Abstract
By 2030, early-onset colorectal cancer (EOCRC) is expected to become the leading cancer-related cause of death for people age 20 to 49. To improve understanding of this phenomenon, we analyzed the geographic determinants of EOCRC in Utah by examining county-level incidence and mortality. We linked data from the Utah Population Database to the Utah Cancer Registry to identify residents (age 18–49) diagnosed with EOCRC between 2000 and 2020, and we used spatial empirical Bayes smoothing to determine county-level hotspots. We identified 1,867 EOCRC diagnoses (52.7% in male patients, 69.2% in non-Hispanic White patients). Ten counties (34%) were classified as hotspots, with high EOCRC incidence or mortality. Hotspot status was unrelated to incidence rates, but non-Hispanic ethnic-minority men (incidence rate ratio, 1.49; 95% CI, 1.15–1.91), Hispanic White men and women (incidence rate ratio, 2.24; 95% CI, 2.00–2.51), and Hispanic ethnic-minority men and women (incidence rate ratio, 4.59; 95% CI, 3.50–5.91) were more likely to be diagnosed with EOCRC. After adjustment for income and obesity, adults living in hotspots had a 31% higher hazard for death (HR, 1.31; 95% CI, 1.02–1.69). Survival was poorest for adults with a late-stage diagnosis living in hotspots (chi square (1) = 4.0; p = .045). Adults who were married or who had a life partner had a lower hazard for death than single adults (HR, 0.73; 95% CI, 0.58–0.92). The risk for EOCRC is elevated in 34% of Utah counties, warranting future research and interventions aimed at increasing screening and survival in the population age 18 to 49.
Early-onset colorectal cancer (EOCRC)—that is, colorectal cancer (CRC) diagnosed in individuals younger than age 50—is the second most common cancer and third leading cause of cancer mortality in people younger than age 50 in the United States1; by 2030, it is projected to become the leading cause of cancer-related death for those age 20 to 49.2,3 Potentially masking the exponential surge in EOCRC incidence over the past 2 decades, CRC incidence in the over-55 age group has declined within the past decade because of an increase in screening rates.4 Moreover, although overall CRC incidence in the United States increased by 1.27% each year between 2001 and 2007, and 3.0% annually between 2012 and 2017, the greatest average annual percentage change in incidence occurred among those age 20 to 24.5 Because EOCRC is more likely than later-onset CRC to lead to poor outcomes, the U.S. Preventive Services Task Force now recommends the initiation of CRC screening at age 45 instead of age 50.6
Experts remain puzzled as to the cause of the alarming rise in EOCRC, but some potential factors include the Western-style diet, obesity, physical inactivity, socioeconomic status, and antibiotic use, especially during the early prenatal to adolescent period.7,8 A family history of CRC and a genetic predisposition for the disease are known risk factors.9 A Utah-focused study by Ochs-Balcom et al10 found that, for patients of any age, having a first-, second-, or third-degree relative with EOCRC conferred a 2.64-fold, 1.96-fold, and 1.3-fold higher risk for developing CRC, respectively. When considering disease-specific risk factors for EOCRC, however, it is important to also examine the intersection of early-life exposures and geographic factors that may contribute to increased EOCRC incidence.11–13
Disparities in EOCRC incidence in the United States are exacerbated when geography is considered.14 For example, disparities are apparent among residents of rural versus urban areas in both CRC risk factors and many social determinants of health (e.g., smoking, obesity, and health care access and utilization). Low rates of CRC screening have been associated with low socioeconomic status, low household income, lack of health insurance, and smoking, among other factors, likely contributing to the increasing disparity in EOCRC incidence in these populations.14–16 Siegel et al17 identified distinct geographic areas in the United States where CRC mortality is highest among those age 50 and older; these regions, as well as additional areas identified in other studies,5,18 have been identified as EOCRC hotspots—that is, counties with high EOCRC mortality rates.19 Specifically, African American men younger than age 50 with a diagnosis of advanced-stage CRC have a significantly higher EOCRC mortality burden and worse survival in hotspots, compared with White men in nonhotspot counties.19 A complementary study, focused on EOCRC in women, found that a higher proportion of the population is African American in EOCRC hotspot counties than in nonhotspot counties.18 These two novel studies provide insight into the contributions of socioeconomic status and community-level factors to the EOCRC epidemic, yet a more granular investigation of factors not captured in these studies is warranted.
Factors contributing to EOCRC disparities include biology/genetics, diet/environment, preventive lifestyle behaviors, rural residence, environmental contamination, and access to high-quality health services, as well as social and political factors.13,14,20–22 To better understand the etiology of EOCRC, this study sought to identify and characterize EOCRC hotspot counties in Utah by examining the variance in EOCRC incidence and survival that could be explained by personal- and county-level factors. We hypothesized that patients with EOCRC residing in hotspot counties in Utah would have significantly worse EOCRC survival compared with patients in coldspot counties. We also hypothesized that rurality and county-level factors would contribute to explaining EOCRC incidence and survival.
MATERIALS AND METHODS
Study Design and Data Sources
We used an ecologic design to examine EOCRC incidence and mortality for the period 2000 to 2020 in women and men age 18 to 49 residing in all 29 Utah counties. Cases and deaths specific for EOCRC were obtained from the Utah Cancer Registry and linked with data from the Utah Population Database. Death-certificate data were collected as available, as well as demographic information, residential histories, clinical characteristics, and survival information. County-level factors were obtained from the U.S. Census Bureau’s 2019 American Community Survey, based on the preceding 5-year period (2015–2019),23 and from the 2019 County Health Rankings.24 This study was approved by the University of Utah Institutional Review Board and by the Resource for Genetic and Epidemiologic Research, the regulatory body overseeing usage of Utah Population Database data.
Study Population
We used the Utah Cancer Registry and SEER site codes (C18.0, C18.2–C18.9, C19.9, C20.9, C26.0) to identify men and women age 18 to 49 who received a primary diagnosis of CRC between the years 2000 and 2020 while living in Utah. A total of 1,867 patients with EOCRC were identified, of whom 52.7% were male, 69.2% non-Hispanic (NH) White, 21.7% Hispanic White, 3.6% NH multiracial, 3.2% Hispanic ethnic-minority (non-White), 1.0% NH Asian American, 0.5% NH Black, 0.5% NH Native Hawaiian/other Pacific Islander, and 0.2% NH American Indian-Alaska Native; 0.1% had unknown race and ethnicity. Eleven patients had residences in more than one county and thus could not be situated within a single county. The remaining 1,856 patients with EOCRC were included in the geospatial analyses.
Incidence and Mortality Rates
County-level incidence and mortality were determined by calculating crude rates, or the total number of EOCRC cases and deaths per county, between 2000 and 2020, divided by the total population at risk (i.e., the total population age 18–49 in each Utah county between 2000 and 2020, according to National Center for Health Statistics data)25 and multiplied by 100,000. For mortality, we identified patients with EOCRC who had a CRC-specific primary cause of death according to the International Classification of Diseases, Tenth Revision (codes C18.0, C18.2–C18.9, C19.9, C20.9, C26.0). Age-adjusted rates were calculated for Utah overall, and within hotspot and coldspot counties, using the 2000 U.S. standard population. Age-adjusted rates could not be reliably estimated for specific counties because certain age categories had a small number of cases and deaths.
County-Level Factors
From the American Community Survey, we extracted for each county the percentages of the following: individuals who completed college, households with an income of less than $25,000, population by race and ethnicity, unemployed individuals, and individuals without health insurance (i.e., uninsured). From the County Health Rankings, we extracted for each county the percentage of individuals with access to exercise opportunities, obesity, and a current smoking status or physical inactivity; the number of primary care physicians per 100,000 persons; the number of violent crimes per 100,000 persons; and the food environment index (values range from 0 = worst to 10 = best). We also calculated the percentage of ZIP codes in each county designated as nonurban (rural) and urban using the 2010 Rural-Urban Commuting Areas ZIP code-based classifications. We aggregated the secondary Rural-Urban Commuting Areas codes into nonurban and urban.26
Geospatial Analysis: Hotspots for Incidence or Mortality
We conducted separate hotspot analyses for EOCRC incidence and mortality. We initially aimed to employ three geospatial methods to determine hotspots across Utah’s 29 counties.27 However, upon inspecting the data, we found that over 40% of EOCRC diagnoses and CRC-specific deaths were clustered in a single county, which limited our ability to test local and global indicators of spatial association. For this reason, we focused our analysis of hotspots using the spatial empirical Bayes smoothing method in GeoDa version 1.18.0,28 while using ArcGIS Pro for mapping (Esri, Redlands, CA). The spatial empirical Bayes smoothing method calculates smoothed rates using the overall population size in each county as weights; thus, the rates of counties with smaller populations are adjusted more than those of counties with larger populations. We categorized the smoothed rates into quartiles and identified counties in the fourth quartile as hotspot counties for EOCRC incidence, mortality, or both. Counties with zero EOCRC cases or deaths were constrained from being classified as a hotspot for incidence or mortality.
