Abstract
Currently, the plastic monomer and plasticizer bisphenol A (BPA) is one of the most widely used chemicals. BPA is present in polycarbonate plastics and epoxy resins, commonly used in food storage and industrial or medical products. However, the use of this synthetic compound is a growing concern, as BPA is an endocrine-disrupting compound and can bind mainly to estrogen receptors, interfering with different functions at the cardiovascular level. Several studies have investigated the disruptive effects of BPA; however, its cardiotoxicity remains unclear. Therefore, this review’s purpose is to address the most recent studies on the implications of BPA on the cardiovascular system. Our findings suggest that BPA impairs cardiac excitability through intracellular mechanisms, involving the inhibition of the main ion channels, changes in Ca2+ handling, the induction of oxidative stress, and epigenetic modifications. Our data support that BPA exposure increases the risk of developing cardiovascular diseases (CVDs) including atherosclerosis and its risk factors such as hypertension and diabetes. Furthermore, BPA exposure is also particularly harmful in pregnancy, promoting the development of hypertensive disorders during pregnancy. In summary, BPA exposure compromises human health, promoting the development and progression of CVDs and risk factors. Further studies are needed to clarify the human health effects of BPA-induced cardiotoxicity.
Keywords: BPA, plasticizer, endocrine disruptor, cardiotoxicity, human health
1. Introduction
According to the World Health Organization, cardiovascular diseases (CVDs) are the leading cause of death worldwide. The majority of CVDs are chronic and asymptomatic over a long time, and usually, the first symptoms only appear as the disease progresses. However, CVDs can also induce immediate sudden death, which is the main cause of premature mortality worldwide. It is estimated that by the year 2030, 23.6 million people will die from CVDs each year. However, there is a slight downward trend in mortality and CVD incidence in north-eastern and Southern Europe [1].
Currently, the influence of environmental contaminants on humans has been proposed as a cause of CVDs [2]. Every year, millions of tons of plastic are produced worldwide which results in continuous daily human exposure to these environmentally toxic chemicals [3]. Endocrine-disrupting compounds (EDCs) are defined by the North American Environmental Protection Agency as a natural or synthetic compounds which can interfere with the actions of the endocrine system. Specifically, EDCs can mimic or antagonize the action of endogenous hormones and alter their synthesis, transport, binding, and elimination. Then, these emerging compounds can disrupt normal hormonal homeostasis, reproduction, and/or behavior [4,5]. Moreover, EDCs are potential modulators of cardiovascular physiology, from which emerges the need for the study of their cardiotoxicity [6].
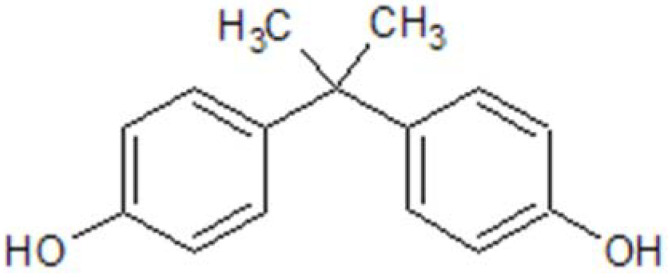
Among the various EDCs, bisphenol A (BPA) (Figure 1) stands out as one of the most widely produced EDCs worldwide [7]. BPA, also designated as 4,4’-ispropylidenediphenol by IUPAC, is a synthetic organic compound formed of two phenol groups, used in polycarbonate plastics and epoxy resins [8]. In 1891, BPA was first synthesized by the chemist Alexender P.Dianin, and about 40 years later, some of its estrogenic effects began to be discovered [9]. Its properties give plastics greater thermal resistance and elasticity, and for this reason, BPA is still in use after 130 years of its discovery.
Figure 1.
BPA chemical structure, drawn in ChemDraw®®.
Regarding its appearance, BPA is a solid, white, crystalline substance whose melting point is 156 °C, with a boiling point of 220 °C (at a pressure of 5 hPa). Furthermore, BPA has a water–octanol coefficient of log Pow = 3.32, indicating that it has good solubility in fats, and contrariwise, low solubility in water (~200 mg/dL3 at 25 °C). The presence of hydroxyl groups determines the good reactivity of BPA. Like other phenols, bisphenol can be converted into ethers, esters, and salts [10]. The structure of BPA is similar to that of 17β-estradiol, and for that reason, this EDC binds to estrogenic receptors such as ERα, ERβ, ERγ, G-protein-coupled estrogen receptor (GPR30), and peroxisome proliferator-activated receptor gamma (PPAR-γ) [11]. Although the mechanisms of action are not yet fully understood, BPA has been shown to induce insulin resistance, adipogenesis, pancreatic β-cell dysfunction, inflammation, and oxidative stress [12].
Therefore, given its ubiquity and endocrine-disrupting (estrogenic) properties, daily exposure to BPA has become a major public health concern, and it is even considered that the cardiovascular system is highly susceptible to the disruptive effects of BPA [13]. Indeed, several studies have associated BPA exposure with an increased risk of developing CVDs via different intracellular mechanisms (as will be described in this review). Thus, our aim is to address the disrupting effects of BPA in the human cardiovascular system, reviewing the current literature based on epidemiological data and experimental studies in humans and animals, with a focus on the underlying molecular mechanisms.
2. Approach to the Review
Recent studies regarding the cardiovascular effects of BPA on animal and human models will be presented in this review. A literature review was carried out for epidemiological and experimental data on the cardiovascular system and supported by in vitro studies. A PubMed search on articles published between the years 2011 and 2022 was carried out. The database search was performed using a combination of terms relating to bisphenol A (“bisphenol A”, ”BPA”, “endocrine disruptor compound”, and “plastic contaminants”), to the cardiovascular system (“cardiovascular system”, “arteries”, “vascular”, “smooth muscle”, “vascular smooth muscle”, “smooth muscle cells”, “endothelium”, and “heart”), and to cardiovascular outcomes (“cardiovascular diseases”, “hypertension”, “endothelial dysfunction”, ”atherosclerosis”, ”myocardial infarction”, “heart failure”, “heart rate variability”, “blood pressure”, and “peripheral vascular disease”). In addition to these terms, we also included in the search relevant citations of the articles used. From all the articles retrieved, duplicates, unrelated, and inaccessible papers were excluded. This review was performed following a weight-of-evidence approach, and the results of the most important studies and those with greater relevance for this paper are described below.
3. Exposure to BPA
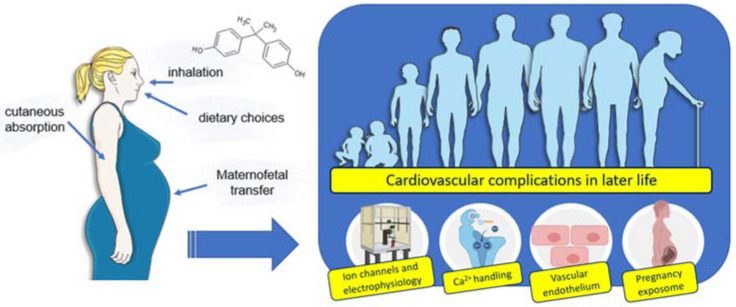
Globally, the use of BPA has progressively increased, reaching more than 10 million tonnes per year [7,10]. BPA is present in 95% of products requiring epoxy resins and polycarbonates, such as food containers, bottles, toys, dental products, CDs, DVDs, and water pipes [14]. The use of BPA in consumables and medical products makes its exposure continuous, having been detected, for example, in urine in over 90% of the United States (US) population [15]. In addition, BPA has also been identified in other biological samples, such as maternal blood (0.3 to 18.9 ng/mL) [16,17,18,19], maternal urine (31.9 μg/L) [19], amniotic liquid (median = 0.26 ng/mL) [17], placental tissue (median = 12.7 ng/g) [16], umbilical cord blood (0.2 to 9.2 ng/mL) [16,18,20], breast milk (0.61 to 0.7 μg/L) [19,21], and human colostrum (3.41 ng/mL) [22]. However, in biomonitoring studies, urinary samples of BPA are often used. The reason is that BPA is a non-persistent chemical, so its chemical concentration is higher in these samples, compared to human plasma or serum [6,23]. Nevertheless, the degree of exposure to BPA is quite variable depending on socioeconomic factors, lifestyle, medical status, and exposure pathways [6]. With regard to this, oral exposure is considered the most prevalent, with BPA levels associated with dietary choices [6,24]. On the other hand, cutaneous absorption and/or inhalation may also be associated with a higher level of exposure to unconjugated or biologically active BPA, which may persist for longer periods (~5.4 h) compared to ingested, subject to first-pass metabolism [23].
BPA, similarly to other EDCs, interacts with receptors activated by estrogens, androgens, thyroid hormones, and peroxisome proliferator, and acts as an agonist or antagonist via a receptor-dependent signaling pathway; this is attributed to its chemical structure. However, its chemical structure may be an advantage, as demonstrated for binding to ER, in which BPA does not achieve proper accommodation in the confines of the hormone-binding site (it only induces a displacement of α-helices forming the ligand-binding domain (LBD)) [11]. Moreover, Tan et al. demonstrated that EDCs share three levels of key fragments: primary and secondary fragments (responsible for the receptors binding, which discriminate active and inactive compounds), and tertiary fragments that determine their activity type (agonist, antagonist, or agonist–antagonist (A-Anta)). This determination is achieved via the interaction of EDCs with the functional lobes, directly affecting the AF-2 surface, which is responsible for coregulator recruitment. In the case of BPA, this EDC contained primary fragments of oxygen-containing aromatics and secondary ones (bisphenol group) [25]. The coexistence of primary and secondary fragments is responsible for activating BPA (active compound). Activation of the estrogen receptor (ER) and androgen receptor (AR) is achieved via interactions of the secondary fragment of stabilized BPA conformations in the LBD by forming hydrogen bonds with R394 amino acid and via van der Waals interactions with N705 amino acid, respectively. The comprehension of secondary fragments forming interacting networks with LBD amino acids is the basis for the activity of BPA. Ligand fragments of BPA interact with LBD and cause changes in the conformation of the AF-2 surface, recruiting two cofactors and, thus, determining its tertiary fragment (A-Anta activity).
Similar to natural hormones, some of the experimental studies with BPA suggest a non-monotonic response, highlighting that risk assessment is required with exposures from ‘lower’ to ‘higher’ doses, given the characteristic U-shaped response also observed by other EDCs [26]. Not surprisingly, this property can complicate BPA toxicity risk assessment, as this EDC can interact with hormone receptors in specific cell types and/or have multiple biological endpoints with linear dose–response that collectively produce a non-monotonic dose–response relationship [27].
Over the past few years, there has been growing concern regarding the adverse effects of BPA exposure on human health. These adverse effects have led countries such as Denmark and Belgium to restrict the use of BPA in food packaging for children between the ages of 0 and 3. Sweden has also limited the use of BPA in varnishes and food packaging coatings for children in the same age group. In addition, Austria has restricted the use of BPA in pacifiers and bottles since October 2010 [28].
Therefore, given the characteristics of BPA as an EDC and its continued exposure to humans, in the following sections, the endocrine-disrupting effects of BPA on the cardiovascular system will be described for animal models (Section 4) and human models (Section 5) (please see below).
4. Effects of BPA on Animal Models
Many studies have linked BPA exposure to adverse effects on health, mainly in reproductive organs; neural, immune, and metabolic systems; and cancer [29,30]. Nevertheless, recent evidence further revealed the relationship between BPA exposure and the incidence of cardiovascular disease, myocardial infarction, hypertension, and altered cardiac electrophysiology [27,31].
Recently, Lind et al. established several key characteristics of cardiovascular toxicants in research: (1) drug discovery, (2) environmental health hazard assessment, (3) research biomarkers (for epidemiological studies and clinical trials), and (4) clinical practice. Thus, the author defined several techniques using in vitro, in vivo, and ex vivo models that are currently used to classify a substance as cardiotoxic. In this review, we will try to address the effects of BPA in these various aspects [32].
4.1. In Vitro Studies
BPA exposure has been extensively studied in rodents, fish, and canine animals. In vitro studies can offer a quicker and more flexible approach to health effects and are also an indispensable tool for studying mechanistic pathways.
As previously mentioned, ion channels play key roles in the excitability of cardiac cells (the sinoatrial node, atria, atrioventricular node, Purkinje fibers, and ventricles cells), and in the regulation of vascular smooth muscle and in endothelial cells. Thus, any alteration in the activity or structures of ion channels in these cells may induce cardiovascular pathologies [33,34].
Regarding the effect of BPA on ion channel activity, Asano et al. was the first to perform a study on human and canine coronary SMC, and showed that BPA (10 µmol/L) activates large conductance Maxi-K channels (BKCa) in a non-genomic pathway. BKCa activation depend on the two main subunits of this channel (α-subunit and β1 subunit). The α-subunit alone was sufficient for the response of these channels to BPA; however, in the presence of the regulatory subunit (β1 subunit), the response to BPA was improved. Thus, the authors concluded that the rapid effect of BPA was similar to that observed for estradiol, and suggested that the effect of BPA could provoke a vasodilatory effect on coronary arteries through the opening of Maxi-K channels [35]. A few years later, in 2014, Rottgen et al. demonstrated, in a genomic study, that BPA (100 µmol/L) activates the BK channels through an increase in α- and β1-subunits expression, corroborating the work of Asano et al. Indeed, it was concluded that BPA activates BK channels via an extracellular binding site and via an intracellular binding site that depends on the presence of the β1 subunit [36]. Both studies, Rottgen et al. and Asano et al., suggested that BPA induces a vasorelaxant effect. More recently, in 2017, in rat aorta, the BPA vasorelaxant effect was proven, and the authors also showed, via patch clamp in A7r5 cells—a vascular smooth muscle cell line obtained from embryonic rat aorta—that BPA induces an inhibition in the voltage-dependent calcium (Ca2+) influx currents via a non-genomic pathway. Moreover, the authors also demonstrated that these Ca2+ currents were due to the L-type Ca2+ channels [37].
Concerning the BPA effect in the ionic channels from cardiomyocytes, Deutschmann et al. showed—in rat GH3 cells, mouse dorsal root ganglion neurons or cardiac myocytes, and recombinant human R-type Ca2+ channels expressed in human embryonic kidney (HEK) 293 cells—that BPA (1–100 µmol/L) can rapidly and reversibly inhibit Ca2+ currents through native L-, N-, P/Q-, T-type Ca2+ channels [38]. The following year, Michaela et al. corroborated this effect of BPA (1–100 μmol/L) on T-type Ca2+ channels, these channels being similar to those expressed in nodal and conduction cardiac cells [39]. The inhibition of L-type Ca2+ channels was also shown by Liang et al. [40] in ventricular cells, and by Hyun et al. in human-induced pluripotent stem-cell-derived cardiomyocytes (hiPSC-CMs). Moreover, this author also observed that BPA (1–100 µmol/L) inhibited Nav1.5 and hERG channel activity [41]. More recently, the effect of BPA was compared with BPA substitutes, BPF and BPS; the inhibitory effect on the voltage-gated sodium channel (Nav1.5), L-type voltage-gated Ca2+ channel (Cav1.2), and the rapidly activating delayed rectifier potassium channel (hERG) was greater for BPA [42]. Moreover, we can mention that the effect of BPA on sodium (Na+) and potassium (K+) channels has been analyzed previously by other authors [41,42,43,44].
The regulation of the cardiovascular system, as mentioned above, does not depend exclusively on the modulation (inhibition or activation) of the ion channels. The phosphorylation of key regulatory proteins is also preponderant in cell signaling pathways that control the concentration of intracellular and extracellular ions, of which Ca2+ is the most important. Thus, it has also been shown by some studies that BPA can act to modify these pathways [27].
