Abstract
Two iron(III)-based molecular receptors employing 1,2-hydroxypyridinone ligands were developed for phosphate recognition and fluorescence sensing via indicator displacement assay (IDA). The tetra- and pentadentate ligands enable anion recognition by the iron(III) center via its remaining one or two open coordination sites. Weak protective coordination of fluorescein at those sites prevents the formation of μ-oxo dimers in aerated solutions. Its rapid and selective displacement by inorganic phosphate results in a 20-fold increase in the fluorescence of the indicator. Both receptors exhibit high affinity for inorganic phosphate and high selectivity over common competing anions, including halides, acetate, carbonate, and, remarkably, pyrophosphate as well as arsenate. Coordination of phosphate to the iron(III) center was confirmed by ATR-IR and 31P NMR spectroscopy.
Keywords: pyrophosphate, phosphate, iron, fluorescence, supramolecular receptor, indicator displacement assay
Introduction
Phosphate is a crucial component of fertilizers needed to maintain the world’s food supply. Unfortunately, most of the phosphate used as fertilizer leaches out into surface water, causing widespread eutrophication and hazardous algal blooms. Over 65% of U.S. estuaries and coastal waters now have moderate to severe eutrophication, with significant consequences to the ecology and industry relying on those systems.1,2 Addressing this issue requires in part facile detection of phosphate in the micromolar range.3,4 The current protocol of the U.S. Environmental Protection Agency (EPA) for measuring phosphate levels, the molybdenum blue method, relies on the formation of a phosphomolybdate Keggin ion followed by its reduction to yield a blue mixed-valence complex.5 The slow kinetics of these reactions renders this multistep protocol laborious. Moreover, the strong acidic conditions necessary for the formation of the Keggin ion does not enable distinction between orthophosphate and other polyphosphates such as pyrophosphate that can also be present in large concentration in surface water but have different impacts on algae growth.6 As such, although much attention has recently been devoted to developing molecular receptors and fluorescent probes for phosphate, effective probes that can readily distinguish between phosphate and pyrophosphate are still needed.7−12
Metal complexes are particularly well-suited for probing phosphates by luminescence. Recognition of the anion can be accomplished either allosterically or via direct coordination. As in the case of the heteroditopic ruthenium(II) bipyridyl complexes, allosteric recognition of phosphate is primarily accomplished by directed hydrogen-bonding interactions. Such probes, however, do not work well with aqueous samples and are rarely selective for phosphate, including over pyrophosphate.13−15 Direct coordination of phosphate is better suited for such applications since the metal ions are able to overcome the high hydration enthalpy of phosphate.7−11,16,17 The requirements for lability and hardness have limited current studies to copper, zinc, and lanthanide complexes,18−23,7 some of which have marked selectivity and affinity for phosphate. Unfortunately, although many of those probes are selective for phosphate over competing anions such as bicarbonate and chloride, selectivity for orthophosphate over polyphosphates such as pyrophosphate has not yet been established.
The presence of iron in the active site of many phosphodiesterases and phosphatases suggest that iron could also be used in the design of receptors for phosphate.24−26 Yet, despite being the most abundant transition metal, iron is rarely explored in the design of molecular receptors, as evidenced by the paucity of iron complexes for anion recognition.27−30 To the best of our knowledge, no iron-based molecular receptors for any oxyanion that function at neutral pH and that is selective over interfering anions has been reported.7 Despite its hardness appropriate for hard anions, coordinatively unsaturated iron(III) complexes present several challenges for such applications that are not yet fully mastered. In particular, iron(III) complexes with open coordination sites have a propensity to form μ-oxo dimers,31,32 which prevents or diminishes further coordination of the targeted anion.33 The development of FeIII-based receptors for anions thus necessitates a re-engineering of the metal center to prevent such dimerization. In heme-based system, formation of μ-oxo dimers can be prevented by increasing the steric hindrance around the iron center with picket fences34,35 or via supramolecular assemblies with cyclodextrins.36 We postulated that, in nonheme iron-based systems, coordination at the open site by a weaker anion could be sufficient to prevent dimerization. Given the propensity of FeIII to quench the fluorescence of organic dyes,37 such metal-based receptors would also function as a fluorescent probe if this weak anion also fluoresces.
