Abstract
The growing use of natural products in cardiovascular (CV) patients has been greatly raising the concerns about potential natural product–CV drug interactions. Some of these may lead to unexpected cardiovascular adverse effects and it is, therefore, essential to identify or predict potential natural product–CV drug interactions, and to understand the underlying mechanisms. Drug transporters are important determinants for the pharmacokinetics of drugs and alterations of drug transport has been recognized as one of the major causes of natural product–drug interactions. In last two decades, many CV drugs (e.g., angiotensin II receptor blockers, beta-blockers and statins) have been identified to be substrates and inhibitors of the solute carrier (SLC) transporters and the ATP-binding cassette (ABC) transporters, which are two major transporter superfamilies. Meanwhile, in vitro and in vivo studies indicate that a growing number of natural products showed cardioprotective effects (e.g., gingko biloba, danshen and their active ingredients) are also substrates and inhibitors of drug transporters. Thus, to understand transporter-mediated natural product–CV drug interactions is important and some transporter-mediated interactions have already shown to have clinical relevance. In this review, we review the current knowledge on the role of ABC and SLC transporters in CV therapy, as well as transporter modulation by natural products used in CV diseases and their induced natural product–CV drug interactions through alterations of drug transport. We hope our review will aid in a comprehensive summary of transporter-mediated natural product–CV drug interactions and help public and physicians understand these type of interactions.
Keywords: Cardiovascular drugs, Natural products, Drug transporters, Natural product-drug interaction, Pharmacokinetics
1. Introduction
Natural products have been widely used among patients with cardiovascular (CV) diseases [1] and many patients often combined natural products with CV medications [2]. However, accumulating clinical evidence indicates that the combination use of natural products and conventional medicines has been paralleled by high risk of harmful natural product–drug interactions [3]. For instance, the patients given anticoagulant agents are at a higher risk of bleeding due to unwanted natural product–drug interactions when co-administered with natural products, such as ginkgo and danshen [4]. Despite increasing recognition of these types of natural product–drug interactions, improved understanding of the underlying mechanisms remain a pressing need for guiding the rational use of such combinational therapies.
Transporter-mediated natural product–drug interactions are increasingly acknowledged to play an important role in changing drug absorption and disposition and thus determine the efficacy and safety of drugs [5]. Generally, two transporter superfamilies including the solute carrier (SLC) transporters and the ATP-binding cassette (ABC) transporters are of considerable pharmacological significance [6]. Potential drug–drug interactions mediated by these two types of transporters are of clinical and regulatory concern [6]. In recent years, a large number of CV drugs have been identified as substrates of both SLC and ABC transporters [6–8]. Altered functions and expressions of these transporters may cause marked changes in the pharmacokinetics of these CV drugs, and many cases have either documented or suspected clinical relevance for patients with CV diseases [7,8]. In parallel, many natural products and their active ingredients were found to display modulatory effects on different drug transporters [5], and some transporter-mediated natural product–CV drug interactions have already shown to have clinical relevance. Aside from metabolizing enzymes [9,10], it is now well established that also modification of transport function is involved in natural product–drug interactions.
Therefore, this paper focuses on the recent understanding regarding transporter-mediated natural product–drug interactions for the treatment of CV diseases. We first briefly summarize the current knowledge on two major transporter families (ABC and SLC transporters) and their role in CV therapy. We then review transporter modulation by natural products used in CV disease and their induced natural product–drug interactions through affecting transporter expressions and functions. Lastly, a brief summary along with future perspectives for studying natural product–drug interactions is presented.
2. Role of drug transporters in cardiovascular therapy
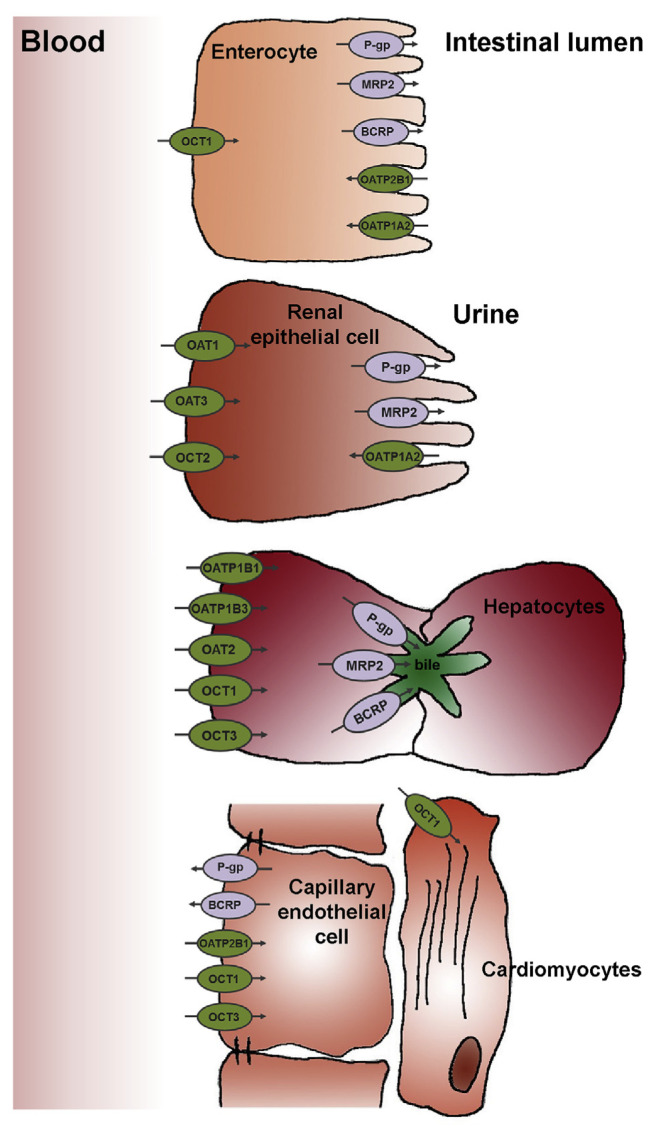
There are more than 400 membrane transporters that have been discovered until now, and generally fall into two classes of transporter proteins: the ABC (efflux) and the SLC (generally influx) transporters [11]. ABC and SLC transporters share a wide distribution in the body, and mediate the influx or bidirectional movement of drugs across the cell membrane. Since the intestine, liver, and kidney are the prime organs that determine drug absorption, distribution, and excretion, and the heart is one of principal target organs in CV diseases, this review focuses on the drug transporters expressed in these organs (Fig. 1).
Fig. 1.
Major SLC and ABC transporters expressed in human enterocytes, renal epithelial cells, hepatocytes, heart capillary endothelial cells and cardiomyocytes.
The role of drug transporters in CV therapy received great interest because many CV drugs with a narrow therapeutic range, such as antiarrhythmic and anticoagulant agents, interacted with the drug transporters [7]. As demonstrated in Table 1, ATP binding cassette (ABC) transporters, organic anion transporting polypeptides (OATPs), organic anion transporters (OATs), and organic cation transporters (OCTs) are four major drug transporters involved in the efflux and uptake of CV drugs.
Table 1.
Summary of the current understanding of human transporters in cardiovascular therapy.
