Abstract
Background
Nitrous oxide has been used for over 160 years for the induction and maintenance of general anaesthesia. It has been used as a sole agent but is most often employed as part of a technique using other anaesthetic gases, intravenous agents, or both. Its low tissue solubility (and therefore rapid kinetics), low cost, and low rate of cardiorespiratory complications have made nitrous oxide by far the most commonly used general anaesthetic. The accumulating evidence regarding adverse effects of nitrous oxide administration has led many anaesthetists to question its continued routine use in a variety of operating room settings. Adverse events may result from both the biological actions of nitrous oxide and the fact that to deliver an effective dose, nitrous oxide, which is a relatively weak anaesthetic agent, needs to be given in high concentrations that restrict oxygen delivery (for example, a common mixture is 30% oxygen with 70% nitrous oxide). As well as the risk of low blood oxygen levels, concerns have also been raised regarding the risk of compromising the immune system, impaired cognition, postoperative cardiovascular complications, bowel obstruction from distention, and possible respiratory compromise.
Objectives
To determine if nitrous oxide‐based anaesthesia results in similar outcomes to nitrous oxide‐free anaesthesia in adults undergoing surgery.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2014 Issue 10); MEDLINE (1966 to 17 October 2014); EMBASE (1974 to 17 October 2014); and ISI Web of Science (1974 to 17 October 2014). We also searched the reference lists of relevant articles, conference proceedings, and ongoing trials up to 17 October 2014 on specific websites (http://clinicaltrials.gov/, http://controlled‐trials.com/, and http://www.centerwatch.com).
Selection criteria
We included randomized controlled trials (RCTs) comparing general anaesthesia where nitrous oxide was part of the anaesthetic technique used for the induction or maintenance of general anaesthesia (or both) with any general anaesthesia using a volatile anaesthetic or propofol‐based maintenance of anaesthesia but no nitrous oxide for adults undergoing surgery. Our primary outcome was inhospital case fatality rate. Secondary outcomes were complications and length of stay.
Data collection and analysis
Two review authors independently assessed trial quality and extracted the outcome data. We used meta‐analysis for data synthesis. Heterogeneity was examined with the Chi² test and by calculating the I² statistic. We used a fixed‐effect model if the measure of inconsistency was low for all comparisons (I² statistic < 50%); otherwise we used a random‐effects model for measures with high inconsistency. We undertook subgroup analyses to explore inconsistency and sensitivity analyses to evaluate whether the results were robust. We assessed the quality of evidence of the main outcomes using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system.
Main results
We included 35 trials (13,872 adult participants). Seven included studies were at low risk of bias. We identified eight studies as awaiting classification since we could not obtain the full texts, and had insufficient information to include or exclude them. We included data from 24 trials for quantitative synthesis. The results of meta‐analyses showed that nitrous oxide‐based techniques increased the incidence of pulmonary atelectasis (odds ratio (OR) 1.57, 95% confidence interval (CI) 1.18 to 2.10, P = 0.002), but had no effects on the inhospital case fatality rate, the incidence of pneumonia, myocardial infarction, stroke, severe nausea and vomiting, venous thromboembolism, wound infection, or the length of hospital stay. The sensitivity analyses suggested that the results of the meta‐analyses were all robust except for the outcomes of pneumonia, and severe nausea and vomiting. Two trials reported length of intensive care unit (ICU) stay but the data were skewed so were not pooled. Both trials reported that nitrous oxide‐based techniques had no effects on the length of ICU stay. We rated the quality of evidence for two outcomes (pulmonary atelectasis, myocardial infarction) as high, four outcomes (inhospital case fatality rate, stroke, venous thromboembolism, length of hospital stay) as moderate, and three (pneumonia, severe nausea and vomiting, wound infection rate) as low.
Authors' conclusions
Given the evidence from this Cochrane review, the avoidance of nitrous oxide may be reasonable in participants with pre‐existing poor pulmonary function or at high risk of postoperative nausea and vomiting. Since there are eight studies awaiting classification, selection bias may exist in our systematic review.
Plain language summary
Nitrous oxide (laughing gas)‐based techniques versus nitrous oxide‐free techniques for general anaesthesia
Review question
We reviewed the evidence about the harmful effects of nitrous oxide on people undergoing general anaesthesia.
Background
Nitrous oxide is an anaesthetic gas which has been used for more than 160 years for inducing anaesthesia and keeping patients anaesthetized throughout an operation. It is also known as 'laughing gas'. It is a colourless non‐flammable gas with a pleasant, faint sweet odour and taste. Its low cost and low toxicity have made nitrous oxide by far the most commonly used general anaesthetic. However, some studies have reported that adding nitrous oxide may lead to harmful effects. This has led many anaesthetists to question its continued routine use in a variety of operating room settings.
We wanted to discover whether using nitrous oxide in general anaesthesia was better or worse than not using nitrous oxide.
Study characteristics
We examined the evidence available up to 17 October 2014. We included 35 trials involving 13,872 adult participants, all of whom were randomized to either receive nitrous oxide or no nitrous oxide. The trials covered a variety of situations during general anaesthesia.
Key results
We found that general anaesthesia with nitrous oxide increased the risk of pulmonary atelectasis (i.e. failure of the lungs to expand fully). When we restricted the results to the highest quality studies only, we found evidence that nitrous oxide may potentially increase the risk of pneumonia and severe nausea and vomiting. However, nitrous oxide had no effect on the patients' survival, the incidence of heart attack, stroke, wound infection, the occurrence of blood clots within veins, the length of hospital stay, or the length of intensive care unit stay.
Quality of the evidence
The evidence related to survival of participants was of moderate quality because we did not have enough data. The evidence related to some harmful effects, such as failure of the lungs to expand fully and heart attack, was of high quality, while for other harmful effects, such as stroke and the occurrence of blood clots within veins, the evidence was of moderate quality. For others, such as pneumonia, severe nausea and vomiting, and wound infection, the evidence was of low quality. The evidence related to the length of time spend in hospital was of moderate quality.
Authors conclusions
The avoidance of nitrous oxide may be reasonable in participants with pre‐existing poor pulmonary function or at high risk of postoperative nausea and vomiting.
Summary of findings
Background
Description of the condition
Nitrous oxide, also known as laughing gas, is a colourless non‐flammable gas with a pleasant, faintly sweet odour and taste. The gas has been in use for more than 160 years for the induction and maintenance of general anaesthesia. It has been used as a sole agent but is most often employed as part of a technique using other anaesthetic gases, intravenous agents, or both. Its low tissue solubility (and therefore rapid kinetics), low cost, and low rate of cardiorespiratory complications have made nitrous oxide by far the most commonly used general anaesthetic. Worldwide, it is given to more than one billion surgical patients annually (Fleischmann 2005).
The accumulating evidence regarding adverse effects of nitrous oxide administration has led many anaesthetists to question its continued routine use in a variety of operating room settings. Adverse events may result from both the biological actions of nitrous oxide and the fact that to deliver an effective dose, nitrous oxide, which is a relatively weak anaesthetic agent, needs to be given in high concentrations that restrict oxygen delivery (for example, a common mixture is 30% oxygen with 70% nitrous oxide).
The disadvantages of nitrous oxide have been reported. Concerns have been raised regarding the risk of compromising the immune system (Parbrook 1967), low blood oxygen levels (Cheney 2007), impaired cognition (mental ability) (Culley 2007; Linde 1969), postoperative cardiovascular complications (Myles 2008b), as well as bowel obstruction from distention and possible respiratory compromise (Eger 1965). In addition, nitrous oxide may increase the risk of developing brain damage from reduced cerebral blood flow (Lehmberg 2008; Pasternak 2009). Finally, nitrous oxide is a proven risk factor for nausea and vomiting (Apfel 2004).
Description of the intervention
As a weak anaesthetic, nitrous oxide is generally not used alone in general anaesthesia. Although there is considerable variation in how this drug is used, a typical scenario would be the maintenance of surgical anaesthesia, for whatever period required, by the administration of 69% nitrous oxide, 29% oxygen, and 2% of a potent volatile anaesthetic agent such as sevoflurane. Alternatively, an intravenous drug could be continuously infused while the patient breathes 70% nitrous oxide and 30% oxygen. The effect of nitrous oxide is to reduce the dose of either a volatile or intravenous anaesthetic that is required to maintain an appropriate level of anaesthesia.
How the intervention might work
As is the case with other gaseous anaesthetic agents, the exact mechanism of action of nitrous oxide is not completely understood. Theories include antagonism at both the N‐methyl‐D‐aspartate (NMDA) excitatory receptors and central nicotinic receptors; and a similar inhibitory effect at the two‐pore K+ channel TWIK‐related potassium channel‐1 (TREK‐1), a potassium channel involved in polymodal pain perception, to display analgesic, anxiolytic, and amnesic properties (Gruss 2004; Jevtović‐Todorović 1998; Yamakura 2000).
As suggested above, nitrous oxide is often used as one component of a balanced anaesthetic approach. This has several potential advantages including a reduction in the requirements for other agents, and consequently a reduced incidence and severity of any adverse effects of those agents, a rapid onset of anaesthetic effect, and a more rapid recovery of consciousness once the anaesthesia is discontinued (Becker 2008). These advantages need to be balanced against the potential disadvantages of nitrous oxide. Mechanistically, many of the adverse effects of nitrous oxide are ascribed to the inactivation of the cobalamin form of vitamin B12, by oxidation, thereby inhibiting the action of methionine synthase, folate metabolism, and deoxyribonucleic acid synthesis. All of these are important for protein production and DNA synthesis (Guirguis 1990; Perry 1983; Rowland 1995). Moreover, nitrous oxide depresses some white cells' ability to respond to various stimuli and reduces the growth of other white cell elements (mononucleocytes) (Kripke 1987).
Why it is important to do this review
As nitrous oxide administration brings both advantages and disadvantages, a systematic review will assist the individual anaesthetist in making the most appropriate choice of anaesthetic technique on an individual patient basis. The balance of risk versus benefit is likely to depend on many factors. The aim of this Cochrane review was to quantitatively evaluate if nitrous oxide was responsible for clinically significant adverse events following general anaesthesia that could be safely avoided by the use of alternative agents. This may have a wide impact on the conduct of general anaesthesia.
Objectives
To determine if nitrous oxide‐based anaesthesia results in similar outcomes to nitrous oxide‐free anaesthesia in adults undergoing surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs).
Types of participants
We included participants, aged 18 years and older, undergoing surgery with standard general anaesthesia.
Types of interventions
Intervention:
General anaesthesia where nitrous oxide was part of the anaesthetic technique used for the induction or maintenance of general anaesthesia, or both.
Control:
General anaesthesia using a volatile anaesthetic or propofol‐based maintenance of anaesthesia but no nitrous oxide.
Types of outcome measures
Primary outcomes
Inhospital case fatality rate (number or proportion of deceased participants after a defined period following anaesthesia).
Secondary outcomes
1. Pulmonary complications: 1.1 Pneumonia: We accepted any definition used by the authors of included papers; 1.2 Pulmonary atelectasis: We accepted any definition used by the authors of included papers.
2. Heart complications: 2.1 Myocardial infarction: We accepted any definition of myocardial infarction used by the authors of included papers.
3. Neurological complications: 3.1 Stroke: We accepted any definition of stroke used by the authors of included papers. Where there was no definition, we accepted in the outcome any participant with new neurological signs (paralysis, weakness or speech difficulties) that persisted for 24 hours or leaded to early death.
4. Other complications: 4.1 Severe nausea and vomiting: We accepted any definition of severe nausea and vomiting made by the authors of included trials. Where there was no definition, we accepted into the outcome any participant with at least two episodes of vomiting or who required at least three doses of antiemetic medication within 24 hours of surgery; 4.2 Venous thromboembolism: We accepted any definition of deep venous thrombosis or pulmonary embolism used by the authors of included papers; 4.3 Wound infection rate: We accepted any definition of wound infection made by the authors of included trials.
5. Length of stay: 5.1 Length of hospital stay: We accepted any definition of length of hospital stay made by the authors of included trials; 5.2 Length of intensive care unit (ICU) stay: We accepted any definition of length of ICU stay made by the authors of included trials.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2014 Issue 10); MEDLINE (1966 to 17 October 2014); EMBASE (1974 to 17 October 2014); and ISI Web of Science (1974 to 17 October 2014).
We developed a specific strategy for each database (Appendix 1 for CENTRAL; Appendix 2 for MEDLINE; Appendix 3 for EMBASE; and Appendix 4 for ISI Web of Science).
Searching other resources
Two review authors (RS, WQJ) examined the reference lists of any retrieved articles for additional relevant publications. In addition, two review authors (BM, YL) manually searched conference proceedings and review articles for relevant studies. We contacted relevant trial authors to identify any additional or ongoing studies. We also searched for relevant trials on specific websites: http://clinicaltrials.gov/; http://controlled‐trials.com/; and http://www.centerwatch.com. We did not apply any language restrictions.
Data collection and analysis
Two review authors (RS, WQJ) developed and used a standardized data extraction form in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors (RS, XFL) independently checked and entered data into RevMan 5.3 for statistical analysis.
Selection of studies
One review author (WQJ) scanned the titles and abstracts of articles retrieved by the search and removed those that did not meet our inclusion criteria. Three review authors (JHT, WQJ, RS) retrieved the full text of all potentially eligible studies. Two review authors (RS, WQJ) independently examined the full text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. We resolved any disagreement as to study eligibility by discussion with a third review author (KHY).
Data extraction and management
We extracted data from eligible studies using a data form we had designed and pilot‐tested (Appendix 5). When a study either overlapped or was a duplicate of another study, WQ Jia and P Zhang contacted the study authors for clarification and, if confirmed, used the publication with the more detailed data for this systematic review and combined the additional data. Two review authors (RS, PZ) contacted the original study authors for additional data for included outcomes that were not published in the study. Two review authors (WQJ, RS) independently extracted the data and resolved any disagreement by consulting a third review author (KHY).
We extracted the following information:
Study design (RCT).
Participants (number, age, gender, American Society of Anesthesiologists (ASA) physical status classification, disease, type of surgery).
Intervention (concentration of nitrous oxide, mixed inhaled anaesthetic, concentration of oxygen, duration of inhaled nitrous oxide).
Quality assessment (sequence generation, allocation concealment, blinding, incomplete outcome data, other issues).
Outcome (primary and secondary outcomes, methods used to assess outcomes, time of follow‐up).
Assessment of risk of bias in included studies
Two review authors (RS, BM) independently assessed the quality of the studies by constructing a 'Risk of bias' table for each study which included sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other bias (Higgins 2011). Any disagreements were resolved by discussion between the two review authors.
We assessed the quality factors of each study separately. These were classified as either 'low', 'high', or 'unclear' risk of bias.
Measures of treatment effect
Considering dichotomous variables, we expressed the difference in the number of events in the nitrous oxide‐based group and the nitrous oxide‐free group as an odds ratio (OR) for complications and Peto odds ratio (Peto OR) for the inhospital case fatality rate. For length of stay, we only pooled the data expressed as mean and standard deviation (SD). The effect size for length of stay was the mean difference (MD). We presented 95% confidence intervals (CIs) for all outcomes.
Unit of analysis issues
Non‐standard design RCTs can present statistical problems. Whilst we did not anticipate including crossover or cluster randomized designs in this Cochrane review, we expected multiple intervention groups. We took care to avoid 'unit of analysis' errors when analysing these types of trials (Higgins 2011).
Dealing with missing data
In the event of missing data, two review authors (WQJ, RS) tried to contact the authors of the original studies in order to obtain the necessary information. Two review authors (XFL, RS) analysed the data on an intention‐to‐treat (ITT) basis as far as possible.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a meaningful summary. Statistically, we examined heterogeneity with the Chi² test and by calculating the I² statistic. We considered heterogeneity to be substantial when the I² statistic > 50% and carefully considered the data before reporting any pooled results (Higgins 2002). If substantial heterogeneity was detected, we explored possible explanations in subgroup analyses.
Assessment of reporting biases
We conducted a comprehensive search for eligible studies. If there were 10 or more studies in an analysis, we used a funnel plot to explore the possibility of publication bias and other reporting biases. In the analyses for dichotomous outcomes we also assessed publication bias statistically with the use of Egger's test (Egger 1997) performed with Stata 11.0. We based evidence of asymmetry on P < 0.05.
Data synthesis
We used meta‐analysis for data synthesis. We used a fixed‐effect model if the measure of inconsistency was low for all comparisons (I² statistic < 50%); otherwise we used a random‐effects model for measures with high inconsistency. Where we did not conduct meta‐analysis, we described the findings of the included studies qualitatively.
We included the following outcomes in the 'Summary of findings' tables:
Inhospital case fatality rate.
Pneumonia.
Pulmonary atelectasis.
Myocardial infarction.
Stroke.
Severe nausea and vomiting.
Length of hospital stay.
Venous thromboembolism.
Wound infection rate.
We rated the quality of evidence for each outcome following the guidelines of Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach (Schünemann 2009) and based on the following five downgrade factors: risk of bias, inconsistency, indirectness, imprecision, and publication bias. For each downgrade factor, a judgment of 'no', 'serious (downgrade the quality of evidence by one level)', or 'very serious (downgrade the quality of evidence by two levels)' was assigned. At the very beginning, we classified all the outcomes as at 'high' quality by default, and after rating, each outcome could receive a grade of either 'high', 'moderate', 'low', or 'very low' quality.
Subgroup analysis and investigation of heterogeneity
We undertook subgroup analyses as follows, as stated in the Cochrane protocol (Yang 2011):
Type of surgery (day‐case procedures/examinations versus intra‐abdominal surgery versus neurosurgery versus vascular surgery versus ophthalmic surgery versus breast surgery).
Different concentrations of inhaled nitrous oxide (high concentration [higher than 50%] versus low concentration [equal to or lower than 50%]).
Different intervention in the nitrous oxide‐free group (propofol‐based maintenance of anaesthesia versus volatile anaesthetic‐based maintenance of anaesthesia).
Sensitivity analysis
To evaluate whether the results of the systematic review were robust, we conducted sensitivity analyses based on the methodological quality (high quality versus low quality) and the percentages of withdrawals (above 10% versus below 10%) of the included RCTs.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; and Characteristics of studies awaiting classification sections.
Results of the search
The number of potential RCTs screened for inclusion in this Cochrane review is outlined in the study flow diagram (Figure 1).
1.
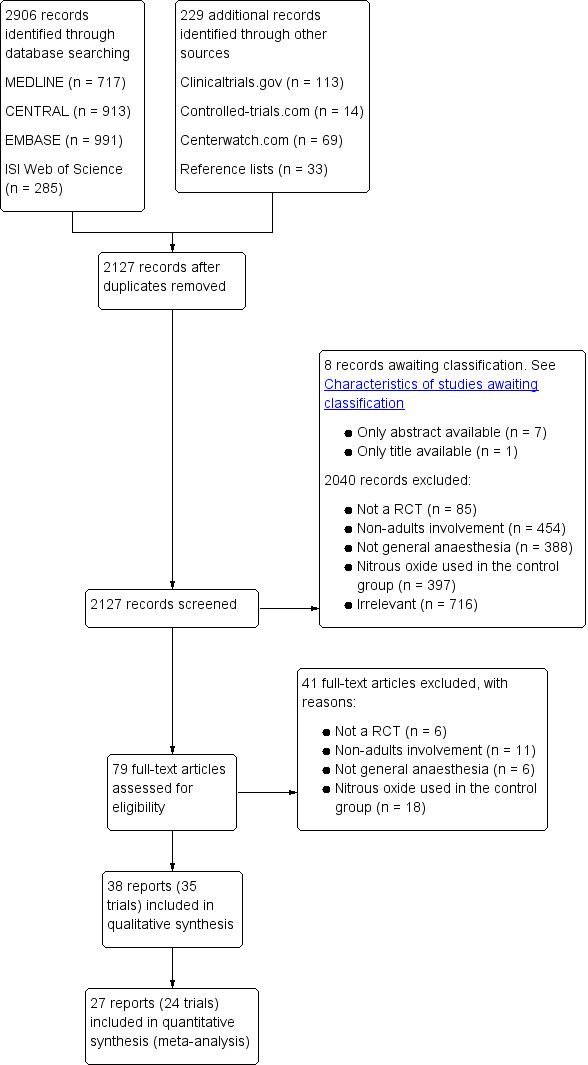
Study flow diagram.
We identified a total of 2906 references through searches of electronic databases and a further 229 through other resources. After removing the duplicates, we screened 2127 unique references. We excluded 2040 records based on titles and abstracts, and a further eight studies are awaiting classification (see Characteristics of studies awaiting classification) as we were unable to obtain their full texts from either our university library, the Danish National Library, or Cochrane Anaesthesia, Critical and Emergency Care Group members. We assessed 79 full text papers, of which 38 reports (consisting of 35 trials) were eligible for inclusion in this Cochrane review.
Included studies
We included 35 trials in this Cochrane review; see Characteristics of included studies.
Four studies included participants who had undergone day‐case procedures or examinations (Arellano 2000; Sengupta 1988; Short 1985; Van Hemelrijck 1991); 14 studies included participants who had undergone intra‐abdominal surgery (Akca 2004; Brodsky 2005; Chen 2013; Fleischmann 2005; Jensen 1992; Jensen 1993a; Jensen 1993b; Krogh 1994; Lee 2005; Lonie 1986; Mraovic 2008; Paredi 1994; Pedersen 1993; Sukhani 1994); three studies included participants who had undergone neurosurgery (Lampe 1990; Singh 2011; Todd 1993); two studies included participants who had undergone vascular surgery (Badner 2000; Kozmary 1990); one study included participants who had undergone ophthalmic surgery (Deleu 2000); one study included participants who had undergone breast surgery (Vanacker 1999); one study included participants who had undergone orthopedic surgery (Alhashemi 1997); and one study included participants who had undergone thoracic surgery (Yoshimura 2014). Eight studies included participants who had undergone different types of surgery (Bloomfield 1988; Eger 1990; ENIGMA II trial 2014; ENIGMA trial 2007; Gilani 2008; Larsen 2000; Leung 2006; Myles 2008a).
Twenty‐six studies used high concentrations of nitrous oxide in the nitrous oxide‐based group (Akca 2004; Alhashemi 1997; Arellano 2000; Badner 2000; Chen 2013; Eger 1990; ENIGMA II trial 2014; ENIGMA trial 2007; Fleischmann 2005; Gilani 2008; Jensen 1992; Jensen 1993a; Jensen 1993b; Kozmary 1990; Krogh 1994; Lampe 1990; Larsen 2000; Lee 2005; Lonie 1986; Myles 2008a; Pedersen 1993; Short 1985; Singh 2011; Todd 1993; Van Hemelrijck 1991; Sukhani 1994); three studies used low concentrations of nitrous oxide (Brodsky 2005; Mraovic 2008; Yoshimura 2014); and one study used both low and high concentrations of nitrous oxide (Sengupta 1988). Five studies did not report the concentration of nitrous oxide (Bloomfield 1988; Deleu 2000; Leung 2006; Paredi 1994; Vanacker 1999).
Ten studies used propofol‐based maintenance of anaesthesia in the nitrous oxide‐free group (Alhashemi 1997; Arellano 2000; Deleu 2000; Jensen 1992; Jensen 1993b; Larsen 2000; Krogh 1994; Sukhani 1994; Todd 1993; Yoshimura 2014); 22 studies used volatile anaesthetic‐based maintenance of anaesthesia in the nitrous oxide‐free group (Akca 2004; Badner 2000; Bloomfield 1988; Brodsky 2005; Chen 2013; Eger 1990; Fleischmann 2005; Gilani 2008; Jensen 1993a; Kozmary 1990; Lampe 1990; Lee 2005; Leung 2006; Lonie 1986; Mraovic 2008; Paredi 1994; Pedersen 1993; Sengupta 1988; Short 1985; Singh 2011; Vanacker 1999; Van Hemelrijck 1991). Three studies used different techniques of anaesthesia in the nitrous oxide‐free group (ENIGMA II trial 2014; ENIGMA trial 2007; Myles 2008a).
Of the 35 included trials, 24 trials reported outcomes identified as of interest for this review (Arellano 2000; Chen 2013; Deleu 2000; Eger 1990; ENIGMA II trial 2014; ENIGMA trial 2007; Fleischmann 2005; Jensen 1992; Jensen 1993a; Jensen 1993b; Kozmary 1990; Krogh 1994; Lampe 1990; Leung 2006; Mraovic 2008; Myles 2008a; Paredi 1994; Pedersen 1993; Sengupta 1988; Short 1985; Singh 2011; Todd 1993; Vanacker 1999; Van Hemelrijck 1991). Of the 11 trials excluded from the quantitative analysis, three reported quality of recovery (Brodsky 2005; Larsen 2000; Sukhani 1994); two reported non‐severe nausea and vomiting (Bloomfield 1988; Lonie 1986); one reported myocardial ischaemia (Badner 2000); one reported bowel distension (Akca 2004); one reported costs of anaesthesia and postoperative care (Alhashemi 1997); one reported postoperative pain (Gilani 2008); one reported postoperative opioid consumption (Lee 2005); and one reported lung collapse score (Yoshimura 2014).
Excluded studies
We excluded 41 studies after full text assessment. We excluded six of those studies because they were not RCTs (Antonini 1994; Barr 1999; Divatia 1996; Dover 1994; Morimoto 1997; Wesner 2005); 11 for including participants aged lower than 18 years (Jastak 1973; Johnson 1997; Lim 1992; Losasso 1992; Nightingale 1992; Ogg 1983; Rocca 2000; Saïssy 2000; Taki 2003; Towey 1979; Van den Berg 1995); six for including participants not undergoing general anaesthesia (Atanassoff 1994; Castéra 2001; Haraguchi 1995; Heath 1996; Kryshtalskyj 1990; Masood 2002); and 18 for using nitrous oxide in the control group (Atassi 2005; Bronco 2010; Cheong 2000; Einarsson 1997; Fredman 1998; Gozdemir 2007; Haessler 1993; Holst 1993; Ishii 1994; Jellish 1996; Nishiyama 1998; Simpson 1977; Sinha 2006; Smith 1993; Vari 2010; Yamakage 2001; Yang 2004; Zuurmond 1986). See Characteristics of excluded studies.
