Abstract
Candida-associated denture stomatitis (CADS) is a common, recurring clinical complication in denture wearers that can lead to serious oral and systemic health problems. Current management strategies are not satisfactory due to short-acting and ineffective therapeutic effects. Here, we describe a new fungal biofilm controlling strategy using polyelectrolyte layer-by-layer (LBL) self-assembly technology on denture materials. Conventional poly (methyl methacrylate) (PMMA) denture material discs were functionalized with negatively charged poly (methacrylic acid) (PMAA) via plasma-initiated surface grafting, followed by repetitive alternating coating with the salivary antimicrobial polypeptide histatin 5 (H-5; cationic polymer) and hyaluronic acid (HA; anionic polymer). On the other hand, the H-5/HA LBL coatings (i.e., the outermost layer was H-5) inhibited fungal attachment/adhesion, significantly reduced fungal biofilm formation, and showed synergistic effects with the antifungal drug miconazole. LBL surface hydrophilicity was not the key mechanism in controlling Candida biofilm formation. The current approach demonstrates the utility of a new design principle for fabricating anticandidal denture materials, as well as potentially other related medical devices, for controlling fungal biofilm formation and combating insidious infections.
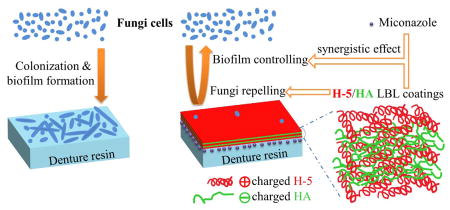
Graphical Abstract
For patients missing multiple teeth or with edentulism, removable partial or full dentures are invaluable for maintenance of overall health, contributing to nutrition, speech, appearance, and quality of life.1 Unfortunately, due to colonization of the dentures by biofilm-forming microorganisms, patients wearing dentures often develop Candida-associated denture stomatitis (CADS), a common, recurring disease that affects up to 67% of denture wearers.2–4 Moreover, Candida and other microorganisms on the denture surface often cause difficulty to control dental diseases (e.g., caries and periodontal disease), oral and systemic infections (e.g., gastrointestinal and pleuropulmonary infections), and even death.4–6
Current management of CADS includes patient compliance with good denture hygiene practices (e.g., denture cleaning, disinfection), ensuring that denture fit is optimized by use of tissue conditioners/liners/rebases, topical or systemic antifungal therapy, and incorporation of antifungal drugs into denture materials.7–9 However, none of these approaches completely eliminates Candida colonization and biofilm formation on the denture surface; this outcome is particularly problematic in the elderly and those who are medically or immunologically compromised and results in poor clinical outcomes.
The current study used a layer-by-layer (LBL) self-assembly approach to functionalize the denture disc surface and provide long-term anticandidal activity. The LBL technology is a simple, yet versatile technique, for alternately depositing polyelectrolytes of opposite charges on top of each other one layer at a time.10–12 Histatin 5 (H-5), a salivary polypeptide, is the smallest cationic peptide with the highest anticandidal activity in the major histatin family.13–16 In clinical applications, topical histatins reduce/prevent oral Candida infection.17 Previous studies have adsorbed synthetic H-5 on denture materials, resulting in low amounts of peptide adsorption and weak antifungal effects.18 Here, we used hyaluronic acid (HA) as the anionic counter ion to increase incorporation of H-5 in a LBL self-assembly multilayer coating process. HA is a water-soluble anionic linear polysaccharide consisting of repeating disaccharide units of N-acetyl-D-glucosamine and D-glucuronic acid.19, 20 As a natural component of the extracellular matrix and synovial fluid, HA, beyond the broad applications in medical practices and cosmetics, has been widely used as a biopolymer for drug delivery, wound dressing, and tissue engineering, due to its excellent biodegradability, biocompatibility, biological activity, and high water sorption and retention ability.19–22
Lucitone 199 (Dentsply Intl, York, PA), a common denture base material, was used for the current studies and is representative of heat-polymerizing poly (methyl methacrylate) (PMMA)-based resins. Denture discs (1.0 cm of diameter and 0.13 cm of thickness) were fabricated following the manufacturer’s recommendations using a standard compression molding technique. Poly (methacrylic acid) (PMAA) was then grafted onto the PMMA denture discs (g-PMAA) for H-5 attachment; this was followed by alternate immersion in HA and H-5 solutions for multilayer self-assembly. Some of the g-PMAA discs were pre-charged with the anticandidal drug, miconazole, prior to H-5/HA self-assembly. The H-5/HA LBL coated discs were tested for activity against C. albicans (e.g., direct contact, adhesion, and biofilm formation assays) and skin fibroblasts (e.g., cytotoxicity) (See detailed procedures in Supporting Materials).
