Summary
One major finding of chronic inflammatory diseases of various origins is the establishment of inflammatory infiltrates, bearing different leukocyte subpopulations, including activated T lymphocytes. Integrins are among the large series of molecular interactions that have been implicated as players in both triggering and maintenance of leukocyte influx from the blood into a given organ parenchyme. Accordingly, blocking the interaction between VLA-6 integrin and laminin, experimentally abrogates heart graft rejection. Many reports have shown that VLA-4 is used by T cells to cross endothelial barriers, as well as to migrate within target tissues. In this respect, a humanized IgG4 anti-VLA-4 monoclonal antibody (specific to the α4-integrin chain of VLA-4) has been successfully applied to treat multiple sclerosis as well as inflammatory bowel disease. Anti-VLA-4 monoclonal antibody has also been applied to block transendothelial passage in other autoimmune diseases, such as rheumatoid arthritis. On this same vein is the action of such a reagent in impairing in vitro transendothial and fibronectin-driven migration of CD4+ and CD8+ T cells expressing high densities of VLA-4 from Duchenne muscular dystrophy patients, thus potentially enlarging the use of this strategy to other diseases. Yet, in a small number of patients, the use of Natalizumab has been correlated with the progressive multifocal leukoencephalopathy, a serious brain infection caused by the John Cunningham virus. This issue restricted the use of the reagent. In this respect, the development of smaller and more specific antibody reagents should be envisioned as a next-generation promising strategy.
Keywords: cell trafficking, immunotherapy, neuroimmunology, T cells
Introduction
A large series of molecular interactions have been implicated as players in triggering and maintenance of the influx of leukocytes, from the blood, through the blood vessel walls, into the given parenchyma. Integrins correspond to a large protein family of membrane receptors involved in this migratory path. They are integral cell membrane proteins; several of them being directly involved in cell migration. Additionally, a number of integrins able to bind to extracellular matrix moieties (e.g. laminin and fibronectin) are relevant for cell migration within tissues in a variety of organs. Lastly, integrin-directed interactions also play a role in the adhesion of leukocytes to cells in various organs, belonging or not to the hemopoietic system.
Chronic inflammatory diseases of various origins collectively correspond to a major public health issue, both in terms of social and economic consequences. One major common finding, in autoimmune and chronic inflammatory diseases, is the establishment of inflammatory infiltrates, bearing different leukocyte sub-populations, including activated T lymphocytes. Such infiltrates, harmful for the targeted tissue, can be either the origin of the illness, and/or play a significant role in the pathophysiological process perpetuating and providing a positive feedback to the disease.
Although steroids and other broad spectrum anti-inflammatory drugs are effective in treating a variety of inflammatory diseases, long-term usage has important side effects, such as bleeding, upper gastrointestinal complications, and opportunistic infections. Therefore, development of drugs that inhibit specific cellular functions without affecting normal immune surveillance is desirable. Herein, we will discuss selected examples, aiming at providing a discussion on the integrin-mediated immunotherapy, potentially applied as a further tool to treat inflammatory diseases as well as in survival of organ or tissue grafts.
Integrins are regarded as central adhesive molecules able to regulate the intricate pathway of leukocyte trafficking into tissues. They correspond to a large protein family of membrane receptors involved in this migratory path. For example, in a variety of tissues and organs, including the central nervous system (CNS), the pancreas and the skeletal muscle, inflammatory cells, particularly T lymphocytes, use the integrins to move toward and within the given tissue, as for example VLA-6 (α6β1, CD49f/CD29 – a laminin receptor) and VLA-4 (α4β1, CD49d/CD29 – which binds to VCAM-1 and fibronectin). In this respect, it is noteworthy that VLA-4 can be applied as a marker of T-cell activation in both humans and mice, being involved in leukocyte cytoskeleton dynamics [1–7]. Other integrins can direct the migration toward one specific tissue, as the integrin α4β7, which directs lymphocyte migration toward and within mucosal cell layers [4].
Consequently, blocking the establishment and/or maintenance of such a deleterious adhesive system in inflammatory reactions by using inhibitors of these integrins, should bring benefits to patients suffering from chronic inflammatory diseases, regardless their pathogenesis, including transplanted patients.
Consequently, blocking the establishment and/or maintenance of such a deleterious adhesive system in inflammatory reactions by using inhibitors of these integrins, should bring benefits to patients suffering from chronic inflammatory diseases, regardless their pathogenesis, including transplanted patients.
Role of VLA-6-mediated interactions in graft rejection
The integrin VLA-6 (α6β1, CD49f/CD29) is a laminin receptor able to bind various laminin isoforms and plays a role in T-cell development, migration, and activation [8]. Previous studies strongly indicated that VLA-6 can be placed as a potential target to abrogate T-cell-mediated immune reaction, as for example graft rejection. Using implants of neonatal hearts into the subcutaneous tissue of the ears from adult syngeneic recipients, we showed that blocking VLA-6-mediated interactions with anti-VLA-6 prevented heart graft rejection by autoreactive spleen-derived CD4+ T lymphocytes obtained from mice previously infected with the parasite Trypanosoma cruzi, the causative agent of Chagas disease [9]. Similar data were observed when we applied an allogeneic transplant into normal adult recipients: both anti-laminin and anti-VLA-6 antibodies could prevent graft rejection [10, 11]. Moreover, specific blockade of the α5 laminin chain in the lymph nodes prevent activated cells to migrate from the lymph node toward the transplanted tissue, thus impacting graft survival [12].
These findings indicate that laminin/VLA-6-mediated infections can be envisioned as immunotherapeutic targets in controlling autoimmunity, as well as graft maintenance, not only at the rejection site, but also in lymph nodes. Yet, despite the consistent experimental data corresponding clinical assays have not yet been developed.
Targeting VLA-4 as immunotherapeutic strategy to treat specific inflammatory diseases
As compared to VLA-6, much more data are available establishing VLA-4-mediated interactions as immunotherapeutic targets. VLA-4 is highly expressed in different activated T cell subsets; having vascular cell adhesion molecule-1 (VCAM-1, CD106), osteopontin, and fibronectin are natural cognate ligands. In this respect, it has been shown that CD49d-overexpressing T cell lines are autoreactive and proliferate in response to antigen-presenting cells, in an MHC class II-dependent manner, even in the absence of the cognate antigen [13].
