibitor; PI = protease inhibitor; SBP = systolic blood pressure
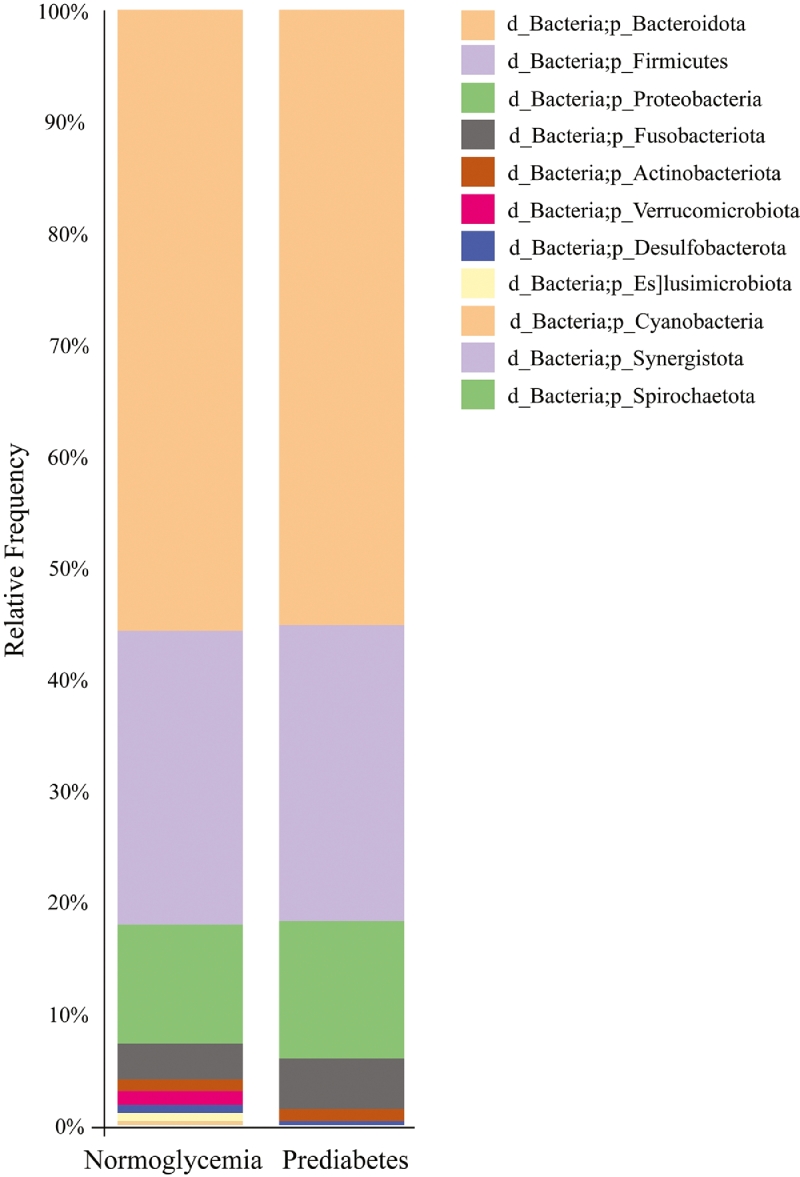
Mean relative abundance of fecal samples showed that Bacteroidota was the most abundant at the phylum level in both groups. This was followed by Firmicutes, Proteobacteria, and Fusobacteriota in both groups. However, Elusimicrobiota, Synergistota, and Spirochaetota were not found in the prediabetes group (Fig. 1 and Supplementary Table 1).
Fig. 1.
