Abstract
Compared to most mammals, human pregnancy is unusual in that it involves chromosomally diverse embryos, cyclical breakdown and regeneration of the uterine mucosa, and intimate integration of fetal and maternal cells at the uteroplacental interface. Not surprisingly, pregnancy often falters in early gestation. Whether these losses result in clinical miscarriages depends on the origins and impacts of chromosomal errors on fetal development and the ability of the decidualizing endometrium to engage in embryo biosensing and selection. Aneuploidy originating in oocytes during meiosis drives the age-related risk of miscarriage. By contrast, the frequency of endometrial cycles with an impaired decidual response may account for the stepwise increase in miscarriage rates with each pregnancy loss independently of maternal age. Additional physiological mechanisms operate in early gestation to ensure that most failing pregnancies are lost before vascular maternal-fetal connections are established by the end of the first trimester. Here, we summarise how investigations into the mechanisms that cause miscarriage led to new insights into the processes that govern maternal selection of human embryos in early gestation.
Keywords: miscarriage, embryo, aneuploidy, endometrium, decidualization, placenta, selection
1. Introduction
Pregnancy begins when the implanting embryo breaches the uterine mucosa. Once the conceptus embeds in the endometrial stroma, maternal serum and urine levels of embryo-derived human chorionic gonadotrophin (hCG) rise, typically around 7 to 9 days after ovulation [1]. Based on sensitive urine hCG measurements, several prospective studies reported miscarriage rates of approximately 30% in young, healthy women trying to conceive [1–3]. Many losses, however, occur soon after implantation and escape detection. The population prevalence of women with one, two or three or more self-reported miscarriages is 10·8%, 1·9%, and 0·7%, respectively [4]. More than 92% of recognised miscarriages occur before 12 weeks of pregnancy [5].
Two independent risk factors, maternal age and the number of previous pregnancy losses, have disproportionate effects on the miscarriage rate [5, 6]. The age-specific risk of miscarriage follows a J-shaped curve [5, 7]. The risk is significantly higher in adolescents, flattens between the ages of 20 and 34 years, and rises again sharply in women over 34 years old (Fig. 1A). Fetal chromosomal errors, or aneuploidies, are the primary driver of age-specific miscarriage risk. The J-shaped curve of age-related miscarriages mirrors the incidences of meiotic chromosome errors in oocytes [8], preimplantation embryos [9, 10], and fetal tissues [11, 12] (Fig. 1A). The impact of previous pregnancy losses on miscarriage rates is also well documented but poorly understood. Several large epidemiological studies have documented a stepwise increase in the recurrence risk of miscarriage by approximately 10% with each additional loss (Fig. 1B), independently of maternal age and other covariates [5, 6].
Figure 1.
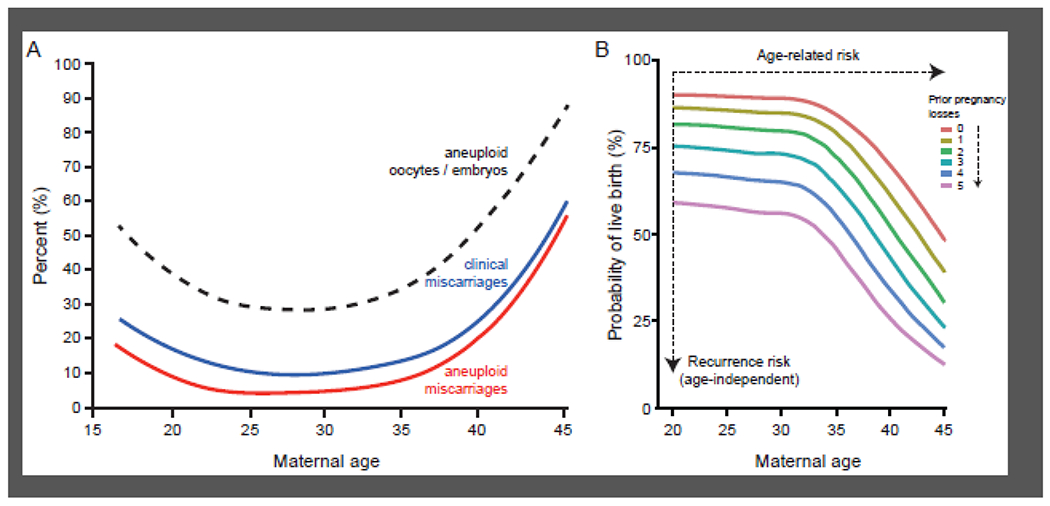
Risks of miscarriage. (A) Composite graph showing risk of miscarriage according to maternal age. Superimposed are the age-dependent incidences of oocyte/embryo aneuploidy and aneuploid miscarriage. The composite graph is based on data extracted from several studies [5, 7–9]. (B) Miscarriage rates increase with maternal age and with each additional miscarriage. The term ‘recurrence risk’ of miscarriage denotes the stepwise increase in miscarriage rates, independently of maternal age, interpregnancy interval, or a previous live birth. Adapted from Kolte and colleagues [6], with permission.
All clinical definitions of recurrent miscarriage are based on an arbitrary number of consecutive or non-consecutive clinical pregnancy losses, usually two or three [13]. Consequently, many studies take a binary approach that does not account for the marked differences in prognoses between individual patients. Numerous pathological mechanisms are invoked to explain recurrent miscarriage [13, 14], although none provide a robust explanation for the stepwise increase in recurrence rate with each additional miscarriage. It is important to emphasize that multiple miscarriages do not preclude a successful pregnancy. Even after four consecutive losses, it remains more likely that a subsequent pregnancy will succeed than fail for women under the age of 36 years (Fig. 1B). Thus, a pivotal challenge is to define the mechanisms that account for recurrence of miscarriage without precluding the possibility of a successful pregnancy.
This review starts with an overview of different paradigms of early pregnancy loss. We then summarise the mechanisms accounting for fetal aneuploidies, beginning with gametes and preimplantation embryos, and assess their contributions to the recurrence risk of miscarriage. Next, we discuss how recent insights into the dynamic nature of embryo-endometrial interactions at implantation have highlighted the importance of maternal checkpoint failures in determining the recurrence risk of miscarriage. Finally, we focus on the pathogenic pathways that converge on the uteroplacental interface in early pregnancy and explore the role of local immune cells.
2. Miscarriage paradigms
The clinical approach to miscarriage is based on the general principle that all physiological processes in the body depend on cooperation between different cell populations in tissues and organs. Early pregnancy loss, discerned or not, is therefore attributed to either inborn errors (i.e., embryonic aneuploidies) or extrinsic pathological processes purported to interfere with the cooperative interactions between embryonic and maternal cells. Consequently, it is common practice to test recurrent miscarriage patients for a host of subclinical disorders, including endocrine perturbations, structural uterine anomalies, thrombophilia, immune disorders, pathogenic mutations, and lifestyle factors [13, 14]. The test results then guide treatment. While this approach appears sensible, two vexing problems remain unresolved. First, no modifiable risk factors are identified in over 50% of couples affected by recurrent miscarriage [13, 14]. Second, there is limited or no evidence that treatments targeting perceived risk factors of miscarriage improve the prognosis for patients, despite numerous clinical trials [14]. Even in the presence of overt chronic disease, including autoimmune disorders, the incidence of miscarriage is largely unaffected [15].
A different perspective on miscarriage emerged from the parent-offspring conflict hypothesis [16], which posits that the interactions between genetically distinct fetal and maternal cells at the mammalian uteroplacental interface are driven by conflict as much as cooperation [17, 18]. Conflicts arise because embryonic genes are evolutionarily selected to maximise the chance of implantation whereas maternal genes evolve to minimise the risk of continued investment of resources in a failing pregnancy or in supporting a fetus with low fitness [18]. This hidden evolutionary tug-of-war between maternal and embryonic genomes is credited for not only the remarkable diversity of placental structures amongst mammals but also the emergence of unique reproductive features [19]. A good example is the vast array of complex chromosomal imbalances in human embryos, many of which do not preclude successful pregnancy [20, 21]. A peculiar maternal reproductive feature, confined mainly to humans and other simians, is the cyclical shedding of the upper endometrial layer at menstruation [22]. Further, comparative studies of placental mammals showed that human trophoblast cells penetrate the uterine wall very deeply, remodel uterine arteries extensively, and induce a marked maternal cardiovascular response [22, 23]. Uterine perfusion increases from 45 mL/minute during the menstrual cycle to 750 mL/minute at term [24]. Maternal energy expenditure over the duration of pregnancy is close to physiological limits and comparable to that of endurance athletes [25].
Thus, human pregnancy uniquely starts with the implantation of a genetically distinct embryo in a freshly regenerated endometrium and leads to the formation of a deeply invading placenta capable of radically altering maternal physiology. At a glance, this reproductive strategy seems reckless. However, several physiological ‘checkpoints’ in early gestation ensure that most pregnancies involving a low-fitness conceptus or an inadequately prepared endometrium fail before the onset of placental perfusion at around 12 weeks (Fig. 2A) [26]. The earlier the pregnancy loss, the smaller the impact on maternal fitness [18, 27]. Thus, the parent-offspring conflict paradigm posits that the high rate of pregnancy loss soon after conception reflects, at least partly, a robust maternal implantation checkpoint. It predicts that failure of this initial checkpoint will lead to more clinically recognised pregnancies but also more miscarriages.
