Abstract
Purpose
To evaluate the safety and efficacy of the Tigertriever 13 (Rapid Medical, Yoqneam, Israel) stent retriever in acute ischemic stroke (AIS) patients with primary or secondary distal, medium vessel occlusions (DMVO).
Methods
We performed a retrospective analysis of all consecutive AIS patients who underwent thrombectomy with the Tigertriever13 for DMVO. Patients’ characteristics were reviewed, procedural complications, angiographic (modified thrombolysis in cerebral infarction score [mTICI]) and clinical (modified Rankin Scale [mRS]) outcomes were documented.
Results
Between November 2019 and November 2020, 16 patients with 17 DMVO were included (40% female, median age 60 [50–65] years). The Tigertriever13 was used in 11/17 (65%, median NIHSS of 8 [6–15]) primary DMVO and in 6/17 (35%, median NIHSS of 20 [13–24]) cases of secondary DMVO after a proximal thrombectomy. The successful reperfusion rate (mTICI 2b, 2c, 3) was 94% (16/17) for the dedicated vessel. At day 1, CT imaging showed a subarachnoid hemorrhage in 29% of the cases and a parenchymal hematoma in 12%. At 3 months, 65% of the patients (11/17) had a favorable outcome (mRS 0–2).
Conclusion
Mechanical thrombectomy using the Tigertriever13 appears to be safe and effective for DMVO. Clinical and anatomical results are in line with those of patients with proximal occlusions.
Keywords: Endovascular recanalization, mild symptoms, distal, thrombectomy, stroke
Introduction
Mechanical thrombectomy is safe and effective for acute ischemic stroke (AIS) patients with an anterior circulation large vessel occlusion (LVO) 1 and is now standard of care for such patients. 2
However, questions remain for patients with distal, medium vessel occlusions (DMVO) as defined recently: anterior cerebral artery [ACA], M2–M4 middle cerebral artery [MCA], posterior cerebral artery [PCA], posterior inferior cerebellar artery [PICA], anterior inferior cerebellar artery [AICA], and superior cerebellar artery [SCA]. 3 DMVO may represent up to 25–40% of AIS (both primary thrombo-emboli or embolies to distal territories after a LVO thrombectomy) and while thrombolytic therapy has shown a better efficacy in DMVO compared to LVO, 3 it still fails to recanalize more than one half of these occlusions. 3
Since those patients were excluded from most previous randomized trials, 1 thrombectomy efficacy and safety for such DMVO remains unclear, especially since distal catheterization and thrombectomy might be associated with higher risks of procedural complications with reported difficulties 4 and risks either while reaching the occluded vessel, crossing the occlusion or retrieving the stent retriever.5,6 While these complications remain hopefully rare, 4 they may be associated with life-threatening situations.
Recently, consensus statements start being proposed on this new endovascular frontier, 3 as potential benefit of thrombectomy for DMVO is suggested,7,8 and several devices are being used with a good efficacy and safety9–11 in such indications.
We aimed to retrospectively assess the safety, technical and clinical efficacy of a new stent retriever designed for DMVO, the Tigertriever 13 12 (Rapid Medical, Yoqneam, Israel) in consecutive patients presenting either with a primary DMVO thrombo-emboli or presenting with embolies to distal territories after a thrombectomy for an AIS with a LVO.
Methods
The present retrospective study received approval from the local ethical standards committee. Informed consent from participants was waived. The data that support the findings of this study are available from the corresponding author upon reasonable request
Population
Data were extracted from our single-center stroke database, including all consecutive patients with an AIS who underwent thrombectomy between November 2019 and November 2020.
Retrospective inclusion criteria were: (1) AIS with an isolated primary DMVO (anterior cerebral artery [ACA], M2–M4 middle cerebral artery [MCA], posterior cerebral artery [PCA]) 3 seen on initial computed tomography (CT); or an AIS with a LVO treated by thrombectomy, with embolies to distal territories; or DMVO complicating a distinct intervention (aneurysm coiling); (2) National Institutes of Health Stroke Scale (NIHSS) ≥1; (3) time from symptom onset to groin puncture <6 h unless a perfusion CT demonstrated a relevant cerebral blood flow to cerebral blood volume (CBF/CBV) mismatch; and (4) mechanical thrombectomy performed with the Tigertriever13 device.
The decision to start the endovascular procedure was made by an interdisciplinary consensus on an individual basis, after consultation with the neurointerventionist, stroke neurologist, and diagnostic neuroradiologist; the technical aspects were left at the discretion of the treating neurointerventionist physician.
Thrombectomy procedures were otherwise carried out using standard of care recommendations. Patient's baseline clinical and radiological characteristics, procedure details, and outcomes were collected using standardized definitions. 13
All cases were reviewed by two board-certified neuroradiologists (A.G. and B.L.), they determined the angiographic treatment success after each attempt. In cases of inconsistency concerning the final result, a decision was made by consensus.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Tigertriever 13 device
The Tigertriever 13 is a stentriever comprised of a fine wire mesh mounted on a flexible shaft that expands when the physician pulls a control wire that is connected to its distal end (see Figure 1). “The basic technical concept comes from the Comaneci Device (Rapid Medical), a compliant manually expandable mesh.”14,15
Figure 1.
