Summary
Though basophils were originally viewed as redundant blood ‘mast cells’, the implementation of flow cytometry has established basophils as unique leukocytes with critical immunomodulatory functions. Basophils play an active role in allergic inflammation, autoimmunity, and hematological malignancies. They are distinguishable from other leukocytes by their characteristic metachromatic deep-purple cytoplasmic, round granules. Mature basophils are phenotypically characterized by surface expression of IL-3Rα (CD123); IL-3 drives basophil differentiation, degranulation, and synthesis of inflammatory mediators including type 2 cytokines. Basophil degranulation is the predominant source of histamine in peripheral blood, promoting allergic responses. Basophils serve as a bridge between innate and adaptive immunity by secreting IL-4 which supports eosinophil migration, monocyte differentiation into macrophages, B-cell activation, and CD4 T-cell differentiation into Th2 cells. Further, basophilia is a key phenomenon in myeloid neoplasms, especially chronic myeloid leukemia (CML) for which it is a diagnostic criterion. Increased circulating basophils, often with aberrant immunophenotype, have been detected in patients with CML and other myeloproliferative neoplasms (MPNs). The significance of basophils’ immunoregulatory functions in malignant and non-malignant diseases is an active area of research. Ongoing and future research can inform the development of immunotherapies that target basophils to impact allergic, autoimmune, and malignant disease states. This review article aims to provide an overview of basophil biology, identification strategies, and roles and dysregulation in diseases.
Keywords: basophils, immunotherapy, mast cells, urticaria, flow cytometry
Introduction and history
Basophils were described more than a century ago in 1879 by Paul Ehrlich, who discovered them by staining their granules with basic aniline dyes. For many years thereafter, basophils were viewed as a blood ‘mast cell’ and redundant cell type because of their paucity; typically, basophils comprise less than 1% of total peripheral blood leukocytes, a barrier that has made them difficult to study. Consequently, understanding of basophil biology was rudimentary until the 1950s [1]. Basophils were primarily associated with immediate hypersensitivity reactions given their expression of high-affinity IgE receptor (FcεRI) and release of bioactive markers such as histamine and leukotrienes [1–3].
The perception of basophils as superfluous cells that only mediated hypersensitivity reactions alongside mast cells and adaptive immune cells persisted until the 1990s. Basophil counting and activation monitoring by flow cytometry elucidated the changes in cell surface markers upon activation and the more extensive immune functions of basophils [1, 4–6]. The advent of the Basophil Activation Test (BAT), which are flow cytometry-assisted assays, further facilitated characterization of basophil identification/activation markers, with CD63 and CD203 being the most commonly used and robust among them [5, 7–9]. Human basophils were subsequently reported to secrete IL-4 and IL-13—a crucial development supporting their role in Th2 immune responses and IgE synthesis [10].
Since their first description as blood ‘mast cells’, basophils have become established as unique leukocytes with important functions in modulating inflammatory responses. Basophils have gained recognition as a link between innate and adaptive immunity in humans. They are critically involved in a wide spectrum of immunologic disorders ranging from allergic inflammation and autoimmunity to malignancies [2, 11–15]. Given this emerging importance of basophils, this review provides an overview of basophil biology and identification strategies with a focus on their roles and dysregulation in malignant and non-malignant disease states.
Basophil morphology and physiology
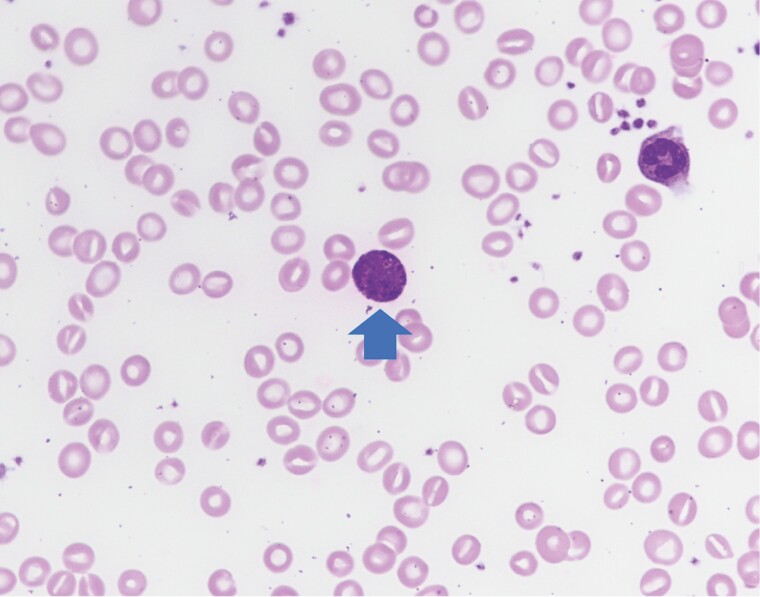
Basophils are spherical cells 5–10 μm in diameter with polylobed nuclei containing condensed chromatin [3, 16, 17]. Basophils are distinguishable from other circulating leukocytes by their characteristic metachromatic deep-purple round granules found in the cytoplasm, that can be visualized with Wright Giemsa or Toluidine blue stains [2]. This characteristic metachromatic staining of basophils is due to proteoglycans comprising chondroitin and chondroitin sulfate, which are glycosaminoglycans that complex with histamine within the granules (Fig. 1) [3, 18].
Figure 1.
Normal basophil (arrow) morphology exhibiting large, basophilic granules. Also seen are one neutrophil and numerous red cells. 1000× magnification.
Histamine (1–2 pg/cell) is the predominant component of basophil granules, and its release contributes to the role of basophils in allergic inflammation [2, 17]. Histamine and the aforementioned proteoglycans are pre-stored within the granules whereas other substances secreted by activated basophils, such as leukotriene C4 (LTC4) and pro-inflammatory cytokines IL-4 and IL-13, are synthesized de novo [3, 19–21]. LTC4 is synthesized from arachidonic acid and is a potent bronchoconstrictor that plays an important role in asthma by binding to cysteinyl leukotriene receptor (CysLTR) 1 and 2 [19]. Basophil-derived IL-4 can support Th2 cell differentiation depending on the study in allergic inflammation and anti-parasitic protective immunity, and stimulates B cells to enhance antibody production in protective immunity and autoimmunity [22]. IL-4, together with IL-13, is fundamental for allergic disease [3]; however, none of these secretions are specific to basophils.
Basogranulin is a highly basic protein (pH = 9.6) believed to be unique to basophils [23, 24]. Found within secretory granules, basogranulin is recognized by monoclonal antibody BB1 and secreted in vitro under the same conditions as those needed for histamine release [23, 25]. Like histamine, basogranulin is released upon both IgE-dependent and IgE-independent stimulation [23, 25]. Release of both histamine and basogranulin was diminished by PI3K inhibitors, indicating that the signaling pathway for basogranulin release passes through PI3K as does the signal for histamine [25]. Plasma basogranulin concentrations have been found to be significantly higher in allergic asthmatic patients than in control subjects [26]. This may suggest a potential role for basogranulin in allergic inflammation.
Agis et al. have shown that BB1 mAb, and consequently its antigen basogranulin, are reliable and sensitive markers for basophil quantification in patients with myeloid neoplasms. The study used BB1 mAb to detect and enumerate basophils in bone marrow biopsy sections from patients with chronic myeloid leukemia (CML), chronic idiopathic myelofibrosis, and other myeloproliferative neoplasms (MPNs). Higher numbers of BB1+ cells were recorded in patients with accelerated phase CML compared to those with chronic phase CML [27]. Thus, the BB1 antibody, and thereby basogranulin, have potential as markers for basophil quantification in various phases of CML and also during treatment with antileukemic drugs [27]. However, further investigation is still needed to elucidate the role of basogranulin and assess its clinical utility as a suitable serum marker.
Activated basophils secrete granule contents into the microenvironment via exocytosis through multiple plasma membrane pores [16]. Basophil activation and subsequent degranulation is stimulated by the cross-linking of high-affinity IgE receptors (FcεRI) with antigen-bound IgE antibodies. FcεRI cross-linking stimulates three main events: (i) first, rapid fusion of granules to the plasma membrane results in histamine release; (ii) activation of lipid metabolism leading to LTC4 production; and (iii) synthesis of immunomodulatory cytokines IL-4 and IL-13 [28].
Alternatively, basophils can be activated to release mediators independently of IgE and FcεRI cross-linking [18, 25, 28]. Basophils express receptors for and thus respond to the binding of IL-3, C3a, and C5a with the release of histamine and production of LTC4 [18, 28]. Combined stimulation with IL-3 and anaphylatoxin C5a also induces basophils to continuously produce IL-4, IL-13, and LTC4 over a prolonged time period, which may be important in maintaining chronic allergic inflammatory diseases such as asthma [21, 28].
