Abstract
Cell therapy is an innovative approach that permits numerous possibilities in the field of cancer treatment. CAR-T cells have been successfully used in patients with hematologic relapsed/refractory. However, the need for autologous sources for T cells is still a major drawback. CAR-NK cells have emerged as a promising resource using allogeneic cells that could be established as an off-the-shelf treatment. NK cells can be obtained from various sources, such as peripheral blood (PB), bone marrow, umbilical cord blood (CB), and induced pluripotent stem cells (iPSC), as well as cell lines. Genetic engineering of NK cells to express different CAR constructs for hematological cancers and solid tumors has shown promising preclinical results and they are currently being explored in multiple clinical trials. Several strategies have been employed to improve CAR-NK-cell expansion and cytotoxicity efficiency. In this article, we review the latest achievements and progress made in the field of CAR-NK-cell therapy.
Keywords: NK cells, chimeric antigen receptor, cell therapy, genetic engineering, cancer
Introduction
Cell therapy using T-cells engineered to express a chimeric antigen receptor (CAR-T cells) has resulted in outstanding improvements in the treatment of patients with hematological cancer [1–3]. CAR structure comprises an extracellular domain, hinge, transmembrane region, and intracellular signaling domains. The use of a single-chain variable fragment (scFv) as the recognition domain directs T-cells to specific tumor antigens, without the need for HLA presentation allowing a broader application [4]. Despite its potential, the wide use of CAR-T-cell therapy presents many challenges in cancer treatment. It can lead to severe side effects, such as cytokine release syndrome (CRS) and neurotoxicity [5, 6]. Moreover, its extensive application is limited by inherent risks such as graft versus host disease (GvHD) and the quality of patient T cells that are used to produce CAR-T cells [7]. A promising alternative is allogeneic cell therapy using NK cells expressing CAR (CAR-NK cells).
NK cells are innate immunity cytotoxic lymphocytes that can eliminate virus-infected and tumor cells [8]. They also secrete cytokines which can activate other immune cells [9]. One of the advantages of using these cells in CAR-based therapies is that they preserve their normal function against cancer cells, which might prevent tumor evasion by downregulation of CAR target [10]. Also, NK cells present a short half-life in circulation, increasing their safety [11]. To exert their function, they are independent of HLA presentation which decreases the risks of GvHD [12]. This allows for the use of NK cells from allogeneic sources and might enable the establishment of CAR-NK cells as an off-the-shelf therapy. Despite the possible benefits with CAR-NK cells, there are still many challenges to attain a robust clinical application.
Some of the issues for CAR-NK cells development are the inefficient in vitro expansion, low efficiency of genetic modification, and that most studies use CAR constructs designed for CAR-T cells.
In addition, due to the NK cells’ relatively short half-life, it may be necessary to administer more than one dose of CAR-NK cells in patients, but long-term follow-up studies with patients treated with CAR-NK still need to be conducted. Lastly, it is still a challenge to overcome the solid tumor microenvironment (TME) and the development of CAR-NK cells that can migrate properly to the tumor and to escape from the immunosuppressive effect of TME is extremely necessary [13]. In this review, we explore improvements in CAR-NK-cell therapy, including genetic engineering strategies to develop NK-cell-specific CAR.
NK sources and expansion
The success of CAR-NK-cell therapy depends on multiple factors, such as the choice of the NK source, the expansion method, the vector used to deliver and express the CAR, and the design of the CAR molecule. Table 1 lists the advantages and disadvantages of the main factors that can affect CAR-NK-cells production that will be discussed in this review.
Table 1.
Advantages and disadvantages of new technologies for CAR-NK production
| Advantages | Disadvantages | |
|---|---|---|
| NK cell type | ||
| NK-92 | • Easy to expand and to engineer; • Homogenous product; • Low in vivo persistence. |
• Safety risk; • Requires irradiation; • No CD16 expression. |
| Peripheral Blood | • Easy to obtain; • Does not require irradiation; • Highly cytotoxic. |
• Difficult expansion; • Low transduction; efficiency; • Sensitive to freeze/thaw cycles; • Non homogenous. |
| Cord blood | • Ease of collection; • Fewer T cells; • Presence of unique NK progenitors; • High proliferation capacity. |
• Difficult expansion; • Non homogenous product; • Immature cells. |
| iPSC | • Yields more cells; • Easy to engineer; • Homogenous product. |
• Longer production period; • Immature phenotype; • Low in vivo persistence; • Potentially immunogenic; • Potentially tumorigenic. |
| NK expansion methods | ||
| Cytokines combination | • Promotes differentiation of memory-like natural killer cells. | • Requires high initial number of cells; • High cost; • Increases the chance of Treg activation. |
| Synthetic beads/antibodies | • Easy handling; • Easy to scale up. |
• Low to moderate expansion; • Expensive. |
| Feeder cells | • Efficient activation and high expansion. | • Complex co-culture system. |
| Membrane particles | • High expansion rates. | • Laborious process of fabrication and characterization; • Risk of residual stimulatory cell material in the final product |
| Gene delivery | ||
| Retroviruses | • Permanent modification of cells. | • Requires actively dividing cells; • Random integration profile (Risk of insertional mutagenesis); • Potential of replication competent retrovirus (RCR); • High manufacturing cost of GMP-grade vectors. |
| Lentiviruses | • Transduction of non-dividing cells; • Permanent modification of cells. |
• Random integration profile (risk of insertional mutagenesis); • Potential of replication competent retrovirus (RCL); • High manufacturing cost of GMP-grade vectors. |
| Transposons | • Cost-effective; • Easier to produce on a large scale; • Large insert capacity; • Stable transgene expression. |
• High cell death rates; • Low integration rate; • Risk of insertional mutagenesis. |
| CRISPR/Cas 9 technology | • Site-specific integration of gene of interest; • Permanent expression of CAR |
• Possible off-target effects; • Low delivery efficiency; • Licensing restrictions |
| mRNA | • Low risk of insertional mutagenesis; • High efficiency of genetic material delivery. |
• Inherently labile; • Short period of expression. |
| Episomes | • Stable expression; • Cost-effective; • Low risk of insertional mutagenesis; • Safety profile compared to viral methods. |
• A good delivery method is still needed. |
Currently, NK cells used in cancer immunotherapy can be manufactured from diverse sources, such as cell lines, peripheral blood (PB), bone marrow, umbilical cord blood (CB), and induced pluripotent stem cells (iPSC) [14–18].
NK cell lines have been used in cancer immunotherapy because of their increased expansion ability in vitro and their relatively simple cultivation conditions. To date, there are seven human NK cell lines: HANK-1, KHYG-1, NK-92, NK-YS, NKL, SNK-6, and YT. Immunophenotypic characterization has revealed the immaturity of the cell lines tested, defined as CD16−CD56+. Only KHYG-1 and NK-92 show significant activity against the MHC-I negative target cell line K562. This discrepancy may be because other NK cell lines have not yet reached the maturity stage where they acquire typical NK cytotoxicity [19]. The only FDA-approved cell line for clinical trials is the NK-92, which is highly cytotoxic [20].
Most studies with CAR-NK cells have used enriched cells from PB from allogeneic donors as their source [21]. In PB, NK cells represent a low percentage of circulating lymphocytes. NK cells are defined as two functionally distinct subsets: CD56bright CD16− and CD56dimCD16+ cells. In PB, the ratio between CD56bright and CD56dim is about 1:9 respectively. The major differences between these two subsets are that CD56dim NK cells show significantly higher cytotoxic activity and contain much more perforin and granzyme while CD56bright are more efficient producers of pro-inflammatory cytokines [22–24]. Also, the pattern of surface receptor expression differs between the CD56bright and CD56dim populations. CD56dim express CD16 and inhibitory KIR while CD56bright are negative for CD16 and KIR but positive for NKG2A and the IL-2 receptor α chain (IL-2Rα/CD25) [25,26].
An alternative source of NK cells to PB is CB. Clinical numbers of CB-derived NK cells (2 × 109) with high purity (92% CD56+) were reached using a bioreactor in a GMP-compliant system [27]. Some benefits of using CB-NK-cells are the relative ease of collection and the reduced risk of GvHD since CB contains reduced T-cell counts [28,29].
Different cytokines have been used to improve NK-cell growth in cell culture, such as IL-2, IL-12, IL-15, IL-18, and IL-27, which are related to cell proliferation, as well as cytotoxic potential [30,31].
NK-cells isolated from PB have been expanded using IL-2 and IL-15, with and without the addition of IL-21, which generated different NK subpopulations. CD56dim population was higher in the presence of IL-21, while NKG2D and NKp44 cell surface markers were downregulated [32]. The combination of IL-12, IL-15, and IL-18 to expand PB-NK cells results in memory-like NK cells with high cytotoxicity and increased production of interferon-γ [33]. Anti-CD19 CAR-NK expanded with these cytokines showed enhanced anti-tumor activity in a target-specific manner [34]. Liu et al. [21]have recently investigated 16 different combinations of the cytokines IL-2, IL-12, IL-15, IL-18, and IL-21 for PB-NK-cell expansion, and the combination of IL-2, IL-15, and IL-18 resulted in improved expansion and cytotoxicity [31]. Another study evaluating PB-NK-cell expansion demonstrated that the cytokines IL-15, IL-18, and IL-27 were the optimal combination leading to enhanced cytotoxicity [30].
CB cells are an excellent source to generate off-the-shelf products, due to their less restrictive requirements for HLA matching and lower risks to cause GvHD [35]. Cord blood is an even better source of NK cells than PB, with 18.2% of lymphocytes being NK cells, while we found 12.7% of NKs in PB [36]. NK cells from CB can be successfully transduced with CAR specific for CD19 and are potentially more cytotoxic to tumor cells from patients with Chronic Lymphocytic Leukemia (CLL) than non-transduced NK cells [37].