Figure 2.

Tests for success. (A) Several physiological mechanisms limit the risk of prolonged maternal investment in a failing pregnancy. The ‘blastocyst checkpoint’ refers to embryo-intrinsic mechanisms that balances developmental arrest with self-correction in response to different levels of mosaicism. The ‘implantation checkpoint’ involves biosensing of the conceptus by encapsulating maternal decidual cells, whereas the ‘fitness checkpoint’ refers to the maintenance of ovarian progesterone production in response to embryonic fitness signals, such as hCG. Marked vascular changes impose a further stress-test on the decidual-placental interface at the end of the first trimester. EpC, epithelial cells; DSC, decidualized stromal cells; SCT, syncytiotrophoblast; VCT, villous cytotrophoblast; CS, cytotrophoblast shell; EVT, extravillous cytotrophoblast; TGC, trophoblast giant cells; LMP, last menstrual period. (B) Transition of the cycling endometrium into the decidua of pregnancy requires cooperation between decidual cells and uNK cells to eliminate senescent decidual cells. SASP, senescence-associated secretory phenotype; IL-15, interleukin 15. (C) Endometrial fate decisions at implantation pivot on the balance between decidual subsets. Lack of BMPC and /or loss of uterine natural killer (uNK) cell activity drive a pro-senescent decidual response that renders uteroplacental interface vulnerable to tissue breakdown.
3. Genetic instability in human embryos
Many human embryos perish during early development, especially prior to the blastocyst stage [28]. Given that early embryonic mortality often coincides with major waves of embryonic genome activation prior to blastocyst formation, genetic causes have been suspected. While direct evidence remains elusive, recent statistical modelling suggests the occurrence of ~0.3–0.4 lethal or nearly lethal de novo point mutations per potential human zygote [29], while others have proposed that the genome-wide burden of weakly deleterious mutations influences embryonic survival [30]. Meanwhile, it has long been recognized that lethal whole chromosome abnormalities are very common in human embryos, with aneuploid miscarriages representing only the tip of the iceberg. In this section, we explore the origins and mechanisms of age-dependent embryonic aneuploidy, the functional and fitness consequences of aneuploidy, and the contribution of aneuploidy to miscarriage.
3.1. Origins of human aneuploidy
The fidelity of chromosome segregation during human gamete formation and early embryonic development is strikingly low. It is estimated that 40-60% of human embryos are lost between fertilization and birth, primarily due to aneuploidy, i.e. extra or missing chromosomes compared to the euploid 46-chromosome set [28]. Although a rigorous phylogenetic perspective on the evolution of embryonic aneuploidy is lacking, the rates in humans are much higher when compared to mice [31], but perhaps comparable to those reported in non-human primates, such as rhesus macaque (~75%) [32]. We note, however, that the practical challenge of controlling for maternal age, ovarian stimulation medications, IVF culture conditions, and other relevant environmental factors complicates such interspecific comparisons.
Preimplantation genetic testing for aneuploidy (PGT-A) seeks to improve the success of in vitro fertilization (IVF) by selecting euploid embryos for transfer [33]. It also offers an exceptional resource for studying human chromosome abnormalities. PGT-A data consistently reveal substantial karyotypic diversity arising from a variety of mechanisms of chromosome mis-segregation [34]. These include maternal meiotic errors, such as classical nondisjunction, precocious separation of sister chromatids (PSSC), and reverse segregation [35]. Nondisjunction is elevated during adolescence and declines through the early twenties, while PSSC and reverse segregation increase exponentially with advancing maternal age, starting around the mid-thirties [8, 36]. The mechanisms of these age associations remain obscure, though several non-mutually exclusive hypotheses have been proposed that generally involve deterioration of chromosome cohesion in oocytes during decades-long meiotic arrest [37]. Paternal meiotic errors are comparatively rare, despite the prevalence of male factor infertility. Aneuploidies affect only 1-5% of sperm and exhibit no discernible age association, though statistical power is limited given this infrequency [10].
While meiotic errors affect all embryonic cells, mitotic errors result in mosaicism, with two or more karyotypically distinct cell lineages (Fig. 2A). Mosaic aneuploidies are thought to arise through mechanisms including mitotic nondisjunction, anaphase lag, or endoreplication [36]. Current estimates of the incidence of mosaicism range from 4% to 90% [38], further fuelling a long-standing debate over the clinical usefulness of PGT-A [39]. While these wide-ranging estimates reflect both technical and biological variability, one crucial limitation of PGT-A is the reliance on biopsies of one or few cells. The resulting sampling variability is further compounded by the unknown spatial distribution of aneuploid cells within the embryo.
One notable observation from aggregated PGT-A data is the large variation in aneuploidy rates among embryos from different women, even after controlling for maternal age. Such patterns of overdispersion imply that aneuploidy rates are influenced by environmental or parental genetic risk factors [40]. Suspected environmental risk factors include exposure to endocrine-disrupting chemicals, such as bisphenol A and its analogues, which disrupt meiosis in model organisms and cultured human tissue [41]. Genetic risk factors for human aneuploidy have proven largely elusive, though recent studies are providing initial headway (Box 1).
Box 1: The search for genetic risk factors for human aneuploidy.
Identification of parental genetic risk factors of embryonic aneuploidy could potentially lead to screening of couples at risk of infertility and miscarriage. The largest genome-wide association study (GWAS) of aneuploidy risk to date reported one significant quantitative trait locus (QTL)—a common set of linked polymorphisms spanning the centrosome regulator PLK4 (polo like kinase 4). The embryos of women carrying the risk haplotype had higher rates of mitotic aneuploidy and were less likely to progress to the blastocyst stage [109]. Tripolar mitosis of diploid cells is a candidate mechanism driving this maternal effect [42] and exemplifies a broader class of complex mosaicism that is compatible with cleavage-stage development but arrests upon embryonic genome activation prior to blastocyst formation [10]. A further study of trisomy 21 reported putative associations at several meiosis-related genes, albeit below genome-wide significance thresholds [45]. Both studies were constrained to the discovery of common genetic variation because of small sample sizes (hundreds to thousands of patients). Population genetic theory predicts that heritability of traits closely tied to fitness will have an outsize contribution from rare and de novo mutations [46]. Characterization of such rare variants will require alternative approaches, such as whole-genome sequencing and meticulous validation in animal models or human cell lines.
3.2. Functional and fitness consequences of aneuploidy
Why certain chromosome abnormalities cause preimplantation embryonic arrest, but others remain viable into later development, is poorly understood. Phenotypic impacts of deletions and duplications, including aneuploidy and sub-chromosomal structural variation, are mediated by effects on gene expression [42]. These include primary dosage effects of chromosome gains and losses, secondary effects of dysregulated transcription factors that propagate to other chromosomes, as well as tertiary effects such as cellular stress responses, all of which have been documented in human embryos [43, 44]. These effects may propagate to the level of translation and elicit the build-up of misfolded proteins, which can aggregate and exert proteotoxic stress on cells [42]. Yet the relationship between gene dosage and activity at protein level is not necessarily linear due to buffering mechanisms at the translational and post-translational stages [45, 46]. Understanding these relationships using quantitative methods is a major goal of genomic research into the functional impacts of aneuploidy.
The fitness consequences of aneuploidy are linked to the number and identity of aneuploid cells and chromosomes. Meiotic aneuploidies arise during egg formation, impact all cells of the embryos, and are unambiguously harmful. Mitotic aneuploidies are restricted to the descendant cells of the erroneous mitotic division. While severe mosaicism may result in early embryonic arrest, lower levels of mosaicism are compatible with normal development and healthy live birth [20, 47, 48]. This observation implies that certain mosaic aneuploidies are subject to negative selection and actively or passively eliminated from the conceptus throughout development. Studies using mouse models and human embryonic stem cells indicate that aneuploid cells are purged from mosaic fetal lineages by autophagy-mediated apoptosis but tolerated within the trophectoderm, which goes on to form the placenta [49]. Analysis of single-cell sequencing data from human embryos indicates that this selection process may intensify during post-implantation development [44]. Additional mechanisms have been identified by which aneuploid cells are extruded from human and non-human primate embryos during blastocyst formation [32].
Recently, extended in vitro embryo culture systems have been used to examine the developmental consequences of specific aneuploidies during and after implantation. Popovic et al. [50], for example, reported that while trisomies of chromosomes 16, 21, and 22 remain viable through day 12 postfertilization, autosomal monosomies tend to arrest at the time of implantation or shortly thereafter. These findings were echoed by Shahbazi et al. [51], who also demonstrated that embryos with trisomy 16 exhibit hypo-proliferation of the trophoblast, potentially driven by cell adhesion defects induced by over-expression of the chromosome 16 gene E-cadherin (ECAD). Future work combining such systems with functional genomic profiling will help identify additional causal genes and mechanisms that drive mortality of aneuploid embryos.