Data Analysis
We tested bivariate differences between hotspot and coldspot counties for personal- and county-level factors using the chi square test for categorical factors and the independent t test for continuous factors. For continuous factors with evidence of non-normal distribution, we used the nonparametric Wilcoxon rank sum test; we also used this test for all county-level factors. A p value of less than .05 was used to infer significance. Survival curves were plotted using the Kaplan-Meier survival estimator. We calculated survival times by subtracting the date of death, or last follow-up visit, from the diagnosis date, transforming the result into months. A threshold of 240 months was applied to estimate the restricted mean survival.
We also tested multivariable models. We used Poisson regression to estimate incidence rate ratios and 95% CIs. We used the county-specific total population between 2000 and 2020, stratified by age groups and race and ethnicity, as the offset or exposure variable in the Poisson model and included county-level factors that reached significance in our bivariate analyses. We estimated four Poisson models and used a final model separately for men and women. Given the smaller number of events across racial and ethnic groups, we did not stratify results by race or ethnicity. We used Cox proportional hazards to estimate hazard ratios and their 95% CIs. We estimated four hazards models and used a final model separately for men and women. For our Cox proportional hazards models, we calculated a generalized R2 following Allison’s adaptation29 of the Cox and Snell method (Rogers et al19). Data analyses were conducted in R Studio, version 1.3.1093 (R version 4.0.5).
RESULTS
Hotspot Characteristics
Between 2000 and 2020, the crude EOCRC incidence rate was 7.03 and the crude EOCRC-specific mortality rate was 1.93 per 100,000; the age-adjusted incidence and mortality rates across all counties were 8.38 and 2.31 per 100,000, respectively. Ten of Utah’s 29 counties (34.48%) were classified as hotspots for EOCRC incidence, mortality, or both (Fig. 1). A list of all counties, their hotspot classifications, and their associated EOCRC rates is shown in Table 1. Average crude rates and empirical smoothed rates by hotspot classification are shown in Table 2. The age-adjusted incidence rates for hotspot and coldspot counties were 8.48 and 8.36 per 100,000, respectively; the age-adjusted mortality rates for hotspot and coldspot counties were 2.60 and 2.25 per 100,000, respectively.
FIGURE 1.
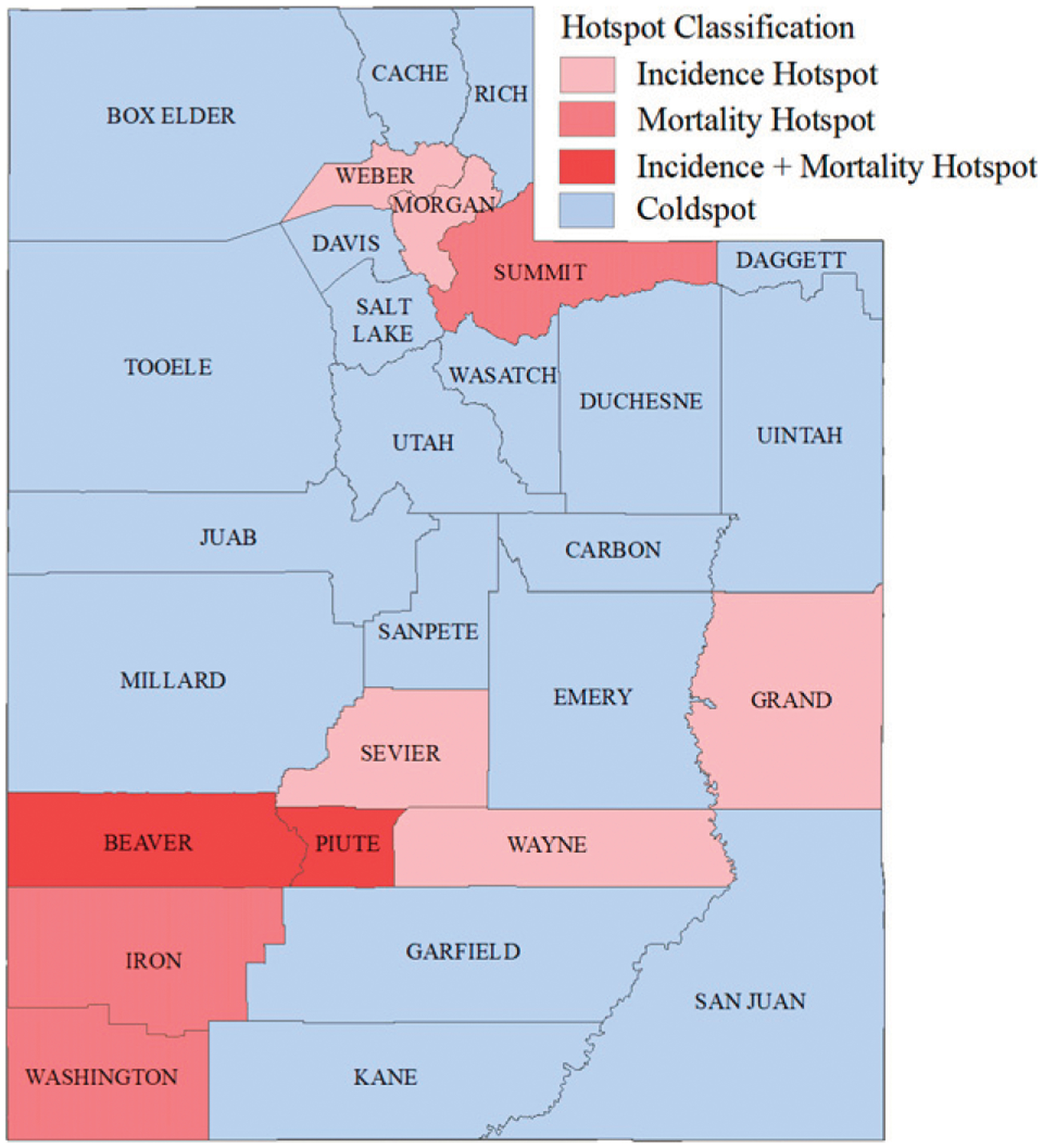
Early-Onset Colorectal Cancer Hotspots for Incidence, Mortality, or Both: Incidence and Mortality Determined Using Spatial Empirical Bayes Smoothed Rates Quartiles for Utah Residents (Male and Female) Age 18 to 49
TABLE 1.
