Highlights
-
•
Native and recombinant Deinococci bio-remediate toxic radioactive waste, heavy metals.
-
•
Deinococcal amylosurase for improvements of natural active compounds.
-
•
Deinococcal antioxidants as radio-protectors in cancer healthcare.
-
•
Deinococci, the perfect guinea pigs for space research.
-
•
Deinococcal proteins for constructing stress resistant organisms.
Keywords: Deinococcaceae, Biotechnological applications, Bioremediation, Antioxidants, Amylosucrase
Abstract
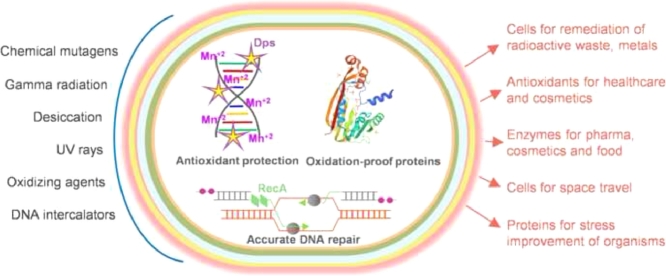
Extremophiles are nature's tiny warriors as they call inhospitable environments their home. They possess special factor(s) that offer an edge over other life forms susceptible to harsh conditions. One such family of extremophiles under discussion here is Deinococcaceae. The microbes belonging to Deinococcaceae are primarily radiophiles, the world's most radiation resistant bacteria, in addition to having resistance to high temperature, metals, cold etc. in specific species. Gamma rays have always been known to be lethal to living cells as it damages DNA, the blueprint of life. But, Deinococci sustain extremely high doses of gamma radiation, about 3000 times more than the dose humans succumb to. This review brings forth the utility of these special factors of Deinococcaceae for a broad range of biotechnological applications.
Graphical abstract
1. Introduction
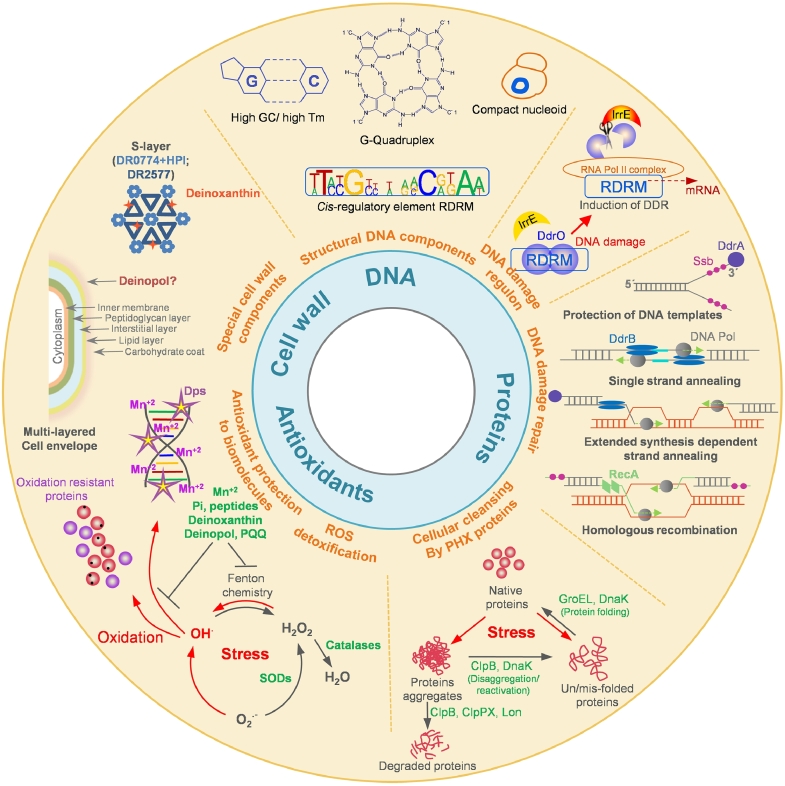
The journey of Deinococcus (initially named Micrococcus) started with the isolation of radiation-resistant bacterium D. radiodurans from a can of spoiled meat that had received a sterilizing dose of gamma radiation (Anderson et al., 1956) and continued with isolation of a sizable number of radiation-resistant species till date. Although the genome of D. radiodurans was completely sequenced in 1999, it did not instantaneously spill all the secrets of radiophily (White et al., 1999). Immense interest in these extremophiles is evident from the fact that as of Feb 2022, The National Center for Biotechnology Information (NCBI) lists completely annotated sequences of 16 Deinococcal species as reference genomes while 217 Deinococcal genomes are at intermediate levels of sequence annotation (htpps://www.ncbi.nlm.nih.gov/datasets/genomes/). Last two decades have put in extensive efforts to understand the molecular basis of gamma radiation resistance in general since these radioresistant cocci can also naturally survive high doses of UV rays, chemical mutagens or prolonged desiccation, all of which compromise structural integrity of DNA. But, with every new piece of research, the enigma of the radiophilic phenotype got even more puzzling and interesting with the focus gradually shifting from highly-accurate DNA repair to metabolic regulation to redundant highly expressed cellular detoxification genes to small-molecule proteome-shields to the intrinsically oxidation-resistant proteins (Basu and Apte, 2012; Battista, 1997; Blasius et al., 2008; Chang et al., 2020; Daly et al., 2004; Daly et al., 2010; Ghosal et al., 2005; Karlin and Mrazek, 2001; Krisko and Radman, 2010; Ujaoney et al., 2017; Ujaoney et al., 2021; Venkateswaran et al., 2000; Zahradka et al., 2006). The well-established signatures that incrementally contribute to the radiophily of Deinococci are graphically represented in Fig. 1. The radiophilic trait appears to be redundant and multivariate in nature since expression of deinococcal protein(s) in heterologous species could not transfer the radioresistance in toto.
Fig. 1.
An overview of various mechanisms important for the radiophily of the Deinococci.
The nature has endowed the Deinococci with a wealth of special factors to endure every inhospitable environmental niche. The innate resistance to radiation, desiccation, UV rays, abundant antioxidant reserves and tough cell walls are common to all Deinococci. Tolerance to high temperature, toxic metals or organic solvents, robust enzymes for industrial applications or strain improvements are found in specific species. This review explores the current status of the biotechnological applications developed around these natural riches of Deinococci, especially the D. radiodurans and D. geothermalis as the best studied models.
2. Deinococci as a host for bioremediation of radioactive waste and toxic heavy metals
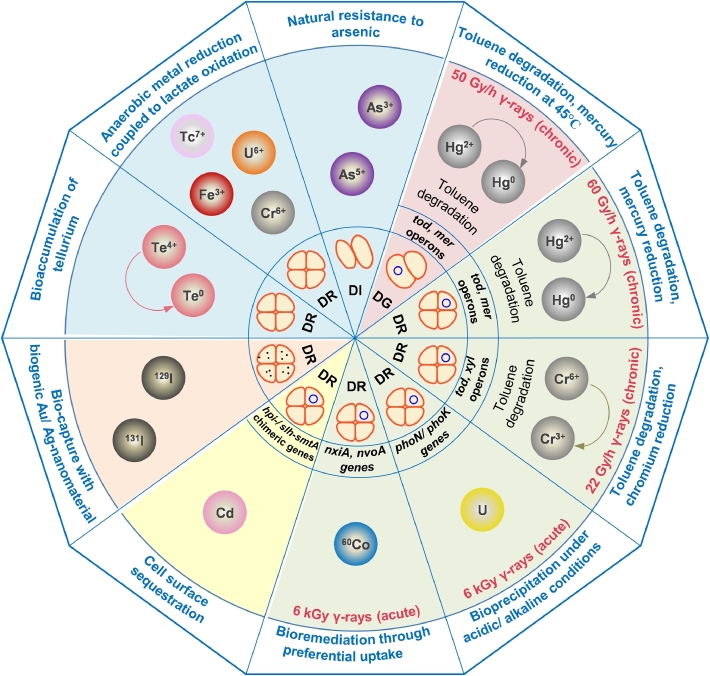
Non-spore forming, non-pathogenic and radio-resistance are the most important attributes of Deinococci that are best suited for remediation of radioactive waste. Add to this, the capability of Deinococci to grow in presence of 60 Gy/h dose of chronic radiation, the level of radiation found in the radioactive waste sites (Lange et al., 1998). Surviving the damaging effects of radiation, an obvious advantage over bioremediating microbes that are instantly killed by radiation, makes Deinococci the most attractive candidate to remediate radioactive waste. The bioremediation approaches explored using various natural or recombinant strains of Deinococci are depicted in Fig. 2.
Fig. 2.
An overview of the bioremediation approaches using native or recombinant Deinococci (DI: D. indicus, DG: D. geothermalis and DR.: D. radiodurans). Blue circle in a cell indicates plasmid construct in a recombinant strain and black dots show nanoparticles.
The nuclear waste contains radionuclides such as uranium (235U), plutonium (238Pu), cesium (137Cs), technetium (99Tc), strontium (90Sr); heavy metals mercury, chromium and lead along with organic solvents like toluene and trichloroethylene at toxic concentrations (Daly, 2000). Some of the deinococcal strains possess natural resistance to metalloids, under aerobic conditions. D. indicus Wt/1aT strain isolated from a shallow arsenic-contaminated aquifer in West Bengal, India was found to be naturally resistant to 10 mM sodium arsenate (Na2HAsO4) or 0.2 mM arsenic trioxide (As2O3), along with the radiation resistance phenotype (Suresh et al., 2004). Similarly, the D. radiodurans cells reduced toxic tellurite (Te4+) to nontoxic metallic tellurium (Te0) and accumulated it intracellularly (Anaganti et al., 2015). D. radiodurans was shown to couple reduction of Fe (III), Cr (VI), U (VI) or Tc (VII) to oxidation of lactate or other organic compounds, under anaerobic conditions (Fredrickson et al., 2000).
