Summary
Co-stimulation is a fundamental component of T cell biology and plays a key role in determining the quality of T cell proliferation, differentiation, and memory formation. T cell-based immunotherapies, such as chimeric antigen receptor (CAR) T cell immunotherapy, are no exception. Solid tumours have largely been refractory to CAR T cell therapy owing to an immunosuppressive microenvironment which limits CAR T cell persistence and effector function. In order to eradicate solid cancers, increasingly sophisticated strategies are being developed to deliver these vital co-stimulatory signals to CAR T cells, often specifically within the tumour microenvironment. These include designing novel co-stimulatory domains within the CAR or other synthetic receptors, arming CAR T cells with cytokines or using CAR T cells in combination with agonist antibodies. This review discusses the evolving role of co-stimulation in CAR T cell therapies and the strategies employed to target co-stimulatory pathways in CAR T cells, with a view to improve responses in solid tumours.
Keywords: chimeric antigen receptor, immunotherapy, T cell immunology, co-stimulation
Introduction
Immunotherapies are an increasingly prevalent therapeutic option for patients with cancer. Chimeric antigen receptor (CAR) T cell immunotherapy is a strategy to genetically engineer patient T cells with a synthetic receptor targeting a specific antigen [1]. The CARs are composed of an antigen binding single-chain fragment variable (scFV) extracellular domain, transmembrane domain, and the intracellular CD3ζ and co-stimulation signalling domains. CAR T cells are currently FDA approved for the treatment of certain B cell malignancies [2]. However the overall responses are disappointing in solid cancers [3]. This is due to several factors such as an immunosuppressive tumour microenvironment (TME), poor trafficking into the tumour and limited persistence of CAR T cells [4].
Optimal T cell activation results from cognate antigen recognition (signal 1), co-stimulation (signal 2), and cytokine support (signal 3). The precise timing and context of co-stimulation signals are understood to ultimately define the effectiveness of the T cell response [5]. Integrating this understanding with CAR T cell design will lead to more robust CAR T cell therapies for solid cancers. This review will summarize the role of co-stimulation in CAR T cell therapies with a focus on strategies to improve responses in solid cancers.
Importance of co-stimulation in CAR design
The first generation of CARs were developed more than 30 years ago. These CARs contained a single CD3ζ chain but did not include any co-stimulation intracellular domain, thus had limited anti-tumour function due to the lack of co-stimulation. In an early phase I study using the first-generation CAR against alpha-folate receptor (FR) in metastatic ovarian cancer, none of the treated patients developed any anti-tumour response, demonstrating the importance in incorporating co-stimulation in the CAR design [6]. The first studies exploring the use of co-stimulation in CAR T cells included a CD28 co-stimulation intracellular domain into the CAR receptor [7]. CD28 co-stimulation domain greatly enhanced CAR T cell function leading to early clinical responses to CAR T cell therapy, highlighting the importance of co-stimulation signalling [8, 9]. In an early trial, a patient with advanced follicular lymphoma was treated with a CD19-CAR that contained a CD28 co-stimulation domain. This patient’s cancerous B cells were eliminated and absent for at least 39 weeks after CAR T cell transfusion. Inspired by the success, other co-stimulatory domains have been included in CARs and some trials have demonstrated great success [8, 9].
Until now only a limited number of co-stimulatory domains have been thoroughly investigated [10]. CD28 and 4-1BB (CD137) are the best characterized domains and the only two included in current FDA approved CAR T cell formulations (Table 1). These domains trigger distinct downstream signalling pathways resulting in either increased persistence or enhanced effector function of CAR T cells [11]. The selection of co-stimulatory domains within the CAR is believed to be key to overcoming barriers imposed by solid tumours. Screening approaches have demonstrated a wide range of novel candidate co-stimulatory domains which can be incorporated into CARs [12]. To this end, many groups are exploring additional domains such as OX40 (CD134), CD27, GITR (CD357), and ICOS (CD278) [13–17] (Fig. 1-1). CARs including one co-stimulatory domain are classified as second generation, while those including two co-stimulatory domains are classified as third generation. Third-generation CARs demonstrated superior anti-tumour responses and magnitude of in vivo expansion compared to second-generation CARs in some studies. Ramos et al. demonstrated that third-generation CAR T cells persisted longer and with superior in vivo expansion compared to second-generation CAR T cells in relapsed/refractory non-Hodgkin lymphoma patients [18]. However, other studies have demonstrated opposing results. For example, a study comparing the second-generation anti-PSCA-CD28 CAR with the third-generation anti-PSCA-CD28-4-1BB CAR indicated that the second-generation CAR was superior in their anti-tumour effect in a human pancreatic cancer xenograft model [19]. The superiority of third-generation CARs is therefore still debatable. Collectively, these studies demonstrated that co-stimulation within the CAR receptor is a key factor determining CAR T cell efficacy.
Table 1.
