Abstract
Pharmaceutical excipients were designed originally to be pharmacologically inert. However, certain excipients were found to have altering effects on drug pharmacodynamics and/or pharmacokinetics. Pharmacokinetic interactions may be caused by modulation of efflux transporter proteins, intercellular tight junctions and/or metabolic enzyme amongst others. In this study, five disintegrants from different chemical classes were evaluated for P-glycoprotein (P-gp) related inhibition and tight junction modulation effects. Bidirectional transport studies of the model compound, Rhodamine 123 (R123) were conducted in the absence (control group) and presence (experimental groups) of four concentrations of each selected disintegrant across excised pig jejunum tissue. The results showed that some of the selected disintegrants (e.g. Ac-di-sol® and Kollidon® CL-M) increased R123 absorptive transport due to inhibition of P-gp related efflux, while another disintegrant (e.g. sodium alginate) changed R123 transport due to inhibition of P-gp in conjunction with a transient opening of the tight junctions in a concentration dependent way. It may be concluded that the co-application of some disintegrants to the intestinal epithelium may lead to pharmacokinetic interactions with drugs that are susceptible to P-gp related efflux. However, the clinical significance of these in vitro permeation findings should be confirmed by means of in vivo studies.
Keywords: Disintegrants, Excipient, Ex vivo, P-glycoprotein, Pharmacokinetic interactions, Rhodamine 123
1. Introduction
The oral delivery route is the most preferred route for drug administration. The safety, ease of administration and flexible dosage form design include some of the advantages that contribute to its popularity [1,2]. Essentially, all oral dosage forms contain excipients, which can be defined as the ingredients other than the active pharmaceutical ingredient(s) that comprise a completed dosage form [3,4]. Disintegrants are included in some immediate release solid oral dosage forms (e.g. tablets) to mediate breakup of the drug delivery system into smaller units, which leads to a larger surface area with increased dissolution, absorption and bioavailability [5,6]. Although most pharmaceutical excipients in solid oral dosage forms have been considered inert, it has been observed that certain excipients can increase membrane permeability of drug molecules or decrease absorption by means of different mechanisms [6,7].
Pharmacokinetic interactions occur when one compound alters the pharmacokinetics (i.e. the absorption, distribution, metabolism and/or excretion) of another compound [8]. Pharmacokinetic interactions may be caused by different mechanisms of action. It has, for example, been demonstrated that metabolic enzymes such as cytochrome P450 as well as active efflux transporters such as P-gp can be modulated (i.e. inhibition, induction or activation) by certain compounds to alter the pharmacokinetics of co-administered drugs [6,7,9]. Efflux transport by members of the ATP-binding cassette (ABC) transporter proteins is responsible for decreasing absorption of substrates from the gastro-intestinal tract after oral administration by actively transporting the molecules back into the lumen from the epithelial cells. This active efflux transport may result in a reduced bioavailability of orally administered drugs that are substrates of these efflux proteins [10,11]. Intentional or unintentional inhibition of P-gp related efflux of a drug in the epithelium of the gastro-intestinal tract would result in the increased uptake of a drug that is a substrate of this efflux transporter when co-administered with the efflux inhibitor [12]. Certain pharmaceutical excipients have shown the ability to inhibit P-gp related efflux and/or modulate tight junctions and thereby enhance the absorption of certain drugs across the intestinal epithelium as well as change the in vivo pharmacokinetic profiles of some drugs [6,7,13,14].
R123 is a known P-gp substrate that has been used as model compound in previous studies to investigate the modulating effect of selected chemicals (e.g. Brijs) on absorptive and secretory transport across Caco-2 cell monolayers as well as intestinal absorption in rats [2]. R123 has also been used as a representative P-gp substrate in an in situ closed loop study in rats to investigate the effect of sodium nitroprusside on absorption and excretion in the ileum [15].