Studies awaiting classification
Eight studies are awaiting classification (Adams 1994; Miralles Pardo 1991; Moussa 1995; Rashchupkin 2011; Röpcke 2001; Schaffranietz 2000; Segatto 1993; Shulunov 2002). We were unable to obtain full text articles of these eight publications from our university library, the Danish National Library, and Cochrane Anaesthesia, Critical and Emergency Care Group members. Of these eight studies, seven were published in non‐English languages (three studies were in German, two studies were in Russian, one study was in Italian, and one study was in Spanish). See Characteristics of studies awaiting classification.
Ongoing studies
We did not identify any ongoing studies.
Risk of bias in included studies
We have summarized our 'Risk of bias' assessments for each included study in Figure 2 and as percentages across all studies in Figure 3. The details and reasons for each assessment are listed in the Characteristics of included studies section. Seven studies were at low risk of bias (Akca 2004; Arellano 2000; ENIGMA II trial 2014; ENIGMA trial 2007; Fleischmann 2005; Lee 2005; Leung 2006).
2.
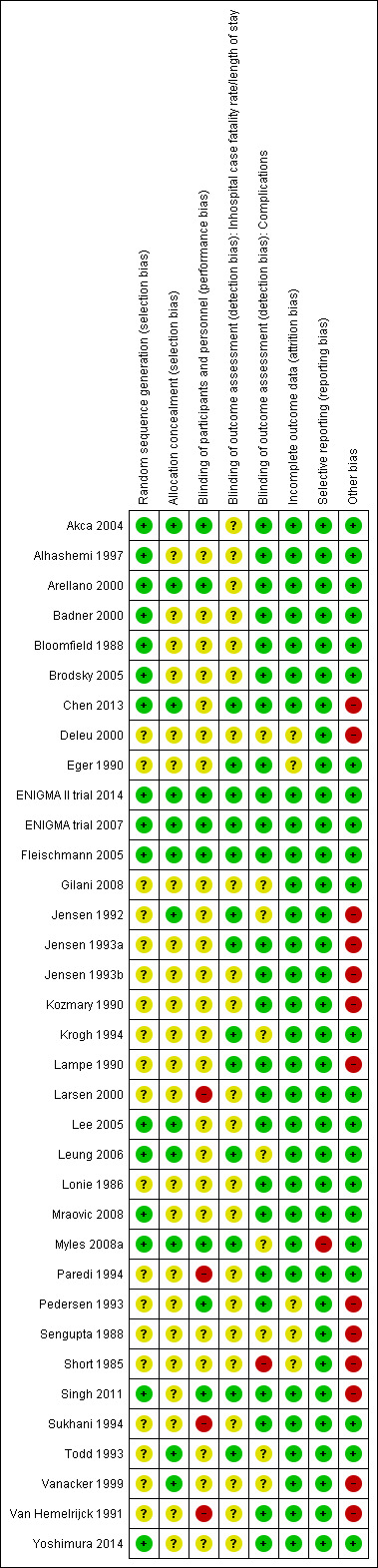
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
3.
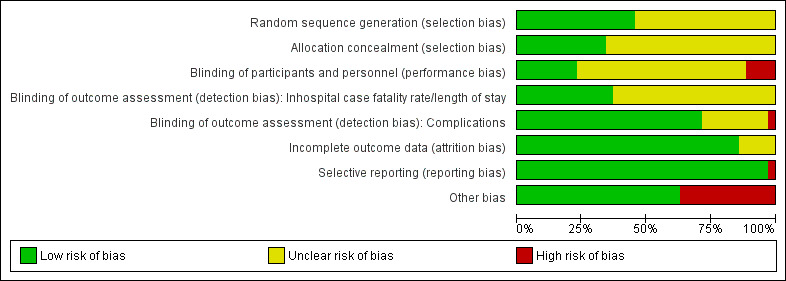
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
Allocation
All included studies mentioned randomization in the methodology, but only 16 trials stated the actual method used for randomization (Akca 2004; Alhashemi 1997; Arellano 2000; Badner 2000; Bloomfield 1988; Brodsky 2005; Chen 2013; ENIGMA II trial 2014; ENIGMA trial 2007; Fleischmann 2005; Lee 2005; Leung 2006; Mraovic 2008; Myles 2008a; Singh 2011; Yoshimura 2014).
In 23 studies, the trial authors did not give the details of the method of concealment of allocation, and we categorized these studies as 'unclear'. Concealment was adequate in 12 studies (Akca 2004; Arellano 2000; Chen 2013; ENIGMA II trial 2014; ENIGMA trial 2007; Fleischmann 2005; Jensen 1992; Lee 2005; Leung 2006; Myles 2008a; Todd 1993; Vanacker 1999).
Blinding
Participants and personnel were blinded in eight studies (Akca 2004; Arellano 2000; ENIGMA II trial 2014; ENIGMA trial 2007; Fleischmann 2005; Myles 2008a; Pedersen 1993; Singh 2011); four studies were not blinded (Larsen 2000; Paredi 1994; Sukhani 1994; Van Hemelrijck 1991); and the remaining studies were unclear.
We have separated 'blinding of outcome assessment (detection bias)' by type of outcome as the impact of outcome assessor knowledge of allocation may vary across different outcomes.
We assessed the 13 studies reporting clinical endpoints of inhospital case fatality rate or length of stay as being at a low risk of detection bias, since the outcome measurements were unlikely to have been influenced by lack of blinding (Chen 2013; Eger 1990; ENIGMA II trial 2014; ENIGMA trial 2007; Fleischmann 2005; Jensen 1992; Jensen 1993a; Krogh 1994; Lampe 1990; Leung 2006; Myles 2008a; Singh 2011; Todd 1993). Of the 32 studies reporting clinical endpoints of complications, the outcome assessors were blinded in 25 studies (Akca 2004; Alhashemi 1997; Arellano 2000; Badner 2000; Bloomfield 1988; Brodsky 2005; Chen 2013; Eger 1990; ENIGMA II trial 2014; ENIGMA trial 2007; Fleischmann 2005; Jensen 1993a; Jensen 1993b; Kozmary 1990; Lampe 1990; Larsen 2000; Lee 2005; Lonie 1986; Mraovic 2008; Paredi 1994; Pedersen 1993; Singh 2011; Sukhani 1994; Van Hemelrijck 1991; Yoshimura 2014); outcome assessors were not blinded in one study (Short 1985); and the remaining studies were unclear (Deleu 2000; Gilani 2008; Jensen 1992; Sengupta 1988; Todd 1993; Vanacker 1999).
Incomplete outcome data
The number of participants entering the trials and the number subjected to analysis, as mentioned in the results, were the same in 21 studies (Akca 2004; Alhashemi 1997; Bloomfield 1988; Brodsky 2005; Gilani 2008; Jensen 1992; Jensen 1993b; Kozmary 1990; Krogh 1994; Lampe 1990; Larsen 2000; Lee 2005; Leung 2006; Lonie 1986; Myles 2008a; Paredi 1994; Sukhani 1994; Todd 1993; Vanacker 1999; Van Hemelrijck 1991; Yoshimura 2014). Of the 14 studies that had withdrawals, the missing outcome data was balanced in numbers across the intervention groups. Nine trials gave similar reasons for missing data across groups (Arellano 2000; Badner 2000; Chen 2013; ENIGMA II trial 2014; ENIGMA trial 2007; Fleischmann 2005; Jensen 1993a; Mraovic 2008; Singh 2011). The remaining five studies had insufficient information to enable us to form a judgment (Deleu 2000; Eger 1990; Pedersen 1993; Sengupta 1988; Short 1985).
Selective reporting
Two studies, ENIGMA II trial 2014 and ENIGMA trial 2007, were registered on ClinicalTrials.gov (NCT00430989 and NCT00164047, respectively). The study protocols were available and all of the pre‐specified (primary and secondary) outcomes that were of interest in the review were reported in the pre‐specified way. Of the 33 studies that had no protocol, one study had not reported all the pre‐specified primary outcomes (Myles 2008a); and the remaining studies reported all the outcomes described in their method sections.
Other potential sources of bias
Given the outcomes of interest in this Cochrane review, such as inhospital death and complications, were at low incidence, most of the included trials were underpowered for these outcomes. We assessed this item as high risk in studies that reported the outcomes of inhospital death or complications, but had fewer than 50 participants per arm (Chaparro 2013). Therefore we assessed 13 studies as at high risk of bias (Chen 2013; Deleu 2000; Jensen 1992; Jensen 1993a; Jensen 1993b; Kozmary 1990; Lampe 1990; Pedersen 1993; Sengupta 1988; Short 1985; Singh 2011; Vanacker 1999; Van Hemelrijck 1991).
Effects of interventions
Summary of findings for the main comparison. Summary of findings’ table 1.
| Nitrous oxide‐based compared to nitrous oxide‐free for general anaesthesia | ||||||
| Patient or population: adult patients 18 years and above undergoing standard general anaesthesia Settings: operating room Intervention: nitrous oxide‐based techniques Comparison: nitrous oxide‐free techniques | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| nitrous oxide‐free | Nitrous oxide‐based | |||||
| Inhospital case fatality rate | Study population | OR 0.87 (0.61 to 1.26) | 10148 (8 studies) | ⊕⊕⊕⊝ moderate1 | — | |
| 12 per 1000 | 11 per 1000 (8 to 16) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Pneumonia | Study population | OR 1.68 (1 to 2.81) | 2699 (8 studies) | ⊕⊕⊝⊝ low2,3 | The sensitivity analysis suggested that the results of meta‐analysis was not robust. | |
| 17 per 1000 | 27 per 1000 (17 to 45) | |||||
| Moderate | ||||||
| 11 per 1000 | 18 per 1000 (11 to 30) | |||||
| Pulmonary atelectasis | Study population | OR 1.57 (1.18 to 2.1) | 2400 (5 studies) | ⊕⊕⊕⊕ high | — | |
| 79 per 1000 | 119 per 1000 (92 to 153) | |||||
| Moderate | ||||||
| 50 per 1000 | 76 per 1000 (58 to 100) | |||||
| Myocardial infarction | Study population | OR 1.01 (0.84 to 1.22) | 9246 (6 studies) | ⊕⊕⊕⊕ high | — | |
| 51 per 1000 | 51 per 1000 (43 to 61) | |||||
| Moderate | ||||||
| 65 per 1000 | 66 per 1000 (55 to 78) | |||||
| Stroke | Study population | OR 1.47 (0.86 to 2.53) | 9142 (4 studies) | ⊕⊕⊕⊝ moderate3 | — | |
| 5 per 1000 | 7 per 1000 (4 to 12) | |||||
| Moderate | ||||||
| 3 per 1000 | 4 per 1000 (3 to 8) | |||||
| Severe nausea and vomiting | Study population | OR 1.44 (0.97 to 2.15) | 11045 (10 studies) | ⊕⊕⊝⊝ low4,5 | The sensitivity analysis suggested that the results of meta‐analysis was not robust. | |
| 95 per 1000 | 131 per 1000 (92 to 184) | |||||
| Moderate | ||||||
| 108 per 1000 | 148 per 1000 (105 to 207) | |||||
| Length of hospital stay | The mean length of hospital stay in the intervention groups was 0.36 higher (0.69 lower to 1.4 higher) | 1103 (6 studies) | ⊕⊕⊕⊝ moderate5 | — | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Serious imprecision: 95% CI of OR includes both 1.0 and 0.75/1.25. Downgraded by one level. 2Serious risk of bias: all studies were described as randomized but details were only provided by three; four studies described allocation concealment. Two studies blinded participants and personnel; six studies blinded outcome assessors. Downgraded by one level. 3Serious imprecision: 95% CI of OR includes both 1.0 and 1.25. Downgraded by one level. 4Serious risk of bias: all studies were described as randomized but details were only provided by three; four studies described allocation concealment. Four studies blinded participants and personnel; seven studies blinded outcome assessors. Downgraded by one level. 5Serious inconsistency: substantial heterogeneity with I² statistic > 50%. Downgraded by one level.
Summary of findings 2. 'Summary of findings' table 2.
| Nitrous oxide‐based compared to nitrous oxide‐free for general anaesthesia | ||||||
| Patient or population: adult patients 18 years and above undergoing standard general anaesthesia Settings: operating room Intervention: nitrous oxide‐based techniques Comparison: nitrous oxide‐free techniques | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| nitrous oxide‐free | Nitrous oxide‐based | |||||
| Venous thromboembolism | Study population | OR 0.73 (0.45 to 1.2) | 9004 (2 studies) | ⊕⊕⊕⊝ moderate1 | — | |
| 8 per 1000 | 6 per 1000 (4 to 10) | |||||
| Moderate | ||||||
| 11 per 1000 | 8 per 1000 (5 to 13) | |||||
| Wound infection rate | Study population | OR 1.22 (0.84 to 1.78) | 9789 (6 studies) | ⊕⊕⊝⊝ low2,3 | — | |
| 88 per 1000 | 106 per 1000 (75 to 147) | |||||
| Moderate | ||||||
| 83 per 1000 | 99 per 1000 (71 to 139) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Serious imprecision: 95% CI of OR includes both 1.0 and 0.75. Downgraded by one level. 2Serious inconsistency: substantial heterogeneity with I² statistic > 50%. Downgraded by one level. 3Serious imprecision: 95% CI of OR includes both 1.0 and 1.25. Downgraded by one level.
Primary outcomes
1. Inhospital case fatality rate (number or proportion of deceased participants after a defined period following anaesthesia)
Eight studies reported inhospital case fatality rate and together included 10,148 participants, 73.2% of the total number of participants included in this Cochrane review (Chen 2013; Eger 1990; ENIGMA II trial 2014; ENIGMA trial 2007; Fleischmann 2005; Lampe 1990; Leung 2006; Todd 1993). Of the included participants, 5076 (50%) were randomized to a nitrous oxide‐based technique and 5072 (50%) to a nitrous oxide‐free technique. Fifty‐five participants died in the nitrous oxide group (1.1%), versus 63 in the nitrous oxide‐free group (1.2%). Pooling of the data showed this small difference was not statistically significant. The Peto OR for the outcome of inhospital case fatality rate was 0.87 (95% CI 0.61 to 1.26; P = 0.47) when nitrous oxide was compared with control (Analysis 1.1). As the 95% CI of Peto OR included both 1.0 and 0.75/1.25, we downgraded the quality of the evidence for this outcome from high to moderate quality due to 'imprecision'.
1.1. Analysis.
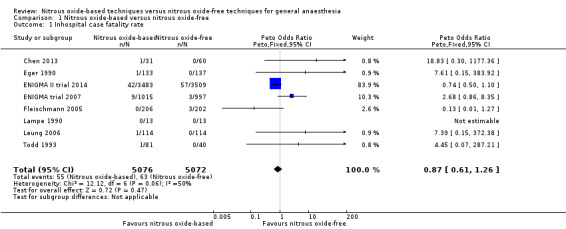
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 1 Inhospital case fatality rate.
We performed subgroup analyses using the prespecified subgroups, and did not detect any significant differences for the following subgroup analyses: type of surgery (Analysis 1.10), test for subgroup differences: Chi² test = 1.02, df = 1 (P value = 0.31); intervention in the nitrous oxide‐free group (Analysis 1.22), test for subgroup differences: Chi² test = 0.37, df = 1 (P value = 0.54). The test for subgroup differences was not applicable when we performed subgroup analysis by concentration of inhaled nitrous oxide. The results showed no significant difference between high‐concentration nitrous oxide‐based group and nitrous oxide‐free group on inhospital case fatality rate (Peto OR 0.86, 95% CI 0.60 to 1.24, I² statistic = 34%, P value = 0.42; seven studies, 9920 participants; Analysis 1.18).
1.10. Analysis.
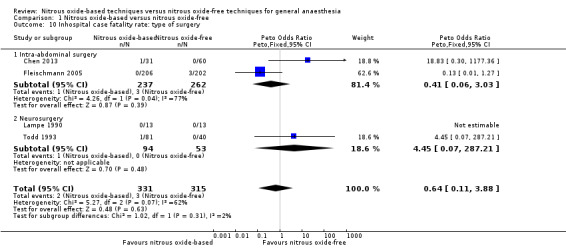
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 10 Inhospital case fatality rate: type of surgery.
1.22. Analysis.
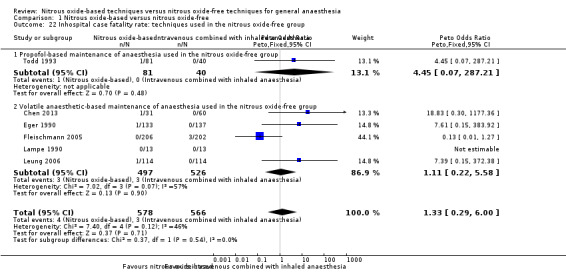
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 22 Inhospital case fatality rate: techniques used in the nitrous oxide‐free group.
1.18. Analysis.
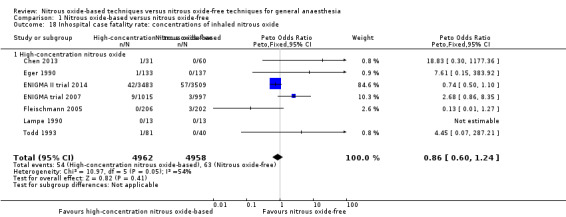
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 18 Inhospital case fatality rate: concentrations of inhaled nitrous oxide.
The sensitivity analysis performed just including the studies at low risk of bias (ENIGMA II trial 2014; ENIGMA trial 2007; Fleischmann 2005; Leung 2006) suggested that the results of meta‐analysis were robust.
As all eight studies had < 10% withdrawals, we did not conduct a sensitivity analysis excluding studies with > 10% withdrawals.
Secondary outcomes
1. Pulmonary complications
1.1 Pneumonia
Eight studies reported pneumonia and together included 2699 participants, 19.5% of the total number of participants included in this review (Chen 2013; Eger 1990; Jensen 1992; Jensen 1993a; Lampe 1990; ENIGMA trial 2007; Singh 2011; Todd 1993). Of the included participants, 1368 (50.7%) were randomized to a nitrous oxide‐based technique and 1331 (49.3%) to a nitrous oxide‐free technique. Thirty‐seven participants caught pneumonia in the nitrous oxide group (2.7%), versus 22 in the nitrous oxide‐free group (1.7%). Pooling of the data showed this small difference was not statistically significant. The OR for the outcome of pneumonia was 1.68 (95% CI 1.00 to 2.81; P = 0.05) when nitrous oxide was compared with control (Analysis 1.2). As the serious risk of bias existed among included studies, and the 95% CI of the OR included both 1.0 and 1.25, we downgraded the quality of the evidence for this outcome from high to low quality due to 'risk of bias' and 'imprecision'.
1.2. Analysis.
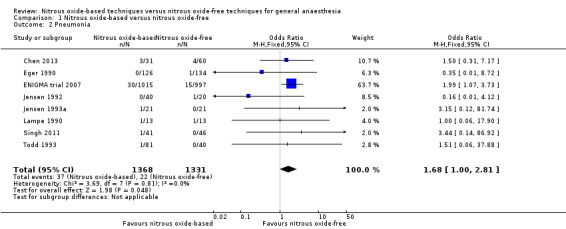
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 2 Pneumonia.
We conducted subgroup analyses using the prespecified subgroups, but could not perform a subgroup analysis of concentration of inhaled nitrous oxide, as all included studies in this analysis used a high concentration. No significant differences were detected for the following subgroup analyses: type of surgery (Analysis 1.11), test for subgroup differences: Chi² test = 0.15, df = 1, P = 0.70; intervention in the nitrous oxide‐free group (Analysis 1.23), test for subgroup differences: Chi² test = 0.84, df = 1, P = 0.36).
1.11. Analysis.
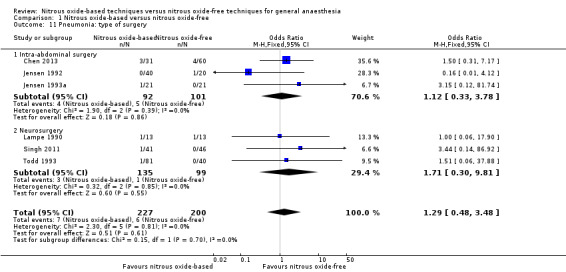
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 11 Pneumonia: type of surgery.
1.23. Analysis.
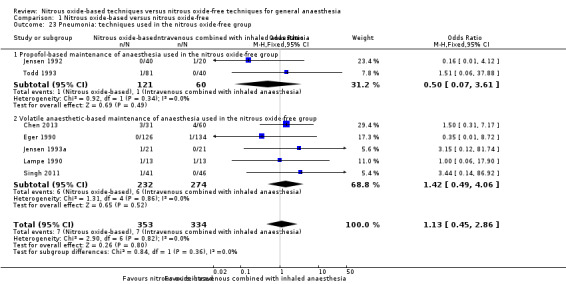
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 23 Pneumonia: techniques used in the nitrous oxide‐free group.
We performed a sensitivity analysis including only the studies at low risk of bias (ENIGMA trial 2007), which suggested that the results of meta‐analysis were not robust. The results changed from OR 1.68 (95% CI 1.00 to 2.81) to OR 1.99 (95% CI 1.07 to 3.73).
The sensitivity analysis excluding studies with more than 10% withdrawals (Singh 2011) suggested that the results of meta‐analysis were robust.
1.2 Pulmonary atelectasis
Five studies reported pulmonary atelectasis and together included 2400 participants, 17.3% of the total number of participants included in this review (Eger 1990; Jensen 1993b; Jensen 1992; Lampe 1990; ENIGMA trial 2007). Of these included participants, 1222 (50.9%) were randomized to a nitrous oxide‐based technique and 1178 (49.1%) to a nitrous oxide‐free technique. One hundred and fifty participants developed pulmonary atelectasis in the nitrous oxide group (12.3%), versus 93 in the nitrous oxide‐free group (7.9%). Pooling of the data showed this difference was statistically significant. The odds of pulmonary atelectasis were significantly increased in the nitrous oxide‐based group (OR 1.57, 95% CI 1.18 to 2.10, I² statistic = 48%, P = 0.002; five studies, 2400 participants; Analysis 1.3). We rated the quality of the evidence for this outcome as high.
1.3. Analysis.
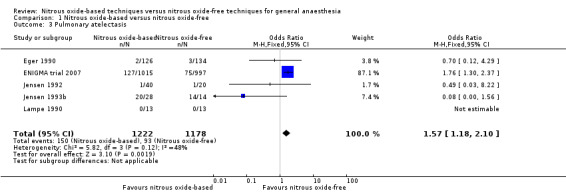
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 3 Pulmonary atelectasis.
We ran subgroup analyses using the prespecified subgroups, but could not perform a subgroup analysis by concentration of inhaled nitrous oxide, as all included studies in this analysis used a high concentration. No significant differences were detected for the subgroup analyses by intervention in the nitrous oxide‐free group (Analysis 1.24), test for subgroup differences: Chi² test = 1.24, df = 1, P = 0.27). The test for subgroup differences was not applicable when we performed subgroup analysis by type of surgery. The results showed no significant difference between the two groups for intra‐abdominal surgery (OR 0.16, 95% CI 0.02 to 1.06, I² statistic = 0%, P value = 0.06; two studies, 102 participants). The subgroup analysis for neurosurgery was not applicable as no pulmonary atelectasis was reported in either the nitrous oxide‐based or nitrous oxide‐free group (Analysis 1.12).
1.24. Analysis.
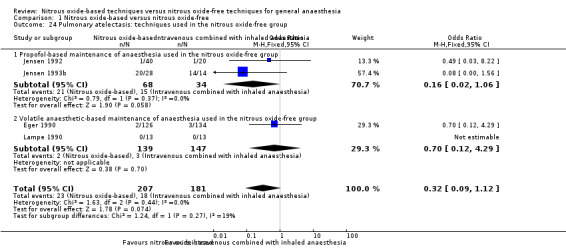
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 24 Pulmonary atelectasis: techniques used in the nitrous oxide‐free group.
1.12. Analysis.
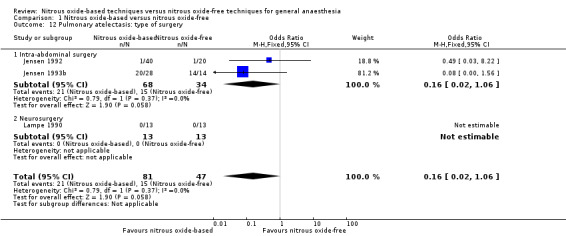
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 12 Pulmonary atelectasis: type of surgery.
We performed a sensitivity analysis including only the studies of low risk of bias (ENIGMA trial 2007), which suggested that the results of meta‐analysis were robust.
As all the five studies had < 10% withdrawals, we did not conduct a sensitivity analysis excluding studies with > 10% withdrawals.
2. Heart complications
2.1 Myocardial infarction
Six studies reported myocardial infarction and together included 9246 participants, 66.7% of the total number of participants included in this review (Chen 2013; Eger 1990; Kozmary 1990; ENIGMA II trial 2014; ENIGMA trial 2007; Singh 2011). Of the included participants, 4602 (49.8%) were randomized to a nitrous oxide‐based technique and 4644 (50.2%) to a nitrous oxide‐free technique. Two hundred and thirty‐five participants developed myocardial infarction in the nitrous oxide group (5.1%), versus 236 in the nitrous oxide‐free group (5.1%). Pooling of the data showed no significant difference in the outcome between groups. The OR for the outcome of myocardial infarction was 1.01 (95% CI 0.84 to 1.22, P = 0.88) when nitrous oxide was compared with control (Analysis 1.4). The quality of the evidence for this outcome was high.
1.4. Analysis.
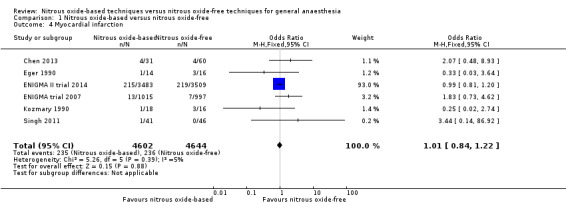
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 4 Myocardial infarction.
