Summary
Vaccination programmes are critically important to suppress the burden of infectious diseases, saving countless lives globally, as emphasised by the current COVID-19 pandemic. Effective adaptive immune responses are complex processes subject to multiple influences. Recent genetic, pre-clinical, and clinical studies have converged to show that availability of iron is a key factor regulating the development of T and B cell responses to infection and immunisation. Lymphocytes obtain iron from circulating transferrin. The amount of iron bound to transferrin is dependent on dietary iron availability and is decreased during inflammation via upregulation of the iron-regulatory hormone, hepcidin. As iron deficiency and chronic inflammatory states are both globally prevalent health problems, the potential impact of low iron availability on immune responses is significant. We describe the evidence supporting the importance of iron in immunity, highlight important unknowns, and discuss how therapeutic interventions to modulate iron availability might be implementable in the context of vaccination and infectious disease.
Keywords: iron, hepcidin, adaptive immunity, vaccination
Introduction – iron deficiency, inflammation, and hepcidin
Iron is an essential cofactor in cellular biochemistry and its catalytic function is thought to be intrinsically linked to the evolution of life [1, 2]. In the form of Fe-S clusters, haem groups, and individual ions, the element supports diverse processes, including energy production via the electron transport chain, DNA replication and repair, oxygen sensing, and demethylation reactions [3–5]. However, both iron deficiency and iron overload are prevalent across the global human population, illuminating iron as a particularly interesting micronutrient in human biology.
The importance of considering iron in the context of immunity lies with the vast prevalence of iron deficiency, particularly in countries heavily burdened by infectious disease. As recorded in 2016, it is estimated that 1.2 billion people worldwide have iron deficiency anaemia [6]. It is a leading cause of years lived with disability in low–middle-income countries (LMICs), and the fourth leading cause globally, particularly affecting children and pre-menopausal women. Our appreciation of the burden of iron deficiency is limited by an overwhelming focus on recording the prevalence of anaemia. Iron deficiency in the absence of anaemia (resolved by low ferritin) is estimated to be even more prevalent, and indeed, iron deficiency impacts on human biology beyond erythropoiesis (e.g. growth and cognitive development [7, 8]). Recent work indicates particular scenarios and populations in whom many cell types may experience a high degree of iron limitation, based on measuring serum iron concentration and transferrin saturation. In high-income settings, serum iron levels in healthy individuals vary relatively little by age, and are generally 10–30 µmol/L [9]. In The Gambia, the prevalence of anaemia among infants is high, and these children also frequently present with extremely low serum iron levels (below 5 µmol/L for much of the first year of life) [10]. Given the well-established high prevalence of iron deficiency anaemia among infants from LMICs, these observations of low serum iron in Gambian infants are likely generalisable to infants in other LMIC settings.
Central to understanding the pathophysiology of iron deficiency is an appreciation of homeostatic iron control by the hepatic iron regulatory hormone, hepcidin. Through its ability to target ferroportin (the iron exporter) for degradation [11], hepcidin maintains iron homeostasis by dictating the location of iron. In conditions of excess iron, hepcidin expression is induced and ferroportin is degraded on enterocytes and erythrophagocytic red-pulp macrophages, preventing dietary iron absorption and the recycling of iron derived from phagocytosis of senescent erythrocytes, respectively. Conversely, inhibition of hepcidin (e.g. during erythropoietic demand or iron deficiency [12, 13]) promotes iron absorption and releases iron from macrophage and hepatocyte storage, thus augmenting serum iron concentration. Yet hepcidin is not only a hormone which homeostatically regulates iron, but also an acute phase response protein. Its expression is induced by inflammation, predominantly via the cytokine, IL-6 [14]. Inflammatory induction of hepcidin is essential for the hypoferremia response to acute inflammation in mouse models [15, 16] and has been observed to correlate with inflammatory hypoferremia in a number of human infections [17–20]. Such hepcidin-mediated hypoferremia has been suggested as a form of nutritional immunity, essential for the control of extracellular siderophilic bacterial pathogens [21, 22].
Systemic iron deficiency can be nutritional in origin, driven by iron-poor diet or diets enriched with factors that antagonise iron absorption [23]. However, systemic iron availability can also be ‘functionally’ low due to chronically raised hepcidin in the context of inflammation, restricting duodenal iron absorption and iron availability in the serum, regardless of sufficiency of ferritin iron stores [24]. Functional iron deficiency can arise secondary to infection, and is common in areas where infection rates are high [25–27]. The global burden of iron deficiency is driven by the combination of poor nutritional uptake (or absorption) and high hepcidin driven by inflammation.
Iron deficiency is not limited to LMICs. In high-income countries, infants, children, pregnant women, and individuals with chronic inflammation are at particular risk of iron deficiency [8, 28]. Worldwide, 20% women of reproductive age and ~40% pregnant women are anaemic [29], with the latter often leading to neonatal iron deficiency [30]. Reportedly, menstruation affects iron stores to a greater extent than dietary intake [31]. In the pathophysiological contexts of chronic inflammation [e.g. cancer, chronic kidney disease (CKD), obesity or autoimmunity], raised hepcidin limits systemic iron availability, contributing to widespread anaemia of inflammation [32–38]. Serum iron levels can also be particularly low in conditions of gastrointestinal disease. For example, coeliac disease poses both systemic inflammation and disrupted gut epithelial integrity, impeding iron absorption [39, 40].
Thus, iron deficiency is commonly nutritional or secondary to infection and inflammation, with a proportion of cases manifesting in anaemia. Many population studies measure rates of iron deficiency anaemia (often defined by low haemoglobin and ferritin concentrations), which likely do not accurately identify individuals with serum iron deficiency. As we will describe, the latter parameter is the more relevant measure from the perspective of iron influencing adaptive immunity.
Serum iron and adaptive immune responses
Adaptive immune responses require dynamic cellular reconfiguration of metabolism and cellular physiology as antigen-specific lymphocytes proliferate, acquire effector functions, and generate immunological memory [41]. Activated T cells express 1 million new copies of transferrin receptor (TFRC) within 24 hours of activation [42], suggesting that increased iron uptake is required to power the T cell response, consistent with the multiple roles of iron for cellular metabolism [1, 2].