The exposure of rat cardiomyocytes to BPA and 17β-oestradiol (E2) shows that these compounds rapidly promoted arrhythmogenesis in cardiac myocytes, and those actions were mediated by the alteration of myocyte Ca2+ cycling. The effects of each compound on contractility were female-specific, that is, the contractility of male cardiomyocytes was not affected by either BPA or E2 [45,46,47]. Thus, Yan et al. demonstrated, for the first time, that acute BPA exposure (10−9 M) increased the duration of sustained ventricular arrhythmias in isolated female rat ventricular cardiomyocytes [45]. These arrhythmias were mediated through rapid Erα- and Erβ-dependent signaling mechanisms through a rapid modulation of Ca2+ handling, particularly via an increase in Ca2+ leakage from the sarcoplasmic reticulum. The previously mentioned effects were abolished when samples were pre-treated with an ER antagonist [45]. In the same sense, Belcher et al. showed that low nanomolar concentrations of BPA (0.001–1 nmol/L) and estrogen (17β-estradiol or E2) could sex-specifically alter estrogen-signaling in cultured adult rodent cardiomyocytes [46]. Two years later Yan et al. showed that acute BPA exposure, alone or combined with 17β-estradiol (E2), induces a double-edged effect in female rat hearts with ischemia–reperfusion (IR) injury. The authors showed that BPA (1 nmol/L) confers a protective effect against infarction, and impairs ventricular arrhythmia after IR injury [47]. In the same year, 2013, Gao et al. performed a study to elucidate the signaling mechanisms underlying the rapid impact of BPA on myocyte Ca2+ handling and arrhythmogenesis in female rat ventricular myocytes. The study shows that BPA (1 nmol/L) activates two parallel signaling pathways, the cAMP/PKA pathway, and the PLC/IP3/Ca2+/CAMKII pathway, which selectively impact two key Ca2+ handling proteins, ryanodine receptors and phospholamban (PLB) [48].
In 2019, Pinto et al., using cell lines, showed that BPA, bisphenol AF (BPAF) and bisphenol C (BPC) were agonists with different potencies for the three zebrafish estrogen receptors [49].
On the other hand, Ramadan et al. used neonatal cardiomyocytes to analyze the effect of BPA exposure [50]. These neonatal populations are more vulnerable to EDCs, so the study of these more vulnerable populations is of utmost importance, to be able to assess the effects of these compounds [51]. The authors exposed the cardiomyocytes to a wide range of BPAs that mimic environmental, clinical, and supraphysiological levels, and showed a reduction in spontaneous beating rate, an increase in heart rate variability (HRV), a reduction in the Ca2+ transient amplitudes, and prolongation of the Ca2+ transient upstroke and the duration time [50]. BPA exposure (1–100 µmol/L) also reduces the Ca2+ transient rise time and decreases the Ca2+ transient amplitude of hiPSC-CM in a dose-dependent manner [41].
Additionally, using mouse embryonic stem cells (ESCs) line R1, derived from 129 mouse strains that are differentiated in cardiomyocytes, Zhou et al. showed, in 2020, that individual and combined exposure to 10 ng/mL of BPA and 100 ng/mL of perfluorooctane sulfonate (PFOS) during embryonic stem cell differentiation could enlarge cardiomyocyte size, increase collagen expression, and damage mitochondria. In summary, combined exposure to PFOS and BPA could lead to adverse effects on heart development, and the interaction between PFOS and BPA may affect the rat fetal heart [52].
In EC, exposure to BPA (0–10 µmol/L) for 24 h increased the necroptosis/apoptosis ratio, the expression of Rat Receptor Interacting Protein 3 (RIP3), and CamKII activation. Moreover, the application of necrostatin-1, an inhibitor of necroptosis, improved BPA-induced cardiac dysfunction and prevented the inflammatory and hemorrhagic response in mice. In conclusion, these authors demonstrated that BPA activates the RIP 3-CamKII necroptotic pathway, leading to endothelial cell death. This mechanism may also be involved in heart failure, as the endothelial barrier loses its function; this leads to the weakening of the vascular wall of the coronary arteries in a hypertensive condition, causing ventricular hemorrhages and cardiac and pulmonary congestion [53].
In summary, it seems clear that BPA exposure induces electrical changes in cardiac muscle cells and vascular SMC, with L-type Ca2+ channels and voltage-dependent K+ channels being the most affected by BPA (Table 1). Thus, we can say that BPA inhibits Ca2+ channels and activates K+ channels, can act as a negative inotropic agent in cardiomyocytes, and may also have a negative chronotopic effect on both SMC and cardiomyocytes. Furthermore, there was also a clear association between exposure to BPA and alterations in Ca2+ handling, and in some parameters that promote oxidative stress, such as NO. Thus, and although much remains to be revealed about the mechanistic effects of BPA on the cardiovascular system, the association between the increase in cardiovascular pathologies, such as arrhythmias, and exposure to BPA seems clear.
Table 1.
Summaries of the disruptive effects of BPA in the animal in vitro studies 1.
| Drugs | Concentration | Animals/Organs/Cells | Results | References |
|---|---|---|---|---|
| BPA and Penitrem |
10 µmol/L 1 µmol/L |
Canine coronary smooth muscle cells AD 293 cells |
|
[35] |
| BPA and/or 17β-estradiol(E2)- | 1 nmol/L | Ventricular myocytes and Sprague Dawley adult mice heart and ERβ knockout mice (Erβ−/−) |
|
[45] |
| BPA and/or E2 | 0.001–1 nmol/L | Rat Sprague Dawley myocytes and female knockout Erβ mice. |
|
[46] |
| BPA | 1–100 µmol/L | HEK293 cells transfected with Human Cardiac Sodium Channel |
|
[43] |
| BPA or BPA and E2 |
1 nmol/L | Adult Sprague Dawley rats’ hearts |
|
[47] |
| BPA | 1 nmol/L | Female rat ventricular myocytes |
|
[48] |
| BPA | 1–100 μmol/L | Mouse cardiac myocytes |
|
[38] |
| BPA membrane-impermeant BPA-monosulfate (BPA-MS) |
100 µmol/L | AD 293 cells expressing α or α + β1 subunits |
|
[36] |
| BPA | 1–100 μmol/L | HEK 293 cells transfected with CaV3.1-CaV3.3 |
|
[39] |
| BPA | 0.1 nmol/L−1–1 μmol/L | Female rat ventricular myocytes |
|
[40] |
| BPA | 0.001–100 µmol/L | Neonatal rat cardiomyocytes |
|
[54] |
| BPA | 0.001–100 µmol/L | A7R5 cells from rat aorta |
|
[37] |
| BPA | 100 µmol/L | Neonatal rat cardiomyocytes |
|
[50] |
| BPA | 1–100 µmol/L | Zebrafish larvae Zebrafish cell lines |
|
[49] |
| BPA and/or PFOS | 25 μmol/L for 14 days | Rat cardiomyocytes |
|
[52] |
| BPA | 0–10 µmol/L BPA for 24 h | Murine aortic ECs (MAECs) and H9c2 cells. |
|
[53] |
| BPA | 1–100 μmol/L | hiPSC-CM |
|
[41] |
| BPA Bisphenol S Bisphenol F |
0.0–100 µmol/L | hiPSC-CM |
|
[42] |
1 Legend: BPA—bisphenol A; Ca2+—calcium; hiPSC-CMs—human-induced pluripotent stem-cell-derived cardiomyocytes; PFOS—perfluorooctane sulfonate.
4.2. Ex Vivo Studies
A few ex vivo studies have shown exposure to BPA in the cardiovascular system. The first ex vivo study was performed in rat atria by Pant et al. in 2011 [55]. The authors analyzed the direct action of BPA (0.1–100 μmol/L) on rat atria and demonstrated that BPA decreases the contractility of beating atria and decreases the rate and force of atrial contractions due to the activation of the NO–guanylyl cyclase pathway [55]. The Posnack group shows that exposure to higher concentrations of BPA (0.1–100 µmol/L) could decrease the rate and force of contractility and cardiac conduction velocity in the hearts of female rats [30] and, to a lesser extent, in the male heart [54]. More specifically, in 2014, it was first demonstrated, in whole hearts of adult female rats, that exposure to BPA adversely affected cardiac electrical conduction in a concentration-dependent manner. In this way, the authors showed that BPA exposure, after acute exposure (≤15 min), results in atrioventricular conduction delay, confirmed by the longer PR segment times and by the decrease in epicardial conduction. Exposure to the highest concentration of BPA resulted in longer QRS breaks and softened heartbeats, resulting in a complete blockage of the heart [30]. In 2015, the Posnack group observed, in female hearts, that during sinus rhythm, BPA exposure decreased left ventricular pressure and contractility activity (inotropic effect) in a dose-dependent manner. In male hearts, BPA exposure also modulated contractile performance, but to a lesser extent. Moreover, the study also showed that BPA exposure (0.001–100 µmol/L) modulated Ca2+ handling by reducing diastolic and systolic Ca2+ [54]. Therefore, the authors concluded that if these results were transposed to live experiments (human exposure), individuals, after chronic exposure, would be expected to show cardiac conduction abnormalities (i.e., bundle branch block, bradycardia, or arrhythmia).
In 2018, Feiteiro et al. performed a study in an organ bath to observe the vasorelaxant effect of BPA in rat aorta rings devoid of endothelium with noradrenaline (1 µmol/L) and potassium chloride (60 mmol/L). Afterward, cumulative concentrations of BPA (0.001–100 µmol/L) were administered to aortic rings, and it was observed that BPA induces rapid and concentration-dependent relaxation. In summary, the authors suggested that BPA inhibits the L-type Ca2+ channels, resulting in the relaxation of vascular smooth muscle. This non-genomic effect is similar to that observed for estradiol and other sex hormones in the same samples [37]. Thus, the authors proved that BPA also modulates the regulation of the vascular smooth muscle, which is essential for vasoreactivity, and may be involved in some cardiovascular diseases such as hypertension and coronary artery disease.
More recently, in 2021, Filice et al. performed ex vivo experiments on excised goldfish hearts. This research group showed that BPA affects the fish heart by inducing time- and concentration-dependent damage. First, they observed that the spontaneous heart rate was unaffected by BPA at 10 µmol/L, but for 25 µmol/L, a significant decrease was verified; this suggests a concentration-dependent chronotropic effect. Moreover, this previous response was also time-dependent, since the effect on animals exposed for 10 days was more pronounced than on those who were exposed for 4 days. In the hearts of goldfish treated with 10 µmol/L BPA, those exposed to 25 µmol/L required a higher preload pressure to achieve the physiological baseline cardiac output, leading the authors to suspect a detrimental effect of the BPA on basal performance [56].
In summary, the ex vivo experiments seem to corroborate the in vitro experiments, especially regarding the association between exposure to BPA and the development of cardiac arrhythmias and hypertension.
4.3. In Vivo Studies
Only a few in vivo mammalian studies have been performed regarding the effect of this EDC on the cardiovascular system. These studies were conducted on rodent and fish models. To simplify the understanding of the studies, we will first address all the studies on rats, chronologically and then those on fish, also chronologically.
The first study performed in vivo was in 2013, where Patel et al. demonstrated, in mice, that long-term exposure to BPA causes increased protein expression of DNMT3a (DNA methyltransferase 3a), and changes in patterns of protein expression and cardiac structure and function, as well as BP [57]. The authors also found a reduction in kidney weight, which might suggest that early exposure to BPA may impair kidney development in male mice. Moreover, the authors also identified differences between male and female rats after BPA exposure, such as concentric remodulation in males, and increased diastolic BP in all females. Some proteins that are essential for Ca2+ homeostasis, such as sodium Ca2+ exchanger-1, PLB, phospho-PLB, and calsequestrin 2, were changed in terms of quantification. Alterations in their expression support increased Ca2+ mobility in males and reduced Ca2+ mobility in females, becoming evidence of cardiac function changes. Finally, DNMT3a expression was increased in all BPA males and females who were given 0.5 µg/kg/day of BPA, and reduced in those females who received 200 µg/kg/day. These changes are suggestive of BPA-directed alterations detected in reproductive tissues and are also targeted in heart tissues [57].
In the next year, Saura et al. used CD1 mice orally administered BPA in their drinking water (4 nmol/L to 400 μmol/L). The authors showed that BPA induces high BP through Ang-II-mediated CaMKII-α uncoupling of eNOS and impairs carotid relaxation in mice. These data suggested that Ang-II-induced activation of CaMKII-α can play a key role in the endothelium dysfunction induced by BPA [58]. In the same year, Kim et al. investigated whether BPA (50 μg/kg body weight/day—12 weeks) would induce atherosclerosis, and; for this, the authors used an animal model of atherosclerosis, apolipoprotein E knockout (ApoE−/−) mice. The data suggested that an increase in non-HDL cholesterol levels may be a major contributing factor to BPA-induced atherosclerosis. The study also showed that expression of TNF-α and IL-6 in the aorta increased, but the serum levels of those inflammatory cytokines, which are the main inductor for atherosclerosis, were not changed [59].
In 2015, BP was studied by Belcher et al. and they showed that exposure to BPA resulted in decreased systolic and mean atrial pressures (MAP) in both male and female mice. Moreover, males exposed to BPA above 5 µg/kg/d presented a significant decrease in systolic BP and MAP, and in female rats, a significant decrease was only observed from the highest BPA exposure group, 300 µg/kg/d. Furthermore, in the same study, changes in the composition of the extracellular matrix of collagen were observed; these consisted of an accumulation of collagen in the heart, which may induce cardiac remodeling and abnormal fibrosis. The authors also performed transcriptome analysis and showed that BPA exposure can induce sex-specific alterations in gene expression, which indicated dysregulation of the collagen extracellular matrix and altered lipid metabolism of the rat heart. Thus, this study allowed us to conclude that BPA presents negative effects at the cardiac level, especially in response to cardiac ischemia [60].
In addition to the effects of BPA on Ca2+ handling, this compound may also have effects on some parameters of oxidative stress, such as NO. With regard to this, in 2015, the research group of Aboul-Ezz et al. studied the effect of BPA exposure, in rats that received a daily oral administration of BPA (25 mg/kg for 6 weeks and 10 mg/kg for 6 and 10 weeks), on the NO levels of male albino rats. The adverse effects of BPA on rat hearts were mainly due to the production of reactive oxygen species. In addition, it is postulated that the decreased level of NO and reduction in antioxidant defenses of the heart may result in vasoconstriction, which may lead to a decreased blood supply to the cardiac tissue and, ultimately, to a state of myocardial ischemia [61].
In the same year, 2015, Patel et al. performed a study to analyze whether BPA exposure (25 ng/mL−5 µg BPA/kg BW/day) is involved in cardiovascular remodeling. For this, C57bl/6n male mice were chronically exposed to BPA and myocardial infarction was induced in the mice. The data showed that chronic BPA exposure reduces remodeling after myocardial infarction by increasing monocyte and macrophage inflammation and reducing myofibroblast repair function [62].
Concerning the effect of BPA on hypertrophy in the NCTR Sprague Dawley rat, the first study to analyze the BPA effect in vivo was performed by Gear et al. in 2017 [63]. Rodent progressive cardiomyopathy is a common background lesion of undetermined etiology, and even though it occurs in both genders, it affects mainly males. This injury is suspected to arise from a localized microvascular dysfunction, and the resulting lesions phenotypically progress from minor to extensive focal mononuclear cell infiltration, myocyte degeneration, and fibrosis [64]. The research work of Gear et al. observed the cardiomyopathy-like lesions in the sections used for the characterization of left ventricular wall thickness and fibrosis. In this study on female rats treated with BPA or EE at 21 days of age (PND21), cardiomyopathy incidence was increased compared to control females, and a significant increase in severity was found for BPA and EE groups which received 2.5, 250, or 25,000 µg of these compounds. When the researchers observed the PND90 at 6 months, 100% of control-sample males and females had cardiomyopathy from both the stop dose and the continuous dose. Remarkably, the greatest morphometric effects observed were due to the fact that the duration of the treatment caused changes in body weight and the accumulation of cardiac collagen. Exposure to BPA caused an increase in the incidence and severity of progressive cardiomyopathy in female rats at 21 days, and increased the severity of cardiomyopathy in both sexes at 90 days [63].
In the same year, Klint et al. used juvenile female Fischer 344 rats to study BPA exposure (5, 50, and 500 μg BPA/kg bodyweight/day) in the cardiovascular function markers and fructose in in vivo cardiac tissues. The markers analyzed were vascular endothelial growth factor (VEGF), eNOS, and angiotensin I-converting enzyme (ACE1), which are known as estrogen-responsive genes in cardiovascular cells and tissues. VEGF exerts its function by targeting VEGFR2 (VEGF receptor 2), in EC. The oral low-dose BPA exposure of rats from pre-adolescence to adulthood up-regulated the expression of genes that control angiogenesis (Vegf and Vegfr2), a gene related to vasoconstriction (Ace1), and a gene related to endothelial dysfunction (eNos) [65].