3.3. The role of aneuploidy in miscarriage
Chromosome abnormalities are a common finding in sporadic miscarriage, with reported rates of 40 to 60 % [52, 53]. Some recent studies reported even higher rates, reflecting the demographic trends in delaying pregnancy [53], and the application of increasingly sensitive platforms for aneuploidy detection [54]. The age-related risk of miscarriage is largely accounted for by increased frequency of meiotic trisomy [7]. The incidence of chromosomal errors in sporadic miscarriage also varies by gestational age, peaking between 9 to 14 weeks of gestation [53]. Counterintuitively, anembryonic miscarriages and clinically recognized losses before 6 weeks of gestation are disproportionally euploid [55–57]. There is no evidence of geographic or ancestry-related variation in the incidence or pattern of chromosomal abnormalities [53], suggesting that the mechanisms causing aneuploid miscarriages are an inherent feature of human reproduction. Alternatively, maternal age may be such a powerful driver of aneuploidy that it masks the impact of ancestry and local environmental factors.
In the context of recurrent miscarriage, there are three pertinent outstanding questions. First, is the underlying rate of embryonic aneuploidy altered in recurrent miscarriage? One PGT-A study, involving 46,439 preimplantation human embryos, observed a small but significant elevation of maternal meiotic (but not mitotic) aneuploidy in blastocyst-stage embryos from recurrent miscarriage patients (age-adjusted odds ratio: 1·14, 95% confidence interval: 1.01 - 1.27) [10]. While intriguing, this modest effect size implies that patient-specific risk of aneuploid conception may be a minor contributor to recurrent miscarriage. Second, is the ratio of euploid versus aneuploid pregnancy losses different in recurrent miscarriage? The literature is replete with contradictory studies reporting mostly reduced, sometimes unchanged, and occasionally elevated rates of chromosome abnormalities in recurrent versus sporadic miscarriage [58–61]. A conspicuous problem with many studies is the failure to account for confounding variables, such as gestational age and the number of previous miscarriages. A final question, with important implications for clinical management, is whether embryonic aneuploidy offers a plausible explanation for the recurrence risk of miscarriage? As illustrated in Box 2, this seems doubtful.
Box 2: Can aneuploid embryos account for the recurrence risk of miscarriage?
The diagnosis of an aneuploid pregnancy loss is often considered a compelling explanation for recurrent miscarriage. Some clinicians recommend no further investigations or treatments, whereas others advocate IVF treatment with PGT-A. However, can the recurrence risk of miscarriage be explained by embryonic aneuploidy alone? To answer this question, consider the following numbers:
At the age of 30, the proportion of embryos that are aneuploid is 30% (Fig. 1A). In healthy women, the sporadic miscarriage rate is approximately 10% (Fig. 1B), half of which are reportedly aneuploid losses. As aneuploid pregnancies rarely result in live births, the aneuploid pregnancy rate will approximate the aneuploid miscarriage rate, that is, 5%. After three prior losses, however, the miscarriage rate rises to 40%. Some studies reported no change in the rate of aneuploid pregnancy losses between sporadic and recurrent miscarriage. If correct, the aneuploid pregnancy rate in recurrent miscarriage patients aged 30-years should approximate to 20%, i.e., half of the reported 40% miscarriage rate.
Is it possible to account for the relative increase in the aneuploid pregnancy rate from 5% to 20% between healthy women and recurrent miscarriage patients? This increase cannot be explained by a proportional increase in aneuploid embryos (since this would be from 30% to 120%). Thus, either the aneuploid miscarriage rate is overestimated or maternal selection at implantation is relaxed.
4. Embryo selection at implantation
The intrinsic genetic instability of human embryos imposes a major challenge onto the endometrium: how to eliminate embryos of low fitness without compromising implantation of high-quality embryos? Here, we describe the cellular events that enable the endometrium to recognise and respond to embryos of different qualities and discuss how pathogenic mechanisms that compromise this endometrial function cause miscarriage, irrespective of the ploidy status of the conceptus.
4.1. Endometrial cyclicity and decidualization
Between menarche and menopause, the endometrium undergoes hundreds of cycles of tissue breakdown, menstrual shedding, and scar-free regeneration in response to the rise and fall of ovarian hormone production. In each cycle, rapid oestrogen-dependent endometrial growth is followed by progesterone-dependent differentiation of glands and stroma. During the midluteal phase, the endometrial stroma remodels intensively, heralding the start of a short implantation window. This process of tissue remodelling, termed decidualization, is driven foremost by the transformation of stromal fibroblasts into epithelial-like decidual cells and accumulation of uterine natural killer (uNK) cells [62].
Decidualization of endometrial stromal cells is a multistep differentiation process, which starts with an acute cellular stress response and release of proinflammatory mediators (Fig. 2B) [63–66]. This initial inflammatory phase coincides with the implantation window [62, 66, 67]. After approximately four days of inflammatory reprogramming, specialized anti-inflammatory decidual cells emerge, highly resistant to metabolic and oxidative stressors, and exquisitely responsive to embryonic signals [62, 68–70]. However, some stromal cells already burdened by replication stress fail to differentiate and are earmarked for cellular senescence [63, 65]. Senescent decidual cells are progesterone-resistant and abundantly secrete a complex mixture of extracellular matrix proteins and proteinases, proinflammatory cytokines, and chemokines (termed senescence associated secretory phenotype), which cause sterile inflammation and induce secondary senescence in neighbouring decidual cells [65]. If left unchecked, spatiotemporal propagation of this senescence phenotype renders the decidua vulnerable to breakdown. Progesterone-dependent decidual cells can escape this default pathway by co-opting uNK cells to eliminate their senescent counterparts through perforin- and granzyme-containing granule exocytosis (Fig. 2B) [63, 65]. This process rejuvenates the endometrium at implantation and enables transformation of the stroma into a tightly adherent, immune-protective decidual matrix that coordinates trophoblast invasion and accommodates the placenta throughout pregnancy. Expansion of the decidua in early pregnancy relies on recruitment and differentiation of circulating bone marrow-derived progenitor cells (BMPC) into decidual cells [71]. In a nonconception cycle, however, progesterone production by the ovarian corpus luteum declines in the late-luteal phase, leading to a preponderance of senescent decidual cells, influx of neutrophiles and macrophages, tissue breakdown and menstrual shedding [72].
4.2. Implantation checkpoints
Decidual transformation of the endometrium occurs in all mammals with an invading placenta. Only in menstruating species is this differentiation process initiated in each cycle, instead of triggered by the implanting embryo [22]. Maternal control of the decidual process imposes multiple checkpoints on the conceptus (Fig. 2A). First, the embryo must implant at the right time in the cycle to direct the decidual reaction away from tissue destruction. Clinically, a delayed rise in hCG levels beyond the putative implantation window is strongly associated with miscarriage in the first two weeks of pregnancy [1]. Once embedded, the conceptus is rapidly encapsulated by migratory decidual cells [73–75]. These cells serve as biosensors of embryo quality, engaging in both negative and positive selection. In response to signals from low-quality embryos, as defined by morphological criteria, decidual cells mount an endoplasmic reticulum stress response, which inhibits secretion of crucial implantation factors and hinders embryo encapsulation [73, 75, 76]. By contrast, secreted factors from successful embryos enhance the expression of maternal implantation and metabolic genes, thus actively promoting further implantation [68]. How human embryos signal their developmental potential remains unclear. However, it is striking that hsa-miR-320a and hyaluronidase 2, two putative embryonic fitness cues that act on decidual and uNK cells, respectively, are also implicated in promoting pre-implantation development [77, 78]. Next, it is incumbent on the embryo to secrete sufficient levels of hCG to rescue ovarian progesterone biosynthesis until the placenta takes over progesterone production around eight weeks of pregnancy. Human embryos are exquisitely adapted to meet this herculean task; the gene encoding the biologically active β-subunit hCG (CGB) is duplicated six times in the genome and the glycosylated protein evolved to have a much longer halflife compared to the ancestral luteinizing hormone [17, 19].
4.3. Implantation checkpoint failure and recurrent miscarriage
Recurrent miscarriage is associated with a pro-senescent decidual response, characterised foremost by a lack of anti-inflammatory decidual cells (Fig. 2B). This presents the embryo with a maternal environment that is easy to invade, devoid of biosensing properties, and prone to breakdown. The term ‘implantation checkpoint failure’ denotes the functional consequences of this pathological state, i.e., an endometrium that neither supports normal embryos nor eliminates abnormal embryos at implantation.
Decidualization is an iterative process, finetuned by cyclical recruitment of uNK cells and BMPC [63, 79, 80]. Hence, the frequency of cycles with an aberrant decidual response, leading to implantation checkpoint failure, is determined by the stringency of these opposing (homeostatic) regulatory mechanisms. For example, recurrent miscarriage is linked to loss of clonogenic BMPC during the implantation window, and the level of depletion correlates with the number of previous miscarriages [79, 81], and thus, the recurrence risk. The abundance of uNK cells varies markedly throughout the luteal phase and between cycles [63], which on the one hand is in keeping with their homeostatic function but, on the other, also accounts for the inconsistent findings in different studies. Nevertheless, there is evidence that lower uNK cell activity in the endometrium and peripheral blood associates with higher miscarriage rates [65, 82–84]. Further, metabolic (e.g., obesity) and endocrine disorders (e.g., hypothyroidism) can perturb homeostatic regulation of the decidual response [4].
