Abstract
There is substantial interest in the potential for traumatic brain injury to result in progressive neurological deterioration. While blood biomarkers such as glial fibrillary acid protein (GFAP) and neurofilament light have been widely explored in characterizing acute traumatic brain injury (TBI), their use in the chronic phase is limited. Given increasing evidence that these proteins may be markers of ongoing neurodegeneration in a range of diseases, we examined their relationship to imaging changes and functional outcome in the months to years following TBI.
Two-hundred and three patients were recruited in two separate cohorts; 6 months post-injury (n = 165); and >5 years post-injury (n = 38; 12 of whom also provided data ∼8 months post-TBI). Subjects underwent blood biomarker sampling (n = 199) and MRI (n = 172; including diffusion tensor imaging). Data from patient cohorts were compared to 59 healthy volunteers and 21 non-brain injury trauma controls. Mean diffusivity and fractional anisotropy were calculated in cortical grey matter, deep grey matter and whole brain white matter. Accelerated brain ageing was calculated at a whole brain level as the predicted age difference defined using T1-weighted images, and at a voxel-based level as the annualized Jacobian determinants in white matter and grey matter, referenced to a population of 652 healthy control subjects.
Serum neurofilament light concentrations were elevated in the early chronic phase. While GFAP values were within the normal range at ∼8 months, many patients showed a secondary and temporally distinct elevations up to >5 years after injury. Biomarker elevation at 6 months was significantly related to metrics of microstructural injury on diffusion tensor imaging. Biomarker levels at ∼8 months predicted white matter volume loss at >5 years, and annualized brain volume loss between ∼8 months and 5 years. Patients who worsened functionally between ∼8 months and >5 years showed higher than predicted brain age and elevated neurofilament light levels.
GFAP and neurofilament light levels can remain elevated months to years after TBI, and show distinct temporal profiles. These elevations correlate closely with microstructural injury in both grey and white matter on contemporaneous quantitative diffusion tensor imaging. Neurofilament light elevations at ∼8 months may predict ongoing white matter and brain volume loss over >5 years of follow-up. If confirmed, these findings suggest that blood biomarker levels at late time points could be used to identify TBI survivors who are at high risk of progressive neurological damage.
Keywords: traumatic brain injury, neurofilament light (NFL), glial fibrillary acid protein (GFAP), outcome, neuroimaging
Newcombe et al. report that serum GFAP and neurofilament light levels remain elevated months to years after traumatic brain injury, and correlate with microstructural injury and atrophy. Biomarker levels may identify survivors at risk of late neurological damage after traumatic brain injury.
See Graham (https://doi.org/10.1093/brain/awac179) for a scientific commentary on this article.
See Graham (https://doi.org/10.1093/brain/awac179) for a scientific commentary on this article.
Introduction
The measurement of protein biomarkers of brain injury in blood has been explored in patients with traumatic brain injury (TBI), where they have been proposed as a basis for patient triage for CT, to monitor disease evolution and detect complications, and as a means of refining prognostication.1,2 While many earlier publications focused on the S100 calcium-binding protein (S100B), its utility is limited by relatively poor diagnostic and prognostic performance and confounded by release from extracranial sources.3 More recently, the development of ultrasensitive assay techniques has generated interest in a new set of protein biomarkers as diagnostic and prognostic aids in TBI. These include glial fibrillary acid protein (GFAP), ubiquitin C-terminal hydrolase-L1 (UCH-L1), neurofilament light (NFL) and total tau.4 Each of these biomarkers has distinctive features and different temporal dynamics, and may provide complementary information about overall injury burden and potentially to specific tissue compartments at different time points post-TBI. All of these have shown promise in recognizing those patients who have visible traumatic abnormalities in conventional imaging (CT/MRI) or in aiding in outcome prediction.1,5 However, additional information is needed in two contexts.
First, a more detailed analysis of the relationship of biomarker levels to long-term MRI findings is, as yet, unavailable. This is an important issue, since diffusion tensor imaging (DTI) is sensitive to disease evolution and prognosis in TBI.6,7 Second, several of these biomarkers are also elevated in chronic neuroinflammatory and neurodegenerative diseases, and some are now being explored as markers of diagnosis and disease progression in patients with chronic neurodegenerative and neuroinflammatory diseases.8 In particular, GFAP, NFL and total tau have been shown to predict cognitive decline and the development of Alzheimer’s disease with a latency of up to 8 years.9 This link between TBI and neurodegenerative diseases is noteworthy, given the increasing interest in TBI as a trigger of progressive neurological deterioration in a significant minority (10–30%) of subjects, and a risk factor for chronic neuroinflammation and/or later neurodegenerative disease in the longer term.10–12 However, data relating late biomarker levels to ongoing brain changes and outcome are limited.
Two recent cohorts have provided important insights into biomarker levels and quantitative metrics of microstructural injury derived from diffusion tensor MRI (DTI) and/or markers of atrophy up to 1 year13 and 5 years after injury.14,15 Shahim et al.15 recruited patients between 30 days and 5 years post-TBI. They showed that both GFAP and NFL were elevated at later time points following TBI, with different temporal profiles. NFL decreased monotonically over the study period, while GFAP showed a biphasic profile with an initial decrease, followed by a secondary increase. They also found that NFL and GFAP levels at 30 days post-injury were associated with changes in functional outcome at 90 days, and that 30-day NFL (but not GFAP) was related to subsequent outcome and grey and white matter loss at 90 days. However, the measurement of NFL and GFAP at 30 days was likely to be strongly driven by the severity of initial injury, and an outcome at 90 days is still heavily dependent on injury severity and acute host response, rather than specifically index chronic progressive pathophysiology. Ongoing elevations in NFL appeared to reflect atrophy longitudinally with serum NFL measured at 6 months associated with white matter volume loss at 1 year, and NFL at 3 years associated with the central corpus callosum volume loss at 4 years. However, follow-up was limited to a maximum of 5 years, and no estimates were made of brain age, a concept that is increasingly seen to be useful in chronic neurodegeneration following TBI.16,17
The multi-centre BIO-AX-TBI study provided a comprehensive assessment of biomarker trajectories form the acute phase of TBI to 1 year post-injury.13 This seminal study found that NFL peaked 10 days to 6 weeks after injury, and was still abnormal at 1 year with peak NFL correlating with the extent of axonal injury defined on DTI and predicting the white matter atrophy rate between 6 and 12 months after injury. Peak NFL and GFAP predicted grey matter atrophy on the first 6 months after injury. These are important results and show that the severity of initial injury (as defined by biomarker levels) predicted grey and white matter loss at 6 months after injury. However, they provide no correlations between imaging metrics of atrophy and late biomarker levels. This is critical since peak NFL levels (which were achieved within 30 days of injury), may simply reflect the severity of acute brain injury, and index the early events after TBI. Inference that chronic processes underlie progressive brain volume loss is dependent on showing that late biomarker elevations (which indicate ongoing neurological injury) are related to brain volume loss.
Much of the data on biomarkers in the context of non-TBI neurodegeneration has concentrated on NFL.8 However, it is also relevant to explore such relationships in the context of GFAP, since we have recently shown that plasma GFAP may be an important marker of amyloid deposition,18 and amyloid deposition is one of the key processes associated with accelerated late neurodegeneration in TBI.10 There is growing interest in the behaviour of these biomarkers in the subacute (months) and chronic phase (years) following TBI. However, the mechanisms and pathological significance of such late biomarker elevations remains unclear, as does their relationship to clinical disease course at these later stages.
These previous studies in TBI and chronic neurodegeneration raise the intriguing possibility that biomarkers, and in particular NFL, may be able to signal ongoing neurogenerative processes after TBI. There is a clear need to find protein biomarkers that identify patients with TBI who suffer late progression of disease, with progressive brain volume loss and functional consequences.
Definitive validation of late biomarker elevation in TBI as a signal of progressive or late neurological disease would require a decade-long longitudinal study, but such studies are difficult (and expensive) to organize and conduct. Leveraging funding and enthusiasm for such studies requires prima facie evidence that late biomarker elevation was indeed associated with, and ideally, predicts, progressive neurological deterioration based on intermediate end points, such as neuroimaging or cognitive changes.