When activated, basophils also release several enzymes, though human basophils appear to be protease-deficient relative to human mast cells and mouse basophils (Table 1) [29]. IL-3 has been reported to induce de novo expression of retinoic acid-generating enzyme RALDH2 (retinaldehyde dehydrogenase 2) and granzyme B, and to upregulate kinase Pim1 in basophils [30–33]. IL-3 induces expression of the serine/threonine kinase Pim1 that correlates with cytokine-enhanced survival, suggesting the potential antiapoptotic role of Pim1 in basophils [31]. IL-3 also induces RALDH2, which was demonstrated to produce retinoic acid that can regulate Th2 polarization of T-helper cells, in activated human basophils under physiologic conditions ex vivo [32].
Table 1.
Comparison of human peripheral blood basophils and murine basophils
| Human basophil | Murine basophil | ||
|---|---|---|---|
| Morphology | Nuclei | Polylobed | Polylobed |
| Granules | Abundant, large, basophilic | Sparse, basophilic | |
| Histamine Content | 1–2 pg/cell | 0.1 pg/cell | |
| Receptors | FcεRI | ++ | ++ |
| TSLPR | + | ++ | |
| CD63 | + | + | |
| CD117 (c-kit) | - | - | |
| CD123 (IL-3Rα) | ++ | ++ | |
| CD193 (CCR3) | ++ | +/- | |
| CD203c | + | + | |
| CRTH2 | ++ | + | |
| Mediators | IL-3 | Promotes development and release of other basophil mediators; autocrine secretion | Promotes development and release of other basophil mediators |
| IL-4 | Secreted | Secreted | |
| IL-6 | Not yet established | Secreted | |
| IL-13 | Secreted | Secreted | |
| IL-18 | Not yet established | Secreted | |
| LTC4 | Secreted | Secreted | |
| Granzyme B | Secreted | Secreted |
CD, cluster of differentiation; CCR3, chemokine receptor type 3; CRTH2, prostaglandin D2 receptor 2; LTC4, leukotriene C4; FcεRI, high-affinity IgE receptor. + indicates expressed; ++ indicates ‘highly expressed’; - indicates ‘not expressed’; +/- indicates ‘potentially expressed’ or ‘poorly expressed’.
Retinoic acid generated by RALDH2 in basophils also has autocrine effects, one of which is to negatively regulate granzyme B [32]. Granzyme B is more commonly associated with the lymphoid lineage. However, granzyme B has been reported as a secretory product of human basophils in vitro in the presence of IL-3 [33]. Tschopp et al. also demonstrated a conditional and temporal correlation between granzyme B and IL-13 secretion by basophils during the allergic late-phase reaction in bronchoalveolar lavages of patients with asthma in vivo. This may suggest a role for basophil-derived granzyme B in the pathogenesis of allergic inflammation and other Th2-type immune responses [33]. Thus, IL-3 potently induces the release of a vast array of mediators from basophils that are important in allergy and inflammation, including, histamine, LTC4, IL-4, IL-13, and RALDH2.
Basophil development
Basophils stem from CD34+ hematopoietic progenitor cells in the bone marrow and are released into circulation as mature cells [3, 17]. They are thought to arise from granulocyte-monocyte progenitors (GMPs) that have the capacity to develop into eosinophils, basophil-mast cell precursors (BMCPs), mast cell precursors (MCPs) and basophil precursors (BaPs) [34]. There are also some reports of basophils developing from peripheral precursors in the setting of inflammation [34,35]. Bone marrow-resident BaPs are reported to differentiate into basophils, while BMCPs are reported to migrate to the spleen but maintain the capacity to return to the bone marrow where they can develop into basophils [35].
Several cytokines and growth factors, such as IL-5, granulocyte-macrophage colony-stimulating factor (GM-CSF), TGF-β, and nerve growth factor (NGF), support basophil differentiation and development [21, 28]. TGF-β has been shown to promote IL-3-induced differentiation of basophils [36]. IL-5 and NGF have been shown to act synergistically with GM-CSF to augment basophil differentiation from myeloid progenitor cells [36, 37]. Though these factors contribute to basophil development, the general consensus is that IL-3 is the key driver [17, 38]. IL-3 is also the most potent of these factors; IL-5, GM-CSF, and NGF induce similar but less robust signaling events in basophils compared to those promoted by IL-3 [39].
Basophils that are not fully mature have been grown in vitro from human cord blood precursors after 3 weeks in a medium containing recombinant IL-3 [37, 39]. Administration of IL-3 in vivo has been reported to potentiate the differentiation of GMPs into basophil lineage-restricted progenitors (BaPs) via a STAT5 (signal transducer and activator of transcription 5) signaling-dependent manner and to increase the number of BMCPs in the spleen [40]. IL‐3 injection not only induces basophilia, but also promotes various changes in other leukocyte populations in humans, suggesting that the effects of IL-3 may not be exclusive to basophil development [37].
Mature basophils are characterized phenotypically by their surface expression of IL-3Rα (CD123), supporting the essential role of IL-3 in basophil development, proliferation, and survival [18, 28]. IL-3 also makes basophils susceptible to stimulation with secretory IgA, C3a, and platelet-activating factor (PAF), none of which alone can sufficiently induce basophil degranulation in the absence of IL-3 [20]. IL-3 has been reported to stimulate in vitro adherence of basophils to vascular endothelial cells, thereby enhancing basophil recruitment from circulation [2, 20].
Thymic stromal lymphopoietin (TSLP) is a cytokine recently recognized for its potential role in basophil development. TSLP is produced primarily by epithelial cells, and as a regulator of Th2 immunity, contributes to the pathogenesis of Th2 diseases (asthma, atopic dermatitis, etc.). Studies in mice in vivo and with human peripheral blood cells ex vivo have suggested heterogeneity in the basophil population, which may be divided into IL-3 elicited- and TSLP-elicited basophils [41]. Basophil progenitors (BaPs) in murine bone marrow, as well as basophils in human peripheral blood have been found to express TSLPR (TSLP-receptor). TSLP has been shown to induce IL-3 independent basophils from bone marrow-resident precursors in mice, promote basophil proliferation, and elicit mature basophil responses in the periphery in both IL-3-sufficient and IL-3-deficient environments [42]. TSLP-derived basophils are also thought to be functionally independent of IgE as they do not degranulate upon FcƐRI cross-linking as IL-3-derived basophils do [2]. Research on the effect of TSLP on human basophil development is limited. One study showed basophil formation from human CD34+ hematopoietic progenitors in response to TSLP in vitro upon co-stimulation with IL-3; more basophils were induced when CD34+ progenitor cells were incubated with TSLP and IL-3 than with IL-3 alone [43]. In patients with allergic asthma, Salter et al. demonstrated increased basophil numbers and upregulated basophil TSLPR expression in peripheral blood post-allergen challenge, in an IL-3-dependent manner [44]. Salabert-Le Guen et al. also showed IL-3 dependence on TSLPR upregulation. These findings in human basophils are in contrast to those in murine basophils, which have been shown to develop and respond to TSLP independently of IL-3 [42]. Furthermore, unlike Salter et al. who found human basophils from allergic asthma patients to be responsive to TSLP in the presence of IL-3. Salabert-Le Guen et al. found that purified basophils from both healthy donors and allergic patients did not respond to TSLP [45]. In light of these differences, additional studies are needed to elucidate the role of TSLP in human basophil development and activation.
Studies have shown that human basophils generate and secrete their own IL-3 upon IgE-dependent stimulation [36, 39]. This suggests the possibility that basophils may be capable of autocrine priming via IL-3. In the context of CML and other MPNs, there is considerable overlap in the signaling pathways of IL-3R and BCR/ABL [27]. Given this overlap, autocrine production of IL-3 may play a role in the differentiation and growth of basophils leading to basophilia in CML, though further investigation is still warranted [36].
IL-3R is linked to the signaling pathway involving STAT5, which has been shown to bind regulatory elements of Gata2 [11]. Transcription factors Gata2 and the CCATT enhancer-binding protein (C/EBPα) are critical for the commitment of precursor populations to the basophil lineage [22, 34, 46]. STAT5, Gata2, and C/EBPα have also been shown to support basophil maintenance, in which the committed cells retain acquired characteristics by transcribing target genes [46]. Transcription factor Ikaros has been reported to limit basophil development by suppressing transcription of the CEBPA gene in basophils [46]. The CEBPA gene, which is highly expressed in basophils but not in mast cells, is necessary, but not sufficient, to drive the differentiation of pre-BMCPs and BaPs into basophils [46].