The Tigertriever 13. Progressive opening of the device (A–D) is shown. (E) The compliant mesh with the inner wire connected to the control handle to open and close the device.
Since the wires of the mesh are completely radiopaque, the physician sees the mesh under fluoroscopy and controls it until it conforms to the vessel diameter. The design of the wire mesh is optimized to penetrate the clot and encapsulate it during retrieval. The mesh is constructed from nitinol wires depending of the size of the device. The distal end of the device consists of a flexible tip, allowing a gentle and safe navigation.14,15
Tigertriever 13's low profile enables using a small microcatheter and hence a small guidewire for safer procedures. Indeed, the Tigertriever 13 is delivered through a microcatheter with an internal diameter of 0.0165 inches (body), measures 20.5 mm in length and can be expanded from 0.5 up to 2.5 mm. Its controllability allows the device to be adapted in order to minimize the resistance during the clot retrieval, which could make it safer.
Outcomes
The primary outcome was successful recanalization (according to the modified Thrombolysis In Cerebral Infarction [mTICI] scale 2b–3 16 at the end of the procedure, considering the territory distal to the targeted occlusion as 100%, 17 reperfusion rates were translated into the mTICI grades.
Secondary efficacy outcomes included good clinical outcome at 3 months, defined as modified Rankin Scale (mRS) of 0–2, excellent clinical outcome (defined by a mRS of 0 or 1) and mortality rate at 3 months. Safety outcomes included the rate of complications, postinterventional subarachnoid hemorrhages (SAH), and hemorrhagic transformation (of any type according to the European Cooperative Acute Stroke Study [ECASS]) at day 1 imaging (dual-energy CT-scan).
Patients were analyzed altogether then separately depending on the thrombectomy approach: Tigertriever13 for patients presenting with a primary DMVO or Tigertriever13 for patients with a secondary DMVO after a proximal thrombectomy with another technique.
Endovascular procedures
All procedures were performed with the patient under general anesthesia or conscious sedation on a biplane digital subtraction angiography unit (Philips Allura Clarity). A combination of a 6F femoral or radial sheath (NeuronMax, Penumbra, Alameda, CA, USA) and an aspiration catheter (SOFIA 6F or 5F, Microvention, Tustin, CA, USA) were placed in the proximal segment of the internal carotid artery (ICA) or the vertebral artery (VA), a diagnostic angiography of the target artery was performed to visualize the occlusion of the intracranial artery.
The occluded artery was then catheterized using a Headway Duo (Microvention) microcatheter. Microcatheterizations were carried out using Traxcess14 (Microvention, Fremont, CA, USA), Synchro14 microguidewires (Stryker, Fremont, CA, USA) or an Hybrid 0.008 (Balt, Montmorency, FRANCE). Distal injections through the microcatheter were performed after crossing the occlusion to ensure its safe position.
The Tigertriever13 was loaded into the microcatheter and advanced towards the occlusion, then we gently unsheathed the Tigertriever13 by pulling back the microcatheter while the delivery wire was maintained in a stable position, distal to the thrombus. The optimal position we tried to obtain aimed to optimize the working length of the device, with the proximal marker of the Tigertriever 13 as close as possible to the proximal part of the thrombus since the device would shorten from the distal end during opening. The device was opened by a stepwise maneuver under permanent fluoroscopy. The stable Tigertriever device was then used to advance the aspiration catheter towards the occlusion14,15 if judged appropriate by the neurointerventionist
The Tigertriever 13 was then pulled into the aspiration catheter under continuous manual aspiration or retrieved with the aspiration catheter simultaneously. The maneuver was repeated in cases of a remaining occlusion.
There was no restriction in the number of attempts conducted with the Tigertriever and the decision to switch to another device was at the operator's discretion. This was likewise in procedures that were initiated with another device and switched to the Tigertriever.
All statistical analyses were performed with XLSTAT (Addinsoft, New York City, NY).
Results
A total of 16 patients with 17 DMVO were included (see the flow-chart, Figure e-1). The Tigertriever13 was used in 11/17 (65%) primary DMVO while used in 6/17 (35%) cases for secondary DMVO after a proximal thrombectomy with another technique.
Overall results
Among 16 patients, median age was 60 (50–65) years, 40% were female, median NIHSS was 11 (7–20), 35% underwent intravenous thrombolysis (IVtPA) (see Table 1).
Table 1.
Baseline characteristics for all patients and depending on the DMVO.