In order to provide such data, we examine both cross-sectional and longitudinal relationships between late (≥6 months post-TBI) GFAP and NFL levels with imaging and functional outcome at two time points. The first of these is 6 months post-injury (a time point conventionally used to define definitive TBI outcome), and the second at over 5 years post-injury, to determine whether biomarker elevation and its relationship to neuroimaging and functional outcome still persist. Finally, we examine a subset of patients with data at both ∼8 months and >5 years to explore whether brain biomarkers at ∼8 months can predict trajectories of brain volume loss and functional recovery over time intervals greater than 5 years.
Materials and methods
We collated a combined total cohort of 204 patients with a range of TBI severity across two centres (Turku University Hospital and Cambridge University) (Supplementary Table 1 and Supplementary Fig. 1). For inclusion patients had to attend a follow-up assessment at least once; ∼8 months at Turku University Hospital and >5 years at Cambridge University. At this follow-up assessment patients were invited to have blood biomarkers taken, MRI scanning, outcome questionnaires and neurocognitive testing. Twelve subjects at Cambridge University attended follow-up sessions at both of these time points. Healthy volunteers (n = 59, scanned at Cambridge University) and 21 orthopaedic trauma controls (who did not sustain a TBI, scanned at Turku University Hospital) were used as comparison groups for both biomarker and imaging analysis for patients imaged at the same site as the particular control group. Fifteen healthy volunteers were imaged on two occasions, at an interval of ∼5 years to provide control data for longitudinal assessments of brain volume loss. Information regarding how these the controls were used in analyses are provided in Supplementary Table 1 and Supplementary Fig. 1, with additional details throughout the methods.
For patients who sustained a TBI, the inclusion criteria were; age ≥ 16 years, a clinical diagnosis of TBI, and indications for acute head CT according to National Institute for Health and Care Excellence Criteria (UK, http://www.nice.org.uk/guidance/cg176). Exclusion criteria were blast-induced or penetrating injury, chronic subdural haematoma, inability to live independently as a result of pre-existing brain disease, TBI or suspected TBI not needing head CT, and no consent obtained. Ethical approval was obtained from the South-West Finland Hospital District Research Ethics Committee (decision 68/180/2011) and the Cambridgeshire 2 Research Ethics Committee (LREC 97/290). Written consent was obtained for all cases and was obtained according to the Declaration of Helsinki.
Biomarker measurement
Blood was collected into serum separator tubes (Sarstedt AG & Co). After coagulation (for 45 ± 15 min) and centrifugation at 1500g for 10 min, the serum was aliquoted into cryovials and stored at −80°C. Serum was transferred between centres and laboratories on dry ice. Blood biomarkers were quantified using commercially available single plex [NF-light™ Advantage Kit (103186); GFAP Discovery Kit (102336)] Simoa assays according to the manufacturer’s instructions (Quanterix). The performance of the assay was determined by internal quality control (iQC) samples. The intermediate precision and repeatability for the high concentration iQC was <8% and <12%, respectively for both biomarkers. The low iQC was demonstrated with an intermediate precision of 4.8% and repeatability 11.3% for NFL. The GFAP low iQC demonstrated an intermediate precision of 3.3% and repeatability 6.7%.
MRI acquisition and analysis
Sequences collected with the imaging protocol included volumetric T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) and diffusion MRI. The MRI acquisition parameters in different contributing studies are described in the Supplementary material. While the precise imaging parameters differed between sites, each of the analyses described was confined to patients with identical imaging protocols (∼8-month analysis was confined to Turku subjects, while the >5-year analysis, and serial ∼8 months to >5 year analysis only included Cambridge subjects). All raw data and pipeline outputs were visually inspected for artefact, excess movement and lesions; and motion parameters for diffusion MRI were calculated. One patient (part of the Cambridge >5-year cohort) was removed from all analyses due to extensive right frontal gliosis, which leads to failure of co-registration.
After neck-cropping and correcting for scanner field inhomogeneities, brain parcellation was performed on T1-weighted images, using MALP-EM (multi-atlas label propagation with expectation-maximization based refinement) which provides robust segmentation of the grey matter even when anatomy is distorted due to trauma.19 The 138 anatomical regions were collapsed into three regions of interest: cortical grey matter (CGM), deep grey matter (DGM) and whole brain white matter (WBWM).
Brain age
To undertake comparisons of predicted brain age difference (PAD) we accessed the Cam-CAN MRI dataset, chosen as the 652 healthy volunteers had a broad age distribution (18 to 88 years).20,21 A machine learning model for brain age regression was developed using the MPRAGE scans in the Cam-CAN repository (see Supplementary material for details).16,20,21 The input to the brain age regressor were MRI–derived estimates of whole brain grey matter (WBGM) and WBWM, spatially normalized to MNI space, obtained with Statistical Parametric Mapping Software (Version SPM12).22 This MRI-based model of ageing was then used to derive predicted brain age in our dataset. The difference between predicted brain age and the actual age was calculated as the PAD, with a positive PAD indicating that the brain was older than expected for the actual age, and a negative PAD implying that the brain was younger than expected for the actual age. We examined the difference between PAD values in our different subject cohorts, and related PAD to biomarker levels in samples obtained contemporaneously (to examine cross-sectional associations with biomarker elevation) and in the past (to examine whether earlier biomarker levels predicted accelerated brain ageing).
Cross-sectional voxel-based morphometry
To assess the global distribution of atrophy in patients scanned >5 years after injury the T1-weighted images were analysed using voxel-based morphometry (SPM 12, updated 13/1/2020, University College London, https://www.fil.ion.ucl.ac.uk/spm/).23,24 This involved tissue classification into grey and white matter segments, creation of study specific templates for grey and white matter, and registration of the images to these templates using the Shoot toolbox. The Shoot toolbox was chosen over other methods [e.g. diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL)] as it is has been shown to achieve more robust solutions in situations where larger deformations are required.25 Images were smoothed with 8 mm full-width at half-maximum Gaussian kernel to improve signal-to-noise ratio and reduce the impact of potential misregistration. Intracranial volume estimates were generated during tissue classification. Each voxel-wise analysis was masked to limit the number of voxels included. Masks for grey and white matter were defined by taking the median of smoothed images for all subjects used in generating the template, and thresholding this median image at ≥ 0.4. Voxel-wise group comparison between the TBI patients and controls used t-tests with age, sex and total intracranial volume as covariates. P < 0.05, corrected for family-wise error rate, was considered significant.
Exploratory analysis: longitudinal voxel-based indices of local volume loss
Jacobian determinants
To provide a more sensitive measure of regional volume loss, we compared biomarker levels at ∼8 months and >5 years with interval-indexed Jacobian determinants (JD), in the 12 patients where imaging and biomarkers were available at both time points. Longitudinal imaging analysis was undertaken in SPM12.23,24 Baseline and follow-up images for each subject were iteratively registered to produce a midpoint reference time-averaged image. The within-patient voxel-level transformation required to transform the baseline image to the cognate follow-up scan image was quantified as the JD.26 Indexing the JD to the inter-scan interval provides an average annualized rate of volume change (the Annual JD Atrophy Index). Voxel-level JDs were averaged for two tissue classes: white matter and grey matter (WBWM and WBGM). The same imaging analysis was performed in 15 controls who underwent imaging at similar intervals with an identical protocol which was important given the changes in scanner to ensure any changes seen were likely to be secondary to the brain injury. As our intent was to compare changes in volume to biomarker levels, and since there was no a priori reason to expect biomarker levels to discriminate between brain regions (rather than tissue classes), we made no attempt in these analyses to identify locations of atrophy (as has been reported in previous publications).27 This decision was also supported by our assessment of the relationship between biomarker levels and DTI metrics, which showed no regional predilection for white matter loss (i.e. all regions were affected).
The grey and white matter volumes obtained from the SPM12 analysis were used to calculate annualised atrophy rates via the below equation:
| (1) |
Diffusion tensor imaging analysis
All diffusion MRI data were corrected for noise,28,29 Gibbs ringing artefacts,29 susceptibility induced distortions,30 head motion and eddy current artefacts,31 and inhomogeneities in the magnetic field.32,33 Diffusion tensors were fitted via weighted least squares to derive mean diffusivity (MD) and fractional anisotropy maps using FSL (https://fsl.fmrib.ox.ac.uk/). The regions of interest were applied to the DTI maps to obtain mean values. White matter parcellation into 72 tracts was performed using TractSeg, a convolutional neural network based approach.34 Mean fractional anisotropy and mean diffusivity MD values were obtained for the grey and white matter parcellations, and TractSeg tracts.