Spanholtz et al. [28] described a cytokine-based culture system for ex vivo expansion of NK-cells from hematopoietic stem cells from CB. CD56+CD3− cells were generated from CD34+ CB-cells. The generated NK-cells expressed NKG2A and KIR receptors, and high levels of NKG2D activating receptors. Functional analysis showed NK-cells are cytotoxic against myeloid leukemia cell lines, melanoma cell lines, and primary Acute Myeloid Leukemia (AML) cells [38].
However, although many cytokines have been implemented to improve NK expansion, cell growth is still very modest [39]. Another approach that has been used for NK-cell expansion is adding feeder cells to its culture, such as K562-mb15-41BBL cells [40]. The K562-mb15-41BBL feeder cell was made by transducing K562-cells with constructs encoding the ‘membrane-bound’ form of IL-15 (mbIL15) and human 4-1BB ligand (4-1BBL) [40,41], which improves NK-cell growth and cytotoxic capacity. NK-cells co-cultivated with K562-mb15-41BBL promoted a mean NK expansion of 277-fold in 21 days [42]. Alternatively, NK cells were co-cultured with K562-based artificial APC expressing membrane-bound IL-21 (mbIL21) or mbIL15, showing a higher fold expansion with mbIL21 after 3 weeks. As well as this, NK-cell expansion with mbIL21 resulted in an increase in telomere length compared to mbIL15, indicating it can diminish NK-cell senescence [43]. K562 engineered to express CD48, 4-1BBL, and mbIL21 has been also used as a potent feeder cell, allowing clinical scale production of NK cells [44]. CD48 is the counter receptor for 2B4, which participates in a variety of cell-to-cell interactions and is an important activator of NK cells [45], while 4-1BBL, the counter-receptor for CD137, mediates NK-cell proliferation and differentiation [46,47].
Other cell types have been tested as feeders for primary NK-cell expansion, and include OCI-AML-3 cells overexpressing mbIL21 (known as NKF cells). NKF-cells showed higher fold expansion at a 5:1 (NKF to NK) ratio and the NK expanded with NKF showed potent cytotoxicity comparable to NK-cells co-cultured with mbIL21-K562 [48].
NK-92 engineered to express OX40L and to secrete neoleukin-2/15 (Neo-2/15) has also been used for expansion of PB-NK cells leading to a 2180-fold expansion in 21 days [49]. Neo-2/15 is a newly engineered protein that mimics the function of both IL-2 and IL-15 [50]. Lastly, NK-cells from CB co-cultivated with irradiated Epstein-Barr virus-transformed lymphoblastoid cell line (EBV-LCL) showed high expansion levels and demonstrated to also be a feasible approach [51].
Although feeder cell-based NK-cell expansion systems can be used to obtain good products [52–54], the use of a feeder cell line may result in unpredictable risks [55]. An alternative method to feeder cells is the use of plasma membrane-derived particles from K562-mbIL15-41BBL, which induced a 250-fold expansion of highly cytotoxic NK-cells after 17 days [56]. An alternative method to promote NK-cell expansion without feeder cells is monoclonal antibodies (mAb), such as the combination of anti-CD52 and anti-CD3, which demonstrated a favorable growth of NK-cells while suppressing CD4+T-cells from peripheral blood mononuclear cell (PBMC) [57]. Moreover, activation using microbeads covered with antibodies against various NK receptors, such as NKp46, 2B4, DNAM-1, CD2, and CD18, is another strategy that can improve NK-cell expansion and activation [58].
Delivery methods for engineering NK-cells
Viral vectors
Lentiviruses and retroviruses are the most used systems to induce stable expression of CAR in NK cells. However, the genetic manipulation of NK cells has historically been limited by the induction of apoptosis in NK cells after genetic manipulation and the low efficiency of transgene delivery compared to T cells [59].
In order to facilitate viral transduction, some approaches have been exploited, including (i) changing the electrical charges of cells, (ii) increasing interaction between virus and target cell via integrin binding, (iii) up-regulation of low-density lipoprotein receptors (LDLR), (iv) changing the viral envelope, and (v) inhibition of innate immune signaling.
Both viral envelope glycoproteins and target cell receptors may contain negative charges that can be detrimental to transduction [60]. Polycationic reagents such as lipids, polymers, and peptides can induce the aggregation of viral particles and facilitate binding to cells through modulation of electrostatic interactions, an example is a polybrene [61]. Retronectin is a chimeric peptide that binds to integrins VLA-4 and VLA-5 and also to the virus. Its use results in an increased transduction efficiency of NK cells with different retroviral platforms. Transduction using alpha-retroviral particles in combination with retronectin allowed stable transduction with an increase of 90% in NKL cell line and up to 60% in primary NK cells [62].
The most used viral envelope is the one pseudotyped with the Vesicular Stomatitis Virus type-G (VSV-G) envelope glycoprotein. The main receptors for this envelope protein are LDLR and phosphatidylserine [63]. However, NK-cell lines and primary NK-cells express low levels of LDLR, and up-regulation of LDLR expression in NK cells by lipophilic drugs, like rosuvastatin, leads to increased transduction rate [64].
In addition, replacing VSV-G with Baboon envelope pseudotyped lentiviral vectors (BaEV-LV) increased the transduction rate of freshly isolated human NK-cells [65].
NK cells are responders to viral infections [66] and this can be reflected in the reduced viral transduction efficiency observed in NK cells. During viral transduction, intracellular antiviral defense mechanisms including one or more of the receptors RIG-I, MDA-5, and TLR3 transmit signals to TBK1 via multiple proteins, leading to the production of IFN-γ that can contribute significantly to the resistance of NK cells to lentiviral genetic modification. Receptors involved in antiviral responses are highly expressed in NK cells and the use of the TBK1/IKKɛ complex inhibitor BX795 improves the transduction rates in NK cells [67,68].
Non-viral vectors
Although viral transduction remains the most employed gene delivery method in NK cells, several limitations still exist and the risk of vector integration into the genomic DNA is the most concerning. Non-viral vectors can be classified into integrative and non-integrative vectors.
Transposons, mRNA, and episomal vectors are examples of non-viral integrative systems. The transposon system, such as Sleeping Beauty (SB), piggyBac, or Tol2, are effective non-viral vectors for delivering genetic material. The transposase enzyme recognizes transposon-specific inverted terminal repeat sequences (ITR) located at both ends of a transposon vector and then simply cuts the sequence and binds it somewhere in the target DNA. This creates stable/permanent genomic integration into the host cell [69].
An SB transposon vector was successfully used to express anti-mesothelin CAR in NK cells derived from iPSC. The resulting CAR-NK-iPSC cells successfully mediate strong anti-tumor activity, repressed tumor growth, and prolonged survival [70]. Wang et al. [71] engineered CAR-NK-cells expressing a chimeric receptor with NKG2D ectodomain and DAP10-CD3ζ signaling domain by transfecting cells with a piggyBac vector complexed with PBAE polymer. With this system, cells maintained high viability after transfection. Combining these CAR-NK-cells with the blockade of CD73 displayed synergic efficacy against CD73+ lung cancer [71]. Transposons are easier to produce on a large scale and they harbor greater transgenic capacity. Their integration occurs generally in AT-rich genomic regions [72] and they are considered biologically safe. However, this year two patients developed malignant lymphoma after treatment with CAR-T-cells genetically modified with a piggyBac vector [73].
New innovative methodologies have been developed to overcome the risk of mutagenesis through the insertion of integrative vectors. Other non-integrative gene delivery methodologies, such as mRNA and episomal vectors, have emerged as a new delivery form that can be used to deliver CAR to NK cells.
The electroporation of mRNA-encoding CARs into NK cells has been successfully demonstrated with an efficiency of up to 81% [74]. This method has also been used to engineer CAR-NK-cells targeting the CD20 antigen in B-cell non-Hodgkin’s lymphoma [75] and Burkitt’s lymphoma [76]. In addition, NK-cells transfected with anti-ROR1 CAR mRNA have been used for metastatic solid tumors treatment [77, 78]. This innovative approach is also being tested in clinical trials with CARs targeting CD19 and NKG2DL (NCT00995137 and NCT03415100) (Table 2).
Table 2.