We conducted subgroup analyses using the prespecified subgroups, but could not perform a subgroup analysis by concentration of inhaled nitrous oxide, as all included studies in this analysis used a high concentration. No significant differences were detected for the subgroup analyses by type of surgery (Analysis 1.13), test for subgroup differences: Chi² test = 2.55, df = 2, P = 0.28, I² statistic = 21.5%. The test for subgroup differences was not applicable when we performed subgroup analysis by interventions in the nitrous oxide‐free group. The results showed no significant difference between nitrous oxide‐based group and volatile anaesthetic‐based group on myocardial infarction (OR 0.96, 95% CI 0.37 to 2.53, I² statistic = 17%, P value = 0.94; four studies, 242 participants; Analysis 1.25).
1.13. Analysis.
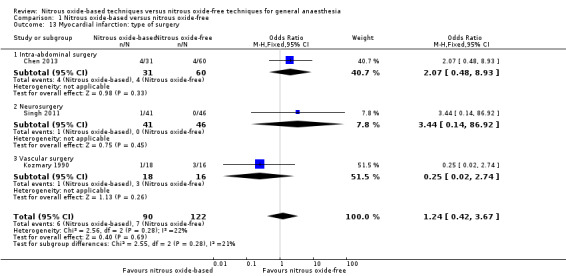
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 13 Myocardial infarction: type of surgery.
1.25. Analysis.

Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 25 Myocardial infarction: techniques used in the nitrous oxide‐free group.
We performed a sensitivity analysis including only studies at low risk of bias (ENIGMA II trial 2014; ENIGMA trial 2007), which suggested that the results of meta‐analysis were robust.
The sensitivity analysis excluding studies with more than 10% withdrawals (Singh 2011) suggested that the results of meta‐analysis were robust.
3. Neurological complications
3.1 Stroke
Four studies reported stroke and together included 9142 participants, 65.9% of the total number of participants included in this review (Deleu 2000; ENIGMA II trial 2014; ENIGMA trial 2007; Singh 2011). Regarding randomization, 4565 (49.9%) were randomized to a nitrous oxide‐based technique and 4577 (50.1%) to a nitrous oxide‐free technique. Thirty‐two participants developed stroke in the nitrous oxide group (0.7%), versus 22 in the nitrous oxide‐free group (0.5%). Pooling of the data showed this small difference was not statistically significant. The OR for the outcome of stroke was 1.47 (95% CI 0.86 to 2.53, P = 0.16) when nitrous oxide was compared with control, with four studies consisting of 9142 participants being analysed (Analysis 1.5). As the 95% CI of OR included both 1.0 and 1.25, we downgraded the quality of the evidence for this outcome from high to moderate quality due to 'imprecision'.
1.5. Analysis.
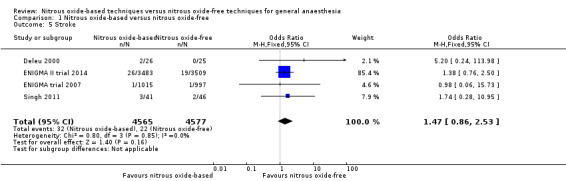
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 5 Stroke.
We performed subgroup analyses using the prespecified subgroups, and no significant differences were detected for the following subgroup analyses: type of surgery (Analysis 1.14), test for subgroup differences: Chi² test = 0.36, df = 1, P = 0.55; intervention in the nitrous oxide‐free group (Analysis 1.26), test for subgroup differences: Chi² test = 0.36, df = 1, P value = 0.55. The test for subgroup differences was not applicable when we performed subgroup analysis by concentrations of inhaled nitrous oxide. The results showed no significant difference between high‐concentration nitrous oxide‐based group and nitrous oxide‐free group on stroke (OR 1.39, 95% CI 0.80 to 2.42; I² statistic = 0%, P value = 0.24; three studies, 9091 participants; Analysis 1.19).
1.14. Analysis.
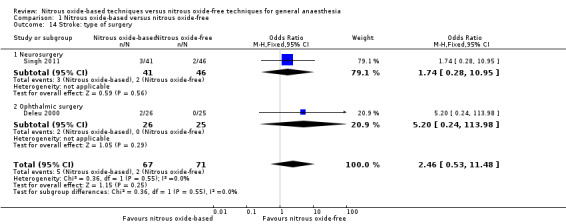
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 14 Stroke: type of surgery.
1.26. Analysis.
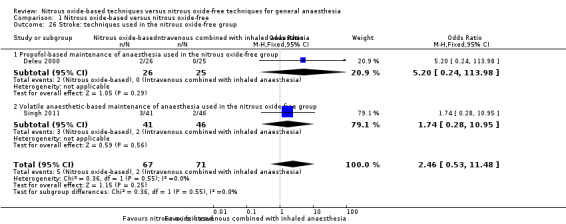
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 26 Stroke: techniques used in the nitrous oxide‐free group.
1.19. Analysis.
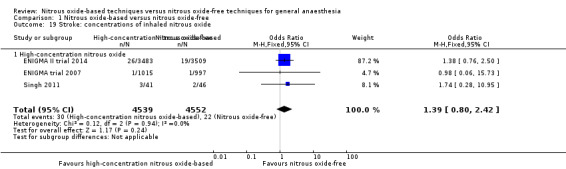
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 19 Stroke: concentrations of inhaled nitrous oxide.
The sensitivity analysis just including the studies of low risk of bias (ENIGMA II trial 2014; ENIGMA trial 2007) suggested that the results of meta‐analysis were robust.
The sensitivity analysis excluding studies with more than 10% withdrawals (Deleu 2000; Singh 2011) suggested that the results of meta‐analysis were robust.
4. Other complications
4.1 Severe nausea and vomiting
Ten studies reported severe nausea and vomiting and together included 11,045 participants, 79.6% of the total number of participants included in this Cochrane review (Arellano 2000; Mraovic 2008; ENIGMA II trial 2014; ENIGMA trial 2007; Paredi 1994; Pedersen 1993; Sengupta 1988; Short 1985; Vanacker 1999; Van Hemelrijck 1991). Of the included participants, 5579 (50.5%) were randomized to a nitrous oxide‐based technique and 5466 (49.5%) to a nitrous oxide‐free technique. Seven hundred and ninety participants had severe nausea and vomiting in the nitrous oxide group (14.2%), versus 518 in the nitrous oxide‐free group (9.5%). Pooling of the data showed this small difference was not statistically significant. The OR for the outcome of severe nausea and vomiting was 1.44 (95% CI 0.97 to 2.15, P = 0.07) when nitrous oxide was compared with control (Analysis 1.6). As serious risk of bias and substantial heterogeneity existed among included studies, we downgraded the quality of the evidence for this outcome from high to low quality due to 'risk of bias' and 'inconsistency'.
1.6. Analysis.
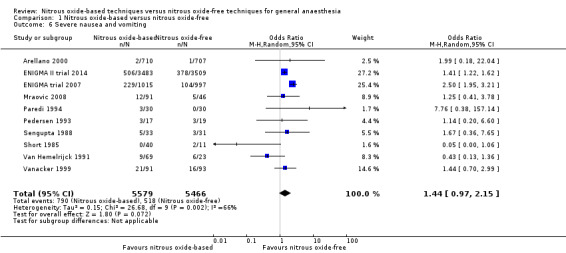
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 6 Severe nausea and vomiting.
We ran subgroup analyses using the prespecified subgroups, and no significant differences were detected for the following subgroup analyses: type of surgery (Analysis 1.15), test for subgroup differences: Chi² test = 2.94, df = 2, P = 0.23); concentration of inhaled nitrous oxide (Analysis 1.20), test for subgroup differences: Chi² test = 0.01, df = 1, P = 0.94); intervention in the nitrous oxide‐free group (Analysis 1.27), test for subgroup differences: Chi² test = 0.22, df = 1, P = 0.64).
1.15. Analysis.
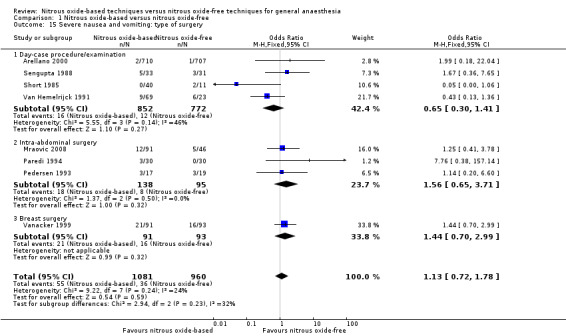
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 15 Severe nausea and vomiting: type of surgery.
1.20. Analysis.
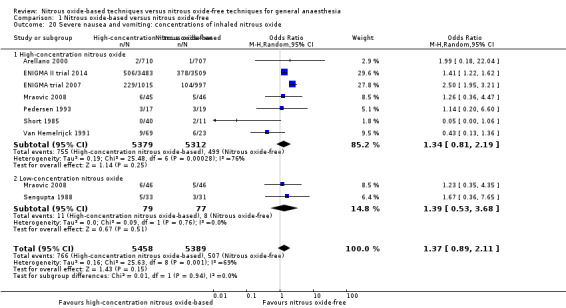
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 20 Severe nausea and vomiting: concentrations of inhaled nitrous oxide.
1.27. Analysis.
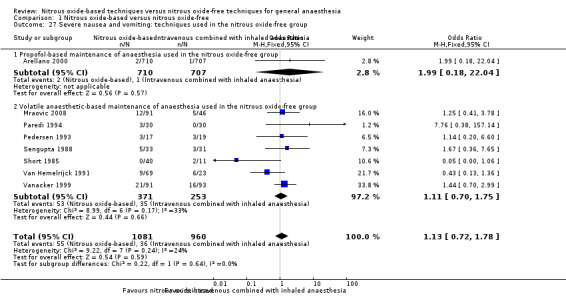
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 27 Severe nausea and vomiting: techniques used in the nitrous oxide‐free group.
The sensitivity analysis just including the studies of low risk of bias (Arellano 2000; ENIGMA II trial 2014; ENIGMA trial 2007) suggested that the results of meta‐analysis were not robust. The results changed from OR 1.44 (95% CI 0.97 to 2.15) to OR 1.86 (95% CI 1.10 to 3.16).
The sensitivity analysis excluding studies with more than 10% withdrawals (Pedersen 1993; Sengupta 1988; Short 1985) suggested that the results of meta‐analysis were not robust. The results changed from OR 1.44 (95% CI 0.97 to 2.15) to OR 1.54 (95% CI 1.02 to 2.33).
Substantial heterogeneity was found in the outcome (Chi² test = 26.68, df = 9; P = 0.002, I² statistic = 66%) and seemed largely attributable to type of surgery and techniques used in the nitrous oxide‐free group.
As the outcome included 10 studies, we generated a funnel plot. The visual inspection of the funnel plot (Figure 4) did not show asymmetry. Egger's test was not statistically significant (P = 0.64).
4.
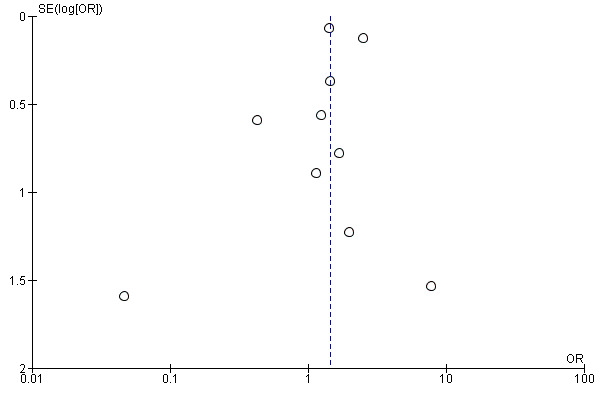
Funnel plot of comparison: 1 Nitrous oxide‐based versus nitrous oxide‐free, outcome: 1.6 Severe nausea and vomiting.
4.2 Venous thromboembolism
Two studies reported venous thromboembolism and together included 9004 participants, 64.9% of the total number of participants included in this review (ENIGMA II trial 2014; ENIGMA trial 2007). Of the included participants, 4498 (50%) were randomized to a nitrous oxide‐based technique and 4506 (50%) to a nitrous oxide‐free technique. Twenty‐eight participants developed venous thromboembolism in the nitrous oxide group (0.6%), versus 38 in the nitrous oxide‐free group (0.8%). Pooling of the data showed this small difference was not statistically significant. The OR for the outcome of venous thromboembolism was 0.73 (95% CI 0.45 to 1.20, P = 0.21) when nitrous oxide was compared with control (Analysis 1.7). As the 95% CI of OR included both 1.0 and 0.75, we downgraded the quality of the evidence for this outcome from high to moderate quality for 'imprecision'.
1.7. Analysis.
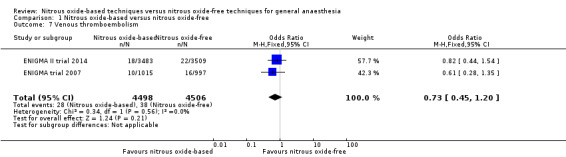
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 7 Venous thromboembolism.
We could not perform subgroup analyses by type of surgery or intervention in the nitrous oxide‐free group, as these were not reported in the studies. Nor could we perform subgroup analysis by concentrations of inhaled nitrous oxide, as all included studies in this analysis used a high concentration.
As all the two studies were of high quality and had < 10% withdrawals, we did not conduct the sensitivity analysis.
4.3 Wound infection rate
Six studies reported wound infection rate and together included 9789 participants, 70.6% of the total number of participants included in this review (Chen 2013; Eger 1990; Fleischmann 2005; Lampe 1990; ENIGMA II trial 2014; ENIGMA trial 2007). Of these participants, 4874 (49.8%) were randomized to a nitrous oxide‐based technique and 4915 (50.2%) to a nitrous oxide‐free technique. Regarding wound infection, 471 participants developed wound infection in the nitrous oxide group (9.7%), versus 434 in the nitrous oxide‐free group (8.8%). Pooling of the data showed this small difference was not statistically significant. The OR for the outcome of wound infection rate was 1.22 (95% CI 0.84 to 1.78, P = 0.30) when nitrous oxide was compared with control (Analysis 1.8). As the 95% CI of OR included both 1.0 and 0.75 as well as substantial heterogeneity existed among included studies, we downgraded the quality of the evidence for this outcome from high to low quality for 'imprecision' and 'inconsistency'.
1.8. Analysis.
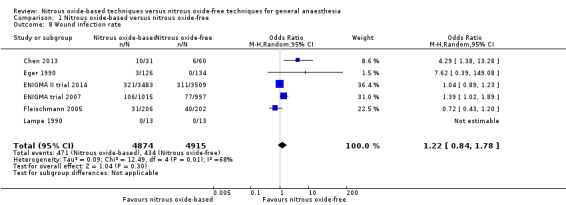
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 8 Wound infection rate.
We ran subgroup analyses using the prespecified subgroups, but could not conduct a subgroup analysis by concentration of inhaled nitrous oxide, as all included studies in this analysis used a high concentration. The test for subgroup differences was not applicable when we performed subgroup analysis by type of surgery or interventions in the nitrous oxide‐free group. The subgroup analysis by types of surgery showed no significant difference between the two groups for intra‐abdominal surgery (OR 1.63, 95% CI 0.28 to 9.33, I² statistic = 87%, P = 0.58; two studies, 499 participants). The subgroup analysis for neurosurgery was not applicable for no wound infection being reported in either nitrous oxide‐based or nitrous oxide‐free group (Analysis 1.16). The subgroup analysis by interventions in the nitrous oxide‐free group showed no significant difference between nitrous oxide‐based group and volatile anaesthetic‐based group (OR 2.13, 95% CI 0.44 to 10.22; I² statistic = 80%, P = 0.34; four studies, 785 participants; Analysis 1.28).
1.16. Analysis.
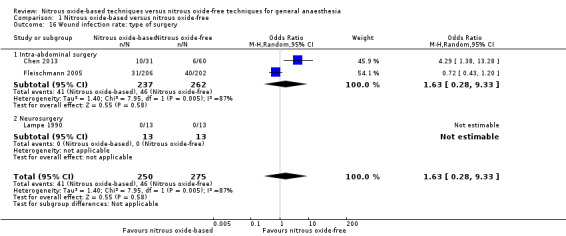
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 16 Wound infection rate: type of surgery.
1.28. Analysis.

Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 28 Wound infection rate: techniques used in the nitrous oxide‐free group.
We performed sensitivity analysis including only the studies at low risk of bias (ENIGMA II trial 2014; ENIGMA trial 2007; Fleischmann 2005), which suggested that the results of meta‐analysis were robust.
As all six studies had less than 10% withdrawals, we did not conduct the sensitivity analysis excluding studies with more than 10% withdrawals.
We found substantial heterogeneity in the trials that reported this outcome (Chi² test = 12.49, df = 4; P = 0.01, I² statistic = 68%), which did not seem to be attributable to type of surgery, concentrations of inhaled nitrous oxide, intervention in the nitrous oxide‐free group, or methodological quality of the included studies.
5. Length of stay
5.1 Length of hospital stay
Thirteen studies reported length of hospital stay (Chen 2013; Eger 1990; Fleischmann 2005; Jensen 1992; Jensen 1993a; Krogh 1994; Lampe 1990; Leung 2006; ENIGMA II trial 2014; ENIGMA trial 2007; Myles 2008a; Singh 2011; Todd 1993). Five studies reported the data as median (interquartile range) (ENIGMA II trial 2014; ENIGMA trial 2007; Jensen 1993a; Krogh 1994; Todd 1993) and two studies reported it as a median (range) value (Jensen 1992; Singh 2011). Only six studies reported the data as mean (SD), and together included 1103 participants, 8.0% of the total number of participants included in this review (Chen 2013; Eger 1990; Fleischmann 2005; Lampe 1990; Leung 2006; Myles 2008a). Of these participants, 546 (49.5%) were randomized to a nitrous oxide‐based technique and 557 (50.5%) to a nitrous oxide‐free technique. Pooling of the data showed no significant difference in the outcome between groups. The MD for the outcome of length of hospital stay was 0.36 days (95% CI ‐0.69 to 1.40 days, P = 0.50) when nitrous oxide was compared with control (Analysis 1.9). Due to the substantial heterogeneity between included studies, we downgraded the quality of the evidence for this outcome from high to moderate quality for 'inconsistency'.
1.9. Analysis.
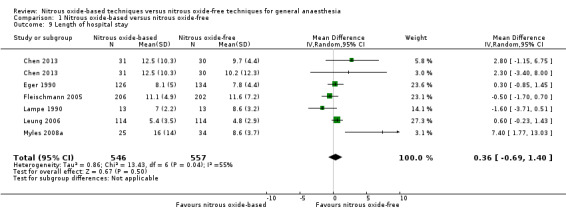
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 9 Length of hospital stay.
We conducted subgroup analyses using the prespecified subgroups, and no significant differences were detected for the subgroup analysis by type of surgery, test for subgroup differences: Chi² test = 1.46, df = 1, P = 0.23). The test for subgroup differences was not applicable when we performed subgroup analysis by concentration of inhaled nitrous oxide and interventions in the nitrous oxide‐free group. The subgroup analysis by concentrations of inhaled nitrous oxide showed no significant difference between high‐concentration nitrous oxide‐based group and nitrous oxide‐free group (MD 0.45 days, 95% CI ‐1.03 to 1.93 days; I² statistic = 59%, P = 0.55; six studies, 875 participants; Analysis 1.21). The subgroup analysis by interventions in the nitrous oxide‐free group showed no significant difference between nitrous oxide‐based group and volatile anaesthetic‐based group (MD 0.20 days, 95% CI ‐0.36 to 0.75 days, I² statistic = 31%, P = 0.49; five studies, 1013 participants; Analysis 1.29).
1.21. Analysis.
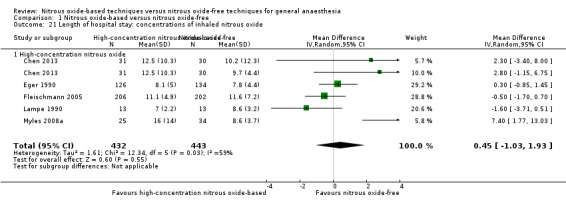
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 21 Length of hospital stay: concentrations of inhaled nitrous oxide.
1.29. Analysis.
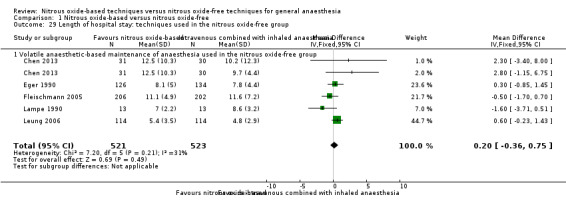
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 29 Length of hospital stay: techniques used in the nitrous oxide‐free group.
The sensitivity analysis including only the studies of low risk of bias (Fleischmann 2005; Leung 2006) suggested that the results of meta‐analysis were robust.
As all the six studies had less than 10% withdrawals, we did not conduct sensitivity analysis excluding studies with more than 10% withdrawals.
We observed substantial heterogeneity for this outcome (Chi² test = 13.43, df = 6; P value = 0.04, I² statistic = 55%) which seemed largely attributable to type of surgery and techniques used in the nitrous oxide‐free group.
5.2. Length of ICU stay
Two studies reported length of ICU stay (ENIGMA trial 2007; Singh 2011). ENIGMA trial 2007 provided only the medians of the ICU stay, but no interquartile ranges. We contacted the study authors via email but found the data were skewed. Singh 2011 reported the data of the ICU stay as median (range) values. Therefore, we did not pool the data. Both trials reported no significant difference in the length of ICU stay between nitrous oxide‐based group and nitrous oxide‐free group.
Discussion
Summary of main results
We included a total of 35 trials; seven of which were of low risk of bias (Akca 2004; Arellano 2000; ENIGMA II trial 2014; ENIGMA trial 2007; Fleischmann 2005; Lee 2005; Leung 2006). The meta‐analyses revealed that nitrous oxide‐based techniques, compared with nitrous oxide‐free techniques, increased the incidence of pulmonary atelectasis but showed no difference in the inhospital case fatality rate, the incidence of pneumonia, myocardial infarction, stroke, severe nausea and vomiting, venous thromboembolism, wound infection, or the length of hospital stay. Compared with nitrous oxide‐free techniques, high‐concentration nitrous oxide‐based techniques increased the incidence of pulmonary atelectasis. Compared with either propofol‐based or volatile anaesthetic‐based anaesthesia, nitrous oxide‐based techniques had no significant effects on the inhospital case fatality rate, complications, or length of stay. The sensitivity analyses suggested that the results of meta‐analyses were all robust except for the outcomes of pneumonia and severe nausea and vomiting.
Overall completeness and applicability of evidence
We included 13,872 adult participants, who were of different ASA status undergoing different surgeries. We compared different concentrations of nitrous oxide with nitrous oxide‐free anaesthesia, and also compared nitrous oxide‐based anaesthesia with either propofol‐based maintenance of anaesthesia or volatile anaesthetic‐based maintenance of anaesthesia. We paid more attention to endpoints and patient‐important outcomes in addressing the question as to whether nitrous oxide was responsible for clinically significant adverse events following general anaesthesia. The meta‐analyses results suggest that nitrous oxide results in more complications. Since the use of nitrous oxide in patients undergoing surgery remains near‐routine (de Vasconcellos 2013), this systematic review may have a wide impact on the conduct of general anaesthesia.
Quality of the evidence
We included 35 RCTs, of which only 16 trials described the methods for randomization and only 12 concealed the allocation sequence. Regarding blinding, eight trials reported they blinded participants and personnel, while 25 trials reported they blinded the outcome assessors. Only seven of the 35 included trials were at low risk of bias.
We identified substantial heterogeneity in the outcomes of severe nausea and vomiting, wound infection rate, and hospital stay, so we downgraded the quality of evidence for inconsistency.
As the 95% CIs of ORs were wide for the outcomes of inhospital case fatality rate, pneumonia, stroke, venous thromboembolism, and wound infection rate, we downgraded the quality of evidence for these outcomes due to imprecision.
Finally, the quality of the evidence for two outcomes (pulmonary atelectasis, myocardial infarction) was rated as high, four outcomes (inhospital case fatality rate, stroke, venous thromboembolism, and length of hospital stay) as moderate, and three (pneumonia, severe nausea and vomiting, wound infection rate) as low; see Table 1 and Table 2.
Potential biases in the review process
We conducted this Cochrane review following the guidelines recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to minimize bias. However, there are two issues that should be of concern. Firstly, we were unable to obtain the full texts of eight publications through either our university library, the Danish National Library, or Cochrane Anaesthesia, Critical or Emergency Care Group members, so we may have missed potential eligible studies. Therefore selection bias may exist in our systematic review. Secondly, substantial heterogeneity was found in the outcome 'wound infection rate', which was not explained by either subgroup analyses or sensitivity analyses. The heterogeneity seemed inexplicable, and we pooled the data using a random‐effects model, which downgraded our confidence in this result.