Strong genetic evidence for a role of iron in human adaptive immunity comes from analysis of members of two families with severe immunodeficiency and susceptibility to infection, who were shown to carry a hypomorphic mutation in TFRC, encoding transferrin receptor 1 [43]. This mutation reduces the efficiency with which immune cells can uptake transferrin-bound iron from serum. These patients had normal numbers of T, B, and NK cells, but lacked circulating IgG and had reduced numbers of circulating memory B cells. Furthermore, ex vivo T and B cell proliferation was defective, but could be rescued by provisioning supraphysiological amounts of elemental iron, thus bypassing the defect in transferrin receptor. Mice with an analogous mutation in TFRC exhibit similar ex vivo and in vivo lymphocyte activation defects [42]. These results are consistent with previous studies in animal models and in vitro, which indicate the importance of transferrin-bound iron uptake for lymphocyte activation [44, 45]. While complete inhibition of transferrin-bound iron uptake blocks lymphocyte development [46, 47], the effect of more subtle changes in iron availability on lymphopoiesis remains unclear [42, 43, 48].
The study by Jabara et al.[43] demonstrates that adaptive immune responses require iron, however, the influence of variable serum iron availability on immune cells remained unaddressed. Our group recently demonstrated that transient acute serum iron deficiency, driven physiologically through enhanced hepcidin activity, suppresses the antigen-specific CD8, CD4, and B cell immune response in mice [42]. Furthermore, in piglets, a natural model of iron deficiency, we found that responses to vaccination were improved by iron interventions that increased serum iron [42]. These results are consistent with earlier observations of impaired humoral immunity and baseline lymphocyte activation ex vivo in rodent models of severe dietary iron deficiency [48, 49]. They also highlight serum iron, regulated by hepcidin, as the factor controlling immune responses. Consistent with this concept, we observed decreased vaccine-inducible pathogen-specific antibody responses in human patients with rare mutations that cause high hepcidin and low serum iron [42].
Inflammatory hypoferremia – benefits and risks
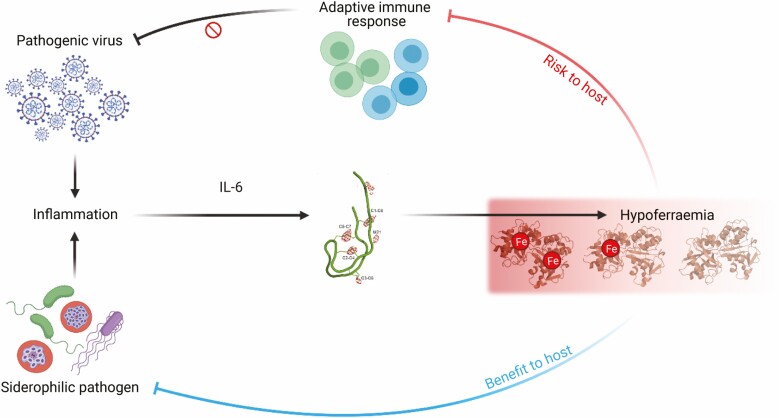
Hepcidin-mediated hypoferremia is a natural part of the acute infection response [50–52]. Hepcidin is commonly elevated during infection and inflammation, preventing macrophagic iron recycling and ‘trapping’ the micronutrient in reticuloendothelial cells, limiting the systemic availability of iron and its potential to support pathogen growth. However, the aforementioned data regarding the iron requirements of lymphocytes imply the existence of a trade-off between hypoferremia as an innate nutritional defence to a subset of infections [50, 53] and the metabolic requirements of the adaptive immune response [42]. The outcome of this trade-off likely depends on the pathogen in question, the persistence of the hypoferraemic response and the quantity and distribution of iron resources within the host. For example, while iron deficiency certainly protects against malaria [54] and certain bacterial infections [21, 22, 50, 55, 56], there is no clear evidence that iron availability directly alters viral replication in vivo [57].
Viruses can induce hypoferraemia in humans, as noted in experimental norovirus infection and acute HIV-1, however, in these instances, the effect may be transient [17, 19]. The COVID-19 pandemic has provided a context in which to investigate how hypoferremia and adaptive immunity may be associated in humans in a more chronic systemic inflammatory setting. Inflammatory hypoferremia has been associated with severe disease and low lymphocyte counts among patients with COVID-19 [58–63]. Emerging evidence indicates that uncoordinated or slowed development of effective adaptive immunity may be a driver of severe disease [64–66]. Interestingly, given the role of IL-6 in systemic iron homeostasis during inflammation [14], its expression is elevated in cases of acute COVID-19 and associates with disease severity [63, 67, 68]. IL-6 levels have also been suggested to correlate inversely with various aspects of the effector T cell response [65]. Clinical trial results indicate that therapeutic blockade of IL-6 with drugs such as tocilizumab and sarilumab may improve patient outcome [69, 70]. Whether disrupted iron homeostasis associates with impaired adaptive immunity in COVID-19, and how this might be approached therapeutically, remains to be investigated. However, inducing inflammatory hypoferremia in a murine model of influenza infection impaired adaptive immunity, delayed viral clearance, worsened the severity of lung inflammation, and slowed recovery from weight loss [42]. This indicates that hypoferremia may predispose to poor outcomes of respiratory infection. This outcome exists in stark contrast to the protective effects of hepcidin-driven hypoferraemia in the context of extracellular bacteria and liver-stage malaria [21, 22, 53]. A potential explanation for this divergence is that denying iron to siderophilic pathogens benefits the host. However, if the iron requirements of the pathogen are relatively low (such as for viral replication), inflammatory hypoferraemia instead risks inhibiting a protective adaptive immune response, allowing infection to persist and potentially exacerbating tissue damage due to unrestrained inflammation (Fig. 1). Notably, this scenario suggests that suppressing hepcidin activity could provide a therapeutic target for restoring iron availability to activated lymphocytes in inflammatory settings.
Figure 1.
Inflammation, often resulting from infection, induces hepcidin expression via IL-6, driving hypoferraemia. Hypoferraemia poses a trade-off to the host, limiting extracellular iron availability and protecting against siderophilic pathogens (e.g. Vibrio vulnificus), but also diverting iron away from the adaptive immune response, impairing control of pathogenic viruses (e.g. Influenza A virus infection).