C/EBPα dysfunction (e.g. reduced expression and activity) has been found in AML patients and as such, been suggested as an important contributor to the pathogenesis of AML: C/EBPα may contribute to the blockage in differentiation along hematopoietic lineages that is characteristic of AML [47, 48]. Lower expression of C/EBPα has also been observed in patients with CML (bone marrow samples) as compared to controls (peripheral blood samples) [49]. Loss of function of Ikaros has been detected in AML that progressed from prior MPNs and in the blast crisis and accelerated phases of CML [50, 51]. These early findings suggest the potential of manipulating these transcription factors for therapeutic purposes.
Basophil markers and quantification
BATs are flow cytometry-based assays used to identify allergens based on the ability of putative allergenic stimuli to crosslink IgE bound to the surface of basophils in patient peripheral blood and induce degranulation. The BAT was first developed as an allergy diagnostic test in 1991, and its development was driven by the discovery of basophil activation marker CD63 [4, 5, 52]. CD63 is a protein associated with basophilic secretory granules and expressed upon degranulation of activated basophils and histamine release. The BAT based on CD63, which is detected by flow cytometry using double labeling with anti-CD63 and anti-IgE mAbs, has been proven to be effective in the diagnosis of IgE-mediated allergy [4, 52].
The utility of BATs has expanded to include various applications and different versions, which are available for research groups and as commercial kits. The various BATs have different sensitivities and specificities for diagnosis of various allergens [53]. Versions of BAT vary in choice of anticoagulant, temperature and duration of blood storage, and the selection and activation markers measured [52]. In addition to CD63, other useful markers are CD203c, CD123, and CD193 (Table 2). Low levels of CD203c are constitutively expressed on resting basophils, and highly expressed upon basophil activation. Studies have also shown increased baseline CD203c expression on basophils in patients with allergies and increased expression after an exacerbation in patients with asthma, indicating the clinical applicability of CD203c [39]. Overall, studies have shown CD63 and CD203c to be the most commonly used, robust, and reliable markers for basophil detection and investigating basophil function [5, 7–9].
Table 2.
Markers commonly present on basophil surface utilize and their function
| Marker | Function/Role | Leukocyte expression | Ref. | |
|---|---|---|---|---|
| Identification markers | CD11b, CD18 | Glycoproteins (α subunit CD11b, ß subunit CD18) for adherence to endothelial cells | Broadly on leukocytes | [20, 101, 102] |
| CD13 | Cleaves nucleotides, monopeptides; pattern of expression similar to that of CD203c | Myeloid cells | [103] | |
| CD45 | Used to phenotype basophilic cells; gating around IgEhigh/CD45low for isolating basophils | Monocytes, platelets (CD45hiigh) | [4, 5, 8] | |
| CD123 (IL-3Rα) | Selection marker used to gate basophils in conjunction with HLA-DRnegative | Plasmacytoid dendritic cells | [38, 53, 101, 103] | |
| CD172a (SIRP-α) |
Low expression on basophils; regulates cytokine-dependent signaling via RTKsa | Neutrophils, monocytes | [102] | |
| CD184 (CXCR4) |
Basophil trans-endothelial migration with CCR3; low expression on basophils | Monocytes, lymphoid cells | [20, 102] | |
| CD193 (CCR3) |
Ligands include eotaxin, monocyte chemotactic protein, etc. High expression independent of activation or atopy. Primes basophil for IgE-mediated histamine release | Th2-type lymphocytes and mast cells (MCs) | [104, 105] | |
| CD203c (ENPP3b) |
High expression unique to basophils; function in basophils still under investigation | MCs, progenitors | [5, 52, 102] | |
| CRTH2c | Highly expressed, important for selective migration, potential role in allergic inflammation. Basophil isolation from Th2 cells achieved with addition of anti-CD3 | Eosinophils, Th2 cells | [5, 12, 104] | |
| Activation markers | CD63 (LAMP3d) |
Located in intracytoplasmic granules; weak expression on resting cells, upregulation mirrors histamine release | Tissue MCs, macrophages, platelets | [5, 7, 52] |
| CD69 | Expressed progressively when stimulated with IL-3 and weakly expressed upon IgE-mediated stimulation | Granulocytes, lymphocytes, monocytes | [2, 38, 53] | |
| CD107a (LAMP1d) |
Pattern of expression similar to that of CD63 (i.e. low levels on resting basophils) | Activated platelets, NK & T cells, MCs | [103] | |
| CD164 | Expression pattern similar to CD63; role in adhesion; utility as marker requires research | MCs, WBC progenitors | [101, 103] |
aTyrosine kinase receptors; bEctonucleotide pyrophosphatase phosphodiesterase 3; cChemoattractant-homologous receptor expressed on Th2 cells; dLysosome-associated membrane protein.
Thus, the BAT has become a sensitive and specific instrument for in vitro diagnosis of allergy, complementary to traditional IgE quantification and skin tests. However, the variation in protocols between sites has been a barrier to mainstream diagnostic application of BATs. Another limitation of the BAT is that it requires fresh blood, initially thought to be 4 hours with a recent study showing maximum storage time of 24 hours to preserve basophil functionality [52, 53]. Lastly, 5–10% of patients have non-responder basophils, which do not upregulate CD203c or CD63 upon IgE-mediated allergen stimulation, further constraining the implementation of BATs [53].
Flow cytometry is a key component of the BAT, and is a well-established method used independently as well for the investigation of basophils. The usefulness of flow cytometry is heavily dependent on the gating markers, which need to be stable markers that do not change their expression during cell activation [54]. This presents a challenge for the quantification of basophils as their identification and activation markers have significant overlap, and their expression levels do change with activation and priming conditions [54, 55]. Studies have found that markers such as CD123 and CD63, whose expression levels change with activation, may contribute to underestimation of basophil cell counts, and therefore false-negatives, by flow cytometry in the context of BAT [54, 56].
These challenges and gating strategies remain active areas of research given the importance of flow cytometry as a widely used, reliable method for basophil identification and quantification. Flow cytometry also plays an essential role in the identification of basophils with aberrant markers. Recent case reports of patients with hematological malignancies have shown basophils with aberrant marker expression that were identified by flow cytometry and may be under-recognized by other less specific cell counting methods [57, 58]. Thus, flow cytometry offers the advantages of precision, analysis of large numbers of cells, high throughput, and the ability to select specific gating markers, and is particularly important for understanding disease states with altered basophil counts and immunophenotype.
Basophil functions
Advancements in identification of more specific markers of basophils and their activation have contributed to the evolving understanding of basophil functions and their role in disease pathophysiology. Despite their limited numbers, basophils have powerful immunoregulatory functions through the effector molecules and cytokines they release upon activation and degranulation.
Basophils are the predominant source of histamine in peripheral blood; basophil degranulation results in release of histamine, which induces vascular permeability, smooth muscle contraction, endothelial cells to release prostacyclin, and so on [3, 59]. Histamine also exerts immunomodulatory effects by increasing production of certain cytokines (IL-6, IL-8, etc.) and decreasing certain cytokines (IL-4, etc.), thereby mediating Th1 and Th2 responses [59]. Basophils are important components of IgE-histamine-mediated anaphylaxis, and unlike mast cells, can also promote an IgG-PAF-mediated anaphylactic reaction [2, 35]. LTC4 is another effector molecule abundantly released by basophils. It has effects similar to those of histamine (increased vascular permeability, smooth muscle contraction, etc.), but is released hours after basophil activation; as such, LTC4 is a major contributor to the late-phase response in allergic reactions [21, 35, 59]. Basophil-derived LTC4 was shown to be a potent pruritogen in an atopic dermatitis-like mouse model by a recent study that elucidated a new basophil-neuronal axis that promotes itch independently of mast cells [60].
As part of the innate immune system themselves, basophils serve as a bridge between innate and adaptive immunity. Basophils are the major innate cells that secrete IL-4—a cytokine that links innate and adaptive immunity [3]. As discussed previously, basophil-derived IL-4 enhances eosinophil migration and the differentiation of monocytes into M2 type macrophages [3, 12, 61]. Basophils have recently been found to be important in lung development as regulators of alveolar macrophage (AM) maturation and function; a novel lung resident basophil, whose unique phenotype is induced by IL-33 and GM-CSF, polarizes AMs toward an anti-inflammatory M2 state, suggesting a role for basophils in inflammation resolution [62]. Basophils have been shown to support plasma cell survival and antibody production in vivo by expressing several B-cell activating factors: B-cell-activating-factor (BAFF), CD40 ligand, IL-4, and IL-6 [12]. Although definitive data are lacking, some have theorized that IL-4 and IL-13 from basophils drive B-cell class switching toward IgE, which may be important in the pathogenesis of anaphylaxis, allergic inflammation/atopy, and autoimmune disease such as chronic urticaria and systemic lupus erythematosus (SLE); further work in this arena is needed [2, 3, 12, 22, 59].
