Abstract
We studied the cross-resistance to three highly toxic Bacillus sphaericus strains, IAB-59 (serotype H6), IAB-881 (serotype H3), and IAB-872 (serotype H48), of four colonies of the Culex pipiens complex resistant to B. sphaericus 2362 and 1593, both of which are serotype H5a5b strains. Two field-selected highly resistant colonies originating from India (KOCHI, 17,000-fold resistance) and France (SPHAE, 23,000-fold resistance) and a highly resistant laboratory-selected colony from California (GeoR, 36,000-fold resistance) showed strong cross-resistance to strains IAB-881 and IAB-872 but significantly weaker cross-resistance to IAB-59 (3- to 43-fold resistance). In contrast, a laboratory-selected California colony with low-level resistance (JRMM-R, 5-fold resistance) displayed similar levels of resistance (5- to 10-fold) to all of the B. sphaericus strains tested. Thus, among the mosquitocidal strains of B. sphaericus we identified a strain, IAB-59, which was toxic to several Culex colonies that were highly resistant to commercial strains 2362 and 1593. Our analysis also indicated that strain IAB-59 may possess other larvicidal factors. These results could have important implications for the development of resistance management strategies for area-wide mosquito control programs based on the use of B. sphaericus preparations.
Bacillus sphaericus has been used to control Culex pipiens pipiens and C. pipiens quinquefasciatus mosquito larvae since the late 1980s, and in some areas it is also used to control Anopheles spp. (7, 10, 11). This organism has several advantages, including low environmental toxicity due to the high specificity of B. sphaericus toxins, high levels of efficacy and environmental persistence, and the ability to overcome resistance developed against conventional insecticides used worldwide. Only a few of the highly larvicidal B. sphaericus strains are sold commercially; strain 2362 (e.g., VectoLex and Spherimos) is sold in the United States and Europe, strain 1593 (e.g., Biocide-S) is sold in India, and strain C3-41 is sold in the People's Republic of China. For unknown reasons, some free-living B. sphaericus strains have strong larvicidal activity directly related to the presence of a paraspore protein crystal produced during sporulation (3, 37). This crystal contains two major polypeptides, a 42-kDa polypeptide and a 51-kDa polypeptide, which are designated BinA and BinB, respectively (21). The mode of action of the toxin complex in susceptible mosquitoes involves highly specific binding to a receptor in the larval midgut (14, 18, 29, 31). The two crystal components act synergistically; the BinB part is responsible for initial binding to the receptor (2), and the BinA component confers toxicity (13, 17).
Resistance to B. sphaericus has been reported in B. sphaericus-treated field populations of the C. pipiens complex in Brazil (32) and India (22) and C. pipiens pipiens in France (33) and China (38). Two independent laboratory selections with California mosquitoes (C. pipiens quinquefasciatus) have also led to resistance (25, 36). Levels of stable laboratory-selected resistance of between 35-fold and more than 100,000-fold have been reported, suggesting that there may be different resistance mechanisms. Investigations of the mechanisms and genetics of resistance to B. sphaericus have been carried out for some of the resistant populations (15, 16, 36).
As resistance to B. sphaericus is likely to occur under certain conditions, further investigation of the variation in the toxic activities and specificities of natural B. sphaericus strains is required. All of the B. sphaericus-resistant C. pipiens populations were selected on strain 2362, 1593, or C3-41 (15, 22, 25, 38); all of these strains belong to the same serotype and have identical genes encoding the binary toxin. However, there are small differences in the amino acid sequences of the B. sphaericus Bin toxins (1, 8, 21), which may be important in the structure and function of the toxin-receptor complex and therefore for larvicidal activity.
We investigated three new B. sphaericus strains which belong to different serotypes and which express binary toxins, whose crystal toxin gene sequences were known or not known at the time of the study (35). These strains were IAB-59 (serotype H6), IAB-872 (serotype H48), and IAB-881 (serotype H3), all of which are highly toxic compared with commercial strain 2362. The sequences of the binary toxin genes of IAB-59 were determined in 1989 (1). The sequences of the binary toxin genes of IAB-881 and IAB-872 were recently determined (after the completion of this study) and were found to be identical to the sequences of IAB-59 (8).
The aim of this study was to test four B. sphaericus-resistant C. pipiens colonies for susceptibility and cross-resistance to the three new highly toxic B. sphaericus strains, which have not been used in the field yet, in order to investigate the possibility of overcoming resistance to B. sphaericus strains 2362 and 1593 by using other B. sphaericus strains. Such strains could be used as alternatives to strains 2362 and 1593 for future management of the development of resistance to strains used commercially.