| All | Primary DMVO | Secondary DMVO | |
|---|---|---|---|
| Number of occlusions | 17 (100%) | 11 (65%) | 6 (35%) |
| Age, years (median, IQR) | 60 (50–65) | 63 (53–69) | 60 (52–60) |
| Female (%) | 7 (40%) | 5 (45%) | 2 (33%) |
| Medical history | |||
| High blood pressure (%) | 12 (71%) | 10 (91%) | 2 (33%) |
| Diabetes (%) | 2 (12%) | 1 (9%) | 1 (17%) |
| Hyperlipidemia (%) | 9 (53%) | 6 (55%) | 3 (50%) |
| Weight (kg) (median, IQR) | 80 (60–85) | 75 (55–83) | 85 (73–85) |
| Antiplatelets (%) | 5 (29%) | 5 (45%) | 0 (0%) |
| Anticoagulants (%) | 3 (18%) | 2 (18%) | 1 (17%) |
| Current smoking (%) | 4 (24%) | 3 (27%) | 1 (17%) |
| Pre stroke mRS 0 (%) | 12 (71%) | 7 (64%) | 5 (83%) |
| Clinical presentation | |||
| Heart rate (bpm) (median, IQR) | 70 (68–80) | 78 (62–80) | 70 (69–75) |
| Systolic blood pressure (mmHg) (median, IQR) | 140 (119–157) | 150 (125–167) | 130 (120–143) |
| Diastolic blood pressure (mmHg) (median, IQR) | 85 (76–90) | 85 (74–90) | 80 (78–84) |
| Temperature (°C) (median, IQR) | 37 (36.6–37) | 37 (36.7–37) | 36.8 (36.6–37) |
| Glycemia (mmol/L) (median, IQR) | 7.1 (5.9–9) | 7.2 (5.9–9.2) | 6.9 (6.1–8.0) |
| Baseline NIHSS (median, IQR) | 11 (7–20) | 8 (6–15) | 20 (13–24) |
| Baseline NIHSS <6 (%) | 3 (18%) | 2 (18%) | 1 (17%) |
| Intravenous tPA (%) | 6 (35%) | 4 (36%) | 2 (33%) |
| Times | |||
| Time onset to puncture in min (median, IQR) | 240 (200–394) | 240 (220–353) | 213 (151–741) |
| Unknown onset (%) | 4 (24%) | 2 (18%) | 2 (33%) |
| Imaging | |||
| Baseline MRI (%) | 1 (6%) | 1 (9%) | 0 (0%) |
| Pc-ASPECTS (median, IQR) | 9 (8–10) | 10 (9–10) | 9 (8–10) |
| Core volume (mL) | 6 (4–23) | 5 (0–6) | 30 (26–40) |
| TMax > 6 s (mL) | 34 (24–60) | 25 (19–32) | 188 (62–188) |
| TMax > 10 s (mL) | 7 (0–19) | 0 (0–5) | 89 (20–89) |
| Mismatch ratio | 4.7 (3.4–6.6) | 4.0 (3.2–7.2) | 4.7 (4.7–4.8) |
| Mismatch volume (mL) | 29 (17–74) | 18 (14–29) | 148 (49–148) |
| Side (left, %) | 13 (76%) | 8 (73%) | 5 (83%) |
| Etiology | |||
| Atherosclerosis (%) | 2 (12%) | 2 (18%) | 0 (0%) |
| Cardio-embolic (%) | 10 (59%) | 7 (64%) | 3 (50%) |
| Dissection (%) | 4 (24%) | 1 (9%) | 3 (50%) |
| Unknown (%) | 1 (6%) | 1 (9%) | 0 (0%) |
Mechanical thrombectomy were mostly performed under direct general anesthesia or general anesthesia after conscious sedation first (16/17, 94%). One was performed through the right radial artery, the others through a femoral approach. In all but one case, a Neuronmax was used as a guiding catheter (one procedure performed in a 9YO child using a 6F femoral sheath, an Envoy 6F [Codman Neuro, Raynham, MA] and a Sofia 5F). A Sofia 6F was used as an aspiration catheter in 5/17 cases while a Sofia 5F was used in 12/17 cases. All Tigertriever 13 were deployed using a Headway Duo over an Hybrid 0.008 guidewire (4/17), a Traxcess 14 (6/17), or a Synchro 14 (7/17).
The overall successful reperfusion rate (mTICI 2b, 2c, 3) was 94% (16/17, see Table 2) for the dedicated vessel after a median of 1 Tigertriever13 pass (IQR of 1–2). Spasm after the Stent retriever pass was seen in 17% (3/17), while a minor procedural bleed occurred in 1 patient (6%) during distal catheterization.
Table 2.
Procedural characteristics, early and long-term outcomes for all patients and depending on the DMVO.