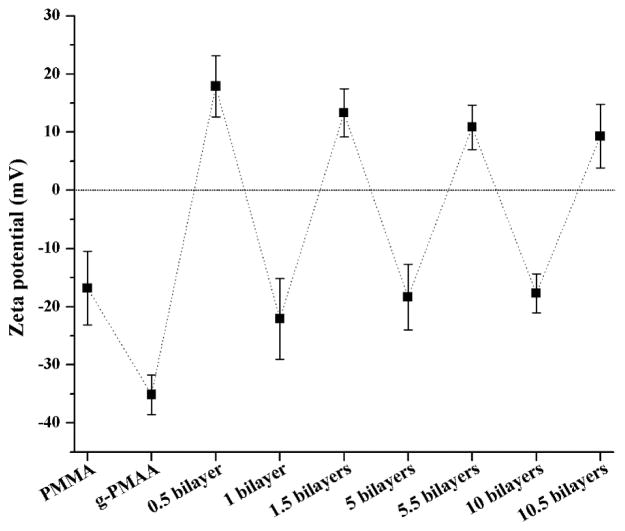
The pristine PMMA surface had a negative charge with a zeta potential of −16.8 ± 6.3mV (Fig. 1). To further increase the anionic character of the PMMA surface for H-5 binding and subsequent H-5/HA LBL coating, PMAA was grafted onto the PMMA discs using plasma-initiated surface grafting as we described previously.23, 24 With a grafting yield of 1.5 ± 0.2 mg/cm2 of PMAA, the resulting denture discs (g-PMAA) had a much lower negative zeta potential (−35.1 ± 3.4 mV). After coating with one layer of H-5 (assigned as 0.5 bilayer), the zeta potential became +17.9 ± 5.3 mV, indicating that positively charged H-5 successfully coated the surface. Further coating with negatively charged HA (1 bilayer) reversed the surface charge (zeta potential = −22.1 ± 6.9 mV). As predicted, surface charge polarity changed with each subsequent LBL cycle (i.e., if the final layer was H-5, a positively charged surface was obtained; if the final layer was HA, the surface carried a negative charge), suggesting that multilayer coatings were successfully built onto the denture disc surface.
Fig. 1.
Zeta potentials of the pristine PMMA, g-PMAA, and the H-5/HA multilayer coatings on g-PMAA
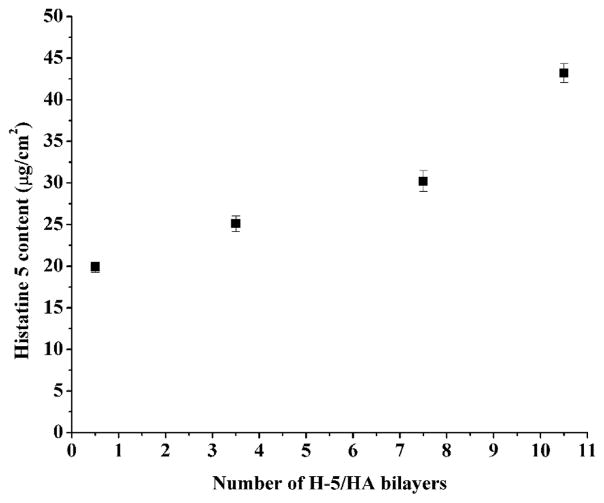
H-5 content in the multilayer coatings was quantified using the colorimetric micro bicinchoninic acid (BCA) protein assay.25, 26 In screening studies, we found that after one cycle of H-5 coating, the pristine PMMA surface adsorbed 1.50 ± 0.39 μg/cm2 of H-5. However, after PMAA grafting, H-5 adsorption on the surface (i.e., g-PMAA) increased dramatically to 19.88 ± 0.61 μg/cm2 of H-5 (0.5 bilayer). Subsequent H-5/HA LBL cycles further increased H-5 content; with 10.5 bilayers of H-5/HA, H-5 content increased to 43.19 ± 1.14 μg/cm2 (Figure 2). The data fitted well to an exponential growth (R2 = 0.958), indicating that the H-5/HA LBL coatings grew exponentially during layer pair deposition. Numerous reports have showed nonlinear growth of multilayer coatings depending on the chemical nature of the polyelectrolytes used, e.g., exponentially growing coatings were commonly observed with polypeptides and polysaccharides; their buildup was based on the diffusion “in” and “out” of at least one of the polyelectrolytes through the coating during layer pair deposition.27–29
Fig. 2.
