Summary
Background
We hypothesised that combining zanubrutinib with obinutuzumab and venetoclax (BOVen) as an initial therapy for chronic lymphocytic leukaemia and small lymphocytic lymphoma would lead to high rates of undetectable minimal residual disease (MRD), and we explored MRD as a biomarker for directing treatment duration.
Methods
This multicenter, investigator-initiated, single-arm, phase 2 trial took place at two two academic medical centres in the USA. Patients were eligible for the primary cohort if they had treatment-naive chronic lymphocytic leukaemia or small lymphocytic lymphoma, required therapy, and were at least 18 years of age with an Eastern Cooperative Oncology Group performance status up to 2. BOVen was administered in 28 day cycles (oral zanubrutinib at 160 mg twice per day starting in cycle 1 on day 1; intravenous obinutuzumab at 1000 mg on day 1 [split over day 1 with 100 mg and day 2 with 900 mg for an absolute lymphocyte count >25 000 cells per µL or lymph nodes >5 cm in diameter], day 8, and day 15 of cycle 1, and day 1 of cycles 2–8; and oral venetoclax ramp up to 400 mg per day starting in cycle 3 on day 1) and discontinued after 8–24 cycles when prespecified undetectable MRD criteria were met in the peripheral blood and bone marrow. The primary endpoint was the proportion of patients that reached undetectable MRD in both the peripheral blood and bone marrow (flow cytometry cutoff less than one chronic lymphocytic leukaemia cell per 10 000 leukocytes [<10−4]) assessed per protocol. This trial is registered at clinicaltrials.gov (NCT03824483). The primary cohort is closed to recruitment, and recruitment continues in the TP53-mutated mantle cell lymphoma cohort.
Findings
Between March 14, 2019, and Oct 10, 2019, 47 patients were screened for eligibility, and 39 patients were enrolled and treated. Median age was 62 years (IQR 52–70) with 30 (77%) of 39 male participants and nine (23%) of 39 female participants. 28 (72%) of 39 patients had unmutated immunoglobulin heavy-chain variable-region and five (13%) of 39 had 17p deletion or TP53 mutation. After a median follow-up of 25.8 months (IQR 24.0–27.3), 33 (89%) of 37 patients (95% CI 75–97) had undetectable MRD in both blood and bone marrow, meeting the prespecified undetectable MRD criteria to stop therapy after a median of ten cycles (IQR 8–12), which includes two cycles of zanubrutinib and obinutuzumab before starting venetoclax. After median surveillance after treatment of 15.8 months (IQR 13.0–18.6), 31 (94%) of 33 patients had undetectable MRD. The most common adverse events were thrombocytopenia (23 [59%] of 39), fatigue (21 [54%]), neutropenia (20 [51%]), and bruising (20 [51%]), and the most common adverse event at grade 3 or worse was neutropenia (seven [18%]) in the intention-to-treat population. One death occurred in a patient with intracranial haemorrhage on day 1 of cycle 1 after initiating intravenous heparin for pulmonary emboli.
Interpretation
BOVen was well tolerated and met its primary endpoint, with 33 (89%) of 37 previously untreated patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma reaching undetectable MRD in both peripheral blood and bone marrow despite a median treatment duration of only 10 months, owing to our undetectable MRD-driven treatment discontinuation design. These data support further evaluation of the BOVen regimen in chronic lymphocytic leukaemia and small lymphocytic lymphoma with treatment duration guided by early MRD response kinetics.
Funding
Beigene, Genentech (Roche), Grais-Cutler Fund, Lymphoma Research Fund, Lymphoma Research Foundation, American Cancer Society, Farmer Family Foundation, and the National Instititutes of Health and National Cancer Institute.
Introduction
Venetoclax, a B-cell lymphoma 2 (BCL2) inhibitor, combined with obinutuzumab, a CD20 antibody, is approved in chronic lymphocytic leukaemia and small lymphocytic lymphoma.1,2 Venetoclax and obinutuzumab is administered as a fixed-duration therapy (1 year) and induces undetectable minimal residual disease (MRD) and durable responses. Given that undetectable MRD is correlated with prolonged progression-free survival, thereby predicting longer remissions after treatment, we explored MRD as a biomarker for directing treatment duration.
Bruton’s tyrosine kinase (BTK) inhibitors sensitise chronic lymphocytic leukaemia cells to venetoclax through increased mitochondrial BCL2 dependence, upregulated proapoptotic Bcl-2-like protein 11, and decreased intracellular myeloid leukaemia-cell differentiation protein 1.3,4 These observations are supported by trials showing that BTK and BCL2 inhibitor combinations are well tolerated, with bone marrow undetectable MRD occurring in 57–77% of patients.5–7
Zanubrutinib (BGB-3111; BTK inhibitor) showed 100% BTK occupancy in lymph nodes and minimal inhibition of interleukin-2-inducible T-cell kinase (essential for antibody-dependent cytotoxicity), and is an appealing BTK inhibitor to combine with venetoclax and obinutuzumab.8–12 We hypothesised that MRD-directed treatment with zanubrutinib, obinutuzumab, and venetoclax (BOVen) would lead to frequent, durable undetectable MRD.
Methods
Study design and participants
This multicentre, investigator-initiated, single-arm, phase 2 trial was done in two academic medical centres in the USA (appendix p 19). This trial was approved by the relevant institutional review boards, and complied with the Declaration of Helsinki and Good Clinical Practice Guidelines. All patients provided written informed consent.
The purpose of this study was to determine the rate of MRD undetectable response of the BOVen regimen in patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma. A separate study of TP53-mutated mantle cell lymphoma and an expansion cohort of treatment-naive patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma were added after the initial cohort was completed, and is now recruiting patients. These outcomes are not reported in this manuscript.
Inclusion criteria were as follows: age at least 18 years; Eastern Cooperative Oncology Group performance status up to 2; diagnosis of chronic lymphocytic leukaemia or small lymphocytic lymphoma (WHO classification);13 treatment naive (localised radiation or corticosteroids permitted); requiring treatment (as established by the 2018 guidelines of the International Workshop for Chronic Lymphocytic Leukaemia [iwCLL]);14 adequate organ function (creatinine clearance >50 mL/min per 1.73 m2, total bilirubin up to two times the upper limit of normal [ULN] or up to three times the ULN with Gilbert’s, and transaminases up to two times the ULN); and adequate haematological function (absolute neutrophil count of ≥500 cells per mL and platelets ≥75 000 cells per mL and haemoglobin ≥9.0 g/dL) unless associated with chronic lymphocytic leukaemia or small lymphocytic lymphoma diagnosis. We excluded patients with HIV, hepatitis B virus (HBV), or hepatitis C virus (occult or previous HBV infection with undetectable HBV DNA was permitted with prophylaxis), patients who were pregnant or breastfeeding (because of the potential for foetal abnormalities and insufficient data on lactation), patients with uncontrolled concurrent medical problems, bleeding diathesis, CNS haemorrhage or stroke within 6 months, major surgery within 4 weeks, QTc higher than 450 ms, active malignancy, or systemic cancer therapy within 3 years (locoregional therapy with curative intent was permitted), and patients who had received coadministration of strong cytochrome P450 3A, 2C8, 2C9, and 2C19 inhibitors or inducers, warfarin, or dual antiplatelets.
Procedures
BOVen was administered in 28 day (monthly) cycles. Zanubrutinib was administered at 160 mg orally twice per day starting on day 1 of cycle 1. Obinutuzumab was administered at 1000 mg intravenously on day 1 (100 mg on day 1 and 900 mg on day 2 of cycle 1 for an absolute lymphocyte count >25000 cells per µL or lymph nodes >5 cm in largest transaxial dimension), day 8, and day 15 of cycle 1, and day 1 of cycles 2–8. Venetoclax was initiated on day 1 of cycle 3 with a 5 week ramp up to 400 mg administered orally per day (20 mg, 50 mg, 100 mg, 200 mg, and 400 mg). Venetoclax and obinutuzumab were provided by Genentech (Roche, San Francisco, CA, USA) and zanubrutinib was provided by Beigene Pharmaceuticals (San Mateo, CA, USA; US Food and Drug Administration Investigational New Drug number 142458).
