Abstract
Cancer is a leading cause of death in patients with kidney transplantation. Patients with kidney transplants are 10- to 200-times more likely to develop cancers after transplant than the general population, depending on the cancer type. Recent advances in cancer therapies have dramatically improved survival outcomes; however, patients with kidney transplants face unique challenges of immunosuppression management, cancer screening, and recurrence of cancer after transplant. Patients with a history of cancer tend to be excluded from transplant candidacy or are required to have long cancer-free wait time before wait-listing. The strategy of pretransplant wait time management may need to be revisited as cancer therapies improve, which is most applicable to patients with a history of multiple myeloma. In this review, we discuss several important topics in transplant onconephrology: the current recommendations for pretransplant wait times for transplant candidates with cancer histories, cancer screening post-transplant, post-transplant lymphoproliferative disorder, strategies for transplant patients with a history of multiple myeloma, and novel therapies for patients with post-transplant malignancies. With emerging novel cancer treatments, it is critical to have multidisciplinary discussions involving patients, caregivers, transplant nephrologists, and oncologists to achieve patient-oriented goals.
Keywords: Kidney transplantation, Cancer, Multiple myeloma, Immunosuppression, Immunotherapy
Graphical Abstract
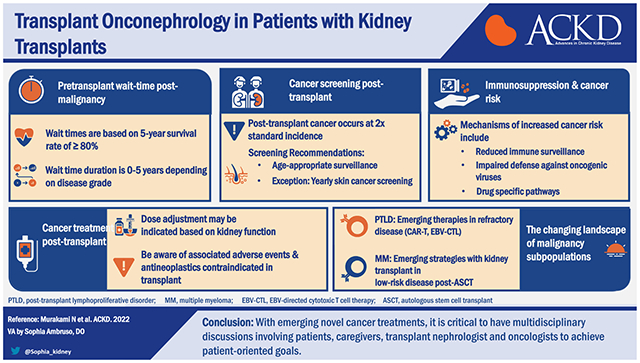
Cancer is a leading cause of death in kidney transplant recipients. Recent advancements in cancer treatment have changed the landscape of pretransplant and post-transplant management. Recently, the Transplant Cancer Match Study showed that cancer incidence has not changed over the past 3 decades and patient outcomes (death with functioning allograft or death-censored allograft failure) have not changed except for patients with non-Hodgkin’s lymphoma.1 Thus, there is an unmet clinical need to improve patient outcomes in kidney transplant recipients with cancer. In this review, we discuss important topics of transplant onconephrology, focusing on pretransplant wait time, management of multiple myeloma, cancer screening post-transplant, and emerging novel cancer therapies.
Pretransplant Wait Time for Prior Malignancies
Timing of transplantation in patients with a history of preexisting malignancy is of significant debate. Several guidelines have been proposed, with the most recent by Al-Adra and colleagues.2,3 The guidelines are based on a consensus conference in 2019 between transplant professionals and oncologists. Although not comprehensive, they cover the most commonly encountered malignancies. The guidelines were predominantly based on cancer outcomes in the general population, as data in transplanted populations remain limited. Cancer risk stratification was based on TNM (tumor, node, metastasis) staging as well as recent advances including tumor marker analysis and epigenetics. In general, wait times are based on a benchmark of expected 5-year cancer survival rate of 80% or more. The suggested wait times range from no wait time to 2 years for low-grade disease and up to 5 years for high-grade disease. Below is a brief summary of proposed wait times based on cancer type (Table 1). Guidelines for stage 4 disease are not covered as in most cases these patients are not candidates for transplantation. For a more comprehensive review, it is suggested to reference the original manuscript.
Table 1.
Recommendation on Cancer-free Wait Time
| Cancer Type | Classification | Recommended Wait* | Comments |
|---|---|---|---|
| Breast | |||
| DCIS/stage 1 | No wait | ||
| Stage 2 | 1-2 years | ||
| Stage 3 | 3-5 years | ||
| Colorectal | |||
| Stage 1 (T1 or T2,N0,M0) | 1-2 years | ||
| Stage 2 (T3,N0,M0) | 2 years | ||
| Stage 2 (T4,N0,M0) | 3-5 years | 5 years for high-risk features | |
| Stage 3 (N+) | 5 years | ||
| Anal | |||
| Invasive, HPV-related | 5 years | ||
| Prostate | |||
| PSA < 10, Gleason < 6,T1-T2a | No wait | If nomogram CS death over 15 yrs < 10% | |
| PSA > 10, Gleason 7 (Grade 2,3) T2b | No wait | ||
| PSA > 20 ng/mL, high volume Gleason 7 | No wait | Treatment suggested | |
| Gleason 8-10, T3 | |||
| Renal cell | |||
| T1a (<4 cm) N0, M0 | No wait | ||
| T1b (>4 cm ≤ 7 cm) N0, M0, Fuhrman grade G1-2 | No wait | ||
| T1b (>4 cm ≤ 7 cm) N0, M0, Fuhrman grade G3-4 | 1-2 years | ||
| T3 or T4 tumors | 2 years minimum | ||
| Bladder | |||
| Low risk NMIBC | 6 months | If no recurrence at 6 months | |
| Intermediate risk NMIBC | 6 months | If no recurrence at 6 months | |
| High risk NMIBC | 2 years | After therapy | |
| Muscle invasive | 2 years | After radical cystectomy | |
| Gynecologic | |||
| Stage 1A, 1B, grade 1-2 endometrial cancer without lymph-vascular space invasion | No wait | ||
| Stage 1A. 1B, 1C grade 1-2 epithelial ovarian cancer | |||
| Stage 1A1, 1A2 squamous/adenocarcinoma of the cervix | |||
| Stage 1 and 2 endometrial cancer with risk factors and Stage 1b cervical cancer | 2-3 years | ||
| Serous, clear cell or carcinosarcoma of the uterus (all stages) | 5 years | ||
| Stage 3 grade 1-3 endometrial cancer of the uterus | |||
| Stage 2,3 epithelial ovarian cancer | |||
| Stage 2,3 squamous cell/adenocarcinoma cervical cancer | |||
| Non-small cell lung | |||
| Early-stage disease (<1b) | 3-5 years | ||
| Stage 1b-3a | 5 years | ||
| Melanoma | |||
| In situ | No wait time | ||
| Stages ≤ 2a | 1 year | ||
| Stage 3a | 1-2 years | ||
| Stage 2b, 2c, 3b | 2-4 years | ||
| Lymphoma | |||
| 2 years after remission |
Abbreviations: CS death, cancer-specific death; DCIS, ductal carcinoma in situ; NMIBC, non–muscle-invasive bladder cancer.
Wait times begin after therapy, if therapy is warranted.
Breast Cancer.
Prognostic staging was based on the American Joint Committee on Cancer (AJCC)’s staging manual, 8th edition and include tumor receptor status (ER+/HER2 +). Based on excellent 5-year survival in low-risk disease, it is suggested that there is no recommended wait time for ductal carcinoma in situ (DCIS) and stage 1 disease. For stage 2 disease, a wait time of 1-2 years was suggested and 3-5 years for stage 3 disease. It is suggested that patients with inflammatory cancer or stage IV disease do not undergo transplantation.
Colorectal Cancer.
Prognostic staging was based on the AJCC’s staging manual which was recently refined with emphasis on histopathologic and molecular features. Low-risk features include microsatellite instability without BRAF mutation, while high-risk features include lymphovascular invasion, perineural invasion, mucinous or signet histology, poorly differentiated histology, bowel obstruction, tumor perforation, < 12 lymph nodes examined, and tumor deposits. The suggested wait time for stage 1 disease is 1 year, 2 years for T3 stage 2 disease, and 3-5 years for T4 stage 2 disease or if high-risk features are present. A wait time of 5 years is suggested for stage 3 disease (node-positive disease).
Anal Cancer.
Squamous cell anal cancer accounts for a small portion of gastrointestinal cancers, but it is often associated with human papilloma virus (HPV). Owing to the possibility of worse viral control through immunosuppression, careful consideration before transplant is suggested. For invasive HPV-related anal cancer, the suggested wait time is 5 years.
Prostate Cancer.
Prostate cancer is considered low risk for progression even with immunosuppression. For this reason, wait imes tend to be very short except for high-grade disease. There is no suggested wait time for cancers limited to the prostate. The authors go on to state that surveillance alone is reasonable for most prostate cancers. Treatment is suggested before transplant for high-volume intermediate risk or high risk. These cancers include prostate-specific antigen (PSA) is > 20 ng/mL, high-volume Gleason 7 disease, or any Gleason 8-10 disease.
Renal Cell Carcinoma.
