Abstract
Moraxella osloensis, a gram-negative bacterium, is associated with Phasmarhabditis hermaphrodita, a nematode parasite of slugs. This bacterium-feeding nematode has potential for the biological control of slugs, especially the grey garden slug, Deroceras reticulatum. Infective juveniles of P. hermaphrodita invade the shell cavity of the slug, develop into self-fertilizing hermaphrodites, and produce progeny, resulting in host death. However, the role of the associated bacterium in the pathogenicity of the nematode to the slug is unknown. We discovered that M. osloensis alone is pathogenic to D. reticulatum after injection into the shell cavity or hemocoel of the slug. The bacteria from 60-h cultures were more pathogenic than the bacteria from 40-h cultures, as indicated by the higher and more rapid mortality of the slugs injected with the former. Coinjection of penicillin and streptomycin with the 60-h bacterial culture reduced its pathogenicity to the slug. Further work suggested that the reduction and loss of pathogenicity of the aged infective juveniles of P. hermaphrodita to D. reticulatum result from the loss of M. osloensis from the aged nematodes. Also, axenic J1/J2 nematodes were nonpathogenic after injection into the shell cavity. Therefore, we conclude that the bacterium is the sole killing agent of D. reticulatum in the nematode-bacterium complex and that P. hermaphrodita acts only as a vector to transport the bacterium into the shell cavity of the slug. The identification of the toxic metabolites produced by M. osloensis is being pursued.
Moraxella osloensis (gamma subdivision: Moraxellaceae) is a gram-negative aerobic bacterium that is coccal or rod shaped but which tends to be pleomorphic. The bacterium can grow in mineral medium with acetate and ammonium salts and produces oxidase and catalase but is sensitive to penicillin. M. osloensis is occasionally isolated from the upper respiratory tract, genitourethral specimens, blood, cerebrospinal fluid, and pyogenic manifestations in joints, bursae, and other sites in humans (1). The bacterium is considered an opportunistic human pathogen and has been found to cause diseases, such as endocarditis (22), osteomyelitis (23), central venous catheter infection (4), and meningitis (11). M. osloensis has also been found to be associated with Phasmarhabditis hermaphrodita (Rhabditida: Peloderinae), a lethal nematode parasite of slugs (26).
This bacterium-feeding nematode has potential for the biological control of mollusk pests, including the grey garden slug, Deroceras reticulatum (Stylommatophora: Limacidae), which is often regarded as the most serious pest of agricultural and horticultural plants (15, 21, 25). As with the entomopathogenic nematodes in the genera Heterorhabditis and Steinernema, the parasitic cycle of P. hermaphrodita is initiated by the third-stage infective juveniles. The infective juveniles invade D. reticulatum through the dorsal integumental pouch immediately posterior to the mantle, enter the shell cavity via a short canal, and then develop into self-fertilizing hermaphrodites that produce progeny, resulting in host death (25). When the food source is depleted, the nematodes form the next generation of infective juveniles, which leave the cadaver to search for new hosts. Unlike Heterorhabditis and Steinernema, which are associated with one particular bacterium each in the genera Photorhabdus and Xenorhabdus, respectively (13), P. hermaphrodita has been found to be associated with many different species of bacteria. Wilson et al. (27) recovered more than 150 bacterial isolates from inside infective juveniles of P. hermaphrodita, from living and dead D. reticulatum, and from xenic foam chip cultures of the nematodes, including M. osloensis. Nine bacterial isolates were selected and studied, and only two isolates from 24-h cultures (Aeromonas hydrophila and Pseudomonas fluorescens isolate 140) were found to be pathogenic to D. reticulatum when they were injected into the hemocoel of the slug. However, nematodes grown with M. osloensis or P. fluorescens (isolate 141) were pathogenic, those grown with Providencia rettgeri produced inconsistent results, and those grown with Serratia proteamaculans and P. fluorescens (isolate 140) were nonpathogenic to the slug. Finally, Wilson et al. (27) selected M. osloensis as the preferred associated bacterium to mass-produce P. hermaphrodita in monoxenic culture.
A commercial product, NemaSlug, based on P. hermaphrodita has been developed in England. However, a high dose of the nematodes (3 × 109 infective juveniles/ha) is required for effective plant protection in the field (17). Pathogenicity of the mass-produced nematodes varies among different batches. Further, aged P. hermaphrodita nematodes are less virulent than young ones. All of these factors restrict further development of the product. Therefore, information on the virulence mechanism of the nematode-bacterium complex is necessary to develop mass-production systems that maintain high and stable nematode pathogenicity. Availability of a high-quality biological control agent is needed to manage slug pests in landscapes, nurseries, and field crops worldwide.