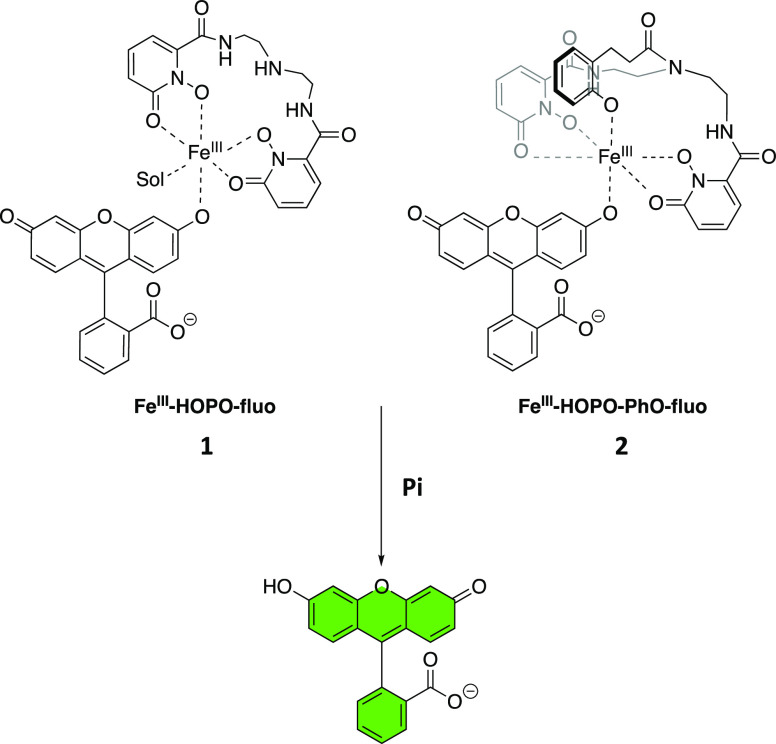
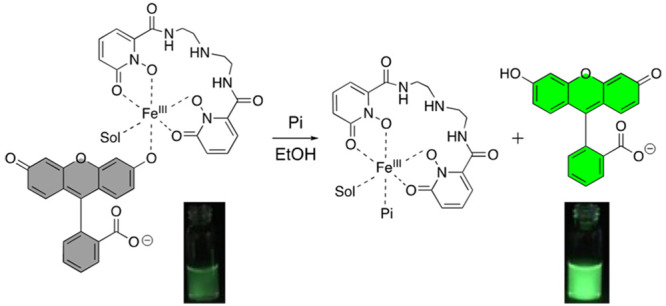
Other parameters should be taken into consideration in the design of the receptor. First, the affinity of receptors for anions are significantly influenced by the overall charge of the metal complex at the pH of interest.21 Highly negatively charged complexes should be avoided. The FeIII complex must also be sufficiently thermodynamically stable to prevent demetalation. The bioinorganic chemistry of siderophores, natural products that are strong iron chelators,38 suggest that both of these requirements can be met with tetra- or pentadentate ligands comprising all oxygen donor such as 1,2-hydroxypyridinone (HOPO). In our corresponding molecular receptors FeIII-HOPO-fluo (1) and FeIII-HOPO-PhO-fluo (2) (Figure 1), the remaining 1 or 2 open coordination sites are protected by fluorescein, a weaker ligand for FeIII than phosphate. We postulated that fluorescein would coordinate sufficiently strongly to iron(III) to prevent formation of μ-oxo dimers, but not too strongly as to enable displacement by phosphate.
Figure 1.
Chemical structures of iron(III)-based luminescent probes for phosphate and detection mechanism. Sol denotes solvent molecules.
Results and Discussion
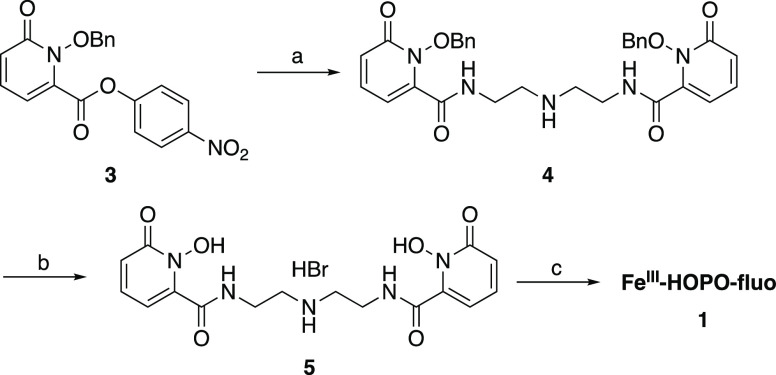
The receptors FeIII-HOPO-fluo and FeIII-HOPO-PhO-fluo were synthesized according to Schemes 1 and 2, respectively. The p-nitrophenol activated ester of the benzyl-protected HOPO podand 3, previously synthesized following literature precedence,39 selectively acylates the primary amino groups of the triamine backbone to yield the protected ligand 4. Deprotection under strong acidic conditions yields the final ligand 5, which was further metalated with FeIII in the presence of fluorescein to give the final receptor FeIII-HOPO-fluo.
Scheme 1. Synthesis of FeIII-HOPO-fluoa.
Experimental conditions: (a) (NH2CH2CH2)2NH, NEt3, CH2Cl2; (b) HBr/AcOH; (c) fluorescein, NaOH(aq), FeBr3, EtOH.
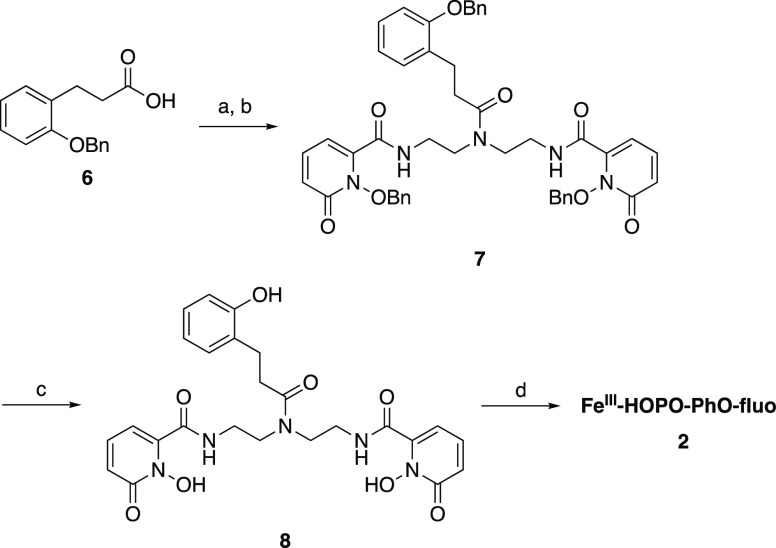
Scheme 2. Synthesis of FeIII-HOPO-PhO-fluo.
Experimental conditions: (a) (COCl)2, DMF (cat.), CH2Cl2; (b) 4, NEt3, CH2Cl2; (c) HBr/AcOH; (d) fluorescein, NaOH (aq), FeBr3, EtOH.
FeIII-HOPO-PhO-fluo employs a pentadentate ligand whose phenolate moiety occupies one more coordination site of the FeIII center. Activation of the benzyl-protected phenol podand 6, previously synthesized according to literature reports,40 with oxalyl chloride enabled coupling to the central secondary amine of 4, thereby yielding the protected ligand 7. Deprotection under strong acidic conditions yielded the final ligand 8 that was further metalated with FeIII in the presence of fluorescein to give the final receptor FeIII-HOPO-PhO-fluo, 2.