The deinococcal cells are inherently incapable of concentrating the radionuclides or dealing with toxicity of the heavy metals and organopollutants. Thus, several genetic engineering approaches have been explored to improve the bioremediating skills of radiation resistant deinococci. Some of the early attempts were focused at heterologous expression of genes that could degrade organic solvents or improve heavy metal tolerance. The recombinant D. radiodurans expressing the tod genes (todC1C2BA of Pseudomonas putida F1) encoding a multicomponent toluene dioxygenase (TDO) could oxidize toluene, chlorobenzene, 3,4-dichloro-1-butene, and indole in presence of 60 Gy/h gamma radiation (Lange et al., 1998). Engineered strains of D. radiodurans expressing the mercury resistance operon (mer) from E. coli BL308 strain reduced highly toxic ionic Hg (II) to less toxic elemental Hg (0) under irradiation conditions (Brim et al., 2000). Further, a D. radiodurans strain co-expressing mer operon as well as tod genes could metabolize toluene and detoxify Hg (II) in presence of chronic radiation (Brim et al., 2000). A combination of P. putida tod and xyl operons imparted D. radiodurans an ability to mineralize toluene (and other fuel hydrocarbons) and couple it with the Cr(VI) reduction (Brim et al., 2006). A similar strategy has been adopted to develop a thermophilic radiophile D. geothermalis for bioremediation of radioactive waste at elevated temperatures. Brim et al., expressed tod genes or mer genes into D. geothermalis, and the recombinant strains were found to be capable of degrading toluene or reducing Hg (II) at 50 °C and in presence of chronic irradiation of 50 Gy/h (Brim et al., 2003). D. geothermalis T27 strain, with a remarkable tolerance to a broad range of solvents and with ability to use them as growth substrates at 45 °C, is another natural candidate for bioremediation of radioactive waste (Kongpol et al., 2008).
D. radiodurans has been actively pursued for bioremediation and recovery of uranium from dilute acid or alkaline nuclear waste. A first recombinant D. radiodurans strain expressing a nonspecific acid phosphatase from Salmonella enterica serovar Typhi (phoN gene) could efficiently precipitate ⁓ 90% of the uranium from a 0.8 mM uranyl nitrate solution in 6 h in presence of 6 kGy gamma radiation (Appukuttan et al., 2006). A radiation inducible promoter (Pssb) has been tagged with phoN gene to augment the expression of PhoN by gamma radiation (Misra et al., 2014). Moreover, the lyophilized D. radiodurans cells expressing PhoN (DrPhoN) exhibited high loading capacity in a batch process (up to 5.7 g uranium/g dry weight of cells) when input uranium concentration was 20 mM, and 6 months of storage at room temperature did not affect the uranium precipitation ability of the lyophilized cells (Appukuttan et al., 2011). The DrPhoN cells also showed cadmium precipitation capability (Misra et al., 2012). Misra et al., developed a user-friendly flow-through system wherein the lyophilized DrPhoN cells were immobilized in polyacrylamide gels were used to remove 70% of uranium from 1 mM input solution which achieved loading of 1 g uranium/g dry weight cells (Misra et al., 2012). Fusion of PhoN with S-layer proteins of D. radiodurans was found to improve uranium precipitation on cell surface (Misra et al., 2021). For bioprecipitation of uranium from alkaline waste, a novel alkaline phosphatase PhoK (from Sphingomonas sp.) was overexpressed in a recombinant D. radiodurans (DrPhoK) cells. The DrPhoK cells could precipitate >90% of uranium within 2 h at 1 mM uranyl concentrations, while at high uranyl concentration (10 mM) an impressive loading capacity of ⁓ 10.7 g U/g of dry weight of cells was achieved (Kulkarni et al., 2013). Interestingly, the extracellular precipitation of uranium occurred in presence of Cs and Sr (common contaminants in liquid radioactive waste) by irradiated DrPhoK cells (Kulkarni et al., 2013). Presence of other metals such as Ca, Mg, Cr, Pb, Ni, and Zn in the nuclear waste may affect the bioprecipitation of uranium. Xu et al. demonstrated that the presence of Cr(VI) halved the U precipitation by DrPhoK cells, which could be restored by co-expression of YieF protein, a chromate reductase from E. coli (Xu et al., 2018). The power generating nuclear reactors produce spent decontamination solution containing 60Co among other radionuclides. Since 60Co has high energy (1.17 and 1.33 MeV) and long half-life of 5.27 years, removal of 60Co is highly beneficial. For the purpose, Gogada et al., cloned Ni/Co transporter (NiCoT for preferential uptake for cobalt) genes nxiA from Rhodopseudomonas palustris CGA009 and nvoA from Novosphingobium aromaticivorans F-199 in D. radiodurans (Gogada et al., 2015). The recombinant strain, wherein the cobalt and nickel induced NiCoT genes, removed > 60% of 60Co within 90 min from 8.5 nM of 60Co containing simulated spent decontamination solution. 60Co removal was unhindered even in the presence of >10 mM of Fe, Cr, and Ni (Gogada et al., 2015). Similarly, D. radiodurans has been used to bioaccumulate cadmium, another toxic heavy metal. A recombinant D. radiodurans expressing a cytosolic metal binding metallothionein protein SmtA from Synechococcus elongates (DrSmtA), showed moderate improvement in cadmium tolerance as well as accumulation (Chaturvedi and Archana, 2014). However, the DrHpi-SmtA and DrSLH-SmtA cells expressing SmtA-S-layer fusion proteins, with SmtA displayed on the cell surface, showed better cadmium removal over DrSmtA cells (Misra et al., 2021).
Radioactive iodine (131I and 129I) is another major byproduct of nuclear reactor. Since iodine has a tendency to accumulate in the thyroid gland, accidental release of radioactive iodine can cause thyroid cancers in the exposed population. In an innovative approach, biogenic silver or gold nanomaterials were synthesized in D. radiodurans cells (Au-Dr or Ag-Dr, respectively) which have been shown to capture > 99% radioactive iodine (Choi et al., 2017; Shim et al., 2018).
A cell-surface deficient mutant of D. radiodurans ∆dr2577, when allowed to colonize the rice roots, could significantly reduce the cadmium and lead contents in the rice roots and shoots, and mitigated heavy metal induced oxidative stress (Dai et al., 2021). A whole cell cadmium biosensor was developed using D. radiodurans ∆crtL mutant expressing crtL as a reporter gene under the control of cadmium inducible promoter Pdr0659, from plasmid (Joe et al., 2012).
3. Deinococcal enzymes as biocatalysts for industrial applications
Future of sustainable technologies largely depends on the use of biocatalysts for the synthesis of expensive value-added chemicals from cheap biomass or agricultural waste. Biocatalysis is environmentally friendly, highly selective and energy-conservative process as compared to their chemical counterparts. A handful of deinococcal enzymes have been exploited for the purpose owing to their better thermostability, higher optimal temperatures etc.
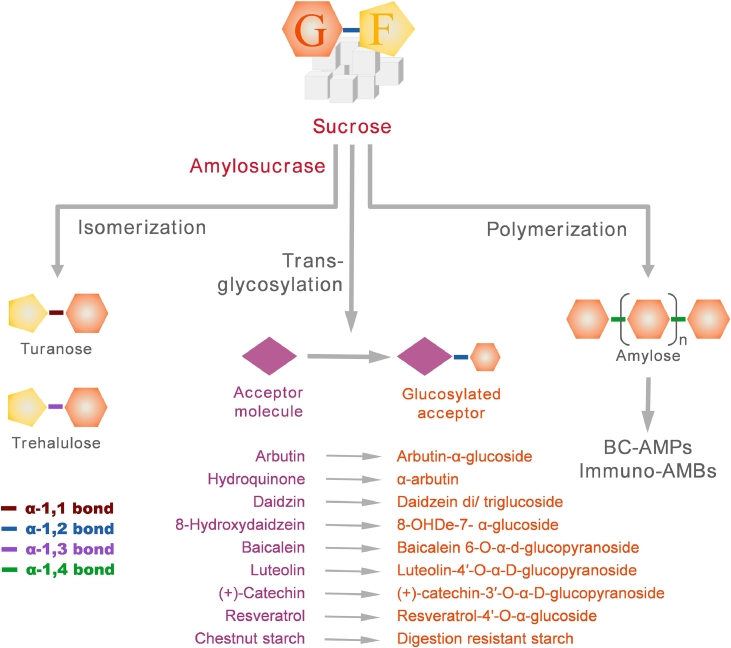
Amylosucrase (or α-transglucosidase or glucansucrase; EC number 2.4.1.4) is one of the industrially important enzymes as it catalyses glucose transfer from an inexpensive donor to synthesize glycoconjugates or transglycosylation products with improved physicochemical or pharmacological properties. The enzyme transfers glucose from sucrose to hydroxyl group (-OH) of a variety of natural acceptor compounds through an α-linked bond. The homodimeric quaternary organization of amylosucrase from D. geothermalis (DgAS) makes it one of the most thermostable amylosucrases identified till date (Guérin et al., 2012). It presents an impressive specific activity of 44 units/mg and 26 h of half-life at its optimum temperature of 50 °C (Emond et al., 2008).