EOCRC Incidence and Mortality in Coldspot and Hotspot Counties and Associated Rates in Utah (2000–2020) Among Adults Age 18 to 49
| County, Utah | Hotspot Classificationa | EOCRC Incidence Rates per 100,000 | EOCRC Mortality Rates per 100,000 | ||
|---|---|---|---|---|---|
| Crude | Spatial Empirical Bayes Smoothedb | Crude | Spatial Empirical Bayes Smoothedb | ||
| Beaver | Hot | 5.99 | 7.62 | 3.99 | 2.16 |
| Box Elder | Cold | 7.94 | 7.34 | 2.65 | 1.91 |
| Cache | Cold | 6.19 | 6.98 | 1.46 | 2.01 |
| Carbon | Cold | 6.85 | 6.31 | 1.14 | 1.49 |
| Daggett | Cold | 0.00 | 6.66 | 0.00 | 1.67 |
| Davis | Cold | 7.40 | 7.40 | 1.74 | 2.08 |
| Duchesne | Cold | 9.33 | 6.63 | 3.33 | 1.53 |
| Emery | Cold | 1.21 | 3.77 | 0.00 | 1.53 |
| Garfield | Cold | 5.49 | 6.26 | 0.00 | 2.09c |
| Grand | Hot | 14.10 | 9.59 | 2.56 | 1.45 |
| Iron | Hot | 7.13 | 6.52 | 2.07 | 2.42 |
| Juab | Cold | 7.28 | 6.19 | 2.43 | 1.49 |
| Kane | Cold | 2.04 | 6.37 | 0.00 | 2.38c |
| Millard | Cold | 8.52 | 6.67 | 1.06 | 1.87 |
| Morgan | Hot | 2.59 | 7.41 | 1.30 | 2.07 |
| Piute | Hot | 10.88 | 8.28 | 10.88 | 2.52 |
| Rich | Cold | 18.33 | 7.38 | 6.11 | 1.93 |
| Salt Lake | Cold | 7.34 | 7.27 | 2.14 | 2.05 |
| San Juan | Cold | 4.17 | 4.57 | 2.50 | 1.30 |
| Sanpete | Cold | 3.54 | 5.89 | 1.18 | 1.46 |
| Sevier | Hot | 9.23 | 7.45 | 2.46 | 1.94 |
| Summit | Hot | 7.11 | 7.38 | 1.18 | 2.14 |
| Tooele | Cold | 6.84 | 7.11 | 1.71 | 1.92 |
| Uintah | Cold | 4.91 | 5.38 | 1.40 | 1.67 |
| Utah | Cold | 6.19 | 6.41 | 1.47 | 1.67 |
| Wasatch | Cold | 10.66 | 7.33 | 2.78 | 1.99 |
| Washington | Hot | 6.54 | 6.56 | 2.67 | 2.42 |
| Wayne | Hot | 10.28 | 7.78 | 0.00 | 1.97 |
| Weber | Hot | 7.89 | 7.49 | 2.28 | 1.91 |
Abbreviation: EOCRC, early-onset colorectal cancer.
Hotspot classification was determined by counties with spatial Empirical Bayes smoothed rates that fell in the top quartile for either incidence or mortality.
Empirical Bayes smoothed rates were weighted by county population of women and men age 18 to 49, between 2000 and 2020.
County had zero events and was constrained to coldspot regardless of the spatial Empirical Bayes smoothed rate.
TABLE 2.
EOCRC Incidence and Mortality in Utah (2000–2020) by Hotspot Classification Among Adults Age 18 to 49
| EOCRC Incidence Rates per 100,000 | EOCRC Mortality Rates per 100,000 | |||
|---|---|---|---|---|
| Crude | Spatial Empirical Bayes Smoothed | Crude | Spatial Empirical Bayes Smoothed | |
| Hotspot Classificationa | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) |
| Coldspot (19 Counties) | 6.54 (0.91) | 6.42 (0.22) | 1.74 (0.33) | 1.79 (0.06) |
| Hotspot (10 Counties) | 8.17 (0.99) | 7.61 (0.28) | 2.94 (0.94) | 2.10 (0.10) |
Abbreviation: EOCRC, early-onset colorectal cancer.
Hotspot classification was determined by counties with spatial Empirical Bayes smoothed rates that fell in the top quartile for either incidence or mortality. Empirical Bayes smoothed rates were weighted by county population of women and men age 18 to 49, between 2000 and 2020.
We found no significant differences in the frequencies of incidence and mortality between hotspot and coldspot counties (Table 3). Mean survival was 7 months lower in hotspot- compared with coldspot counties (167 vs. 174 months), but this difference did not meet the significance threshold (p < .05; Table 3). Of the personal-level factors, more divorced, separated, or widowed adults (hotspot, 15.45% vs. coldspot, 11.66%) and fewer single or never-married adults (hotspot, 12.12% vs. coldspot, 17.43%) lived in hotspot counties. Of the county-level factors (Table 4), hotspot counties had a significantly lower percentage of adults with obesity than did coldspot counties (24.20% vs. 27.84%).
TABLE 3.
Summary of Personal-Level Factors by Hotspot Classification for EOCRC in Utah (2000–2020) Among Adults Age 18 to 49
| EOCRC Hotspot Countiesa | ||||
|---|---|---|---|---|
| Patient Characteristic | Number (%)b or Mean (SE) | Hotspot Number (%)b or Mean (SE) | Coldspot Number (%)b or Mean (SE) | pc |
| Total Cases | 1,856 (100) | 330 (17.78) | 1,526 (82.22) | .890 |
| Total Deaths | 511 (100) | 101 (19.77) | 410 (80.23) | .945 |
| Mean Survival (Months) d | 172.27 (2.51) | 166.51 (5.98) | 173.59 (2.76) | .240 |
| Age in Years, Mean | 41.11 (0.16) | 41.51 (0.38) | 41.02 (0.17) | .130 |
| Age at Diagnosis (%) | .118 | |||
| 18–29 Years | 136 (7.33) | 25 (7.58) | 111 (7.27) | |
| 30–39 Years | 489 (26.35) | 72 (21.82) | 417 (27.33) | |
| 40–49 Years | 1,231 (66.33) | 233 (70.61) | 998 (65.40) | |
| Race and Ethnicity (%) | .101 | |||
| NH American Indian-Alaska Native | * | * | * | |
| NH Asian | 19 (1.02) | * | 19 (1.25) | |
| NH Black | * | * | * | |
| NH Islander | * | * | * | |
| NH Multiple | 64 (3.45) | 11 (3.33) | 53 (3.48) | |
| NH White | 1,286 (69.33) | 247 (74.85) | 1,039 (68.13) | |
| Hispanic White | 404 (21.78) | 57 (17.27) | 347 (22.75) | |
| Hispanic Ethnic Minority | 59 (3.18) | 13 (3.94) | 46 (3.02) | |
| Sex (%) | .680 | |||
| Female | 878 (47.31) | 160 (48.48) | 718 (47.05) | |
| Male | 978 (52.69) | 170 (51.52) | 808 (52.95) | |
| BMI (%) | .912 | |||
| Underweight | 32 (1.92) | * | 25 (1.83 | |
| Normal | 608 (36.41) | 113 (37.29) | 495 (36.21) | |
| Overweight | 541 (32.40) | 97 (32.01) | 444 (32.48) | |
| Obese | 489 (29.28) | 86 (28.38) | 403 (29.48) | |
| Marital Status (%) | .048 | |||
| Single or Never Married | 306 (16.49) | 40 (12.12) | 266 (17.43) | |
| Married or Life Partnered | 1,279 (68.91) | 231 (70.00) | 1,048 (68.68) | |
| Divorced, Separated, or Widowed | 229 (12.34) | 51 (15.45) | 178 (11.66) | |
| Unknown | 42 (2.26) | * | 34 (2.23) | |
| Tumor Side (%) e | .360 | |||
| Right | 415 (22.36) | 67 (20.30) | 348 (22.80) | |
| Left | 1,441 (77.64) | 263 (79.70) | 1,178 (77.20) | |
| Stage (%) | .325 | |||
| Local | 679 (36.58) | 121 (36.67) | 558 (36.57) | |
| Regional | 603 (32.50) | 114 (34.55) | 489 (32.04) | |
| Distant/Metastatic | 424 (22.84) | 64 (19.39) | 360 (23.59) | |
| Unknown | 150 (8.08) | 31 (9.39) | 119 (7.80) | |
| Year of Diagnosis (%) | .422 | |||
| 2000–2004 | 352 (18.97) | 68 (20.61) | 284 (18.61) | |
| 2005–2009 | 408 (21.98) | 77 (23.33) | 331 (21.69) | |
| 2010–2014 | 526 (29.09) | 96 (29.09) | 430 (28.18) | |
| 2015–2019 | 570 (30.71) | 89 (26.97) | 481 (31.52) | |
Abbreviations: EOCRC, early-onset colorectal cancer; NH, non-Hispanic; BMI, body mass index.
Hotspot classification was determined by counties with spatial Empirical Bayes smoothed rates that fell in the top quartile for either incidence or mortality.
In accordance with Utah Department of Health Data Suppression Guidelines, an asterisk (*) denotes counts of < 11 (see https://uofuhealth.utah.edu/utah-cancer-registry/research/).
The p value was derived from the chi square test, independent t test, Wilcoxon rank sum test, or log-rank test, as appropriate.
Calculated using the Kaplan-Meier method.
