Abstract
Background
COVID-19 is a respiratory illness caused by SARS-CoV-2. The most recent variant is Omicron (line B.1.1.529), which was first identified in South Africa in November 2021. The concern with this variant is the ineffectiveness of vaccines currently available. We aim to systematically evaluate the effectiveness of the currently available COVID-19 vaccines and boosters for the Omicron variant.
Methods
We searched the PubMed, Embase, the Cochrane Library and Web of Science databases from inception to June 5th, 2022. Studies that examined the effectiveness of SARS-CoV-2 vaccines against the Omicron variant infection were included. Random-effects model was used to estimate the pooled vaccine effectiveness against the Omicron variant.
Results
A total of 13 studies were included to evaluate the effectiveness of the vaccine against the Omicron variant, and 11 studies were included to compare the effectiveness between the two-dose and three-dose (booster) vaccinations. Full vaccination (two-dose with or without booster) showed a protective effect against the Omicron variant compared to no vaccination (OR = 0.62, 95% CI: 0.56–0.69), while the effectiveness decreased significantly over 6 months after the last dose. The two-dose vaccination plus booster provided better protection against the Omicron variant compared to the two-dose vaccination without booster (OR = 0.60, 95% CI: 0.52–0.68). Additional analysis was performed for the most commonly used vaccines in the United Staes: BNT162b2(Pfizer) (OR = 0.65, 95% CI: 0.52–0.82) and mRNA-1273(Moderna) (OR = 0.67, 95% CI: 0.58–0.88) vaccines in the US, which showed similar effectiveness compared to no vaccination.
Conclusions
The full dose of SARS-CoV-2 vaccination effectively reduces infection from the SARS-CoV-2 Omicron variant; however, the effectiveness wanes over time. The booster vaccine provides additional protection against the Omicron variant.
Keywords: booster, infection, meta-analysis, Omicron variant, vaccination
Introduction
Coronavirus disease 2019 (COVID-19) is caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) (1). Since the first reported case in December 2019, in Wuhan, China, the virus has spread through China and many other countries worldwide in a short period of time (2). The COVID-19 pandemic has changed the lives of billions of people all over the world and has significantly weakened the global economy. Approximately 400 million people worldwide have been infected by this virus, resulting in around 6 million deaths (3). Consequently, there is an urgent need and of great importance to prevent COVID-19 infections with the implementation of a safe and effective vaccination program.
International researches proceeded at an unprecedented speed in the pursuit of an effective and safe vaccine against COVID-19. In this regards, a variety of different types of vaccines is currently being administered to people of various ages all around the world (4, 5). It is commonly recognized that the mRNA vaccine is the most effective type of vaccine, followed by the viral vector vaccines and inactivated virus vaccines (6, 7). Nonetheless, all vaccines are effective tools in preventing severe COVID-19 symptoms, hospitalization, and death (4).
COVID-19 has become increasingly virulent due to the emergence of variants (8, 9). Recently, a new variant of SARS-CoV-2 was reported from South Africa. On November 26th, 2021, the World Health Organization (WHO) designated this mutant as a variant of concern—Omicron (line B.1.1.529) (10). The B.1.1.529 Omicron variant has enhanced transmissibility and immune evasion, and has been observed in over 77 countries (11). This raises a concern regarding the efficacy of existing COVID-19 vaccines against the Omicron variant (12), and also stimulates the debate for a booster vaccine (13). Our study aims to provide a comprehensive understanding of the effectiveness of the existing COVID-19 vaccines and whether the booster provides additional protection against the B.1.1.529 Omicron variant. Clinically, our findings will help to develop vaccination strategies against the Omicron variant.
Methods
Study Design That Contains Inclusion and Exclusion Criteria
Human studies were included and studies with specifically targeted participants (e.g., patients with severe systemic diseases, patients with prolonged antibiotic therapy, pregnant women, cancer patients, frontline workers, nursing home employees) were excluded. Studies focused on the effectiveness of COVID-19 vaccines against the Omicron variant, and the effectiveness on vaccinated participants vs. unvaccinated participants were included. No restrictions were applied to the age of participants, the types of vaccination, or the number of participants.