In 2018, Sui et al. performed a study to analyze the effect of perinatal BPA exposure on atherosclerosis development in offspring. To this end, they developed a mouse model—PXR-humanized apolipoprotein E-deficient (huPXR•ApoE−/−)—to study BPA’s atherogenic effect. The data showed that perinatal BPA exposure (50 mg/kg) impaired atherosclerosis in adult male huPXR•ApoE−/− offspring but had no effects on their control newborn rats. The BPA perinatal exposure did not modify the plasma lipid levels; nevertheless, it increased aortic and atherosclerotic lesional CD36 expression, potentially through pregnane X receptor-dependent epigenetic regulation [66].
In 2019, Bruno et al. demonstrated that exposure to high doses of BPA increases the risk of female mice suffering from myocarditis, an inflammatory heart disease, with an inverted-U dose–effect curve. The data also showed that exposure to BPA significantly increased inflammatory mediators such as CD4+ T cells, IFNγ, IL-17A, TLR4, caspase-1, and IL-1β in the heart. The increase in cardiac fibrosis compared to the controls was also shown. To analyze the effect of BPA in cardiac remodeling, the effect of BPA in the mast cells was also analyzed, and an increase in the number and degranulation of these cells was observed, mainly in the pericardium. In summary, it was demonstrated that BPA exposure (0.5, 5, and 50 µg BPA/kg body weight) increases the risk of viral pericarditis, an inflammation of the pericardial layers, due to mast cell degranulation [67].
More recently, Reventun et al., in 2020, exposed mice to a more prolonged exposure; they were orally exposed to 4 × 10−5 mol/L of BPA in their drinking water for 16 weeks. The authors observed that BPA induces an increased heart rate, prolonged PQ interval, PR segment, and impaired cardiac contractility. Moreover, and as expected, BPA increased systolic and diastolic BP after 4 weeks, which was further elevated at 16 weeks [53]. In the same year, Zhou et al. demonstrated the effect of the exposure of pregnant rats to BPA, PFOS, or their combination for 19 days on fetal hearts. The authors observed that BPA alone, and in combination with PFOS, induced an increase in septal thickness in the ventricular tissue, and also increased myocardial collagen content. Additionally, these in vivo experiments’ results were confirmed by in vitro experiments, and the thickening of the ventricular septum may be related to the effects of this mixture exposure on mitochondrial metabolism [52].
The zebrafish (Danio rerio) model has been used on studies to assess cardiovascular development and function [68,69,70]. Indeed, although the zebrafish heart is composed of a single ventricle, the cardiac electrophysiologic system and characteristics are similar to that of a four-chambered vertebrate [70]. Furthermore, zebrafish have a high homology of thyroid hormone signaling with mammals, enabling extrapolation to human health effects and the extensive accumulated knowledge on their thyroid signaling pathways [71]. Moreover, the model is also very important to study environmental pollutants, mainly EDCs, since these models have three ERs (zfERα, zfERβ2, and zfERβ1) that are 50% homologous in their amino-acid sequence identity to the human ER [72]. Therefore, by studying the implications of exposure to EDCs on the cardiovascular system of this model, we can extrapolate the observed adverse outcomes to human health.
In a study carried out on zebrafish embryos, Cypher et al. analyzed the cardiovascular response during early development and discovered that it was altered by the presence of BPA and hypoxia (0.25, 1 and 5 mg/L and 1.0 mg O2/L). The results showed that all the cardiovascular parameters analyzed, except for venous diameter, reduced more during exposure to BPA and hypoxia together than to BPA and hypoxia alone. This joint effect was synergistically superior, and there was an interaction between both parameters. Thus, the authors demonstrated, for the first time, that BPA exposure modifies the cardiovascular system during hypoxia more so than during normoxia [73].
In the same year, 2015, in a study performed on adult zebrafish, Lombo et al. assessed the potential effects of BPA in paternal exposure on offspring development. Adult zebrafish males were exposed to BPA during spermatogenesis and reproduced with control females. The results demonstrated that exposure to BPA increases the rate of heart failure in the progeny to F2 and decreases the gene expression of cardiac development in F1 embryos. Furthermore, it was also observed that after exposure to 2000 µg/L BPA, an increased percentage of cardiac malformations occurred in future generations (F1 and F2), such as cardiac edema and incorrect looping, and showed disorganized heart walls [74]. Thus, this work allowed us to conclude, for the first time, that exposure to adult males may promote adverse cardiovascular effects in the following two generations.
Three years later, in 2018, Cypher et al. developed the study discussed above, and observed that co-exposure to BPA (0.001–100 µg/L) and hypoxia could interfere with cardiovascular function, and vascular parameters were the most affected, particularly at lower BPA concentrations. BPA and oxygen concentration interacted to affect vascular parameters, particularly arterial red-blood-cell velocity [75]. In the same year, another study investigated the estrogenic responses and estrogen receptor signaling mechanisms by which BPA and its metabolite 4-Methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene (MBP) act. The data showed that MBP in zebrafish has an effect several orders of magnitude greater than the effect observed for BPA. Furthermore, it was also demonstrated that the development of atrioventricular valves and bulbus arteriosus are major constituents in the heart, both for BPA and its metabolite. Estrogenic signal transduction for both bisphenols is mediated via an estrogen receptor 1-dependent pathway [76]. The following year, in 2019, the same research group demonstrated that exposure to BPA (100 and 1000 µg/L) and MBP (2.5 and 25 µg/L) activates the Estrogen Response Element (ERE) that acts primarily on heart valves. The integrity of the heart valves after exposure was compromised, with extra-cellular matrix collagen deficiency being the main reason for this, resulting in a modification of vascular function (reduced ventricular beat rate and blood flow) [77].
On the other hand, Pinto et al. demonstrated the estrogenic activity of BPA, BPAF, and BPC in zebrafish, and showed zfERα selectivity via the activation of the GFP reporter in the heart valves of zebrafish larvae. The authors also concluded that BPAF and BPC have a bigger affinity for zebrafish receptors than that observed by BPA [49].
Later, the same research group, Lombó and Herráez, observed that exposure to BPA during the early stages of development seriously affects the development of the heart. The exposure of BPA (2000 and 4000 µg/L) on embryos led to changes in cardiac phenotype; induced overexpression of hand2, a crucial factor for cardiomyocyte differentiation; increased the ER expression (esr2b); promoted an overexpression of histone acetyltransferase (kat6a); and also caused an increase in histone acetylation, estrogenic and epigenetic mechanisms; the latter are closely related, might act in synergy, and could be responsible for the upregulation observed in the transcription factor hand2, crucial for cardiac formation [78]. In the last year, in 2021, the same authors investigated the underlying molecular mechanisms of paternal exposure to BPA (100 and 2000 μg/L) to induce long-term effects on F1 cardiogenesis. The results showed that male exposure induces an increase in sperm histone acetylation (which is inherited by the F1) and, thus, alters the chromatin structure of the genes essential for heart development and those of the HAT in charge of maintaining the profile. Furthermore, when F1 embryos obtained from BPA-exposed males were treated with EGCG, histone acetylation levels were restored (which prevent ER overexpression and transcription factors) and, thus, reduced the likelihood of heart disease [79].
More recently, in 2022, Ji et al. studied the developmental vascular toxicity of BPA (0.25–12 mg L−1) and three predominant substitutes (BPF, BPS, and BPAF) in zebrafish embryos. They demonstrated that all drugs induce adverse effects on early vascular development. Moreover, BPAF showed the highest vascular toxicity, followed by BPF and BPA, while BPS exhibited the weakest toxicity [80].
Concerning the experiments with other fishes, a study on the role of BPA on tissue stress was performed by Filice et al. in the goldfish heart, by analyzing stress and pro-apoptotic markers, such as HSPs, Bax, and Cytochrome c, in cardiac extracts. The results showed that despite the unchanged expression of Hsp70 and Hsp90 in the presence of BPA 10 µmol/L, it significantly decreased in animals exposed to 25 µmol/L for the same period. However, they revealed the hypothesis that apoptosis is activated at low concentrations of the EDC; this is explained by the increased levels of the pro-apoptotic markers Bax and Cytochrome c which were detected after exposure at 10 µmol/L of BPA, but not at 25 µmol/L. This same study showed unchanged lipid peroxidation levels, increased OMP (oxidatively modified proteins) levels, and increased SOD (superoxide dismutase) activity in goldfish hearts exposed to 10 µmol/L, which suggests that in the presence of low BPA concentrations, increased SOD activity may contribute to counteracting lipid peroxidation. On the other hand, at high BPA concentrations, not only may lipid peroxidation and OMP decrease, but so may the activity of the antioxidant enzyme [56]. In 2022, Schönemann et al. showed that 7 days of exposure to 10 μg/L of the BPA metabolite, MBP, altered the liver proteome of male Cyprinodon variegatus fish. Furthermore, MBP enhanced ribosomal activity, protein synthesis, and transport, with upregulation of 91% of the ribosome-related proteins, and 12 proteins whose expression is regulated by ERE. Moreover, the acidic protein (WAP) was the protein most affected by MBP exposure, indicating that WAP may be a good new biomarker for xenoestrogens [81].
In conclusion, in vivo studies corroborate in vitro and ex vivo studies, and demonstrate that BPA and its metabolites can cause alterations in the cardiovascular system, leading to various pathologies such as higher BP, arrhythmias, atherosclerosis, endothelial dysfunction, cardiac ischemia, myocardial infarction, cardiomyopathy, myocarditis, congenital heart defects or cardiac anomalies, which may develop up to the second generation (F2). Further studies are, therefore, required to determine the exact mechanisms by which this bisphenol induces adverse cardiovascular effects. Summaries of the disruptive effects of BPA in the animal in vitro, ex vivo, and in vivo studies can be seen in Table 1, Table 2 and Table 3, respectively.
Table 2.
Summaries of the disruptive effects of BPA in animal ex vivo studies 1.
| Drugs | Concentration | Animals/Organs/Cells | Results | References |
|---|---|---|---|---|
| BPA | 0.1–100 μmol/L | Adult albino rats of Charles Foster strain |
|
[55] |
| BPA | 0.1–100 µmol/L | Sprague Dawley rat adult hearts |
|
[30] |
| BPA | 0.001–100 µmol/L | Sprague Dawley rat hearts |
|
[54] |
| BPA | 0.001–100 µmol/L | Male Wistar aorta rats |
|
[37] |
| BPA | 10 µmol/L and 25 µmol/L | Goldfish (C. auratus) adults hearts |
|
[56] |
1 Legend: BPA—bisphenol A; Ca2+—calcium.
Table 3.
Summaries of the disruptive effects of BPA in the animal in vivo studies 1.
| Drugs | Concentration | Animals/Organs/Cells | Results | References |
|---|---|---|---|---|
| BPA | 0.5, 5.0 and 200 µg/kg day | Rats |
|
[57] |
| BPA | 50 μg/kg body weight/day–12 weeks | ApoE−/− male mice |
|
[59] |
| BPA | 4 nmol/L–400 µmol/L | Mice CD1 |
|
[58] |
| BPA or EE |
0.15–5000 µg/kg/day | CD1 mice |
|
[60] |
| BPA | 25 mg/kg 10 mg/kg |
Adult male Wistar albino rats |
|
[61] |
| BPA | 25 ng/mL–5 µg BPA/kg BW/day | C57bl/6n mice |
|
[62] |
| BPA | 100 and 2000 µg/L | Zebra fish |
|
[74] |
| BPA and/or hipóxia | 0.25, 1 and 5 mg/L 1.0 mg O2/L |
Zebra fish embryos |
|
[73] |
| BPA or EE |
BPA (2.5–25,000 µg/kg day) EE (0.05 or 0.5 µg/kg/day) |
PND21, PND90, PND180 Sprague Dawley rat |
|
[63] |
| BPA | 5, 50, and 500 μg BPA/kg bodyweight/day | Juvenile female Fischer 344 rats |
|
[65] |
| BPA | 50 mg/kg | Adult PXR-Humanized Mice |
|
[66] |
| BPA | 0.1 and 1.0 mg/L | Zebrafish embryos |
|
[76] |
| BPA and/or hypoxia | 0.001–100 µg/L | Zebrafish larvae |
|
[75] |
| BPA | 0.5, 5, 50 µg BPA/kg body weight | BALB/c Mice |
|
[67] |
| BPA and/or EGCG | 2000 and 4000 µg/L BPA 50 and 100 µmol/L EGCG |
Zebrafish embryos |
|
[78] |
| BPA or metabolite MBP |
100 and 1000 µg/L 2.5 and 25 µg/L |
Embryo—larval zebrafish |
|
[77] |
| BPA | 1–100 µmol/L | Zebrafish larvae |
|
[49] |
| BPA | Orally exposed to 4 × 10−5 mol/L of BPA in drinking water for 4, 8, and 16 weeks | Wild-type CD1 mice |
|
[53] |
| BPA | 2 and 100 μg/L BPA | Pregnant rats |
|
[52] |
| BPA EGCG |
100 and 2000 μg/L BPA 50 µmol/L EGCG |
Zebrafish male |
|
[79] |
| BPA | BPA (0.25–12 mg L−1) | Zebrafish embryos |
|
[80] |
| BPA | 10 µmol/L and 25 µmol/L |
Goldfish (C. auratus) adult hearts |
|
[56] |
| BPA and metabolite MBP | 7 d exposure to 10 μg/L of BPA and MBP | Male Cyprinodon variegatus fish |
|
[81] |
1 Legend: BPA—bisphenol A; EE—17α- ethinylestradiol; EGCG—epigallocatechin gallate; MBP—4-Methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene.
5. Effects of BPA on Humans
Several studies have shown that there is an increased risk of premature CVDs positively associated with environmental factors, such as exposure to EDCs [31]. Environmental exposure to BPA appears to be harmful to human health; however, there are still few studies assessing the toxicity of this EDC in humans. This poses some concern because BPA has a potentially disruptive effect, but also because exposure to this compound is ubiquitous [82]. Additionally, BPA’s ability to cause adverse effects on humans is well documented in epidemiological studies, supporting a positive association between higher exposure to BPA and an increased risk of CVDs or risk factors for them. Therefore, this section will address the main disrupting effects of BPA described in humans through in vitro studies (namely on ion channels and electrophysiology, Ca2+ handling, vascular endothelium, and pregnancy exposome) and the epidemiological evidence supporting a relationship between BPA and CVDs (including risk factors and pregnancy exposure) (please see below).
5.1. In Vitro Studies
5.1.1. Effects of BPA on Ion Channels and Electrophysiology
Chemical exposure to BPA has been associated with changes in cardiac excitability, notably through changes in HRV and/or electrical conduction [27]. As recently reviewed by Cooper and Posnack [27] and Ramadan et al. [6] several studies have shown that BPA has an inhibitory effect on individual ion channels, with an important role in the regulation of cardiac action potentials.
As heart toxicity may derive from modified cardiac electrophysiology, O’Reilly et al., in 2012, investigated the interaction between BPA and the human Nav1.5 channel (the predominant voltage-gated Na+ channel subtype expressed in the human heart and responsible for the action potential upstroke). The electrophysiology results in the HEK-transfected cell line showed that BPA (1–100 µmol/L) blocks the channel (Kd = 25.4 ± 1.3 µmol/L). Docking predictions suggested that BPA-induced blockage involves the local anesthetic receptor and may enter the closed-state pore via membrane-located side fenestrations, possibly via a cavity delimited by F1760 and contiguous with the DIII–IV pore fenestration [43]. In cardiac tissue, this effect on Na+ channel currents will reduce the rate of depolarization and slow cardiac conduction velocity [6]. According to these authors, Prudencio et al. also recently reported, in 2021, that BPA has a half-maximal inhibitory concentration (IC50) of 55.3 µmol/L and 23.6 µmol/L BPA for fast/peak and late Na+ channel currents, respectively, using the same cell type [42]. In the same year, Ae Hyun et al. also demonstrated that BPA (1–100 µmol/L) significantly inhibited sodium current (INa) channels (IC50 = 56.5 µmol/L) [41]. Using both optical and microelectrode array methodologies, the authors suggested that BPA exposure altered cardiac function in human-induced pluripotent stem-cell-derived cardiomyocytes (hiPSC-CMs), as a slowing of the action potential upstroke (1–100 µmol/L BPA) and a reduction in the action potential amplitude (30–100 µmol/L BPA) were observed. These alterations may be due to the inhibition of INa channels [41]. As recently reviewed by Horváth et al., the late sodium currents’ (INa, late) inhibitors are potential antiarrhythmic agents. Increased late INa seems to play an important pathophysiological role in cardiac diseases, including rhythm disorders. Regarding the BPA-induced blockade, as the late INa channels are active during the plateau action potential phase, it is expected that this EDC shortens the repolarization time and, in turn, reduces the ICa channels [44].