Right was defined using International Classification of Diseases for Oncology, Third Edition (ICD-0–3) codes 180, 181, 182, 183, and 184, and left was defined using codes 185, 186, 187, 188, 189, 199, 209, and 260. In accordance with Utah Department of Health Data Suppression Guidelines, an asterisk (*) denotes counts of < 11 (see https://uofuhealth.utah.edu/utah-cancer-registry/research/).
TABLE 4.
Summary of County-Level Factors by Hotspot Classification for EOCRC in Utah (2000–2020) Among Adults Age 18 to 49
| County Characteristic | EOCRC Hotspot Countiesa | |||
|---|---|---|---|---|
| Hotspot Mean (SE) | Coldspot Mean (SE) | Pb | Spearman Correlation (ρ)c | |
| Race and Ethnicity | ||||
| % NH American Indian-Alaska Native | 1.07 (0.35) | 3.78 (2.44) | .513 | −0.13 |
| % NH Asian | 0.68 (0.20) | 0.89 (0.22) | .818 | −0.05 |
| % NH Black | 0.50 (0.10) | 0.43 (0.11) | .313 | 0.20 |
| % NH Islander | 0.35 (0.16) | 0.32 (0.08) | .765 | −0.06 |
| % NH Multiple | 1.39 (0.24) | 1.46 (0.20) | .731 | −0.07 |
| % NH White | 87.14 (1.99) | 83.62 (2.53) | .429 | 0.16 |
| % NH Other | 0.06 (0.03) | 0.08 (0.02) | .543 | −0.12 |
| % Hispanic/Latinx | 8.81 (1.52) | 9.42 (0.89) | .668 | −0.09 |
| % Household Income < $25,000 | 18.10 (2.11) | 15.75 (1.36) | .247 | 0.23 |
| % Completed College | 25.78 (3.13) | 21.71 (1.82) | .211 | 0.24 |
| % Unemployed | 3.75 (0.44) | 4.13 (0.51) | .875 | −0.03 |
| % Uninsured | 12.23 (1.39) | 10.56 (1.01) | .377 | 0.17 |
| % Access to Exercise Opportunities | 71.70 (8.29) | 67.26 (4.73) | .383 | 0.17 |
| Food Environment Index d | 7.16 (0.46) | 7.12 (0.27) | .783 | 0.06 |
| % Obesity | 24.20 (1.50) | 27.84 (0.67) | .024 | −0.43 |
| % Smoking | 9.50 (0.43) | 9.89 (0.44) | .623 | −0.10 |
| % Physical Inactivity | 17.70 (1.18) | 18.47 (0.65) | .562 | −0.11 |
| PCP per 100,000 Persons | 63.90 (12.74) | 44.56 (4.21) | .314 | 0.20 |
| Violent crimes per 100,000 Persons | 163.25 (32.04) | 166.17 (27.35) | .978 | 0.01 |
| % Nonurban (Rural) | 66.92 (14.90) | 63.73 (10.31) | 1.0 | 0.00 |
Abbreviations: EOCRC, early-onset colorectal cancer; NH, non-Hispanic; PCP, primary care physician.
Hotspot classification was determined by counties with spatial Empirical Bayes smoothed rates that fell in the top quartile for either incidence or mortality.
The p value was derived from the Wilcoxon rank sum test.
Spearman correlation with being classified as a hotspot for EOCRC incidence or mortality.
Indicator of access to healthy foods, from 0 = worst to 10 = best.
Multivariable Analyses
Hotspot classification was not significantly associated with EOCRC incidence (Table 5). However, age at diagnosis and race and ethnicity were associated with EOCRC incidence, regardless of hotspot classification. Adults age 40 to 49 had a 93% and 67% higher incidence, respectively, than adults age 18 to 29 (incidence rate ratio, 0.07; 95% CI, 0.06–0.08) and adults age 30 to 39 (incidence rate ratio, 0.33; 95% CI, 0.30–0.37). Non-Hispanic ethnic-minority adults (incidence rate ratio, 1.26; 95% CI, 1.03–1.53) and Hispanic White adults (incidence rate ratio, 2.24; 95% CI, 2.00–2.51) had a higher incidence than NH White adults, whereas Hispanic ethnic-minority adults had a more than threefold higher incidence than NH White adults (incidence rate ratio, 4.59; 95% CI, 3.50–5.91).
TABLE 5.
Multivariate Poisson Regression Models for EOCRC Incidence in Utah (2000–2020) Among Adults Age 18 to 49
| Predictors | EOCRC Incidence | |||
|---|---|---|---|---|
| Model 1 IRR [95% CI] | Model 2 IRR [95% CI] | Model 3 IRR [95% CI] | Model 4 IRR [95% CI] | |
| Hotspot Classification a | ||||
| Coldspot | Ref | Ref | Ref | Ref |
| Hotspot | 1.08 [0.96–1.21] | 1.03 [0.91–1.15] | 1.06 [0.94–1.19] | 1.01 [0.89–1.15] |
| Age at Diagnosis | ||||
| 18–29 Years | 0.07 [0.06–0.08] | 0.07 [0.06–0.08] | 0.07 [0.06–0.08] | |
| 30–39 Years | 0.34 [0.30–0.37] | 0.33 [0.30–0.37] | 0.33 [0.30–0.37] | |
| 40–49 Years | Ref | Ref | Ref | |
| Race and Ethnicity (%) b | ||||
| NH White | Ref | Ref | ||
| NH Ethnic Minority | 1.26 [1.03–1.53] | 1.26 [1.03–1.53] | ||
| Hispanic White | 2.24 [2.00–2.50] | 2.24 [2.00–2.51] | ||
| Hispanic Ethnic Minority | 4.58 [3.49–5.89] | 4.59 [3.50–5.91] | ||
| % Household Income < $25,000 | 1.02 [1.00–1.03] | |||
| % Obesity | 1.00 [0.98–1.02] | |||
Abbreviations: EOCRC, early-onset colorectal cancer; IRR, incidence rate ratio; NH, non-Hispanic.
Bold indicates significance at p < .05.
Hotspot classification was determined by counties with spatial Empirical Bayes smoothed rates that fell in the top quartile for either incidence or mortality.
Racial and ethnic categories were based on the available population breakdown on U.S. Centers for Disease Control and Prevention WONDER online database bridged-race population estimates, which did not include a multiracial category, and which grouped Asian Americans and Pacific Islanders.
When we stratified the results by sex, the findings for men were similar to the overall model (Table 6). Among women, although we found no evidence of a difference in incidence between NH White and NH ethnic-minority women, we found a significant association between EOCRC incidence and the percentage of households with income below $25,000. Women living in counties where a greater percentage of households had incomes under $25,000 had a 3% higher incidence of EOCRC (incidence rate ratio, 1.03; 95% CI, 1.004–1.06).
TABLE 6.
Multivariate Poisson Regression Models for EOCRC Incidence in Utah (2000–2020) Stratified by Sex Among Adults Age 18 to 49
| Predictors | EOCRC Incidence | |||
|---|---|---|---|---|
| Women | Men | |||
| Number of Cases | IRR [95% CI] | Number of Cases | IRR [95% CI] | |
| Hotspot Classification a | ||||
| Coldspot | 718 (81.78) | Ref | 808 (82.62) | Ref |
| Hotspot | 160 (18.22) | 0.97 [0.80–1.18] | 170 (17.38) | 1.08 [0.90–1.31] |
| Age at Diagnosis | ||||
| 18–29 Years | 62 (7.06) | 0.07 [0.05–0.08] | 74 (7.57) | 0.08 [0.06–0.09] |
| 30–39 Years | 221 (25.17) | 0.32 [0.28–0.38] | 268 (27.40) | 0.35 [0.30–0.41] |
| 40–49 Years | 595 (67.77) | Ref | 636 (65.03) | Ref |
| Race andEthnicity (%) b | ||||
| NH White | 612 (69.78) | Ref | 674 (68.92) | Ref |
| NH Ethnic Minority | 41 (4.68) | 1.09 [0.78–1.48] | 65 (6.65) | 1.49 [1.15–1.91] |
| Hispanic White | 195 (22.23) | 2.43 [2.06–2.85] | 209 (21.37) | 2.19 [1.87–2.55] |
| Hispanic Ethnic Minority | 29 (3.31) | 5.67 [3.82–8.07] | 30 (3.07) | 4.17 [2.83–5.90] |
| % Household Income < $25,000 | NA | 1.03 [1.004–1.06] | NA | 1.02 [0.99–1.04] |
| % Obesity | NA | 1.01 [0.98–1.04] | NA | 0.99 [0.96–1.01] |
Abbreviations: EOCRC, early-onset colorectal cancer; IRR, incidence rate ratio; NH, non-Hispanic; NA, not available.