Taxonomic profile at the phylum level between participants with prediabetes and normoglycemia
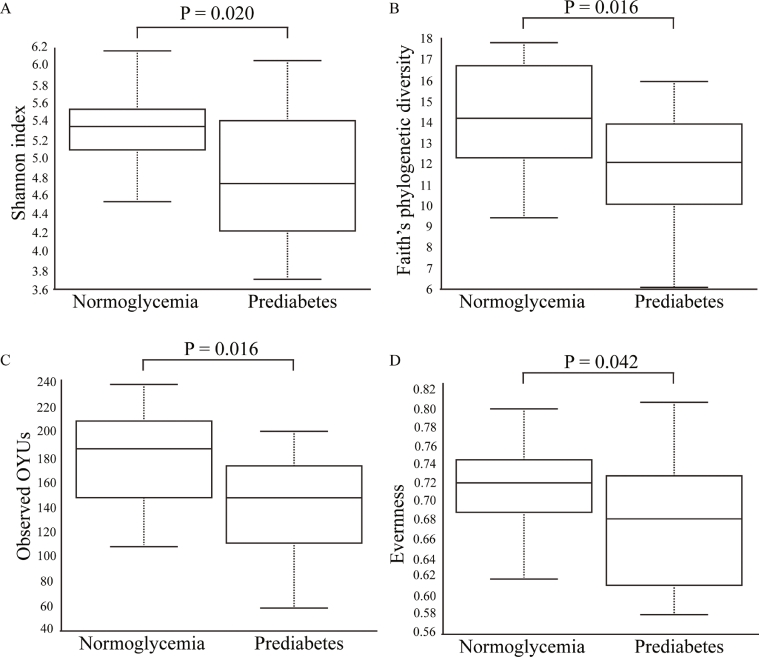
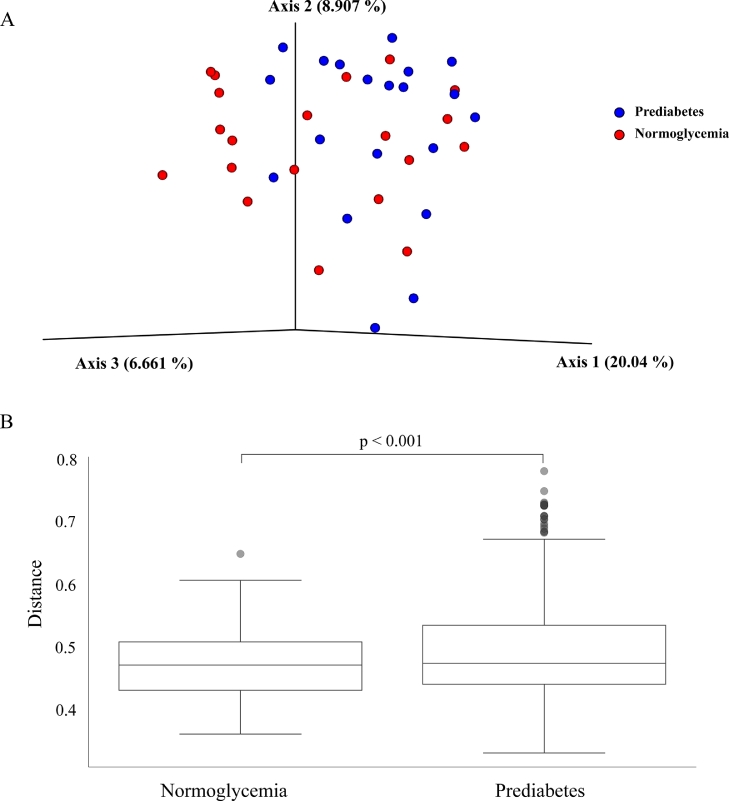
Alpha-diversity (within-sample microbial diversity for each measurement) was significantly lower in the prediabetes group than in the normoglycemia group: Shannon index (p=0.020), Faith's phylogenetic diversity (p=0.016), observed OTUs richness (p=0.016), and Evenness (p=0.042) (Fig. 2). Beta-diversity (similarity or dissimilarity between the two groups) in the fecal microbiome was evaluated by unweighted UniFrac at OTU levels. Principal coordinates analysis illustrated the clustering of fecal samples between gut microbiomes of the prediabetes group and the normoglycemia group (Fig. 3a). PerMANOVA showed a significant difference between samples obtained from the prediabetic group and the normoglycemia group by unweighted UniFrac (p=0.001) (Fig. 3b), but no significant difference between the two groups by Bray-Curtis (p=0.315), Jaccard (p=0.065), and weighted UniFrac (p=0.584) (Supplementary Fig. 1).
Fig. 2.
Alpha diversity of microbial composition in participants with normoglycemia and prediabetes measured by (a) Shannon index, (b) Faith's phylogenetic diversity, (c) observed OTUs, and (d) Evenness Box-and-whisker plots represented median and IQR.
Figure 3.
Beta-diversity of microbial composition in participants with normoglycemia and prediabetes by (a) principal coordinates analysis (PCoA) of unweighted UniFrac and (b) perMANOVA-observed differences of unweighted UniFrac.