The present study was conducted to discern the real virulent agent in the nematode-bacterium complex. Wilson et al. (27) reported that a 24-h culture of M. osloensis that is injected into the D. reticulatum hemocoel is not pathogenic. As the shell cavity in the posterior mantle region, and not the mouth or genital pore, serves as the main portal of entry for P. hermaphrodita (24), it is predicted that the nematode carries M. osloensis first into the shell cavity and not the hemocoel. Since important organs, including the kidney, lung, and heart, are located in the mantle region, it is possible that M. osloensis alone may kill the slug without entering the hemocoel. Therefore, we hypothesized that M. osloensis vectored into the shell cavity could be pathogenic to the slug. We also hypothesized that the pathogenicity of M. osloensis may vary with the age and number of the bacteria.
MATERIALS AND METHODS
Sources of bacteria, nematodes, and slugs.
Pure culture of M. osloensis and the foam or powder formulation of monoxenic culture of P. hermaphrodita with its associated bacterium M. osloensis were supplied by MicroBio Ltd. (Cambridge, United Kingdom). Nematodes were removed from the formulation by mixing them in tap water. Infective stages were separated from the noninfective stages by treating the nematode suspension with a 5% solution of hand soap (AJAX; Colgate-Palmolive Company, New York, N.Y.) for 5 h. Nematode infective stages withstand exposure to detergents, but noninfective stages do not (20). The treated suspension was filtered through two layers of tissue paper loaded on an aluminum sieve. The sieve with the nematodes (on the tissue paper) was then put on a petri dish containing tap water. The living infective juveniles that migrated through the tissue paper into the water were used in all the following experiments.
All adult D. reticulatum were collected from the field and allowed to feed on pieces of fresh carrots and cabbage leaves at room temperature for at least 12 days. Only healthy adult slugs were then used in the subsequent experiments.
Pathogenicity of M. osloensis to D. reticulatum after injection into the shell cavity.
Two experiments were conducted to determine the pathogenicity of M. osloensis to D. reticulatum. In the first experiment, the pure culture of M. osloensis was inoculated into nutrient agar plates and incubated at 25°C for 40 h (in log phase). The bacteria were then washed off the plates into a sterile petri dish using sterile saline solution (0.85% NaCl). The total numbers of bacteria in the suspension were measured with a spectrophotometer with a wavelength at 600 nm and estimated using a standard curve of the bacteria. The bacterial suspensions were then diluted serially into different concentrations, namely, 1.01 × 104, 1.01 × 105, 1.01 × 106, 1.01 × 107, 1.01 × 108, 1.01 × 109, and 1.01 × 1010 CFU/ml. A 50-μl volume of suspension of each concentration was injected into the shell cavity of D. reticulatum as described by Tan and Grewal (24). Twenty-four slugs were treated with each concentration and were then separated into three petri dishes (eight slugs per dish) as three replicates for the calculation of slug mortality. Slugs injected with the saline solution served as controls. All slugs were fed pieces of fresh carrots and cabbage leaves and incubated at 18°C. The numbers of dead slugs were recorded every day for 16 days.
Bacteria in stationary phase usually secrete and/or accumulate more exotoxin(s) than those in log phase (5, 7, 19). In order to determine whether M. osloensis bacteria in the stationary phase are more toxic to the slug, we repeated the experiment described above with a 60-h bacterial culture except that slightly different concentrations of bacteria (1.23 × 102, 1.23 × 104, 1.23 × 106, 1.23 × 108, and 1.23 × 1010 CFU/ml) were used.
We decided to grow the bacteria on plates rather than in broth culture because (i) it is easier to monitor bacterial contamination on plates than in broth culture, (ii) it is easier to monitor the growth of bacteria on plates than in broth culture since isolated colonies are visible to the naked eye in the third phase of a three-phase streak on a nutrient agar plate for a 60-h bacterial culture but not for a 40-h bacterial culture, and (iii) culturing the bacteria on plates may eliminate the potential adverse effect of broth medium on slug mortality.