Statistical analysis
Unless specified, statistical analyses were conducted using R (version 3·6·2, https://www.R-project.org/) in RStudio (version 1·2·5033, http://www.rstudio.com). The serum biomarker values were significantly skewed and were therefore log transformed (log2 of raw biomarker values) for analyses except where specifically noted. However, where plots show biomarker values on a log scale, labels signify actual levels of biomarkers measured (rather than log transformations of these values), to facilitate clinical interpretation. For parametric data, comparisons were performed using t-tests. For non-parametric data, comparisons were performed using Mann-Whitney U and for correlations Spearman’s Rho. To enable adjustment for age, sex and time from injury to assessment where appropriate associations were assessed with a general linear model. Benjamini-Hochberg correction for multiple comparisons was used for group wise comparisons within sets of correlations.
We compared biomarker levels between controls and individual study cohorts separately at each time point, and serially for the 12 patients in whom biomarker levels were available at both ∼8 months and >5 years. Linear mixed effects models were fitted for the later group of 12 patients to assess the effects of time between samples, age and sex on biomarker level. In order to understand whether biomarker levels correlated with imaging findings, within each cohort at the relevant time point (∼8 months for Turku patients and >5 years for Cambridge patients), we compared biomarker levels to MRI variables. These included MD and fractional anisotropy from regions of interest defined using TractSeg; PAD and annualized atrophy index derived from JDs; and regional variations in grey and white matter loss, quantified using voxel-based morphometry SPM.23,24 Finally, in the subset of 12 patients where serial imaging and biomarker levels were available, we explored whether biomarker levels at ∼8 months predicted subsequent imaging changes.
Data availability
Anonymized data are available upon request conditional on an approved study proposal and a signed data access agreement; there are no end dates to the availability. Please contact the corresponding authors to request. Data from the Cam-CAN repository are available by submitting a request to the Cam-CAN data access portal (http://www.mrc-cbu.cam.ac.uk/datasets/camcan/).20,21 The software code for brain age regression will be made freely available on GitHub (https://github.com/biomedia-mira/brain-age-cnn).
Results
Analysis of biomarker levels was based on 35 samples from healthy controls, and a total of 211 samples from patients after TBI (Supplementary Table 1 and Supplementary Fig. 1). MRI data were available for 134 patients at ∼8 months, and for 38 patients at >5 years post-TBI. Twelve patients had serial biomarker measurements and MRI at both ∼8 months and >5 years post-TBI.
Biomarker levels are elevated at ∼8 months and remain elevated beyond 5 years post-TBI in some subjects
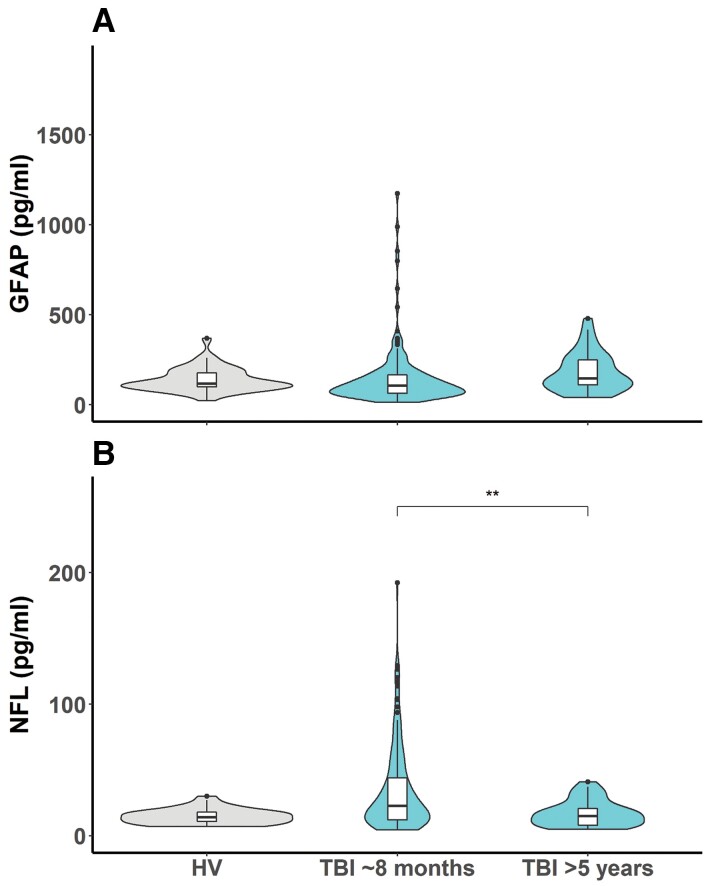
While GFAP values were not significantly different from healthy controls in patients with TBI at ∼8 months, NFL levels were elevated (Fig. 1, P < 0.001). In a group level comparison, the 34 patients studied at >5 years post-TBI showed GFAP and NFL levels that were no different from healthy control values.
Figure 1.
Comparison of healthy volunteer levels of GFAP (A) and NFL (B) (plotted on a linear scale) compared to patients approximately 8 months and >5 years after a TBI. GFAP: healthy volunteer (HV) versus TBI ∼8 months P = 0.086, HV versus TBI >5 years P = 0.11, TBI ∼8 months versus TBI >5 years P = 0.0087. NFL: HV versus TBI ∼6months P < 0.0001, HV versus TBI >5 years P = 0.55, TBI ∼8 months versus TBI >5 years P = 0.0025. ns P > 0.05, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
There were significant associations between age when blood sample taken, and levels of NFL and GFAP for patients ∼8 months and >5 years after injury and with NFL levels in healthy volunteers (Supplementary Fig. 2). There were no significant associations with sex (P = 0.32) or Glasgow Coma Score (GCS, P = 0.45) at the time of injury.
GFAP and NFL are correlated at each time point with the two biomarkers showing specific temporal patterns
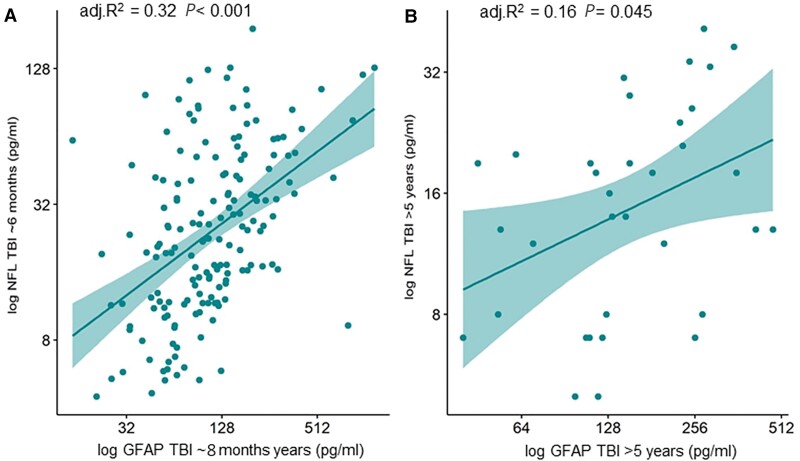
At both time points (∼8 months, and >5 years post-TBI) the levels of GFAP and NFL were significantly correlated with each other, but the strength of this correlation decreased over time (adjusted R2 = 0.32, P < 0.001; and adjusted R2 = 0.16, P = 0.045, at 6 months and >5 years, respectively; Fig. 2).
Figure 2.
Log GFAP and log NFL levels correlate at each time point post-TBI (A: ~6 to 8 months; and B: >5 years after injury), but the strength of this correlation decreases over time. The R2 are shown adjusted for age, sex, and time since injury. GFAP and NFL are shown on log scales, but figures denote actual concentrations in pg/ml.