2. Materials and methods
2.1. Materials
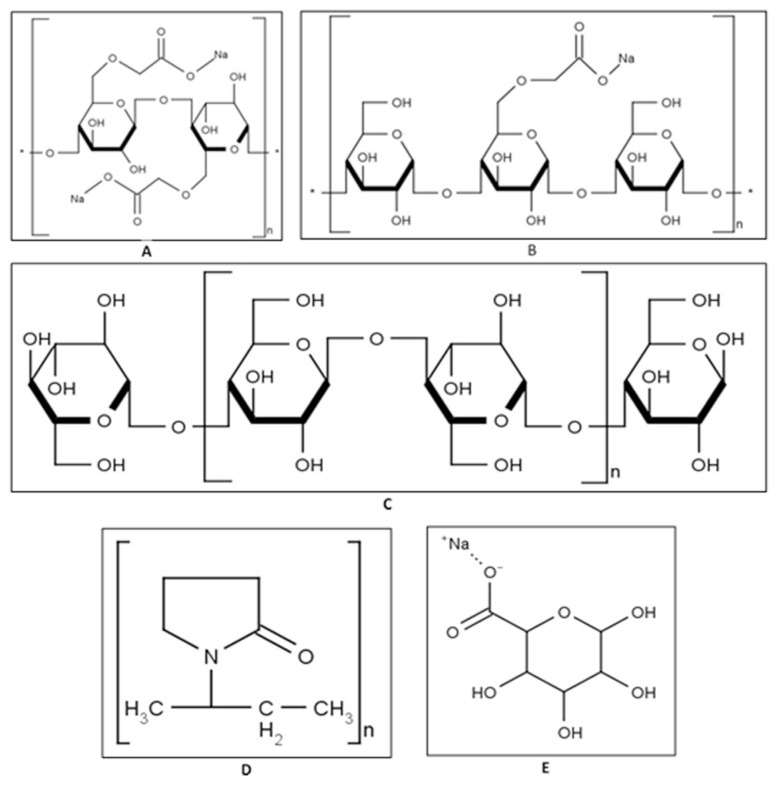
R123, Krebs–Ringer bicarbonate (KRB) buffer and sodium alginate (batch number MKBN7680V) were purchased from Sigma–Aldrich (Johannesburg, South Africa). Cro-scarmellose sodium (Ac-di-sol®, batch number T017C) and microcrystalline cellulose (Avicel® PH-200, batch number M939C) were purchased from FMC Corporation (Cork, Ireland). Sodium starch glycolate (Explotab®, batch number SSGP0601) was purchased from Mirren (PTY) LTD and crospovidone (Kollidon® CL-M, batch number 91416136W0) was purchased from BASF (Ludwigshafen, Germany). Costar® 96-well plates (lot number 07914036) were purchased from The Scientific Group (Randburg, South Africa). Pig intestinal tissue was collected at a local abattoir in Potchefstroom, South Africa. The chemical structures of the selected disintegrants are shown in Fig. 1.
Fig. 1.
Chemical structures of the selected disintegrants: A) Ac-di-sol®, B) Explotab®, C) Avicel® PH-200, D) Kollidon® CL-M and E) sodium alginate.
2.2. Preparation of pig intestinal tissue for ex vivo transport studies
Before every transport experiment, a piece of approximately 20 cm of pig proximal jejunum was collected from an abattoir (Potchefstroom, South Africa) directly after slaughter of the animal. Jejunum tissue was obtained from Landrace pigs, which forms part of the domestic pig family (Sus scrofa domesticus), that are bred on farms in South African and routinely slaughtered for meat production. All animals used were designated for slaughter by the abattoir on behalf of independent providers. Feeder pigs ranging from approximately 4 to 6 months were selected as far as possible for tissue sample collection. After excision of this intestinal segment, it was rinsed and submerged in freshly prepared, ice-cold KRB buffer. On arrival at the laboratory, the intestinal segment was pulled over a glass tube and the serosal layer was stripped off via blunt dissection. The jejunum tissue was cut along the mesenteric border and the resulting sheet of tissue was spread open onto filter paper. The intestinal tissue sheet was cut into smaller segments, which were mounted between the half-cells of six Sweetana-Grass diffusion chambers. Peyer’s patches were avoided when selecting jejunal tissue pieces suitable for mounting between the half-cells of the diffusion apparatus [1,14].
3. Bi-directional transport studies
3.1. Preparation of solutions
All the selected disintegrants were evaluated for transport effects at four different concentrations based on the recommended minimum and maximum quantities to be incorporated into tablet formulations [16–20] as well as taking into account that a tablet (e.g. 200 mg as a typical example) is commonly taken with 100 ml (scenario 1) to 200 ml (scenario 2) of fluid [7]. The mass of each selected disintegrant that was used to prepare 50 ml of each test solution/suspension for the transport studies are given in Table 1.
Table 1.
The recommended minimum (Min) and maximum (Max) quantities of each selected disintegrant for tablet formulations and the mass of each disintegrant per 50 ml volume of each test solution prepared for the transport studies.
| Disintegrant | Recommended concentration (% w/w) in tablet | Mass (mg) | ||
|---|---|---|---|---|
|
| ||||
| Scenario 1 | Scenario 2 | |||
| Ac-di-sol® (ADS) (Croscarmellose sodium) | Min | 0.50 | 0.50 | 0.25 |
| Max | 5.00 | 5.00 | 2.50 | |
| Avicel® PH-200 (AVC) (Microcrystalline cellulose) | Min | 5.00 | 5.00 | 2.50 |
| Max | 15.00 | 15.00 | 7.50 | |
| Explotab® (XPT) (Sodium starch glycolate) | Min | 2.00 | 2.00 | 1.00 |
| Max | 8.00 | 8.00 | 4.00 | |
| Kollidon® CL-M (KLM) (Crospovidone) | Min | 2.00 | 2.00 | 1.00 |
| Max | 5.00 | 5.00 | 2.50 | |
| Sodium alginate (SAL) | Min | 2.50 | 2.50 | 1.25 |
| Max | 10.00 | 10.00 | 5.00 | |
R123 was used as model compound and was completely dissolved in KRB to a final concentration of 5 μM in the bidirectional transport studies, since it is a fluorescence marker that is also a substrate of the P-gp efflux transporter [2,15,21,22]. For the transport experiments in the apical-to-basolateral (absorptive) direction, the R123 and required amount of disintegrant was mixed and placed in the apical chamber, while KRB was placed in the basolateral chamber. For the transport studies in the basolateral-to-apical (secretory) direction, two separate solutions were applied to the different half cells of each diffusion chamber. A clear solution of R123 (5 μM) in KRB buffer was inserted in the basolateral side, while a solution/suspension of each disintegrant in KRB buffer was placed in the apical side.