In both syntheses, the formation and purity of the ternary complexes 1 and 2 were confirmed by HPLC and ESI-MS (Figures S1, S3, S4, and S6). No μ-oxo diiron dimers were detected, confirming that coordination of the fluorescein ligand is sufficient to protect the FeIII center and prevent the formation of bimetallic species. In contrast, in the absence of fluorescein, the μ-oxo diiron dimer is the predominant species observed by MS. The significant line broadening observed in the 1H NMR of the ternary complexes in solution (Figures S2 and S5), which is typical of paramagnetic Fe(III) species, further confirmed coordination of fluorescein to the receptors 1 and 2. Both FeIII·fluorescein complexes were stable as solids and in ethanol for weeks; both can tolerate up to 10 vol % water with pH adjusted to 7 without significant fluorescein dissociation (<1%) in ethanol.
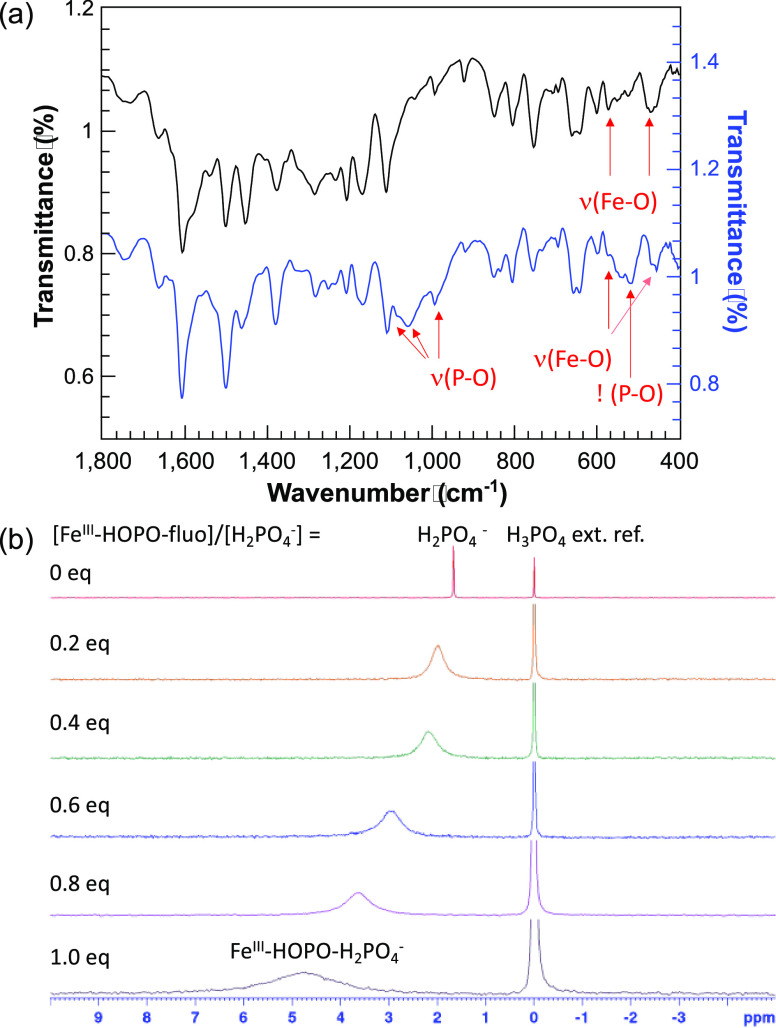
Direct coordination of phosphate to the iron centers of the receptors concomitant with displacement of the fluorescein moiety upon addition of the oxyanion was first confirmed from attenuated total reflection-infrared (ATR-IR) spectroscopic analysis of the precipitate obtained from FeIII-HOPO-fluo+Pi and FeIII-HOPO-PhO-fluo+Pi. The iron complex FeIII-HOPO-Pi displays the characteristic ν(Fe–O) vibrations at 571 and 461 cm–1, ν(P–O) bands at 1088, 1067, and 968 cm–1 and δ (O–P–O) bands at (541) cm–1 (Figure 2a).41−44 Each of those bands was also observed for the FeIII-HOPO-PhO-Pi adduct (Figure S7). These observations are in agreement with the formation of the postulated ternary complexes.
Figure 2.
Spectroscopic analyses of FeIII-HOPO-fluo + Pi. (a) ATR-IR spectra of FeIII-HOPO-fluo and FeIII-HOPO-Pi. (b) 31P NMR of Bu4N·H2PO4* titrated with FeIII-HOPO-fluo (DMSO-d6, 162 MHz). Experimental conditions: Samples for ATR-IR analysis was prepared by isolating, rinsing, and drying the precipitate formed from FeIII-HOPO-fluo + 1 equiv of Pi. [Bu4N·H2PO4] = 0.11 M in DMSO-d6. External reference: 85% H3PO4 diluted to 4% with DMSO. *Bu4N·H2PO4 was used due to the low solubility of inorganic phosphate in DMSO, and low solubility of FeIII-HOPO-Pi in CD3OD.