The catalytic activities of DgAS on a wide range of acceptor molecules yield products with improved activities or physicochemical properties (Table 1, Fig. 3). DgAS-synthesized arbutin-α-glucoside exhibited stronger tyrosinase inhibitor activity and thus, improved skin lightening property than parent arbutin (Seo et al., 2009; Sugimoto et al., 2003). Further, the action of DgAS on an antioxidant hydroquinone produced α-arbutin with better skin lightening property than arbutin-α-glucoside (Lee et al., 2018; Seo et al., 2012; Sugimoto et al., 2003). Both the compounds are of great interest to the cosmetic industry. Lee et al. have used immobilized DgAS to produce glucosides of resorcinol and catechol (Lee et al., 2018). These dihydroxybenzene glucosides have better stability, water solubility and biological activity than their parent phenolic compounds (Lee et al., 2018). DgAS was used to synthesize glycation products of surfactant compounds glyceryl caprylate and glyceryl caprate, with improved water solubility (Kim et al., 2021). These surfactants have better antimicrobial activity and skin moisture retention ability than other non-ionic surfactants, properties of interest to pharma and cosmetic industries (Kim et al., 2021).
Table 1.
Catalytic activities of D. geothermalis amylosucrase (DgAS) with various acceptor molecules.
| Acceptor molecule | Activity or uses | Reaction products (Conversion yield) | Improvement in pharmacological or physicochemical properties | Industrial application | References |
|---|---|---|---|---|---|
| Arbutin | Tyrosinase inhibitor, skin lightening | α-d-glucopyranosyl-(1→4)-β-arbutin (Ab-α-glucoside)(over 98.0%) | Stronger inhibitory activity than arbutin | Cosmetic | Sugimoto et al., 2003; Seo et al., 2009 |
| Hydroquinone | Antioxidant, skin depigmentation | Hydroquinone-O-α-d-glucopyranoside (α-arbutin) (90.0%, in presence of ascorbic acid) | Powerful skin whitening Agent; stronger inhibitory activity than arbutin-α-glucoside |
Cosmetic | Sugimoto et al., 2003; Seo et al., 2012 |
| Hydroquinone | Antioxidant, skin depigmentation | Hydroquinone glucoside (α-arbutin) (> 95%) | – | Cosmetic | Seo et al., 2012 |
| Catechol | Anti-cancer Compound; cosmeceutical agent |
Catechol glucoside (> 90%) | – | Cosmeceutical | Lee et al., 2018 |
| Resorcinol | Skin depigmentation; relief from pain and itching |
Resorcinol glucoside (> 70%) | – | Cosmeceutical | Lee et al., 2018 |
| Glyceryl caprylate, glyceryl caprate, and polyglyceryl-2 caprate | Biosurfactant | Glyceryl caprylate glycoside Glyceryl caprate glycoside |
– | Cosmeceutical | Kim et al., 2021 |
| Salicin |
Analgesic and antipyretic | α-d-glucopyranosyl-(1 → 4)-salicin (79.0%) α-d-glucopyranosyl-(1 → 4)-α-d-glucopyranosyl-(1 → 4)-salicin (5.0%) |
– | Pharma | Jung et al., 2009 |
| Daidzin | Antidipsotropic | Daidzein diglucoside and triglucoside (99.0%) | Improved solubility | Pharma | Kim et al., 2019 |
| Daidzin | Antidipsotropic | Daidzein-7-O-α-d-glucopyranosyl-(4 → 1)-O-β-d-glucopyranoside (daidzin-4-O-α-d-glucopyranoside, DA2) and Daidzein-4-O-α-d-glucopyranosyl-7-O-α-d-glucopyranosyl-(1 → 4)-O-β-d-glucopyranoside (daidzin-4,4-O-α-d-diglucopyranoside, DA3) (89%) | Improved solubility | Pharma | Rha et al., 2019b |
| 8-Hydroxydaidzein | Potent anti-inflammatory activity | 8-OHDe-7- α-glucoside (89.3%) | Higher solubility and alkali stability, retained 20% of anti-inflammatory activity | Pharma | Chang et al., 2019 |
| Baicalein | Anti-inflammatory effects | Baicalein 6-O-α-d-glucopyranoside (59.1%) | Improved water solubility, stability in buffer/ media, bioavailability | Pharma | Kim et al., 2014 |
| Caffeic acid phenethyl ester | Anti-inflammatory activity | Caffeic acid phenethyl ester-4-O-α-d-glucopyranoside | Improved solubility, stability and bioavailability | Pharma | Moon et al., 2017 |
| Quercetin | Anti-oxidant and anti-inflammatory effects | Quercetin 4′ -O-α-d-glucopyranoside (QG1) Quercetin 4′ - O-α-d-isomaltoside (QG2′, 74%) Quercetin diglucoside (QG2), Quercetin triglucoside and tetraglucoside |
Improved water solubility | Pharma | Rha et al., 2020 |
| Luteolin | Antioxidant, anti-inflammatory, anti-tumor, and immune-modulatory agent | Luteolin-4′-O-α-d-glucopyranoside (86.0%) | – | Pharma | Jang et al., 2018 |
| (+)-Catechin# | Antioxidant, anti-tumor | (+)-catechin-3′-O-α-d-glucopyranoside (97.0%); (+)-catechin-3′-O-α-d-maltoside | Better water solubility | Pharma | Cho et al., 2011 |
| Resveratrol | Antioxidant, anti-tumor, phytoalexin (anti-bacterial, anti-fungal) | Resveratrol-4′-O-α-glucoside Resveratrol-3-O-α-glucoside |
Improved water solubility; buffer stability; bioaviability; tyrosinase inhibitory activity | Pharma | Moon et al., 2021 |
| Isoquercitrin | Anti-oxidant and anti-cancer activity | Isoquercitrin glucoside (IQ-G1, 14.6%) Isoquercitrin diglucoside (IQ-G2, 25.3%) Isoquercitrin diglucoside isomer (11.3%) Isoquercitrin triglucoside (IQ-G3, 46.5%) |
IQ-G3 is the most bioavailable form |
Pharma, food | Rha et al., 2019a |
| Genistein | Antineoplastic activity | Genistein monoglucoside, Genistein diglucoside, Genistein triglucoside (98.2%) | – | Pharma | Jung et al., 2020 |
| Daidzein | Agonist of the GPER | Daidzein monoglucoside, Daidzein diglucoside, Daidzein triglucoside, Daidzein tetraglucoside (96.9%) | – | Pharma | Jung et al., 2020 |
| Glycitein | Phytoestrogen | Glycitein monoglucoside (88.8%) | – | Pharma | Jung et al., 2020 |
| Rutina | Antioxidant | Glucosyl-α-(1 → 4)-rutin (ND) | – | Pharma, food | Kim et al., 2016 |
| Sucrose | Inexpensive substrate | β-carotene embedded amylose microparticles (BC-AmMPs) | Safe food material | Food | Letona et al., 2017 |
| Sucrose | Inexpensive substrate | Amylose magnetic beads (AMBs) | Efficient separation and concentration of E. coliO157:H7 from milk | Food | Lim et al., 2016 |
| Sucrose | Inexpensive substrate | Turanose and trehalulose | Next generation sweetener, lower glycemic index | Food | Emond et al., 2008 |
| Chestnut starch | Inexpensive substrate | Modified chestnut starch with increased proportion of resistant starch | Prebiotic, anti-obesity effects | Functional food | Lee et al., 2020 |
D. radiopugnans amylosucrase.
higher transglycosylation efficiency than all other published sources.
Fig. 3.
An overview of the catalytic activities of Deinococcal amylosucrase. Abbreviations: Glucose; F, Fructose; BC-AMPS, β-carotene encapsulated Amylose microparticles; Immuno-AMBs, antibody coated amylose magnetic beads.
Several natural compounds have important pharmacological properties; however, their active use is limited by poor solubility or stability in water resulting in low bioavailability. Glycosylation has favorable effect on these important physicochemical and biological properties. Transglycosylation of analgesic and antipyretic salicin (Jung et al., 2009) and antidipsotropic daidzin (Kim et al., 2019; Rha et al. 2019b) with DgAS improved water solubility. Similar effect of glycation through DgAS was observed on a number of anti-inflammatory compounds (8-hydroxydaidzein, baicalein, caffeic acid phenethyl ester, quercetin), anti-neoplastic compounds (luteolin, (+)-catechin, Resveratrol, isoquercitrin, genistein, daidzein); phytoestrogen glycitein and anti-oxidant rutin (Chang et al., 2019; Cho et al., 2011; Jang et al., 2018; Jung et al., 2020; Kim et al., 2014, 2016; Moon et al., 2017; Moon et al., 2021; Rha et al., 2019a; Rha et al., 2020).
Action of DgAS on inexpensive substrate sucrose has been effectively exploited for innovative approaches relevant to food industry. Amylose synthesized from sucrose by DgAS is a safe food material. It was further used to prepare β-carotene encapsulated Amylose microparticles (BC-AMPs) with enhanced stability against UV light and oxidation (Letona et al., 2017). Such approach is speculated to have a wide range of applications for long term storage or transportation of expensive active food ingredients. Another innovative approach was antibody coated amylose magnetic beads (Immuno-AMBs) for efficient separation and concentration of E. coli O157:H7 from milk (Lim et al., 2016). The sophisticated system utilizes intrinsic affinity of maltose-binding protein (MBP) to di-glucose moieties on amylose while SPG-tag on MBP specifically bind Fc region of the anti-Escherichia coli O157 antibodies (Lim et al., 2016). An inexpensive substrate chestnut starch could also be modified with DgAS to increase its digestion-resistant starch contents with potential pre-biotic and anti-obesity effects which have applications in functional food industry (Lee et al., 2020). Conversion of sucrose to next-generation sweeteners such as turanose and trehalulose was achieved with DgAS (Emond et al., 2008).
