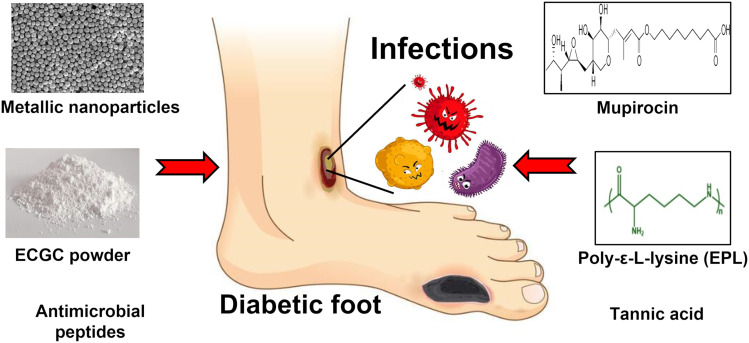
Abstract
Diabetic chronic wounds or amputation, which are complications of diabetes mellitus (DM), are a cause of great suffering for diabetics. In addition to the lack of oxygen, elevated reactive oxygen species (ROS) and reduced vascularization, microbial invasion is also a critical factor that induces non-healing chronic diabetic wounds, ie, wounds still remaining in the stage of inflammation, after which the wound tissue begins to age and becomes necrotic. To clear up the infection, alleviate the inflammation in the wound and prevent necrosis, many kinds of hydrogel have been fabricated to eliminate infections with pathogens. The unique properties of hydrogels make them ideally suited to wound dressings because they provide a moist environment for wound healing and act as a barrier against bacteria. This review article will mainly cover the recent developments and innovations of antibacterial hydrogels for diabetic chronic wound healing.
Keywords: antibacterial, hydrogel, diabetic chronic wound, infection, inflammation, wound dressing
Graphical Abstract
Introduction
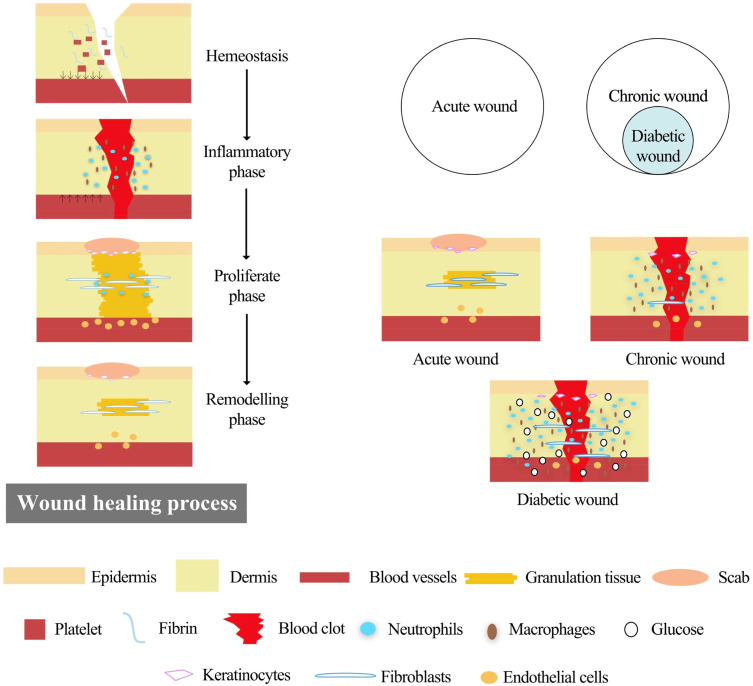
Diabetes mellitus (DM), a common metabolic disease with over 400 million sufferers worldwide, puts a great burden on society, economies, and health care systems.1 Deaths among diabetics are mainly due to chronic complications caused by severe hyperglycemia.2 Non-healing diabetic wounds, especially those in the lower extremities, are common complications, and are called diabetic foot ulcer (DFU), which lead to 15–25% of diabetes patients needing amputation and suffering from disability during their lifetimes.3,4 Normal wound healing is a dynamic and complicated biological process involving four typical phases: hemostasis, inflammation, proliferation and remodeling, in which many types of cells, cytokines and the extracellular matrix (ECM) are involved (Figure 1).5–7 However, a wound with a micro-environment featuring high glycemic levels is more easily infected by bacteria, and macrophages produce more reactive oxygen species (ROS) to defend against foreign pathogens.8–10 Excessive ROS impairs normal cells and tissues resulting in lack of nutrients, impaired angiogenesis, hypoxia and neuropathy, which finally induce persistent inflammation and a long-time non-healing chronic wound (Figure 2).11–14 Therefore, clearing the infection and alleviating the inflammation are crucial for the management of diabetic chronic wound, and subsequently providing other growth factors (GFs) such as vascular endothelial growth factor (VEGF), stromal cell-derived factor-1a (SDF-1a) to promotes wound healing.15,16
Figure 1.
An illustration of how wounds heal and the major differences between an acute wound and diabetic chronic wound. Reprinted from Tan CT, Liang K, Ngo ZH, Dube CT, Lim CY. Application of 3D bioprinting technologies to the management and treatment of diabetic foot ulcers. Biomedicines. 2020;8:10. Creative Commons Attribution License.4
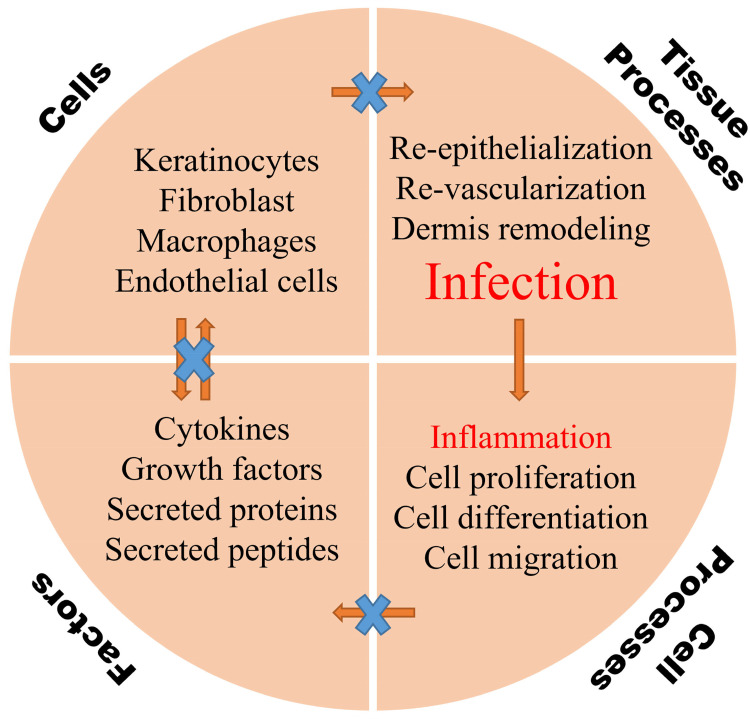
Figure 2.
An illustration of major factors that contribute to the pathophysiology of diabetic chronic wounds.
Using wound dressings is the most direct and convenient way to prevent the wound from being infected by microorganism and has been used for wound management for a very long time. A traditional form of wound dressing is a gauze made from cotton, which has been widely used in clinical treatment. However, it is unsuitable for the management of diabetic wound due to the secondary damage from each time of the dressing is exchanged, which is painful for the patients. Besides, traditional wound dressings have no bioactive promotion and moisturizing effects on wound healing.9,17,18 With the development of biomaterials, several kinds of biopolymers or synthetic polymers have been utilized in wound dressings, such as chitosan,19,20 collagen,21 gelatin,22 hyaluronic acid,23,24 cellulose,25,26 alginate,27 poly-(vinyl alcohol) (PVA),28 poly(lactic-co-glycolic acid) (PLGA)29 polylactide (PLA)30 and others. These polymers with properties such as non-toxicity, biodegradability and non-immunogenicity are conducive to wound management. Nevertheless, commercially available wound dressings for the treatment of diabetic wounds still have multitudinous limitations. For example, a kind of silver ion dressing, Biatain Alginate Ag® cannot be used for dry wounds due to the need for an external fixation dressing. Mepiform® is a kind of soft silicone dressing that should not be used for infected wounds, dry wounds or wounds with eschar, at the same time it is opaque and therefore is not convenient for viewing wounds.