IL-6 from basophils has been reported to induce CD4+ T cells to Th17 differentiation; the role of basophils in augmenting Th17 response has been highlighted in lung and bowel inflammatory diseases and SLE [11, 61]. More recently, interactions between basophils and regulatory T cells in the setting of allergen-specific immunotherapy are being examined to further elucidate the immunoregulatory roles of basophils [61]. Thus, not only do basophils enhance innate and adaptive immune cell migration to inflamed tissues but they also support B- and T-cell maturation, differentiation, and function.
Th2-responses are thought to be the linchpin of protective immunity against helminths. Basophils, recruited in the skin, are activated in response to helminth antigens; basophils from patients infected with Toxocara, Ascaris, Strongyloides, Schistosoma, and so on have been shown to release histamine in response to parasite antigens and secrete IL-4 [59]. Mice infected with helminths have been valuable models for studying basophil function. Mouse models have reported that basophils accumulate in the skin and small intestine and induce rapid expansion of Th2 cells via IL-4, protecting against Nippostrongylus brasiliensis and forming granulomas to prevent tissue damage in S. mansoni infection; IL-4 from skin-infiltrating basophils also generated M2 macrophages that led to larval trapping in the skin, impeding migration of larvae to the lung and intestine [11]. By upregulating Notch receptor expression in mice, basophils support Th2 cell responses that promote expulsion of Trichuris muris, which is similar to the human parasite Tichuris trichiuria [63]. Perrigoue et al. showed that basophils promote MHC class II-dependent parasitic antigen-specific CD4+ T cell proliferation and Th2 cytokine response against T. muris [64]. N. brasiliensis-induced basophils have been reported to accumulate in the lungs after parasite larvae exit from the tissue [65]. Inclan-Rico et al. showed exacerbated helminth-induced group 2 innate lymphoid cell (ILC2) responses in basophil-depleted mice, resulting in increased inflammation and diminished lung function. They also demonstrated that basophils enhanced expression of a neuropeptide receptor on ILC2s that limited type 2 inflammation in the lungs to maintain tissue integrity [65]. These findings suggest that basophils may promote healing of helminth-affected tissues rather than directly acting on parasites [65]. Thus, the roles of basophils in anti-helminth immunity tend to be diverse: they promote immune responses against parasites and may even help maintain tissue integrity and function after parasitic infection.
Basophilia
Basophilia has been defined by various benchmarks: (i basophil count greater than the upper reference limit, which is generally 0.1 × 109/L, and (ii) >2% of all WBCs in peripheral blood [66, 67]. According to the Clinical & Laboratory Standards Institute guidelines, the reference method for basophil count is microscopic slide examination with a 400 cell count; and at present, flow cytometry is regarded as the most reliable method for basophil identification [66].
Basophilia is a well-established phenomenon in myeloid neoplasms, especially CML for which it is a diagnostic criterion, and has been described in non-malignant conditions as well. Of the non-malignant conditions associated with basophilia (non-clonal basophilia), atopy is the most well established. Studies have consistently found elevated basophil counts in patients with atopy when compared with controls, and correlation of greater numbers of basophils to increased symptoms of allergic rhinitis [68, 69]. Experimental allergen challenges and anaphylaxis studies have shown increased basophil migration to circulation, resulting in basophilia, and increased expression of activation markers (CD63, CD203c, etc.), indicating degranulation [13]. Basophilia in allergic inflammation, particularly in Th2-mediated response, is believed to be promoted by IL-3 and TSLP; patients with gain-of-function polymorphism in TSLP were found to present with peripheral basophilia [42, 70]. Thus, increased basophil numbers and degranulation are important components of allergic inflammation.
Moderate increases in basophil count have been reported in patients with iron deficiency anemia, though not much research has been done in this area since the 1980s to further support and characterize this association [67]. Elevated basophil count has been observed in patients with diabetes (both type 1 and type 2) when compared with non-diabetic controls and was found to be even higher in patients with diabetic ketoacidosis [71]. Though there is evidence of benign basophilia in disease states such as diabetes and anemia, its impact on pathogenesis and its clinical utility have not been studied in depth in these non-malignant states.
The role of basophils in the immune response against parasitic infection is widely studied, though primarily in mouse models [6]. Much of the basophilia in parasitic infections has been reported in mice and ex vivo studies. One of the few studies looking at the relationship between basophilia and helminth/protozoa infections in humans did not find a statistically significant difference in basophil counts from parasite-infected patients (n = 668) and uninfected controls (n = 50) [72]. This is not to say that basophils are not an important component of the immune response against parasites; simply that their role may not include an increased cell count to the point of basophilia.
Most notably, basophilia, especially hyperbasophilia (basophil count > 1 × 109/L), and aberrant basophils are most frequently markers of underlying malignancy [66]. Basophilia is the most common complete blood count (CBC) abnormality in the three phases of CML (chronic, accelerated, and blast). It has been established as a prognostic marker in CML, and is even a diagnostic criterion in the accelerated phase of CML (>20% basophils in CBC), which is characterized as more aggressive disease and less responsive to therapy [73, 74]. Magnitude of basophilia is thought to be a potential indicator of disease severity, and higher percentage of circulating basophils may be associated with lower survival [74]. Basophilia may also be used to measure treatment response with basophils <5% indicating complete hematologic response to treatment in CML [73]. In CML, basophilia has great diagnostic, prognostic, and potential treatment response monitoring utility.
In addition to CML, increased numbers of circulating basophils and dysplastic basophils are often seen in patients with the other MPNs (Polycythemia Vera, Essential Thrombocythemia, and Myelofibrosis), MDS (myelodysplastic syndrome), and less frequently in AML and APL [2, 57, 66]. Neoplastic basophils from patients with CML, idiopathic myelofibrosis, and MDS have been noted to contain elevated tryptase in their granules and expressed α‐tryptase mRNA, whereas normal blood basophils only express trace amounts of tryptase; mature, peripheral blood basophils in healthy subjects have been shown to express tryptase at levels less than 1% of those of tissue mast cells, which are the predominant source of tryptase [14, 37, 75]. Mast cells are widely considered to be the culprits in conditions with tryptasemia [76]. However, it is important to note that immature basophils (as seen in malignancies like CML and MDS) can release abundant tryptase, which has also been demonstrated to have prognostic significance in CML [14, 77]. In addition to aberrant tryptase release, basophils atypical in morphology have been reported in with a case of accelerated phase CML: the patient had 70% basophils in peripheral blood which were only detected by flow cytometry due to the morphologic atypicality of the basophils [78]. In a cohort study of 1008 patients with MDS, basophilia (>0.25 × 109/L) was associated with significantly reduced overall survival [15]. Therefore, basophilia in these hematological malignancies is not only characterized by aberrant basophils, but may also be a harbinger of lower survival.
Lastly, hyperbasophilia has been established as a key component of acute and chronic basophilic leukemias. Both are rare conditions that may present independently (primary basophilic leukemias) or with a pre-existing underlying myeloid neoplasm (secondary basophilic leukemias). Acute Basophilic Leukemia is becoming an increasingly recognized disease that has recently been added to the WHO classification which is the most widely used classification of hematopoietic disease. As there are no widely accepted diagnostic criteria yet, a series of them have been proposed by a cohort of scientists and clinicians: (i) hyperbasophilia, (ii) >40% basophils on CBC, and (iii) basophils derived from a malignant clone as evidenced by atypical morphology, underlying myeloid neoplasm, and clonal molecular or cytogenetic marker [79]. This working definition will potentially inform clinical practice and diagnosis, and aid in standardization of terminology for future research.
Basopenia
Basopenia is a rare phenomenon that is difficult to detect without flow cytometry given the low number of basophils normally circulating in peripheral blood; basophils comprise 0.5–1% of total white blood cell count. Basopenia has been observed in a select number of conditions, including after corticosteroids administration, and in disease states, such as chronic urticaria and lupus [80, 81].
Investigating the effects of intravenous corticosteroids on circulating levels of leukocytes in 11 patients with atopy, Dunsky et al. reported a rise in total WBCs with a significant reduction (by 72%) in basophil counts. Their findings demonstrated that corticosteroids induce a prominent decrease in histamine by depleting basophils without reduction in histamine content per basophil and without changes in antigen histamine release sensitivity and total skin histamine [82]. Grattan et al. found reduced basophil counts after administering corticosteroids to healthy controls. These results may be explained by enhanced apoptosis of basophils by steroids; steroids have been shown to accelerate basophil apoptosis by Yoshimura et al [83, 84]. Therefore, basopenia is a consistently observed effect of steroid administration, though the exact mechanism remains unclear, and is not a very active area of research at present.