Clinical trials using CAR-NK cells for gene therapy
| Reference | NK source | Gene transfer | Target molecule | Target tumor | CAR construct | Status | Sponsor | Study Phase |
|---|---|---|---|---|---|---|---|---|
| NCT04796675 | CB | RV | CD19 | CD19+ B-cell malignancies | Unknown | Recruiting | Wuhan Union Hospital, China | I |
| NCT03056339 [125] | CB | RV | CD19 | B Lymphoid Malignancies | CD19-CD28-zeta-2A-iCasp9-IL15 | Recruiting | M.D. Anderson Cancer Center | I/II |
| NCT03656705 | NK92 | RV/LV | Unknown | Non-small Cell Lung Cancer | Unknown | Enrolling by invitation | Xinxiang medical university | I |
| NCT02944162 [156] | NK92 | LV | CD33 | AML | ScFv-CD28-CD137-CD3z | Unknown | PersonGen BioTherapeutics (Suzhou) Co., Ltd. | I/II |
| NCT02839954 | NK92 | LV | MUCI | Solid tumor | ScFv-CD28-CD137-CD3z | Unknown | PersonGen BioTherapeutics (Suzhou) Co., Ltd. | I/II |
| NCT02892695 | NK92 | LV | CD19 | Lymphoma, leukemia | ScFv-CD28-CD137-CD3z | Unknown | PersonGen BioTherapeutics (Suzhou) Co., Ltd. | I/II |
| NCT03383978 [157] | NK92 | LV | HER2 | GBM | ScFv-CD28-CD3z | Recruiting | Johann Wolfgang Goethe University Hospital | I |
| NCT03940833 | NK92 | LV | BCMA | Multiple myeloma | Unknown | Recruiting | Asclepius Technology Company Group (Suzhou) Co., Ltd. | I/II |
| NCT03941457 | NK92 | LV | ROBO1 | Pancreatic Cancer | Unknown | Recruiting | Asclepius Technology Company Group (Suzhou) Co., Ltd. | I/II |
| NCT03940820 | NK92 | LV | ROBO1 | Solid Tumor | Unknown | Recruiting | Asclepius Technology Company Group (Suzhou) Co., Ltd. | I/II |
| NCT04245722 [147] | iPSC | LV | CD19 | B-cell lymphoma, CLL | scFv-NKG2D-2B4-CD3z-IL-15/RhnCD16 | Recruiting | Fate Therapeutics | I |
| NCT00995137 | PB-NK | mRNA electroporation | CD19 | B-ALL | ScFv-CD8aTM-CD137-CD3z | completed | St. Jude Children’s Research Hospital | I |
| NCT02742727 | NK92 | Electroporation | CD7 | Lymphoma, leukaemia | ScFv-CD28-CD137-CD3z | Unknown | PersonGen BioTherapeutics (Suzhou) Co., Ltd. | I/II |
| NCT03415100 [115] | PB-NK | mRNA electroporation | NKG2DL | Metastatic solid tumor | ScFv-CD8aTM-CD3z; ScFvCD8aTM-DAP12 | Unknown | The Third Affiliated Hospital of Guangzhou Medical University | I |
Episomal vectors are emerging as a safer alternative to integrating vectors [79]. Plasmid vectors containing S/MAR (matrix support and fixation region) enable plasmid retention and replication within the host cell nucleus [80]. Recently, this type of vector was used to genetically modify T-cells with an anti-CD19 CAR resulting in CAR-T-cells with long-term transgene expression and in vivo cytotoxicity [81].
CRISPR-Cas9 is a technology that can also be used in non-viral vectors and unlike transposons, the insertion of genetic material takes place in a specific location. In NK cells, knock-in with CRISPR-Cas9 has been implemented by combining with adeno-associated virus (AAV) delivery of template DNA for homologous repair [82]. Kararoudi et al. used AAV delivery of template DNA with CRISPR-Cas9 to generate anti-CD33 CAR-NK cells, which demonstrated maintenance of CAR expression after 2 weeks in culture and efficient targeting of AML cells [83]. For non-viral DNA template delivery, the combination of truncated Cas9 target sequences (tCTS) added to the end of homology-directed repair (HDR) template with an anionic polymer can increase knock-in efficiency in NK cells from 3.09% to 16.6% [84], although this strategy has not been used for CAR delivery yet.
Methods to delivery non-viral vectors
There are different methods to physically deliver naked genetic material, those methods can be combined with gene carriers or enhancement buffers, such as biphasic polymers, liposomes, or other vehicles [85–87]. These strategies create a temporary pore in the cell membrane or provide carriers that can fuse with the cell membrane allowing incorporation.
Electroporation-based methods are well known for transfecting mammalian cells with foreign genetic material. However, the possibility of permanent cell damage due to the electroporation process is still a challenge [88]. To minimize the damage caused by the electroporation, other methods to permeabilize the cell membrane have been developed and employed to produce CAR-NK cells. Cell squeezing is an intracellular delivery method based on rapid mechanical deformation of the cell’s membrane as the cell flows at high speed through a narrow microchannel in a silicon chip, the transient pores created during the ‘squeezing’ allow for molecules in the cell’s surroundings to diffuse into its cytosol [89]. The technique has been shown to deliver a large panel of small or uncharged molecules such as dextrans, proteins, RNPs, nanoparticles, and siRNA [90–92], and does not disrupt cell function [90]. Squeezing cells followed by an electrical field enhanced the delivery of nucleic acids. This technique could allow cell transduction with mRNAs for safer CAR delivery in the context of NK cells. Chang et al. have developed a technique of nanochannel-electroporation and when used to deliver a CAR/GFP-reporter plasmid in NK cells, it led to around 80% of GFP expression with the maintenance of cell viability [93].
Each of the delivery methods discussed has a set of advantages and disadvantages compared with each other (Table 1). But the choice of an efficient delivery method also depends on other factors such as NK-cell source and CAR construct [94]. Table 2 lists CAR-NK-cells used in the clinical trials registered on Clinicaltrials.gov that provide information on the delivery method. Among the studies whose type of delivery system is known, three studies were performed with retroviral vectors, eight studies with lentiviral vectors, and three studies by electroporation.
Specific CARs molecules for NK cells
Initially, CAR-NK-cells were constructed with only CD3ζ (first-generation CAR) as a signaling domain, and these were shown to be efficient in eliminating target cells [95, 96]. As for CAR-T cells, CAR-NK with one or two additional co-stimulatory domains (second- and third-generation), such as CD28 and/or 4-1BB along with CD3ζ, have also been successfully applied in NK cells [40, 97, 98]. Recently, fourth- and fifth-generations CAR have been described, both are based on second-generation CAR. Fourth-generation secretes cytokines, while the fifth contains an intracellular domain of a cytokine receptor [99,100].
Optimization with more specific signaling domains for NK cells has been pursued to increase its cytotoxicity. NK cell activation results from simultaneous stimulation of NK cell-activating receptors, such as natural cytotoxicity receptors (NCR), NKG2D, 2B4 (CD244), and DNAM-1 (CD226). NCR NKp30 and NKp46 associate with CD3ζ and FcRγ, while NKp44 interacts with DNAX-activating protein 12 (DAP12). These adaptors mediate NK-cell activation via their immunoreceptor tyrosine-based activation motifs (ITAM) [101–103]. CD3ζ signaling domain is also co-associated with CD16 in NK cells [104]. NKG2D interacts with DAP10, which presents a phosphatidylinositol-3 kinase (PI3K) binding motif [105]. 2B4 and DNAM-1 are not associated with adaptors containing ITAM and act as co-receptors amplifying signals induced by NCR and NKG2D [106, 107].
First-generation constructs with DAP12 or FcεR1γ (high-affinity immunoglobulin epsilon receptor subunit gamma) as the CAR signaling domain instead of CD3ζ, demonstrated that these specific molecules were efficient in activating CAR-NK cells [108, 109]. Most studies evaluate constructions with additional signaling domains to achieve better signaling potency. The incorporation of 2B4 in CAR-NK cells against CD19 or G2D resulted in increased cytotoxicity compared to a CAR containing only CD3ζ. It was also observed that CAR-NK-cells containing only 2B4 failed to induce activation [110]. A study comparing 4-1BB-CD3ζ and 2B4-CD3ζ CAR-NK cells against CD5 demonstrated that even though both constructs were functional, 2B4-CD3ζ CAR-NK cells had enhanced cytotoxicity [111]. A third-generation CAR constructs with the NK-cell-activating molecules 2B4 and DNAM-1 displayed greater cytotoxicity against hepatocellular cancer cells expressing GPC3 compared to CAR with only CD3ζ or CD28-CD3ζ [112].
The most thorough investigation of CAR constructs for NK cells, assessed combinations of CD16, NKp44, NKp46, and NKG2D transmembrane regions, as well as costimulatory domains 2B4, DAP10, DAP12, and 4-1BB in several combinations with CD3ζ. Among the second and third-generation constructs evaluated, the one with NKG2D transmembrane domain and 2B4 as co-stimulatory signaling demonstrated higher cytotoxicity against target cells [70]. Conversely, a study comparing second-generation CAR with 4-1BB or 2B4 or a third-generation with both, demonstrated equal cytotoxicity among constructs in targeting GD2+ Ewing sarcoma cells [113].
In addition to intracellular domain constructions, a chimeric receptor using NKG2D instead of a specific scFv has also been investigated. A NKG2D-DAP10-CD3ζ construct was able to increase NK-cells cytotoxicity against tumor cells without increasing activity against normal cells [114]. Chimeric receptors in NK cells using NKG2D ectodomain combined with DAP12 or CD3ζ signaling domains were both functional, but NK cells with the DAP12 construct had the highest cytolytic activity and were shown to be effective in patients with colorectal cancer [115]. Whereas a study comparing chimeric receptors for NK cells with NKG2D ectodomain combined with CD3ζ, CD28-CD3ζ or 4-1BB-CD28-CD3ζ showed higher cytotoxicity against ovarian cancer with the construct containing only CD3ζ [116]. Together these comparative studies indicate that CAR constructs including NK-specific molecules present a promising path in the development of CAR-NK-cell therapy (Fig. 1).
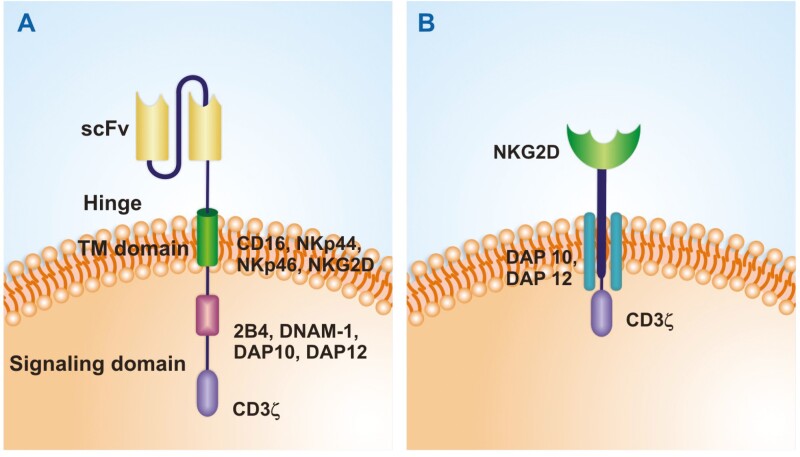
Figure 1.