MATERIALS AND METHODS
B. sphaericus strains.
The experiments were conducted with four B. sphaericus strains. Three of these strains were highly toxic and were isolated in Ghana, and they were members of the following serotypes: IAB-59, serotype H6; IAB-872, serotype H48; and IAB-881, serotype H3 (35). The fourth strain was commercial B. sphaericus reference strain 2362 (serotype H5a5b), which was isolated in Nigeria. All strains were obtained from the Pasteur Institute Collection of Entomopathogenic Bacilli. Strains IAB-59, IAB-872, and IAB-881 were prepared as lactose-precipitated acetone powders (4) from 72-h sporulated cultures in MBS medium in 5-liter fermentors (9) at the Entomopathogenic Bacteria Unit of the Pasteur Institute. A standard B. sphaericus powder, SPH-88, consisting of a lyophilized whole culture of strain 2362 from the Pasteur Institute, was used as a positive reference strain in all bioassays. This preparation has an activity of 1,200 International Toxic Units (ITU)/mg against the C. pipiens pipiens IP strain (Pasteur Institute).
Protein analysis.
Protein contents were determined by using 100 mg of each powder, which was solubilized by incubation for 1 h in 10 ml of 50 mM NaOH at 37°C with shaking and then centrifuged at 8,000 × g for 30 min. The protein concentrations of the supernatants were determined by the Bradford protein assay (6), using bovine serum albumin as a standard. The equivalent of 250 μg of solubilized powder for each strain was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12) in a 10% polyacrylamide gel. We used broad-range protein molecular weight standards obtained from New England BioLabs (reference no. 7701L) in this analysis.
Insect colonies.
The following three susceptible and four resistant colonies of C. pipiens pipiens and C. pipiens quinquefasciatus were investigated in this study: (i) JRMM-R, a laboratory-selected field colony of C. pipiens quinquefasciatus with fivefold resistance, and its parental susceptible colony, JRMM-S (F-S and F-SEL, respectively) (25, 26); (ii) KOCHI, a C. pipiens quinquefasciatus colony with high-level (>2,000-fold) resistance to B. sphaericus that was field selected from an area of southern India treated with B. sphaericus strain 1593M (22), and Madurai, a susceptible laboratory colony of C. pipiens quinquefasciatus; (iii) SPHAE, a C. pipiens pipiens colony with high-level (>50,000fold) resistance that was generated by field selection from an area of southern France treated with B. sphaericus strain 2362 (16, 33); and (iv) GeoR, a C. pipiens quinquefasciatus colony with high-level (>50,000-fold) resistance that was produced by G. P. Georghiou, who selected field-collected larvae from California with B. sphaericus 2362 in the laboratory (15, 36). The SPHAE and GeoR colonies were established from egg rafts kindly provided by Nicole Pasteur (University of Montpellier II, Montpellier, France) (SPHAE) and by G. P. Georghiou and Margareth Wirth (University of California, Riverside) (GeoR) and were reared at the Pasteur Institute in the Entomopathogenic Bacteria Unit. A C. pipiens pipiens colony (IP) that originated from southern France and was reared at the Pasteur Institute for more than 15 years was used as a susceptible reference colony when GeoR and SPHAE colonies were tested.
Insect toxicity assays.
Bioassays were carried out during 1995 and 1996 in the following three laboratories: Centre for Research in Medical Entomology, Madurai, India, for the resistant KOCHI and susceptible Madurai colonies; Department of Entomology, University of California, Riverside, for the resistant JRMM-R and susceptible JRMM-S colonies; and the Pasteur Institute, where the resistant SPHAE and GeoR colonies were compared with the susceptible IP colony. Identical bioassay protocols were used in all of the laboratories, and the test materials for the three laboratories were produced from the same B. sphaericus powders. According to the 1985 World Health Organization protocol, 50 mg of IAB-59, IAB-872, IAB-881, or 2362 powder per 10 ml was shaken with glass beads. Bioassays were conducted with duplicate groups of 25 L4 instars by using two replicates per concentration and five or six concentrations per test in three experiments carried out in plastic cups with 150-ml (final volume) portions of serial dilutions of the bacterial powder preparations; controls were exposed to only water. Mortality was recorded 48 h after treatment. For each strain tested, the five concentrations used were determined as required for determination of 50% lethal concentrations (LC50) and were then adapted to each colony, with some overlap. Strains IAB-881 and IAB-872 were not tested at concentrations greater than 267 mg/liter with the KOCHI colony or greater than 800 mg/liter with the GeoR and SPHAE colonies due to the high levels of resistance of the colonies to these strains.
Statistical analysis.
Probit regression analysis was carried out with POLO-PC (28) (LeOra Software POLO-PC, Berkeley, Calif.), and resistance ratios and 95% confidence intervals (CI) were calculated as described by Robertson and Preisler (24) for toxicity tests with JRMM-S and JRMM-R. For the other three colonies, resistance ratios and CI were determined by using the Probit software described by Raymond et al. (23), which tests the linearity of dose responses and estimates slopes, calculates lethal concentrations and 95% CI, tests whether two or more dose-mortality lines are parallel, and calculates resistance ratios and 95% CI. A resistance ratio was considered significantly different from 1 (P < 0.05) if its 95% CI did not include the value 1. Statistical analyses of LC50 and LC90 for different B. sphaericus strains within and between insect colonies were performed by one-way analysis of variance (ANOVA) and nonparametric one-way Kruskal-Wallis analysis (30), using the free version of R1.2.2 Splus software. Differences among strains and colonies were significant if P was less than 0.05.