Treatment consisted of 8–24 cycles (including two lead-in cycles of zanubrutinib and obinutuzumab) with the duration determined by prespecified MRD criteria. Beginning on day 1 of cycle 7 and then every other cycle, patients with undetectable MRD in the peripheral blood (cutoff less than one chronic lymphocytic leukaemia cell per 10 000 leukocytes [<10−4]) underwent bone marrow biopsy and aspiration within 14 days to assess MRD status. If patients had undetectable MRD in the bone marrow, then MRD testing was repeated in peripheral blood after two additional cycles. Patients with confirmed undetectable MRD discontinued therapy. Dose interruptions or reductions were permitted for zanubrutinib (80 mg twice per day and 80 mg once per day) and venetoclax (300 mg, 200 mg, 100 mg, 50 mg, 20 mg, and 10 mg once per day) in the case of adverse events. Treatment was continued with growth-factor support for grade 3 or 4 neutropenia in the absence of fever or evidence of infection. Dose modification for obinutuzumab was not permitted. Zanubrutinib and venetoclax were held for 3–7 days before and after surgical procedures.
Patients who met MRD-retreatment criteria or biopsy-confirmed progressive disease resumed treatment with zanubrutinib and venetoclax. MRD-retreatment criteria were peripheral blood MRD comprising more than 1% of the total leukocytes for patients with previous undetectable MRD-directed discontinuation, and a 1 log increase in peripheral blood MRD for those who completed therapy with persistent detectable MRD.
MRD was evaluated for the primary endpoint with at least ten-colour flow cytometry. On the basis of iwCLL recommendations, the MRD flow-cytometry assay was considered undetectable if it was lower than a threshold of 10−4. Assessment of peripheral blood MRD occurred at baseline, at every other cycle starting on day 1 of cycle 3, at the end of treatment, and during surveillance after treatment (every 3 months). Assessment of bone marrow MRD occurred at baseline, on day 1 of cycle 3, and when peripheral blood undetectable MRD was shown. Bone marrow was obtained on day 1 of cycle 3 to facilitate MRD concordance data. Peripheral blood MRD assessment on day 1 of cycle 5 was used to measure the decrease in MRD as assessed by immunosequencing from baseline to day 1 of cycle 5 (ΔMRD). Local MRD flow cytometry was used for the primary endpoint and MRD was also evaluated at each MRD-assessment timepoint, with a central 14-colour MRD flow-cytometry assay (Memorial Sloan Kettering Cancer Center Clinical Flow Cytometry Laboratory, New York, NY, USA) and with immunosequencing (clonoSEQ, Adaptive Biotechnologies, Seattle, WA) at all MRD assessments (limit of detection of less than one leukaemic cell per 1000 000 leukocytes given sufficient input).
Patients were examined for investigator-assessed response (iwCLL guidelines 2018)14 with complete blood counts and CT scan of the neck, chest, abdomen, and pelvis at baseline, before cycles 3 and 7, after bone marrow undetectable MRD was confirmed, 30 days after discontinuation, then every 6 months until progressive disease. Bone marrow biopsy and aspiration was required at baseline, on day 1 of cycle 3, and for MRD and response assessment in patients that had peripheral blood undetectable MRD. Patients who received up to two cycles of therapy and underwent response assessment or exhibited progressive disease before initial restaging were examined for response. Overall response, complete response, complete response with incomplete marrow recovery, and partial response were defined as per the 2018 iwCLL guidelines.14
Toxicity assessments included history, vitals, examination, and laboratories, including complete blood counts with differential and serum chemistries at all visits (appendix p 20). Adverse events were assessed at every study visit using the Common Terminology Criteria version 5.0. All patients who received any amount of therapy were examined for toxicity. Adverse events were tabulated in the manuscript irrespective of the relationship to protocol therapy.
Patients were examined at baseline with a genetic assessment, which included immunoglobulin heavy-chain variable-region (IGHV) gene-mutation analysis, fluorescence in-situ hybridisation (deletion [del]11q, trisomy 12, del13q, and del17p), and MSK-IMPACT targeted DNA sequencing (all exons) of 400 genes associated with haematological malignancies.15 Molecular assessments were not repeated.
Criteria for removal from the study included progressive disease while on treatment, unacceptable toxicity, withdrawal of consent, or non-adherence to protocol treatment or follow-up.
Outcomes
The primary endpoint was undetectable MRD, defined as the proportion of patients reaching undetectable MRD in both the peripheral blood and bone marrow (flow cytometry cutoff <10−4]). Secondary activity endpoints were time to undetectable MRD from treatment initiation, recommended phase 2–3 duration of therapy, proportion of patients successfully discontinuing therapy after reaching the undetectable MRD endpoint, and durability of clinical benefit after treatment discontinuation, as measured by duration of peripheral blood undetectable MRD response and treatment-free survival. Recommended phase 2–3 duration of therapy was not established (appendix p 13). Treatment-free survival is not reported in this manuscript, because only one patient required subsequent treatment. Secondary safety endpoints included safety and tolerability (number of haematological and non-haematological adverse-event rates) and reduction in tumour lysis syndrome risk with two cycles of zanubrutinib and obinutuzumab before venetoclax.16
Statistical analysis