Compared with other types of novel wound dressings, such as porous sponges, biocompatible membranes and electrospun nanofibers, hydrogels are superior candidates, due to their unique properties, for example, good flexibility, biocompatibility, moisturizing qualities and great sensitivity to physiological environments. Relative to other dressings, hydrogels can not only increase wound humidity, absorb wound exudate and reduce wound temperature, but are also comfortable, non-irritating, easy to change, and importantly, have analgesic effect for the injured tissue.31–33 With the development of biomaterial science, many functional hydrogels have been created by scientists to improve the ability of hydrogels for wound healing promotion.14,32,34 Different strategies exist for hydrogel preparation such as physical cross-linking, the radiation-induced gelation of polymer-water systems, chemical cross-linking, enzyme-catalyzed reactions and su-pramolecular interactions.14,32,34 Hydrogels are fabricated to work as intelligent drug-carriers and even adjust to the local wound microenvironment, but as a wound dressing, it is still highly important that they defend against microorganisms, especially in diabetic chronic wounds. Accordingly, to endow hydrogels with antibacterial activity, some antibacterial agents, metal nanoparticles, biopolymers and natural bioactive ingredients have been incorporated into the design of antibacterial multifunctional hydrogels.
In this review, we summarize and discuss the latest progress on multifunctional hydrogels with antibacterial ability, that decrease infection and promote diabetic chronic wound healing.
Loss of Wound Healing Ability in Diabetes Mellitus
The body responds to an injury in multiple ways to protect itself through restoring the integrity of damaged tissues, which involves a complex set of mechanisms. The wound healing process consists of four distinct phases: hemostasis, inflammation, proliferation, and remodeling.35 In healthy people, minor acute wounds heal within 2–3 weeks. However, when the physiological mechanism of healing is out of balance, diabetic wounds may become chronic and fail to heal within six to eight weeks.2,36,37
In the case of diabetes patients, the normal process of wound healing is interrupted, resulting in tissue damage, persistent infection and peripheral vascular problems. The imbalance between angiogenic factors such as TGF-β, FGF2, VEGF, angiogenin, angioinhibitory factors and abnormal apoptotic potential in diabetic patients may lead to disturbed angiogenesis.38 Diabetic wounds are in the stage of chronic inflammation and do not progress to the stage of proliferation and remodeling, thus obstructing the normal wound healing process.39 Due to normal phase interference, various parameters, including growth factors in the wound microenvironment, and immune cell circulation are interfered with, and the wound bed receives less energy, inhibiting the activation of caspase-3 and affecting metabolism here. Therefore, diabetic wounds are characterized by delayed wound healing, which is usually associated with infections caused by disrupted levels of microcirculating cell and decreased levels of endogenous growth factor, leading to the development of unhealing chronic ulcers. Further infection of the wound often leads to limb amputation.40
Antibacterial Hydrogels Based on Metal Ions
In the natural world, many kinds of metallics occur that possess antibacterial activity such as silver,41 copper42,43 and zinc,44 which also have excellent potential against multidrug-resistant bacteria. Metal elements act as antibacterial agents in their ionic form. In environments with relatively high concentrations of metal ions, the survival of microorganisms is affected in many aspects.45
Firstly, outside the membrane, the high concentration of metal cations alters the polarization state within and outside the biofilm, resulting in a new ion concentration difference, which blocks or disrupts the transport of small and large molecules required for cell maintenance, such as glucose and amino acid transport driven by the Na+/K+ pump.46 Some metal ions can also enter microbial cells. It has been demonstrated that heavy metals can inactivate most of the enzymes, although the mechanism of inactivation is still unclear.41,46 Some scholars consider that heavy metal ions with positive valence complexed with the N and O elements of protein destroy the spatial conformation of enzyme protein molecules.47 It is also possible that heavy metal ions react with -SH groups to replace protons, or even destroy or replace metal ions such as Mg2+, Fe3+ and Ca2+, which are necessary to maintain enzyme activity. Enzymes are the catalyst of all biological processes, also controlling microbial biochemical reactions.48 Once an enzyme is inactivated, it will cause the reduction of catalytic efficiency and performance, such that the biochemical reactions cannot be carried out normally, and affect related biochemical reactions, resulting in the blocked energy metabolism and material metabolism of microorganisms, to achieve the purpose of antibacterial effect. In addition, metal ions entering cells can combine with nucleic acids, destroying the ability of cells to divide and reproduce.48
Hydrogels Loaded with Silver Nanoparticles (AgNPs) for Diabetic Wound Management
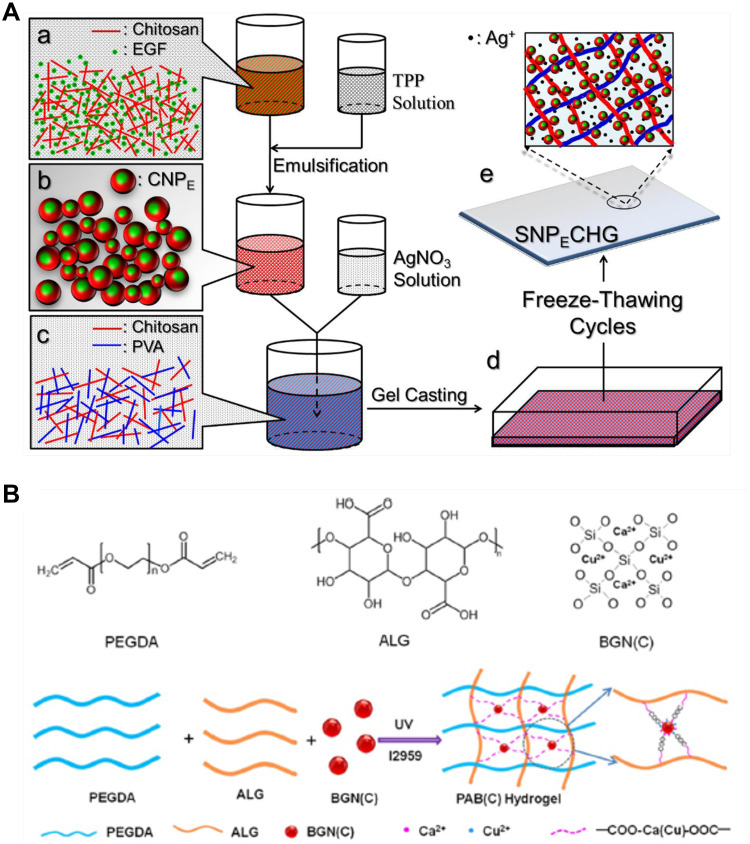
Among the different metal nanoparticles, silver nanoparticles are the most dynamic nanoparticles for wound care management because of their antimicrobial activity, even against hospital strains of multidrug resistant microorganisms, thereby promoting wound healing.49,50 AgNPs have been mixed into many kinds of healthcare products, such as textiles,51 cosmetics,52 and wound dressings,53 as they have excellent antimicrobial properties and are electrically conductive. Recently, AgNPs were also applied in the fabrication of antibacterial hydrogel for the treatment of diabetic chronic wound. A composite hydrogel encapsulating silver nanoparticles and epidermal growth factor (EGF) co-loaded with chitosan was developed by Yu-Hsiang Lee et al and was named SNPECHG,54 The production process is shown in Figure 3A. They eventually found that the dosages of 24 mM Ag+ had an optimal antimicrobial effect, The antimicrobial activity of SNPECHG was demonstrated by the significant bactericidal effect of Ag+ on S. aureus and S. epidermidis, while CNP-coated EGF promoted the growth of NIH/3T3 cells, thus verifying its proliferation-promoting function. In addition, the optimized SNPECHG provides sustained release and excellent hydration of Ag+ and EGF in high ionic strength media, indicating that the developed composite hydrogel was highly suited to the exudate environment at the wound site.54 Nosheen Masood also observed that for AgNPs-loaded chitosan-polyethylene glycol hydrogels, 0.1g of AgNO3 was added into chitosan solution, which had superior antioxidant and antibacterial properties compared to bare chitosan-polyethylene glycol hydrogels, prompting researchers to use them to treat wounds in diabetic rabbits.55 They found that AgNP-loaded hydrogel, which released AgNPs slowly and continuously for at least seven days, had remarkable antibacterial ability against E. coli, P. aeruginosa, B. subtilis and S. aureus. Finally, the AgNP-loaded chitosan PEG hydrogel well promoted the healing of diabetic wound.55
Figure 3.