Specific CAR molecules for NK cells. (A) CAR with NK-specific signaling molecules. A second-generation CAR is represented, constructs specific for NK cells may contain 2B4, DNAM-1, DAP10, and/or DAP12 as co-stimulatory signaling domains with CD3ζ. The TM domain can also contain specific NK molecules such as CD16, NKp44, NKp46, or NKG2D. (B) NKG2D ectodomain as the recognition domain. A chimeric receptor containing NKG2D with DAP10/DAP12 and CD3ζ is represented. Constructions may contain the entire NKG2D protein as shown or only its ectodomain with a CD8 hinge and transmembrane region.
Genetic engineering strategies beyond CAR target
NK-cells engineered to express a non-cleavable CD16 Fc receptor
In the presence of IgG antibodies, NK-cells recognize target cells through the interaction between CD16A receptor and IgG, resulting in antibody-dependent cell-mediated cytotoxicity (ADCC) [117, 118].
The metalloproteinase ADAM17 can cleave CD16A after NK cell activation by multiple stimuli [119]. This regulation can affect the ADCC efficiency response decreasing IFN-γ production by NK cells [120, 121].
An approach to block CD16 shedding involves the modification of the ADAM17 cleavage site in CD16A (between Val196 and Ser197), substituting Ser197 with Pro197 creating a cleavage resistance, referred to as non-cleavable CD16A (ncCD16A) (Fig. 2a) [122]. NK-cells expressing ncCD16A presented higher levels of ADCC and cytokine production in co-culture with various therapeutic mAb and tumor types, including ovarian cancer, Burkitt’s lymphoma, and lung adenocarcinoma [123]. Currently, a Phase I clinical trial is employing iPSC-NK cells expressing ncCD16A (FT516) in the treatment of acute myeloid leukemia (NCT04023071), as well as iPSC-NK cells expressing ncCD16A and anti-CD19CAR (FT596), as a combined targeting approach for the treatment of B-cell lymphoma (BCL) and CLL (NCT04245722) (Table 2) [123–125].
Figure 2.
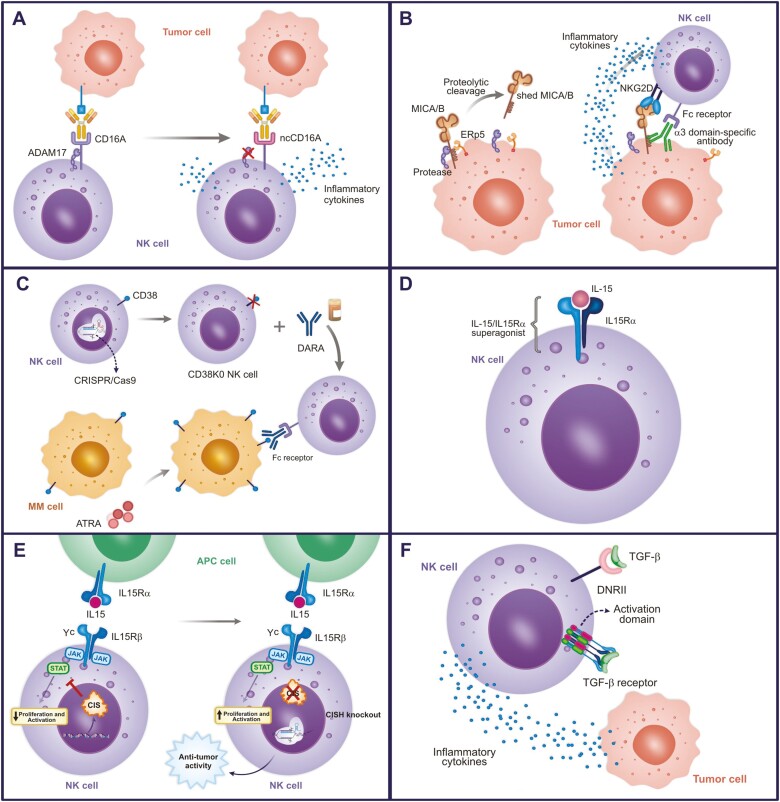
Genetic engineering strategies beyond CAR target. (A) NK-cells modified to express a non-cleavable CD16 Fc receptor through ADAM17 blocking. (B) Stabilization of MICA/B on the tumor cell surface using antibody against MICA α3 domain. Anti-MICA α3 domain further enhances NK function by NK cell Fc receptor recognition. (C) NK-cells CD38 KO associated with DARA for cancer treatment. CD38 is expressed in neoplastic B cells as well as in NK cells, monocytes, regulatory T cells, regulatory B cells, and myeloid-derived suppressor cells. Thus, knocking out CD38 in NK cells prevents NK cell depletion after treatment with DARA. (D) NK cells engineered to express IL-15 receptor fusion (IL-15/IL-15Rα) on cell surface. IL-15/IL-15-Rα complex is extremely important for generation, activation, and proliferation of NK cells. (E) NK CISH KO. The knock out of the CISH gene is another way of exploring the role of IL-15, it enhances sensitivity of NK cells to IL-15. (F) NK-cells expressing modified TGF-β receptor. TGF-β suppresses the function of NK cells and the use of the TGF-β receptor extracellular domain coupled to the intracellular domain of NK-cell-activating receptors has been associated with activation of these cells. In addition, TGF-β-DNRII expression in NK-cells resulted in the inhibition of TGF-β signaling.
NK-cells engineered to stabilize MICA/B on the tumor cell surface
NK-cells can recognize and eliminate tumor cells expressing MICA/B by the NKG2D receptor. MICA and MICB are stress-inducible ligands of NKG2D expressed on the cell surface. Their expression is strongly induced by cellular stress conditions due to DNA damage, viral infections, and neoplastic transformation, being absent in normal tissues [126, 127]. To escape NKG2D-mediated immune surveillance, tumors can prevent the receptor recognition by proteolytic cleavage of MICA/B from the cell surface, generating soluble MICA/B in a process called MICA/B shedding. In addition, soluble MICA/B can bind to NKG2D, which in turn induces NKG2D internalization and degradation [128].
Avoiding MICA/B shedding has been investigated as a potential target for cancer immunotherapy. Ferrari de Andrade et al. inhibited MICA/B shedding by blocking the initiation of release through antibodies binding to key epitopes on the MICA and MICB α3 domain (a membrane-proximal domain). Such binding prevents the shed action of the proteases but does not interfere with the interaction of NKG2D with the α1 and α2 domains of MICA (Fig. 2b) [129]. The study reported that after treatment with MICA α3 domain-specific antibodies there was an increase in the binding of NKG2D to target ligands, thus inducing greater tumor immunity mediated by NK cells. This strategy could be applied in combination with CAR-NK-cells to additionally support their activity.
NK-cells CD38KO
Recently, the high expression of CD38 on malignant cells has prompted the development of targeted immunotherapies, especially in multiple myeloma (MM) [130]. The FDA has approved a new immunotherapy for MM, a mAb targeting CD38, called daratumumab (DARA) [131]. Despite the well-established clinical benefits of DARA, some patients experience disease relapse, and a possible explanation is the rapid depletion of NK cells after treatment with DARA since NK cells also express relatively high levels of CD38 [132,133]. The reduction of circulating CD38+ NK cells results in an inefficient ADCC against MM cells [134].
Aiming to overcome this problem, Kararoudi et al. proposed to delete CD38 in NK cells, by using a DNA-free method with Cas9 ribonucleoprotein complexes (Cas9/RNP) and associated this CD38 knockout (KO) NK cells with DARA in the treatment (Figure 2c) [135]. The authors reported that these cells were resistant to DARA-induced conjugation and fratricide, and persisted in the presence of DARA in vivo, in addition to showing superior ADCC activity against MM cell lines and primary samples when compared with the paired CD38 wild-type cells.
NK-cells engineered to express IL-15 receptor fusion
Recently, strategies for cancer treatment using IL-15 alone or associated with other therapies have been reported [136, 137]. It was demonstrated that soluble IL-15 treatment can induce NK and CD8 T-cell proliferation in patients, but toxicities were reported [136,138]. Studies demonstrated that the binding of IL-15 to the IL-15 alpha subunit (IL-15Rα) is important to its function and prolongs its half-life in circulation [139, 140]. In addition, animal models and Phase 1 clinical trials have shown that IL-15/IL-15Rα complex is less toxic than IL-15 and was not associated with severe adverse events [137, 141, 142].
IL-15 secreting CAR19-NK-cells were successfully developed by Rezvani’s group. In this study, 73% of patients with CD19+ tumors responded to CAR19-NK cells treatment and serious adverse effects were not reported [125]. Recently, Ma et al. demonstrated that the combination of oncolytic virus expressing IL-15/IL-15Rα sushi domain fusion protein with EGFR-CAR NK-cells improved efficiency and prolonged survival compared to EGFR-CAR NK cells alone in a glioblastoma model. Interestingly, soluble IL-15/IL15-Rα also improves CAR-NK persistence without inducing exhaustion [143].
According to these results, an interesting strategy for cancer treatment would be the development of NK-cells expressing membrane-bound IL-15/IL-15Rα superagonists (Fig. 2d). Indeed, CAR-NK for different targets (such as CD19, MICA/B, B7H3, and BCMA) in combination with IL-15/IL-15Rα have been tested in pre-clinical studies [137,144].