| All | Primary DMVO | Secondary DMVO | |
|---|---|---|---|
| Number of patients | 17 (100%) | 11 (65%) | 6 (35%) |
| Mechanical thrombectomy | |||
| Admission mothership (%) | 11 (65%) | 6 (55%) | 5 (83%) |
| Type of anesthesia | |||
| General anesthesia (%) | 15 (88%) | 9 (82%) | 6 (100%) |
| Conscious sedation (%) | 1 (6%) | 1 (9%) | 0 (0%) |
| Conscious then general (%) | 1 (6%) | 1 (9%) | 0 (0%) |
| Associated treatment | |||
| Nimodipine (%) | 4 (24%) | 2 (18%) | 2 (33%) |
| Antiplatelets (%) | 1 (6%) | 1 (9%) | 0 (0%) |
| Number of Tigertriever13 passes (median, IQR) | 1 (1–2) | 2 (1–2) | 1 (1–2) |
| Number of passes (median, IQR) | 2 (2–3) | 2 (2–3) | 3 (2–4) |
| Final TICI 2b/2c/3 (%) | 16 (94%) | 10 (91%) | 6 (100%) |
| Final TICI 2c/3 (%) | 12 (71%) | 6 (55%) | 6 (100%) |
| Procedural complication (%) | 4 (23%) | 3 (27%) | 1 (17%) |
| Spasm (%) | 3 (17%) | 2 (18%) | 1 (17%) |
| Procedural bleed (%) | 1 (6%) | 1 (9%) | 0 (0%) |
| Subarachnoid hyperdensity on post procedural XPerCT | 8 (47%) | 4 (36%) | 4 (67%) |
| Times | |||
| Time puncture to recanalization in min (median, IQR) | 70 (44–90) | 55 (30–85) | 73 (59–86) |
| Time onset to recanalization in min (median, IQR) | 310 (259–419) | 314 (274–374) | 274 (237–805) |
| Early outcomes | |||
| Day 1 NIHSS (median, IQR) | 8 (2–9) | 7 (2–11) | 8 (6–8) |
| NIHSS shift (median, IQR) | −3 (−8 to 0) | −2 (−4 to −1) | −9 (−12 to −2) |
| Discharge mRS (median, IQR) | 3 (3–4) | 3 (3–4) | 3 (3–4) |
| Day 1 hemorrhagic transformation (any type, %) | 7 (41%) | 5 (45%) | 2 (33%) |
| ECASS PH-type (%) | 2 (12%) | 1 (9%) | 1 (16%) |
| ECASS SAH-type (%) | 5 (29%) | 4 (36%) | 1 (16%) |
| 3 months outcomes | |||
| mRS (median, IQR) | 2 (1–4) | 2 (1–4) | 2 (1–2) |
| Good outcome (mRS 0–2) (%) | 11 (65%) | 6 (55%) | 5 (83%) |
| Excellent outcome (mRS 0–1) (%) | 6 (35%) | 3 (27%) | 3 (50%) |
| Mortality (%) | 1 (6%) | 0 (0%) | 1 (17%) |
At day 1, a control CT-scan was performed and showed 29% of SAH in the corresponding territory beyond the occlusion site, two (12%) parenchymal-type hemorrhage (PH1-type) were depicted. None of them were considered symptomatic as patients did not deteriorate of more than 4 points on the NIHSS (one deteriorate of 1 point while the other improved of 14 points at day 1).
At discharge from the neurology department, median mRS was 3 (IQR of 3–4).
At 3 months, median mRS was 2 (1–4), with 65% showing a favorable outcome (11/17), only one patient died (6%).
Tigertriever 13 as a first-line strategy
Patients considered for a primary DMVO (65%, 11/17) with a Tigertriever 13 had a lower NIHSS (8 vs. 20) than patients with a secondary DMVO after proximal thrombectomy (see Table 1), their core volume was small (5 vs. 30 mL), with a lower mismatch volume (18 vs. 148 mL). Median number of Tigertriever 13 passes was 2 (1–2) in this group, while the total number of passes was 2 (2–3) as the Tigertriver 13 was sometimes used after another distal stent retriever (see Table 2). Time from puncture to recanalization was shorter as less maneuvers were needed and no proximal occlusion had to be recanalized first (55 min vs. 73 min). Their median day 1 NIHSS was 7 (IQR of 2–11), and median discharge mRS was 3 (IQR 3–4). At 3 months, 55% of them were independent and none of them died.
Occlusions locations
Patients had different clinical and angiographic presentations, most of them were M2 occlusions (7/17), while M3–M4 (4/17), anterior (3/17), and posterior cerebral artery (2) occlusions were treated too (see Table 3). One 9YO child with a left M1 (diameter of 2.4 mm) occlusion was also included because the procedure was performed via a left posterior communicating artery because of a proximal left common carotid occlusion. Therefore, this anatomical pattern was considered as a DMVO.
Table 3.
Baseline characteristics of treated occlusions.
| Localization (number) | Reperfusion (TICI 2b-3) | Attempts (median, IQR) | 3 months mRS (0–2) |
|---|---|---|---|
| Anterior cerebral artery A2 (2) | 2 (100%) | 1 (1–1) | 0 (0%) |
| Anterior cerebral artery A3 (1) | 1 (100%) | 2 (2–2) | 0 (0%) |
| Middle cerebral artery M1 (1) | 0 (0%) | 1 (1–1) | 1 (100%) |
| Middle cerebral artery M2 (7) | 7 (100%) | 2 (1–2) | 6 (86%) |
| Middle cerebral artery M3–M4 (4) | 4 (100%) | 2 (1–2) | 3 (75%) |
| Posterior cerebral artery P2–P3 (2) | 2 (100%) | 1 (1–1) | 1 (50%) |
Examples of tigertriever 13
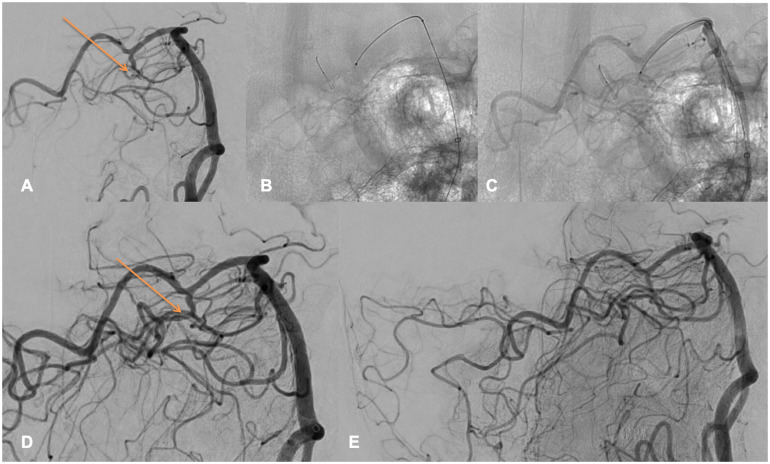
Figure 2 depicts the thrombectomy of a 63YO male, who presented an acute AIS with a baseline NIHSS of 15. The baseline head CT showed a left P1 occlusion (core/TMax 6 s mismatch), IVtPa administration was not performed as the patient was under active anticoagulation. Femoral puncture was performed 240 min after symptom onset, under general anesthesia. A 80 cm Neuronmax was positioned in the left subclavian artery, a Sofia 5F was advanced in the left V4 vertebral artery. After 2 passes of another stent retriever in the left P1 and P1/P2 segment, a remaining left P3 occlusion was seen (A, orange arrow). After discussion with the stroke neurologist and considering the eloquent territory, we decided to catheterize the P3 segment thanks to an Headway Duo over a Traxcess 14 microwire. A complete reperfusion (mTICI 3) was achieved after 1 pass of Tigertriever13 (B-C-D-E), 74 min after femoral puncture, a mild SAH was seen on the post procedural XPerCT. Day 1 NIHSS was 1, 3 months mRS was of 1.