Sample size was calculated to provide adequate power for detecting an improvement in the primary endpoint (proportion of patients with undetectable MRD confirmed in both peripheral blood and bone marrow by flow cytometry at <10−4) using an exact single-stage phase 2 design.17 With 37 patients, there was 80% power to detect an improvement in the primary endpoint from 50% (null) to 70% (alternative) using a one-sided significance level of 0.05. This design required that at least 24 patients reached the primary endpoint for their results to be declared promising and eligible for further investigation. The per-protocol population included patients who received at least two cycles of treatment and underwent MRD and response assessment or exhibited progressive disease. The intention-to-treat population included all patients who were enrolled and treated. Patients who were ineligible for the per-protocol analysis were replaced, but were included in the intention-to-treat analysis. Activity data are presented per protocol, and the primary endpoint is also presented in an intention-to-treat analysis. A TP53-mutated mantle cell lymphoma cohort and a treatment-naive chronic lymphocytic leukaemia or small lymphocytic lymphoma expansion cohort were added (total 38 patients) after the initial cohort was completed and met the primary endpoint. This manuscript includes patients in the primary chronic lymphocytic leukaemia and small lymphocytic lymphoma cohort (39 patients) and excludes the expansion cohorts.
Patient and disease characteristics were summarised using descriptive statistics (numbers and percentages for categorical variables; median and range for continuous variables). Toxicities were summarised by maximum grade per patient and reported irrespective of the relationship to protocol therapy. Responses were tabulated and summarised descriptively, and we calculated frequencies and proportions of peripheral blood undetectable MRD, bone marrow undetectable MRD, overall response, complete response or complete response with incomplete marrow recovery, and treatment discontinuation. We calculated time to undetectable MRD and progression-free survival from the start of treatment and MRD-free survival from end of treatment using the Kaplan-Meier method. Successful treatment discontinuation was defined as sustained undetectable MRD for at least 6 months after treatment discontinuation. We assessed MRD concordance between MRD methods and compartments (peripheral blood vs bone marrow) using Lin’s concordance correlation coefficient. The biphasic nature of the time to undetectable MRD curve prompted a post-hoc MRD-kinetics analysis. ΔMRD was measured as the decrease in MRD by immunosequencing from baseline to day 1 of cycle 5. The Youden Index was used to identify an optimal ΔMRD cutoff to predict bone marrow undetectable MRD within 8 months. Statistical analyses were done with R, version 4.1.0. This trial is registered at ClinicalTrials.gov (NCT03824483).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between March 14, 2019, and Oct 10, 2019, 47 patients signed informed consent and were screened for eligibility (figure 1). 39 patients at Memorial-Sloan Kettering Cancer Center (n=21) and Massachusetts General Hospital Cancer Center (n=18) were eligible and initiated treatment. Participants had a median age of 62 years (IQR 52–70) and were predominantly male (table 1). Of the 39 patients, 26 (67%) had chronic lymphocytic leukaemia or small lymphocytic lymphoma with high or very high International Prognostic Index for chronic lymphocytic leukaemia,18 28 (72%) had unmutated IGHV and five (13%) had 17p deletion or TP53 mutation.16
Figure 1:
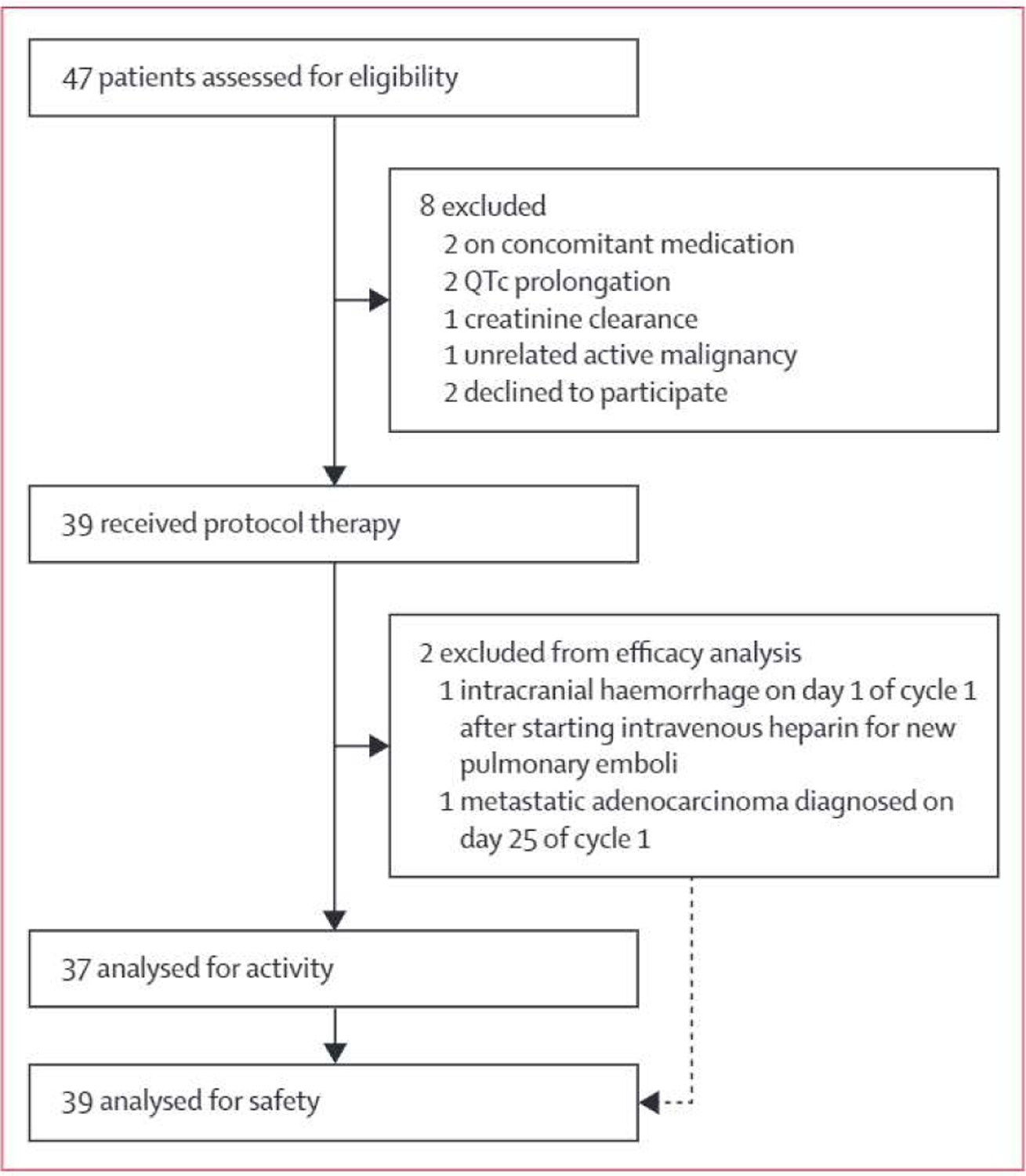
Study profile
Table 1:
Baseline characteristics
| Patients (n=39) | |
|---|---|
| Age, years | 62 (52–70) |
| Sex | |
| Female | 9 (23%) |
| Male | 30(77%) |
| Race and ethnicity | |
| White and non-Hispanic | 35 (90%) |
| Unknown | 4 (10%) |
| Median lymphocyte count (per µL) | 43400 (20450–103300) |
| Immunoglobulin heavy-chain variable region unmutated | 28 (72%) |
| Chronic lymphocytic leukaemia with high-risk or very-high-risk CLL-IPI | 26 (67%) |
| 17p deletion or TP53 mutation | 5 (13%) |
| 17p deletion | 2/39(5%) |
| TP53 mutation | 5/38 (13%) |
| NOTCH1 mutation | 6/38 (16%) |
| SF3B1 mutation | 5/38(13%) |
| Fluorescence in-situ hybridisation (hierarchical) | |
| 17p deletion | 2(5%) |
| 11q deletion | 6(15%) |
| Normal | 17(44%) |
| Trisomy 12 | 5 (13%) |
| 13q deletion | 9 (23%) |
Data are n (%), n/N (%) or median (IQR). CLL-IPI=lnternationaI Prognostic Index for chronic lymphocytic leukaemia.
Cutoff for data analysis was on July 1, 2021, median follow-up was 25.8 months (IQR 24.0–27.3), and median follow-up after treatment was 15.4 months (IQR 10.8–17.3). Of the 39 patients enrolled (intention-to-treat population), 37 (95%) received at least two cycles of therapy and underwent MRD and response assessment, and thus could be assessed for MRD and response (per-protocol population). Two patients received fewer than two cycles of therapy because of intracranial haemorrhage occurring on day 1 of cycle 1 (n=l) and metastatic adenocarcinoma on day 25 of cycle 1 (n=l) and did not undergo MRD or response assessment. Undetectable MRD in both peripheral blood and bone marrow (primary endpoint) occurred in 33 (89%) of 37 patients (95% CI 75–97; figure 2A) in the per-protocol analysis and 33 (85%) of 39 patients (70–94) in the intention-to-treat analysis. Median time to bone marrow undetectable MRD was 8 months (IQR 6–10). 37 (100%) of 37 patients had an overall response and 21 (57%) of 37 had a complete response or complete response with incomplete marrow recovery (figure 2B).
Figure 2: MRD and iwCLL response.
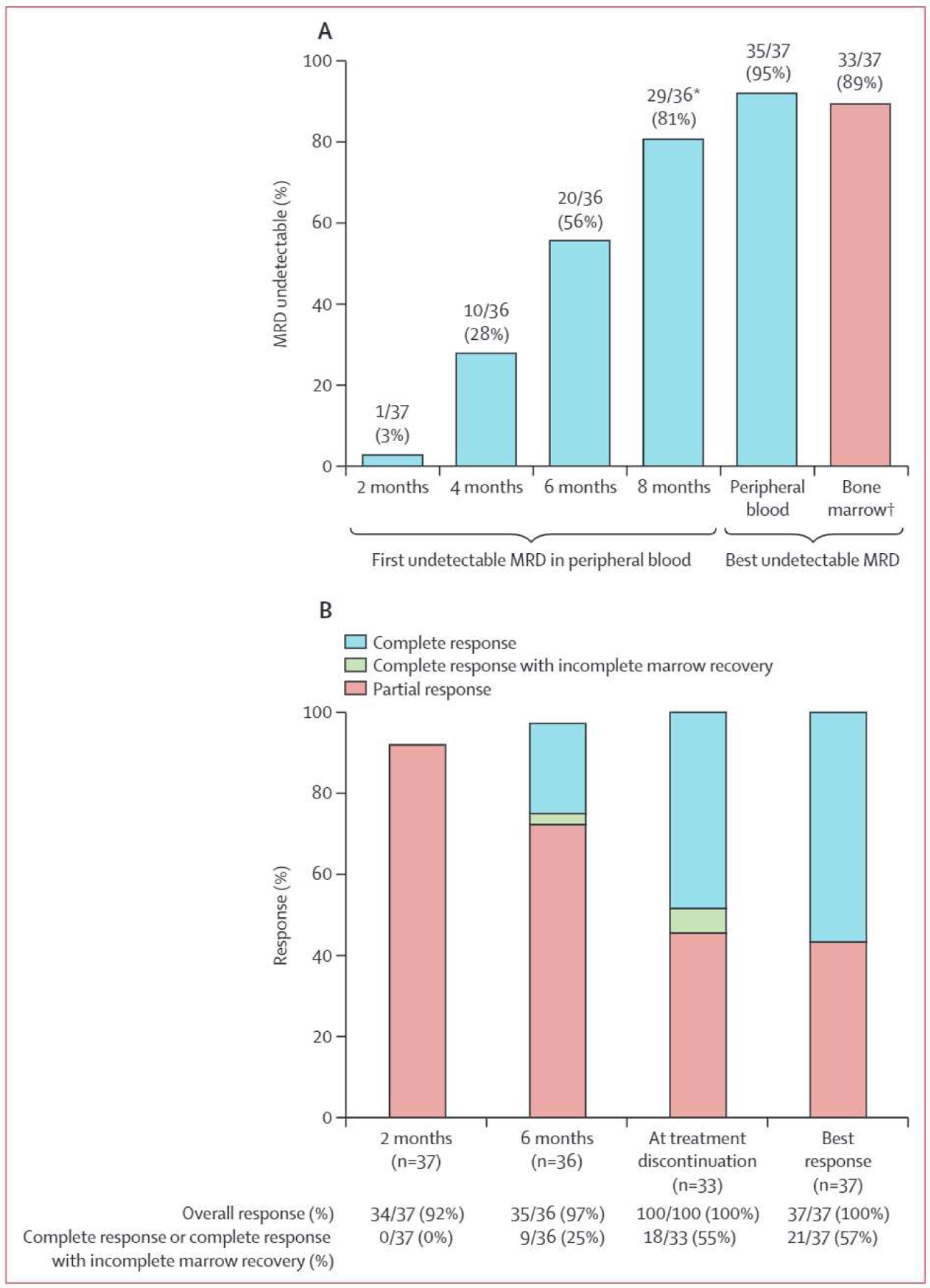
(A) MRD response as assessed by flow cytometry in evaluable patients. (B) iwCLL response in evaluable patients. MRD=minimum residual disease. iwCLL=lnternational Workshop for Chronic Lymphocytic Leukaemia. *One patient was initially ascertained as having peripheral-blood undetectable MRD, but subsequent serial testing confirmed MRD positivity at the threshold of detection, so the patient was excluded from the proportion of patients reaching undetectable MRD. †All 33 patients who reached bone marrow undetectable MRD met the prespecified endpoint, with undetectable MRD confirmed in both peripheral blood and bone marrow.