The process of making antibacterial hydrogel. (A) Schematic diagram of the SNPECHG fabrication procedures. Chitosan nanoparticles loaded with EGF were first prepared through a modified emulsification method (a-b), following the production of CNPE, AgNO3 was added to a chitosan-PVA solution, which was vigorously stirred afterward (c), an eight-cycle freezing/thawing procedure was performed on the polymeric mixture (d) to obtain SNPECHG (e); Reproduced from Lee YH, Hong YL, Wu TL. Novel silver and nanoparticle-encapsulated growth factor co-loaded chitosan composite hydrogel with sustained antimicrobility and promoted biological properties for diabetic wound healing. Mater Sci Eng C. 2021;118:111385. Copyright 2021, with permission from Elsevier.54 (B) Synthesis and potential wound healing application of PABC hydrogel: Main components of PABC hydrogel including PEGDA, ALG and BGN; Reproduced from Li Y, Xu T, Tu Z, et al. Bioactive antibacterial silica-based nanocomposites hydrogel scaffolds with high angiogenesis for promoting diabeticwound healing and skin repair. Theranostics. 2020;10(11):4929–4943. Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/).62
Zinc Oxide-Based Hydrogels for Diabetic Wound Management
Various wound dressings use zinc oxide nanoparticles as active ingredients, as they have antibacterial properties and promote fibroblast proliferation and angiogenesis.56–58 A study conducted by Rashid Ahmed et al discovered that chitosan /PVA/ZnO nanofibrous membranes had higher antibacterial potential against E. coli, Pseudomonas aeruginosa, Bacillus subtilis and Staphylococcus aureus compared with chitosan /PVA nanofiber mats. Furthermore, unlike chitosan/PVA/ZnO nanofibre mats, chitosan/PVA/ZnO nanofibrous membranes had higher antioxidant capacity and can facilitate diabetic wound healing in vivo.59
Recently a novel kind of ZnO particles, tetrapod-shaped ZnO particles, were integrated into 3D-printed GelMA hydrogels with the ability of light-controlled release of growth factors,60 tetrapod-shaped ZnO particles have better cytocompatibility than spherical ZnO nanoparticles. VEGF can be decorated onto the surface of t-ZnO, then treated with H2O2 and the surface turned into rough and porous.60 In vivo experiments have shown that the t-ZnO-laden composite hydrogels had a good effect, with lower immunogenicity and better wound healing Therefore, they can be also applied to diabetic wound healing due to their excellent antibacterial ability and controllable release of VEGF under ultraviolet/ visible light exposure.
Cu2+-Based Hydrogels for Diabetic Wound Management
Copper ions (Cu2+) with excellent antibacterial property can reduce wound infections and speed up wound healing.61 Recently, a novel bioactive, self-healing, antibacterial, dual-network nanocomposite hydrogel was developed, which significantly promoted diabetic wound healing/skin tissue formation by enhancing early angiogenesis without the addition of bioactive factors.62 Nanocomposite hydrogel consists of a network of polyethylene glycol diacrylate (PEGDA) and an auxiliary dynamic network between bioactive glass nanoparticles containing copper (BGNC) and sodium alginate (ALG), also named as PABC scaffolds, with the production process shown in Figure 3B.62 PABC scaffolds exhibit the mechanical properties of biomimetic elastomer with good injectability, self-repair, and strong broad-spectrum antibacterial activity. A significant increase in the proliferation and angiogenesis of endothelial progenitor cells (EPCs) was observed in vitro after the application of PABC hydrogels.62 In vivo, PABC hydrogels promoted wound healing and skin tissue regeneration in full-layer diabetic wounds by significantly increasing HIF-1*/VEGF expression and collagen matrix deposition.62 In addition, Sun et al developed a wound dressing from antibacterial nanocomposites based on chitosan, copper, and gallic acid61 and a novel hydrogel dressing (HKUST-Hs)63 containing copper metal organic framework nanoparticles can also be utilized in diabetic wound treatment, because both of them have dual effects as antibacterial and antioxidant.
Metal-Organic Framework (MOF)-Based Hydrogels for Diabetic Wound Management
When discussing the antibacterial effects of metal ions, a crucial aspect to cover is cytotoxicity. MOFs have emerged as a kind of porous, solid and adsorptive material, which are composed of metal ions and organic ligands. MOFs can carry drugs, enzymes, biological macromolecules and other substances to gain functionality while retaining low cytotoxicity, thus it is conducive to incorporated them into antibacterial wound dressings. Do Nam Lee et al conducted in-depth research in the field of MOFs. In 2020, they published a paper entitled “Novel Metal-Organic Frameword-based Photocrosslinked Hydrogel System for Efficient Antibacterial Applications” to present a hydrogel made of diacrylated polyethylene glycol (PEG), 4-arm-thiolated PEG, and MOFs. Their main contribution was to compare the structure and antibacterial performance of three kinds of MOF-based hydrogels: @Cu-MOF, @Co-MOF, and @Zn-MOF. The results showed that @Cu-MOF hydrogel the most stable 3D structure, low cytotoxicity, and high antibacterial activity, which could be attributed to Cu2+ and the excellent MOF system.64
Metal ions have favorable antibacterial ability, but when the concentration of metal ions in the body becomes toxic, their usage should be controlled at safe levels that still yield sufficient antibacterial activity.
Antibacterial Hydrogels Based on Natural Bioactive Ingredients
Antimicrobial compounds are emerging as a potential chemical alternative to conventional antibiotics, which consist an antibacterial strategy that is free from the problems of overuse and resistance associated with synthetic antibiotics.65
Epigallocatechin-3-Gallate (EGCG)-Based Hydrogels for Diabetic Wound Management
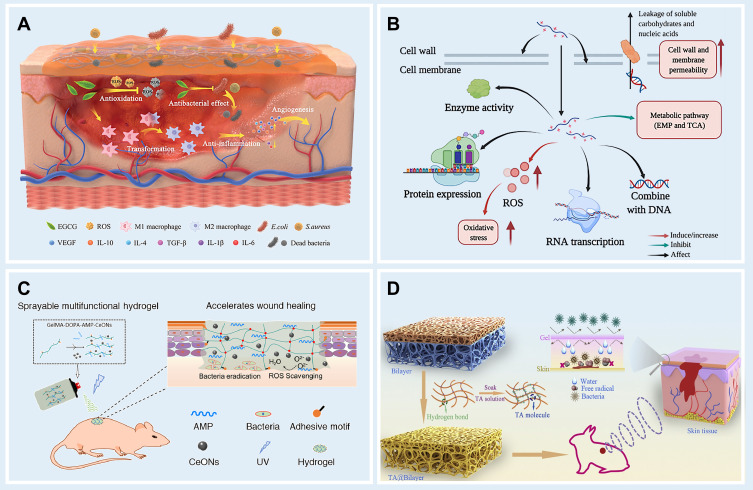
In recent years, EGCG has been the subject of extensive research,66,67 and its anticancer, anti-inflammatory, antioxidant, and anti-aging properties have led to its wide application in a number of fields.66 EGCG and its wound dressings play various roles in different wound healing stages, such as increasing hemadsorption, inhibiting neutrophil infiltration and monocyte migration and adhesion, promoting re-epithelialization, stimulating angiogenesis, altering collagen synthesis, and reducing ECM formation (Figure 4A).66 A team from Xi’an Jiao-tong University produced a smart hydrogel dressing,68 which can be conveniently obtained through copolymerization of the complex formed by EGCG and 3-acrylamido phenyl boronic acid (APBA) (resulting in the formation of boronate ester bond) with acrylamide. Scine E-A complexes are dynamic, the resulting hydrogels have good mechanical strength, moderate tissue adhesiveness and excellent self-regeneration capacity, which largely facilitates regeneration and self-healing. Besides its anti-oxidation and antibacterial properties, this functional hydrogel was shown to also anti-inflammatory, anti-inflammatory, and proangiogenic effects, as well as to modulate macrophage polarization. EGCG, however, also reduced the adhesive strength of tissue to facilitate dressing changes, all of which resulted in outstanding wound healing efficiency. in the chronic diabetic wound bed.68
Figure 4.