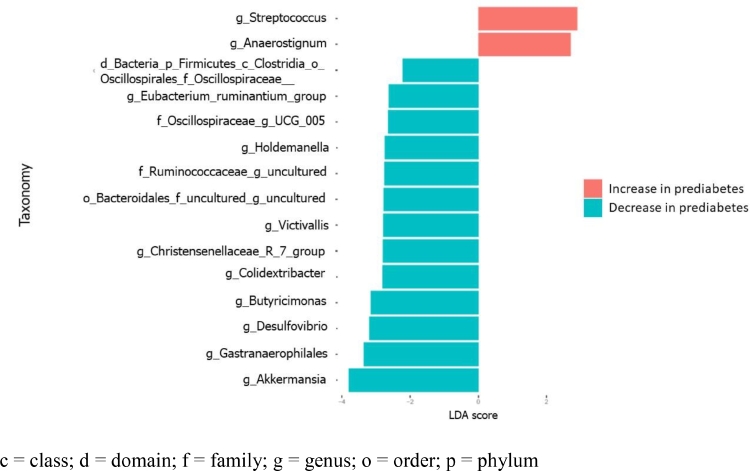
Differential analysis in taxa between fecal microbiome between the prediabetes and normoglycemia groups was conducted by LDA effect size (LEfSe) (Fig. 4). At the genus level, the differential abundance analysis demonstrated 15 genera were associated with prediabetes in the PLWH (Supplementary Table 2). Two genera in Firmicutes (Streptococcus and Anaerostignum) became significantly more abundant in the prediabetes group than in the normoglycemia group. Interestingly, Firmicutes, together with Bacteroidota, Cyanobacteria, Desulfobacerota, and Verrucomicrobiota were significantly more abundant in the normoglycemia group than in the prediabetes group. Akkermansia, Gastranaerophilales, Desulfovibrio, Butyricimonas, Colidextribacter, Christensenellaceae R 7 group, Victivallis, Uncultured Bacteroidota, Uncultured phylum Firmicutes, Holdemanella, UCG-005, Eubacterium ruminantium group, and family Oscillospiraceae-associated group were more abundant in the normoglycemia group.
Fig. 4.
Differentially abundant bacterial taxa of participants with prediabetes and normoglycemia illustrated by linear discriminant analysis (LDA) effect size (LEfSe) plot c = class; d = domain; f = family; g = genus; o = order; p = phylum
4. Discussion
Gut microbiota has been known to be associated with various metabolic syndromes, especially T2DM (Wu et al., 2020). Nonetheless, this association has not been well established in prediabetes, particularly among PLWH. This cross-sectional study evaluated the association between gut microbiota and prediabetes in PLWH. We found that both the diversity and composition of microbiomes between PLWH with prediabetes and those with normoglycemia were significantly dissimilar. Compared to PLWH with normoglycemia, those with prediabetes had less genus and diversity of gut microbiomes. Additionally, the percentages of abundance were higher in two particular genera and lower in 13 other genera among PLWH with prediabetes.
We have demonstrated that alpha diversity and beta diversity were significantly different between PLWH with prediabetes and those with normoglycemia. The alpha diversity was significantly lower in PLWH with prediabetes. In the general population, the diversity of gut microbiota composition was changed in individuals with hyperglycemia (Larsen et al., 2010). Previous works reported that the alpha diversity of gut microbiota was lower in patients with T2DM and prediabetes (Lambeth et al., 2015; Li et al., 2020). However, alpha diversity was not significantly different in patients with T2DM and without diabetes in a study conducted in Mexican Americans (Kitten et al., 2021). Another study showed a decreased alpha diversity in patients with newly diagnosed diabetes, but not in those with prediabetes, when compared with those without diabetes (Gaike et al., 2020). The diversity of gut microbiota composition could be affected by multiple factors (Lozupone et al., 2012), including the types of diet and health status (Senghor et al., 2018). Regarding the diversity of microbiota composition in PLWH, a previous study in women with or at high risk for HIV infection showed no significant differences in the diversity of microbial communities between those with and without diabetes; nonetheless, relative abundances of genus Finegoldia, Anaerococcus, Sneathia, and Adlercreutzia were decreased in those with diabetes (Moon et al., 2018).