There is no required wait time for small (< 4 cm) incidental renal cell carcinoma (RCC) after resection. For cancers staged T1b (tumor > 4 cm ≤ 7 cm) with Fuhrman grade 1-2, there is nowait time. With 3-4 Fuhrman grade, 1-2 years ofwait time is recommended. For T3 or T4 tumors, a minimum wait time of 2 years is suggested.
Bladder Cancer.
In patients with localized non–muscle-invasive bladder cancer (NMIBC), ecurrence rate is very high, but progression is low. These cancers can often be treated locally. For low-risk NMIBC, patients should have surveillance at 6 months after resection. If no recurrence, transplantation can be performed without further wait. The same is suggested for intermediate-risk NMIBC. For high-risk NMIBC, a wait time of at least 2 years is advised after local and intravesical therapy. In patients with muscle-invasive bladder cancer, transplantation can be considered 2 years after radical cystectomy.
Gynecological Cancers (Endometrial, Ovarian, and Cervical).
These cancers were grouped together based on classification by the International Federation of Gynecologic Oncology. These cancers were classified by 5-year risk of recurrence: low risk (<5%), intermediate risk (5-15%), high risk (>30%), and very high (>80%). There is no suggested waiting time for low-risk disease, which included most stage 1 gynecologic cancers without risk factors, 2-3 years for intermediate-risk disease which included stage I and II endometrial cancer with risk factors and Ib cervical cancer, and 5 years for high-risk disease. Transplantation was not recommended for very high-risk cancers.
Non-Small Cell Lung Cancer.
These cancers remain the leading cause of cancer-related mortality in the US, with most recurrences occurring in the first 2 years after treatment. However, late metastatic recurrences are not uncommon. Therefore, it is suggested that patients with early-stage disease that responded to treatment can be considered transplant candidates after 3-5 years but with caution.
Melanoma.
Owing to recent advances, the prognosis of melanoma has dramatically improved. Wait time of 1 year is suggested in all stages IIa or less, whereas 2-4 years of wait time is suggested for stages IIIb or less. There was no consensus reached for stages above IIIb. These wait times are in stark difference to prior recommendations. It is important to note that much of the improvement in melanoma therapy may be attributed to immune checkpoint inhibitors (ICIs) which increase risks of rejection after transplant. Response to ICIs also suggests that melanoma is a particularly immune-sensitive disease, meaning there is significant potential to worsen melanoma after initiating immunosuppression for transplantation.
Lymphoma.
Several types of lymphoma exist from acute and aggressive to indolent. In general, treated patients with progression-free survival at 2 years have above 80% 5-year cancer-free survival. For this reason, it is suggested that patients with a history of most lymphomas may be transplanted after 2 years from therapy.
Screening for Cancer After Transplantation
After kidney transplantation, cancer occurs at twice the standardized incidence of the general population, and therefore, screening for cancer after transplantation is of utmost importance.4 Society recommendations for cancer screening vary.5 In general, cancer screening should follow the recommendations for the general population with a few exceptions.
Skin and Lip Cancer.
The most common skin cancers seen are cutaneous squamous cell carcinoma (cSCC) and basal cell carcinoma. Melanoma also occurs more commonly after transplantation. It is therefore advised that skin cancer screening occur annually in all transplant recipients by a physician with experience in skin cancer.6
Urologic Malignancies.
No clear guidelines exist for RCC screening of native kidneys after transplantation. Patients with retransplant acquired cystic kidney disease, prior RCC, and analgesic abuse may benefit from screening ultrasonography every 1-3 years after transplantation.7,8 Transplant recipients with new-onset hematuria should also be evaluated for a urologic malignancy.
Table 2 summarizes recommendations from the American Society of Transplantation (AST) and Kidney Disease: Improving Global Outcomes (KDIGO).6,9
Table 2.
Recommendation on Cancer Screening After Kidney Transplantation
| Screening | KDIGO | AST |
|---|---|---|
| Skin/lip | Annual examination | Annual examination |
| Anogenital | Annual examination | |
| Pelvic exam/Pap smear | Every 3 years | Annually |
| Mammogram | Annually > age 40 | Every 1-2 years |
| PSA/DRE | Not recommended | Annually age > 50 |
| Colorectal | Same as general population | Same as general population |
| Lymph node exam | Every 3 months in the first year then annually | |
Abbreviations: AST, American Society of Transplantation; DRE, digital rectal exam; KDIGO, Kidney Disease: Improving Global Outcomes; PSA, prostate-specific antigen.
Immunosuppression and Cancer Risk
One of the most important risks for cancer after transplantation relates to immunosuppression. There are several manners in which immunosuppression can increase the risk for cancer including reduced immune surveillance, impaired defense mechanism against oncogenic viruses, and drug-specific pathways. The importance of total immunosuppression in cancer risk is highlighted by historic data that suggest heart transplant patients have a higher incidence of malignancy than kidney transplant recipients.10,11 Similarly, several studies have suggested increased risk for malignancy, in particular post-transplant lymphoproliferative disorders (PTLDs), in patients who have received lymphocyte depletion induction therapy.12,13 Higher doses of antilymphocyte preparations may also predispose them to post-transplant malignancy.14 Lymphodepleting induction agents are in contrast to nondepleting agents, such as the IL-2 receptor antibodies, which have not been associated with an increased risk of cancer.
Although net immunosuppression is a risk for post-transplant malignancy, maintenance immunosuppressives differ in neoplastic potential.
Calcineurin Inhibitors.
Both tacrolimus and cyclosporine are associated with an increased risk for malignancy. Several mechanisms have been implicated such as increased production of cytokines including transforming growth factor (TGF)-beta, vascular endothelial growth factor (VEGF), and IL-6. Calcineurin inhibitors also reduce the ability to repair radiation-induced DNA damage important in the pathogenesis of skin cancer.15 Dantal and colleagues found a lower incidence of malignancy with low-dose cyclosporine than with high-dose cyclosporine.16 Another study initially suggested a higher incidence of malignancy with tacrolimus over cyclosporine, but was later thought to be related to tacrolimus dose rather than a drug-specific effect.17
Antimetabolites.
Azathioprine has been implicated in the development of post-transplant malignancy, especially nonmelanoma skin cancer. This may be related to impaired radiation-induced DNA repair and increased development of microsatellite DNA instability.18,19 Mycophenolate analogs however (mycophenolate mofetil [MMF] and mycophanolic acid [MPA]) have not been found to independently increase the risk for malignancy. In fact, it has been suggested that the incidence of post-transplant malignancy has decreased after introduction of MMF.20 It is unclear if there are specific antitumor properties of MMF or if simply less rejection results in less overall net immunosuppression.
Mammalian Target of Rapamycin Inhibitors.
Several registry-based studies suggest that mammalian target of rapamycin (mTOR) inhibitors may decrease the incidence of post-transplant malignancy compared with other immunosuppressive regimens.21,22 In fact, everolimus has been used to treat hormone receptor–positive, HER2-negative breast cancer, neuroendocrine tumors, and RCC (brand name Afinitor®). In addition, several case series have reported full resolution of post-transplant Kaposi sarcoma by switching from a calcineurin inhibitor to sirolimus.23 In the TUMORAPA study, conversion to sirolimus significantly reduced the risk for cSCC relapse compared with calcineurin inhibitors.24 However, a recent study found higher mortality in transplant patients on mTOR inhibitors despite a lower rate of malignancy.25 Antitumor mechanisms include reduced TGF-beta and IL-10 production, direct antitumor effect by inhibiting the mTOR pathway, inhibition of p70 S6K cancer cell proliferation, and inhibition of tumor angiogenesis by decreasing VEGF signaling.26–31
Belatacept.