Thermostable transaminase from D. geothermalis has been used to synthesize a commercially expensive compound β-hydroxypyruvate from L‑serine and α-ketoglutaric acid while the thermostable transketolases from D. geothermalis or D. radiodurans produced L‑gluco-heptulose from L-arabinose and β-hydroxypyruvate (Bawn et al., 2018). L-arabinose is a major monosaccharide present in sugar beet pulp, a low value animal feed while L‑gluco-heptulose has therapeutic potential in hypoglycaemia and cancer (Cárdenas-Fernández et al., 2017). There is a great interest in an in-built, low-cost, single-enzyme ATP regeneration system for ATP-dependent enzymes mediated industrial processes, eg. bioconversion of L-aspartic acid (L-Asp) to L-asparagine (L-Asn). Class III polyphosphate kinase 2 (PPK2) catalyze polyphosphate-dependent pyrophosphorylation to regenerate ATP from AMP using cheap pyrophosphate as a phosphate donor. PPK2 from D. ficus, D. radiodurans and D. proteolyticus NBRC 101906T have been employed for the purpose (Luo et al., 2021; Ogawa et al., 2019; Suzuki et al., 2018). Microbial lipases and proteases are versatile biocatalysts catering to the needs of detergents, textile, leather, food, medical, pharmaceuticals industries and more. For detailed information on industrial applications of microbial lipases and proteases, please refer to Chandra et al., 2020 and Razzaq et al., 2019. Microbial lipases generally hydrolyze esters of glycerol with specific preference for long-chain fatty acids, some of the lipases are implicated in the virulence of pathogenic bacteria (Jaeger et al., 1994). Although D. radiodurans is a non-pathogenic organism, it encodes lipases with industrially desirable properties with respect to thermostability, broad temperature range and better organic solvent tolerance (Shao et al., 2014), however, their potential for industrial applications is yet to be unlocked. Deinococci lead a proteolytic lifestyle. As Deinococci are dependent on extraneous proteinaceous compounds as the sources of amino acids, they possess a large number of highly active proteases (Basu and Apte, 2008). Commercial value of these proteases has not been explored yet.
4. Deinococcal antioxidants with potential applications in healthcare
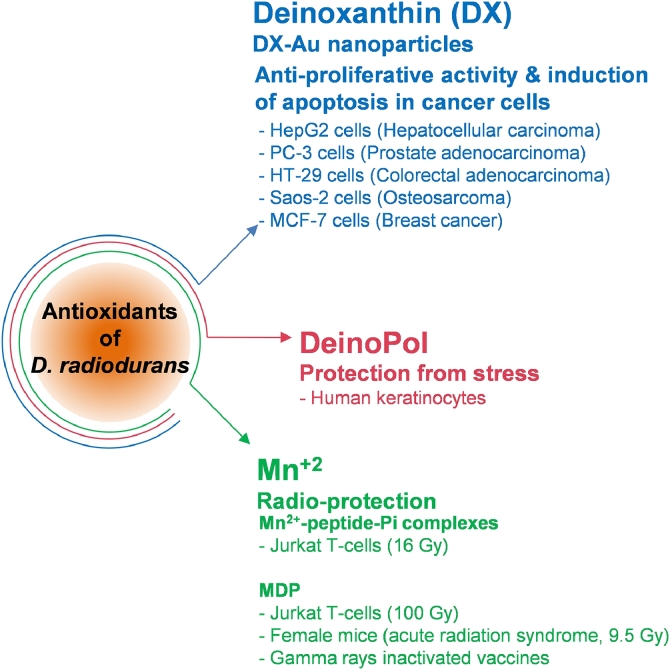
Deinococci possess a wide array of anti-oxidants to combat radiation-induced oxidative stress (Slade and Radman, 2011). It has been proposed that the radiation resistance of these extremophiles is a function of its anti-oxidant reserves since they maintain the DNA repair enzymes in a functionally competent form to achieve complete repair of massively damaged DNA (Slade and Radman, 2011). These antioxidants comprise of both enzymatic as well as non-enzymatic components, however, the non-enzymatic antioxidants (also referred to as extremolytes) have grabbed more attention for their prospective applications to healthcare (Fig. 4).
Fig. 4.
An overview of the potential healthcare applications of the non-enzymatic antioxidants of D. radiodurans.
The crude secondary metabolite extract (CSME) from D. radiodurans showed anti-proliferative effect and induced classical apoptotic markers in triple-negative human breast carcinoma (MDA-MB-231) cells (Maqbool et al., 2020). Interestingly, the CSME contained 23 bioactive compounds, some of which [pyrroloquinoline quinine (PQQ), ethyl cyclodocosane, n-hexadecanoic acid, hentriacontane and methoxyacetic acid octyl ester, etc.] have known anticancer properties (Maqbool et al., 2020 and references thereof). Oxidative stress is one of the important mediators of apoptosis and dietary carotenoids are known to exert pro-apoptotic and anti-proliferative effects on the cancer cells. Possible mechanisms of action include carotenoid-triggered ROS production, activation of pro-apoptotic signaling by ROS, and apoptotic cell death in cancer cells leading to the anti-proliferative effect (Shin et al., 2020). The tetraterpenoid carotenoid deinoxanthin (2,1-dihydroxy-3,4-didehydro-1,2-dihydro-β,ψ-caroten-4-one), a strong antioxidant from D. radiodurans, was shown to induce apoptosis in HepG2, PC-3, HT-29 and Saos-2 cancer cells (Choi et al., 2014; Tapia et al., 2021). Further, Tian et al. reported production of functionalized DX−AuNPs (deinoxanthin − gold nanoparticles) with stronger inhibitory activity against MCF-7 cancer cells but no toxic effects on normal cells (Tian et al., 2018). Considering the potent anticancer property of deinoxanthin, a metabolically engineered strain of D. radiodurans was developed for efficient deinoxanthin production from sucrose as a carbon source (Jeong et al., 2021). Deinococcal exopolysaccharide (DeinoPol), a component of the cell wall, is a safe and attractive ROS scavenger useful for cosmetics and pharmaceutical industries as it effectively protected human keratinocytes from stress-induced apoptosis (Lin et al., 2020).
The cellular extracts of D. radiodurans contains a soup of extremolytes such as Mn2+, PQQ, orthophosphate (Pi) and peptides, capable of functionally substituting enzymatic antioxidants like superoxide dismutase and catalase, and contributing to the protein-oxidation resistance of the organism (Culotta and Daly, 2013; Misra et al., 2004). The ultrafiltrate of D. radiodurans containing Mn2+-peptide-Pi complexes was first shown to protect cultured human Jurkat T-cells from 16 Gy gamma rays and could preserve the enzymes activities after gamma irradiation (Daly et al., 2010). A synthetic decapeptide complexed with Mn2+ and Pi (MDP) could not protect DNA or RNA, but it preserved the activity of T4 DNA ligase following exposure to 60 kGy, protected Jurkat T-cells (100 Gy) and protected female mice (B6D2F1/J) from acute radiation syndrome (9.5 Gy) (Daly et al., 2010; Gaidamakova et al., 2012; Gupta et al., 2016). Thus, the protein-oxidation protection attribute of MDP needs to be explored further for reducing the side-effects of cancer radiotherapy or during radiation emergencies. The radio-protective effects of MDP have been used to develop gamma rays inactivated vaccines against polio virus or bacterial pathogen Acinetobacter baumannii (Dollery et al., 2021; Tobin et al., 2020). Another novel manganese and glutathione antioxidant complex DT(GS)2Mn(II) [Diethylene triamine pentaacetate-bis-glutathione manganese complex] could also protect NIH/3T3 cells from radio-toxicity (Khurana et al., 2019). PQQ, a powerful redox modulator with anti-oxidant and anti-inflammatory properties, has potential applications in nutritional supplements and cosmetics (Ikemoto et al., 2012). A skincare company Cosmax has claimed development of Solarbiome sunscreen, from Bacillus subtilis and D. radiodurans, which reverses the damage to skin cells and prevents skin aging.
5. Miscellaneous applications
PprI encodes a metalloprotease that acts on a negative regulator DdrO and activates the induction of DNA damage regulon during recovery from exposure to a number of DNA damaging agents (Anaganti et al., 2016, 2017; Narasimha and Basu, 2021). The heterologous expression of pprI (inducer of pleiotropic proteins promoting DNA repair, also known as irrE) gene has been found beneficial in terms of improved stress resistance. Expression of PprIDR in recombinant yeast Saccharomyces cerevisiae improved tolerance to high concentrations of salt (1.2 M), ethanol (11% v/v) and butanol which in turn boosted the yield of biofuel production from ligonocellulosic substrates (Hossein Helalat et al., 2019). Similarly, recombinant Lactococcus lactis strain MG1363 expressing PprIDR had better resistance to oxidative stress and high osmotic pressure, and enhanced lactate dehydrogenase activity resulting in net increase in lactic acid production (Dong et al., 2015). Differential expression of genes induced by PprIDR expression in Pseudomonas aeruginosa PAO1, a model organism for electrochemically active bacteria, remarkably improved the bioelectricity generation by the recombinant strain (Luo et al., 2018). At molecular level, improved stress resistance in the heterologous strains expressing deinococcal PprI protein is attributed to differential expression of genes related to stress-response, DNA repair, enzymatic antioxidants, phenazines core biosynthesis, biofilm formation or general cellular homeostasis that impacted the tolerance and sustainability of the recombinant strains. The exact action of metalloprotease PprI on heterologous transcription factors that lead to modulation of gene expression remains to be explored. In another novel application, PprA protein of D. radiodurans which binds DNA strand breaks was used to develop an immune-fluorescence technique to visualize radiation induced DNA strand breaks in mammalian cells (Satoh et al., 2006).