Our study revealed that Akkermansia spp. was significantly reduced in PLWH with prediabetes. This finding is consistent with several previous studies showing that Akkermansia spp. could mainly contribute to reducing the risk of diabetes and other metabolic syndromes (Ouyang et al., 2020b; Xu et al., 2020; Zhou et al., 2021). A purified membrane protein of Akkermansia muciniphila has been shown to reduce the expression of hepatic flavin monooxygenase 3 (Fmo3) (Plovier et al., 2017). A knockout of this gene prevented the development of hyperglycemia in the mouse model (Miao et al., 2015). Regarding glucose metabolism, Akkermansia muciniphila increases thermogenesis by induction of uncoupling protein 1 in brown adipose tissue and regulates appetite by stimulating L-cells (enteroendocrine cells) to release glucagon-like peptide-1 (GLP-1). However, the data of bioactive molecules involving GLP-1 secretion are lacking (Derrien et al., 2017; Yoon et al., 2021). Akkermansia muciniphila also played a role in maintaining a healthy gut barrier and reducing inflammation in mice (Schneeberger et al., 2015). The increased gut permeability increased gram-negative bacteria-derived lipopolysaccharide leakage into the systemic circulation with subsequent inflammation and metabolic dysfunction, including insulin resistance (Utzschneider et al., 2016). This may emphasize that Akkermansia spp. can be a potential probiotic for T2DM. Metformin was also shown to increase the abundance of Akkermansia muciniphila in PLWH (Isnard et al., 2020; Ouyang et al., 2020a), thus metformin might be a potential treatment for modifying the progression to diabetes in PLWH.
In addition to Akkermansia, our findings revealed the significantly reduced abundance of Christensenellaceae in PLWH with prediabetes compared to those with normoglycemia. It has been assumed that the appropriate abundance of Christensenellaceae can improve metabolic syndrome. The reduction in Christensenellaceae abundance was observed in prediabetes individuals (He et al., 2018), while the normal abundance of Christensenellaceae was associated with healthy glucose metabolism (Lippert et al., 2017). Furthermore, Christensenellaceae was enriched following healthy lifestyle behavior, including regular consumption of fruits and vegetables (Bowyer et al., 2018; Klimenko et al., 2018). This change could also be observed when feeding rodents with dietary fiber (Ferrario et al., 2017). Interestingly, Christensenellaceae significantly increased in normal body mass index (BMI) (18.5-24.9 kg/m2) individuals as compared to people with obesity (BMI >30 kg/m2) (Goodrich et al., 2014; Waters and Ley, 2019). A clinical trial to improve metabolic syndrome using Christensenellaceae has been conducted (clinical trial.gov: NCT04663139). However, our findings did not show a difference in BMI and other body component analysis between the prediabetes and normoglycemia groups. It remains unclear how Christensenellaceae is involved in the hyperglycemic status. One possible mechanism is that Christensenellaceae can produce short-chain fatty acids, which are known to reduce the risk of diabetes by various mechanisms, for example, increased insulin sensitivity and suppression of appetite (Lau and Vaziri, 2019; Waters and Ley, 2019; Zhou et al., 2021). Additionally, inflammation has been considered one of the causes of both type 1 and type 2 diabetes (Tsalamandris et al., 2019). An in vitro study showed that the supernatant obtained from Christensenellaceae culture can maintain the integrity of intestinal epithelia and suppress inflammatory response (Kropp et al., 2021). Taken together, this suggests that a decreased abundance of Christensenellaceae may lead to a hyperglycemic/prediabetic status.
Our study in PLWH revealed a decrease in the abundance of Akkermansia and an increase in the abundance of Streptococcus in those with prediabetes. A recent systematic review on bacteria involved in T2DM reported that the genera of Ruminococcus, Fusobacterium, and Blautia were positively associated with T2DM, while the genera of Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia, and Roseburia were negatively associated with T2DM (M et al., 2020). Another case-control study showed a decreased abundance of the genus Clostridium, but an increased abundance of Dorea (Ruminococcus), Suterella, and Streptococcus in prediabetics as compared to age- and sex-matched normoglycemic persons (Allin et al., 2018). A damage of gut epithelial barrier in PLWH is a potential factor of microbial translocation and inflammation in PLWH (Ellis et al., 2021). This might contribute to the different abundance of bacterial taxa between PLWH and the general population.