Belatacept was initially found to result in an elevated risk for PTLDs, especially with central nervous system involvement.32 Many of these cases occurred in patients who were Epstein-Barr virus (EBV) seronegative, and belatacept now carries a black box warning against its use in such individuals. Long-term extension studies in EBV-positive patients suggest a similar incidence of malignancy as other immunosuppressive agents.33
Post-Transplant Lymphoproliferative Disorder
Post-transplant lymphoproliferative disorder (PTLD) is a serious complication after transplantation, associated with cumulative exposure to immunosuppression. The prevalence of PTLD is 1-2%, and the mortality is as high as 50%. PTLD occurs in two phases, with one peak in early phase (predominantly EBV-positive, 1-2 years after transplant), and the other peak in late phase (mostly EBV-negative, 7-10 years after transplantation).34 The clinical presentation of PTLD varies from asymptomatic mononucleosis to advanced lymphoma. Lymphoproliferative disease is commonly found in the gastrointestinal tract and central nervous system, and the symptoms at presentation can be nonspecific. Risk factors for PTLD include EBV status mismatch (ie, donor EBV positive to recipient EBV negative), lymphocyte-depleting induction, and pediatric transplantation.35 Treatment approach involves the reduction of immunosuppression, which may achieve objective response rate of 30-40% with an accompanied risk of acute rejection.36,37 With no randomized trials to guide the reduction of immunosuppression, discontinuation of antimetabolite agents and reduction of calcineurin inhibitors are common.38 If there is no improvement with immunosuppression reduction, additional therapy with chemo/targeted therapy should be considered. The therapeutic approach includes an anti-CD20 agent (eg, rituximab) with or without cytotoxic chemotherapy. A recent phase II study showed rituximab alone or in combination with R–CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) achieved complete remission in 25% of patients.37
More recently, cellular therapies have become available for PTLD: EBV-directed cytotoxic T-cell therapy (EBV-CTL) and chimeric antigen receptor (CAR)-T therapy. EBV-CTL is indicated in patients with EBV-positive PTLD, refractory to other conventional chemotherapy/targeted therapy. Engineered allogeneic EBV-CTL is manufactured and infused in the patients. One product uses HLA-matched EBV-CTL, which achieved objective response rate of 64% at 5 weeks and 52% in 6 months.39 Another product of pooled third party EBV-CTL was tested in pediatric patients, achieving complete remission in 7 and partial response in 1 out of 11 patients.40 Another emerging cellular therapy for PTLD is CAR-T therapy. CAR-T cells are the manufactured cellular product derived from patients’ T cells, expressing engineered chimeric antigen receptors (CARs) to target cancer cells. CAR-T therapy has been approved by the FDA for diffuse large B-cell lymphoma (DLBCL)41 and multiple myeloma42 in nontransplant patients and showed promise in refractory cases. However, CAR-T therapy in solid organ transplant patients is challenging owing to presumed higher risk of rejection while on minimal immunosuppression and concern for concomitant immunosuppression therapy, which could theoretically increase in the chance of manufacturing failure of CAR-T therapy. CAR-T therapy consists of multiple steps (Fig 1): leukocyte mobilization, leukapheresis, ex vivo CAR-T manufacturing, lymphodepleting chemotherapy, and CAR-T infusion. In nontransplant patients, acute kidney injury (AKI) and electrolyte abnormalities are common after CAR-T.43,44 In patients with kidney transplants, in addition to AKI risk, the risk of rejection is thought to be highest in the peri-infusion process. To mitigate rejection while keeping CAR-T effective, several immunosuppression modifications are considered. First, to collect functional T cells by leukapheresis, the immunosuppression should be reduced beforehand, as T cells may be functionally exhausted in the presence of immunosuppression. Some experts suggest reduction to a single agent, prednisone 5-10 mg daily. Second, several days before CAR-T cell infusion, patients receive lymphocyte-depleting therapy with cyclophosphamide with or without fludarabine, which can work as immunosuppressants and may help mitigate the risk of rejection. Third, after the infusion, cytokine release syndrome (CRS) is a common complication. Anti-IL-6 agent (eg, tocilizumab) is approved to treat severe, life-threatening CRS,45 and it could potentially help prevent rejection in transplant patients. Krishnamoorthy and colleagues reported 3 cases of PTLD where immunosuppression was not reduced, but all suffered from AKI and poor tumor response.46 Mamlouk and colleagues reported another 3 cases with relatively good response in cancer without rejection.47 Another group reported similar efficacy without allograft rejection48 (Table 3). The data of CAR-T therapy in transplant are evolving, and more studies are needed.
Figure 1.
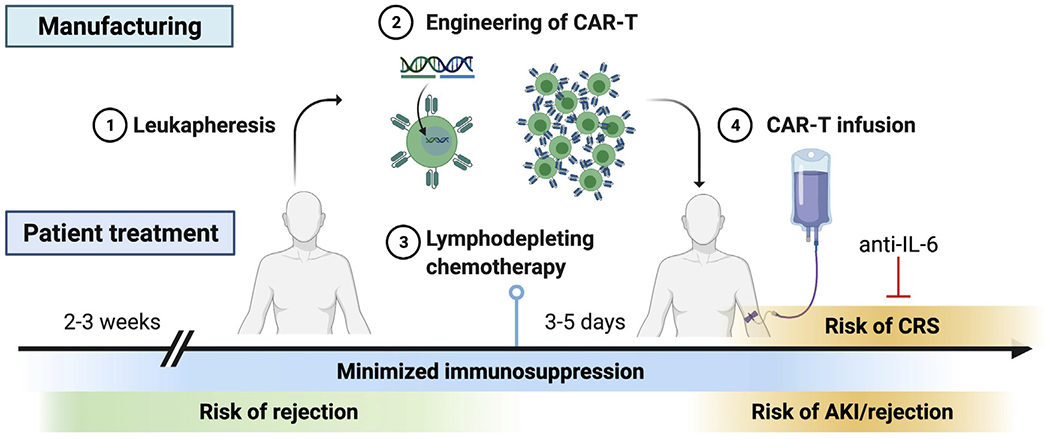
CAR-T therapy in patient with kidney transplants. Immunosuppression agents need to be minimized 2-3 weeks before leukapheresis (1), to ensure the efficacy of CAR-T manufacturing (2). Lymphodepleting chemotherapy (3) will be administered 3-5 days before CAR-T infusion (4). Risk of acute kidney injury (AKI) and rejection is high during peri-infusion period, along with cytokine release syndrome (CRS). Anti-IL-6 treatment can be used for treatment of CRS.
Table 3.
Case Series of CAR-T Therapy in Solid Organ Transplant
| Baseline Characteristics | Disease Characteristics | Therapies Before CAR-T | Immunosuppression Management | CAR-T Adverse Events and Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Publication | Case # | Age, Gender | Organ Transplant | Immunosuppression | Time from tx (years) | Timor EBV Static | Pathology | CRS Grade | Outcomes | ||
| Krishnamoorthy | 1 | 54M | DDKT/Pancreas | Tac/Aza/Pred | 20 | Negative | DLBCL-GCB | Rituximab alone, R–CHOP | Low-dose tac, pred | 1 | Death at day 115 due to refractory PTLD |
| 2 | 54F | Heart | Tac/MMF/Pred | 26 | Negative | DLBCL-non-GCB | R–CHOP, R-ICE, lenalidomide | switched from tac tosirolimus | 2 | AKI requiring RRT, death at day 44 due to GI bleed from refractory PTLD | |
| 3 | 71M | DDKT | Tac/Aza/Pred | 10 | Negative | DLBCL-non-GCB | R–CHOP, R-DHAX, ibrutinib. | Stopped | 3 | AKI requiring RRT, aspergillus pneumonia, VRE bacteremia, death at day 15 from refractory PTLD | |
| Mamlouk | 4 | 38M | Kidney | Tac/MMF/Pred | 10 | Negative | DLBCL-GCB | R-EPOCH, R-GEM-Ox | M MF. On low dose pred | 1 | Rejection at 16 weeks, CR at 1 month, up to 7 mo. |
| 5 | 44M | Kidney | Sirolimus/Pred | 10 | Negative | DLBCL-GCB | R–CHOP | Stopped | 1 | Pneumonia, AW, CR at 1 mo. Relapse at 34 week | |
| 6 | 41M | Kidney | Sirolimus/Pred | 7 | Negative | DLBCL-GCB | R-EPOCH, R-GDP, R- ESHAP, Pola-BR | Stopped sirolimus, on low dose pred | None | PR at 1 mo. PD at 3 mo. | |
| Luttwak | 7 | 69 M | DDKT | Tac | 25 | Negative | DLBCL-GCB | R-daEPOCH, R-GDP, Pola-BR | Tac (2 ng/mL) | 1 | CR at 1 mo. Relapse at 3 mo. |
| 8 | 50F | DDKT | Tac | 5 | Negative | DLBCL-GCB | R–CHOP, R-ICE + ASCT, gemcitabine | Tac (2 ng/mL) | 2 | CR at 1 mo. Sustained at 3 mo. | |
| 9 | 66M | Liver | Tac | 8 | Negative | DLBCL-non-GCB | R–CHOP, ICE, radiotherapy | Tac (2 ng/mL) | 1 | PR at 1 mo. Sustained at 3 mo. | |
| Dang | 10 | 17F | Heart | Tac/Pred | 0.3 | Positive | DLBCL-non-GCB | CYVE, O-ICE, radiation, EBV-CTL, radiation | Pred, low dose tac (1-2 ng/mL) | 1 | CR at 1 mo. Sustained at 6 mo. |
| Wang | 11 | 3M | Liver | Tac/Pred | 1.8 | Positive | Burkitt lymphoma | R–CP, CP, radiation | Pred | 2 | CR at 2 mo. Sustained at 16 mo. |
Abbreviations: ASCT, autologous stem cell transplantation; Aza, azathioprine; CR, complete response; DDKT, deceased kidney transplant; DLBCL, diffuse large B-cell lymphoma; EBV-CTL, EB virus-specific cytotoxic T cell therapy; GCB, germinal center B cell; GDP, gemcitabine, carboplatin and dexamethasone; MMF, mycophenolate mofetil; PR, partial response; PD, progressive disease; Pola-BR, polatuzumab vedotin, bendamustine, rituximab; R–CHOP: rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisone; R-daEPOCH, rituximab, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin; R-DHAX, Rituximab, dexamethasone, cytarabine, and oxaliplatin; R-EPOCH, rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin; R-ESHAP, rituximab, etoposide, methylprednisolone,cytarabine, cisplatin; R-GEM-Ox, rituximab, gemcitabine, oxaliplatin; R-ICE, Rituximab, ifosfamide, carboplatin, and etoposide; RRT, renal replacement therapy; Tac, tacrolimus.