The implantation checkpoint failure hypothesis leads to several predictions. First, as implantation is the rate limiting step for pregnancy, lack of embryo selection at implantation should lead to more rapid conception or “supcrfcrtility”. which is consistent with clinical observations [85, 86]. Second, the frequency of implantation checkpoint failure should determine the recurrence risk of miscarriage [65, 81]. While this conjecture warrants further investigation, the predicted impact on miscarriage rates aligns with the reported stepwise increase in recurrence risk with each additional miscarriage (Fig. 3 and Supplementary file 1). Finally, checkpoint failure implies that the endometrium does not select efficiently against aneuploid embryos at implantation. Hence, the incidence of aneuploid pregnancy loss in recurrent miscarriage is predicted to mirror the incidence of meiotic errors in preimplantation embryos, i.e., a preponderance of losses should be euploid in younger but not older patients [61].
Figure 3.

Predicted impact of implantation checkpoint failure on recurrence risk of miscarriage. (A) The implantation checkpoint hypothesis posits that the decidualizing endometrium mounts a tailored response to individual embryos, eliminating developmentally compromised embryos (leading to occult losses) but supporting competent embryos (leading to successful pregnancies). Checkpoint failure means that aneuploid embryos will escape detection (leading to aneuploid miscarriages), whilst euploid pregnancies will fail because the endometrium is unsupportive and prone to breakdown (leading to euploid miscarriages). Four scenarios can be considered in which the embryo is either euploid (E) or aneuploid (A) and the endometrium has either a normal (N) or failed (F) checkpoint, leading to three clinical outcomes: no pregnancy (occult loss), miscarriage, or live birth. (B) Predicted miscarriage rates with increasing frequency of cycles with implantation checkpoint failure. The predicted rates are compared to reported miscarriage rates associated with increasing number of pregnancy losses. *Based on 30% aneuploidy rate; †indicated number of previous miscarriages or more; ‡two and three previous miscarriages were combined in this study.
5. The uteroplacental interface
The development of the placenta in early pregnancy involves a sequence of events, starting with the emergence of different placental cell lineages and plugging of the uterine spiral arteries by invading trophoblast. Consequently, the early conceptus develops under hypoxic conditions, supported by endometrial gland secretions, until the onset of placental perfusion around 12 weeks of pregnancy. This section highlights how this sequence of events is disrupted in miscarriage.
5.1. Placental defects and miscarriage
Human placentation involves complex fetal-maternal interactions that are more extensive than in most other mammalian species [87]. Various placental cell lineages proliferate rapidly, and numerous villi cover the chorionic sac shortly after implantation. Each villus consists of a mesodermal core surrounded by an inner layer of progenitor cytotrophoblast cells and an outer layer of syncytiotrophoblast. At the tip of the villus, cytotrophoblast cells interrupt the syncytiotrophoblast and first form a columnar structure before spreading laterally to surround the entire conceptus (Fig. 2A) [88]. This cytotrophoblastic shell anchors the placenta but also plugs the terminal branches of the uterine spiral arteries [89]. Consequently, the conceptus is sealed off and develops under low oxygen tension, protected against a variety of stressors during the critical period of organogenesis [87]. During this phase, profuse glandular secretions, rich in growth factors, lipids, and carbohydrates, nourish the placenta and fetus [90, 91]. Invasive trophoblast cells emerge from the decidual surface of the shell and migrate through the decidua and into the inner myometrium where they fuse into multinucleated trophoblast giant cells [88].
Towards the end of the first trimester, the trophoblast plugs in the spiral arteries dislocate progressively, allowing gradual perfusion of the intervillous space. During this period, oxygen tension rises steeply [89, 92]. The ensuing wave of reactive oxygen species stress-tests the resilience the placental-decidual interface [93]. Once the uteroplacental circulation is established around 12 weeks, the pregnancy has successfully negotiated the final checkpoint, and miscarriage rates drop sharply [5]. However, remodelling of the maternal spiral arteries is not yet complete. This process requires continued cooperation between interstitial and endovascular trophoblast cells to convert the decidual and inner myometrial portions of these arteries into large-bore vessels devoid of smooth muscle and elastin [94].
In 70% of first-trimester miscarriages, the cytotrophoblastic shell surrounding the conceptus is thin and fragmented across the placenta [95]. Deficient endovascular trophoblast invasion causes incomplete plugging of spiral arteries, precocious placental perfusion, and oxidative damage to immature villi [87, 92, 93, 95]. These pathogenic events underpin both euploid and aneuploid miscarriages, suggesting orchestration by an aberrant decidual environment. Lesions confined to the placental edge may cause significant bleeding without necessarily imperilling the pregnancy and present clinically as a threatened miscarriage [87].
5.2. Decidual immune system and miscarriage
The first trimester decidua is rich in immune cells, principally uNK cells (~70%), macrophages (~20%), and T cells (~10%) [96]. Decidual cells control the influx and expansion of local immune cells [97, 98]. Conversely, cooperation between different innate immune cell populations is essential for optimal decidualization and development of the uteroplacental interface (Box 3). Through this interdependency, impaired decidualization adversely impacts local immune populations and vice versa, hampering the distinction between cause and effect.
Box 3: Examples of immune cell cooperation in early pregnancy.
Three distinct uNK cell subpopulations at the feto-matemal interface exert diverse roles in tissue homeostasis, spiral arteries remodelling through secretion of IFNγ and VEGF, and trophoblast invasion [96].
In early pregnancy, macrophages acquire an anti-inflammatory (M2) phenotype, secrete TGFβ, IL-10, and IDO (indoleamine 2,3-dioxygenase), and contribute to decidual angiogenesis, vascular transformation, and trophoblast invasion [110].
Dendritic cells (DCs) are also directly involved in the decidualization process, promoting proliferation and differentiation [111]
Activated regulatory T cells (Tregs) interact with DCs through CTLA4 (cytotoxic T-lymphocyte-associated protein 4), reducing the expression of co-stimulatory molecules (CD80 and CD86) and inhibiting effector T cell differentiation. Tregs also suppress cytotoxic uNK cell activity and regulate M2 macrophages and tissue DCs through release of heme oxygenase-1 [101].
Because the semi-allogeneic conceptus expresses ‘foreign’ paternal proteins, miscarriage is frequently attributed to a breakdown in maternal T cell tolerance, akin to graft-versus-host disease in transplant immunology [99]. However, placental mammals arose at least 75 million years ago [100], and multiple adaptations have evolved to preclude placental trophoblast killing by decidual or systemic T cells [101]. uNK cells also engage in allo-recognition of fetal cells through binding of their killer immunoglobulin-like receptors (KIR) to human leukocyte antigen-C (HLA-C) molecules on invading trophoblast [101]. The genes encoding maternal KIR and fetal HLA-C are highly polymorphic, with different haplotype combinations stimulating or inhibiting uNK cell activity. Immunogenetic studies have consistently associated excessively inhibitory KIR and HLA-C combinations with adverse pregnancy outcome, including recurrent miscarriage [102, 103]. Thus, uNK cell activity is essential for pregnancy. Nevertheless, the misconception that pregnancies fail because of an ‘overreactive’ immune response continues to resonate with many clinicians, fuelling a plethora of immune tests of uncertain value. Clinical trials have not demonstrated that immunosuppressive therapies (e.g., prednisolone, intralipids, intravenous immunoglobulin) increase live birth rates in recurrent miscarriage [13].
The uteroplacental interface is vulnerable to autoimmune disorders, most prominently antiphospholipid syndrome (APS), defined by the presence of antiphospholipid antibodies (aPL), such as lupus anticoagulant, anticardiolipin or anti-β2-glycoprotein 1 [104]. Since the main antigen of aPL, β2-glycoprotein 1, is expressed constitutively on decidual and trophoblast cells, the matemal-fetal interface is a major target for these autoantibodies [105]. Unlike systemic APS, which is athrombotic disorder, obstetric APS is primarily an inflammatory disorder [105], linked to fetal death and late pregnancy complications, such as preeclampsia, fetal growth restriction and preterm birth [106].
6. Perspective
Mammalian reproduction is characterised by remarkable diversification in pregnancy traits [19]. Based on comparative transcriptomics, hundreds of genes have been identified in pregnant endometrium that were gained or lost in primate and human lineages [107]. These genes not only underly the emergence of novel reproductive traits, such as spontaneous decidualization, menstruation, and deep haemochorial placentation, but also play a disproportionate role in reproductive disorders [107]. Despite these emerging insights, clinical practice remains firmly grounded in the misconception that early gestation represents a ‘vulnerable’ period, easily disrupted by a host of subclinical disorders [4, 14]. Here, we discussed how spontaneous decidualization enables active selection of embryos at implantation and elaborated the clinical consequences of implantation checkpoint failure. Endometrial fate decisions at implantation, that is, menstruation-like breakdown or transformation into a robust decidual matrix of pregnancy, pivot on balancing anti-inflammatory decidual cells and pro-inflammatory senescent decidual cell. While developmentally competent embryos promote cooperation between anti-inflammatory decidual cells and extra-uterine uNK cells and BMPC to form a robust decidua, low-fitness embryos subvert these interactions, thereby engineering their own demise [78]. However, the fitness consequences of aneuploidy depend on the identity of chromosomes; and embryos harbouring trisomies of smaller chromosomes appear particularly adept at evading maternal recognition at implantation, thus accounting for most age-related miscarriages. We posit that recurrence risk reflects the frequency of menstrual cycles resulting in implantation checkpoint failure, a paradigm that is already pointing towards novel therapeutic approaches. For example, a recent double-blind placebo-controlled feasibility trial demonstrated that sitagliptin, a dipeptidyl-peptidase IV (DPP4) inhibitor used in the management of diabetes, improves the decidual response at implantation by enhancing recruitment of BMPC [108]. Whether prepregnancy interventions, with sitagliptin or other drugs, lead to higher live birth rates in recurrent miscarriage patients will have to await further clinical trials.