Exploration of space, for exploitation of natural resources and human settlement, has gained pace in the 21st century. However, planets other than the Earth pose challenges in the form of harsh environments, intense radiation, no water or oxygen and low gravity. Resistance to high doses of UV rays and years of desiccation are the most important attributes that make D. radiodurans a favourite organism for space travel to Moon or Mars. D. radiodurans has been a part of a number of experiments that simulated space environment. Initial experiments assessed the effect of microgravity on DNA repair capacity of D. radiodurans and reported that the organism was able to repair damaged DNA under microgravity environment (Harada et al., 1998; Kobayashi et al., 2000). In real and simulated space conditions, D. radiodurans survived 15 days of desiccation but the extreme UV radiation (10 – 100 nm) reduced the cell viability by 4-fold (Saffary et al., 2002). Combining extreme UV radiation with space vacuum had deleterious effects on cell viability (Saffary et al., 2002). However, when several martian environmental parameters were simulated in an experiment, the diurnal cycles of temperature and relative humidity severely affected the viability of D. radiodurans (de la Vega et al., 2007). While martian UV climate was found to have most deleterious effect, the cells could be protected by hematite (de la Vega et al., 2007). Later, the biofilms of D. geothermalis were shown to better withstand the desiccation, extraterrestrial UV rays, space and Mars-like conditions compared to planktonic cells (Panitz et al., 2019).
6. Concluding remarks and future prospects
Microorganisms have evolved over millions of years specializing sustenance under diverse environmental conditions and acquiring newer traits occasionally to score advantages over their neighbours. Isolation of such microbes for sustainable biotechnology have opened up a new and exciting era that is set to replace chemical processes with biological ones with better efficiency, in situ reactions at physiological conditions. The radiophiles of Deinococcaceae are poised to impact the biotechnological advances in virtually unlimited ways. The natural reserves of these radiophiles have been tapped by pharma, cosmetic and food industry to improve physicochemical properties and application potential of various natural compounds. Use of natural antioxidants as radioprotectors is an upcoming field with far reaching health implications for use of radiation in healthcare sans the damage they cause. With the robust cell envelope, high cellular reduction potential and ease of genetic engineering, the Deinococcal cells could become mini-factories of remediation and recycling of radioactive waste. Our current knowledge on industrial applicability of Deinococci is based on fundamental research in a handful of strains. Hundreds of Deinococcal strains and other radiation resistant genera, still holding their time-tested secrets, assure us of an exciting future for biotechnology.
CRediT authorship contribution statement
Bhakti Basu: Conceptualization, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anaganti N., Basu B., Gupta A., Joseph D., Apte S.K. Depletion of reduction potential and key energy generation metabolic enzymes underlies tellurite toxicity in Deinococcus radiodurans. Proteomics. 2015;15(1):89–97. doi: 10.1002/pmic.201400113. [DOI] [PubMed] [Google Scholar]
- Anaganti N., Basu B., Apte S.K. In situ real-time evaluation of radiation-responsive promoters in the extremely radioresistant microbe Deinococcus radiodurans. J. Biosci. 2016;41(2):193–203. doi: 10.1007/s12038-016-9608-y. [DOI] [PubMed] [Google Scholar]
- Anaganti N., Basu B., Mukhopadhyaya R., Apte S.K. Proximity of radiation desiccation response motif to the core promoter is essential for basal repression as well as gamma radiation-induced gyrB gene expression in Deinococcus radiodurans. Gene. 2017;615:8–17. doi: 10.1016/j.gene.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Anderson A.W., Nordon H.C., Cain R.F., Parrish G., Duggan D. Studies on a radio-resistant micrococcus. I. Isolation, morphology, cultural characteristics, and resistance to gamma radiation. Food Technol. 1956;10:575–578. [Google Scholar]
- Appukuttan D., Rao A.S., Apte S.K. Engineering of Deinococcus radiodurans R1 for bioprecipitation of uranium from dilute nuclear waste. Appl. Environ. Microbiol. 2006;72(12):7873–7878. doi: 10.1128/AEM.01362-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appukuttan D., Seetharam C., Padma N., Rao A.S., Apte S.K. PhoN-expressing, lyophilized, recombinant Deinococcus radiodurans cells for uranium bioprecipitation. J. Biotechnol. 2011;154(4):285–290. doi: 10.1016/j.jbiotec.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Basu B., Apte S.K. A novel serralysin metalloprotease from Deinococcus radiodurans. Biochim. Biophys. Acta. 2008;1784(9):1256–1264. doi: 10.1016/j.bbapap.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Basu B., Apte S.K. Gamma radiation-induced proteome of Deinococcus radiodurans primarily targets DNA repair and oxidative stress alleviation. Mol. Cell. Proteomics. 2012;11(1) doi: 10.1074/mcp.M111.011734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista J.R. Against all odds: the survival strategies of Deinococcus radiodurans. Annu. Rev. Microbiol. 1997;51:203–224. doi: 10.1146/annurev.micro.51.1.203. [DOI] [PubMed] [Google Scholar]
- Bawn M., Subrizi F., Lye G.J., Sheppard T.D., Hailes H.C., Ward J.M. One-pot, two-step transaminase and transketolase synthesis of L-gluco-heptulose from L-arabinose. Enzyme Microb. Technol. 2018;116:16–22. doi: 10.1016/j.enzmictec.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Blasius M., Sommer S., Hübscher U. Deinococcus radiodurans: what belongs to the survival kit? Crit. Rev. Biochem. Mol. Biol. 2008;43(3):221–238. doi: 10.1080/10409230802122274. [DOI] [PubMed] [Google Scholar]
- Brim H., McFarlan S.C., Fredrickson J.K., Minton K.W., Zhai M., Wackett L.P., Daly M.J. Engineering Deinococcus radiodurans for metal remediation in radioactive mixed waste environments. Nat. Biotechnol. 2000;18(1):85–90. doi: 10.1038/71986. [DOI] [PubMed] [Google Scholar]
- Brim H., Venkateswaran A., Kostandarithes H.M., Fredrickson J.K., Daly M.J. Engineering Deinococcus geothermalis for bioremediation of high-temperature radioactive waste environments. Appl. Environ. Microbiol. 2003;69(8):4575–4582. doi: 10.1128/AEM.69.8.4575-4582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim H., Osborne J.P., Kostandarithes H.M., Fredrickson J.K., Wackett L.P., Daly M.J. Deinococcus radiodurans engineered for complete toluene degradation facilitates Cr(VI) reduction. Microbiology (Reading) 2006;152(Pt 8):2469–2477. doi: 10.1099/mic.0.29009-0. [DOI] [PubMed] [Google Scholar]
- Cárdenas-Fernández M., Bawn M., Hamley-Bennett C., Bharat P.K.V., Subrizi F., Suhaili N., Ward D.P., Bourdin S., Dalby P.A., Hailes H.C., Hewitson P., Ignatova S., Kontoravdi C., Leak D.J., Shah N., Sheppard T.D., Ward J.M., Lye G.J. An integrated biorefinery concept for conversion of sugar beet pulp into value-added chemicals and pharmaceutical intermediates. Faraday Discuss. 2017;202:415–431. doi: 10.1039/c7fd00094d. [DOI] [PubMed] [Google Scholar]
- Chandra P., Enespa, Singh R., Arora P.K. Microbial lipases and their industrial applications: a comprehensive review. Microb. Cell Fact. 2020;19(1):169. doi: 10.1186/s12934-020-01428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T.S., Wang T.Y., Yang S.Y., Kao Y.H., Wu J.Y., Chiang C.M. Potential industrial production of a well-soluble, alkaline-stable, and anti-inflammatory isoflavone glucoside from 8-hydroxydaidzein glucosylated by recombinant amylosucrase of Deinococcus geothermalis. Molecules. 2019;24(12):2236. doi: 10.3390/molecules24122236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R.L., Stanley J.A., Robinson M.C., Sher J.W., Li Z., Chan Y.A., Omdahl A.R., Wattiez R., Godzik A., Matallana-Surget S. Protein structure, amino acid composition and sequence determine proteome vulnerability to oxidation-induced damage. EMBO J. 2020;39(23) doi: 10.15252/embj.2020104523. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi R., Archana G. Cytosolic expression of synthetic phytochelatin and bacterial metallothionein genes in Deinococcus radiodurans R1 for enhanced tolerance and bioaccumulation of cadmium. Biometals. 2014;27(3):471–482. doi: 10.1007/s10534-014-9721-z. [DOI] [PubMed] [Google Scholar]