Agreements and disagreements with other studies or reviews
In this Cochrane review we compared nitrous oxide‐based techniques with nitrous oxide‐free techniques on adult surgical participants, to determine whether nitrous oxide was responsible for clinically significant adverse events following general anaesthesia and whether nitrous oxide could be avoided. There are also three systematic reviews comparing general anaesthesia techniques with or without nitrous oxide but they focus on postoperative nausea and vomiting and intraoperative awareness. Two of these systematic reviews were published in 1996 (Divatia 1996; Tramèr 1996). Tramèr 1996 analysed the data on 2,478 participants from 24 studies and concluded that omitting nitrous oxide from general anaesthetics significantly decreased the incidence of postoperative vomiting for patients at high risk of vomiting preoperatively, but had no effect on the incidence of nausea. They also found that omitting nitrous oxide increased the risk of intraoperative awareness. Divatia 1996 included 26 trials and reported that omission of nitrous oxide reduced the odds of postoperative nausea and vomiting by 37%, a reduction in risk of 28%. Fernández‐Guisasola 2010 is another systematic review, and unlike the former systematic reviews, Fernández‐Guisasola 2010 excluded paediatric reports. The authors included 30 studies with 4598 adult participants, and concluded that avoiding nitrous oxide reduces the risk of postoperative nausea and vomiting, especially in women, but the overall impact was modest. In this Cochrane review we also evaluated the effects of nitrous oxide on postoperative nausea and vomiting. However, we focused on the incidence of severe nausea and vomiting. We found that avoiding nitrous oxide may have no effects on the incidence of severe nausea and vomiting, but the sensitivity analysis suggested that the result was not robust. Imberger 2014 conducted a systematic review with meta‐analysis and trial sequential analysis, focusing on the effects of nitrous oxide on mortality and cardiovascular morbidity. The authors analysed the data of 13 trials and found that nitrous oxide did not affect either short term (within 30 days after operation) or long term (starting from 30 days after operation) mortality. However, trial sequential analysis demonstrated that the data were far too sparse to make any conclusions. They did not perform meta‐analysis for cardiovascular complications (i.e. stroke, myocardial infarct, pulmonary embolus, cardiac arrest) due to insufficient data. Consistent with Imberger 2014, we also found that nitrous oxide‐based anaesthesia resulted in similar inhospital mortality compared with nitrous oxide‐free anaesthesia. Moreover, we pooled the data of cardiovascular complications (i.e. myocardial infarction). The results showed no significant difference in the outcome between groups. The beneficial effects were also explored by several studies. When used as one component of general anaesthesia, nitrous oxide enables a reduction in the requirements for other agents, which are usually more expensive and could have other side effects (Becker 2008). Moreover, a follow‐up study showed that nitrous oxide reduced the risk of persistent pain after surgery (Chan 2011). These outcomes were not assessed in our Cochrane review but should be taken into consideration in clinical practice.
Authors' conclusions
Implications for practice.
This Cochrane review shows that adding nitrous oxide in general anaesthesia increases the risk of pulmonary atelectasis and may potentially increase the incidence of pneumonia and severe nausea and vomiting. However, it also reveals that nitrous oxide neither increases the risk of death, myocardial infarction, stroke, venous thromboembolism, wound infection, nor prolongs the hospital stay. Given the evidence from this review, avoidance of nitrous oxide may be reasonable in participants with pre‐existing poor pulmonary function or at high risk of postoperative nausea and vomiting.
Implications for research.
Most of the included studies did not report the methods for randomization, allocation concealment, or blinding, which made it difficult for us to determine their methodological quality. Future studies would benefit from improved reporting, and we strongly recommend that future studies be reported according to the CONSORT statement (Consolidated Standards of Reporting Trials) (www.consort‐statement.org).
To improve research transparency and ultimately strengthen the validity and value of the scientific evidence base, study authors are encouraged to register their clinical trials in the registry platform. However in this systematic review, only two included trials were registered (ENIGMA II trial 2014; ENIGMA trial 2007). This should be improved in any future studies.
In this systematic review we focused on endpoints and patient‐important outcomes, but some studies did not report them, and so we excluded them from quantitative synthesis. Outcome reporting is another concern in future studies.
Many outcomes we focused on had a low incidence and were downgraded for 'imprecision'. Large‐scale, multicentre studies are still needed to enable us to draw a reliable conclusion. Another approach of study design may be to establish prospective registries or a multi‐database for a large cohort (Khan 2013).
Another suggestion for future studies is that they should pay more attention to the outcome of economic factors, such as total costs of hospitalization and costs of nursing after discharge. It could answer the question whether adding nitrous oxide reduces the total costs of hospitalization or not.
Acknowledgements
We thank the following people:
All the participants and clinical researchers who were involved in the publications mentioned in this Cochrane review.
The Cochrane Anaesthesia, Critical and Emergency Care Group for the support that they have provided, especially Jane Cracknell for her enthusiastic support from the very beginning and her careful and constructive advice throughout.
Mike Bennett (content editor), Cathal Walsh (statistical editor), Mark D Neuman, Kate Leslie (peer reviewers), and Robert Wyllie (consumer referee) for their help and editorial advice during the preparation of this systematic review.
Alejandro Gonzalez Garay, Anna Lee, Anthony Messina, Brenda NG Silva, Cristina Martinelli, Davide Chiumello, Ingrid Arévalo Rodriguez, Marc Van de Velde, Massimo Lamperti, Melissa Giraldo Duque, Michael Holzer, Miguel Coral Pabon, Mukadder Orhan Sungur, Rintaro Mori, Stephan Kettner, and Yuu Tanaka for providing full‐text papers.
Bernard Coronel, Brenda NG Silva, Christian Byhann, Federico Bilotta, Martin Hellmich, Massimo Lamperti, Mukadder Orhan Sungur, Nicola Petrucci, Patrick Brass, Souhayl Dahmani, Hongliang Tian, and Wenyu Zhao for help in abstracting data from papers.
Professor Paul Myles for providing additional data from the ENIGMA trial 2007.
Yi Kang, Li Lun, and Jiang Lei for their help and advice during the preparation of this Cochrane review.
Appendices
Appendix 1. CENTRAL, the Cochrane Library
#1MeSH descriptor: [Nitrous Oxide] explode all trees #2(laughing gas or nitrous oxide or dinitrogen monoxide or dinitrogen oxide or factitious air or hyponitrous acid anhydride or nitrogen protoxide or N2O):ti,ab #3#1 or #2 #4MeSH descriptor: [Anesthesia, General] explode all trees #5general an?esth*:ti,ab #6surg*:ti,ab #7MeSH descriptor: [General Surgery] explode all trees #8(#4 or #5) and (#6 or #7) and #3
Appendix 2. MEDLINE (Ovid SP)
#1 exp Nitrous oxide/ or (laughing gas or nitrous oxide or dinitrogen monoxide or dinitrogen oxide or factitious air or hyponitrous acid anhydride or nitrogen protoxide or N2O).ti,ab. #2 (General anesthesia/ or general an?esthesia.mp.) and (General surgery/ or surg*.mp.) #3 ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. #4 #1 and #2 and #3
Appendix 3. EMBASE (Ovid SP)
#1 exp nitrous oxide/ or (laughing gas or nitrous oxide or dinitrogen monoxide or dinitrogen oxide or factitious air or hyponitrous acid anhydride or nitrogen protoxide or N2O).ti,ab. #2 (general anesthesia/ or general an?esthesia.ti,ab.) and (general surgery/ or surg*.ti,ab.) #3 (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh. #4 #1 and #2 and #3
Appendix 4. ISI Web of Science
TS=(laughing gas or nitrous oxide or dinitrogen monoxide or dinitrogen oxide or factitious air or hyponitrous acid anhydride or nitrogen protoxide or N2O) and TS=((general an?esth*) and surg*) and TS=(random* or (trial* SAME (control* or clinical)) or placebo* or multicenter* or prospective or ((blind* or mask*) SAME (single or double or triple or treble)))
Appendix 5. Data extraction form
Study selection form
| First author | Journal/Conference proceedings etc | Year |
Study eligibility
| RCT | Relevant participants (age over 18 years, having standard general anaesthesia) | Relevant interventions (nitrous oxide‐based throughout duration of anaesthesia compared with nitrous oxide‐free) | Relevant outcomes: Inhospital case fatality rate, pulmonary complications (pneumonia and pulmonary atelectasis), heart complications (myocardial infarction), neurological complications (stroke), other complications (venous thromboembolism, wound infection rate and severe nausea and vomiting), length of stay (length of hospital stay and length of ICU stay) |
| Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear |
Issue relates to selective reporting ‐ when study authors may have taken measurements for particular outcomes, but not reported these within the paper(s). Review authors should contact study authors for information on possible non‐reported outcomes and reasons for exclusion from publication. Study should be listed in 'Studies awaiting assessment' section until clarified. If no clarification is received after three attempts, the review authors should exclude the study.
| Do not proceed if any of the above answers are "No". If study is to be included in the 'Excluded studies' section of the review, record below the information to be inserted into 'Table of excluded studies' section. |
References to trial
Check other references identified in searches. If there are further references to this trial link the papers now and list below. All references to a trial should be linked under one Study ID in RevMan 5.3.
| Code each paper | Author(s) | Journal/Conference proceedings etc | Year |
| A | The paper listed above | ||
| B | Further papers |
Participants and trial characteristics
| Participant and trial characteristics | |
| Characteristics | Further details |
| Age (mean, median, range, etc) | |
| Number of participants in each intervention group | |
| Sex of participants (numbers/%, etc) | |
| Disease status/type, etc? (if applicable) | |
| ASA physical status classification | |
| Type of surgery | |
| Single centre/multi‐centre | |
| Country/Countries | |
| Number of participants who were analysed | |
| Trial design (e.g. parallel/cross‐over*) | |
| Other | |
Abbreviations: ASA: American Society of Anesthesiologists.
Details of intervention
| Groups | Details of intervention |
| Nitrous oxide based group(concentration of inhaled N2O, O2 separately, duration of inhaled N2O) | |
| Nitrous oxide‐free group (inhaled gas, concentration of inhaled O2) |
Methodological quality
| Allocation of intervention | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| Comment on allocation by review authors or included study quote concerning allocation | Adequate (random) |
| Inadequate (e.g. alternate) | |
| Unclear | |
| Concealment of allocation | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| Comment on allocation by review authors or included study quote concerning allocation | Adequate (random) |
| Inadequate (e.g. alternate) | |
| Unclear | |
| Blinding | |
| Person responsible for participants' care | Yes/No |
| Participant | Yes/No |
| Outcome assessor | Yes/No |
| Other (please specify) | Yes/No |
| Intention‐to‐treat | |
| All participants entering trial | |
| 15% or fewer excluded | |
| More than 15% excluded | |
| Not analysed as "intention‐to‐treat" | |
| Unclear | |
Were withdrawals described? Yes/No/Unclear
Discuss if appropriate
| For "duration of stay" data | |||||||
| Code of paper | Outcomes | Unit of measurement | N2O‐based group | N2O‐free group | Details if outcome only described in text | ||
| n | Mean | n | Mean | ||||
| Secondary outcomes | |||||||
| Duration of hospital stay | Yes/No | ||||||
| Duration of ICU stay | |||||||
| For dichotomous data | |||
| Code of paper | Outcomes | N2O‐based group(n) n = number of participants, not number of events | N2O‐free group(n) n = number of participants, not number of events |
| Primary outcomes | |||
| Inhospital case fatality rate | |||
| Secondary outcomes | |||
| Pneumonia | |||
| Pulmonary atelectasis | |||
| Myocardial infarction | |||
| Stroke | |||
| Severe nausea and vomiting | |||
| Venous thromboembolism | |||
| Wound infection rate | |||
|
Other information which you feel is relevant to the results Indicate if: any data were obtained from the primary study author; if results were estimated from graphs etc; or calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained this should be made clear here to be cited in review. |
Data and analyses
Comparison 1. Nitrous oxide‐based versus nitrous oxide‐free.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Inhospital case fatality rate | 8 | 10148 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.61, 1.26] |
| 2 Pneumonia | 8 | 2699 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.00, 2.81] |
| 3 Pulmonary atelectasis | 5 | 2400 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.18, 2.10] |
| 4 Myocardial infarction | 6 | 9246 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.84, 1.22] |
| 5 Stroke | 4 | 9142 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.86, 2.53] |
| 6 Severe nausea and vomiting | 10 | 11045 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.97, 2.15] |
| 7 Venous thromboembolism | 2 | 9004 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.45, 1.20] |
| 8 Wound infection rate | 6 | 9789 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.84, 1.78] |
| 9 Length of hospital stay | 6 | 1103 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.69, 1.40] |
| 10 Inhospital case fatality rate: type of surgery | 4 | 646 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.11, 3.88] |
| 10.1 Intra‐abdominal surgery | 2 | 499 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.41 [0.06, 3.03] |
| 10.2 Neurosurgery | 2 | 147 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.45 [0.07, 287.21] |
| 11 Pneumonia: type of surgery | 6 | 427 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.48, 3.48] |
| 11.1 Intra‐abdominal surgery | 3 | 193 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.33, 3.78] |
| 11.2 Neurosurgery | 3 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.30, 9.81] |
| 12 Pulmonary atelectasis: type of surgery | 3 | 128 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.06] |
| 12.1 Intra‐abdominal surgery | 2 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.06] |
| 12.2 Neurosurgery | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Myocardial infarction: type of surgery | 3 | 212 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.42, 3.67] |
| 13.1 Intra‐abdominal surgery | 1 | 91 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.48, 8.93] |
| 13.2 Neurosurgery | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.44 [0.14, 86.92] |
| 13.3 Vascular surgery | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.02, 2.74] |
| 14 Stroke: type of surgery | 2 | 138 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.53, 11.48] |
| 14.1 Neurosurgery | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.28, 10.95] |
| 14.2 Ophthalmic surgery | 1 | 51 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.20 [0.24, 113.98] |
| 15 Severe nausea and vomiting: type of surgery | 8 | 2041 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.72, 1.78] |
| 15.1 Day‐case procedure/examination | 4 | 1624 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.30, 1.41] |
| 15.2 Intra‐abdominal surgery | 3 | 233 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.65, 3.71] |
| 15.3 Breast surgery | 1 | 184 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.70, 2.99] |
| 16 Wound infection rate: type of surgery | 3 | 525 | Odds Ratio (M‐H, Random, 95% CI) | 1.63 [0.28, 9.33] |
| 16.1 Intra‐abdominal surgery | 2 | 499 | Odds Ratio (M‐H, Random, 95% CI) | 1.63 [0.28, 9.33] |
| 16.2 Neurosurgery | 1 | 26 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Length of hospital stay: type of surgery | 3 | 556 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐1.44, 0.54] |
| 17.1 Intra‐abdominal surgery | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐1.25, 1.00] |
| 17.2 Neurosurgery | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐3.71, 0.51] |
| 18 Inhospital case fatality rate: concentrations of inhaled nitrous oxide | 7 | 9920 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.60, 1.24] |
| 18.1 High‐concentration nitrous oxide | 7 | 9920 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.60, 1.24] |
| 19 Stroke: concentrations of inhaled nitrous oxide | 3 | 9091 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.80, 2.42] |
| 19.1 High‐concentration nitrous oxide | 3 | 9091 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.80, 2.42] |
| 20 Severe nausea and vomiting: concentrations of inhaled nitrous oxide | 8 | 10847 | Odds Ratio (M‐H, Random, 95% CI) | 1.37 [0.89, 2.11] |
| 20.1 High‐concentration nitrous oxide | 7 | 10691 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.81, 2.19] |
| 20.2 Low‐concentration nitrous oxide | 2 | 156 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.53, 3.68] |
| 21 Length of hospital stay: concentrations of inhaled nitrous oxide | 5 | 875 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐1.03, 1.93] |
| 21.1 High‐concentration nitrous oxide | 5 | 875 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐1.03, 1.93] |
| 22 Inhospital case fatality rate: techniques used in the nitrous oxide‐free group | 6 | 1144 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.33 [0.29, 6.00] |
| 22.1 Propofol‐based maintenance of anaesthesia used in the nitrous oxide‐free group | 1 | 121 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.45 [0.07, 287.21] |
| 22.2 Volatile anaesthetic‐based maintenance of anaesthesia used in the nitrous oxide‐free group | 5 | 1023 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.22, 5.58] |
| 23 Pneumonia: techniques used in the nitrous oxide‐free group | 7 | 687 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.45, 2.86] |
| 23.1 Propofol‐based maintenance of anaesthesia used in the nitrous oxide‐free group | 2 | 181 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.07, 3.61] |
| 23.2 Volatile anaesthetic‐based maintenance of anaesthesia used in the nitrous oxide‐free group | 5 | 506 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.49, 4.06] |
| 24 Pulmonary atelectasis: techniques used in the nitrous oxide‐free group | 4 | 388 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.09, 1.12] |
| 24.1 Propofol‐based maintenance of anaesthesia used in the nitrous oxide‐free group | 2 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.06] |
| 24.2 Volatile anaesthetic‐based maintenance of anaesthesia used in the nitrous oxide‐free group | 2 | 286 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.12, 4.29] |
| 25 Myocardial infarction: techniques used in the nitrous oxide‐free group | 4 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.37, 2.53] |
| 25.1 Volatile anaesthetic‐based maintenance of anaesthesia used in the nitrous oxide‐free group | 4 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.37, 2.53] |
| 26 Stroke: techniques used in the nitrous oxide‐free group | 2 | 138 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.53, 11.48] |
| 26.1 Propofol‐based maintenance of anaesthesia used in the nitrous oxide‐free group | 1 | 51 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.20 [0.24, 113.98] |
| 26.2 Volatile anaesthetic‐based maintenance of anaesthesia used in the nitrous oxide‐free group | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.28, 10.95] |
| 27 Severe nausea and vomiting: techniques used in the nitrous oxide‐free group | 8 | 2041 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.72, 1.78] |
| 27.1 Propofol‐based maintenance of anaesthesia used in the nitrous oxide‐free group | 1 | 1417 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.18, 22.04] |
| 27.2 Volatile anaesthetic‐based maintenance of anaesthesia used in the nitrous oxide‐free group | 7 | 624 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.70, 1.75] |
| 28 Wound infection rate: techniques used in the nitrous oxide‐free group | 4 | 785 | Odds Ratio (M‐H, Random, 95% CI) | 2.13 [0.44, 10.22] |
| 28.1 Volatile anaesthetic‐based maintenance of anaesthesia used in the nitrous oxide‐free group | 4 | 785 | Odds Ratio (M‐H, Random, 95% CI) | 2.13 [0.44, 10.22] |
| 29 Length of hospital stay: techniques used in the nitrous oxide‐free group | 5 | 1044 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.36, 0.75] |
| 29.1 Volatile anaesthetic‐based maintenance of anaesthesia used in the nitrous oxide‐free group | 5 | 1044 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.36, 0.75] |
1.17. Analysis.
Comparison 1 Nitrous oxide‐based versus nitrous oxide‐free, Outcome 17 Length of hospital stay: type of surgery.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akca 2004.
| Methods | Multi‐centre RCT | |
| Participants |
Setting: two Viennese hospitals Inclusion criteria: ASA I‐III patients, 18 to 80 years of age, scheduled for elective colon resection scheduled to last more than 2 hours Exclusion criteria: patients with bowel obstruction or having minor colon surgery (e.g. polypectomy, isolated colostomy) Participant numbers: 344 randomly assigned; 344 analysed |
|
| Interventions | Intervention: anaesthetic management was standardized. Sodium thiopental (3 to 5 mg/kg) and vecuronium (0.1 mg/kg) were used for induction; anaesthesia subsequently was maintained with isoflurane (0.5 to 1.0% in 65% nitrous oxide), vecuronium, and remifentanil (0.2 mg/kg/min). Control: anaesthetic management was standardized. Sodium thiopental (3 to 5 mg/kg) and vecuronium (0.1 mg/kg) were used for induction; anaesthesia subsequently was maintained with isoflurane (0.5 to 1.0% in air), vecuronium, and remifentanil (0.2 mg/kg/min). | |
| Outcomes | Other outcomes: Bowel distension | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned to one of two groups using a reproducible set of computer‐generated random numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "The assignments were kept in sealed, sequentially numbered opaque envelopes that were opened after induction of anaesthesia." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Great care was exercised to prevent the surgeons from observing the administered gas mixture." |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "The rater was blinded to anaesthesia management." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Alhashemi 1997.
| Methods | Single‐centre RCT | |
| Participants |
Setting: the Ottawa General Hospital, Canada Inclusion criteria: ASA I‐II patients, scheduled to undergo arthroscopic knee surgery, and electing general anaesthesia Exclusion criteria: patient preference for regional anaesthesia; age < 20 or > 60 years; body mass index either < 20 or > 30 kg/m²; current or chronic use of benzodiazepines or other sedative‐hypnotics; excessive alcohol intake; moderate or severe cardiac or respiratory disease; severe or uncontrolled hypertension; known allergy to any of the study medications; or chronic use of drugs known to interfere with the metabolism or clinical effects of the study medications Participant numbers: 93 randomly assigned; 93 analysed |
|
| Interventions | Intervention: patients received nitrous oxide 70% supplemented with isoflurane 0.5 to 1.0% or with intermittent boluses of 7 to 15 μg/kg iv alfentanil every 10 to 15 min. Control: patients received intermittent boluses of 7 to 15 μg/kg iv alfentanil every 10 to 15 min in conjunction with a continuous infusion of propofol. | |
| Outcomes | Other outcomes: Costs of anaesthesia and postoperative care | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated according to a computer generated randomization schedule." |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "Blinding was achieved by precluding the trained observer, who recorded all post‐operative data, from gaining any knowledge of the intra‐operative anaesthetic care." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Arellano 2000.
| Methods | Multi‐centre RCT | |
| Participants |
Setting: Toronto General Hospital, Toronto Western Hospital, North York General Hospital and Women's College Hospital, Canada Inclusion criteria: ASA I‐II patients, aged 18 to 55 years, undergoing termination of pregnancy and laparoscopy Exclusion criteria: patients undergoing other ambulatory gynaecologic procedures; there was a history of psychiatric disease, narcotic/sedative use, drug abuse, or morbid obesity (> 30% above ideal body weight) Participant numbers: 1490 randomly assigned; 1417 analysed |
|
| Interventions | Intervention: for patients undergoing termination of pregnancy, they received fentanyl 0.7 mg/kg intravenously. After denitrogenation of the lungs with 100% oxygen, 20 mg lidocaine and 2.0 mg/kg propofol were infused intravenously over 40 s with further increments of propofol titrated to loss of lid reflex. Nitrous oxide and oxygen 65% to 35% were administered by mask. Anaesthesia was maintained with intermittent bolus doses of 20 mg propofol in response to clinical signs of light anaesthesia (movement, tearing, or phonation in response to surgical stimuli, or increases in blood pressure, pulse rate, or respiratory rate of ≥ 20%). For patients undergoing laparoscopy, they received fentanyl 1.5 mg/kg and d‐tubocurare 3 mg intravenously. After denitrogenation of the lungs with 100% oxygen, 20 mg lidocaine and 2 mg/kg propofol were infused intravenously over 40 s with further increments of propofol titrated to loss of lid reflex. After the administration of succinylcholine 1.5 mg/kg intravenously, subjects were intubated orally. After induction, patients were paralysed with 0.075 to 0.1 mg/kg vecuronium intravenously and mechanically ventilated. Patients received 65% nitrous oxide‐35% oxygen and the anaesthesia was maintained with an infusion of propofol 100 to 200 μg/kg/min supplemented by intermittent bolus doses of 20 mg propofol in response to clinical signs of light anaesthesia (movement or tearing in response to surgical stimuli or increases in blood pressure, or pulse rate of ≥ 20%). At the end of surgery, neuromuscular blockade was reversed with atropine 0.02 mg/kg and neostigmine 0.04 mg/kg. Control: for patients undergoing termination of pregnancy, they received fentanyl 0.7 mg/kg intravenously. After denitrogenation of the lungs with 100% oxygen, 20 mg lidocaine and 2.0 mg/kg propofol were infused intravenously over 40 s with further increments of propofol titrated to loss of lid reflex. 100% oxygen were administered by mask. Anaesthesia was maintained with intermittent bolus doses of 20 mg propofol in response to clinical signs of light anaesthesia (movement, tearing, or phonation in response to surgical stimuli, or increases in blood pressure, pulse rate, or respiratory rate of ≥ 20%). For patients undergoing laparoscopy, they received fentanyl 1.5 mg/kg and d‐tubocurare 3 mg intravenously. After denitrogenation of the lungs with 100% oxygen, 20 mg lidocaine and 2 mg/kg propofol were infused intravenously over 40 s with further increments of propofol titrated to loss of lid reflex. After the administration of succinylcholine 1.5 mg/kg intravenously, subjects were intubated orally. After induction, patients were paralysed with 0.075 to 0.1 mg/kg vecuronium intravenously and mechanically ventilated. Patients received 100% oxygen and the anaesthesia was maintained with an infusion of propofol 100 to 200 μg/kg/min supplemented by intermittent bolus doses of 20 mg propofol in response to clinical signs of light anaesthesia (movement or tearing in response to surgical stimuli or increases in blood pressure, or pulse rate of ≥ 20%). At the end of surgery, neuromuscular blockade was reversed with atropine 0.02 mg/kg and neostigmine 0.04 mg/kg. | |
| Outcomes | Secondary outcomes: Severe nausea and vomiting: no specific definition | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated by computer‐generated random numbers in blocks of four, and stratification by hospital site and surgical procedure." |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The personnel were not blinded to treatment allocation to ensure safe anaesthetic care. Biased administration of the aesthetics and unblinding of the research assistants were prevented by the following: (1) pre‐enrolment training of anaesthesiologists to standardize anaesthetic administration; (2) random visits by the principal investigator to discuss the anaesthetic protocol with the anaesthesiologists; (3) ongoing review of the anaesthetic study sheets by the principal investigator; (4) restricting the research assistants from access to the operating rooms or patients' charts." |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "Four research assistants blinded to treatment allocation postoperative data." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Badner 2000.
| Methods | Single‐centre RCT | |
| Participants |
Setting: McMaster University, Canada Inclusion criteria: ASA I‐III patients, age > 18 years, presenting for elective carotid endarterectomy Exclusion criteria: patients were excluded if they had received an anaesthetic within 30 days before their scheduled surgery, if they were currently taking medications known to affect plasma homocysteine (vitamins B12 and B6, folic acid, penicillamine, methotrexate, azaurodine, isoniazid, cycloserine, phenelzine, or procarbazine); if they were vitamin B12 or folate deficient, malnourished or cirrhotic; or if they had a pace‐maker or left bundle branch block on electrocardiogram (ECG) Participant numbers: 90 randomly assigned; 86 analysed |
|
| Interventions | Intervention: anaesthesia was maintained with opioid (fentanyl or sufentanil), isoflurane, and nitrous oxide/oxygen (inspired nitrous oxide 50%). Control: anaesthesia was maintained with opioid (fentanyl and sufentanil), isoflurane, and oxygen/air. | |
| Outcomes | Other outcomes: Myocardial ischaemia | |
| Notes | Four patients did not complete the 48‐h study period, two required reoperation for hematoma formation (both non‐nitrous oxide), and two patients had Holter monitoring inappropriately discontinued (one from each group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized using a computer‐generated random number table." |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "Myocardial ischemia was determined by a blinded technician." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Bloomfield 1988.