The rationale behind the application of two different solutions to the chambers for the transport studies in the basolateral-to-apical direction as described above, was to ensure that the disintegrants were always exposed to the apical side of the excised tissue to simulate normal conditions as it would occur in vivo. Most disintegrants are not absorbed into the systemic circulation from the gastro-intestinal tract due to their large molecular weight and poor water solubility characteristics.
3.2. Transport studies
The chambers (assembled half-cells) of the Sweetana-Grass diffusion apparatus were connected to a heating block (37 °C) and carbogen (95% O2:5% CO2) was bubbled through the buffer in each half-cell. Thereafter, pre-heated (37 °C) KRB buffer was incubated on both sides of the mounted excised intestinal tissue for 15 min to accustom the excised tissues to the experimental conditions. The KRB buffer was then aspirated and the test solutions/suspensions were applied as described above. Samples of 180 μl were collected from the acceptor side at 20 min intervals for a total of 120 min and replaced with an equal volume of KRB buffer [14]. All the transport studies were performed in triplicate.
3.3. Fluorescent spectroscopic analysis
A validated fluorescence spectroscopic method was used to analyze the transport samples for R123 content on a Spectramax Paradigm® multi-mode detection platform plate reader. Excitation and emission wavelengths were set to 480 nm and 520 nm respectively [21,22].
3.4. Data analysis
The concentration of R123 in the transport samples were corrected for dilution and its transport was expressed as a percentage of the initial concentration of R123 applied to the donor chamber (Equation (1)). The percentage transport was plotted as a function of time to produce percentage transport curves.
| (1) |
The apparent permeability coefficient (Papp) values for R123 were calculated from the percentage transport curves in the absence and presence of the selected disintegrants (Equation (2)) [2,14,22].
| (2) |
where Papp is the apparent permeability coefficient (cm·s−1), is the permeability rate (amount permeated per minute), A is the diffusion area of the membrane (cm2) and C0 is the initial concentration of R123 [2,14,22].
The Papp values in both transport directions were used to calculate the efflux ratio (ER) of R123 in the presence and absence of the selected disintegrants (Equation (3)) [2,14].
| (3) |
where Papp (B-A) is the permeability coefficient for permeation in the basolateral to the apical direction and Papp (A–B) the same variable in the apical to basolateral direction.
Analysis of variance (ANOVA) was performed on all data gathered to determine if there were any statistically significant differences between the Papp values of the experimental groups when compared to that of the control group (R123 alone). Post-hoc tests such as the Dunnett’s t-test and the Kruskal–Wallis test were used to analyze all non-parametric data to determine if any statistically significant differences were evident. When p ≤ 0.05, it was considered to be indicative of statistically significant differences.
4. Results and discussion
The effects of each selected disintegrant on the transport of R123 in two directions (i.e. absorptive and secretory) across excised pig intestinal tissues are reported below.
4.1. Ac-di-sol® (ADS)
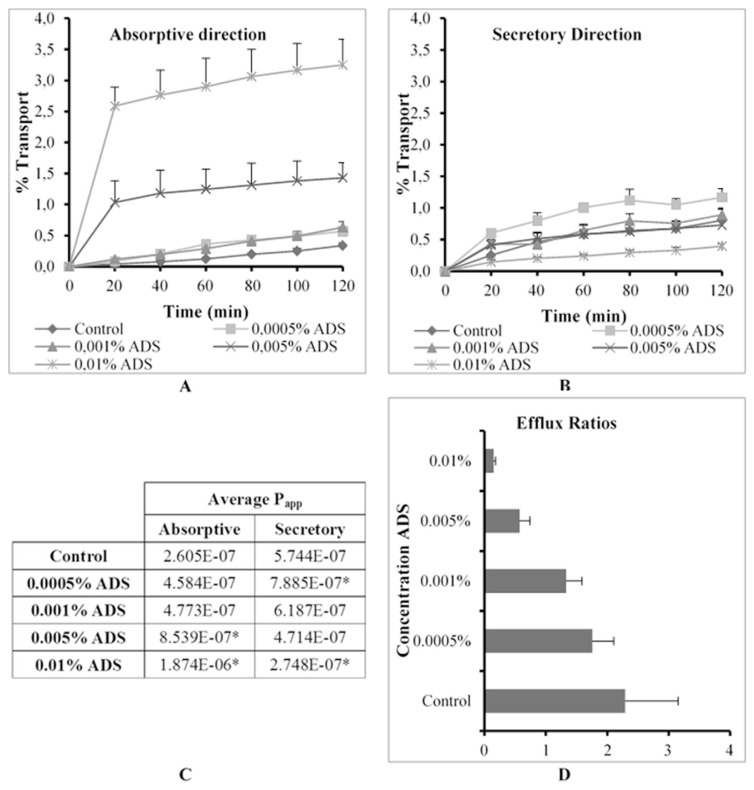
The % transport, apparent permeability coefficient (Papp) values in the absorptive and secretory directions as well as ER values for R123 across excised pig intestinal tissues in the absence (control group) and presence of Ac-di-sol® (ADS) at four concentrations are shown in Fig. 2.