In this sense, some studies have also shown that BPA can inhibit ICa. In 2013, Deutschmann et al. reported that BPA (1–100 µmol/L) acts as a potent blocker of voltage-activated Ca2+ channels. Briefly, the authors determined the mechanisms of blockage and the structural elements of BPA essential for its action, and verified that BPA rapidly and reversibly inhibited the recombinant human R-type Ca2+ channels expressed in HEK 293 cells. BPA binding to the channel occured in the extracellular part (outside the pore formation region), not involving intracellular signaling pathways. Moreover, this binding was voltage independent and did not affect channel gating, indicating that binding occurs with the channel in its resting state [38]. On the other hand, as T-type Ca2+ channels are important regulatory elements in the cardiovascular system, in the same year, Michaela et al. also performed electrophysiological studies to evaluate the effects of BPA on these channels in HEK 293 cells. The authors observed a concentration-dependent inhibition of T-type Ca2+ channels; for nanomolar concentrations of BPA, they observed inhibition of the channels (order of efficiency: CaV3.2 ≥ CaV3.1 > CaV3.3) without affecting the voltage dependence and kinetics of channel gating. However, BPA at micromolar concentrations accelerated the current-decay kinetics, shifted the voltage dependence of steady-state inactivation to more negative values, and inhibited the current amplitudes. Thus, the authors suggested that BPA (1–100 μmol/L) appears to act as a modifier of channel gating and directly plugs the pores of the conductive channel at high concentrations. The concentration range in which BPA-induced inhibition was observed corresponds to concentrations detected in human fluids; therefore, it may be relevant for the evaluation of the effects of this EDC on cardiovascular health, not least because T-type Ca2+ channels are expressed in nodal and conduction cells [39]. Regarding L-type Ca2+ channels, in 2021, Ae Hyun et al. also demonstrated that BPA dose-dependently inhibited ICa channels in hiPSC-CMs (IC50 = 6.9 µmol/L). These results seem to suggest that BPA can lead to cardiac dysfunction and cardiac risk factors (e.g., arrhythmias) [41]. In this context, it is expected that the effects of BPA on cardiac physiology are mediated by a Ca2+-dependent mechanism, given the importance that these channels have both for cardiac excitability and contractility. Moreover, due to the inhibitory effects that BPA exerts on L-type Ca2+ channels, and given the role of these channels in the plateau phase of action potentials, it is expected that BPA can also reduce the duration of the action potential. Concordantly, Ae Hyun et al. also revealed that acute exposure to BPA can shorten the optic action potential (10–100 µmol/L) [41]. Moreover, BPA can also exert effects on other ion channels; for example, Asano et al. reported that 100 µmo/L of BPA increases the large-conductance Ca2+/voltage-sensitive K+ channel (Maxi-K) current in human coronary artery SMC [35].
In summary, and taken together, the literature agrees that BPA has a dose-dependent monotonic effect on voltage-gated Na+ and Ca2+ channels [27,83], impairing cardiac electrophysiology (please see Table 4). Scientific evidence agrees that BPA has effects on Ca2+ currents, directly impairing cardiac automaticity and electrical conduction. However, further studies are needed to clarify whether these effects are sex-specific and what the dose–response relationships are with the application of BPA alone or in combination with other bisphenols. Furthermore, it is still important to clarify whether the effects induced by BPA are direct or involve other intracellular signaling pathways, as well as to unveil what role this EDC plays in cardiac development [6].
Table 4.
Summaries of the disruptive effects of BPA in in vitro studies using human cell lines 1.
| Topic | Studied Mechanism | Concentration | Type of Cells | Observed Effects | References |
|---|---|---|---|---|---|
| Ion channels and electrophysiology | Nav1.5 channels | 1–100 µmol/L | HEK-transfected cell line |
|
[43] |
| Nav1.5 channels | 0.0–100 µmol/L | HEK-transfected cell line |
|
[42] | |
| Nav1.5 channels | 1–100 µmol/L | hiPSC-CMs |
|
[41] | |
| Recombinant human R-type Ca2+ channels | 1–100 µmol/L | HEK 293 cells |
|
[38] | |
| T-type Ca2+ channels | 1–100 μmol/L | HEK 293 cells |
|
[39] | |
| L-type Ca2+ channels Cav1.2 | 1–100 µmol/L | hiPSC-CMs |
|
[41] | |
| Maxi-K channels | 100 µmol/L | HCASMC |
|
[35] | |
| Ca2+ handling | Ca2+ current channels, Ca2+ transients and contraction | 1–100 µmol/L | hiPSC-CMs |
|
[41] |
| Cardiac hypertrophy by disrupting Ca2+ homeostasis | 8 ng/mL | Human embryonic stem-cell-derived cardiomyocytes |
|
[86] | |
| Vascular endothelium | Endothelial dysfunction, inflammation, and angiogenesis | 0.1–1 μmol/L | HUVECs |
|
[88] |
| Cell division and chromosomal segregation | 0.5–10 ng/mL | HUVECs |
|
[89] | |
| Senescence | 10 ng/mL and 1 µg/mL | HUVECs |
|
[90] | |
| Accelerating atherosclerosis | 0.1–10 nmol/L | HUVECs |
|
[59] | |
| Pregnancy exposome | Epigenetic disruption | 35.4–56.1 ng/g | Human fetal liver samples |
|
[91] |
| 0.57 and 0.78 ng/mL | Maternal urine samples and Infant cord blood |
|
[92] | ||
| Pregnancy physiology | 1 nmol/L | BeWo trophoblast cell line, placental explant cultures, placental perfusions, and skin diffusion models |
|
[95] | |
| 0, 0.09, 0.9, and 9.0 μmol/L | BeWo trophoblast cell line |
|
[96] | ||
| 1 × 10−15 to 1 × 10−7 mol/L | Human trophoblast cells HTR-8/SVneo |
|
[97] | ||
| 1 nmol/L | Human trophoblast cells HTR-8/SVneo |
|
[98] |
1 Legend: BPA—bisphenol A; CnAβ—calcineurin; DRP1—dynamin-related protein 1; HCASMC—human coronary artery smooth muscle cells; HEK—human embryonic kidney; hiPSC-CMs–human-induced pluripotent stem-cell-derived cardiomyocytes; HUVECs—human umbilical vein endothelial cells; IC50—half-maximal inhibitory concentration; LINE-1—long interspersed nuclear element-1 or L1; Maxi-K—large conductance Ca2+/voltage-sensitive K+ channel; XME—xenobiotic-metabolizing enzymes.
5.1.2. Effects of BPA on Ca2+ Handling
Some research on rodent models has demonstrated the effects that BPA exposure has on intracellular Ca2+ handling, contractility, and relaxation, as previously described in Section 4. Most studies associate these effects with the inhibition of ionic currents and/or phosphorylation of key regulatory proteins induced by BPA [27], not least because BPA can inhibit Ca2+ ion influx through interaction with voltage-gated Ca2+ channels. Thus, and since cardiomyocyte contractility is proportional to the magnitude of this slow inward current, BPA may act as a negative inotropic agent [27,84].
In humans, BPA exposure has also been associated with alterations in both Ca2+ release and/or sequestration back to the sarcoplasmic reticulum [85]. With regard to this, Ae Hyun et al. demonstrated that BPA dose-dependently inhibited ICa channels, Ca2+ transients, and contraction in hiPSC-CMs. The results showed that acute exposure to BPA (1–100 µmol/L) in a dose-dependent manner slows the Ca2+ transient rise time and decreases the Ca2+ transient amplitude of hiPSC-CMs [41]. In another perspective, Cheng et al. analyzed how BPA at low doses of BPA (equivalents to human internal exposure levels) could induce cardiac hypertrophy via the calcineurin (CnAβ)-dynamin-related protein 1 (DRP1) signaling pathway by disrupting Ca2+ homeostasis. Using human embryonic stem-cell-derived cardiomyocytes (XX and XY karyotypes), the authors discovered that BPA (8 ng/mL) significantly elevated hypertrophic-related mRNA expression levels, enhanced cellular area, and reduced ATP supplementation, evidencing a hypertrophic cardiomyocyte phenotype in vitro. Additionally, BPA-induced excessive fission was promoted via CnAβ-mediated dephosphorylation of DRP1. At the molecular level, the increase in cytosolic Ca2+ levels due to low doses of BPA could discriminate between karyotyped-derived cardiomyocytes. Thus, because the results are more prominent in XX-karyotyped cells, these results are suggestive that there exists a potential BPA-induced sex-specific hypertrophic risk in terms of abnormal mitochondrial fission and ATP production through the impairment of CnAβ-DRP1 signaling [86].
In summary, to our knowledge, these were the only two studies in human cells that evaluated the effects of BPA on Ca2+ handling (please see Table 4), and there are also no in vivo studies that address the effects of BPA on cardiac contractility. Nevertheless, the literature is in agreement with studies in rodent models, suggesting that human exposure to BPA also has disruptive effects on intracellular Ca2+ handling. Further studies are needed to clarify the role of this EDC on cardiac contractility at this point.
5.1.3. Effects on Vascular Endothelium
Several investigations have suggested that urinary BPA levels are associated with the pathogenesis of age-related pathologies (where CVDs is included). Vascular endothelium dysfunction may be implicated in CVDs [87], including atherosclerosis. The literature describes that there exists an association between elevated exposure to BPA and CVDs; however, little is known about the effects of BPA on the human endothelium. With regard to this, Andersson and Brittebo (2012) were the first authors to investigate the effects of BPA (0.1 nmol/L–1 μmol/L) on selected biomarkers of endothelial dysfunction, inflammation, and angiogenesis in human umbilical vein endothelial cells (HUVECs). The authors demonstrated that BPA (≤1 μmol/L) increased the mRNA expression of the proangiogenic genes (VEGFR-2, VEGF-A, eNOS, and Cx43) and increased NO production in HUVECs. Moreover, the results also showed that BPA increased the expression of phosphorylated eNOS and endothelial tube formation in the cells. Thus, the authors suggested that exposure to BPA has direct proangiogenic effects on human primary EC in vitro and, more importantly, human endothelium may be an important target for BPA [88]. Ribeiro-Varandas et al. also demonstrated that BPA at plasma concentrations (0.5–10 ng/mL) induces aneugenic effects on EC. The authors found alterations in micronucleus formation and cell division processes (leading to mitotic abnormalities), as well as positive regulation of the genes of encoded proteins associated with chromosomal segregation [89]. In the following year (2014), the same authors also reported that BPA (10 ng/mL and 1 µg/mL) impairs transcription and decreases viability in aging vascular EC. The authors suggested that BPA is associated with the etiology of age-related human pathologies, such as atherosclerosis, by interfering with senescence in primary vascular EC [90]. Kim et al. also demonstrated that BPA (0.1–10 nmol/L) appears to be involved in accelerating atherosclerosis but found no changes in HUVEC proliferation or migration [59].
Taken together, these findings suggest that BPA exposure may induce endothelial dysfunction, promoting the development of age-related diseases such as atherosclerosis (please see Table 4). Further studies are needed to clarify the adverse effects adjacent to BPA exposure and the mechanisms of toxicity by which the vascular endothelium is impaired. In this way, better targeting in clinical practice will be achieved.
5.1.4. Effects in Pregnancy Exposome
In recent years, there has been increased interest in the endocrine-disrupting effects of BPA on human pregnancy and fetal development. As recently reviewed by Lorigo and Cairrao, several studies have reported the presence of BPA in various biological matrices important in pregnancy (including maternal blood and urine, amniotic liquid, placenta, umbilical cord blood, breast milk, and human colostrum) [51]. Usually, detected concentrations of BPA are higher in urine and, therefore, urine is preferentially the most-used biological sample (not least because BPA is a non-persistent compound) [6,82]. Nevertheless, different analytical techniques have been performed to detect BPA in human samples [7].
Worryingly, concerning pregnancy, studies have shown that BPA can not only penetrate, but also accumulate, in the human placenta, with studies even demonstrating higher levels of this EDC in the placenta than in maternal plasma [6]. Consequently, this maternal–fetal transfer may be one of the causes of BPA-induced cardiovascular disorders in adulthood [51].
Indeed, BPA is one of the most-studied EDCs, resulting in epigenetic disruption, including changes in DNA methylation, acetylation, genomic imprinting, and modifications in the expression of microRNAs and non-coding RNAs [51]. These changes are particularly important in those who are pregnant, a susceptible populational group, as the fetus has a high rate of DNA synthesis. In 2014, Nahar et al. investigated the hypothesis that in utero exposure to BPA influences the expression and epigenetic regulation of phase I and II xenobiotic-metabolizing enzymes (XME) genes during development. The authors found an association of higher levels of BPA (35.4–56.1 ng/g) with significantly reduced expression of XME genes, and with increased site-specific methylation at COMT and increased average methylation at SULT2A1 promoters [91]. Later, in 2018, Montrose et al. also investigated whether maternal exposure to BPA (0.57 and 0.78 ng/mL) in the first trimester of pregnancy is associated with infant cord blood DNA methylation. The authors found decreases in the methylation of imprinted (H19, IGF2) and unprinted (PPARA, ESR1) genes and of repetitive element LINE-1 (long interspersed nuclear element-1 or L1), with increasing BPA concentrations [92]. Overall, it seems that BPA accumulation in the maternal placenta is responsible for global methylation, which may lead to fetal growth retardation [93,94].
In addition to BPA-induced epigenetic changes, studies are reporting a direct effect on pregnancy physiology. In 2010, Mørck et al. conducted studies in the BeWo trophoblast cell line, placental explant cultures, placental perfusions, and skin diffusion models, all of human origin, to assess the effects of BPA exposure during pregnancy. The authors demonstrated that there is BPA cytotoxicity in these cells (EC50 = 100–125 µmol/L). BPA exposure (1 nmol/L) significantly increased β-hCG secretion and caspase-3 expression in placental explants. A rapid transfer of this EDC through the term placentae and the BeWo cell monolayer was also observed, as well as transdermal transport. Thus, these results seem to indicate that placental transfer of BPA (1 nmol/L) to the fetus occurs, with potentially adverse effects on both placental and fetal development [95]. Concordantly, and using the same cells, Ponniah et al. also demonstrated, in 2015, that BPA exposure (0–9.0 μmol/L) may have implications on placental trophoblasts during development, as this EDC induced trophoblast cell death under conditions of cellular stress [96]. Additionally, in the same year, Spagnoletti et al. evaluated the effect of BPA (1 × 10−15 to 1 × 10−7 mol/L) on the main physiological processes which characterize the extravillous trophoblast in human trophoblast cells HTR-8/SVneo. The results showed that BPA acts on these cells, altering key physiological processes in placenta development. Although the exact mechanism by which BPA acts on human trophoblasts needs further clarification, this study showed that there are reductions in the processes of cell migration and invasion as well as a differentiation of HTR-8/SVneo towards polyploidy via the process of endoreduplication induced by BPA [97]. More recently, in 2018, Basak et al. also demonstrated that low concentrations of BPA (1 nmol/L) can not only affect cellular growth and development and angiogenic activities, but also, concordantly with previous studies, induce alterations in DNA methylation of the stress response and down-regulation of angiogenic growth factors. These alterations were observed using HTR8/SVneo cells, during the first trimester of pregnancy [98].
Therefore, the mentioned studies are concordant in their statements that exposure to BPA plays a determinant role in pregnancy physiology. Additionally, exposure to BPA has also been associated with adverse placental outcomes v multiple mechanisms of action (MOAs) [99,100,101]. Consequently, BPA exposure may also induce several fetal and obstetric outcomes. Risk of increased pregnancy loss, longer pregnancies or preterm birth, metabolic dysfunction, altered somatometric parameters, and newborn weight are some of the demonstrated outcomes (please see reviews [100,102]).
In summary, all the studies agree that BPA exposure plays a determining role in pregnancy, and consequently, may increase susceptibility to CVDs in later life (please see Table 4). In addition to BPA-induced epigenetic changes, this EDC also directly affects the physiology of pregnancy, which, taken together, highlight the need for future studies to assess the risk of BPA-induced reproductive toxicity.