To the best of our knowledge, the present study is the first to reveal the association between gut dysbiosis and prediabetes in PLWH. None of the participants were receiving diabetic treatment that could have affected the results. The study was conducted under a well-designed protocol and standard technique. Nevertheless, our study has some limitations that should be considered when interpreting these results: (1) the sample size is relatively modest; (2) since the study design is a cross-sectional study, the causal relationship between gut microbiota and prediabetes in PLWH cannot be evaluated directly; (3) as the participants are only PLWH who received ART with an undetectable plasma viral load, we might not be able to apply the results to the ART-naïve PLWH or those without successful ART; and (4) some potential factors such as route of HIV infection, sex preferences, other sexually transmitted infections, history of antibiotics and antacid usage, and dietary intakes can affect gut microbiota change; nonetheless, we did not include these factors as covariates in our study data.
In conclusion, our study demonstrated the association between gut microbiota and prediabetes in PLWH receiving ART with an undetectable plasma viral load. Diversity of gut microbiota composition decreased in PLWH with prediabetes. The abundances of Akkermansia spp. and Christensenellaceae R 7 group were also decreased in PLWH with prediabetes. We also found that PLWH with prediabetes had increased abundances of two genera in Firmicutes (Streptococcus and Anaerostignum). Further studies on the mechanism that contributed to the development of dysglycemia by these two genera in PLWH are warranted.
Funding
This work was supported by grants from the Faculty of Medicine Ramathibodi Hospital, Mahidol University, and the Thailand Research Fund (grant number RTA6080009).
CRediT authorship contribution statement
KJ, AP, SR, SS conceived and designed the study. SS obtained funding. KJ, AP, PPo, PPr analyzed and interpreted the data. HN, SS contributed to data collection. KJ, AP, PPo drafted the manuscript. HN, SR, SS revised the manuscript. All authors critically revised the manuscript, agreed to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript before submission.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful to all patients for participating in this study and Worramin Suksuwan for the laboratory assistant. We thank Mr Brian Daniels for his help in English grammar review and editing.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.crmicr.2022.100143.
Appendix. Supplementary materials
References
- Aekplakorn W., Chariyalertsak S., Kessomboon P., Sangthong R., Inthawong R., Putwatana P., Taneepanichskul, Thai National Health Examination Survey IV Study Group, 2011 Prevalence and management of diabetes and metabolic risk factors in Thai adults: the Thai national health examination survey IV. Diabetes Care. 2009;34:1980–1985. doi: 10.2337/dc11-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allin K.H., Tremaroli V., Caesar R., Jensen B.A.H., Damgaard M.T.F., Bahl M.I., Licht T.R., Hansen T.H., Nielsen T., Dantoft T.M., et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia. 2018;61:810–820. doi: 10.1007/S00125-018-4550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Classification and diagnosis of diabetes: standards of medical care in diabetesd 2019. Diabetes Care. 2019;42:S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- Bokulich N.A., Kaehler B.D., Rideout J.R., Dillon M., Bolyen E., Knight R., Huttley G.A., Gregory Caporaso J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2′s q2-feature-classifier plugin. Microbiome. 2018;6:90. doi: 10.1186/s40168-018-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer R.C.E., Jackson M.A., Pallister T., Skinner J., Spector T.D., Welch A.A., Steves C.J. Use of dietary indices to control for diet in human gut microbiota studies. Microbiome. 2018;6:77. doi: 10.1186/s40168-018-0455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T.T., Cole S.R., Li X., Kingsley L.A., Palella F.J., Riddler S.A., Visscher B.R., Margolick J.B., Dobs A.S. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- Brunner E.J., Shipley M.J., Witte D.R., Fuller J.H., Marmot M.G. Relation between blood glucose and coronary mortality over 33 years in the Whitehall study. Diabetes Care. 2006;29:26–31. doi: 10.2337/diacare.29.01.06.dc05-1405. [DOI] [PubMed] [Google Scholar]
- Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantrathamachart P., Sungkanuparph S., Kietiburanakul S., Malathum K. Diabetes mellitus and hypertension in HIV-infected patients receiving antiretroviral therapy: a pilot study. J Infect Dis Antimicrob Agents. 2006;22:131–138. [Google Scholar]
- Chen J., Guo Y., Gui Y., Xu D. Physical exercise, gut, gut microbiota, and atherosclerotic cardiovascular diseases. Lipids Health Dis. 2018;17:17. doi: 10.1186/s12944-017-0653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien M., Belzer C., de Vos W.M. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog. 2017;106:171–181. doi: 10.1016/j.micpath.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Ellis R.J., Iudicello J.E., Heaton R.K., Isnard S., Lin J., Routy J.P., Gianella S., Hoenigl M., Knight R. Markers of gut barrier function and microbial translocation associate with lower gut microbial diversity in people with HIV. Viruses. 2021;13 doi: 10.3390/v13101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith D.P. Conservation evaluation and phylogenetic diversity. Biol Conserv. 1992;61:1–10. doi: 10.1016/0006-3207(92)91201-3. [DOI] [Google Scholar]
- Feng Q., Chen W.D., Wang Y.D. Gut microbiota: an integral moderator in health and disease. Front. Microbiol. 2018;9:151. doi: 10.3389/fmicb.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C., Statello R., Carnevali L., Mancabelli L., Milani C., Mangifesta M., Duranti S., Lugli G.A., Jimenez B., Lodge S., et al. How to feed the mammalian gut microbiota: bacterial and metabolic modulation by dietary fibers. Front Microbiol. 2017;8:1749. doi: 10.3389/fmicb.2017.01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaike A.H., Paul D., Bhute S., Dhotre D.P., Pande P., Upadhyaya S., Reddy Y., Sampath R., Ghosh D., Chandraprabha D., et al. The gut microbial diversity of newly diagnosed diabetics but not of prediabetics is significantly different from that of healthy nondiabetics. mSystems. 2020;5 doi: 10.1128/MSYSTEMS.00578-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardes M., Bruns T.D. ITS primers with enhanced specificity for basidiomycetes–application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993;2:113–118. doi: 10.1111/J.1365-294X.1993.TB00005.X. [DOI] [PubMed] [Google Scholar]
- Goodrich J.K., Waters J.L., Poole A.C., Sutter J.L., Koren O., Blekhman R., Beaumont M., Van Treuren W., Knight R., Bell J.T., et al. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Wu W., Wu S., Zheng H.M., Li P., Sheng H.F., Chen M.X., Chen Z.H., Ji G.Y., Zheng Z.D., et al. Linking gut microbiota, metabolic syndrome and economic status based on a population-level analysis. Microbiome. 2018;6:172. doi: 10.1186/s40168-018-0557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoel H., Hove-Skovsgaard M., Hov J.R. Impact of HIV and type 2 diabetes on gut microbiota diversity, tryptophan catabolism and endothelial dysfunction. Sci Rep. 2018;8:6725. doi: 10.1038/s41598-018-25168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnard S., Lin J., Fombuena B., Ouyang J., Varin T.V., Richard C., Marette A., Ramendra R., Planas D., Raymond Marchand L., et al. Repurposing metformin in nondiabetic people with HIV: influence on weight and gut microbiota. Open Forum Infect Dis. 2020;7:ofaa338. doi: 10.1093/ofid/ofaa338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen S., McDonald D., Gonzalez A., Navas-Molina J.A., Jiang L., Xu Z.Z., Winker K., Kado D.M., Orwoll E., Manary M., et al. Phylogenetic placement of exact amplicon sequences improves associations with clinical information. mSystems. 2018;3:e00021–e000218. doi: 10.1128/mSystems.00021-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitten A.K., Ryan L., Lee G.C., Flores B.E., Reveles K.R. Gut microbiome differences among Mexican Americans with and without type 2 diabetes mellitus. PLoS ONE. 2021;16 doi: 10.1371/journal.pone.0251245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimenko N.S., Tyakht A.V., Popenko A.S., Vasiliev A.S., Altukhov I.A., Ischenko D.S., Shashkova T.I., Efimova D.A. Microbiome responses to an uncontrolled short-term diet intervention in the frame of the citizen science project. Nutrients. 