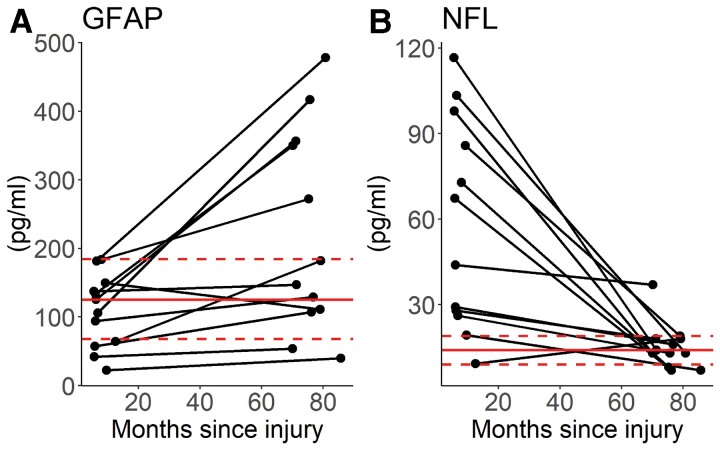
In contrast to the larger group results, and albeit in small numbers where serial biomarker measurements were available in patients, these showed clearly different behaviour for GFAP and NFL over time (Fig. 3). GFAP was within (or below) the range of values seen in the control group in all of the subjects at ∼8 months, but tended to rise, and was above the normal range in five subjects >5 years after injury. The levels of NFL showed a reverse pattern, with elevated values in most patients at ∼8 months, all but one of which had returned to control range at the >5-year time point. On average the NFL decreased by 0.39 pg/month [standard error (SE) 0.15, P = 0.0001] and GFAP increased by 1.47 pg/month (SE 0.54, P = 0.007). Age and sex were not significantly associated with biomarker levels (NFL: Age − β = −0.39, SE = 0.37, P = 0.30, Sex − β = −7.20, SE = 21.53, P = 0.74; GFAP Age − β = 1.51, SE = 1.97, P = 0.44, Sex − β = 50.7, SE = 113.8, P = 0.66).
Figure 3.
Temporal changes in GFAP (A) and NFL (B) in patient subset with data at ∼8 months and >5 years post-TBI (absolute values). The solid horizontal line represents the mean value for healthy volunteers and the dotted lines the standard deviations.
Although the levels of GFAP were not significantly elevated at ∼8 months at a group level in patients with TBI and trauma controls, in the subset of patients where biomarker levels were available at both time points, GFAP levels within individuals predicted GFAP levels >5 years after TBI (adjusted R2 0.39, P < 0.001). There was no similar temporal relationship observed for NFL.
GFAP and NFL levels at both ∼8 months and >5 years are related to contemporaneous DTI metrics of injury
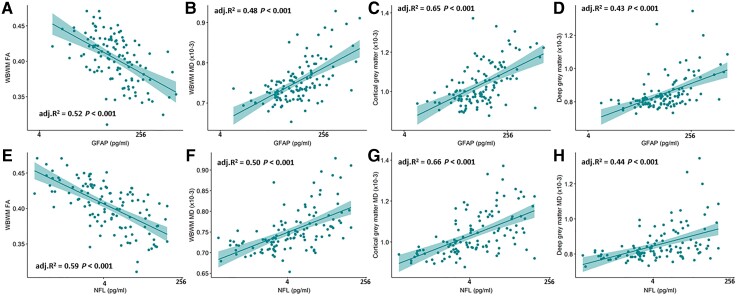
At 6 months post-TBI, levels of both GFAP and NFL were associated with higher MD in the CGM, DGM and WBWM, and inversely with fractional anisotropy in WBWM (Fig. 4). These associations were pervasive throughout the white matter, with significant associations for the majority of white matter tracts (Supplementary Tables 2–13). At the later time point of >5 years, NFL levels still remained strongly correlated with MD in WBWM (P < 0.001; Supplementary Fig. 3).
Figure 4.
Log GFAP and log NFL levels at ∼8 months significantly correlate with fractional anisotropy and mean diffusivity in WBWM (A, B, E and F), mean diffusivity in the cortical grey matter (C and G) and the deep grey matter (D and H). The R2 values shown are adjusted for age, sex, and time since injury. GFAP and NFL are shown on log scales, but figures denote actual concentrations in pg/ml.
NFL levels at ∼8 months predict DTI metrics at >5 years
In the subset of 12 patients where serial biomarker and MRI data were available, we found that NFL levels at ∼8 months were associated with WBWM MD at >5 years after injury (Supplementary Fig. 3). GFAP at ∼8 months was not significantly associated with WBGM MD at >5 years post-injury (Supplementary Fig. 3).
Metrics of brain ageing in TBI survivors and their relationship to biomarker levels and clinical outcome
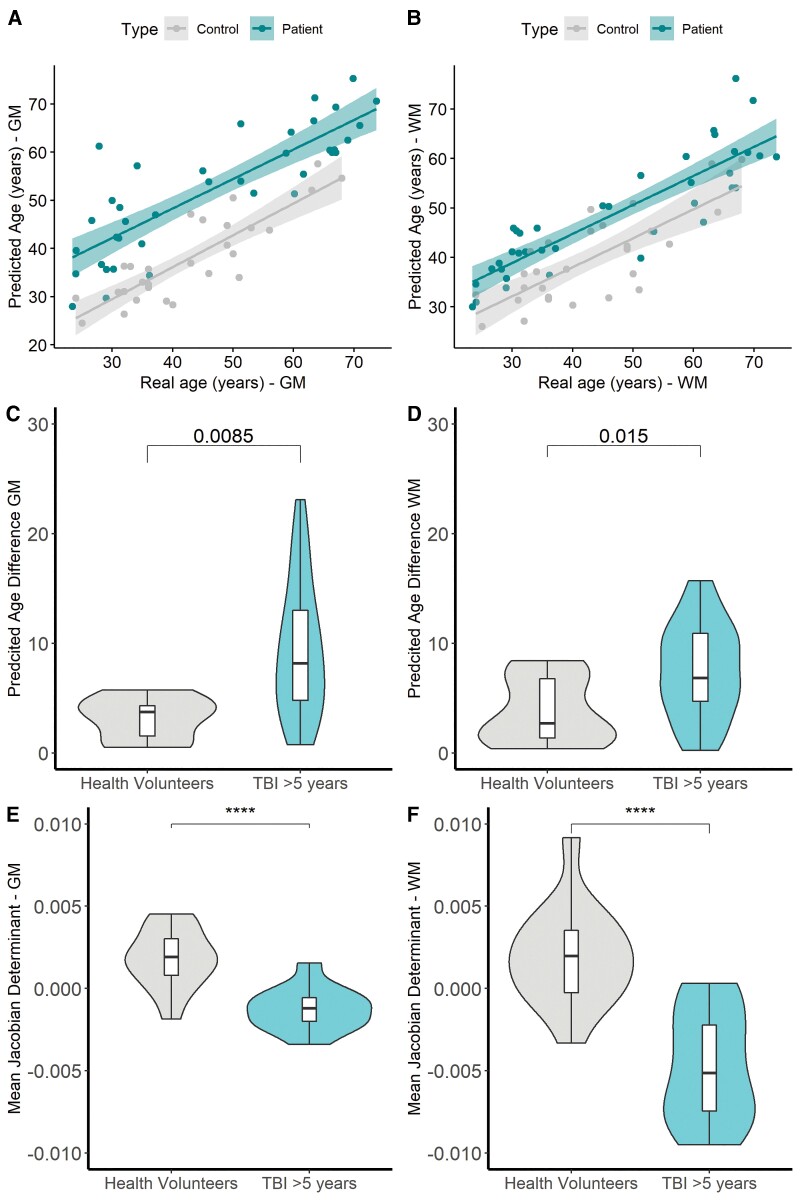
For patients imaged at ∼8 months post-TBI the median (IQR) PAD was significantly higher than in the orthopaedic control group for both grey matter [TBI 6.1 (4.0–9.8) years, orthopaedic controls 5.4 (3.2–6.3) years, P = 0.002] and white matter [TBI 8.2 (3.3–13.3) years, orthopaedic 3.1 (1.2–6.0) years, P = 0.02] (Supplementary Fig. 4). This difference appeared more marked for patients with TBI >5 years post-injury compared to health volunteers with the median (IQR) PAD significantly, both for grey matter [TBI 7.6 (4.8–12.7) years, healthy volunteers 3.7 (1.1–3.9) years, P = 0.0085] and for white matter [TBI 6.7 (4.3–10.1) years, healthy volunteers 2.5 (1.2–6.3) years, P = 0.015] (Fig. 5). Due to the cohorts at each time point being collected on different sites (including differing orthopaedic controls and healthy volunteers), and scanners formal statistics were not performed between the two time points.
Figure 5.
Predicted brain age, predicted brain age difference for patients imaged >5 years after injury, and the mean JD for the subset of patients and controls imaged longitudinally. Predicted brain age versus actual for grey matter (WBGM) (A: Healthy volunteers R = 0.85 P < 0.0001, Patients R = 0.83, P < 0.0001) and WBWM (B: Healthy Volunteers 0.77, P < 0.001, Patients R = 0.87 P < 0.001). Comparison of PAD and mean JD for WBGM and WBWM between healthy volunteers and patients >5 years after injury (C and D). Comparison of the mean JD for WBGM and WBWM between patients imaged from ∼8 months and >5 years after injury compared to controls imaged twice over the same period (E and F). ****P < 0.00001.