FDA-approved CAR T therapies and their associated co-stimulatory domains. Data collected from Clinicaltrials.gov and fda.gov
| Product | Company | Target | Disease | Co-stimulatory domain | Clinical Trial |
|---|---|---|---|---|---|
| KYMRIAH (tisagenlecleucel) | Novartis | CD19 | Diffuse large B cell lymphoma (DLBCL), high grade B-cell lymphoma and DLBCL arising from follicular lymphoma. | 4-1BB | NCT02445248 |
| YESCARTA (axicabtagene ciloleucel) |
Kite Pharma | CD19 | DLBCL not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma. | CD28 | NCT02348216 |
| BREYANZI (lisocabtagene maraleucel) | Juno Therapeutics | CD19 | DLBCL, high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B | 4-1BB | NCT02631044 NCT03484702 NCT03744676 NCT03310619 NCT03483103 NCT03331198 NCT03743246 NCT03435796 |
| ABECMA (Idecabtagene vicleucel) |
Celgene Corporation | BCMA | Relapsed/refractory multiple myeloma | 4-1BB |
NCT03361748 NCT02215967 NCT02658929 |
| TESCARTUSa (brexucabtagene autoleucel) |
Kite Pharma | CD19 | Relapsed/refractory mantle cell lymphoma | CD28 | NCT02601313 |
aTESCARTUS employs the identical retroviral vector to YESCARTA however is manufactured using a distinct protocol which enriches for T cells.
Figure 1.
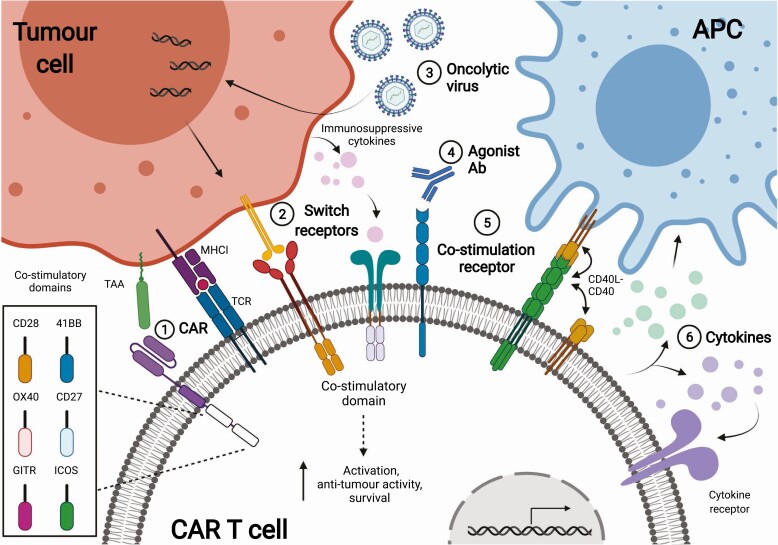
Strategies enhancing co-stimulation of CAR T cells. 1-1: Co-stimulation and synthetic signalling domains can be integrated directly within the CAR receptor. These domains provide co-stimulatory signals when the CAR is activated. 1-2: Switch receptors (e.g. PDL1-CD28) and inverted cytokine receptors (e.g. GM-CSF-IL-18) transduce a co-stimulation signal when ligating immunosuppressive cytokines such as PDL1 or GM-CSF. 1-3: Oncolytic viruses target tumor cells and remodel the TME with immunostimulatory molecules such as OX40. 1-4: CAR T cell secreting agonist antibodies against CD40 or 4-1BB activate CAR T or endogenous immune cells. 1-5: CD40 (represented by the yellow molecule) can act in cis and trans when expressed on CAR T cells. 1-6: CAR T cells secreting cytokines such as IL-18 or IL-12 license APCs or act on T cells to drive an antitumor response.
Co-stimulation delivered intrinsically within the CAR can be coupled with other methods of co-stimulation to overcome the key barriers imposed by solid cancers. Some novel designs include co-stimulatory domains from certain signalling pathways. CARs incorporating MyD88 domains along with intracellular domains of CD40 demonstrated improved efficacy. The incorporation of these ‘MC’ co-stimulatory domains resulted in increased long-lived central memory CAR T cells associated with improved clinical outcomes [20, 21]. Coupling co-stimulation and CAR engagement affords precise control over when and how co-stimulation is delivered. Other strategies may include transducing additional genes that code for cytokines, synthetic signalling domains and receptors into the CAR T cells. For example, a study included a JAK-STAT signalling domain into a CAR to resemble γ-chain cytokine signalling and resulted in increased CAR T cell proliferation in vivo in a model of oesophageal cancer [22]. Including domains such as this within the CAR circumvents potential cytokine release syndrome (CRS) associated with non-specific secretion of cytokine and avoids administration of toxic cytokines. Toll-like receptors (TLR) are known co-receptors in T cells, and CARs incorporating TLR domains are being developed [23]. TLR2 is expressed on memory T cell subsets and detects pathogen-associated molecular pattern (PAMPs) and endogenous danger-associated molecular patterns (DAMPs), such as heat shock protein (HSPs) and amyloids [24, 25]. Unlike commonly used domains, TLR2 signals through MyD88 to improve cytokine secretion and effector function in T cells [26, 27]. The incorporation of TLR2 domains improved the efficacy of MUC1-CAR T cell function in a solid tumour model [28].
