Abstract
Glucuronidation is one of the major metabolic pathways for flavonoids. However, quantification of flavonoid glucuronides in biological samples, especially in the bile, is sometimes challenging due to signal suppression by bile acids. The purpose of this study is to establish a robust LC-MS/MS method for directly measuring flavonoid glucuronides in bile and blood. Wogonoside (wogonin-7-O-glucuronide), baicalin (baicalein-7-O-glucuronide) and apigenin-7-O-glucuronide were used as the model compounds and taurocholic acid (T-CA) were used as the model bile acid to establish the method. Bile samples were processed using solid phase extraction (SPE) and blood samples were prepared using protein precipitation method. The analytes were separated on a Resteck HPLC (50 mm × 2.1 mm ID, 1.7 μm) column using acetonitrile and 0.1% formic acid in water as the mobile phases. The mass analysis was performed in an AB Sciex 5500 Qtrap mass spectrometer via multiple reaction monitoring (MRM) in the positive mode. The results showed that the linear range of the above three analytes were 10 nM–5000 nM in the bile and 1.56 nM–4000 nM in the blood, respectively. The recoveries of three glucuronides were> 85% and the matrix effects were <20% at low, medium and high concentrations in the bile and the blood. The results also showed that >90% of these bile acids were removed by the selected SPE procedure to facilitate glucuronide analysis. The validated method was successfully applied to a portal vein infusion study using rats to quantify baicalin, wogonoside, and apigenin-glucuronide in bile and blood samples.
Keywords: Wogonoside, Baicalin, Apigenin-7-O-Glucuronide, Glucuronide quantification, Bile, Blood, SPE, LC-MS/MS
1. Introduction
Flavonoids, which consists of two phenyl rings and a heterocyclic ring in the structures, are widely distributed in many fruits and vegetables [1]. For example, apigenin, a 5,7,4′-trihydroxyl-flavone, is found as one of the major ingredients in chamomile tea and the contents of apigenin is up to 0.8%–1.2% in a typical chamomile tea drink [2]. Other examples include genistein in soy, quercetin in oranges, and anthocyanidins in blueberries [3,4]. Flavonoids possess multiple pharmacological effects, including anti-cancer, anti-inflammation, and anti-oxidation [5–7], probably due to their anti-oxidation property from hydroxyls. Therefore, this class of compounds is believed to be the active components in nutritional and herbal materials. For example, wogonin was reported as the key active components against inflammation in Scutellaria Radix [8].
Although bio-activities of flavonoids are promising, their oral bioavailabilities are usually poor [9,10], which hampers flavonoids being developed as therapeutic drugs. Pharmaceutical research has demonstrated that one of the major reasons for poor oral bioavailability for flavonoids is glucuronidation in the intestine and the liver [11,12]. Interestingly, some flavonoid-glucuronides are good substrates of certain efflux transporters (e.g., BCRP, MRP2) [13] and can be secreted through bile into the intestine, where glucuronides can be hydrolyzed by microflora to release the aglycones, followed by re-absorption in the intestine to form a enterohepatic recycling (EHR) [11,12]. Many studies have been conducted to study enterohepatic recycling in order to have a better understanding and utilization of this physiological phenomenon. Thus, sensitive and robust analytical method to quantify flavonoids and their glucuronides in the bile and blood samples are required.
A few LC-MS methods for flavonoid-glucuronides quantification in the bile and blood have been published previously [14–20]. However, these methods are not widely cited probably because of low sensitivity and complex sample preparation procedures. Further studies suggested that low signal sensitivity in the bile might be caused by ion suppression from bile acids [21]. In addition, bile acids can form micelles and encapsulate the analytes, resulting in low extraction recovery [22,23]. Alternatively, a common approach to analyze glucuronides in the bile is to hydrolyze the conjugates and quantify the aglycones [24,25]. However, this indirect quantification method is not robust and may not be accurate because stability is a concern for some flavonoids during hydrolysis. Moreover, this indirect quantification can’t quantify both glucuronides and aglycones at the same time, which is required in many studies.
This paper developed and validated a LC-MS method for glucuronides quantification in the bile and blood samples and apply the method in a portal vein infusion study, where both bile and blood samples are collected.
2. Experiments
2.1. Chemicals and reagents
Wogonoside was purchased from Meilunebio (Dalian, China). Apigenin-7-O-glucuronide was purchased from HWI Analytik GmbH (Rheinzaberner, Rülzheim, German). Rutin (internal standard, I.S.) was purchased from Cayman Chemistry Company. Baicalin was purchased from Indofine (NJ, USA). DMSO was purchased from Sigma-Aldrich. Acetonitrile and methanol were from Omni Solv (CA, US). HLB cartridge and MCX cartridge were purchased from Waters Oasis (MA, USA). X-AW, C18–W and SCX cartridge were purchased from Strata Phenomenex (CA, USA.). Other chemicals were used as received.
2.2. Instrument and conditions
2.2.1. UPLC conditions
UPLC condition: system, Waters Acquity with diode array; column, Restek Ultra BPh (5 μm, 100 mm × 2.1 mm); mobile phase, A 0.1% formic acid in water, mobile phase B acetonitrile; gradient, 10% B (0–0.5 min), 10% B–34% B (0.5–1.0 min), 34% B–60% B (1.0–2.5 min), 60% B–95% B (2.5–6.0 min), 95% B–10% B (6.0–7.0 min); flow rate, 0.45 ml/min; column temperature, 45 °C; injection volume, 10 μL.