H-5 content in the H-5/HA multilayer coatings on g-PMAA
The effect of LBL coating on surface hydrophilicity/hydrophobicity was assessed using water contact angle measurements (see Fig. S1 in Supporting Materials). Pristine PMMA resin discs had a water contact angle of 85.1 ± 4.0°. After grafting PMAA to the surface, which introduced hydrophilic PMAA polymer chains, the water contact angle of g-PMAA significantly decreased to 66.9 ± 1.0°. After deposition of one layer of H-5 onto the g-PMAA (0.5 bilayer) surface, the contact angle was not significantly changed; however, after one complete cycle of H-5/HA (HA was the outermost layer), the contact angle was significantly reduced to 53.9 ± 5.2°, suggesting that HA, containing numerous -OH and -COOH groups, was more hydrophilic than H-5. If we keep HA as the outermost layer, further increase in coating layers did not significantly affect contact angle values (around 54°) with each additional bilayer (1, 5, and 10 bilayers). However, if the outermost layer was H-5 (0.5, 5.5, and10.5 bilayers), the contact angle gradually decreased from 69.0 ± 2.5° to 54.9 ± 5.5°, suggesting that when H-5 was the outermost layer, with the increase of coating layers, the HA chains might protrude through adjacent layers (i.e., H-5) during LBL self-assembly and increase the hydrophilicity of the surface. If HA did not protrude, H-5 outermost layer (0.5, 5.5 and 10.5 bilayers) should have similar contact angles. This observation correlated well with the diffusion-based buildup mechanism of the exponentially growing H-5/HA LBL coatings as discussed above (Fig. 2). Also, the hydrophilicity may not account for fungal repelling of H-5 (see below).
Candida albicans (C. albicans, ATCC 10231) was selected as a representative fungus for evaluating the anticandidal activity of the denture materials. This strain can be isolated from patients with CADS and readily forms biofilms on various biomaterials.2–4 A direct contact mode “sandwich assay” (i.e., incubation of C. albicans between two denture discs) was used to test the fungicidal effects of the multilayer coatings on g-PMAA,30, 31 using pristine PMMA discs as controls. Interestingly, LBL coatings with H-5 as the outermost layer (e.g., 0.5 bilayer, 5.5 bilayers, and 10.5 bilayers of H-5/HA) did not show any change in colony-forming units (CFUs) with up to 8 h of contact. Since the fungicidal action of H-5 relies on its internalization into the cells to reach intracellular targets,14–16, 32–34 these results suggest that H-5 remained bound to the coatings and did not diffuse into the surrounding environment to kill Candida.
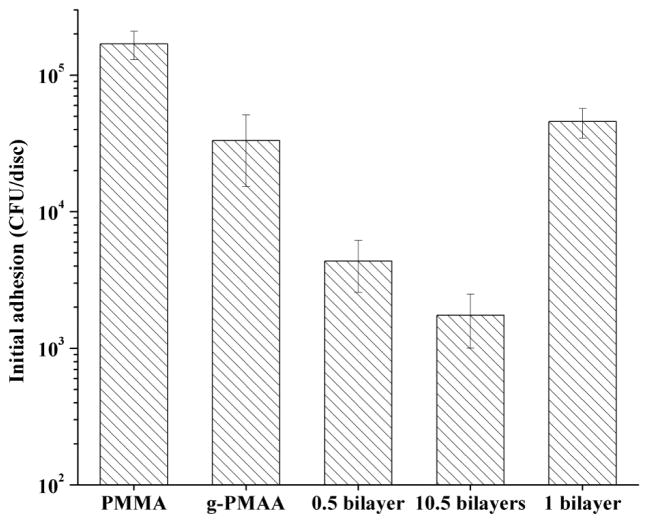
Although H-5 in the LBL coatings did not provide direct fungicidal activity, it significantly reduced Candida initial adhesion. Initial adhesion is the first stage of biofilm formation, which is mainly affected by the surface properties of the solid materials. As shown in Fig. 3, after 1 h of immersion in a Candida suspension in PBS, (1.69 ± 0.39) x 105 CFUs of adherent Candida could be recovered from the pristine PMMA control. The g-PMAA disc showed a significantly lower level of Candida adherence, which could be caused by the hydrophilic surface.30, 35
Fig. 3.