VLA-4 is broadly expressed in cells of both innate and adaptive immune responses. Accordingly, this integrin is constitutively seen on the membranes of eosinophils and monocytes, as well as on T and B lymphocytes [14, 15]. Furthermore, it is expressed in all stages in intrathymic T-cell differentiation, particularly in the immature CD4/CD8 double-negative thymocytes [16].
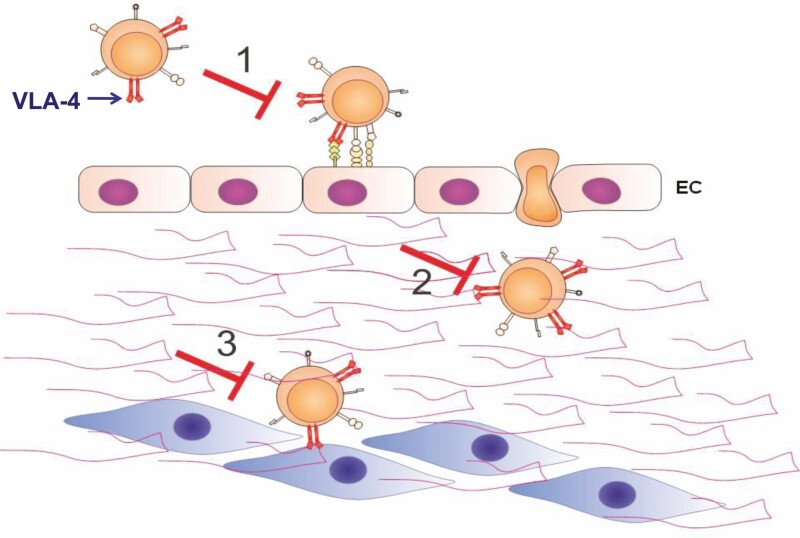
Additionally, VLA-4 overexpression in circulating T lymphocytes is associated with an increased in vitro adhesion to endothelial cells [3]. Accordingly (Fig. 1), blockade of VLA-4/VCAM-1 interaction should impair transendothelial migration of leukocytes to inflammation sites. Moreover, the blockade of VLA-4/fibronectin interaction should impair migration of leukocytes within the given target tissue, as well as binding to putative target cells. Of note, VLA-4 has an anti-apoptotic role in T lymphocytes [17]. In keeping with these findings, induction of apoptosis in lymphocytes has been shown as consequence of corresponding antibody therapy, in the model of autoimmune neuritis [18].
Figure 1.
Anti-VLA-4 antibodies can potentially abrogate transendothelial and intra-tissular T cell migration and adhesion. 1. Extravasation of lymphocytes can be impaired by blocking VLA-4/VCAM-1 interaction at the endothelial of blood vessels; 2. Intratissular migration of lymphocytes in the inflammatory sites can be significantly diminished by blocking VLA-4/fibronectin interaction; 3. Adhesion of the activated lymphocyte to a potential organ specific cell type can be abolished by blocking fibronectin/VCAM-1/VLA-4 mediated cell–cell interaction. EC: endothelial cells; fibronectin is represented by the double waves in the extracellular space.
Therefore, biological products able to inhibit the alpha-4 integrin subunit represent potential immunotherapeutic agents to be applied in a larger spectrum of T-cell-related inflammatory diseases. Actually, since several years, large numbers of pre-clinical and clinical data strongly placed the integrin VLA-4 as a target for immunotherapy, particularly with the use of the anti- α4 integrin antibody [19–22].
Lastly, it is noteworthy that, as briefly mentioned above, the integrin α4β7, that binds to MAdCAM-1 (Mucosal Addressin Cell Adhesion Molecule-1), and also to VCAM-1 and fibronectin, is involved in lymphocyte migration to mucosal inflammatory sites, and has been a target for immunotherapy in gut inflammation [23, 24].
Natalizumab: therapeutic role in multiple sclerosis and inflammatory bowel disease
Multiple sclerosis (MS) is a neurological autoimmune disease, being highly frequent in northern countries such as USA, Canada, and western European countries, and to a lesser extent in Latinoamerican and African countries, varying from less than 30 patients per 100,000 population in Brazil, to more than 150 per 100,000 in Canada. Additionally, it is more prevalent in females than males and represents the most common autoimmune disease in young adults. The disease has three main forms: relapsing and remitting MS, characterized by episodes of neurological dysfunction interspersed with periods of stability; primary-progressive MS, in which progressive neurological disability occurs from the outset; and secondary-progressive MS, in which progressive neurological disability occurs later in the course of the disease [25]. Axonal loss is the major determinant of the accumulation of irreversible (progressive) disability as a result of inflammation during both the relapsing and remitting and progressive phases of MS [26, 27].
MS is considered to be initiated by activated, self-reactive CD4+ T lymphocytes that recognize components of the myelin sheath, which surrounds and insulates nerve fibers. T cells enter the CNS through postcapillary venules and are reactivated by antigen-presenting cells in the perivascular space. These steps are followed by the recruitment of additional inflammatory cells, such as macrophages, which cause inflammation, edema and, eventually, destruction of the myelin sheath (28).
A largely used animal model for human MS is the experimental autoimmune encephalomyelitis (EAE), which can be induced in mice by immunization with myelin-derived peptide aa139-151. Animals develop clinical signs of disease, with intermittent episodes. This occurs with leukocyte infiltration within the CNS. Such an infiltration can be abrogated by anti-VLA-4 antibodies [19, 29–31]. Interestingly, not only VLA-4 is involved in the migration of T lymphocytes through the brain endothelium, but also in the recruitment of immature dendritic cells [32].