| Methods | Single‐centre RCT | |
| Participants |
Setting: the University of Colorado Health Science Center, USA Inclusion criteria: ASA I‐II patients, aged 18 to 60 years Participant numbers: 63 randomly assigned; 63 analysed |
|
| Interventions | Intervention: nitrous oxide/oxygen and 0.25% isoflurane with or without sufentanil Control: oxygen and 0.5% isoflurane with or without sufentanil | |
| Outcomes |
Other outcomes: Non‐severe nausea and vomiting |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated using random number tables." |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "Nausea and vomiting were recorded by an observer unaware of the anaesthetic technique used." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Brodsky 2005.
| Methods | Single‐centre RCT | |
| Participants |
Setting: Stanford University Medical Center, USA Inclusion criteria: patients undergoing either laparoscopic Roux‐en‐Y gastric bypass or gastric banding operations Participant numbers: 50 randomly assigned; 50 analysed |
|
| Interventions | Intervention: the lungs of patients were ventilated with the volatile anaesthetic, oxygen (50%) and nitrous oxide (50%). Control: the lungs of patients were ventilated with the volatile anaesthetic, oxygen (50%) and air. | |
| Outcomes |
Other outcomes: Quality of recovery |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomization table was used to assign each patient to one of two groups." |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "The surgeon was blinded as to whether the patient was receiving air or nitrous oxide, was asked whether nitrous oxide was being used." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Chen 2013.
| Methods | Single‐centre RCT | |
| Participants |
Setting: Prince of Wales Hospital, Hong Kong, China Inclusion criteria: ASA I‐IV patients, aged > 18 years, scheduled for elective open colorectal surgery Exclusion criteria: patients with ongoing infection and those with fever in the 24 h before surgery; patients with marked impairment of gaseous exchange; surgery for which primary wound closure; in the opinion of the attending anaesthesiologist that nitrous oxide administration was contraindicated Participant numbers: 93 randomly assigned; 91 analysed |
|
| Interventions | Intervention: anaesthesia was induced with propofol 1 to 2.5 mg/kg. Patients received sevoflurane targeted to achieve a bispectral index value between 40 and 60. Intraoperative analgesia was provided by remifentanil infusion 0.1 to 0.5 μg/kg/min and intravenous morphine 0.1 to 0.15 mg/kg, 30 min before completion. Muscle relaxation was facilitated by rocuronium. The lungs were ventilated through a tracheal tube using 70% nitrous oxide and 30% oxygen. Control: anaesthesia was induced with propofol 1 to 2.5 mg/kg. Patients received sevoflurane targeted to achieve a bispectral index value between 40 and 60. Intraoperative analgesia was provided by remifentanil infusion 0.1 to 0.5 μg/kg/min and intravenous morphine 0.1 to 0.15 mg/kg, 30 min before completion. Muscle relaxation was facilitated by rocuronium. The lungs were ventilated through a tracheal tube using either 30% oxygen with 70% nitrogen or 80% oxygen with 20% nitrogen. | |
| Outcomes |
Primary outcomes: Inhospital case fatality rate Secondary outcomes: Myocardial infarction: The diagnosis of myocardial infarction required any one of the following criteria:
This criterion also required that one of the following must also exist:
Pneumonia: The definition of pneumonia required any one of the following criteria:
Wound infection: diagnosed by ASEPSIS > 20 Length of hospital stay |
|
| Notes | Two patients were excluded after randomization because their surgeries were cancelled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated from a computer‐generated list." |
| Allocation concealment (selection bias) | Low risk | The random sequence was accessed through an intranet system. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Low risk | The outcome measurement is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "The complications were examined by ward medical staff who were unaware of the allocated group identity." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | High risk | Fewer than 50 participants per arm. |
Deleu 2000.
| Methods | Single‐centre RCT | |
| Participants |
Setting: a hospital in Sultanate of Oman Inclusion criteria: patients aged 55 years or above undergoing ophthalmic surgery Exclusion criteria: patients had suffering from any major organ failure; patients had clinical signs or symptoms of cobalamin or folate deficiency; patients had macrocytosis (mean corpuscular volume lower than 96 fl) or anaemia (haematocrit higher than 0.30); or patients had cobalamin and/or folate substitution therapy during the preceding months Participant numbers: 69 randomly assigned; 51 analysed |
|
| Interventions | Intervention: patients were premedicated with midazolam 5 to 7.5 mg by mouth 1 h before being transferred to the operating theatre. Anaesthesia was induced and maintained nitrous oxide‐based with propofol. Control: patients were premedicated with midazolam 5 to 7.5 mg by mouth 1 h before being transferred to the operating theatre. Anaesthesia was induced and maintained nitrous oxide‐free with propofol. | |
| Outcomes |
Secondary outcomes: Stroke: new neurological signs (paralysis, weakness or speech difficulties) that persisted for 24 hours |
|
| Notes | 18 patients were either lost to follow‐up (n = 6), had one or more laboratory values missing (n = 9) or had taken folic acid or cobalamin‐containing vitamins during the interval between surgery and re‐evaluation (n = 3). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind but no further details. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Unclear risk | Described as double‐blind but no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | High risk | Fewer than 50 participants per arm. |
Eger 1990.
| Methods | Single‐centre RCT | |
| Participants |
Setting: a hospital in San Francisco, USA Inclusion criteria: patients scheduled for elective total hip arthroplasty, carotid endarterectomy, or transsphenoidal hypophysectomy Participant numbers: 270 randomly assigned; 260 analysed |
|
| Interventions | Intervention: patients received isoflurane, thiopental, vecuronium and 60% nitrous oxide/40% oxygen. The concentration of isoflurane was determined by the attending anaesthesiologist. Fentanyl and edrophonium/atropine were administered at the anaesthesiologist's discretion. Ventilation was controlled, and total gas flows of 5 L/min were maintained throughout surgery. Control: patients received isoflurane, thiopental, vecuronium and 100% oxygen. The concentration of isoflurane was determined by the attending anaesthesiologist. Fentanyl and edrophonium/atropine were administered at the anaesthesiologist's discretion. Ventilation was controlled, and total gas flows of 5 L/min were maintained throughout surgery. | |
| Outcomes |
Primary outcomes: Inhospital case fatality rate Secondary outcomes: Pneumonia: based on chest x‐ray Pulmonary atelectasis: based on chest x‐ray Myocardial infarction: new abnormalities in postoperative creatine kinase isoenzymes or Q‐wave development Wound infection: determined by the surgeon in the setting of suspected infection. Length of hospital stay |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Low risk | Quote: "All data collection, analysis, and patient interviews were performed by medical personnel blinded to the anaesthetic regimen." |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "All data collection, analysis, and patient interviews were performed by medical personnel blinded to the anaesthetic regimen." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
ENIGMA II trial 2014.
| Methods | Multicentre RCT | |
| Participants |
Setting: 45 participating centres from 10 countries Inclusion criteria:
Exclusion criteria:
Participant numbers: 7112 randomly assigned; 6992 analysed |
|
| Interventions | Intervention: 70% nitrous oxide Control: no nitrous oxide | |
| Outcomes |
Primary outcomes: The primary endpoint is a composite of death and cardiovascular events (clinical and silent MI, cardiac failure, cardiac arrest, pulmonary embolism, and stroke) measured at 30 days after surgery. Secondary outcomes: Myocardial infarction:
Wound infection At least one of the following:
Stroke
Severe nausea and vomiting
Pulmonary embolism
Hospital stay |
|
| Notes | ClinicalTrials.gov identifier: NCT00430989 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done with a computer‐generated code." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization sequence was accessed via an automated telephone voice‐recognition service." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The attending anaesthetists were aware of the patients' group assignments, but the patients, their surgical team were not." |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Low risk | Quote: "The postoperative interviewers, and endpoint adjudicators were unaware of the patients' group assignments." |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "The postoperative interviewers, and endpoint adjudicators were unaware of the patients' group assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | Protocol was registered as NCT00430989 and outcomes were reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
ENIGMA trial 2007.
| Methods | Multi‐centre RCT | |
| Participants |
Setting: 19 hospitals from 6 countries Inclusion criteria: patients were aged 18 years or older, were scheduled to undergo general anaesthesia for surgery that included a skin incision and that was anticipated to exceed 2 h, and were expected to be in the hospital for at least 3 days after surgery Exclusion criteria: patients undergoing cardiac surgery, or thoracic surgery requiring one‐lung ventilation; patients that the anaesthesiologist considered that nitrous oxide was contraindicated (e.g. a history of post‐operative emesis or if the anaesthesiologist wanted to use supplemental oxygen for colorectal surgery) Participant numbers: 2050 randomly assigned; 2012 analysed |
|
| Interventions | Intervention: patients were administered a gas mixture of 70% nitrous oxide with 30% oxygen after induction of anaesthesia and until completion of surgery. Control: patients were administered a gas mixture of 80% oxygen with 20% nitrogen after induction of anaesthesia and until completion of surgery. | |
| Outcomes |
Primary outcomes: Inhospital case fatality rate Secondary outcomes: Pneumonia: radiologic infiltrate confirmed by chest x‐ray or computed tomography, in association with at least one of the following: temperature greater than 38°C, leukocyte count greater than 12,000/mL, or positive sputum culture that was not heavily contaminated with oral flora or that corresponded with positive blood cultures Pulmonary atelectasis: confirmed by chest x‐ray or computed tomography Myocardial infarction: confirmed by ECG and/or troponin or CK‐MB enzyme rise Stroke: a new neurological deficit persisting for 24 hours, confirmed by neurologist assessment and/or computed tomography scan or magnetic resonance imaging Severe nausea and vomiting: at least 2 episodes > 6 hrs apart, or if requiring > 2 doses of antiemetic medication Venous thromboembolism: symptomatic deep venous thrombosis, confirmed by venography, duplex ultrasonography, V‐Q scan or spiral computed tomography, or autopsy Wound infection: if associated with purulent discharge or a positive microbial culture Length of hospital stay Length of ICU stay |
|
| Notes | ClinicalTrials.gov identifier: NCT00164047 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned using a computer‐generated code." |
| Allocation concealment (selection bias) | Low risk | Quote: "The random sequence was accessed via an automated telephone voice recognition service." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All research staff, including those responsible for postoperative data collection and outcome assessment, were precluded by protocol from accessing the anaesthetic record and so were blinded to group identity." |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Low risk | Quote: "All research staff, including those responsible for postoperative data collection and outcome assessment, were precluded by protocol from accessing the anaesthetic record and so were blinded to group identity." |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "All research staff, including those responsible for postoperative data collection and outcome assessment, were precluded by protocol from accessing the anaesthetic record and so were blinded to group identity." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | Protocol was registered as NCT00164047 and outcomes were reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Fleischmann 2005.
| Methods | Multi‐centre RCT | |
| Participants |
Setting: three hospitals in Austria and Hungary Inclusion criteria: 418 ASA I‐III patients, aged 18 to 80 years, scheduled for elective colon resection expected to last more than 2 h Exclusion criteria: patients with acute bowel obstruction or those having minor colon surgery (e.g. polypectomy, isolated colostomy); patients in whom the surgeon did not anticipate primary wound closure; patients with a history of fever or infection within 24 h of surgery Participant numbers: 418 randomly assigned; 408 analysed |
|
| Interventions | Intervention: anaesthetic management was standardized. Thiopental sodium (3 to 5 mg/kg) or propofol (2 to 3 mg/kg), fentanyl (1 to 3 μg/kg), and vecuronium (0.1 mg/kg) or rocuronium (0.6 mg/kg) were used for induction; anaesthesia was maintained with isoflurane (0.6%) in 65% nitrous oxide, with vecuronium or rocuronium. An infusion of remifentanil (0.2 µg/kg/min) was subsequently started. Control: anaesthetic management was standardized. Thiopental sodium (3 to 5 mg/kg) or propofol (2 to 3 mg/kg), fentanyl (1 to 3 μg/kg), and vecuronium (0.1 mg/kg) or rocuronium (0.6 mg/kg) were used for induction; anaesthesia was maintained with isoflurane (0.6%) in nitrogen, with vecuronium or rocuronium. An infusion of remifentanil (0.2 µg/kg/min) was subsequently started. | |
| Outcomes |
Primary outcomes: Inhospital case fatality rate Secondary outcomes: Wound infection: pus was expressed from the surgical incision or aspirated from a loculated mass inside the wound; the culture of pus was positive for pathogenic bacteria Length of hospital stay |
|
| Notes | The data cover sheets were lost for 4 patients; thus, their group assignment was unknown. Surgical complications occurred in 2 patients in the nitrous oxide group that required stopping the study. 4 patients in the nitrogen group were excluded from the analysis: 1 patient was excluded when the attending physician refused to allow the patient to participate; the other 3 patients that were excluded did not meet the inclusion criteria. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The assignments were based on computer‐generated random numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "The random sequence was kept in sealed, sequentially numbered envelopes until used." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients were not informed of their group assignments." |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Low risk | The outcome measurement is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "Surgical wounds were examined daily by a physician unaware of group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Gilani 2008.
| Methods | Single‐centre RCT | |
| Participants |
Setting: King Fahad National Guard Hospital, Saudi Arabia Inclusion criteria: patients above age of 18 years undergoing various elective and emergency surgical procedures under general anaesthesia Exclusion criteria: patients undergoing cardiac surgery or thoracic surgery Participant numbers: 200 randomly assigned; 200 analysed |
|
| Interventions | Intervention: general anaesthesia was maintained by 40% oxygen (FiO2 0.4) with nitrous oxide and volatile anaesthetic sevoflurane (MAC 1.2‐1.3) through oral endotracheal tube or laryngeal mask depending on the type of surgery. All patients received standard anaesthetic care and monitoring. Control: general anaesthesia was maintained by 40% oxygen (FiO2 0.4) with air and volatile anaesthetic sevoflurane (MAC 1.2‐1.3) through oral endotracheal tube or laryngeal mask depending on the type of surgery. All patients received standard anaesthetic care and monitoring. | |
| Outcomes |
Other outcomes: Postoperative pain |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Unclear risk | This was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Jensen 1992.
| Methods | Single‐centre RCT | |
| Participants |
Setting: University Hospital Linköping, Linköping, Sweden Inclusion criteria: patients aged 18 to 85 years, scheduled for intraabdominal operations of the colon and rectum Participant numbers: 60 randomly assigned; 60 analysed |
|
| Interventions | Intervention: two anaesthetic protocols were used for induction and maintenance of anaesthesia. In protocol 1, patients received thiopentone 4 mg/kg and fentanyl 2 μg/kg intravenous for induction of anaesthesia; additional thiopentone was given if needed. During the operation the lungs were ventilated with isoflurane and 30% oxygen in nitrous oxide; fentanyl intravenous was added in amounts to ensure adequate anaesthesia. Whenever needed, the inhaled concentration of isoflurane was changed based on the use of precisely defined clinical signs of inadequate anaesthesia. In protocol 2, patients received a modified total intravenous anaesthesia; Propofol 2 mg/kg intravenous was given for induction of sleep and an infusion of propofol was given at a rate of 6 mg/kg/h for the first 30 min and then reduced to 4 mg/kg/h. Fentanyl was given for induction in a bolus dose of 2 μg/kg, followed by an infusion of 5 μg/kg/h. After 30 rain, this infusion rate was reduced to 2.5 μg/kg/h. During anaesthesia the lungs of these patents were ventilated with 30% oxygen in nitrous oxide. Whenever needed, the infusion rates of both propofol and fentanyl was changed based on the use of precisely defined clinical signs of inadequate anaesthesia. Control: patients received total intravenous anaesthesia, with sleep induction by propofol 2 mg/kg followed immediately by an initial infusion of 9 mg/kg/h of propofol, reduced to 6 mg/kg/h after 30 min. Fentanyl was given in a bolus dose of 2 μg/kg, followed by an infusion of 7.5 μg/kg/h. The rate of fentanyl infusion was reduced after 30 min to 3.75 μg/kg/h. Ventilation was with oxygen in air to give an inspiratory fraction of oxygen of 0.3. Whenever needed, the infusion rates of both propofol and fentanyl was changed based on the use of precisely defined clinical signs of inadequate anaesthesia. | |
| Outcomes |
Secondary outcomes: Pneumonia: no specific definition Pulmonary atelectasis: no specific definition Length of hospital stay |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Low risk | Quote: "A set of numbered envelopes was used." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The patients were blinded, but insufficient information on the personnel. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Low risk | The outcome measurement is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Complications | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | High risk | Fewer than 50 participants per arm. |
Jensen 1993a.
| Methods | Single‐centre RCT | |
| Participants |
Setting: University Hospital Linköping, Linköping, Sweden Inclusion criteria: patients > 18 years, scheduled for laparoscopic cholecystectomy Participant numbers: 42 randomly assigned; 42 analysed for the outcome of pneumonia; 40 analysed for the outcome of length of hospital stay |
|
| Interventions | Intervention: patients received meperidine 1 mg/kg and atropine 6 μg/kg im for premedication approximately 45 min prior to anaesthetic induction. Anaesthesia was induced intravenously with fentanyl 2 μg/kg, and thiopental 4 to 6 mg/kg was administered until loss of eyelash reflex. Tracheal intubation was facilitated by the use of succinylcholine 1 mg/kg after pretreatment with vecuronium 1 mg. The patients received isoflurane with nitrous oxide in oxygen for maintenance of anaesthesia. An inspiratory fraction of oxygen of 0.3 was used. Control: patients received meperidine 1 mg/kg and atropine 6 μg/kg im for premedication approximately 45 min prior to anaesthetic induction. Anaesthesia was induced intravenously with fentanyl 2 μg/kg, and thiopental 4 to 6 mg/kg was administered until loss of eyelash reflex. Tracheal intubation was facilitated by the use of succinylcholine 1 mg/kg after pretreatment with vecuronium 1 mg. The patients received isoflurane with air in oxygen for maintenance of anaesthesia. An inspiratory fraction of oxygen of 0.3 was used. | |
| Outcomes |
Secondary outcomes: Pneumonia: no specific definition Length of hospital stay |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind but no further details. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Low risk | The outcome measurement is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "Postoperative data was assessed by the postoperative ward staff and surgeon blinded to the anaesthetic technique." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | High risk | Fewer than 50 participants per arm. |
Jensen 1993b.
| Methods | Single‐centre RCT | |
| Participants |
Setting: University Hospital Linköping, Linköping, Sweden Inclusion criteria: patients scheduled for major operations on the intestines, and with an expected duration of surgery of more than 1 h Participant numbers: 42 randomly assigned; 42 analysed |
|
| Interventions | Intervention: patients received flunitrazepam 0.5 to 1 mg by mouth as premedication 1 h before induction of anaesthesia. Two anaesthetic protocols were used for induction and maintenance of anaesthesia. In protocol 1, patients received intravenous thiopentone 4 mg/kg and fentanyl 2 μg/kg for induction of anaesthesia. Fentanyl supplements were given as needed during the operation. Isoflurane (0.5 to 1.5%) in 70% nitrous oxide/30% oxygen was used for the maintenance of anaesthesia. In protocol 2, propofol 2 mg/kg was used for induction, and anaesthesia was maintained using propofol at a rate of 6 mg/kg/h for the first 30 min and 4 mg/kg/h thereafter. At induction, fentanyl was given in a bolus dose of 2 μg/kg followed by an infusion rate of 5 μg/kg/h. After 30 min this was reduced to 2.5 μg/kg/h. The lungs were ventilated with 30% oxygen in nitrous oxide. For all the patients, vecuronium 1 mg was given for precurarization followed by suxamethonium 1 mg/kg for tracheal intubation. Ventilation was adjusted to give an arterial carbon dioxide tension of 44.5 kPa. Control: patients received flunitrazepam 0.5 to 1 mg by mouth as premedication 1 h before induction of anaesthesia. Anaesthesia was induced with propofol 2 mg/kg and maintained by a total intravenous technique using propofol 9 mg/kg/h for the first 30 min, followed by propofol 6 mg/kg/h. Fentanyl was given in a bolus dose of 2 μg/kg, followed by an infusion of 7.5 μg/kg/h. The fentanyl infusion was also reduced after 30 min to 3.75 μg/kg/h. Oxygen in air was used for ventilation (FiO2, 0.3). The patients received vecuronium 1 mg for precurarization followed by suxamethonium 1 mg/kg for tracheal intubation. Ventilation was adjusted to give an arterial carbon dioxide tension of 44.5 kPa. | |
| Outcomes |
Secondary outcome: Pulmonary atelectasis: defined by CT scans |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "The CT scans for the diagnosis of pulmonary atelectasis were reviewed blind to the anaesthetic given." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | High risk | Fewer than 50 participants per arm. |
Kozmary 1990.
| Methods | Single‐centre RCT | |
| Participants |
Setting: a hospital in California, USA Inclusion criteria: patients scheduled for carotid endarterectomy or other surgery on the carotid artery Participant numbers: 70 randomly assigned; 70 analysed |
|
| Interventions | Intervention: patients received isoflurane, fentanyl (2 to 5 μg/kg), thiopental (2 to 5 mg/kg), vecuronium, and 60% nitrous oxide/40% oxygen. The patients were mechanically ventilated with tidal volumes of 10 mL/kg at a rate sufficient to produce an end‐tidal carbon dioxide of 30 to 35 mmHg. Control: patients received isoflurane, fentanyl (2 to 5 μg/kg), thiopental (2 to 5 mg/kg), vecuronium, and 100% oxygen. The patients were mechanically ventilated with tidal volumes of 10 mL/kg at a rate sufficient to produce an end‐tidal carbon dioxide of 30 to 35 mmHg. | |
| Outcomes |
Secondary outcomes: Myocardial infarction: defined by creatine kinase enzyme changes. |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The patients were blinded, but insufficient information on the personnel. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "Experts unaware of the choice of anaesthetic analysed the data for diagnosis of myocardial infarction." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | High risk | Fewer than 50 participants per arm. |
Krogh 1994.
| Methods | Single‐centre RCT | |
| Participants |
Setting: a hospital in Odense, Denmark Inclusion criteria: ASA I‐II patients scheduled for elective major colonic surgery Participant numbers: 139 randomly assigned; 139 analysed |
|
| Interventions | Intervention: premedication comprised diazepam 0.2 mg/kg orally. Anaesthesia was induced with fentanyl 2 to 5 μg/kg intravenous. Propofol was given as a bolus dose of 1 mg/kg. All patients breathed 100% oxygen during induction. The patient's lungs were ventilated with oxygen via a face mask until the trachea had been intubated. Tracheal intubation was facilitated by administration of pancuronium 0.1mg/kg. Anaesthesia was maintained with fentanyl 2 to 4 μg/kg/h and propofol 1 to 2 mg/kg/h. The lungs of the patients were ventilated with nitrous oxide in oxygen. The inspiratory oxygen concentration was maintained at 30%. Neuromuscular block was maintained with pancuronium 1 to 2 mg if train‐of‐four showed one or two twitches. Before induction of anaesthesia, a lumbar extradural catheter was inserted and extradural bupivacaine given. Control: premedication comprised diazepam 0.2 mg/kg orally. Anaesthesia was induced with fentanyl 2 to 5 μg/kg intravenous. Propofol was given as a bolus dose of 1 mg/kg. All patients breathed 100% oxygen during induction. The patient's lungs were ventilated with oxygen via a face mask until the trachea had been intubated. Tracheal intubation was facilitated by administration of pancuronium 0.1mg/kg. Anaesthesia was maintained with fentanyl 2 to 4 μg/kg/h and propofol 4 to 6 mg/kg/h. The lungs of the patients were ventilated with oxygen and air. The inspiratory oxygen concentration was maintained at 30%. Neuromuscular block was maintained with pancuronium 1 to 2 mg if train‐of‐four showed one or two twitches. Before induction of anaesthesia, a lumbar extradural catheter was inserted and extradural bupivacaine given. | |
| Outcomes |
Secondary outcomes: Length of hospital stay |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Low risk | The outcome measurement is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Complications | Unclear risk | — |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Lampe 1990.
| Methods | Single‐centre RCT | |
| Participants |
Setting: a hospital in California, USA Inclusion criteria: patients presenting for elective resection of acoustic neuroma Participant numbers: 26 randomly assigned; 26 analysed |
|
| Interventions | Intervention: premedication (triazolam, and/or morphine, or none) and intraoperative fentanyl and edrophonium/atropine were administered at the discretion of the anaesthesiologist. Patients received thiopental and vecuronium and the lungs were ventilated with 50 to 60% nitrous oxide/30 to 40% oxygen and isoflurane. Inhaled isoflurane concentrations were adjusted to maintain clinically acceptable levels of anaesthesia as determined by the attending anaesthesiologist. Ventilation was controlled, and total gas flows of 5 L/min were maintained throughout surgery. Control: premedication (triazolam, and/or morphine, or none) and intraoperative fentanyl and edrophonium/atropine were administered at the discretion of the anaesthesiologist. Patients received thiopental and vecuronium and the lungs were ventilated with 100% oxygen and isoflurane. Inhaled isoflurane concentrations were adjusted to maintain clinically acceptable levels of anaesthesia as determined by the attending anaesthesiologist. Ventilation was controlled, and total gas flows of 5 L/min were maintained throughout surgery. | |
| Outcomes |
Primary outcomes: Inhospital case fatality rate Secondary outcomes: Pneumonia: based on radiographic evidence and increased white blood cell count plus fever Pulmonary atelectasis: based on radiographic evidence Wound infection: as defined by the surgeon Length of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Low risk | Quote: "All data collection (including patient interviews) and analyses were performed by individuals unaware of the patient's anaesthetic regimen." |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "All data collection (including patient interviews) and analyses were performed by individuals unaware of the patient's anaesthetic regimen." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | High risk | Fewer than 50 participants per arm. |
Larsen 2000.