33 (89%) of 37 patients (95% CI 75–97) reached the prespecified MRD endpoint and discontinued therapy after a median of 10 cycles (IQR 8–12; figure 3); of 33 patients who reached the prespecified MRD endpoint, iwCLL complete response or complete response with incomplete marrow recovery response at time of MRD-directed treatment discontinuation occurred in 18 patients (55%; 95% CI 36–72) and partial response in 15 patients (45%; 28–64). Three (8%) of 37 patients completed 24 cycles and stopped therapy with detectable bone marrow MRD. One (3%) of 37 patients withdrew consent with an ongoing MRD detectable partial response after two cycles of therapy. Of 15 patients with MRD undetectable partial response at treatment discontinuation, 14 (93%) had borderline lymphadenopathy or splenomegaly, one (7%) had substantial intra-abdominal soft tissue measuring 6.6×2.5 cm with biopsy showing no evidence of chronic lymphocytic leukaemia or small lymphocytic lymphoma. Of these 15 patients, 13 (87%) had continued reduction in lymphadenopathy or splenomegaly after treatment discontinuation (including two who later had complete responses), one had no change in disease, and one had biopsy-confirmed progressive disease.
Figure 3: Patient-level outcomes.
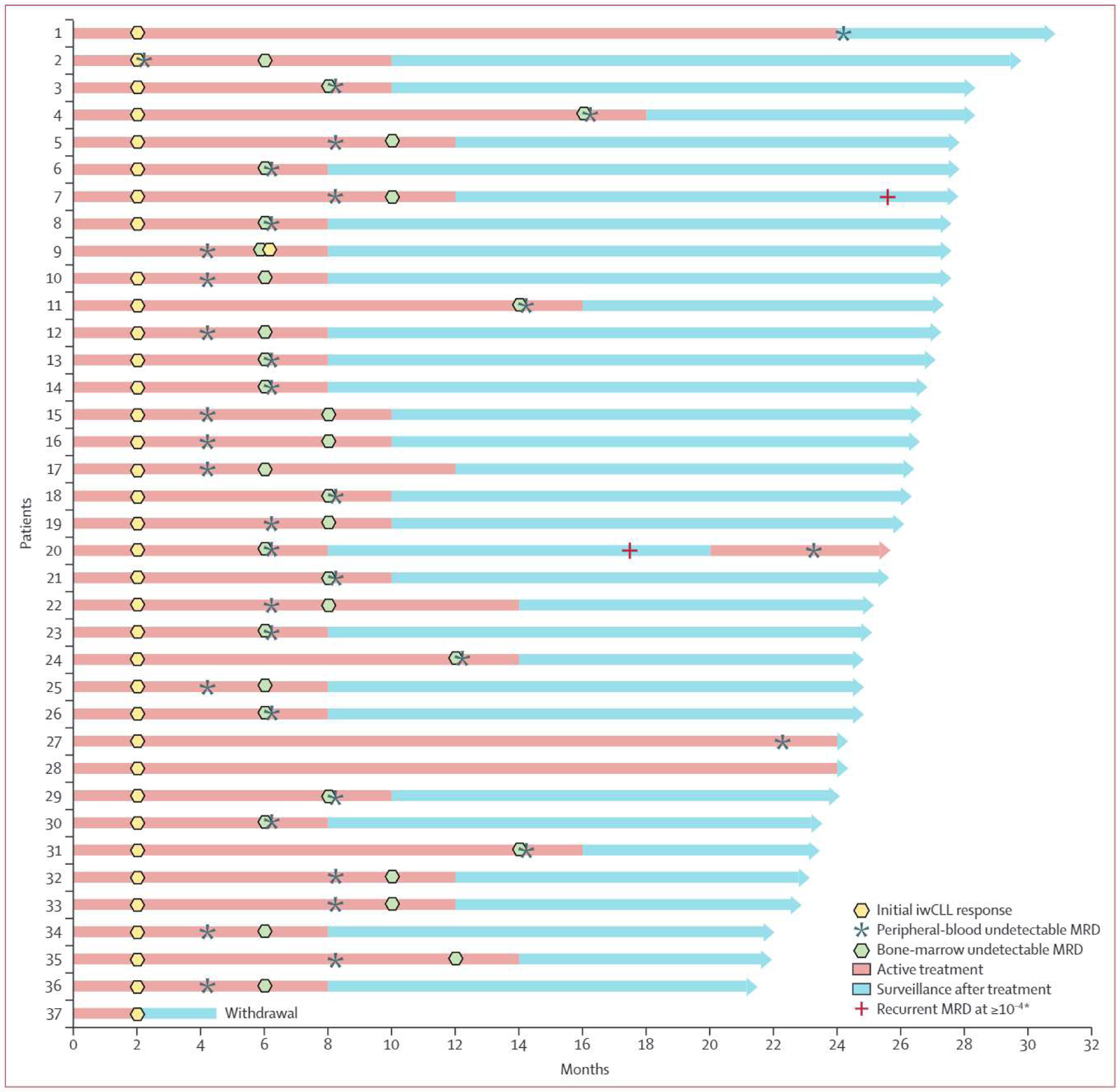
Swimmer’s plot of patient-level outcomes. iwCLL response was inclusive of initial complete response, complete response with incomplete marrow recovery, or partial response. iwCLL=lnternationai Workshop for Chronic Lymphocytic Leukaemia. MRD=minimal residual disease. *As assessed by flow cytometry.
Median progression-free survival was not reached, with only one patient having progressive disease (figure 3; appendix p 3). Of 33 patients who met the prespecified undetectable MRD endpoint and discontinued therapy, 33 (100%) met the prespecified definitition of successful treatment discontinuation, and 31 (94%) had ongoing undetectable MRD in the peripheral blood as established by flow cytometry following a median time of 15.8 months (IQR 13–18.6) during surveillance after treatment (appendix p 12). Two patients had recurrent detectable MRD as established by flow cytometry (figure 3), including one with detectable MRD (0.22% in peripheral blood) and progressive lymphadenopathy (biopsy confirmed chronic lymphocytic leukaemia or small lymphocytic lymphoma) 12 months after treatment, who then reached peripheral blood undetectable MRD with venetoclax and zanubrutinib after 3 months retreatment.
The most common all-cause adverse events (table 2) were thrombocytopenia (23 [59%] of 39), fatigue (21 [54%]), neutropenia (20 [51%]), bruising (20 [51%]), diarrhoea (18 [46%]), infusion-related reaction (17 [44%]), anaemia (16 [41%]), cough (14 [36%]), rash (13 [33%]), and nausea (12 [31%]). Adverse events of grade 3 or worse in 5% of patients or more were neutropenia (5 [18%]), thrombocytopenia (3 [8%]), rash (3 [8%]), lung infection (3 [8%]), and infusion-related reaction (2 [5%]). Nine (23%) of 39 patients received granulocyte colony-stimulating factor (G-CSF) for grade 3–4 (five patients) or grade 2 neutropenia (four patients). One grade 1 atrial-fibrillation event occurred in a patient with previous paroxysmal atrial fibrillation. One death occurred due to intracranial haemorrhage on day 1 of cycle 1 after initiating intravenous heparin for pulmonary emboli, following one dose of zanubrutinib and day 1 of split-dose obinutuzumab. No additional grade 3 or worse bleeding or bruising occurred. One death occurred in a patient who was diagnosed with metastatic adenocarcinoma on day 25 of cycle 1 and opted for hospice. Of four patients who required dose reduction for toxicity, three required dose reduction of zanubrutinib and venetoclax for grade 2 diarrhoea after 5.9 months, 6.4 months, and 8.2 months, and one patient required dose reduction of venetoclax for grade 3 lung infection after 5.9 months.