Iron deficiency and vaccine responses in humans
When considering studies seeking to link iron status and responses to vaccines in humans, as previously reviewed by Oppenheimer [49], early investigations tended to provide inconsistent results. Vaccination trials for diseases such as diphtheria, tetanus [71], and typhoid [72] did not indicate an association between iron deficiency and vaccine efficacy, but were likely underpowered [73]. A larger study involving 1554 Ecuadorian infants showed that anaemic children generate low diphtheria antibody titres (frequently below the protective level) following DTP vaccination compared with control [74]. More recently, anaemia was linked to altered development of the immune system in children from Mozambique and Tanzania [75]. It was recently demonstrated that variable combinations of anaemia, haemoglobin, and soluble transferrin receptor measurements in Kenyan infants predicted poor responses to diphtheria, pertussis, measles, and pneumococcal vaccines [76]. However, anaemia is a multifactorial condition, which can be driven by influences other than iron deficiency [77]. Direct evidence that serum iron deficiency regulates responses to vaccines in humans is limited because this parameter is rarely reported. One small study found that among elderly hospitalised patients receiving influenza vaccines, non-response was clearly associated with suppressed serum iron concentrations (mean, 8.34 µmol/L vs. 16.00 µmol/L in responders) [78]. More significantly, iron supplementation at time of vaccination, which would be expected to raise serum iron, improved the antibody response to the measles vaccine in Kenyan infants [76].
Lower vaccine efficacy has been observed for certain vaccines, including measles and live attenuated influenza vaccine, among infants in LMICs [79, 80]. Certain high-income populations, including individuals with coeliac disease [81], obesity [82], and CKD [83] are reported to generate poor responses to the hepatitis B virus vaccine, and the inactivated flu vaccine has been reported to perform poorly in CKD patients [84] and the elderly [85]. However, whether the high prevalence of iron deficiency and low serum iron in these populations contributes to low vaccine efficacy has not yet been explored, to our knowledge.
Iron and immunometabolism
Iron deficiency has been shown to have broad effects on metabolism and function of different types of cells [86–91]. In general, cellular iron homeostasis is maintained by iron-regulatory protein-1 and -2 [92]. Loss of the genes encoding these proteins in activated T cells impairs iron uptake, proliferation, and effector functions [42]. However, the precise iron-requiring processes disrupted in T cells by iron-restricted conditions, critically impairing activity, remain unclear. In vitro, both low-iron culture conditions and genetic disruption of tetrahydrobiopterin synthesis (which impacts T cell proliferation in an iron-dependent manner) impair T-cell mitochondrial oxidative metabolism [42, 93]. DNA and histone demethylases are iron-dependent, and modulation of their activity through iron chelation results in altered cell cycle behaviour in B cells [48, 94]. Serum iron deficiency prevents acquisition of effector functions by T cells [42]. In CD4 T cells, iron controls expression of proinflammatory cytokines IL-2 and GM-CSF (but not IFNγ and TNFα) via interactions with the RNA binding protein, PCBP1 [44]. Iron has also been suggested to modulate the responsiveness of lymphocytes to IL-2R signalling [42, 95]. The effects of low iron on T cells are likely to be complex and there may be different levels of sensitivity depending upon the iron requirements and metabolic states of different T cell subsets.
Transient serum iron deficiency during the expansion phase of the T cell response impairs the quality of CD8 T cell memory 35 days post-immunisation (assessed by cytokine production and magnitude of secondary recall response) [42]. This result suggests that iron availability may influence the trajectory of T cell differentiation and polarisation of the immune response. However, more work is required to establish how the negative effects of iron deficiency persist over time, and how the cell-intrinsic and extrinsic regulators of memory differentiation are thus perturbed [96].
Although this review focuses on iron deficiency, we should note that genetic diseases which suppress hepcidin (hereditary hemochromatosis and thalassemia [97]) and lead to systemic iron loading have also been proposed to influence immunity. In HFE hemochromatosis patients, this impact is complicated by the strong association between HFE mutations and the major histocompatibility complex gene complex. The effect of HFE mutations on baseline CD8:CD4 ratios and lymphocyte activation phenotypes has been extensively investigated [98–100], yet it is not well understood how HFE mutations alter the quality of adaptive immunity in vivo.
Correcting iron deficiency to improve immunity – challenges and potential
The suggestion that iron deficiency may contribute to suboptimal adaptive immunity makes the normalisation of iron status an attractive potential ‘immunotherapeutic’ intervention (Fig. 2). However, the key evidence that therapeutically targeting iron can support adaptive immunity is currently limited to the improved T cell and antibody responses seen in hypoferraemic mice and piglets, respectively, upon iron supplementation [42], and the improved seroconversion and antibody avidity to measles vaccination in a retrospective analysis of Kenyan infants receiving iron [76]. It is critically important that prospective randomised controlled trials are performed to more rigorously test the idea that iron could boost immune responses to vaccines in iron-deficient individuals, and research in this area is ongoing.
Figure 2.
Dietary factors and constitutively high hepcidin expression can drive low serum iron availability. Sufficient iron concentrations are required to generate a robust adaptive immune response. In particular settings, it may be possible to acutely supplement serum iron availability to support immunity, thereby improving vaccine efficacy – this concept remains to be investigated.
Of relevance to this concept, the resolution of iron deficiency remains a challenge in itself for several reasons. Oral iron formulations may exacerbate some infections, including malaria, and can increase episodes of diarrhoea, although these results are not universally observed in all settings [101–106]. Furthermore, the efficacy of oral iron in restoring iron status can be variable. A major issue in populations with high infection burden is that persistent mild inflammation can drive high hepcidin concentrations, disabling efficient dietary iron absorption [107, 108]. High-dose oral iron supplementation regimes may also increase hepcidin to a point where the majority of iron is not absorbed [109, 110]. Future improvements in dosing schedules [111–113], new iron formulations that are more absorbable [114, 115], and potentially the use of intravenous iron [116], could counteract the current difficulties associated with nutritional iron interventions. Other molecular mechanistic approaches, aimed at inhibiting hepcidin and so enhancing iron absorption and release of iron from cellular sequestration [117–121], may also be beneficial, particularly in the context of inflammatory anaemias.