2.2.2. Mass spectrometry conditions
The MS analysis was performed on a Sciex 5500 triple quadrupole mass spectrometer (AB Sciex LLC, Framingham, MA) equipped with an ESI source. The detection was conducted using MRM scan type in positive ion mode. The instrument dependent parameters were: ionspray voltage, 5.5 kV; ion source temperature, 400 °C; nebulizer gas (gas 1), nitrogen, 20 psi; turbo gas (gas 2), nitrogen 20 psi; curtain gas, nitrogen 20 psi. Unit mass resolution was set in both mass-resolving quadruples Q1 and Q3. Compound-dependent parameters were listed in Table 1.
Table 1.
Compound dependent parameter of the analytes and I.S.
| Compound | Q1/Q3 | DP | CE | EP | CXP |
|---|---|---|---|---|---|
| Wogonosidea | 461.0/285.0 | 30 | 26 | 10 | 16 |
| Wogonosideb | 461.0/270.0 | 34 | 21 | 10 | 11 |
| Baicalina | 447.0/271.0 | 31 | 37 | 10 | 17 |
| Baicalinb | 447.0/175.0 | 36 | 28 | 10 | 12 |
| Apigenin-7-O-glucuronidea | 447.0/271.0 | 31 | 37 | 10 | 17 |
| Apigenin-7-O-glucuronideb | 447.0/175.0 | 36 | 28 | 10 | 12 |
| T-CA | 514.0/80.0 | 120 | 78 | 10 | 16 |
| Rutin (I.S.) | 611/303 | 120 | 47 | 10 | 13 |
This ion pair was used for quantification for its highest signal intensity.
This ion pair was used to verify the results in case of false positive results.
2.3. Preparation of stock solution, calibration curves, and QC samples in the bile and blood
Stock solutions (1.0 mM) of wogonoside, baicalin, apigenin-7-O-glucuronide, and rutin were prepared in water:acetonitrile = 1:1 (containing 0.1% formic acid). The working solution for bile samples preparation were prepared by serial dilution of the stock solution into 50% methanol to afford 200, 100, 50, 25, 12.5, 6.25, 3.12, 1.56, 0.78, 0.39 μM, respectively. To prepare calibration curve samples in the bile, these working solutions (5 μL each) were spiked into 5 μL of blank bile, after which the mixtures were further diluted in 1 mL of water (containing 0.1 μM rutin as internal standard)and loaded to SPE columns. After eluted from the SPE column with elution solutions described below, samples were collected, and solvents were removed under N2 flow. The residues were reconstituted into 20% acetonitrile (200 μL) for LC-MS analysis to reach a final concentration of 5,000, 2,500, 1,250, 625, 312.5, 156.3, 78, 39, 20, 10 nM. The quality control (QC) samples for each analyte were prepared at three different concentrations (10.0 nM as low, 156.25 nM as medium, and 2500.0 nM as high) following the same procedures as described above. Standard curves were prepared every time when a new batch of samples were prepared and injected with samples.
The working solution for blood samples preparation were prepared by serial dilution of the stock solution into 50% methanol to afford 4,000, 2,000, 1,000, 500, 250, 125, 62.5, 31.2, 15.6 nM, respectively. To prepare standard curve in the blood, blank blood (10 μL) was mixed with 10 μL of standard curve solutions. Then, the mixture was further added with 200 μL of acetonitrile (containing 100 nM rutin as internal standard). The mixture was further vortex for 1min and centrifuged at 15,000 rpm for 15min. Then, 200 μL of supernatant was collected and dried under nitrogen flow. The residues were reconstituted into 20% acetonitrile (200 μL) to reach a final concentration of 400, 200, 100, 50, 25, 12.5, 6.25, 3.12, 1.56 nM for LC-MS analysis. The quality control (QC) samples for each analyte were prepared at three different concentrations (1.56 nM as low, 25.0 nM as medium, and 200.0 nM as high) following the same procedures as described above.
2.4. Sample preparation
Bile samples preparation.
The bile samples were prepared following the procedures described in the calibration curve samples preparation. Briefly, 5 μL of bile samples was spiked into 5 μL of 50% acetonitrile, which was further diluted into 1 mL of water for SPE extraction.
Blood samples preparation.
Blood samples were also prepared following the procedures described in the calibration curve samples preparation. Briefly, 10 μL of blood samples was spiked into 10 μL of 50% acetonitrile, which was further precipitated by 200 μL of Methanol (with 0.1% formic acid and IS).
2.5. Liquid-liquid extraction (LLE) and solid phase extraction (SPE) of bile samples
To establish a reliable extraction method, wogonoside was selected as model compound and T-CA, the most abundant bile acid in rat bile [26], was selected as the marker of bile acid. Methanol, which is a typical solvent used for protein precipitation [27–29], was used in LLE. Briefly, 5 μL of QC samples at three concentrations (low, medium and high) were spiked with 5 μL of blank bile. Then, 1 mL of methanol (with 0.1% formic acid and IS) was applied to precipitate the bile acids and proteins. After vortex and sonicate for 15min, samples were further centrifuged at 15,000 rpm for 15min, after which 950 μL of supernatant was taken and dried under nitrogen flow. Then, the residue was reconstituted into 200 μL of 20% acetonitrile for LC-MS analysis.
To establish an appropriate SPE procedure, five commonly used SPE cartridges were tested. Briefly, wogonoside (5 μL) was mixed with blank bile (5 μL) and diluted to 1 mL of water with 0.1% formic acid at the concentration of 1 μM. Internal standard (rutin) was added in and diluted at 0.5 μM in the mixture. Then, samples were loaded onto the cartridges, which were eluted sequentially with 1 mL of water with 0.1%formic acid (loading fraction) and then 1 mL of methanol with 0.1% formic acid twice (MeOH elution). The collected fractions were dried under nitrogen flow and reconstituted with 200 μL of 20% acetonitrile for LC-MS analysis.