Four kinds of natural bioactive ingredients based antibacterial hydrogel. (A) Mechanism of EGCG promoting wound healing; Reproduced from Zhao X, Pei D, Yang Y, et al. Green tea derivative driven smart hydrogels with desired functions for chronic diabetic wound treatment. Adv Funct Mater. 2021;31(18):2009442. Copyright 2021, John Wiley and sons.68 (B) Several antimicrobial pathways of EPL; Reproduced from Wang L, Zhang C, Zhang J, et al. Epsilon-poly-L-lysine: recent advances in biomanufacturing and applications. Front Bioeng Biotechnol.2021;9:748976. Copyright © 2021 Wang, Zhang, Zhang, Rao, Xu, Mao and Chen. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY).72 (C) Composition and action mode of the Gelma-dopa-amp-Ceons dressing; Reproduced from Cheng H, Shi Z, Yue K, et al. Sprayable hydrogel dressing accelerates wound healing with combined reactive oxygen species-scavenging and antibacterial abilities. Acta Biomater. 2021;124:219–232. Copyright 2021, with permission from Elsevier.76 (D) Structure of TA@bilayer hydrogel and its interaction with wound; Reproduced from Li Y, Fu R, Zhu C, Fan D. An antibacterial bilayer hydrogel modified by tannic acid with oxidation resistance and adhesiveness to accelerate woundrepair. Colloids Surf B. 2021;205:111869. Copyright 2021, with permission from Elsevier.78
Polyε-L-Lysine (EPL)-Based Hydrogel for Diabetic Wound Management
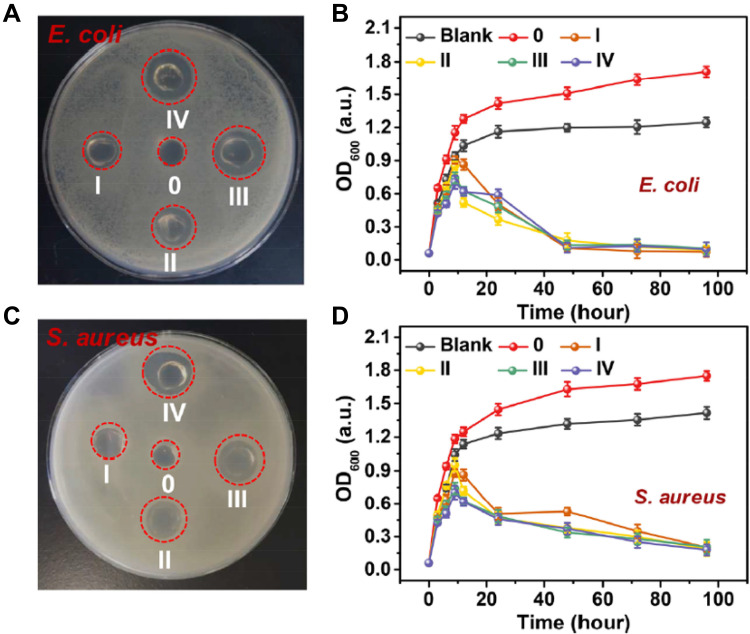
EPL is biodegradable, antibacterial, and biocompatible naturally occurring cationic polypeptide produced by S. albus.69–71 EPL acts as a cationic surface-active compound that is known to inhibit the proliferation of microorganisms by effecting on the outer membrane of bacteria, Specifically, in EPL-treated cells, electrostatic adsorption of EPL and external membrane stripping, accompanied by abnormal cytoplasm distribution, resulted in physiological damage. (Figure 4B).72 A representative example of the antibacterial effect of EPL in vitro is shown in Figure 5. It was found to be active against both Gram-positive and Gram-negative bacteria.73 Two kinds of multifunctional antibacterial hydrogels have been developed that contain EPL. Firstly, an injectable, self-healing and antimicrobial peptide-based FHE hydrogel (F127/OHA-EPL) featuring the stimuli-responsive release of adipose derived mesenchymal stem cell exosomes (AMSCs-exo) was proposed by Chenggui Wang for the synergistic enhancement of chronic wound healing and relative tissue regeneration. FHE@exo hydrogel with 5% (wt/vol) of EPL and 10% (wt/vol) of EPL both had excellent antibacterial activity.72 Further in vivo studies confirmed that neo-vascular formation and cell proliferation were promoted in FHE@ exo hydrogel-treated wounds, leading to faster granulation tissue formation, re-epithelialization and collagen remodeling within the wound site, which accelerated the healing process of diabetic wounds.72 Secondly, a polyacrylamide, gelatin, and ε-polylysine dressing that is temperature tolerant (−20 to 60℃) was prepared and called G-PAGL, which displayed good heat resistance and anti-freezing properties. They established that the G-PAGL with 20% (wt/vol) EPL exerted the highest antibacterial activity against E. coli and S. aureus and the inherent and long-lasting antimicrobial properties conferred by ε-PL were considered essential for G-PAGL hydrogels used as DFU wound dressings.74
Figure 5.
Digital image of E. coli (A) and S. aureus (C) clones on an AGAR plate after exposure; reproduced from Xu Z, Xu Z, Feng X, Xu D, Liang J, Xu H. Recent advances in the biotechnological production of microbial poly(ɛ-L-lysine) and understanding of its biosynthetic mechanism. Appl Microbiol Biotechnol. 2016;100(15):6619–6630. Copyright 2016, Springer Nature.73 Red circles indicate bacterial clones killed by hydrogel. In the presence of blank LB medium, G-PAGL-0, G-PAGL-I, G-PAGL-II, G-PAGL-III and G-PAGL-IV, the growth curves of (B) Escherichia coli and (D) Staphylococcus aureus varied with culture time.
Antimicrobial Peptides (AMPs)-Based Hydrogel for Diabetic Wound Management
AMPs are a type of natural bioactive ingredients with a wide range of antimicrobial and immunomodulatory activities to combat drug resistance, which inhibit the survival of bacteria through targeting the bacterial cell membrane by electrostatic interactions.75 To date, a number of antimicrobial bioactive hydrogel designs have been based on AMPs on account of their low resistance, high biocompatibility and antibacterial benefits.75
Wang et al developed a sprayable hydrogel containing GelMA functionalized by DOPA and encapsulating AMP HC-36 and CeONs, which possesses antibacterial, ROS scavenging and wound healing effects by (Figure 4C).76 By comparing the antibacterial performance of nano-silver, vancomycin and non-AMP loaded hydrogels, hydrogels loaded with AMP could ablate approximately 100% (and GT; 99%) of bacterias especially S. aureus and S. epidermidis.76 Antibacterial hydrogels based on AMPs have shown great antibacterial benefits, which attributes a certain instructive meaning to the investigation of infection wound healing.