Despite the unresolved issues noted above, one specific consideration for the possible future use of iron to improve immune responses is worth highlighting: less iron is likely to be needed to boost immune responses than is needed to improve systemic iron deficiency. The latter often requires iron interventions over long periods, with oral iron regimes lasting for several months. The nature of this exposure (time and quantity) likely contributes to the noted gastrointestinal side effects and predisposition to infections. However, to achieve the goal of enhancing the immune response to specific vaccines, only short-term supplementation during the metabolically active acute expansion phase of adaptive immunity may be necessary, as quiescent memory cells are likely to have low iron requirements. As each red blood cell contains ~100-fold more iron atoms than a T cell [122–124], it is likely that less iron administration would be required to support an immune response than for longer-term resolution of anaemia. Furthermore, over two thirds of the total body iron content exists in the erythroid compartment [123], highlighting that the number of red blood cells required to sufficiently increase haemoglobin during anaemia greatly exceeds the number of antigen-specific T cells generated at the peak of even a large immune response [125]. Acute iron treatment likely presents less infection risk than long-term oral-iron regimes, although accompanying short-term prophylactic measures may be required to further reduce infection risk.
Concluding remarks
Driven by our increasing knowledge of systemic iron homeostasis and immunity, iron has been revealed as an important immunometabolite. Serum iron status influences the adaptive immune response to infections and vaccinations. The protective innate immune effect of low serum iron in the context of extracellular siderophilic bacterial infections is offset by the possible impairment of adaptive immunity. Our knowledge of hepcidin regulation could potentially be leveraged to swing this risk–benefit balance in favour of improving immunity. The highly variable nature of serum iron status warrants deeper research into which immune cell types have low iron levels in the varying contexts of systemic iron deficiency outlined above. The high global prevalence of iron deficiency, particularly in populations at risk of poor vaccine responses, highlights the hypothetical value of therapeutically optimising serum iron levels to improve vaccination efficacy. Trials will be required to ascertain which vaccines are particularly impaired by iron deficiency and could be improved by iron supplementation, the optimal iron delivery regime for maximising a durable vaccine response, and in which populations we should target iron interventions.
Acknowledgement
The Editor-in-Chief, Tim Elliott, would like to thank Marianne Boes and Linda Sinclair for their contribution to the peer review of this article.
Glossary
Abbreviations
- CKD
Chronic kidney disease
- LMIC
Low- and middle-income countries
- TFRC
Transferrin receptor
Funding
This work was supported by the UK Medical Research Council (MRC Human Immunology Unit core funding to H.D., award no. MC_UU_12010/10).
Author contributions
A.E.P. and J.N.F. drafted the manuscript; H.D. revised the draft; all authors approved the final draft.
Conflict of interest
The authors declare no competing interests.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Muchowska KB, Varma SJ, Moran J. Synthesis and breakdown of universal metabolic precursors promoted by iron. Nature 2019;569(7754):104–7. doi: 10.1038/s41586-019-1151-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andreini C, Putignano V, Rosato A, Banci L. The human iron-proteome. Metallomics 2018;10(9):1223–31. doi: 10.1039/c8mt00146d [DOI] [PubMed] [Google Scholar]
- 3. Rouault TA. Mammalian iron-sulphur proteins: Novel insights into biogenesis and function. Nat Rev Mol Cell Biol 2015;16(1):45–55. doi: 10.1038/nrm3909 [DOI] [PubMed] [Google Scholar]
- 4. Kim HJ, Khalimonchuk O, Smith PM, Winge DR. Structure, function, and assembly of heme centers in mitochondrial respiratory complexes. Biochim Biophys Acta - Mol Cell Res 2012;1823(9):1604–16. doi: 10.1016/j.bbamcr.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poulos TL. Heme enzyme structure and function. Chem Rev 2014;114(7):3919–62. doi: 10.1021/cr400415k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vos T, Abajobir AA, Abbafati C, et al. . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390(10100):1211–59. doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armitage AE, Moretti D. The importance of iron status for young children in low- and middle-income countries: A narrative review. Pharmaceuticals 2019;12(2). doi: 10.3390/ph12020059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lynch S, Pfeiffer CM, Georgieff MK, et al. . Biomarkers of Nutrition for Development (BOND)-Iron review. J Nutr 2018;148(suppl_1):1001S–67S. doi: 10.1093/jn/nxx036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY. Reference distributions for serum iron and transferrin saturation: a practical, simple, and clinically relevant approach in a large cohort. J Clin Lab Anal 2002;16(5):237–45. doi: 10.1002/jcla.10048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Armitage AE, Agbla SC, Betts M, et al. . Rapid growth is a dominant predictor of hepcidin suppression and declining ferritin in Gambian infants. Haematologica 2019;104(8):1542–53. doi: 10.3324/haematol.2018.210146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nemeth E, Tuttle MS, Powelson J, et al. . Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science (80-) 2004;306(5704):2090–3. doi: 10.1126/science.1104742 [DOI] [PubMed] [Google Scholar]