| ABC | SLC | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||
| P-gp | BCRP | MRP2 | OAT1 | OAT3 | OATP1A1 | OATP1B1 | OATP1B3 | OATP2B1 | OCT1 | OCT2 | OCT3 | MATE1 | MATE2K | ||
| ACE inhibitors | Captopril | – | – | – | ● | – | – | – | – | – | – | – | – | – | – |
| Enalapril | – | – | ● | – | – | ● | ● | ● | – | – | – | – | – | – | |
| Fosinopril | – | – | ● | – | – | – | – | – | – | – | – | – | – | – | |
| Quinaprilat | – | – | – | ● | ● | – | – | – | – | – | – | – | – | – | |
| Ramipril | – | – | – | ● | – | – | – | – | – | – | – | – | – | – | |
| ARBs | Eprosartan | – | – | ● | – | – | – | ● | – | – | – | – | – | – | – |
| Losartan | ●▼ | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Olmesartan | ● | ● | ● | – | ● | – | ● | ● | – | – | – | – | – | – | |
| Telmisartan | ▼ | – | – | – | – | – | – | ● | – | – | – | – | – | – | |
| Valsartan | – | – | ● | – | – | – | ● | ● | – | – | – | – | – | – | |
| Antiarrhythmics | Amiodarone | ▼ | – | – | – | – | – | – | – | ▴ | – | – | – | – | – |
| Bepridil | ● | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Dronedarone | ▼ | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Digoxin | ● | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Felodipine | ▼ | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Propafenone | ▼ | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Quinidine | ●▼ | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Anticoagulants | Apixaban | ● | ● | – | – | – | – | – | – | – | – | – | – | – | – |
| Dabigatran | ● | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Edoxaban | ●▽ | – | – | ○ ▽ | ○ ▽ | – | ○ ▽ | ▽ | – | ▽ | ○ ▽ | – | – | – | |
| Rivaroxaban | ● | ● | – | – | – | – | – | – | – | – | – | – | – | – | |
| Warfarin | ●▼ | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Antihypertensive agents | Aliskiren | ● | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Celiprolol | ● | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Chlorothiazide | – | – | – | ● | ● | – | – | – | – | – | – | – | – | – | |
| Reserpine | ▼ | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Antilipemics | Atorvastatin | ●▼ | ● | ●▼ | – | ▼ | – | ● | ● | ● | – | – | – | – | – |
| Lovastatin | ●▼ | – | ●▼ | – | – | – | – | – | – | – | – | – | – | – | |
| Pravastatin | ▽ | ● | ●▼ | – | ▼ | – | ● | – | ● | – | – | – | – | – | |
| Rosuvastatin | – | ● | ●▼ | – | ●▼ | – | ● | – | ● | – | – | – | – | – | |
| Simvastatin | ▼ | – | ●▼ | ▼ | ▼ | – | ● | – | – | – | – | – | – | – | |
| Antiplatelets | Clopidogrel | ● | – | – | – | – | – | – | – | – | ○▼ | ○ | – | – | – |
| Ticagrelor | ●▼ | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Timolol | ● | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Beta-blockers | Atenolol | ▵ | – | – | – | – | – | – | – | – | ● | ● | – | ● | ● |
| Bisoprolol | ▼ | – | – | – | – | – | – | – | – | – | ▼ | – | – | – | |
| Carvedilol | ●▼ | – | – | – | – | – | – | – | – | ▼ | ▼ | – | – | – | |
| Labetalol | ● | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Nadolol | ●▼ | – | – | – | – | – | ○ | ○ | – | ● | ● | – | ● | ● | |
| Propranolol | ●▼ | – | – | – | – | – | – | – | – | ▼ | ▼ | ▼ | – | – | |
| Talinolol | ●▼ | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| CCBs | Diltiazem | ●▼ | ▽ | – | – | – | – | – | – | – | ▼ | – | – | – | – |
| Isradipine | ▼ | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Mibefradil | ●▼ | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Nifedipine | ▼ | – | – | – | – | – | – | – | – | ▼ | – | – | – | – | |
| Verapamil | ●▼ | ▽ | – | – | – | – | – | – | – | ▼ | – | ▼ | – | – | |
| Diuretics | Bendrofluazide | – | – | – | ● | ● | – | – | – | – | – | – | – | – | – |
| Bumetanide | – | – | – | ▼ | – | – | – | – | – | – | – | – | – | – | |
| Chlorothiazide | – | – | – | ● | ● | – | – | – | – | – | – | – | – | – | |
| Furosemide | – | – | – | ●▼ | ● | – | – | – | – | – | – | – | – | – | |
| Hydrochlorothiazide | – | – | – | ●▼ | – | – | – | – | – | – | – | – | – | – | |
| ERAs | Ambrisentan | ● | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Bosentan | – | – | – | – | – | – | ● | ● | – | – | – | – | – | – | |
| Neprilysin inhibitor | Sacubitril | – | – | – | – | – | – | ● | ● | – | – | – | – | – | – |
Numerous in vitro and in vivo experiments have lead to the identification of many of currently marketed CV drugs including angiotensin receptor blockers, antiarrhythmics, anticoagulants, antihypertensive agents, statins, anti-platelets, beta-blockers, calcium channel blockers, and endothelin receptor antagonist as P-glycoprotein (P-gp, also known as ABCB1 or MDR1) substrates and inhibitors [7,12–14]. Most of statins including atorvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin are substrates and inhibitors of multidrug resistance-associated protein 2 (MRP2, also known as ABCC2) [15]. Furthermore, MRP2-mediated transport of enalapril, fosinopril, eprosartan, olmesartan, and valsartan has also been shown [16–20]. In addition, five CV drugs including olmesartan, apixaban, rivaroxaban, pravastatin, and rosuvastatin were shown to be substrates for Breast Cancer Resistance Protein (BCRP, also known as ABCG2) [8,21–23].
Beyond ABC transporter, SLC transporters also play an important role in CV drugs uptake. For example, OATP-mediated transport of angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, statins and sacubitril was shown in OATP1A1-, OATP1B1-, OATP1B3-, and OATP2B1-expressing HEK293 cells [8,16–18,24,25]. Meanwhile, many ACE inhibitors and diuretics were found to be substrates of OATs [26–29]. Bumetanide, furosemide, hydrochlorothiazide also inhibit OAT1 substantially in a cell model generated by transfection of S2 cells with the human OAT1 gene. Several statins are also reported to be inhibitors of OATs [30]. Recently, the other influx transporters OCTs and multi-antimicrobial extrusion protein (MATEs) were also identified to transport beta blockers, atenolol and nadolol [31,32]. Several other beta blockers including bisoprolol, carvedilol, and propranolol, as well as calcium channel blockers including diltiazem, nifedipine, and verapamil are reported to be inhibitors of OCTs [33,34].
Although many in vitro and in vivo studies demonstrate a comprehensive interaction between CV drugs and transporters, the clinical relevance of this relationship in terms of absorption and disposition of CV drugs still need confirmation. To date, there are several clinical studies have been conducted in human subjects to evaluate the effect of transporter gene polymorphisms on the pharmacokinetics of CV drugs. For example, the P-gp polymorphism C3435T is correlate with lower intestinal P-gp expression [35] and has been shown to increase digoxin plasma levels in health volunteers [36]. The BCRP polymorphism C421A is associated with lower protein expression in vitro [37,38] and has also been shown to increase exposure to atorvastatin and rosuvastatin in human [39]. In addition, the BCRP polymorphism −24CT is related to lower protein expression [40] and appeared to strongly increase systemic exposure to telmisartan and olmesartan in human individuals [41]. Moreover, the CV patients often receive polymedications, and many clinical pharmacokinetic studies have identified the transporter-related CV drug–drug interactions. One of the most widely studied transporter-related CV drug interactions is the interaction between digoxin and quinidine. Since the interaction was first published in 1978, research 20 years later demonstrated that the effect of quinidine on plasma digoxin concentrations was the result of quinidine-induced inhibition of P-gp in the intestine and kidneys, resulting in greater absorption and decreased elimination of digoxin [42]. From then on, many studies have been conducted to evaluate the P-gp-mediated CV drug–drug interactions (reviewed in Ref. [7]). BCRP and OATPs have also been evaluated for their clinical significance in CV drug–drug interactions, especially statins [8]. However, other transporters such as MRPs, OCTs and OATs have not been evaluated for their clinical relevance in CV drug–drug interactions. Thus, further basic and clinical research is still needed to fully understand the involvement of all the transporters in CV medication and guide the safe use of these CV drugs in patients.
3. Modulation of transporters by natural products used in cardiovascular diseases
The effects of natural products and the derived phytochemicals on transporter expression and function have been extensively studied in recent years (reviewed in Ref. [5]). In this section, we highlighted some popular natural products used in CV diseases, and their potential interactions with drug transporters (Table 2). Eight natural products (ginseng, gingko biloba, danshen, green tea, resveratrol, curcumin, berberine and grapefruit juice) will be included and discussed individually below.
Table 2.
Summary of transporters involved in interactions with selected natural products.
| ABC | SLC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| P-gp | BCRP | MRP2 | OAT1 | OAT3 | OATP1A2 | OATP1B1 | OATP1B3 | OATP2B1 | OCT1 | OCT2 | MATE1 | MATE2K | ||
| Ginseng | 20(S)-ginsenoside Rh2 | ●▼ | – | – | – | – | – | – | – | – | – | – | – | – |
| Compound K | ▼ | – | – | – | – | – | – | – | – | – | – | – | – | |
| 20(S)-protopanaxadiol (PPD) | ▼ | ▼ | – | – | – | – | – | – | – | – | – | – | – | |
| 20(S)-protopanaxatriol (PPT) | ▼ | ▼ | – | – | – | – | – | – | – | – | – | – | – | |
| 20(S)-ginsenoside Rg3 | ▼ | ▽ | – | – | – | – | – | – | – | – | – | – | – | |
| 20(S)-ginsenoside Rb1 | ▽ | – | – | – | – | – | ▼ | ▼ | – | – | – | – | – | |
| 20(S)-ginsenoside Rc | ▽ | – | – | – | – | – | ▼ | ▼ | – | – | – | – | – | |
| 20(S)-ginsenoside Rd | ▽ | – | – | – | – | – | ▼ | ▼ | – | – | – | – | – | |
| Gingko biloba | Kaempferol | ●▼ | ●▼ | – | – | – | ○▼ | ○ | ○ | ○▼ | ○ | – | – | – |
| Quercetin | ●▼ | ●▼ | ● | – | – | ●▼ | ●▼ | – | ●▼ | ● | – | – | – | |
| Isorhamnetin | ● | – | – | – | – | – | – | – | – | – | – | – | – | |
| Ginkgolic acids I/II | ●▼ | – | – | – | – | – | – | – | – | – | – | – | ||
| Ginkgolide A | ▴ | – | – | – | – | – | – | – | – | – | – | – | – | |
| Ginkgolide B | ▴ | – | – | – | – | – | – | – | – | – | – | – | – | |
| Danshen | Cryptotanshinone | ●▼▴ | – | – | – | – | – | – | – | – | – | – | – | – |
| Danshensu | ● | – | – | – | – | – | – | – | – | – | – | – | – | |
| Tanshinone I | ●▼ ▵ | – | – | – | – | – | – | – | – | – | – | – | – | |
| Tanshinone II A | ● ▽▴ | – | – | – | – | – | – | – | – | – | – | – | – | |
| Tanshinone II B | ●▼ | – | – | – | – | – | – | – | – | – | – | – | – | |
| Lithospermic acid | – | – | – | ▼ | ▼ | – | – | – | – | – | – | – | – | |
| Rosmarinic acid | – | – | – | ▼ | ▼ | – | – | – | – | – | – | – | – | |
| Salvianolic acid A | – | – | – | ▼ | ▼ | – | – | – | – | – | – | – | – | |
| Salvianolic acid B | – | ▽ | ▽ | ▼ | ▼ | – | – | – | – | – | – | – | – | |
| Tanshinol | – | – | – | ▼ | ▼ | – | ▼ | ▼ | – | – | – | – | – | |
| Green tea | (−)-Epicatechin-3-gallate (ECG) | ●▼ | – | ● | – | – | ●▼ | ▼ | ● | ▼ | – | ▼ | – | – |
| (−)-Epigallocatechin gallate (EGCG) | ▼ | – | ● | – | – | ●▼ | ▼ | ●▼ | ▼ | ▼ | ▼ | ▼ | ▼ | |
| (−)-Catechin gallate (CG) | ▼ | – | – | – | – | – | – | – | – | – | – | – | – | |
| (−)-Epicatechin (EC) | ▽ | – | ● | – | – | – | – | – | ▼ | – | – | – | – | |
| Grapes/Red wine | Resveratrol | ▼ | – | ▼ | ▼ | ▼ | – | – | – | – | – | – | – | – |
| Turemic | Curcumin | ▼ | ▼ | – | ●▼ | ● | – | ●▼ | ●▼ | ● | – | – | – | – |
| Goldenseal/Goldthread | Berberine | ●▼ | – | – | – | – | – | – | ● | – | ▼ | ▼ | ● | – |
| Grapefruit juice | 6′,7′-Epoxybergamottin | ▼ | – | – | – | – | – | – | – | – | – | – | – | – |
| 6′,7′-Dihydroxybergamottin | ▼ | – | – | – | – | – | – | – | – | – | – | – | – | |
| Naringenin | ▼ | – | – | – | – | – | – | – | – | – | – | – | – | |
| Naringin | ▼ | – | – | – | – | ▼ | – | – | – | – | – | – | – | |
3.1. Ginseng
Ginseng is a well-known natural product widely used for the treatment of CV diseases [43]. Its major active components are ginsenosides and the modulatory effects of many of ginsenosides on transporters have been investigated [5]. For example, ginsenoside Rh2, compound K, protopanaxadiol (PPD) and protopanaxatriol (PPT) showed an inhibitory effect toward both P-gp and BCRP in Caco-2 or MCF-7/MX cell lines [44–46]. Ginsenoside Rh2 is also a substrate of P-gp identified in MDR1a/b knockout mice [47]. In addition to the interplay of efflux transporters and ginsenosides, the inhibition of OATPs by ginsenosides has also been shown [48]. Ginsenosides Rb1, Rc, and Rd appear to be potent inhibitors of OATP1B1 and OATP1B3 [48].