Bold indicates significance at p < .05.
Hotspot classification was determined by counties with spatial Empirical Bayes smoothed rates that fell in the top quartile for either incidence or mortality.
Racial and ethnic categories were based on the available population breakdown on U.S. Centers for Disease Control and Prevention WONDER online database bridged-race population estimates, which did not include a multiracial category, and which grouped Asian Americans and Pacific Islanders.
Hotspot classification was significantly associated with EOCRC-specific mortality when accounting for stage at diagnosis (Table 7). Adults living in hotspot counties had a 31% higher hazard for EOCRC-specific death compared with those in coldspot counties (HR, 1.31; 95% CI, 1.02–1.69). Likewise, compared with localized tumors, the hazard for CRC-specific death was higher for regional (HR, 4.42; 95% CI, 3.10–6.30), distant/metastatic (HR, 40.41; 95% CI, 28.79–56.73), and unknown tumor stages (HR, 6.28; 95% CI, 3.54–11.16). When we examined differences in survival probabilities in individuals with late-stage diagnoses (i.e., distant/metastatic disease), adults living in hotspot counties had a lower probability of survival than did their counterparts in coldspot counties (chi square (1) = 4.0; p = .045; Fig. 2). We found no significant differences in late-stage survival between hotspot- and coldspot counties when stratified by sex (Fig. 2).
TABLE 7.
Multivariate Cox Proportional Hazards Regression Models for EOCRC-Specific Deaths in Utah (2000–2020) Among Adults Age 18 to 49
| Predictors | Number of Deathsa | EOCRC Survival | |||
|---|---|---|---|---|---|
| Model 1 HR [95% CI] | Model 2 HR [95% CI] | Model 3 HR [95% CI] | Model 4 HR [95% CI] | ||
| Hotspot Classification b | |||||
| Coldspot | 410 (80.23) | Ref | Ref | Ref | Ref |
| Hotspot | 101 (19.77) | 1.14 [0.92–1.41] | 1.38 [1.11–1.73] | 1.39 [1.11–1.74] | 1.31 [1.02–1.69] |
| Age at Diagnosis | |||||
| 18–29 Years | 37 (7.24) | 1.40 [1.00–1.98] | 1.28 [0.90–1.81] | 1.28 [0.90–1.81] | |
| 30–39 Years | 131 (25.64) | 1.14 [0.93–1.40] | 1.12 [0.91–1.37] | 1.12 [0.91–1.38] | |
| 40–49 Years | 343 (67.12) | Ref | Ref | Ref | |
| Race and Ethnicity | |||||
| NH American Indian-Alaska Native | * | 0.26 [0.04–1.89] | 0.26 [0.04–1.88] | 0.20 [0.03–1.53] | |
| NH Asian | * | 1.18 [0.44–3.16] | 1.23 [0.46–3.30] | 1.23 [0.46–3.31] | |
| NH Black | * | 0.38 [0.12–1.20] | 0.40 [0.13–1.28] | 0.40 [0.13–1.26] | |
| NH Islander | * | 2.06 [0.66–6.45] | 2.13 [0.68–6.67] | 2.13 [0.68–6.66] | |
| NH Multiple | 15 (2.94) | 0.85 [0.51–1.42] | 0.82 [0.49–1.38] | 0.82 [0.49–1.38] | |
| NH White | 370 (72.41) | Ref | Ref | Ref | |
| Hispanic White | 100 (19.57) | 0.90 [0.72–1.13] | 0.90 [0.72–1.12] | 0.91 [0.72–1.13] | |
| Hispanic Ethnic Minority | 15 (2.94) | 1.11 [0.66–1.86] | 1.07 [0.64–1.80] | 1.09 [0.65–1.84] | |
| Stage | |||||
| Local | 40 (7.83) | Ref | Ref | Ref | |
| Regional | 132 (25.83) | 4.44 [3.11–6.32] | 4.42 [3.10–6.30] | 4.42 [3.10–6.30] | |
| Distant/Metastatic | 322 (63.01) | 40.88 [29.14–57.34] | 40.31 [28.72–56.58] | 40.41 [28.79–56.73] | |
| Unknown | 17 (3.33) | 6.36 [3.58–11.29] | 6.28 [3.54–11.16] | 6.32 [3.56–11.23] | |
| Marital Status | |||||
| Single or Never Married | 97 (18.98) | Ref | Ref | ||
| Married or Life Partner | 335 (65.56) | 0.73 [0.58–0.92] | 0.73 [0.58–0.92] | ||
| Divorced, Separated, or Widowed | 77 (15.07) | 0.82 [0.60–1.12] | 0.81 [0.59–1.11] | ||
| Unknown | * | 0.18 [0.04–0.72] | 0.18 [0.04–0.72] | ||
| % Household Income < $25,000 | NA | 1.02 [0.98–1.05] | |||
| % Obesity | NA | 1.00 [0.97–1.03] | |||
| Generalized R 2 | 0.07% | 37.32% | 37.81% | 37.84% | |
Abbreviations: EOCRC, early-onset colorectal cancer; NH, non-Hispanic; NA, not available.
Bold indicates statistical significance (p < .05).
In accordance with Utah Department of Health Data Suppression Guidelines, an asterisk (*) denotes counts of < 11 (see https://uofuhealth.utah.edu/utah-cancer-registry/research/).
Hotspot classification was determined by counties with spatial Empirical Bayes smoothed rates that fell in the top quartile for either incidence or mortality.
FIGURE 2.
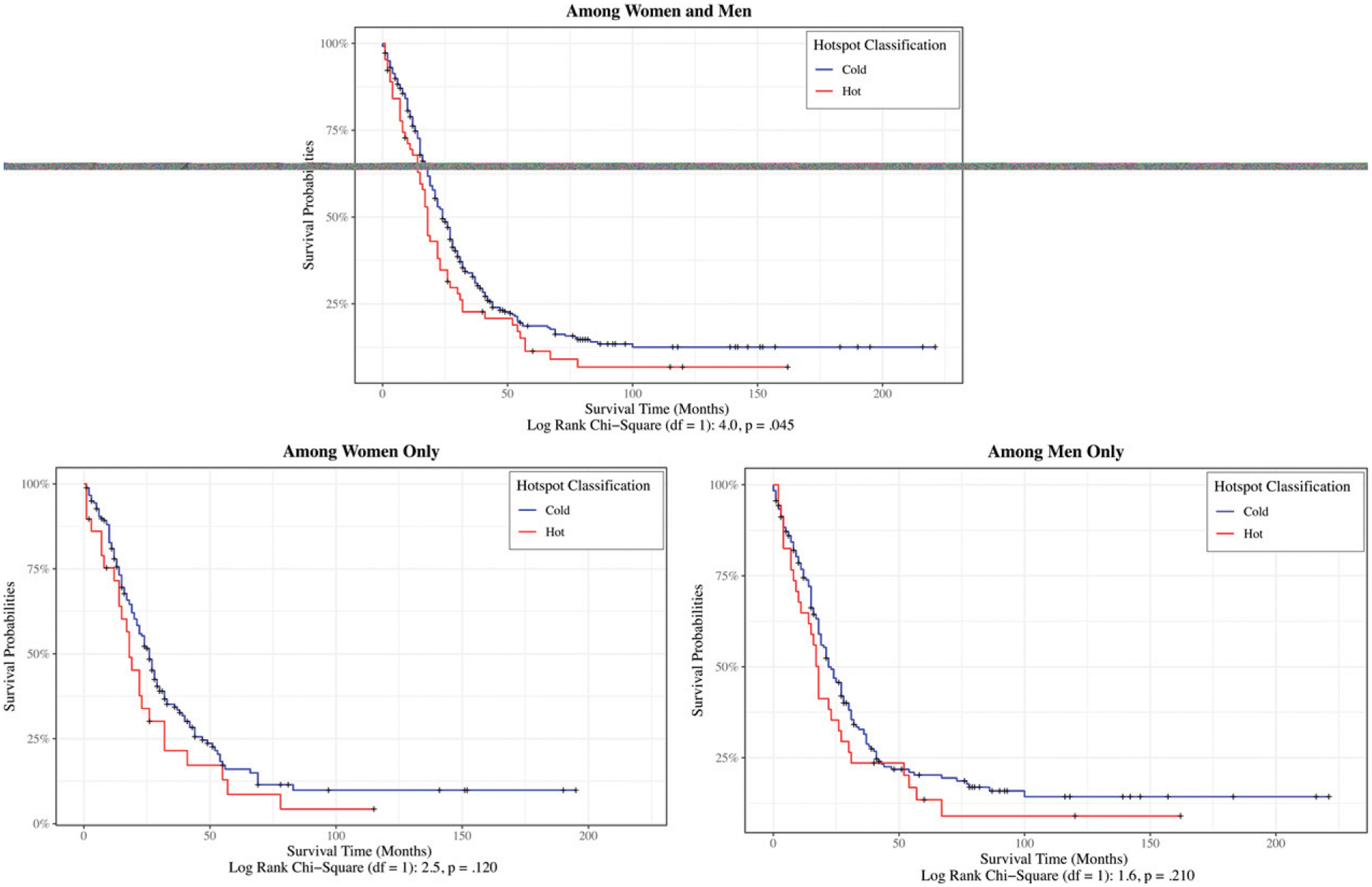
Kaplan-Meier-Specific Survival Curves for Early-Onset Colorectal Cancer (Patients Age 18–49) With Late-Stage Diagnosis (Distant/Metastatic Disease) Among Women and Men, Women Only, and Men Only, by Hotspot-County Classification in Utah (2000–2020)
Ticks represent censored data.