Valamehr’s group has successfully worked on multi-engineered CAR-NK-cells for MM treatment. These iPSC-NK cells are specific for BCMA and besides a membrane-bound IL-15/IL-15Rα complex, they are KO for CD38 and express ncCD16A [145,146]. An anti-CD19 CAR engineered with IL-15/IL-15Rα and ncCD16A has also been developed with iPSC-NK cells, a clinical trial for dose determination of this construct to be used alone or in combination with anti-CD20 mAb is currently recruiting patients (NCT04245722) (Table 2) [147].
NK CISH KO
The edition of the CISH gene is another way of exploring the role of IL-15 in NK activation and mitigating the toxicities associated with intravenous IL-15 application (Fig. 2e). CIS is an inhibitory intracellular protein that blocks the binding of STATs to cytokine receptors [148, 149]. Therefore, due to its role as an immune checkpoint inhibitor, recent studies have explored the potential of knocking out the CISH gene to enhance NK cell sensitivity to IL-15.
Bernard and collaborators developed a conditional mouse model for CISH gene depletion in NK cells to better understand its role in NK regulation. CISH depletion did not affect the maturation or immunophenotypic profile of NK cells, but NK-cells over-express genes associated with cell-cycling and activation, resulting in increased production of IFN-γ and CD107a expression. Interestingly, CISH KO NK cells were able to proliferate at a low concentration of IL-15 in vitro, demonstrating more sensitivity to IL-15. The conditional depletion of CISH in NK cells improves in vivo response against breast cancer cells and decreases TIGIT expression, a receptor that is associated with NK cell exhaustion [149]. Similar results were achieved in human CISH KO iPSC-NK cells by Kaufman’s group. CISH KO iPSC-NK cells expanded at low IL-15 levels in vitro and presented better antitumor response and long persistence in vivo [150].
Recently, Daher and collaborators demonstrated that CISH KO CAR19-NK-cells secreting IL-15 had enhanced antitumor response compared to IL-15-secreting CAR19-NK in vitro and in vivo. Depletion of the CISH gene resulted in increased expression of activator receptors and proteins related to cytotoxicities, such as granzyme B, perforin, TRAIL, CD3z, DAP12, DNAM-1, CD25, and Ki67. The RNA-sequencing analysis also demonstrated the upregulation of genes of tumor necrosis factor TNF and IFN signaling and genes of cytokine signaling after CISH KO. It is important to note that treatment with CISH KO CAR19-NK did not result in toxicities or abnormal NK expansion and that it was dependent on the IL-15 gene in the CAR construct, suggesting it is a safe product for clinical use [148].
NK-cells engineered with modified TGF-β receptor
Targeting the TGF-β receptor is another approach to enhance the cellular metabolism and the antitumor response of NK cells. TGF-β is an important cytokine for cell differentiation, migration, apoptosis, wound healing, and angiogenesis. It has been postulated that TGF-β suppresses NK function by reprogramming the metabolism [151]. Thus, different strategies for modulation of TGF-β signaling in NK cells have been proposed.
Recently, Yvon et al. developed NK-cells engineered to express a TGF-β-dominant-negative receptor II (DNRII), which resulted in the inhibition of TGF-β signaling. In vitro studies demonstrated that DNRII CB-NK-cells can efficiently kill tumor cells and can increase the expression of perforin, IFN-γ, NKG2D, and DNAM-1, even in the presence of TGF-β [152]. Animals with lung metastasis treated with NK-cells expressing DNRII had similar results showing decreased tumor growth [153].
Furthermore, the construction of a chimeric TGF-β receptor coupled to activating molecules has also been shown to be a promising strategy (Fig. 2f). NK-cells engineered to express TGF-β type II receptor coupled to the NKG2D intracellular domain, or a receptor containing the truncated TGFβRII domain linked to the synthetic Notch-like receptor coupled to the transcription factor RELA (which activates NK cells), had their antitumor activity enhanced [154, 155].
Conclusion
Even though there are many difficulties in developing CAR-NK-cells, a lot of improvements in their manufacturing have been made. We are at the moment testing several methods to expand and activate primary NK-cells, among these the use of feeders, beads, and cytokines enables the production of enough cells for clinical use. In addition to viral vectors, non-viral delivery methods are being optimized to produce a safer option for clinical use, including electroporation and/or cell squeezing. Non-viral vectors such as transposons, episomes, and CRISPR-Cas9, have been successfully implemented in pre-clinical studies and mRNA is already undergoing CAR-NK clinical trials.
Regarding CAR structure, the use of NK-specific signaling molecules in CAR signaling domains is a strategy that enables us to increase the cytotoxicity of CAR-NK cells. Furthermore, we have presented several engineering strategies that might help to increase CAR-NK-cell antitumor efficiency. Stabilizing MICA/B in the tumor cell membrane in combination with CAR-NK-cells has not yet been tested, but this could be an interesting approach for treating MICA/B+ tumors. The use of chimeric receptors to convert TGF-β inhibitory signaling into an activating signal could help to escape microenvironment TGF-β inhibition. CAR-NK-cells combining the CD38 KO with ncCD16A and also expressing membrane-bound IL-15/IL-15Rα are already being tested. The expression of IL-15/IL-15Rα is an interesting approach due to its capacity to increase proliferation, efficiency, and persistence of CAR-NK cells, which can be further improved by CISH KO.
Considering the many advances in CAR-NK-cells engineering, their clinical application as an off-the-shelf product is imminent. We are heading to a future where non-viral vectors may become the main delivery method for CAR expression, and NK cells can permit a ready-to-use targeted cell therapy with a safer profile. Taking advantage of the several existing engineering options can allow to develop more robust CAR-NK-cells. Moreover, their combination with other therapies such as monoclonal antibodies against CTLA-4 or PD-1/PDL-1 could increase CAR-NK-cells effector function. Therefore, CAR-NK-cells could allow more cancer patients to benefit from cell therapy.
Acknowledgments
We thank Sandra Navarro Besciane for the graphic design and Andrew Cumming for the English language review. The Editor-in-Chief, Tim Elliott, and handling editor, Adriana Bonomo, would like to thank the following reviewer, Norberto Zwirner, and an anonymous reviewer, for their contribution to the publication of this article.
Glossary
Abbreviations
- AAV
Adeno-associated virus
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- AML
Acute myeloid leukemia
- BaEV-LV
Baboon envelope pseudotyped lentiviral vectors
- CAR
Chimeric antigen receptor
- CB
Cord blood
- CLL
Chronic lymphocytic leukemia
- CRS
Cytokine release syndrome
- GvHD
Graft versus host disease
- MP
Good manufacturing practice
- HLA
Human leucocyte antigen
- IL
Interleukin
- iPSC
induced pluripotent stem cells
- ITAM
Immunoreceptor tyrosine-based activation motif
- ITR
inverted terminal repeat sequences
- KO
Knockout
- KIR
Killer-cell immunoglobulin-like receptor
- LDLR
Low-density lipoprotein receptors
- mAb
monoclonal antibody
- MM
Multiple myeloma
- NK
Natural killer
- PB
Peripheral blood
- PBMC
Peripheral blood mononuclear cell
- RV
Retroviral vector
- SB
Sleeping beauty
- scFv
single-chain variable fragment
- S/MAR
Scaffold/matrix attachment region
- TM
Transmembrane domain
- TME
Tumor microenvironment
- VSV-G
Vesicular stomatitis virus type-G
Author contributions
Conceptualization: J.T.C.A. and V.P. Writing – original draft: all authors. Writing – review and editing: D.S., S.E., J.T.C.A., and V.P. Supervision: D.T.C and V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed by Brazilian foundations: grant #2019/25309-0, São Paulo Research Foundation (FAPESP): grant #2013/08135-2, CTC Center for Cell-based Therapy, São Paulo Research Foundation (FAPESP); National Institute of Science and Technology in Stem Cell and Cell Therapy (grant #573754-2008-0 CNPq and grant #2008/578773 FAPESP) and grant #442484/2020-8, CNPq. DS declares support from Coordination for the Improvement of Higher Education Personnel (CAPES) finance code 001. SE was supported by Coordination for the Improvement of Higher Education Personnel (CAPES) finance code 001. KRG acknowledges support from São Paulo Research Foundation (FAPESP), grant #2020/09206-4. MCT was supported by the National Council for Scientific and Technological Development (CNPq) (grant #140819/2021-5). RNS declares support from the Improvement of Higher Education Personnel (CAPES) finance code 001. JTCA was supported by grant #2020/08279-8, São Paulo Research Foundation (FAPESP).
Conflict of Interest
The authors declare that they have no conflict of interest.
Data Availability
Statement: No data available as this is a review article.