Clinical study has been conducted to determine the P-gp-mediated ginseng–drug interaction [49]. No clinically significant pharmacokinetic change was observed with concomitant administration of Panax ginseng and P-gp substrates fexofenadine [49]. One explanation for these negative interactions is that fexofenadine is also a substrate of OATPs [50,51] and ginsenosides could inhibit OATP1B1 and OATP1B3 in addition to P-gp, thus these efflux and influx transporters were altered by Panax ginseng in opposite directions, resulting in no systemic change in fexofenadine pharmacokinetics. Another possible explanation is that ginsenosides have relatively low bioavailability [52], which means that ginseng did not truly affect the activity of any of the transporters involved in fexofenadine transport. The clinical significance of transporter-mediated ginseng–drug interactions remains to be further elucidated.
3.2. Gingko biloba
Leaf extracts of Ginkgo biloba (GBE) is one of the most widely used natural products, and there is an increasing evidence of the potential role of GBE in treating CV diseases [53]. The main active constituents of GBE include ginkgolides, bilobalides, and flavonoids [53]. The individual constituents of GBE have been studied to identify the candidate active ingredients for transporter modulation. Quercetin, one essential flavonoid in GBE, is potential substrate and inhibitor of efflux transporters P-gp and BCRP, but also substrate and inhibitor of influx transporter OATP1A2, OATP1B1 and OATP2B1 [54–57]. Similar to quercetin, another flavonoid in GBE kaempferol also showed an inhibitory effect on P-gp, BCRP, OATP1A2 and OATP2B1 in vitro [54–56]. Kaempferol is also a substrate of P-gp and BCRP, but not substrate of OATP1A2 and OATP2B1 [54–56]. In addition, ginkgolide A and B, another group of essential ingredients in GBE can induce P-gp mRNA expression by activated PXR in LS180 cell model [58]. Furthermore, ginkgolic acids in GBE have also been reported to be substrates and weak inhibitors of P-gp and BCRP [59].
Although the interactions between GBE and transporters are limited to in vitro data, its influence on talinolol suggests that the clinical relevance may prove significant. Long-term ingestion of GBE increases serum talinolol levels up to 36%, which may through inhibition of P-gp activity [60]. It should be noted that GBE has been found to has no relevant effect on the in vivo activity of the major CYP enzymes in humans and thus has no relevant potential to cause CYP enzymes-mediated drug interactions [61]. On the other hand, transporter-mediated GBE–drug interactions should be considered and remains to be elucidated.
3.3. Danshen
Danshen is one of the most versatile natural products that have been widely used in China as well as in United States in addition to usual medicinal therapy in treatment of CV diseases [62]. Over 70 lipophilic and hydrophilic compounds have been isolated and identified from danshen [63]. Of these, lipophilic compounds including tanshinone I, tanshinone II A, tanshinone II B, and cryptotanshinone and hydrophilic compounds including danshensu, lithospermic acid, rosmarinic acid, salvianolic acid A, salvianolic acid and tanshinol are considered to be major components [64,65]. The interactions between these major components of danshen and transporters have been observed in many independent studies [65]. Cryptotanshinone, danshensu, tanshinone I, tanshinone II A, and tanshinone II B have been identified as P-gp substrates in vitro and in vivo [66–70]. Cryptotanshinone, tanshinone I and tanshinone II B also inhibit P-gp substantially in Caco-2 cells [68,71,72]. Second, another study identified active hydrophilic components of danshen including lithospermic acid, rosmarinic acid, salvianolic acid A, salvianolic acid B, and tanshinol are potential inhibitors of OAT1 and OAT3 [65]. In addition, cryptotanshinone and tanshinone II A can induce P-gp mRNA expression after long term exposure in human hepatocytes [73]. Furthermore, the in vivo effect of danshen extract on the pharmacokinetics of fexofenadine in healthy volunteers has also been evaluated. Repeated ingestion of danshen extract for 10 days significantly reduced plasma concentrations of fexofenadine (37% decrease in fexofenadine AUC), probably by the induction of P-gp-mediated efflux in humans [73]. However, few clinical investigations have been conducted to determine the potential effect of danshen on these transporters in humans, and further in vivo and clinical investigations are warranted.
3.4. Green tea
Green tea, made from the leaves of Camellia sinensis, is the most widely-consumed beverage worldwide and its extract is also one of the most common natural products. The predominant constituents of green tea are polyphenols, which are reported to have diverse effects on CV health [74]. Thus, the potential concomitant use of green tea and CV drugs is thought to be increasing. The main polyphenols (−)-epicatechin (EC), (−)-epicatechin-3-gallate (ECG), (−)-epigallocatechin-3-gallate (EGCG), catechin gallate (CG) except (−)-epigallocatechin (EGC) in green tea have been found to interact with both ABC and SLC transporters [75–82]. First, ECG, EGCG, and CG are reported to be inhibitors of P-gp [75,76], and ECG is also a P-gp substrate [77]. It should be noted that the inhibition of P-gp transport by these polyphenols was found to be a reversible process. Second, MRP2-mediated transport of ECG, EGCG, and EC has also been identified in Caco-2 cells [77–79]. Third, EGCG also inhibit many influx transporters including OATP1A1, OATP1B1, OATP1B3, OATP2B1 OCT1, OCT2, MATE1, and MATE2K in vitro [80–82]. Meanwhile, OATP1A1, OATP1B1, OATP2B1, and OCT2 were also identified to be inhibited by ECG in vitro [81,82]. Interestingly, ECG and EGCG were not only found to be inhibitors but also substrates of OATPs. Both of them are transported by OATP1A2 and OATP1B3 [76,77].
Although several in vitro studies demonstrate an interaction between green tea polyphenols and P-gp transporters, the clinical relevance of the interaction between green tea polyphenols and P-gp substrates has not been fully documented and remains to be elucidated. In contrast, the effect of green tea on the disposition of OATP substrates has been evaluated in humans. Repeated consumption of green tea with exceptionally high polyphenols content showed an OATP inhibitory effect in healthy human subjects [83]. However, the green tea used in this clinical trial study contains an exceptionally high total polyphenols, which is two to five times higher than that of typical bottled green tea [84]. The OATP-mediated green tea–drug interaction has the potential to be clinically important. Future studies need to measure the actual green tea consumption for assessing the potential risks associated with the combined use of green tea and selected medications.
3.5. Resveratrol
Resveratrol is a natural polyphenol found in grapes and red wine. It is commonly believed to play a potential protective role against CV diseases. Since an increasing variety of resveratrol natural supplements is widely used, the topic of potential resveratrol–drug interactions is of great interest. Transporter interactions with resveratrol have been observed in several in vitro and in vivo studies. In one study, resveratrol was reported to potently inhibit P-gp, MRP2, and OAT1/OAT3 in vitro and in vivo, and enhanced methotrexate absorption in intestine and decreased methotrexate renal elimination [85]. Another study also reported that resveratrol significantly enhanced the exposure of P-gp substrate fexofenadine in rats likely through the inhibition of intestinal P-gp [86]. However, dose-dependent resveratrol stimulation of P-gp mediated efflux of saquinavir in MDCKII-MDR1 cells was also observed in a later study [87]. The interactions of resveratrol with transporters are still not fully elucidated. Furthermore, few clinical study was conducted to determine transporter-mediated resveratrol–drug interaction. Whether the modulation effects of resveratrol on transporter functions is clinically relevant warrants further investigation.