RESULTS AND DISCUSSION
Our studies of four larvicidal B. sphaericus strains assayed with four B. sphaericus-resistant Culex colonies with different genetic backgrounds in three laboratories in different geographical locations gave similar results for all highly resistant colonies; cross-resistance to strain IAB-59 was weak, whereas cross-resistance to IAB-881 and IAB-872 was strong. In contrast, strong cross-resistance to all strains was observed for the colony with low-level resistance.
Larval toxicity tests.
The larval toxicity tests were performed with B. sphaericus powders, and lethal concentrations were expressed in milligrams of powder per liter. The protein contents of the strains differed. Strains IAB-881 and IAB-59 had more protein than IAB-872 and 2362 (20 ± 2 μg of protein per mg of powder for strain 2362, 29 ± 2 μg/mg for strain IAB-59, 10 ± 1 μg/mg for strain IAB-872, and 32 ± 2 μg/mg for strain IAB-881). However, both similar productivities and similar larvicidal activities were observed by Thiéry et al. (35) when they compared several IAB strains. The apparent differences in protein (toxin) content could influence the activity of the powder and the LC50 and LC90. However, ANOVA when the LC50 were compared indicated that there were not significant differences either among the three susceptible colonies (F = 1.707, P = 0.235, as determined by ANOVA) or among the four B. sphaericus strains (F = 1.388, P = 0.315, as determined by ANOVA). Thus, all four strains had similar levels of activity when they were tested with susceptible colonies. Equivalent results were found when LC90 were analyzed.
The comparative toxicities of the four B. sphaericus strains for the susceptible and resistant C. pipiens pipiens and C. pipiens quinquefasciatus colonies are shown in Tables 1 to 3, and resistance ratios for each B. sphaericus strain are shown in Table 4. The ratios are based on comparisons of the LC50 and LC90 for a B. sphaericus-resistant colony and a susceptible reference colony, carried out in each laboratory; the two colonies were tested at the same time. The absence of a significant difference between colonies susceptible to B. sphaericus strains made it possible to compare resistance ratios between resistant colonies.
TABLE 1.
Susceptibilities of a nonselected laboratory colony of C. pipiens quinquefasciatus (JRMM-S) and a selected field-collected resistant colony of C. pipiens quinquefasciatus (JRMM-R) to B. sphaericus spore crystal suspensions
| Colony | B. sphaericus strain | Slope (mean ± SE) | LC50 (95%CI) (mg/liter) | LC90 (mg/liter) | X2 (df) |
|---|---|---|---|---|---|
| JRMM-S (susceptible) | 2362 | 3.6 ± 0.3 | 0.010 (0.009–0.011) | 0.022 | 0.6 (3) |
| IAB-59 | 2.1 ± 0.1 | 0.018 (0.009–0.029) | 0.072 | 21.9 (3) | |
| IAB-881 | 1.8 ± 0.1 | 0.113 (0.072–0.172) | 0.571 | 19.8 (4) | |
| IAB-872 | 2.0 ± 0.2 | 0.021 (0.010–0.030) | 0.093 | 12.6 (4) | |
| JRMM-R (resistant) | 2362 | 2.0 ± 0.1 | 0.044 (0.011–0.082) | 0.189 | 33.5 (3) |
| IAB-59 | 2.3 ± 0.1 | 0.163 (0.109–0.220) | 0.588 | 12.6 (3) | |
| IAB-881 | 2.0 ± 0.2 | 0.647 (0.522–0.770) | 2.868 | 4.1 (4) | |
| IAB-872 | 1.9 ± 0.1 | 0.212 (0.190–0.234) | 0.992 | 1.9 (4) |
TABLE 3.
Susceptibilities of a nonselected laboratory colony C. pipiens quinquefasciatus (Madurai) and a selected field-collected resistant colony of C. pipiens quinquefasciatus (KOCHI) to B. sphaericus spore crystal suspensions
| Colony | B. sphaericus strain | Slope (mean ± SE) | LC50 (95% CI) (mg/liter) | LC90 (mg/liter) | X2 (df) |
|---|---|---|---|---|---|
| Madurai (susceptible) | 2362 | 1.32 ± 0.19 | 0.012 (0.007–0.019) | 0.108 | 16.6 (4) |
| IAB-59 | 1.11 ± 0.09 | 0.069 (0.058–0.085) | 0.992 | 2.79 (4) | |
| IAB-881 | 0.55 ± 0.8 | 1.31 (0.79–1.88) | 257.38 | 1.62 (4) | |
| IAB-872 | 0.54 ± 0.1 | 0.66 (0.398–0.971) | 163.6 | 0.5 (4) | |
| KOCHI (resistant) | 2362 | 1.02 ± 0.2 | 20.17 (10.97–37.06) | 368.59 | 19.4 (4) |
| IAB-59 | 1.88 ± 0.1 | 3.04 (2.69–13.45) | 14.56 | 7.2 (3) | |
| IAB-881 | >270 | ||||
| IAB-872 | >270 |
TABLE 4.