Fig. 2.
Graphical presentation of the percentage transport of Rhodamine 123 in the (A) absorptive and (B) secretory directions (C) apparent permeability coefficient (Papp) values for bi-directional transport and (D) the efflux ratio (ER) values in the presence of Ac-di-sol® (ADS) at four concentrations across excised pig intestinal tissues (* indicates statistically significant differences from the control, p ≤ 0.05).
ADS mediated a concentration dependent increase in R123 transport in the absorptive direction across the pig intestinal tissue. Statistically significant increases in absorptive transport were evident at the two highest concentrations tested (namely 0.005% and 0.01% w/v) in comparison with the control group of R123 alone (Fig. 2C). ADS also mediated a simultaneous, concentration dependent, decrease in secretory R123 transport. However, the lower two concentrations did cause an increase in secretory transport of R123 as compared to the control group. This may be explained by a simultaneous reduction in the trans-epithelial electrical resistance (TEER). TEER reflects the ionic conductance of the paracellular pathway in the membrane and may be used to deduce the extent of paracellular transport. The TEER reduction for the transport studies in the secretory direction was 42.31%, 55.29%, 21.6% and 54.20%, respectively for each concentration tested. The decrease in TEER is indicative of a partial opening of the intercellular tight junctions. It has previously been reported in the literature that R123 is capable of being transported via the paracellular route [15]. Therefore, it can be concluded that the increased secretory transport of R123 in the presence of ADS at 0.0005% (w/v) and 0.001% (w/v) may be attributed to the contribution of the increased transport via the paracellular route. On the other hand, the statistically significant reduction in the secretory transport of R123 at the highest concentration of ADS may be explained by the overwhelming inhibition effect on P-gp related efflux, which makes the contribution of paracellular transport negligible.
The efflux ratio (ER) values (Fig. 2D) may be used to identify the main transport modulation mechanism of the co-applied disintegrant. When ER >> 1, it indicates that a compound is susceptible to active efflux transport, while an ER << 1 is indicative of active absorptive uptake while an ER value equal or close to 1 indicates that passive diffusion is the main transport mechanism of the compound [23]. Fig. 2D shows that R123 in the control group was actively transported via an efflux based mechanism with a corresponding ER value of 2.20. A concentration dependent ER reduction was observed when ADS was co-applied with R123, which indicated a concentration dependent inhibition of P-gp related efflux.
4.2. Avicel® PH-200 (AVC)
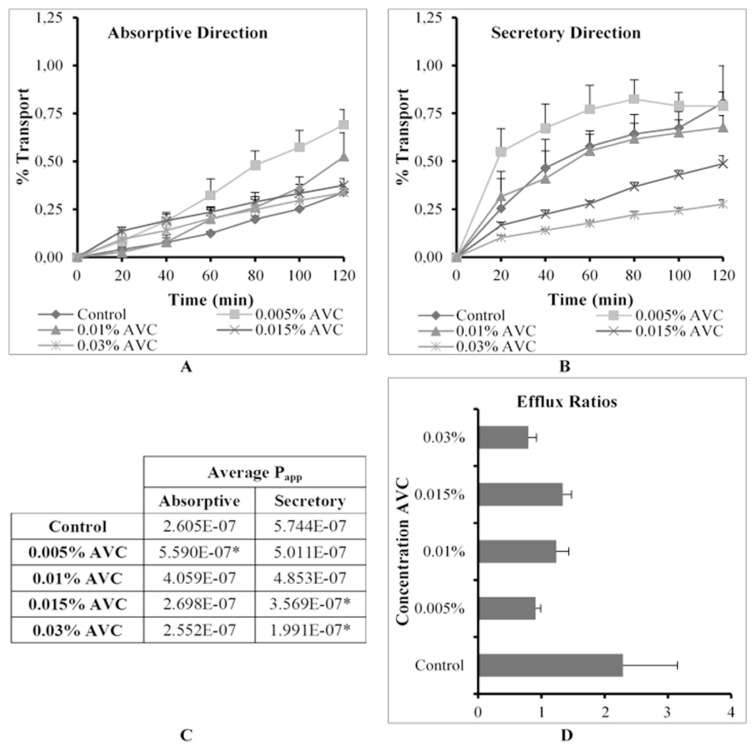
The % transport, apparent permeability coefficient (Papp) values in the absorptive and secretory directions as well as ER values for R123 across excised pig intestinal tissues in the absence (control group) and presence of Avicel® PH-200 (AVC) at four concentrations are shown in Fig. 3.