2018;10:576. doi: 10.3390/nu10050576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp C., Le Corf K., Relizani K., Tambosco K., Martinez C., Chain F., Rawadi G., Langella P., Claus S.P., Martin R. The Keystone commensal bacterium Christensenella minuta DSM 22607 displays anti-inflammatory properties both in vitro and in vivo. Sci Rep. 2021;11:11494. doi: 10.1038/s41598-021-90885-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth S.M., Carson T., Lowe J., Ramaraj T., Leff J.W., Luo L., Bell C.J., Shah V.O. Composition, diversity and abundance of gut microbiome in prediabetes and type 2 diabetes. J Diabetes Obes. 2015;2:1–7. doi: 10.15436/2376-0949.15.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen N., Vogensen F.K., van den Berg F.W., Nielsen D.S., Andreasen A.S., Pedersen B.K., Al-Soud W.A., Sørensen S.J., Hansen L.H., Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau W.L., Vaziri N.D. Gut microbial short-chain fatty acids and the risk of diabetes. Nat Rev Nephrol. 2019;15:389–390. doi: 10.1038/s41581-019-0142-7. [DOI] [PubMed] [Google Scholar]
- Li Q., Chang Y., Zhang K., Chen H., Tao S., Zhang Z. Implication of the gut microbiome composition of type 2 diabetic patients from northern China. Sci Rep. 2020;10:5450. doi: 10.1038/s41598-020-62224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippert K., Kedenko L., Antonielli L., Kedenko I., Gemeier C., Leitner M., Kautzky-Willer A., Paulweber B., Hackl E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef Microbes. 2017;8:545–556. doi: 10.3920/BM2016.0184. [DOI] [PubMed] [Google Scholar]
- Lozupone C., Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–8235. doi: 10.1128/aem.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C.A., Hamady M., Kelley S.T., Knight R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl Environ Microbiol. 2007;73:1576–1585. doi: 10.1128/aem.01996-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J., Ling A.V., Manthena P.V., Gearing M.E., Graham M.J., Crooke R.M., Croce K.J., Esquejo R.M., Clish C.B., Torrecilla E., et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6 doi: 10.1038/ncomms7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J.Y., Zolnik C.P., Wang Z., Qiu Y., Usyk M., Wang T., Kizer J.R., Landay A.L., Kurland I.J., Anastos K., et al. Gut microbiota and plasma metabolites associated with diabetes in women with, or at high risk for, HIV infection. EBioMedicine. 2018;37:392–400. doi: 10.1016/j.ebiom.2018.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D.M., Chew E., Christophi C.A., Davis M.D., Fowler S., Goldstein B.J., Hamman R.F., Hubbard L.D., Knowler W.C., Molitch M.E. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the diabetes prevention program. Diabet Med. 2007;24:137–144. doi: 10.1111/j.1464-5491.2007.02043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang J., Isnard S., Lin J., Fombuena B., Marette A., Routy B., Chen Y., Routy J.P. Metformin effect on gut microbiota: insights for HIV-related inflammation. AIDS Res Ther. 2020;17:10. doi: 10.1186/s12981-020-00267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang J., Lin J., Isnard S., Fombuena B., Peng X., Marette A., Routy B., Messaoudene M., Chen Y., Routy J.P. The bacterium Akkermansia muciniphila: A sentinel for gut permeability and its relevance to HIV-related inflammation. Front Immunol. 2020;11:645. doi: 10.3389/fimmu.2020.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palella F.J., Delaney K.M., Moorman A.C., Loveless M.O., Fuhrer J., Satten G.A., Aschman D.J., Holmberg S.D. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/nejm199803263381301. [DOI] [PubMed] [Google Scholar]
- Phuphuakrat A., Nimitphong H., Reutrakul S., Sungkanuparph S. Prediabetes among HIV-infected individuals receiving antiretroviral therapy: prevalence, diagnostic tests, and associated factors. AIDS Res Ther. 2020;17:25. doi: 10.1186/s12981-020-00284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga L.C., Crews D.C., Coresh J., Miller E.R., Saran R., Yee J., Hedgeman E., Pavkov M., Eberhardt M.S., Williams D.E., Powe N.R. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010;5:673–682. doi: 10.2215/CJN.07891109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plovier H., Everard A., Druart C., Depommier C., Van Hul M., Geurts L., Chilloux J. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- Prioreschi A., Munthali R.J., Soepnel L., Goldstein J.A., Micklesfield L.K., Aronoff D.M., Norris S.A. Incidence and prevalence of type 2 diabetes mellitus with HIV infection in Africa: a systematic review and meta-analysis. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger M., Everard A., Gómez-Valadés A.G., Matamoros S., Ramírez S., Delzenne N.M., Gomis R., Claret M., Cani P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedighi M., Razavi S., Navab-Moghadam F., Khamseh M.E., Alaei-Shahmiri F., Mehrtash A., Amirmozafari N. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb Pathog. 2017;111:362–369. doi: 10.1016/j.micpath.2017.08.038. [DOI] [PubMed] [Google Scholar]