- 12. Kautz L, Jung G, Valore E V., Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet 2014;46(7):678–84. doi: 10.1038/ng.2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pasricha SR, Lim PJ, Duarte TL, et al. . Hepcidin is regulated by promoter-associated histone acetylation and HDAC3. Nat Commun 2017;8(1):1–15. doi: 10.1038/s41467-017-00500-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nemeth E, Rivera S, Gabayan V, et al. . IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 2004;113(9):1271–6. doi: 10.1172/jci20945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Armitage AE, Lim PJ, Frost JN, et al. . Induced Disruption of the Iron-Regulatory Hormone Hepcidin Inhibits Acute Inflammatory Hypoferraemia. J Innate Immun 2016;8(5):517–28. doi: 10.1159/000447713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fillebeen C, Wilkinson N, Charlebois E, Katsarou A, Wagner J, Pantopoulos K. Hepcidin-mediated hypoferremic response to acute inflammation requires a threshold of Bmp6/Hjv/Smad signaling. Blood 2018;132(17):1829–41. doi: 10.1182/blood-2018-03-841197 [DOI] [PubMed] [Google Scholar]
- 17. Armitage AE, Stacey AR, Giannoulatou E, et al. . Distinct patterns of hepcidin and iron regulation during HIV-1, HBV, and HCV infections. Proc Natl Acad Sci USA 2014;111(33):12187–92. doi: 10.1073/pnas.1402351111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Darton TC, Blohmke CJ, Giannoulatou E, et al. . Rapidly escalating hepcidin and associated serum iron starvation are features of the acute response to typhoid infection in humans. Baker S, ed. PLoS Negl Trop Dis. 2015;9(9):e0004029. doi: 10.1371/journal.pntd.0004029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williams AM, Ladva CN, Leon JS, et al. . Changes in micronutrient and inflammation serum biomarker concentrations after a norovirus human challenge. Am J Clin Nutr 2019;110(6):1456–64. doi: 10.1093/ajcn/nqz201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spottiswoode N, Armitage AE, Williams AR, et al. . Role of activins in hepcidin regulation during malaria. Infect Immun 2017;85(12). doi: 10.1128/IAI.00191-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arezes J, Jung G, Gabayan V, et al. . Hepcidin-induced hypoferremia is a critical host defense mechanism against the siderophilic bacterium vibrio vulnificus. Cell Host Microbe 2015;17(1):47–57. doi: 10.1016/j.chom.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stefanova D, Raychev A, Arezes J, et al. . Endogenous hepcidin and its agonist mediate resistance to selected infections by clearing non–transferrin-bound iron. Blood 2017;130(3):245–57. doi: 10.1182/blood-2017-03-772715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet 2007;370(9586):511–20. doi: 10.1016/S0140-6736(07)61235-5 [DOI] [PubMed] [Google Scholar]
- 24. Wang CY, Babitt JL. Hepcidin regulation in the anemia of inflammation. Curr Opin Hematol 2016;23(3):189–97. doi: 10.1097/MOH.0000000000000236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prentice AM, Bah A, Jallow MW, et al. . Respiratory infections drive hepcidin-mediated blockade of iron absorption leading to iron deficiency anemia in African children. Sci Adv. 2019;5(3):eaav9020. doi: 10.1126/sciadv.aav9020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ganz T. Anemia of Inflammation. Longo DL, ed. N Engl J Med. 2019;381(12):1148–57. doi: 10.1056/NEJMra1804281 [DOI] [PubMed] [Google Scholar]
- 27. Shaw JG, Friedman JF. Iron deficiency anemia: Focus on infectious diseases in lesser developed countries. Anemia 2011;2011. doi: 10.1155/2011/260380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gupta PM, Perrine CG, Mei Z, Scanlon KS. Iron, anemia, and Iron deficiency anemia among young children in the United States. Nutrients 2016;8(6). doi: 10.3390/nu8060330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prevalence of anemia among pregnant women (%) | Data. https://data.worldbank.org/indicator/sh.Prg.Anem. Accessed February 24, 2021.
- 30. Juul SE, Derman RJ, Auerbach M. Perinatal iron deficiency: Implications for mothers and infants. Neonatology 2019;115(3):269–74. doi: 10.1159/000495978 [DOI] [PubMed] [Google Scholar]
- 31. Harvey LJ, Armah CN, Dainty JR, et al. . Impact of menstrual blood loss and diet on iron deficiency among women in the UK. Br J Nutr 2005;94(4):557–64. doi: 10.1079/bjn20051493 [DOI] [PubMed] [Google Scholar]
- 32. Macciò A, Madeddu C, Gramignano G, et al. . The role of inflammation, Iron, And nutritional status in cancer-related anemia: Results of a large, Prospective, Observational study. Haematologica 2015;100(1):124–32. doi: 10.3324/haematol.2014.112813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hershko C, Camaschella C. How I treat unexplained refractory iron deficiency anemia. Blood 2014;123(3):326–33. doi: 10.1182/blood-2013-10-512624 [DOI] [PubMed] [Google Scholar]
- 34. Song SNJ, Iwahashi M, Tomosugi N, et al. . Comparative evaluation of the effects of treatment with tocilizumab and TNF-α inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Res Ther 2013;15(5):R141. doi: 10.1186/ar4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shu W, Pang Z, Xu C, et al. . Anti-TNF- α Monoclonal Antibody Therapy Improves Anemia through Downregulating Hepatocyte Hepcidin Expression in Inflammatory Bowel Disease. Mediators Inflamm 2019;2019. doi: 10.1155/2019/4038619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ludwig H Prof., Müldür E, Endler G, Hübl W. Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol 2013;24(7):1886–92. doi: 10.1093/annonc/mdt118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gardenghi S, Renaud TM, Meloni A, et al. . Distinct roles for hepcidin and interleukin-6 in the recovery from anemia in mice injected with heat-killed Brucella abortus. Blood 2014;123(8):1137–45. doi: 10.1182/blood-2013-08-521625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim A, Fung E, Parikh SG, et al. . A mouse model of anemia of inflammation: Complex pathogenesis with partial dependence on hepcidin. Blood 2014;123(8):1129–36. doi: 10.1182/blood-2013-08-521419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Corazza GR, Valentini RA, Andreani ML, et al. . Subclinical coeliac disease is a frequent cause of iron-deficiency anaemia. Scand J Gastroenterol 1995;30(2):153–6. doi: 10.3109/00365529509093254 [DOI] [PubMed] [Google Scholar]