TA-Based Hydrogel for Diabetic Wound Management
An inevitable issue in developing antibacterial hydrogels based on natural active ingredients is biocompatibility. Some researchers have found that a phenolic non-cytotoxic natural plant extract, tannic acid (TA), works by attaching to bacteria, inhibiting the uptake of sugars and amino acids, and interfering with their metabolism.77 A research group led by Chenhui Zhu, from Northwest University, changed the structure of the hydrogel by TA and proposed the TA@bilayer hydrogel, which showed excellent properties including adhesion, self-healing, antibacterial and antioxidant, which made the product become a multifunctional antibacterial hydrogel with great advantages (Figure 4D).78
Special Antibiotic Based Antibacterial Hydrogel
Mupirocin antibiotics have been used to treat secondary skin infections caused by S. aureus and S. pyogenes.79–81 However, recent reports indicated a rise in Staphylococci with mupirocin resistance.82,83 Therefore, it is necessary to develop alternative anti-microbial drugs or to improve the efficacy of mupirocin.
Golmohammadi et al created the Selenium-chitosan-Mupirocin (M-SeNPs-CCH) complex, which is a nanohybrid system, prepared using chitosan-cetyltrimethylammonium bromide (CTAB)-based hydrogel (CCH) with mupirocin (M) and selenium nanoparticles (SeNPs) entrapped.84 Its antibacterial activity and toxicity were evaluated on the L929 mouse fibroblast cell line. The concentration of M 20 mg/mL had the best antibacterial ability against S. aureus (MRSA), and the wounds were subsequently treated by M-SeNPs-CCH nanohybrid system with concentrations of M; 20 mg/mL, CCH; 2 mg/mL and SeNPs; 512 μg/mL in two times/day for 21 days.84 It was discovered that this system could play a crucial role in the formation and contraction of wounds, angiogenesis, fibroblastosis, and collagenesis, as well as the proliferation of hair follicles and epidermis.84
An ROS-scavenging hydrogel to promote the healing of infected diabetic wounds was put forward by Jian Wang and his team which was fabricated by using polyvinyl alcohol (PVA) cross-linked by a ROS-responsive linker. This hydrogel could allow the release of GM-CSF and therapeutics, including mupirocin to kill bacteria.84 The doses of M and GM-CSF were 100 μg/wound and 0.5 μg/wound, after treatment with PBS, Hydrogel, M@Hydrogel, G@Hydrogel or M+G@Hydrogel under infection with S. aureus, and the M@Hydrogel and M+G@Hydrogel groups showed more powerful antibacterial activity.84 This work provided an antibacterial ROS-scavenging hydrogel with different therapeutic ingredients such as mupiroxacin, which is expected to be used to treat chronic wounds including infected diabetic wounds.14
However, it is undeniable that the use of antibacterial drugs will bring the serious consequence of antibiotic resistance, therefore, when during developing antibiotic based antibacterial hydrogels, one needs to pay special attention to the limitation of dosage and indications.
Biopolymer-Based Antibacterial Hydrogels
In addition to adding antibacterial substances to hydrogels, some of the biopolymers used to make hydrogels also have antibacterial properties.
Using the dynamic Schiff-based reaction, Qian Xu and Wenxin Wang’s team developed a self-healing hydrogel system made of chitosan (CTS) and dialdehyde chitosan (CTS-CHO), which prevents infection during wound healing. The results showed that the antibacterial properties of chitosan and aldehyde chitosan exhibited in the hydrogel system significantly impaired bacterial growth upon contact.85 However, the antibacterial mechanism of chitosan and its derivatives is still not clear. There is a generally accepted theory that the large number of positively charged amino groups in their molecular structure plays a vital role, they can also absorb bacteria and enter bacterial cells to inhibit bacterial growth by interfering with the transcription of bacterial DNA and block the absorption of trace elements and nutrients necessary for cell growth. These kind of hydrogels with antibacterial action based on biopolymers has the advantages of convenient preparation, high economic benefit and great development prospect.
The Future Perspectives
Chronic wounds fall into different categories,86 such as diabetic foot ulcers (DFU), venous leg ulcers (VLU) and pressure ulcers (PU), surgical site infections (SSI), abscesses, or traumatic ulcers, in which colonization by pathogenic bacteria at the wound site results in wound chronicity.87 Therefore, it is important to perform antibacterial treatment in the process of chronic wound therapy. As we have previously reviewed, many kinds of antibacterial hydrogel have been created and applied for diabetic chronic wound treatment, owing to the special properties of hydrogel (Table 1). With the development of biopolymers, hydrogel synthetic procedures are becoming more mature, along with the diversification of synthetic pathways and products, which is conducive to the further industrialization of hydrogel application and faster translation into clinical treatments. Meanwhile, combining antibacterial metal ions and natural antibacterial substances into hydrogel for antibacterial treatment can help to reduce the use of antibiotics and prevent the generation of bacterial resistance, ultimately reducing the risk of superbug emergence. Photodynamic and photothermal are also widely used in wound antibacterial applications by combining with hydrogels.88,89 Effective antibacterial hydrogels can help to heal patients’ wounds and reduce the pain from chronic diabetic wounds as well as reduce the healthcare burden.
Table 1.
The Roles of Antibiotic Substance and Their Wound Dressings
| Examples | Antibiotic Substance | Other Therapeutic Effects |
|---|---|---|
| SNPECHG hydrogel54 | Ag+ | Re-epithelization, sufficient collagen deposition, and accelerated collagen maturation |
| Chitosan-PEG-Silver Nitrate based hydrogel55 | Antioxidant | |
| Chitosan/PVA/ZnO nanofibrous membranes | Zn2+ | Antioxidant |
| VEGF-decorated t-ZnO-laden hydrogel patches59 | Improved angiogenesis | |
| PABC scaffolds61 | Cu2+ | Enhancing HIF-1α/VEGF expression, improved angiogenesis and collagen matrix deposition |
| HKUST-Hs62 | Inhibiting oxidative stress (antioxidant) | |
| @Cu-MOF hydrogel63 | Low cytotoxicity and high antibacterial activity | |
| Green Tea Derivative Driven Smart Hydrogels65 | EGCG | Antioxidation, antiinflammatory improved angiogenesis, and modulation of macrophage polarization |
| FHE@exosomes (FHE@exo) hydrogel71 | Poly-ε-L-lysine (EPL) | Improving the proliferation, migration, and tube formation ability of HUVECs (angiogenesis) |
| The double-network (DN) G-PAGL hydrogel dressing73 | Improving collagen deposition, angiogenesis, and inhibiting bacterial breed | |
| GelMA-DOPA-AMP-CeONs Hydrogel75 | AMPs | ROS scavenging and wound healing |
| TA@bilayer hydrogel77 | TA | Adhesion, self-healing, and antioxidant |
| Selenium-chitosan-Mupirocin nanohybrid System83 | Mupirocin | Improving angiogenesis, fibroblastosis, collagenesis, proliferation of hair follicle, and epidermis growth |
| Chitosan-Based Self-Healable Hydrogel System84 | Chitosan (CTS) | Biocompatibility, injectability, and self-healable properties |
Currently, Hydrosorb®, 3M™Tegaderm™, AQUACELAg®, TenderWet® and Comfeel® are the main hydrogel dressings available on the market (Table 2), whose components include PU, CHG, CMC, PEA, or other biological large molecules, and Ag+. The antibacterial activity of these hydrogels is mainly attributed to the action of Ag+ and the adsorption of bacteria. However, most of the commercially available products are not multifunctional, lack biological activity, are difficult to cut, are costly, etc. Although the scientific achievements on multi-functional antibacterial hydrogels are increasing, there are too many ideas to be selected from and some of the scholars lack a business aptitude, which leads to few products being commercialized. However, there is still a potential market and extensive prospects of antibacterial hydrogel dressings. Therefore, the development of antibacterial hydrogel dressings needs to rely on the joint efforts of researchers, doctors, patients, and medical device manufacturers to transform emerging technological achievements into products, in order to help more patients that suffer from chronic wounds and realize a win-win situation.