Adapted from Schreiber et al.110
Multiple Myeloma, Amyloid Light-chain Amyloidosis, and Kidney Transplantation
Patients with end-stage kidney disease (ESKD) from multiple myeloma (MM) have poor outcomes on dialysis.49 Decourt and colleagues showed an adjusted hazard ratio of death of 2.18 in patients with ESKD from monoclonal gammopathies compared with controls.49 MM has traditionally been a contraindication to kidney transplantation, owing to reported high relapse rates and high risk of allograft loss.50
Over the past 2 decades, there have been extraordinary advancements in the management of this disease with 11 new drugs approved by the FDA improving the average median survival of MM from 20 months to 8 years.51,52 These drugs can be divided into three groups: proteasome inhibitors (PIs), immunomodulatory drugs, and monoclonal antibodies. The current treatment algorithm for newly diagnosed MM relies on eligibility for auto-stem cell transplantation (ASCT). Eligibility is affected by age, comorbidities, and performance status.53 In ASCT-eligible patients, induction with bortezomib, lenalidomide, and dexamethasone (VRD) is preferred, followed by ASCT and lenalidomide maintenance. In patients with AKI, cyclophosphamide is used for induction instead of lenalidomide.53 In a recent study, the addition of a fourth agent for induction, the monoclonal antibody against CD38 (daratumumab) to VRD, showed added benefit, with more than 90% of patients achieving a very good partial remission (VGPR) or better.54
In addition, a powerful prognostic staging system for MM, the Revised International Staging System for Multiple Myeloma (R–ISS) has emerged as the predominant staging tool used by hematologists. Patients with R–ISS stages 1, 2, and 3 had 5-year overall survival rates of 82%, 62%, and 40%, respectively.55 Furthermore, sensitive minimal residual disease (MRD) assays detecting low levels of disease in bone marrow samples with next-generation flow cytometry or next-generation VDJ (variability, diversity, and joining) sequencing have been added to response criteria used by hematologists.56
Now armed with effective treatment regimens achieving high remission rates, excellent prognostic tools that can aid in risk stratification, and assays that can assess remission on molecular levels, transplant centers should revisit kidney transplantation as feasible and, in many cases, the optimal treatment for ESKD in patients with MM.
Unfortunately, most of the available data on kidney transplantation for patients with ESKD from MM have come from case reports and small case series. Several of the reports include patients who have not been treated with ASCT, or whose regimens did not include the current standard of care. Supplemental Table is adapted from a recent descriptive literature review of all case reports and series and lists the MM and kidney transplant outcomes of patients treated with ASCT57 (Supplemental Table).
Similar to MM, amyloid light-chain (AL) amyloidosis has also been a contraindication to kidney transplantation owing to its reported high relapse rates and worse overall survival.58 However, also similar to MM, the prognosis for patients with AL amyloidosis has improved as it has become a more treatable disease using many of the same agents as those used for MM. A recent study evaluated the outcomes of 49 patients with AL amyloidosis and kidney transplants and found 1-, 3-, and 5-year graft survival to be 94%, 89%, and 81%, respectively.59 The median patient survival from kidney transplantation was 10.5 years. Those patients who achieved a complete remission (CR) or VGPR fared better than those with partial response or no response (NR) after treatment for amyloidosis, which is confirmed in another observational study from UK.60
No streamlined protocols are available for the pre–kidney transplant clearance of and post–kidney transplant management of patients with ESKD from multiple myeloma or AL amyloidosis. In the next section, we will illustrate UCSF’s approach to patients with ESKD from MM. A similar strategy is used for patients with ESKD from AL amyloidosis, but given the variable involvement of multiple organ systems in AL amyloidosis, our approach is less protocolized with these patients and, along with our oncology colleagues, clearance is on case-by-case basis.
Medical Evaluation and Kidney Transplant Clearance for Patients with ESKD from MM.
Timing of kidney transplantation for patients with ESKD from MM is critical. For most malignancies, the more time that has transpired since achieving a remission, the less likely the malignancy will relapse. On the contrary, MM is a disease characterized by multiple remissions and relapses, with a first relapse usually occurring approximately 3-4 years after the initial diagnosis.53,61 Therefore, according to Huskey and colleagues, a strategy of transplanting patients with low-risk myeloma earlier after ASCT and higher risk patients later (or never) should be implemented.62 The strategy at UCSF is to wait 1 year after a successful ASCT with the patient having achieved a VGPR or CR before kidney transplantation. Clearance by hematology is required using their expertise to risk stratify and select appropriate candidates (Table 4).
Table 4.
Kidney Transplant Candidate Criteria and Post-Transplant Management for Individuals with Plasma Cell Dyscrasias109
| Transplant Candidate Criteria |
|---|
| 1. Living donor or estimated time to deceased donor transplant less than or equal to 2 years |
| 2. No ABO incompatible donors or positive crossmatch |
| 3. Confirmed CR or VGPR for 1 year post-ASCT |
| 4. Bone marrow biopsy within 4 months of kidney transplant (within 1 year if deceased donor transplant) |
| 5. PCD labs within 4 weeks of kidney transplant (within 6 months if deceased donor transplant) |
| 6. Clearance by oncology |
| 7. Fat pad biopsy and cardiac MRI to evaluate for amyloidosis |
| 8. Kidney and bladder ultrasound to evaluate for GU malignancy |
| 9. Hold IMiD for 4 weeks and proteasome inhibitor for 2 weeks pre-transplant (avoid regimens containing IMiDs if possible in deceased donor recipients) |
| Post-Transplant Management |
|
|
| 1. Induction therapy for kidney transplant per PRA |
| 2. Maintenance immunosuppressive regimen consisting of tacrolimus, mycophenolate, and prednisone. Lower dose mycophenolate after re-initiation of PCD-directed therapy |
| 3. Use of PCP prophylaxis and HSV prophylaxis indefinitely due to increased state of immunosuppression from PCD-directed therapy |
| 4. Preferentially treat with proteasome inhibitor or CD38 inhibitor for maintenance, restarting at 2-3 weeks post-transplant |
| 5. More aggressive screening for malignancies including kidney/bladder ultrasound every 1-3 years to rule out urologic malignancy |
Abbreviations: ASCT, autologous stem cell transplant; CR, complete response; GU, genitourinary; ImiD, imide; MRI, magnetic resonance imaging; PCD, plasma cell dyscrasia; PCP, pneumocystis pneumonia; PRA, panel reactive antibody; VGPR, very good partial response.
All candidates for kidney transplantation at UCSF will have undergone the current recommended 3-4 drug regimen for induction followed by ASCT.53 Consideration of the number of early relapses and treatment regimens needed to achieve a VGPR or CR is important as treatment regimens for relapses usually need to include 2 or more drugs that the patient is not refractory to.53,63–65 Therefore, having multiple available agents after kidney transplantation to treat relapses is essential to optimizing kidney transplant outcomes.
For patients with a living donor, IMIDs (thalidomide, lenalidomide, pomalidomide) are held for 4 weeks before their surgery to minimize the risk of thrombotic-related complications perioperatively.66 To minimize cytopenias immediately after transplantation, PIs are held (bortezomib, carfilzomib, and ixazomib) for 2 weeks before transplantation. Induction therapy with anti-thymocyte globulin or basiliximab is based on the current institutions’ protocols and is based primarily on calculated panel reactive antibody (cPRA). All patients are maintained on triple therapy with a calcineurin inhibitor, antimetabolite, and prednisone. Steroid-inclusive regimens are implemented, and mTOR inhibitors are avoided mainly to combat the cytopenias that are likely to develop in the setting of the combination of myeloma therapies and antirejection therapies. The antimetabolite dose is reduced once maintenance therapy for myeloma is reinstituted after transplantation, which is usually at 2-4 weeks after transplant.