References
- 1. Locke FL, Neelapu SS, Bartlett NL.et al. Clinical and biologic covariates of outcomes in ZUMA-1: A pivotal trial of axicabtagene ciloleucel (axi-cel; KTE-C19) in patients with refractory aggressive non-Hodgkin lymphoma (r-NHL). J Clin Oncol 2017;35:7512. doi: 10.1200/JCO.2017.35.15_suppl.7512 [DOI] [Google Scholar]
- 2. Maude SL, Laetsch TW, Buechner J.et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang M, Munoz J, Goy A.et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2020;382:1331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sadelain M, Brentjens R, Rivière I.. The basic principles of chimeric antigen receptor design. Cancer Discov 2013;3:388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee DW, Gardner R, Porter DL.et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santomasso BD, Park JH, Salloum D.et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov 2018;8:958–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Themeli M, Rivière I, Sadelain M.. New cell sources for T cell engineering and adoptive immunotherapy. Cell Stem Cell 2015;16:357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waldhauer I, Steinle A.. NK cells and cancer immunosurveillance. Oncogene 2008;27:5932–43. [DOI] [PubMed] [Google Scholar]
- 9. Pallmer K, Oxenius A.. Recognition and regulation of T cells by NK cells. Front Immunol 2016;7:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oei VYS, Siernicka M, Graczyk-Jarzynka A, et al. Intrinsic functional potential of NK-cell subsets constrains retargeting driven by chimeric antigen receptors. Cancer Immunol Res 2018;6:467–80. [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y, Wallace DL, de Lara CM.et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology 2007;121:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoon SR, Lee YS, Yang SH, et al. Generation of donor natural killer cells from CD34(+) progenitor cells and subsequent infusion after HLA-mismatched allogeneic hematopoietic cell transplantation: a feasibility study. Bone Marrow Transplant 2010;45:1038–46. [DOI] [PubMed] [Google Scholar]
- 13. Wrona E, Borowiec M, Potemski P.. CAR-NK cells in the treatment of solid tumors. Int J Mol Sci 2021;22:5899. doi: 10.3390/ijms22115899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehta RS, Shpall EJ, Rezvani K.. Cord blood as a source of natural killer cells. Front Immunol 2016;11:584099. doi: 10.3389/fmed.2015.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verneris MR, Miller JS.. The phenotypic and functional characteristics of umbilical cord blood and peripheral blood natural killer cells. Br J Haematol 2009;147:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luevano M, Madrigal A, Saudemont A.. Generation of natural killer cells from hematopoietic stem cells in vitro for immunotherapy. Cell Mol Immunol 2012;9:310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woll PS, Martin CH, Miller JS.et al. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J Immunol 2005;175:5095–103. [DOI] [PubMed] [Google Scholar]
- 18. Knorr DA, Ni Z, Hermanson D.et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med 2013;2:274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsuo Y, Drexler HG.. Immunoprofiling of cell lines derived from natural killer-cell and natural killer-like T-cell leukemia-lymphoma. Leuk Res 2003;27:935–45. [DOI] [PubMed] [Google Scholar]
- 20. Zhang J, Zheng H, Diao Y.. Natural killer cells and current applications of chimeric antigen receptor-modified NK-92 cells in tumor immunotherapy. Int J Mol Sci 2019; 20:317. doi: 10.3390/ijms20020317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gong Y, Klein Wolterink RGJ, Wang J, et al. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol 2021;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cooper MA, Fehniger TA, Caligiuri MA.. The biology of human natural killer-cell subsets. Trends Immunol 2001;22:633–40. [DOI] [PubMed] [Google Scholar]
- 23. Jacobs R, Hintzen G, Kemper A.et al. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol 2001;31:3121–7. [DOI] [PubMed] [Google Scholar]
- 24. Poli A, Michel T, Thérésine M.et al. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology 2009;126:458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fehniger TA, Cooper MA, Nuovo GJ.et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 2003;101:3052–7. [DOI] [PubMed] [Google Scholar]
- 26. Ferlazzo G, Thomas D, Lin SL.et al. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol 2004;172:1455–62. [DOI] [PubMed] [Google Scholar]
- 27. Spanholtz J, Preijers F, Tordoir M.. et al. Clinical-grade generation of active NK Cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS One 2011;6:e20740. doi: 10.1371/journal.pone.0020740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oran B, Shpall E.. Umbilical cord blood transplantation: a maturing technology. Hematology Am Soc Hematol Educ Program 2012;2012:215–22. [DOI] [PubMed] [Google Scholar]
- 29. Nomura A, Takada H, Jin CH.et al. Functional analyses of cord blood natural killer cells and T cells: a distinctive interleukin-18 response. Exp Hematol 2001;29:1169–76. [DOI] [PubMed] [Google Scholar]
- 30. Choi YH, Lim EJ, Kim SW.et al. IL-27 enhances IL-15/IL-18-mediated activation of human natural killer cells. J Immunother Cancer 2019;7:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu M, Meng Y, Zhang L.et al. High-efficient generation of natural killer cells from peripheral blood with preferable cell vitality and enhanced cytotoxicity by combination of IL-2, IL-15 and IL-18. Biochem Biophys Res Commun 2021;534:149–56. [DOI] [PubMed] [Google Scholar]
- 32. de Rham C, Ferrari-Lacraz S, Jendly S.et al. The proinflammatory cytokines IL-2, IL-15 and IL-21 modulate the repertoire of mature human natural killer cell receptors. Arthritis Res Ther 2007;9:R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Romee R, Rosario M, Berrien-Elliott MM.et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med 2016;8:357ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gang M, Marin ND, Wong P.et al. CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood 2020;136:2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sarvaria A, Jawdat D, Madrigal JA.et al. Umbilical cord blood natural killer cells, their characteristics, and potential clinical applications. Front Immunol 2017;8:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luevano M, Daryouzeh M, Alnabhan R.et al. The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Hum Immunol 2012;73:248–57. [DOI] [PubMed] [Google Scholar]
- 37. Herrera L, Santos S, Vesga MA.et al. Adult peripheral blood and umbilical cord blood NK cells are good sources for effective CAR therapy against CD19 positive leukemic cells. Sci Rep 2019;9:18729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spanholtz J, Tordoir M, Eissens D.et al. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. Plos One 2010;5:e9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cho D, Campana D.. Expansion and activation of natural killer cells for cancer immunotherapy. Korean J Lab Med 2009;29:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Imai C, Iwamoto S, Campana D.. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 2005;106:376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Michen S, Frosch J, Füssel M.et al. Artificial feeder cells expressing ligands for killer cell immunoglobulin-like receptors and CD94/NKG2A for expansion of functional primary natural killer cells with tolerance to self. Cytotherapy 2020;22:354–68. [DOI] [PubMed] [Google Scholar]
- 42. Fujisaki H, Kakuda H, Shimasaki N.et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 2009;69:4010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Denman CJ, Senyukov VV, Somanchi SS.et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. Plos One 2012;7:e30264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu E, Ang SOT, Kerbauy L.et al. GMP-compliant universal antigen presenting cells (uAPC) Promote the metabolic fitness and antitumor activity of armored cord blood CAR-NK cells. Front Immunol 2021;12:626098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Latchman Y, McKay PF, Reiser H.. Identification of the 2B4 molecule as a counter-receptor for CD48. J Immunol 1998;161:5809–12. [PubMed] [Google Scholar]
- 46. Wilcox RA, Tamada K, Strome SE.et al. Signaling through NK cell-associated CD137 promotes both helper function for CD8+ cytolytic T cells and responsiveness to IL-2 but not cytolytic activity. J Immunol 2002;169:4230–6. [DOI] [PubMed] [Google Scholar]
- 47. Wang X, Lee DA, Wang Y.et al. Membrane-bound interleukin-21 and CD137 ligand induce functional human natural killer cells from peripheral blood mononuclear cells through STAT-3 activation. Clin Exp Immunol 2013;172:104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ojo EO, Sharma AA, Liu R.et al. Membrane bound IL-21 based NK cell feeder cells drive robust expansion and metabolic activation of NK cells. Sci Rep 2019;9:14916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guo M, Sun C, Qian Y.et al. Proliferation of highly cytotoxic human natural killer cells by OX40L armed NK-92 with secretory neoleukin-2/15 for cancer immunotherapy. Front Oncol 2021;11:632540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Quijano-Rubio A, Ulge UY, Walkey CD.et al. The advent of de novo proteins for cancer immunotherapy. Curr Opin Chem Biol 2020;56:119–28. [DOI] [PubMed] [Google Scholar]
- 51. Vasu S, Berg M, Davidson-Moncada J.et al. A novel method to expand large numbers of CD56(+) natural killer cells from a minute fraction of selectively accessed cryopreserved cord blood for immunotherapy after transplantation. Cytotherapy 2015;17:1582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ciurea SO, Schafer JR, Bassett R.et al. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood 2017;130:1857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shah NN, Szabo A, Huntington SF.et al. R-CHOP versus dose-adjusted R-EPOCH in frontline management of primary mediastinal B-cell lymphoma: a multi-centre analysis. Br J Haematol 2018;180:534–44. [DOI] [PubMed] [Google Scholar]
- 54. Silla L, Valim V, Pezzi A.et al. Adoptive immunotherapy with double-bright (CD56bright /CD16bright) expanded natural killer cells in patients with relapsed or refractory acute myeloid leukaemia: a proof-of-concept study. Br J Haematol 2021;195:710–21. [DOI] [PubMed] [Google Scholar]
- 55. Geraghty RJ, Capes-Davis A, Davis JM.et al.; Cancer Research UK. Guidelines for the use of cell lines in biomedical research. Br J Cancer 2014;111:1021–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oyer JL, Igarashi RY, Kulikowski AR.et al. Generation of highly cytotoxic natural killer cells for treatment of acute myelogenous leukemia using a feeder-free, particle-based approach. Biol Blood Marrow Transplant 2015;21:632–9. [DOI] [PubMed] [Google Scholar]