As mentioned above, beneficial effects of α4-integrin blockade were demonstrated in animal models as well as in clinical trials with MS patients. Based on these findings, the humanized whole monoclonal antibody Natalizumab [33], targeting the α4-integrin subunit, and that has been approved for treating relapsing-remitting MS. It has been demonstrated that Natalizumab was able to reduce the annual rate of MS relapse by 2/3 and to decrease the development of new gadolinium-enhancing lesions by ±90%, as ascertained by magnetic resonance imaging of MS patients [34]. Natalizumab is part of the therapeutic arsenal of drugs efficient against MS. Actually, a comparative study revealed that was not only more efficacious than fingolimod and dimethyl fumarate, but also was better tolerated by the patients [35]. Nonetheless, the use of Natalizumab has been correlated with the appearance of progressive multifocal leukoencephalopathy (PML), a serious and rare opportunistic infection of the brain caused by the John Cunningham virus (JCV). Since PML is a viral disease, such an adverse effect is likely to be due to the induction of immunodeficiency. These studies revealed that, despite the good general tolerability and sustained efficacy of Natalizumab for patients with severe MS, the risk of PML remained a concern [36]. At present, the use of Natalizumab has been restricted, as a monotherapy for MS patients presenting a highly active progressing disease in Europe and in the USA [37–40].
Natalizumab has also been applied in inflammatory bowel disease (IBD), a group of inflammatory conditions of the colon and small intestine of unknown etiology. The two major autologous types of IBD are Crohn’s disease (CD) and ulcerative colitis (UC), in which the immune system recognizes gastrointestinal tract moieties, causing what is considered an autoimmune inflammation. Treatment of CD comprises anti-inflammatory biologicals such as TNF antagonists, that target inflammatory pathways to induce remission in CD patients. However, approximately one third patients do not respond to anti-TNF therapy, and in some cases severe systemic side-effects have been reported [41]. In this context, Natalizumab has been applied to target a pathway other than TNF inhibition. The drug has demonstrated efficacy in inducing and maintaining remission in moderate-to-severe refractory CD patients with active inflammation [42]. However, due to possible occurrence of PML, the use of Natalizumab as a therapeutic strategy to treat CD is also rather restricted.
Migration to the intestines involves the presence of α4β7 and α4β1 integrins on the lymphocyte membrane [43, 44]. In this respect, it is noticeable that treatment with specific humanized anti-α4β7 antibodies revealed consistent good results in patients with UC [45, 23, 46, 47].
As α4β7 integrin is a central molecule for selective migration of T lymphocytes to the intestines [44], the treatment with specific humanized antibodies that target the interaction of MadCAM with α4β7 seems to overcome such a restriction. Interestingly, several clinical studies reported consistent good results in both UC and CD patients [47] and elicit new comprehensive studies on the migration-targeting immunotherapy of IBD [48].
Potential use of anti-VLA-4 immunotherapy in other inflammatory diseases
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a systemic inflammatory disease affecting the joint lining tissue called synovium. The chronic character of autoimmune diseases has an important socio-economic impact. RA is the most frequent autoimmune disease with a prevalence of about 0.3 to 1% of the population worldwide and often associated with reduced mobility, increased social dependency, and finally work disability. RA patients are frequently at working age and the inability to work causes major financial and psychological issues for the person with the disease and their family. There is also the social and economic burden placed on the community resulting from a person’s incapacity to maintain employment.
RA patients are in general treated with a group of small molecular drugs called disease-modifying antirheumatic drugs (DMARDs). DMARDs suppress the body’s overactive immune and/or inflammatory systems in some way, thereby slowing down disease progression. RA patients not responding to DMARDs are treated with biological agents such as tumor necrosis factor (TNF) antagonists. Though TNF antagonists are effective in about two-thirds of the patients, the responding patients frequently become non-responsive within 5 years. Therefore, alternative treatments are required.
The synovium is normally a relatively acellular structure with a delicate intimal lining that is one or two cell layers deep. It covers the lubricating synovial fluid found in the cavities of synovial joints. The rheumatoid synovial tissue is characterized by hyperproliferation of fibroblast-like synoviocytes in the intimal lining layer and infiltration of the sublining by macrophages, T and B cells, which promote inflammation and destruction of bone and cartilage. Most leukocytes express VLA-4 on their surface and they interact with VCAM-1 expressed on synoviocytes and endothelial cells. Moreover, it has been shown that VLA-4/VCAM interactions between B lymphocytes and synovial fibroblasts upregulating expression of the anti-apoptotic protein Bcl-xL in B cells thus promoting B cell survival in the inflamed synovium [49]. Disruption of these VLA-4-mediated interactions between lymphocytes, synoviocytes and endothelial cells should put an end to the cycle of chronic inflammation, which is the hallmark of rheumatoid arthritis [50]. Accordingly, Natalizumab has been applied to treat RA patients. Nevertheless, a phase II, multicenter, double-blind, placebo-controlled clinical trial designed to determine the safety, tolerability and efficacy of Natalizumab in subjects diagnosed with moderate to severe RA receiving concomitant treatment with methotrexate. No statistically significant differences were found between Metothrexate-treated patients, in the presence or absence of Natalizumab [51]. Deeper investigation on mechanisms, as well as new tools should then be searched for.