Figure 2.
Left P3 occlusion.
Figure 2 highlights perfectly how the Tigertriever has to be opened. The orange arrow on Figure 2(a) is depicting the proximal part of the thrombus, exactly where the proximal part of the opened Tigertriever 13 is positioned on Figure 2(c), using the full working length of the device.
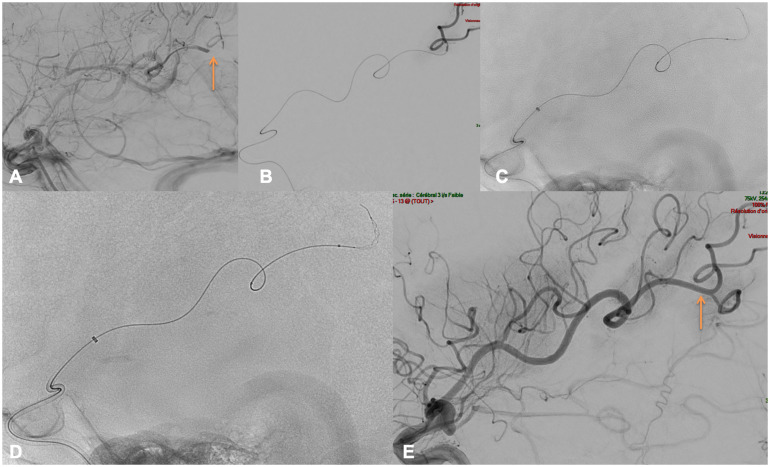
Figure 3 depicts the case of a 73YO male presenting with an NIHSS of 17, M1 occlusion was described on baseline CTA (core/TMax mismatch but large area with TMax > 10 s), IVtPa was started 210 min after symptom onset and the patient was transferred to the angiosuite for thrombectomy. The procedure was performed under general anesthesia, puncture was obtained 240 min after symptom onset. A 90 cm Neuronmax was placed in the left common carotid artery, with a Sofia 5F in the left internal carotid artery. The diagnostic DSA depicted multiple DMVO. We decided to treat the largest, occluding a large fronto-parietal M3–M4 branch ((a), orange arrow). Using a Headway Duo placed over an Hybrid 0.008, the left M3–M4 branch was catheterized, a distal run was performed to confirm the correct positioning (b) distal to the thrombus. After three passes with the Tigertriever 13 ((c)–(d)) we obtained a successful TICI 2b (e) recanalization of the targeted branch, 90 min after femoral access. A SAH was seen at day 1 with a concomitant NIHSS of 13. The 3 months mRS was of 3.
Figure 3.
Left M3–M4 occlusion.
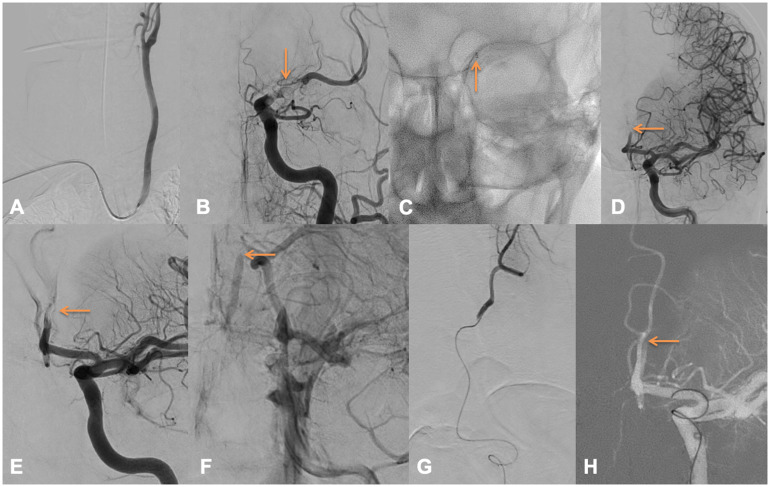
Figures 4 and 5 depict the case of a 50YO male presenting with an NIHSS of 29 with a left terminal ICA occlusion on CT (with a core/TMax 6 s mismatch), IVTpA was not performed because of an active anticoagulation. A right radial puncture was obtained 241 min after symptom onset, under general anesthesia. A 90 cm Neuronmax was placed in the left common carotid artery (Figure 4(a)) over a 5F JB2 (Cordis, Cardinal Health), then a Sofia 6F was advanced in the left internal carotid artery (Figure 4(b) and (c)). The terminal internal carotid artery and middle cerebral artery were recanalized after one aspiration over the Sofia 6F. A left A2 occlusion (Figure 4(d)–(f)) was left, and we decided to catheterize the anterior cerebral artery using a Headway Duo over an Hybrid 0.008. A distal run was performed to confirm the correct positioning (Figure 4(g)). The Tigertriever13 was then progressively opened (Figure 5(a)–(c)). As it was not possible to advance the Sofia 6F in the A1 segment we used the Traxcess 14 in parallel of the Headway Duo to improve support (Figure 5(d)). One pass of Tigertriever13 obtained a successful TICI 2c recanalization 47 min after radial puncture (Figure 5(f)–(h)). A mild SAH was described at Day 1, while his NIHSS increased to 30. The patient developed a malignant sylvian infarction and craniectomy was performed. Discharge mRS was 5, and he died at 3 months.