Supplementary Material
Funding Sources:
The work was funded by Tommy’s Baby Charity, United Kingdom. RCM is supported by NIH grant R35GM133747. JJB holds a Wellcome Trust Investigator Award (212233/Z/18/Z). The funders had no role in the writing of the article; or in the decision to submit the paper for publication.
Abbreviations:
- aPL
anti-phospholipid antibodies
- APS
antiphospholipid syndrome
- BMPC
bone marrow-derived progenitor cells
- hCG
human chorionic gonadotrophin
- KIR
killer immunoglobulin-like receptors
- HLA-C
human leukocyte antigen-C
- PGT-A
pre-implantation genetic testing for aneuploidy
- PSSC
precocious separation of sister chromatids
- uNK cells
uterine natural killer cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests:
None
References
- [1].Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC, Incidence of early loss of pregnancy, The New England journal of medicine 319(4) (1988): 189–94, 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- [2].Wang X, Chen C, Wang L, Chen D, Guang W, French J, Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study, Fertility and sterility 79(3) (2003): 577–84, 10.1016/s0015-0282(02)04694-0. [DOI] [PubMed] [Google Scholar]
- [3].Zinaman MJ, Clegg ED, Brown CC, O’Connor J, Selevan SG, Estimates of human fertility and pregnancy loss, Fertility and sterility 65(3) (1996): 503–9. [PubMed] [Google Scholar]
- [4].Quenby S, Gallos ID, Dhillon-Smith RK, Podesek M, Stephenson MD, Fisher J, Brosens JJ, Brewin J, Ramhorst R, Lucas ES, McCoy RC, Anderson R, Daher S, Regan L, Al-Memar M, Bourne T, MacIntyre DA, Rai R, Christiansen OB, Sugiura-Ogasawara M, Odendaal J, Devall AJ, Bennett PR, Petrou S, Coomarasamy A, Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss, Lancet 397(10285) (2021): 1658–1667, 10.1016/S0140-6736(21)00682-6. [DOI] [PubMed] [Google Scholar]
- [5].Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Haberg SE, Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study, Bmj 364 (2019): l869, 10.1136/bmj.l869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kolte AM, Westergaard D, Lidegaard O, Brunak S, Nielsen HS, Chance of live birth: a nationwide, registry-based cohort study, Hum Reprod 36(4) (2021): 1065–1073, 10.1093/humrep/deaa326. [DOI] [PubMed] [Google Scholar]
- [7].Hassold T, Chiu D, Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy, Hum Genet 70(1) (1985): 11–7, 10.1007/bf00389450. [DOI] [PubMed] [Google Scholar]
- [8].Gruhn JR, Zielinska AP, Shukla V, Blanshard R, Capalbo A, Cimadomo D, Nikiforov D, Chan AC, Newnham LJ, Vogel I, Scarica C, Krapchev M, Taylor D, Kristensen SG, Cheng J, Ernst E, Bjorn AB, Colmorn LB, Blayney M, Elder K, Liss J, Hartshorne G, Grondahl ML, Rienzi L, Ubaldi F, McCoy R, Lukaszuk K, Andersen CY, Schuh M, Hoffmann ER, Chromosome errors in human eggs shape natural fertility over reproductive life span, Science 365(6460) (2019): 1466–1469, 10.1126/science.aav7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT Jr., The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening, Fertility and sterility 101(3) (2014): 656–663 e1, 10.1016/j.fertnstert.2013.11.004. [DOI] [PubMed] [Google Scholar]
- [10].McCoy RC, Demko ZP, Ryan A, Banjevic M, Hill M, Sigurjonsson S, Rabinowitz M, Petrov DA, Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development, PLoS genetics 11(10) (2015): e1005601, 10.1371/journal.pgen.1005601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hassold T, Chen N, Funkhouser J, Jooss T, Manuel B, Matsuura J, Matsuyama A, Wilson C, Yamane JA, Jacobs PA, A cytogenetic study of 1000 spontaneous abortions, Ann Hum Genet 44(2) (1980): 151–78. [DOI] [PubMed] [Google Scholar]
- [12].Nagaoka SI, Hassold TJ, Hunt PA, Human aneuploidy: mechanisms and new insights into an age-old problem, Nature reviews. Genetics 13(7) (2012): 493–504, 10.1038/nrg3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Coomarasamy A, Dhillon-Smith RK, Papadopoulou A, Al-Memar M, Brewin J, Abrahams VM, Maheshwari A, Christiansen OB, Stephenson MD, Goddijn M, Oladapo OT, Wijeyaratne CN, Bick D, Shehata H, Small R, Bennett PR, Regan L, Rai R, Bourne T, Kaur R, Pickering O, Brosens JJ, Devall AJ, Gallos ID, Quenby S, Recurrent miscarriage: evidence to accelerate action, Lancet 397(10285) (2021): 1675–1682, 10.1016/S0140-6736(21)00681-4. [DOI] [PubMed] [Google Scholar]
- [14].Dimitriadis E, Menkhorst E, Saito S, Kutteh WH, Brosens JJ, Recurrent pregnancy loss, Nat Rev Dis Primers 6(1) (2020):98, 10.1038/s41572-020-00228-z. [DOI] [PubMed] [Google Scholar]
- [15].Magnus MC, Morken NH, Wensaas KA, Wilcox AJ, Haberg SE, Risk of miscarriage in women with chronic diseases in Norway: A registry linkage study, PLoS Med 18(5) (2021): e1003603, 10.1371/journal.pmed.1003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Trivers RL, Parent-offspring conflict, American Zoologist 14(1) (1974): 249–264. [Google Scholar]
- [17].Haig D, Genetic conflicts in human pregnancy, Q Rev Biol 68(4) (1993): 495–532. [DOI] [PubMed] [Google Scholar]
- [18].Haig D, Cooperation and conflict in human pregnancy, Current biology : CB 29(11) (2019): R455–R458, 10.1016/j.cub.2019.04.040. [DOI] [PubMed] [Google Scholar]
- [19].McCoy DE, Haig D, Embryo Selection and Mate Choice: Can ‘Honest Signals’ Be Trusted?, Trends Ecol Evol 35(4) (2020): 308–318, 10.1016/j.tree.2019.12.002. [DOI] [PubMed] [Google Scholar]
- [20].Greco E, Minasi MG, Fiorentino F, Healthy Babies after Intrauterine Transfer of Mosaic Aneuploid Blastocysts, N Engl J Med 373(21) (2015): 2089–90, 10.1056/NEJMc1500421. [DOI] [PubMed] [Google Scholar]
- [21].McCoy RC, Mosaicism in Preimplantation Human Embryos: When Chromosomal Abnormalities Are the Norm, Trends Genet 33(7) (2017): 448–463, 10.1016/j.tig.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Emera D, Romero R, Wagner G, The evolution of menstruation: a new model for genetic assimilation: explaining molecular origins of maternal responses to fetal invasiveness, BioEssays : news and reviews in molecular, cellular and developmental biology 34(1) (2012): 26–35, 10.1002/bies.201100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Carter AM, Enders AC, Pijnenborg R, The role of invasive trophoblast in implantation and placentation of primates, Philosophical transactions of the Royal Society of London. Series B, Biological sciences 370(1663) (2015): 20140070, 10.1098/rstb.2014.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kliman HJ, Uteroplacental blood flow. The story of decidualization, menstruation, and trophoblast invasion, The American journal of pathology 157(6) (2000): 1759–68, 10.1016/S0002-9440(10)64813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Thurber C, Dugas LR, Ocobock C, Carlson B, Speakman JR, Pontzer H, Extreme events reveal an alimentary limit on sustained maximal human energy expenditure, Sci Adv 5(6) (2019): eaaw0341, 10.1126/sciadv.aaw0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ewington LJ, Tewary S, Brosens JJ, New insights into the mechanisms underlying recurrent pregnancy loss, The journal of obstetrics and gynaecology research 45(2) (2019): 258–265, 10.1111/jog.13837. [DOI] [PubMed] [Google Scholar]
- [27].Haig D, Fertile soil or no man’s land: cooperation and conflict in the placental bed, In Placental Bed Disorders, Pijnenborg R, Brosens I, and Romero R, eds (Cambridge University Press; ) (2010). [Google Scholar]
- [28].Hassold T, Hunt P, To err (meiotically) is human: the genesis of human aneuploidy, Nature reviews. Genetics 2(4) (2001): 280–91, 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- [29].Dukler N, Mughal MR, Ramani R, Huang Y-F, Siepel A, Extreme purifying selection against point mutations in the human genome, bioRxiv (2021): 2021.08.23.457339, 10.1101/2021.08.23.457339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Popadin K, Peischl S, Garieri M, Sailani MR, Letourneau A, Santoni F, Lukowski SW, Bazykin GA, Nikolaev S, Meyer D, Excoffier L, Reymond A, Antonarakis SE, Slightly deleterious genomic variants and transcriptome perturbations in Down syndrome embryonic selection, Genome research 28(1) (2018): 1–10, 10.1101/gr.228411.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lightfoot DA, Kouznetsova A, Mahdy E, Wilbertz J, Hoog C, The fate of mosaic aneuploid embryos during mouse development, Developmental biology 289(2) (2006): 384–94, 10.1016/j.ydbio.2005.11.001. [DOI] [PubMed] [Google Scholar]