For elution optimization, different percentage of methanol applied in elution solutions were tested. Briefly, after sample loading, 30%, 40%, 50%, or 60% of methanol (1.0 mL) were eluted respectively to afford the pre-elute fractions (Fr. 1). Then, 100% of MeOH was used to eluted to afford MeOH fraction (Fr. 2). After that, solvents were dried under nitrogen blowing and the residues were re-constituted into 20% of acetonitrile (200 μL) for LC-MS analysis.
2.6. Method validation
The analytical method was validated according to the “Guidance for Industry, Bioanalytical Method Validation” presented by the US Food and Drug Administration (2018) [30].
2.6.1. Specificity
The specificity of the method was determined by injecting processed pooled blank bile and pooled blank blood samples.
2.6.2. Linearity and lowest limit of quantification (LLOQ)
Calibration curves were prepared as described before. After sample analysis, a least-square linear regression (1/X2 weights) was applied to determine the slope, intercept and correlation coefficient factor. The lowest limit of quantification (LLOQ) was determined accurately and precisely with a signal-noise ratio (S/N) of at least 5:1.
2.6.3. Recovery, matrix effect, and carry-over
The recovery was evaluated by comparing the peak areas obtained from samples prepared from blank bile/blood spiked with QC samples with those from samples prepared from water spiked with the same concentrations.
The matrix effect was determined by comparing the peak areas of samples prepared from residues of pooled bile/blood spiked with QC samples with those from residues of water spiked with the same concentration of QC samples.
The carryover impact was evaluated by inject blank matrix (50% acetonitrile, no analyte and IS.) right after the injection of the highest concentration of calibration standards.
2.6.4. Effect of T-CA on wogonoside’s ionization
The effect of T-CA on wogonoside’s ionization was evaluated by comparing the peak areas of QC samples at a low (156.2 nM) and a high concentration (2500 nM) spiked with T-CA (20 mg/mL) with those spiked with water.
2.6.5. Precision and accuracy
Precision and accuracy were conducted by replicate the analysis of QC samples (n = 3). The intra-day accuracy was conducted by performing the analysis in same day and 3-day accuracy was conducted by performing the analysis in consecutive 3 days.
2.7. Application to a portal vein infusion experiment
Animals.
The animal protocol used in this study was approved by University of Houston Institution of Animal Care and Use Committee. Male Wistar rats (270–320 g) were housed in an environmentally controlled room (temperature: 25 ± 2 °C, humidity: 50% ± 5%, 12 h day-night time cycle) at least a week before the experiment.
Portal vein infusion:
The portal vein infusion study has been performed using a method was previously published by us [31]. Briefly, rats were fasted over-night with free access of water before the day of experiment. After a rat was anesthetized, its superior mesenteric vein in the mesentery of jejunum, which is directly connected to the portal vein, was exposed. The superior mesenteric vein was punctured by a venous indwelling needle. Then, rat’s bile duct was located and separated from adjacent tissues. After that, a small cut was made with a micro vascular scissor and a tubing (PE10) was inserted and secured with a surgical suture. Its bile duct was also cannulated as described [31]. Drug solutions were infused from the portal vein cannulation and bile samples were collected from bile duct cannulation. The samples were collected every 0.5 h.
3. Results
3.1. Method development
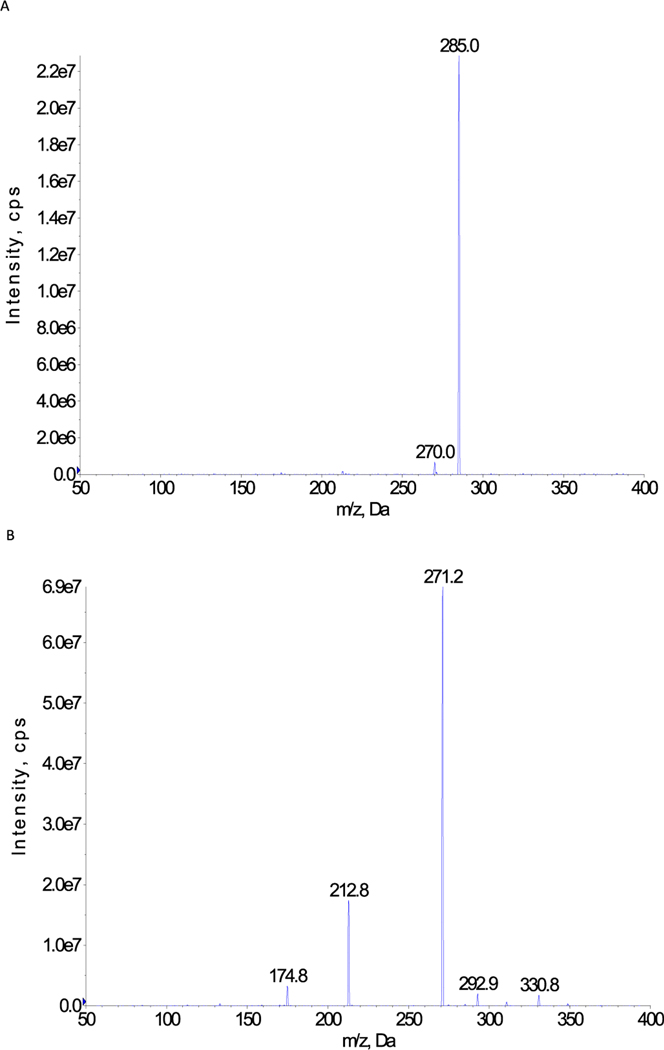
Both UPLC and MS conditions were optimized. For LC condition, formic acid (0.1%) in water and acetonitrile were used as the mobile phases. For MS conditions, positive mode was selected for all analytes based on the method specificity and signal intensity comparing with negative mode. MRM scan type was used to increase the specificity of the method. Each compound was tuned and optimized separately to improve the analysis sensitivity. The parent ions and fragment ions with highest abundance were selected as the ion pairs for each analyte. The compound dependent parameters were summarized in Table 1. Rutin was selected as internal standard (I.S.) and added into the solvent used for extraction. Loss of the glucuronic acid unit (−176) is the major fragment ions for a glucuronide as the bond between the aglycone and the glucuronic acid is the easiest one to be broken in MS/MS. This fragment is commonly used in glucuronide quantification in LC-MS/MS in literature [32–34]. The MS/MS spectrum of 3 analytes were also presented in Fig. 1.