- Cho H.K., Kim H.H., Seo D.H., Jung J.H., Park J.H., Baek N.I., Kim M.J., Yoo S.H., Cha J., Kim Y.R., Park C.S. Biosynthesis of (+)-catechin glycosides using recombinant amylosucrase from Deinococcus geothermalis DSM 11300. Enzyme Microb. Technol. 2011;49(2):246–253. doi: 10.1016/j.enzmictec.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Choi M.H., Jeong S.W., Shim H.E., Yun S.J., Mushtaq S., Choi D.S., Jang B.S., Yang J.E., Choi Y.J., Jeon J. Efficient bioremediation of radioactive iodine using biogenic gold nanomaterial-containing radiation-resistant bacterium, Deinococcus radiodurans R1. Chem. Commun. (Camb.) 2017;53(28):3937–3940. doi: 10.1039/c7cc00720e. [DOI] [PubMed] [Google Scholar]
- Choi Y.J., Hur J.M., Lim S., Jo M., Kim D.H., Choi J.I. Induction of apoptosis by deinoxanthin in human cancer cells. Anticancer Res. 2014;34(4):1829–1835. [PubMed] [Google Scholar]
- Culotta V.C., Daly M.J. Manganese complexes: diverse metabolic routes to oxidative stress resistance in prokaryotes and yeast. Antioxid. Redox Signal. 2013;19(9):933–944. doi: 10.1089/ars.2012.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S., Chen Q., Jiang M., Wang B., Xie Z., Yu N., Zhou Y., Li S., Wang L., Hua Y., Tian B. Colonized extremophile Deinococcus radiodurans alleviates toxicity of cadmium and lead by suppressing heavy metal accumulation and improving antioxidant system in rice. Environ. Pollut. 2021;284 doi: 10.1016/j.envpol.2021.117127. [DOI] [PubMed] [Google Scholar]
- Daly M.J. Engineering radiation-resistant bacteria for environmental biotechnology. Curr. Opin. Biotechnol. 2000;11(3):280–285. doi: 10.1016/s0958-1669(00)00096-3. [DOI] [PubMed] [Google Scholar]
- Daly M.J., Gaidamakova E.K., Matrosova V.Y., Vasilenko A., Zhai M., Venkateswaran A., Hess M., Omelchenko M.V., Kostandarithes H.M., Makarova K.S., Wackett L.P., Fredrickson J.K., Ghosal D. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science. 2004;306(5698):1025–1028. doi: 10.1126/science.1103185. [DOI] [PubMed] [Google Scholar]
- Daly M.J., Gaidamakova E.K., Matrosova V.Y., Kiang J.G., Fukumoto R., Lee D.Y., Wehr N.B., Viteri G.A., Berlett B.S., Levine R.L. Small-molecule antioxidant proteome-shields in Deinococcus radiodurans. PLoS ONE. 2010;5(9):e12570. doi: 10.1371/journal.pone.0012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Vega U.P., Rettberg P., Reitz G. Simulation of the environmental climate conditions on Martian surface and its effect on Deinococcus radiodurans. Adv. Space Res. 2007;40(11):1672–1677. doi: 10.1016/j.asr.2007.05.022. [DOI] [Google Scholar]
- Dollery S.J., Zurawski D.V., Gaidamakova E.K., Matrosova V.Y., Tobin J.K., Wiggins T.J., Bushnell R.V., MacLeod D.A., Alamneh Y.A., Abu-Taleb R., Escatte M.G., Meeks H.N., Daly M.J., Tobin G.J. Radiation-inactivated Acinetobacter baumannii vaccine candidates. Vaccines (Basel) 2021;9(2):96. doi: 10.3390/vaccines9020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Tian B., Dai S., Li T., Guo L., Tan Z., Jiao Z., Jin Q., Wang Y., Hua Y. Expression of PprI from Deinococcus radiodurans improves lactic acid production and stress tolerance in Lactococcus lactis. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0142918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emond S., Mondeil S., Jaziri K., André I., Monsan P., Remaud-Siméon M., Potocki-Véronèse G. Cloning, purification and characterization of a thermostable amylosucrase from Deinococcus geothermalis. FEMS Microbiol. Lett. 2008;285(1):25–32. doi: 10.1111/j.1574-6968.2008.01204.x. [DOI] [PubMed] [Google Scholar]
- Fredrickson J.K., Kostandarithes H.M., Li S.W., Plymale A.E., Daly M.J. Reduction of Fe(III), Cr(VI), U(VI), and Tc(VII) by Deinococcus radiodurans R1. Appl. Environ. Microbiol.. 2000;66(5):2006–2011. doi: 10.1128/AEM.66.5.2006-2011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidamakova E.K., Myles I.A., McDaniel D.P., Fowler C.J., Valdez P.A., Naik S., Gayen M., Gupta P., Sharma A., Glass P.J., Maheshwari R.K., Datta S.K., Daly M.J. Preserving immunogenicity of lethally irradiated viral and bacterial vaccine epitopes using a radio- protective Mn2+-Peptide complex from Deinococcus. Cell Host Microbe. 2012;12(1):117–124. doi: 10.1016/j.chom.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., Omelchenko M.V., Gaidamakova E.K., Matrosova V.Y., Vasilenko A., Venkateswaran A., Zhai M., Kostandarithes H.M., Brim H., Makarova K.S., Wackett L.P., Fredrickson J.K., Daly M.J. How radiation kills cells: survival of Deinococcus radiodurans and Shewanella oneidensis under oxidative stress. FEMS Microbiol. Rev. 2005;29(2):361–375. doi: 10.1016/j.femsre.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Gogada R., Singh S.S., Lunavat S.K., Pamarthi M.M., Rodrigue A., Vadivelu B., Phanithi P.B., Gopala V., Apte S.K. Engineered Deinococcus radiodurans R1 with NiCoT genes for bioremoval of trace cobalt from spent decontamination solutions of nuclear power reactors. Appl. Microbiol. Biotechnol. 2015;99(21):9203–9213. doi: 10.1007/s00253-015-6761-4. [DOI] [PubMed] [Google Scholar]
- Guérin F., Barbe S., Pizzut-Serin S., Potocki-Véronèse G., Guieysse D., Guillet V., Monsan P., Mourey L., Remaud-Siméon M., André I., Tranier S. Structural investigation of the thermostability and product specificity of amylosucrase from the bacterium Deinococcus geothermalis. J. Biol. Chem. 2012;287(9):6642–6654. doi: 10.1074/jbc.M111.322917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P., Gayen M., Smith J.T., Gaidamakova E.K., Matrosova V.Y., Grichenko O., Knollmann-Ritschel B., Daly M.J., Kiang J.G., Maheshwari R.K. MDP: a Deinococcus Mn2+-decapeptide complex protects mice from ionizing radiation. PLoS ONE. 2016;11(8) doi: 10.1371/journal.pone.0160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K., Sugahara T., Ohnishi T., Ozaki Y., Obiya Y., Miki S., Miki T., Imamura M., Kobayashi Y., Watanabe H., Akashi M., Furusawa Y., Mizuma N., Yamanaka H., Ohashi E., Yamaoka C., Yajima M., Fukui M., Nakano T., Takahashi S., Amano T., Sekikawa K., Yanagawa K., Nagaoka S. Inhibition in a microgravity environment of the recovery of Escherichia coli cells damaged by heavy ion beams during the NASDA ISS phase I program of NASA Shuttle/Mir mission no. 6. Int. J. Mol. Med. 1998;1(5):817–822. doi: 10.3892/ijmm.1.5.817. [DOI] [PubMed] [Google Scholar]
- Hossein Helalat S., Bidaj S., Samani S., Moradi M. Producing alcohol and salt stress tolerant strain of Saccharomyces cerevisiae by heterologous expression of pprI gene. Enzyme Microb. Technol. 2019;124:17–22. doi: 10.1016/j.enzmictec.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Ikemotom K., Sakamotom H., Nakanom M. Crystal structure and characterization of pyrroloquinoline quinone disodium trihydrate. Chem. Cent. J. 2012;6(1):57. doi: 10.1186/1752-153X-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger K.E., Ransac S., Dijkstra B.W., Colson C., van Heuvel M., Misset O. Bacterial lipases. FEMS Microbiol. Rev. 1994;15(1):29–63. doi: 10.1111/j.1574-6976. [DOI] [PubMed] [Google Scholar]
- Jang S.W., Cho C.H., Jung Y.S., Rha C., Nam T.G., Kim D.O., Lee Y.G., Baek N.I., Park C.S., Lee B.H., Lee S.Y., Shin H.S., Seo D.H. Enzymatic synthesis of α-flavone glucoside via regioselective transglucosylation by amylosucrase from Deinococcus geothermalis. PLoS ONE. 2018;13(11) doi: 10.1371/journal.pone.0207466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S.W., Kim J.H., Kim J.W., Kim C.Y., Kim S.Y., Choi Y.J. Metabolic engineering of extremophilic bacterium Deinococcus radiodurans for the production of the novel carotenoid deinoxanthin. Microorganisms. 2021;9(1):44. doi: 10.3390/microorganisms9010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe M.H., Lee K.H., Lim S.Y., Im S.H., Song H.P., Lee I.S., Kim D.H. Pigment-based whole-cell biosensor system for cadmium detection using genetically engineered Deinococcus radiodurans. Bioprocess. Biosyst. Eng. 2012;35(1–2):265–272. doi: 10.1007/s00449-011-0610-3. [DOI] [PubMed] [Google Scholar]