Synthetic and combinatorial co-stimulatory receptors enhance CAR T cell function
CAR T cells can be transduced to express additional synthetic receptors, which act in trans or parallel with CAR receptors to provide co-stimulation to CAR T cells. These receptors often target molecules overexpressed by the TME. Switch receptors link a checkpoint extracellular domain to a co-stimulatory intracellular domain, for example, PD-1 (CD279) and CD28 [29] (Fig. 1-2). This PD-1-CD28 receptor delivers CD28 co-stimulation to CAR T cells when ligating PD-L1 (CD274), which is overexpressed by solid tumours. The ligation leads to enhanced cytokine secretion and restimulation of the switch CAR cells. In two models of mesothelin and several PSCA+ solid tumours, the switch CAR anti-tumour effect is stronger than the non-switch CAR cells used in combination with pembrolizumab (anti-PD-1), indicating that signalling through CD28 of the switch receptor is driving this effect [30]. Inverted cytokine receptors (ICR) function similarly to switch receptors but leverage the abundance of immunosuppressive cytokines in the TME [31, 32]. ICRs couple an extracellular domain of an immunosuppressive cytokine receptor such as IL-4 with an intracellular signalling domain of a pro-survival cytokine receptor such as IL-7. These receptors deliver pro-survival cytokine signals (signal 3) in the presence of suppressive cytokines in the TME (Fig. 1-2). For example, CAR T cells expressing a GM-CSF-IL-18 ICR were able to mediate tumour regression in HER2 and EphA2 solid tumour models. In this design, the ICR contained an extracellular domain of the GM-CSF receptor and the signalling domains of the IL-18 receptor (GM18). GM18 can be activated in the tumour by endogenous GM-CSF of the TME, leading to enhanced CAR T cell survival and tumour cell clearance [33]. GM-CSF has also been targeted with an IL-2-based ICR [34]. These additional co-stimulatory triggers may synergize with CAR signalling by including distinct domains, effectively augmenting CAR signalling localized within tumour tissues. Additional synthetic co-stimulatory receptors also flip key interactions within the TME to deliver additional pro-survival signals to CAR T cells.
CD40 is a receptor expressed on antigen presenting cells (APCs) and is central to developing tumour-specific T cell responses [35]. When expressed on T cells, CD40 is able to act in cis and trans by binding to CD40-L expressed on T cells, ultimately enhancing the survival of CAR T cells in tumours [36] (Fig. 1-5). Solid tumours evade the immune system through a number of mechanisms including a large degree of antigen heterogeneity, as well as their immunosuppressive microenvironment. Enhancing co-stimulation of both CAR T and endogenous T cells may boost endogenous immune responses to recognise neoantigens and reduce tumour immune escape. CD40L+ CAR T cells are shown to be superior in their anti-tumour effect and provide a rational to incorporate CD40-CD40-L signal in CAR T design [36].
Cytokine co-stimulation is a crucial component of a CAR T cell response
Cytokines are secreted proteins with a range of effects on all sets of immune cells. In the context of CAR T cells, these proteins constitute the ‘signal 3’ checkpoint for activation. γ-chain cytokines such as IL-2 and IL-15 have essential non-redundant roles in supporting the survival and differentiation of T cells, as well as CAR T cells [37]. Ex vivo production of CAR T cells using these γ-chain cytokines drives CAR T cell differentiation to effective subtypes for solid cancers, and these cytokines have also been used as direct therapies in vivo [38]. Cytokines such as IL-2 and IL-12 have been used to activate and expand tumour-infiltrating lymphocytes (TILs) in solid cancers resulting in some curative responses, but are associated with toxicity [39]. Therefore, ‘armoured CAR’ T cells have been developed to secrete such cytokines specifically within the TME to reduce toxicity as well as recruit endogenous T cells to overcome tumour heterogeneity [40] (Fig. 1-6). CAR T cells transduced to secrete IL-12 increased macrophage and innate cell-mediated clearance of TAA-negative cells, leading to enhanced control of tumours [41]. However, excessive cytokine co-stimulation with IL-12 has been documented to drive CART cell exhaustion [42]. IL-1 family cytokines are a group of proinflammatory cytokines including IL-1, IL-18, and IL-36γ [43]. These cytokines are generally proinflammatory and can act on both T cells and dendritic cells (DCs) to drive a Th1 type response and increase IFNγ secretion by T cells [43]. IL-18, best known for inducing antigen-independent bystander T cell activation, can act synergistically with IL-12 to inhibit solid cancer progression [44, 45]. CAR T cells expressing IL-18 were able to mediate effective responses in a model of colon cancer while also activating endogenous TILs [46]. Similarly, CAR T cells expressing IL-36γ also mediated tumour regression but with different kinetics to previously tested IL-1 family cytokines, demonstrating non-redundant signalling within this cytokine family [47]. Chemotactic cytokines, or chemokines, can also be used to enhance trafficking of CAR T cells to solid tumours. CAR T cells secreting IL-7 and CCL19 provide both pro-survival signals to CAR T cells in the tumour as well as recruit and license intertumoral APCs in a model of lung cancer [48]. This resulted in increased immune cell infiltration and memory formation as cured mice were resistant to tumour re-challenge, and these results have now been extended to human xenograft models. Manipulation of the cytokine milieu by direct CAR T cell secretion has demonstrated effects directly on the function of CAR T cells and endogenous cells, remodelling the TME to a more permissive immune environment. Understanding the role of cytokines in sustaining, improving or hampering intra-tumoral immune response will facilitate their optimal incorporation into CAR T cell therapy regimes.