Search Strategy
A comprehensive search was conducted for academic research studies that reported the effectiveness of COVID-19 vaccines against the B.1.1.529 Omicron variant published from inception to June 5th, 2022 by searching the following electronic bibliographic databases: PubMed, Embase, the Cochrane Library, and Web of Science. The following search terms were used: (“Covid19 variant” OR “Omicron” OR “B.1.1.529” OR “BA lineages” OR “21H variant” OR “BA.2” OR “21K variant”) AND (“Vaccine” OR “vaccination” OR “BNT162b2” OR “Pfizer” OR “BNT-162C2” OR “Tozinameran” OR “Comirnaty” OR “mRNA-1273” OR “Moderna” OR “Elasomeran” OR “TAK-919” OR “M-1273”).
Data Collection and Analysis
Two independent investigators (Y.Z., D.H.) assessed the articles and extracted data according to the inclusion and exclusion criteria. These two independent investigators (Y.Z., D.H.) also assessed the methodological quality of the trials included in this review. A Microsoft Excel database was created to record all available information, including vaccine type, doses, and vaccination rate in infection and control groups. Discrepancies were discussed and resolved by consensus. The Newcastle-Ottawa assessment scale was used to assess the quality of evidence.
Statistical Analysis
Stata version 16.0 (Stata corp., college station, TX, USA) was used to perform the meta-analyses. Pooled odds ratio (OR) with 95% confidence intervals (CI) were calculated by the random-effects model to accommodate heterogeneity across studies. Heterogeneity was assessed by I2 statistics.
Results
Description of Included Studies
The search of databases yielded 6,413 results, of which 13 studies (12, 14–25) met the criteria and were included in this study. Andrews et al. (26) also reported effectiveness of COVID-19 vaccines against the Omicron variant in 2021, but the included cases could be reported in his another article published in 2022 according to the timetable and the inclusion criteria. Figure 1 shows the detail selection procedures to identify the included studies. Among them, five included studies have low risk of bias and eight have high risk of bias (Supplementary Table 1). The included studies had a total sample of around one hundred million (N = 148260342) participants and of these, 74.2% were fully vaccinated (n = 110076947) with at least two-doses of COVID-19 vaccines (Table 1). Among the 13 studies, 1 study reported the effectiveness of the BNT162b2 (Pfizer–BioNTech), ChAdOx1 (AstraZeneca), mRNA-1273 (Moderna) and JNJ-78436735 vaccines (25); 1 study reported the effectiveness of the BNT162b2 and ChAdOx1 vaccines (24); 3 studies reported the effectiveness of the BNT162b2, ChAdOx1, and mRNA-1273 vaccines (12, 21, 22); 5 studies reported the effectiveness of the BNT162b2, and mRNA-1273 vaccines (14, 16, 17, 20, 23); 2 studies reported the effectiveness of the BNT162b2 vaccine (18, 19), and 1 study reported the effectiveness of the mRNA-1273 vaccine (15).
Figure 1.
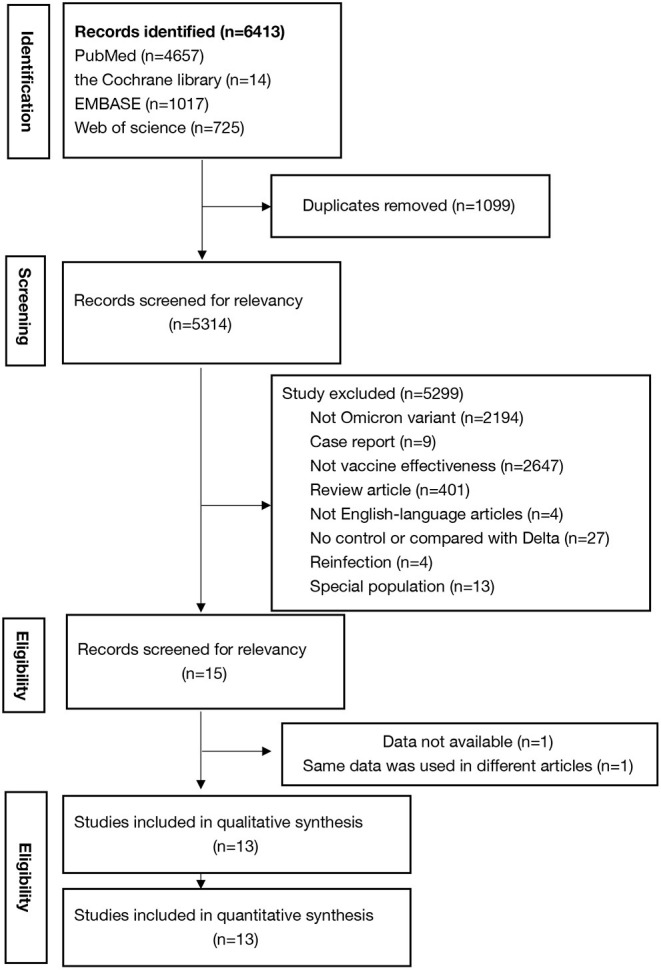
Steps of the study selection procedures.