Resistance ratios for susceptible (JRMM-S, IP, Madurai) and resistant (JRMM-R, GeoR, KOCHI, SPHAE) C. pipiens colonies tested with different B. sphaericus strains
| Strain | Resistance ratiosa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Lab-selected JRMM-R (United States)
|
Lab-selected GeoR (United States)
|
Field-selected KOCHI (India)
|
Field-selected SPHAE (France)
|
|||||
| LC50 | LC90 | LC50 | LC90 | LC50 | LC90 | LC50 | LC90 | |
| 2362 | 4.5 (4.0–5.1)b | 8.5 (6.9–10.4)b | 35,999 (31,498–41,145)b | 59,442 (45,737–77,254)b | 1,745 (1,271–2,392)b | 4,128 (41,473–1,569)b | 23,322 (20,174–26,961)b | 50,475 (38,825–65,620)b |
| IAB-59 | 9.2 (7.9–10.7)b | 8.2 (6.6–10.2)b | 2.6 (2.1–3.1)b | 0.5 (0.3–0.7)b | 43.5 (38.3–50.3)b | 14.7 (10.7–20.2)b | 2.7 (2.3–3.2)b | 0.6 (0.4–0.9)b |
| IAB-881 | 5.7 (4.7–6.9)b | 5.1 (3.7–6.9)b | ND (800)cd | ND | ND (267)d | ND | ND (800)d | ND |
| IAB-872 | 10.2 (8.3–12.6)b | 10.6 (8.5–13.3)b | ND (800)d | ND | ND (267)d | ND | ND (800)d | ND |
The resistance ratios for JRMM-R are the ratios determined with reference to JRMM-S; the resistance ratios for GeoR and SPHAE are the ratios determined with reference to IP; and the resistance ratios for KOCHI are the ratios determined with reference to Madurai.
The values in parentheses are 95% confidence intervals.
ND, ratio not determined, but the resistance ratio should be greater than the highest dose tested with the resistant Culex colony.
The value in parentheses is the concentration (in milligrams per liter) at which 10% of exposed resistant larvae were killed.
The colony with low-level resistance (JRMM-R) and the JRMM-S colony (Table 1) showed similar susceptibilities to the various strains; the resistance ratios were 4.5 to 10.2 at the LC50 and 5.1 to 10.6 at the LC90. The colony with low-level resistance showed strong cross-resistance to all three IAB strains, particularly IAB-59 and IAB-872 (10-fold). In contrast, the three highly resistant colonies showed greater variation depending on the strain used. GeoR and SPHAE were tested in the same laboratory, and the IP colony was used as the susceptible reference colony (Table 2). The LC50 and LC90 of these colonies were almost identical, and these colonies displayed 36,000- and 23,000-fold resistance to strain 2362, respectively, at the LC50. High-level cross-resistance to strains IAB-881 and IAB-872 was observed. The precise level of cross-resistance was not determined as the highest dose tested (800 mg/liter) caused only 10% larval mortality. However, crossresistance to strain IAB-59 was low, with a resistance ratio of about 3 at the LC50. The third resistant colony, KOCHI, was compared with the Madurai colony (Table 3). The resistance ratio for these two colonies was 1,745 at the LC50 (Table 4), and strong cross-resistance to strains IAB-872 and IAB-881 was observed (10% mortality occurred at the highest dose tested [267 mg/liter]). However, with the GeoR and SPHAE colonies, cross-resistance to strain IAB-59 was low (three-fold) at the LC50.
TABLE 2.