3.6. Curcumin
Curcumin, the active component of turmeric, is a well-known natural product with a surprisingly wide range of beneficial properties, including anti-inflammatory, antioxidant, chemopreventive and chemotherapeutic activity [88]. Furthermore, there are newly discovered pharmacological activities for curcumin in CV diseases [89]. The potential clinical application of curcumin leads to essential assessments regarding its drug interactions. Transporter-mediated interactions with curcumin have been investigated. Curcumin was firstly reported to dose-dependently inhibit P-gp activity in primary rat hepatocytes [90]. Similarly, curcumin also inhibit P-gp activity in Caco-2 and KB-V1 cells [91,92]. Interestingly, curcumin was also found to decrease the expression of P-gp in KB-V1 cells [93]. Further, curcumin strongly inhibited BCRP in vitro and in vivo [94]. In addition to the interplay of ABC transporters, curcumin as well as its metabolites, curcumin-O-glucuronide and curcumin-O-sulfate, are also found to be substrates and inhibitors of OATPs and OATs, particularly OATP1B1 and OATP1B3 [95,96]. However, there is only one clinical trial which proved the involvement of transporter in curcumin–conventional drug interaction [97]. The transporter-mediated curcumin–drug interactions are largely unknown in clinic. Well-designed clinical studies in healthy volunteers as the first step and in patients as the second step are essential to provide adequate evidence needed to demonstrate the curcumin–drug interactions in humans.
3.7. Berberine
Berberine is an alkaloid isolated from medicinal plants such as Goldenseal and Chinese Goldthread. Although this natural product was traditionally used for various infectious disorders for a long time [98], the beneficial effects in metabolic and CV diseases have been shown in many preclinical and clinical studies during past decades [99]. Therefore, berberine is a promising complementary agent for the treatment of CV diseases. The potential of a combined use berberine and CV drugs raise an interest in its drug interactions. P-gp has been identified as a major transporter responsible for the efflux of berberine in Caco-2 cells [100,101]. OATP1B3 and MATE1 have also been identified as influx transporters for the uptake of berberine in vitro and in vivo [102,103]. In addition, it also has been reported that berberine acts as inhibitor of P-gp, OCT 1 and OCT2 in vitro and in vivo [104,105]. However, there were few clinical reports about transporter-mediated berberine–drug interactions. The berberine–drug interactions in humans need to be further investigated.
3.8. Grapefruit juice
Grapefruit Juice is rich in flavonoids, which have been consumed widely as a preventive measure against CV diseases. However, grapefruit juice is known to cause serious interactions with many CV drugs, such as statins, beta-blockers and calcium channel blockers [106–108]. In most cases, the drugs on which grapefruit juice had a significant effect were undergoes extensive first-pass metabolism by intestinal CYP3A4 [109,110]. Furanocoumarins have been demonstrated to be the main ingredients in grapefruit juice to inhibit CYP3A4. In addition, transporter-mediated grapefruit juice–drug interactions have also been reported. Four compounds 6′,7′-dihydroxybergamottin, 6′,7′-epoxybergamottin, naringin, and naringenin present in grapefruit juice were shown to be able to inhibit the P-gp activity in Caco-2 cells [111]. Naringin also inhibit OATP1A2 in a cell model generated by transfection of HeLa cells with the human OATP1A2 cDNA [112]. In addition to preclinical investigations, several clinical studies were conducted to determine the transporter-mediated grapefruit juice and drug interactions [113–116]. One clinical study reported that the interaction between grapefruit juice and digoxin, a well-known P-gp substrate undergoing little metabolism was modest [114]. One potential explanation for this negative result is the high oral bioavailability of digoxin, which makes the interpretation difficult. Another study found that the exposure of talinolol, another non-metabolized P-gp substrate, was doubled in the presence of grapefruit juice, which likely through the inhibition of intestinal P-gp activity [115]. In contrast, grapefruit juice was later found to be able to decrease the plasma concentrations of talinolol in human [116]. As these phenomena cannot be explained by the known inhibitory effect of grapefruit juice on P-gp, the researchers conducting these studies suggested that constituents in grapefruit juice preferentially inhibited an intestinal uptake process rather than P-gp. Indeed, OATP1A2 was identified as a key intestinal uptake transporter for the grapefruit juice–fexofenadine interaction in another clinical study [117]. However, the expression of OATP1A2 in the intestine has been debated in the literature because some studies reported no OATP1A2 detection, whereas other groups identified relatively low SLC1A2 mRNA expression in the intestine [118,119]. The contribution of OATP1A2 to in vivo OATP-mediated grapefruit juice–drug interactions remains further investigation.
4. Transporter-mediated interaction of natural products with cardiovascular drugs in human
Transporter-mediated interactions between natural products and CV drugs have been reported in several clinical studies [60,83,97,116,120,121] (Table 3). The inhibition of P-gp and OATPs by natural products occurs more often, and shows clinical significance. Curcumin has been widely explored for its inhibitory effect on P-gp. Concomitant use of curcumin with P-gp substrate talinolol, caused an increased plasma level and a reduced elimination of talinolol [97]. Gingko biloba also showed a P-gp inhibitory effect in healthy human subjects. Consecutive oral administration of Gingko biloba (360 mg/day) to healthy subjects for 14 days resulted in significant increase of plasma level of talinolol [60]. In contrast, natural product–drug interaction study with another P-gp substrate digoxin and Gingko biloba (80 mg three times daily for one week) did not result in any pharmacokinetic changes of digoxin [122]. It should be emphasized that the two studies discussed above utilized a dosage regimen of Gingko biloba in their interaction assessment. Also, note that different content of the active ingredients in Gingko biloba and different P-gp substrates used might also contribute to these contradictory results. In addition, quercetin, one of the most important components in Ginkgo biloba, has been shown to inhibit intestinal P-gp-mediated drug efflux, but quercetin co-administration provoked a tendency to reduced talinolol bioavailability which was unexpected with respect to the in vitro P-gp inhibitory effects of quercetin [120]. The researchers conducting these studies anticipated that these interactions might be mediated by a new mechanism involving inhibition of OATPs. Similarly, grapefruit juice was also found to be able to decrease the plasma concentrations of talinolol and this interaction was attributed to the inhibitory effect of grapefruit Juice on OATPs, not the known inhibition of P-gp [116]. Moreover, green tea and its major ingredient EGCG have also been found to decrease the plasma level of nadolol and rosuvastatin through inhibition of OATPs, respectively [83,121]. Thus, the clinical significance of natural product–CV drug interactions mediated by P-gp and OATPs has been demonstrated in these clinical studies. However, there is limited clinical information on natural product–CV drug interactions mediated by other transporters, including BCRP, MRPs, OATs, OCTs and MATEs. The potential effect of natural products on these transporters in humans has not been established, and further clinical investigations are warranted.
Table 3.
Summary of transporter-mediated natural product–CV drug interactions in human.
| Natural products | CV drugs | Effects | Transporters | Reference(s) |
|---|---|---|---|---|
| Curcumin | ||||
| 500 mg capsule (Amazon, USA): 1000 mg QD for 14 days | Talinolol 100 mg QD |
Cmax and AUC of talinolol ↑ CLoral/F ↓ |
Inhibition of P-gp | [97] |
| Gingko biloba | ||||
| Standardized G. biloba extract: Single dose, 120 mg QD; multiple doses, 120 mg TID for 14 days | Talinolol 100 mg QD |
Cmax and AUC of talinolol ↑ | Inhibition of P-gp | [60] |
| Quercetin | ||||
| 500 mg capsules (Mecoline B.V., Winschoten, Netherlands): Single dose, 1500 mg QD; multiple doses, 500 mg TID on Day 1–6; 1500 mg QD on Day 7 | Talinolol 100 mg QD |
Cmax and AUC of talinolol ↓ | Inhibition of OATP2B1 | [120] |
| Grapefruit juice | ||||
| Commercial grapefruit juice (Paradiso–Succo di pompelmo, Cologne, Germany; 100% pure at a normal strength): Single dose, 300 mL daily; multiple doses, 900 mg daily for 6 days | Talinolol 50 mg QD |
Cmax and AUC of talinolol ↓ | Inhibition of OATP | [116] |
| Green tea | ||||
| Commercial green tea beverage (Healthya; Kao, Tokyo, Japan): 700 mL daily for 7 days | Nadolol 30 mg QD |
Cmax and AUC of nadolol ↓ | Inhibition of OATP1A2 | [83] |
| EGCG | ||||
| Teavigo™, 94% pure crystalline EGCG: Single dose, 300 mg QD; multiple doses, 300 mg QD for 10 days | Rosuvastatin 20 mg QD |
Cmax and AUC of rosuvastatin ↓ | Inhibition of intestinal OATP2B1 or OATP1A2 | [121] |
5. Conclusion and future perspectives
The use of natural products is prevalent among CV patients who are taking prescription medications. It can be, therefore, speculated that with the combination use of natural products and conventional medicines, the risk of natural product–drug interactions increases. The natural product–drug interactions are especially relevant when CV drugs with a narrow therapeutic index, such as digoxin and warfarin, are co-administered with natural products that can potentially reduce pharmacologic effects. It is important to understand the potential mechanisms about these natural product–drug interactions. Aside from drug metabolizing enzymes, it is now greatly acknowledged that also modification of transport function is involved in these natural product–drug interactions. Therefore, both preclinical and clinical reports on the roles of transporters in CV therapy, natural product modulation of transporters and its induced natural product—CV drug interactions summarized in this review. The interactions of major transporters including efflux transporters such as P-gp, MRPs and BCRP, and uptake transporters such as OATs, OATPs, OCTs and MATEs with both CV drugs and natural products have been extensively studied in preclinical models and clinical trials. However, only a few direct evaluations have been performed to identify transporter-mediated natural product–CV drug interactions, which mainly focus on P-gp and OATPs. Therefore, more evaluations should be performed to understand the potential natural product–CV drug interactions associated with the interactions of other transporters.