- 40. Halfdanarson TR, Litzow MR, Murray JA. Hematologic manifestations of celiac disease. Blood 2007;109(2):412–21. doi: 10.1182/blood-2006-07-031104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Phan AT, Goldrath AW, Glass CK. Metabolic and Epigenetic Coordination of T Cell and Macrophage Immunity. Immunity 2017;46(5):714–29. doi: 10.1016/j.immuni.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frost JN, Tan TK, Abbas M, et al. . Hepcidin-mediated hypoferremia disrupts immune responses to vaccination and infection. Med 2020;2(2):164–79.e12. doi: 10.1016/j.medj.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jabara HH, Boyden SE, Chou J, et al. . A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nat Genet 2015;48(1):74–8. doi: 10.1038/ng.3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang Z, Yin W, Zhu L, et al. . Iron Drives T Helper Cell Pathogenicity by Promoting RNA-Binding Protein PCBP1-Mediated Proinflammatory Cytokine Production. Immunity 2018;49(1):80–92.e7. doi: 10.1016/j.immuni.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 45. Neckers LM, Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. Proc Natl Acad Sci USA 1983;80(11 I):3494–8. doi: 10.1073/pnas.80.11.3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brekelmans P, Van Soest P, Leenen PJM, van Ewijk W. Inhibition of proliferation and differentiation during early T cell development by anti-transferrin receptor antibody. Eur J Immunol 1994;24(11):2896–902. doi: 10.1002/eji.1830241147 [DOI] [PubMed] [Google Scholar]
- 47. Ned RM, Swat W, Andrews NC. Transferrin receptor 1 is differentially required in lymphocyte development. Blood 2003;102(10):3711–8. doi: 10.1182/blood-2003-04-1086 [DOI] [PubMed] [Google Scholar]
- 48. Jiang Y, Li C, Wu Q, et al. . Iron-dependent histone 3 lysine 9 demethylation controls B cell proliferation and humoral immune responses. Nat Commun 2019;10(1):1–15. doi: 10.1038/s41467-019-11002-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oppenheimer SJ. Iron and its relation to immunity and infectious disease. In: Journal of Nutrition. Vol 131. Oxford Academic; 2001:616S–635S. doi: 10.1093/jn/131.2.616s [DOI] [PubMed] [Google Scholar]
- 50. Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science (80-) 2012;338(6108):768–72. doi: 10.1126/science.1224577 [DOI] [PubMed] [Google Scholar]
- 51. Cartwright GE, Lauritsen MA. The anemia of infection; hypoferremia, hypercupremia, and alterations in porphyrin metabolism in patients. J Clin Invest 1946;25(1):65–80. doi: 10.1172/JCI101690 [DOI] [PubMed] [Google Scholar]
- 52. Cartwright GE, Lauritsen MA. The anemia of infection; the experimental production of hypoferremia and anemia in dogs. J Clin Invest 1946;25:81–6. doi: 10.1172/JCI101691 [DOI] [PubMed] [Google Scholar]
- 53. Portugal S, Carret C, Recker M, et al. . Host-mediated regulation of superinfection in malaria. Nat Med 2011;17(6):732–7. doi: 10.1038/nm.2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gwamaka M, Kurtis JD, Sorensen BE, et al. . Iron deficiency protects against severe Plasmodium falciparum malaria and death in young children. Clin Infect Dis 2012;54(8):1137–44. doi: 10.1093/cid/cis010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Harrington-Kandt R, Stylianou E, Eddowes LA, et al. . Hepcidin deficiency and iron deficiency do not alter tuberculosis susceptibility in a murine M.tb infection model. Pantopoulos K, ed. PLoS One. 2018;13(1):e0191038. doi: 10.1371/journal.pone.0191038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lim D, Kim KS, Jeong JH, et al. . The hepcidin-ferroportin axis controls the iron content of Salmonella-containing vacuoles in macrophages. Nat Commun 2018;9(1):1–12. doi: 10.1038/s41467-018-04446-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Drakesmith H, Prentice A. Viral infection and iron metabolism . Nat Rev Microbiol 2008;6(7):541–52. doi: 10.1038/nrmicro1930 [DOI] [PubMed] [Google Scholar]
- 58. Shah A, Frost JN, Aaron L, et al. . Systemic hypoferremia and severity of hypoxemic respiratory failure in COVID-19. Crit Care 2020;24(1):320. doi: 10.1186/s13054-020-03051-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sonnweber T, Boehm A, Sahanic S, et al. . Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: a prospective observational cohort study. Respir Res 2020;21(1):276. doi: 10.1186/s12931-020-01546-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nai A, Lorè NI, Pagani A, et al. . Hepcidin levels predict <scp>Covid-19</scp> severity and mortality in a cohort of hospitalized Italian patients. Am J Hematol 2021;96(1):E32–5. doi: 10.1002/ajh.26027 [DOI] [PubMed] [Google Scholar]
- 61. Bolondi G, Russo E, Gamberini E, et al. . Iron metabolism and lymphocyte characterisation during Covid-19 infection in ICU patients: An observational cohort study. World J Emerg Surg 2020;15(1). doi: 10.1186/s13017-020-00323-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Serum iron level as a potential predictor of coronavirus disease 2019 severity and mortality: A retrospective study. Open Forum Infect Dis 2020;7(7). doi: 10.1093/ofid/ofaa250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hippchen T, Altamura S, Muckenthaler MU, Merle U. Hypoferremia is associated with increased hospitalization and oxygen demand in COVID-19 patients. HemaSphere 2020;4(6):e492. doi: 10.1097/HS9.0000000000000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021;184(4):861. doi: 10.1016/j.cell.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. . Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 2020;183(4):996–1012.e19. doi: 10.1016/j.cell.2020.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lucas C, Klein J, Sundaram M, et al. . Kinetics of antibody responses dictate COVID-19 outcome. medRxiv. December 2020:2020.12.18.20248331. doi: 10.1101/2020.12.18.20248331 [DOI] [Google Scholar]
- 67. Chen LYC, Hoiland RL, Stukas S, Wellington CL, Sekhon MS. Assessing the importance of interleukin-6 in COVID-19. Lancet Respir Med 2021;9(2):e13. doi: 10.1016/S2213-2600(20)30600-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gordon AC, Mouncey PR, Al-Beidh F, et al. . Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19-Preliminary report The REMAP-CAP Investigators Author and Group Information Writing Committee: Corresponding Author. medRxiv January 2021:2021.01.07.21249390. doi: 10.1101/2021.01.07.21249390 [DOI] [Google Scholar]