Table 2.
The Commercially Available Hydrogels Used for Wound Dressings
| Trade Name | Composition | Feature |
|---|---|---|
| Hydrosorb® | Polyurethane (PU) | Adsorption capacity, moisturizing, pain relief, reduce scar formation, breathable, waterproof, avoid adhesion |
| 3M™ Tegaderm™ | 2% (w/w) Chlorhexidine Gluconate (CHG) | Effective under compression, safe on fragile tissue, protect the wound, maintains moisture balance |
| AQUACEL Ag® | Carboxymethylcellulose sodium (CMC)/Ag+ | Antibacteria, promoting wound healing |
| TenderWet® | Polyacrylate (PEA)/Ringer’s solution | Continue active debridement, adsorb bacteria and toxins, adjust the balance of seepage, high compliance |
| Comfeel® | CMC/Calcium alginate/Purified water | Transparent, flexible, adsorb bacteria |
Acknowledgment
This work was supported by grants from Scientific Research Fund of Zhejiang Hospital (No. Z210057).
Disclosure
There are no conflicts of interest to declare.
References
- 1.Patty Y, Nita Y, Nita Y. Cost of illness of diabetes mellitus in Indonesia: a systematic review. J Basic Clin Physiol Pharmacol. 2021;32(4):285–295. doi: 10.1515/jbcpp-2020-0502 [DOI] [PubMed] [Google Scholar]
- 2.Clements JM, West BT, Yaker Z, et al. Disparities in diabetes-related multiple chronic conditions and mortality: the influence of race. Diabetes Res Clin Pract. 2020;159:107984. doi: 10.1016/j.diabres.2019.107984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Wahbi A. Operative versus non-operative treatment in diabetic dry toe gangrene. Diabetes Metab Syndr. 2019;13(2):959–963. doi: 10.1016/j.dsx.2018.12.021 [DOI] [PubMed] [Google Scholar]
- 4.Tan CT, Liang K, Ngo ZH, Dube CT, Lim CY. Application of 3D bioprinting technologies to the management and treatment of diabetic foot ulcers. Biomedicines. 2020;8:10. doi: 10.3390/biomedicines8100441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broughton G, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7Suppl):1e-S-32e-S. doi: 10.1097/01.prs.0000222562.60260.f9 [DOI] [PubMed] [Google Scholar]
- 6.Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599–610. doi: 10.1007/s12325-017-0478-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528–1542. doi: 10.1177/147323000903700531 [DOI] [PubMed] [Google Scholar]
- 8.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–1743. doi: 10.1016/S0140-6736(05)67700-8 [DOI] [PubMed] [Google Scholar]
- 9.Lim JZ, Ng NS, Thomas C. Prevention and treatment of diabetic foot ulcers. J R Soc Med. 2017;110(3):104–109. doi: 10.1177/0141076816688346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nouvong A, Ambrus AM, Zhang ER, Hultman L, Coller HA. Reactive oxygen species and bacterial biofilms in diabetic wound healing. Physiol Genomics. 2016;48(12):889–896. doi: 10.1152/physiolgenomics.00066.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thangarajah H, Vial IN, Grogan RH, et al. HIF-1alpha dysfunction in diabetes. Cell Cycle. 2010;9(1):75–79. doi: 10.4161/cc.9.1.10371 [DOI] [PubMed] [Google Scholar]
- 12.Tandara AA, Mustoe TA. Oxygen in wound healing–more than a nutrient. World J Surg. 2004;28(3):294–300. doi: 10.1007/s00268-003-7400-2 [DOI] [PubMed] [Google Scholar]
- 13.Thangarajah H, Yao D, Chang EI, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci U S A. 2009;106(32):13505–13510. doi: 10.1073/pnas.0906670106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao H, Huang J, Li Y, et al. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials. 2020;258:120286. doi: 10.1016/j.biomaterials.2020.120286 [DOI] [PubMed] [Google Scholar]
- 15.Hong HS, Kim S, Jin Y, Son Y. Substance P enhances the therapeutic effect of MSCs by modulating their angiogenic potential. J Cell Mol Med. 2020;24(21):12560–12571. doi: 10.1111/jcmm.15804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–870. doi: 10.1152/physrev.2003.83.3.835 [DOI] [PubMed] [Google Scholar]
- 17.Fang QQ, Wang XF, Zhao WY, et al. Development of a chitosan-vaseline gauze dressing with wound-healing properties in murine models. Am J Trop Med Hyg. 2020;102(2):468–475. doi: 10.4269/ajtmh.19-0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rekha PD, Rao SS, Sahana TG, Prabhu A. Diabetic wound management. Br J Community Nurs. 2018;23(Sup9):S16–S22. doi: 10.12968/bjcn.2018.23.Sup9.S16 [DOI] [PubMed] [Google Scholar]
- 19.Matica MA, Aachmann FL, Tøndervik A, Sletta H, Ostafe V. Chitosan as a wound dressing starting material: antimicrobial properties and mode of action. Int J Mol Sci. 2019;20:23. doi: 10.3390/ijms20235889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JU, Song EH, Jeong SH, Song J, Kim HE, Kim S. Chitosan-based dressing materials for problematic wound management. Adv Exp Med Biol. 2018;1077:527–537. [DOI] [PubMed] [Google Scholar]
- 21.Chattopadhyay S, Raines RT, Glick GD. Review collagen-based biomaterials for wound healing. Biopolymers. 2014;101(8):821–833. doi: 10.1002/bip.22486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hubner P, Donati N, Quines LKM, Tessaro IC, Marcilio NR. Gelatin-based films containing clinoptilolite-Ag for application as wound dressing. Mater Sci Eng C Mater Biol Appl. 2020;107:110215. doi: 10.1016/j.msec.2019.110215 [DOI] [PubMed] [Google Scholar]
- 23.Graça MFP, Miguel SP, Cabral CSD, Correia IJ. Hyaluronic acid-Based wound dressings: a review. Carbohydr Polym. 2020;241:116364. doi: 10.1016/j.carbpol.2020.116364 [DOI] [PubMed] [Google Scholar]
- 24.Cortes H, Caballero-Florán IH, Mendoza-Muñoz N, et al. Hyaluronic acid in wound dressings. Cell Mol Biol. 2020;66(4):191–198. doi: 10.14715/cmb/2020.66.4.23 [DOI] [PubMed] [Google Scholar]
- 25.Portela R, Leal CR, Almeida PL, Sobral RG. Bacterial cellulose: a versatile biopolymer for wound dressing applications. Microb Biotechnol. 2019;12(4):586–610. doi: 10.1111/1751-7915.13392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alven S, Aderibigbe BA. Chitosan and cellulose-based hydrogels for wound management. Int J Mol Sci. 2020;21:24. doi: 10.3390/ijms21249656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varaprasad K, Jayaramudu T, Kanikireddy V, Toro C, Sadiku ER. Alginate-based composite materials for wound dressing application: a mini review. Carbohydr Polym. 2020;236:116025. doi: 10.1016/j.carbpol.2020.116025 [DOI] [PubMed] [Google Scholar]
- 28.Zheng C, Liu C, Chen H, et al. Effective wound dressing based on Poly (vinyl alcohol)/Dextran-aldehyde composite hydrogel. Int J Biol Macromol. 2019;132:1098–1105. doi: 10.1016/j.ijbiomac.2019.04.038 [DOI] [PubMed] [Google Scholar]
- 29.Tang KC, Yang KC, Lin CW, et al. Human adipose-derived stem cell secreted extracellular matrix incorporated into electrospun poly(lactic-co-glycolic acid) nanofibrous dressing for enhancing wound healing. Polymers. 2019;11:10. doi: 10.3390/polym11101609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giammona G, Craparo EF. Biomedical Applications of Polylactide (PLA) and its copolymers. Molecules. 2018;23(4). doi: 10.3390/molecules23040980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francesko A, Petkova P, Tzanov T. Hydrogel dressings for advanced wound management. Curr Med Chem. 2018;25(41):5782–5797. doi: 10.2174/0929867324666170920161246 [DOI] [PubMed] [Google Scholar]