Antibody-based approaches utilizing co-stimulation in CAR T cell therapies
Checkpoint blockade therapies are an indirect method of modulating T cell co-stimulation by utilising antibodies to inhibit negative regulators of co-stimulatory molecules. These therapies have demonstrated to enhance CAR T cell efficacy and have been reviewed elsewhere [49, 50]. Antibodies directly targeting co-stimulatory molecules can also boost the immune response to cancer. CD40 antibodies are approved therapeutics for cancer and have both T cell intrinsic and pleiotropic effects [51]. When used in combination with IL-15, CD40 agonists were able to increase CD8 T cell and NK cell infiltration into pancreatic cancers, leading to establishment of immune memory response [52]. In a novel approach, CAR T cells were engineered to secrete CD40 agonist antibodies. Compared with traditional CAR T cells, these anti-CD40 secreting CAR T cells demonstrated elevated cytotoxic effect on cancer cells and increased proportion of central memory phenotype [53]. 4-1BB agonist antibodies have also been investigated in the context of solid cancers and were able to increase the cytokine secretion of CAR T cells as well as remodelling of endogenous T cells in a model of breast cancer [54] (Fig. 1-4). However, these agonist antibodies have not progressed beyond clinical trials due to systemic toxicity and requirement of FcγRIII to facilitate hyper clustering of 4-1BB [55].
Co-stimulatory bispecific antibodies have been developed which combine two antibody or ligand specificities [56]. This strategy allows for agonist antibodies being targeted to the TME by coupling with an antibody specific for a TAA [57, 58]. For example, a bispecific composed of 4-1BBL (CD137L) and fibroblast activator protein was able to provide co-stimulation to T cells [59]. Similarly, coupling antibodies to collagen factors in tumour-associated vasculature has been used to deliver checkpoint antibodies, IL-2 or chemokine factors to the TME, leading to APC recruitment [60, 61]. A CD27-PD-L1 bispecific was able to simultaneously deliver co-stimulation and checkpoint blockade, leading to increased T cell function [62]. These bispecific antibodies have great potential to be used together with CAR T cells to boost CAR T cell anti-tumour effect. For example, bispecific engager antibodies targeting CD40 and the c-Myc tag expressed within CAR was able to eliminate tumours in mouse models of breast cancer [63]. The eradication of tumour was due to enhanced co-stimulation of CAR T cells by APCs mediated by this bispecific antibody. Currently CD27, CD28, CD40, and 4-1BB co-stimulation have been tested in the form of a bispecific engagers.
Antibody-based therapies offer precise dose control and targeting to the TME to limit toxicity. Additionally, antibody therapies offer a high degree of flexibility for combination with many CAR T formats already in use and have pleiotropic effects to enhance both CAR T cell and endogenous immune responses.
Non-antibody-based approaches utilizing co-stimulation in CAR T cell therapies
Nanotechnology and biotechnology are increasingly utilized in health and medicine. In the context of CAR T cell therapies, these fields offer alternative methods of delivering co-stimulation to antibody-based methods. Nanoparticle vaccines have been demonstrated to engage the host APCs to activate T cells and can be used in cancer immunotherapy [64]. For example, a nanoparticle targeting CLEC-9A was able to effectively deliver antigen to host cross presenting DCs promoting the activation of CAR-TCR dual-specific cells [65]. Additionally, a nanoparticle RNA vaccine enabled claudin-presentation by APCs to claudin-specific CAR T cells, and enhanced CAR T cell trafficking to tumour tissues, leading to eradication of disease [66]. A similar technology utilised APC targeting ‘amph ligands’ to direct CAR T cell interactions with endogenous DCs. This platform utilises the CAR-specific ligand attached to a DC targeting phospholipid polymer, resulting in CAR T cell and DCs interactions [67]. The co-stimulatory signals delivered by DCs to CAR T cells leads to increased proliferation and tumour control [68].