Table 1.
Characteristics of the selected studies.
| Study | Country | Study design | Vaccine type | Positive | Negative | ||
|---|---|---|---|---|---|---|---|
| Fully vaccinated* | Not vaccinated | Fully vaccinated* | Not vaccinated | ||||
| Lauring et al. (14) | USA | Case-control | BNT162b2, mRNA-1273 | 291 | 268 | 3908 | 2054 |
| Tseng et al. (15) | USA | Case-control | mRNA-1273 | 13412 | 8590 | 32882 | 17051 |
| Accorsi et al. (16) | USA | Case-control | BNT162b2, mRNA-1273 | 9686 | 3412 | 38043 | 8721 |
| Thompson et al. (17) | USA | Case-control | BNT162b2, mRNA-1273 | 3272 | 3572 | 8876 | 3884 |
| Collie et al. (18) | South Africa | Case-control | BNT162b2 | 9700 | 7889 | 35957 | 18442 |
| Andrews et al. (12) | UK | Case-control | ChAdOx1, BNT162b2, mRNA-1273 | 753437 | 101109 | 1285532 | 107238 |
| Klein et al. (19) | USA | Case-control | BNT162b2 | 1050 | 4434 | 1527 | 5203 |
| Ferdinands et al. (20) | USA | Case-control | BNT162b2, mRNA-1273 | 10289 | 13991 | 20464 | 10808 |
| Kodera et al. (23) | Japan | observatory | BNT162b2, mRNA-1273 | 25187 | 12681 | 103065994 | 35329428 |
| Acuti Martellucci et al. (25) | Italy | Cohort | ChAdOx1, BNT162b2, mRNA-1273, JNJ-78436735 | 95714 | 41281 | 827293 | 252421 |
| Horne et al. (24) | UK | Cohort | ChAdOx1, BNT162b2 | 845048 | 90451 | 1858123 | 2033092 |
| Kirsebom et al. (22) | UK | Case-control | ChAdOx1, BNT162b2, mRNA-1273 | 437276 | 59793 | 561848 | 37280 |
| Sheikh et al. (21) | UK | Cohort | ChAdOx1, BNT162b2, mRNA-1273 | 12067 | 1003 | 120071 | 9299 |
| Total | n = 13 | 2216429 | 348474 | 107860518 | 37834921 | ||
Fully vaccinated group included people had received at least two vaccine doses.