In chronic urticaria, which presents with recurrent erythematous wheals and with or without angioedema for >6 weeks, basopenia is well documented and associated with disease severity. Basophil counts are reduced during active phases of urticaria, and restored to baseline when hives are under control [85, 86]. This basopenia is attributed to increased migration of basophils from the circulation into wheals and dysregulated expression of signaling proteins on basophils; these manifest as distinct degranulation phenotypes of basophils in chronic urticaria [83, 86, 87]. Grattan et al. reported increased peripheral basophil counts after corticosteroid administration in patients with chronic urticaria. This increase was attributed to inhibition of recruitment of basophils into the skin by reducing activation and adhesion marker expression in chronic urticaria patients [83]. Based on these findings, peripheral basophil count is a promising marker for assessing disease severity and response to treatment in patients with chronic urticaria.
In SLE, basophils amplify autoantibody synthesis by accumulating in secondary lymphoid organs. This increased migration of basophils has been linked to increased CXCR4 expression, resulting in peripheral basopenia. Studies have shown that basopenia is a hallmark of active SLE in patients and that magnitude of basopenia negatively correlates with SLE disease activity, even in patients with lupus nephritis [12, 88, 89]. Basopenia due to increased extravasation was found to correlate with overexpression of CXCR4 and CD164, both of which are markers that promote basophil migration and were induced by prostaglandin D2, in patients with SLE (n = 222) [12]. Given these data, basophil count may prove beneficial as a biomarker for SLE disease activity and prostaglandin antagonists as potential therapeutic options for SLE.
Basophils as therapeutic targets
Given their multifaceted role in the pathogenesis of allergy, helminth infections, autoimmune diseases, and malignancies, basophils present an attractive target for potential therapies. Because basophils are functionally diverse, many options for therapeutic targeting exist: receptors such as FcεRI and IL-3Rα (CD123), mediators such as histamine and LTC4, and so on. Antihistamines and leukotriene receptor antagonists have been used as allergy treatments for decades. However, they only counteract the effects of a few of the many mediators that cause allergic inflammation. Immunotherapies that modulate the FcεRI signaling pathway may prevent the release of mediators more broadly.
In food allergy, oral immunotherapy (OIT) has been shown to promote peanut desensitization in patients; one mechanism by which peanut OIT works is by decreasing basophil activation, which is also used to study the efficacy of OIT response [90–92]. Repeated exposures to low-dose antigen during OIT cause IgE endocytosis and actin rearrangement that is thought to render basophils hypo-responsive to allergen [91]. Omalizumab, which sequesters free IgE, causes downregulation of surface FcεRI expression on basophils and mast cells, making them less sensitive to allergen-mediated activation; there is some evidence suggesting that omalizumab results in greater suppression of basophils than mast cells [70, 92]. The utility of omalizumab as an adjuvant therapy with OIT for food allergy is being evaluated in trials [92]. Ligelizumab, another humanized anti-IgE monoclonal antibody like Omalizumab, has demonstrated superior and more durable suppression of basophil surface FcεRI expression in humans compared to omalizumab [92]. Inhibitors of kinases, such as Bruton’s tyrosine kinase (BTK) which is involved in the FcεRI pathway, have been shown to suppress IgE-mediated basophil activation to aeroallergens and food allergens in preliminary human studies [92, 93]. While the aforementioned therapies do not target basophils exclusively, basophils are invaluable for measuring treatment efficacy because of their availability in peripheral blood and utility of the BAT.
In the context of atopy, therapies such as omalizumab are effective treatment options for asthma and allergic rhinitis working by the same IgE pathway mechanism as for food allergy. In atopic dermatitis, anti-IgE therapy has produced mixed results and has not advanced beyond phase 2 trials in humans [60]. Beyond the IgE pathway, the TSLP-TSLPR pathway is associated with the pathogenesis of asthma and atopic dermatitis [94]. Tezepelumab, an anti-TSLP antibody that prevents TSLP-TSLPR interactions, has shown greater but not statistically significant clinical improvement (compared to placebo) in atopic dermatitis patients; the efficacy of tezepelumab is thought to be partly due to TSLP effects on basophils [94]. While many of these therapies have multifaceted immunomodulatory effects, the newly elucidated basophil-neuronal axis that highlighted a mast-cell independent form of basophil IgE-mediated itch presents a potential target for therapeutic intervention in atopic dermatitis [60]. Lung resident basophils, which induce alveolar macrophages to adopt an anti-inflammatory M2 state, may have therapeutic potential to promote inflammation resolution in asthma and allergic rhinitis [65].
Other disease states in which therapies can modulate basophils’ role in pathology are autoimmunity and malignancy. The utility of omalizumab in SLE is currently being investigated given the pathogenic role of basophils and IgE in this disease; it has been shown to be well tolerated in SLE patients and associated with improvement in disease activity [95, 96]. CSL362 or talacotuzumab, an anti-CD123 (IL-3Rα) antibody originally developed for AML, depletes CD123+ cells in peripheral blood and also inhibits IL-3-dependent signaling by preventing the binding of IL-3 to its receptor. Ooon et al. showed that talacotuzumab depletes basophils and plasmacytoid dendritic cells ex vivo and confirmed these effects in cells derived from SLE donors, interferon-dependent autoimmune diseases, and healthy controls [97]. In addition to SLE, anti-CD123 antibodies like talacotuzumab are being investigated in clinical trials for AML, MDS, and so on; the reduction in basophils has been noted in these trial data [98]. In polycythemia vera, a myeloproliferative neoplasm with increased peripheral basophil counts, treatment with a JAK2 inhibitor attenuated basophil activation ex vivo; patients with aquagenic pruritus had greater numbers of circulating activated basophils, suggesting a possible role for JAK2 inhibitors in targeting basophils [99]. Therefore, therapies that target basophils have the potential to impact allergic, autoimmune, and malignant disease states.
Basophil versus mast cell
Basophils and mast cells have long been grouped together as cells with similar properties and purposes. However, over the years, their distinct identities and functions have been better characterized. Their first point of distinction is their development, though they share common progenitor cells. Mast cell precursors are released from bone marrow and their differentiation occurs after they enter tissue, whereas basophils differentiate within the bone marrow and are released into circulation as mature basophils [17, 28]. Additionally, in contrast to basophils, mast cells require stem cell factor for differentiation. Basophils are thought to be more closely related to eosinophils [17]. Other aspects of basophil and mast cell development are compared in Table 3.
Table 3.
Delineation of similarities and key differences between mast cells and basophils
| Mast cell | Basophil | Ref. | |
|---|---|---|---|
| Morphology | □ Mono-lobed nucleus, partially condensed chromatin □ Small, numerous granules □ Granules complex with heparin |
□ Multilobed nucleus, condensed chromatin □ Large, few granules □ Granules complex with chondroitin sulfate |
[18, 106] |
| Granule contents | Histamine, tryptase, chymase, heparin, cytokines | Histamine, tryptase (low), chymase, basogranulin, cytokines | [17] |
| Development | □ Regulated by transcription factors: Gata2/Gata3, MITF (TF for lineage commitment) □ Dependent on c-kit primarily, TGF- β, IL-4, IL-9 □ Site of maturation: tissues □ Lifespan: Weeks to months |
□ Regulated by transcription factors: Gata2, C/EBPα (TF for lineage commitment) □ Dependent on IL-3 primarily, GM-CSF, TGF- β, NGF, IL-5 □ Site of maturation: bone marrow □ Lifespan: Days |
[17, 22, 46, 107] |
| CD markers | CD117 (c-kit, receptor for SCF), CCR3, CD64 (FcγRI), MRGPRX2, etc. | CD123 (IL-3 receptor α chain), CCR3, CRTH2, etc. | |
| Function | □ TNF-α, LTC4, LTB4, PGD2, Thromboxanes released in response to FcεRI cross-linking □ Pro- and anti-inflammatory effects via interactions with B, Treg and Th17 cells |
□ Produces IL-4, IL-13, and LTC4 in response to FcεRI cross-linking and stimulation with IL-3 and C5a – Maintain inflammatory response during late-phase reactions via enhancement of Th2 immunity |
[17, 18, 21] |
| Diseases | □ Autoimmune urticarial □ Allergic diseases □ Multiple Sclerosis □ Rheumatoid Arthritis □ Primary mastocytosis □ Cardiovascular disease (e.g. MI, atherogenesis, etc.) |
□ Autoimmune urticaria □ SLE, lupus nephritis □ CML □ MDS □ Acute/Chronic Basophilic leukemia |
[2, 18, 108, 109] |
CD, cluster of differentiation; CCR3, chemokine receptor type 3; CML, chronic myeloid leukemia; CRTH2, prostaglandin D2 receptor 2; FcεRI, high-affinity IgE receptor; MRGPRX2, Mas-related G protein-coupled receptor-X2; MDS, myelodysplastic syndrome; LTC4, leukotriene C4; LTB4, leukotriene B4; PGD2, prostaglandin D2; SCF, stem cell factor; SLE, systemic lupus erythematosus; TNF-α, tumor necrosis factor-α.