- Jung J.H., Seo D.H., Ha S.J., Song M.C., Cha J., Yoo S.H., Kim T.J., Baek N.I., Baik M.Y., Park C.S. Enzymatic synthesis of salicin glycosides through transglycosylation catalyzed by amylosucrases from Deinococcus geothermalis and Neisseria polysaccharea. Carbohydr. Res. 2009;344(13):1612–1619. doi: 10.1016/j.carres.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Jung Y.S., Kim Y.J., Kim A.T., Jang D., Kim M.S., Seo D.H., Nam T.G., Rha C.S., Park C.S., Kim D.O. Enrichment of polyglucosylated isoflavones from soybean isoflavone aglycones using optimized amylosucrase transglycosylation. Molecules. 2020;25(1):181. doi: 10.3390/molecules25010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin S., Mrazek J. Predicted highly expressed and putative alien genes of Deinococcus radiodurans and implications for resistance to ionizing radiation damage. Proc. Natl. Acad. Sci. U. S. A. 2001;98(9):5240–5245. doi: 10.1073/pnas.081077598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana H., Hazari P.P., Mishra A.K. Radioprotective efficacy of GSH based peptidomimetic complex of manganese against radiation induced damage: DT(GS)2Mn(II) Free Radic. Biol. Med. 2019;145:161–174. doi: 10.1016/j.freeradbiomed.2019.09.023. [DOI] [PubMed] [Google Scholar]
- Kim E.R., Rha C.S., Jung Y.S., Choi J.M., Kim G.T., Jung D.H., Kim T.J., Seo D.H., Kim D.O., Park C.S. Enzymatic modification of daidzin using heterologously expressed amylosucrase in Bacillus subtilis. Food Sci. Biotechnol. 2019;28(1):165–174. doi: 10.1007/s10068-018-0453-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.H., Park Y.D., Park H., Moon K.O., Ha K.T., Baek N.I., Park C.S., Joo M., Cha J. Synthesis and biological evaluation of a novel baicalein glycoside as an anti-inflammatory agent. Eur. J. Pharmacol. 2014;744:147–156. doi: 10.1016/j.ejphar.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Kim M.D., Jung D.H., Seo D.H., Jung J.H., Seo E.J., Baek N.I., Yoo S.H., Park C.S. Acceptor specificity of amylosucrase from Deinococcus radiopugnans and its application for synthesis of rutin derivatives. J. Microbiol. Biotechnol. 2016;26(11):1845–1854. doi: 10.4014/jmb.1606.06036. [DOI] [PubMed] [Google Scholar]
- Kim Y.J., Siziya I.N., Hong S., Lee G.Y., Seo M.J., Kim Y.R., Yoo S.H., Park C.S., Seo D.H. Biosynthesis of glyceride glycoside (nonionic surfactant) by amylosucrase, a powerful glycosyltransferase. Food Sci. Biotechnol. 2021;30(2):267–276. doi: 10.1007/s10068-020-00861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Watanabe H., Kikuchi M., Narumi I. Effect of the space environment on the induction of DNA-repair related proteins and recovery from radiation damage. Adv. Space Res. 2000;25(10):2103–2106. doi: 10.1016/s0273-1177(99)01061-3. [DOI] [PubMed] [Google Scholar]
- Kongpol A., Kato J., Vangnai A.S. Isolation and characterization of Deinococcus geothermalis T27, a slightly thermophilic and organic solvent-tolerant bacterium able to survive in the presence of high concentrations of ethyl acetate. FEMS Microbiol. Lett. 2008;286(2):227–235. doi: 10.1111/j.1574-6968.2008.01273.x. [DOI] [PubMed] [Google Scholar]
- Krisko A., Radman M. Protein damage and death by radiation in Escherichia coli and Deinococcus radiodurans. Proc. Natl. Acad. Sci. U. S. A. 2010;107(32):14373–14377. doi: 10.1073/pnas.1009312107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S., Ballal A., Apte S.K. Bioprecipitation of uranium from alkaline waste solutions using recombinant Deinococcus radiodurans. J. Hazard. Mater. 2013;262:853–861. doi: 10.1016/j.jhazmat.2013.09.057. [DOI] [PubMed] [Google Scholar]
- Lange C.C., Wackett L.P., Minton K.W., Daly M.J. Engineering a recombinant Deinococcus radiodurans for organopollutant degradation in radioactive mixed waste environments. Nat. Biotechnol. 1998;16(10):929–933. doi: 10.1038/nbt1098-929. [DOI] [PubMed] [Google Scholar]
- Lee E.S., Song E.J., Nam Y.D., Nam T.G., Kim H.J., Lee B.H., Seo M.J., Seo D.H. Effects of enzymatically modified chestnut starch on the gut microbiome, microbial metabolome, and transcriptome of diet-induced obese mice. Int. J. Biol. Macromol. 2020;145:235–243. doi: 10.1016/j.ijbiomac.2019.12.169. [DOI] [PubMed] [Google Scholar]
- Lee H.S., Kim T.S., Parajuli P., Pandey R.P., Sohng J.K. Sustainable production of dihydroxybenzene glucosides using immobilized amylosucrase from Deinococcus geothermalis. J. Microbiol. Biotechnol. 2018;28(9):1447–1456. doi: 10.4014/jmb.1805.05054. [DOI] [PubMed] [Google Scholar]
- Letona C.A.M., Park C.S., Kim Y.R. Amylosucrase-mediated β-carotene encapsulation in amylose microparticles. Biotechnol. Prog. 2017;33(6):1640–1646. doi: 10.1002/btpr.2521. [DOI] [PubMed] [Google Scholar]
- Lim M.C., Lee G.H., Huynh D.T.N., Hong C.E., Park S.Y., Jung J.Y., Park C.S., Ko S., Kim Y.R. Biological preparation of highly effective immunomagnetic beads for the separation, concentration, and detection of pathogenic bacteria in milk. Colloids Surf. B Biointerfaces. 2016;145:854–861. doi: 10.1016/j.colsurfb.2016.05.077. [DOI] [PubMed] [Google Scholar]
- Lin S.M., Baek C.Y., Jung J.H., Kim W.S., Song H.Y., Lee J.H., Ji H.J., Zhi Y., Kang B.S., Bahn Y.S., Seo H.S., Lim S. Antioxidant activities of an exopolysaccharide (DeinoPol) produced by the extreme radiation-resistant bacterium Deinococcus radiodurans. Sci. Rep. 2020;10(1):55. doi: 10.1038/s41598-019-56141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Wang T., Li X., Yang Y., Zhou M., Li M., Yan Z. Enhancement of bioelectricity generation via heterologous expression of IrrE in Pseudomonas aeruginosa-inoculated MFCs. Biosens. Bioelectron. 2018;117:23–31. doi: 10.1016/j.bios.2018.05.052. [DOI] [PubMed] [Google Scholar]
- Luo W., Xu J., Chen H., Zhang H., Yang P., Yu X. Synthesis of L-asparagine catalyzed by a novel asparagine synthase coupled with an ATP regeneration system. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.747404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqbool I., Sudharsan M., Kanimozhi G., Alrashood S.T., Khan H.A., Prasad N.R. Crude cell-free extract from Deinococcus radiodurans exhibit anticancer activity by inducing apoptosis in triple-negative breast cancer cells. Front. Cell Dev. Biol. 2020;8:707. doi: 10.3389/fcell.2020.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra C.S., Appukuttan D., Kantamreddi V.S.S., Rao A.S., Apte S.K. Recombinant D. radiodurans cells for bioremediation of heavy metals from acidic/neutral aqueous wastes. Bioeng. Bugs. 2012;3(1):44–48. doi: 10.4161/bbug.3.1.18878. [DOI] [PubMed] [Google Scholar]
- Misra C.S., Mukhopadhyaya R., Apte S.K. Harnessing a radiation inducible promoter of Deinococcus radiodurans for enhanced precipitation of uranium. J. Biotechnol. 2014;189:88–93. doi: 10.1016/j.jbiotec.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Misra C.S., Sounderajan S., Apte S.K. Metal removal by metallothionein and an acid phosphatase PhoN, surface-displayed on the cells of the extremophile, Deinococcus radiodurans. J. Hazard. Mater. 2021;419 doi: 10.1016/j.jhazmat.2021.126477. [DOI] [PubMed] [Google Scholar]
- Misra H.S., Khairnar N.P., Barik A., Indira Priyadarsini K., Mohan H., Apte S.K. Pyrroloquinoline-quinone: a reactive oxygen species scavenger in bacteria. FEBS Lett. 2004;578(1–2):26–30. doi: 10.1016/j.febslet.2004.10.061. [DOI] [PubMed] [Google Scholar]
- Moon K.O., Park S., Joo M., Ha K.T., Baek N.I., Park C.S., Cha J. Glycosylation enhances the physicochemical properties of caffeic acid phenethyl ester. J. Microbiol. Biotechnol. 2017;27(11):1916–1924. doi: 10.4014/jmb.1706.06017. [DOI] [PubMed] [Google Scholar]