Maintenance Therapies for Myeloma After Kidney Transplantation.
Multiple studies have demonstrated improved progression-free and overall survival with maintenance therapy after ASCT with IMIDs and PIs.67–69 Studies evaluating the role of monoclonal antibodies for maintenance after ASCT are ongoing.67 In addition to antirejection therapies, most patients with kidney transplants with ESKD from MM will require maintenance therapy for myeloma as well. Intensified immunosuppression in this context may lead to increased risk of infectious complications as well as cytopenias. For the increased infectious risk, patients remain indefinitely on herpes simplex virus (HSV) prophylaxis and pneumocystis prophylaxis after kidney transplant.
There is also the added risk of rejection that has been reported in solid organ transplant recipients treated with immunomodulatory agents after transplantation.70–73 Despite this documented risk, not all transplant patients treated with IMIDs after solid organ transplant reject.62 Transplant centers can still consider using these agents, with careful monitoring, after transplantation.62 Bortezomib has been studied in patients with kidney transplants for the treatment of antibody-mediated rejection, and for this reason, PIs are the preferred class of drugs for maintenance therapy after kidney transplant at UCSF.74 The anti-CD38 monoclonal antibody daratumumab is also being actively studied in patients with kidney transplants for desensitization and for antibody-mediated rejection and may play a role in maintenance therapy for MM.67,75,76 However, its potential to inhibit T regulatory cells may promote alloimmune activity against the graft in the form of cell-mediated rejection.27 Finally, anti–B-cell maturation antigen (BCMA) CAR T cell is an exciting advancement in the field of MM; however, owing to its immunomodulatory effects, it may not be an appropriate agent for solid organ transplant recipients because of the risk of rejection.42,46,77
Other Considerations: Malignancy Screening.
Several of the agents used to treat MM are also associated with secondary malignancies.78,79 For patients with kidney transplants with MM, the risk for de novo malignancies may be compounded by these factors and current guidelines for screening after kidney transplant may not be sufficient. In addition to currently recommended cancer screening, at UCSF, we screen patients with MM for urologic malignancies with urinalyses looking for microscopic hematuria and native and kidney transplant ultrasounds every 1-3 years.5,80
Advancements in risk stratification, molecular detection of residual disease, and therapies associated with high remission rates have made transplant centers reconsidering kidney transplantation as a feasible option for the treatment of ESKD in patients with MM and AL amyloidosis. A multidisciplinary team of transplant nephrologists and hematologists is critical for the identification of ideal candidates before transplantation and the complex management of patients after transplantation.
Treatment of Cancers After Transplantation: Novel Cancer Therapies
Treatment of cancer after transplant can be challenging owing to kidney allograft dysfunction. It is common to develop cancer more than 10 years after transplant, and kidney function may be marginal by the time cancer therapy begins. Cytotoxic chemotherapy, molecular targeted therapy, and radiotherapy are the most common therapeutic approaches in patients with kidney transplants with cancer. As kidneys are a major elimination pathway for many antineoplastic drugs and their metabolites, dose adjustment may be necessary depending on kidney function. In nontransplant patients, dose adjustment is made based on the creatinine clearance (Cockcroft-Gault), Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI), or Modification of Diet in Renal Disease (MDRD).81,82 However, these estimations have not been validated in kidney transplant recipients with one functioning kidney Thus, unintentional overdosing or underdosing of chemotherapy may be common. Besides the dose adjustment, nephrologists and oncologists should be aware of the treatment-associated adverse events. For example, platinum agents can cause tubular injury, hypomagnesemia, and proximal tubular dysfunction (ie, Fanconi-like syndrome), as it does in nontransplant patients. Tyrosine kinase inhibitors (TKIs), including anti-VEGF antibodies, can be associated with hypertension and proteinuria. The mechanism of proteinuria caused by TKIs are thought to be due to the combination of reduction of nitric oxide production and endothelial injury83 and thus often accompanied with hypertension. This is important when patients develop proteinuria while on mTOR inhibitor, as proteinuria due to mTOR inhibitor is not usually associated with hypertension. Furthermore, it is notable that many patients with kidney transplants are on calcineurin inhibitors and may be at a higher risk of endothelial injury/thrombotic microangiopathy (TMA) when treated with chemotherapeutic agents such as gemcitabine (Table 5).
Table 5.
Anticancer Agents and Major Renal Complications
| Anticancer Agents | Renal Complications |
|---|---|
| Gemcitabine, VEGF inhibitors, TKIs | Thrombotic microangiopathy (TMA) |
| CAR-T therapy | CRS, acute kidney injury, vasodilation, decreased cardiac output, capillary leak syndrome. |
| High dose IL-2 | Acute allograft rejection |
| IMiD (lenalidomide, pomalidomide, thalidomide) | Acute allograft rejection |
| Interferon alpha | Acute allograft rejection |
| Immune checkpoint inhibitor (anti-CTLA-4, anti-PD-1, anti-PD-L1) | Acute allograft rejection, glomerulonephritis |
Abbreviations: CAR, chimeric antigen receptor; CLTA-4, cytotoxic T-lymphocyte-associated protein 4; CRS, cytokine release syndrome; IMiD, immunomodulatory drug; PD-1, program cell death protein-1; PD-L1, program death ligand-1; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor.
Besides cytotoxic chemotherapy and molecular targeted therapy, immunomodulating agents have been introduced and associated with an increased risk of rejection, such as immunomodulatory drugs (IMiD, eg, lenalidomide) for MM,70,73,84,85 and interferon alpha86,87 for RCC. Lenalidomide contributes to direct phosphorylation of CD28 and induce T-cell activation.88 Moderate- to high-dose IL-2 therapy has been used in metastatic RCC and melanoma and is associated with cytokine storm and capillary leak. Given well-described roles of IL-2 in maintaining allograft tolerance, high-dose IL-2 is considered a relative contraindication in transplant patients89 (Table 5).
Immune checkpoint inhibitors (ICIs) have become standard of therapy for many cancers.90 However, ICIs are associated with high risk of acute rejection of the transplant organ. Initially, ipilimumab (anti-CTLA4) was reported to be safe to use in transplant,91 but later, pembrolizumab (anti-PD-1) was associated with a higher risk of rejection.92 This has been supported by mouse transplant models suggesting both PD-1 and PD-L1 are essential to achieve allograft tolerance.93–95 More recently, Abdel-Wahab and colleagues reported the risk factors and survival benefit in solid organ transplant patients.96 The rejection rate was 41%, and the overall survival was worse in the patients with allograft rejection. A meta-analysis by d’Izarny-Gargas and colleagues also confirmed the high risk of acute rejection (39.8%) in patients with kidney transplants.97 The median overall survival of the patients receiving ICIs seemed comparable (36 weeks) with historical cohorts. More recently, a multicenter observational study investigated the efficacy and safety of ICIs in kidney transplant recipients.98 Estimated risk of acute rejection was 42%, with median ICI to rejection time being 24 days. The rejection risk was lower if the patients were on a higher number of immunosuppressants (eg, three-agent immunosuppression) and mTOR inhibitors. In patients with advanced cSCC, the overall survival in those receiving ICIs was significantly longer than that of historical cohorts of advanced cSCC who did not receive ICIs. Currently, there is no consensus on how to manage immunosuppression to mitigate acute rejection while achieving effective tumor response. There are several ongoing clinical trials to investigate immunosuppression strategies in patients with kidney transplants. One approach is to continue tacrolimus (NCT03816332)99 for those with skin cancer (cSCC, melanoma, basal cell carcinoma, Merkel cell carcinoma) treated with ipilimumab and/or nivolumab. Another approach includes combination of mTOR inhibitor and dynamic steroid dosing for cSCC treated with cemiplimab (NCT04339062).100 In this study, the patients receive prednisone minipulse synchronizing with the infusion, modified from a previously reported approach.101 Another safety monitoring study of those with various cancer species treated with nivolumab is ongoing (ANZCTR CA209-993ISR).102 A targeted gene expression analysis of kidney allograft biopsies suggested that interferon signaling pathway, particularly the expression level of IFI27, is associated with ICI-associated rejection,103 and further research on biomarkers is awaited to better understand the mechanism of ICI-associated rejection. Donor-derived cell-free DNA has been used as a biomarker for rejection in setting of ICI use,104,105 although the optimal interval of monitoring is unclear at this point. Interestingly, acute rejection can still occur in patients on dialysis with previously failed allograft. In these cases, patients developed allograft pain and fever 2-4 weeks after the first cycle of checkpoint therapy, owing to intolerant allograft symptoms.106–108 In summary, as therapeutic options for cancer expand, specific considerations of immunomodulatory effects of cancer treatment are needed in patients with kidney transplants (Fig 2).