Basophils and mast cells both play important roles in allergic responses; they are strongly responsive to activation through allergen-induced crosslinking of IgE molecules bound to their high-affinity cell surface receptors (FcεRI). Though both are key players in allergy, a recent study, which showed the mast-cell independent effects of basophil-derived LTC4 on itch, provides further evidence that mast cells and basophils have evolutionarily distinct roles in allergy [60]. Research efforts over recent decades have uncovered that basophils and mast cells are strongly implicated in conditions beyond allergic response, such as autoimmune diseases, malignancy, and so on (Table 3). The active involvement of these two cells has also been demonstrated in host protection against bacterial, fungal, and parasitic infiltration [100]. Research to further explain the roles of basophils and mast cells may enhance understanding of disease pathophysiology and inform treatment development.
Summary and conclusions
Our understanding of basophils’ immunoregulatory functions has vastly increased in the last two decades as basophil research is in a period of transition from pre-clinical models to human investigations. This rapidly growing knowledgebase has been made possible largely by advancements in flow cytometry and BAT which have facilitated identification and isolation of basophils. This has enabled us to study the role of basophils in pathologic states and in response to therapeutic interventions. Aberrations in basophil counts, immunophenotype, and secretions have been consistently reported in hematological malignancies, allergic, and autoimmune conditions in cohort studies and case reports. These aberrations and the core immunoregulatory functions of basophils require further characterization and investigation in humans as they may help inform diagnosis, prognosis, and treatment decisions in a myriad of disease states. Greater understanding of basophils will enable us to further harness their power as a target for future immunotherapies.
Acknowledgement
The Editor-in-Chief, Tim Elliott, and handling editor, Stephanie Dougan, would like to thank the following reviewer, Brian Kim, and an anonymous reviewer, for their contribution to the publication of this article.
Glossary
Abbreviations
- AM
Alveolar macrophage
- BAFF
B-cell-activating-factor
- BaP
Basophil precursor
- BAT
Basophil activation test
- BMCP
Basophil-mast cell precursor
- BTK
Bruton’s tyrosine kinase
- CML
Chronic myeloid leukemia
- CysLTR
Cysteinyl leukotriene receptor
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- GMP
Granulocyte-monocyte progenitor
- LTC4
Leukotriene C4
- MCP
Mast cell precursor
- MDS
Myelodysplastic syndrome
- MPN
Myeloproliferative neoplasm
- NGF
Nerve growth factor
- OIT
Oral immunotherapy
- PAF
Platelet-activating factor
- RALDH2
Retinaldehyde dehydrogenase 2
- SLE
Systemic lupus erythematosus
- TSLP
Thymic stromal lymphopoietin
Funding
The authors have no relevant funding to disclose.
Author contributions
H.S. wrote the original manuscript, S.E. provided critical edits, C.A.T. co-conceptualized the project and provided critical edits, and A.J.S. co-conceptualized the project, provided critical edits, and supervised the project.
Conflict of interest
The authors declare no conflicts of interest.
Data availability
No new data were generated or analyzed in support of this research.
References
- 1. Steiner M, Huber S, Harrer Aet al. The evolution of human basophil biology from neglect towards understanding of their immune functions. Biomed Res Int 2016;2016:8232830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cromheecke JL, Nguyen KT, Huston DP. Emerging role of human basophil biology in health and disease. Curr Allergy Asthma Rep 2014;14:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chirumbolo S. State-of-the-art review about basophil research in immunology and allergy: is the time right to treat these cells with the respect they deserve? Blood Transfus 2012;10:148–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ocmant A, Peignois Y, Mulier Set al. Flow cytometry for basophil activation markers: the measurement of CD203c up-regulation is as reliable as CD63 expression in the diagnosis of cat allergy. J Immunol Methods 2007;320:40–8. [DOI] [PubMed] [Google Scholar]
- 5. Boumiza R, Debard AL, Monneret G. The basophil activation test by flow cytometry: recent developments in clinical studies, standardization and emerging perspectives. Clin Mol Allergy 2005;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lantz CS, Boesiger J, Song CHet al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature 1998;392:90–3. [DOI] [PubMed] [Google Scholar]
- 7. Tammaro A, Narcisi A, Amodeo Ret al. CD63 cell expression detected by flow-cytometric determination of basophil activation in allergic patients. Int J Immunopathol Pharmacol 2012;25:1143–7. [DOI] [PubMed] [Google Scholar]
- 8. Chirumbolo S, Vella A, Ortolani Ret al. Differential response of human basophil activation markers: a multi-parameter flow cytometry approach. Clin Mol Allergy 2008;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bühring HJ, Streble A, Valent P. The basophil-specific ectoenzyme E-NPP3 (CD203c) as a marker for cell activation and allergy diagnosis. Int Arch Allergy Immunol 2004;133:317–29. [DOI] [PubMed] [Google Scholar]
- 10. Gibbs BF, Haas H, Falcone FHet al. Purified human peripheral blood basophils release interleukin-13 and preformed interleukin-4 following immunological activation. Eur J Immunol 1996;26:2493–8. [DOI] [PubMed] [Google Scholar]
- 11. Voehringer D. Recent advances in understanding basophil functions in vivo. F1000Res 2017;6:1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pellefigues C, Dema B, Lamri Yet al. Prostaglandin D2 amplifies lupus disease through basophil accumulation in lymphoid organs. Nat Commun 2018;9:725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Korošec P, Gibbs BF, Rijavec Met al. Important and specific role for basophils in acute allergic reactions. Clin Exp Allergy 2018;48:502–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samorapoompichit P, Kiener HP, Schernthaner GHet al. Detection of tryptase in cytoplasmic granules of basophils in patients with chronic myeloid leukemia and other myeloid neoplasms. Blood 2001;98:2580–3. [DOI] [PubMed] [Google Scholar]
- 15. Wimazal F, Germing U, Kundi Met al. Evaluation of the prognostic significance of eosinophilia and basophilia in a larger cohort of patients with myelodysplastic syndromes. Cancer 2010;116:2372–81. [DOI] [PubMed] [Google Scholar]
- 16. Dvorak AM. Similarities in the ultrastructural morphology and developmental and secretory mechanisms of human basophils and eosinophils. J Allergy Clin Immunol 1994;94:1103–34. [DOI] [PubMed] [Google Scholar]
- 17. Knol EF, Olszewski M. Basophils and mast cells: underdog in immune regulation? Immunol Lett 2011;138:28–31. [DOI] [PubMed] [Google Scholar]
- 18. Varricchi G, Raap U, Rivellese Fet al. Human mast cells and basophils-How are they similar how are they different? Immunol Rev 2018;282:8–34. [DOI] [PubMed] [Google Scholar]
- 19. Harvima IT, Levi-Schaffer F, Draber Pet al. Molecular targets on mast cells and basophils for novel therapies. J Allergy Clin Immunol 2014;134:530–44. [DOI] [PubMed] [Google Scholar]
- 20. Yamaguchi M, Koketsu R, Suzukawa Met al. Human basophils and cytokines/chemokines. Allergol Int 2009;58:1–10. [DOI] [PubMed] [Google Scholar]
- 21. Ochensberger B, Tassera L, Bifrare Det al. Regulation of cytokine expression and leukotriene formation in human basophils by growth factors, chemokines and chemotactic agonists. Eur J Immunol 1999;29:11–22. [DOI] [PubMed] [Google Scholar]
- 22. Karasuyama H, Yamanishi Y. Basophils have emerged as a key player in immunity. Curr Opin Immunol 2014;31:1–7. [DOI] [PubMed] [Google Scholar]
- 23. McEuen AR, Calafat J, Compton SJet al. Mass, charge, and subcellular localization of a unique secretory product identified by the basophil-specific antibody BB1. J Allergy Clin Immunol 2001;107:842–8. [DOI] [PubMed] [Google Scholar]
- 24. Beck SC, Wilding T, Buka RJet al. Biomarkers in human anaphylaxis: a critical appraisal of current evidence and perspectives. Front Immunol 2019;10:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mochizuki A, McEuen AR, Buckley MGet al. The release of basogranulin in response to IgE-dependent and IgE-independent stimuli: validity of basogranulin measurement as an indicator of basophil activation. J Allergy Clin Immunol 2003;112:102–8. [DOI] [PubMed] [Google Scholar]