Formation of a FeIIIL·Pi ternary complex was also supported by NMR spectroscopy. The 31P NMR spectrum of FeIII-HOPO-Pi is nearly featureless (Figure S8), an observation that is attributed to the shortened transverse relaxation times, T2, of the 31P nucleus by the strongly paramagnetic FeIII. As is apparent in Figure 2b, when referenced to an external standard of H3PO4, in a titration monitored by NMR, the 31P signal of phosphate progressively shifts downfield from 1.61 to 4.72 ppm upon gradual addition of FeIII-HOPO-fluo (1). This shift is accompanied by a significant line broadening corresponding to a decrease in T2 of the phosphorus nuclei from 0.11 s (no FeIII-HOPO-fluo) to 1.95 ms (1 equiv of FeIII-HOPO-fluo). Both of those observations are attributable to coordination of orthophosphate to the strongly paramagnetic FeIII center.45,46 Of note, the presence of a single peak in the 31P also suggests the presence of a rapid equilibrium between bound and free phosphate. FeIII-HOPO-PhO-fluo (2), which employs a pentadentate ligand, displays similar behavior with the coordination of phosphate to the FeIII center confirmed from both the ATR-IR and the 31P NMR spectra (Figures S7 and S8, respectively). Unfortunately, further attempts to characterize the ternary phosphate complexes by mass spectrometry were unsuccessful due to the their low solubility and the known ability of phosphate to suppress ionization.47,48
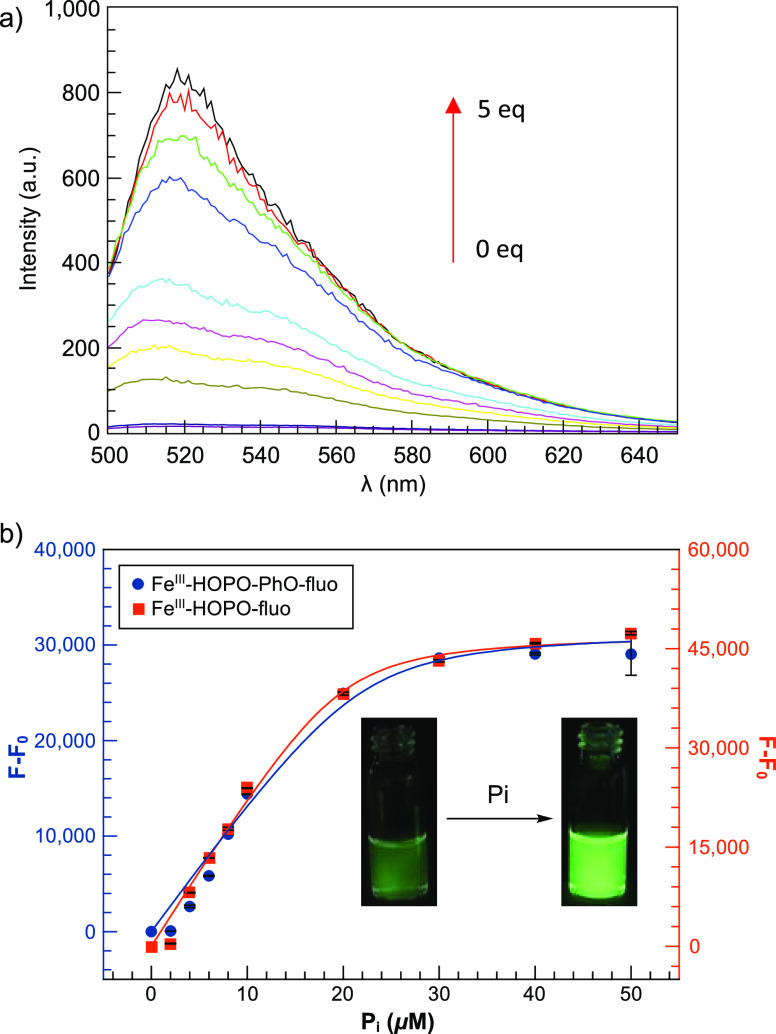
The indicator displacement assay (IDA) was evaluated by both UV–visible and fluorescence spectroscopy. A 20-fold turn-on fluorescence was observed upon gradual addition of 1 equiv of orthophosphate (Figure 3a). The fluorescence titrations (Figures 3 and S15) of both receptors were best fitted to a 1:1 binding model from which the equilibrium constants were derived (Table 1). This 1:1 stoichiometry was determined first by evaluating the fit of the titrations and subsequently confirmed by Job plots (Figures S11 and S12 for FeIII-HOPO-fluo and FeIII-HOPO-PhO-fluo, respectively). Interestingly, the use of a tetradentate ligand in FeIII-HOPO-fluo does not appear to favor coordination of two phosphate anions to the metal center. The two receptors display a similar turn-on response (20-fold at 1 equiv) and similar equilibrium constants for phosphate: 8.8 × 105 and 1.1 × 106 M–1 for FeIII-HOPO-fluo and FeIII-HOPO-PhO-fluo, respectively. This similarity in both turn-on response and apparent equilibrium constants could be attributed to the comparable core structure of both receptors. Interestingly, the extra phenolate podand of 2 does not appear to affect displacement of the fluorescein moiety by phosphate. A likely coordinated solvent molecule appears to have a similar effect.
Figure 3.
Fluorescence titration of FeIII-HOPO-fluo and FeIII-HOPO-PhO-fluo with phosphate (Pi): (a) fluorescence spectra of FeIII-HOPO-fluo with phosphate. (b) Increase in emission intensity. Experimental conditions: [FeIII-HOPO-PhO-fluo] and [FeIII-HOPO-fluo] = 10 μM in wet ethanol. pH = 7. λexcitation = 456 nm, excitation and emission slit widths = 5 nm, voltage = 600 V. T = 25 °C. F = integrated fluorescence intensity from 500 to 650 nm in the presence of anions, Fo = integrated fluorescence intensity in the absence of anions. Fluorescence spectra were obtained 5 min after mixing to ensure that thermodynamic equilibrium was reached. The pH of all solutions was adjusted to 7 carefully by addition of either HCl or NaOH, as necessary.