PAD linearly correlated with chronological age in both healthy volunteers and patients with TBI examined ∼8 months and >5 years post-injury. However, the mean regression line for the TBI cohort was shifted above the line for the control for across the entire age range, for both grey and white matter (Supplementary Figs 4 and 5). These data suggest that patients with TBI examined >5 years post-injury had, as a group, brains with grey and white matter compartments 8–10 years older than age-matched controls.
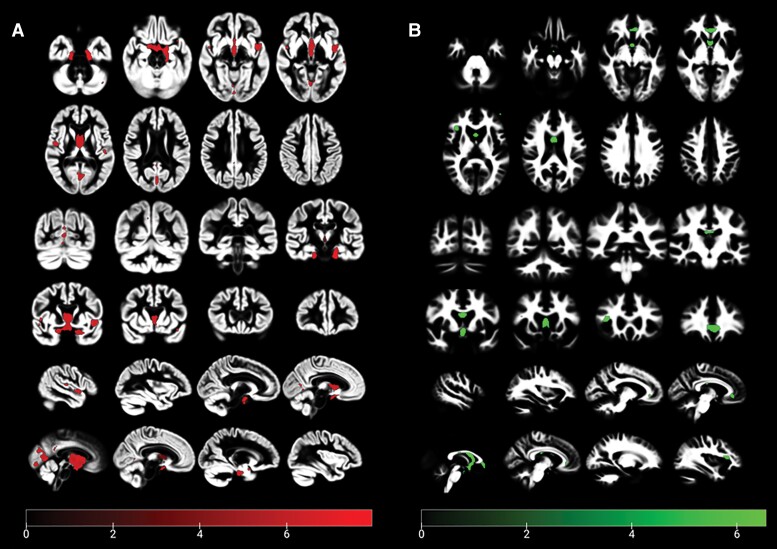
Voxel-based morphometry showed that when compared to healthy controls, maximal areas of grey matter loss were in the hippocampus, dorsolateral prefrontal cortex and striatum; while the most prominent areas of white matter loss were in the corpus callosum, pyramidal tracts and arcuate fasciculus (Fig. 6). We used the volumes obtained from voxel-based morphometry for WBGM, WBWM and ventricular size to calculate annualized atrophy rates (percentage change per year). When compared to controls, patients showed greater volume loss in WBGM [controls 0.02 (−0.08 to 0.16) versus patients −0.23 (−0.41 to −0.03); P = 0.047]; and in white matter [controls 0.04 (−0.05 to 0.17) versus patients −0.72 (−1.2 to −0.54); P = 0.039]. These differences resulted in a 5-fold annualized increase in the ventricular volume in patients when compared to controls [controls 0.5 (−0.9 to 1.5) versus patients 2.7 (−0.02 to 3.57); P = 0.020].
Figure 6.
Grey (A) and white matter (B) VBM for patients >5 years after TBI and control subjects. Results are corrected for FWE P < 0.05. The covariates in the model were age, sex and total intracranial volume.
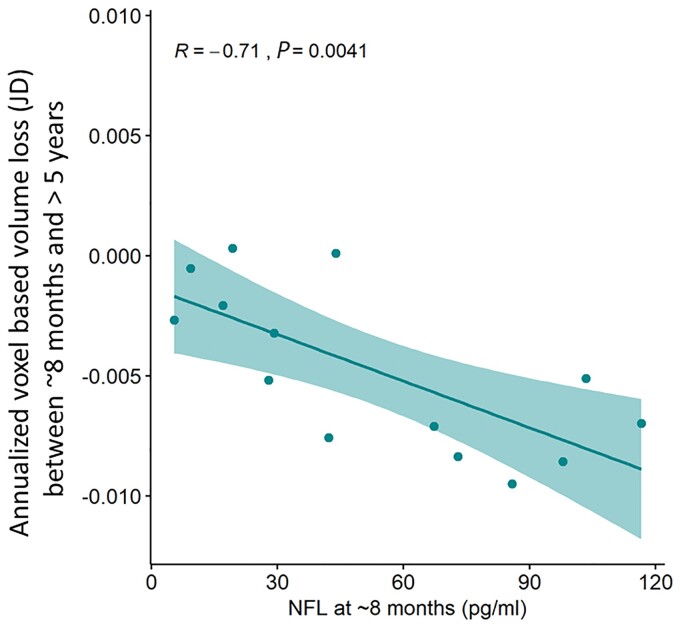
Voxel-based assessment of volume loss using JD corroborated the finding of greater annualized volume loss in patients imaged at >5 years when compared to controls, for both WBGM and WBWM. NFL level at ∼8 months, adjusted for age, sex and duration of follow-up, predicted white matter loss per year of follow-up, defined using the annualized JD between ∼8 months and >5 years (Fig. 7; adjusted R2 = 0.41, P = 0.04).
Figure 7.
NFL levels at ∼8 months post-TBI, adjusted for age, sex, and time post-TBI, predict WBWM rate of volume loss between ∼8 months and >5 years defined using the JD. Adjusted R2 = 0.41, P = 0.04. Absolute values for NFL shown (pg/ml).
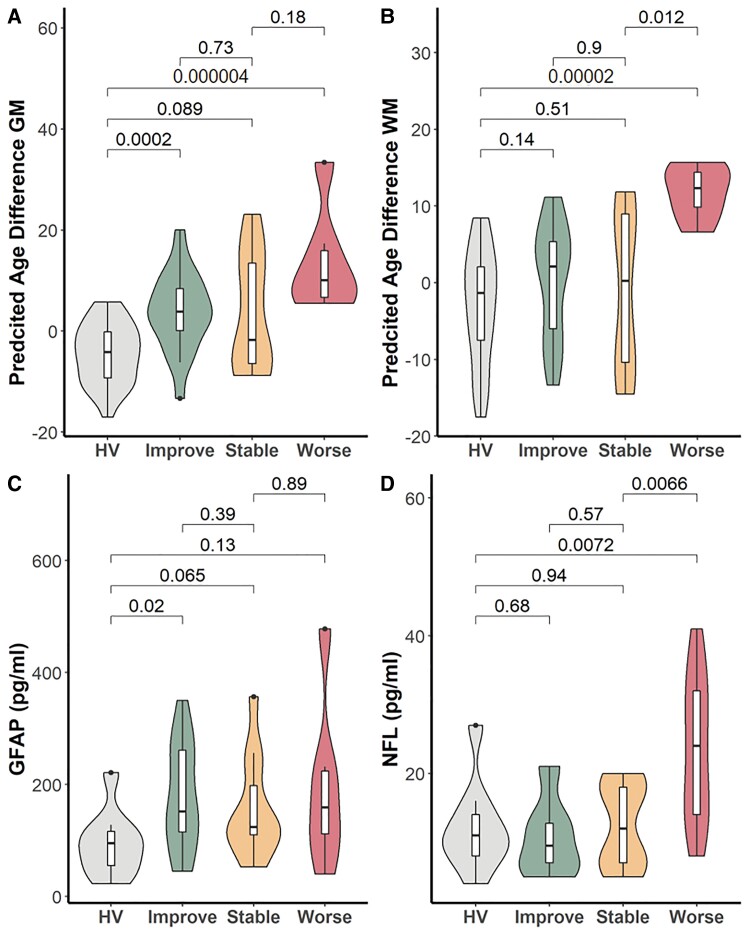
Patients recruited >5 years post TBI showed a median (range) Glasgow Outcome Score Extended (GOSE) of 6 (3–8). For the 12 patients for whom data were also available at ∼8 months, GOSE showed variable trajectories, with improvements in five, no change in three, and worsening in four subjects. NFL (but not GFAP) levels at >5 years post-TBI were significantly higher in those patients who showed worsening GOSE from an ∼8-month baseline compared to those whose GOSE remained stable or got worse (Fig. 8). There was no significant association between GOSE trends and biomarker levels at ∼8 months (Supplementary Fig. 5). Similarly, PAD at >5 years was significantly higher in patients who showed worsening GOSE, both in grey and white matter (Fig. 8).
Figure 8.