Effect of antibiotics on the pathogenicity of M. osloensis to D. reticulatum
Penicillin can interfere with the formation of cell walls of bacteria, while streptomycin can block the synthesis of bacterial proteins. The two antibiotics were used to inhibit the growth and metabolism of M. osloensis, thus testing their effect on the pathogenicity of the bacterium to D. reticulatum. A bacterial suspension (1.23 × 1010 CFU/ml) from the 60-h culture was prepared as described above. Fifty microliters of the suspension was injected into the shell cavity of each slug with or without penicillin (500 U/ml) and streptomycin (500 μg/ml). Twenty-four slugs were maintained for each treatment and were then separated into three petri dishes (eight slugs per dish) as three replicates for the calculation of slug mortality. At the same time, slugs injected with the antibiotics or the saline solution served as controls. All slugs were fed pieces of fresh carrots and cabbage leaves and incubated at 18°C. The numbers of dead slugs were recorded every day for 12 days.
Pathogenicity of M. osloensis to D. reticulatum after injection into the hemocoel.
It is possible that the pathogenicity of M. osloensis to D. reticulatum varies with the age of the bacterial cultures. Therefore, the pathogenicity to D. reticulatum of cultures of the bacterium of different ages after injection into the hemocoel was determined. Pure culture of the bacterium was inoculated in nutrient agar plates, and the plates were incubated at 25°C for 24, 40, and 60 h. Bacterial suspensions were prepared, and their concentrations were estimated as described above for the shell cavity injection experiment. The bacteria from the three cultures of different ages were then diluted, and their concentrations were adjusted to 1.0 ×109 CFU/ml. A 20-μl suspension was injected into the hemocoel from the middle of the hind dorsal portion of each D. reticulatum slug. Eighteen slugs were treated with bacteria of each culture age and were then separated into three petri dishes (six slugs per dish) as three replicates for the determination of slug mortality. At the same time, slugs injected with 20 μl of sterile saline solution served as controls. All slugs were fed pieces of fresh carrots and cabbage leaves and incubated at 18°C. The numbers of dead slugs were recorded every day for 16 days.
Numbers of viable M. osloensis bacteria in the fresh and aged infective juveniles of P. hermaphrodita
As aged P. hermaphrodita nematodes are less virulent to slugs, the numbers of viable M. osloensis bacteria in the infective juveniles from a fresh, a 3-month-old, and an 8-month-old batch were determined. Infective juveniles from the three batches were surface sterilized by immersion in 0.1% thimerosal for 3 h. Twenty surface-sterilized infective juveniles were ground using an autoclaved mortar for 30 s, and the nematode homogenate was plated on a 9-cm-diameter nutrient agar plate. Ten replicates were prepared for each batch. All of the nutrient agar plates were incubated at 25°C for 2 days. The numbers of CFU were then counted.
Pathogenicity of axenic and aged P. hermaphrodita to D. reticulatum
It was very difficult to culture P. hermaphrodita without bacteria (data not shown); however, axenic juvenile nematodes at stage J1 or J2 (J1/J2) were obtained by immersing nematode eggs in 0.1% thimerosal for 3 h. They were then transferred, through two changes of the sterile saline solution, to a sterile petri dish containing sterile saline solution with penicillin (500 U/ml) and streptomycin (500 μg/ml). Five axenic J1/J2 infective juveniles (n = 3) were ground, and the nematode homogenate was then plated on a nutrient agar plate at 25°C for 2 days to verify the axenicity of the nematodes. Infective juveniles from the fresh, 3-month old, and 8-month old batches were surface sterilized as described above. Five surface-sterilized infective juveniles from the three batches (only the 8-month-old batch with or without the antibiotics) and five axenic J1/J2 infective juveniles with the antibiotics were injected into the shell cavity of the slug. The antibiotics were used to maintain the aseptic environment for the aged or axenic nematodes. Twenty-four slugs were maintained for each treatment and were then separated into three petri dishes (eight slugs per dish) as three replicates for the calculation of slug mortality. At the same time, slugs injected with the antibiotics or the saline solution served as controls. All slugs were fed pieces of fresh carrots and cabbage leaves and incubated at 18°C. The numbers of dead slugs were recorded after 12 days.
Statistical analyses.
All data presented in percentage values were arcsine transformed and subjected to one-way, or repeated-measure, analysis of variance using the statistical software program STATISTICA Kernel release 5.5 (StatSoft Inc.; 2000). Significant differences among treatment results were determined using Tukey's honestly significant difference tests at a P of 0.05.
RESULTS
Pathogenicity of M. osloensis to D. reticulatum after injection into the shell cavity.