Initial adhesion levels of Candida on pristine PMMA, g-PMAA, and H-5/HA LBL coatings on g-PMAA after 1 h of immersion in a Candida suspension.
In the study of the LBL coatings, both layer numbers and the nature of the outermost polymer affected Candida initial adhesion. For instance, when H-5 was the outermost layer, the g-PMAA discs coated with 0.5 bilayer and 10.5 bilayers further significantly reduced Candida adhesion, demonstrating that the presence of H-5, not hydrophilicity, was responsible for this level of reduction in Candida adhesion. The 10.5 bilayers had significantly lower Candida initial adhesion than the 0.5 bilayer, suggesting that more H-5 would lead to higher anti-adhesion activity. On the other hand, when the outermost layer was changed from H-5 to HA, Candida adhesion on the 1 bilayer coating was significantly higher than that on the 0.5 bilayer coating (although H-5 content was the same in the 0.5 bilayer and the 1 bilayer coatings), confirming that the reduction in Candida adhesion was provided by H-5, not the hydrophilic HA.
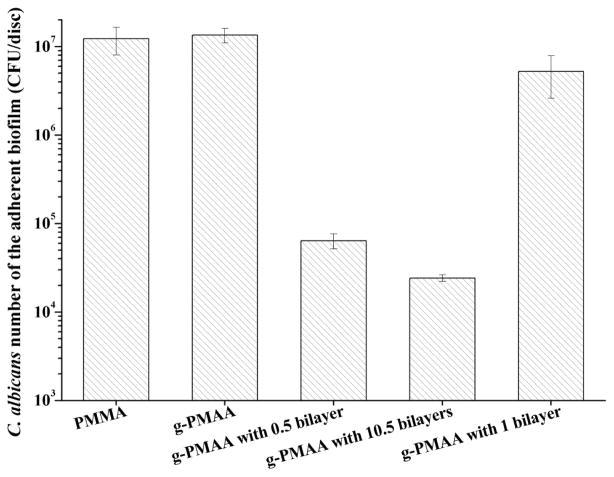
After 1 h of initial adhesion, the Candida-containing discs were individually incubated in YM broth for 2 days to allow biofilm formation. As shown in Fig. 4, (1.23 ± 0.42) x 107 CFUs of Candida were recovered from the adherent biofilm on the pristine PMMA control discs, which was nearly the same as recovered from the g-PMAA disc.
Fig. 4.
Levels of recoverable adherent Candida in biofilms on different denture discs after two days of culture in YM broth
These results suggest that although the hydrophilic surface was able to temporarily reduce Candida initial adhesion, it was ineffective in preventing biofilm formation. A similar result was obtained with the g-PMAA disc coated with 1 bilayer (HA was the outermost layer), further confirming that the HA layer (although hydrophilic) could not control Candida colonization and biofilm formation. On the other hand, g-PMAA discs coated with 0.5 bilayer and 10.5 bilayers (H-5 was the outermost layer in both) had much lower levels of C. albicans [> 100 times less; (6.40 ± 1.24) x 104 CFUs for 0.5 bilayer and (2.42 ± 0.22) x 104 CUFs for 10.5 bilayers, respectively] than on the pristine PMMA, suggesting that the biofilm-controlling effect was provided by H-5. As predicted, the biofilm level on 10.5 bilayers was significantly lower than that on 0.5 bilayer. These results were consistent with the initial adhesion studies.
The biofilm-controlling performance was also preliminarily evaluated in artificial saliva and 50% human saliva, respectively, and the results were presented in Table S1 in Supporting Materials. Exposure to artificial saliva or human saliva did not significantly affect the anticandidal activity of the H-5/HA LBL coatings against biofilm formation, suggesting that the biofilm-controlling potency of H-5 in the LBL coatings was maintained in the presence of either artificial saliva or human saliva. These findings demonstrated the stability and efficacy of the H-5/HA LBL coatings in the presence of oral fluid, pointing to great potentials for real applications.