Marital status was also associated with EOCRC survival; adults who were married or who had a life partner had a 27% lower hazard for EOCRC-specific death compared with single or never-married adults (HR, 0.73; 95% CI, 0.58–0.92). Stratification of the models by sex revealed several differences from the overall model (Table 8). First, hotspot classification was not associated with EOCRC survival when examined separately for women and men. Second, marital status was not associated with EOCRC survival for women. Third, we saw a significant association between race and ethnicity and EOCRC survival among men. Non-Hispanic Native Hawaiian/other Pacific Islander men had a 265% higher hazard for CRC-specific death compared with NH White men (HR, 3.65; 95% CI, 1.16–11.52).
TABLE 8.
Multivariate Cox Proportional Hazards Regression Models for EOCRC-Specific Deaths in Utah (2000–2020) Among Women and Men Age 18 to 49
| Predictors | EOCRC Survival | |||
|---|---|---|---|---|
| Women | Men | |||
| Number of Deathsa | HR [95% CI] | Number of Deathsa | HR [95% CI] | |
| Hotspot Classification b | ||||
| Coldspot | 188 (80.34) | Ref | 222 (80.14) | Ref |
| Hotspot | 46 (19.66) | 1.39 [0.96–2.01] | 55 (19.86) | 1.29 [0.88–1.88] |
| Age at Diagnosis | ||||
| 18–29 Years | 15 (6.41) | 1.19 [0.69–2.05] | 22 (7.94) | 1.26 [0.79–2.02] |
| 30–39 Years | 55 (23.50) | 1.18 [0.86–1.62] | 76 (27.44) | 1.08 [0.82–1.43] |
| 40–49 Years | 164 (70.09) | Ref | 179 (64.62) | Ref |
| Race and Ethnicity | ||||
| NH American Indian-Alaska Native | * | 0.72 [0.08–6.13] | * | — |
| NH Asian | * | 0.86 [0.21–3.47] | * | 2.02 [0.50–8.22] |
| NH Black | * | 0.53 [0.06–4.33] | * | 0.31 [0.07–1.26] |
| NH Islander | * | — | * | 3.65 [1.16–11.52] |
| NH Multiple | * | 1.27 [0.59–2.73] | * | 0.59 [0.29–1.22] |
| NH White | 172 (73.50) | Ref | 198 (71.48) | Ref |
| Hispanic White | 44 (18.80) | 0.95 [0.68–1.33] | 56 (20.22) | 0.87 [0.64–1.18] |
| Hispanic Ethnic Minority | * | 0.93 [0.43–2.02] | * | 1.34 [0.66–2.75] |
| Stage | ||||
| Local | 13 (5.56) | Ref | 27 (9.75) | Ref |
| Regional | 58 (24.79) | 6.60 [3.61–12.07] | 74 (26.71) | 3.30 [2.12–5.14] |
| Distant/Metastatic | 155 (66.24) | 68.41 [37.99–123.18] | 167 (60.29) | 29.63 [19.48–45.09] |
| Unknown | * | 9.85 [4.04–24.04] | * | 4.70 [2.18–10.13] |
| Marital Status | ||||
| Single or Never Married | 38 (16.24) | Ref | 59 | Ref |
| Married or Life Partner | 154 (65.81) | 0.92 [0.64, 1.32] | 181 | 0.65 [0.47, 0.88] |
| Divorced, Separated, or Widowed | 40 (17.09) | 0.78 [0.49, 1.23] | 37 | 1.01 [0.64, 1.59] |
| Unknown | * | 0.41 [0.09. 1.88] | * | - |
| % Household Income < $25,000 | NA | 1.04 [1.00, 1.09] | NA | 0.99 [0.94, 1.04] |
| % Obesity | NA | 0.99 [0.94, 1.04] | NA | 1.00 [0.96, 1.05] |
| Generalized R 2 | 40.83% | 36.44% | ||
Abbreviations: EOCRC, early-onset colorectal cancer; NH, non-Hispanic; NA, not available.
Bold indicates significance at p < .05.
In accordance with Utah Department of Health Data Suppression Guidelines, an asterisk (*) denotes counts of < 11 (see https://uofuhealth.utah.edu/utah-cancer-registry/research/).
Hotspot classification was determined by counties with spatial Empirical Bayes smoothed rates that fell in the top quartile for either incidence or mortality.
DISCUSSION
In this ecological study, we aimed to identify and characterize EOCRC hotspot counties in Utah by examining the variance in EOCRC incidence and survival that could be explained by personal- and county-level factors. Of the 1,856 patients included in our study, all age 18 to 49 and diagnosed with EOCRC between 2000 and 2020, those living in hotspot counties had notably worse survival, with a 31% higher hazard of CRC-specific mortality than their counterparts in coldspot counties. Outcome determinants were specific to patients diagnosed with CRC, which varied across counties; up to 34% of counties had higher incidence or mortality relative to other counties in Utah. Age and race and ethnicity were found to be predictive of CRC incidence rather than hotspot classification. When accounting for stage at diagnosis, individuals living in hotspots had worse EOCRC survival than those residing in coldspot counties.
Although fewer single or never-married adults resided in hotspot counties than divorced, separated, or widowed adults, they had similar survival rates; this could suggest that formerly partnered individuals differ in risk in more nuanced ways than the never-married. The distribution of frequencies across marital status is consistent with a nationwide analysis of EOCRC hotspots across the United States.19 Marital status is a known predictor of cancer-related prevention behavior and survival outcomes,30 including CRC prevention and survival,31,32 which may stem from spousal/social support.33 In our study, hotspot counties had fewer adults with obesity than coldspot counties. These findings contrast with those of a prior nationwide analysis that found a higher percentage of adults with obesity in hotspot counties.19 However, the percentage of adults with obesity at the county level was unassociated with EOCRC incidence and survival. Therefore, this county-level factor does not explain EOCRC outcomes in Utah.
Our findings demonstrate the impacts of known EOCRC determinants, as well as of unique findings, on the intersectionality of sex and socioeconomic status. Results of our Poisson regression analyses indicate that younger age groups (18–29 and 30–39) are less likely to be diagnosed with EOCRC compared with the older age group (40–49); these findings are consistent with those of prior U.S.-based studies reporting EOCRC incidence by age at diagnosis. For example, using SEER 18 data, Ansa et al34 reported an EOCRC incidence rate of 2.3 per 100,000 (95% CI, 2.3–2.4) among individuals younger than age 40 and 22.5 per 100,000 (95% CI, 22.1–22.8) among those age 40 to 49. With respect to race and ethnicity, NH White adults in our study were less likely than ethnic-minority adults to be diagnosed with EOCRC. The Non-Hispanic Black population has long been known to have the highest burden of CRC and now also has a higher incidence of EOCRC.35,36 As recommended by Muller et al,14 population-level data analysis is warranted to determine whether the recent U.S. Preventive Services Task Force recommendation that the CRC screening age be lowered to 45 is affecting EOCRC incidence in populations that often face cancer disparities.