Ideally, all potential natural product–drug interactions can be evaluated by clinical study, which gives direct evidence for understanding natural product–drug interactions with the goal to prevent adverse natural product–drug interactions. However, current food and drug laws allow natural products to be marketed as dietary supplements not subject to the same regulations required for prescription drugs. Thus, individual natural products from different manufacturers may not have same purity, efficacy, and safety, and even not contain the same amount of active ingredients listed on the label. Furthermore, the absorption, distribution, renal excretion, and/or hepatic elimination of active ingredients from natural products in human is often unknown. Although many interactions or potential interactions were suggested by in vitro or animal studies, successful extrapolations of in vitro and animal studies and accurate prediction of natural product–drug interactions remain challenging. The FDA guidance on drug–drug interactions should be applied to study natural product–drug interactions. Using in vitro assays, decision-tree models to determine whether major ingredients from natural products are substrates or inhibitors of transporters and when a subsequent in vivo clinical study is needed. Researchers are also highly recommended to provide the detail information about the amount of active ingredients and pharmacokinetics of major bioactive components from natural products, which will help understand in vivo clinical natural product–drug interaction results.
In spite of lacking systematically assessed potential interactions between natural products and CV drugs, potentially serious consequences might be avoided by carefully monitor and early identify possible natural product–drug interactions. Thus, clinicians are encouraged to carefully question patients about their use of natural products, and also have a sound knowledge base about transporter-mediated natural product–CV drug interactions. Especially, they must take proper caution and perform close monitoring for possible natural product–CV drug interactions when using natural products that may bear mechanism-based P-gp inhibitors. To minimize natural product–CV drug interactions involving transporters, it is necessary to choose safe natural products and drug combinations, adjust drug dosages appropriately and conduct therapeutic drug monitoring for drugs with narrow therapeutic indices.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Liperoti R, Vetrano DL, Bernabei R, Onder G. Herbal medications in cardiovascular medicine. J Am Coll Cardiol. 2017;69:1188–99. doi: 10.1016/j.jacc.2016.11.078. [DOI] [PubMed] [Google Scholar]
- 2. Vogel JH, Bolling SF, Costello RB, Guarneri EM, Krucoff MW, Longhurst JC, et al. Integrating complementary medicine into cardiovascular medicine. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (Writing committee to develop an expert consensus document on complementary and integrative medicine) J Am Coll Cardiol. 2005;46:184–221. doi: 10.1016/j.jacc.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 3. Elmer GW, Lafferty WE, Tyree PT, Lind BK. Potential interactions between complementary/alternative products and conventional medicines in a Medicare population. Ann Pharmacother. 2007;41:1617–24. doi: 10.1345/aph.1K221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tachjian A, Maria V, Jahangir A. Use of herbal products and potential interactions in patients with cardiovascular diseases. J Am Coll Cardiol. 2010;55:515–25. doi: 10.1016/j.jacc.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu X, Ma J, Ye Y, Lin G. Transporter modulation by Chinese herbal medicines and its mediated pharmacokinetic herb-drug interactions. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1026:236–53. doi: 10.1016/j.jchromb.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 6. Nigam SK. What do drug transporters really do? Nat Rev Drug Discov. 2015;14:29–44. doi: 10.1038/nrd4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wessler JD, Grip LT, Mendell J, Giugliano RP. The P-glycoprotein transport system and cardiovascular drugs. J Am Coll Cardiol. 2013;61:2495–502. doi: 10.1016/j.jacc.2013.02.058. [DOI] [PubMed] [Google Scholar]
- 8. Hua WJ, Hua WX, Fang HJ. The role of OATP1B1 and BCRP in pharmacokinetics and DDI of novel statins. Cardiovasc Ther. 2012;30:e234–41. doi: 10.1111/j.1755-5922.2011.00290.x. [DOI] [PubMed] [Google Scholar]
- 9. Wanwimolruk S, Prachayasittikul V. Cytochrome P450 enzyme mediated herbal drug interactions (part 1) EXCLI J. 2014;13:347–91. [PMC free article] [PubMed] [Google Scholar]
- 10. Wanwimolruk S, Phopin K, Prachayasittikul V. Cytochrome P450 enzyme mediated herbal drug interactions (part 2) EXCLI J. 2014;13:869–96. [PMC free article] [PubMed] [Google Scholar]
- 11. Sugano K, Kansy M, Artursson P, Avdeef A, Bendels S, Di L, et al. Coexistence of passive and carrier-mediated processes in drug transport. Nat Rev Drug Discov. 2010;9:597–614. doi: 10.1038/nrd3187. [DOI] [PubMed] [Google Scholar]
- 12. Sakaeda T, Takara K, Kakumoto M, Ohmoto N, Nakamura T, Iwaki K, et al. Simvastatin and lovastatin, but not pravastatin, interact with MDR1. J Pharm Pharmacol. 2002;54:419–23. doi: 10.1211/0022357021778493. [DOI] [PubMed] [Google Scholar]
- 13. Bachmakov I, Werner U, Endress B, Auge D, Fromm MF. Characterization of beta-adrenoceptor antagonists as substrates and inhibitors of the drug transporter P-glycoprotein. Fundam Clin Pharmacol. 2006;20:273–82. doi: 10.1111/j.1472-8206.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 14. Rigalli JP, Ruiz ML, Perdomo VG, Villanueva SS, Mottino AD, Catania VA. Pregnane X receptor mediates the induction of P-glycoprotein by spironolactone in HepG2 cells. Toxicology. 2011;285:18–24. doi: 10.1016/j.tox.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 15. Ellis LC, Hawksworth GM, Weaver RJ. ATP-dependent transport of statins by human and rat MRP2/Mrp2. Toxicol Appl Pharmacol. 2013;269:187–94. doi: 10.1016/j.taap.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 16. Liu L, Cui Y, Chung AY, Shitara Y, Sugiyama Y, Keppler D, et al. Vectorial transport of enalapril by Oatp1a1/Mrp2 and OATP1B1 and OATP1B3/MRP2 in rat and human livers. J Pharmacol Exp Ther. 2006;318:395–402. doi: 10.1124/jpet.106.103390. [DOI] [PubMed] [Google Scholar]
- 17. Sun P, Wang C, Liu Q, Meng Q, Zhang A, Huo X, et al. OATP and MRP2-mediated hepatic uptake and biliary excretion of eprosartan in rat and human. Pharmacol Rep. 2014;66:311–9. doi: 10.1016/j.pharep.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 18. Nakagomi-Hagihara R, Nakai D, Kawai K, Yoshigae Y, Tokui T, Abe T, et al. OATP1B1, OATP1B3, and mrp2 are involved in hepatobiliary transport of olmesartan, a novel angiotensin II blocker. Drug Metab Dispos. 2006;34:862–9. doi: 10.1124/dmd.105.008888. [DOI] [PubMed] [Google Scholar]
- 19. Yamashiro W, Maeda K, Hirouchi M, Adachi Y, Hu Z, Sugiyama Y. Involvement of transporters in the hepatic uptake and biliary excretion of valsartan, a selective antagonist of the angiotensin II AT1-receptor, in humans. Drug Metab Dispos. 2006;34:1247–54. doi: 10.1124/dmd.105.008938. [DOI] [PubMed] [Google Scholar]
- 20. Green BR, Bain LJ. Mrp2 is involved in the efflux and disposition of fosinopril. J Appl Toxicol. 2013;33:458–65. doi: 10.1002/jat.1767. [DOI] [PubMed] [Google Scholar]
- 21. Yamada A, Maeda K, Kamiyama E, Sugiyama D, Kondo T, Shiroyanagi Y, et al. Multiple human isoforms of drug transporters contribute to the hepatic and renal transport of olmesartan, a selective antagonist of the angiotensin II AT1-receptor. Drug Metab Dispos. 2007;35:2166–76. doi: 10.1124/dmd.107.017459. [DOI] [PubMed] [Google Scholar]
- 22. Zhang D, He K, Herbst JJ, Kolb J, Shou W, Wang L, et al. Characterization of efflux transporters involved in distribution and disposition of apixaban. Drug Metab Dispos. 2013;41:827–35. doi: 10.1124/dmd.112.050260. [DOI] [PubMed] [Google Scholar]