Table 1. Apparent Equilibrium Constants of FeIII-HOPO-fluo (1) and FeIII-HOPO-PhO-fluo (2) with Orthophosphate.
| Ka (M–1) | |
|---|---|
| FeIII-HOPO-fluo | 8.8 ± 3.4 × 105 |
| FeIII-HOPO-PhO-fluo | 1.1 ± 0.5 × 106 |
The limit of detection (LOD) of phosphate by the two FeIII receptors, commonly estimated as three times the standard deviation of measurement (3σ), are 3.5 and 4.1 μM for FeIII-HOPO-fluo (1) and FeIII-HOPO-PhO-fluo (2), respectively (Table S1). Although not as sensitive as prior EuIII probes previously developed by our group,21,22 these iron receptors are sensitive enough to detect problematic phosphate levels in eutrophic samples (2–10 μM).49,50
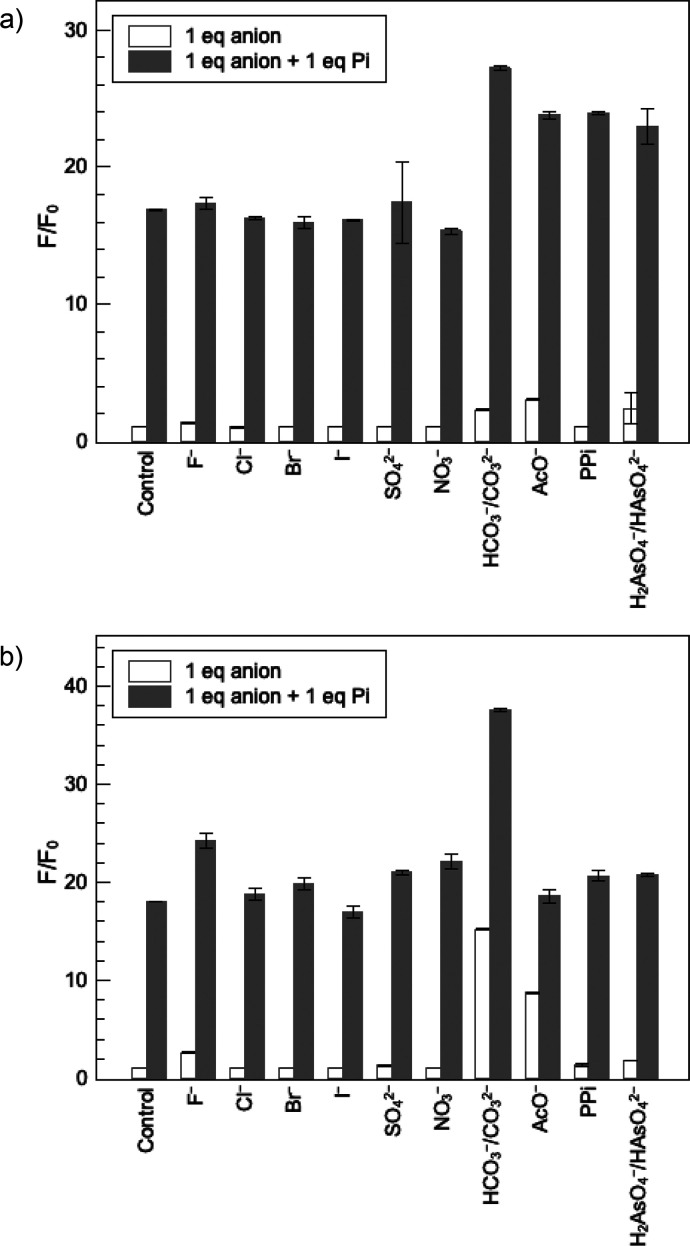
The selectivity of the two iron receptors for phosphate over competing anions commonly found in environmental samples was also evaluated by fluorescence spectroscopy. As shown in the white bars of Figure 4, the fluorescence intensity of both probes is not affected by the addition of 1 equiv of common competing anions including halides, sulfate, and nitrate. Subsequent addition of 1 equiv of phosphate restores the luminescence of the indicator (Figure 4, gray bars), further indicating that these competing anions do not interfere with detection of phosphate. Interestingly, FeIII-HOPO-fluo is more selective over bicarbonate and acetate than FeIII-HOPO-PhO-fluo. A more sterically hindered recognition site therefore does not appear to generate higher selectivity for the targeted anion.
Figure 4.
Fluorescence response of (a) FeIII-HOPO-fluo and (b) FeIII-HOPO-PhO-fluo to competing anions. White bars represent the relative fluorescence intensity after addition of 1 equiv of the appropriate anions (NaF, NaCl, NaBr, NaI, Na2SO4, NaNO3, NaHCO3, NaOAc, Na4P2O7, and Na2HAsO4·7H2O). Gray bars represent the relative fluorescence intensity after subsequent addition of 1 equiv of phosphate (Pi). PPi denotes pyrophosphate. Experimental conditions: [FeIII-HOPO-fluo] = 10 μM in wet ethanol, pH 7, λexcitation = 456 nm, excitation and emission slit widths = 5 nm, F = integrated fluorescence intensity from 500 to 650 nm in the presence of anions, Fo = integrated fluorescence intensity in the absence of anions. T = 25 °C. The pH of all solutions was adjusted to 7 carefully using 0.01 N HCl and 0.01N NaOH. Fluorescence spectra were obtained 5 min after mixing to ensure that thermodynamic equilibrium was reached. Control denotes the same volume of water was used in replacement of anions.
Uniquely, and importantly, both FeIII-HOPO-fluo (1) and FeIII-HOPO-PhO-fluo (2) are selective for phosphate over pyrophosphate. Whereas numerous probes selective for pyrophosphate over phosphates have been described in the literature,39−41 to the best of our knowledge, complexes 1 and 2 are unique in their reverse selectivity for phosphate over pyrophosphate. This selectivity likely stems from the preferred bidentate binding mode of pyrophosphate and likely steric hindrance at the coordination site.51,52 Since only one displaceable fluorescein is present, bidentate binding is disfavored. The slightly softer anion, arsenate, also does not displace fluorescein despite its structural similarity to phosphate. This is an unusual selectivity given that most metal probes for phosphate also respond to arsenate.7 As such, these fluorescent iron(III) probes offer a distinctive ability to rapidly monitor the level of the most important phosphorus species causing nutrient pollution in surface water: phosphate.