Potential use of anti-VLA-4 immunotherapy in Duchenne muscular dystrophy
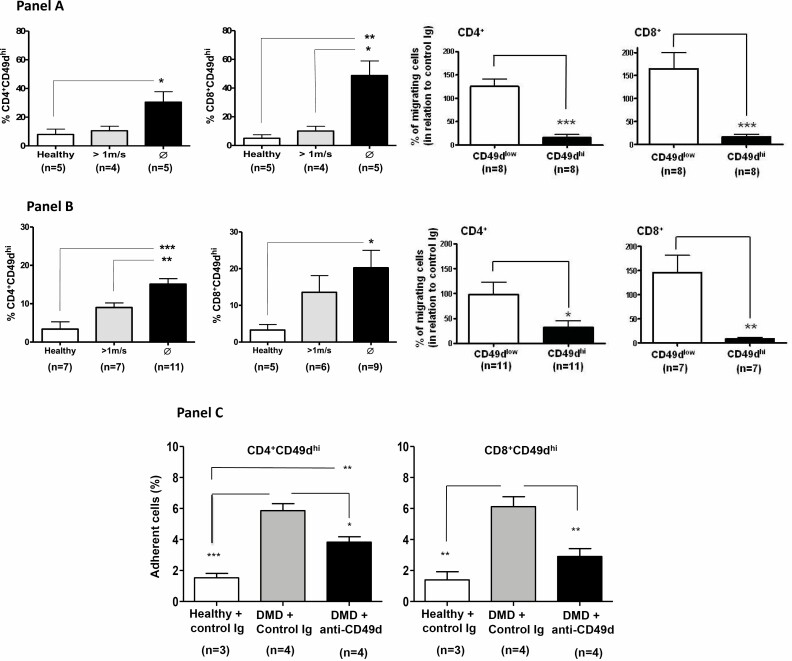
Muscular dystrophies are inherited diseases of the muscle that are characterized clinically by progressive muscle weakness, and pathologically by muscle degeneration. Among them, Duchenne muscular dystrophy (DMD) is the most common form of muscular dystrophy affecting 1 in 3500 newborn boys. DMD presents a progressive muscle weakness resulting in a loss of ambulation usually in the early teens and death around 30 years of age if modern standard care is applied. Despite the genetic cause for DMD, several studies in humans and animal models have suggested that the immune system is implicated in the pathophysiology of the muscular lesions [52–55]. We studied 74 DMD patients at different stages of disease and assayed for CD49d (the α4-integrin subunit) expression in circulating and intramuscular T-cells. Functionally, we tested transendothelial and fibronectin-driven migration, and adhesion to myotube monolayers. Increased percentages of circulating CD4+CD49dhigh and CD8+CD49dhigh T lymphocytes correlated with the more rapid disease progression. Moreover, CD49d+CD4+ and CD49d+CD8+ T cells were found in muscular inflammatory infiltrates. Importantly, T cells from severely affected patients exhibited higher transendothelial and fibronectin-driven migratory responses and increased adhesion to myotubes, when compared with control individuals [3]. As shown in Fig. 2, these responses were blocked with an anti-CD49d monoclonal antibody.
Figure 2.
Role of VLA-4 in migration and adhesion of T-cells from Duchenne Muscular Dystrophy patients: blockade by anti-VLA-4 monoclonal antibody. Panel A reveals that transendothelial migration of CD4+ and CD8+ T cells expressing high densities of CD49d from DMD patients and unable to walk migrate more than the patients able to walk (upper graphics). Importantly, migration of CD49dhi T cells is largely impaired in the presence of anti-VLA-4 antibody (bottom graphics). Similar enhancement of fibronectin-driven T cell migration is seen in panel B, which also shows that migration of CD49dhi T cell subsets is largely impaired in the presence of anti-VLA-4 antibody. Finally, panel C provides evidence showing that both CD4+CD49dhi and CD8+CD49dhi T cells subsets adhere more to cultured human myoblasts, and that such an increase is abrogated by anti-VLA-4 antibody, as compared to unrelated Immunoglobulin. Groups were statistically compared using the Kruskal–Wallis test followed by Dunn’s multiple comparison test. *P < 0.05; **P < 0.01; ***P < 0.001. Modified from Pinto Mariz et al. 2015.
We also found increased numbers of circulating CD49hiCD4+ T lymphocytes in the Golden Retriever Muscular Dystrophy (GRMD) dog, a useful pre-clinical model for DMD, since it mimics the human disease in many aspects more closely than other existing mammalian models of dystrophin deficiency, including the classical mdx mouse. A significant increase was more important in rapid progressors as compared to slow progressors of the diseases. Similarly, CD49hiCD4+ T cells were present in the inflammatory infiltrates within the muscular tissue [56].
Overall, the data discussed in this item tell us that disease progression in DMD correlates with the increase in the relative numbers of CD49dhi T cells (CD4 and CD8) in the blood. Moreover, increased numbers of CD49dhi T cells (both CD4s and CD8s) predict a rapid progression of the disease. Additionally, CD49d expression on T lymphocytes can be used as a biomarker of disease progression in DMD (both in humans and in dystrophic dogs), including the stratification of patients before various clinical trials for other therapeutic strategies. Finally, VLA-4-directed interactions are potential therapeutic targets for selected patients in DMD aiming to improve their quality of life. Thus, the use of anti-VLA-4 antibodies is theoretically a promising approach to ameliorate the quality of life of DMD patients.
Toward a second generation of anti-VLA-4 antibodies
Although the use of monoclonal antibodies as therapeutic tools is quite well established, most marketed antibodies are full-length humanized IgG molecules [57] that provide long half-lives and effector functions. However, there is a range of therapeutic applications in which other antibody formats may be more desirable. For instance, in some conditions, a long antibody serum half-life results in poor contrast in imaging applications, and inappropriate activation of Fc receptor-expressing cells may lead to massive cytokine release and associated toxic effects [58]. Furthermore, some IgG4 antibodies, as Natalizumab, may undergo half-antibody exchange in vivo, which can compromise the performance of the antibody even without damages on the clinical effects [59]. In addition, due to high molecular weight (~150 kDa), IgG antibodies are known to diffuse poorly into solid tissues and clear slowly from the body. By contrast, antibody fragments with specific antigen-recognition sites seem to be versatile stable, cost-effective, and efficient, therapeutic solutions for a range of autoimmune and potentially other inflammatory diseases [60].
Antibody fragments are, in general, less immunogenic due to the absence of Fc component of immunoglobulin [61]. This comprises a huge advantage in comparison to full-length antibodies, which induce the production of human anti-human antibodies (HAHAs) and therefore, activation of the immune system [62].
Besides their reduced immunogenicity, one of the main aspects of using antibody fragments is their ability to cross the blood–brain barrier (BBB) [63]. Most of the antibodies currently used for treating neurodegenerative diseases act mainly in the periphery, therefore, antibody fragments could be useful to perform directly in the CNS [64]. In addition, fragments can be applied to drug delivery, intracellular targeting, and labeling for imaging and diagnosis [64, 64]. In terms of cost-effectivity, it is well known that heterologous proteins produced by bacteria and yeast are cheaper, faster, and easier to produce comparing with mammalian, plant, and insect systems [65]. While full antibodies are recommended to be produced by mammalian cells, antibody fragments are produced in bacteria and yeast systems, are versatile and compatible with all the heterologous systems, which may reduce the costs of production by choosing bacteria as expression platform.