PAD and biomarker levels at 5-year MRI in patient subgroups. (A and B) PAD in WBGM and WBWM and (C and D) levels of GFAP and NFL at >5-year MRI in subgroups of patients who showed improving (Improve; increase in GOSE ≥ 1 point), stable (no change in GOSE), or worsening (Worse; reduction in GOSE ≥ 1 point) between ∼8 months and >5 years post-injury. HV = healthy volunteers. Figures above box plots show unadjusted P-values for comparisons (Mann-Whitney U).
Discussion
We have used multiple complementary cohorts of patients (total n = 203) to examine the levels of GFAP and NFL up to 13 years after TBI (Supplementary Fig. 6). We show that many patients show persistent and temporally distinct elevation in these biomarkers up to 13 years after TBI. While the two biomarkers show persistent correlation with each other at all time points, the strength of this correlation fades over time, suggesting an evolving heterogeneity of pathophysiology. In the subgroup of patients where data were available at both late time points, we found that GFAP levels were initially normal at ∼8 months but tended to rise by >5 years; while NFL levels showed the reverse—showing elevation at ∼8 months, which settled to normal levels by >5 years. The persistent elevation of GFAP and NFL at ∼8 months was significantly related to contemporaneous metrics of microstructural injury on DTI, as measured by MD and fractional anisotropy in WBWM, and MD in CGM and DGM. We confirm that patients with TBI show a greater PAD than normal (suggesting accelerated brain ageing in the TBI cohort).16 Critically, in patients where data were available at both ∼8 months and >5 years, we show that NFL levels at ∼8 months predicted white matter volume loss at >5 years, and indexed JD (as a voxel-based measure of annual brain volume loss) between ∼8 months and 5 years. Finally, we show that late protein biomarker and imaging changes are potentially clinically relevant, since patients who worsened functionally between ∼8 months and >5 years showed a higher PAD and elevated levels of NFL compared to those who improved or remained stable.
Our finding of persistent elevation in NFL at ∼8 months post-TBI, and a secondary elevation of GFAP >5 years post-TBI provide objective evidence of ongoing injury for several years after TBI; though this needs replication given the small numbers involved and the lack of significance in the larger cross-sectional analyses. While the elevation in the two biomarkers were correlated at both time points, the strength of this correlation diminished over time (with R2 values of 0.32, and 0.16; Fig. 2). The initial strong correlation between the two biomarkers is in keeping with the proposition that they reflect different facets of severity of the acute injury (possibly the glial and axonal tissue compartments). However, we speculate that, over time, host factors become more dominant, with progressive separation of glial and axonal pathophysiology at later time points. This last point is clearly illustrated in the subgroup of 12 patients where biomarkers were available at both late time points, where the temporal behaviour of the two biomarkers is diametrically opposite. The late GFAP elevation that we observe at >5 years is open to one of two possible explanations. It is possible that this represents the emergence of new pathology many years after TBI and/or astrogliosis. However, interestingly, GFAP levels at ∼8 months (although largely within normal ranges), closely correlated with subsequent elevation in GFAP levels at >5 years. This suggests that the processes that result in GFAP elevation at >5 years may already have been activated at ∼8 months, and/or represent a host specific (possibly genetically driven) propensity for the processes responsible for such elevation.
The pathology and neurobiology that underlie these late biomarker elevations are, as yet, unclear, but our correlations with DTI at late time points provide some insight. At 6 months, we find that both GFAP and NFL levels are related to DTI metrics of microstructural injury, both in grey matter and in white matter. At 8 months post-TBI, both GFAP and NFL levels correlated inversely with fractional anisotropy and directly with MD WBWM, suggesting that they reflected different facets of ongoing axonal pathology, with NFL possibly reflecting ongoing axonal loss while GFAP represents glial responses to this evolving injury. At the later time point of >5 years, the only significant correlation we observed was between NFL and WBWM MD. While this suggests that NFL elevations at these time points reflect ongoing axonal pathology, the relative normalization of NFL levels at this time point in the group with serial samples may indicate a less active underlying process. A continued decline towards normal values is consistent with Shahim et al. who found that NFL decreased linearly over a 5-year period.14,15 Despite this, the clear and persistent correlations with DTI parameters provide evidence that the biomarker elevations at late time points reflect ongoing neural damage. While other authors have described such late MRI changes,7 in this study we were also able to demonstrate relationship of these DTI changes to blood biomarkers.
The late and progressive changes that we demonstrate using DTI and volumetric analysis of T1-weighted MRI replicate prior studies which show evolving brain injury and volume loss months to years post-TBI.27,35,36 These studies show significant overall volume loss, white matter loss, or accelerated ageing of the brain in TBI survivors. In many studies however, the progressive neuroimaging changes have been limited to a substantial minority (10–30%) of patients rather than affecting all subjects.35,36 Our imaging data replicate results from two recent publications,13,15 and the correlations that we demonstrate between late biomarker levels and contemporaneous imaging metrics are consistent with the results provided by Shahim et al.15 However, we also show that ongoing white matter loss continues to occur beyond 5 years and is related to NFL levels at ∼8 months.
It is useful to consider what pathological changes underlie these late changes on neuroimaging. Late pathology after TBI is complex, and includes tau, amyloid-β, and TDP-43 deposition; neuroinflammation, axonal degeneration, white matter degradation, neuronal loss, and blood–brain barrier disruption.10,37 Neuroimaging reports of progressive white matter loss, in particular, are also seen in neuropathological studies,12 and may be driven by microglial activation,38 detrimental adaptive immune responses against neural antigens,11 or Wallerian degeneration.39 The changes in NFL levels that we observe, and their dominant correlations to progressive white matter injury on DTI, suggest that they may provide circulating biomarkers that denote these processes. Further, the presence of reactive astrocytes has been known to be a hallmark of late TBI pathology for years post-injury,40 and astrogliosis has been shown to both correlate with DTI abnormalities41 and be a major component of the glial response months following focal TBI.15,42 While a direct link of astrogliosis to blood levels of GFAP is not well established, increase in astroglial GFAP immunoreactivity on histological sections is the hallmark of reactive astrogliosis.43–45 However, as with some imaging studies, the white matter loss and microglial pathological changes do not appear to be uniform across the TBI population at follow-up—but are prominent in a minority of subjects; for example, microglial activation accompanying while matter loss is observed in about 30% of subjects.12
While post-mortem histology provides definitive descriptions of the eventual pathological consequences of these processes, it is not suited to study their dynamic course. While MRI can document progressive changes, addressing the underlying pathophysiology requires other tools such as PET, which can image tau46 and amyloid47 deposition and map microglial activation.38 However, both MRI and (even more so) PET are expensive research and clinical tools, and not appropriate for universal use following TBI. If blood biomarkers could, as our results suggest, be used to identify enriched populations of subjects who are more likely to suffer progressive neurological damage, this could allow a more rational choice of subjects and timing for MRI and PET studies, and, in turn, selection of patients for more intensive follow up and/or recruitment to therapeutic trials.
Regardless of the underlying pathology, it seems clear that these biomarker elevations reflect processes that have consequences in the brain. Our demonstration of increased PAD provides evidence of accelerated brain ageing in TBI and confirms past reports in this context.16,26 However, in addition, we show, that blood biomarker levels may provide a more accessible predictive biomarker of such ageing. The fact that imaging metrics of brain volume loss were not abnormal at ∼8 months suggests that this is a slowly evolving secondary process, and the relationship with circulating protein biomarkers only declares itself over a period of years. A predictive role for biomarkers is more strongly supported by the fact that NFL elevation at 6 months correlated with annualized JD over the next 5–9 years. These findings recapitulate recent reports that GFAP, NFL and tau elevation in older patients reflect the development of cognitive decline, MCI and AD with a latency of ∼8 years.48 Our data add TBI to a growing list of diseases, including several canonical neurodegenerative conditions (such as Alzheimer’s disease and other forms of dementia) where peripheral levels of GFAP, tau, NFL, and phosphorylated tau, are being explored as markers for diagnosis and disease progression.8,48–52
However, we need to acknowledge that the study has several limitations. The sample sizes were relatively small at some time points, and serial data were only available in a minority of patients with the imaging data analysis limited due to a change in scanner used. While we found no correlation between late biomarker elevation and patient age or initial injury severity, our sample size was too small to formally model the effects of these (and other) covariates. Confirmation of these findings will require a prospective study in a larger sample of well-characterised patients, careful correction for confounding covariates, imaging data collection ensuring sequence and scanner stability, and perhaps involving a larger panel of biomarkers. Finally, Graham et al. also showed correlations between serum Tau levels and grey matter loss, but as we did not measure this biomarker, we could not attempt to replicate this result.13
This study shows preliminary evidence that GFAP and NFL can remain elevated months to years after TBI and show distinct temporal profiles. These elevations correlate closely with microstructural injury in both grey and white matter on contemporaneous quantitative DTI. NFL elevations at ∼8 months may predict ongoing white matter and brain volume loss over the succeeding 5–9 years of follow-up. If confirmed, these findings suggest that blood biomarker levels at late time points could be used to identify TBI survivors who are at high risk of progressive neurological damage, triggered by their initial TBI.