Figure 4.
Left A2 occlusion (1/2).
Figure 5.
Left A2 occlusion (2/2).
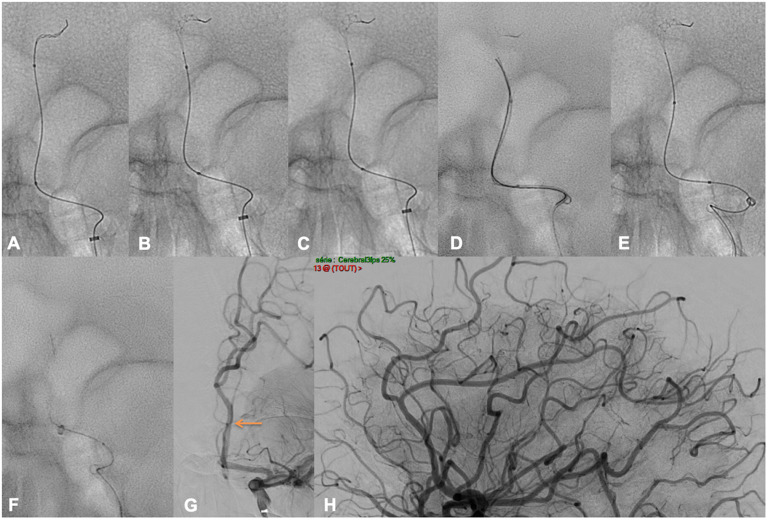
Figure e-2 depicts the case of a 45YO male addressed for a mild AIS. The baseline CT imaging realized 541 min after symptom onset (core/TMax 6 s mismatch) showed a left M2 sub-occlusion, the NIHSS was of 2 (mild motor aphasia), no IVtPa was given. Thrombectomy was decided after discussion with the stroke team, the family and the patient, femoral puncture was obtained 600 min after onset under general anesthesia. A 90 cm Neuronmax was placed in the left common carotid artery, then a Sofia 5F was advanced in the left internal carotid artery. A Headway Duo was advanced over an Hybrid 0.008, a distal run was performed to confirm the correct positioning (Figure e-2, image B). The Tigertriever 13 was positioned across the occlusion and progressively opened ((c) and (d)), the Sofia 5F was advanced in the M1–M2 segment using the Tigertriever 13 as distal anchor (e). After adapting the Tigertriever 13 opening (f), two passes of Tigertriever13 achieved a TICI 3 recanalization (g) 55 min after puncture. No SAH was seen on the post procedural XPerCT, the day 1 NIHSS was 0, and the patient was mRS 0 at 3 months.
Discussion
Our study shows that mechanical thrombectomy using the Tigertriever 13 device appears to be safe and effective either for primary or secondary DMVO.
In this small series of selected patients, the clinical outcome is encouraging because 11/17 patients (65%) were functionally independent at 3 months and only one had a fatal outcome (6%). Although a comparison is not feasible, one must remember that in patients with LVO, 46% have a functional independence at 3 months with a 15% mortality rate. 1 These good clinical results are related to the distal occlusion site and a high rate of successful recanalization (94%) compared to the rates of 71% achieved for LVO in early thrombectomy trials, 1 despite the rate of subarachnoid hyperdensities (47%) on post procedural XPerCT and SAH at day 1 (29%). The rate of PH-type hemorrhagic transformation was 12%, no hemorrhagic infarct type 1 or 2, PH2 type or symptomatic PH-type were observed while 5.1% of PH2 type are described for LVO 1 and up to 16% of any ECASS type hemorrhagic transformation after thrombolysis. 18
Compared to other series focusing on DMVO, baseline characteristics of our patients with a primary DMVO as well as their angiographic and clinical results were comparable.