A finding that, to our knowledge, has not been previously identified is that women living in areas in which a greater proportion of households have incomes below $25,000 are more likely to be diagnosed with EOCRC. Prior research by Aloysius et al37 has focused on the impacts of socioeconomic determinants of health (e.g., high school completion, poverty, household income, employment status, insurance status) on EOCRC survival. In increasing knowledge of the risk factors that may contribute to EOCRC disparities, our findings highlight the importance of addressing socioeconomic barriers across the continuum of EOCRC, from diagnosis to treatment.
When we stratified by stage at diagnosis (or when stage at diagnosis was similar between hotspot- and coldspot counties), we observed worse survival for patients with EOCRC living in hotspot counties, supporting our hypothesis that EOCRC survival, accounting for stage, would differ between hotspot- and coldspot counties. These survival differences are likely attributable to variations in socioeconomic and social factors. The confluence of insurance status, household income, and marital status significantly affects CRC diagnostic stage, treatment, and survival.38–40 Numerous studies have confirmed that individuals with health insurance and those who are married are more likely to be diagnosed with CRC at an earlier stage, receive definitive treatment, and have longer survival,38,39,41–43 whereas lower household income is associated with higher cancer-specific mortality.40 Therefore, interventions aimed at reducing CRC risk, or increasing rates of CRC screening, should focus on strengthening community resources to help overcome the structural barriers often associated with household income and insurance status.44
Among all determinants of EOCRC survival, stage at diagnosis explained the largest proportion of variance, regardless of hotspot classification. However, those living in hotspots were more likely to die sooner after receiving a late-stage EOCRC diagnosis than their counterparts living in coldspots. Future studies will be needed to identify causal contributors to this relationship. For example, those residing in hotspot counties who are diagnosed with late-stage EOCRC may fare worse because of differences in access to or quality of CRC treatment or treatment adherence. We agree with Wang et al42 that these possibilities highlight a potential role of both individual-level sociodemographic variables and psychosocial characteristics, as well as county-level factors. This may be explained, in part, by factors such as travel time, distance, financial strain, and screening resistance, which merit further investigation.
Our multivariable analyses revealed that participants in our sample who were married or who had a life partner had a greater likelihood of EOCRC survival compared with adults who never married or were single, regardless if they lived in a hotspot- or coldspot county. These results are consistent with other literature highlighting the prognostic significance of marital status for cancer survival.32,45 Even after controlling for disease stage, age, race, and surgery, the survival benefits associated with marital status are seen across multiple cancers—including breast, colorectal, esophageal, prostate, and other cancers with greater impact among men than women.41,42 For CRC specifically, marriage is found to increase the odds of undergoing CRC screening and has a protective effect on survival31,43,46; research is limited, however, on the influence of marital status on survival for patients with EOCRC. Increased CRC survival among those who are married, have a life partner, or have other forms of high social support is likely attributable to psychosocial factors, including the availability of emotional support, assistance in monitoring health status, and aid in coping with treatment and shaping preventive health behaviors.46–48 Moreover, individuals with established support systems often experience reduced cortisol levels associated with chronic psychological stress, thereby reducing various inflammatory markers that may provide the opportunity for precancerous cells to grow and flourish.32,49,50 Although social support has a positive impact on health and health-promoting behaviors, more interventional research is warranted to focus on increasing the survival benefit associated with EOCRC for single or never-married individuals, and for patients with poor social relationships.
Limitations
Our study is not without limitations. First, very rural and small counties may not have been identified as hotspots because of a limited number of events/cases. However, a key strength of the Bayesian approach is that, for counties with very limited data, we can borrow strength from the observed data and derive reasonable posterior estimates for EOCRC incidence and mortality. In turn, we estimated smoothed mortality rates for counties with a small number of deaths (i.e., < 10), a limitation of our prior geospatial analyses.19,51 Second, the limited number of events among underrepresented racial and ethnic groups included in our study may have underestimated the true incidence and burden of disease within these populations. However, our study includes results for specific racial and ethnic groups to provide a foundation for future efforts to examine racial and ethnic differences in EOCRC survival among Utahans. Third, the use of each patient’s residence at baseline did not permit us to account for time-varying changes in residence and associated-county characteristics. Yet by including county-level factors, we were able to advance understanding of how multiple levels within an ecological system contribute to both EOCRC risk and survival. Finally, we did not focus on individual cancer-prevention determinants (e.g., screening reluctance, family history of CRC, distance to care) or temporal changes relevant to CRC during our study timeframe (e.g., patient navigation, advancements in screening options and treatment).
CONCLUSION
Using spatial analytical methodology, we identified 10 Utah counties (34%) as hotspots with high EOCRC incidence, EOCRC mortality, or both. The incidence of EOCRC was elevated among NH ethnic-minority men, Hispanic White men and women, and Hispanic ethnic-minority men and women compared with their NH White counterparts. Among Utahan adults younger than age 50, residing in a hotspot county increased the risk of EOCRC mortality independent of late-stage diagnosis, whereas marital status was a key social determinant of survival. These findings provide insight for future research, policy changes, and interventions aimed at improving CRC survival and increasing CRC screening uptake in individuals younger than age 50.
PRACTICAL APPLICATIONS.
Future researchers may use our spatial analytical method to identify state-specific geographic determinants of colorectal cancer incidence and mortality.
The higher incidence of early-onset colorectal cancer (EOCRC) among non-Hispanic ethnic-minority men, as well as among Hispanic White and Hispanic ethnic-minority men and women, suggests that racial- and ethnicity-specific colorectal cancer screening strategies should be considered for patients younger than the previously recommended initiation age of 50.
Although healthy behaviors are prominent among the White population in western states, EOCRC incidence is rising most rapidly in the West. Our Utah-focused study provides insight into personal- and county-level factors that may be contributing to disturbing trends in EOCRC incidence and mortality across the United States.
Adults in Utah with a late-stage EOCRC diagnosis who live in the 10 counties we identified as EOCRC hotspots are at increased risk of dying, emphasizing the need for researchers and health care providers to better understand how to serve these communities.
Marital status appears to be a key social determinant of EOCRC survival, suggesting an important role for social support. Further work is needed to better understand the role of support by family members and/or non-household members.
ACKNOWLEDGMENT
The authors extend gratitude to the participants who made the study possible; to Eleanor Mayfield, ELS, for editorial support; and to other members of our #iBeatCRC Advocate-Survivor team for study insight. Research reported in this publication was supported by 5 For the Fight and the Huntsman Cancer Institute; the V Foundation for Cancer Research; the National Cancer Institute (Grant K01CA234319); and the National Institute on Minority Health and Health Disparities (Grant K01MD015304), entities of the National Institutes of Health (NIH). Partial support for all data sets within the Utah Population Database was provided by the University of Utah Huntsman Cancer Institute and the Huntsman Cancer Institute Cancer Center Support (Grant P30 CA2014) from the National Cancer Institute. The Utah Cancer Registry is funded by the National Cancer Institute’s SEER Program (Contract No. HHSN261201800016I), the U.S. Centers for Disease Control and Prevention’s National Program of Cancer Registries (Cooperative Agreement No. NU58DP006320), with additional support from the University of Utah and Huntsman Cancer Foundation. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, 5 For the Fight, V Foundation for Cancer Research, Huntsman Cancer Institute, the Centers for Disease Control and Prevention, the Huntsman Cancer Foundation, or the University of Utah.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
Disclosures provided by the authors and data availability statement (if applicable) are available with this article at DOI https://doi.org/10.1200/EDBK_350241.