Figure 2.
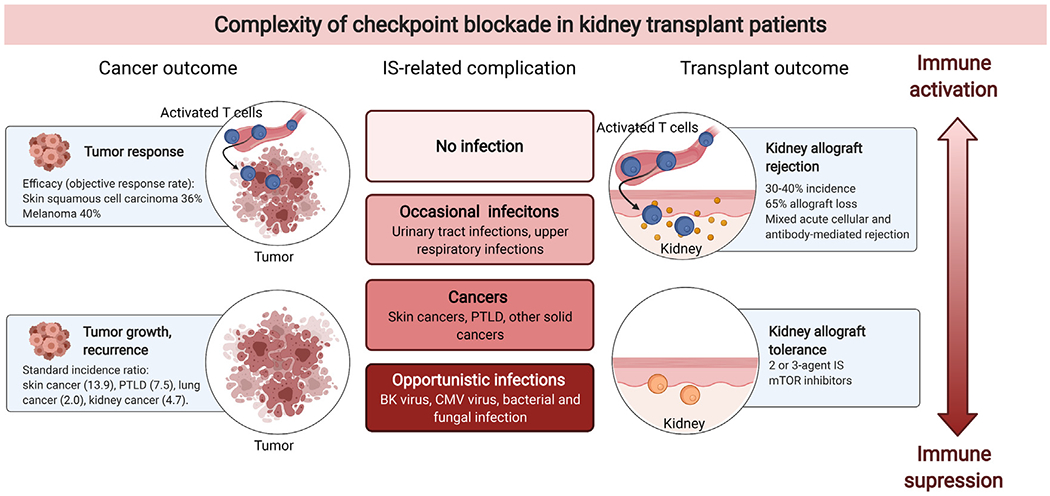
Complexity of immune checkpoint inhibitor use in patients with kidney transplants. The figure highlights the complexity of immune suppression–associated adverse effects (eg, cancers [left] and infections [middle]) and benefits (eg, achieving allograft tolerance [right]). Abbreviation: PTLD, post-transplant lymphoproliferative disorder.
CONCLUDING REMARKS
The treatment options for cancers are rapidly evolving. Transplant centers should revisit their current protocols regarding pretransplant cancer-free wait times and reassess patient populations that were previously excluded from kidney transplantation owing to histories of cancer. Multidisciplinary decision-making among the patient, caretaker, oncologist, and transplant nephrologist is extremely important in ensuring appropriate pretransplant and post-transplant care.
Supplementary Material
CLINICAL SUMMARY.
Cancer-free wait time and cancer screening strategies after transplant are opinion-based, and more data are needed.
Careful strategies may enable kidney transplantation in patients with multiple myeloma.
Novel cancer therapies with potential immunomodulatory effects need to be used with caution.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at https://doi.org/10.1053/j.ackd.2021.09.002.
Contributor Information
Naoka Murakami, Division of Renal Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Allison B. Webber, Divisino of Nephrology, Kidney Transplant Service, University of California San Francisco, San Francisco, CA
Vinay Nair, Division of Kidney Disease and Hypertension, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY.
REFERENCES
- 1.Blosser CD, Haber G, Engels EA. Changes in cancer incidence and outcomes among kidney transplant recipients in the United States over a thirty-year period. Kidney Int. 2021;99(6):1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Adra DP, Hammel L, Roberts J, et al. Pretransplant solid organ malignancy and organ transplant candidacy: a consensus expert opinion statement. Am J Transpl. 2021;21(2):460–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Adra DP, Hammel L, Roberts J, et al. Preexisting melanoma and hematological malignancies, prognosis, and timing to solid organ transplantation: a consensus expert opinion statement. Am J Transpl. 2021;21(2):475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engels EA, Pfeiffer RM, Fraumeni JF, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acuna SA, Huang JW, Scott AL, et al. Cancer screening recommendations for solid organ transplant recipients: a systematic review of clinical practice guidelines. Am J Transpl. 2017;17(1):103–114. [DOI] [PubMed] [Google Scholar]
- 6.Kasiske BL, Vazquez MA, Harmon WE, et al. Recommendations for the outpatient surveillance of renal transplant recipients. American Society of Transplantation. J Am Soc Nephrol. 2000;11(Suppl 15):S1–S86. [PubMed] [Google Scholar]
- 7.Doublet JD, Peraldi MN, Gattegno B, Thibault P, Sraer JD. Renal cell carcinoma of native kidneys: prospective study of 129 renal transplant patients. J Urol. 1997;158(1):42–44. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz A, Vatandaslar S, Merkel S, Haller H. Renal cell carcinoma in transplant recipients with acquired cystic kidney disease. Clin J Am Soc Nephrol. 2007;2(4):750–756. [DOI] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transpl. 2009;9(Suppl 3):S1–S155. [DOI] [PubMed] [Google Scholar]
- 10.Mihalov ML, Gattuso P, Abraham K, Holmes EW, Reddy V. Incidence of post-transplant malignancy among 674 solid-organ-transplant recipients at a single center. Clin Transpl. 1996;10(3):248–255. [PubMed] [Google Scholar]
- 11.Opelz G, Henderson R. Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients. Lancet. 1993;342(8886-8887):1514–1516. [DOI] [PubMed] [Google Scholar]
- 12.Cherikh WS, Kauffman HM, McBride MA, Maghirang J, Swinnen LJ, Hanto DW. Association of the type of induction immunosuppression with posttransplant lymphoproliferative disorder, graft survival, and patient survival after primary kidney transplantation. Transplantation. 2003;76(9):1289–1293. [DOI] [PubMed] [Google Scholar]
- 13.Bustami RT, Ojo AO, Wolfe RA, et al. Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transpl. 2004;4(1):87–93. [DOI] [PubMed] [Google Scholar]
- 14.Marks WH, Ilsley JN, Dharnidharka VR. Posttransplantation lymphoproliferative disorder in kidney and heart transplant recipients receiving thymoglobulin: a systematic review. Transpl Proc. 2011;43(5):1395–1404. [DOI] [PubMed] [Google Scholar]
- 15.Herman M, Weinstein T, Korzets A, et al. Effect of cyclosporin A on DNA repair and cancer incidence in kidney transplant recipients. J Lab Clin Med. 2001;137(1):14–20. [DOI] [PubMed] [Google Scholar]
- 16.Dantal J, Hourmant M, Cantarovich D, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351(9103):623–628. [DOI] [PubMed] [Google Scholar]
- 17.Dharnidharka VR, Ho P-L, Stablein DM, Harmon WE, Tejani AH. Mycophenolate, tacrolimus and post-transplant lymphoproliferative disorder: a report of the North American pediatric renal transplant Cooperative study. Pediatr Transpl. 2002;6(5):396–399. [DOI] [PubMed] [Google Scholar]
- 18.Swann PF, Waters TR, Moulton DC, et al. Role of postreplicative DNA mismatch repair in the cytotoxic action of thioguanine. Science. 1996;273(5278):1109–1111. [DOI] [PubMed] [Google Scholar]
- 19.Offman J, Opelz G, Doehler B, et al. Defective DNA mismatch repair in acute myeloid leukemia/myelodysplastic syndrome after organ transplantation. Blood. 2004;104(3):822–828. [DOI] [PubMed] [Google Scholar]
- 20.Robson R, Cecka JM, Opelz G, Budde M, Sacks S. Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transpl. 2005;5(12):2954–2960. [DOI] [PubMed] [Google Scholar]
- 21.Campistol JM, Eris J, Oberbauer R, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. J Am Soc Nephrol. 2006;17(2):581–589. [DOI] [PubMed] [Google Scholar]
- 22.Luan FL, Hojo M, Maluccio M, Yamaji K, Suthanthiran M. Rapamycin blocks tumor progression: unlinking immunosuppression from antitumor efficacy. Transplantation. 2002;73(10):1565–1572. [DOI] [PubMed] [Google Scholar]
- 23.Stallone G, Schena A, Infante B, et al. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J Med. 2005;352(13):1317–1323. [DOI] [PubMed] [Google Scholar]
- 24.Euvrard S, Morelon E, Rostaing L, et al. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367(4):329–339. [DOI] [PubMed] [Google Scholar]
- 25.Santos AH, Chen C, Leghrouz MA, Bueno EP, Lee JJ, Wen X. Association of HLA mismatch and MTOR inhibitor regimens with malignancy and mortality after kidney transplantation. Transpl Immunol. 2021;66:101391. [DOI] [PubMed] [Google Scholar]
- 26.Muthukkumar S, Ramesh TM, Bondada S. Rapamycin, a potent immunosuppressive drug, causes programmed cell death in B lymphoma cells. Transplantation. 1995;60(3):264–270. [DOI] [PubMed] [Google Scholar]
- 27.Luan FL, Ding R, Sharma VK, Chon WJ, Lagman M, Suthanthiran M. Rapamycin is an effective inhibitor of human renal cancer metastasis. Kidney Int. 2003;63(3):917–926. [DOI] [PubMed] [Google Scholar]
- 28.Nepomuceno RR, Balatoni CE, Natkunam Y, Snow AL, Krams SM, Martinez OM. Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Epstein Barr virus B-cell lymphomas. Cancer Res. 2003;63(15):4472–4480. [PubMed] [Google Scholar]
- 29.García-Morales P, Hernando E, Carrasco-García E, Menéndez-Gutierrez MP, Saceda M, Martínez-Lacaci I. Cyclin D3 is down-regulated by rapamycin in HER-2-overexpressing breast cancer cells. Mol Cancer Ther. 2006;5(9):2172–2181. [DOI] [PubMed] [Google Scholar]
- 30.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8(2):128–135. [DOI] [PubMed] [Google Scholar]
- 31.Huber S, Bruns CJ, Schmid G, et al. Inhibition of the mammalian target of rapamycin impedes lymphangiogenesis. Kidney Int. 2007;71(8):771–777. [DOI] [PubMed] [Google Scholar]