Effectiveness of the COVID-19 Vaccines Against the Omicron Variant
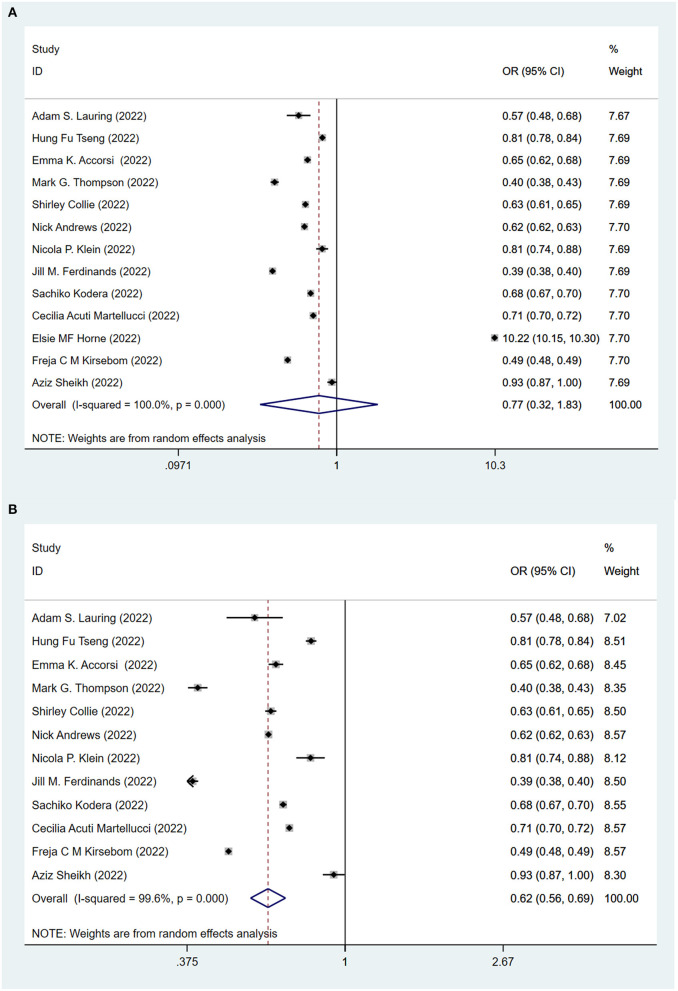
To evaluate the protective role of COVID-19 vaccination, we first compare fully vaccinated (at least two standard doses) and unvaccinated populations. Figure 2A shows the effectiveness of the COVID-19 vaccines against the Omicron variant after at least two doses of the vaccination. All (12, 14–23, 25) but one study (24) showed that vaccines played a significant role in reducing the risk of B.1.1.529 Omicron variant infection among the fully vaccinated population (two doses with or without boosters), compared to the unvaccinated population. The pooling of 13 studies showed that no significant difference between fully vaccinated population and unvaccinated population (OR = 0.77, 95% CI: 0.32–1.83). After excluding the one study (24) looking at the long term effectiveness of the vaccinated population, the pooling of the remaining 12 studies showed that full vaccination significantly lowered the risk (OR = 0.62, 95% CI: 0.56–0.69) of infection against the Omicron variant (Figure 2B).
Figure 2.
Effectiveness of the fully COVID-19 vaccines (at least two doses) against the Omicron variant compared to the unvaccination. (A) 13 studies included. (B) 12 studies included and excluded one study about effectiveness of vaccines over 6 months since second dose.
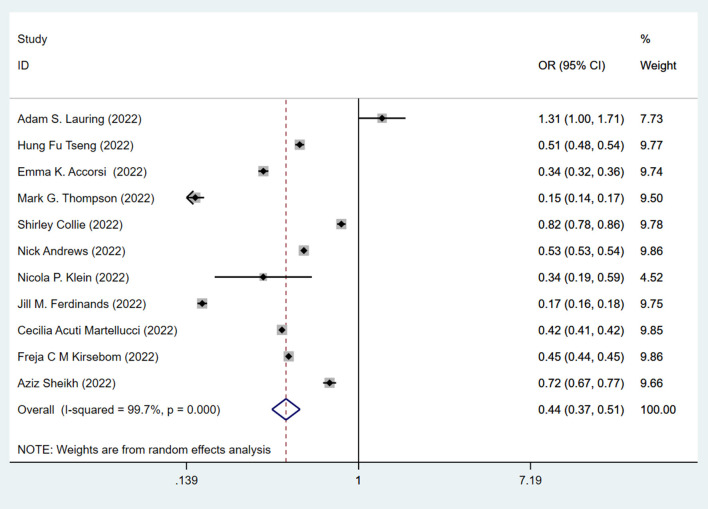
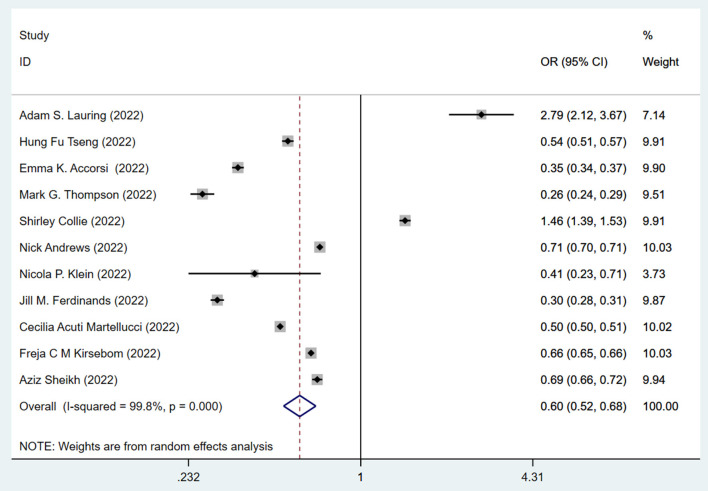
This data prompted us to examine the effectiveness of the booster vaccination. The effectiveness of the standard two-dose vaccination plus one booster (total three doses) against the Omicron variant is shown in Figure 3. The pooling of 11 studies showed that two-dose vaccination plus booster significantly lowered the risk (OR = 