Table 2:
Adverse events
| Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|
| Thrombocytopenia | 20 (51%) | 3 (8%) | 0 | 0 |
| Fatigue | 20 (51%) | 1(3%) | 0 | 0 |
| Neutropenia | 13(33%) | 2(5%) | 5 (13%) | 0 |
| Bruising | 20(51%) | 0 | 0 | 0 |
| Diarrhoea | 18 (46%) | 0 | 0 | 0 |
| Infusion-related reaction | 15(39%) | 1(3%) | 1(3%) | 0 |
| Anaemia | 16(41%) | 0 | 0 | 0 |
| Cough | 14(36%) | 0 | 0 | 0 |
| Rash | 10 (26%) | 3(8%) | 0 | 0 |
| Nausea | 12 (31%) | 0 | 0 | 0 |
| Constipation | 11 (28%) | 0 | 0 | 0 |
| Nasal congestion | 10 (26%) | 0 | 0 | 0 |
| Gastroesophageal reflux disease | 10 (26%) | 0 | 0 | 0 |
| Insomnia | 9 (23%) | 0 | 0 | 0 |
| Myalgia | 9(23%) | 0 | 0 | 0 |
| Arthralgia | 8 (21%) | 0 | 0 | 0 |
| Abdominal pain | 7(18%) | 0 | 0 | 0 |
| Anxiety | 7(18%) | 0 | 0 | 0 |
| Dyspnea | 7(18%) | 0 | 0 | 0 |
| Sinusitis | 7(18%) | 0 | 0 | 0 |
| Alkaline phosphatase increased | 5(13%) | 1(3%) | 0 | 0 |
| Dizziness | 6(15%) | 0 | 0 | 0 |
| Dry skin | 6(15%) | 0 | 0 | 0 |
| Postnasal drip | 6(15%) | 0 | 0 | 0 |
| Back pain | 6(15%) | 0 | 0 | 0 |
| Weight loss | 6(15%) | 0 | 0 | 0 |
| Hypocalcaemia | 6(15%) | 0 | 0 | 0 |
| Hyperglycaemia | 5 (13%) | 0 | 0 | 0 |
| Sore throat | 5 (13%) | 0 | 0 | 0 |
| Hypertension | 5(13%) | 0 | 0 | 0 |
| Lung infection | 2(5%) | 3(8%) | 0 | 0 |
| Mucositis oral | 3(8%) | 1(3%) | 0 | 0 |
| Aspartate aminotransferase increased | 4 (10%) | 0 | 0 | 0 |
| Headache | 4 (10%) | 0 | 0 | 0 |
| Bloating | 4 (10%) | 0 | 0 | 0 |
| Dyspepsia | 4 (10%) | 0 | 0 | 0 |
| Edema limbs | 4(10%) | 0 | 0 | 0 |
| Non-cardiac chest pain | 4 (10%) | 0 | 0 | 0 |
| Paresthesia | 4 (10%) | 0 | 0 | 0 |
| Weight gain | 4(10%) | 0 | 0 | 0 |
| Creatinine increased | 4(10%) | 0 | 0 | 0 |
| Skin infection | 2(5%) | 1(3%) | 0 | 0 |
| Blood bilirubin increased | 1(3%) | 1(3%) | 0 | 0 |
| Febrile neutropaenia | 0 | 1(3%) | 0 | 0 |
| Heart failure | 0 | 1(3%) | 0 | 0 |
| Hypophosphataemia | 0 | 1(3%) | 0 | 0 |
| Intracranial haemorrhage | 0 | 0 | 0 | 1(3%) |
Data are n (%). All-cause grade 1–2 adverse events occurring in at least 10% of patients and all grade 3,4, and 5 events are shown, irrespective of the relationship to protocol therapy.
16 (43%) of 37 patients were at high risk for tumour lysis syndrome at baseline.16 After the two-cycle lead in, two (5%) remained at high risk for TLS and four (11%) initiated venetoclax treatment as an inpatient (appendix p 2). No patients had laboratory or clinical TLS during the venetoclax ramp up (Howard criteria).
Of 35 patients who achieved peripheral blood undetectable MRD, all (100%) had undetectable MRD as established by immunosequencing at less than 10−4, 33 (94%) had undetectable MRD as established by immunosequencing at less than one leukaemic cell per 100 000 leukocytes (<10−5), and five (14%) had undetectable MRD as established by immunosequencing at less than 10−6 (table 2; appendix p 16). Of 30 patients who reached bone marrow undetectable MRD as established by flow cytometry (<10−4) and had concurrent evaluation by immunosequencing, 24 (80%) had undetectable MRD as established by immunosequencing at less than 10−4, 12 (40%) had undetectable MRD as established by immunosequencing at less than 10−5, and one (3%) was undetectable by immunosequencing at less than 10−6 (denominator includes two patients were indeterminate for presence of residual disease at <10−6). Among patients meeting the prespecified undetectable MRD endpoint permitting treatment discontinuation, the rate of peripheral blood undetectable MRD as established by immunosequencing at less than 10−5 improved from 20 (61%) in 33 patients to 26 (87%) in 30 patients during the two cycles of therapy preceding treatment discontinuation, suggesting declining MRD at treatment discontinuation (appendix p 16).
We evaluated MRD concordance by method (14-colour flow cytometry or immunosequencing) and compartment (peripheral blood or bone marrow) in a preplanned secondary analysis using Lin’s concordance coefficient (appendix pp 4, 5, and 15). Among paired peripheral blood and bone marrow sample collections, good concordance was shown between peripheral blood and bone marrow MRD measurements using either flow cytometry (r=0.77, 95% CI 0.70–0.82) or immune-sequencing (r=0.87, 0.82–0.91), but MRD was higher in bone marrow than peripheral blood, leading to 13 (11%) of 117 discordant pairs (MRD status) with immune-sequencing and 18 (15%) of 118 discordant pairs (MRD status) with flow cytometry. Flow cytometry and immune-sequencing both showed stronger concordance in peripheral blood (r=0.90, 0.88–0.92) than bone marrow (r=0.84, 0.79–0.88) and discordant pairs (MRD status) were less frequent in peripheral blood (ten [3%] of 396 paired sample collections) than bone marrow (12 [10%] of 116 paired sample collections).
We did a post-hoc analysis to find out whether ΔMRD (decrease in MRD by immunosequencing from baseline to day 1 of cycle 5) predicts early bone marrow undetectable MRD. Median ΔMRD was 2.8 log (IQR 2.1 log to 4 log). Day 1 of cycle 5 was selected because it was the time at which venetoclax had been delivered at the target dose for 1 month. A ΔMRD reduction to 1/400th of the baseline ΔMRD (2.6 log, ΔMRD400) was identified as the optimal cutoff point for predicting undetectable MRD by the end of cycle 8 (sensitivity 21 [88%] of 24 patients, specificity 11 [100%] of 11, positive predictive value 21 [100%] of 21, negative predictive value 11 [79%] of 14; appendix p 6 and 14). Among 21 patients who reached ΔMRD400, all had bone marrow undetectable MRD after a median of six cycles (IQR 6–6), and later met the prespecified MRD treatment-discontinuation criterion to stop therapy, 20 (95%) of 21 patients required 12 or fewer cycles of therapy, and median duration of therapy was eight cycles (IQR 8–10; appendix p 14). Among 14 patients who did not reach ΔMRD400, seven (50%) required more than 12 cycles of therapy, and median duration of therapy was 13 cycles (IQR 12–17.5). Traditional biomarkers of chronic lymphocytic leukaemia or small lymphocytic lymphoma (eg, IGHV status, del17p, and TP53 mutation) did not appear to be associated with ΔMRD400 (appendix p 17).
Of 33 patients who discontinued therapy after reaching the prespecified MRD endpoint, MRD was evaluated every 3 months by immunosequencing in 31 (94%) patients for a median observed time of 12 months (IQR 6–15) measured from end of treatment to recurrent detectable MRD at equal or greater than 10−5 or last immunosequencing assessment. Median time to recurrent detectable MRD at 10−5 was not reached and was longer among patients who had ΔMRD400 (log rank p<0.001; appendix p 7 and 8). Of 20 patients who reached ΔMRD400 and later discontinued therapy after reaching the prespecified MRD endpoint, the 1 year rate of recurrent detectable MRD at equal or greater than 10−5 was 5% (95% CI 0–15). Of 11 patients who did not reach ΔMRD400 and later discontinued therapy after reaching the prespecified MRD endpoint, the 1 year rate of recurrent detectable MRD at equal or greater than 10−5 was 75% (19–92).