- 26. Wong CK, Leung TF, Chu IMet al. Aberrant expression of regulatory cytokine IL-35 and pattern recognition receptor NOD2 in patients with allergic asthma. Inflammation 2015;38:348–60. [DOI] [PubMed] [Google Scholar]
- 27. Agis H, Krauth MT, Böhm Aet al. Identification of basogranulin (BB1) as a novel immunohistochemical marker of basophils in normal bone marrow and patients with myeloproliferative disorders. Am J Clin Pathol 2006;125:273–81. [DOI] [PubMed] [Google Scholar]
- 28. Falcone FH, Haas H, Gibbs BF. The human basophil: a new appreciation of its role in immune responses. Blood 2000;96:4028–38. [PubMed] [Google Scholar]
- 29. Caughey GH. Mast cell proteases as pharmacological targets. Eur J Pharmacol 2016;778:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kämpfer SS, Odermatt A, Dahinden CAet al. Late IL-3-induced phenotypic and functional alterations in human basophils require continuous IL-3 receptor signaling. J Leukoc Biol 2017;101:227–38. [DOI] [PubMed] [Google Scholar]
- 31. Didichenko SA, Spiegl N, Brunner Tet al. IL-3 induces a Pim1-dependent antiapoptotic pathway in primary human basophils. Blood 2008;112:3949–58. [DOI] [PubMed] [Google Scholar]
- 32. Spiegl N, Didichenko S, McCaffery Pet al. Human basophils activated by mast cell-derived IL-3 express retinaldehyde dehydrogenase-II and produce the immunoregulatory mediator retinoic acid. Blood 2008;112:3762–71. [DOI] [PubMed] [Google Scholar]
- 33. Tschopp CM, Spiegl N, Didichenko Set al. Granzyme B, a novel mediator of allergic inflammation: its induction and release in blood basophils and human asthma. Blood 2006;108:2290–9. [DOI] [PubMed] [Google Scholar]
- 34. Siracusa MC, Perrigoue JG, Comeau MRet al. New paradigms in basophil development, regulation and function. Immunol Cell Biol 2010;88:275–84. [DOI] [PubMed] [Google Scholar]
- 35. Siracusa MC, Comeau MR, Artis D. New insights into basophil biology: initiators, regulators, and effectors of type 2 inflammation. Ann N Y Acad Sci 2011;1217:166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Valent P, Dahinden CA. Role of interleukins in the regulation of basophil development and secretion. Curr Opin Hematol 2010;17:60–6. [DOI] [PubMed] [Google Scholar]
- 37. Arock M, Schneider E, Boissan Met al. Differentiation of human basophils: an overview of recent advances and pending questions. J Leukoc Biol 2002;71:557–64. [PubMed] [Google Scholar]
- 38. Siracusa MC, Wojno ED, Artis D. Functional heterogeneity in the basophil cell lineage. Adv Immunol 2012;115:141–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schroeder JT. Basophils: emerging roles in the pathogenesis of allergic disease. Immunol Rev 2011;242:144–60. [DOI] [PubMed] [Google Scholar]
- 40. Ohmori K, Luo Y, Jia Yet al. IL-3 induces basophil expansion in vivo by directing granulocyte-monocyte progenitors to differentiate into basophil lineage-restricted progenitors in the bone marrow and by increasing the number of basophil/mast cell progenitors in the spleen. J Immunol 2009;182:2835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nakashima C, Otsuka A, Kabashima K. Recent advancement in the mechanism of basophil activation. J Dermatol Sci 2018;91:3–8. [DOI] [PubMed] [Google Scholar]
- 42. Siracusa MC, Saenz SA, Hill DAet al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 2011;477:229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hui CC, Rusta-Sallehy S, Asher Iet al. The effects of thymic stromal lymphopoietin and IL-3 on human eosinophil-basophil lineage commitment: relevance to atopic sensitization. Immun Inflamm Dis 2014;2:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Salter BM, Oliveria JP, Nusca Get al. Thymic stromal lymphopoietin activation of basophils in patients with allergic asthma is IL-3 dependent. J Allergy Clin Immunol 2015;136:1636–44. [DOI] [PubMed] [Google Scholar]
- 45. Salabert-Le Guen N, Hémont C, Delbove Aet al.. Thymic stromal lymphopoietin does not activate human basophils. Journal of Allergy and Clinical Immunology 2018;141(4):1476–9.e6. [DOI] [PubMed] [Google Scholar]
- 46. Huang H, Li Y, Liu B. Transcriptional regulation of mast cell and basophil lineage commitment. Semin Immunopathol 2016;38:539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nerlov C. C/EBPα mutations in acute myeloid leukaemias. Nature Reviews Cancer. 2004;4(5):394–400. [DOI] [PubMed] [Google Scholar]
- 48. Koschmieder S, Halmos B, Levantini Eet al. Dysregulation of the C/EBPalpha differentiation pathway in human cancer. J Clin Oncol 2009;27:619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dong F, Zhang G, Zhang Xet al. Aberrantly expressed transcription factors C/EBP and SOX4 have positive effects in the development of chronic myeloid leukemia. Molecular Medicine Reports 2017;16:7131–7. [DOI] [PubMed] [Google Scholar]
- 50. Beer PA, Knapp DJ, Miller PHet al. Disruption of IKAROS activity in primitive chronic-phase CML cells mimics myeloid disease progression. Blood 2015;125:504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Theocharides AP, Dobson SM, Laurenti Eet al. Dominant-negative Ikaros cooperates with BCR-ABL1 to induce human acute myeloid leukemia in xenografts. Leukemia 2015;29:177–87. [DOI] [PubMed] [Google Scholar]
- 52. Mukai K, Gaudenzio N, Gupta Set al. Assessing basophil activation by using flow cytometry and mass cytometry in blood stored 24 hours before analysis. J Allergy Clin Immunol 2017;139:889–899.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hemmings O, Kwok M, McKendry Ret al. Basophil activation test: old and new applications in allergy. Curr Allergy Asthma Rep 2018;18:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Salvatore Chirumbolo AUGBAUAV. Using a CD45dim/CD123bright/HLA-DRneg phenotyping protocol to gate basophils in FC for airway allergy. CD123 does not decrease. Using a CD45dim/CD123bright/HLA-DRneg phenotyping protocol to gate basophils in FC for airway allergy CD123 does not decrease. 2017;85(4):193-201-193-201. [DOI] [PubMed] [Google Scholar]
- 55. Kim Z, Choi BS, Kim JKet al. Basophil markers for identification and activation in the indirect basophil activation test by flow cytometry for diagnosis of autoimmune urticaria. Ann Lab Med 2016;36:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Santos AF, Bécares N, Stephens Aet al. The expression of CD123 can decrease with basophil activation: implications for the gating strategy of the basophil activation test. Clin Transl Allergy 2016;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shameli A, Jamani K. Acute promyelocytic leukemia presenting with atypical basophils. Clin Case Rep 2020;8:584–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tormey CA, Siddon AJ. Morphology and flow cytometry of atypical basophils. Blood 2018;132:552. [DOI] [PubMed] [Google Scholar]
- 59. Mitre E, Nutman TB. Basophils, basophilia and helminth infections. Chem Immunol Allergy 2006;90:141–56. [DOI] [PubMed] [Google Scholar]
- 60. Wang F, Trier AM, Li Fet al. A basophil-neuronal axis promotes itch. Cell 2021;184:422–440.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chirumbolo S, Bjørklund G, Sboarina Aet al. The role of basophils as innate immune regulatory cells in allergy and immunotherapy. Hum Vaccin Immunother 2018;14:815–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cohen M, Giladi A, Gorki ADet al. Lung single-cell signaling interaction map reveals basophil role in macrophage imprinting. Cell 2018;175:1031–1044.e18. [DOI] [PubMed] [Google Scholar]
- 63. Webb LM, Oyesola OO, Früh SPet al. The Notch signaling pathway promotes basophil responses during helminth-induced type 2 inflammation. J Exp Med 2019;216:1268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Perrigoue JG, Saenz SA, Siracusa MCet al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol 2009;10:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Inclan-Rico JM, Ponessa JJ, Valero-Pacheco Net al. Basophils prime group 2 innate lymphoid cells for neuropeptide-mediated inhibition. Nat Immunol 2020;21:1181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Feriel J, Depasse F, Geneviève F. How I investigate basophilia in daily practice. Int J Lab Hematol 2020;42:237–45. [DOI] [PubMed] [Google Scholar]