Candida that remained in the broth solution (i.e., not in the biofilm on disc surface) after two days of culture was also assessed and quantified. No significant differences in broth levels of C. albicans were observed after immersion with pristine PMMA, g-PMAA, or g-PMAA coated with H-5/HA multilayers; Candida levels in all cases were around (1.3 ± 0.2) x 107 CFUs/ml. The biofilm-controlling results suggest that while Candida readily colonized pristine PMMA, g-PMAA, and the g-PMAA discs coated with HA outermost layer, the yeast cells stayed in the broth and avoided the surface of g-PMAA discs coated with H-5 as the outermost layer, indicating that H-5 on the surface might have acted as a “fungal repellent” and prevented biofilm formation.
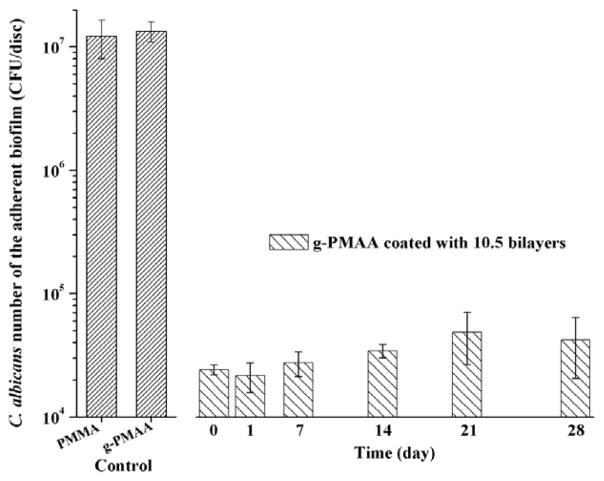
The “fungal repellent” effect of H-5 in the coatings was long-lasting. A series of g-PMAA discs coated with 10.5 bilayers of H-5/HA were immersed in PBS at 37°C with constant shaking up to 4 weeks. PBS was changed daily. The biofilm-controlling activity as demonstrated with C. abicans initial adhesion and YM cultures was essentially unchanged during the entire time of immersion (Fig. 5). As clinical treatments for CADS are often within 1–2 weeks,36 this level of durability is relevant for managing CADS in real applications.
Fig. 5.
Biofilm-controlling effects of pristine PMMA, g-PMAA, and g-PMAA coated with 10.5 bilayers of H-5/HA after different periods of immersion in PBS at 37°C with constant shaking
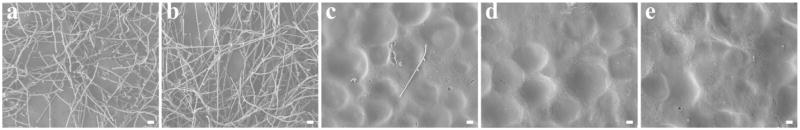
The biofilm-controlling properties of the LBL coatings were further confirmed by scanning electron microscopy (SEM). After 2 days of incubation in YM broth, pristine PMMA and the g-PMAA discs were covered with copious amounts of layered Candida colonies containing filamentous cells, highly indicative of fungal biofilm formation (Fig. 6a and b). On g-PMAA discs with 0.5 bilayer of H-5/HA, only scattered adherent Candida could be observed (Fig. 6c). When the number of bilayers was increased to 10.5, the disc surface appeared clear and no Candida biofilm present (Fig. 6d). Furthermore, after immersion in PBS at 37°C for 4 weeks, the biofilm-controlling function of the 10.5 LBL coating was maintained (Fig. 6e), suggesting that the coating had potential long-term fungal repellent/biofilm-controlling activity and clinical prevention of development of CADS. Since CADS commonly occurs on the oral mucosa underneath the dentures, in real applications the coatings will present on the tissue side of the dentures. For properly fitted dentures, the tissue side has limited exposure to oral fluids with low friction/scratch with the denture for patient comfort/compliance. Thus, this level of stability of the LBL coatings on the denture materials points to good likelihood of the new technology for clinical applications. If, future clinical tests find that longevity of the coating should be further improved, one strategy would be to use covalent bonding or cross-linking to improve the durability of the coating. Another option will be to “recharge” the H-5/HA multilayer coatings, since dentures are removable and can be easily taken out for re-coating.
Fig. 6.