- 57. Masuyama J, Murakami T, Iwamoto S.et al. Ex vivo expansion of natural killer cells from human peripheral blood mononuclear cells co-stimulated with anti-CD3 and anti-CD52 monoclonal antibodies. Cytotherapy 2016;18:80–90. [DOI] [PubMed] [Google Scholar]
- 58. Zamai L, Del Zotto G, Buccella F.. et al. Understanding the synergy of NKp46 and co-activating signals in various NK cell subpopulations: paving the way for more successful NK-cell-based immunotherapy. Cells 2020;9:753. doi: 10.3390/cells9030753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Carlsten M, Childs RW.. Genetic manipulation of NK cells for cancer immunotherapy: techniques and clinical implications. Front Immunol 2015;6:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Davis HE, Rosinski M, Morgan JR, et al. Charged polymers modulate retrovirus transduction via membrane charge neutralization and virus aggregation. Biophys J 2004;86:1234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Davis HE, Morgan JR, Yarmush ML.. Polybrene increases retrovirus gene transfer efficiency by enhancing receptor-independent virus adsorption on target cell membranes. Biophys Chem 2002;97:159–72. [DOI] [PubMed] [Google Scholar]
- 62. Suerth JD, Morgan MA, Kloess S.et al. Efficient generation of gene-modified human natural killer cells via alpharetroviral vectors. J Mol Med (Berl) 2016;94:83–93. [DOI] [PubMed] [Google Scholar]
- 63. Finkelshtein D, Werman A, Novick D.et al. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci U S A 2013;110:7306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gong Y, Klein Wolterink RGJ, Janssen I.et al. Rosuvastatin enhances VSV-G lentiviral transduction of NK cells via upregulation of the low-density lipoprotein receptor. Mol Ther Methods Clin Dev 2020;17:634–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Colamartino ABL, Lemieux W, Bifsha P.et al. Efficient and robust NK-cell transduction with baboon envelope pseudotyped lentivector. Front Immunol 2019;10:2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brandstadter JD, Yang Y.. Natural killer cell responses to viral infection. J Innate Immun 2011;3:274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sutlu T, Nyström S, Gilljam M.et al. Inhibition of intracellular antiviral defense mechanisms augments lentiviral transduction of human natural killer cells: implications for gene therapy. Hum Gene Ther 2012;23:1090–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Allan DSJ, Chakraborty M, Waller GC.. et al. Systematic improvements in lentiviral transduction of primary human natural killer cells undergoing ex vivo expansion. Mol Ther – Methods Clin Dev 2021;20:559–71. doi: 10.1016/j.omtm.2021.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tipanee J, Chai YC, VandenDriessche T, Chuah MK.. Preclinical and clinical advances in transposon-based gene therapy. Biosci Rep 2017;37:BSR20160614. doi: 10.1042/BSR20160614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li Y, Hermanson DL, Moriarity BS, Kaufman DS.. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell 2018;23:181–92.e5. doi: 10.1016/J.STEM.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang J, Lupo KB, Chambers AM, Matosevic S.. Purinergic targeting enhances immunotherapy of CD73+ solid tumors with piggyBac-engineered chimeric antigen receptor natural killer cells 11 Medical and Health Sciences 1107 Immunology. J Immunother Cancer 2018;7:155. doi: 10.1186/s40425-018-0441-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sandoval-Villegas N, Nurieva W, Amberger M, Ivics Z.. Contemporary transposon tools: a review and guide through mechanisms and applications of sleeping beauty, piggyBac and Tol2 for genome engineering. Int J Mol Sci 2021;2:5084. doi: 10.3390/ijms22105084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Micklethwaite KP, Gowrishankar K, Gloss BS.et al. Investigation of product-derived lymphoma following infusion of piggyBac-modified CD19 chimeric antigen receptor T cells. Blood 2021;138:1391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li L, Liu LN, Feller S, et al. Expression of chimeric antigen receptors in natural killer cells with a regulatory-compliant non-viral method. Cancer Gene Ther 2010;17:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chu Y, Hochberg J, Yahr A, et al. Targeting CD20+ aggressive B-cell non-hodgkin lymphoma by anti-CD20 CAR mRNA-modified expanded natural killer cells in vitro and in NSG mice. Cancer Immunol Res 2015;3:333–44. [DOI] [PubMed] [Google Scholar]
- 76. Chu Y, Yahr A, Ayello J.. et al. Anti-CD20 chimeric antigen receptor (CAR) modified expanded natural killer (NK) cells significantly mediate rituximab sensitive and resistant Burkitt lymphoma (BL) regression and improve survival in human BL xenografted NSG mice. Biol Blood Marrow Transplant 2014;20:S257. doi: 10.1016/j.bbmt.2013.12.433 [DOI] [Google Scholar]
- 77. Elmacken M, Awasthi A, Ayello J.. et al. Neuroblastoma and Ewing’s sarcoma associated with ROR1 expression can be effectively targeted with NK cells modified to express an anti ROR1 chimeric antigen receptor. Biol Blood Marrow Transplant 2015;21:S95–S97. doi: 10.1016/J.BBMT.2014.11.117 [DOI] [Google Scholar]
- 78. Oberoi P, Wels WS.. Arming NK cells with enhanced antitumor activity: CARs and beyond. Oncoimmunology 2013;2:e25220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mulia GE, Picanço-Castro V, Stavrou EF.et al. Advances in the development and the applications of nonviral, episomal vectors for gene therapy. Hum Gene Ther 2021;32:1076–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Picanço-Castro V, Pereira CG, Covas DT.et al. Emerging patent landscape for non-viral vectors used for gene therapy. Nat Biotechnol 2020;38:151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bozza M, De Roia A, Correia MP.. et al. A nonviral, nonintegrating DNA nanovector platform for the safe, rapid, and persistent manufacture of recombinant T cells. Sci Adv 2021;7:eabf1333. doi: 10.1126/sciadv.abf1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pomeroy EJ, Hunzeker JT, Kluesner MG, et al. A genetically engineered primary human natural killer cell platform for cancer immunotherapy. Mol Ther 2020;28:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kararoudi MN, Likhite S, Elmas E.et al. CRISPR-targeted CAR gene insertion using Cas9/RNP and AAV6 enhances anti-AML activity of primary NK cells. bioRxiv 2021; preprint: doi: 10.1101/2021.03.17.435886 [DOI] [Google Scholar]
- 84. Nguyen DN, Roth TL, Li PJ.et al. Polymer-stabilized Cas9 nanoparticles and modified repair templates increase genome editing efficiency. Nat Biotechnol 2020;38:44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Al-Dosari MS, Gao X.. Nonviral gene delivery: principle, limitations, and recent progress. Aaps J 2009;11:671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Begum AA, Toth I, Hussein WM.et al. Advances in targeted gene delivery. Curr Drug Deliv 2019;16:588–608. [DOI] [PubMed] [Google Scholar]
- 87. Marofi F, Rahman HS, Thangavelu L.et al. Renaissance of armored immune effector cells, CAR-NK cells, brings the higher hope for successful cancer therapy. Stem Cell Res Ther 2021;12:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shi J, Ma Y, Zhu J, Chen Y, Sun Y, Yao Y, Yang Z, Xie J.. A review on electroporation-based intracellular delivery. Molecules 2018;23:3044. doi: 10.3390/MOLECULES23113044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sharei A, Zoldan J, Adamo A.et al. A vector-free microfluidic platform for intracellular delivery. Proc Natl Acad Sci U S A 2013;110:2082–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. DiTommaso T, Cole JM, Cassereau L.et al. Cell engineering with microfluidic squeezing preserves functionality of primary immune cells in vivo. Proc Natl Acad Sci U S A 2018;115:E10907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Szeto GL, Van Egeren D, Worku H.et al. Microfluidic squeezing for intracellular antigen loading in polyclonal B-cells as cellular vaccines. Sci Rep 2015;5:10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kollmannsperger A, Sharei A, Raulf A.et al. Live-cell protein labelling with nanometre precision by cell squeezing. Nat Commun 2016;7:10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chang L, Gallego-Perez D, Zhao X.et al. Dielectrophoresis-assisted 3D nanoelectroporation for non-viral cell transfection in adoptive immunotherapy. Lab Chip 2015;15:3147–53. [DOI] [PubMed] [Google Scholar]
- 94. Schmidt P, Raftery MJ, Pecher G.. Engineering NK cells for CAR therapy-recent advances in gene transfer methodology. Front Immunol 2020;11:611163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Müller T, Uherek C, Maki G.et al. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol Immunother 2008;57:411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Boissel L, Betancur-Boissel M, Lu W.et al. Retargeting NK-92 cells by means of CD19- and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology 2013;2:e26527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kruschinski A, Moosmann A, Poschke I.et al. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proc Natl Acad Sci U S A 2008;105:17481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen KH, Wada M, Pinz KG.et al. Preclinical targeting of aggressive T-cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia 2017;31:2151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chmielewski M, Kopecky C, Hombach AA.et al. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res 2011;71:5697–706. [DOI] [PubMed] [Google Scholar]
- 100. Kagoya Y, Tanaka S, Guo T.et al. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med 2018;24:352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cantoni C, Bottino C, Vitale M.et al. NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. J Exp Med 1999;189:787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pende D, Parolini S, Pessino A.et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med 1999;190:1505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pessino A, Sivori S, Bottino C.et al. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J Exp Med 1998;188:953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lanier LL, Yu G, Phillips JH.. Co-association of CD3 zeta with a receptor (CD16) for IgG Fc on human natural killer cells. Nature 1989;342:803–5. [DOI] [PubMed] [Google Scholar]
- 105. Wu J, Song Y, Bakker AB.et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science 1999;285:730–2. [DOI] [PubMed] [Google Scholar]
- 106. Sivori S, Parolini S, Falco M.et al. 2B4 functions as a co-receptor in human NK cell activation. Eur J Immunol 2000;30:787–93. [DOI] [PubMed] [Google Scholar]