Fig. 1.
A The MS/MS spectrum of wogonoside (461.0) B The MS/MS spectrum of baicalin/apigenin-7-O-glucuronide (447.0).
3.2. Liquid-liquid extraction (LLE) for bile sample preparation
The results of LLE were summarized in Table 2. The recovery and matrix effect were out of the acceptable ranges (85–115%), especially at low concentration, suggesting components in the bile suppressed ionization of the analytes. Protein precipitation using organic solvent was not an appropriate method for bile sample preparation.
Table 2.
The recovery and matrix effect of wogonoside in Liquid-liquid extraction in the bile.
| Concentration | Recovery (%) | Matrix effect (%) |
|---|---|---|
| Low | ND | ND |
| Medium | 70.6 ± 2.3 | 61.3 ± 5.2 |
| High | 76.7 ± 6.4 | 69.1 ± 1.3 |
ND: not detected.
3.3. Solid phase extraction (SPE) for bile sample preparation
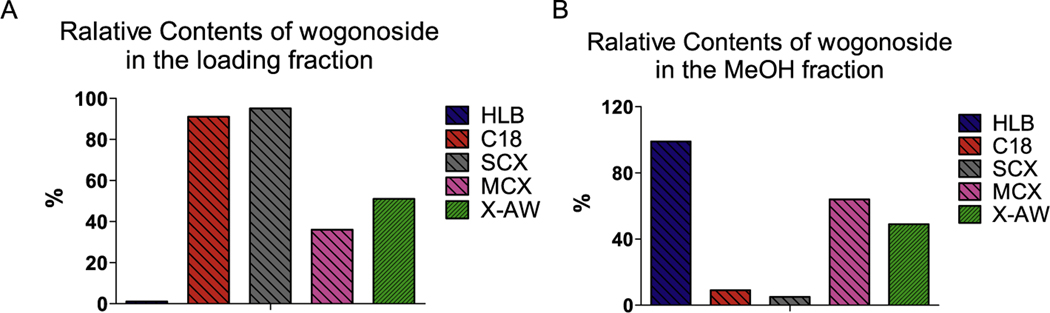
First, the retention of wogonoside on the SPE cartridges were tested. The results showed that < 15%, which is the acceptable analytical error range, of the loading wogonoside was detected in the loading fraction when HLB cartridge used (Fig. 2A) and > 85% of wogonoside was in the MeOH elution (Fig. 2B). Based on this finding, HLB cartridge was selected as the SPE cartridge.
Fig. 2.
The retention of wogonoside on different SPE cartridges.
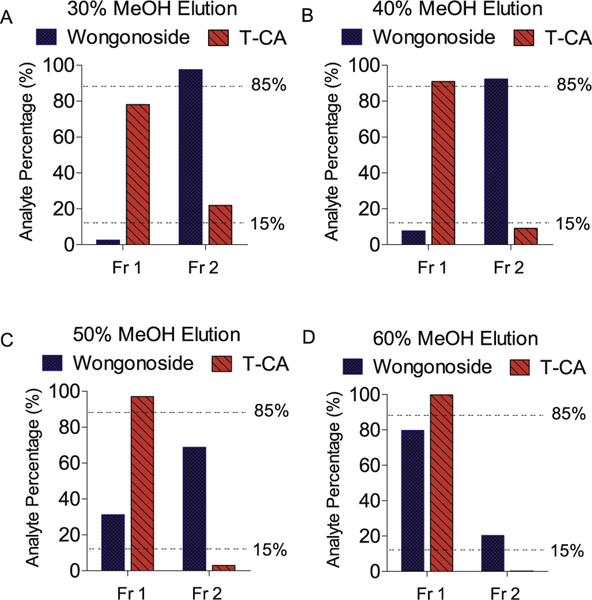
Then, the elution condition was optimized based on the wogonoside’s recovery and its separation with T-CA in the elution. The results showed that when 40% of MeOH was used as the pre-elution solvent, TCA can be eluted entirely (> 85%, Fr. 1 in Fig. 3) and only tiny amount of T-CA (< 15%) was in the final elution (Fr. 2 in Fig. 3). While for wogonoside, 40% MeOH pre-elution couldn’t elute wogonoside (< 15%, Fr. 1 in Fig. 3) and most of wogonoside (> 85%) was in the final elution (Fr. 2, Fig. 3). These findings revealed that with 40% of MeOH pre-elution, wogonoside can be separated with T-CA on HLB cartridge. Therefore, of MeOH pre-elution will be used in the preparation of bile samples.
Fig. 3.
Recovery and separation of wogonoside in SPE extraction with HLB cartridge. (Fr. 1, pre-eluted fraction, Fr. 2100% MeOH eluted fraction).