SEM images of biofilms formed on (a) pristine PMMA; (b) g-PMAA; (c) g-PMAA with 0.5 bilayer coating; (d) g-PMAA with 10.5 bilayer coating; and (e) g-PMAA with 10.5 bilayers coating after immersion in PBS at 37°C for 4 weeks (bar = 10 μm )
An attractive feature of H-5 in the LBL coatings was that its antifungal properties were synergistic with clinical anticandidal drugs and provided potent biofilm-controlling effects. To test combination of H-5/HA LBL coatings and anticandidal drugs, g-PMAA discs were pre-charged with miconazole following a procedure previously reported by us that resulted in binding of 126.8 ± 10.5 μg of miconazole per disc.23 After drug charging was complete, 0.5 bilayer and 10.5 bilayers were built on the miconazole-containing g-PMAA discs.
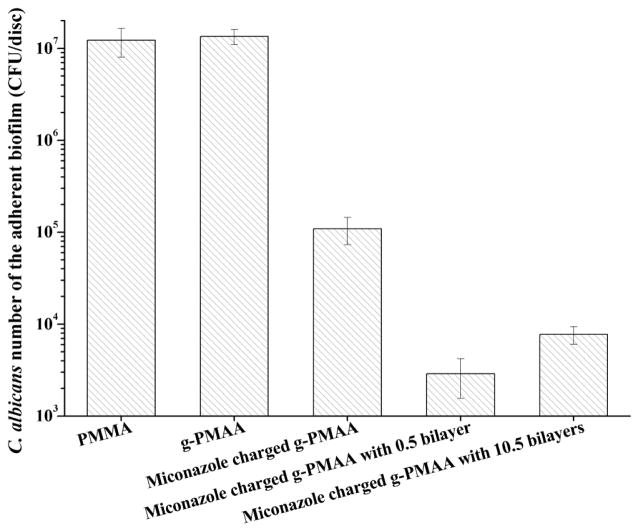
The combination of H-5/HA coatings with miconazole significantly reduced Candida biofilm formation more than using either one alone (Fig. 7 as well as data presented in Fig. 4), suggesting that H-5/HA coatings synergized with miconazole to provide more potent anticandidal activity. Interestingly, the disc with 0.5 bilayer plus miconazole showed a greater reduction in biofilm formation than the sample with 10.5 bilayers and miconazole (Fig. 7, p < 0.05). We showed in our previous studies23 that the anticandidal effects of miconazole-containing denture materials were achieved by the sustained release of miconazole, which was due to the specific interactions (e.g., hydrogen bonding) between miconazole and the grafted PMAA chains. The drug release profiles of miconazole-containing discs without coating and with 0.5 and 10.5 bilayers are presented in Table S2 in Supporting Materials. With 10.5 bilayers coating, the discs released much less miconazole compared with discs either with 0.5 bilayer coating or without coating in the biofilm testing period. Thus, the 10.5 bilayers coating might have acted as a barrier to miconazole release,10 thereby reducing biofilm-controlling potency. These results provide important insights for the design of anticandidal dentures and other related medical devices in the future and suggest the possibility of using multiple classes of antifungal agents to control biofilm formation.
Fig. 7.
Levels of recoverable adherent Candida in biofilms on different denture discs after two days of culture in YM broth
The potential cytotoxic effects of H-5/HA LBL coatings on denture materials were evaluated using rat skin fibroblasts (ATCC CRL-1213) and the XTT assay as specified by ISO 10993-5 with Triton X-100 as a positive control. Pristine PMMA and g-PMAA discs, with or without 10.5 bilayers H-5/HA coatings, were individually immersed in culture media for 1 or 3 days and the conditioned media were then added to fibroblast cultures to assess their effect. Compared to control media, fibroblast viability was not significantly affected by any of the conditioned media, suggesting that H-5/HA LBL coatings had no cytotoxic effects on the cells (see Fig. S2 in Supporting Materials). This result was not completely unexpected since PMMA is one of the most commonly used denture base resins and has a proven safety record.37, 38 PMAA has also been widely used in the preparation of drug delivery systems and surface modification of various biomaterials.39, 40 H-5 is a component of saliva, and HA is a component of extracellular matrix and synovial fluid. All of these factors contribute to the outstanding biocompatibility of the LBL coatings on the denture materials. Nevertheless, the long-term safety of the coatings should be further evaluated using animal models and potentially clinical trials in future studies.