Viruses can alter the TME to enhance CAR T cell infiltration, activation, and anti-tumour effects. Oncolytic viruses (OV) naturally infect malignant cells and are therefore good theoretical candidates for synergy with CAR T cell therapy. OV can remodel the TME, as well as cause tumour cell death and release of neoantigens [69, 70]. Some studies armed OVs with molecules such as cytokines or co-stimulatory ligands, which are expressed by tumours after OV infection. The expression of these immune modulatory molecules subsequently drives CAR T cell activation (Fig. 1-3). OV-mediated expression of a bispecific engager worked synergistically with CAR T cell activity in two tumour models [71]. In a tumour model of B16 melanoma, modified OV expressing IL-21 enhanced the survival of mice compared to a panel of co-stimulatory molecules including CD86 and 4-1BB [72]. Other therapies utilising OVs to express molecules such as OX40, IL-2, and CD40 have also been studied [73, 74]. OV therapies can be further refined to enhance tropism for tumour cells through the inclusion of tumour-specific promoters such as survivin or hTERT, or modification of OV capsid proteins [75]. For example, a chimeric OV created from vesicular stomatitis virus (VSV) and Newcastle disease virus generated potent anti-tumour effect with greatly reduced hepatotoxicity and neurotoxicity compared to wild-type VSV OV. [69].
Platforms for delivering co-stimulation specifically to the TME or specific subsets of APC within the immune system can be used to drive CAR T cell proliferation and persistence in vivo. These methods offer several advantages over antibody-based methods, including delivering flexible payloads or antigens. Therefore, these technologies should be developed further to deliver specific co-stimulatory payloads for each tumour type.
Conclusions and future directions
The understanding of the role of co-stimulation for the design of immunotherapies including CAR T cell therapies has expanded rapidly. Co-stimulatory pathways are demonstrating potential to overcome barriers specifically associated with the TME such as impeded cell trafficking, persistence and exhaustion. The identification and thorough characterisation of novel co-stimulatory pathways and their potential role in improving CAR T cell persistence and avoiding exhaustion in the solid tumour TME is one of the most pressing areas to develop for CAR T cell research. To date, the majority of CARs have incorporated CD28 or 4-1BB domains, but T cells are known to utilize a multitude of co-stimulatory signals to develop a potent immune response. Novel platforms such as OV, nano-emulsion vaccines and combination therapies with antibody therapeutics offer bespoke strategies for delivering such broad co-stimulatory signals to allow CAR T cells to overcome these barriers. Solid tumours continue to be a major human and economic toll in our society. Understanding and refining the use of co-stimulation in CAR T cell design is critical for the future application of CAR T cell therapy enabling all of us to live longer, healthier lives.
Acknowledgements
The Editor-in-Chief, Tim Elliott, and handling editor, Tao Dong, would like to thank the following reviewers, Alexander McLellan and Ricardo Fernandes, for their contribution to the publication of this article.
Glossary
Abbreviations
- APC
Antigen presenting cell
- CAR
Chimeric antigen receptor
- CD
Cluster of differentiation
- DAMP
Danger-associated molecular pattern
- DC
Dendritic cell
- FDA
American Food and Drug Administration
- HSP
Heat shock protein
- ICR
Inverted cytokine receptor
- OV
Oncolytic virus
- PAMP
Pathogen-associated molecular pattern
- scFV
Single-chain fragment variable
- Th1
T helper type 1
- TME
Tumour microenvironment
- TLR
Toll-like receptor
- TIL
Tumour-infiltrating lymphocyte
- NK
Natural killer
- VSV
Vesicular stomatitis virus
Funding
This work was supported by grants from the National Health and Medical Research Council (NHMRC) of Australia (1103352 and 1176935) and the National Breast Cancer Foundation (NBCF) of Australia (IIRS-18–064 and IIRS-20–073).
Author contributions
Conception and design: A.J.H. and C.S. Write, review, and revision of the manuscript: A.J.H., Bv.S., X.D., C.S., and M.K.
Conflict of interest
None declared.
Ethical approval information
Not applicable.
Data availability
Not applicable.
References
- 1. Kershaw MH, Teng MW, Smyth MJet al. Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol 2005;5(12):928–40. 10.1038/nri1729 [DOI] [PubMed] [Google Scholar]
- 2. Calmes-Miller J. FDA approves second CAR T-cell therapy. Cancer Discov 2018;8(1):5–6. 10.1158/2159-8290.CD-NB2017-155 [DOI] [PubMed] [Google Scholar]
- 3. Chan JD, Harrison AJ, Darcy PKet al. Chimeric antigen receptor T cell therapies for thoracic cancers-challenges and opportunities. J Thorac Dis 2020;12(8):4510–5. 10.21037/jtd.2020.03.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tantalo DG, Oliver AJ, von Scheidt Bet al. Understanding T cell phenotype for the design of effective chimeric antigen receptor T cell therapies. J Immunother Cancer 2021;9. 10.1136/jitc-2021-002555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Azuma M. Co-signal molecules in T-cell activation: historical overview and perspective. Adv Exp Med Biol 2019;1189:3–23. 10.1007/978-981-32-9717-3_1 [DOI] [PubMed] [Google Scholar]