Supplementary Material
Acknowledgements
This work was supported by researchers at the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre. Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge acted as the sponsor for this study, with responsibility for study conduct and management.
We thank all our patients and their families, and healthy volunteers for participating in this study.
Abbreviations
- DTI =
diffusion tensor imaging
- GOSE =
Glasgow Outcome Score Extended
- JD =
Jacobian determinants
- PAD =
predicted brain age difference
- MD =
mean diffusivity
- NFL =
neurofilament light
- TBI =
traumatic brain injury
- WBWM =
whole brain white matter
- WBGM =
whole brain grey matter
Contributor Information
Virginia F J Newcombe, University Division of Anaesthesia, Department of Medicine, University of Cambridge, Cambridge, UK.
Nicholas J Ashton, Wallenberg Centre for Molecular and Translational Medicine, University of Gothenburg, Gothenburg, Sweden; Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, The Sahlgrenska Academy at the University of Gothenburg, Mölndal, Sweden; King’s College London, Institute of Psychiatry, Psychology and Neuroscience, Maurice Wohl Institute Clinical Neuroscience Institute, London, UK; Mental Health and Biomedical Research Unit for Dementia, Maudsley NIHR Biomedical Research Centre, London, UK.
Jussi P Posti, Neurocenter, Department of Neurosurgery, Turku University Hospital and University of Turku, Turku, Finland; Turku Brain Injury Center, Turku University Hospital and University of Turku, Turku, Finland.
Ben Glocker, Biomedical Image Analysis Group, Department of Computing, Imperial College London, London, UK.
Anne Manktelow, University Division of Anaesthesia, Department of Medicine, University of Cambridge, Cambridge, UK.
Doris A Chatfield, University Division of Anaesthesia, Department of Medicine, University of Cambridge, Cambridge, UK.
Stefan Winzeck, University Division of Anaesthesia, Department of Medicine, University of Cambridge, Cambridge, UK; Biomedical Image Analysis Group, Department of Computing, Imperial College London, London, UK.
Edward Needham, University Division of Anaesthesia, Department of Medicine, University of Cambridge, Cambridge, UK.
Marta M Correia, MRC (Medical Research Council) Cognition and Brain Sciences Unit, University of Cambridge, Cambridge, UK.
Guy B Williams, Wolfson Brain Imaging Centre, Department of Clinical Neurosciences, Cambridge, UK.
Joel Simrén, Institute of Neuroscience and Physiology, Department of Psychiatry and Neurochemistry, The Sahlgrenska Academy at the University of Gothenburg, Mölndal, Sweden; Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden.
Riikka S K Takala, Perioperative Services, Intensive Care Medicine and Pain Management, Department of Anesthesiology and Intensive Care, Turku University Hospital, University of Turku, Turku, Finland.
Ari J Katila, Perioperative Services, Intensive Care Medicine and Pain Management, Department of Anesthesiology and Intensive Care, Turku University Hospital, University of Turku, Turku, Finland.
Henna Riikka Maanpää, Neurocenter, Department of Neurosurgery, Turku University Hospital and University of Turku, Turku, Finland; Turku Brain Injury Center, Turku University Hospital and University of Turku, Turku, Finland.
Jussi Tallus, Turku Brain Injury Center, Turku University Hospital and University of Turku, Turku, Finland.
Janek Frantzén, Neurocenter, Department of Neurosurgery, Turku University Hospital and University of Turku, Turku, Finland.
Kaj Blennow, Institute of Neuroscience and Physiology, Department of Psychiatry and Neurochemistry, The Sahlgrenska Academy at the University of Gothenburg, Mölndal, Sweden; Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden.
Olli Tenovuo, Turku Brain Injury Center, Turku University Hospital and University of Turku, Turku, Finland.
Henrik Zetterberg, Institute of Neuroscience and Physiology, Department of Psychiatry and Neurochemistry, The Sahlgrenska Academy at the University of Gothenburg, Mölndal, Sweden; Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden; Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK; UK Dementia Research Institute at UCL, University College London, London, UK; Hong Kong Center for Neurodegenerative Disease, Hong Kong, China.
David K Menon, University Division of Anaesthesia, Department of Medicine, University of Cambridge, Cambridge, UK.
Funding
This work was partly funded by the European Commission under the 7th Framework Programme (FP7-270259-TBIcare), a Medical Research Council (UK) Program Grant [Acute brain injury: heterogeneity of mechanisms, therapeutic targets and outcome effects (G9439390 ID 65883)], the UK National Institute of Health Research (NIHR) Biomedical Research Centre at Cambridge and the Technology Platform funding provided by the UK Department of Health. Data collection and sharing for this project was provided by the Cambridge Centre for Ageing and Neuroscience (CamCAN). CamCAN funding was provided by the UK Biotechnology and Biological Sciences Research Council (grant number BB/H008217/1), together with support from the UK Medical Research Council and University of Cambridge, UK.
Individual sources of funding were Academy of Medical Sciences/The Health Foundation (V.F.J.N.). the Engineering and Physical Sciences Research Council (EP/R511547/1, B.G.) and the National Institute for Health Research (D.K.M.). J.P.P. received funding from Academy of Finland (#17379) and Government’s Special Financial Transfer tied to academic research in Health Sciences (#11123 and #11129). K.B. is supported by the Swedish Research Council (#2017-00915), the Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB-201809-2016615), the Swedish Alzheimer Foundation (#AF-742881), Hjärnfonden, Sweden (#FO2017-0243), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986), the European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236), and the National Institute of Health (NIH), USA, (grant #1R01AG068398-01). H.Z. is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG-720931), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer's Association (#ADSF-21-831376-C, #ADSF-21-831381-C and #ADSF-21-831377-C), the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2019-0228), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), and the UK Dementia Research Institute at UCL.