3.4. Effect of T-CA on wogonoside’s ionization
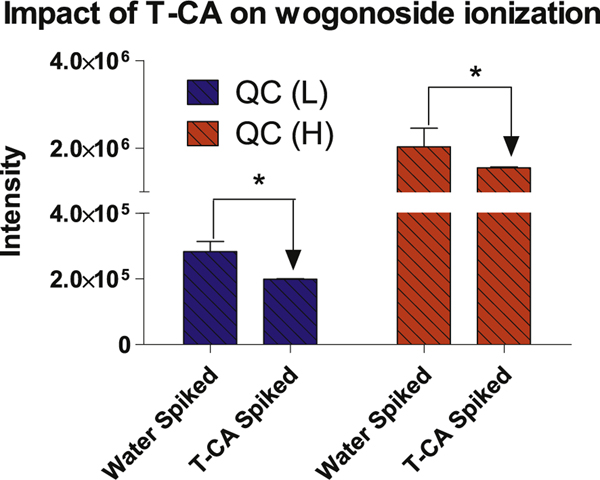
The effect of bile acid on wogonoside’s ionization was determined by spiking T-CA into QC sample. The spiked T-CA was 20 mg/mL, which is a concentration lower than the real bile samples but close to the solubility limit of T-CA in water [26,35]. The results showed that in the presence of T-CA, the intensity of wogonoside was decreased when compared to those spiked with water (Fig. 4). This result suggested that components in the bile (e.g., bile acid) suppress glucuronide’s ionization. Therefore, remove bile acids in a key step in sample preparation in LC-MS analysis.
Fig. 4.
The impact of T-CA on wogonoside ionization. The addition of T-CA significantly decreased the signal intensity of wogonoside. Statistics were conducted by Student t test p < 0.05.
3.5. Method validation
3.5.1. Selectivity
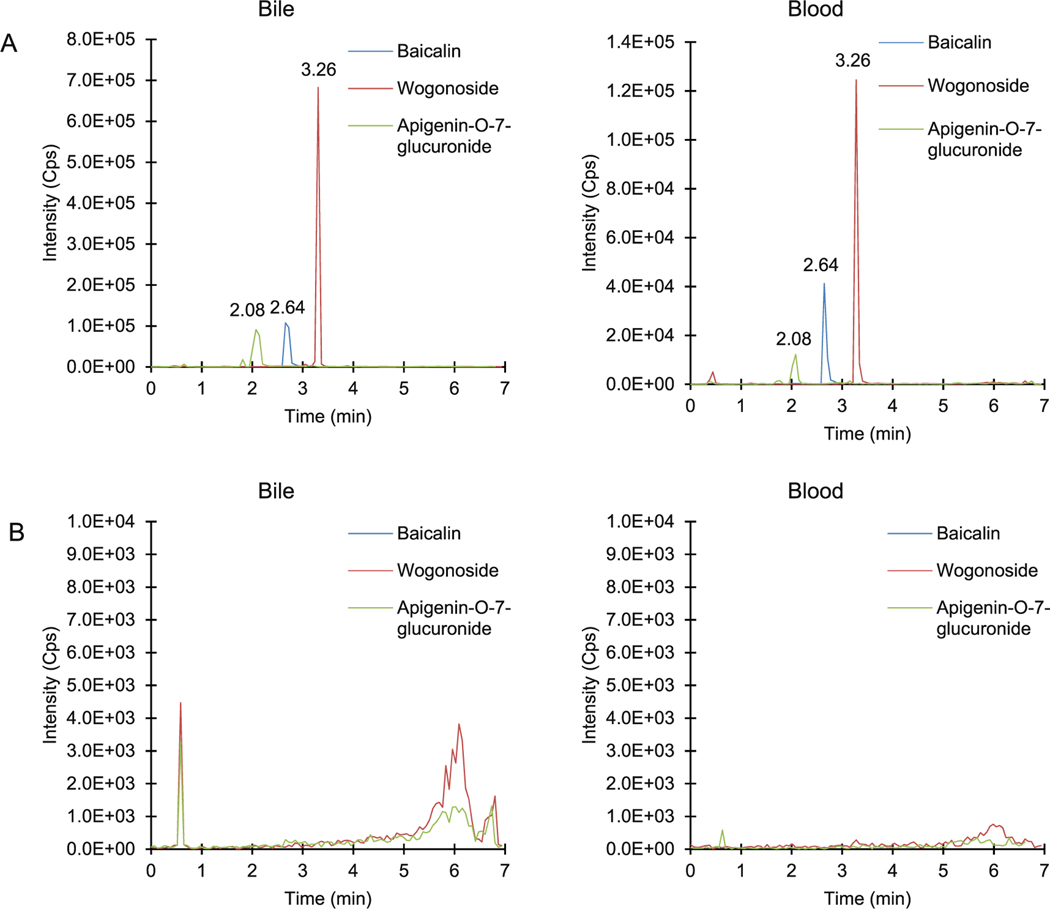
The specificity was determined by comparing the samples at LLOQ with those from blank bile and blood samples pooled from six animals. As shown in Fig. 2, no significant interfering was observed with the analytes including baicalin, wogonoside and apigenin-7-O-glucuronide. The chromatographs where 3 analytes in bile and blood matrix were compared to corresponding blank matrix were summarized in Fig. 6. The comparison using second transitions was summarized in supplement Fig. S1.
Fig. 6.
A The LC/MS chromatographs of apigenin-7-O-glucuronide (green), baicalin (blue) and wogonoside (red) in bile and blood matrix. The retention time of each peak was marked in the chromatographs. B The LC/MS chromatographs of blank bile and blood matrix without any spiked analytes. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5.2. Linearity, LLOQ, precision and accuracy
In the bile, the calibration curves were linear from 10.0 nM to 5000.0 nM for wogonoside, from 10.0 nM to 5000 nM for baicalin, and from 10.0 nM to 5000.0 nM for apigenin-7-O-glucuronide. The correlation coefficient (r2) was at least 0.98 for these glucuronides (Table 3). The lower limit of quantification (LLOQ) for these 3 glucuronides are 10 nM. In the blood, calibration curves were linear from 1.56 nM to 4000.0 nM for wogonoside, from 1.56 nM to 4000.0 nM for baicalin, and from 1.56 nM to 4000.0 nM for apigenin-7-O-glucuronide. The correlation coefficient (r2) was at least 0.99 for these glucuronides (Table 3). The lower limit of quantification (LLOQ) for these 3 glucuronides are 1.56 nM. The accuracies (%) of each concentration in standard curves were also summarized in Table 4.The intra-day and 3-day precisions and accuracies were summarized in Table 5. All the accuracies and precisions were within the acceptance values (100% ± 15%).