- 32.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transpl. 2010;10(3):535–546. [DOI] [PubMed] [Google Scholar]
- 33.Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. 2016;374(4):333–343. [DOI] [PubMed] [Google Scholar]
- 34.Dharnidharka VR, Webster AC, Martinez OM, Preiksaitis JK, Leblond V, Choquet S. Post-transplant lymphoproliferative disorders. Nat Rev Dis Primers. 2016;2:15088. [DOI] [PubMed] [Google Scholar]
- 35.Allen UD, Preiksaitis JK, AST Infectious Diseases Community of Practice. Post-transplant lymphoproliferative disorders, Epstein-barr virus infection, and disease in solid organ transplantation: guidelines from the American Society of transplantation infectious diseases Community of practice. Clin Transpl. 2019;33(9):e13652. [DOI] [PubMed] [Google Scholar]
- 36.Reshef R, Vardhanabhuti S, Luskin MR, et al. Reduction of immunosuppression as initial therapy for posttransplantation lymphoproliferative disorder(☆). Am J Transpl. 2011;11(2):336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trappe RU, Dierickx D, Zimmermann H, et al. Response to rituximab induction is a Predictive marker in B-cell post-transplant lymphoproliferative disorder and Allows successful stratification into rituximab or R-CHOP Consolidation in an International, prospective, multicenter phase II trial. J Clin Oncol. 2017;35(5):536–543. [DOI] [PubMed] [Google Scholar]
- 38.Zimmermann H, Trappe RU. EBV and posttransplantation lymphoproliferative disease: what to do? Hematol Am Soc Hematol Educ Program. 2013;2013:95–102. [DOI] [PubMed] [Google Scholar]
- 39.Haque T, Wilkie GM, Jones MM, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110(4):1123–1131. [DOI] [PubMed] [Google Scholar]
- 40.Chiou FK, Beath SV, Wilkie GM, Vickers MA, Morland B, Gupte GL. Cytotoxic T-lymphocyte therapy for post-transplant lymphoproliferative disorder after solid organ transplantation in children. Pediatr Transpl. 2018;22(2). 10.1111/petr.13133 [DOI] [PubMed] [Google Scholar]
- 41.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New Engl J Med. 2017;377(26):2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanduri SR, Cheungpasitporn W, Thongprayoon C, et al. Systematic review of risk factors and incidence of acute kidney injury among patients treated with CAR-T cell therapies. Kidney Int Rep. 2021;6(5):1416–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta S, Seethapathy H, Strohbehn IA, et al. Acute kidney injury and electrolyte abnormalities after chimeric antigen receptor T-cell (CAR-T) therapy for diffuse large B-cell lymphoma. Am J Kidney Dis. 2020;76(1):63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23(8):943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krishnamoorthy S, Ghobadi A, Santos RD, et al. CAR-T therapy in solid organ transplant recipients with treatment refractory posttransplant lymphoproliferative disorder. Am J Transpl. 2021;21(2):809–814. [DOI] [PubMed] [Google Scholar]
- 47.Mamlouk O, Nair R, Iyer SP, et al. Safety and efficacy of CAR T-cell therapy in kidney transplant recipients. Blood. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luttwak E, Hagin D, Perry C, et al. Anti-CD19 CAR-T therapy for EBV-negative posttransplantation lymphoproliferative disease-a single center case series. Bone Marrow Transpl. 2020;56(5):1031–1037. [DOI] [PubMed] [Google Scholar]
- 49.Decourt A, Gondouin B, Delaroziere JC, et al. Trends in survival and renal Recovery in patients with multiple myeloma or light-chain amyloidosis on Chronic dialysis. Clin J Am Soc Nephrol. 2016;11(3):431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leung N, Lager DJ, Gertz MA, Wilson K, Kanakiriya S, Fervenza FC. Long-term outcome of renal transplantation in light-chain deposition disease. Am J Kidney Dis. 2004;43(1):147–153. [DOI] [PubMed] [Google Scholar]
- 51.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fonseca R, Abouzaid S, Bonafede M, et al. Trends in overall survival and costs of multiple myeloma, 2000-2014. Leukemia. 2017;31(9):1915–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajkumar SV, Kumar S. Multiple myeloma current treatment algorithms. Blood Cancer J. 2020;10(9):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International staging system for multiple myeloma: a report from International myeloma working group. J Clin Oncol. 2015;33(26):2863–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munshi NC, Avet-Loiseau H, Rawstron AC, et al. Association of minimal residual disease with Superior survival outcomes in patients with multiple myeloma: a meta-analysis. JAMA Oncol. 2017;3(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chitty DW, Hartley-Brown MA, Abate M, et al. Kidney transplantation in patients with multiple myeloma: narrative analysis and review of the last 2 decades. Nephrol Dial Transpl. 2020;gfaa361. 10.1093/ndt/gfaa361 [DOI] [PubMed] [Google Scholar]
- 58.Pasternack A, Ahonen J, Kuhlbäck B. Renal transplantation in 45 patients with amyloidosis. Transplantation. 1986;42(6):598–601. [DOI] [PubMed] [Google Scholar]
- 59.Angel-Korman A, Stern L, Sarosiek S, et al. Long-term outcome of kidney transplantation in AL amyloidosis. Kidney Int. 2019;95(2):405–411. [DOI] [PubMed] [Google Scholar]
- 60.Law S, Cohen O, Lachmann HJ, et al. Renal transplant outcomes in amyloidosis. Nephrol Dial Transpl. 2021;36(2):355–365. [DOI] [PubMed] [Google Scholar]
- 61.Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79(7):867–874. [DOI] [PubMed] [Google Scholar]
- 62.Huskey JL, Heilman RL, Khamash H, Fonseca R. Kidney transplant in the Era of modern therapy for multiple myeloma. Transplantation. 2018;102(12):1994–2001. [DOI] [PubMed] [Google Scholar]
- 63.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–152. [DOI] [PubMed] [Google Scholar]
- 64.Siegel DS, Dimopoulos MA, Ludwig H, et al. Improvement in overall survival with carfilzomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36(8):728–734. [DOI] [PubMed] [Google Scholar]
- 65.Moreau P, Masszi T, Grzasko N, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374(17):1621–1634. [DOI] [PubMed] [Google Scholar]
- 66.Carrier M, Le Gal G, Tay J, Wu C, Lee AY. Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: a systematic review and meta-analysis. J Thromb Haemost. 2011;9(4):653–663. [DOI] [PubMed] [Google Scholar]
- 67.Dimopoulos MA, Jakubowiak AJ, McCarthy PL, et al. Developments in continuous therapy and maintenance treatment approaches for patients with newly diagnosed multiple myeloma. Blood Cancer J. 2020;10(2):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dimopoulos MA, Gay F, Schjesvold F, et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2019;393(10168):253–264. [DOI] [PubMed] [Google Scholar]
- 69.Jagannath S, Abonour R, Durie BGM, et al. Impact of post-ASCT maintenance therapy on outcomes in patients with newly diagnosed multiple myeloma in Connect MM. Blood Adv. 2018;2(13):1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lum E, Huang E, Bunnapradist S, Pham T, Danovitch G. Acute kidney allograft rejection Precipitated by lenalidomide treatment for multiple myeloma. Am J Kidney Dis. 2017;69(5):701–704. [DOI] [PubMed] [Google Scholar]
- 71.Meyers DE, Adu-Gyamfi B, Segura AM, et al. Fatal cardiac and renal allograft rejection with lenalidomide therapy for light-chain amyloidosis. Am J Transpl. 2013;13(10):2730–2733. [DOI] [PubMed] [Google Scholar]
- 72.Vaxman I, Eaton J, Lee HE, Gertz MA. Acute liver rejection in a multiple myeloma patient treated with lenalidomide. Case Rep Transpl. 2020;2020:8894922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walavalkar V, Adey DB, Laszik ZG, Jen K-Y. Severe renal allograft rejection resulting from lenalidomide therapy for multiple myeloma: case report. Transplant Proc. 2018;50(3):873–876. [DOI] [PubMed] [Google Scholar]