0.44, 95% CI: 0.37–0.51; I2 = 99.7%, p < 0.001) of infection against the Omicron variant compared to the unvaccinated group (Figure 3). The pooling of these same studies also showed that the standard two-dose vaccination plus a booster significantly lowered the risk (OR = 0.60, 95% CI: 0.52–0.68) of infection against the Omicron variant compared to the two-dose vaccination without booster (Figure 4), with a significant heterogeneity (I2 = 99.8%, p < 0.001). Collectively, these analyses reveal that full vaccination builds a protective effect against the Omicron variant, and booster vaccines provide additional protection against the Omicron variant.
Figure 3.
Effectiveness of the three doses COVID-19 vaccines against the Omicron variant compared to the unvaccination.
Figure 4.
Effectiveness of the three doses COVID-19 vaccines against the Omicron variant compared to the two doses only vaccination.
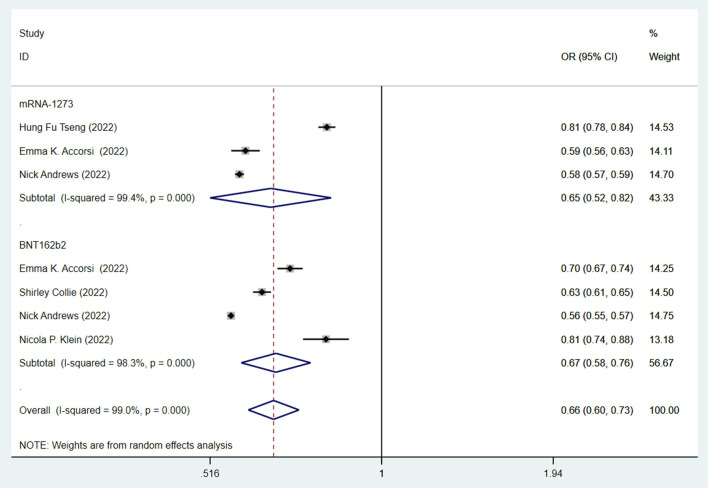
Since mRNA vaccines were commonly recognized as the most effective vaccines (6, 7), we then studied the efficacy of mRNA vaccines against the Omicron variant. Both the BNT162b2 (OR = 0.65, 95% CI: 0.52–0.82; I2 = 99.4%, p < 0.001) and mRNA-1273 (OR = 0.67, 95% CI: 0.58–0.88; I2 = 98.3%, p < 0.001) vaccines have similar effectiveness and significantly lowered the risk of infection against the Omicron variant compared to no vaccination (Figure 5).
Figure 5.
Effectiveness of fully mRNA vaccines (BNT162b2 and mRNA-1273) against the Omicron variant compared to the unvaccination.
In summary, our data clearly indicated the effectiveness of vaccination and the promoter role of booster vaccination against the Omicron variant. The results also confirmed the effectiveness of the mRNA vaccines in preventing the Omicron variant infections.