5.2. Epidemiological Studies
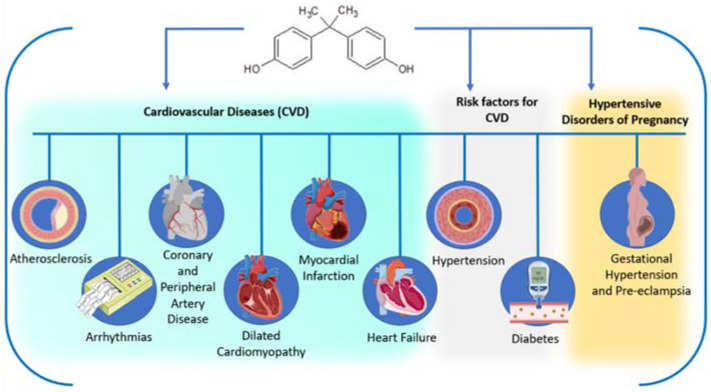
Several epidemiological investigations have been developed to assess the cardiotoxic effects of BPA. The evidence is consistent, reporting that there is an association between exposure to BPA and an increased prevalence of CVDs [103,104] including atherosclerosis [105], coronary artery disease (CAD) [104,106,107,108,109], peripheral artery disease (PAD) [110], dilated cardiomyopathy (DCM) [109], and myocardial infarction (MI) [104,111]; heart failure; and angina pectoris [104] and its risk factors, such as hypertension [112,113,114,115] and diabetes [103,108] (Figure 2). There is a multiplicity of different analytical techniques to quantify BPA concentrations in human tissues. However, due to their short half-lives (<24 h), the concentration of this EDC is measured preferentially in urine, the matrix where the concentration detected is higher (the concentration ranges from 1.6 to 946 μg/L) [51]. Regarding the association between BPA and adverse effects on the cardiovascular system, the maximum mean concentration detected was 4.66 ng/mL. Furthermore, exposure to BPA is also particularly harmful in more sensitive windows of development, such as pregnancy, which are, in these cases, related to the development of hypertensive disorders of pregnancy (HDPs). The serum range levels detected in pregnant women are from 0.2 to 20 ng/mL. and in placental tissue the concentration detected was much higher, at around 100 ng/g. In urine samples, the concentration ranged from 0.87 to 31.9 μg/L [51,116] for the pregnant women, and 1.65 ng/mL when there was an association between urinary BPA concentrations and CVDs (Table 5). With regard to this, epidemiological studies reporting the effects of BPA exposure and its association with CVDs and risk factors, as well as the implications of exposure during pregnancy, will be discussed in detail in the next two sections.
Figure 2.
Summary representation of the endocrine-disrupting effects of bisphenol A on the cardiovascular system.
Table 5.
Summaries of the disruptive effects of BPA in human epidemiological studies 1.
| Topic | Studied Population | Gender | Concentration | Observed Effects | References |
|---|---|---|---|---|---|
| Cardiovascular diseases | NHANES from 2003–2004 1455 adults aged 18 through 74 years |
694 men 761 women |
4.53 ng/mL (urinary) 4.66 ng/mL (urinary) |
|
[103] |
| NHANES 2003–2006 data, separately between 2003/2004 and 2005/2006 and pooled n = 1455 (2003/04) and n = 1493 (2005/06) adults aged 18–74 years |
694 men and 761 women in 2003/04 720 men and 773 women in 2005/06 |
2.49 ng/mL (urinary) 1.79 ng/mL (urinary) |
|
[108] | |
| Population-based Prospective Investigation of the Vasculature in Uppsala Seniors study (1016 subjects all aged 70) | 510 women and 506 men | 3.76 ng/mL (serum) |
|
[105] | |
| 758 incident CAD cases and 861 controls followed for 10.8 years from the European Prospective Investigation of Cancer—Norfolk, UK | 534 men and 327 women in control group 501 men and 257 women in CAD group |
Control vs. CAD group (1.24 ng/mL vs. 1.35 ng/mL) (urinary) |
|
[106] | |
| 591 patients participating in The Metabonomics and Genomics in Coronary Artery Disease study in Cambridgeshire, UK | 120 controls (62 women and 58 men) 385 patients (301 men and 84 women) 86 Intermediate diseases (43 men and 43 women) |
Control vs. CAD vs. intermediate (2.13 vs. 3.82 vs. 3.31 ng/mL) (urinary) |
|
[107] | |
| 745 participants in the NHANES 2003–2004 | 361 women and 392 men | 2.30 ng/mL (urinary) |
|
[110] | |
| 88 DCM patients and 88 age-and gender-matched healthy controls | 59 men and 29 women with DCM 55 men and 33 women Control |
DCM vs. control group (6.9 ± 2.7 vs. 3.8 ± 1.9 ng/mL) (serum) |
|
[109] | |
| NHANES 2003–2014 (n = 9139, aged ≥20 years) | 4467 men and 4672 women | - |
|
[104] | |
| Hypertension | 1380 subjects from NHANES 2003–2004 | 700 women and 680 men (580 with hypertension) |
1.5–4.0 ng/mL (urinary) |
|
[112] |
| 560 noninstitutionalized elderly citizens from August 2008 to August 2010 in Seoul from Korean Elderly Environmental Panel Study | 521 participants were included (138 men and 383 women) | (men) and 1.3 (women) μg/g of creatinine (urinary) |
|
[113] | |
| 60 noninstitutionalized elderly participants, who were aged ≥60 years between February 2014 and March 2014 | 60 participants, 56 were women and 4 male | 1.13 ± 1.76 μg/L (urinary) |
|
[114] | |
| A subsample of 2558 randomly selected from the Thai National Health Examination Survey IV, 2009 | 1275 men and 1283 women | 0.35 ng/mL (men) and 0.33 ng/mL (women) (serum) |
|
[115] | |
| Pregnancy exposure | 645 children at the age of 4 who were born from women who participated, midterm during their pregnancy, in a birth cohort study from August 2008 to July 2011 | 486 mother–child pairs were included in the present analysis | Maternal urinary: 0.9 μg/L (urinary) |
|
[121] |
| 152 female volunteer participants in the Human Early-Life Exposome project | 152 pregnant women | 3.1 μg/g creatinine (urinary) |
|
[120] | |
| 1064 mother-child pairs/childhood at a mean age of 9.7 years old | 1064 mother-child pais | 6.0 nmol/L (boys) and 7.2 nmol/L (girls) (urinary) |
|
[122] | |
| 58 pregnancies, including 35 normotensive and 23 preeclamptic women | 35 normotensive pregnant women and 23 preeclamptic pregnant women | Control vs. PE (3.00 vs. 2.80 ng/mL–maternal serum; 2.17 vs. 2.23 ng/mL–fetal serum; 3.00 vs. 9.40–placental homogenate) |
|
[124] | |
| A nested case-control population consisting of 130 mothers who delivered preterm and 352 who delivered term from a prospective birth cohort | 130 women and 352 controls | 7.08% change (adjusted with soluble fms-like tyrosine kinase-1) (urinary) |
|
[125] | |
| A nested case-control study of preterm birth was performed in 2011 from women enrolled in a prospective birth cohort study at Women’s Hospitals in Brigham and in Boston (included 50 cases of PE) | 482 women (50 with PE) | Cases vs. control (1.56 vs. 1.38 ng/mL) (urinary) |
|
[126] | |
| 1233 women excluding those without any BP measurement or with pre-existing hypertension | 1233 women | 1.65 ng/mL (urinary) |
|
[127] | |
| 1 year postpartum among 199 women in Mexico City | 199 women | 1.18 ng/mL (urinary) |
|
[129] |
1 Legend: BP—blood pressure; BPA—bisphenol A; CAD—coronary artery disease; CVDs—cardiovascular diseases; DCM—dilated cardiomyopathy; EDC—endocrine-disrupting compound; HRV—heart rate variability; MI—myocardial infarction; NHANES—National Health and Nutrition Examination Survey; PE—preeclampsia.
5.2.1. Cardiovascular Diseases and Risk Factors
Several epidemiological studies have been carried out, mostly from a National Health and Nutrition Examination Survey (NHANES) dataset. The assessment of cardiovascular parameters was performed using urine samples from the participants for whom BPA concentrations were analyzed [13]. The first study was conducted in 2008 by Lang et al., who analyzed data from NHANES from 2003 to 2004 and showed that there is an association between CVDs and elevated urinary BPA levels (OR = 1.39), after adjusting for the confounding factors age and sex. The authors also found a relationship between higher BPA concentrations and diabetes (OR = 1.39) [103]. On the other hand, a relationship between BPA exposure and PAD and/or CAD was also assessed and found. With regard to this, Melzer et al., in 2010, also analyzed BPA concentrations with heart diseases by analyzing NHANES 2003–2006 data, separately between 2003/2004 and 2005/2006, and pooled. The results of urinary BPA concentrations were smaller for the 2005/2006 data (mean 1.79 ng/mL) than those from 2003/3004 (mean 2.49 ng/mL). After adjustment for confounding factors, the authors found that there was an association between higher BPA levels (2003/3004 data) with CAD (OR = 1.33), but the same was not observed with diabetes. Contrarily, when data were pooled, an association between higher BPA concentrations and these diseases was found (OR = 1.42 for CAD and OR = 1.24 for diabetes) [108]. In summary, the authors verified that higher levels of urinary BPA were cross-sectionally associated with heart disease in NHANES 2003–2004 and NHANES 2005–2006, independent of traditional risk factors. Thus, and due to BPA levels having previously been associated with CAD (which is an atherosclerotic disease), Lind et al. also evaluated, in 2011, a possible relationship between exposure to BPA and atherosclerosis in a cross-sectional study. The results showed that elevated levels of BPA were related to the echogenicity of the plaques, suggesting a role for BPA in atherosclerosis [105]. One year later, Melzer et al. continued their previous studies, using data for 10.8 years from the European Prospective Investigation of Cancer—Norfolk, UK. The authors showed that urinary BPA concentrations were low (median value, 1.3 ng/mL) and that an increased BPA concentration was associated with incident CAD, after adjustment for the confounding factors age, sex, and urinary creatinine (OR = 1.13) [106]; similar trends were obtained to the previous study by the same authors [108]. Additionally, in the same year, but in a different study, Melzer et al. also studied the possible association between BPA exposure with grades of severity of CAD on angiography [107] in 591 patients participating in The Metabonomics and Genomics in Coronary Artery Disease study in Cambridgeshire, UK. The authors found that there was a higher urinary concentration of BPA in patients with severe CAD compared to patients with normal coronary arteries (OR = 1.43; 95% CI = 1.03–1.98, p = 0.033). Furthermore, for the case of patients with intermediate disease, significant differences were also almost obtained (OR = 1.69; 95% CI = 0.98–2.94, p = 0.061). Thus, it can be concluded that BPA exposure was associated with the severity of angiography-defined coronary artery stenosis and may contribute to a future risk of developing CAD in the adult population [107]. Taken together, these findings highlight the need for further studies to accurately estimate the prospective exposure–response curve and, thus, establish the underlying mechanisms [106,107].
Additionally, in 2012, another study by Shankar et al. examined the relationship between urinary BPA levels and peripheral arterial disease (PAD) (which is a subclinical measure of atherosclerotic vascular disease and a strong independent risk factor for CVDs and mortality) in a nationally representative sample of U.S. adults [110]. This study, which was conducted with participants from NHANES 2003–2004, also showed that there is a positive association between increased levels of BPA with DBP, independent of traditional CVD risk factors (OR = 2.69; 95% CI = 1.02–7.09; p = 0.01) [110]. Although they cannot provide definitive conclusions, these results suggest that environmental exposure to BPA may promote the development of PAD [110]. Additionally, in 2015, Xiong et al. performed a case-control study evaluating the concentrations of BPA in patients with DCM, and demonstrated higher levels of BPA in DCM patients compared with the healthy group (6.9 ± 2.7 ng/mL vs. 3.8 ± 1.9 ng/mL, p < 0.001), suggesting that BPA exposure may promote the development of this pathology [109]. On the other hand, if a positive association between BPA exposure and CAD has been shown, this has not been evaluated in patients with type 2 diabetes (T2D). With regard to this, in 2019, Hu et al. evaluated this possible relationship through two nested case-control studies in two independent European cohorts (SURDIAGENE and ESTHER). The authors demonstrated the exposure to BPA in the two studies (ESTHER cohort: 31% and SURDIAGENE cohort: 38%). The meta-analysis of the results showed that in booth cohorts, there exists a positive association between MI and the detection of BPA in urine (OR = 1.97; 95% CI = 1.05–3.70, p = 0.04) [111]. More recently, in 2020, Cai et al. evaluated the relationship between urinary BPA levels and CVDs in the U.S. adult population using data from NHANES 2003–2014. With this study, the authors demonstrated a positive association with heart failure, CAD, angina pectoris, MI, and CVDs, which was more evident in males [104].
On the other hand, hypertension is a risk factor for CVDs and has also been associated with high levels of BPA. Shankar and Teppala (2012) defined hypertension as “blood pressure-reducing medication use and/or blood pressures >140/90 mm of Hg” [112]. With regard to this, these authors analyzed the relationship between urinary levels of BPA and hypertension in a Multi-ethnic Sample of U.S. Adults. The study was also conducted with participants from NHANES 2003–2004. In agreement with the previous studies mentioned, the results demonstrated a positive association between elevated urinary BPA levels and hypertension, independent of traditional risk factors (OR = 1.50; 95% CI = 1.12–2.00, p = 0.007) [112]. Moreover, Bae et al., in 2012, investigated the associations of BPA exposure with HRV and blood pressure (BP) in elderly citizens in Seoul, and demonstrated that urinary BPA was associated negatively with HRV and positively with BP. Furthermore, the authors also found an association with hypertension (OR = 1.27; CI 95% = 0.85–1.88 [113]. Later, in 2015, the same authors also analyzed a possible association between exposure to BPA (from consumption of canned beverages) and BP and HRV. The main findings were that consuming canned beverages (and the consequent increase in BPA exposure) is related to BP increase, but differences in HRV were not found [114]. In the same year, Aekplakorn et al. also determined the association of serum BPA with hypertension in the Thai Population. The authors demonstrated that there is an association between serum BPA levels with hypertension in women (OR = 2.16; 95% CI = 1.31–3.56) [115].
From the studies mentioned above, it is clear that the NHANES dataset has been widely used to draw conclusions about BPA exposure and adverse cardiovascular health outcomes. However, in 2012, La Kind et al. questioned whether this approach is the most appropriate, especially for a chemical with a short physiological half-life such as BPA, and chronic diseases with multifactorial etiologies. With regard to this, analyzing four datasets (2003/04; 2005/06; 2007/08 and 2009/10) and selecting a priori consistent methods, the authors tried to clarify the association between urinary BPA concentrations and diabetes, CAD, and/or MI. After adjustment for confounding factors, the results showed no association between urinary BPA levels and heart disease and diabetes, thus, not supporting previous studies wherein different methods and definitions were used. Thus, the authors suggested that using the NHANES data to study the association of BPA with CVDs is not appropriate, and may lead to inconsistent results due to this discrepancy between methodologies. Further epidemiological studies should be conducted to toxicologically evaluate the role of BPA in the association with CVD development [117], as the experimental design of the epidemiological studies developed so far is constraining our clear comprehension of this topic [118]. In addition, Olsén et al. also assessed the associations between circulating levels of BPA and coronary risk in an elderly population [119] using the Framingham risk score to predict CVD risk. Contrary to previous studies, but corroborating the studies of La Kind et al. [117,118], the authors found no strong associations between BPA and FRS in the population, thus suggesting that BPA exposure is not a risk factor for CVDs [119].
In summary, the set of epidemiological studies presented suggests that BPA exposure seems to be related to the development of CVDs or risk factors for CVDs, such as hypertension. Most studies are conducted using NHANES data, evidencing that this causal relationship is consistent with higher urinary BPA levels. The causal relationship with diabetes has also been established. However, due to the discrepancy in methodologies performed (including exclusion criteria, definitions, and scoring systems) caution is needed in their comparative analysis.