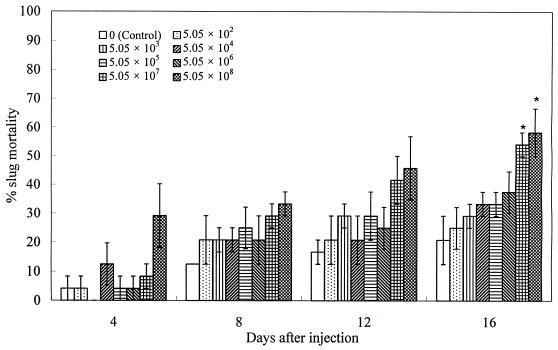
The pathogenicity of different concentrations of M. osloensis from a 40-h culture to D. reticulatum is shown in Fig. 1. There is a trend that slug mortality increased with the increase of the bacterial concentration over time. Both the highest and the second-highest concentrations (5.05 × 108 and 5.05 × 107 CFU/slug) of the bacteria had significant effects (P < 0.05) on slug mortality at 16 days after treatment compared with the results for the control. Moreover, there is a significant linear relationship (R2 = 0.57) between transformed slug mortality and the log of bacterial concentrations at this time.
FIG. 1.
Percentage mortality (mean ± SE, n = 3) of D. reticulatum following injection of different concentrations (CFU/slug) of 40-h M. osloensis culture into the shell cavity. The asterisk indicates results that are significantly different at a P of <0.05 from those for the control.
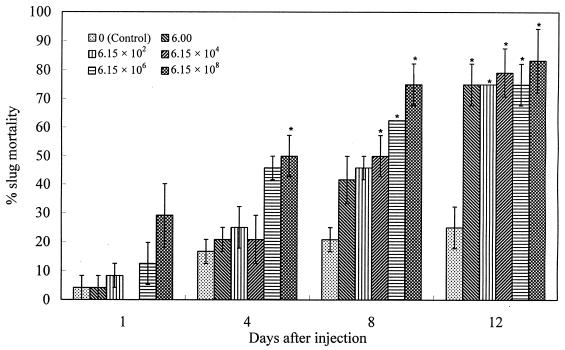
The 60-h bacterial culture is highly pathogenic to the slug after injection into the shell cavity (Fig. 2). Compared with the control, the highest concentration (6.15 × 108 CFU/slug) caused significant slug mortality (P < 0.05) as early as 4 days after treatment. The second- and third-highest concentrations (6.15 × 106 and 6.15 × 104 CFU/slug) also had significant effects on slug mortality at 8 days after treatment. In addition, all of the concentrations of the 60-h bacterial culture, from 6 CFU per slug to 6.15 × 108 CFU per slug, resulted in similar and significant slug mortalities (around 80%) at 12 days after treatment. There are significant linear relationships between transformed slug mortality and the log of bacterial concentrations at 4 and 8 days after treatment (R2 = 0.54 and 0.73 for 4 and 8 days, respectively).
FIG. 2.
Percentage mortality (mean ± SE, n = 3) of D. reticulatum following injection of different concentrations (CFU/slug) of 60-h M. osloensis culture into the shell cavity. The asterisk indicates results that are significantly different at a P of <0.05 from those for the control.
Effect of antibiotics on the pathogenicity of M. osloensis to D. reticulatum
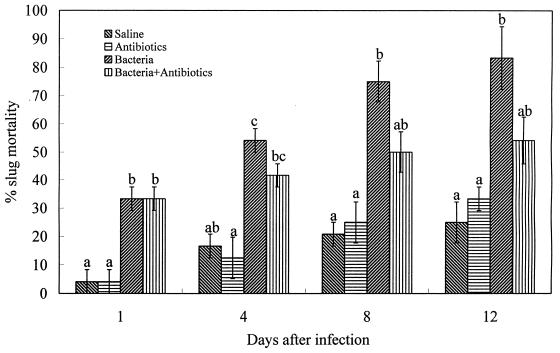
Compared with the two groups of controls, the 60-h culture of M. osloensis with and without the antibiotics, overall, had a significant effect (P < 0.05) on slug mortality after injection into the shell cavity (Fig. 3). Both treatments caused significant slug mortality at 1 day after treatment compared with the results for the controls. After that, coinjection of the antibiotics with the culture reduced the pathogenicity of the bacteria to the slug. Only treatment with the bacteria without the antibiotics resulted in slug mortalities that differed significantly from that of the controls after 1 day. There are no significant differences (P > 0.05) between the results for the two groups of controls (saline and antibiotic treatments).