- 23. Gong IY, Mansell SE, Kim RB. Absence of both MDR1 (ABCB1) and breast cancer resistance protein (ABCG2) transporters significantly alters rivaroxaban disposition and central nervous system entry. Basic Clin Pharmacol Toxicol. 2013;112:164–70. doi: 10.1111/bcpt.12005. [DOI] [PubMed] [Google Scholar]
- 24. Ishiguro N, Maeda K, Kishimoto W, Saito A, Harada A, Ebner T, et al. Predominant contribution of OATP1B3 to the hepatic uptake of telmisartan, an angiotensin II receptor antagonist, in humans. Drug Metab Dispos. 2006;34:1109–15. doi: 10.1124/dmd.105.009175. [DOI] [PubMed] [Google Scholar]
- 25. Ayalasomayajula S, Han Y, Langenickel T, Malcolm K, Zhou W, Hanna I, et al. In vitro and clinical evaluation of OATP-mediated drug interaction potential of sacubitril/valsartan (LCZ696) J Clin Pharm Ther. 2016;41:424–31. doi: 10.1111/jcpt.12408. [DOI] [PubMed] [Google Scholar]
- 26. Juhasz V, Beery E, Nagy Z, Bui A, Molnar E, Zolnerciks JK, et al. Chlorothiazide is a substrate for the human uptake transporters OAT1 and OAT3. J Pharm Sci. 2013;102:1683–7. doi: 10.1002/jps.23491. [DOI] [PubMed] [Google Scholar]
- 27. Vallon V, Rieg T, Ahn SY, Wu W, Eraly SA, Nigam SK. Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. Am J Physiol Renal Physiol. 2008;294:F867–73. doi: 10.1152/ajprenal.00528.2007. [DOI] [PubMed] [Google Scholar]
- 28. Sekine T, Cha SH, Endou H. The multispecific organic anion transporter (OAT) family. Pflügers Archiv. 2000;440:337–50. doi: 10.1007/s004240000297. [DOI] [PubMed] [Google Scholar]
- 29. Yuan H, Feng B, Yu Y, Chupka J, Zheng JY, Heath TG, et al. Renal organic anion transporter-mediated drug-drug interaction between gemcabene and quinapril. J Pharmacol Exp Ther. 2009;330:191–7. doi: 10.1124/jpet.108.149476. [DOI] [PubMed] [Google Scholar]
- 30. Windass AS, Lowes S, Wang Y, Brown CD. The contribution of organic anion transporters OAT1 and OAT3 to the renal uptake of rosuvastatin. J Pharmacol Exp Ther. 2007;322:1221–7. doi: 10.1124/jpet.107.125831. [DOI] [PubMed] [Google Scholar]
- 31. Yin J, Duan H, Shirasaka Y, Prasad B, Wang J. Atenolol renal secretion is mediated by human organic cation transporter 2 and multidrug and Toxin extrusion proteins. Drug Metab Dispos. 2015;43:1872–81. doi: 10.1124/dmd.115.066175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Misaka S, Knop J, Singer K, Hoier E, Keiser M, Muller F, et al. The nonmetabolized beta-blocker nadolol is a substrate of OCT1, OCT2, MATE1, MATE2-K, and P-Glycoprotein, but not of OATP1B1 and OATP1B3. Mol Pharm. 2016;13:512–9. doi: 10.1021/acs.molpharmaceut.5b00733. [DOI] [PubMed] [Google Scholar]
- 33. Bachmakov I, Glaeser H, Endress B, Morl F, Konig J, Fromm MF. Interaction of beta-blockers with the renal uptake transporter OCT2. Diabetes Obes Metab. 2009;11:1080–3. doi: 10.1111/j.1463-1326.2009.01076.x. [DOI] [PubMed] [Google Scholar]
- 34. Grube M, Ameling S, Noutsias M, Kock K, Triebel I, Bonitz K, et al. Selective regulation of cardiac organic cation transporter novel type 2 (OCTN2) in dilated cardiomyopathy. Am J Pathol. 2011;178:2547–59. doi: 10.1016/j.ajpath.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schwab M, Schaeffeler E, Marx C, Fromm MF, Kaskas B, Metzler J, et al. Association between the C3435T MDR1 gene polymorphism and susceptibility for ulcerative colitis. Gastroenterology. 2003;124:26–33. doi: 10.1053/gast.2003.50010. [DOI] [PubMed] [Google Scholar]
- 36. Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97:3473–8. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kondo C, Suzuki H, Itoda M, Ozawa S, Sawada J, Kobayashi D, et al. Functional analysis of SNPs variants of BCRP/ABCG2. Pharm Res. 2004;21:1895–903. doi: 10.1023/b:pham.0000045245.21637.d4. [DOI] [PubMed] [Google Scholar]
- 38. Imai Y, Nakane M, Kage K, Tsukahara S, Ishikawa E, Tsuruo T, et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther. 2002;1:611–6. [PubMed] [Google Scholar]
- 39. Keskitalo JE, Zolk O, Fromm MF, Kurkinen KJ, Neuvonen PJ, Niemi M. ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2009;86:197–203. doi: 10.1038/clpt.2009.79. [DOI] [PubMed] [Google Scholar]
- 40. Laechelt S, Turrini E, Ruehmkorf A, Siegmund W, Cascorbi I, Haenisch S. Impact of ABCC2 haplotypes on transcriptional and posttranscriptional gene regulation and function. Pharmacogenomics J. 2011;11:25–34. doi: 10.1038/tpj.2010.20. [DOI] [PubMed] [Google Scholar]
- 41. Kim CO, Cho SK, Oh ES, Park MS, Chung JY. Influence of ABCC2, SLCO1B1, and ABCG2 polymorphisms on the pharmacokinetics of olmesartan. J Cardiovasc Pharmacol. 2012;60:49–54. doi: 10.1097/FJC.0b013e3182576098. [DOI] [PubMed] [Google Scholar]
- 42. Fromm MF, Kim RB, Stein CM, Wilkinson GR, Roden DM. Inhibition of P-glycoprotein-mediated drug transport: a unifying mechanism to explain the interaction between digoxin and quinidine [seecomments] Circulation. 1999;99:552–7. doi: 10.1161/01.cir.99.4.552. [DOI] [PubMed] [Google Scholar]
- 43. Karmazyn M, Moey M, Gan XT. Therapeutic potential of ginseng in the management of cardiovascular disorders. Drugs. 2011;71:1989–2008. doi: 10.2165/11594300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 44. Zhang J, Zhou F, Wu X, Gu Y, Ai H, Zheng Y, et al. 20(S)-ginsenoside Rh2 noncompetitively inhibits P-glycoprotein in vitro and in vivo: a case for herb-drug interactions. Drug Metab Dispos. 2010;38:2179–87. doi: 10.1124/dmd.110.034793. [DOI] [PubMed] [Google Scholar]
- 45. Li N, Wang D, Ge G, Wang X, Liu Y, Yang L. Ginsenoside metabolites inhibit P-glycoprotein in vitro and in situ using three absorption models. Planta Med. 2014;80:290–6. doi: 10.1055/s-0033-1360334. [DOI] [PubMed] [Google Scholar]
- 46. Jin J, Shahi S, Kang HK, van Veen HW, Fan TP. Metabolites of ginsenosides as novel BCRP inhibitors. Biochem Biophys Res Commun. 2006;345:1308–14. doi: 10.1016/j.bbrc.2006.04.152. [DOI] [PubMed] [Google Scholar]
- 47. Yang Z, Gao S, Wang J, Yin T, Teng Y, Wu B, et al. Enhancement of oral bioavailability of 20(S)-ginsenoside Rh2 through improved understanding of its absorption and efflux mechanisms. Drug Metab Dispos. 2011;39:1866–72. doi: 10.1124/dmd.111.040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jiang R, Dong J, Li X, Du F, Jia W, Xu F, et al. Molecular mechanisms governing different pharmacokinetics of ginsenosides and potential for ginsenoside-perpetrated herb-drug interactions on OATP1B3. Br J Pharmacol. 2015;172:1059–73. doi: 10.1111/bph.12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Malati CY, Robertson SM, Hunt JD, Chairez C, Alfaro RM, Kovacs JA, et al. Influence of Panax ginseng on cytochrome P450 (CYP)3A and P-glycoprotein (P-gp) activity in healthy participants. J Clin Pharmacol. 2012;52:932–9. doi: 10.1177/0091270011407194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shimizu M, Fuse K, Okudaira K, Nishigaki R, Maeda K, Kusuhara H, et al. Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2005;33:1477–81. doi: 10.1124/dmd.105.004622. [DOI] [PubMed] [Google Scholar]
- 51. Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J Pharmacol Exp Ther. 2004;308:438–45. doi: 10.1124/jpet.103.060194. [DOI] [PubMed] [Google Scholar]
- 52. Leung KW, Wong AS. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5:20. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou W, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Clinical use and molecular mechanisms of action of extract of Ginkgo biloba leaves in cardiovascular diseases. Cardiovasc Drug Rev. 2004;22:309–19. doi: 10.1111/j.1527-3466.2004.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 54. Wang Y, Cao J, Zeng S. Involvement of P-glycoprotein in regulating cellular levels of Ginkgo flavonols: quercetin, kaempferol, and isorhamnetin. J Pharm Pharmacol. 2005;57:751–8. doi: 10.1211/0022357056299. [DOI] [PubMed] [Google Scholar]
- 55. An G, Gallegos J, Morris ME. The bioflavonoid kaempferol is an Abcg2 substrate and inhibits Abcg2-mediated quercetin efflux. Drug Metab Dispos. 2011;39:426–32. doi: 10.1124/dmd.110.035212. [DOI] [PubMed] [Google Scholar]