Discussion
The recent emergence of the SARS-CoV-2 Omicron variant has attracted worldwide attention and became a global concern (11). Among the different variants of SARS-CoV-2, the Omicron variant has a remarkably increased transmissibility (27, 28). It is also difficult to evaluate the effectiveness of the currently available COVID19 vaccines against the Omicron variant in the absence of strong data (29). Besides, there are a lot of debates about the necessity of the boosters, and the new emerging Omicron variant with the significantly increased transmissibility supercharges the debates (13). The goal of this study is to investigate the roles of COVID-19 vaccination in preventing the Omicron variant infections. The results showed the effectiveness of full vaccination against the Omicron variant was significantly but waned over time. The booster dose could induce higher levels of effectiveness compared to the standard two-dose vaccination, which may be related to the shorter length of time between the last dose and the study data collection since the vaccination effectiveness wanes over time. These findings will facilitate vaccination strategies and help develop booster schedules.
Full vaccination with the currently available vaccines provides a statistically significant protection against the Omicron variant (OR = 0.62, 95% CI: 0.56–0.69) within a period of time, but it is not as effective as it is against the Delta variant (RR = 0.15, 95% CI: 0.07–0.31) (30). The booster provides additional protection against Omicron (OR = 0.44, 95% CI: 0.37–0.51); however, it is also less effective compared to the reported protection effectiveness against Delta (OR = 0.065, 95% CI: 0.059–0.071) (16). It is expected that new variants will continue to emerge as viruses mutates. Although the effectiveness of the currently available vaccines against new variants may decrease, vaccination still provides protection against severe COVID-19 caused by different variants (31, 32) and may also decrease the emergence of new variants.
While Omicron has been less virulent than the Alpha and Delta variants, which was likely related to higher vaccination rates and thus reduced disease severity (33), it has still caused tremendous morbidity and mortality (14). In the absence of an Omicron variant-specific vaccines, the currently existing vaccines remain the best option to reduce infection and disease severity against the currently circulating SARS-CoV-2 Omicron variant. The booster is also recommended given the increased transmissibility of the Omicron variant and reduced protective effectiveness of the standard two-dose vaccination compared to the three-dose vaccination with booster, especially for the high-risk populations. Additionally, those who received the two-dose vaccination plus booster were also reported to have fewer hospitalizations and lower disease severity compared to the unvaccinated population (14, 15).
Several COVID-19 vaccines (BNT162b2, ChAdOx1, mRNA-1273, and JNJ-78436735) were included in this analysis since the availability of different types of vaccinations were varied across the world. Different vaccines may have various protective effects again COVID-19 variants. An additional analysis was performed to assess the effectiveness of mRNA vaccines (BNT162b2 and mRNA-1273), which are recognized as the most effective (6, 7) against the Omicron variant. Although the results showed both the BNT162b2 and mRNA-1273 vaccines significantly lowered the risk of infection against the Omicron variant, interpretation of the results must consider the small number of included studies (3 for mRNA-1273 and 4 for BNT162b2). These studies did not evaluate the time between the second or third vaccinated doses and symptom onset, which might influence the results as the vaccine effectiveness decreased with time (15).
In this analysis, the effectiveness of the vector vaccine (ChAdOx1) was reported by in five studies (12, 21, 22, 24, 25). The pooled effectiveness of the vector vaccine was unable to be evaluated as most of these studies did not report the effectiveness of each vaccine individually. The ChAdOx1 vector vaccine followed by boosting with a mRNA vaccine (BNT162b2 or mRNA-1273) is currently recommended in Germany. This vaccination strategy is reported to induce higher or comparable humoral and cellular immune responses than homologous mRNA vaccines (34). More studies are needed in the future to assess the effectiveness of a specific vaccine and vaccination strategy. Moreover, the misclassification of disease status could be a potential source of bias. Several studies (12, 15, 18, 21, 22, 25) used highly specific and sensitive PT-PCR tests, which minimized misclassification and is recommended for future studies in this field.