Considering that beneficial effects of blocking VLA-4 are evident, at least in severe MS and CD, it seems clear that novel α4-integrin blocking antibodies should be developed, particularly taking into that other autoimmune and chronic inflammatory diseases could benefit from such treatment. Therefore, smaller antibody molecules such as the antigen-binding fragment (Fab) or the variable fragment (Fv) should be envisioned as further anti-VLA-4 therapeutic agents [66–68]. Single-chain Fv (scFv) molecules are fragments of antibodies composed of the VH and VL domains of the corresponding immunoglobulin, joined by a flexible linker peptide of variable size. These proteins have an average molecular weight of 30 kDa [69], and have two disulfide bridges, one related to VH and the other to VL sequences. Because scFvs are formed by variable domains, they have all six CDRs that make up their antigen recognition region [70]. In addition to the composition, the size of the binding peptide is fundamental to the scFv molecule. Studies have shown that when comparing different scFvs made up of ligand peptides of varying sizes, the reactivity and specificity properties of scFvs have been can change [71]. Therefore, not only the CDRs of these antibody fragments influence the affinity and specificity of scFv, but also its structural conformation and type of peptide linker.
In this context, we recently produced a scFv antibody fragment able to specifically recognize VLA-4 (pending patent deposited at the Brazilian National Institute of Intelectual Property – INPI –, number BR 10 2020 016890 8). The scFv nucleotide sequence was previously designed by using in silico tools as database search, molecular modeling and docking, site-directed mutagenesis, and molecular dynamics. Docking results showed that the scFv presented favorable parameters, namely Haddock Score, Cluster size and RMSD, for its interaction with VLA-4, as compared with two other integrins (LPAM-1 and VLA-5). Molecular dynamics confirmed the docking results and further showed that the main interactions involved are salt bridges, electrostatic and Van der Waals interactions. The scFv sequence thus obtained was cloned and expressed in Escherichia coli. Functionally, this scFv antibody was able to significantly impair the adhesion of T cells (Jurkat T cell line), on surfaces coated with VCAM-1 (Table 1). Furthermore, experiments performed under flow conditions showed that the scFv reduced the adhesion frequency of primary T lymphocytes over VLA-4 ligands. This scFv product also interfered with the pattern of distribution of actin and phosphotyrosine in CD8+ T cells activated by anti-CD3 and fibronectin. Overall, this reagent seems promising, although it still needs validation in relevant pre-clinical models of selected autoimmune and chronic inflammatory diseases is necessary.
Table 1.
Migration of Jurkat T cells over transwell chambers coated with VCAM-1: blockage by Natalizumab and anti-VLA-4 scFv antibodies
| Coating liganda | Cell treatmentb | Number of transmigrating cellsc | P value: versus controld | P value: versus natalizumabd |
|---|---|---|---|---|
| VCAM-1 | None | 30.41 ± 13.25 | ----- | 0.0058 |
| VCAM-1 | Anti VLA-4 scFv | 9.50 ± 4.24 | 0.0440 | 0.582 |
| VCAM-1 | Natalizumab | 1.92 ± 0.29 | 0.0058 | ----- |
| BSA | None | 3.10 ± 3.10 | 0.0073 | 0.993 |
aVCAM-1 concentration = 2.5 µg/ml; BSA concentration: 2 µg/ml; bAnti VLA-4 scFv and Natalizumab concentration = 20 µg/ml; cCell numbers × 103 ± standard deviation. Means of three independent experiments. dDunnett’s multiple comparisons test. Statistically significant P values are shown in bold.
Concluding remarks
Although steroids and other anti-inflammatory drugs with broad-spectrum activities are effective in treating a variety of inflammatory diseases, long-term usage is known to have unacceptable side effects, such as greater risk of bleeding, upper gastro-intestinal complications, and infection caused by alteration of phagocytic leukocyte migration and function. Therefore, in the treatment of chronic inflammatory diseases, it is desirable to develop drugs that inhibit more selectively specific cellular functions without affecting normal immune surveillance.
The data summarized above provide evidence showing that targeting VLA-4, by applying humanized anti-VLA-4 antibody was a relevant therapeutic strategy to treat at least severe refractory inflammatory diseases. Nevertheless, a new generation of inhibitors will certainly be welcome, and the development of smaller and more selective antibodies should be envisioned. In this respect, the design of similar molecules, but only containing the single chain variable fragment of the α4 integrin chain seems to be a promising strategy.
Using antibody fragments may overcome some limitations related to full anti-VLA-4 antibodies. The absence of Fc portion avoids unnecessary immune system activation and allows penetration across the BBB, which could increase the antibody performance directly within the CNS. In addition, antibody fragments can be cleared from the body faster than full antibodies, which may be relevant to prevent harmful effects related to permanent VLA-4 blockade.
Specificity for such a reagent could be further improved by constructing double-specific scFVs directing the anti-integrin to target specific cells.
Having said that, it should be pointed out that VLA-4 is not the only α4-containing integrin that deserves more research and technology improvement. As mentioned above, target α4β7 integrin using the same antibody strategy has been proven to be efficient in gut associated autoimmune diseases.
Lastly, other integrin-directed cellular interactions should be better investigated, so that to enlarge the possibility of reagents to tackle specific and potentially harmful activated T lymphocytes in chronic inflammatory diseases and controlling organ transplantation.
Acknowledgements
The authors thank Dr. Fernanda Pinto Mariz for providing the artwork for Fig. 2 of this manuscript. The Editor-in-Chief, Tim Elliott, and handling editor, Stefan Barth, would like to thank the reviewer Mehmet Tur, and two anonymous reviewers, for their contribution to the publication of this article.
Glossary
Abbreviations
- BBB
Blood–brain barrier
- CD
Crohn’s disease
- DMARDs
Disease-modifying antirheumatic drugs
- DMD
Duchenne muscular dystrophy
- Fab
Antigen-binding fragment
- Fv
Variable fragment
- IBD
Inflammatory bowel disease
- MS
Multiple sclerosis
- PML
Progressive multifocal leukoencephalopathy
- RA
Rheumatoid arthritis
- scFv
Single-chain Fv
- TNF
Tumor necrosis factor
- UC
Ulcerative colitis
- VH
Variable portion of the heavy chain of immunoglobulin
- VLA
Very late antigen
- VL
Variable portion of the light chain of Immunoglobulin
Funding
This work was supported by Fiocruz, CNPq, CAPES and FAPERJ (Brazil), as well as the MercoSur Fund for Structural Convergence (FOCEM/MercoSur). This work was developed in the frameworks of the Brazilian National Institute of Science and Technology on Neuroimmunomodulation and the Rio de Janeiro Research Network on Neuroinflammation.