5.2.2. Pregnancy Exposure
HDPs are one of the leading causes of maternofetal mortality and morbidity. Exposure to EDCs is suspected to increase BP, but few studies have investigated the impact of these chemicals on pregnant women [120]. Due to BPA being suspected to cross the placenta during pregnancy, possibly affecting children’s health, Bae et al. also analyzed maternal urinary concentrations of BPA during midterm pregnancy (around 20 weeks) and in the BP of children at age 4. The authors demonstrated that there exists a positive association between the diastolic (and not systolic) BP of the children with a maternal urinary concentration of BPA above a threshold level (4.5 μg/g creatinine) [121]. Moreover, Warembourg et al. also found that BPA exposure was associated with a significant decrease in systolic and/or diastolic BP during pregnancy (most frequently observed during the second trimester) [120]. Recently, in 2020, Sol et al. also evaluated the association between maternal BPA urine concentrations during pregnancy and BP in childhood in a population-based prospective cohort study. The authors demonstrated that this association is sex-dependent, as evidenced by higher BP in boys after fetal BPA exposure. However, these results highlight the need for further studies to unravel the underlying mechanisms and long-term outcomes [122].
According to the recently reviewed studies, BPAs (as well as phthalates) are the class of EDCs that appear to mostly harm the fetoplacental vasculature during pregnancy and have been associated with the development of pregnancy complications such as preeclampsia (PE) [51]. However, these specific exposure–response relationships are not yet fully established [123]. In 2014, Leclerc et al. were the first authors to demonstrate that there was an association between PE and BPA accumulation in the placenta. The authors found that placentas from women with PE had a higher accumulation of this EDC compared to placentas from normotensive women [124]. In the following year, Fergunson et al. also observed that there is an association between urinary BPA concentrations and angiogenic biomarkers during pregnancy, which may be indicative of this pathology through placental disruption development and/or function during gestation [125]. Concordantly, Spagnoletti et al. also demonstrated that BPA acts on trophoblasts and alters physiological processes involved in normal placental development [97], and Cantonwine et al. also reported that early pregnancy (~10 weeks gestation) appears to be a window of increased susceptibility for the development of this HDP associated with BPA exposure [126]. Furthermore, in 2017, Philips et al., in their findings, suggested that there is probably a greater association of bisphenols in early pregnancy with a risk of PE than with gestational hypertension (as no significant changes in BP were found) [127]. The same authors also recently described that early exposure to BPA during pregnancy appears to be related to changes in cardiometabolic health later in life [128].
Additionally, the postpartum period may be a vulnerable life stage for a woman’s cardiometabolic health [129]. According to the recent review by Lorigo and Cairrao, considering the postpartum period is equally as important as considering the pregnancy period when talking about endocrine disruption. The associations between elevated levels of EDCs in general, including BPA, and vascular changes in children or adults are not yet demonstrated; they require further study, because pregnancy and postpartum are both susceptible windows of endocrine exposure. Thus, further studies must be developed to clarify these mechanisms and, thus, understand whether the risk of CVDs is temporary or permanent in later life [51]. With regard to this, in 2020, Perng et al. analyzed the effect of BPA during pregnancy on weight from delivery through 1 year postpartum among 199 women in Mexico City. Through their prospective cohort study, the authors demonstrated that prenatal exposure to BPA is inversely associated with weight at delivery, but there exists a slower rate of weight loss through the first postpartum year [129]. However, to our knowledge, these authors were the only ones to evaluate the effects of BPA in the postpartum period.
Therefore, taken together, these studies indicate that there is an environmental contribution by EDCs in the development of HDPs; therefore, urgent action should be taken to decrease exposure to these emerging compounds to reduce the mortality and morbidity associated with these conditions.
6. Conclusions
The analysis of several studies of BPA-induced cardiotoxicity led us to conclude that exposure to this EDC is associated with an increased prevalence of CVDs. It has become clear that BPA, even acting at lower doses as an EDC, can cause adverse effects on cardiovascular health, especially at more susceptible stages of development, such as pregnancy. BPA exposure impairs not only cardiac electrophysiology, but also Ca2+ signaling, leading to arrhythmias and changes in contractile functions (contraction and relaxation) (Figure 3). However, the concentrations used in most in vitro studies are relatively high. Therefore, the choice of concentrations to be used in the in vitro, ex-vivo, and in vivo studies is one of the greatest challenges of toxicology, and this choice should always be dependent on the concentrations detected in epidemiological studies (a concentration range from 1.6 to 946 μg/L) [51] and environmental studies (effluent ranging from non-detect to 370 μg/L, soil ranging from <0.01 to 1000 μg/kg, and sediments ranging from 3400 to 20,136 μg/kg DW [130]. The use of higher concentrations (20–200 times higher than therapeutic Cmax) in in vitro studies is consensual. These concentrations are also designated using pharmacological concentrations and cause more accurate results than lower concentrations [131]. The ranges of these pharmacological concentrations are those that are required to cause effects at the cellular level, and are normally bigger than those required to cause harmful effects in vivo. This interrelationship is called the in vitro–in vivo scaling factor. However, this factor depends mainly on the mechanism of action of the substance, the metabolization route, and the mechanism of toxicity. Thus, only these high concentrations may be predictive of the effects induced by these substances [131,132]. In summary, the use of a high concentration range is crucial to determine the occurrence of toxic effects of substances, such as for BPA.
Figure 3.
Summary representation of Bisphenol A (BPA) exposure pathways and main mechanisms involved in BPA-induced cardiotoxicity.
Another very important parameter that remains under great controversy is the daily amount considered safe for human exposure, which is also referred to as the acceptable daily intake (ADI) by the Food and Drug Administration (FDA), and as the reference dose by the Environmental Protection Agency (EPA). This dose is currently at 50 µg/kg/day [133] and assumes that even if a daily exposure of this amount occurs, no adverse effects occur. However, recent studies have shown that the lowest observed adverse effect level (LOAEL), which serves as the reference ADI, is much lower than currently accepted (20,000-fold lower). These studies have led several members of the Endocrine Society to notify the FDA and EPA that there are several incorrect assumptions regarding the calculation of AID for BPA: (1) the responses to BPA are likely to be monotonic, which, in reality, is not the case for EDC; (2) the threshold at which there are no effects, which is also very controversial and questioned, as it is known that it can develop changes in the next generations; (3) a similar response for both sexes, which is also not true for many responses to BPA; and (4) only toxicological guideline studies are valid [133].
Thus, it seems clear that the human current daily exposure is not safe and that the problems arising from the massive use of this EDC continue to be a reality. The controversial studies can be justified through the different experimental approaches that are applied, as well as changes in the levels of doses, duration of exposure, or even the experimental model used. With regard to this, more studies are needed to further clarify the mechanism of BPA toxicity in the cardiovascular system. We emphasize that it is essential to use a wide range of concentrations to adequately address the dose–response relationship of BPA.
Furthermore, as humans are widely exposed to multiple bisphenols, this underscores the need for and importance of studying co-exposure patterns and/or the ‘exposome’, always considering multiple exposures and, thus, the best way to address the adverse effects of this EDC on cardiovascular health. The development of new BPA analogues, as well as the study of the safety of some analogues currently already developed, are a key point in the evolution of research on these compounds. Environmental and human exposure to these compounds should be free of health risks, especially in the most vulnerable groups such as children and pregnant women. Finally, it is also important that public health approaches are taken to clarify which are the best practices for reducing exposure to BPA (e.g., recommending the use of glass containers for food storage, reducing the use of canned products and plastic containers, etc.), thus improving human lifestyles and, consequently, decreasing the risk of BPA-induced cardiotoxicity.
Abbreviations
| Ang-II | angiotensin II |
| BP | blood pressure |
| BPA | bisphenol A |
| BPAF | bisphenol AF |
| BPC | bisphenol C |
| Ca2+ | calcium |
| CAD | coronary artery disease |
| CnAβ | calcineurin |
| CVDs | cardiovascular diseases |
| DCM | dilated cardiomyopathy |
| DRP1 | dynamin-related protein 1 |
| EC | endothelial cells |
| EDC | endocrine-disrupting compound; |
| EDHF | endothelium-derived hyperpolarizing factor |
| ER | estrogen receptors |
| ET-1 | endothelin 1 |
| GPR30 | G-protein-coupled estrogen receptor |
| HDPs | hypertensive disorders of pregnancy |
| HEK | human embryonic kidney |
| hiPSC-CMs | human-induced pluripotent stem-cell-derived cardiomyocytes |
| HRV | heart rate variability |
| HUVECs | human umbilical vein endothelial cells |
| IC50 | half-maximal inhibitory concentration |
| iPSCs | induced pluripotent stem cells |
| IR | ischemia–reperfusion |
| K+ | potassium |
| MAP | mean atrial pressures |
| MBP | 4-Methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene |
| MI | myocardial infarction |
| MOA | mechanisms of action |
| Na+ | sodium |
| NHANES | National Health and Nutrition Examination Survey |
| NO | nitric oxide |
| PAD | peripheral artery disease |
| PE | preeclampsia |
| PFOS | perfluorooctane sulfonate; |
| PGI-2 | prostacyclin |
| PLB | phospholamban |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| RIP3 | Rat Receptor-Interacting Protein 3 |
| SMC | smooth muscle cells |
| TxA2 | thromboxane |
| U.S. | United States |
| XME | xenobiotic-metabolizing enzymes |
Author Contributions
Conceptualization, E.C.; writing—original draft preparation, M.I.F., M.L. and E.C.; writing—review and editing, M.L. and E.C.; supervision, M.L. and E.C.; funding acquisition, E.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
M.L. acknowledges the Ph.D. fellowship from FCT (Reference: 2020.06616.BD). This work was developed within the scope of the CICS-UBI projects UIDB/00709/2020 and UIDP/00709/2020, financed by national funds through the Portuguese Foundation for Science and Technology/MCTES.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Francula-Zaninovic S., Nola I.A. Management of Measurable Variable Cardiovascular Disease’ Risk Factors. Curr. Cardiol. Rev. 2018;14:153–163. doi: 10.2174/1573403X14666180222102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatnagar A. Environmental Determinants of Cardiovascular Disease. Circ. Res. 2017;121:162–180. doi: 10.1161/CIRCRESAHA.117.306458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leslie H.A., van Velzen M.J.M., Brandsma S.H., Vethaak A.D., Garcia-Vallejo J.J., Lamoree M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022;163:107199. doi: 10.1016/j.envint.2022.107199. [DOI] [PubMed] [Google Scholar]
- 4.Diamanti-Kandarakis E., Bourguignon J.P., Giudice L.C., Hauser R., Prins G.S., Soto A.M., Zoeller R.T., Gore A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kabir E.R., Rahman M.S., Rahman I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015;40:241–258. doi: 10.1016/j.etap.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Ramadan M., Cooper B., Posnack N.G. Bisphenols and phthalates: Plastic chemical exposures can contribute to adverse cardiovascular health outcomes. Birth Defects Res. 2020;112:1362–1385. doi: 10.1002/bdr2.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandenberg L.N., Hauser R., Marcus M., Olea N., Welshons W.V. Human exposure to bisphenol A (BPA) Reprod. Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Campanale C., Massarelli C., Savino I., Locaputo V., Uricchio V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health. 2020;17:1212. doi: 10.3390/ijerph17041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodds E.C., Lawson W. Synthetic strogenic Agents without the Phenanthrene Nucleus. Nature. 1936;137:996. doi: 10.1038/137996a0. [DOI] [Google Scholar]
- 10.Michałowicz J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014;37:738–758. doi: 10.1016/j.etap.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Acconcia F., Pallottini V., Marino M. Molecular Mechanisms of Action of BPA. Dose Response. 2015;13:1559325815610582. doi: 10.1177/1559325815610582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee O., Singh S., Saha I., Pal S., Banerjee M., Kundu S., Syamal A.K., Maji B.K., Mukherjee S. Molecular dissection of cellular response of pancreatic islet cells to Bisphenol-A (BPA): A comprehensive review. Biochem. Pharmacol. 2022;201:115068. doi: 10.1016/j.bcp.2022.115068. [DOI] [PubMed] [Google Scholar]
- 13.Gao X., Wang H.-S. Impact of Bisphenol A on the Cardiovascular System—Epidemiological and Experimental Evidence and Molecular Mechanisms. Int. J. Environ. Res. Public Health. 2014;11:8399–8413. doi: 10.3390/ijerph110808399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rancière F., Lyons J.G., Loh V.H., Botton J., Galloway T., Wang T., Shaw J.E., Magliano D.J. Bisphenol A and the risk of cardiometabolic disorders: A systematic review with meta-analysis of the epidemiological evidence. Environ. Health A Glob. Access Sci. Source. 2015;14:46. doi: 10.1186/s12940-015-0036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandenberg Laura N., Chahoud I., Heindel Jerrold J., Padmanabhan V., Paumgartten Francisco J.R., Schoenfelder G. Urinary, Circulating, and Tissue Biomonitoring Studies Indicate Widespread Exposure to Bisphenol A. Environ. Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schönfelder G., Wittfoht W., Hopp H., Talsness C.E., Paul M., Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.021100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada H., Furuta I., Kato E.H., Kataoka S., Usuki Y., Kobashi G., Sata F., Kishi R., Fujimoto S. Maternal serum and amniotic fluid bisphenol A concentrations in the early second trimester. Reprod. Toxicol. 2002;16:735–739. doi: 10.1016/S0890-6238(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 18.Kuroda N., Kinoshita Y., Sun Y., Wada M., Kishikawa N., Nakashima K., Makino T., Nakazawa H. Measurement of bisphenol A levels in human blood serum and ascitic fluid by HPLC using a fluorescent labeling reagent. J. Pharm. Biomed. Anal. 2003;30:1743–1749. doi: 10.1016/S0731-7085(02)00516-2. [DOI] [PubMed] [Google Scholar]