| Methods | Single‐centre RCT | |
| Participants |
Setting: University of Saarland, Germany Inclusion criteria: ASA I‐II patients, aged 18–65 year, scheduled for elective operative procedures Exclusion criteria: a history of a significant cardiac, pulmonary, hepatic, or renal disease; chronic drug or alcohol abuse; morbid obesity; disabling neuropsychiatric disorders; hypersensitivity to aesthetics or familial history of malignant hyperthermia; women who were pregnant or breast‐feeding; patients who refused to give consent Participant numbers: 60 randomly assigned; 60 analysed |
|
| Interventions | Intervention: before the induction of anaesthesia, all patients received fentanyl 2 μg/kg IV, then breathed 100% oxygen for 3 min. Anaesthesia was induced with propofol 2 mg/kg IV. After loss of consciousness, patients received either desflurane at an endtidal concentration of 5% or sevoflurane 1.7% and rocuronium 0.6 mg/kg to facilitate endotracheal intubation. Maintenance of anaesthesia was provided with the respective volatile anaesthetic (0.85 MAC concentration with nitrous oxide 65% in oxygen; the inspired concentration was adjusted to maintain mean arterial pressure within 20% of baseline values. Control: patients were infused with remifentanil at a rate of 0.5 μg/kg/min until they felt dazed. Thereafter, anaesthesia was induced by propofol in a dose adequate for loss of eye‐lash reflex, followed by rocuronium 0.6 mg/kg for tracheal intubation. After intubation, remifentanil infusion was reduced to 0.25 μg/kg/min, and a propofol infusion was started at a rate of 3 mg/kg/min and maintained throughout surgery. During the maintenance of anaesthesia, patients were ventilated with a fresh gas flow of 2 L/min of oxygen 35% in air by using a semiclosed circle system. No inhaled anaesthetics were given. | |
| Outcomes |
Other outcomes: Quality of recovery |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The assignment of patients was single blinded." |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "Observer was blinded to the anaesthesia the patients had received." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Lee 2005.
| Methods | Single‐centre RCT | |
| Participants |
Setting: University of Hong Kong, China Inclusion criteria: patients undergoing open colorectal surgery Exclusion criteria: patients were excluded from the study if they had known allergy to remifentanil or morphine, had abnormal preoperative renal or hepatic function, regularly took analgesics or had consumed any kind of opioid within the past 24 h, had a history of drug or alcohol abuse, were unable to use patient‐controlled analgesia, were less than 18 yr old, or had a body weight that was not within 20% of ideal Participant numbers: 60 randomly assigned; 60 analysed |
|
| Interventions | Intervention: patients received isoflurane at an end tidal concentration of 0.5 to 1.5% (according to clinical requirement), delivered with 70% nitrous oxide in oxygen. Control: patients received isoflurane at an end tidal concentration of 0.5 to 1.5% (according to clinical requirement) delivered in an oxygen‐air gas mixture and they also received an intravenous infusion of remifentanil at 0.05 to 0.5 μg/kg/min. | |
| Outcomes |
Other outcomes: Postoperative opioid consumption |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was based on computer‐generated codes." |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Nurses and acute pain team members who were not involved in the study and were unaware of the patients' intraoperative randomization conducted observation and management in the postanaesthesia care unit and subsequently the ward. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Leung 2006.
| Methods | Single‐centre RCT | |
| Participants |
Setting: University of California, San Francisco Medical Centre, USA Inclusion criteria: consecutive men or women who were > 65 years of age, undergoing non‐cardiac surgery, requiring general anaesthesia, who were expected to remain in the hospital after operation for > 48 h Exclusion criteria: patients who could not complete the neuropsychological testing such as those who were expected to remain intubated after operation; patients who not able to provide informed consent; surgical cases in which the use of nitrous oxide was contraindicated Participant numbers: 228 randomly assigned; 228 analysed |
|
| Interventions | Intervention: pre‐medication was limited to fentanyl up to 2 μg/kg intravenous. During operation, mechanical ventilation was initiated to maintain normocarbia and oxygen saturation > 95%. Anaesthetists were requested to control intraoperative heart rate and blood pressure to within ± 30% of preoperative baseline measurements. Intraoperative monitoring was not controlled by the study but was measured. Additional intravenous morphine sulfate or fentanyl was allowed to be titrated to maintain spontaneous ventilatory frequencies of 10 to 20 bpm and end‐tidal CO2 between 45 and 55 mm Hg while the inhalational agents were discontinued at the conclusion of surgery. The intraoperative anaesthetic management was consisted of nitrous oxide with oxygen plus a potent inhalational agent. In order to make the study clinically feasible, the study allowed the anaesthetists to adjust the percentages of inspired concentrations of oxygen during surgery as clinically indicated. Control: pre‐medication was limited to fentanyl up to 2 μg/kg intravenous. During operation, mechanical ventilation was initiated to maintain normocarbia and oxygen saturation > 95%. Anaesthetists were requested to control intraoperative heart rate and blood pressure to within ± 30% of preoperative baseline measurements. Intraoperative monitoring was not controlled by the study but was measured. Additional intravenous morphine sulfate or fentanyl was allowed to be titrated to maintain spontaneous ventilatory frequencies of 10 to 20 bpm and end‐tidal CO2 between 45 and 55 mm Hg while the inhalational agents were discontinued at the conclusion of surgery. The intraoperative anaesthetic management was consisted of oxygen plus a potent inhalational agent. In order to make the study clinically feasible, the study allowed the anaesthetists to adjust the percentages of inspired concentrations of oxygen during surgery as clinically indicated. | |
| Outcomes |
Primary outcomes: Inhospital case fatality rate Secondary outcomes: Length of hospital stay |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computerized random number list was created to designate the two anaesthetic group assignments." |
| Allocation concealment (selection bias) | Low risk | Quote: "The assignment of the anaesthetic group for each study patient was contained in a sealed envelope." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Low risk | The outcome measurement is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Complications | Unclear risk | — |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Lonie 1986.
| Methods | Single‐centre RCT | |
| Participants |
Setting: Manchester Royal Infirmary and St Mary's Hospital, UK Inclusion criteria: ASA I‐II patients who were scheduled for elective inpatient laparoscopy Participant numbers: 93 randomly assigned; 93 analysed |
|
| Interventions | Intervention: nitrous oxide 67% in oxygen and 1.25% MAC end tidal enflurane (0.7%) Control: 33% oxygen in nitrogen and 1.25 MAC end tidal enflurane (2.1%) | |
| Outcomes |
Other outcomes: Non‐severe nausea and vomiting |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "Patients were interviewed by a senior nurse who was unaware of which anaesthetic the patient had received." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Mraovic 2008.
| Methods | RCT | |
| Participants |
Setting: unknown Inclusion criteria: 150 ASA I‐II patients, between 19 and 75 years old, undergoing elective laparoscopic gynaecological surgery (removal of ovarian tumours and cysts, myomectomy, laparoscopic‐assisted vaginal hysterectomy, and infertility surgery) Exclusion criteria: obesity (body mass index > 33 kg/m²), pregnancy, breast‐feeding, known hypersensitivity to drugs used in the study protocol, use of antiemetics, psychotropic drugs and steroids within 72 h before surgery; patients with known comorbidities that could increase the incidence of postoperative nausea and vomiting, i.e. diseases which impaired gastric motility (diabetes mellitus, chronic cholecystitis, gastric and intestinal disease, neuromuscular disorders, neuropathies, and liver dysfunction), vestibular disease, history of migraine headache, central nervous system injury, renal impairment, irregular menstrual cycle (duration of < 21 or > 35 days and/or variations between cycles > 4 days), alcoholism, and opioid addiction Participant numbers: 150 randomly assigned; 137 analysed |
|
| Interventions | Intervention: patients received 7.5 mg of midazolam by mouth 1 h before the surgery with no prophylactic antiemetics. After induction of anaesthesia with thiopental 5 mg/kg and fentanyl 1 to 2 μg/kg, patients were manually ventilated with oxygen via facemask. Endotracheal intubation was facilitated with vecuronium 0.1 mg/kg IV. Patients then received either 50% nitrous oxide with oxygen or 70% nitrous oxide with oxygen. Anaesthesia was maintained with sevoflurane (end‐tidal concentration approximately 1 MAC) and supplemental bolus doses of fentanyl intravenous (1 μg/kg) to keep heart rate and arterial blood pressure within 20% of baseline values and additional vecuronium was administered to maintain 1 or 2 twitches on the train‐of‐four monitor. All patients received 10 mL/kg of crystalloids intraoperatively. Control: patients received 7.5 mg of midazolam by mouth 1 h before the surgery with no prophylactic antiemetics. After induction of anaesthesia with thiopental 5 mg/kg and fentanyl 1 to 2 μg/kg, patients were manually ventilated with oxygen via facemask. Endotracheal intubation was facilitated with vecuronium 0.1 mg/kg IV. Patients then received air and oxygen, FiO230%. Anaesthesia was maintained with sevoflurane (end‐tidal concentration approximately 1 MAC) and supplemental bolus doses of fentanyl intravenous (1 μg/kg) to keep heart rate and arterial blood pressure within 20% of baseline values and additional vecuronium was administered to maintain 1 or 2 twitches on the train‐of‐four monitor. All patients received 10 mL/kg of crystalloids intraoperatively. | |
| Outcomes |
Secondary outcomes: Severe nausea and vomiting: 2 or more episodes of vomiting and retching within a period of 30 min or total number of 3 or more emetic episodes during 24 h postoperatively. |
|
| Notes | 13 patients were excluded from the analysis. 4 patients were excluded in nitrous oxide‐free group: 1 patient was treated with corticosteroids for urticaria at induction of anaesthesia, 1 patient had an anaesthesia time < 30 min, 2 patients had a protocol violation. 4 patients were excluded in nitrous oxide‐based group 1: 1 patient had a conversion to laparotomy, 1 patient's anaesthesia time was < 30 min, and 2 patients had a protocol violation. 5 patients were excluded from the nitrous oxide‐based group 2: 2 patients' surgery was converted to laparotomy, 1 patient each had severe hypotension after induction, which lasted more than 5 mins, acute coronary syndrome postoperatively, and anaesthesia time < 30 min. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized by computer‐generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "Clinical nurses specifically trained for the study collected the data and were blinded to the anaesthesia technique used and randomizations." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Myles 2008a.
| Methods | Muli‐centre RCT | |
| Participants |
Setting: 2 hospitals from Australia and Hong Kong Inclusion criteria: patients undergoing elective noncardiac surgery, with risk factors or a known history of coronary artery disease (hypertension, diabetes, age older than 60 years, or preexisting history of coronary artery disease) Exclusion criteria: patients expected to require a high inspired oxygen concentration intraoperatively or with any relative contraindication to nitrous oxide (volvulus, pulmonary hypertension, increased intracranial pressure) Participant numbers: 59 randomly assigned; 59 analysed |
|
| Interventions | Intervention: patients had maintenance of general anaesthesia with nitrous oxide and FiO2 0.3, and one of three other hypnotic agents (isoflurane, sevoflurane, or propofol) at the discretion of the anaesthesiologist. Control: patients had their anaesthesia maintained with FiO2 0.8 or FiO2 0.3, but without nitrous oxide. | |
| Outcomes |
Secondary outcomes: Length of hospital stay |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated via a computer‐generated random list." |
| Allocation concealment (selection bias) | Low risk | Quote: "The random sequence was concealed in opaque, sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Administration and group identity were concealed from the surgeon and research staff." |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Low risk | The outcome measurement is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Complications | Unclear risk | — |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | High risk | Not all of the study's pre‐specified primary outcomes (e.g. myocardial infarction) had been reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Paredi 1994.
| Methods | Single‐centre RCT | |
| Participants |
Setting: a hospital in Italy Inclusion criteria: ASA I‐II female patients, aged older than 18, undergoing total hysterectomy Participant numbers: 184 randomly assigned; 184 analysed |
|
| Interventions | Intervention: enflurane 1.3% in nitrous oxide and oxygen Control: enflurane 2% in air and oxygen | |
| Outcomes |
Secondary outcomes: Severe nausea and vomiting: no specific definition. |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participants and personnel had knowledge of nitrous oxide exposure." |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "Nausea and vomiting were assessed by an investigator other than the anaesthetist or the surgeon." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Pedersen 1993.
| Methods | Single‐centre RCT | |
| Participants |
Setting: Herlev hospital, Herlev, Denmark Inclusion criteria: 44 ASA I‐II patients, aged 30 to 65 years, scheduled for elective abdominal hysterectomy with or without salpingo oophorectomy Exclusion criteria: patients with gastrointestinal disease of any kind, malignancy, or weight < 45 kg or > 90 kg; preoperative medication known to interfere with bowel function; contraindications against any of the anaesthetics used; insertion of a nasogastric tube; surgical complications; administration of laxatives or enemas before the fourth day postoperatively (the operation day being day 0) Participant numbers: 44 randomly assigned; 36 analysed |
|
| Interventions | Intervention: diazepam 0.15 mg/kg administered orally 1 h before anaesthesia was used as premedication. Anaesthesia was induced with fentanyl 3 μg/kg and atracurium as precurarization followed by thiopentone 3 to 5 mg/kg. Intubation was facilitated by suxamethonium 1.5 mg/kg. Anaesthesia was maintained with fentanyl 2 μg/kg/h and isoflurane with nitrous oxide in 30% oxygen. Ventilation was adjusted to maintain end‐tidal carbon dioxide tension between 4 and 4.5 kPa. After the disappearance of the effect of suxamethonium, neuromuscular block was achieved with a bolus of atracurium, 0.3 mg/kg, and maintained with infusion of atracurium. Control: diazepam 0.15 mg/kg administered orally 1 h before anaesthesia was used as premedication. Anaesthesia was induced with fentanyl 3 μg/kg and atracurium as precurarization followed by thiopentone 3 to 5 mg/kg. Intubation was facilitated by suxamethonium 1.5 mg/kg. Anaesthesia was maintained with fentanyl 2 μg/kg/h and isoflurane in 30% oxygen. Ventilation was adjusted to maintain end‐tidal carbon dioxide tension between 4 and 4.5 kPa. After the disappearance of the effect of suxamethonium, neuromuscular block was achieved with a bolus of atracurium, 0.3 mg/kg, and maintained with infusion of atracurium. | |
| Outcomes |
Secondary outcomes: Severe nausea and vomiting: patient rated |
|
| Notes | 8 patients were excluded during the study: 3 patients because of per‐ or postoperative surgical complications, 1 patient because of the surgeon's wish for insertion of a nasogastric tube due to distension of the intestines (the patient received nitrous oxide), 3 patients due to erroneous administration of laxative on the second postoperative day and 1 patient because of severe gastrointestinal discomfort on the third day postoperatively requiring an enema (the patient received nitrous oxide). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The mixture of gas administered was blinded for everyone other than the anaesthetist." |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "Nausea and vomiting were assessed by an investigator other than the anaesthetist or the surgeon." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | High risk | Fewer than 50 participants per arm. |
Sengupta 1988.
| Methods | Single‐centre RCT | |
| Participants |
Setting: a hospital in London, UK Inclusion criteria: 80 ASA I‐II patients older than 18 years undergoing a standard anaesthetic technique for day‐case laparoscopy Exclusion criteria: patients with a history of excessive nausea and vomiting after previous anaesthetics Participant numbers: 80 randomly assigned; 64 analysed |
|
| Interventions | Intervention: patients were given fentanyl 1.5 μg/kg intravenous and anaesthesia was induced with propofol 2 mg/kg intravenous followed by vecuronium 0.06 mg/kg intravenous. The patients received an inspired gas mixture of 33% nitrous oxide and 1% enflurane in oxygen. Control: patients were given fentanyl 1.5 μg/kg intravenous and anaesthesia was induced with propofol 2 mg/kg intravenous followed by vecuronium 0.06 mg/kg intravenous. The patients received an inspired gas mixture of 1% enflurane in oxygen. | |
| Outcomes |
Secondary outcomes: Severe nausea and vomiting: patient rated |
|
| Notes | 16 patients had not returned questionnaires | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Unclear risk | This was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | High risk | Fewer than 50 participants per arm. |
Short 1985.
| Methods | Single‐centre RCT | |
| Participants |
Setting: a hospital in London, UK Inclusion criteria: ASA I‐III patients scheduled for either minor gynaecological procedures such as dilatation and curettage, or urological procedures such as cysto‐urethroscopy Exclusion criteria: surgery lasted less than 30 minutes; alternative anaesthetic techniques were required; use of opioids for postoperative pain relief Participant numbers: 60 randomly assigned; 47 analysed |
|
| Interventions | Intervention: two anaesthetic protocols were used for induction and maintenance of anaesthesia. In protocol 1, patients received alfentanil 5 μg/kg over 1 minute, followed by methohexitone 1.5 mg/kg. The patients then breathed nitrous oxide and oxygen (FiO2 = 0.3). Supplements of alfentanil 2.5 μg/kg were given every 8 minutes until cessation of surgery, and increments of methohexitone 20 mg were given as clinically required. In protocol 2, patients received methohexitone 1.5 mg/kg as induction, followed by up to 5% isoflurane with 66% nitrous oxide in oxygen via a Magill system. When anaesthesia was satisfactory, the isoflurane concentration was decreased to 1.5 to 2%. Control: patients received methohexitone 1.5 mg/kg as induction, followed by up to 5% isoflurane in oxygen via a Magill system. When anaesthesia was satisfactory, the isoflurane concentration was decreased to 1.5 to 2%. | |
| Outcomes |
Secondary outcomes: Severe nausea and vomiting: no specific definition |
|
| Notes | The results from 13 patients were excluded from the study because either surgery lasted more than 30 minutes, or alternative anaesthetic techniques were required, such as tracheal intubation or the use of opioids for postoperative pain relief. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | High risk | Quote: "The observer was aware of which anaesthetic had been used." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | High risk | Fewer than 50 participants per arm. |
Singh 2011.
| Methods | Single‐centre RCT | |
| Participants |
Setting: a hospital in New Delhi, India Inclusion criteria: 116 ASA I‐II patients between 18 and 60 years of age, either gender, scheduled for elective supratentorial tumour surgery, with anticipated duration of anaesthesia more than 4 hours Exclusion criteria: patients with history of smoking, patients with history of megaloblastic anaemia, those requiring postoperative mechanical ventilation, patients receiving vitamin B12/folic acid supplementation, history of exposure to general anaesthesia in the last one month, history of motion sickness/postoperative emesis, evidence of pneumothorax/pneumocephalus, and bleeding disorders Participant numbers: 116 randomly assigned; 87 analysed |
|
| Interventions | Intervention: patients were preoxygenated with 100% oxygen for 3 minutes. General anaesthesia was induced with fentanyl 2 mcg/kg and thiopentone 4 to 6 mg/kg and tracheal intubation facilitated with rocuronium 1 mg/kg. Additional dose of thiopentone 1 to 2 mg/kg was given before laryngoscopy and intubation to prevent the pressor response. Anaesthesia was maintained using 60% nitrous oxide and 40% oxygen as carrier gases, as well as isoflurane at end‐tidal concentration of 0.7%. The flow rate of inhaled gas mixture was kept at 2 L/min in both the groups. Flow rate of nitrous oxide and oxygen were 1.2 and 0.8 L/min, respectively. Intermittent doses of fentanyl (1 mcg/kg) and vecuronium (0.01 mg/kg) were repeated as and when required. Use of other drugs and intravenous fluids was at the discretion of the attending anaesthesiologist. Control: patients were preoxygenated with 100% oxygen for 3 minutes. General anaesthesia was induced with fentanyl 2 mcg/kg and thiopentone 4 to 6 mg/kg and tracheal intubation facilitated with rocuronium 1 mg/kg. Additional dose of thiopentone 1 to 2 mg/kg was given before laryngoscopy and intubation to prevent the pressor response. Anaesthesia was maintained using 60% medical air and 40% oxygen as carrier gases, as well as isoflurane at end‐tidal concentration of 1.2%. The flow rate of inhaled gas mixture was kept at 2 L/min in both the groups. Flow rates of medical air and oxygen were 1.5 and 0.5 L/min, respectively. Intermittent doses of fentanyl (1 mcg/kg) and vecuronium (0.01 mg/kg) were repeated as and when required. Use of other drugs and intravenous fluids was at the discretion of the attending anaesthesiologist. | |
| Outcomes |
Secondary outcomes: Pneumonia: radiologic infiltrate confirmed by chest X‐ray or computed tomography, in association with at least one of the following: temperature greater than 38°C, leukocyte count greater than 12000 cell/mm³, or positive sputum culture that corresponds with positive culture Myocardial infarction: confirmed by a typical rise and fall in cardiac enzymes (troponin or CK‐MB fraction) with at least one of the following: typical ischaemic symptoms, new Q‐wave or ST‐segment electrocardiographic changes Stroke: a new neurological deficit persisting for 24 hours or longer, confirmed by neurologist assessment, and/or computed tomography, or magnetic resonance imaging Length of hospital stay Length of ICU stay |
|
| Notes | 29 patients could not be tracheally extubated at the end of surgery (15 patients in nitrous oxide‐based group and 14 in nitrous oxide‐free group), so the data of these patients were excluded from final analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly divided into two groups by a computer‐generated randomization chart." |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Attending anaesthesiologist was aware of the group identity (for safe administration of anaesthesia), but it was concealed from the surgeons." |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Low risk | Quote: "Staff conducting the postoperative follow‐ups (i.e., those responsible for postoperative data collection and outcome assessment) was blinded to the group identity." |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "Staff conducting the postoperative follow‐ups (i.e., those responsible for postoperative data collection and outcome assessment) was blinded to the group identity." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | High risk | Fewer than 50 participants per arm. |
Sukhani 1994.
| Methods | Single‐centre RCT | |
| Participants |
Setting: Loyola University Medical Center, USA Inclusion criteria: nonpregnant patients, 19 to 40 years of age, ASA I‐II, scheduled for ambulatory gynaecologic laparoscopy Exclusion criteria: patients were excluded from the study if they weighed more than 150% of their ideal body weight or had predisposing factors for delayed gastric emptying, such as diabetes, chronic cholecystitis, scleroderma, neuropathies, and neuromuscular disorders. Patients who demonstrated significant anxiety and who, in the anaesthesiologist's judgment, required preoperative anxiolytic therapy were also excluded Participant numbers: 70 randomly assigned; 70 analysed |
|
| Interventions | Intervention: patients were ventilated with a mixture of oxygen and nitrous oxide and the inspired oxygen concentration was maintained at 30%. Control: patients were ventilated with a mixture of oxygen and air and the inspired oxygen concentration was maintained at 30%. | |
| Outcomes |
Other outcomes: Quality of recovery |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The patients were assigned using a non‐blinded study design." |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "Intermediate recovery variables were recorded by recovery room nurses and the attending anaesthesiologist blinded to anaesthetic technique." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Todd 1993.
| Methods | Single‐centre RCT | |
| Participants |
Setting: a hospital in Iowa, USA Inclusion criteria: ASA II‐III patients, aged 18‐75 years, scheduled for elective craniotomy for resection of a supratentorial mass lesion Exclusion criteria: patients with known aneurysms, arteriovenous malformations, or posterior fossa tumours; patients who suffered from severe ischaemic heart disease, congestive heart failure, renal or hepatic dysfunction, severe chronic respiratory disease, medically controlled hypertension, stable angina, diabetes mellitus, or mild chronic obstructive lung disease; a rapid post‐operative return to normal consciousness was unlikely due to the location or size of the lesion (e.g. large hypothalamic lesions) or if postoperative sedation and mechanical ventilation were planned Participant numbers: 121 randomly assigned; 121 analysed |
|
| Interventions | Intervention: two anaesthetic protocols were used for induction and maintenance of anaesthesia. In protocol 1, anaesthesia was induced with 4 to 6 mg/kg thiopental, followed by 0.1 mg/kg vecuronium. Mask ventilation was begun with gradually increasing inspired concentrations of isoflurane in 60% nitrous oxide/balance oxygen, and continued for 10 min. The trachea was intubated, mechanical ventilation begun with nitrous oxide/oxygen (fraction of inspired oxygen 0.4), and the administered concentration of isoflurane was adjusted thereafter according to the judgment of the attending anaesthesiologist. In protocol 2, anaesthesia was induced with 4 to 6 mg/kg thiopental, followed by 0.1 mg/kg vecuronium. Mask ventilation was begun with 60% nitrous oxide/balance oxygen, and incremental doses of fentanyl were given, with a target loading dose of 10 μg/kg fentanyl to be given over 10 min. This could be varied at the discretion of the anaesthesiologist. After 10 min, the trachea was intubated, and mechanical ventilation begun with 60% nitrous oxide/oxygen (fraction of inspired oxygen 0.4). An infusion of fentanyl was started at the rate of 2 μg/kg/h, and paralysis was maintained with vecuronium or pancuronium. Control: anaesthesia was induced with 1 to 2 mg/kg propofol, followed by 0.1 mg/kg vecuronium. Simultaneously with the start of induction, a propofol infusion was begun at an initial rate of 200 μg/kg / min. Manual mask ventilation with 40% oxygen (as an oxygen/air mixture) was begun, and incremental doses of fentanyl were given, with a target loading dose of 10 μg/kg fentanyl to be given over 10 mins. The rate of fentanyl administration and the propofol infusion could be varied at the discretion of the attending anaesthesiologist. The patients' lungs mechanically ventilated with oxygen/air (FiO2 0.4). A fentanyl infusion was started and maintained at a rate of 2 μg/kg/h. The propofol infusion rate was varied according to clinical need. | |
| Outcomes |
Primary outcomes: Inhospital case fatality rate Secondary outcomes: Pneumonia: no specific definition Length of hospital stay |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Low risk | The outcome measurement is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Complications | Unclear risk | This was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Van Hemelrijck 1991.