FIG. 3.
Percentage mortality (mean ± SE, n = 3) of D. reticulatum following injection of M. osloensis (60-h culture) with or without the antibiotics into the shell cavity. Values differ significantly at a P of <0.05 as indicated by different letters.
Pathogenicity of M. osloensis to D. reticulatum after injection into the hemocoel.
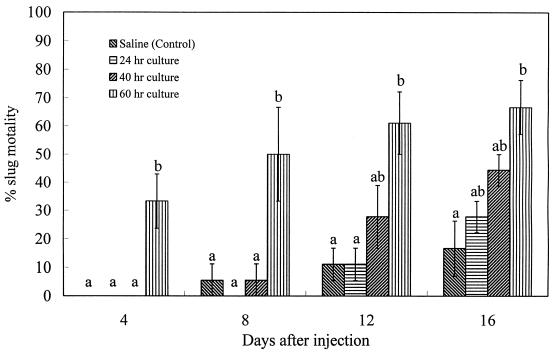
Since M. osloensis bacteria from the 60-h culture were more pathogenic than those from the 40-h culture, it is possible that the bacteria did not kill the slug just because the 24-h bacterial culture was nonpathogenic in the study of Wilson et al. (27). D. reticulatum slug mortality caused by cultures of M. osloensis of different ages after injection into the hemocoel is shown in Fig. 4. Only M. osloensis from the 60-h culture had a significant effect (P < 0.05) on slug mortality up to 16 days after treatment compared with the results for the control. In addition, there is a significant linear relationship (R2 = 0.66) between the total slug mortality after 16 days of treatment and the culture age of M. osloensis at the time of treatment. The 24-h bacterial culture had no significant effect (P > 0.05) on slug mortality during the entire experimental period.
FIG. 4.
Percentage mortality (mean ± SE, n = 3) of D. reticulatum following injection of cultures of M. osloensis of different ages into the hemocoel. Values differ significantly at a P of <0.05 as indicated by different letters.
Numbers of viable M. osloensis bacteria in the fresh and aged infective juveniles of P. hermaphrodita
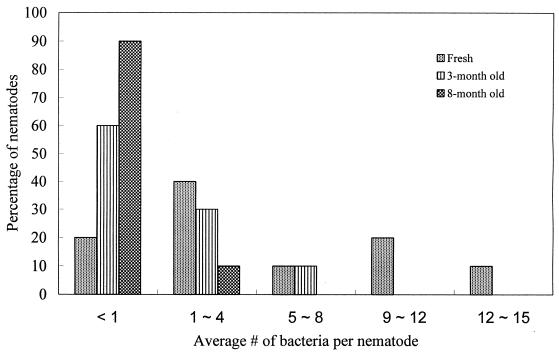
The numbers of viable M. osloensis bacteria in 20 infective juveniles of P. hermaphrodita were 96 ± 30 (mean ± standard error [SE]) for the fresh batch, 43 ± 16 for the 3-month-old batch, and 7 ± 6 for the 8-month-old batch. The bacterial numbers in the fresh batch and the 8-month-old batch were significantly different (P < 0.05). According to the average number of M. osloensis bacteria per nematode, the infective juveniles were divided into five groups: group 1(<1 bacterium/nematode), group 2 (1 to 4 bacteria/nematode), group 3 (5 to 8 bacteria/nematode), group 4 (9 to 12 bacteria/nematode), and group 5 (12 to 15 bacteria/nematode). The percentages of the nematodes from the three batches in the five groups are presented in Fig. 5. No nematodes from the 8-month-old batch fell into groups 3 to 5, whereas 10% of the nematodes from the 3-month-old batch and 40% of the nematodes from the fresh batch fell into these groups. In addition, only nematodes from the fresh batch fell into groups 4 and 5 (20 and 10% of the nematodes fell into groups 4 and 5, respectively). In contrast, only 20% of the nematodes from the fresh batch fell into group 1, whereas 60% of the nematodes from the 3-month-old batch and 90% of the nematodes from the 8-month-old batch belonged to group 1.
FIG. 5.
Percentages of infective juveniles of P. hermaphrodita from a fresh, a 3-month-old, and an 8-month old batch in different groups.