Table 3.
The linearity, LLOQ, and R2 of wogonoside, apigenin-7-O-glucuronide and baicalin in the bile and blood. The accuracies (%) at each concentrations of the standard curves were presented.
| Analytes | Linearity | LLOQ (nM) | R2 | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Bile | Blood | Bile | Blood | Bile | Blood | |
| Wogonoside | 10.0–5000.0 | 1.56–4000.0 | 10.0 | 1.56 | 0.983 | 0.991 |
| Apigenin-7-O-glucuronide | 10.0–5000.0 | 1.56–4000.0 | 10.0 | 1.56 | 0.996 | 0.989 |
| Baicalin | 10.0–5000.0 | 1.56–4000.0 | 10.0 | 1.56 | 0.995 | 0.992 |
| Conc.(nM) | Accuracy in Blood (%) | Conc.(nM) | Accuracy in Bile (%) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Wogonoside | Apigenin-O-7-glucuronide | Baicalin | Wogonoside | Apigenin-O-7-glucuronide | Baicalin | ||
| 1.56 | 92.7 | 98.2 | 112 | 10 | 90.8 | 86.9 | 97.9 |
| 3.12 | 111 | 135 | 81.1 | 20 | 110 | 119 | 86.7 |
| 6.25 | 112 | 118 | 90.7 | 39 | 109 | 108 | 124 |
| 12.5 | 86.1 | 93 | 102 | 78 | 111 | 98.9 | 100 |
| 25 | 112 | 111 | 94.3 | 156 | 113 | 91 | 103 |
| 50 | 88.1 | 77.2 | 109 | 312 | 108 | 118 | 115 |
| 100 | 98.7 | 91.4 | 102 | 625 | 96.7 | 108 | 100 |
| 200 | 101 | 100 | 110 | 1250 | 98.1 | 107 | 96.9 |
| 400 | 98 | 85.1 | 100 | 2500 | 85.2 | 95.5 | 86.8 |
| 5000 | 79.5 | 80.9 | 86.6 | ||||
Table 4.
The recovery and matrix effect of wogonoside, apigenin-7-O-glucuronide and baicalin in bile and blood matrix.
| Analytes | QCa | Recovery (%) | Matrix effect (%) | ||
|---|---|---|---|---|---|
|
| |||||
| Bile | Blood | Bile | Blood | ||
| Wogonoside | Low | 88.3 ± 13.2 | 99.8 ± 8.1 | 99.2 ± 12.4 | 103.1 ± 3.3 |
| Medium | 100.5 ± 15.1 | 86.7 ± 7.5 | 98.4 ± 15.7 | 104.8 ± 8.2 | |
| High | 114.4 ± 1.3 | 87.3 ± 2.4 | 85.6 ± 3.7 | 102.1 ± 1.1 | |
| Apigenin-7-O-glucuronide | Low | 88.2 ± 7.5 | 88.7 ± 7.2 | 103.6 ± 10.4 | 103.1 ± 3.9 |
| Medium | 103.2 ± 5.2 | 90.7 ± 8.3 | 101.5 ± 19.1 | 104.4 ± 8.2 | |
| High | 92.5 ± 4.5 | 92.2 ± 3.3 | 98.1 ± 11.6 | 101.3 ± 1.4 | |
| Baicalin | Low | 103.3 ± 5.3 | 89.4 ± 7.2 | 104.4 ± 7.5 | 103.5 ± 3.3 |
| Medium | 105.2 ± 5.7 | 98.1 ± 4.7 | 92.8 ± 4.4 | 103.6 ± 7.8 | |
| High | 94.2 ± 5.2 | 100.8 ± 3.3 | 90.4 ± 3.3 | 101.8 ± 1.2 | |
QC: Low, medium, and high were 10, 156, 2500 nM in the bile and 1.56, 25, and 2000 nM in the blood, respectively.
Table 5.
The accuracy and intra-day, inter-day precision of wogonosides, apigenin-7-O-glucuronide, and baicalin in the bile and blood matrix.
| Analytes | QCa | Intra-day | Inter-day | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Accuracy (%) | Precision (RSD %) | Accuracy (%) | Precision (RSD %) | ||||||
|
| |||||||||
| Bile | Blood | Bile | Blood | Bile | Blood | Bile | Blood | ||
| Wogonoside | Low | 108.4 | 99.9 | 2.0 | 11.2 | 105.9 | 94.4 | 4.4 | 6.1 |
| Medium | 102.5 | 99.1 | 4.3 | 5.6 | 104.1 | 96.3 | 2.7 | 7.7 | |
| High | 99 | 96.7 | 8.3 | 3.1 | 99.7 | 94.1 | 4.0 | 4.4 | |
| Apigenin-7-O-glucuronide | Low | 107.5 | 111.1 | 1.7 | 2.0 | 111.4 | 94.5 | 3.2 | 5.2 |
| Medium | 113.5 | 98.7 | 6.9 | 12.0 | 108.0 | 96.4 | 4.9 | 11.0 | |
| High | 88.1 | 94.8 | 4.7 | 9.2 | 95.2 | 93.0 | 3.0 | 10.6 | |
| Baicalin | Low | 105.9 | 99.5 | 4.7 | 9.9 | 104.2 | 92.9 | 3.2 | 7.7 |
| Medium | 98.0 | 94.2 | 4.8 | 6.7 | 102.0 | 88.5 | 3.7 | 10.6 | |
| High | 95.3 | 97.1 | 7.9 | 6.9 | 94.0 | 94.4 | 4.8 | 8.3 | |
Low, medium, and high were 10.0, 156.0, 2500.0 nM in the bile and 1.56, 25.0, and 2000.0 nM in the blood, respectively.