- Moon K., Lee S., Park H., Cha J. Enzymatic synthesis of resveratrol α-glucoside by amylosucrase of Deinococcus geothermalis. J. Microbiol. Biotechnol. 2021;31(12):1692–1700. doi: 10.4014/jmb.2108.08034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimha A., Basu B. New insights into the activation of radiation desiccation response regulon in Deinococcus radiodurans. J. Biosci. 2021;46:10. [PubMed] [Google Scholar]
- Ogawa M., Uyeda A., Harada K., Sato Y., Kato Y., Watanabe H., Honda K., Matsuura T. Class III polyphosphate kinase 2 enzymes catalyze the pyrophosphorylation of adenosine-5′-monophosphate. ChemBioChem. 2019;20(23):2961–2967. doi: 10.1002/cbic.201900303. [DOI] [PubMed] [Google Scholar]
- Panitz C., Frösler J., Wingender J., Flemming H.C., Rettberg P. Tolerances of Deinococcus geothermalis biofilms and planktonic cells exposed to space and simulated Martian conditions in low earth orbit for almost two years. Astrobiology. 2019;19(8):979–994. doi: 10.1089/ast.2018.1913. [DOI] [PubMed] [Google Scholar]
- Razzaq A., Shamsi S., Ali A., Ali Q., Sajjad M., Malik A., Ashraf M. Microbial proteases applications. Front. Bioeng. Biotechnol. 2019;7:110. doi: 10.3389/fbioe.2019.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rha C.S., Choi J.M., Jung Y.S., Kim E.R., Ko M.J., Seo D.H., Kim D.O., Park C.S. High-efficiency enzymatic production of α-isoquercitrin glucosides by amylosucrase from Deinococcus geothermalis. Enzyme Microb. Technol. 2019;120:84–90. doi: 10.1016/j.enzmictec.2018.10.006. [DOI] [PubMed] [Google Scholar]
- Rha C.S., Kim E.R., Kim Y.J., Jung Y.S., Kim D.O., Park C.S. Simple and efficient production of highly soluble daidzin glycosides by amylosucrase from Deinococcus geothermalis. J. Agric. Food Chem. 2019;67(46):12824–12832. doi: 10.1021/acs.jafc.9b05380. [DOI] [PubMed] [Google Scholar]
- Rha C.S., Kim H.G., Baek N.I., Kim D.O., Park C.S. Amylosucrase from Deinococcus geothermalis can be modulated under different reaction conditions to produce novel quercetin 4′-O-α-D-isomaltoside. Enzyme Microb. Technol. 2020;141 doi: 10.1016/j.enzmictec.2020.109648. [DOI] [PubMed] [Google Scholar]
- Saffary R., Nandakumar R., Spencer D., Robb F.T., Davila J.M., Swartz M., Ofman L., Thomas R.J., DiRuggiero J. Microbial survival of space vacuum and extreme ultraviolet irradiation: strain isolation and analysis during a rocket flight. FEMS Microbiol. Lett. 2002;215(1):163–168. doi: 10.1111/j.1574-6968.2002.tb11386.x. [DOI] [PubMed] [Google Scholar]
- Satoh K., Wada S., Kikuchi M., Funayama T., Narumi I., Kobayashi Y. Method for detecting DNA strand breaks in mammalian cells using the Deinococcus radiodurans PprA protein. Mutat. Res. 2006;596(1–2):36–42. doi: 10.1016/j.mrfmmm.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Seo D.H., Jung J.H., Ha S.J., Song M.C., Cha J., Yoo S.H., Kim T.J., Baek N.I., Park C.S. Highly selective biotransformation of arbutin to arbutin-α-glucoside using amylosucrase from Deinococcus geothermalis DSM 11300. J. Mol. Catal. - B Enzym. 2009;60(3–4):113–118. doi: 10.1016/j.molcatb.2009.04.006. [DOI] [Google Scholar]
- Seo D.H., Jung J.H., Ha S.J., Cho H.K., Jung D.H., Kim T.J., Baek N.I., Yoo S.H., Park C.S. High-yield enzymatic bioconversion of hydroquinone to α-arbutin, a powerful skin lightening agent, by amylosucrase. Appl. Microbiol. Biotechnol. 2012;94(5):1189–1197. doi: 10.1007/s00253-012-3905-7. [DOI] [PubMed] [Google Scholar]
- Shao H., Xu L., Yan Y. Thermostable lipases from extremely radioresistant bacterium Deinococcus radiodurans: cloning, expression, and biochemical characterization. J. Basic Microbiol. 2014;54(9):984–995. doi: 10.1002/jobm.201300434. [DOI] [PubMed] [Google Scholar]
- Shim H.E., Yang J.E., Jeong S.W., Lee C.H., Song L., Mushtaq S., Choi D.S., Choi Y.J., Jeon J. Silver nanomaterial-immobilized desalination systems for efficient removal of radioactive iodine species in water. Nanomaterials (Basel) 2018;8(9):660. doi: 10.3390/nano8090660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Song M.H., Oh J.W., Keum Y.S., Saini R.K. Pro-oxidant actions of carotenoids in triggering apoptosis of cancer cells: a review of emerging evidence. Antioxidants (Basel) 2020;9(6):532. doi: 10.3390/antiox9060532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade D., Radman M. Oxidative stress resistance in Deinococcus radiodurans. Microbiol. Mol. Biol. Rev. 2011;75(1):133–191. doi: 10.1128/MMBR.00015-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Nishimura T., Nomura K., Sugimoto K., Kuriki T. Syntheses of arbutin-alpha-glycosides and a comparison of their inhibitory effects with those of alpha-arbutin and arbutin on human tyrosinase. Chem. Pharm. Bull. (Tokyo) 2003;51(7):798–801. doi: 10.1248/cpb.51.798. [DOI] [PubMed] [Google Scholar]
- Suresh K., Reddy G.S.N., Sengupta S., Shivaji S. Deinococcus indicus sp. nov., an arsenic-resistant bacterium from an aquifer in West Bengal, India. Int. J. Syst. Evol. Microbiol. 2004;54(Pt 2):457–461. doi: 10.1099/ijs.0.02758-0. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Hara R., Kino K. Production of aminoacyl prolines using the adenylation domain of nonribosomal peptide synthetase with class III polyphosphate kinase 2-mediated ATP regeneration. J. Biosci. Bioeng. 2018;125(6):644–648. doi: 10.1016/j.jbiosc.2017.12.023. [DOI] [PubMed] [Google Scholar]
- Tapia C., López B., Astuya A., Becerra J., Gugliandolo C., Parra B., Martínez M. Antiproliferative activity of carotenoid pigments produced by extremophile bacteria. Nat. Prod. Res. 2021;35(22):4638–4642. doi: 10.1080/14786419.2019.1698574. [DOI] [PubMed] [Google Scholar]
- Tian B., Li J., Pang R., Dai S., Li T., Weng Y., Jin Y., Hua Y. Gold nanoparticles biosynthesized and functionalized using a hydroxylated tetraterpenoid trigger gene expression changes and apoptosis in cancer cells. ACS Appl. Mater. Interfaces. 2018;10(43):37353–37363. doi: 10.1021/acsami.8b09206. [DOI] [PubMed] [Google Scholar]
- Tobin G.J., Tobin J.K., Gaidamakova E.K., Wiggins T.J., Bushnell R.V., Lee W.M., Matrosova V.Y., Dollery S.J., Meeks H.N., Kouiavskaia D., Chumakov K., Daly M.J. A novel gamma radiation-inactivated sabin-based polio vaccine. PLoS ONE. 2020;15(1) doi: 10.1371/journal.pone.0228006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujaoney A.K., Padwal M.K., Basu B. Proteome dynamics during post-desiccation recovery reveal convergence of desiccation and gamma radiation stress response pathways in Deinococcus radiodurans. Biochim. Biophys. Acta Proteins Proteom. 2017;1865(9):1215–1226. doi: 10.1016/j.bbapap.2017.06.014. [DOI] [PubMed] [Google Scholar]
- Ujaoney A.K., Padwal M.K., Basu B. An in vivo interaction network of DNA-repair proteins: a snapshot at double strand break repair in Deinococcus radiodurans. J. Proteome Res. 2021;20(6):3242–3255. doi: 10.1021/acs.jproteome.1c00078. [DOI] [PubMed] [Google Scholar]
- Venkateswaran A., McFarlan S.C., Ghosal D., Minton K.W., Vasilenko A., Makarova K., Wackett L.P., Daly M.J. Physiologic determinants of radiation resistance in Deinococcus radiodurans. Appl. Environ. Microbiol. 2000;66(6):2620–2626. doi: 10.1128/AEM.66.6.2620-2626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White O., Eisen J.A., Heidelberg J.F., Hickey E.K., Peterson J.D., Dodson R.J., Haft D.H., Gwinn M.L., Nelson W.C., Richardson D.L., Moffat K.S., Qin H., Jiang L., Pamphile W., Crosby M., Shen M., Vamathevan J.J., Lam P., McDonald L., Utterback T., Zalewski C., Makarova K.S., Aravind L., Daly M.J., Minton K.W., Fleischmann R.D., Ketchum K.A., Nelson K.E., Salzberg S., Smith H.O., Venter J.C., Fraser C.M. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science. 1999;286(5444):1571–1577. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Wu K., Han H., Ling Z., Chen Z., Liu P., Xiong J., Tian F., Zafar Y., Malik K., Li X. Co-expression of YieF and PhoN in Deinococcus radiodurans R1 improves uranium bioprecipitation by reducing chromium interference. Chemosphere. 2018;211:1156–1165. doi: 10.1016/j.chemosphere.2018.08.061. [DOI] [PubMed] [Google Scholar]
- Zahradka K., Slade D., Bailone A., Sommer S., Averbeck D., Petranovic M., Lindner A.B., Radman M. Reassembly of shattered chromosomes in Deinococcus radiodurans. Nature. 2006;443(7111):569–573. doi: 10.1038/nature05160. [DOI] [PubMed] [Google Scholar]