- 67. May ME, Waddell CC. Basophils in peripheral blood and bone marrow. A retrospective review. Am J Med 1984;76:509–11. [DOI] [PubMed] [Google Scholar]
- 68. Hirsch SR, Kalbfleisch JH. Circulating basophils in normal subjects and in subjects with hay fever. J Allergy Clin Immunol 1976;58:676–82. [DOI] [PubMed] [Google Scholar]
- 69. Reilly KM, Yap PL, Dawes Jet al. Circulating basophil counts in atopic individuals. Int Arch Allergy Appl Immunol 1987;84:424–6. [DOI] [PubMed] [Google Scholar]
- 70. Siracusa MC, Kim BS, Spergel JMet al. Basophils and allergic inflammation. J Allergy Clin Immunol 2013;132:789–801; quiz 788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xu W, Wu HF, Ma SGet al. Correlation between peripheral white blood cell counts and hyperglycemic emergencies. Int J Med Sci 2013;10:758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mitre E, Nutman TB. Lack of basophilia in human parasitic infections. The American Journal of Tropical Medicine and Hygiene. 2003;69(1):87–91. [PubMed] [Google Scholar]
- 73. Hochhaus A, Saussele S, Rosti Get al. . Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Annals of Oncology. 2017;28:iv41–iv51. [DOI] [PubMed] [Google Scholar]
- 74. Valent P, Horny HP, Arock M. The underestimated role of basophils in Ph+ chronic myeloid leukaemia. Eur J Clin Invest 2018;48:e13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jogie-Brahim S, Min H-K, Fukuoka Y, Xia H-Z, Schwartz LB. Expression of α-tryptase and β-tryptase by human basophils. Journal of Allergy and Clinical Immunology. 2004;113(6):1086–92. [DOI] [PubMed] [Google Scholar]
- 76. Valent P, Akin C, Nedoszytko Bet al.. Diagnosis, classification and management of mast cell activation syndromes (MCAS) in the era of personalized medicine. Int J Mol Sci. 2020;21(23):9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sperr WR, Pfeiffer T, Hoermann Get al. Serum-tryptase at diagnosis: a novel biomarker improving prognostication in Ph(+) CML. Am J Cancer Res 2015;5:354–62. [PMC free article] [PubMed] [Google Scholar]
- 78. Stacchini A, Demurtas A, Godio L. Flow cytometric detection of degranulated basophils in chronic myeloid leukemia in accelerated phase. Cytometry B Clin Cytom 2011;80:122–4. [DOI] [PubMed] [Google Scholar]
- 79. Valent P, Sotlar K, Blatt Ket al. Proposed diagnostic criteria and classification of basophilic leukemias and related disorders. Leukemia 2017;31:788–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shelley WB, Parnes HM. The absolute basophil count. JAMA 1965;192:368–70. [DOI] [PubMed] [Google Scholar]
- 81. Juhlin L. Basophil and eosinophil leukocyted in various internal disorders. Acta Med Scand 1963;174:249–54. [DOI] [PubMed] [Google Scholar]
- 82. Dunsky EH, Zweiman B, Fischler Eet al. Early effects of corticosteroids on basophils, leukocyte histamine, and tissue histamine. J Allergy Clin Immunol 1979;63:426–32. [DOI] [PubMed] [Google Scholar]
- 83. Grattan CE, Dawn G, Gibbs Set al. Blood basophil numbers in chronic ordinary urticaria and healthy controls: diurnal variation, influence of loratadine and prednisolone and relationship to disease activity. Clin Exp Allergy 2003;33:337–41. [DOI] [PubMed] [Google Scholar]
- 84. Yoshimura C, Miyamasu M, Nagase Het al. Glucocorticoids induce basophil apoptosis. J Allergy Clin Immunol 2001;108:215–20. [DOI] [PubMed] [Google Scholar]
- 85. Kishimoto I, Kambe N, Ly NTMet al. Basophil count is a sensitive marker for clinical progression in a chronic spontaneous urticaria patient treated with omalizumab. Allergol Int 2019;68:388–90. [DOI] [PubMed] [Google Scholar]
- 86. Rauber MM, Pickert J, Holiangu Let al. Functional and phenotypic analysis of basophils allows determining distinct subtypes in patients with chronic urticaria. Allergy 2017;72:1904–11. [DOI] [PubMed] [Google Scholar]
- 87. Jain S. Pathogenesis of chronic urticaria: an overview. Dermatol Res Pract 2014;2014:674709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liang P, Tang Y, Fu Set al. Basophil count, a marker for disease activity in systemic lupus erythematosus. Clin Rheumatol 2015;34:891–6. [DOI] [PubMed] [Google Scholar]
- 89. Liang P, Tang Y, Lin Let al. Low level of circulating basophil counts in biopsy-proven active lupus nephritis. Clin Rheumatol 2018;37:459–65. [DOI] [PubMed] [Google Scholar]
- 90. Tsai M, Mukai K, Chinthrajah RSet al. Sustained successful peanut oral immunotherapy associated with low basophil activation and peanut-specific IgE. J Allergy Clin Immunol 2020;145:885–896.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kulis MD, Patil SU, Wambre Eet al. Immune mechanisms of oral immunotherapy. J Allergy Clin Immunol 2018;141:491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dispenza MC, Bochner BS, MacGlashan DW Jr. Targeting the FcεRI pathway as a potential strategy to prevent food-induced Anaphylaxis. Front Immunol 2020;11:614402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Regan JA, Cao Y, Dispenza MCet al. Ibrutinib, a Bruton’s tyrosine kinase inhibitor used for treatment of lymphoproliferative disorders, eliminates both aeroallergen skin test and basophil activation test reactivity. J Allergy Clin Immunol 2017;140:875–879.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nakajima S, Kabata H, Kabashima Ket al. Anti-TSLP antibodies: targeting a master regulator of type 2 immune responses. Allergol Int 2020;69:197–203. [DOI] [PubMed] [Google Scholar]
- 95. Lamri Y, Charles N. IgE in the Pathogenesis of SLE: from pathogenic role to therapeutic target. Antibodies (Basel). 2020;9(4):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hasni S, Gupta S, Davis Met al. Safety and tolerability of omalizumab: a randomized clinical trial of humanized Anti-IgE monoclonal antibody in systemic lupus Erythematosus. Arthritis Rheumatol 2019;71:1135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Oon S, Huynh H, Tai TYet al. A cytotoxic anti-IL-3Rα antibody targets key cells and cytokines implicated in systemic lupus erythematosus. JCI Insight 2016;1: e86131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Montesinos P, Roboz GJ, Bulabois CEet al. Safety and efficacy of talacotuzumab plus decitabine or decitabine alone in patients with acute myeloid leukemia not eligible for chemotherapy: results from a multicenter, randomized, phase 2/3 study. Leukemia 2021;35:62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Pieri L, Bogani C, Guglielmelli Pet al. The JAK2V617 mutation induces constitutive activation and agonist hypersensitivity in basophils from patients with polycythemia vera. Haematologica 2009;94:1537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hallgren J, Hellman L, Maurer Met al. Novel aspects of mast cell and basophil function: highlights from the 9th meeting of the European Mast Cell and Basophil Research Network (EMBRN)-A Marcus Wallenberg Symposium. Allergy 2020;75:707–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Anna W-M, Wojciech B, Wojciech M. CD164 as a Basophil Activation Marker. Current Pharmaceutical Design. 2011;17(34):3786–96. [DOI] [PubMed] [Google Scholar]
- 102. Ghannadan M, Hauswirth AW, Schernthaner GHet al. Detection of novel CD antigens on the surface of human mast cells and basophils. Int Arch Allergy Immunol 2002;127:299–307. [DOI] [PubMed] [Google Scholar]
- 103. Hennersdorf F, Florian S, Jakob Aet al. Identification of CD13, CD107a, and CD164 as novel basophil-activation markers and dissection of two response patterns in time kinetics of IgE-dependent upregulation. Cell Res 2005;15:325–35. [DOI] [PubMed] [Google Scholar]
- 104. Hausmann OV, Gentinetta T, Fux Met al. Robust expression of CCR3 as a single basophil selection marker in flow cytometry. Allergy 2011;66:85–91. [DOI] [PubMed] [Google Scholar]
- 105. Khanolkar A, Burden SJ, Hansen Bet al. Evaluation of CCR3 as a basophil activation marker. Am J Clin Pathol 2013;140:293–300. [DOI] [PubMed] [Google Scholar]
- 106. Dvorak AM. Ultrastructural studies of human basophils and mast cells. J Histochem Cytochem 2005;53:1043–70. [DOI] [PubMed] [Google Scholar]
- 107. Voehringer D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol 2013;13:362–75. [DOI] [PubMed] [Google Scholar]
- 108. Conti P, Kempuraj D. Important role of mast cells in multiple sclerosis. Mult Scler Relat Disord 2016;5:77–80. [DOI] [PubMed] [Google Scholar]
- 109. Xu Y, Chen G. Mast cell and autoimmune diseases. Mediators Inflamm 2015;2015:246126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.