REFERENCES
- 1.Hofseth LJ, Hebert JR, Chanda A, et al. Early-onset colorectal cancer: initial clues and current views. Nat Rev Gastroenterol Hepatol. 2020;17:352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahib L, Wehner MR, Matrisian LM, et al. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open. 2021;4:e214708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 5.Shah RR, Millien VO, da Costa WL Jr., et al. Trends in the incidence of early-onset colorectal cancer in all 50 United States from 2001 through 2017. Cancer. 2022;128:299–310. [DOI] [PubMed] [Google Scholar]
- 6.Davidson KW, Barry MJ, Mangione CM, et al. ; US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965–1977. [DOI] [PubMed] [Google Scholar]
- 7.Akimoto N, Ugai T, Zhong R, et al. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol. 2021;18:230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danos D, Leonardi C, Wu XC. Geographic determinants of colorectal cancer in Louisiana. Cancer Causes Control. 2022;33:525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdelsattar ZM, Wong SL, Regenbogen SE, et al. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer. 2016;122:929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochs-Balcom HM, Kanth P, Cannon-Albright LA. Early-onset colorectal cancer risk extends to second- and third-degree relatives. Cancer Epidemiol. 2021;73:101973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015;150:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis L, Abrahão R, McKinley M, et al. Colorectal cancer incidence trends by age, stage, and racial/ethnic group in California, 1990–2014. Cancer Epidemiol Biomarkers Prev. 2018;27:1011–1018. [DOI] [PubMed] [Google Scholar]
- 13.Hinshaw T, Lea S, Arcury J, et al. Racial and geographic disparities in stage-specific incidence and mortality in the colorectal cancer hotspot region of eastern North Carolina, 2008–2016. Cancer Causes Control. 2021;32:271–278. [DOI] [PubMed] [Google Scholar]
- 14.Muller C, Ihionkhan E, Stoffel EM, et al. Disparities in early-onset colorectal cancer. Cells. 2021;10:1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh GK, Williams SD, Siahpush M, et al. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part I—all cancers and lung cancer and part II—colorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warren Andersen S, Blot WJ, Lipworth L, et al. Association of race and socioeconomic status with colorectal cancer screening, colorectal cancer risk, and mortality in southern US adults. JAMA Netw Open. 2019;2:e1917995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel RL, Sahar L, Robbins A, et al. Where can colorectal cancer screening interventions have the most impact? Cancer Epidemiol Biomarkers Prev. 2015;24:1151–1156. [DOI] [PubMed] [Google Scholar]
- 18.Holowatyj AN, Langston ME, Han Y, et al. Community health behaviors and geographic variation in early-onset colorectal cancer survival among women. Clin Transl Gastroenterol. 2020;11:e00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers CR, Moore JX, Qeadan F, et al. Examining factors underlying geographic disparities in early-onset colorectal cancer survival among men in the United States. Am J Cancer Res. 2020;10:1592–1607. [PMC free article] [PubMed] [Google Scholar]
- 20.Abolhassani M, Asadikaram G, Paydar P, et al. Organochlorine and organophosphorous pesticides may induce colorectal cancer; a case-control study. Ecotoxicol Environ Saf. 2019;178:168–177. [DOI] [PubMed] [Google Scholar]
- 21.El-Tawil AM. Colorectal cancer and pollution. World J Gastroenterol. 2010;16:3475–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mainous AG III, King DE, Garr DR, et al. Race, rural residence, and control of diabetes and hypertension. Ann Fam Med. 2004;2:563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Census Bureau. 2015–2019. American Community Survey 5-Year Estimates. https://data.census.gov/cedsci. Accessed January 31, 2022.
- 24.University of Wisconsin Population Health Institute. County Health Rankings & Roadmaps 2019. https://www.countyhealthrankings.org. Accessed January 31, 2022.
- 25.Centers for Disease Control and Prevention. Bridged-Race Population Estimates, United States, July 1, Resident Population by State, County, Age, Sex, Bridged-Race, and Hispanic Origin. http://wonder.cdc.gov/bridged-race-v2020.html. Accessed January 31, 2022.
- 26.Kirchhoff AC, Hart G, Campbell EG. Rural and urban primary care physician professional beliefs and quality improvement behaviors. J Rural Health. 2014;30:235–243. [DOI] [PubMed] [Google Scholar]
- 27.Rogers CR, Brooks E, Curtin K, et al. Protocol for #iBeatCRC: a community-based intervention to increase early-onset colorectal cancer awareness using a sequential explanatory mixed-methods approach. BMJ Open. 2021;11:e048959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anselin L, Syabri I, Kho Y. GeoDa: an introduction to spatial data analysis. Geogr Anal. 2006;38:5–22. [Google Scholar]
- 29.Allison PD. Survival Analysis Using SAS: A Practical Guide. 2nd ed. Cary, North Carolina: SAS Press;1995. [Google Scholar]
- 30.Kravdal O The impact of marital status on cancer survival. Soc Sci Med. 2001;52:357–368. [DOI] [PubMed] [Google Scholar]
- 31.El-Haddad B, Dong F, Kallail KJ, et al. Association of marital status and colorectal cancer screening participation in the USA. Colorectal Dis. 2015;17:O108–O114. [DOI] [PubMed] [Google Scholar]
- 32.Li Q, Gan L, Liang L, et al. The influence of marital status on stage at diagnosis and survival of patients with colorectal cancer. Oncotarget. 2015;6: 7339–7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nausheen B, Gidron Y, Peveler R, et al. Social support and cancer progression: a systematic review. J Psychosom Res. 2009;67:403–415. [DOI] [PubMed] [Google Scholar]
- 34.Ansa BE, Coughlin SS, Alema-Mensah E, et al. Evaluation of colorectal cancer incidence trends in the United States (2000–2014). J Clin Med. 2018;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Araghi M, Fidler MM, Arnold M, et al. The future burden of colorectal cancer among US blacks and whites. J Natl Cancer Inst. 2018;110:791–793. [DOI] [PubMed] [Google Scholar]
- 36.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst. 2017;109 10.1093/jnci/djw322. 28376186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aloysius MM, Goyal H, Shah NJ, et al. Impact of race and socioeconomics disparities on survival in young-onset colorectal adenocarcinoma—a SEER registry analysis. Cancers (Basel). 2021;13:3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun W, Cheng M, Zhuang S, et al. Impact of insurance status on stage, treatment, and survival in patients with colorectal cancer: a population-based analysis. Med Sci Monit. 2019;25:2397–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tawk R, Abner A, Ashford A, et al. Differences in colorectal cancer outcomes by race and insurance. Int J Environ Res Public Health. 2015;13:h13010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Berg I, Buettner S, van den Braak RRJC, et al. Low socioeconomic status is associated with worse outcomes after curative surgery for colorectal cancer: results from a large, multicenter study. J Gastrointest Surg. 2020;24:2628–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31:3869–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Wilson SE, Stewart DB, et al. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: does marriage affect cancer survival by gender and stage? Cancer Epidemiol. 2011;35:417–422. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Cao W, Zheng C, et al. Marital status and survival in patients with rectal cancer: an analysis of the Surveillance, Epidemiology and End Results (SEER) database. Cancer Epidemiol. 2018;54:119–124. [DOI] [PubMed] [Google Scholar]
- 44.Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W, Chen W, Lin J, et al. Incidence and characteristics of young-onset colorectal cancer in the United States: an analysis of SEER data collected from 1988 to 2013. Clin Res Hepatol Gastroenterol. 2019;43:208–215. [DOI] [PubMed] [Google Scholar]
- 46.Yang CC, Cheng LC, Lin YW, et al. The impact of marital status on survival in patients with surgically treated colon cancer. Medicine (Baltimore). 2019;98:e14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gray NM, Hall SJ, Browne S, et al. Predictors of anxiety and depression in people with colorectal cancer. Support Care Cancer. 2014;22:307–314. [DOI] [PubMed] [Google Scholar]
- 48.Kroenke CH, Paskett ED, Cené CW, et al. Prediagnosis social support, social integration, living status, and colorectal cancer mortality in postmenopausal women from the women’s health initiative. Cancer. 2020;126:1766–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baritaki S, de Bree E, Chatzaki E, et al. Chronic stress, inflammation, and colon cancer: a CRH system-driven molecular crosstalk. J Clin Med. 2019;8:1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reblin M, Uchino BN. Social and emotional support and its implication for health. Curr Opin Psychiatry. 2008;21:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore KJ, Sussman DA, Koru-Sengul T. Age-specific risk factors for advanced stage colorectal cancer, 1981–2013. Prev Chronic Dis. 2018;15:E106. [DOI] [PMC free article] [PubMed] [Google Scholar]