Fig. 3.
Graphical presentation of the percentage transport of Rhodamine 123 in the (A) absorptive and (B) secretory directions (C) apparent permeability coefficient (Papp) values for bi-directional transport and (D) the efflux ratio (ER) values in the presence of Avicel® PH-200 (AVC) at four concentrations across excised pig intestinal tissues (* indicates statistically significant differences from the control, p ≤ 0.05).
When considering the results of the secretory transport (Fig. 3B) of R123 in the presence of AVC, it appears that P-gp related efflux was inhibited in a concentration dependent manner. The results also showed a general improvement of R123 transport in the absorptive direction in the presence of AVC, but this transport enhancement is accompanied with a concentration dependent decrease (Fig. 3A). Statistical analysis of the data showed that AVC at the lowest concentration (0.005% w/v) yielded a statistically significant increase in the transport of R123 in the absorptive direction, while also decreasing the transport of R123 in the secretory direction (i.e. an efflux inhibition with an ER value of 0.90) (Fig. 3C and D).
A probable explanation for the concentration dependent decrease in R123 transport in the absorptive direction may be due to physical or chemical interactions between R123 molecules and AVC particles (e.g. the formation of complexes or adsorption onto the surface of particles). The results suggest that the extent of the complex formation was concentration dependent and that complexes only formed above a certain AVC concentration, which would explain the increase in R123 transport at the lower two concentrations, but not at the two higher concentrations. These findings are in accordance with the results from a similar study [2], where a reduction in R123 transport across rat intestinal tissue was reported in the presence of certain surfactants at concentrations higher than the critical micelle concentration. In addition to the above, it is known that AVC is poorly water soluble [18] and forms suspensions in aqueous media of which the particles may aggregate. It is possible that when AVC particles aggregate together to form clumps, that some R123 molecules can get trapped within these structures.
4.3. Explotab® (XPT)
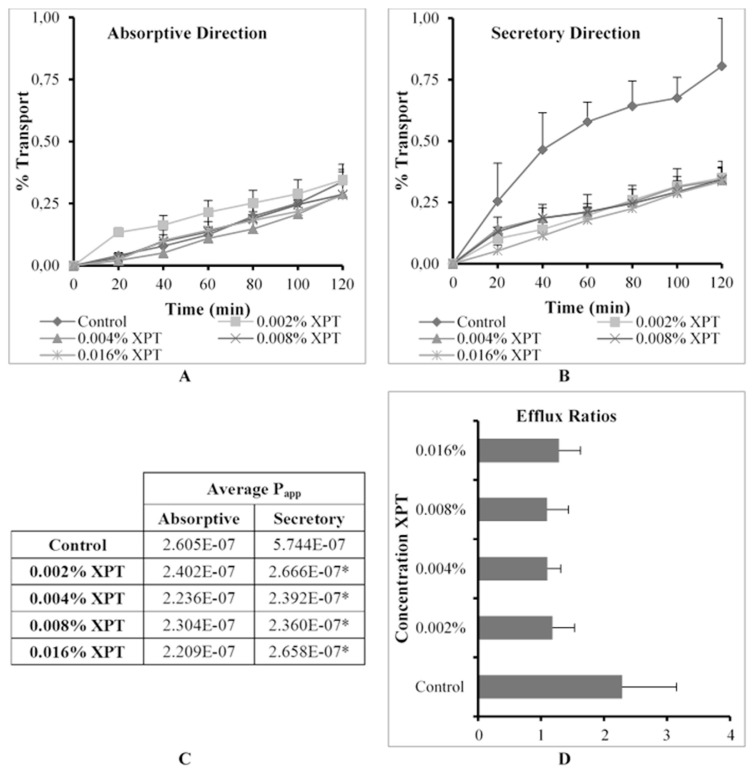
The % transport, apparent permeability coefficient (Papp) values in the absorptive and secretory directions as well as ER values for R123 across excised pig intestinal tissues in the absence (control group) and presence of Explotab® (XPT) at four concentrations are shown in Fig. 4.
Fig. 4.
Graphical presentation of the percentage transport of Rhodamine 123 in the (A) absorptive and (B) secretory directions (C) apparent permeability coefficient (Papp) values for bi-directional transport and (D) the efflux ratio (ER) values in the presence of Explotab® (XPT) at four concentrations across excised pig intestinal tissues (* indicates statistically significant differences from the control, p ≤ 0.05).
R123 transport in both directions was limited to relatively low Papp values (i.e. below about 2.40 × 10−7 cm.s−1) for all XPT concentrations that were co-applied (Fig. 4C). Although the P-gp related efflux (i.e. transport in the secretory direction) was inhibited statistically significantly, the absorptive transport of R123 was not increased at all. This lack of increase in absorptive transport can possibly be explained by the experimental setup where XPT was applied in the apical chamber together with the R123 for the absorptive transport experiment. According to the literature [17], XPT is virtually insoluble in water and has the ability to swell to approximately 300 times its initial size. The swollen XPT particles may have formed a hydrated layer with increased viscosity on the tissue surfaces, which mediated a reduction in the diffusion rate according to Fick’s law of diffusion for the absorptive transport studies. Physical or chemical interactions between R123 molecules and XPT particles may also have contributed to this effect. Since the R123 was applied alone to the basolateral side for the secretory transport experiments, these effects did not occur in the secretory transport direction where R123 was applied to the basolateral side. Inhibition of P-gp transporters by the XPT applied to the apical side decreased the secretory transport of R123, but it did not result in an increased absorptive transport due to the reasons described above.