- 19.Hines E.P., Mendola P., von Ehrenstein O.S., Ye X., Calafat A.M., Fenton S.E. Concentrations of environmental phenols and parabens in milk, urine and serum of lactating North Carolina women. Reprod. Toxicol. 2015;54:120–128. doi: 10.1016/j.reprotox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan B.L., Ali Mohd M. Analysis of selected pesticides and alkylphenols in human cord blood by gas chromatograph-mass spectrometer. Talanta. 2003;61:385–391. doi: 10.1016/S0039-9140(03)00281-9. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y., Irie M., Kishikawa N., Wada M., Kuroda N., Nakashima K. Determination of bisphenol A in human breast milk by HPLC with column-switching and fluorescence detection. Biomed. Chromatogr. 2004;18:501–507. doi: 10.1002/bmc.345. [DOI] [PubMed] [Google Scholar]
- 22.Kuruto-Niwa R., Tateoka Y., Usuki Y., Nozawa R. Measurement of bisphenol A concentrations in human colostrum. Chemosphere. 2007;66:1160–1164. doi: 10.1016/j.chemosphere.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 23.Völkel W., Colnot T., Csanády G.A., Filser J.G., Dekant W. Metabolism and Kinetics of Bisphenol A in Humans at Low Doses Following Oral Administration. Chem. Res. Toxicol. 2002;15:1281–1287. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- 24.Cimmino I., Fiory F., Perruolo G., Miele C., Beguinot F., Formisano P., Oriente F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020;21:5761. doi: 10.3390/ijms21165761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan H., Wang X., Hong H., Benfenati E., Giesy J.P., Gini G.C., Kusko R., Zhang X., Yu H., Shi W. Structures of Endocrine-Disrupting Chemicals Determine Binding to and Activation of the Estrogen Receptor α and Androgen Receptor. Environ. Sci. Technol. 2020;54:11424–11433. doi: 10.1021/acs.est.0c02639. [DOI] [PubMed] [Google Scholar]
- 26.Vandenberg L.N. Non-Monotonic Dose Responses in Studies of Endocrine Disrupting Chemicals: Bisphenol a as a Case Study. Dose-Response. 2014;12:259–276. doi: 10.2203/dose-response.13-020.Vandenberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper B.L., Posnack N.G. Characteristics of Bisphenol Cardiotoxicity: Impaired Excitability, Contractility, and Relaxation. Cardiovasc. Toxicol. 2022;22:273–280. doi: 10.1007/s12012-022-09719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boudalia S., Oudir M. Bisphenol-A: Legislation in Industrials Countries and in Algeria. Res. J. Environ. Toxicol. 2016;10:189–192. doi: 10.3923/rjet.2016.189.192. [DOI] [Google Scholar]
- 29.Xing J., Zhang S., Zhang M., Hou J. A critical review of presence, removal and potential impacts of endocrine disruptors bisphenol A. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022;254:109275. doi: 10.1016/j.cbpc.2022.109275. [DOI] [PubMed] [Google Scholar]
- 30.Posnack N.G., Jaimes R., 3rd, Asfour H., Swift L.M., Wengrowski A.M., Sarvazyan N., Kay M.W. Bisphenol A exposure and cardiac electrical conduction in excised rat hearts. Environ. Health Perspect. 2014;122:384–390. doi: 10.1289/ehp.1206157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y.-F., Shan C., Wang Y., Qian L.-L., Jia D.-D., Zhang Y.-F., Hao X.-D., Xu H.-M. Cardiovascular toxicity and mechanism of bisphenol A and emerging risk of bisphenol S. Sci. Total Environ. 2020;723:137952. doi: 10.1016/j.scitotenv.2020.137952. [DOI] [PubMed] [Google Scholar]
- 32.Lind L., Araujo J.A., Barchowsky A., Belcher S., Berridge B.R., Chiamvimonvat N., Chiu W.A., Cogliano V.J., Elmore S., Farraj A.K., et al. Key Characteristics of Cardiovascular Toxicants. Environ. Health Perspect. 2021;129:95001. doi: 10.1289/EHP9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cumberland M.J., Riebel L.L., Roy A., O’Shea C., Holmes A.P., Denning C., Kirchhof P., Rodriguez B., Gehmlich K. Basic Research Approaches to Evaluate Cardiac Arrhythmia in Heart Failure and Beyond. Front. Physiol. 2022;13:806366. doi: 10.3389/fphys.2022.806366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verkerk A.O., Marchal G.A., Zegers J.G., Kawasaki M., Driessen A.H.G., Remme C.A., de Groot J.R., Wilders R. Patch-Clamp Recordings of Action Potentials From Human Atrial Myocytes: Optimization Through Dynamic Clamp. Front. Pharmacol. 2021;12:649414. doi: 10.3389/fphar.2021.649414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asano S., Tune J.D., Dick G.M. Bisphenol A activates Maxi-K (K(Ca)1.1) channels in coronary smooth muscle. Br. J. Pharmacol. 2010;160:160–170. doi: 10.1111/j.1476-5381.2010.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rottgen T.S., Fancher I.S., Asano S., Widlanski T.S., Dick G.M. Bisphenol A activates BK channels through effects on α and β1 subunits. Channels. 2014;8:249–257. doi: 10.4161/chan.27709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feiteiro J., Mariana M., Glória S., Cairrao E. Inhibition of L-type calcium channels by Bisphenol A in rat aorta smooth muscle. J. Toxicol. Sci. 2018;43:579–586. doi: 10.2131/jts.43.579. [DOI] [PubMed] [Google Scholar]
- 38.Deutschmann A., Hans M., Meyer R., Häberlein H., Swandulla D. Bisphenol A Inhibits Voltage-Activated Ca2+ Channels in Vitro: Mechanisms and Structural Requirements. Mol. Pharmacol. 2013;83:501. doi: 10.1124/mol.112.081372. [DOI] [PubMed] [Google Scholar]
- 39.Michaela P., Mária K., Silvia H., Ľubica L. Bisphenol A differently inhibits CaV3.1, CaV3.2 and CaV3.3 calcium channels. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014;387:153–163. doi: 10.1007/s00210-013-0932-6. [DOI] [PubMed] [Google Scholar]
- 40.Liang Q., Gao X., Chen Y., Hong K., Wang H.-S. Cellular Mechanism of the Nonmonotonic Dose Response of Bisphenol A in Rat Cardiac Myocytes. Environ. Health Perspect. 2014;122:601–608. doi: 10.1289/ehp.1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hyun S.-A., Lee C.Y., Ko M.Y., Chon S.-H., Kim Y.-J., Seo J.-W., Kim K.K., Ka M. Cardiac toxicity from bisphenol A exposure in human-induced pluripotent stem cell-derived cardiomyocytes. Toxicol. Appl. Pharmacol. 2021;428:115696. doi: 10.1016/j.taap.2021.115696. [DOI] [PubMed] [Google Scholar]
- 42.Prudencio T.M., Swift L.M., Guerrelli D., Cooper B., Reilly M., Ciccarelli N., Sheng J., Jaimes R., III, Posnack N.G. Bisphenol S and Bisphenol F Are Less Disruptive to Cardiac Electrophysiology, as Compared with Bisphenol A. Toxicol. Sci. 2021;183:214–226. doi: 10.1093/toxsci/kfab083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Reilly A.O., Eberhardt E., Weidner C., Alzheimer C., Wallace B.A., Lampert A. Bisphenol A Binds to the Local Anesthetic Receptor Site to Block the Human Cardiac Sodium Channel. PLoS ONE. 2012;7:e41667. doi: 10.1371/journal.pone.0041667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horváth B., Hézső T., Kiss D., Kistamás K., Magyar J., Nánási P.P., Bányász T. Late Sodium Current Inhibitors as Potential Antiarrhythmic Agents. Front. Pharmacol. 2020;11:413. doi: 10.3389/fphar.2020.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan S., Chen Y., Dong M., Song W., Belcher S.M., Wang H.-S. Bisphenol A and 17β-Estradiol Promote Arrhythmia in the Female Heart via Alteration of Calcium Handling. PLoS ONE. 2011;6:e25455. doi: 10.1371/journal.pone.0025455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belcher S.M., Chen Y., Yan S., Wang H.-S. Rapid Estrogen Receptor-Mediated Mechanisms Determine the Sexually Dimorphic Sensitivity of Ventricular Myocytes to 17β-Estradiol and the Environmental Endocrine Disruptor Bisphenol A. Endocrinology. 2012;153:712–720. doi: 10.1210/en.2011-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan S., Song W., Chen Y., Hong K., Rubinstein J., Wang H.S. Low-dose bisphenol A and estrogen increase ventricular arrhythmias following ischemia-reperfusion in female rat hearts. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013;56:75–80. doi: 10.1016/j.fct.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao X., Liang Q., Chen Y., Wang H.-S. Molecular Mechanisms Underlying the Rapid Arrhythmogenic Action of Bisphenol A in Female Rat Hearts. Endocrinology. 2013;154:4607–4617. doi: 10.1210/en.2013-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinto C., Hao R., Grimaldi M., Thrikawala S., Boulahtouf A., Aït-Aïssa S., Brion F., Gustafsson J., Balaguer P., Bondesson M. Differential activity of BPA, BPAF and BPC on zebrafish estrogen receptors in vitro and in vivo. Toxicol. Appl. Pharmacol. 2019;380:114709. doi: 10.1016/j.taap.2019.114709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramadan M., Sherman M., Jaimes R., Chaluvadi A., Swift L., Posnack N.G. Disruption of neonatal cardiomyocyte physiology following exposure to bisphenola. Sci. Rep. 2018;8:7356. doi: 10.1038/s41598-018-25719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lorigo M., Cairrao E. Fetoplacental vasculature as a model to study human cardiovascular endocrine disruption. Mol. Aspects Med. 2021;87:101054. doi: 10.1016/j.mam.2021.101054. [DOI] [PubMed] [Google Scholar]
- 52.Zhou R., Cheng W., Feng Y., Wang W., Liang F., Luo F., Yang S., Wang Y. Combined effects of BPA and PFOS on fetal cardiac development: In vitro and in vivo experiments. Environ. Toxicol. Pharmacol. 2020;80:103434. doi: 10.1016/j.etap.2020.103434. [DOI] [PubMed] [Google Scholar]
- 53.Reventun P., Sanchez-Esteban S., Cook A., Cuadrado I., Roza C., Moreno-Gomez-Toledano R., Muñoz C., Zaragoza C., Bosch R.J., Saura M. Bisphenol A induces coronary endothelial cell necroptosis by activating RIP3/CamKII dependent pathway. Sci. Rep. 2020;10:4190. doi: 10.1038/s41598-020-61014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Posnack N.G., Brooks D., Chandra A., Jaimes R., Sarvazyan N., Kay M. Physiological response of cardiac tissue to bisphenol a: Alterations in ventricular pressure and contractility. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H267–H275. doi: 10.1152/ajpheart.00272.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pant J., Ranjan P., Deshpande S.B. Bisphenol A decreases atrial contractility involving NO-dependent G-cyclase signaling pathway. J. Appl. Toxicol. 2011;31:698–702. doi: 10.1002/jat.1647. [DOI] [PubMed] [Google Scholar]
- 56.Filice M., Leo S., Mazza R., Amelio D., Garofalo F., Imbrogno S., Cerra M.C., Gattuso A. The heart of the adult goldfish Carassius auratus as a target of Bisphenol A: A multifaceted analysis. Environ. Pollut. 2021;269:116177. doi: 10.1016/j.envpol.2020.116177. [DOI] [PubMed] [Google Scholar]
- 57.Patel B.B., Raad M., Sebag I.A., Chalifour L.E. Lifelong Exposure to Bisphenol A Alters Cardiac Structure/Function, Protein Expression, and DNA Methylation in Adult Mice. Toxicol. Sci. 2013;133:174–185. doi: 10.1093/toxsci/kft026. [DOI] [PubMed] [Google Scholar]
- 58.Saura M., Marquez S., Reventun P., Olea-Herrero N., Arenas M.I., Moreno-Gómez-Toledano R., Gómez-Parrizas M., Muñóz-Moreno C., González-Santander M., Zaragoza C., et al. Oral administration of bisphenol A induces high blood pressure through angiotensin II/CaMKII-dependent uncoupling of eNOS. FASEB J. 2014;28:4719–4728. doi: 10.1096/fj.14-252460. [DOI] [PubMed] [Google Scholar]
- 59.Kim M.J., Moon M.K., Kang G.H., Lee K.J., Choi S.H., Lim S., Oh B.-C., Park D.J., Park K.S., Jang H.C., et al. Chronic Exposure to Bisphenol A can Accelerate Atherosclerosis in High-Fat-Fed Apolipoprotein E Knockout Mice. Cardiovasc. Toxicol. 2014;14:120–128. doi: 10.1007/s12012-013-9235-x. [DOI] [PubMed] [Google Scholar]
- 60.Belcher S.M., Gear R.B., Kendig E.L. Bisphenol A alters autonomic tone and extracellular matrix structure and induces sex-specific effects on cardiovascular function in male and female CD-1 mice. Endocrinology. 2015;156:882–895. doi: 10.1210/en.2014-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aboul Ezz H.S., Khadrawy Y.A., Mourad I.M. The effect of bisphenol A on some oxidative stress parameters and acetylcholinesterase activity in the heart of male albino rats. Cytotechnology. 2015;67:145–155. doi: 10.1007/s10616-013-9672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel B.B., Kasneci A., Bolt A.M., Di Lalla V., Di Iorio M.R., Raad M., Mann K.K., Chalifour L.E. Chronic Exposure to Bisphenol A Reduces Successful Cardiac Remodeling After an Experimental Myocardial Infarction in Male C57bl/6n Mice. Toxicol. Sci. 2015;146:101–115. doi: 10.1093/toxsci/kfv073. [DOI] [PubMed] [Google Scholar]
- 63.Gear R., Kendziorski J.A., Belcher S.M. Effects of bisphenol A on incidence and severity of cardiac lesions in the NCTR-Sprague-Dawley rat: A CLARITY-BPA study. Toxicol. Lett. 2017;275:123–135. doi: 10.1016/j.toxlet.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hailey J.R., Maleeff B.E., Thomas H.C., Pearse G., Klapwijk J.C., Cristofori P.G., Berridge B., Kimbrough C.L., Parker G.A., Morton D., et al. A Diagnostic Approach for Rodent Progressive Cardiomyopathy and Like Lesions in Toxicology Studies up to 28 Days in the Sprague Dawley Rat (Part 1 of 2) Toxicol. Pathol. 2017;45:1043–1054. doi: 10.1177/0192623317743938. [DOI] [PubMed] [Google Scholar]
- 65.Klint H., Lejonklou M.H., Karimullina E., Rönn M., Lind L., Lind P.M., Brittebo E. Low-dose exposure to bisphenol A in combination with fructose increases expression of genes regulating angiogenesis and vascular tone in juvenile Fischer 344 rat cardiac tissue. Upsala J. Med. Sci. 2017;122:20–27. doi: 10.1080/03009734.2016.1225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sui Y., Park S.-H., Wang F., Zhou C. Perinatal Bisphenol A Exposure Increases Atherosclerosis in Adult Male PXR-Humanized Mice. Endocrinology. 2018;159:1595–1608. doi: 10.1210/en.2017-03250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruno K.A., Mathews J.E., Yang A.L., Frisancho J.A., Scott A.J., Greyner H.D., Molina F.A., Greenaway M.S., Cooper G.M., Bucek A., et al. BPA Alters Estrogen Receptor Expression in the Heart After Viral Infection Activating Cardiac Mast Cells and T Cells Leading to Perimyocarditis and Fibrosis. Front. Endocrinol. 2019;10:598. doi: 10.3389/fendo.2019.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Freeman J.L., Weber G.J., Peterson S.M., Nie L.H. Embryonic ionizing radiation exposure results in expression alterations of genes associated with cardiovascular and neurological development, function, and disease and modified cardiovascular function in zebrafish. Front. Genet. 2014;5:268. doi: 10.3389/fgene.2014.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vargas R., Vásquez I.C. Cardiac and somatic parameters in zebrafish: Tools for the evaluation of cardiovascular function. Fish Physiol. Biochem. 2016;42:569–577. doi: 10.1007/s10695-015-0160-8. [DOI] [PubMed] [Google Scholar]
- 70.Sedmera D., Reckova M., deAlmeida A., Sedmerova M., Biermann M., Volejnik J., Sarre A., Raddatz E., McCarthy R.A., Gourdie R.G., et al. Functional and morphological evidence for a ventricular conduction system in zebrafish and Xenopus hearts. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1152–H1160. doi: 10.1152/ajpheart.00870.2002. [DOI] [PubMed] [Google Scholar]
- 71.Murk A.J., Rijntjes E., Blaauboer B.J., Clewell R., Crofton K.M., Dingemans M.M., Furlow J.D., Kavlock R., Köhrle J., Opitz R., et al. Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals. Toxicol. In Vitro Int. J. Publ. Assoc. BIBRA. 2013;27:1320–1346. doi: 10.1016/j.tiv.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 72.Menuet A., Pellegrini E., Anglade I., Blaise O., Laudet V., Kah O., Pakdel F. Molecular Characterization of Three Estrogen Receptor Forms in Zebrafish: Binding Characteristics, Transactivation Properties, and Tissue Distributions1. Biol. Reprod. 2002;66:1881–1892. doi: 10.1095/biolreprod66.6.1881. [DOI] [PubMed] [Google Scholar]
- 73.Cypher A.D., Ickes J.R., Bagatto B. Bisphenol A alters the cardiovascular response to hypoxia in Danio rerio embryos. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015;174–175:39–45. doi: 10.1016/j.cbpc.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 74.Lombó M., Fernández-Díez C., González-Rojo S., Navarro C., Robles V., Herráez M.P. Transgenerational inheritance of heart disorders caused by paternal bisphenol A exposure. Environ. Pollut. 2015;206:667–678. doi: 10.1016/j.envpol.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 75.Cypher A.D., Fetterman B., Bagatto B. Vascular parameters continue to decrease post-exposure with simultaneous, but not individual exposure to BPA and hypoxia in zebrafish larvae. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018;206–207:11–16. doi: 10.1016/j.cbpc.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 76.Moreman J., Takesono A., Trznadel M., Winter M.J., Perry A., Wood M.E., Rogers N.J., Kudoh T., Tyler C.R. Estrogenic Mechanisms and Cardiac Responses Following Early Life Exposure to Bisphenol A (BPA) and Its Metabolite 4-Methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene (MBP) in Zebrafish. Environ. Sci. Technol. 2018;52:6656–6665. doi: 10.1021/acs.est.8b01095. [DOI] [PubMed] [Google Scholar]