Susceptibilities of a nonselected laboratory colony C. pipiens pipiens (IP) and field-collected selected resistant colonies of C. pipiens quinquefasciatus (GeoR) and C. pipiens pipiens (SPHAE) to B. sphaericus spore crystal suspensions
| Colony | B. sphaericus strain | Slope (mean ± SE) | LC50 (95% CI) (mg/liter) | LC90 (mg/liter) | X2 (df) |
|---|---|---|---|---|---|
| IP (susceptible) | 2362 | 3.89 ± 0.19 | 0.009 (0.009–0.095) | 0.019 | 6.57 (4) |
| IAB-59 | 0.91 ± 0.08 | 0.347 (0.258–0.483) | 9.065 | 2.39 (4) | |
| IAB-881 | 0.09 ± 0.23 | 0.35 (0.14–0.94) | 7.58 | 15.34 (4) | |
| IAB-872 | 1.56 ± 0.43 | 0.23 (0.07–0.77) | 1.54 | 10.51 (2) | |
| GeoR (resistant) | 2362 | 2.34 ± 0.18 | 326 (293–363) | 1.151 | 5.12 (3) |
| IAB-59 | 1.87 ± 0.15 | 0.89 (0.73–1.06) | 4.30 | 1.01 (3) | |
| IAB-881 | >800 | ||||
| IAB-872 | >800 | ||||
| SPHAE (resistant) | 2362 | 2.02 ± 0.33 | 197 (116–334) | 853 | 17.16 (3) |
| IAB-59 | 1.61 ± 0.09 | 0.95 (0.81–1.12) | 5.95 | 5.11 (3) | |
| IAB-881 | >800 | ||||
| IAB-872 | >800 |
To determine whether the results of the mortality test were significant, we performed a statistical analysis (one-way ANOVA) of LC50 and LC90. Surprisingly, there were not clearly significant differences between resistant colonies as determined by ANOVA (F = 2.729, P = 0.09), but a significant difference was observed with the Kruskal-Wallis test (nonparametric one-way analysis; P = 0.026). This difference was due to the colony with low-level resistance, JRMM-R, which displayed susceptibility similar to that of the susceptible colonies (F = 1.291, P = 0.322, as determined by ANOVA). This colony also accounted for the lack of highly significant differences in tests with the resistant colonies and B. sphaericus strains (F = 2.771, P = 0.087, as determined by ANOVA). However, ANOVA that included the three highly resistant colonies revealed a clearly significant difference between strains; the IAB-59 strain was significantly more toxic to the resistant colonies than strains 2362, IAB-872, and IAB-881 were (F = 8.587, P = 0.007, as determined by ANOVA).
Cross-resistance and mechanisms of resistance.
For the colony with low-level laboratory-selected resistance (JRMM-R), no significant differences were observed in the resistance ratios for the B. sphaericus strains tested. For this colony, which was reported to exhibit stable resistance to strain 2362 (Abbott technical powder) that was 31 times stronger than the resistance of JRMM-S (25, 26), the resistance ratio was only about 4 to 9 in this study, when the test was performed with B. sphaericus standard strain 2362. To improve our understanding of the difference in the levels of resistance to strain 2362 and to confirm the strong cross-resistance, we recently repeated these tests with the same powders (stored at 4°C since 1995). Interestingly, in the latter tests we observed 29-fold resistance to strain 2362 and strong resistance to strain IAB-59 (42-fold at the LC50). It therefore seems clear that for the colony with low-level resistance, strain IAB-59 cannot reduce the level of resistance. This may be because the mechanism of resistance to B. sphaericus in the JRMM-R colony is different from that in the other colonies. This is consistent with the fact that the level of resistance of JRMM-R has never reached high values, even under strong selection pressure and with homozygous resistant colonies (26, 27). It is important to understand the mechanisms of resistance if we are to predict resistance and cross-resistance. Binding between the toxin and the larval midgut membrane receptor is an important step in the mode of action of and mechanisms of resistance to most Bacillus thuringiensis Cry toxins and the binary toxins of B. sphaericus (5, 14, 15). In most cases, Cry toxin resistance is due to a lack of toxin-receptor binding. However, this has been shown for the highly resistant GeoR (15) colony but not for the SPHAE colony, whose mechanism of resistance remains unknown (16). Toxin-receptor binding assays were done with midgut membranes from the JRMM-R (31-fold resistance) and JRMM-S colonies some years ago, and the receptors of the two colonies displayed similar affinities for the toxin (Nielsen-LeRoux, unpublished data). It is therefore likely that there are various mechanisms of B. sphaericus resistance even in areas located close together geographically, as both GeoR and JRMM-R originated from C. pipiens quinquefasciatus collected in the field in California. Additionally, since both the GeoR and SPHAE colonies are susceptible to IAB-59, the data may indicate that the mechanism of resistance in JRMM-R is different from those in GeoR and SPHAE.
Cross-resistance to other strains.
Consistent with our results, it has been reported that the IAB-59 strain has only low-level cross-resistance to the laboratory B. sphaericus 2362-selected C. pipiens quinquefasciatus Bsph-R colony (36), from which GeoR originated, and that an Indian C. pipiens quinquefasciatus colony with low-level resistance (20-fold) to B. sphaericus 1593 displays cross-resistance to IAB-59 (20). Other cross-resistance studies with highly toxic B. sphaericus strains have shown strong cross-resistance to strains 1593 and 2297 for the JRMM-R colony (26) and also strong cross-resistance to strain 2297 for the KOCHI colony from India (19). In addition, since it has recently been shown that B. sphaericus LP1-G (serotype H3) is able to overcome resistance in B. sphaericus-resistant C. pipiens quinquefasciatus larvae from China (38), then B. sphaericus strains other than IAB-59 may overcome resistance to strains 1593 and 2362. All reported Culex populations with field resistance to B. sphaericus have been tested for susceptibility to the widely used bacterial larvicide B. thuringiensis subsp. israelensis. No cross-resistance to B. thuringiensis subsp. israelensis was observed (22, 32, 38), as expected, because the multitoxin complex of this bacterium does not share a receptor binding site with the B. sphaericus binary toxins (14).