Pathogenicity of axenic and aged P. hermaphrodita to D. reticulatum
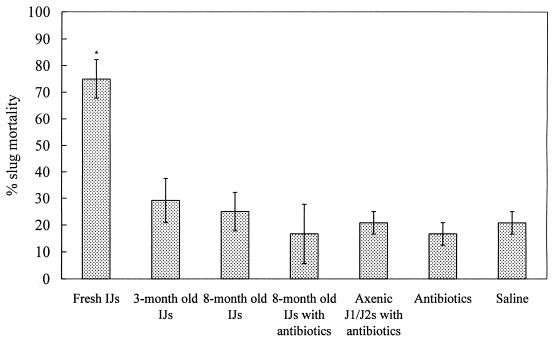
Percentages of mortality of D. reticulatum caused by the axenic and aged P. hermaphrodita organisms are shown in Fig. 6. Only the fresh infective juveniles caused significant slug mortality (P < 0.05) compared with the results for controls. Neither the 8-month-old infective juveniles with or without the antibiotics nor the axenic J1/J2 P. hermaphrodita juveniles with antibiotics had observable effects on slug mortality.
FIG. 6.
Percentage mortality (mean ± SE, n = 3) of D. reticulatum following injection of axenic and aged P. hermaphrodita into the shell cavity. IJs, infective juveniles. The asterisk indicates results that are significantly different at a P of <0.05 from those of the control.
DISCUSSION
The present results demonstrate that M. osloensis alone can kill D. reticulatum adults after injection into the shell cavity or hemocoel. The pathogenicity of M. osloensis to the slug, however, varied with the age of the bacterial cultures: 24-h cultures were nonpathogenic, whereas 40- and 60-h cultures were pathogenic. Wilson et al. (27) injected only a 24-h-old M. osloensis culture (with unknown concentration) into the hemocoel of D. reticulatum and did not observe a significant effect on slug mortality after 8 days of treatment. Their results are consistent with what we found when bacteria of the same culture age were used. However, our results demonstrate that the aged cultures of M. osloensis (e.g., 60-h-old cultures) are pathogenic to D. reticulatum both in the shell cavity and in the hemocoel.
Axenic J1/J2 nematodes were nonpathogenic after injection into the shell cavity, and the pathogenicity of the infective juveniles depended on the number of viable bacteria carried by the nematodes. In addition, the 8-month-old batch of the nematodes was almost axenic, since only 7 CFU of M. osloensis was found in 180 nematodes and these nematodes did not have an observable effect on slug mortality following injection into the shell cavity. Therefore, we conclude that P. hermaphrodita alone is unable to kill the slug host, that the nematode acts only as a vector to transport its associated bacterium, M. osloensis, into the shell cavity of D. reticulatum, and that the bacterium appears to be the only killing agent in the nematode-bacterium complex.
Our results strongly suggest that M. osloensis may produce a toxin(s) to kill D. reticulatum. The 60-h bacterial cultures were more pathogenic than the 40-h bacterial cultures, as indicated by the higher and more rapid mortality of the slugs injected with the former. It is highly possible that the 60-h culture, which is in stationary phase, may have produced and/or accumulated more exotoxin(s) before injection into the shell cavity or hemocoel. Further, reduction in the pathogenicity of the bacteria by the addition of the antibiotics also suggests the involvement of a toxin(s), since the antibiotics may inhibit production of the bacterial toxin(s) by blocking the multiplication and metabolism of M. osloensis. Moreover, M. osloensis coinjected with the antibiotics caused significant slug mortality that was the same as that caused by the bacteria injected alone 1 day after treatment, which suggests that the toxin(s) may have been produced before the addition of the antibiotics and the antibiotics did not have any effect on the toxicity of the toxin(s). A related bacterium, Moraxella (Branhamella) catarrhalis, is regarded as the third most common pathogen of the respiratory tract for humans (9). The liberated endotoxin, histamine, and chemotactically active factors are considered the major pathogenic factors of M. catarrhalis (6). Enright and McKenzie (9) reported that three serotypes of lipooligosaccharide, fimbriae, and a possible capsule might be related to the pathogenicity of the bacterium. Hemagglutinin also might be a marker of pathogenicity for M. catarrhalis (12). In addition, Hoiczyk et al. (18) indicated that two adhesins on the outer membrane of M. catarrhalis are established pathogenicity factors. The identification of the toxic metabolites produced by M. osloensis is being pursued.