Discussion
In this multicentre, phase 2 trial, we investigated MRD-directed, time-limited treatment with BOVen as a first-line therapy in chronic lymphocytic leukaemia or small lymphocytic lymphoma. The primary endpoint of the trial was met, with 33 (89%) of 37 patients attaining undetectable MRD as established by flow cytometry at a cutoff of less than 10−4, confirmed in both peripheral blood and bone marrow, all of whom met the prespecified undetectable MRD endpoint treatment-discontinuation criterion and stopped therapy after a median of 10 months of treatment. MRD and clinical responses were durable; one patient with recurrent detectable MRD and clinical progression had venetoclax and zanubrutinib retreatment and reached an undetectable MRD response in peripheral blood for a second time. ΔMRD400 identified a cohort of patients (40%) exhibiting delayed bone marrow MRD clearance, despite longer treatment duration, and this warrants further study as a predictive biomarker for treatment duration.
The high rates of undetectable MRD in both peripheral blood and bone marrow are highly encouraging and compare favourably with other drug combinations in the frontline treatment of chronic lymphocytic leukaemia and small lymphocytic lymphoma. In the CLL14 trial,2 which led to the approval of venetoclax combined with obinutuzumab, the frequency of undetectable MRD was 76% in the peripheral blood and 57% in the bone marrow in the group treated with venetoclax and obinutuzumab.1,2 In two phase 2 trials evaluating ibrutinib combined with venetoclax in previously untreated chronic lymphocytic leukaemia, the frequency of bone marrow undetectable MRD was 72–75%, and one trial reported undetectable MRD in both peripheral blood and bone marrow in 58% patients.6, 19 Among patients who were treatment naive on the phase 2 trial that ibrutinib-venetoclax-obinutuzumab for 14 cycles followed by ibrutinib monotherapy, the rate of undetectable MRD in both peripheral blood and bone marrow was 67%,7 and in the phase 2 trial investigating acalabrutinib, venetoclax, and obinutuzumab treatment, which permitted treatment discontinuation after completing 15 cycles for patients in undetectable MRD complete response, the rate of bone marrow undetectable MRD was 77%.20 A concern of our study design was that the extended duration of therapy up to 24 months, compared with 12–24 months in other studies, would naturally result in a higher undetectable MRD rate. However, BOVen resulted in bone marrow undetectable MRD after a median of only 8 months (IQR 6–10), showing that the undetectable MRD rate was not related to extended treatment duration. Importantly, although CD20 antibody therapy can interfere with MRD evaluations that rely on flow cytometric assessment of CD20 expression, our flow cytometric MRD assay panels was comprised of more than ten antibodies, did not rely on CD20 expression, and exhibited high concordance with immunosequencing.21 Comparisons across trials are fraught with selection bias resulting in differences in treated patient populations, and randomised data are needed to establish the optimal BTK inhibitor to combine with venetoclax with or without obinutuzumab, and to establish whether BOVen improves progression-free survival and overall survival compared with current standard first-line therapy (eg, venetoclax and obinutuzumab, or a BTK inhibitor, such acalabrutinib or ibrutinib, with or without obinutuzumab).1,2,22–24
BOVen was associated with a low rate of grade 3 or worse neutropenia (18%) and one episode of febrile neutropenia (3%), which compared favourably with grade 3 or worse neutropenia rates reported in patients on ibrutinib and venetoclax with or without obinutuzumab (35–56%).6,7,19 This observation was unexpected given previous randomised data reporting a higher rate of grade 3 or worse neutropenia with zanubrutinib than with ibrutinib, albeit in largely relapsed or refractory populations.8 Further, the rate of grade 3 or worse neutropenia was 10.1% in a treatment-naive population with 17p del chronic lymphocytic leukaemia who were receiving zanubrutinib.10 G-CSF administration could partially account for the low incidence of severe neutropenia with BOVen, but the proportion of patients developing grade 3 or worse neutropenia with or without receiving G-CSF was just 11 (28%) of 39 patients. Additionally, cardiac toxicities were infrequent and included one (3%) of 39 patients with a history of paroxysmal atrial fibrillation who developed recurrent atrial fibrillation and five (13%) with hypertension. These preliminary safety data are encouraging and compare favorably to ibrutinib-venetoclax-obinutuzumab, which was associated with frequent grade 3–4 neutropenia (56%), thrombocytopenia (40%), and hypertension (48%). On the basis of our results in the initial cohort reported here, expansion cohorts were added. We will expand upon the BOVen safety results in these ongoing expansion studies within the current protocol evaluating the BOVen regimen in previously untreated TP53-mutated mantle cell lymphoma and a treatment-naive chronic lymphocytic leukaemia expansion cohort.
Although MRD is a promising surrogate for survival in chronic lymphocytic leukaemia and small lymphocytic lymphoma, its real potential is as a biomarker for guiding treatment. MRD has been applied in prospective trials to evaluate its use to escalate or de-escalate therapy, analogous to FDG-PET imaging in Hodgkin lymphoma. Early MRD kinetics, as measured in the peripheral blood of patients receiving venetoclax-based therapy, has been shown to correlate with progression-free survival.25,26 MRD is an appealing early endpoint that does not require bone marrow biopsy. We showed that ΔMRD400 can identify a cohort of patients who had bone marrow undetectable MRD within eight cycles. This finding is similar to those in the CLARITY chronic lymphocytic leukaemia study26 of ibrutinib-venetoclax in relapsed or refractory chronic lymphocytic leukaemia and small lymphocytic lymphoma, which showed that a 2 log depletion following 2 months of venetoclax was associated with higher rates of bone marrow undetectable MRD.26 We observed about 10–15% discordance between peripheral blood and bone marrow with both MRD modalities, which cannot be overcome by simply adjusting the peripheral blood MRD threshold (appendix p 5). We observed a relatively higher discordance between MRD modalities in the confirmatory bone marrow as a result of MRD levels near the limit of detection. Had bone marrow biopsies been done at uniform timepoints, for instance at 12 months or 15 months, as opposed to those of our study, which required bone marrow biopsies to be done within 14 days of initial peripheral blood undetectable MRD, we would expect less discordance at this timepoint and fewer repeat bone marrow biopsies. These data support our decision to require confirmation of undetectable MRD in both peripheral blood and bone marrow to discontinue therapy. Although ΔMRD400 provides prognostic insight for patients receiving BOVen, and might overcome the limitation of peripheral blood and bone marrow discordance, thereby obviating the need for bone marrow biopsy, this potential surrogate endpoint requires external validation in prospective trials and should be validated with different MRD modalities, such as flow cytometry-based MRD assays.
We observed a strong association between ΔMRD400 and undetectable MRD response duration among patients who met the prespecified undetectable MRD endpoint for treatment discontinuation. This finding cannot be explained by end-of-treatment MRD, because MRD depth was similar between patients who reached or did not reach ΔMRD400. Moreover, because ΔMRD400 was associated with earlier bone marrow undetectable MRD, patients who reached ΔMRD400 received less therapy than those who did not meet ΔMRD400. As such, our MRD-driven treatment-discontinuation design probably underestimated the association between ΔMRD400 and undetectable MRD response duration. These data suggest that ΔMRD400 defines a cohort of patients with high-risk biology independent of traditional chronic lymphocytic leukaemia and small lymphocytic lymphoma biomarkers (appendix p 9–11). We hypothesise that ΔMRD400 can identify patients for an abbreviated treatment with BOVen therapy (8–12 months), and flag patients at higher risk who require a longer course of therapy and could be included in further research into mechanisms of resistance. Similar assessment of early MRD kinetics should be explored among patients receiving venetoclax and obinutuzumab, which might define a similar cohort of patients with an unfavourable MRD-response kinetics signature who are more likely to develop early MRD and ultimately clinical recurrence and might benefit from extended therapy.
We did not require complete response to discontinue therapy. Among patients with undetectable MRD, many have only a partial response owing to borderline lymphadenopathy or splenomegaly, which could represent fibrosis after treatment rather than viable tumour. This assertion is supported by post-hoc analyses from prospective trials that investigate CAR T-cell therapy and venetoclax-based combinations, suggesting that undetectable MRD is a better predictor of progression-free survival than iwCLL-assessed complete response.27–30 In our study, 15 patients were in undetectable MRD partial response when therapy was discontinued, including 13 with borderline lymphadenopathy and splenomegaly and one patient with a large residual mass that was biopsied without viable chronic lymphocytic leukaemia and small lymphocytic lymphoma. 13 of 15 patients with at least two consecutive scans after treatment had continued reduction, supporting our hypothesis that residual radiographic abnormalities often do not reflect viable tumour. One patient with undetectable MRD partial response subsequently had progressive disease, but immunosequencing revealed a bone marrow MRD of 0.1% at time of treatment discontinuation for this patient.