- 74.Everly MJ, Everly JJ, Susskind B, et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. 2008;86(12):1754–1761. [DOI] [PubMed] [Google Scholar]
- 75.Doberer K, Kläger J, Gualdoni GA, et al. CD38 antibody daratumumab for the treatment of Chronic active antibody-mediated kidney allograft rejection. Transplantation. 2021;105(2):451–457. [DOI] [PubMed] [Google Scholar]
- 76.Kwun J, Matignon M, Manook M, et al. Daratumumab in Sensitized kidney transplantation: Potentials and Limitations of Experimental and clinical Use. J Am Soc Nephrol. 2019;30(7):1206–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.D’Agostino M, Raje N. Anti-BCMA CAR T-cell therapy in multiple myeloma: can we do better? Leukemia. 2020;34(1):21–34. [DOI] [PubMed] [Google Scholar]
- 78.Musto P, Anderson KC, Attal M, et al. Second primary malignancies in multiple myeloma: an overview and IMWG consensus. Ann Oncol. 2017;28(2):228–245. [DOI] [PubMed] [Google Scholar]
- 79.Yamasaki S, Yoshimoto G, Kohno K, et al. Risk of secondary primary malignancies in multiple myeloma patients with or without autologous stem cell transplantation. Int J Hematol. 2019;109(1):98–106. [DOI] [PubMed] [Google Scholar]
- 80.Hickman LA, Sawinski D, Guzzo T, Locke JE. Urologic malignancies in kidney transplantation. Am J Transpl. 2018;18(1):13–22. [DOI] [PubMed] [Google Scholar]
- 81.Casal MA, Nolin TD, Beumer JH. Estimation of kidney function in oncology: Implications for Anticancer drug selection and dosing. CJASN. 2019;14(4):587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chancharoenthana W, Wattanatorn S, Vadcharavivad S, Eiam-Ong S, Leelahavanichkul A. Agreement and Precision Analyses of various estimated Glomerular Filtration rate Formulae in cancer patients. Sci Rep. 2019;9(1):19356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kandula P, Agarwal R. Proteinuria and hypertension with tyrosine kinase inhibitors. Kidney Int. 2011;80(12):1271–1277. [DOI] [PubMed] [Google Scholar]
- 84.Nadeau Nguyen M, Nayernama A, Jones SC, Kasamon YL, Waldron PE. Solid organ transplant rejection associated with the Use of the immunomodulatory drugs (IMIDs). Blood. 2019;134(Supplement_1):2189. [Google Scholar]
- 85.Qualls DA, Lewis GD, Sanchorawala V, Staron A. Orthotopic heart transplant rejection in association with immunomodulatory therapy for AL amyloidosis: a case series and review of the literature. Am J Transpl. 2019;19(11):3185–3190. [DOI] [PubMed] [Google Scholar]
- 86.Magnone M, Holley JL, Shapiro R, et al. Interferon-α-induced acute renal allograft rejection. Transplantation. 1995;59(7):1068–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saab S, Kalmaz D, Gajjar NA, et al. Outcomes of acute rejection after interferon therapy in liver transplant recipients. Liver Transplant. 2004;10(7):859–867. [DOI] [PubMed] [Google Scholar]
- 88.LeBlanc R, Hideshima T, Catley LP, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004;103(5):1787–1790. [DOI] [PubMed] [Google Scholar]
- 89.Dutcher JP, Schwartzentruber DJ, Kaufman HL, et al. High dose interleukin-2 (Aldesleukin) - expert consensus on best management practices-2014. J ImmunoTherapy Cancer. 2014;2(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. [DOI] [PubMed] [Google Scholar]
- 91.Lipson EJ, Bodell MA, Kraus ES, Sharfman WH. Successful administration of ipilimumab to two kidney transplantation patients with metastatic melanoma. J Clin Oncol. 2014;32(19):e69–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lipson EJ, Bagnasco SM, Moore J, et al. Tumor Regression and allograft rejection after administration of anti-PD-1. N Engl J Med. 2016;374(9):896–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ito T, Ueno T, Clarkson MR, et al. Analysis of the role of negative T cell Costimulatory pathways in CD4 and CD8 T cell-mediated Alloimmune responses in vivo. J Immunol. 2005;174(11):6648–6656. [DOI] [PubMed] [Google Scholar]
- 94.Yang J, Popoola J, Khandwala S, et al. Critical role of donor tissue expression of programmed death ligand-1 in regulating cardiac allograft rejection and vasculopathy. Circulation. 2008;117(5):660–669. [DOI] [PubMed] [Google Scholar]
- 95.Riella LV, Watanabe T, Sage PT, et al. Essential role of PDL1 expression on nonhematopoietic donor cells in acquired tolerance to vascularized cardiac allografts. Am J Transpl. 2011;11(4):832–840. [DOI] [PubMed] [Google Scholar]
- 96.Abdel-Wahab N, Safa H, Abudayyeh A, et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer. 2019;7(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.d’Izarny-Gargas T, Durrbach A, Zaidan M. Efficacy and tolerance of immune checkpoint inhibitors in transplant patients with cancer: a systematic review. Am J Transpl. 2020;10(9):2457–2465. [DOI] [PubMed] [Google Scholar]
- 98.Murakami N, Mulvaney P, Danesh M, et al. A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant. Kidney Int. 2020;100(1):196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.National Cancer Institute (NCI). Immune Checkpoint Blockade for Kidney Transplant Recipients With Selected Unresectable or Metastatic Cancers. clinicaltrials.gov; 2021. Accessed September 25, 2021. https://clinicaltrials.gov/ct2/show/NCT03816332
- 100.Hanna GJ. Safety and efficacy of cemiplimab (PD-1 Blockade) in Selected Organ Transplant Recipients With Advanced Cutaneous Squamous Cell Carcinoma (CONTRAC). clinicaltrials.gov; 2020. Accessed September 25, 2021. https://clinicaltrials.gov/ct2/show/NCT04339062
- 101.Barnett R, Barta VS, Jhaveri KD. Preserved renal-allograft function and the PD-1 pathway inhibitor nivolumab. N Engl J Med. 2017;376(2):191–192. [DOI] [PubMed] [Google Scholar]
- 102.ANZCTR - Registration. Accessed September 25, 2021. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372928
- 103.Adam B, Murakami N, Reid G, et al. Gene expression Profiling in kidney transplants with immune checkpoint inhibitor-associated adverse Events. Clin J Am Soc Nephrol. 2021;16(9):1376–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hurkmans DP, Verhoeven JGHP, de Leur K, et al. Donor-derived cell-free DNA detects kidney transplant rejection during nivolumab treatment. J Immunother Cancer. 2019;7(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lakhani L, Alasfar S, Bhalla A, et al. Utility of serial donor-derived cell-free DNA measurements for detecting allograft rejection in a kidney transplant recipient after PD-1 checkpoint inhibitor administration. Transpl Direct. 2021;7(2):e656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duni A, Kitsos A, Liapis G, Tatsis V, Pappas C, Dounousi E. Acute kidney transplant rejection after administration of nivolumab in a dialysis patient with a failed graft. Kidney Int Rep. 2021;6(5):1459–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mejia CD, Frank AM, Singh P, Yadav A. Immune checkpoint inhibitor therapy-associated graft intolerance syndrome in a failed kidney transplant recipient. Am J Transpl. 2021;21(3):1322–1325. [DOI] [PubMed] [Google Scholar]
- 108.Kitchlu A, Jhaveri KD, Sprangers B, Yanagita M, Wanchoo R. Immune-checkpoint inhibitor use in patients with end-stage kidney disease: an analysis of reported cases and literature review. Clin Kidney J. 2021;14(9):2012–2022, sfab090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dinh AR, Wong SW, Martin TG, Wolf JL, Webber AB. Outcomes of kidney transplant recipients with ESKD due to plasma cell dyscrasia: a case series. Clin Transplant. 2021;36(3):e14541. [DOI] [PubMed] [Google Scholar]
- 110.Schreiber B, Abdelrahim M, Abudayyeh A, Murakami N. Emerging concepts in managing malignancy in kidney transplant patients. Semin Nephrol. 2022;42(1):63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