- 6. Kershaw MH, Westwood JA, Parker LLet al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res 2006;12(20 Pt 1):6106–15. 10.1158/1078-0432.CCR-06-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maher J, Brentjens RJ, Gunset Get al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol 2002;20(1):70–5. 10.1038/nbt0102-70 [DOI] [PubMed] [Google Scholar]
- 8. Kochenderfer JN, Wilson WH, Janik JEet al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010;116(20):4099–102. 10.1182/blood-2010-04-281931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grupp SA, Kalos M, Barrett Det al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368(16):1509–18. 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinkove R, George P, Dasyam Net al. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin Transl Immunology 2019;8(5):e1049. 10.1002/cti2.1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng Z, Wei R, Ma Qet al. In vivo expansion and antitumor activity of coinfused CD28- and 4-1BB-engineered CAR-T cells in patients with B cell leukemia. Mol Ther 2018;26(4):976–85. 10.1016/j.ymthe.2018.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duong CP, Westwood JA, Yong CSet al. Engineering T cell function using chimeric antigen receptors identified using a DNA library approach. PLoS One 2013;8(5):e63037. 10.1371/journal.pone.0063037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guedan S, Posey AD, Shaw C Jret al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight 2018;3:1. 10.1172/jci.insight.96976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hombach AA, Abken H. Of chimeric antigen receptors and antibodies: OX40 and 41BB costimulation sharpen up T cell-based immunotherapy of cancer. Immunotherapy 2013;5(7):677–81. 10.2217/imt.13.54 [DOI] [PubMed] [Google Scholar]
- 15. Song DG, Powell DJ. Pro-survival signaling via CD27 costimulation drives effective CAR T-cell therapy. Oncoimmunology 2012;1(4):547–9. 10.4161/onci.19458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song DG, Ye Q, Poussin Met al. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood 2012;119(3):696–706. 10.1182/blood-2011-03-344275 [DOI] [PubMed] [Google Scholar]
- 17. Golubovskaya VM, Berahovich R, Xu Qet al. GITR domain inside CAR co-stimulates activity of CAR-T cells against cancer. Front Biosci (Landmark Ed) 2018;23:2245–54. [DOI] [PubMed] [Google Scholar]
- 18. Ramos CA, Rouce R, Robertson CSet al. In vivo fate and activity of second- versus third-generation CD19-specific CAR-T cells in B cell Non-Hodgkin’s Lymphomas. Mol Ther 2018;26(12):2727–37. 10.1016/j.ymthe.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abate-Daga D, Lagisetty KH, Tran Eet al. A novel chimeric antigen receptor against prostate stem cell antigen mediates tumor destruction in a humanized mouse model of pancreatic cancer. Hum Gene Ther 2014;25(12):1003–12. 10.1089/hum.2013.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Collinson-Pautz MR, Chang WC, Lu Aet al. Constitutively active MyD88/CD40 costimulation enhances expansion and efficacy of chimeric antigen receptor T cells targeting hematological malignancies. Leukemia 2019;33(9):2195–207. 10.1038/s41375-019-0417-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prinzing B, Schreiner P, Bell Met al. MyD88/CD40 signaling retains CAR T cells in a less differentiated state. JCI Insight, 2020;5:21. 10.1172/jci.insight.136093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kagoya Y, Tanaka S, Guo Tet al. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med 2018;24(3):352–9. 10.1038/nm.4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Komai-Koma M, Jones L, Ogg GSet al. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci USA 2004;101(9):3029–34. 10.1073/pnas.0400171101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cottalorda A, Mercier BC, Mbitikon-Kobo FMet al. TLR2 engagement on memory CD8(+) T cells improves their cytokine-mediated proliferation and IFN-gamma secretion in the absence of Ag. Eur J Immunol 2009;39(10):2673–81. 10.1002/eji.200939627 [DOI] [PubMed] [Google Scholar]
- 25. Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol 2010;87(6):989–99. 10.1189/jlb.1209775 [DOI] [PubMed] [Google Scholar]
- 26. Rohrs JA, Siegler EL, Wang Pet al. ERK activation in CAR T cells is amplified by CD28-mediated increase in CD3ζ phosphorylation. Iscience 2020;23(4):101023. 10.1016/j.isci.2020.101023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun C, Shou P, Du Het al. THEMIS-SHP1 recruitment by 4-1BB Tunes LCK-mediated priming of chimeric antigen receptor-redirected T cells. Cancer Cell 2020;37(2):216–25.e6. 10.1016/j.ccell.2019.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lai Y, Weng J, Wei Xet al. Toll-like receptor 2 costimulation potentiates the antitumor efficacy of CAR T Cells. Leukemia 2018;32(3):801–8. 10.1038/leu.2017.249 [DOI] [PubMed] [Google Scholar]