- 32.Li W, Wang S, Zhong D, Du Z, Zhou M. A bioactive living hydrogel: photosynthetic bacteria mediated hypoxia elimination and bacteria‐killing to promote infected wound healing. Adv Ther. 2020;4(1):2000107. [Google Scholar]
- 33.Sepantafar M, Maheronnaghsh R, Mohammadi H, et al. Stem cells and injectable hydrogels: synergistic therapeutics in myocardial repair. Biotechnol Adv. 2016;34(4):62–379. doi: 10.1016/j.biotechadv.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 34.Lee PY, Li Z, Huang L. Thermosensitive hydrogel as a Tgf-β1 gene delivery vehicle enhances diabetic wound healing. Pharm Res. 2003;20(12):1995–2000. doi: 10.1023/B:PHAM.0000008048.58777.da [DOI] [PubMed] [Google Scholar]
- 35.Piperigkou Z, Götte M, Theocharis AD, Karamanos NK. Insights into the key roles of epigenetics in matrix macromolecules-associated wound healing. Adv Drug Deliv Rev. 2018;129:16–36. doi: 10.1016/j.addr.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 36.Taub A, Bucay V, Keller G, Williams J, Mehregan D. Multi-center, double-blind, vehicle-controlled clinical trial of an alpha and beta defensin-containing anti-aging skin care regimen with clinical, histopathologic, immunohistochemical, photographic, and ultrasound evaluation. J Drugs Dermatol. 2018;17(4):426–441. [PubMed] [Google Scholar]
- 37.Peppa M, Raptis SA. Glycoxidation and wound healing in diabetes: an interesting relationship. Curr Diabetes Rev. 2011;7(6):416–425. doi: 10.2174/157339911797579188 [DOI] [PubMed] [Google Scholar]
- 38.Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2020;226:S1019–S1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vagesjo E, Ohnstedt E, Mortier A, et al. Accelerated wound healing in mice by on-site production and delivery of CXCL12 by transformed lactic acid bacteria. Proc Natl Acad Sci U S A. 2018;115(8):1895–1900. doi: 10.1073/pnas.1716580115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shankhdhar K. Diabetic foot amputation prevention during COVID-19. Adv Skin Wound Care. 2021;34(5):1–4. doi: 10.1097/01.ASW.0000741532.29113.78 [DOI] [PubMed] [Google Scholar]
- 41.Yin IX, Zhang J, Zhao IS, Mei ML, Li Q, Chu CH. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int J Nanomed. 2020;15:2555–2562. doi: 10.2147/IJN.S246764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zong M, Bai L, Liu Y, et al. Antibacterial ability and angiogenic activity of Cu-Ti-O nanotube arrays. Mater Sci Eng C. 2017;71:93–99. doi: 10.1016/j.msec.2016.09.077 [DOI] [PubMed] [Google Scholar]
- 43.Yang L, Chen L, Chen YC, et al. Homogeneously alloyed nanoparticles of immiscible Ag-Cu with ultrahigh antibacterial activity. Colloids Surf B. 2019;180:466–472. doi: 10.1016/j.colsurfb.2019.05.018 [DOI] [PubMed] [Google Scholar]
- 44.Prado-Prone G, Silva-Bermudez P, Bazzar M, et al. Antibacterial composite membranes of polycaprolactone/gelatin loaded with zinc oxide nanoparticles for guided tissue regeneration. Biomed Mater. 2020;15(3):035006. doi: 10.1088/1748-605X/ab70ef [DOI] [PubMed] [Google Scholar]
- 45.Wyszogrodzka G, Marszałek B, Gil B, Dorożyński P. Metal-organic frameworks: mechanisms of antibacterial action and potential applications. Drug Discov Today. 2016;21(6):1009–1018. doi: 10.1016/j.drudis.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Liu S, Li M, et al. The synergistic antibacterial activity and mechanism of multicomponent metal ions-containing aqueous solutions against Staphylococcus aureus. J Inorg Biochem. 2016;163:214–220. doi: 10.1016/j.jinorgbio.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 47.Sharma SK, Goloubinoff P, Christen P. Heavy metal ions are potent inhibitors of protein folding. Biochem Biophys Res Commun. 2008;372(2):341–345. doi: 10.1016/j.bbrc.2008.05.052 [DOI] [PubMed] [Google Scholar]
- 48.Kulakovskaya T. Inorganic polyphosphates and heavy metal resistance in microorganisms. World J Microbiol Biotechnol. 2018;34(9):139. doi: 10.1007/s11274-018-2523-7 [DOI] [PubMed] [Google Scholar]
- 49.Kalantari K, Mostafavi E, Afifi AM, et al. Wound dressings functionalized with silver nanoparticles: promises and pitfalls. Nanoscale. 2020;12(4):2268–2291. doi: 10.1039/C9NR08234D [DOI] [PubMed] [Google Scholar]
- 50.Pangli H, Vatanpour S, Hortamani S, Jalili R, Ghahary A. Incorporation of silver nanoparticles in hydrogel matrices for controlling wound infection. J Burn Care Res. 2021;42(4):785–793. doi: 10.1093/jbcr/iraa205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gauger A. Silver-coated textiles in the therapy of atopic eczema. Curr Probl Dermatol. 2006;33:152–164. [DOI] [PubMed] [Google Scholar]
- 52.Arroyo GV, Madrid AT, Gavilanes AF, et al. Green synthesis of silver nanoparticles for application in cosmetics. J Environ Sci Health A. 2020;55(11):1304–1320. doi: 10.1080/10934529.2020.1790953 [DOI] [PubMed] [Google Scholar]
- 53.Probst S, Saini C, Rosset C, Skinner MB. Superabsorbent charcoal dressing versus silver foam dressing in wound area reduction: a randomised controlled trial. J Wound Care. 2022;31(2):140–146. doi: 10.12968/jowc.2022.31.2.140 [DOI] [PubMed] [Google Scholar]
- 54.Lee YH, Hong YL, Wu TL. Novel silver and nanoparticle-encapsulated growth factor co-loaded chitosan composite hydrogel with sustained antimicrobility and promoted biological properties for diabetic wound healing. Mater Sci Eng C. 2021;118:111385. doi: 10.1016/j.msec.2020.111385 [DOI] [PubMed] [Google Scholar]
- 55.Masood N, Ahmed R, Tariq M, et al. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int J Pharm. 2019;559:23–36. doi: 10.1016/j.ijpharm.2019.01.019 [DOI] [PubMed] [Google Scholar]
- 56.Vijayakumar V, Samal SK, Mohanty S, Nayak SK. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int J Biol Macromol. 2019;122:137–148. doi: 10.1016/j.ijbiomac.2018.10.120 [DOI] [PubMed] [Google Scholar]
- 57.Gharpure S, Ankamwar B. Synthesis and antimicrobial properties of zinc oxide nanoparticles. J Nanosci Nanotechnol. 2020;20(10):5977–5996. doi: 10.1166/jnn.2020.18707 [DOI] [PubMed] [Google Scholar]
- 58.Mishra PK, Mishra H, Ekielski A, Talegaonkar S, Vaidya B. Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug Discov Today. 2017;22(12):1825–1834. doi: 10.1016/j.drudis.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 59.Ahmed R, Tariq M, Ali I, et al. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int J Biol Macromol. 2018;120(Pt A):385–393. doi: 10.1016/j.ijbiomac.2018.08.057 [DOI] [PubMed] [Google Scholar]