Authors contribution
W.S., B.C., A.B., and V.C.A. – conceptualization and writing.
Conflict of interest
The authors declare no competing interests.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Vicente-Manzanares M, Sánchez-Madrid F. Role of the cytoskeleton during leukocyte responses. Nat Rev Immunol. 2004;4:110–22. 10.1038/nri1268 [DOI] [PubMed] [Google Scholar]
- 2. Morrot A, Terra-Granado E, Pérez ARet al.. Chagasic thymic atrophy does not affect negative selection but results in the export of activated CD4+CD8+ T cells in severe forms of human disease. PLoS Negl Trop Dis. 2011;5(8):e1268. 10.1371/journal.pntd.0001268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pinto-Mariz F, Rodrigues Carvalho L, Prufer De Queiroz Campos Araujo Aet al.. CD49d is a disease progression biomarker and a potential target for immunotherapy in Duchenne muscular dystrophy. Skelet Muscle. 2015;5(1):45. 10.1186/s13395-015-0066-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bertoni A, Alabiso O, Galetto ASet al.. Integrins in T cell physiology. Int J Mol Sci. 2018;19(2):485. 10.3390/ijms19020485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jankowska KI, Williamson EK, Roy NHet al. Integrins modulate T cell receptor signaling by constraining actin flow at the immunological synapse. Front Immunol. 2018;9:25. 10.3389/fimmu.2018.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martín-Cófreces NB, Vicente-Manzanares M, Sánchez-Madrid F. Adhesive interactions delineate the topography of the immune synapse. Front Cell Dev Biol. 2018;6:149. 10.3389/fcell.2018.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Janahú LTA, Da Costa CA, Vallinoto ACRet al. CD49d is upregulated in circulating T lymphocytes from HTLV-1-infected patients. Neuroimmunomodulation. 2020;27(2):113–22. 10.1159/000507086 [DOI] [PubMed] [Google Scholar]
- 8. Savino W, Mendes-da-Cruz DA, Ferreira Golbert DCet al. Laminin-mediated interactions in thymocyte migration and development. Front Immunol. 2015;6:579. 10.3389/fimmu.2015.00579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silva-Barbosa 1 SD, Cotta-de-Almeida V, Riederer Iet al. Involvement of laminin and its receptor in abrogation of heart graft rejection by autoreactive T cells from Trypanosoma cruzi-infected mice. J Immunol. 1997;159:997–1003. [PubMed] [Google Scholar]
- 10. Silva-Barbosa SD, Riederer I, Savino W. Laminin-mediated interactions in heart graft rejection. Transplantation. 2001;72:172–3. 10.1097/00007890-200107150-00034 [DOI] [PubMed] [Google Scholar]
- 11. Riederer I, Silva-Barbosa SD, Rodrigues MLet al. Local antilaminin antibody treatment alters the rejection pattern of murine cardiac allografts: Correlation between cellular infiltration and extracellular matrix. Transplantation. 2002;74(11):1515–22. 10.1097/00007890-200212150-00007 [DOI] [PubMed] [Google Scholar]
- 12. Warren KJ, Iwami D, Harris DGet al. Laminins affect T cell trafficking and allograft fate. J Clin Invest. 2014;124(5):2204–18. 10.1172/JCI73683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mo R-R, Eisenbraun JK, Sonstein Jet al. CD49d overexpression and T cell autoimmunity. J Immunol. 2003;171(2):745–53. 10.4049/jimmunol.171.2.745 [DOI] [PubMed] [Google Scholar]
- 14. Gonzalez-Amaro R, Mittelbrunn M, Sanchez-Madrid F. Therapeutic anti-integrin (alpha4 and alphaL) monoclonal antibodies: two-edged swords? Immunology. 2005;116(3):289–96. 10.1111/j.1365-2567.2005.02225.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rice GPA, Hartung HP, Calabresi PA. Anti-α4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64:1336–42. 10.1212/01.WNL.0000158329.30470.D0 [DOI] [PubMed] [Google Scholar]
- 16. Dalmau 1 S R, C S Freitas WS. Upregulated expression of fibronectin receptors underlines the adhesive capability of thymocytes to thymic epithelial cells during the early stages of differentiation: lessons from sublethally irradiated mice. Blood. 1999;93:974–90. [PubMed] [Google Scholar]
- 17. Koopman G, Keehnen RM, Lindhout Eet al. Adhesion through the LFA-1 (CD11a/CD18)-ICAM-1 (CD54) and the VLA-4 (CD49d)-VCAM-1 (CD106) pathways prevents apoptosis of germinal center B cells. J Immunol. 1994;152(8):3760–7. [PubMed] [Google Scholar]
- 18. Leussink VI, Zettl UK, Jander Set al. Blockade of signaling via the very late antigen (VLA-4) and its counterligand vascular cell adhesion molecule-1 (VCAM-1) causes increased T cell apoptosis in experimental autoimmune neuritis. Acta Neuropathol. 2002;103(2):131–6. 10.1007/s004010100444 [DOI] [PubMed] [Google Scholar]
- 19. Yednock TA, Cannon C, Fritz LCet al. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4βl integrin. Nature. 1992;356(6364):63–6. 10.1038/356063a0 [DOI] [PubMed] [Google Scholar]
- 20. Yang XD, Karin N, Tisch Ret al. Inhibition of insulitis and prevention of diabetes in nonobese diabetic mice by blocking L-selectin and very late antigen 4 adhesion receptors. Proc Natl Acad Sci USA. 1993;90(22):10494–8. 10.1073/pnas.90.22.10494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsukamoto K, Yokono K, Amano Ket al. Administration of monoclonal antibodies against vascular cell adhesion molecule-1/very late antigen-4 abrogates predisposing autoimmune diabetes in NOD mice. Cell Immunol. 1995;165(2):193–201. 10.1006/cimm.1995.1205 [DOI] [PubMed] [Google Scholar]
- 22. Vajkoczy P, Laschinger M, Engelhardt B. α4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest. 2001;108(4):557–65. 10.1172/JCI12440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Danese S, De la Rue SA, Gasbarrini A. Antibody to α 4 β 7 integrin for ulcerative colitis. N Engl J Med. 2005;353(11):1180–1. 10.1056/nejmc051937 [DOI] [PubMed] [Google Scholar]