- 29. Ankri C, Shamalov K, Horovitz-Fried Met al. Human T cells engineered to express a programmed death 1/28 costimulatory retargeting molecule display enhanced antitumor activity. J Immunol 2013;191(8):4121–9. 10.4049/jimmunol.1203085 [DOI] [PubMed] [Google Scholar]
- 30. Liu X, Ranganathan R, Jiang Set al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T Cells in advanced solid Tumors. Cancer Res 2016;76(6):1578–90. 10.1158/0008-5472.CAN-15-2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bajgain P, Tawinwung S, D’Elia Let al. CAR T cell therapy for breast cancer: harnessing the tumor milieu to drive T cell activation. J Immunother Cancer 2018;6(1):34. 10.1186/s40425-018-0347-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weimin S, Abula A, Qianghong Det al. Chimeric cytokine receptor enhancing PSMA-CAR-T cell-mediated prostate cancer regression. Cancer Biol Ther 2020;21(6):570–80. 10.1080/15384047.2020.1739952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lange S, Sand LGL, Bell Met al. A chimeric GM-CSF/IL18 receptor to sustain CAR T-cell function. Cancer Discov 2021;11:1661–71. 10.1158/2159-8290.CD-20-0896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Evans LS, Witte PR, Feldhaus ALet al. Expression of chimeric granulocyte-macrophage colony-stimulating factor/interleukin 2 receptors in human cytotoxic T lymphocyte clones results in granulocyte-macrophage colony-stimulating factor-dependent growth. Hum Gene Ther 1999;10(12):1941–51. 10.1089/10430349950017301 [DOI] [PubMed] [Google Scholar]
- 35. Elgueta R, Benson MJ, de Vries VCet al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 2009;229(1):152–72. 10.1111/j.1600-065X.2009.00782.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuhn NF, Purdon TJ, van Leeuwen DGet al. CD40 Ligand-modified chimeric antigen receptor T Cells enhance antitumor function by eliciting an endogenous antitumor response. Cancer Cell 2019;35(3):473–88.e6. 10.1016/j.ccell.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leonard WJ, Lin JX, O’Shea JJ. The gammac family of cytokines: Basic biology to therapeutic ramifications. Immunity 2019;50(4):832–50. 10.1016/j.immuni.2019.03.028 [DOI] [PubMed] [Google Scholar]
- 38. Sabatino M, Hu J, Sommariva Met al. Generation of clinical-grade CD19-specific CAR-modified CD8+ memory stem cells for the treatment of human B-cell malignancies. Blood 2016;128(4):519–28. 10.1182/blood-2015-11-683847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lacy MQ, Jacobus S, Blood EAet al. Phase II study of interleukin-12 for treatment of plateau phase multiple myeloma (E1A96): a trial of the Eastern Cooperative Oncology Group. Leuk Res 2009;33(11):1485–9. 10.1016/j.leukres.2009.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chmielewski M, Hombach AA, Abken H. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol Rev 2014;257(1):83–90. 10.1111/imr.12125 [DOI] [PubMed] [Google Scholar]
- 41. Chmielewski M, Kopecky C, Hombach AAet al. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res 2011;71(17):5697–706. 10.1158/0008-5472.CAN-11-0103 [DOI] [PubMed] [Google Scholar]
- 42. Wijewarnasuriya D, Bebernitz C, Lopez AVet al. Excessive costimulation leads to dysfunction of adoptively transferred T cells. Cancer Immunol Res 2020;8(6):732–42. 10.1158/2326-6066.CIR-19-0908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Muñoz-Wolf N, Lavelle EC. A guide to IL-1 family cytokines in adjuvanticity. Febs J 2018;285(13): 2377–401. 10.1111/febs.14467 [DOI] [PubMed] [Google Scholar]
- 44. Kim TS, Shin EC. The activation of bystander CD8+ T cells and their roles in viral infection. Exp Mol Med 2019;51(12):1–9. 10.1038/s12276-019-0316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Coughlin CM, Salhany KE, Wysocka Met al. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J Clin Invest 1998;101(6):1441–52. 10.1172/JCI1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chmielewski M, Abken H. CAR T Cells releasing IL-18 convert to T-Bethigh FoxO1low effectors that exhibit augmented activity against advanced solid Tumors. Cell Rep 2017;21(11):3205–19. 10.1016/j.celrep.2017.11.063 [DOI] [PubMed] [Google Scholar]
- 47. Li X, Daniyan AF, Lopez AVet al. Cytokine IL-36γ improves CAR T-cell functionality and induces endogenous antitumor response. Leukemia 2021;35(2):506–21. 10.1038/s41375-020-0874-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Adachi K, Kano Y, Nagai Tet al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol 2018;36(4):346–51. 10.1038/nbt.4086 [DOI] [PubMed] [Google Scholar]
- 49. Rafiq S, Yeku OO, Jackson HJet al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol 2018;36(9):847–56. 10.1038/nbt.4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359(6382):1350–5. 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vonderheide RH. CD40 agonist antibodies in cancer immunotherapy. Annu Rev Med 2020;71:47–58. 10.1146/annurev-med-062518-045435 [DOI] [PubMed] [Google Scholar]