4.4. Kollidon® CL-M (KLM)
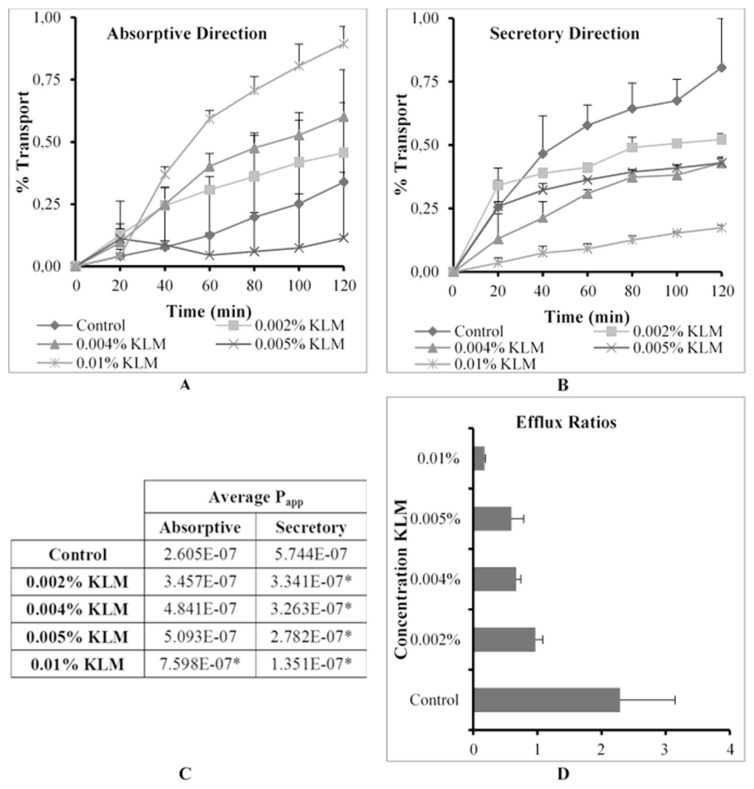
The % transport, apparent permeability coefficient (Papp) values in the absorptive and secretory directions as well as ER values for R123 across excised pig intestinal tissues in the absence (control group) and presence of Kollidon® CL-M (KLM) at four concentrations are shown in Fig. 5.
Fig. 5.
Graphical presentation of the percentage transport of Rhodamine 123 in the (A) absorptive and (B) secretory directions (C) apparent permeability coefficient (Papp) values for bi-directional transport and (D) the efflux ratio (ER) values in the presence of Kollidon® CL-M (KLM) at four concentrations across excised pig intestinal tissues (* indicates statistically significant differences from the control, p ≤ 0.05).
From Fig. 5 it is clear that KLM inhibited P-gp related efflux transport of R123 in the secretory direction in a concentration dependent manner (Fig. 5B), which resulted in a corresponding concentration dependent increase in R123 absorptive transport (Fig. 5A). At the highest KLM concentration tested (i.e. 0.01% w/v) statistically significant enhancement of absorptive transport was found to be as much as 3 fold higher than that of the control group (Fig. 5C). In the secretory direction it is shown that KLM inhibited P-gp to such an extent that all transport of R123 was statistically significantly lower than the control group. Furthermore, the reduction in ER values confirmed an inhibition of P-gp related efflux transport by KLM (Fig. 5D), especially when taken into consideration that the TEER measurements had shown that no effects were elicited on tight junctions and paracellular transport (results not shown).
4.5. Sodium alginate (SAL)
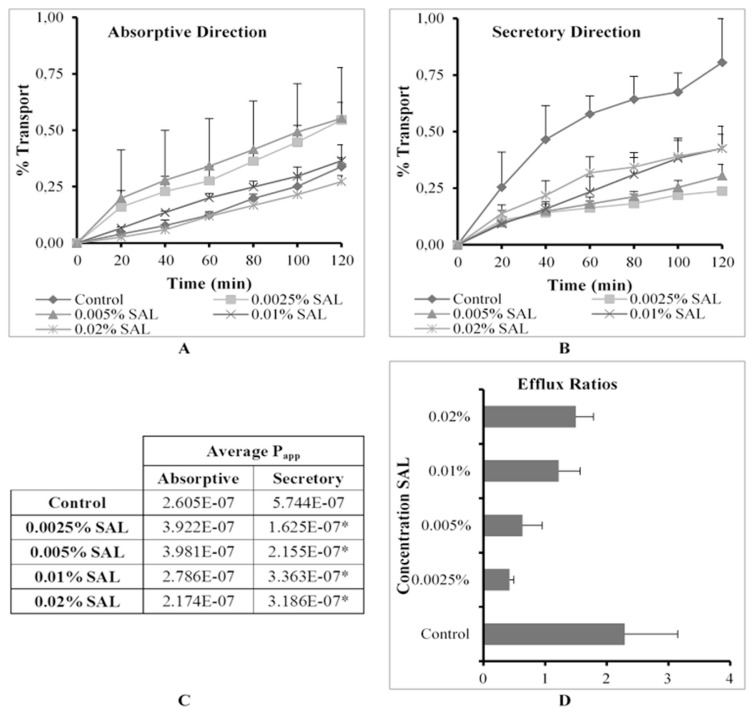
The % transport, apparent permeability coefficient (Papp) values in the absorptive and secretory directions as well as ER values for R123 across excised pig intestinal tissues in the absence (control group) and presence of sodium alginate at four concentrations are shown in Fig. 6.