- 56. Mandery K, Bujok K, Schmidt I, Keiser M, Siegmund W, Balk B, et al. Influence of the flavonoids apigenin, kaempferol, and quercetin on the function of organic anion transporting polypeptides 1A2 and 2B1. Biochem Pharmacol. 2010;80:1746–53. doi: 10.1016/j.bcp.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 57. Wu LX, Guo CX, Chen WQ, Yu J, Qu Q, Chen Y, et al. Inhibition of the organic anion-transporting polypeptide 1B1 by quercetin: an in vitro and in vivo assessment. Br J Clin Pharmacol. 2012;73:750–7. doi: 10.1111/j.1365-2125.2011.04150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Satsu H, Hiura Y, Mochizuki K, Hamada M, Shimizu M. Activation of pregnane X receptor and induction of MDR1 by dietary phytochemicals. J Agric Food Chem. 2008;56:5366–73. doi: 10.1021/jf073350e. [DOI] [PubMed] [Google Scholar]
- 59. Li L, Yao QQ, Xu SY, Hu HH, Shen Q, Tian Y, et al. Cyclosporin A affects the bioavailability of ginkgolic acids via inhibition of P-gp and BCRP. Eur J Pharm Biopharm. 2014;88:759–67. doi: 10.1016/j.ejpb.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 60. Fan L, Tao GY, Wang G, Chen Y, Zhang W, He YJ, et al. Effects of Ginkgo biloba extract ingestion on the pharmacokinetics of talinolol in healthy Chinese volunteers. Ann Pharmacother. 2009;43:944–9. doi: 10.1345/aph.1L656. [DOI] [PubMed] [Google Scholar]
- 61. Zadoyan G, Rokitta D, Klement S, Dienel A, Hoerr R, Gramatte T, et al. Effect of Ginkgo biloba special extract EGb 761(R) on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur J Clin Pharmacol. 2012;68:553–60. doi: 10.1007/s00228-011-1174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cheng TO. Cardiovascular effects of Danshen. Int J Cardiol. 2007;121:9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 63. Li YG, Song L, Liu M, Hu ZB, Wang ZT. Advancement in analysis of Salviae miltiorrhizae Radix et Rhizoma (Danshen) J Chromatogr A. 2009;1216:1941–53. doi: 10.1016/j.chroma.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 64. Wang X, Morris-Natschke SL, Lee KH. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med Res Rev. 2007;27:133–48. doi: 10.1002/med.20077. [DOI] [PubMed] [Google Scholar]
- 65. Wang L, Sweet DH. Competitive inhibition of human organic anion transporters 1 (SLC22A6), 3 (SLC22A8) and 4 (SLC22A11) by major components of the medicinal herb Salvia miltiorrhiza (Danshen) Drug Metab Pharmacokinet. 2013;28:220–8. doi: 10.2133/dmpk.dmpk-12-rg-116. [DOI] [PubMed] [Google Scholar]
- 66. Zhou ZW, Chen X, Liang J, Yu XY, Wen JY, Zhou SF. Involvement of P-glycoprotein and multidrug resistance associated protein 1 in the transport of tanshinone IIB, a primary active diterpenoid quinone from the roots of Salvia miltiorrhiza, across the blood-brain barrier. Drug Metab Lett. 2007;1:205–17. doi: 10.2174/187231207781369807. [DOI] [PubMed] [Google Scholar]
- 67. Yu XY, Lin SG, Chen X, Zhou ZW, Liang J, Duan W, et al. Transport of cryptotanshinone, a major active triterpenoid in Salvia miltiorrhiza Bunge widely used in the treatment of stroke and Alzheimer’s disease, across the blood-brain barrier. Curr Drug Metab. 2007;8:365–78. doi: 10.2174/138920007780655441. [DOI] [PubMed] [Google Scholar]
- 68. Li XX, Zhou ZW, Zhou SF. Role of P-glycoprotein in the transport of tanshinone I, one active triterpenoid from Salvia miltiorrhiza. Drug Metab Lett. 2008;2:223–30. doi: 10.2174/187231208785425746. [DOI] [PubMed] [Google Scholar]
- 69. Chen X, Zhou ZW, Xue CC, Li XX, Zhou SF. Role of P-glycoprotein in restricting the brain penetration of tanshinone IIA, a major active constituent from the root of Salvia miltiorrhiza Bunge, across the blood-brain barrier. Xenobiotica. 2007;37:635–78. doi: 10.1080/00498250701411258. [DOI] [PubMed] [Google Scholar]
- 70. Yu PF, Wang WY, Eerdun G, Wang T, Zhang LM, Li C, et al. The role of P-Glycoprotein in transport of Danshensu across the blood-brain barrier. Evid Based Complement Alternat Med. 2011;2011:713523. doi: 10.1155/2011/713523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yu XY, Zhou ZW, Lin SG, Chen X, Yu XQ, Liang J, et al. Role of ATP-binding cassette drug transporters in the intestinal absorption of tanshinone IIB, one of the major active diterpenoids from the root of Salvia miltiorrhiza. Xenobiotica. 2007;37:375–415. doi: 10.1080/00498250701230559. [DOI] [PubMed] [Google Scholar]
- 72. Hu T, To KK, Wang L, Zhang L, Lu L, Shen J, et al. Reversal of P-glycoprotein (P-gp) mediated multidrug resistance in colon cancer cells by cryptotanshinone and dihydrotanshinone of Salvia miltiorrhiza. Phytomedicine. 2014;21:1264–72. doi: 10.1016/j.phymed.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 73. Qiu F, Zeng J, Liu S, He M, Zhu L, Ye Y, et al. Effects of danshen ethanol extract on the pharmacokinetics of fexofenadine in healthy volunteers. Evid Based Complement Alternat Med. 2014;2014:473213. doi: 10.1155/2014/473213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Babu PV, Liu D. Green tea catechins and cardiovascular health: an update. Curr Med Chem. 2008;15:1840–50. doi: 10.2174/092986708785132979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kitagawa S, Nabekura T, Kamiyama S. Inhibition of P-glycoprotein function by tea catechins in KB-C2 cells. J Pharm Pharmacol. 2004;56:1001–5. doi: 10.1211/0022357044003. [DOI] [PubMed] [Google Scholar]
- 76. Jodoin J, Demeule M, Beliveau R. Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols. Biochim Biophys Acta. 2002;1542:149–59. doi: 10.1016/s0167-4889(01)00175-6. [DOI] [PubMed] [Google Scholar]
- 77. Vaidyanathan JB, Walle T. Cellular uptake and efflux of the tea flavonoid (−)epicatechin-3-gallate in the human intestinal cell line Caco-2. J Pharmacol Exp Ther. 2003;307:745–52. doi: 10.1124/jpet.103.054296. [DOI] [PubMed] [Google Scholar]
- 78. Vaidyanathan JB, Walle T. Transport and metabolism of the tea flavonoid (−)-epicatechin by the human intestinal cell line Caco-2. Pharm Res. 2001;18:1420–5. doi: 10.1023/a:1012200805593. [DOI] [PubMed] [Google Scholar]
- 79. Hong J, Lambert JD, Lee SH, Sinko PJ, Yang CS. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (−)-epigallocatechin-3-gallate and its methyl metabolites. Biochem Biophys Res Commun. 2003;310:222–7. doi: 10.1016/j.bbrc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 80. Knop J, Misaka S, Singer K, Hoier E, Muller F, Glaeser H, et al. Inhibitory effects of green tea and (−)-Epigallocatechin gallate on transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2-K and P-Glycoprotein. PLoS One. 2015;10:e0139370. doi: 10.1371/journal.pone.0139370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Roth M, Timmermann BN, Hagenbuch B. Interactions of green tea catechins with organic anion-transporting polypeptides. Drug Metab Dispos. 2011;39:920–6. doi: 10.1124/dmd.110.036640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jaiyen C, Jutabha P, Anzai N, Lungkaphin A, Soodvilai S, Srimaroeng C. Interaction of green tea catechins with renal organic cation transporter 2. Xenobiotica. 2015:1–10. doi: 10.3109/00498254.2015.1107785. [DOI] [PubMed] [Google Scholar]
- 83. Misaka S, Yatabe J, Muller F, Takano K, Kawabe K, Glaeser H, et al. Green tea ingestion greatly reduces plasma concentrations of nadolol in healthy subjects. Clin Pharmacol Ther. 2014;95:432–8. doi: 10.1038/clpt.2013.241. [DOI] [PubMed] [Google Scholar]
- 84. Ide K, Park M, Yamada H. The effect of green tea with exceptionally high catechin content on nadolol plasma concentration. Clin Pharmacol Ther. 2014;95:588. doi: 10.1038/clpt.2014.36. [DOI] [PubMed] [Google Scholar]
- 85. Jia Y, Liu Z, Wang C, Meng Q, Huo X, Liu Q, et al. P-gp, MRP2 and OAT1/OAT3 mediate the drug-drug interaction between resveratrol and methotrexate. Toxicol Appl Pharmacol. 2016;306:27–35. doi: 10.1016/j.taap.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 86. Bedada SK, Yellu NR, Neerati P. Effect of resveratrol on the pharmacokinetics of fexofenadine in rats: involvement of P-glycoprotein inhibition. Pharmacol Rep. 2016;68:338–43. doi: 10.1016/j.pharep.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 87. Li J, Liu Y, Zhang J, Yu X, Wang X, Zhao L. Effects of resveratrol on P-glycoprotein and cytochrome P450 3A in vitro and on pharmacokinetics of oral saquinavir in rats. Drug Des Devel Ther. 2016;10:3699–706. doi: 10.2147/DDDT.S118723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–52. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jiang S, Han J, Li T, Xin Z, Ma Z, Di W, et al. Curcumin as a potential protective compound against cardiac diseases. Pharmacol Res. 2017;119:373–83. doi: 10.1016/j.phrs.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 90. Romiti N, Tongiani R, Cervelli F, Chieli E. Effects of curcumin on P-glycoprotein in primary cultures of rat hepatocytes. Life Sci. 1998;62:2349–58. doi: 10.1016/s0024-3205(98)00216-1. [DOI] [PubMed] [Google Scholar]