- Senghor B., Sokhna C., Ruimy R., Lagier J.-C. Gut microbiota diversity according to dietary habits and geographical provenance. Human Microbiome Journal. 2018;7-8:1–9. doi: 10.1016/j.humic.2018.01.001. [DOI] [Google Scholar]
- Sommer F., Anderson J.M., Bharti R., Raes J., Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 2017;15:630–638. doi: 10.1038/nrmicro.2017.58. [DOI] [PubMed] [Google Scholar]
- Srivanich N., Ngarmukos C., Sungkanuparph S. Prevalence of and risk factors for pre-diabetes in HIV-1-infected patients in Bangkok, Thailand. J Int Assoc Physicians AIDS Care (Chic) 2010;9:358–361. doi: 10.1177/1545109710373832. [DOI] [PubMed] [Google Scholar]
- Sumner C.J., Sheth S., Griffin J.W., Cornblath D.R., Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60:108–111. doi: 10.1212/WNL.60.1.108. [DOI] [PubMed] [Google Scholar]
- Tabák A.G., Herder C., Rathmann W., Brunner E.J., Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/s0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalamandris S., Antonopoulos A.S., Oikonomou E., Papamikroulis G.A., Vogiatzi G., Papaioannou S., Deftereos S., Tousoulis D. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. 2019;14:50–59. doi: 10.15420/ecr.2018.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utzschneider K.M., Kratz M., Damman C.J., Hullar M. Mechanisms linking the gut microbiome and glucose metabolism. J Clin Endocrinol Metab. 2016;101:1445–1454. doi: 10.1210/jc.2015-4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes A.M., Walter J., Segal E., Spector T.D. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:36–44. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J.L., Ley R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019;17:83. doi: 10.1186/s12915-019-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Tremaroli V., Schmidt C., Lundqvist A., Olsson L.M., Krämer M., Gummesson A., Perkins R., Bergström G., Bäckhed F. The gut microbiota in prediabetes and diabetes: a population-based cross-sectional study. Cell Metab. 2020;32:379–390. doi: 10.1016/j.cmet.2020.06.011. e3. [DOI] [PubMed] [Google Scholar]
- Xu M., Li X.Y., Wang J.G., Wang X.J., Huang Y., Cheng Q., Huang H.E., Li R., Xiang J., Tan J.R., Dai M., Ning G. Retinol-binding protein 4 is associated with impaired glucose regulation and microalbuminuria in a Chinese population. Diabetologia. 2009;52:1511–1519. doi: 10.1007/s00125-009-1386-8. [DOI] [PubMed] [Google Scholar]
- Xu Y., Wang N., Tan H.Y., Li S., Zhang C., Feng Y. Function of Akkermansia muciniphila in obesity: interactions with lipid metabolism, immune response and gut systems. Front Microbiol. 2020;11:219. doi: 10.3389/fmicb.2020.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H.S., Cho C.H., Yun M.S., Jang S.J., You H.J., Kim J.H., Han D., Cha K.H., Moon S.H., Lee K. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat Microbiol. 2021;6:563–573. doi: 10.1038/s41564-021-00880-5. [DOI] [PubMed] [Google Scholar]
- Zhao J., Zhang X., Liu H., Brown M.A., Qiao S. Dietary protein and gut microbiota composition and function. Curr Protein Pept Sci. 2018;20:145–154. doi: 10.2174/1389203719666180514145437. [DOI] [PubMed] [Google Scholar]
- Zhou H., Yu B., Sun J., Liu Z., Chen H., Ge L., Chen D. Short-chain fatty acids can improve lipid and glucose metabolism independently of the pig gut microbiota. J Anim Sci Biotechnol. 2021;12:61. doi: 10.1186/s40104-021-00581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Pang G., Zhang Z., Yuan H., Chen C., Zhang N., Yang Z., Sun L. Association between gut Akkermansia and metabolic syndrome is dose-dependent and affected by microbial interactions: a cross-sectional study. Diabetes Metab Syndr Obes. 2021;14:2177–2188. doi: 10.2147/dmso.s311388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.