- [32].Daughtry BL, Rosenkrantz JL, Lazar NH, Fei SS, Redmayne N, Torkenczy KA, Adey A, Yan M, Gao L, Park B, Nevonen KA, Carbone L, Chavez SL, Single-cell sequencing of primate preimplantation embryos reveals chromosome elimination via cellular fragmentation and blastomere exclusion, Genome Res 29(3) (2019): 367–382, 10.1101/gr.239830.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vermeesch JR, Voet T, Devriendt K, Prenatal and pre-implantation genetic diagnosis, Nature reviews. Genetics 17(10) (2016): 643–56, 10.1038/nrg.2016.97. [DOI] [PubMed] [Google Scholar]
- [34].Vanneste E, Voet T, Le Caignec C, Ampe M, Konings P, Melotte C, Debrock S, Amyere M, Vikkula M, Schuit F, Fryns JP, Verbeke G, D’Hooghe T, Moreau Y, Vermeesch JR, Chromosome instability is common in human cleavage-stage embryos, Nature medicine 15(5) (2009): 577–83, 10.1038/nm.1924. [DOI] [PubMed] [Google Scholar]
- [35].Ottolini CS, Newnham L, Capalbo A, Natesan SA, Joshi HA, Cimadomo D, Griffin DK, Sage K, Summers MC, Thornhill AR, Housworth E, Herbert AD, Rienzi L, Ubaldi FM, Handyside AH, Hoffmann ER, Genome-wide maps of recombination and chromosome segregation in human oocytes and embryos show selection for maternal recombination rates, Nature genetics 47(7) (2015): 727–735, 10.1038/ng.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mantikou E, Wong KM, Repping S, Mastenbroek S, Molecular origin of mitotic aneuploidies in preimplantation embryos, Biochimica et biophysica acta 1822(12) (2012): 1921–30, 10.1016/j.bbadis.2012.06.013. [DOI] [PubMed] [Google Scholar]
- [37].Webster A, Schuh M, Mechanisms of Aneuploidy in Human Eggs, Trends Cell Biol 27(1) (2017): 55–68, 10.1016/j.tcb.2016.09.002. [DOI] [PubMed] [Google Scholar]
- [38].Capalbo A, Ubaldi FM, Rienzi L, Scott R, Treff N, Detecting mosaicism in trophectoderm biopsies: current challenges and future possibilities, Hum Reprod 32(3) (2017): 492–498, 10.1093/humrep/dew250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rosenwaks Z, Handyside AH, Fiorentino F, Gleicher N, Paulson RJ, Schattman GL, Scott RT Jr., Summers MC, Treff NR, Xu K, The pros and cons of preimplantation genetic testing for aneuploidy: clinical and laboratory perspectives, Fertility and sterility 110(3) (2018): 353–361, 10.1016/j.fertnstert.2018.06.002. [DOI] [PubMed] [Google Scholar]
- [40].Delhanty JD, Harper JC, Ao A, Handyside AH, Winston RM, Multicolour FISH detects frequent chromosomal mosaicism and chaotic division in normal preimplantation embryos from fertile patients, Hum Genet 99(6) (1997): 755–60, 10.1007/s004390050443. [DOI] [PubMed] [Google Scholar]
- [41].Horan TS, Pulcastro H, Lawson C, Gerona R, Martin S, Gieske MC, Sartain CV, Hunt PA, Replacement Bisphenols Adversely Affect Mouse Gametogenesis with Consequences for Subsequent Generations, Current biology CB 28(18) (2018): 2948–2954 e3, 10.1016/j.cub.2018.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lyle R, Gehrig C, Neergaard-Henrichsen C, Deutsch S, Antonarakis SE, Gene expression from the aneuploid chromosome in a trisomy mouse model of down syndrome, Genome research 14(7) (2004): 1268–74, 10.1101/gr.2090904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Licciardi F, Lhakhang T, Kramer YG, Zhang Y, Heguy A, Tsirigos A, Human blastocysts of normal and abnormal karyotypes display distinct transcriptome profiles, Scientific reports 8(1) (2018): 14906, 10.1038/s41598-018-33279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Starostik MR, Sosina OA, McCoy RC, Single-cell analysis of human embryos reveals diverse patterns of aneuploidy and mosaicism, Genome research 30(6) (2020): 814–825, 10.1101/gr.262774.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bhattacharya A, Bense RD, Urzua-Traslavina CG, de Vries EGE, van Vugt M, Fehrmann RSN, Transcriptional effects of copy number alterations in a large set of human cancers, Nat Commun 11(1) (2020): 715, 10.1038/s41467-020-14605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ishikawa K, Makanae K, Iwasaki S, Ingolia NT, Moriya H, Post-Translational Dosage Compensation Buffers Genetic Perturbations to Stoichiometry of Protein Complexes, PLoS genetics 13(1) (2017): e1006554, 10.1371/journal.pgen.1006554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Munne S, Blazek J, Large M, Martinez-Ortiz PA, Nisson H, Liu E, Tarozzi N, Borini A, Becker A, Zhang J, Maxwell S, Grifo J, Babariya D, Wells D, Fragouli E, Detailed investigation into the cytogenetic constitution and pregnancy outcome of replacing mosaic blastocysts detected with the use of high-resolution next-generation sequencing, Fertility and sterility 108(1) (2017): 62–71 e8, 10.1016/j.fertnstert.2017.05.002. [DOI] [PubMed] [Google Scholar]
- [48].Capalbo A, Poli M, Rienzi L, Girardi L, Patassini C, Fabiani M, Cimadomo D, Benini F, Farcomeni A, Cuzzi J, Rubio C, Albani E, Sacchi L, Vaiarelli A, Figliuzzi M, Findikli N, Coban O, Boynukalin FK, Vogel I, Hoffmann E, Livi C, Levi-Setti PE, Ubaldi FM, Simon C, Mosaic human preimplantation embryos and their developmental potential in a prospective, non-selection clinical trial, American journal of human genetics 108(12) (2021): 2238–2247, 10.1016/j.ajhg.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Singla S, Iwamoto-Stohl LK, Zhu M, Zernicka-Goetz M, Autophagy-mediated apoptosis eliminates aneuploid cells in a mouse model of chromosome mosaicism, Nat Commun 11(1) (2020): 2958, 10.1038/s41467-020-16796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Popovic M, Dhaenens L, Taelman J, Dheedene A, Bialecka M, De Sutter P, Chuva de Sousa Lopes SM, Menten B, Heindryckx B, Extended in vitro culture of human embryos demonstrates the complex nature of diagnosing chromosomal mosaicism from a single trophectoderm biopsy, Hum Reprod 34(4) (2019): 758–769, 10.1093/humrep/dez012. [DOI] [PubMed] [Google Scholar]
- [51].Shahbazi MN, Wang T, Tao X, Weatherbee BAT, Sun L, Zhan Y, Keller L, Smith GD, Pellicer A, Scott RT Jr., Seli E, Zernicka-Goetz M, Developmental potential of aneuploid human embryos cultured beyond implantation, Nat Commun 11(1) (2020): 3987, 10.1038/s41467-020-17764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Eiben B, Bartels I, Bahr-Porsch S, Borgmann S, Gatz G, Gellert G, Goebel R, Hammans W, Hentemann M, Osmers R, et al. , Cytogenetic analysis of 750 spontaneous abortions with the direct-preparation method of chorionic villi and its implications for studying genetic causes of pregnancy wastage, American journal of human genetics 47(4) (1990): 656–63. [PMC free article] [PubMed] [Google Scholar]
- [53].Hardy K, Hardy PJ, Jacobs PA, Lewallen K, Hassold TJ, Temporal changes in chromosome abnormalities in human spontaneous abortions: Results of 40 years of analysis, American journal of medical genetics. Part A 170(10) (2016): 2671–80, 10.1002/ajmg.a.37795. [DOI] [PubMed] [Google Scholar]
- [54].Pauta M, Grande M, Rodriguez-Revenga L, Kolomietz E, Borrell A, Added value of chromosomal microarray analysis over karyotyping in early pregnancy loss: systematic review and meta-analysis, Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 51(4) (2018): 453–462, 10.1002/uog.18929. [DOI] [PubMed] [Google Scholar]
- [55].Morikawa M, Yamada H, Kato EH, Shimada S, Yamada T, Minakami H, Embryo loss pattern is predominant in miscarriages with normal chromosome karyotype among women with repeated miscarriage, Hum Reprod 19(11) (2004): 2644–7, 10.1093/humrep/deh451. [DOI] [PubMed] [Google Scholar]
- [56].Romero ST, Geiersbach KB, Paxton CN, Rose NC, Schisterman EF, Branch DW, Silver RM, Differentiation of genetic abnormalities in early pregnancy loss, Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 45(1) (2015): 89–94, 10.1002/uog.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yoneda S, Shiozaki A, Yoneda N, Sameshima A, Ito M, Shima T, Nakashima A, Yoshino O, Kigawa M, Takamori R, Shinagawa Y, Saito S, A Yolk Sac Larger Than 5 mm Suggests an Abnormal Fetal Karyotype, Whereas an Absent Embryo Indicates a Normal Fetal Karyotype, Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine 37(5) (2018): 1233–1241, 10.1002/jum.14467. [DOI] [PubMed] [Google Scholar]