Protein analysis.
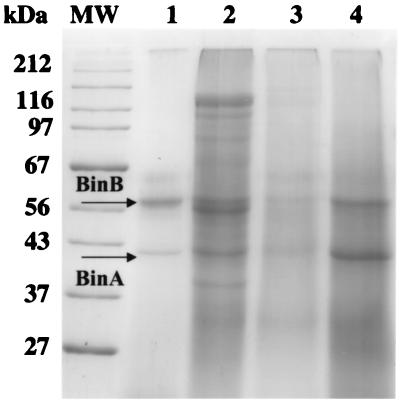
The toxicity of strain IAB-59 to the highly resistant colonies is unlikely to be due to the binary toxin of this strain (Bin1), whose amino acid sequence is slightly different from the amino acid sequence of Bin2 of strains 1593 and 2362 (1), because it was recently shown that the binary toxins of IAB-881, IAB-872, and IAB-59 are identical (8). This suggests that other toxic factors may be present in strain IAB-59, which apparently are not present in strains IAB-872 and IAB-881. We investigated this possibility by comparing the protein profiles of the four B. sphaericus strains. The equivalent of 250 μg of powder of each strain was analyzed by SDS-PAGE (Fig. 1). The differences in protein concentration observed were consistent with differences in the amounts of protein in the 250-μg portions analyzed. Although the protein bands were only weakly stained and the gel was misstained (due to the presence of lactose in the powders), the binary toxin was nonetheless clearly present in all strains, which is consistent with their larvicidal toxicity. In addition to the binary toxin, a few other major proteins seem to be specifically present in strain IAB-59; on gels one of these occurs at about 120 kDa and one occurs between the 56-kDa (BinB) and 42-kDa (BinA) proteins of the binary toxin. We are currently investigating whether the activity of strain IAB-59 depends on one of these additional proteins and are paying particular attention to the protein just below BinB (Fig. 1).
FIG. 1.
SDS–10% PAGE analysis of 250-μg portions of whole-culture dried powders of B. sphaericus 2362 (lane 1), IAB-59 (lane 2), IAB-872 (lane 3), and IAB-881 (lane 4). The gel was stained with Coomassie blue. Broad-range molecular weight standards (lane MW) were obtained from New England BioLabs. The arrows labeled BinB and BinA indicate the 56- and 42-kDa polypeptides of the binary toxins, respectively.
Our results indicate that strain IAB-59 may be used as an alternative to strains 1593 and 2362, but certainly not at the same level as B. thuringiensis subsp. israelensis, because there is some cross-resistance. Whether the IAB-59 strain has commercial value as an alternative to 2362 and 1593 will depend on the productivity and activity of this strain. Studies to validate the low level of cross-resistance to IAB-59 are currently under way; in these studies workers are selecting for resistance to this strain under laboratory conditions in order to evaluate the potential of this strain for use in resistance management programs in which B. sphaericus is the main insecticide used against Culex mosquito larvae.
Our results also suggest that although the insecticidal B. sphaericus strains currently known have limitations, both in terms of their activity spectrum (Diptera) and in terms of toxin variation compared with B. thuringiensis, the activities of the mosquitocidal factors expressed in some strains of B. sphaericus may extend beyond the activities of the known binary crystal toxins and vegetatively expressed Mtx toxins (34). The insecticidal activities of these strains against members of other insect groups, such as the Lepidoptera, remain to be investigated.
ACKNOWLEDGMENTS
This work was supported in part by a grant from Danish Development Assistance (DANIDA) to C.N.-L.
We thank the referees for their constructive suggestions in response to an earlier draft of the manuscript and Nayer S. Zahiri, University of California, Riverside, for performing the latest bioassays.