Competing interests
V.F.J.N. reports an Academy of Medical Sciences/The Health Foundation Clinician Scientist Fellowship, during the conduct of this study; and a grant from Roche Pharmaceuticals, outside the submitted work. B.G. has received grants from European Commission and UK Research and Innovation Engineering and Physical Sciences Research Council, during the conduct of this study; and is Scientific Advisor for Kheiron Medical Technologies, Advisor and Scientific Lead of the HeartFlow-Imperial Research Team, and Visiting Researcher at Microsoft Research. K.B. has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. H.Z. has served at scientific advisory boards for Eisai, Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, Nervgen, AZTherapies and CogRx, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. O.T. has received personal fees from NeuroTraumaSciences. D.K.M. reports grants from GlaxoSmithKline and personal fees from NeuroTraumaSciences, Pfizer, Calico, PressuraNeuro, Lantmannen, Integra Neurosciences, Gryphon, and Cortirio, outside the submitted work. All other authors declare no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Czeiter E, Amrein K, Gravesteijn BY, et al. . Blood biomarkers on admission in acute traumatic brain injury: Relations to severity, CT findings and care path in the CENTER-TBI study. EBioMedicine. 2020;56:102785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maas AIR, Menon DK, Adelson PD, et al. . Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. [DOI] [PubMed] [Google Scholar]
- 3. Thelin EP, Nelson DW, Bellander BM. A review of the clinical utility of serum S100B protein levels in the assessment of traumatic brain injury. Acta Neurochir (Wien). 2017;159(2):209–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zetterberg H, Blennow K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat Rev Neurol. 2016;12(10):563–574. [DOI] [PubMed] [Google Scholar]
- 5. Yue JK, Yuh EL, Korley FK, et al. . Association between plasma GFAP concentrations and MRI abnormalities in patients with CT-negative traumatic brain injury in the TRACK-TBI cohort: A prospective multicentre study. Lancet Neurol. 2019;18(10):953–961. [DOI] [PubMed] [Google Scholar]
- 6. Richter S, Winzeck S, Kornaropoulos EN, et al. . Neuroanatomical substrates and symptoms associated with magnetic resonance imaging of patients with mild traumatic brain injury. JAMA Netw Open. 2021;4(3):e210994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jolly AE, Bălăet M, Azor A, et al. . Detecting axonal injury in individual patients after traumatic brain injury. Brain. 2021;144(1):92–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ashton NJ, Janelidze S, Al Khleifat A, et al. . A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun. 2021;12(1):3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rajan KB, Aggarwal NT, McAninch EA, et al. . Remote blood biomarkers of longitudinal cognitive outcomes in a population study. Ann Neurol. 2020;88(6):1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson L, Stewart W, Dams-O’Connor K, et al. . The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017;16(10):813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Needham EJ, Stoevesandt O, Thelin EP, et al. . Complex autoantibody responses occur following moderate to severe traumatic brain injury. J Immunol. 2021;207(1):90–100. [DOI] [PubMed] [Google Scholar]
- 12. Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136(Pt 1):28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Graham NSN, Zimmerman KA, Moro F, et al. . Axonal marker neurofilament light predicts long-term outcomes and progressive neurodegeneration after traumatic brain injury. Sci Transl Med. 2021;13(613):eabg9922. [DOI] [PubMed] [Google Scholar]
- 14. Shahim P, Politis A, van der Merwe A, et al. . Neurofilament light as a biomarker in traumatic brain injury. Neurology. 2020;95(6):e610––e622.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shahim P, Politis A, van der Merwe A, et al. . Time course and diagnostic utility of NfL, tau, GFAP, and UCH-L1 in subacute and chronic TBI. Neurology. 2020;95(6):e623–e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cole JH, Leech R, Sharp DJ. Alzheimer’s disease neuroimaging I. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol. 2015;77(4):571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graham NS, Sharp DJ. Understanding neurodegeneration after traumatic brain injury: From mechanisms to clinical trials in dementia. J Neurol Neurosurg Psychiatry. 2019;90(11):1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pereira JB, Janelidze S, Smith R, et al. . Plasma glial fibrillary acidic protein is an early marker of Aβ pathology in Alzheimer’s disease. Brain. 2021;144:3505–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ledig C, Heckemann RA, Hammers A, et al. . Robust whole-brain segmentation: Application to traumatic brain injury. Med Image Anal. 2015;21(1):40–58. [DOI] [PubMed] [Google Scholar]
- 20. Taylor JR, Williams N, Cusack R, et al. . The Cambridge centre for ageing and neuroscience (Cam-CAN) data repository: Structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. Neuroimage. 2017;144(Pt B):262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shafto MA, Tyler LK, Dixon M, et al. . The Cambridge centre for ageing and Neuroscience (Cam-CAN) study protocol: A cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 2014;14:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11(6 Pt 1):805–821. [DOI] [PubMed] [Google Scholar]
- 23. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. [DOI] [PubMed] [Google Scholar]
- 24. Ashburner J, Ridgway GR. Symmetric diffeomorphic modeling of longitudinal structural MRI. Front Neurosci. 2012;6:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ashburner J, Friston KJ. Diffeomorphic registration using geodesic shooting and Gauss-Newton optimisation. Neuroimage. 2011;55(3):954–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cole JH, Jolly A, de Simoni S, et al. . Spatial patterns of progressive brain volume loss after moderate-severe traumatic brain injury. Brain. 2018;141(3):822–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Graham NSN, Jolly A, Zimmerman K, et al. . Diffuse axonal injury predicts neurodegeneration after moderate-severe traumatic brain injury. Brain. 2020;143(12):3685–3698. [DOI] [PubMed] [Google Scholar]
- 28. Manjon JV, Coupé P, Concha L, Buades A, Collins DL, Robles M. Diffusion weighted image denoising using overcomplete local PCA. PLoS One. 2013;8(9):e73021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage. 2003;20(2):870–888. [DOI] [PubMed] [Google Scholar]
- 31. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jeurissen B, Tournier JD, Dhollander T, Connelly A, Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage. 2014;103:411–426. [DOI] [PubMed] [Google Scholar]
- 33. Tustison NJ, Avants BB, Cook PA, et al. . N4ITK: Improved N3 bias correction. IEEE Trans Med Imaging. 2010;29(6):1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wasserthal J, Neher P, Maier-Hein KH. TractSeg - Fast and accurate white matter tract segmentation. Neuroimage. 2018;183:239–253. [DOI] [PubMed] [Google Scholar]
- 35. Newcombe VF, Correia MM, Ledig C, et al. . Dynamic changes in white matter abnormalities correlate with late improvement and deterioration following TBI: A diffusion tensor imaging study. Neurorehabil Neural Repair. 2016;30(1):49–62. [DOI] [PubMed] [Google Scholar]
- 36. Castano-Leon AM, Cicuendez M, Navarro B, et al. . Longitudinal analysis of corpus callosum diffusion tensor imaging metrics and its association with neurological outcome. J Neurotrauma. 2019;36(19):2785–2802. [DOI] [PubMed] [Google Scholar]
- 37. Kenney K, Iacono D, Edlow BL, et al. . Dementia after moderate-severe traumatic brain injury: Coexistence of multiple proteinopathies. J Neuropathol Exp Neurol. 2018;77(1):50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ramlackhansingh AF, Brooks DJ, Greenwood RJ, et al. . Inflammation after trauma: Microglial activation and traumatic brain injury. Ann Neurol. 2011;70(3):374–383. [DOI] [PubMed] [Google Scholar]
- 39. Hill CS, Coleman MP, Menon DK. Traumatic axonal injury: Mechanisms and translational opportunities. Trends Neurosci. 2016;39(5):311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maxwell WL, MacKinnon MA, Smith DH, McIntosh TK, Graham DI. Thalamic nuclei after human blunt head injury. J Neuropathol Exp Neurol. 2006;65(5):478–488. [DOI] [PubMed] [Google Scholar]
- 41. Braeckman K, Descamps B, Pieters L, Vral A, Caeyenberghs K, Vanhove C. Dynamic changes in hippocampal diffusion and kurtosis metrics following experimental mTBI correlate with glial reactivity. Neuroimage Clin. 2019;21:101669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yasmin A, Pitkanen A, Jokivarsi K, Poutiainen P, Grohn O, Immonen R. MRS reveals chronic inflammation in T2w MRI-negative perilesional cortex - A 6-months multimodal imaging follow-up study. Front Neurosci. 2019;13:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Susarla BT, Villapol S, Yi JH, Geller HM, Symes AJ. Temporal patterns of cortical proliferation of glial cell populations after traumatic brain injury in mice. ASN Neuro. 2014;6(3):159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Onyszchuk G, LeVine SM, Brooks WM, Berman NE. Post-acute pathological changes in the thalamus and internal capsule in aged mice following controlled cortical impact injury: A magnetic resonance imaging, iron histochemical, and glial immunohistochemical study. Neurosci Lett. 2009;452(2):204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brenner M, Messing A. Regulation of GFAP Expression. ASN Neuro. 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takahata K, Kimura Y, Sahara N, et al. . PET-detectable tau pathology correlates with long-term neuropsychiatric outcomes in patients with traumatic brain injury. Brain. 2019;142(10):3265–3279. [DOI] [PubMed] [Google Scholar]
- 47. Hong YT, Veenith T, Dewar D, et al. . Amyloid imaging with carbon 11-labeled Pittsburgh compound B for traumatic brain injury. JAMA Neurol. 2014;71(1):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moscoso A, Grothe MJ, Ashton NJ, et al. . Longitudinal associations of blood phosphorylated Tau181 and neurofilament light chain with neurodegeneration in Alzheimer disease. JAMA Neurol. 2021;78(4):396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Simren J, Leuzy A, Karikari TK, et al. . The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer’s disease. Alzheimers Dement. 2021;17(7):1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Karikari TK, Pascoal TA, Ashton NJ, et al. . Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19(5):422–433. [DOI] [PubMed] [Google Scholar]
- 51. Chatterjee P, Pedrini S, Stoops E, et al. . Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl Psychiatry. 2021;11(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cicognola C, Janelidze S, Hertze J, et al. . Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimers Res Ther. 2021;13(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data are available upon request conditional on an approved study proposal and a signed data access agreement; there are no end dates to the availability. Please contact the corresponding authors to request. Data from the Cam-CAN repository are available by submitting a request to the Cam-CAN data access portal (http://www.mrc-cbu.cam.ac.uk/datasets/camcan/).20,21 The software code for brain age regression will be made freely available on GitHub (https://github.com/biomedia-mira/brain-age-cnn).