3.5.3. Matrix effect, recovery and carryover effects
In the bile, the extraction recoveries were evaluated using QC samples (n = 6) at low (10.0 nM), medium (156.2 nM) and high (2500 nM) concentrations. The mean recovery was over 95%, suggesting that SPE could extract these glucuronides from bile. The matrix effect after SPE preparations were within the range of 85%–105% for wogonoside, 95%–105% for baicalin, and 95%–105% for apiginen-7-O-glucuronide (Table 2).
In the blood, the matrix effect and recoveries in all three samples were all close to 100%, indicating that the method was good enough to analyze these glucuronides in blood samples without hydrolysis.
To determine carryover, blank matrix samples were injected right after the highest concentration of calibration standards. The results showed that the analytes and IS peaks detected in the blank matrix in both blood and bile are < 20% of LLOQ.
3.6. Application of the LC-MS method to analyze bile and blood samples derived from a portal vein infusion experiment in rats
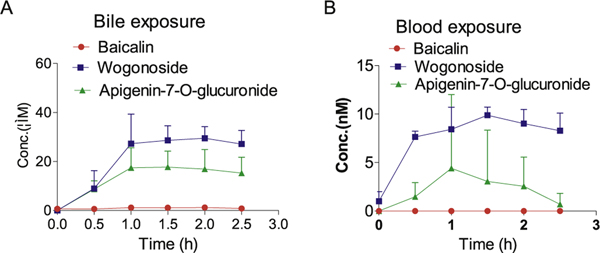
The application of the established method was demonstrated by conducting rat portal vein infusion experiment. Wogonoside, baicalin and apigenin-7-O-glucuronide. The results showed that when the compounds were infused through the portal vein, the analytes can be detected in both the bile and blood (Fig. 5), suggesting that these three flavonoid glucuronides were taken up by the hepatocytes and then secreted to the apical side into the bile or basolateral side into the blood. Since glucuronides are highly hydrophilic, uptake transporter may be involved in the liver. Further studies need to focus on transporter identification to fully elucidate the mechanism. The recovery of wogonoside in the bile was significantly higher than the other two compounds, suggesting that the efficiency of enterohepatic recycling of wogonoside is better than the other two compounds. For baicalin, both biliary and blood exposure were very low, indicating that this compound might have a very fast clearance through urine.
Fig. 5.
The concentrations of wogonoside, apigenin-7-O-glucuronide, and baicalin in the bile and blood in a portal vein infusion study.
4. Conclusion
In conclusion, a sensitive LC-MS method for directly analyzing flavonoid glucuronides in the bile and blood was established and validated. The method was applied in a portal vein infusion study. The current data suggested that bile acids suppress glucuronides ionization and SPE with HLB can efficiently separate flavonoid-glucuronides from bile acid in bile matrix. More studies are needed to further investigate the mechanism of enterohepatic recycling and clearance of these glucuronides.
Supplementary Material
Acknowledgements
Funder: National Institutes of Health, Funding number: GM070737
Footnotes
CRediT authorship contribution statement
Yifan Tu: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing - original draft, Writing - review & editing. Lei Zhou: Methodology, Software. Li Li: Methodology, Software. Lu Wang: Methodology, Software, Writing - review & editing. Song Gao: Conceptualization, Methodology, Software, Resources, Supervision, Writing - original draft, Writing - review & editing. Ming Hu: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing - review & editing.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ab.2020.113723.
References
- [1].Kumar S, Pandey AK, Chemistry and biological activities of flavonoids: an over-view, Sci. World J. 2013 (2013) 162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhan M, Liu X, Li J, Clinical significance of multidrug resistance-associated protein(MRP) gene expression in non-small cell lung cancer, Zhonghua Zhongliu Zazhi 21 (2) (1999) 112–113. [PubMed] [Google Scholar]
- [3].Shankar E, et al. , Plant flavone apigenin: an emerging anticancer agent, Curr. Pharmacol. Rep. 3 (6) (2017) 423–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Panche AN, Diwan AD, Chandra SR, Flavonoids: an overview, J. Nutr. Sci. 5 (2016) e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hollman PC, Katan MB, Dietary flavonoids: intake, health effects and bioavailability, Food Chem. Toxicol. 37 (9–10) (1999) 937–942. [DOI] [PubMed] [Google Scholar]
- [6].Halliwell B, Antioxidants in human health and disease, Annu. Rev. Nutr. 16 (1996) 33–50. [DOI] [PubMed] [Google Scholar]
- [7].Birt DF, Hendrich S, Wang W, Dietary agents in cancer prevention: flavonoids and isoflavonoids, Pharmacol. Ther. 90 (2–3) (2001) 157–177. [DOI] [PubMed] [Google Scholar]
- [8].Chi YS, et al. , Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: in vivo regulation of inflammation-associated gene expression, Biochem. Pharmacol. 66 (7) (2003) 1271–1278. [DOI] [PubMed] [Google Scholar]
- [9].Thilakarathna SH, Rupasinghe HP, Flavonoid bioavailability and attempts for bioavailability enhancement, Nutrients 5 (9) (2013) 3367–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hu M, Commentary: bioavailability of flavonoids and polyphenols: call to arms, Mol. Pharm. 4 (6) (2007) 803–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang L, Zuo Z, Lin G, Intestinal and hepatic glucuronidation of flavonoids, Mol. Pharm. 4 (6) (2007) 833–845. [DOI] [PubMed] [Google Scholar]