Dobrocky et al. 9 described patients with a baseline M2 occlusion with a median NIHSS of 7. They achieved 74% of successful recanalization with 65% of good clinical outcome using the Mindframe Capture low profile (LP) device (Medtronic, Minneapolis, MN, USA). Hofmeister et al. 11 used a different device, the Catch Mini (CM) (Balt, Montmorency, France) in different DMVO with 78% successful recanalization and 82.4% of good clinical outcome. Interestingly, they described 19.5% of per-procedural vasospasm, compared to 17% in our series. Kurre et al. 19 described an interesting experience with the pREset LITE (phenox GmbH, Bochum, Germany) device, with a wide variety of occlusions, achieving 70% of successful recanalization with low rates of vasospasm (5.6%) and 13.3% SAH. Kühn et al. used the 3 × 20 mm Trevo XP ProVue (“Baby Trevo” Stryker Neurovascular, Fremont, California, USA) and achieved a successful reperfusion in 86% with 53% in one pass with however a high rate of mortality (20%).
Our single-center series includes a large heterogeneity of DMVO, both in the anterior and posterior circulation, showing the efficiency and adaptability of a 0.0165 inch system using low profile microcatheters and guidewires for distal catheterization. However, several specific issues, related to the distal location of the occlusion, need to be emphasized.
- First, distal thrombectomy does not show the same level of evidence than MT for proximal occlusions. Several devices have already been used in DMVO with a good efficacy and safety,8–11 with high rates of successful recanalization (70–80%) and favorable outcome (60–65%),7,20 in line with our angiographic and clinical results. However, thrombectomy for DMVO still needs larger randomized trials to validate its place in the therapeutic armamentarium of AIS.
- Second, distal catheterization requires to navigate tortuous, thin and fragile intracranial vessels; a more gentle and cautious approach, compared to proximal thrombectomy, is thus needed to prevent vessel perforation or dissection. A small microcatheter and microwire should be used, Headway Duo and Hybrid 0.008 are more and more considered as the best option in our center as it allows a distal navigation with devices we are used to for the endovascular treatment of intracranial aneurysms or arterio-venous malformations.
- Third, the anesthesia will probably be the cornerstone of thrombectomy for DMVO. While general and conscious sedation21,22 have been compared with different results for LVO thrombectomy, we think that navigating distal vessels needs to be performed under general anesthesia. In our experience, only one MT was performed under local anesthesia for a right A3 occlusion as the patient was not agitated and with contra-indications for general anesthesia, 2 passes resulted in a successful recanalization.
- Fourth, the risk to perform a distal thrombectomy after IVtPA has to be analyzed. Our series gathers too few patients to analyze a possible association between SAH, hemorrhagic transformation, or outcome and IVtPA administration. Indeed, only 6/17 patients had IVtPA while we performed the distal thrombectomy, 3 were under anticoagulation and 5 under antiplatelet therapy.
- Fifth, the number of passes that has to be performed to recanalize the artery is difficult to estimate. In our experience, the median number of Tigertriever passes was 1 (1–2) for all patients and 2 (1–2) for primary DMVO. The rate of first-pass effect is not evaluated in our series as the technique we use does not favor first-pass effect. Indeed, as the opening of the Tigertriever13 in distal vessels may expose to excessive forces during retrieval, we tend to open it widely then close it until no resistance or straightening of the arteries is felt during retrieval. Then in case of failure, we open the device more for the next pass. However, we never tried more than 3 passes and would probably not, even in case of recanalization failure after 3 passes.
- Sixth, the need and safety of aspiration during the Stent retriever retrieval has to be discussed. Whether it should be performed distally or proximally, with a balloon guiding catheter or a dedicated distal intermediate catheter will need further trials. Yet, the risk of vessel collapse 23 has to be emphasized. Distal aspiration may lead to higher rates of collapse especially if the intermediate catheter is not in contact with the clot; this may increase vessel straightening during retrieval and lead to an increased risk of hemorrhagic complications.
Limitations
There are several limitations to our study, the single-center design, the low number of patients and the lack of a control group do not permit generalization of our results.
Conclusions
Thrombectomy using the Tigertriever 13 appears to be safe and effective for DMVO in case of adequate case selection and device manipulation as clinical and anatomical results are at least in line with those of patients with proximal occlusions. Larger cohort studies are however needed to validate its clinical benefit.
Acknowledgements
We thank Mr Bernd Van Egmond for the Tigertriever 13 images (Figure 1).
APPENDIX 1: AUTHORS’ CONTRIBUTIONS
| Name | Location | Contribution(s) |
|---|---|---|
| Adrien GUENEGO | Erasme University Hospital (Belgium) | Design and conceptualized study; analyzed the data; drafted the manuscript for intellectual content |
| Benjamin MINE | Erasme University Hospital (Belgium) | Interpreted the data; revised the manuscript for intellectual content |
| Thomas BONNET | Erasme University Hospital (Belgium) | Interpreted the data; revised the manuscript for intellectual content |
| Stephanie ELENS | Erasme University Hospital (Belgium) | Interpreted the data; revised the manuscript for intellectual content |
| Juan VAZQUEZ SUAREZ | Erasme University Hospital (Belgium) | Interpreted the data; revised the manuscript for intellectual content |
| Lise JODAITIS | Erasme University Hospital (Belgium) | Interpreted the data; revised the manuscript for intellectual content |
| Noémie LIGOT | Erasme University Hospital (Belgium) | Interpreted the data; revised the manuscript for intellectual content |
| Gilles NAEIJE | Erasme University Hospital (Belgium) | Interpreted the data; revised the manuscript for intellectual content |
| Boris LUBICZ | Erasme University Hospital (Belgium) | Design and conceptualized study; analyzed the data (statistical analyses); drafted the manuscript for intellectual content |
Footnotes
Authors contributions: All authors participated to study design, data collection, data analysis, and writing.