Conclusion
We describe two nonheme iron(III) complexes, FeIII-HOPO-fluo and FeIII-HOPO-PhO-fluo, for selective recognition of inorganic phosphate via indicator displacement assay. In both cases, the open coordination sites were sufficiently protected by weakly coordinating fluorescein to prevent dimerization in aerated solutions. Coordination of inorganic phosphate concomitant with displacement of the fluorescein moiety increases the emission of the latter by 20-fold. Uniquely, these probes distinguish themselves from other receptors that function by direct metal coordination in that they are highly selective for phosphate over pyrophosphate. They are also highly selective over common competing endogenous anions such as carbonate, nitrate, sulfate, halides, and, unusually, arsenate. The limit of detection of the iron(III) receptors, 3.5 and 4.1 μM for FeIII-HOPO-fluo and FeIII-HOPO-PhO-fluo, respectively, enables detection of phosphate typical of eutrophic water samples. On this basis, the two iron(III) probes enable rapid and facile detection of phosphate in eutrophic samples. To the best of our knowledge, these are the first examples employing nonheme FeIII-based molecular receptors for anions. These results thus also provide a blueprint for the development of inorganic phosphate probes that use iron, an earth abundant and economical element.
Acknowledgments
We thank Dr. Marc Hillmyer for the use of the ATR-IR spectrophotometer.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.2c00200.
Experimental details and characterization data, including 1H and 13C NMR spectra of intermediates and final ligands, ESI-MS data of the intermediates, ligands, and FeIII complexes, HPLC chromatograms of the ligands and FeIII complexes, ultraviolet–visible spectra, fluorescence spectra, and ATR-IR spectra of the FeIII complexes in the absence and presence of phosphate, Job plots of the FeIII complexes with phosphate (PDF)
The authors acknowledge the support of the National Institutes of Health provided by R01 DK124333-01A1. S.-Y.H. was supported in part by a John Wertz Fellowship from the Department of Chemistry of the University of Minnesota and from a Doctoral Dissertation Fellowship from the University of Minnesota.
The authors declare no competing financial interest.
Supplementary Material
References
- National Oceanic and Atmospheric Administration . What is eutrophication? https://oceanservice.noaa.gov/facts/eutrophication.html (accessed Nov 9, 2020).
- Bricker S. B.Effects of Nutrient Enrichment in the Nation’s Estuaries: A Decade of Change: National Estuarine Eutrophication Assessment Update. NOAA Coastal Ocean Program decision analysis series; no. 26; 328; Silver Spring, MD, 2007.
- 5.6 Phosphorus; Monitoring & Assessment; US EPA. https://archive.epa.gov/water/archive/web/html/vms56.html (accessed Nov 23, 2020).
- Boyd C. E.Water Quality: An Introduction, 3rd ed.; Springer International Publishing, 2020. [Google Scholar]
- Rice E. W.; Baird R. B.; Eaton A. D.. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation, 2017. [Google Scholar]
- Sundareshwar P. V.; Morris J. T.; Pellechia P. J.; Cohen H. J.; Porter D. E.; Jones B. C. Occurrence and Ecological Implications of Pyrophosphate in Estuaries. Limnol. Oceanogr 2001, 46 (6), 1570–1577. 10.4319/lo.2001.46.6.1570. [DOI] [Google Scholar]
- Ramakrishnam Raju M. V.; Harris S. M.; Pierre V. C. Design and Applications of Metal-Based Molecular Receptors and Probes for Inorganic Phosphate. Chem. Soc. Rev. 2020, 49 (4), 1090–1108. 10.1039/C9CS00543A. [DOI] [PubMed] [Google Scholar]
- Meng Q.; Wu M.; Shang Z.; Zhang Z.; Zhang R. Responsive Gadolinium(III) Complex-Based Small Molecule Magnetic Resonance Imaging Probes: Design, Mechanism and Application. Coord. Chem. Rev. 2022, 457, 214398. 10.1016/j.ccr.2021.214398. [DOI] [Google Scholar]
- Macreadie L. K.; Gilchrist A. M.; McNaughton D. A.; Ryder W. G.; Fares M.; Gale P. A. Progress in Anion Receptor Chemistry. Chem. 2022, 8 (1), 46–118. 10.1016/j.chempr.2021.10.029. [DOI] [Google Scholar]
- Chen L.; Berry S. N.; Wu X.; Howe E. N. W.; Gale P. A. Advances in Anion Receptor Chemistry. Chem. 2020, 6 (1), 61–141. 10.1016/j.chempr.2019.12.002. [DOI] [Google Scholar]
- Bodman S. E.; Butler S. J. Advances in Anion Binding and Sensing Using Luminescent Lanthanide Complexes. Chem. Sci. 2021, 12 (8), 2716–2734. 10.1039/D0SC05419D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre V. C.; Wilharm R. K. Design Principles and Applications of Selective Lanthanide-Based Receptors for Inorganic Phosphate. Front. Chem. 2022, 10, 821020. 10.3389/fchem.2022.821020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen B.; Kumar Patra S.; Rabha M.; Kumar Sheet S.; Aguan K.; Samanta D.; Khatua S. Luminescence Detection of Ag+ and Phosphate Ions by a Ruthenium(II) Complex-Based Multianalyte Probe: A Combined Spectroscopic, Crystallographic, and Theoretical Approach. Eur. J. Inorg. Chem. 2021, 2021 (35), 3549–3560. 10.1002/ejic.202100293. [DOI] [Google Scholar]