The mutual association between P. hermaphrodita and M. osloensis seems parallel to the association between the entomopathogenic nematodes and their associated bacteria. The infective juveniles of the entomopathogenic nematodes also carry their symbiotic Xenorhabdus or Photorhabdus bacteria into insect hosts, in which the latter multiply and kill the hosts within 24 to 48 h (16). Further, it is the symbiotic bacteria of the entomopathogenic nematodes that produce toxins to kill the insect hosts. Like many other gram-negative bacteria, Xenorhabdus spp. produce endotoxins. The Xenorhabdus nematophilus endotoxins are lipopolysaccharide components of the cell wall that are toxic for the hemocytes of Galleria mellonella (8). Exotoxin activity has also been demonstrated for Photorhabdus luminescens, X. nematophilus, and Xenorhabdus bovienii by injecting the culture supernatant into insects (14). A high-molecular-weight extracellular insecticidal protein complex in P. luminescens has been found and purified. The purified toxin complex contained no protease, phospholipase, or hemolytic activity and only a trace of lipase activity but was found to be active in nanogram concentrations against insects representing four orders of the class Insecta (2). Further, the genes encoding the toxin complex have been cloned. The toxin complex loci tca, tcb, tcc, and tcd encode a series of four native complexes. Both tca and tcb encode complexes with high oral toxicity for Manduca sexta so that they may represent potential alternatives to the deployment of Bacillus thuringiensis toxins in transgenic plants (3). Otherwise, similar toxin complex gene sequences from X. nematophilus have been found (10).
It is not fully clear why M. osloensis from cultures of different ages had significantly different effects on slug mortality when they were injected into the shell cavity or hemocoel. As stated above, it is strongly suggested that the pathogenicity of M. osloensis to the slug is related to the toxicity of the bacterium. As the 24-, 40-, and 60-h bacterial cultures were in the early log phase, late log phase, and stationary phase, respectively, it is possible that the bacteria from cultures of different ages have different capacities for producing toxin(s) and/or accumulate different amounts of toxin(s) before the injection. For example, the 24-, 40-, and 60-h cultures of M. osloensis may have produced and/or accumulated small, medium, and large amounts of toxin(s) before injection into the shell cavity or hemocoel, respectively. Furthermore, compared with M. osloensis in log phase (e.g., the 24- and 40-h cultures), the bacterium in stationary phase (e.g., the 60-h culture) may have specific physiological, biochemical, and morphological characteristics enabling better and more rapid slug colonization, evading or overcoming host defense mechanisms.
ACKNOWLEDGMENTS
This work was supported by a Matching Fund Grant from the Ohio Agricultural Research and Development Center and MicroBio Ltd.
REFERENCES
- 1.Bovre K. Genus II. Moraxella Lwoff 1939, 173 emend, Henriksen and Bovre 1986, 391AL. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: Williams & Wilkins; 1984. pp. 296–303. [Google Scholar]
- 2.Bowen D J, Ensign J C. Purification and characterization of a high-molecular-weight insecticidal protein complex produced by the entomopathogenic bacterium Photorhabdus luminescens. Appl Environ Microbiol. 1998;64:3029–3035. doi: 10.1128/aem.64.8.3029-3035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowen D J, Rocheleau T A, Blackburn M, Andreev O, Golubeva E, Bhartia R, ffrench-Constant R H. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science. 1998;280:2129–2132. doi: 10.1126/science.280.5372.2129. [DOI] [PubMed] [Google Scholar]
- 4.Buchman A L, Pickett M J, Ament M E. Central venous catheter infection caused by Moraxella osloensis in a patient receiving home parenteral-nutrition. Diagn Microbiol Infect Dis. 1993;17:163–166. doi: 10.1016/0732-8893(93)90028-6. [DOI] [PubMed] [Google Scholar]
- 5.Chaussee M S, Phillips E R, Ferretti J J. Temporal production of streptococcal erythrogenic toxin B (streptococcal cysteine proteinase) in response to nutrient depletion. Infect Immun. 1997;65:1956–1959. doi: 10.1128/iai.65.5.1956-1959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullmann W. Moraxella catarrhalis: mechanisms of virulence and antibiotic resistance. Med Klin. 1997;92:162–166. doi: 10.1007/BF03043274. [DOI] [PubMed] [Google Scholar]