This study is limited by its relatively small sample size, therefore we did not have the statistical power to detect differences in outcomes between subgroups, particularly by presence of 17p deletion or TP53 mutation. Additionally, we enrolled relatively fewer women (23%) limiting our ability to find potential differences in outcomes by biological sex. This study is further limited by relatively short follow-up, and additional observation after treatment is needed to assess the feasibility of MRD-driven discontinuation. By using an MRD endpoint to guide treatment discontinuation, an additional limitation is that we probably underestimated the true proportion of patients treated with BOVen who would reach undetectable MRD at less than 10−6 with continuous therapy. Among 35 patients who reached peripheral blood undetectable MRD at less than 10−4, 33 (94%) had undetectable disease at less than 10−5 and five (14%) had undetectable disease at less than 10−6. Importantly, the rate of undetectable MRD at less than 10−5 increased from 61% to 87% with the last 2 months of therapy, with some reaching less than 10−5 only after treatment discontinuation. These data suggest a higher proportion of patients would reach undetectable MRD at less than 10−6 with extended therapy. Notwithstanding, we do not have prospective data to establish the clinically relevant cutoff for treatment discontinuation (10−4, 10−5, or 10−6) to prolong progression-free survival or overall survival, and we will assess this question with longer follow-up. If we learn that attaining bone marrow undetectable MRD at less than 10−5 or less than 10−6 leads to improved outcomes, future studies should take this into account. These data were analysed on a per-protocol basis to facilitate an accurate assessment of the median duration of treatment to reach undetectable MRD. However, our study would also have met its primary endpoint on an intention-to-treat basis (85%, 95% CI 70–94). Another potential limitation of our study is that more recent data, published after our study was initiated, showed that venetoclax-based combinations can reach bone marrow undetectable MRD higher than the null hypothesis.6–7, 19–20 However, BOVen resulted in undetectable MRD in both peripheral blood and bone marrow in 89% (75–97) of patients, which is encouraging given that patients treated with BOVen received less therapy than other reported venetoclax-based combinations, with a median treatment duration of only 10 months (IQR 8–12).
In summary, the results of this phase 2 study in previously untreated chronic lymphocytic leukaemia or small lymphocytic lymphoma show that MRD-directed therapy with BOVen has a favourable safety profile in comparison to combinations of venetoclax and other BTK inhibitors, with or without a CD20 antibody. The study met its primary endpoint, with 89% of patients reaching undetectable MRD in both peripheral blood and bone marrow, meeting the prespecified undetectable MRD treatment-discontinuation criterion, and discontinuing therapy after a median of 10 months. In this proof-of-concept study, we have identified ΔMRD400 as a potential biomarker to guide duration of therapy. ΔMRD400 identifies patients who reach early bone marrow undetectable MRD and defines a cohort of patients with high-risk biology at increased risk for MRD recurrence after treatment discontinuation. Given that these data support further development of BOVen in chronic lymphocytic leukaemia and small lymphocytic lymphoma, we plan to prospectively evaluate ΔMRD400 as a biomarker to guide treatment duration.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed on July 1, 2021, for the terms “chronic lymphocytic leukaemia/small lymphocytic lymphoma” and “untreated”, “venetoclax”, and “ibrutinib”, “acalabrutinib”, or “zanubrutinib” and restricted the search to “clinical trial” with no language or date restrictions. This search identified a single-centre, phase 2 study of ibrutinib and venetoclax (fixed duration of 24 months) in high-risk and older patients with previously untreated chronic lymphocytic leukaemia and a single-centre, phase 2 study of ibrutinib, venetoclax, and obinutuzumab (14 months followed by ibrutinib maintenance) in a treatment-naive cohort. We next searched the archives of abstracts presented at the 2018–20 Annual Meetings of the American Society of Hematology. This search identified a multicentre, phase 2 study of ibrutinib and venetoclax (minimum 15 months) in previously untreated chronic lymphocytic leukaemia and a single-centre, phase 2 study of acalabrutinib, venetoclax, and obinutuzumab (minimum 15 months) in previously untreated chronic lymphocytic leukaemia. These regimens were associated with undetectable minimal residual disease (MRD) in the bone marrow in 57–77% of patients, but only 58–67% of patients had undetectable MRD in both blood and bone marrow. Although grade 3 or worse neutropenia occurred in 32–56% patients in these studies, infectious complications were uncommon.
Added value of this study
To our knowledge, this multicentre, phase 2 study is the first reported prospective trial in chronic lymphocytic leukaemia or small lymphocytic lymphoma to use MRD as the principal determinant of treatment duration and discontinuation, and the first to report the safety, tolerability, and activity of venetoclax and obinutuzumab combined with the second-generation Bruton’s tyrosine kinase inhibitor zanubrutinib in this disease setting. Additionally, a post-hoc analysis revealed that early-MRD-response kinetics defined a discrete cohort of patients (40%) with high-risk biology, which appeared independent of traditional biomarkers for chronic lymphocytic leukaemia or small lymphocytic lymphoma, which predicted MRD outcomes after treatment.
Implications of all the available evidence
These results support MRD-directed, time-limited therapy with zanubrutinib, obinutuzumab, and venetoclax as a well tolerated regimen for previously untreated patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma, with a low rate of grade 3 or worse neutropenia, which resulted in undetectable MRD in the blood and bone marrow in 89% (95% CI 75–97) of patients with a median treatment duration of 10 cycles (IQR 8–12). These data warrant further study of this regimen as a first-line therapy for chronic lymphocytic leukaemia or small lymphocytic lymphoma, and we plan to prospectively validate early-MRD-response kinetics as a biomarker to guide treatment duration.
Acknowledgments
This study was funded by BeiGene, Genentech (Roche), the Lymphoma Research Foundation under Clinical Investigator Career Development Award (JDS 549904), the Lymphoma Research Fund (ADZ), the Grais-Cutler Fund (ADZ), the American Cancer Society under Institutional Research Grant (JDS 232293), the Farmer Family Foundation (AD), and in part by the National Institutes of Health and the National Cancer Institute Core Grant (P30CA008748). The authors are grateful to the patients for participating in this study.