- Deetz M. J.; Smith B. D. Heteroditopic Ruthenium(II) Bipyridyl Receptor with Adjacent Saccharide and Phosphate Binding Sites. Tetrahedron Lett. 1998, 39 (38), 6841–6844. 10.1016/S0040-4039(98)01492-0. [DOI] [Google Scholar]
- Kumar P.; Kumar S.. Detection of Bio-Relevant Metal Ions by Luminescent Ru(II)-Polypyridyl Based Sensors; IntechOpen, 2021. [Google Scholar]
- Marcus Y. Thermodynamics of Solvation of Ions. Part 5.—Gibbs Free Energy of Hydration at 298.15 K. J. Chem. Soc., Faraday Trans. 1991, 87 (18), 2995–2999. 10.1039/FT9918702995. [DOI] [Google Scholar]
- Beer P. D.; Gale P. A. Anion Recognition and Sensing: The State of the Art and Future Perspectives. Angew. Chem., Int. Ed. 2001, 40 (3), 486–516. . [DOI] [PubMed] [Google Scholar]
- Han M. S.; Kim D. H. Naked-Eye Detection of Phosphate Ions in Water at Physiological PH: A Remarkably Selective and Easy-To-Assemble Colorimetric Phosphate-Sensing Probe. Angew. Chem., Int. Ed. 2002, 41 (20), 3809–3811. . [DOI] [PubMed] [Google Scholar]
- Tobey S. L.; Jones B. D.; Anslyn E. V. C3v Symmetric Receptors Show High Selectivity and High Affinity for Phosphate. J. Am. Chem. Soc. 2003, 125 (14), 4026–4027. 10.1021/ja021390n. [DOI] [PubMed] [Google Scholar]
- Harris S. M.; Nguyen J. T.; Pailloux S. L.; Mansergh J. P.; Dresel M. J.; Swanholm T. B.; Gao T.; Pierre V. C. Gadolinium Complex for the Catch and Release of Phosphate from Water. Environ. Sci. Technol. 2017, 51 (8), 4549–4558. 10.1021/acs.est.6b05815. [DOI] [PubMed] [Google Scholar]
- Huang S.-Y.; Qian M.; Pierre V. C. A Combination of Factors: Tuning the Affinity of Europium Receptors for Phosphate in Water. Inorg. Chem. 2019, 58 (23), 16087–16099. 10.1021/acs.inorgchem.9b02650. [DOI] [PubMed] [Google Scholar]
- Huang S.-Y.; Qian M.; Pierre V. C. The Ligand Cap Affects the Coordination Number but Not Necessarily the Affinity for Anions of Tris-Bidentate Europium Complexes. Inorg. Chem. 2020, 59 (6), 4096–4108. 10.1021/acs.inorgchem.0c00137. [DOI] [PubMed] [Google Scholar]
- Ramakrishnam Raju M. V.; Wilharm R. K.; Dresel M. J.; McGreal M. E.; Mansergh J. P.; Marting S. T.; Goodpaster J. D.; Pierre V. C. The Stability of the Complex and the Basicity of the Anion Impact the Selectivity and Affinity of Tripodal Gadolinium Complexes for Anions. Inorg. Chem. 2019, 58 (22), 15189–15201. 10.1021/acs.inorgchem.9b02133. [DOI] [PubMed] [Google Scholar]
- Schenk G.; Mitić N.; Gahan L. R.; Ollis D. L.; McGeary R. P.; Guddat L. W. Binuclear Metallohydrolases: Complex Mechanistic Strategies for a Simple Chemical Reaction. Acc. Chem. Res. 2012, 45 (9), 1593–1603. 10.1021/ar300067g. [DOI] [PubMed] [Google Scholar]
- Rodriguez F.; Lillington J.; Johnson S.; Timmel C. R.; Lea S. M.; Berks B. C. Crystal Structure of the Bacillus Subtilis Phosphodiesterase PhoD Reveals an Iron and Calcium-Containing Active Site. J. Biol. Chem. 2014, 289 (45), 30889–30899. 10.1074/jbc.M114.604892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi F.; Trebacz M.; Kokot T.; Hoermann B.; Rios P.; Barabas O.; Köhn M. Effects of Stably Incorporated Iron on Protein Phosphatase-1 Structure and Activity. FEBS Lett. 2018, 592 (24), 4028–4038. 10.1002/1873-3468.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano K.; Kitagishi H.; Tamura S.; Yamada A. Anion Binding to a Ferric Porphyrin Complexed with Per-O-Methylated β-Cyclodextrin in Aqueous Solution. J. Am. Chem. Soc. 2004, 126 (46), 15202–15210. 10.1021/ja045472i. [DOI] [PubMed] [Google Scholar]
- Watanabe K.; Kitagishi H.; Kano K. Supramolecular Ferric Porphyrins as Cyanide Receptors in Aqueous Solution. ACS Med. Chem. Lett. 2011, 2 (12), 943–947. 10.1021/ml200231x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P. K.; Bhattacharya S. G.; Banerjee R.; Banerjea D. Thermodynamic and Kinetic Studies on the Iron(III)-Hydroxamate Interaction in Acid Media. J. Coord. Chem. 1989, 19 (4), 311–320. 10.1080/00958978909408834. [DOI] [Google Scholar]
- Gabričević M.; Crumbliss A. L. Kinetics and Mechanism of Iron(III)–Nitrilotriacetate Complex Reactions with Phosphate and Acetohydroxamic Acid. Inorg. Chem. 2003, 42 (13), 4098–4101. 10.1021/ic026281o. [DOI] [PubMed] [Google Scholar]