In summary, we demonstrated that fungal biofilm-controlling functionality can be introduced onto PMMA-based denture materials by grafting PMAA to the surface, followed by H-5/HA bilayer coating using LBL self-assembly technology. We found that hydrophilic surface alone was ineffective in controlling Candida biofilm formation. Although H-5 in the multilayer coatings did not provide fungicidal activity in the direct contact anticandidal tests, its antifungal activity significantly reduced the initial adhesion of fungal cells and effectively prevented biofilm formation. Thus, H-5 in the coatings acted as a “fungal repellent” to control Candida colonization and biofilm formation. Furthermore, the anti-adhesion and biofilm-controlling effects were stable for longer than 4 weeks and H-5/HA bilayer coatings synergized with clinical anticandidal drugs, such as miconazole, to provide even more potent anticandidal activity. In addition, the H-5/HA LBL coatings on denture materials demonstrated excellent biocompatibility toward mammalian cells. These results suggest that this strategy has great potential for use in developing new anticandidal denture materials and a wide range of other related medical devices to prevent fungal biofilm formation.
Supplementary Material
Acknowledgments
We are grateful to Dr. David D. Dean (Professor, Comprehensive Dentistry, UTHSCSA) for his critical review and editing of the manuscript. This study was supported by the National Institutes of Health (NIH), NIDCR (R01 DE021084) and VA Merit Review (I01BX001103).
References
- 1.Douglass CW, Shih A, Ostry L. J Prosthet Dent. 2002;87:5–8. doi: 10.1067/mpr.2002.121203. [DOI] [PubMed] [Google Scholar]
- 2.Ramage G, Tomsett K, Wickes BL, López-Ribot JL, Redding SW. Oral Surg, Oral Med, Oral Pathol, Oral Radiol, Endod. 2004;98:53–59. doi: 10.1016/j.tripleo.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Pereira-Cenci T, Del Bel Cury AA, Crielaard W, Ten Cate JM. J Appl Oral Sci. 2008;16:86–94. doi: 10.1590/S1678-77572008000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikawa H, Hamada T, Yamamoto T. J Dent. 1998;26:299–304. doi: 10.1016/s0300-5712(97)00026-2. [DOI] [PubMed] [Google Scholar]
- 5.Webb BC, Thomas CJ, Willcox MDP, Harty DWS, Knox KW. Aust Dent J. 1998;43:160–166. doi: 10.1111/j.1834-7819.1998.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 6.Radford DR, Challacombe S, Walter JD. Crit Rev Oral Biol Med. 1999;10:99–116. doi: 10.1177/10454411990100010501. [DOI] [PubMed] [Google Scholar]
- 7.Lamb DJ, Douglas CWI. J Dent. 1988;16:219–221. doi: 10.1016/0300-5712(88)90075-9. [DOI] [PubMed] [Google Scholar]
- 8.Cross LJ, Williams DW, Sweeney CP, Jackson MS, Lewis MAO, Bagg J. Oral Surg, Oral Med, Oral Pathol, Oral Radiol, Endod. 2004;97:351–358. doi: 10.1016/j.tripleo.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Epstein JB. Oral Surg, Oral Med, Oral Pathol. 1990;69:32–41. doi: 10.1016/0030-4220(90)90265-t. [DOI] [PubMed] [Google Scholar]
- 10.Keeney M, Jiang X, Yamane M, Lee MJ, Goodman SB, Yang F. J Mater Chem B. 2015;3:8757–8770. doi: 10.1039/c5tb00450k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borges J, Mano JF. Chem Rev. 2014;114:8883–8942. doi: 10.1021/cr400531v. [DOI] [PubMed] [Google Scholar]
- 12.Decher G. Science. 1997;277:1232–1237. [Google Scholar]
- 13.Tsai H, Bobek LA. Crit Rev Oral Biol Med. 1998;9:480–497. doi: 10.1177/10454411980090040601. [DOI] [PubMed] [Google Scholar]
- 14.Gyurko C, Lendenmann U, Helmerhorst EJ, Troxler RF, Oppenheim FG. Antonie van Leeuwenhoek. 2001;79:297–309. doi: 10.1023/a:1012070600340. [DOI] [PubMed] [Google Scholar]
- 15.Helmerhorst EJ, van’t Hof W, Breeuwer P, Veerman ECI, Abee T, Troxler RF, Amerongen AVN, Oppenheim FG. J Biol Chem. 2001;276:5643–5649. doi: 10.1074/jbc.M008229200. [DOI] [PubMed] [Google Scholar]