- 60.Siebert L, Luna‐Cerón E, García‐Rivera LE, et al. Light‐controlled growth factors release on tetrapodal ZnO‐incorporated 3D‐printed hydrogels for developing smart wound scaffold. Adv Funct Mater. 2021;31(22):2007555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun X, Dong M, Guo Z, et al. Multifunctional chitosan-copper-gallic acid based antibacterial nanocomposite wound dressing. Int J Biol Macromol. 2021;167:10–22. doi: 10.1016/j.ijbiomac.2020.11.153 [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Xu T, Tu Z, et al. Bioactive antibacterial silica-based nanocomposites hydrogel scaffolds with high angiogenesis for promoting diabetic wound healing and skin repair. Theranostics. 2020;10(11):4929–4943. doi: 10.7150/thno.41839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang M, Huang H, Ma X, Huang C, Peng X. Copper metal-organic framework embedded carboxymethyl chitosan-g-glutathione/polyacrylamide hydrogels for killing bacteria and promoting wound healing. Int J Biol Macromol. 2021;187:699–709. doi: 10.1016/j.ijbiomac.2021.07.139 [DOI] [PubMed] [Google Scholar]
- 64.Gwon K, Han I, Lee S, Kim Y, Lee DN. Novel metal-organic framework-based photocrosslinked hydrogel system for efficient antibacterial applications. ACS Appl Mater Interfaces. 2020;12(18):20234–20242. doi: 10.1021/acsami.0c03187 [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Yang Y, Shi Y, Song H, Yu C. Antibiotic-free antibacterial strategies enabled by nanomaterials: progress and perspectives. Adv Mater. 2020;32(18):e1904106. doi: 10.1002/adma.201904106 [DOI] [PubMed] [Google Scholar]
- 66.Xu FW, Lv YL, Zhong YF, et al. Beneficial effects of green tea EGCG on skin wound healing: a comprehensive review. Molecules. 2021;26:20. doi: 10.3390/molecules26206123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steinmann J, Buer J, Pietschmann T, Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol. 2013;168(5):1059–1073. doi: 10.1111/bph.12009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao X, Pei D, Yang Y, et al. Green tea derivative driven smart hydrogels with desired functions for chronic diabetic wound treatment. Adv Funct Mater. 2021;31(18):2009442. doi: 10.1002/adfm.202009442 [DOI] [Google Scholar]
- 69.Chen S, Huang S, Li Y, Zhou C. Recent advances in epsilon-Poly-L-lysine and L-lysine-based dendrimer synthesis, modification, and biomedical applications. Front Chem. 2021;9:659304. doi: 10.3389/fchem.2021.659304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nishikawa M, Ogawa K. Inhibition of epsilon-poly-L-lysine biosynthesis in Streptomycetaceae bacteria by short-chain polyols. Appl Environ Microbiol. 2006;72(4):2306–2312. doi: 10.1128/AEM.72.4.2306-2312.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang L, Zhang C, Zhang J, et al. Epsilon-poly-L-lysine: recent advances in biomanufacturing and applications. Front Bioeng Biotechnol. 2021;9:748976. doi: 10.3389/fbioe.2021.748976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang C, Wang M, Xu T, et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9(1):65–76. doi: 10.7150/thno.29766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu Z, Xu Z, Feng X, Xu D, Liang J, Xu H. Recent advances in the biotechnological production of microbial poly(ɛ-L-lysine) and understanding of its biosynthetic mechanism. Appl Microbiol Biotechnol. 2016;100(15):6619–6630. doi: 10.1007/s00253-016-7677-3 [DOI] [PubMed] [Google Scholar]
- 74.Liu H, Li Z, Zhao Y, et al. Novel diabetic foot wound dressing based on multifunctional hydrogels with extensive temperature-tolerant, durable, adhesive, and intrinsic antibacterial properties. ACS Appl Mater Interfaces. 2021;13(23):26770–26781. doi: 10.1021/acsami.1c05514 [DOI] [PubMed] [Google Scholar]
- 75.Boparai JK, Sharma PK. Mini review on antimicrobial peptides, sources, mechanism and recent applications. Protein Pept Lett. 2020;27(1):4–16. doi: 10.2174/0929866526666190822165812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheng H, Shi Z, Yue K, et al. Sprayable hydrogel dressing accelerates wound healing with combined reactive oxygen species-scavenging and antibacterial abilities. Acta Biomater. 2021;124:219–232. doi: 10.1016/j.actbio.2021.02.002 [DOI] [PubMed] [Google Scholar]
- 77.Kaczmarek B. Tannic acid with antiviral and antibacterial activity as a promising component of Biomaterials-A minireview. Materials. 2020;13:14. doi: 10.3390/ma13143224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Y, Fu R, Zhu C, Fan D. An antibacterial bilayer hydrogel modified by tannic acid with oxidation resistance and adhesiveness to accelerate wound repair. Colloids Surf B. 2021;205:111869. doi: 10.1016/j.colsurfb.2021.111869 [DOI] [PubMed] [Google Scholar]
- 79.Tucaliuc A, Blaga AC, Galaction AI, Cascaval D. Mupirocin: applications and production. Biotechnol Lett. 2019;41(4–5):495–502. doi: 10.1007/s10529-019-02670-w [DOI] [PubMed] [Google Scholar]
- 80.Sritharadol R, Hamada M, Kimura S, Ishii Y, Srichana T, Tateda K. Mupirocin at subinhibitory concentrations induces biofilm formation in Staphylococcus aureus. Microb Drug Resist. 2018;24(9):1249–1258. doi: 10.1089/mdr.2017.0290 [DOI] [PubMed] [Google Scholar]
- 81.Kotloff KL, Shirley DT, Creech CB, et al. Mupirocin for Staphylococcus aureus decolonization of infants in neonatal intensive care units. Pediatrics. 2019;143(1). doi: 10.1542/peds.2018-1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thomas CM, Hothersall J, Willis CL, Simpson TJ. Resistance to and synthesis of the antibiotic mupirocin. Nat Rev Microbiol. 2010;8(4):281–289. doi: 10.1038/nrmicro2278 [DOI] [PubMed] [Google Scholar]
- 83.Hetem DJ, Bonten MJ. Clinical relevance of mupirocin resistance in Staphylococcus aureus. J Hosp Infect. 2013;85(4):249–256. doi: 10.1016/j.jhin.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 84.Golmohammadi R, Najar-Peerayeh S, Tohidi Moghadam T, Hosseini SMJ. Synergistic antibacterial activity and wound healing properties of selenium-chitosan-mupirocin nanohybrid system: an in vivo study on rat diabetic Staphylococcus aureus wound infection model. Sci Rep. 2020;10(1):2854. doi: 10.1038/s41598-020-59510-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X, Song R, Johnson M, He C, Milne X, Wang I. An injectable chitosan-based self-healable hydrogel system as an antibacterial wound dressing. Materials. 2021;14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dissemond J, Kröger K, Storck M, Risse A, Engels P. Topical oxygen wound therapies for chronic wounds: a review. J Wound Care. 2015;24(2):3–4, 56–60, 62–3. doi: 10.12968/jowc.2015.24.2.53 [DOI] [PubMed] [Google Scholar]
- 87.Rahim K, Saleha S, Zhu X, Huo L, Basit A, Franco OL. Bacterial contribution in chronicity of wounds. Microb Ecol. 2017;73(3):710–721. doi: 10.1007/s00248-016-0867-9 [DOI] [PubMed] [Google Scholar]
- 88.Guo N, Xia Y, Zeng W, et al. Alginate-based aerogels as wound dressings for efficient bacterial capture and enhanced antibacterial photodynamic therapy. Drug Deliv. 2022;29(1):1086–1099. doi: 10.1080/10717544.2022.2058650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y, Fu R, Duan Z, Zhu C, Fan D. Artificial nonenzymatic antioxidant MXene nanosheet-anchored injectable hydrogel as a mild photothermal-controlled oxygen release platform for diabetic wound healing. ACS Nano. 2022;16(5):7486–7502. doi: 10.1021/acsnano.1c10575 [DOI] [PubMed] [Google Scholar]