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Adrien Guenego https://orcid.org/0000-0001-7281-1652
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 3.Saver JL, Chapot R, Agid R, et al. Thrombectomy for distal, medium vessel occlusions: a consensus statement on present knowledge and promising directions. Stroke 2020; 51: 2872–2884. [DOI] [PubMed] [Google Scholar]
- 4.Mokin M, Fargen KM, Primiani CT, et al. Vessel perforation during stent retriever thrombectomy for acute ischemic stroke: technical details and clinical outcomes. J Neurointerv Surg 2017; 9: 922–928. [DOI] [PubMed] [Google Scholar]
- 5.Schwaiger BJ, Gersing AS, Zimmer Cet al. et al. The curved MCA: influence of vessel anatomy on recanalization results of mechanical thrombectomy after acute ischemic stroke. AJNR Am J Neuroradiol 2015; 36: 971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rai AT, Hogg JP, Cline Bet al. et al. Cerebrovascular geometry in the anterior circulation: an analysis of diameter, length and the vessel taper. J Neurointerv Surg 2013; 5: 371–375. [DOI] [PubMed] [Google Scholar]
- 7.Sarraj A, Sangha N, Hussain MS, et al. Endovascular therapy for acute ischemic stroke with occlusion of the middle cerebral artery M2 segment. JAMA Neurol 2016; 73: 1291–1296. [DOI] [PubMed] [Google Scholar]
- 8.Rikhtegar R, Mosimann PJ, Weber R, et al. Effectiveness of very low profile thrombectomy device in primary distal medium vessel occlusion, as rescue therapy after incomplete proximal recanalization or following iatrogenic thromboembolic events. J Neurointerv Surg 2021; 5: 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobrocky T, Bellwald S, Kurmann R, et al. Stent retriever thrombectomy with mindframe capture LP in isolated M2 occlusions. Clin Neuroradiol 2020; 30: 51–58. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn AL, Wakhloo AK, Lozano JD, et al. Two-year single-center experience with the “Baby Trevo” stent retriever for mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg 2017; 9: 541–546. [DOI] [PubMed] [Google Scholar]
- 11.Hofmeister J, Kulcsar Z, Bernava G, et al. The Catch Mini stent retriever for mechanical thrombectomy in distal intracranial occlusions. J Neuroradiol 2018; 45: 305–309. [DOI] [PubMed] [Google Scholar]
- 12.Ho FL, Chapot R. Removal of distal fragments of liquid embolic agents during arteriovenous malformation embolization using the TIGERTRIEVER 13: a technical report. J Neurointerv Surg 2020; 12: 794–797. [DOI] [PubMed] [Google Scholar]
- 13.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 14.Fischer S, Weber A, Carolus Aet al. et al. Coiling of wide-necked carotid artery aneurysms assisted by a temporary bridging device (comaneci): preliminary experience. J Neurointerv Surg 2017; 9: 1039–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Will L, Maus V, Maurer Cet al. et al. Mechanical thrombectomy in acute ischemic stroke using a manually expandable stent retriever (tigertriever): preliminary single center experience. Clin Neuroradiol 2020; 5: 272–276. [DOI] [PubMed] [Google Scholar]
- 16.Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013; 44: 2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maus V, Styczen H, Liman J, et al. Intracranial mechanical thrombectomy of large vessel occlusions in the posterior circulation using SAVE. BMC Neurol 2019; 19: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed N, Wahlgren N, Grond M, et al. Implementation and outcome of thrombolysis with alteplase 3–4.5 h after an acute stroke: an updated analysis from SITS-ISTR. Lancet Neurol 2010; 9: 866–874. [DOI] [PubMed] [Google Scholar]
- 19.Kurre W, Aguilar-Perez M, Martinez-Moreno Ret al. et al. Stent retriever thrombectomy of small caliber intracranial vessels using pREset LITE: safety and efficacy. Clin Neuroradiol 2017; 27: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CJ, Wang C, Buell TJ, et al. Endovascular mechanical thrombectomy for acute middle cerebral artery M2 segment occlusion: a systematic review. World Neurosurg 2017; 107: 684–691. [DOI] [PubMed] [Google Scholar]
- 21.Schonenberger S, Henden PL, Simonsen CZ, et al. Association of general anesthesia vs procedural sedation with functional outcome among patients with acute ischemic stroke undergoing thrombectomy: a systematic review and meta-analysis. JAMA 2019; 322: 1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cappellari M, Pracucci G, Forlivesi S, et al. General anesthesia versus conscious sedation and local anesthesia during thrombectomy for acute ischemic stroke. Stroke 2020; 51: 2036–2044. [DOI] [PubMed] [Google Scholar]
- 23.Nikoubashman O, Wischer D, Hennemann HMet al. et al. Under pressure: comparison of aspiration techniques for endovascular mechanical thrombectomy. AJNR Am J Neuroradiol 2018; 39: 905–909. [DOI] [PMC free article] [PubMed] [Google Scholar]