REFERENCES
- 1.Berry C, Jackson-Yap J, Oei C, Hindley J. Nucleotide sequence of 2 toxin genes from Bacillus sphaericus Iab59—sequence comparisons between 5 highly toxinogenic strains. Nucleic Acids Res. 1989;17:7516. doi: 10.1093/nar/17.18.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charles J-F, Silva-Filha M-H, Nielsen-LeRoux C, Humphreys M-J, Berry C. Binding of 51- and 42-kDa individual components from Bacillus sphaericus crystal toxin to mosquito larval midgut membranes from Culex and Anopheles sp. (Diptera: Culicidae) FEMS Microbiol Lett. 1997;156:153–159. doi: 10.1111/j.1574-6968.1997.tb12721.x. [DOI] [PubMed] [Google Scholar]
- 3.de Barjac H, Charles J-F. Une nouvelle toxine active sur les moustiques, présente dans des inclusions cristallines produites par Bacillus sphaericus. C R Acad Sci Ser III. 1983;296:905–910. [Google Scholar]
- 4.Dulmage H T, Correa J A. Coprecipitation with lactose as a means of recovering the spore-crystal complex of Bacillus thuringiensis. J Invertebr Pathol. 1970;15:15–20. doi: 10.1016/0022-2011(70)90093-5. [DOI] [PubMed] [Google Scholar]
- 5.Ferré J, Escriche B, Bel Y, Van Rie J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis insecticidal crystal proteins. FEMS Microbiol Lett. 1995;132:1–7. [Google Scholar]
- 6.Finney D J. Probit analysis. A statistical treatment of the sigmoid response curve. Cambridge, United Kingdom: University Press; 1971. [Google Scholar]
- 7.Hougard J M, Mbentengam R, Lochouarn L, Escaffre H, Darriet F, Barbazan P, Quillevere D. Control of Culex quinquefasciatus by Bacillus sphaericus: results of a pilot campaign in a large urban area in equatorial Africa. Bull W H O. 1993;71:367–375. [PMC free article] [PubMed] [Google Scholar]
- 8.Humphreys M J, Berry C. Variants of the Bacillus sphaericus binary toxin: implications for differential toxicity strains. J Invertebr Pathol. 1998;71:184–185. doi: 10.1006/jipa.1997.4711. [DOI] [PubMed] [Google Scholar]
- 9.Kalfon A, Larget-Thiéry I, Charles J-F, de Barjac H. Growth, sporulation and larvicidal activity of Bacillus sphaericus. Eur J Appl Microbiol Biotechnol. 1983;18:168–173. [Google Scholar]
- 10.Karch S, Asidi N, Manzambi M, Salaun J J. Efficacy of Bacillus sphaericus against the malaria vector Anopheles gambiae and other mosquitoes in swamps and rice fields in Zaire. J Am Mosq Control Assoc. 1992;8:376–380. [PubMed] [Google Scholar]
- 11.Kumar A, Sharma V P, Thavaselvam D, Sumodan D, Kama R H, Audi S S, Surve B N. Control of Culex quinquefasciatus with Bacillus sphaericus in Vasoco City, Goa. J Am Mosq Control Assoc. 1996;12:409–413. [PubMed] [Google Scholar]
- 12.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Nicolas L, Nielsen-LeRoux C, Charles J-F, Delécluse A. Respective role of the 42- and 51-kDa components of the Bacillus sphaericus toxin overexpressed in Bacillus thuringiensis. FEMS Lett. 1993;106:275–280. doi: 10.1111/j.1574-6968.1993.tb05976.x. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen-LeRoux C, Charles J-F. Binding of Bacillus sphaericus binary toxin to a specific receptor on midgut brush-border membranes from mosquito larvae. Eur J Biochem. 1992;210:585–590. doi: 10.1111/j.1432-1033.1992.tb17458.x. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen-LeRoux C, Charles J-F, Thiéry I, Georghiou G P. Resistance in a laboratory population of Culex quinquefasciatus (Diptera: Culicidae) to Bacillus sphaericus binary toxin is due to a change in the receptor on midgut brush-border membranes. Eur J Biochem. 1995;228:206–210. doi: 10.1111/j.1432-1033.1995.tb20251.x. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen-LeRoux C, Pasquier F, Charles J-F, Sinègre G, Gaven B, Pasteur N. Resistance to Bacillus sphaericus involves different mechanisms in Culex pipiens (Diptera: Culicidae) larvae. J Med Entomol. 1997;34:321–327. doi: 10.1093/jmedent/34.3.321. [DOI] [PubMed] [Google Scholar]
- 17.Oei C, Hindley J, Berry C. Binding of purified Bacillus sphaericus binary toxin and its deletion derivates to Culex quinquefasciatus gut: elucidation of functional binding domains. J Gen Microbiol. 1992;138:1515–1526. doi: 10.1099/00221287-138-7-1515. [DOI] [PubMed] [Google Scholar]
- 18.Oppert B, Kramer K J, Beeman R W, Johnson D E, McGaughey W H. Protein-mediated insect resistance. J Biol Chem. 1997;272:23473–23476. doi: 10.1074/jbc.272.38.23473. [DOI] [PubMed] [Google Scholar]