| Methods | Single‐centre RCT | |
| Participants |
Setting: A hospital in Missouri, USA Inclusion criteria: 92 non‐pregnant gynaecologic patients, 19 to 46 years of age, ASA I‐II, scheduled for out‐patient laparoscopic surgery Participant numbers: 92 randomly assigned; 92 analysed |
|
| Interventions | Intervention: all patients breathed 100% oxygen for 2 min after receiving a preinduction dose of fentanyl 1.5 μg/kg intravenous and dtubocurarine 3 mg intravenous. Three anaesthetic protocols were used for induction and maintenance of anaesthesia. In protocol 1, anaesthesia was induced with propofol 2.5 mg/kg administered over 2.5 minutes using a syringe‐type infusion pump. After loss of consciousness, succinylcholine 1.5 mg/kg intravenous was administered to facilitate intubation. Anaesthesia was maintained with nitrous oxide 60% and a continuous infusion of propofol at an initial infusion rate of 160 μg/kg/min, which subsequently was titrated within the range of 50 to 200 μg/kg/min. The maintenance infusion rate of propofol was adjusted to maintain an adequate depth of anaesthesia, adjudged by clinical signs and hemodynamic responses. In protocol 2, anaesthesia was induced with propofol 2.5 mg/kg administered over 2.5 minutes using a syringe‐type infusion pump. After loss of consciousness, succinylcholine 1.5 mg/kg intravenous was administered to facilitate intubation. Anaesthesia was maintained with desflurane 4 to 7% inspired concentration in combination with 60% nitrous oxide. The inspired desflurane concentration were adjusted to maintain an adequate depth of anaesthesia, adjudged by clinical signs and hemodynamic responses. In protocol 3, anaesthesia was induced by inhalation of desflurane with nitrous oxide 60% in oxygen. After loss of consciousness, succinylcholine 1.5 mg/kg intravenous was administered to facilitate intubation. Anaesthesia was maintained with desflurane 4 to 7% inspired concentration in combination with 60% nitrous oxide. The inspired desflurane concentration were adjusted to maintain an adequate depth of anaesthesia, adjudged by clinical signs and hemodynamic responses. Control: anaesthesia was induced by inhalation of desflurane with 100% oxygen. After loss of consciousness, succinylcholine 1.5 mg/kg intravenous was administered to facilitate intubation. Anaesthesia was maintained with desflurane 4 to 7% inspired concentration in combination with 100% oxygen. The inspired desflurane concentration were adjusted to maintain an adequate depth of anaesthesia, adjudged by clinical signs and hemodynamic responses. | |
| Outcomes |
Secondary outcomes: Severe nausea and vomiting: persistent nausea with repeated episodes of vomiting, requiring treatment |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study used an open (non blinded) design." |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "The outcome was assessed by research nurse who was blinded to as to the anaesthetic treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | High risk | Fewer than 50 participants per arm. |
Vanacker 1999.
| Methods | RCT | |
| Participants |
Inclusion criteria: 60 female in‐patients (ASA I‐II), aged 18 to 65 years, scheduled for breast surgery with a duration of 1 to 3 hours Exclusion criteria: patients had body weight 20% outside normal weight, history of motion sickness or of postoperative nausea and vomiting, pregnant or breastfeeding patients, history of alcohol or drug abuse, sensitivity to narcotics, impaired renal or hepatic function, recent (< 30 days) participation in another study Participant numbers: 30 randomly assigned; 30 analysed |
|
| Interventions | Intervention: patients were breathing 100% oxygen with a fresh gas flow of 7 L/min for 2 to 3 minutes. A standardized anaesthetic technique consisting of propofol for induction (2 mg/kg) followed by desflurane with nitrous oxide for maintenance of anaesthesia was used in all patients. The concentration of anaesthetic given to the patients was based on previously determined MAC values and adjusted to the patient's needs as clinically indicated with the objective to maintain the heart rate and blood pressure within 20% of the baseline values. They received a pre‐induction dose of fentanyl 2 μg/kg; additional doses of fentanyl 1 μg/kg were given if there were signs of inadequate anaesthesia (i.e. movement, swallowing, tearing, salivation) despite changes in inhalation concentration. Muscle relaxation for intubation was achieved by a single dose of vecuronium 0.1 mg/kg. Mechanical ventilation was instituted in all patients and ventilatory settings were adjusted to achieve normocapnia; the fresh gas flow was reduced to 2 L/ min during maintenance of anaesthesia. At the end of surgery, desflurane and nitrous oxide were discontinued and the patients received 100% oxygen (7 L/min fresh gas flow). Control: patients were breathing 100% oxygen with a fresh gas flow of 7 L/min. for 2 to 3 minutes. A standardized anaesthetic technique consisting of propofol for induction (2 mg/kg) followed by desflurane for maintenance of anaesthesia was used in all patients. The concentration of anaesthetic given to the patients was based on previously determined MAC values and adjusted to the patient's needs as clinically indicated with the objective to maintain the heart rate and blood pressure within 20% of the baseline values. They received a pre‐induction dose of fentanyl 2 μg/kg; additional doses of fentanyl 1 μg/kg were given if there were signs of inadequate anaesthesia (i.e. movement, swallowing, tearing, salivation) despite changes in inhalation concentration. Muscle relaxation for intubation was achieved by a single dose of vecuronium 0.1 mg/kg. Mechanical ventilation was instituted in all patients and ventilatory settings were adjusted to achieve normocapnia; the fresh gas flow was reduced to 2 L/ min during maintenance of anaesthesia. At the end of surgery, desflurane was discontinued and the patients received 100% oxygen (7 L/min fresh gas flow). | |
| Outcomes |
Secondary outcomes: Severe nausea and vomiting: vomiting requiring at least three doses of antiemetic medication within 24 hours of surgery. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Unclear risk | This was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | High risk | Fewer than 50 participants per arm. |
Yoshimura 2014.
| Methods | Single‐centre RCT | |
| Participants |
Setting: Teikyo University, Japan Inclusion criteria: Adult patients scheduled for elective thoracotomy or thoracoscopic surgery Exclusion criteria: Patients were excluded if pleural adhesion was anticipated during preoperative assessment or if they had evidence of bullae on their chest computed tomography scans Participant numbers: 50 randomly assigned; 50 analysed |
|
| Interventions | Intervention: patients received a gas mixture of oxygen and nitrous oxide (FiO2 = 0.5). Anaesthesia was induced with propofol (1 to 2 mg/kg), remifentanil (0.3 to 0.5 μg/kg/min), and rocuronium (1 mg/kg) and was maintained with propofol infusion (120 to 200 μg/kg/min) and intermittent boluses of rocuronium. Control: patients received 100% oxygen for three minutes for thorough denitrogenation. Anaesthesia was induced with propofol (1 to 2 mg/kg), remifentanil (0.3 to 0.5 μg/kg/min), and rocuronium (1 mg/kg) and was maintained with propofol infusion (120 to 200 μg/kg/min) and intermittent boluses of rocuronium. | |
| Outcomes |
Other outcomes: Lung collapse score |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated by random number." |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Inhospital case fatality rate/length of stay | Unclear risk | — |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: "Surgeons were blinded to the gas mixture and were instructed to assess the lung collapse scale." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | No other potential sources of bias were detected. |
Abbreviations: ASA: American Society of Anesthesiologists; ASEPSIS: Additional treatment, Serous discharge, Erythema, Purulent exudate, Separation of deep tissues, Isolation of bacteria, and duration of inpatient Stay; bpm: breaths per minute; CABG: Coronary Artery Bypass Grafting; CK‐MB: Creatine Kinase, MB Form; CT: Computed Tomography; ECG: Electrocardiogram; h: hour(s); ICU: Intensive Care Unit; IM: intramuscular injection; IV: intravenous injection; kPa: kilopascals; MAC: Minimum Alveolar Concentration; N: number; PTCA: Percutaneous Transluminal Coronary Angioplasty; ST: ST‐segment; TIA: Transient Ischaemic Attack; V‐Q: Ventilation/Perfusion.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Antonini 1994 | Not a RCT. |
| Atanassoff 1994 | Not general anaesthesia. |
| Atassi 2005 | Nitrous oxide used in the control group. |
| Barr 1999 | Not a RCT. |
| Bronco 2010 | Nitrous oxide used in the control group. |
| Castéra 2001 | Not general anaesthesia. |
| Cheong 2000 | Nitrous oxide used in the control group. |
| Divatia 1996 | Not a RCT. |
| Dover 1994 | Not a RCT. |
| Einarsson 1997 | Nitrous oxide used in the control group. |
| Fredman 1998 | Nitrous oxide used in the control group. |
| Gozdemir 2007 | Nitrous oxide used in the control group. |
| Haessler 1993 | Nitrous oxide used in the control group. |
| Haraguchi 1995 | Not general anaesthesia. |
| Heath 1996 | Not general anaesthesia. |
| Holst 1993 | Nitrous oxide used in the control group. |
| Ishii 1994 | Nitrous oxide used in the control group. |
| Jastak 1973 | Non‐adults involvement. |
| Jellish 1996 | Nitrous oxide used in the control group. |
| Johnson 1997 | Non‐adults involvement. |
| Kryshtalskyj 1990 | Not general anaesthesia. |
| Lim 1992 | Non‐adults involvement. |
| Losasso 1992 | Non‐adults involvement. |
| Masood 2002 | Not general anaesthesia. |
| Morimoto 1997 | Not a RCT. |
| Nightingale 1992 | Non‐adults involvement. |
| Nishiyama 1998 | Nitrous oxide used in the control group. |
| Ogg 1983 | Non‐adults involvement. |
| Rocca 2000 | Non‐adults involvement. |
| Saïssy 2000 | Non‐adults involvement. |
| Simpson 1977 | Nitrous oxide used in the control group. |
| Sinha 2006 | Nitrous oxide used in the control group. |
| Smith 1993 | Nitrous oxide used in the control group. |
| Taki 2003 | Non‐adults involvement. |
| Towey 1979 | Non‐adults involvement. |
| Van den Berg 1995 | Non‐adults involvement. |
| Vari 2010 | Nitrous oxide used in the control group. |
| Wesner 2005 | Not a RCT. |
| Yamakage 2001 | Nitrous oxide used in the control group. |
| Yang 2004 | Nitrous oxide used in the control group. |
| Zuurmond 1986 | Nitrous oxide used in the control group. |
Characteristics of studies awaiting assessment [ordered by study ID]
Adams 1994.
| Methods | RCT |
| Participants | 20 ASA 1 to 2 patients 18 to 60 years of age scheduled for orthopaedic surgery |
| Interventions |
Intervention : intravenous combined with inhaled anaesthesia ventilated with 1.2 to 2.4 volume % isoflurane in nitrous oxide and oxygen Control : total intravenous anaesthesia ventilated with air and oxygen, FiO2 33% |
| Outcomes | Unknown |
| Notes |
Miralles Pardo 1991.
| Methods | Controlled study |
| Participants | 20 ASA 1 to 2 patients |
| Interventions |
Intervention : thiopental and nitrous oxide in oxygen Control : propofol |
| Outcomes | Unknown |
| Notes |
Moussa 1995.
| Methods | Controlled study |
| Participants | Patients scheduled for dental day surgery |
| Interventions |
Intervention : anaesthesia ventilated with nitrous oxide Control : total intravenous anaesthesia |
| Outcomes | Unknown |
| Notes |
Rashchupkin 2011.
| Methods | Controlled study |
| Participants | 60 patients undergoing open cholecystectomy |
| Interventions |
Intervention : nitrous oxide Control : xenon |
| Outcomes | Unknown |
| Notes |
Röpcke 2001.
| Methods | RCT |
| Participants | 25 female patients during gynaecological laparotomies |
| Interventions |
Intervention : sevoflurane in air and oxygen Control : sevoflurane in nitrous oxide and oxygen |
| Outcomes | Unknown |
| Notes |
Schaffranietz 2000.
| Methods | Controlled study |
| Participants | 40 patients undergoing an elective craniotomy for brain tumour resection |
| Interventions |
Intervention : general anaesthesia with nitrous oxide Control : general anaesthesia without nitrous oxide |
| Outcomes | Unknown |
| Notes |
Segatto 1993.
| Methods | RCT |
| Participants | 200 pregnant patients |
| Interventions |
Intervention : thiopental‐nitrous oxide anaesthesia Control : total intravenous anaesthesia |
| Outcomes | Unknown |
| Notes | Only title available |
Shulunov 2002.
| Methods | Controlled study |
| Participants | 44 patients undergoing cholecystectomy |
| Interventions |
Intervention : nitrous oxide and oxygen Control : xenon and oxygen |
| Outcomes | Unknown |
| Notes |
Abbreviations; ASA: American Society of Anesthesiologists.
Differences between protocol and review
Authors: we have changed the author list from the published protocol (Yang 2011). Rao Sun and Akira Kuriyama joined the review author team.
Types of interventions: we replaced 'total intravenous anaesthesia' and 'inhaled anaesthesia' with the more precise descriptions of 'propofol‐based maintenance of anaesthesia' and 'volatile anaesthetic‐based maintenance of anaesthesia', respectively.
Data collection and analysis: we used the most recent of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) for 'Risk of bias' assessment, and we generated 'Risk of bias' tables. We also used RevMan 5.3 for statistical analyses.
Selection of studies: the full texts we obtained provided sufficient information for us to determine their eligibility. Therefore, we did not correspond with the original study investigators.
Measures of treatment effect: the data expressed as median and the interquartile range values may be skewed. To avoid introducing potential bias, we only pooled the data expressed as mean and standard deviation for length of stay.
Assessment of reporting biases: we conducted Egger's test to examine asymmetry of the funnel plot.
Data synthesis: where we did not conduct meta‐analysis, we described the findings of the included studies qualitatively. We stated the implementation of GRADE methods and the selection of outcomes in the 'Summary of findings' tables.
Subgroup analysis and investigation of heterogeneity: we stated the details of grouping in the subgroup analysis.
Sensitivity analysis: we conducted sensitivity analyses based on the percentages of withdrawals (above 10% versus below 10%) of the included RCTs.
Contributions of authors
Rao Sun (RS), Wen Qin Jia (WQJ), KeHu Yang (KHY), Jin Hui Tian (JHT), Bin Ma (BM), Yali Liu (YL), Run H Jia (RHJ), Peng Zhang (PZ), Xiao F Luo (XFL), Akira Kuriyama (AK):
KHY and WQJ conceived the review.
KHY and JHT coordinated the review.
BM and YL undertook manual searches of the literature.
WQJ and RS screened search results.
WQJ, JHT, and RS organized retrieval of papers.
WQJ and RS screened retrieved papers against the inclusion criteria.
RS and BM appraised the quality of the papers.
RS, WQJ, and AK: abstracted data from papers.
RS, PZ, and AK wrote to authors of papers for additional information.
WQJ, RS, PZ, and AK provided additional data about papers.
YL and BM obtained and screened data on unpublished studies.
RS and JHT performed data management for the review.
XFL and RS entered data into RevMan 5.3.
XFL and RS performed statistical analyses using RevMan 5.3.
XFL and RS performed statistical analyses using Stata 11.0.
RS, JHT, and WQJ performed other statistical analyses without RevMan 5.3.
JHT and RS performed double entry of data: person one: JHT; person two: RS.
WQJ and RS interpreted the data.
RS and XFL assessed statistical inferences.
KHY, RS, and RHJ wrote the review.
KHY secured funding for the review.
JHT and WQJ performed previous work that was the foundation of the present review.
KHY is guarantor for this Cochrane review.
KHY and RS were responsible for reading and checking the review before submission.
Sources of support
Internal sources
Evidence‐Based Medicine Center, School of Basic Medical Sciences, Lanzhou University, China.
External sources
No sources of support supplied
Declarations of interest
Rao Sun: none known. Wen Qin Jia: none known. KeHu Yang: none known. Jin Hui Tian: none known. Bin Ma: none known. Yali Liu: none known. Run H Jia: none known. Peng Zhang: none known. Xiao F Luo: none known. Akira Kuriyama: none known.
New
References
References to studies included in this review
Akca 2004 {published data only}
- Akca O, Lenhardt R, Fleischmann E, Treschan T, Greif R, Fleischhackl R, et al. Nitrous oxide increases the incidence of bowel distension in patients undergoing elective colon resection. Acta Anaesthesiologica Scandinavica 2004;48(7):894‐8. [PUBMED: 15242436] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alhashemi 1997 {published data only}
- Alhashemi JA, Miller DR, O'Brien HV, Hull KA. Cost‐effectiveness of inhalational, balanced and total intravenous anaesthesia for ambulatory knee surgery. Canadian Journal of Anaesthesia 1997;44(2):118‐25. [PUBMED: 9043722] [DOI] [PubMed] [Google Scholar]
Arellano 2000 {published data only}
- Arellano RJ, Pole ML, Rafuse SE, Fletcher M, Saad YG, Friedlander M, et al. Omission of nitrous oxide from a propofol‐based anesthetic does not affect the recovery of women undergoing outpatient gynecologic surgery. Anesthesiology 2000;93(2):332‐9. [PUBMED: 10910478] [DOI] [PubMed] [Google Scholar]
Badner 2000 {published data only}
- Badner NH, Beattie WS, Freeman D, Spence JD. Nitrous oxide‐induced increased homocysteine concentrations are associated with increased postoperative myocardial ischemia in patients undergoing carotid endarterectomy. Anesthesia and Analgesia 2000;91(5):1073‐9. [PUBMED: 11049886] [DOI] [PubMed] [Google Scholar]
Bloomfield 1988 {published data only}
- Bloomfield E, Hilberman M, Brown P, Noe H, Gaydos J, Miller G, et al. Postoperative nausea and vomiting. A comparison of sufentanil, nitrous oxide, and isoflurane. Cleveland Clinic Journal of Medicine 1988;55(6):549‐52. [PUBMED: 2976325] [DOI] [PubMed] [Google Scholar]
Brodsky 2005 {published data only}
- Brodsky JB, Lemmens HJ, Collins JS, Morton JM, Curet MJ, Brock‐Utne JG. Nitrous oxide and laparoscopic bariatric surgery. Obesity Surgery 2005;15(4):494‐6. [PUBMED: 15946427] [DOI] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen Y, Liu X, Cheng CH, Gin T, Leslie K, Myles P, et al. Leukocyte DNA damage and wound infection after nitrous oxide administration: a randomized controlled trial. Anesthesiology 2013;118(6):1322‐31. [PUBMED: 23549382] [DOI] [PubMed] [Google Scholar]
Deleu 2000 {published data only}
- Deleu D, Louon A, Sivagnanam S, Sundaram K, Okereke P, Gravell D, et al. Long‐term effects of nitrous oxide anaesthesia on laboratory and clinical parameters in elderly Omani patients: A randomized double‐blind study. Journal of Clinical Pharmacy and Therapeutics 2000;25(4):271‐7. [PUBMED: 10971777] [DOI] [PubMed] [Google Scholar]
Eger 1990 {published data only}
- Eger EI 2nd, Lampe GH, Wauk LZ, Whitendale P, Cahalan MK, Donegan JH. Clinical pharmacology of nitrous oxide: an argument for its continued use. Anesthesia and Analgesia 1990;71(6):575‐85. [PUBMED: 2240627] [DOI] [PubMed] [Google Scholar]
ENIGMA II trial 2014 {published and unpublished data}
- Myles PS, Leslie K, Chan MT, Forbes A, Peyton PJ, Paech MJ, et al. The safety of addition of nitrous oxide to general anaesthesia in at‐risk patients having major non‐cardiac surgery (ENIGMA‐II): a randomised, single‐blind trial. Lancet 2014;384(9952):1446‐54. [PUBMED: 25142708] [DOI] [PubMed] [Google Scholar]
- Myles PS, Leslie K, Peyton P, Paech M, Forbes A, Chan MT, et al. ANZCA Trials Group. Nitrous oxide and perioperative cardiac morbidity (ENIGMA‐II) Trial: rationale and design. American Heart Journal 2009;157(3):488‐94.e1. [PUBMED: 19249419] [DOI] [PubMed] [Google Scholar]
ENIGMA trial 2007 {published and unpublished data}
- Chan MT, Wan AC, Gin T, Leslie K, Myles PS. Chronic postsurgical pain after nitrous oxide anesthesia. Pain 2011;152(11):2514‐20. [PUBMED: 21889262] [DOI] [PubMed] [Google Scholar]
- Graham AM, Myles PS, Leslie K, Chan MT, Paech MJ, Peyton P, et al. A cost‐benefit analysis of the ENIGMA trial. Anesthesiology 2011;115(2):265‐72. [PUBMED: 21681081] [DOI] [PubMed] [Google Scholar]
- Myles PS, Leslie K, Chan MTV, Forbes A, Paech MJ, Peyton P, et al. Avoidance of nitrous oxide for patients undergoing major surgery: a randomized controlled trial. Anesthesiology 2007;107(2):221‐31. [PUBMED: 17667565] [DOI] [PubMed] [Google Scholar]
Fleischmann 2005 {published data only}
- Fleischmann E, Lenhardt R, Kurz A, Herbst F, Fülesdi B, Greif R, et al. Outcomes Research Group. Nitrous oxide and risk of surgical wound infection: a randomised trial. Lancet 2005;366(9491):1101‐7. [PUBMED: 16182898] [DOI] [PubMed] [Google Scholar]
Gilani 2008 {published data only}
- Gilani SM, Sofi K. Is nitrous oxide necessary for general anaesthesia?. Journal of Ayub Medical College Abbottabad 2008;20(4):149‐52. [PUBMED: 19999230] [PubMed] [Google Scholar]
Jensen 1992 {published data only}
- Jensen AG, Kalman SH, Nyström PO, Eintrei C. Anaesthetic technique does not influence postoperative bowel function: a comparison of propofol, nitrous oxide and isoflurane. Canadian Journal of Anaesthesia 1992;39(9):938‐43. [PUBMED: 1451222] [DOI] [PubMed] [Google Scholar]
Jensen 1993a {published data only}
- Jensen AG, Prevedoros H, Kullman E, Anderberg B, Lennmarken C. Peroperative nitrous oxide does not influence recovery after laparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica 1993;37(7):683‐6. [PUBMED: 8249558] [DOI] [PubMed] [Google Scholar]
Jensen 1993b {published data only}
- Jensen AG, Kalman SH, Eintrei C, Fransson SG, Morales O. Atelectasis and oxygenation in major surgery with either propofol with or without nitrous oxide or isoflurane anaesthesia. Anaesthesia 1993;48(12):1094‐6. [PUBMED: 8285335] [DOI] [PubMed] [Google Scholar]
Kozmary 1990 {published data only}
- Kozmary SV, Lampe GH, Benefiel D, Cahalan MK, Wauk LZ, Whitendale P, et al. No finding of increased myocardial ischemia during or after carotid endarterectomy under anesthesia with nitrous oxide. Anesthesia and Analgesia 1990;71(6):591‐6. [PUBMED: 2240629] [DOI] [PubMed] [Google Scholar]
Krogh 1994 {published data only}
- Krogh B, Jørn Jensen P, Henneberg SW, Hole P, Kronborg O. Nitrous oxide does not influence operating conditions or postoperative course in colonic surgery. British Journal of Anaesthesia 1994;72(1):55‐7. [PUBMED: 8110552] [DOI] [PubMed] [Google Scholar]
Lampe 1990 {published data only}
- Lampe GH, Wauk LZ, Donegan JH, Pitts LH, Jackler RK, Litt LL, et al. Effect on outcome of prolonged exposure of patients to nitrous oxide. Anesthesia and Analgesia 1990;71(6):586‐90. [PUBMED: 2240628] [PubMed] [Google Scholar]
Larsen 2000 {published data only}
- Larsen B, Seitz A, Larsen R. Recovery of cognitive function after remifentanil‐propofol anesthesia: a comparison with desflurane and sevoflurane anesthesia. Anesthesia and Analgesia 2000;90(1):168‐74. [PUBMED: 10624999] [DOI] [PubMed] [Google Scholar]
Lee 2005 {published data only}
- Lee LH, Irwin MG, Lui SK. Intraoperative remifentanil infusion does not increase postoperative opioid consumption compared with 70% nitrous oxide. Anesthesiology 2005;102(2):398‐402. [PUBMED: 15681957] [DOI] [PubMed] [Google Scholar]
Leung 2006 {published data only}
- Leung JM, Sands LP, Vaurio LE, Wang Y. Nitrous oxide does not change the incidence of postoperative delirium or cognitive decline in elderly surgical patients. British Journal of Anaesthesia 2006;96(6):754‐60. [PUBMED: 16670110] [DOI] [PubMed] [Google Scholar]
Lonie 1986 {published data only}
- Lonie DS, Harper NJN. Nitrous oxide anaesthesia and vomiting. The effect of nitrous oxide anaesthesia on the incidence of vomiting following gynaecological laparoscopy. Anaesthesia 1986;41(7):703‐7. [PUBMED: 2944432] [DOI] [PubMed] [Google Scholar]
Mraovic 2008 {published data only}