- 70. Landray M. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. medRxiv. February 2021:2021.02.11.21249258. doi: 10.1101/2021.02.11.21249258 [DOI] [Google Scholar]
- 71. Bagchi K, Mohanram M, Reddy V. Humoral immune response in children with iron-deficiency anaemia. Br Med J 1980;280(6226):1249. doi: 10.1136/bmj.280.6226.1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Macdougall LG, Jacobs MR, Ch MBB. The Immune Response in Iron-Deficient Children Isohaemagglutinin Titres and Antibody Response to Immunization. South African Med J 1978;53(11):405–7. [PubMed] [Google Scholar]
- 73. Savy M, Edmond K, Fine PEM, et al. . Landscape analysis of interactions between nutrition and vaccine responses in children. J Nutr 2009;139(11):2154S–218S. doi: 10.3945/jn.109.105312 [DOI] [PubMed] [Google Scholar]
- 74. Brussow H, Sidoti J, Dirren H, Freire WB. Effect of malnutrition in Ecuadorian children on titers of serum antibodies to various microbial antigens. Clin Diagn Lab Immunol 1995;2(1):62–8. doi: 10.1128/cdli.2.1.62-68.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hill DL, Carr EJ, Rutishauser T, et al. . Immune system development varies according to age, location, and anemia in African children. Sci Transl Med 2020;12(529). doi: 10.1126/scitranslmed.aaw9522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stoffel NU, Uyoga MA, Mutuku FM, et al. . Iron deficiency anemia at time of vaccination predicts decreased vaccine response and iron supplementation at time of vaccination increases humoral vaccine response: A birth cohort study and a randomized trial follow-up study in Kenyan infants. Front Immunol 2020;11:1313. doi: 10.3389/fimmu.2020.01313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Balarajan Y, Ramakrishnan U, Özaltin E, Shankar AH, Subramanian S V. Anaemia in low-income and middle-income countries. Lancet 2011;378(9809):2123–35. doi: 10.1016/S0140-6736(10)62304-5 [DOI] [PubMed] [Google Scholar]
- 78. Fülöp T, Wagner JR, Khalil A, Weber J, Trottier L, Payette H. Relationship between the response to influenza vaccination and the nutritional status in institutionalized elderly subjects. Journals Gerontol - Ser A Biol Sci Med Sci 1999;54(2). doi: 10.1093/gerona/54.2.M59 [DOI] [PubMed] [Google Scholar]
- 79. Prendergast AJ. Malnutrition and vaccination in developing countries. Philos Trans R Soc B Biol Sci 2015;370(1671). doi: 10.1098/rstb.2014.0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mupere E, Karamagi C, Zirembuzi G, et al. . Measles vaccination effectiveness among children under 5 years of age in Kampala, Uganda. Vaccine 2006;24(19):4111–5. doi: 10.1016/j.vaccine.2006.02.038 [DOI] [PubMed] [Google Scholar]
- 81. Passanisi S, Dipasquale V, Romano C. Vaccinations and immune response in celiac disease. Vaccines 2020;8(2):1–10. doi: 10.3390/vaccines8020278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Young KM, Gray CM, Bekker LG. Is obesity a risk factor for vaccine non-responsiveness? PLoS One 2013;8(12):82779. doi: 10.1371/journal.pone.0082779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Martin P, Friedman LS. Chronic Viral Hepatitis and the Management of Chronic Renal Failure Hepatitis B 1995. doi: 10.1038/ki.1995.177 [DOI] [PubMed] [Google Scholar]
- 84. Watcharananan SP, Thakkinstian A, Srichunrasmee C, Chuntratita W, Sumethkul V. Comparison of the immunogenicity of a monovalent influenza A/H1N1 2009 vaccine between healthy individuals, patients with chronic renal failure, and immunocompromised populations. Transplant Proc 2014;46(2):328–31. doi: 10.1016/j.transproceed.2013.11.063 [DOI] [PubMed] [Google Scholar]
- 85. Cunningham AL, McIntyre P, Subbarao K, Booy R, Levin MJ. Vaccines for older adults. BMJ 2021;372:n188. doi: 10.1136/bmj.n188 [DOI] [PubMed] [Google Scholar]
- 86. Muckenthaler MU, Rivella S, Hentze MW, Galy B. A Red Carpet for Iron Metabolism. Cell 2017;168(3):344–61. doi: 10.1016/j.cell.2016.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li H, Liu Y, Shang L, et al. . Iron regulatory protein 2 modulates the switch from aerobic glycolysis to oxidative phosphorylation in mouse embryonic fibroblasts. Proc Natl Acad Sci USA 2019;116(20):9871–6. doi: 10.1073/pnas.1820051116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Weber RA, Yen FS, Nicholson SP V, Abu-Remaileh M, Molina H. Maintaining Iron Homeostasis Is the Key Role of Lysosomal Acidity for Cell Proliferation. Mol Cell 2020;77:645–655.e7. doi: 10.1016/j.molcel.2020.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Müller S, Sindikubwabo F, Cañeque T, et al. . CD44 regulates epigenetic plasticity by mediating iron endocytosis. Nat Chem 2020;12(10):929–38. doi: 10.1038/s41557-020-0513-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Oexle H, Gnaiger E, Weiss G. Iron-dependent changes in cellular energy metabolism: Influence on citric acid cycle and oxidative phosphorylation . Biochim Biophys Acta - Bioenerg 1999;1413(3):99–107. doi: 10.1016/S0005-2728(99)00088-2 [DOI] [PubMed] [Google Scholar]
- 91. Pereira M, Chen T Di, Buang N, et al. . Acute Iron Deprivation Reprograms Human Macrophage Metabolism and Reduces Inflammation In Vivo. Cell Rep 2019;28(2):498–511.e5. doi: 10.1016/j.celrep.2019.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Crooks DR, Maio N, Lane AN, et al. . Acute loss of iron–sulfur clusters results in metabolic reprogramming and generation of lipid droplets in mammalian cells. J Biol Chem 2018;293(21):8297–311. doi: 10.1074/jbc.RA118.001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cronin SJF, Seehus C, Weidinger A, et al. . The metabolite BH4 controls T cell proliferation in autoimmunity and cancer. Nature 2018;563(7732):564–8. doi: 10.1038/s41586-018-0701-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet 2006;7(9):715–27. doi: 10.1038/nrg1945 [DOI] [PubMed] [Google Scholar]
- 95. Yarosz EL, Ye C, Kumar A, et al. . Cutting Edge: Activation-Induced Iron Flux Controls CD4 T Cell Proliferation by Promoting Proper IL-2R Signaling and Mitochondrial Function . J Immunol 2020;204(7):1708–13. doi: 10.4049/jimmunol.1901399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 2012;12(11):749–61. doi: 10.1038/nri3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ganz T, Nemeth E. Hepcidin and Disorders of Iron Metabolism. Annu Rev Med 2011;62(1):347–60. doi: 10.1146/annurev-med-050109-142444 [DOI] [PubMed] [Google Scholar]