- 24. Xu YZ, Smith JL, Semko CMet al. Orally available and efficacious α4β1/α4β7 integrin inhibitors. Bioorganic Med Chem Lett. 2013;23(15):4370–3. 10.1016/j.bmcl.2013.05.076 [DOI] [PubMed] [Google Scholar]
- 25. Wallin MT, Culpepper WJ, Nichols Eet al. Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269–85. 10.1016/S1474-4422(18)30443-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2(9):762–4. 10.1038/ni0901-762 [DOI] [PubMed] [Google Scholar]
- 27. Absinta M, Lassmann H, Trapp BD. Mechanisms underlying progression in multiple sclerosis. Curr Opin Neurol. 2020;33(3):277–85. 10.1097/WCO.0000000000000818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sallusto F, Impellizzieri D, Basso Cet al. T-cell trafficking in the central nervous system. Immunol Rev. 2012;248(1):216–27. 10.1111/j.1600-065X.2012.01140.x [DOI] [PubMed] [Google Scholar]
- 29. Engelhardt B, Laschinger M, Schulz Met al. The development of experimental autoimmune encephalomyelitis in the mouse requires α4-integrin but not α4β7-integrin. J Clin Invest. 1998;102(12):2096–105. 10.1172/JCI4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laschinger M, Engelhardt B. Interaction of alpha4-integrin with VCAM-1 is involved in adhesion of encephalitogenic T cell blasts to brain endothelium but not in their transendothelial migration in vitro. J Neuroimmunol. 2000;102:32–43. 10.1172/JCI12440 [DOI] [PubMed] [Google Scholar]
- 31. Bauer M, Brakebusch C, Coisne Cet al. β1 integrins differentially control extravasation of inflammatory cell subsets into the CNS during autoimmunity. Proc Natl Acad Sci USA. 2009;106(6):1920–5. 10.1073/pnas.0808909106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jain P, Coisne C, Enzmann Get al. α 4 β 1 integrin mediates the recruitment of immature dendritic cells across the blood-brain barrier during experimental autoimmune encephalomyelitis. J Immunol. 2010;184(12):7196–206. 10.4049/jimmunol.0901404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Léger OJ, Yednock TA, Tanner Let al. Humanization of a mouse antibody against human alpha-4 integrin: a potential therapeutic for the treatment of multiple sclerosis. Human Antibodies. 1997;8(1):3–16. [PubMed] [Google Scholar]
- 34. Engelhardt B, Kappos L. Natalizumab: targeting αlpha4-integrins in multiple sclerosis. Neurodegener Dis. 2008;5(1):16–22. 10.1159/000109933 [DOI] [PubMed] [Google Scholar]
- 35. Vollmer BL, Nair KV, Sillau Set al. Natalizumab versus fingolimod and dimethyl fumarate in multiple sclerosis treatment. Ann Clin Transl Neurol. 2019;6(2):252–62. 10.1002/acn3.700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 2010;61:35–47. 10.1146/annurev.med.080708.082655 [DOI] [PubMed] [Google Scholar]
- 37. Monaco MCG, Major EO. The link between VLA-4 and JC virus reactivation. Expert Rev Clin Immunol. 2012;8:63–72. 10.1586/eci.11.85 [DOI] [PubMed] [Google Scholar]
- 38. Schwab N, Schneider-Hohendorf T, Wiendl H. Therapeutic uses of anti- 4-integrin (anti-VLA-4) antibodies in multiple sclerosis. Int Immunol. 2015;27(1):47–53. 10.1093/intimm/dxu096 [DOI] [PubMed] [Google Scholar]
- 39. Schwab N, Schneider-Hohendorf T, Melzer Net al. Natalizumab-associated PML: Challenges with incidence, resulting risk, and risk stratification. Neurology. 2017;88:1197–205. 10.1212/WNL.0000000000003739 [DOI] [PubMed] [Google Scholar]
- 40. Khoy K, Mariotte D, Defer Get al. Natalizumab in multiple sclerosis treatment: from biological effects to immune monitoring. Front Immunol. 2020;11:2468. 10.3389/fimmu.2020.549842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Atreya R, Neurath MF, Siegmund B. Personalizing treatment in IBD: hype or reality in 2020? Can we predict response to anti-TNF? Front Med. 2020;7:517. 10.3389/fmed.2020.00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pagnini C, Arseneau KO, Cominelli F. Natalizumab in the treatment of Crohn’s disease patients. Expert Opin Biol Ther. 2017;17(11):1433–8. 10.1080/14712598.2017.1366444 [DOI] [PubMed] [Google Scholar]
- 43. Zundler S, Fischer A, Schillinger Det al. The 4β1 homing pathway is essential for ileal homing of Crohn’s disease effector T cells in vivo. Inflamm Bowel Dis. 2017;23(3):379–91. 10.1097/MIB.0000000000001029 [DOI] [PubMed] [Google Scholar]
- 44. Allner C, Melde M, Becker Eet al. Baseline levels of dynamic CD4+ T cell adhesion to MAdCAM-1 correlate with clinical response to vedolizumab treatment in ulcerative colitis: a cohort study. BMC Gastroenterol. 2020;20(1):103. 10.1186/s12876-020-01253-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feagan BG, Greenberg GR, Wild Get al. Treatment of ulcerative colitis with a humanized antibody to the α 4 β 7 integrin. N Engl J Med. 2005;352(24):2499–507. 10.1056/nejmoa042982 [DOI] [PubMed] [Google Scholar]
- 46. Milch C, Wyant T, Xu Jet al. Vedolizumab, a monoclonal antibody to the gut homing α4β7 integrin, does not affect cerebrospinal fluid T-lymphocyte immunophenotype. J Neuroimmunol. 2013;264(1–2):123–6. 10.1016/j.jneuroim.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 47. Panés J, Salas A. Past, present and future of therapeutic interventions targeting leukocyte trafficking in inflammatory bowel disease. J Crohn’s Colitis. 2018;12(suppl_2):S633–40. 10.1093/ecco-jcc/jjy011 [DOI] [PubMed] [Google Scholar]