- 7.Dewanti R, Doyle M P. Influence of cultural conditions on cytotoxin production by Salmonella enteritidis. J Food Prot. 1992;55:28–33. doi: 10.4315/0362-028X-55.1.28. [DOI] [PubMed] [Google Scholar]
- 8.Dunphy G B, Webster J M. Lipopolysaccharides of Xenorhabdus nematophilus (Enterobacteriaceae) and their haemocyte toxicity in non-immune Galleria mellonella (Insecta: Lepidoptera) larvae. J Gen Microbiol. 1988;134:1017–1028. [Google Scholar]
- 9.Enright M C, McKenzie H. Moraxella (Branhamella) catarrhalis: clinical and molecular aspects of a rediscovered pathogen. J Med Microbiol. 1997;46:360–371. doi: 10.1099/00222615-46-5-360. [DOI] [PubMed] [Google Scholar]
- 10.ffrench-Constant R H, Bowen D J. Novel insecticidal toxins from nematode symbiotic bacteria. Cell Mol Life Sci. 2000;57:828–833. doi: 10.1007/s000180050044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fijen C A P, Kuijper E J, Tjia H G, Daha M R, Dankert J. Complement deficiency predisposes for meningitis due to nongroupable meningococci and Neisseria-related bacteria. Clin Infect Dis. 1994;18:780–784. doi: 10.1093/clinids/18.5.780. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald M, Murphy S, Mulcahy R, Keane C, Coakley D, Scott T. Tissue culture adherence and haemagglutination characteristics of Moraxella (Branhamella) catarrhalis. FEMS Immunol Med Microbiol. 1999;24:105–114. doi: 10.1111/j.1574-695X.1999.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 13.Forst S, Nealson K. Molecular biology of the symbiotic-pathogenic bacteria Xenorhabdus spp. and Photorhabdus spp. Microbiol Rev. 1996;60:21–43. doi: 10.1128/mr.60.1.21-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Georgis R, Kelly J. Novel pesticidal substances from the entomopathogenic nematode-bacterium complex. In: Hedin P A, editor. Phytochemicals for pest control. Washington, D.C.: American Chemical Society; 1997. pp. 135–143. [Google Scholar]
- 15.Godan D. Pest slugs and snails: biology and control. Berlin, Germany: Springer-Verlag; 1983. [Google Scholar]
- 16.Grewal P S, Georgis R. Entomopathogenic nematodes. In: Hall F R, Menn J J, editors. Biopesticides: use and delivery. Totowa, N.J: Humana Press; 1999. pp. 271–279. [Google Scholar]
- 17.Grewal P S, Grewal S K, Taylor R A J, Hammond R B. Application of molluscicidal nematodes to slug shelters: a novel approach to economic biological control of slugs. Biol Control. 2001;22:72–80. [Google Scholar]
- 18.Hoiczyk E, Roggenkamp A, Reichenbecher M, Lupas A, Heesemann J. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 2000;19:5989–5999. doi: 10.1093/emboj/19.22.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karunkaran T, Devi B G. Characterization of hemolytic-activity from Aeromonas caviae. Epidemiol Infect. 1994;112:291–298. doi: 10.1017/s0950268800057708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riddle D L. The dauer larva. In: Wood W B, editor. The nematode Caenorhabditis elegans. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1988. pp. 393–412. [Google Scholar]
- 21.South A. Terrestrial slugs: biology, ecology and control. London, England: Chapman & Hall; 1992. [Google Scholar]
- 22.Stryker T D, Stone W J, Savage A M. Renal-failure secondary to Moraxella osloensis endocarditis. Johns Hopkins Med J. 1982;151:217–219. [PubMed] [Google Scholar]
- 23.Sugarman B, Clarridge J. Osteomyelitis caued by Moraxella osloensis. J Clin Microbiol. 1982;15:1148–1149. doi: 10.1128/jcm.15.6.1148-1149.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan, L., and P. S. Grewal. Infection behavior of the rhabditid nematode Phasmarhabditis hermaphrodita to the grey garden slug Deroceras reticulatum. J. Parasitol, in press. [DOI] [PubMed]
- 25.Wilson M J, Glen D M, George S K. The rhabditid nematode Phasmarhabditis hermaphrodita as a potential biological control agent for slugs. Biocontrol Sci Technol. 1993;3:503–511. [Google Scholar]
- 26.Wilson M J, Glen D M, Pearce J D, Rodgers P B. Monoxenic culture of the slug parasite Phasmarhabditis hermaphrodita (Nematoda: Rhabditidae) with different bacteria in liquid and solid phase. Fundam Appl Nematol. 1995;18:159–166. [Google Scholar]
- 27.Wilson M J, Glen D M, George S K, Pearce J D. Selection of a bacterium for the mass production of Phasmarhabditis hermaphrodita (Nematoda: Rhabditidae) as a biocontrol agent for slugs. Fundam Appl Nematol. 1995;18:419–425. [Google Scholar]