- Nishid Y.; Ito S. Structures and Reactivities of Several Iron(III) Complexes in the Presence of Hydrogen Peroxide: Relevance to Induction of Tissue Damage Caused by Iron(III) Chelates in Rats. Polyhedron 1995, 14 (17), 2301–2308. 10.1016/0277-5387(95)00109-6. [DOI] [Google Scholar]
- Mizuno R.; Kawabata T.; Sutoh Y.; Nishida Y.; Okada S. Oxidative Renal Tubular Injuries Induced by Aminocarboxylate-Type Iron (III) Coordination Compounds as Candidate Renal Carcinogens. Biometals 2006, 19 (6), 675–683. 10.1007/s10534-006-9004-4. [DOI] [PubMed] [Google Scholar]
- Gabričević M.; Crumbliss A. L. Kinetics and Mechanism of Iron(III)–Nitrilotriacetate Complex Reactions with Phosphate and Acetohydroxamic Acid. Inorg. Chem. 2003, 42 (13), 4098–4101. 10.1021/ic026281o. [DOI] [PubMed] [Google Scholar]
- Jones R. D.; Summerville D. A.; Basolo F. Synthetic Oxygen Carriers Related to Biological Systems. Chem. Rev. 1979, 79 (2), 139–179. 10.1021/cr60318a002. [DOI] [Google Scholar]
- Shikama K. Stability Properties of Dioxygen-Iron(II) Porphyrins: An Overview from Simple Complexes to Myoglobin. Coord. Chem. Rev. 1988, 83, 73–91. 10.1016/0010-8545(88)80019-5. [DOI] [Google Scholar]
- Huang X.; Groves J. T. Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev. 2018, 118 (5), 2491–2553. 10.1021/acs.chemrev.7b00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M. A.; Hoyland B.; Carter S.; Zhang C.; Haugland R. P. Fluorescent Ion Indicators for Detecting Heavy Metals. Proc. SPIE 1995, 2388, 238–244. 10.1117/12.208483. [DOI] [Google Scholar]
- Raymond K. N.; Allred B. E.; Sia A. K. Coordination Chemistry of Microbial Iron Transport. Acc. Chem. Res. 2015, 48 (9), 2496–2505. 10.1021/acs.accounts.5b00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérard F.; Beyler M.; Lee Y.-S.; Tripier R.; Gestin J.-F.; Brechbiel M. W. Investigation of the Complexation of NatZr(IV) and 89Zr(IV) by Hydroxypyridinones for the Development of Chelators for PET Imaging Applications. Dalton Trans 2017, 46 (14), 4749–4758. 10.1039/C6DT04625H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsberry K. L.; Borchardt R. T. The Lactonization of 2’-Hydroxyhydrocinnamic Acid Amides: A Potential Prodrug for Amines. J. Org. Chem. 1990, 55 (23), 5867–5877. 10.1021/jo00310a019. [DOI] [Google Scholar]
- Arai Y.; Sparks D. L. ATR–FTIR Spectroscopic Investigation on Phosphate Adsorption Mechanisms at the Ferrihydrite–Water Interface. J. Colloid Interface Sci. 2001, 241 (2), 317–326. 10.1006/jcis.2001.7773. [DOI] [Google Scholar]
- Rabie M. S.; Balkees H. Infrared Study of FeO·OH→Fe2O3 Thermal Transformation. J. Chem. Sci. 1986, 96 (5), 315–320. 10.1007/BF02895727. [DOI] [Google Scholar]
- Hewkin D. J.; Griffith W. P. Infrared Spectra of Binuclear Complexes. J. Chem. Soc. A 1966, 0, 472–475. 10.1039/j19660000472. [DOI] [Google Scholar]
- Aqdim S.; Ouchetto M. Elaboration and Structural Investigation of Iron (III) Phosphate Glasses. Adv. Mater. Phys. Chem. 2013, 3 (8), 332–339. 10.4236/ampc.2013.38046. [DOI] [Google Scholar]
- Quin L. D.; Williams A. J.. Practical Interpretation of P-31 NMR Spectra and Computer Assisted Structure Verification; Advanced Chemistry Development: Toronto, 2004. [Google Scholar]
- Merkx D. W.H.; Delic F.; Wierenga P. A.; Hennebelle M.; van Duynhoven J. P.M. 31P NMR Assessment of the Phosvitin-Iron Complex in Mayonnaise. Magn. Reson. Chem. 2019, 57 (9), 540–547. 10.1002/mrc.4808. [DOI] [PubMed] [Google Scholar]
- King R.; Bonfiglio R.; Fernandez-Metzler C.; Miller-Stein C.; Olah T. Mechanistic Investigation of Ionization Suppression in Electrospray Ionization. J. Am. Soc. Mass Spectrom. 2000, 11 (11), 942–950. 10.1016/S1044-0305(00)00163-X. [DOI] [PubMed] [Google Scholar]
- Mallet C. R.; Lu Z.; Mazzeo J. R. A Study of Ion Suppression Effects in Electrospray Ionization from Mobile Phase Additives and Solid-Phase Extracts. Rapid Commun. Mass Spectrom. 2004, 18 (1), 49–58. 10.1002/rcm.1276. [DOI] [PubMed] [Google Scholar]
- Boyd C. E.Eutrophication. In Water Quality: An Introduction; Boyd C. E., Ed.; Springer International Publishing: Cham, 2020; pp 311–322. [Google Scholar]
- Boyd C. E.Phosphorus. In Water Quality: An Introduction; Boyd C. E., Ed.; Springer International Publishing: Cham, 2020; pp 291–309. [Google Scholar]
- Ikotun O. F.; Marino N.; Kruger P. E.; Julve M.; Doyle R. P. Coordination Complexes Incorporating Pyrophosphate: Structural Overview and Exploration of Their Diverse Magnetic, Catalytic and Biological Properties. Coord. Chem. Rev. 2010, 254 (7), 890–915. 10.1016/j.ccr.2009.12.015. [DOI] [Google Scholar]
- Gupta A.; Pratt R.; Mishra B. Physicochemical Characterization of Ferric Pyrophosphate Citrate. Biometals 2018, 31 (6), 1091–1099. 10.1007/s10534-018-0151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.