- [58].Choi TY, Lee HM, Park WK, Jeong SY, Moon HS, Spontaneous abortion and recurrent miscarriage: A comparison of cytogenetic diagnosis in 250 cases, Obstet Gynecol Sci 57(6) (2014): 518–25, 10.5468/ogs.2014.57.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ogasawara M, Aoki K, Okada S, Suzumori K, Embryonic karyotype of abortuses in relation to the number of previous miscarriages, Fertil Steril 73(2) (2000): 300–4. [DOI] [PubMed] [Google Scholar]
- [60].Ozawa N, Ogawa K, Sasaki A, Mitsui M, Wada S, Sago H, Maternal age, history of miscarriage, and embryonic/fetal size are associated with cytogenetic results of spontaneous early miscarriages, Journal of assisted reproduction and genetics 36(4) (2019): 749–757, 10.1007/s10815-019-01415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Stephenson MD, Awartani KA, Robinson WP, Cytogenetic analysis of miscarriages from couples with recurrent miscarriage: a case-control study, Hum Reprod 17(2) (2002): 446–51. [DOI] [PubMed] [Google Scholar]
- [62].Gellersen B, Brosens JJ, Cyclic decidualization of the human endometrium in reproductive health and failure, Endocr Rev 35(6) (2014): 851–905, 10.1210/er.2014-1045. [DOI] [PubMed] [Google Scholar]
- [63].Brighton PJ, Maruyama Y, Fishwick K, Vrljicak P, Tewary S, Fujihara R, Muter J, Lucas ES, Yamada T, Woods L, Lucciola R, Hou Lee Y, Takeda S, Ott S, Hemberger M, Quenby S, Brosens JJ, Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium, Elife 6 (2017), 10.7554/eLife.31274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Erkenbrack EM, Maziarz JD, Griffith OW, Liang C, Chavan AR, Nnamani MC, Wagner GP, The mammalian decidual cell evolved from a cellular stress response, PLoS Biol 16(8) (2018): e2005594, 10.1371/journal.pbio.2005594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lucas ES, Vrljicak P, Muter J, Diniz-da-Costa MM, Brighton PJ, Kong CS, Lipecki J, Fishwick KJ, Odendaal J, Ewington LJ, Quenby S, Ott S, Brosens JJ, Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window, Commun Biol 3(1) (2020): 37, 10.1038/s42003-020-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Salker MS, Nautiyal J, Steel JH, Webster Z, Sucurovic S, Nicou M, Singh Y, Lucas ES, Murakami K, Chan YW, James S, Abdallah Y, Christian M, Croy BA, Mulac-Jericevic B, Quenby S, Brosens JJ, Disordered IL-33/ST2 activation in decidualizing stromal cells prolongs uterine receptivity in women with recurrent pregnancy loss, PLoS One 7(12) (2012): e52252, 10.1371/journal.pone.0052252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mor G, Cardenas I, Abrahams V, Guller S, Inflammation and pregnancy: the role of the immune system at the implantation site, Annals of the New York Academy of Sciences 1221 (2011): 80–7, 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Brosens JJ, Salker MS, Teklenburg G, Nautiyal J, Salter S, Lucas ES, Steel JH, Christian M, Chan YW, Boomsma CM, Moore JD, Hartshorne GM, Sucurovic S, Mulac-Jericevic B, Heijnen CJ, Quenby S, Koerkamp MJ, Holstege FC, Shmygol A, Macklon NS, Uterine selection of human embryos at implantation, Scientific reports 4 (2014): 3894, 10.1038/srep03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Muter J, Alam MT, Vrljicak P, Barros FSV, Ruane PT, Ewington LJ, Aplin JD, Westwood M, Brosens JJ, The Glycosyltransferase EOGT Regulates Adropin Expression in Decidualizing Human Endometrium, Endocrinology 159(2) (2018): 994–1004, 10.1210/en.2017-03064. [DOI] [PubMed] [Google Scholar]
- [70].Teklenburg G, Salker M, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BA, Quenby S, Kuijk EW, Kavelaars A, Heijnen CJ, Regan L, Brosens JJ, Macklon NS, Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation, PLoS One 5(4) (2010): e10258, 10.1371/journal.pone.0010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Diniz-da-Costa M, Kong CS, Fishwick KJ, Rawlings T, Brighton PJ, Hawkes A, Odendaal J, Quenby S, Ott S, Lucas ES, Vrljicak P, Brosens JJ, Characterization of highly proliferative decidual precursor cells during the window of implantation in human endometrium, Stem Cells 39(8) (2021): 1067–1080, 10.1002/stem.3367. [DOI] [PubMed] [Google Scholar]
- [72].Muter J, Kong C-S, Brosens JJ, The Role of Decidual Subpopulations in Implantation, Menstruation and Miscarriage, Frontiers in Reproductive Health 3 (2021), 10.3389/frph.2021.804921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Berkhout RP, Lambalk CB, Huirne J, Mijatovic V, Repping S, Hamer G, Mastenbroek S, High-quality human preimplantation embryos actively influence endometrial stromal cell migration, Journal of assisted reproduction and genetics 35(4) (2018): 659–667, 10.1007/s10815-017-1107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Rawlings TM, Makwana K, Taylor DM, Molè MA, Fishwick KJ, Tryfonos M, Odendaal J, Hawkes A, Zernicka-Goetz M, Hartshorne GM, Brosens JJ, Lucas ES, Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids, bioRxiv (2021):2021.03.02.433560, 10.1101/2021.03.02.433560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Weimar CH, Kavelaars A, Brosens JJ, Gellersen B, de Vreeden-Elbertse JM, Heijnen CJ, Macklon NS, Endometrial stromal cells of women with recurrent miscarriage fail to discriminate between high- and low-quality human embryos, PloS one 7(7) (2012): e41424, 10.1371/journal.pone.0041424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Macklon NS, Brosens JJ, The human endometrium as a sensor of embryo quality, Biology of reproduction 91(4) (2014): 98, 10.1095/biolreprod.114.122846. [DOI] [PubMed] [Google Scholar]
- [77].Berkhout RP, Keijser R, Repping S, Lambalk CB, Afink GB, Mastenbroek S, Hamer G, High-quality human preimplantation embryos stimulate endometrial stromal cell migration via secretion of microRNA hsa-miR-320a, Hum Reprod 35(8) (2020): 1797–1807, 10.1093/humrep/deaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kong CS, Ordonez AA, Turner S, Tremaine T, Muter J, Lucas ES, Salisbury E, Vassena R, Tiscornia G, Fouladi-Nashta AA, Hartshorne G, Brosens JJ, Brighton PJ, Embryo biosensing by uterine natural killer cells determines endometrial fate decisions at implantation, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 35(4) (2021): e21336, 10.1096/fj.202002217R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Diniz-da-Costa M, Kong CS, Fishwick KJ, Rawlings T, Brighton PJ, Hawkes A, Odendaal J, Quenby S, Ott S, Lucas ES, Vrljicak P, Brosens JJ, Characterization of highly proliferative decidual precursor cells during the window of implantation in human endometrium, Stem Cells 39 (2021): 1067–1080, 10.1002/stem.3367. [DOI] [PubMed] [Google Scholar]
- [80].Tal R, Shaikh S, Pallavi P, Tal A, Lopez-Giraldez F, Lyu F, Fang YY, Chinchanikar S, Liu Y, Kliman HJ, Alderman M 3rd, Pluchino N, Kayani J, Mamillapalli R, Krause DS, Taylor HS, Adult bone marrow progenitors become decidual cells and contribute to embryo implantation and pregnancy, PLoS Biol 17(9) (2019): e3000421, 10.1371/journal.pbio.3000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lucas ES, Dyer NP, Murakami K, Lee YH, Chan YW, Grimaldi G, Muter J, Brighton PJ, Moore JD, Patel G, Chan JK, Takeda S, Lam EW, Quenby S, Ott S, Brosens JJ, Loss of Endometrial Plasticity in Recurrent Pregnancy Loss, Stem Cells 34(2) (2016): 346–56, 10.1002/stem.2222. [DOI] [PubMed] [Google Scholar]
- [82].Fukui A, Funamizu A, Fukuhara R, Shibahara H, Expression of natural cytotoxicity receptors and cytokine production on endometrial natural killer cells in women with recurrent pregnancy loss or implantation failure, and the expression of natural cytotoxicity receptors on peripheral blood natural killer cells in pregnant women with a history of recurrent pregnancy loss, The journal of obstetrics and gynaecology research 43(11) (2017): 1678–1686, 10.1111/jog.13448. [DOI] [PubMed] [Google Scholar]