- 107. Bryceson YT, March ME, Ljunggren HG.et al. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 2006;107:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Töpfer K, Cartellieri M, Michen S.et al. DAP12-based activating chimeric antigen receptor for NK cell tumor immunotherapy. J Immunol 2015;194:3201–12. [DOI] [PubMed] [Google Scholar]
- 109. Robbins Y, Greene S, Friedman J.et al. Tumor control via targeting pd-l1 with chimeric antigen receptor modified NK cells. Elife 2020;9:1–18. doi: 10.7554/eLife.54854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Altvater B, Landmeier S, Pscherer S.et al. 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clin Cancer Res 2009;15:4857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Xu Y, Liu Q, Zhong M.et al. 2B4 costimulatory domain enhancing cytotoxic ability of anti-CD5 chimeric antigen receptor engineered natural killer cells against T cell malignancies. J Hematol Oncol 2019;12:1–13. doi: 10.1186/s13045-019-0732-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Huang Y, Zeng J, Liu T.et al. DNAM1 and 2B4 costimulatory domains enhance the cytotoxicity of anti-gpc3 chimeric antigen receptor-modified natural killer cells against hepatocellular cancer cells in vitro. Cancer Manag Res 2020;12:3247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kailayangiri S, Altvater B, Spurny C.et al. Targeting Ewing sarcoma with activated and GD2-specific chimeric antigen receptor-engineered human NK cells induces upregulation of immune-inhibitory HLA-G. Oncoimmunology 2017;6:e1250050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chang YH, Connolly J, ShimasakiN,.et al. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res 2013;73:1777–86. [DOI] [PubMed] [Google Scholar]
- 115. Xiao L, Cen D, Gan H.et al. Adoptive transfer of NKG2D CAR mRNA-engineered natural killer cells in colorectal cancer patients. Mol Ther 2019;27:1114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ng YY, Tay JCK, Wang S.. CXCR1 expression to improve anti-cancer efficacy of intravenously injected CAR-NK cells in mice with peritoneal xenografts. Mol Ther Oncolytics 2020;16:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yoon SR, Kim TD, Choi I.. Understanding of molecular mechanisms in natural killer cell therapy. Exp Mol Med 2015;47:e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mota G, Moldovan I, Calugaru A.et al. Interaction of human immunoglobulin G with CD16 on natural killer cells: ligand clearance, FcgammaRIIIA turnover and effects of metalloproteinases on FcgammaRIIIA-mediated binding, signal transduction and killing. Scand J Immunol 2004;59:278–84. [DOI] [PubMed] [Google Scholar]
- 119. Wu J, Mishra HK, Walcheck B.. Role of ADAM17 as a regulatory checkpoint of CD16A in NK cells and as a potential target for cancer immunotherapy. J Leukoc Biol 2019;105:1297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Romee R, Foley B, Lenvik T.et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood 2013;121:3599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mishra HK, Pore N, Michelotti EF.et al. Anti-ADAM17 monoclonal antibody MEDI3622 increases IFNγ production by human NK cells in the presence of antibody-bound tumor cells. Cancer Immunol Immunother 2018;67:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jing Y, Ni Z, Wu J.et al. Identification of an ADAM17 cleavage region in human CD16 (FcγRIII) and the engineering of a non-cleavable version of the receptor in NK cells. Plos One 2015;10:e0121788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhu H, Blum RH, Bjordahl R.et al. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood 2020;135:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Dixon KJ, Wu J, Walcheck B.. Engineering anti-tumor monoclonal antibodies and Fc receptors to enhance ADCC by human NK scells. Cancers (Basel) 2021;13:312. doi: 10.3390/cancers13020312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Liu E, Marin D, Banerjee P.et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med 2020;382:545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bauer S, Groh V, Wu J.et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999;285:727–9. [DOI] [PubMed] [Google Scholar]
- 127. Raulet DH, Gasser S, Gowen BG.et al. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol 2013;31:413–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Xing S, Ferrari de Andrade L.. NKG2D and MICA/B shedding: a ‘tag game’ between NK cells and malignant cells. Clin Transl Immunology 2020;9:e1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ferrari de Andrade L, Tay RE.et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell–driven tumor immunity. Science (80-) 2018;359:1537–1542. doi: 10.1126/science.aao0505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hashmi H, Husnain M, Khan A.et al. CD38-directed therapies for management of multiple myeloma. Immunotargets Ther 2021;10:201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. McKeage K. Daratumumab: first global approval. Drugs 2016;76:275–81. [DOI] [PubMed] [Google Scholar]
- 132. Krejcik J, Casneuf T, Nijhof IS.et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016;128:384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Casneuf T, Xu XS, Adams HC 3rd. et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv 2017;1:2105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wang Y, Zhang Y, Hughes T.et al. Fratricide of NK cells in daratumumab therapy for multiple myeloma overcome by ex vivo-expanded autologous NK cells. Clin Cancer Res 2018;24:4006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Naeimi Kararoudi M, Nagai Y, Elmas E.et al. CD38 deletion of human primary NK cells eliminates daratumumab-induced fratricide and boosts their effector activity. Blood 2020;136:2416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Miller JS, Morishima C, McNeel DG.et al. A first-in-human phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin Cancer Res 2018;24:1525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Romee R, Cooley S, Berrien-Elliott MM.et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 2018;131:2515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Conlon KC, Lugli E, Welles HC.et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol 2015;33:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Stoklasek TA, Schluns KS, Lefrançois L.. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol 2006;177:6072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Tamzalit F, Barbieux I, Plet A.. et al. IL-15.IL-15R complex shedding following trans-presentation is essential for the survival of IL-15 responding NK and T cells. Proc Natl Acad Sci 2014;11:8565–8570. doi: 10.1073/pnas.1405514111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Guo Y, Luan L, Rabacal W.et al. IL-15 Superagonist-mediated immunotoxicity: role of NK cells and IFN-γ. J Immunol 2015;195:2353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wrangle JM, Velcheti V, Patel MR.et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol 2018;19:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ma R, Lu T, Li Z.et al. An oncolytic virus expressing IL15/IL15Rα combined with off-the-Shelf EGFR-CAR NK cells targets glioblastoma. Cancer Res 2021;81:3635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Margolin K, Morishima C, Velcheti V.et al. Phase I Trial of ALT-803, a novel recombinant IL15 complex, in patients with advanced solid tumors. Clin Cancer Res 2018;24:5552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Bjordahl R, Gaidarova S, Goodridge JP.et al. FT576: a novel multiplexed engineered off-the-shelf natural killer cell immunotherapy for the dual-targeting of CD38 and Bcma for the treatment of multiple myeloma. Blood 2019;134:3214. doi: 10.1182/blood-2019-131373 [DOI] [Google Scholar]
- 146. Goodridge JP, Bjordahl R, Mahmood S.et al. FT576: multi-specific off-the-shelf CAR-NK cell therapy engineered for enhanced persistence, avoidance of self-fratricide and optimized Mab combination therapy to prevent antigenic escape and elicit a deep and durable response in multiple myeloma. Blood 2020;136:4–5. doi: 10.1182/blood-2020-14275032614961 [DOI] [Google Scholar]
- 147. Bachanova V, Cayci Z, Lewis D.et al. Initial clinical activity of FT596, a first-in-class, multi-antigen targeted, off-the-shelf, iPSC-derived CD19 CAR NK cell therapy in relapsed/refractory B-cell lymphoma. Blood 2020;136:8–8. doi: 10.1182/blood-2020-14160632614959 [DOI] [Google Scholar]
- 148. Daher M, Basar R, Gokdemir E.et al. Targeting a cytokine checkpoint enhances the fitness of armored cord blood CAR-NK cells. Blood 2021;137:624–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Bernard P-L, Delconte RB, Laletin V.. et al. CISH targeting in NK cells activates natural cytotoxicity receptor signaling and reduce cell exhaustion to unsilence primary anti-tumor response. bioRxiv 2021; preprint: doi: 10.1101/2021.03.16.435571 [DOI] [Google Scholar]
- 150. Zhu H, Blum RH, Bernareggi D.et al. Metabolic reprograming via deletion of CISH in human iPSC-derived NK cells promotes in vivo persistence and enhances anti-tumor activity. Cell Stem Cell 2020;27:224–237.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Slattery K, Gardiner CM.. NK cell metabolism and TGFβ – implications for immunotherapy. Front Immunol 2019;10:2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Yvon ES, Burga R, Powell A.et al. Cord blood natural killer cells expressing a dominant negative TGF-β receptor: implications for adoptive immunotherapy for glioblastoma. Cytotherapy 2017;19:408–18. [DOI] [PubMed] [Google Scholar]
- 153. Yang B, Liu H, Shi W.et al. Blocking transforming growth factor-β signaling pathway augments antitumor effect of adoptive NK-92 cell therapy. Int Immunopharmacol 2013;17:198–204. [DOI] [PubMed] [Google Scholar]
- 154. Wang Z, Guo L, Song Y.. et al. Augmented anti-tumor activity of NK-92 cells expressing chimeric receptors of TGF-βR II and NKG2D. Cancer Immunol Immunother 2017;66:537–548. doi: 10.1007/s00262-017-1959-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Burga RA, Yvon E, Chorvinsky E.et al. Engineering the TGFβ receptor to enhance the therapeutic potential of natural killer cells as an immunotherapy for neuroblastoma. Clin Cancer Res 2019;25:4400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Tang X, Yang L, Li Z.et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res 2018;8:1083–1089. Available at: http://www.ncbi.nlm.nih.gov/pubmed/30034945 [PMC free article] [PubMed] [Google Scholar]
- 157. Burger MC, Zhang C, Harter PN.et al. CAR-engineered NK cells for the treatment of glioblastoma: turning innate effectors into precision tools for cancer immunotherapy. Front Immunol 2019;10:2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Statement: No data available as this is a review article.