- 48. Sandborn WJ, Vermeire S, Tyrrell Het al. Etrolizumab for the treatment of ulcerative colitis and Crohn’s disease: an overview of the phase 3 clinical program. Adv Ther. 2020;37(7):3417–31. 10.1007/s12325-020-01366-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Silverman MD, Haas CS, Rad AMet al. The role of vascular cell adhesion molecule 1/very late activation antigen 4 in endothelial progenitor cell recruitment to rheumatoid arthritis synovium. Arthritis Rheum. 2007;56(6):1817–26. 10.1002/art.22706 [DOI] [PubMed] [Google Scholar]
- 50. Mori M, Hashimoto M, Matsuo Tet al. Cell-contact-dependent activation of CD4+ T cells by adhesion molecules on synovial fibroblasts. Mod Rheumatol. 2017;27(3):448–56. 10.1080/14397595.2016.1220353 [DOI] [PubMed] [Google Scholar]
- 51. Natalizumab in the Treatment of Rheumatoid Arthritis in Subjects Receiving Methotrexate - Full Text View - ClinicalTrials.gov. [cited 18 December 2020]. https://clinicaltrials.gov/ct2/show/NCT00083759
- 52. De Paepe B, De Bleecker JL. Cytokines and chemokines as regulators of skeletal muscle inflammation: presenting the case of Duchenne muscular dystrophy. Mediators Inflamm. 2013;2013:540370. 10.1155/2013/540370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nitahara-Kasahara Y, Takeda S, Okada T. Inflammatory predisposition predicts disease phenotypes in muscular dystrophy. Inflammation and Regeneration. 2016;36:14. 10.1186/s41232-016-0019-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tidball JG, Welc SS, Wehling-Henricks M. Immunobiology of inherited muscular dystrophies. Compr Physiol. 2018;8(4):1313–56. 10.1002/cphy.c170052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bonomo AC, Pinto-Mariz F, Riederer Iet al. Crosstalk between innate and T cell adaptive immunity with(in) the muscle. Frontiers in Physiology. 2020;11:573347. 10.3389/fphys.2020.573347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barthélémy I, Pinto-Mariz F, Yada Eet al. Predictive markers of clinical outcome in the GRMD dog model of Duchenne muscular dystrophy. DMM Dis Model Mech. 2014;7(11):1253–61. 10.1242/dmm.016014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ley K, Rivera-Nieves J, Sandborn WJet al. Integrin-based therapeutics: Biological basis, clinical use and new drugs. Nat Rev Drug Discov. 2016;15:173–83. 10.1038/nrd.2015.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Harding FA, Stickler MM, Razo Jet al. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs. 2010;2(3):256–65. 10.4161/mabs.2.3.11641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shapiro RI, Plavina T, Schlain BRet al. Development and validation of immunoassays to quantify the half-antibody exchange of an IgG4 antibody, natalizumab (Tysabri ®) with endogenous IgG4. J Pharm Biomed Anal. 2011;55(1):168–75. 10.1016/j.jpba.2011.01.006 [DOI] [PubMed] [Google Scholar]
- 60. Fernandes JC. Therapeutic application of antibody fragments in autoimmune diseases: current state and prospects. Drug Discov Today. 2018;23(12):1996–2002. 10.1016/j.drudis.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 61. Kholodenko RV, Kalinovsky DV, Doronin IIet al. Antibody fragments as potential biopharmaceuticals for cancer therapy: success and limitations. Curr Med Chem. 2019;26(3):396–426. 10.2174/0929867324666170817152554 [DOI] [PubMed] [Google Scholar]
- 62. Nechansky A. HAHA - nothing to laugh about. Measuring the immunogenicity (human anti-human antibody response) induced by humanized monoclonal antibodies applying ELISA and SPR technology. J Pharm Biomed Anal. 2010;51(1):252–4. 10.1016/j.jpba.2009.07.013 [DOI] [PubMed] [Google Scholar]
- 63. Yu YJ, Watts RJ. Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics. 2013;10:459–72. 10.1007/s13311-013-0187-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bates A, Power CA. David vs. Goliath: the structure, function, and clinical prospects of antibody fragments. Antibodies. 2019;8(2):28. 10.3390/antib8020028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gomes1 AR, Byregowda1 SM, Belamaranahally. et al. An overview of heterologous expression host systems for the production of recombinant proteins. Adv Anim Vet Sci. 2016;4(7):346–56. 10.14737/journal.aavs/2016/4.7.346.356 [DOI] [Google Scholar]
- 66. Nelson AL. Antibody fragments. MAbs. 2010;2(1):77–83. 10.4161/mabs.2.1.10786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ahmad ZA, Yeap SK, Ali AMet al. scFv antibody: principles and clinical application. Clin Dev Immunol. 2012;2012:1–15. 10.1155/2012/980250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cheng M, Santich BH, Xu Het al. Successful engineering of a highly potent single-chain variable-fragment (scFv) bispecific antibody to target disialoganglioside (GD2) positive tumors. Oncoimmunology. 2016;5(6):e1168557. 10.1080/2162402X.2016.1168557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Le Gall F F Le, Bove J-M, Garnier M. Engineering of a single-chain variable-fragment (scFv) antibody specific for the stolbur phytoplasma (Mollicute) and its expression in Escherichia coli and tobacco plants. Appl Environ Microbiol. 1998;64(11):4566–72. 10.1128/aem.64.11.4566-4572.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shen Z, Stryker GA, Mernaugh RLet al. Single-chain fragment variable antibody piezoimmunosensors. Anal Chem. 2005;77(3):797–805. 10.1021/ac048655w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yusakul G, Sakamoto S, Pongkitwitoon Bet al. Effect of linker length between variable domains of single chain variable fragment antibody against daidzin on its reactivity. Biosci Biotechnol Biochem. 2016;80(7):1306–12. 10.1080/09168451.2016.1156482 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.