- [83].Hiby SE, Regan L, Lo W, Farrell L, Carrington M, Moffett A, Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage, Hum Reprod 23(4) (2008): 972–6, 10.1093/humrep/den011. [DOI] [PubMed] [Google Scholar]
- [84].Katano K, Suzuki S, Ozaki Y, Suzumori N, Kitaori T, Sugiura-Ogasawara M, Peripheral natural killer cell activity as a predictor of recurrent pregnancy loss: a large cohort study, Fertility and sterility 100(6) (2013): 1629–34, 10.1016/j.fertnstert.2013.07.1996. [DOI] [PubMed] [Google Scholar]
- [85].Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BA, Quenby S, Kuijk EW, Kavelaars A, Heijnen CJ, Regan L, Macklon NS, Brosens JJ, Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss, PloS one 5(4) (2010): e10287, 10.1371/journal.pone.0010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ticconi C, Pietropolli A, D’Ippolito S, Chiaramonte C, Piccione E, Scambia G, Di Simone N, Time-to-Pregnancy in Women with Unexplained Recurrent Pregnancy Loss: A Controlled Study, Reprod Sci 27(5) (2020): 1121–1128, 10.1007/s43032-019-00122-4. [DOI] [PubMed] [Google Scholar]
- [87].Burton GJ, Jauniaux E, The cytotrophoblastic shell and complications of pregnancy, Placenta 60 (2017): 134–139, 10.1016/j.placenta.2017.06.007. [DOI] [PubMed] [Google Scholar]
- [88].Turco MY, Moffett A, Development of the human placenta, Development 146(22) (2019), 10.1242/dev.163428. [DOI] [PubMed] [Google Scholar]
- [89].Burton GJ, Jauniaux E, Watson AL, Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited, American journal of obstetrics and gynecology 181(3) (1999): 718–24. [DOI] [PubMed] [Google Scholar]
- [90].Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E, Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy, The Journal of clinical endocrinology and metabolism 87(6) (2002): 2954–9, 10.1210/jcem.87.6.8563. [DOI] [PubMed] [Google Scholar]
- [91].Jones CJ, Aplin JD, Burton GJ, First trimester histiotrophe shows altered sialylation compared with secretory phase glycoconjugates in human endometrium, Placenta 31(7) (2010): 576–80, 10.1016/j.placenta.2010.04.011. [DOI] [PubMed] [Google Scholar]
- [92].Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ, Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure, The American journal of pathology 157(6) (2000): 2111–22, 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Burton GJ, Jauniaux E, Placental oxidative stress: from miscarriage to preeclampsia, J Soc Gynecol Investig 11(6) (2004): 342–52, 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- [94].Brosens JJ, Pijnenborg R, Brosens IA, The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature, American journal of obstetrics and gynecology 187(5) (2002): 1416–23, 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- [95].Jauniaux E, Hustin J, Histological examination of first trimester spontaneous abortions: the impact of materno-embryonic interface features, Histopathology 21(5) (1992): 409–14, 10.1111/j.1365-2559.1992.tb00424.x. [DOI] [PubMed] [Google Scholar]
- [96].Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, Park JE, Stephenson E, Polanski K, Goncalves A, Gardner L, Holmqvist S, Henriksson J, Zou A, Sharkey AM, Millar B, Innes B, Wood L, Wilbrey-Clark A, Payne RP, Ivarsson MA, Lisgo S, Filby A, Rowitch DH, Bulmer JN, Wright GJ, Stubbington MJT, Haniffa M, Moffett A, Teichmann SA, Single-cell reconstruction of the early maternal-fetal interface in humans, Nature 563(7731) (2018): 347–353, 10.1038/s41586-018-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A, Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface, Science 336(6086) (2012): 1317–21, 10.U26/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Silasi M, You Y, Simpson S, Kaislasuo J, Pal L, Guller S, Peng G, Ramhorst R, Grasso E, Etemad S, Durosier S, Aldo P, Mor G, Human Chorionic Gonadotropin modulates CXCL10 Expression through Histone Methylation in human decidua, Scientific reports 10(1) (2020): 5785, 10.1038/s41598-020-62593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Mor G, Aldo P, Alvero AB, The unique immunological and microbial aspects of pregnancy, Nat Rev Immunol 17(8) (2017): 469–482, 10.1038/nri.2017.64. [DOI] [PubMed] [Google Scholar]
- [100].Liu L, Zhang J, Rheindt FE, Lei F, Qu Y, Wang Y, Zhang Y, Sullivan C, Nie W, Wang J, Yang F, Chen J, Edwards SV, Meng J, Wu S, Genomic evidence reveals a radiation of placental mammals uninterrupted by the KPg boundary, Proceedings of the National Academy of Sciences of the United States of America 114(35) (2017): E7282–E7290, 10.1073/pnas.1616744114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Moffett A, Chazara O, Colucci F, Maternal allo-recognition of the fetus, Fertility and sterility 107(6) (2017): 1269–1272, 10.1016/j.fertnstert.2017.05.001. [DOI] [PubMed] [Google Scholar]
- [102].Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, Claas FH, Walker JJ, Redman CW, Morgan L, Tower C, Regan L, Moore GE, Carrington M, Moffett A, Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2, The Journal of clinical investigation 120(11) (2010): 4102–10, 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Hiby SE, Walker JJ, O’Shaughnessy K M, Redman CW, Carrington M, Trowsdale J, Moffett A, Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success, J Exp Med 200(8) (2004): 957–65, 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA, Antiphospholipid syndrome, Lancet 376(9751) (2010): 1498–509, 10.1016/S0140-6736(10)60709-X. [DOI] [PubMed] [Google Scholar]
- [105].Abrahams VM, Chamley LW, Salmon JE, Emerging Treatment Models in Rheumatology: Antiphospholipid Syndrome and Pregnancy: Pathogenesis to Translation, Arthritis Rheumatol 69(9) (2017): 1710–1721, 10.1002/art.40136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Schreiber K, Sciascia S, de Groot PG, Devreese K, Jacobsen S, Ruiz-Irastorza G, Salmon JE, Shoenfeld Y, Shovman O, Hunt BJ, Antiphospholipid syndrome, Nat Rev Dis Primers 4 (2018): 18005, 10.1038/nrdp.2018.5. [DOI] [PubMed] [Google Scholar]
- [107].Mika K, Marinic M, Singh M, Muter J, Brosens JJ, Lynch VJ, Evolutionary transcriptomics implicates new genes and pathways in human pregnancy and adverse pregnancy outcomes, eLife 10 (2021), 10.7554/eLife.69584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Tewary S, Lucas ES, Fujihara R, Kimani PK, Polanco A, Brighton PJ, Muter J, Fishwick KJ, Da Costa M, Ewington LJ, Lacey L, Takeda S, Brosens JJ, Quenby S, Impact of sitagliptin on endometrial mesenchymal stem-like progenitor cells: A randomised, double-blind placebo-controlled feasibility trial, EBioMedicine 51 (2020): 102597, 10.1016/j.ebiom.2019.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].McCoy RC, Demko Z, Ryan A, Banjevic M, Hill M, Sigurjonsson S, Rabinowitz M, Fraser HB, Petrov DA, Common variants spanning PLK4 are associated with mitotic-origin aneuploidy in human embryos, Science 348(6231) (2015): 235–8, 10.1126/science.aaa3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Schumacher A, Sharkey DJ, Robertson SA, Zenclussen AC, Immune Cells at the Fetomaternal Interface: How the Microenvironment Modulates Immune Cells To Foster Fetal Development, J Immunol 201(2) (2018): 325–334, 10.4049/jimmunol.1800058. [DOI] [PubMed] [Google Scholar]
- [111].Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, Mor G, Keshet E, Dekel N, Neeman M, Jung S, Uterine DCs are crucial for decidua formation during embryo implantation in mice, The Journal of clinical investigation 118(12) (2008): 3954–65, 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Coomarasamy A, Devall AJ, Brosens JJ, Quenby S, Stephenson MD, Sierra S, Christiansen OB, Small R, Brewin J, Roberts TE, Dhillon-Smith R, Harb H, Noordali H, Papadopoulou A, Eapen A, Prior M, Di Renzo GC, Hinshaw K, Mol BW, Lumsden MA, Khalaf Y, Shennan A, Goddijn M, van Wely M, Al-Memar M, Bennett P, Bourne T, Rai R, Regan L, Gallos ID, Micronized vaginal progesterone to prevent miscarriage: a critical evaluation of randomized evidence, American journal of obstetrics and gynecology (2020), 10.1016/j.ajog.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