- 16.Helmerhorst EJ, Breeuwer P, van’t Hof W, Walgreen-Weterings E, Oomen LCJM, Veerman ECI, Amerongen AVN, Abee T. J Biol Chem. 1999;274:7286–7291. doi: 10.1074/jbc.274.11.7286. [DOI] [PubMed] [Google Scholar]
- 17.Kavanagh K, Dowd S. J Pharm Pharmacol. 2004;56:285–289. doi: 10.1211/0022357022971. [DOI] [PubMed] [Google Scholar]
- 18.Yoshinari M, Kato T, Matsuzaka K, Hayakawa T, Inoue T, Oda Y, Okuda K, Shimono M. J Biomed Mater Res Part B Appl Biomater. 2006;77B:47–54. doi: 10.1002/jbm.b.30393. [DOI] [PubMed] [Google Scholar]
- 19.Luo Y, Kirker KR, Prestwich GD. J Control Release. 2000;69:169–184. doi: 10.1016/s0168-3659(00)00300-x. [DOI] [PubMed] [Google Scholar]
- 20.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Biomacromolecules. 2005;6:386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia X, Yeo Y, Clifton RJ, Jiao T, Kohane DS, Kobler JB, Zeitels SM, Langer R. Biomacromolecules. 2006;7:3336–3344. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- 22.Messager L, Portecop N, Hachet E, Lapeyre V, Pignot-Paintrand I, Catargi B, Auzely-Velty R, Ravaine V. J Mater Chem B. 2013;1:3369–3379. doi: 10.1039/c3tb20300j. [DOI] [PubMed] [Google Scholar]
- 23.Wen J, Jiang F, Yeh CK, Sun Y. Colloids Surf B. 2016;140:19–27. doi: 10.1016/j.colsurfb.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen J, Yeh CK, Sun Y. ACS Biomater Sci Eng. 2016;2:224–230. doi: 10.1021/acsbiomaterials.5b00416. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C, Liu X, Nomizu M, Nishi N. Biomaterials. 2003;24:3747–3755. doi: 10.1016/s0142-9612(03)00250-3. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Takai M, Ishihara K. Biomaterials. 2009;30:4930–4938. doi: 10.1016/j.biomaterials.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Hübsch E, Ball V, Senger B, Decher G, Voegel JC, Schaaf P. Langmuir. 2004;20:1980–1985. [Google Scholar]
- 28.Burke SE, Barrett CJ. Biomacromolecules. 2003;4:1773–1783. doi: 10.1021/bm034184w. [DOI] [PubMed] [Google Scholar]
- 29.Picart C, Mutterer J, Richert L, Luo Y, Prestwich GD, Schaaf P, Voegel JC, Lavalle P. Proc Natl Acad Sci USA. 2002;99:12531–12535. doi: 10.1073/pnas.202486099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang F, Yeh CK, Wen J, Sun Y. Adv Healthcare Mater. 2015;4:469–475. doi: 10.1002/adhm.201400428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun X, Cao Z, Yeh CK, Sun Y. Colloids Surf B. 2013;110:96–104. doi: 10.1016/j.colsurfb.2013.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li XS, Reddy MS, Baev D, Edgerton M. J Biol Chem. 2003;278:28553–28561. doi: 10.1074/jbc.M300680200. [DOI] [PubMed] [Google Scholar]
- 33.Helmerhorst EJ, Troxler RF, Oppenheim FG. Proc Natl Acad Sci USA. 2001;98:14637–14642. doi: 10.1073/pnas.141366998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar R, Chadha S, Saraswat D, Bajwa JS, Li RA, Conti HR, Edgerton M. J Biol Chem. 2011;286:43748–43758. doi: 10.1074/jbc.M111.311175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nett J, Andes D. Curr Opin Microbiol. 2006;9:340–345. doi: 10.1016/j.mib.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Vasconcellos AAd, Vasconcellos AAd, Chagas RB, Gonçalves LM. Clin Microbial. 2014;3:1000160. [Google Scholar]
- 37.Navarro M, Michiardi A, Castaño O, Planell JA. J R Soc Interface. 2008;5:1137–1158. doi: 10.1098/rsif.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaeblon T. J Am Acad Orthop Surg. 2010;18:297–305. doi: 10.5435/00124635-201005000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Yoo HS, Kim TG, Park TG. Adv Drug Deliv Rev. 2009;61:1033–1042. doi: 10.1016/j.addr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Cheng R, Meng F, Deng C, Klok HA, Zhong Z. Biomaterials. 2013;34:3647–3657. doi: 10.1016/j.biomaterials.2013.01.084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.