- 19.Poncet S, Bernard C, Dervyn E, Cayley J, Klier A, Rapoport G. Improvement of Bacillus sphaericus toxicity against Diperan larvae by integration, via homologous recombination, of the Cry11A toxin gene from Bacillus thuringiensis subsp. israelensis. Appl Environ Microbiol. 1997;63:4413–4420. doi: 10.1128/aem.63.11.4413-4420.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poopathi S, Mani T, Rao R D, Baskaran G, Kabilan L. Cross-resistance to Bacillus sphaericus strains in Culex quinquefasciatus resistant to B. sphaericus 1593M. Southeast Asian J Trop Med Public Health. 1999;30:477–481. [PubMed] [Google Scholar]
- 21.Priest F G, Ebdrup L, Zahner V, Carter P. Distribution and characterization of mosquitocidal toxin genes in some strains of Bacillus sphaericus. Appl Environ Microbiol. 1997;63:1195–1198. doi: 10.1128/aem.63.4.1195-1198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao D R, Mani T R, Rajendran R, Joseph A S, Gajanana A. Development of high level resistance to Bacillus sphaericus in a field population of Culex quinquefasciatus from Kochi, India. J Am Mosq Control Assoc. 1995;11:1–5. [PubMed] [Google Scholar]
- 23.Raymond M, Prato G, Raysia D. PROBIT. Analysis of mortality assays displaying quantal response. Praxeme (licence N) L93019. France: Saint Georges d'Orque; 1993. [Google Scholar]
- 24.Robertson J L, Preisler H K. Pesticide bioassays with arthropods. Boca Raton, Fla: CRC Press; 1992. [Google Scholar]
- 25.Rodcharoen J, Mulla M S. Resistance development in Culex quinquefasciatus (Diptera: Culicidae) to the microbial agent Bacillus sphaericus. J Econ Entomol. 1994;87:1133–1140. [Google Scholar]
- 26.Rodcharoen J, Mulla M S. Cross-resistance to Bacillus sphaericus strains in Culex quinquefasciatus. J Am Mosq Control Assoc. 1996;12:247–250. [PubMed] [Google Scholar]
- 27.Rodcharoen J, Mulla M S. Biological fitness of Culex quinquefasciatus (Diptera: Culicidae) susceptible and resistant to Bacillus sphaericus. J Med Entomol. 1997;34:5–10. doi: 10.1093/jmedent/34.1.5. [DOI] [PubMed] [Google Scholar]
- 28.Russel R, Robertson J L, Savin N E. POLO: a new computer program for probit analysis. Bull Entomol Soc Am. 1977;23:202–213. [Google Scholar]
- 29.Sanchis V, Ellar D J. Identification and partial purification of a Bacillus thuringiensis CryIC ∂-endotoxin binding protein from Spodoptera littoralis gut membranes. FEBS Lett. 1993;316:264–268. doi: 10.1016/0014-5793(93)81305-j. [DOI] [PubMed] [Google Scholar]
- 30.Siegel S, Castellan J. Nonparametric statistics for the behavioral sciences. Sydney, Australia: McGraw-Hill International Editors; 1956. [Google Scholar]
- 31.Silva-Filha M-H, Nielsen-LeRoux C, Charles J-F. Binding kinetics of Bacillus sphaericus binary toxin to midgut brush border membranes of Anopheles and Culex spp. mosquito larvae. Eur J Biochem. 1997;247:754–761. doi: 10.1111/j.1432-1033.1997.00754.x. [DOI] [PubMed] [Google Scholar]
- 32.Silva-Filha M-H, Regis L, Nielsen-leRoux C, Charles J-F. Low level resistance to Bacillus sphaericus in a field-treated population of Culex quinquefasciatus (Diptera: Culicidae) J Econ Entomol. 1995;88:525–530. [Google Scholar]
- 33.Sinègre G, Babinot M, Quermel J M, Gaven B. Society for Vector Proceedings. Control, Santa Ana, Calif. 1994. First field occurrence of Culex pipiens resistance to Bacillus sphaericus in southern France; p. 17. [Google Scholar]
- 34.Thanabalu T, Porter A G. A Bacillus sphaericus gene encoding a novel type of mosquitocidal toxin of 31.8 kDa. Gene. 1996;170:85–89. doi: 10.1016/0378-1119(95)00836-5. [DOI] [PubMed] [Google Scholar]
- 35.Thiéry I, Ofori J, Cosmao Dumanoir V, Hamon S, de Barjac H. New mosquitocidal strains from Ghana belonging to serotypes H3, H6 and H48 of Bacillus sphaericus. Appl Microbiol Biotechnol. 1992;37:718–722. [Google Scholar]
- 36.Wirth M C, Georghiou G P, Malik J I, Abro G H. Laboratory selection for resistance to Bacillus sphaericus in Culex quinquefasciatus (Diptera: Culicidae) from California, USA. J Med Entomol. 2000;37:534–540. doi: 10.1603/0022-2585-37.4.534. [DOI] [PubMed] [Google Scholar]
- 37.Yousten A A, Davidson E W. Ultrastructural analysis of spores and parasporal crystals formed by Bacillus sphaericus 2297. Appl Environ Microbiol. 1982;44:1449–1455. doi: 10.1128/aem.44.6.1449-1455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan Z, Zhang Y, Cai Q, Liu E Y. High-level field resistance to Bacillus sphaericus C3–41 in Culex quinquefasciatus from southern China. Biocontrol Sci Technol. 2000;10:41–49. [Google Scholar]