Footnotes
Declaration of interests
JDS reports consulting fees from Abbvie, AstraZeneca, Beigene, Bristol Myers Squibb, TG Therapeutics, and Verastem, and research funding from Adaptive Biotechnologies, Beigene, BostonGene, Genentech (Roche), GSK, Moderna, and TG Therapeutics. ARM reports consulting fees from Curio, Dava, Medscape, GenMab, PeerView, AstraZeneca, Abbvie, Adaptive, Beigene, Genentech, LOXO, Janssen, Pharmacyclics, and TG Therapeutics, research funding from DTRM, Nurix, GenMab, AstraZeneca, Abbvie, Adaptive, Beigene, Genentech, LOXO, Janssen, Pharmacyclics, and TG Therapeutics, and Data Safety Monitoring Board membership for TG Therapeutics. AD reports consulting fees from Physician’s Education Resource, Seattle Genetics, Takeda, EUSA Pharma, and Abbvie, and research funding from Roche and Takeda. EJ reports consulting fees from AstraZeneca and Epizyme. EH reports consulting fees from Leuko and Intervention Insights. JSA reports consulting fees from Abbvie, Bayer, Celgene, Gilead, Juno Therapeutics, Kite Pharma, Genentech, Amgen, Novartis, Karyopharm, Verastem, Janssen, Merck, and Seattle Genetics. CLB reports consulting fees from Life Sd, GLG, Juno (Celgene), Seattle Genetics, Kite, Karyopharm, TG Therapeutics, and ADC Therapeutics, honoraria from Dava Oncology, TouchIME, and Medscape, stock ownership in BMS, Pfizer, Viatris, Regeneron, Moderna, and Novavax, and research funding from Autolus, Bayer, Janssen, Novartis, Epizyme, Xynomics, Bayer, Autolus, and Genentech (Roche). MJM reports consulting fees from Genentech (Roche), Merck, Bayer, Juno, Teva, Rocket Medical, Seattle Genetics, Daiichi Sankyo, Takeda, honoraria from Genentech (Roche), GSK, Bayer, Pharmacyclics, Janssen, Seattle Genetics, Immunovaccine Technologies, and Takeda, travel, accommodation, or other expenses from Genentech (Roche), Bayer, and Seattle Genetics, and research funding from Genentech (Roche), Bayer, GlaxoSmithKline, IGM Biosciences, Rocket Medical, Seattle Genetics, Janssen, Pharmacyclics, and Immunovaccine Technologies, and minority ownership interest in Merck. AN reports consulting fees from Epizyme, Janssen, Pharmacyclics, Medscape, Targeted Oncology, and Morphosys, and research funding from Pharmacyclics and Rafael Pharma. MLP reports consulting fees from Beigene, Celgene, Novartis, Merck, and Synthekine, research funding from Pharmacyclics, Genentech, Juno, and Regeneron, and an immediate family member who receives royalties from Juno and Seres. AK reports consulting fees from Kite Pharmaceuticals, AstraZeneca, and Janssen, and research funding from Abbvie, Adaptive, Celgene, Pharmacyclics, Seattle Genetics, and AstraZeneca, and serves on a steering committee for mantle cell lymphoma Registry for AstraZeneca. LER reports consulting fees from Abbvie, AstraZeneca, Beigene, Janssen, Loxo Oncology, Pfizer, Pharmacyclics, TG Therapeutics, and Vaniam Group, minority ownership interest in Abbott Laboratories and Abbvie, and research funding from Pfizer. MT reports honoraria from the Massachusetts Medical Society, the Brazilian Association of Haematology, MJH Life Sciences, Curio Science, and VJHemeOne. MR reports consulting fees from Celgene, Auron Therapeutics, and Physician’s Education Resource, research funding from Cellularity, NGM, Genentech, and Beat AML, and ownership or equity interest in Auron Therapeutics. JH reports employment from Beigene. JB reports employment from Genentech. QW reports employment from Genentech. AJ reports employment from Adaptive Biotechnologies. OA-W reports consulting fees from H3 Biomedicine, Envisagenics, Janssen, and Merck. ADZ reports consulting fees from Adaptive Biotechnologies, Abbvie, Amgen, AstraZeneca, Beigene, Bristol Myers Squibb, Celgene, Genentech (Roche), Gilead, MEI Pharma, MorphoSys, NCCN, Novartis, and Verastem, data safety monitoring committee membership for BMS, Celgene, and Juno, data safety monitoring committee membership chair for Beigene, and research funding from MEI Pharma, Gilead, Beigene, and Roche. All other authors declare no competing interests.
Data sharing
The study protocol is available upon email request to the corresponding author. All data collected for the study, including individual deidentified participant data and a data dictionary defining each field in the set, will be made available at study closeout.
Contributor Information
Jacob D Soumerai, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Anthony R Mato, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Ahmet Dogan, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Venkatraman E Seshan, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Erel Joffe, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Kelsey Flaherty, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Jason Carter, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Ephraim Hochberg, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Jeffrey A Barnes, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Audrey M Hamilton, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Jeremy S Abramson, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Connie L Batlevi, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Matthew J Matasar, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Ariela Noy, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Colette N Owens, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
M Lia Palomba, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Anita Kumar, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Tak Takvorian, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Ai Ni, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Morgan Choma, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Chaya Friedman, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Puja Chadha, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Elizabeth Simkins, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Jade Ruiters, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Sidney Sechio, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Daneal Portman, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Lauren Ramos, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Natascha Nolet, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Neena Mahajan, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Rosalba Martignetti, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Joanna Mi, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Krista Scorsune, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Julia Lynch, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Brianne McGree, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Stephanie Hughes, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Clare Grieve, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Lindsey E Roeker, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Meghan Thompson, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
P Connor Johnson, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Mikhail Roshal, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Jane Huang, Beigene, San Mateo, CA, USA.
Juliana Biondo, Genentech, San Francisco, CA, USA.
Qun Wu, Genentech, San Francisco, CA, USA.
Allison Jacob, Adaptive Biotechnologies, Seattle, WA, USA.
Omar Abdel-Wahab, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Andrew D Zelenetz, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
References
- 1.Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med 2019; 380: 2225–36. [DOI] [PubMed] [Google Scholar]
- 2.Al-Sawaf O, Zhang C, Tandon M, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2020; 21:1188–200. [DOI] [PubMed] [Google Scholar]
- 3.Deng J, Isik E, Fernandes SM, Brown JR, Letai A, Davids MS. Bruton’s tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia. Leukemia 2017; 31: 2075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cervantes-Gomez F, Lamothe B, Woyach JA, et al. Pharmacological and protein profiling suggests venetoclax (ABT-199) as optimal partner with ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res 2015; 21: 3705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hillmen P, Rawstron AC, Brock K, et al. Ibrutinib plus venetoclax in relapsed/refractory chronic lymphocytic leukemia: the CLARITY study.J Clin Oncol 2019; 37: 2722–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain N, Keating M, Thompson P, et al. Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med 2019; 380: 2095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers KA, Huang Y, Ruppert AS, et al. Phase II study of combination obinutuzumab, ibrutinib, and venetoclax in treatment-naïve and relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol 2020; 38: 3626–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tam CSL, Opat S, D’Sa S, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenstrom macroglobulinemia: the ASPEN study. Blood 2020; 136: 2038–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flinsenberg TWH, Tromedjo CC, Hu N, et al. Differential effects of BTK inhibitors ibrutinib and zanubrutinib on NK-cell effector function in patients with mantle cell lymphoma. Haematologica 2020; 105: e76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tam CS, Robak T, Ghia P, et al. Efficacy and safety of zanubrutinib in patients with treatment-naive chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) with del(17p): initial results from arm C of the Sequoia (BGB-3111–304) trial. Blood 2019; 134 (suppl 1): 499 (abstr). [Google Scholar]
- 11.Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 2019; 134: 851–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FDA. Label review. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/2132l7s000lbl.pdf (accessed Jan 7, 2021).
- 13.Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: World Health Organization, 2017 [Google Scholar]
- 14.Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018; 131: 2745–60. [DOI] [PubMed] [Google Scholar]
- 15.FDA. Evaluation of automatic class III designation for MSK- IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets). Silver Spring: US Food and Drug Administration, 2017. [Google Scholar]
- 16.AbbVie. Venclexta (venetoclax). 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208573s013lbl.pdf (accessed Jan 7, 2021).
- 17.A’Hern RP. Sample size tables for exact single-stage phase II designs. Stat Med 2001; 20: 859–66. [DOI] [PubMed] [Google Scholar]
- 18.International CLL-IPI Working Group. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data. Lancet Oncol 2016; 17: 779–90. [DOI] [PubMed] [Google Scholar]
- 19.Wierda WG, Tam CS, Allan JN, et al. Ibrutinib (Ibr) plus venetoclax (Ven) for first-line treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): 1-year disease-free survival (DFS) results from the MRD cohort of the phase 2 CAPTIVATE study. Blood 2020; 123 (suppl 1): 16–17 (abstr). [Google Scholar]
- 20.Davids MS, Lampson BL, Tyekucheva S, et al. Updated safety and efficacy results from a phase 2 study of acalabrutinib, venetoclax and obinutuzumab (AVO) for frontline treatment of chronic lymphocytic leukemia (CLL). Blood 2020; 136 (suppl 1): 20–21 (abstr). [Google Scholar]
- 21.Rawstron AC, Bottcher S, Letestu R, et al. Improving effidency and sensitivity: European Research Initiative in CLL (ERIC) update on the international harmonised approach for flow cytometric residual disease monitoring in CLL. Leukemia 2013; 27:142–49. [DOI] [PubMed] [Google Scholar]
- 22.Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet 2020; 395:1278–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N EnglJ Med 2019; 381: 432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N EnglJ Med 2018; 379: 2517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregory W, Alexiou D, Pivonka D, Rawstron A, Sail K, Hillmen P. Extrapolating progression free survival curves in CLL using peripheral blood MRD measurements from venetoclax trials. HemaSphere 2020; 4: EP708. [Google Scholar]
- 26.Hillmen P, Boucher RH, Webster N, et al. Continued long term responses to ibrutinib + venetoclax treatment for relapsed/refractory CLL in the blood cancer UK TAP clarity trial. Blood 2020; 136 (suppl 1): 17–18 (abstr). [Google Scholar]
- 27.Turtle CJ, Hay KA, Hanafi LA, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-spedfic chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol 2017; 35: 3010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seymour JF, Ma S, Brander DM, et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase lb study. Lancet Oncol 2017; 18: 230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 2018; 378:1107–20. [DOI] [PubMed] [Google Scholar]
- 30.Kater AP, Seymour JF, Hillmen P, et al. Fixed duration of venetoclax-rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: post-treatment follow-up of the MURANO phase III study. J Clin Oncol 2019; 37: 269–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