- 98. Costa M, Cruz E, Oliveira S, et al. . Lymphocyte Gene Expression Signatures from Patients and Mouse Models of Hereditary Hemochromatosis Reveal a Function of HFE as a Negative Regulator of CD8+ T-Lymphocyte Activation and Differentiation In Vivo. Pantopoulos K, ed. PLoS One 2015;10(4):e0124246. doi: 10.1371/journal.pone.0124246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Porto G, De Sousa M. Iron overload and immunity. World J Gastroenterol 2007;13(35):4707–15. doi: 10.3748/wjg.v13.i35.4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Reuben A, Phénix M, Santos MM, Lapointe R. The WT hemochromatosis protein HFE inhibits CD8+ T-lymphocyte activation. Eur J Immunol 2014;44(6):1604–14. doi: 10.1002/eji.201343955 [DOI] [PubMed] [Google Scholar]
- 101. Sazawal S, Black RE, Ramsan M, et al. . Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 2006;367(9505):133–43. doi: 10.1016/S0140-6736(06)67962-2 [DOI] [PubMed] [Google Scholar]
- 102. Jaeggi T, Kortman GAM, Moretti D, et al. . Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015;64(5):731–42. doi: 10.1136/gutjnl-2014-307720 [DOI] [PubMed] [Google Scholar]
- 103. Soofi S, Cousens S, Iqbal SP, et al. . Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: A cluster-randomised trial. Lancet. 2013;382(9886):29–40. doi: 10.1016/S0140-6736(13)60437-7 [DOI] [PubMed] [Google Scholar]
- 104. Gera T, Sachdev HPS. Effect of iron supplementation on incidence of infectious illness in children: Systematic review. Br Med J 2002;325(7373):1142–4. doi: 10.1136/bmj.325.7373.1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Paganini D, Uyoga MA, Zimmermann MB. Iron fortification of foods for infants and children in low-income countries: Effects on the gut microbiome, gut inflammation, and diarrhea. Nutrients 2016;8(8). doi: 10.3390/nu8080494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tolkien Z, Stecher L, Mander AP, Pereira DIA, Powell JJ. Ferrous Sulfate Supplementation Causes Significant Gastrointestinal Side-Effects in Adults: A Systematic Review and Meta-Analysis. Strnad P, ed. PLoS One 2015;10(2):e0117383. doi: 10.1371/journal.pone.0117383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Pasricha SR, Atkinson SH, Armitage AE, et al. . Expression of the iron hormone hepcidin distinguishes different types of Anemia in African children. Sci Transl Med 2014;6(235):235re3-235re3. doi: 10.1126/scitranslmed.3008249 [DOI] [PubMed] [Google Scholar]
- 108. Prentice AM, Doherty CP, Abrams SA, et al. . Hepcidin is the major predictor of erythrocyte iron incorporation in anemic African children. Blood 2012;119(8):1922–8. doi: 10.1182/blood-2011-11-391219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Moretti D, Goede JS, Zeder C, et al. . Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015;126(17):1981–9. doi: 10.1182/blood-2015-05-642223 [DOI] [PubMed] [Google Scholar]
- 110. Uyoga MA, Mikulic N, Paganini D, et al. . The effect of iron dosing schedules on plasma hepcidin and iron absorption in Kenyan infants. Am J Clin Nutr 2020;112(4):1132–41. doi: 10.1093/ajcn/nqaa174 [DOI] [PubMed] [Google Scholar]
- 111. Stoffel NU, von Siebenthal HK, Moretti D, Zimmermann MB. Oral iron supplementation in iron-deficient women: How much and how often? Mol Aspects Med 2020;75:100865. doi: 10.1016/j.mam.2020.100865 [DOI] [PubMed] [Google Scholar]
- 112. Stoffel NU, Zeder C, Brittenham GM, Moretti D, Zimmermann MB. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica 2020;105(5):1232–9. doi: 10.3324/HAEMATOL.2019.220830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Stoffel NU, Cercamondi CI, Brittenham G, et al. . Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol 2017;4(11):e524–33. doi: 10.1016/S2352-3026(17)30182-5 [DOI] [PubMed] [Google Scholar]
- 114. Pereira DIA, Bruggraber SFA, Faria N, et al. . Nanoparticulate iron(III) oxo-hydroxide delivers safe iron that is well absorbed and utilised in humans. Nanomedicine Nanotechnology, Biol Med 2014;10(8):1877–86. doi: 10.1016/j.nano.2014.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pereira DIA, Mohammed NI, Ofordile O, et al. . A novel nano-iron supplement to safely combat iron deficiency and anaemia in young children: The IHAT-GUT double-blind, randomised, placebo-controlled trial protocol. Gates Open Res 2018;2. doi: 10.12688/gatesopenres.12866.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Vanobberghen F, Lweno O, Kuemmerle A, et al. . Efficacy and safety of intravenous ferric carboxymaltose compared with oral iron for the treatment of iron deficiency anaemia in women after childbirth in Tanzania: a parallel-group, open-label, randomised controlled phase 3 trial. Lancet Glob Heal 2021;9(2):e189–8. doi: 10.1016/S2214-109X(20)30448-4 [DOI] [PubMed] [Google Scholar]
- 117. Katsarou A, Pantopoulos K. Hepcidin therapeutics. Pharmaceuticals 2018;11(4):127. doi: 10.3390/PH11040127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fung E, Nemeth E. Manipulation of the hepcidin pathway for therapeutic purposes. Haematologica 2013;98(11):1667–76. doi: 10.3324/haematol.2013.084624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Renders L, Budde K, Rosenberger C, et al. . First-in-human Phase I studies of PRS-080#22, a hepcidin antagonist, in healthy volunteers and patients with chronic kidney disease undergoing hemodialysis. Eller K, ed. PLoS One 2019;14(3):e0212023. doi: 10.1371/journal.pone.0212023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Vadhan-Raj S, Abonour R, Goldman JW, et al. . A first-in-human phase 1 study of a hepcidin monoclonal antibody, LY2787106, in cancer-associated anemia. J Hematol Oncol 2017;10(1):73. doi: 10.1186/s13045-017-0427-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kovac S, Böser P, Cui Y, et al. . Anti-hemojuvelin antibody corrects anemia caused by inappropriately high hepcidin levels. Haematologica 2016;101(5):e173–6. doi: 10.3324/haematol.2015.140772 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.