Fig. 6.
Graphical presentation of the percentage transport of Rhodamine 123 in the (A) absorptive and (B) secretory directions (C) apparent permeability coefficient (Papp) values for bi-directional transport and (D) the efflux ratio (ER) values in the presence of sodium alginate (SAL) at four concentrations across excised pig intestinal tissues (* indicates statistically significant differences from the control, p ≤ 0.05).
The lower three concentrations (0.0025%, 0.005% w/v and 0.01% w/v) of SAL caused an increase in R123 absorptive transport, while the highest concentration caused a decrease in R123 absorptive transport when compared to the control (Fig. 6A). In the secretory direction, however, all the Papp values for R123 were statistically significantly lower than that of the control group. A gradual apparent increase in the Papp values for R123 transport in the secretory direction was evident as the SAL concentration increased (Fig. 6B).
To explain these permeability results, it is necessary to take the TEER values into consideration. During the transport studies in the absorptive direction, the TEER value decreased by approximately 18% at the lowest concentration of SAL, while the TEER increased by 5% at the highest SAL concentration. A similar result was found with TEER values in the secretory direction where a 41% decrease in TEER was caused by SAL at the lowest concentration and a 31% increase in TEER was observed for the highest concentration. These results indicate that tight junctions were modulated to open up at the lower concentrations, but tightened at the higher concentrations of SAL. It therefore seemed that both efflux inhibition and tight junction opening played a role in R123 transport at lower concentrations of co-applied SAL, while only efflux inhibition played a role at higher concentrations of co-applied SAL.
5. Conclusions
Based on the in vitro permeation results from this study it is clear that certain excipients may alter drug absorption via various mechanisms such as alterations in P-gp related efflux, tight junction modulation or by interactions with the drug molecules. Ac-di-sol® showed a tendency to inhibit P-gp related efflux and simultaneously increased paracellular transport by means of modulating tight junctions. The results showed that Avicel® PH-200 has an inhibitory effect on P-gp related efflux, but a complex interaction was probably involved to reduce the absorptive transport of R123 in a concentration dependent manner even though a relatively high increase in absorptive transport was observed at its lowest concentration tested. Although Explotab® seemed to inhibit P-gp related efflux; it did not increase absorptive transport of R123 possibly due to other interactions. Kollidon® CL-M also inhibited P-gp related efflux, but did not exhibit noticeable effects on tight junction as revealed by TEER results. Sodium alginate most likely altered R123 transport by tight junction modulation, which presented with an apparent increase in R123 efflux transport. These in vitro permeation studies showed that certain excipients such as selected disintegrants may have an effect on drug transport across intestinal epithelia via different mechanisms of action.
Acknowledgements
This work was carried out with the financial support of the National Research Foundation of South Africa (Grant no: 103479). Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors and therefore the NRF do not accept any liability with regard thereto.
Funding Statement
This work was carried out with the financial support of the National Research Foundation of South Africa (Grant no: 103479).