Except for the vaccinated doses, type and time, the intrinsic host factors (age, sex, genetics, comorbidities and so on), and extrinsic factors (preexisting immunity, microbiota, infections, antibiotics and so on) also influence humoral and cellular vaccine responses in humans (35). Besides, vaccine adjuvant and administration factors (schedule, site, route, and coadministered vaccines and other drugs) are also influential (35). Additional research will provide more information about these factors to help create effective vaccination strategies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
Study design: CC and QJ. Data acquisition and analysis of data: YZ and DH. Manuscript drafting and manuscript editing: YZ, DH, QJ, YG, and CC. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from National Institute of Dental and Craniofacial Research, National Institutes of Health, Department of Health and Human Services (R00DE025915 and R03DE028026 to CC).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.940956/full#supplementary-material
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. (2020) 382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sidiq Z, Hanif M, Dwivedi KK, Chopra KK. Benefits and limitations of serological assays in Covid-19 infection. Indian J Tuberc. (2020) 67:S163–s6. 10.1016/j.ijtb.2020.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nugent MA. The future of the Covid-19 pandemic: how good (or Bad) Can the SARS-CoV2 spike protein get? Cells. (2022) 11:855. 10.3390/cells11050855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing Covid-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. (2022) 28:202–21. 10.1016/j.cmi.2021.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tregoning JS, Brown ES, Cheeseman HM, Flight KE, Higham SL, Lemm NM, et al. Vaccines for Covid-19. Clin Exp Immunol. (2020) 202:162–92. 10.1111/cei.13517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling Y, Zhong J, Luo J. Safety and effectiveness of SARS-CoV-2 vaccines: a systematic review and meta-analysis. J Med Virol. (2021) 93:6486–95. 10.1002/jmv.27203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sathian B, Asim M, Banerjee I, Roy B, Pizarro AB, Mancha MA, et al. Development and implementation of a potential Coronavirus Disease 2019 (Covid-19) vaccine: a systematic review and meta-analysis of vaccine clinical trials. Nepal J Epidemiol. (2021) 11:959–82. 10.3126/nje.v11i1.36163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirby T. New Variant of SARS-CoV-2 in Uk causes surge of Covid-19. Lancet Respir Med. (2021) 9:e20–e1. 10.1016/S2213-2600(21)00005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reardon S. How the delta variant achieves its ultrafast spread. Nature. (2021). 10.1038/d41586-021-01986-w [DOI] [PubMed] [Google Scholar]
- 10.Kannan S, Shaik Syed Ali P. Sheeza A. Omicron (B11529) - variant of concern - molecular profile and epidemiology: a mini review. Eur Rev Med Pharmacol Sci. (2021) 25:8019–22. 10.26355/eurrev_202112_27653 [DOI] [PubMed] [Google Scholar]
- 11.Thakur V, Ratho RK. Omicron (B.1.1.529): A new SARS-CoV-2 variant of concern mounting worldwide fear. J Med Virol. (2022) 94:1821–4. 10.1002/jmv.27541 [DOI] [PubMed] [Google Scholar]
- 12.Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. (2022) 386:1532–46. 10.1056/NEJMoa2119451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolgin E. Omicron is supercharging the Covid vaccine booster debate. Nature. (2021). 10.1038/d41586-021-03592-2. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14.Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T, et al. Clinical severity and mrna vaccine effectiveness for Omicron, Delta, and Alpha SARS-CoV-2 Variants in the United States: a prospective observational study. MedRxiv. (2022). 10.1101/2022.02.06.22270558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Tian Y, et al. Effectiveness of Mrna-1273 against SARS-CoV-2 Omicron and Delta Variants. Nat Med. (2022). 10.1101/2022.01.07.22268919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G, et al. Association between 3 Doses of Mrna Covid-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA. (2022) 327:639–51. 10.1001/jama.2022.0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson MG, Natarajan K, Irving SA, Rowley EA, Griggs EP, Gaglani M, et al. Effectiveness of a third dose of mrna vaccines against Covid-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron Variant Predominance - vision network, 10 states, august 2021-January 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:139–45. 