- Mraovic B, Simurina T, Sonicki Z, Skitarelic N, Gan TJ. The dose‐response of nitrous oxide in postoperative nausea in patients undergoing gynecologic laparoscopic surgery: a preliminary study. Anesthesia and Analgesia 2008;107(3):818‐23. [PUBMED: 18713890] [DOI] [PubMed] [Google Scholar]
Myles 2008a {published data only}
- Myles PS, Chan MT, Kaye DM, McIlroy DR, Lau CW, Symons JA, et al. Effect of nitrous oxide anesthesia on plasma homocysteine and endothelial function. Anesthesiology 2008;109(4):657‐63. [PUBMED: 18813045] [DOI] [PubMed] [Google Scholar]
Paredi 1994 {published data only}
- Paredi G, Sofi G, Bonazzi M, Bianchi de Grazia L, Giacomello R, Paladini C, et al. [Role of nitrous oxide on the incidence of postoperative emesis: Randomized, double blinded study]. Acta Anaesthesiologica Italica 1994;45:239‐42. [EMBASE: 1995129835] [Google Scholar]
Pedersen 1993 {published data only}
- Pedersen FM, Wilken‐Jensen C, Knudsen F, Lindekaer AL, Svare EI. The influence of nitrous oxide on recovery of bowel function after abdominal hysterectomy. Acta Anaesthesiologica Scandinavica 1993;37(7):692‐6. [PUBMED: 8249560] [DOI] [PubMed] [Google Scholar]
Sengupta 1988 {published data only}
- Sengupta P, Plantevin OM. Nitrous oxide and day‐case laparoscopy: effects on nausea, vomiting and return to normal activity. British Journal of Anaesthesia 1988;60(5):570‐3. [PUBMED: 2967712] [DOI] [PubMed] [Google Scholar]
Short 1985 {published data only}
- Short SM, Rutherfoord CF, Sebel PS. A comparison between isoflurane and alfentanil supplemented anaesthesia for short procedures. Anaesthesia 1985;40(12):1160‐4. [PUBMED: 3936374] [DOI] [PubMed] [Google Scholar]
Singh 2011 {published data only}
- Singh GP, Prabhakar H, Bithal PK, Dash HH. A comparative evaluation of nitrous oxide‐isoflurane vs isoflurane anesthesia in patients undergoing craniotomy for supratentorial tumors: A preliminary study. Neurology India 2011;59(1):18‐24. [PUBMED: 21339653] [DOI] [PubMed] [Google Scholar]
Sukhani 1994 {published data only}
- Sukhani R, Lurie J, Jabamoni R. Propofol for ambulatory gynecologic laparoscopy: does omission of nitrous oxide alter postoperative emetic sequelae and recovery?. Anesthesia and Analgesia 1994;78(5):831‐5. [PUBMED: 8160978] [DOI] [PubMed] [Google Scholar]
Todd 1993 {published data only}
- Todd MM, Warner DS, Sokoll MD, Maktabi MA, Hindman BJ, Scamman FL, et al. A prospective, comparative trial of three anesthetics for elective supratentorial craniotomy. Propofol/fentanyl, isoflurane/nitrous oxide, and fentanyl/nitrous oxide. Anesthesiology 1993;78(6):1005‐20. [PUBMED: 8512094] [DOI] [PubMed] [Google Scholar]
Vanacker 1999 {published data only}
- Vanacker BF. The impact of nitrous oxide on postoperative nausea and vomiting after desflurane anesthesia for breast surgery. Acta anaesthesiologica Belgica 1999;50(2):77‐81. [PUBMED: 10418646] [PubMed] [Google Scholar]
Van Hemelrijck 1991 {published data only}
- Hemelrijck J, Smith I, White PF. Use of desflurane for outpatient anesthesia. A comparison with propofol and nitrous oxide. Anesthesiology 1991;75(2):197‐203. [PUBMED: 1830462] [DOI] [PubMed] [Google Scholar]
Yoshimura 2014 {published data only}
- Yoshimura T, Ueda K, Kakinuma A, Sawai J, Nakata Y. Bronchial blocker lung collapse technique: nitrous oxide for facilitating lung collapse during one‐lung ventilation with a bronchial blocker. Anesthesia and Analgesia 2014;118(3):666‐70. [PUBMED: 24557112] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Antonini 1994 {published data only}
- Antonini M, D'Errico RR, Della Rocca G, Oliva P, Coccia C, Pastore E. [Anesthesia in laparoscopic cholecystectomy: Evaluation of the use of nitrous oxide]. Acta Anaesthesiologica Italica 1994;45(2 Suppl):243‐9. [EMBASE: 1995129836] [Google Scholar]
Atanassoff 1994 {published data only}
- Atanassoff PG, Alon E, Weiss BM. Intercostal nerve block for lumpectomy: superior postoperative pain relief with bupivacaine. Journal of Clinical Anesthesia 1994;6(1):47‐51. [PUBMED: 8142099] [DOI] [PubMed] [Google Scholar]
Atassi 2005 {published data only}
- Atassi K, Mangiapan G, Fuhrman C, Lasry S, Onody P, Housset B. Prefixed equimolar nitrous oxide and oxygen mixture reduces discomfort during flexible bronchoscopy in adult patients: a randomized, controlled, double‐blind trial. Chest 2005;128(2):863‐8. [PUBMED: 16100179] [DOI] [PubMed] [Google Scholar]
Barr 1999 {published data only}
- Barr G, Jakobsson JG, Owall A, Anderson RE. Nitrous oxide does not alter bispectral index: study with nitrous oxide as sole agent and as an adjunct to i.v. anaesthesia. British Journal of Anaesthesia 1999;82(6):827‐30. [PUBMED: 10562773] [DOI] [PubMed] [Google Scholar]
Bronco 2010 {published data only}
- Bronco A, Ingelmo PM, Aprigliano M, Turella M, Sahillioğlu E, Bucciero M, et al. Xenon anaesthesia produces better early postoperative cognitive recovery than sevoflurane anaesthesia. European Journal of Anaesthesiology 2010;27(10):912‐6. [PUBMED: 20523212] [DOI] [PubMed] [Google Scholar]
Castéra 2001 {published data only}
- Castéra L, Nègre I, Samii K, Buffet C. Patient‐administered nitrous oxide/oxygen inhalation provides safe and effective analgesia for percutaneous liver biopsy: A randomized placebo‐controlled trial. American Journal of Gastroenterology 2001;96(5):1553‐7. [PUBMED: 11374698] [DOI] [PubMed] [Google Scholar]
Cheong 2000 {published data only}
- Cheong KF, Choy JM. Sevoflurane‐fentanyl versus etomidate‐fentanyl for anesthetic induction in coronary artery bypass graft surgery patients. Journal of Cardiothoracic Vascular Anesthesia 2000;14(4):421‐4. [PUBMED: 10972608] [DOI] [PubMed] [Google Scholar]
Divatia 1996 {published data only}
- Divatia JV, Vaidya JS, Badwe RA, Hawaldar RW. Omission of nitrous oxide during anesthesia reduces the incidence of postoperative nausea and vomiting. A meta‐analysis. Anesthesiology 1996;85(5):1055‐62. [PUBMED: 8916823] [DOI] [PubMed] [Google Scholar]
Dover 1994 {published data only}
- Dover SB, Plenderleith L, Moore MR, McColl KEL. Safety of general anaesthesia and surgery in acute hepatic porphyria. Gut 1994;35(8):1112‐5. [PUBMED: 7926916] [DOI] [PMC free article] [PubMed] [Google Scholar]
Einarsson 1997 {published data only}
- Einarsson S, Cerne A, Bengtsson A, Stenqvist O, Bengtson JP. Should nitrous oxide be discontinued before desflurane after anaesthesia with desflurane/N2O?. Acta Anaesthesiologica Scandinavica 1997;41(10):1285‐91. [PUBMED: 9422294] [DOI] [PubMed] [Google Scholar]
Fredman 1998 {published data only}
- Fredman B, Zohar E, Philipov A, Olsfanger D, Shalev M, Jedeikin R. The induction, maintenance, and recovery characteristics of spinal versus general anesthesia in elderly patients. Journal of Clinical Anesthesia 1998;10(8):623‐30. [PUBMED: 9873961] [DOI] [PubMed] [Google Scholar]
Gozdemir 2007 {published data only}
- Gozdemir M, Sert H, Yilmaz N, Kanbak O, Usta B, Demircioglu RI. Remifentanil‐propofol in vertebral disk operations: Hemodynamics and recovery versus desflurane‐n(2)O inhalation anesthesia. Advances in Therapy 2007;24(3):622‐31. [PUBMED: 17660173] [DOI] [PubMed] [Google Scholar]
Haessler 1993 {published data only}
- Haessler R, Schwender D, Leppmeier U, Klasing S, Rindfleisch F, Peter K. Anaesthesia for coronary artery bypass grafting: opioid‐analgesia combined with either flunitrazepam, propofol or isoflurane. Acta Anaesthesiologica Scandinavica 1993;37(6):532‐40. [PUBMED: 8213015] [DOI] [PubMed] [Google Scholar]
Haraguchi 1995 {published data only}
- Haraguchi N, Furusawa H, Takezaki R, Oi K. Inhalation sedation with sevoflurane: a comparative study with nitrous oxide. Journal of Oral and Maxillofacial Surgery 1995;53(1):24‐6; discussion 26‐7. [PUBMED: 7799117] [DOI] [PubMed] [Google Scholar]
Heath 1996 {published data only}
- Heath KJ, Sadler P, Winn JH, McFadzean WA. Nitrous oxide reduces the cost of intravenous anaesthesia. European Journal of Anaesthesiology 1996;13(4):369‐72. [PUBMED: 8842658] [DOI] [PubMed] [Google Scholar]
Holst 1993 {published data only}
- Holst D, Anger C, Bauch HJ. [N2O‐supplemented intravenous anesthesia versus inhalation anesthesia. A comparative study of the sympathoadrenergic reaction and postoperative vigilance]. Anästhesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie: AINS 1993;28(1):18‐22. [PUBMED: 8467026] [DOI] [PubMed] [Google Scholar]
Ishii 1994 {published data only}
- Ishii K, Kobayashi T, Kitajima T, Ogata H. [Neuromuscular blockade with vecuronium and its reversal with edrophonium during total intravenous anesthesia, neuroleptanalgesia and sevoflurane anesthesia]. Masui. The Japanese Journal of Anesthesiology 1994;43(8):1196‐200. [PUBMED: 7933501] [PubMed] [Google Scholar]
Jastak 1973 {published data only}
- Jastak JT, Gorretta C. Ketamine HCl as a continuous‐drip anesthetic for outpatients. Anesthesia and Analgesia 1973;52(3):341‐4. [PUBMED: 4735984] [PubMed] [Google Scholar]
Jellish 1996 {published data only}
- Jellish WS, Lien CA, Jerrel Fontenot H, Hall R. The comparative effects of sevoflurane versus propofol in the induction and maintenance of anesthesia in adult patients. Anesthesia and Analgesia 1996;82(3):479‐85. [PUBMED: 8623947] [DOI] [PubMed] [Google Scholar]
Johnson 1997 {published data only}
- Johnson GW, John Gray H. Nitrous oxide inhalation as an adjunct to intravenous induction of general anaesthesia with propofol for day surgery. European Journal of Anaesthesiology 1997;14(3):295‐9. [PUBMED: 9202917] [DOI] [PubMed] [Google Scholar]
Kryshtalskyj 1990 {published data only}
- Kryshtalskyj B, Direnfeld VN, Johnson TW. Use of low‐dose ketamine hydrochloride in outpatient oral surgery. Oral Surgery, Oral Medicine, and Oral Pathology 1990;69(4):413‐9. [PUBMED: 2326032] [DOI] [PubMed] [Google Scholar]
Lim 1992 {published data only}
- Lim BL, Low TC. Total intravenous anaesthesia versus inhalational anaesthesia for dental day surgery. Anaesthesia and Intensive Care 1992;20(4):475‐8. [PUBMED: 1463176] [DOI] [PubMed] [Google Scholar]
Losasso 1992 {published data only}
- Losasso TJ, Muzzi DA, Dietz NM, Cucchiara RF. Fifty percent nitrous oxide does not increase the risk of venous air embolism in neurosurgical patients operated upon in the sitting position. Anesthesiology 1992;77(1):21‐30. [PUBMED: 1609997] [DOI] [PubMed] [Google Scholar]
Masood 2002 {published data only}
- Masood J, Shah N, Lane T, Andrews H, Simpson P, Barua JM. Nitrous oxide (Entonox) inhalation and tolerance of transrectal ultrasound guided prostate biopsy: a double‐blind randomized controlled study. Journal of Urology 2002;168(1):116‐20; discussion 120. [PUBMED: 12050503] [PubMed] [Google Scholar]
Morimoto 1997 {published data only}
- Morimoto Y, Matsumoto S, Nakamura M, Makino A, Tamura T, Oka H, et al. [Total intravenous anesthesia with propofol and fentanyl for laparoscopic cholecystectomy]. Masui. The Japanese Journal of Anesthesiology 1997;46(9):1242‐5. [PUBMED: 9311219] [PubMed] [Google Scholar]
Nightingale 1992 {published data only}
- Nightingale JJ, Lewis IH. Recovery from day‐case anaesthesia: comparison of total i.v. anaesthesia using propofol with an inhalation technique. British Journal of Anaesthesia 1992;68(4):356‐9. [PUBMED: 1642912] [DOI] [PubMed] [Google Scholar]
Nishiyama 1998 {published data only}
- Nishiyama T, Hanaoka K. [Anesthesia induction for laryngeal mask insertion‐‐comparison among sevoflurane, isoflurane and propofol]. Masui. The Japanese Journal of Anesthesiology 1998;47(11):1320‐4. [PUBMED: 9852695] [PubMed] [Google Scholar]
Ogg 1983 {published data only}
- Ogg TW, Jennings RA, Morrison CG. Day‐case anaesthesia for termination of pregnancy. Evaluation of a total intravenous anaesthetic technique. Anaesthesia 1983;38(11):1042‐6. [PUBMED: 6638451] [DOI] [PubMed] [Google Scholar]
Rocca 2000 {published data only}
- Rocca G, Montecchi C, Baisi F, Monaco S, Romboli D, Gasparetto A. [N2O‐free sevoflurane anesthesia. Clinical evaluation]. Minerva Anestesiologica 2000;66(9):611‐9. [PUBMED: 11070960] [PubMed] [Google Scholar]
Saïssy 2000 {published data only}
- Saïssy JM. Simplified use of mixed propofol and alfentanil for anesthesia in remote locations. Military Medicine 2000;165(3):195‐9. [PUBMED: 10741082] [PubMed] [Google Scholar]
Simpson 1977 {published data only}
- Simpson P, Forsling M. The effects of halothane on plasma vasopressin during cardio‐pulmonary bypass. Clinical Endocrinology (Oxford) 1977;7(1):33‐9. [PUBMED: 328188] [DOI] [PubMed] [Google Scholar]
Sinha 2006 {published data only}
- Sinha PK, Neema PK, Unnikrishnan KP, Varma PK, Jaykumar K, Rathod RC. Effect of lung ventilation with 50% oxygen in air or nitrous oxide versus 100% oxygen on oxygenation index after cardiopulmonary bypass. Journal of Cardiothoracic and Vascular Anesthesia 2006;20(2):136‐42. [PUBMED: 16616650] [DOI] [PubMed] [Google Scholar]
Smith 1993 {published data only}
- Smith I, Ding Y, White PF. Muscle pain after outpatient laparoscopy‐‐influence of propofol versus thiopental and enflurane. Anesthesia and Analgesia 1993;76(6):1181‐4. [PUBMED: 8498652] [DOI] [PubMed] [Google Scholar]
Taki 2003 {published data only}
- Taki K, Sugimura M, Morimoto Y, Hirose Y, Ueda J, Okada K, et al. [The influences of nitrous oxide inhalation during general anesthesia on postoperative nausea and vomiting]. Journal of Japanese Dental Society of Anesthesiology 2003;31(3):268‐73. [EMBASE: 2003322122] [Google Scholar]
Towey 1979 {published data only}
- Towey RM, Stanford BJ, Ballard RM, Gilbert JR. Morbidity of day‐case gynaecological surgery. A comparison of thiopentone and Althesin. British Journal of Anaesthesia 1979;51(5):453‐5. [PUBMED: 444346] [DOI] [PubMed] [Google Scholar]
Van den Berg 1995 {published data only}
- Berg AA, Savva D, Honjol NM, Prabhu NV. Comparison of total intravenous, balanced inhalational and combined intravenous‐inhalational anaesthesia for tympanoplasty, septorhinoplasty and adenotonsillectomy. Anaesthesia and Intensive Care 1995;23(5):574‐82. [PUBMED: 8787257] [DOI] [PubMed] [Google Scholar]
Vari 2010 {published data only}
- Vari A, Gazzanelli S, Cavallaro G, Toma G, Tarquini S, Guerra C, et al. Post‐Operative Nausea and Vomiting (PONV) after thyroid surgery: A prospective, randomized study comparing totally intravenous versus inhalational anesthetics. The American Surgeon 2010;76(3):325‐8. [PUBMED: 20349666] [PubMed] [Google Scholar]
Wesner 2005 {published data only}
- Wesner L, Marone LK, Dennehy KC. Anesthesia for lower extremity bypass. International Anesthesiology Clinics 2005;43(1):93‐109. [PUBMED: 15632519] [DOI] [PubMed] [Google Scholar]
Yamakage 2001 {published data only}
- Yamakage M, Chen X, Kamada Y, Tsujiguchi N, Namiki A. [Changes in sedative level during induction of anesthesia using a single volatile anesthetic]. Masui. The Japanese Journal of Anesthesiology 2001;50(4):383‐6. [PUBMED: 11345750] [PubMed] [Google Scholar]
Yang 2004 {published data only}
- Yang H, Choi PTL, McChesney J, Buckley N. Induction with sevoflurane‐remifentanil is comparable to propofol‐fentanyl‐rocuronium in PONV after laparoscopic surgery. Canadian Journal of Anaesthesia 2004;51(7):660‐7. [PUBMED: 15310632] [DOI] [PubMed] [Google Scholar]
Zuurmond 1986 {published data only}
- Zuurmond WW, Leeuwen L. Alfentanil v. isoflurane for outpatient arthroscopy. Acta Anaesthesiologica Scandinavica 1986;30(4):329‐31. [PUBMED: 3090845] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Adams 1994 {published data only}
- Adams HA, Schmitz CS, Baltes‐Götz B. [Endocrine stress reaction, hemodynamics and recovery in total intravenous and inhalation anesthesia. Propofol versus isoflurane]. Der Anaesthesist 1994;43(11):730‐7. [PUBMED: 7840401] [DOI] [PubMed] [Google Scholar]
Miralles Pardo 1991 {published data only}
- Miralles Pardo FS, Robles García E, Moreno Cuesta J, Iranzo Reverter J, Sánchez Ortega JL, López Rodríguez F. [Intravenous perfusion of 2,6‐diisopropylphenol (propofol) in the maintenance of emergency anesthesia]. Revista Española de Anestesiología y Reanimación 1991;38(3):162‐6. [PUBMED: 1961960] [PubMed] [Google Scholar]
Moussa 1995 {published data only}
- Moussa AM, Geaisa KN. Comparison of the recovery characteristics of propofol total intravenous anaesthesia and isoflurane inhalation anaesthesia for dental day surgery. Egyptian Dental Journal 1995;41(4):1451‐5. [PUBMED: 9497696] [PubMed] [Google Scholar]
Rashchupkin 2011 {published data only}
- Rashchupkin AB, Burov NE. [Low‐flow xenon anesthesia in surgical patients with hypertension]. Anesteziologiia i reanimatologiia 2011;2(2):4‐7. [PUBMED: 21692217] [PubMed] [Google Scholar]
Röpcke 2001 {published data only}
- Röpcke H, Wartenberg HC, Konen‐Bergmann M, Hoeft A. [The interaction of desflurane and nitrous oxide on EEG during surgery is additive]. Anästhesiologie und Intensivmedizin 2001;42(1):16‐22. [EMBASE: 2001064587] [Google Scholar]
Schaffranietz 2000 {published data only}
- Schaffranietz L, Rudolph C, Heinke W, Olthoff D. [Is the combination of nitrous oxide and hyperventilation in elective neurosurgical operations useful?]. Anaesthesiologie und Reanimation 2000;25(4):88‐95. [PUBMED: 11132399] [PubMed] [Google Scholar]
Segatto 1993 {published data only}
- Segatto A, Vincenti E, Valenti S, Sammons AW, Bianchi E, Morossi M, et al. [TIVA with propofol vs thiopental‐nitrous oxide anaesthesia for uterine cerclage (A prospective randomized clinical study on 200 pregnant patients)]. Acta Anaesthesiologica Italica 1993;44(Suppl 1):59‐65. [EMBASE: 1994014491] [Google Scholar]
Shulunov 2002 {published data only}
- Shulunov MV, Burov NE, Ibragimova GV. [Neurohumoral effect of xenon]. Anesteziologiia i reanimatologiia 2002;3:32‐5. [PUBMED: 12221873] [PubMed] [Google Scholar]
Additional references
Apfel 2004
- Apfel CC, Roewer N. [Postoperative nausea and vomiting]. Der Anaesthesist 2004;53(4):377‐89. [PUBMED: 15190867] [DOI] [PubMed] [Google Scholar]
Becker 2008
- Becker DE, Rosenberg M. Nitrous oxide and the inhalation anesthetics. Anesthesia Progress 2008;55(4):124‐30. [PUBMED: 19108597] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2011
- Chan MT, Wan AC, Gin T, Leslie K, Myles PS. Chronic postsurgical pain after nitrous oxide anesthesia. Pain 2011;152(11):2514‐20. [PUBMED: 21889262] [DOI] [PubMed] [Google Scholar]
Chaparro 2013
- Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD008307.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheney 2007
- Cheney FW. An early example of evidence‐based medicine: hypoxemia due to nitrous oxide. Anesthesiology 2007;106(1):186‐8. [PUBMED: 17197861] [DOI] [PubMed] [Google Scholar]
Culley 2007
- Culley DJ, Raghavan SV, Waly M, Baxter MG, Yukhananov R, Deth RC, et al. Nitrous oxide decreases cortical methionine synthase transiently but produces lasting memory impairment in aged rats. Anesthesia and Analgesia 2007;105(1):83‐8. [PUBMED: 17578961] [DOI] [PubMed] [Google Scholar]
de Vasconcellos 2013
- Vasconcellos K, Sneyd JR. Nitrous oxide: are we still in equipoise? A qualitative review of current controversies. British Journal of Anaesthesia 2013;111(6):877‐85. [PUBMED: 23801743] [DOI] [PubMed] [Google Scholar]
Eger 1965
- Eger EI 2nd, Saidman LJ. Hazards of nitrous oxide anaesthesia in bowel obstruction and pneumothorax. Anesthesiology 1965;26:61‐6. [PUBMED: 14257336] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629–34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernández‐Guisasola 2010
- Fernández‐Guisasola J, Gómez‐Arnau JI, Cabrera Y, Valle SG. Association between nitrous oxide and the incidence of postoperative nausea and vomiting in adults: a systematic review and meta‐analysis. Anaesthesia 2010;65(4):379‐87. [PUBMED: 20151955] [DOI] [PubMed] [Google Scholar]
Gruss 2004
- Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP. Two‐pore‐domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Molecular Pharmacology 2004;65(2):443‐52. [PUBMED: 14742687] [DOI] [PubMed] [Google Scholar]
Guirguis 1990
- Guirguis SS, Pelmear PL, Roy ML, Wong L. Health effects associated with exposure to anaesthetic gases in Ontario hospital personnel. British Journal of Industrial Medicine 1990;47(7):490‐7. [PUBMED: 2383519] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Imberger 2014
- Imberger G, Orr A, Thorlund K, Wetterslev J, Myles P, Møller AM. Does anaesthesia with nitrous oxide affect mortality or cardiovascular morbidity? A systematic review with meta‐analysis and trial sequential analysis. British Journal of Anaesthesia 2014;112(3):410‐26. [PUBMED: 24408738] [DOI] [PubMed] [Google Scholar]
Jevtović‐Todorović 1998
- Jevtović‐Todorović V, Todorović SM, Mennerick S, Powell S, Dikranian K, Benshoff N, et al. Nitrous oxide (laughing gas) is an NMDA antagonist, neuroprotectant and neurotoxin. Nature Medicine 1998;4(4):460‐3. [PUBMED: 9546794] [DOI] [PubMed] [Google Scholar]
Khan 2013
- Khan FA, Ullah H. Pharmacological agents for preventing morbidity associated with the haemodynamic response to tracheal intubation. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD004087.pub2] [DOI] [PubMed] [Google Scholar]
Kripke 1987
- Kripke BJ, Kupferman A, Luu KC. Suppression of chemotaxis to corneal inflammation by nitrous oxide. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi [Chinese Journal of Microbiology and Immunology] 1987;20(4):302‐10. [PUBMED: 3130236] [PubMed] [Google Scholar]
Lehmberg 2008
- Lehmberg J, Waldner M, Baethmann A, Uhl E. Inflammatory response to nitrous oxide in the central nervous system. Brain Research 2008;1246:88‐95. [PUBMED: 18929548] [DOI] [PubMed] [Google Scholar]
Linde 1969
- Linde HW, Bruce DL. Occupational exposure of anesthetists to halothane, nitrous oxide and radiation. Anesthesiology 1969;30(4):363‐8. [PUBMED: 5773945] [DOI] [PubMed] [Google Scholar]
Myles 2008b
- Myles PS, Chan MT, Leslie K, Peyton P, Paech M, Forbes A. Effect of nitrous oxide on plasma homocysteine and folate in patients undergoing major surgery. British Journal of Anaesthesia 2008;100(6):780‐6. [PUBMED: 18400808] [DOI] [PubMed] [Google Scholar]
Parbrook 1967
- Parbrook GD. Techniques of inhalational analgesia in the postoperative period. British Journal of Anaesthesia 1967;39(9):730‐5. [PUBMED: 6051234 ] [DOI] [PubMed] [Google Scholar]
Pasternak 2009
- Pasternak JJ, McGregor DG, Lanier WL, Schroeder DR, Rusy DA, Hindman B, et al. IHAST Investigators. Effect of nitrous oxide use on long‐term neurologic and neuropsychological outcome in patients who received temporary proximal artery occlusion during cerebral aneurysm clipping surgery. Anesthesiology 2009;110(3):563‐73. [PUBMED: 19212259] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perry 1983
- Perry J, Chanarin I, Deacon R, Lumb M. Chronic cobalamin inactivation impairs folate polyglutamate synthesis in the rat. The Journal of Clinical Investigation 1983;71(5):1183‐90. [PUBMED: 6853707] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rowland 1995
- Rowland AS, Baird DD, Shore DL, Weinberg CR, Savitz DA, Wilcox AJ. Nitrous oxide and spontaneous abortion in female dental assistants. American Journal of Epidemiology 1995;141(6):531‐8. [PUBMED: 7900720] [DOI] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann H, Brożek J, Oxman A (editors). GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2 [updated March 2009]. The GRADE Working Group, 2009. Available from www.cc‐ims.net/gradepro.
Stata 11.0 [Computer program]
- StataCorp LP. Stata. Version 11.0. College Station: StataCorp LP, 2009.
Tramèr 1996
- Tramèr M, Moore A, McQuay H. Omitting nitrous oxide in general anaesthesia: meta‐analysis of intraoperative awareness and postoperative emesis in randomized controlled trials. British Journal of Anaesthesia 1996;76(2):186‐93. [PUBMED: 8777095] [DOI] [PubMed] [Google Scholar]
Yamakura 2000
- Yamakura T, Borghese C, Harris RA. A transmembrane site determines sensitivity of neuronal nicotinic acetylcholine receptors to general anesthetics. The Journal of Biological Chemistry 2000;275(52):40879‐86. [PUBMED: 11020384] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Yang 2011
- Yang K, Jia WQ, Tian J, Ma B, Liu Y, Jia RH, et al. Nitrous oxide‐based techniques versus nitrous oxide‐free techniques for general anaesthesia. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008984] [DOI] [PMC free article] [PubMed] [Google Scholar]