- [12].Day AJ, et al. , Human metabolism of dietary flavonoids: identification of plasma metabolites of quercetin, Free Radic. Res. 35 (6) (2001) 941–952. [DOI] [PubMed] [Google Scholar]
- [13].Peng X, Feng F, Zhang W, Expression of multidrug resistance-associated protein (MRP) and lung resistance protein (LRP) in human rectal carcinomas and its clinical significance, Zhonghua Zhongliu Zazhi 21 (3) (1999) 193–195. [PubMed] [Google Scholar]
- [14].Shan G, Zhong H, Zhang F, Expression and prognostic significance of multidrug resistance associated protein (MRP) gene in non-small cell lung cancer by in situ hybridization, Zhonghua Zhongliu Zazhi 22 (1) (2000) 27–29. [PubMed] [Google Scholar]
- [15].Peng X, Feng F, Zhang W, Expression of multidrug resistance-associated protein in human non-small cell lung cancer, Zhonghua Jiehe He Huxi Zazhi 22 (11) (1999) 655–658. [PubMed] [Google Scholar]
- [16].Bagrij T, et al. , Influences of glutathione on anionic substrate efflux in tumour cells expressing the multidrug resistance-associated protein, MRP1, Biochem. Pharmacol. 62 (2) (2001) 199–206. [DOI] [PubMed] [Google Scholar]
- [17].Mottino AD, et al. , Expression of multidrug resistance-associated protein 2 in small intestine from pregnant and postpartum rats, Am. J. Physiol. Gastrointest. Liver Physiol. 280 (6) (2001) G1261–G1273. [DOI] [PubMed] [Google Scholar]
- [18].Harbottle A, et al. , Role of glutathione S-transferase P1, P-glycoprotein and multidrug resistance-associated protein 1 in acquired doxorubicin resistance, Int. J. Canc. 92 (6) (2001) 777–783. [DOI] [PubMed] [Google Scholar]
- [19].Komdeur R, et al. , Expression of P-glycoprotein, multidrug resistance-associated protein 1, and lung resistance-related protein in human soft tissue sarcomas before and after hyperthermic isolated limb perfusion with tumor necrosis factor-alpha and melphalan, Cancer 91 (10) (2001) 1940–1948. [PubMed] [Google Scholar]
- [20].Docampo M, et al. , Glucuronidated flavonoids in neurological protection: structural analysis and approaches for chemical and biological synthesis, J. Agric. Food Chem. 65 (35) (2017) 7607–7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fang N, et al. , Matrix effects break the LC behavior rule for analytes in LC-MS/MS analysis of biological samples, Exp. Biol. Med. 240 (4) (2015) 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Klaassen CD, Watkins JB 3rd, Mechanisms of bile formation, hepatic uptake, and biliary excretion, Pharmacol. Rev. 36 (1) (1984) 1–67. [PubMed] [Google Scholar]
- [23].Boyer JL, Bile formation and secretion, Comp. Physiol. 3 (3) (2013) 1035–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Trontelj J, Quantification Of Glucuronide Metabolites In Biological Matrices By LC-MS/ MS Tandem Mass Spectrometry - Applications and Principles, (2012), p. 29. [Google Scholar]
- [25].Adnan A, Kadi MMH, Biological Fluids: Glucuronides from LC/MS, (2012). [Google Scholar]
- [26].Yang T, et al. , Quantitative profiling of 19 bile acids in rat plasma, liver, bile and different intestinal section contents to investigate bile acid homeostasis and the application of temporal variation of endogenous bile acids, J. Steroid Biochem. Mol. Biol. 172 (2017) 69–78. [DOI] [PubMed] [Google Scholar]
- [27].Polson C, et al. , Optimization of protein precipitation based upon effectiveness of protein removal and ionization effect in liquid chromatography-tandem mass spectrometry, J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 785 (2) (2003) 263–275. [DOI] [PubMed] [Google Scholar]
- [28].Dong SAM, Handbook of Pharmaceutical Analysis by HPLC, (2005). [Google Scholar]
- [29].Silberring PCJ, Proteomic Profiling and Analytical Chemistry, The Crossroads, 2016. [Google Scholar]
- [30].Bioanalytical Method Validation Guidance for Industry, F.a.D.A. U.S. Department of Health and Human Services, Center for Drug Evaluation and Research (CDER),Center for Veterinary Medicine (CVM), 2018. [Google Scholar]
- [31].Zeng M, et al. , Disposition of flavonoids via recycling: direct biliary excretion of enterically or extrahepatically derived flavonoid glucuronides, Mol. Nutr. Food Res. 60 (5) (2016) 1006–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].MacGregor JT, Genetic and carcinogenic effects of plant flavonoids: an overview, Adv. Exp. Med. Biol. 177 (1984) 497–526. [DOI] [PubMed] [Google Scholar]
- [33].Luszczki JJ, et al. , Pharmacodynamic and pharmacokinetic interaction studies of loreclezole with felbamate, lamotrigine, topiramate, and oxcarbazepine in the mouse maximal electroshock seizure model, Epilepsia 46 (3) (2005) 344–355. [DOI] [PubMed] [Google Scholar]
- [34].Kernohan AF, et al. , An oral yohimbine/L-arginine combination (NMI 861) for the treatment of male erectile dysfunction: a pharmacokinetic, pharmacodynamic and interaction study with intravenous nitroglycerine in healthy male subjects, Br. J. Clin. Pharmacol. 59 (1) (2005) 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Perwaiz S, et al. , Determination of bile acids in biological fluids by liquid chromatography-electrospray tandem mass spectrometry, J. Lipid Res. 42 (1) (2001) 114–119. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.