10.15585/mmwr.mm7104e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of Bnt162b2 vaccine against Omicron Variant in South Africa. N Engl J Med. (2022) 386:494–6. 10.1056/NEJMc2119270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein NP, Stockwell MS, Demarco M, Gaglani M, Kharbanda AB, Irving SA, et al. Effectiveness of Covid-19 Pfizer-Biontech Bnt162b2 Mrna vaccination in preventing Covid-19-associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5-17 years - vision network, 10 states, april 2021-january 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:352–8. 10.15585/mmwr.mm7109e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferdinands JM, Rao S, Dixon BE, Mitchell PK, DeSilva MB, Irving SA, et al. Waning 2-Dose and 3-dose effectiveness of Mrna Vaccines against Covid-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron Variant Predominance - vision network, 10 states, august 2021-January 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:255–63. 10.15585/mmwr.mm7107e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheikh A, Kerr S, Woolhouse M, McMenamin J, Robertson C, Simpson CR, et al. Severity of Omicron Variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland (Eave Ii): a National cohort study with nested Test-negative design. Lancet Infect Dis. (2022). 10.1016/S1473-3099(22)00141-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirsebom FCM, Andrews N, Stowe J, Toffa S, Sachdeva R, Gallagher E, et al. Covid-19 vaccine effectiveness against the Omicron (Ba.2) Variant in England. Lancet Infect Dis. (2022) 22:931–3. 10.1101/2022.03.22.22272691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kodera S, Rashed EA, Hirata A. Estimation of real-world vaccination effectiveness of Mrna Covid-19 Vaccines against Delta and Omicron Variants in Japan. Vaccines. (2022) 10:430. 10.3390/vaccines10030430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horne EM, Hulme WJ, Keogh RH, Palmer TM, Williamson EJ, Parker EP, et al. Waning effectiveness of Bnt162b2 and Chadox1 Covid-19 vaccines over six months since second dose: a cohort study using linked electronic health records. MedRxiv. (2022) 10.1101/2022.03.23.22272804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acuti Martellucci C, Flacco ME, Soldato G, Di Martino G, Carota R, Caponetti A, et al. Effectiveness of Covid-19 vaccines in the general population of an Italian Region before and During the Omicron Wave. Vaccines. (2022) 10:662. 10.3390/vaccines10050662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Effectiveness of Covid-19 Vaccines against the Omicron (B.1.1.529) Variant Concern. MedRxiv. (2021). [Google Scholar]
- 27.He X, Hong W, Pan X, Lu G, Wei X. SARS-CoV-2 Omicron Variant: characteristics and prevention. MedComm. (2021) 2:838–45. 10.1002/mco2.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingraham NE, Ingbar DH. The Omicron Variant of SARS-CoV-2: understanding the known and living with unknowns. Clin Transl Med. (2021) 11:e685. 10.1002/ctm2.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Callaway E. Omicron likely to weaken Covid Vaccine protection. Nature. (2021) 600:367–8. 10.1038/d41586-021-03672-3 [DOI] [PubMed] [Google Scholar]
- 30.Mahumud RA, Ali MA, Kundu S, Rahman MA, Kamara JK, Renzaho AMN. Effectiveness of Covid-19 vaccines against Delta Variant (B.1.617.2): a meta-analysis. Vaccines. (2022) 10:10020277. 10.3390/vaccines10020277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Aghayari Sheikh Neshin S, Khatami A, et al. Effectiveness of Covid-19 Vaccines against Delta (B.1.617.2) Variant: a systematic review and meta-analysis of clinical studies. Vaccines. (2021) 10:10010023. 10.3390/vaccines10010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puranik A, Lenehan PJ, Silvert E, Niesen MJM, Corchado-Garcia J, O'Horo JC, et al. comparison of two highly-effective Mrna Vaccines for Covid-19 during periods of Alpha and Delta Variant prevalence. MedRxiv. (2021). 10.1101/2021.08.06.21261707 [DOI] [Google Scholar]
- 33.Iuliano AD, Brunkard JM, Boehmer TK, Peterson E, Adjei S, Binder AM, et al. Trends in disease severity and health care utilization during the early Omicron Variant Period compared with previous SARS-CoV-2 High Transmission Periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:146–52. 10.15585/mmwr.mm7104e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt T, Klemis V, Schub D, Mihm J, Hielscher F, Marx S, et al. Immunogenicity and reactogenicity of heterologous Chadox1 Ncov-19/Mrna vaccination. Nat Med. (2021) 27:1530–5. 10.1038/s41591-021-01464-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. (2019) 32:e00084–18. 10.1128/CMR.00084-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.