- 77.Brown A.R., Green J.M., Moreman J., Gunnarsson L.M., Mourabit S., Ball J., Winter M.J., Trznadel M., Correia A., Hacker C., et al. Cardiovascular Effects and Molecular Mechanisms of Bisphenol A and Its Metabolite MBP in Zebrafish. Environ. Sci. Technol. 2019;53:463–474. doi: 10.1021/acs.est.8b04281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lombó M., González-Rojo S., Fernández-Díez C., Herráez M.P. Cardiogenesis impairment promoted by bisphenol A exposure is successfully counteracted by epigallocatechin gallate. Environ. Pollut. 2019;246:1008–1019. doi: 10.1016/j.envpol.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Lombó M., Herráez M.P. Paternal Inheritance of Bisphenol A Cardiotoxic Effects: The Implications of Sperm Epigenome. Int. J. Mol. Sci. 2021;22:2125. doi: 10.3390/ijms22042125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ji G., Gu J., Guo M., Zhou L., Wang Z., Shi L., Gu A. A systematic comparison of the developmental vascular toxicity of bisphenol A and its alternatives in vivo and in vitro. Chemosphere. 2022;291:132936. doi: 10.1016/j.chemosphere.2021.132936. [DOI] [PubMed] [Google Scholar]
- 81.Schönemann A.M., Moreno Abril S.I., Diz A.P., Beiras R. The bisphenol A metabolite MBP causes proteome alterations in male Cyprinodon variegatus fish characteristic of estrogenic endocrine disruption. Environ. Pollut. 2022;300:118936. doi: 10.1016/j.envpol.2022.118936. [DOI] [PubMed] [Google Scholar]
- 82.Rochester J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 83.Soriano S., Ripoll C., Alonso-Magdalena P., Fuentes E., Quesada I., Nadal A., Martinez-Pinna J. Effects of Bisphenol A on ion channels: Experimental evidence and molecular mechanisms. Steroids. 2016;111:12–20. doi: 10.1016/j.steroids.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 84.Katz A.M., Lorell B.H. Regulation of Cardiac Contraction and Relaxation. Circulation. 2000;102:Iv-69–Iv-74. doi: 10.1161/circ.102.suppl_4.IV-69. [DOI] [PubMed] [Google Scholar]
- 85.Edwards J.N., Blatter L.A. Cardiac alternans and intracellular calcium cycling. Clin. Exp. Pharmacol. Physiol. 2014;41:524–532. doi: 10.1111/1440-1681.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng W., Yang S., Li X., Liang F., Zhou R., Wang H., Feng Y., Wang Y. Low doses of BPA induced abnormal mitochondrial fission and hypertrophy in human embryonic stem cell-derived cardiomyocytes via the calcineurin-DRP1 signaling pathway: A comparison between XX and XY cardiomyocytes. Toxicol. Appl. Pharmacol. 2020;388:114850. doi: 10.1016/j.taap.2019.114850. [DOI] [PubMed] [Google Scholar]
- 87.Mangana C., Lorigo M., Cairrao E. Implications of Endothelial Cell-Mediated Dysfunctions in Vasomotor Tone Regulation. Biologics. 2021;1:231–251. doi: 10.3390/biologics1020015. [DOI] [Google Scholar]
- 88.Andersson H., Brittebo E. Proangiogenic effects of environmentally relevant levels of bisphenol A in human primary endothelial cells. Arch. Toxicol. 2012;86:465–474. doi: 10.1007/s00204-011-0766-2. [DOI] [PubMed] [Google Scholar]
- 89.Ribeiro-Varandas E., Viegas W., Sofia Pereira H., Delgado M. Bisphenol A at concentrations found in human serum induces aneugenic effects in endothelial cells. Mutat. Res. 2013;751:27–33. doi: 10.1016/j.mrgentox.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 90.Ribeiro-Varandas E., Pereira H.S., Monteiro S., Neves E., Brito L., Ferreira R.B., Viegas W., Delgado M. Bisphenol A Disrupts Transcription and Decreases Viability in Aging Vascular Endothelial Cells. Int. J. Mol. Sci. 2014;15:15791–15805. doi: 10.3390/ijms150915791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nahar M.S., Kim J.H., Sartor M.A., Dolinoy D.C. Bisphenol A-associated alterations in the expression and epigenetic regulation of genes encoding xenobiotic metabolizing enzymes in human fetal liver. Environ. Mol. Mutagen. 2014;55:184–195. doi: 10.1002/em.21823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Montrose L., Padmanabhan V., Goodrich J.M., Domino S.E., Treadwell M.C., Meeker J.D., Watkins D.J., Dolinoy D.C. Maternal levels of endocrine disrupting chemicals in the first trimester of pregnancy are associated with infant cord blood DNA methylation. Epigenetics. 2018;13:301–309. doi: 10.1080/15592294.2018.1448680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Troisi J., Mikelson C., Richards S., Symes S., Adair D., Zullo F., Guida M. Placental concentrations of bisphenol A and birth weight from births in the Southeastern U.S. Placenta. 2014;35:947–952. doi: 10.1016/j.placenta.2014.08.091. [DOI] [PubMed] [Google Scholar]
- 94.Nahar M.S., Liao C., Kannan K., Harris C., Dolinoy D.C. In utero bisphenol A concentration, metabolism, and global DNA methylation across matched placenta, kidney, and liver in the human fetus. Chemosphere. 2015;124:54–60. doi: 10.1016/j.chemosphere.2014.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mørck T.J., Sorda G., Bechi N., Rasmussen B.S., Nielsen J.B., Ietta F., Rytting E., Mathiesen L., Paulesu L., Knudsen L.E. Placental transport and in vitro effects of Bisphenol A. Reprod. Toxicol. 2010;30:131–137. doi: 10.1016/j.reprotox.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 96.Ponniah M., Billett E.E., De Girolamo L.A. Bisphenol A increases BeWo trophoblast survival in stress-induced paradigms through regulation of oxidative stress and apoptosis. Chem. Res. Toxicol. 2015;28:1693–1703. doi: 10.1021/acs.chemrestox.5b00093. [DOI] [PubMed] [Google Scholar]
- 97.Spagnoletti A., Paulesu L., Mannelli C., Ermini L., Romagnoli R., Cintorino M., Ietta F. Low concentrations of Bisphenol A and para-Nonylphenol affect extravillous pathway of human trophoblast cells. Mol. Cell Endocrinol. 2015;412:56–64. doi: 10.1016/j.mce.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 98.Basak S., Srinivas V., Duttaroy A.K. Bisphenol-A impairs cellular function and alters DNA methylation of stress pathway genes in first trimester trophoblast cells. Reprod. Toxicol. 2018;82:72–79. doi: 10.1016/j.reprotox.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 99.Vrooman L.A., Xin F., Bartolomei M.S. Morphologic and molecular changes in the placenta: What we can learn from environmental exposures. Fertil. Steril. 2016;106:930–940. doi: 10.1016/j.fertnstert.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Strakovsky R.S., Schantz S.L. Impacts of bisphenol A (BPA) and phthalate exposures on epigenetic outcomes in the human placenta. Environ. Epigenet. 2018;4:dvy022. doi: 10.1093/eep/dvy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Strakovsky R.S., Schantz S.L. Using Experimental Models to Assess Effects of Bisphenol A (BPA) and Phthalates on the Placenta: Challenges and Perspectives. Toxicol. Sci. Off. J. Soc. Toxicol. 2018;166:250–268. doi: 10.1093/toxsci/kfy224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Basak S., Das M.K., Duttaroy A.K. Plastics derived endocrine-disrupting compounds and their effects on early development. Birth Defects Res. 2020;112:1308–1325. doi: 10.1002/bdr2.1741. [DOI] [PubMed] [Google Scholar]
- 103.Lang I.A., Galloway T.S., Scarlett A., Henley W.E., Depledge M., Wallace R.B., Melzer D. Association of Urinary Bisphenol A Concentration with Medical Disorders and Laboratory Abnormalities in Adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 104.Cai S., Rao X., Ye J., Ling Y., Mi S., Chen H., Fan C., Li Y. Relationship between urinary bisphenol a levels and cardiovascular diseases in the U.S. adult population, 2003–2014. Ecotoxicol. Environ. Saf. 2020;192:110300. doi: 10.1016/j.ecoenv.2020.110300. [DOI] [PubMed] [Google Scholar]
- 105.Lind P.M., Lind L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis. 2011;218:207–213. doi: 10.1016/j.atherosclerosis.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 106.Melzer D., Osborne N.J., Henley W.E., Cipelli R., Young A., Money C., McCormack P., Luben R., Khaw K.-T., Wareham N.J., et al. Urinary Bisphenol A Concentration and Risk of Future Coronary Artery Disease in Apparently Healthy Men and Women. Circulation. 2012;125:1482–1490. doi: 10.1161/CIRCULATIONAHA.111.069153. [DOI] [PubMed] [Google Scholar]
- 107.Melzer D., Gates P., Osborn N.J., Henley W.E., Cipelli R., Young A., Money C., McCormack P., Schofield P., Mosedale D., et al. Urinary Bisphenol A Concentration and Angiography-Defined Coronary Artery Stenosis. PLoS ONE. 2012;7:e43378. doi: 10.1371/annotation/5f293018-48a3-40ae-96b7-04438d1d9cb9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Melzer D., Rice N.E., Lewis C., Henley W.E., Galloway T.S. Association of Urinary Bisphenol A Concentration with Heart Disease: Evidence from NHANES 2003/06. PLoS ONE. 2010;5:e8673. doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xiong Q., Liu X., Shen Y., Yu P., Chen S., Hu J., Yu J., Li J., Wang H.-S., Cheng X., et al. Elevated serum Bisphenol A level in patients with dilated cardiomyopathy. Int. J. Environ. Res. Public Health. 2015;12:5329–5337. doi: 10.3390/ijerph120505329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shankar A., Teppala S., Sabanayagam C. Bisphenol A and Peripheral Arterial Disease: Results from the NHANES. Environ. Health Perspect. 2012;120:1297–1300. doi: 10.1289/ehp.1104114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu C., Schöttker B., Venisse N., Limousi F., Saulnier P.J., Albouy-Llaty M., Dupuis A., Brenner H., Migeot V., Hadjadj S. Bisphenol A, Chlorinated Derivatives of Bisphenol A and Occurrence of Myocardial Infarction in Patients with Type 2 Diabetes: Nested Case-Control Studies in Two European Cohorts. Environ. Sci. Technol. 2019;53:9876–9883. doi: 10.1021/acs.est.9b02963. [DOI] [PubMed] [Google Scholar]
- 112.Shankar A., Teppala S. Urinary Bisphenol A and Hypertension in a Multiethnic Sample of US Adults. J. Environ. Public Health. 2012;2012:481641. doi: 10.1155/2012/481641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bae S., Kim J.H., Lim Y.-H., Park H.Y., Hong Y.-C. Associations of Bisphenol A Exposure with Heart Rate Variability and Blood Pressure. Hypertension. 2012;60:786–793. doi: 10.1161/HYPERTENSIONAHA.112.197715. [DOI] [PubMed] [Google Scholar]
- 114.Bae S., Hong Y.C. Exposure to bisphenol A from drinking canned beverages increases blood pressure: Randomized crossover trial. Hypertension. 2015;65:313–319. doi: 10.1161/HYPERTENSIONAHA.114.04261. [DOI] [PubMed] [Google Scholar]
- 115.Aekplakorn W., Chailurkit L.-O., Ongphiphadhanakul B. Association of Serum Bisphenol A with Hypertension in Thai Population. Int. J. Hypertens. 2015;2015:594189. doi: 10.1155/2015/594189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mehlsen A., Høllund L., Boye H., Frederiksen H., Andersson A.-M., Bruun S., Husby S., Jensen T.K., Timmermann C.A.G. Pregnancy exposure to bisphenol A and duration of breastfeeding. Environ. Res. 2022;206:112471. doi: 10.1016/j.envres.2021.112471. [DOI] [PubMed] [Google Scholar]
- 117.LaKind J.S., Goodman M., Naiman D.Q. Use of NHANES Data to Link Chemical Exposures to Chronic Diseases: A Cautionary Tale. PLoS ONE. 2012;7:e51086. doi: 10.1371/journal.pone.0051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.LaKind J.S., Goodman M., Mattison D.R. Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: A systematic review of epidemiologic research. Crit. Rev. Toxicol. 2014;44:121–150. doi: 10.3109/10408444.2013.860075. [DOI] [PubMed] [Google Scholar]
- 119.Olsén L., Lind L., Lind P.M. Associations between circulating levels of bisphenol A and phthalate metabolites and coronary risk in the elderly. Ecotoxicol. Environ. Saf. 2012;80:179–183. doi: 10.1016/j.ecoenv.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 120.Warembourg C., Basagaña X., Seminati C., de Bont J., Granum B., Lyon-Caen S., Manzano-Salgado C.B., Pin I., Sakhi A.K., Siroux V., et al. Exposure to phthalate metabolites, phenols and organophosphate pesticide metabolites and blood pressure during pregnancy. Int. J. Hyg. Environ. Health. 2019;222:446–454. doi: 10.1016/j.ijheh.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 121.Bae S., Lim Y.H., Lee Y.A., Shin C.H., Oh S.Y., Hong Y.C. Maternal Urinary Bisphenol A Concentration During Midterm Pregnancy and Children’s Blood Pressure at Age 4. Hypertension. 2017;69:367–374. doi: 10.1161/HYPERTENSIONAHA.116.08281. [DOI] [PubMed] [Google Scholar]
- 122.Sol C.M., Santos S., Asimakopoulos A.G., Martinez-Moral M.-P., Duijts L., Kannan K., Trasande L., Jaddoe V.W.V. Associations of maternal phthalate and bisphenol urine concentrations during pregnancy with childhood blood pressure in a population-based prospective cohort study. Environ. Int. 2020;138:105677. doi: 10.1016/j.envint.2020.105677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Varshavsky J., Smith A., Wang A., Hom E., Izano M., Huang H., Padula A., Woodruff T.J. Heightened susceptibility: A review of how pregnancy and chemical exposures influence maternal health. Reprod. Toxicol. 2020;92:14–56. doi: 10.1016/j.reprotox.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leclerc F., Dubois M.F., Aris A. Maternal, placental and fetal exposure to bisphenol A in women with and without preeclampsia. Hypertens. Pregnancy. 2014;33:341–348. doi: 10.3109/10641955.2014.892607. [DOI] [PubMed] [Google Scholar]
- 125.Ferguson K.K., McElrath T.F., Cantonwine D.E., Mukherjee B., Meeker J.D. Phthalate metabolites and bisphenol-A in association with circulating angiogenic biomarkers across pregnancy. Placenta. 2015;36:699–703. doi: 10.1016/j.placenta.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cantonwine David E., Meeker John D., Ferguson Kelly K., Mukherjee B., Hauser R., McElrath Thomas F. Urinary Concentrations of Bisphenol A and Phthalate Metabolites Measured during Pregnancy and Risk of Preeclampsia. Environ. Health Perspect. 2016;124:1651–1655. doi: 10.1289/EHP188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Philips E.M., Trasande L., Kahn L.G., Gaillard R., Steegers E.A.P., Jaddoe V.W.V. Early pregnancy bisphenol and phthalate metabolite levels, maternal hemodynamics and gestational hypertensive disorders. Hum. Reprod. 2019;34:365–373. doi: 10.1093/humrep/dey364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Philips E.M., Jaddoe V.W.V., Trasande L. Effects of early exposure to phthalates and bisphenols on cardiometabolic outcomes in pregnancy and childhood. Reprod. Toxicol. 2017;68:105–118. doi: 10.1016/j.reprotox.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Perng W., Kasper N.M., Watkins D.J., Sanchez B.N., Meeker J.D., Cantoral A., Solano-González M., Tellez-Rojo M.M., Peterson K. Exposure to Endocrine-Disrupting Chemicals During Pregnancy Is Associated with Weight Change Through 1 Year Postpartum Among Women in the Early-Life Exposure in Mexico to Environmental Toxicants Project. J. Women’s Health. 2020;29:1419–1426. doi: 10.1089/jwh.2019.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Corrales J., Kristofco L.A., Steele W.B., Yates B.S., Breed C.S., Williams E.S., Brooks B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose-Response. 2015;13:1559325815598308. doi: 10.1177/1559325815598308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hengstler J.G., Sjögren A.-K., Zink D., Hornberg J.J. In Vitro prediction of organ toxicity: The challenges of scaling and secondary mechanisms of toxicity. Arch. Toxicol. 2020;94:353–356. doi: 10.1007/s00204-020-02669-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Albrecht W. Which concentrations are optimal for in vitro testing? EXCLI J. 2020;19:1172–1173. doi: 10.17179/excli2020-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vom Saal F.S., Vandenberg L.N. Update on the Health Effects of Bisphenol A: Overwhelming Evidence of Harm. Endocrinology. 2021;162:bqaa171. doi: 10.1210/endocr/bqaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.