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
REFERENCES
- 1. Pietzonka P, Walter E, Duda-Johner S, Langguth P, Merkle HP. Compromised integrity of excised porcine intestinal epithelium obtained from the abattoir affects the outcome of in vitro particle uptake studies. Eur J Pharm Sci. 2002;15:39–47. doi: 10.1016/s0928-0987(01)00203-2. [DOI] [PubMed] [Google Scholar]
- 2. Zhao W, Uehera S, Tanaka K, Tadokoro S, Kusamori K, Katsumi H, et al. Effects of polyoxyethylene alkyl ethers on the intestinal transport and absorption of rhodamine 123: a P-glycoprotein substrate by in vitro and in vivo studies. J Pharm Sci. 2016;105:1526–34. doi: 10.1016/j.xphs.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 3. Bele MH, Derle DV. Effect of polacrilin potassium as disintegrant on bioavailability of diclofenac potassium in tablets: a technical note. AAPS PharmSciTech. 2012;13(3):756–9. doi: 10.1208/s12249-012-9802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dave VS, Saoji SD, Raut NA, Haware RV. Excipient variability and its impact on dosage form functionality. J Pharm Sci. 2015;104:906–15. doi: 10.1002/jps.24299. [DOI] [PubMed] [Google Scholar]
- 5. Desai PM, Liew CV, Heng PWS. Review of disintegrants and disintegration phenomena. J Pharm Sci. 2016;105:2545–55. doi: 10.1016/j.xphs.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 6. García-Arieta A. Interactions between active pharmaceutical ingredients and excipients affecting bioavailability: impact on bioequivalence. Eur J Pharm Sci. 2014;65:89–97. doi: 10.1016/j.ejps.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 7. Takizawa Y, Kishimoto H, Nakagawa M, Sakamoto N, Tobe Y, Furuya T, et al. Effects of pharmaceutical excipients on membrane permeability in rat small intestine. Int J Pharm. 2013;453:363–70. doi: 10.1016/j.ijpharm.2013.05.055. [DOI] [PubMed] [Google Scholar]
- 8. Corrie K, Hardman JG. Mechanisms of drug interactions: pharmacodynamics and pharmacokinetics. Anaesth Intensive Care. 2017;18(7):331–4. [Google Scholar]
- 9. Ondieki G, Nyagblordzro M, Kikete S, Liang R, Wang L, He X. Cytochrome P450 and P-glycoprotein-mediated interactions involving African herbs indicated for common noncommunicable diseases. Evid Based Compl Alternat Med. 2017;2582463 doi: 10.1155/2017/2582463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varma MVS, Ashokraj Y, Dey CS, Panchagnula R. P-glycoprotein inhibitors and their screening: a perspective from bioavailability enhancement. Pharmacol Res. 2003;48:347–59. doi: 10.1016/s1043-6618(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 11. Wang S-w, Monagle J, McNulty C, Putnam D, Chen H. Determination of P-glycoprotein inhibition by excipients and their combinations using an integrated high-throughput process. J Pharm Sci. 2004;93(11):2755–67. doi: 10.1002/jps.20183. [DOI] [PubMed] [Google Scholar]
- 12. Darby RAJ, Callaghan R, McMahon RM. P-glycoprotein inhibition: the past, the present and the future. Curr Drug Metabol. 2011;12:722–31. doi: 10.2174/138920011798357006. [DOI] [PubMed] [Google Scholar]
- 13. Cornaire G, Woodley J, Hermann P, Cloarec A, Arellano C, Houin G. Impact of excipients on the absorption of P-glycoprotein substrates in vitro and in vivo. Int J Pharm. 2004;278:119–31. doi: 10.1016/j.ijpharm.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 14. Legen I, Salobir M, Kerč J. Comparison of different intestinal epithelia as models for absorption enhancement studies. Int J Pharm. 2005;291:183–8. doi: 10.1016/j.ijpharm.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 15. Takizawa Y, Kitazato T, Ishizaka H, Kamiya N, Ito Y, Kishimoto H, et al. Changes in absorption and excretion of rhodamine 123 by sodium nitroprusside. Int J Pharm. 2013;450:31–5. doi: 10.1016/j.ijpharm.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Cable CG. Sodium alginate. In: Rowe RC, Sheskey PJ, Owen SC, editors. Handbook of pharmaceutical excipients. 5th ed. London: Pharmaceutical Press; 2006. pp. 656–8. [Google Scholar]
- 17.Edge S, Miller RW. Sodium starch glycolate. In: Rowe RC, Sheskey PJ, Owen SC, editors. Handbook of pharmaceutical excipients. 5th ed. London: Pharmaceutical Press; 2006. pp. 701–4. [Google Scholar]
- 18.Galichet LY. Cellulose, microcrystalline. In: Rowe RC, Sheskey PJ, Owen SC, editors. Handbook of pharmaceutical excipients. 5th ed. London: Pharmaceutical Press; 2006. pp. 132–5. [Google Scholar]
- 19.Guest RT. Croscarmellose sodium. In: Rowe RC, Sheskey PJ, Owen SC, editors. Handbook of pharmaceutical excipients. 5th ed. London: Pharmaceutical Press; 2006. pp. 211–3. [Google Scholar]
- 20.Kibbe AH. Crospovidone. In: Rowe RC, Sheskey PJ, Owen SC, editors. Handbook of pharmaceutical excipients. 5th ed. London: Pharmaceutical Press; 2006. pp. 214–6. [Google Scholar]
- 21. Kaprelyants AS, Kell DB. Rapid assessment of bacterial viability and vitality by rhodamine 123 and flow cytometry. J Appl Bacteriol. 1992;72:410–22. [Google Scholar]
- 22. Wang X, Meng M, Gao L, Liu T, Xu Q, Zeng S. Permeation of astilbin and taxifolin in Caco-2 cell and their effects on the P-gp. Int J Pharm. 2009;378:1–8. doi: 10.1016/j.ijpharm.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 23. Bock U, Kottke T, Gindorf C, Haltner E. Validation of caco-2 cell monolayer system for determining the permeability of drug substances according to the Biopharmaceutics Classification System (BCS) Across Barriers GmbH. 2003:1–7. [Unpublished] [Google Scholar]