- 91. Ampasavate C, Sotanaphun U, Phattanawasin P, Piyapolrungroj N. Effects of Curcuma spp. on P-glycoprotein function. Phytomedicine. 2010;17:506–12. doi: 10.1016/j.phymed.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 92. Chearwae W, Anuchapreeda S, Nandigama K, Ambudkar SV, Limtrakul P. Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from turmeric powder. Biochem Pharmacol. 2004;68:2043–52. doi: 10.1016/j.bcp.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 93. Anuchapreeda S, Leechanachai P, Smith MM, Ambudkar SV, Limtrakul PN. Modulation of P-glycoprotein expression and function by curcumin in multidrug-resistant human KB cells. Biochem Pharmacol. 2002;64:573–82. doi: 10.1016/s0006-2952(02)01224-8. [DOI] [PubMed] [Google Scholar]
- 94. Shukla S, Zaher H, Hartz A, Bauer B, Ware JA, Ambudkar SV. Curcumin inhibits the activity of ABCG2/BCRP1, a multidrug resistance-linked ABC drug transporter in mice. Pharm Res. 2009;26:480–7. doi: 10.1007/s11095-008-9735-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhou X, Zhang F, Chen C, Guo Z, Liu J, Yu J, et al. Impact of curcumin on the pharmacokinetics of rosuvastatin in rats and dogs based on the conjugated metabolites. Xenobiotica. 2017;47:267–75. doi: 10.1080/00498254.2016.1183060. [DOI] [PubMed] [Google Scholar]
- 96. Sun X, Li J, Guo C, Xing H, Xu J, Wen Y, et al. Pharmacokinetic effects of curcumin on docetaxel mediated by OATP1B1, OATP1B3 and CYP450s. Drug Metab Pharmacokinet. 2016;31:269–75. doi: 10.1016/j.dmpk.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 97. He X, Mo L, Li ZY, Tan ZR, Chen Y, Ouyang DS. Effects of curcumin on the pharmacokinetics of talinolol in human with ABCB1 polymorphism. Xenobiotica. 2012;42:1248–54. doi: 10.3109/00498254.2012.697590. [DOI] [PubMed] [Google Scholar]
- 98. Zhu XZ, Li XY, Liu J. Recent pharmacological studies on natural products in China. Eur J Pharmacol. 2004;500:221–30. doi: 10.1016/j.ejphar.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 99. Affuso F, Mercurio V, Fazio V, Fazio S. Cardiovascular and metabolic effects of Berberine. World J Cardiol. 2010;2:71–7. doi: 10.4330/wjc.v2.i4.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Maeng HJ, Yoo HJ, Kim IW, Song IS, Chung SJ, Shim CK. P-glycoprotein-mediated transport of berberine across Caco-2 cell monolayers. J Pharm Sci. 2002;91:2614–21. doi: 10.1002/jps.10268. [DOI] [PubMed] [Google Scholar]
- 101. Pan GY, Wang GJ, Liu XD, Fawcett JP, Xie YY. The involvement of P-glycoprotein in berberine absorption. Pharmacol Toxicol. 2002;91:193–7. doi: 10.1034/j.1600-0773.2002.t01-1-910403.x. [DOI] [PubMed] [Google Scholar]
- 102. Chen C, Wu ZT, Ma LL, Ni X, Lin YF, Wang L, et al. Organic anion-transporting polypeptides contribute to the hepatic uptake of berberine. Xenobiotica. 2015;45:1138–46. doi: 10.3109/00498254.2015.1042537. [DOI] [PubMed] [Google Scholar]
- 103. Yang G, Zhao Z, Zhang X, Wu A, Huang Y, Miao Y, et al. Effect of berberine on the renal tubular epithelial-to-mesenchymal transition by inhibition of the Notch/snail pathway in diabetic nephropathy model KKAy mice. Drug Des Devel Ther. 2017;11:1065–79. doi: 10.2147/DDDT.S124971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Xin HW, Tang X, Ouyang M, Zhong JX, Li WL. Effects of berberine on pharmacokinetics of midazolam and rhodamine 123 in rats in vivo. Springerplus. 2016;5:380. doi: 10.1186/s40064-016-2013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kwon M, Choi YA, Choi MK, Song IS. Organic cation transporter-mediated drug-drug interaction potential between berberine and metformin. Arch Pharm Res. 2015;38:849–56. doi: 10.1007/s12272-014-0510-6. [DOI] [PubMed] [Google Scholar]
- 106. Lee JW, Morris JK, Wald NJ. Grapefruit juice and statins. Am J Med. 2016;129:26–9. doi: 10.1016/j.amjmed.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 107. Sica DA. Interaction of grapefruit juice and calcium channel blockers. Am J Hypertens. 2006;19:768–73. doi: 10.1016/j.amjhyper.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 108. Mertens-Talcott SU, Zadezensky I, De Castro WV, Derendorf H, Butterweck V. Grapefruit-drug interactions: can interactions with drugs be avoided? J Clin Pharmacol. 2006;46:1390–416. doi: 10.1177/0091270006294277. [DOI] [PubMed] [Google Scholar]
- 109. Karmakar S, Biswas S, Bera R, Mondal S, Kundu A, Ali MA, et al. Beverage-induced enhanced bioavailability of carbamazepine and its consequent effect on antiepileptic activity and toxicity. J Food Drug Anal. 2015;23:327–34. doi: 10.1016/j.jfda.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Garg SK, Kumar N, Bhargava VK, Prabhakar SK. Effect of grapefruit juice on carbamazepine bioavailability in patients with epilepsy. Clin Pharmacol Ther. 1998;64:286–8. doi: 10.1016/S0009-9236(98)90177-1. [DOI] [PubMed] [Google Scholar]
- 111. de Castro WV, Mertens-Talcott S, Derendorf H, Butterweck V. Grapefruit juice-drug interactions: grapefruit juice and its components inhibit P-glycoprotein (ABCB1) mediated transport of talinolol in Caco-2 cells. J Pharm Sci. 2007;96:2808–17. doi: 10.1002/jps.20975. [DOI] [PubMed] [Google Scholar]
- 112. Bailey DG, Dresser GK, Leake BF, Kim RB. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther. 2007;81:495–502. doi: 10.1038/sj.clpt.6100104. [DOI] [PubMed] [Google Scholar]
- 113. de Castro WV, Mertens-Talcott S, Derendorf H, Butterweck V. Effect of grapefruit juice, naringin, naringenin, and bergamottin on the intestinal carrier-mediated transport of talinolol in rats. J Agric Food Chem. 2008;56:4840–5. doi: 10.1021/jf0728451. [DOI] [PubMed] [Google Scholar]
- 114. Parker RB, Yates CR, Soberman JE, Laizure SC. Effects of grapefruit juice on intestinal P-glycoprotein: evaluation using digoxin in humans. Pharmacotherapy. 2003;23:979–87. doi: 10.1592/phco.23.8.979.32881. [DOI] [PubMed] [Google Scholar]
- 115. Spahn-Langguth H, Langguth P. Grapefruit juice enhances intestinal absorption of the P-glycoprotein substrate talinolol. Eur J Pharm Sci. 2001;12:361–7. doi: 10.1016/s0928-0987(00)00191-3. [DOI] [PubMed] [Google Scholar]
- 116. Schwarz UI, Seemann D, Oertel R, Miehlke S, Kuhlisch E, Fromm MF, et al. Grapefruit juice ingestion significantly reduces talinolol bioavailability. Clin Pharmacol Ther. 2005;77:291–301. doi: 10.1016/j.clpt.2004.11.111. [DOI] [PubMed] [Google Scholar]
- 117. Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- 118. Obaidat A, Roth M, Hagenbuch B. The expression and function of organic anion transporting polypeptides in normal tissues and in cancer. Annu Rev Pharmacol Toxicol. 2012;52:135–51. doi: 10.1146/annurev-pharmtox-010510-100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos. 2007;35:1333–40. doi: 10.1124/dmd.107.014902. [DOI] [PubMed] [Google Scholar]
- 120. Nguyen MA, Staubach P, Wolffram S, Langguth P. Effect of single-dose and short-term administration of quercetin on the pharmacokinetics of talinolol in humans - Implications for the evaluation of transporter-mediated flavonoid-drug interactions. Eur J Pharm Sci. 2014;61:54–60. doi: 10.1016/j.ejps.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 121. Kim TE, Ha N, Kim Y, Kim H, Lee JW, Jeon JY, et al. Effect of epigallocatechin-3-gallate, major ingredient of green tea, on the pharmacokinetics of rosuvastatin in healthy volunteers. Drug Des Devel Ther. 2017;11:1409–16. doi: 10.2147/DDDT.S130050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Mauro VF, Mauro LS, Kleshinski JF, Khuder SA, Wang Y, Erhardt PW. Impact of ginkgo biloba on the pharmacokinetics of digoxin. Am J Ther. 2003;10:247–51. doi: 10.1097/00045391-200307000-00003. [DOI] [PubMed] [Google Scholar]