- 52. Van Audenaerde JR, Marcq E, von Scheidt Bet al. Novel combination immunotherapy for pancreatic cancer: potent anti-tumor effects with CD40 agonist and interleukin-15 treatment. Clin Transl Immunology 2020;9(8):e1165. 10.1002/cti2.1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang Y, Wang P, Wang Tet al. Chimeric antigen receptor T cells engineered to secrete CD40 agonist antibodies enhance antitumor efficacy. J Transl Med 2021;19(1):82. 10.1186/s12967-021-02750-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mardiana S, John LB, Henderson MAet al. A multifunctional role for adjuvant Anti-4-1BB Therapy in augmenting antitumor response by Chimeric Antigen Receptor T Cells. Cancer Res 2017;77(6):1296–309. 10.1158/0008-5472.CAN-16-1831 [DOI] [PubMed] [Google Scholar]
- 55. Li F, Ravetch JV. Antitumor activities of agonistic anti-TNFR antibodies require differential FcγRIIB coengagement in vivo. Proc Natl Acad Sci USA 2013;110(48):19501–6. 10.1073/pnas.1319502110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res 2009;69(12):4941–4. 10.1158/0008-5472.CAN-09-0547 [DOI] [PubMed] [Google Scholar]
- 57. A novel antibody-4-1BBL fusion protein for targeted costimulation in cancer immunotherapy. 2008;31(8):714–22. 10.1097/CJI.0b013e31818353e9 [DOI] [PubMed] [Google Scholar]
- 58. Aigner M, Janke M, Lulei Met al. An effective tumor vaccine optimized for costimulation via bispecific and trispecific fusion proteins. Int J Oncol 2008;32(4):777–89. [PubMed] [Google Scholar]
- 59. Claus C, Ferrara C, Xu Wet al. Tumor-targeted 4-1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Sci Transl Med 2019;11:496. 10.1126/scitranslmed.aav5989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ishihara J, Ishihara A, Sasaki Ket al. Targeted antibody and cytokine cancer immunotherapies through collagen affinity. Sci Transl Med 2019;11:487. 10.1126/scitranslmed.aau3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Williford JM, Ishihara J, Ishihara Aet al. Recruitment of CD103+ dendritic cells via tumor-targeted chemokine delivery enhances efficacy of checkpoint inhibitor immunotherapy. Sci Adv 2019;5(12):eaay1357. 10.1126/sciadv.aay1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vitale LA, He LZ, Thomas LJet al. Development of CDX-527: a bispecific antibody combining PD-1 blockade and CD27 costimulation for cancer immunotherapy. Cancer Immunol Immunother 2020;69(10):2125–37. 10.1007/s00262-020-02610-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. von Scheidt B, Wang M, Oliver AJet al. Enterotoxins can support CAR T cells against solid tumors. Proc Natl Acad Sci USA 2019;116(50):25229–35. 10.1073/pnas.1904618116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kranz LM, Diken M, Haas Het al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016;534(7607):396–401. 10.1038/nature18300 [DOI] [PubMed] [Google Scholar]
- 65. Chan JD, von Scheidt B, Zeng Bet al. Enhancing chimeric antigen receptor T-cell immunotherapy against cancer using a nanoemulsion-based vaccine targeting cross-presenting dendritic cells. Clin Transl Immunology 2020;9(7):e1157. 10.1002/cti2.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Reinhard K, Rengstl B, Oehm Pet al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science 2020;367(6476):446–53. 10.1126/science.aay5967 [DOI] [PubMed] [Google Scholar]
- 67. Liu H, Moynihan KD, Zheng Yet al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 2014;507(7493):519–22. 10.1038/nature12978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ma L, Dichwalkar T, Chang JYHet al. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 2019;365(6449):162–8. 10.1126/science.aav8692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Abdullahi S, Jakel M, Behrend SJet al. A Novel Chimeric Oncolytic Virus vector for improved safety and efficacy as a platform for the treatment of Hepatocellular Carcinoma. J Virol 2018;92:23. 10.1128/JVI.01386-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zheng M, Huang J, Tong Aet al. Oncolytic viruses for cancer therapy: barriers and recent advances. Mol Ther Oncolytics 2019;15:234–47. 10.1016/j.omto.2019.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wing A, Fajardo CA, Posey AD Jret al. Improving CART-Cell therapy of solid tumors with Oncolytic Virus-driven production of a bispecific T-cell Engager. Cancer Immunol Res 2018;6(5):605–16. 10.1158/2326-6066.CIR-17-0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen T, Ding X, Liao Qet al. IL-21 arming potentiates the anti-tumor activity of an oncolytic vaccinia virus in monotherapy and combination therapy. J Immunother Cancer 2021;9(1). 10.1136/jitc-2020-001647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Andarini S, Kikuchi T, Nukiwa Met al. Adenovirus vector-mediated in vivo gene transfer of OX40 ligand to tumor cells enhances antitumor immunity of tumor-bearing hosts. Cancer Res 2004;64(9): 3281–7. 10.1158/0008-5472.can-03-3911 [DOI] [PubMed] [Google Scholar]
- 74. Feder-Mengus C, Schultz-Thater E, Oertli Det al. Nonreplicating recombinant vaccinia virus expressing CD40 ligand enhances APC capacity to stimulate specific CD4+ and CD8+ T cell responses. Hum Gene Ther 2005;16(3):348–60. 10.1089/hum.2005.16.348 [DOI] [PubMed] [Google Scholar]
- 75. Ulasov IV, Zhu ZB, Tyler MAet al. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum Gene Ther 2007;18(7):589–602. 10.1089/hum.2007.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.