Abstract
Besides the most prominent peptide toxin, microcystin, the cyanobacteria Microcystis spp. have been shown to produce a large variety of other bioactive oligopeptides. We investigated for the first time the oligopeptide diversity within a natural Microcystis population by analyzing single colonies directly with matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). The results demonstrate a high diversity of known cyanobacterial peptides such as microcystins, anabaenopeptins, microginins, aeruginosins, and cyanopeptolins, but also many unknown substances in the Microcystis colonies. Oligopeptide patterns were mostly related to specific Microcystis taxa. Microcystis aeruginosa (Kütz.) Kütz. colonies contained mainly microcystins, occasionally accompanied by aeruginosins. In contrast, microcystins were not detected in Microcystis ichthyoblabe Kütz.; instead, colonies of this species contained anabaenopeptins and/or microginins or unknown peptides. Within a third group, Microcystis wesenbergii (Kom.) Kom. in Kondr., chiefly a cyanopeptolin and an unknown peptide were found. Similar patterns, however, were also found in colonies which could not be identified to species level. The significance of oligopeptides as a chemotaxonomic tool within the genus Microcystis is discussed. It could be demonstrated that the typing of single colonies by MALDI-TOF MS may be a valuable tool for ecological studies of the genus Microcystis as well as in early warning of toxic cyanobacterial blooms.
Freshwater and marine cyanobacteria are known to produce a variety of bioactive compounds, among them potent hepatotoxins and neurotoxins (for an overview, see reference 45). Many of the toxic species of cyanobacteria tend to massive proliferation in eutrophicated water bodies and thus have been the cause for considerable hazards for animal and human health (3, 23). One of the most widespread bloom-forming cyanobacteria is the genus Microcystis, a well-known producer of the hepatotoxic peptide microcystin (45). Microcystins are a group of closely related cyclic heptapeptides sharing the common structure cyclo(d-Ala-l-X-d-MeAsp-l-Z-Adda-d-Glu-Mdha), in which MeAsp is d-erythro-β-methylaspartic acid, Mdha is N-methyldehydroalanine, Adda is 2S,3S,8S,9S-3-amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4E,6E-dienoic acid, and X and Z are variable l-amino acids, e.g., microcystin-LR (MC-LR) contains leucine (L) and arginine (R) (5). So far, more than 60 derivatives of microcystins have been identified, varying largely by the degree of methylation, peptide sequence, and toxicity (for an overview, see reference 45).
The hepatotoxicity of microcystins is based on their inhibition of protein phosphatases 1 and 2A in combination with transport into hepatocytes via the bile acid carrier, leading to acute liver failure due to the disruption of hepatocyte cytoskeletal components (11, 26). The widespread occurrence and acute toxicity of microcystins and their tumor-promoting properties imply the need for identification and prediction of toxic blooms (23).
The traditional botanical code describes the genus Microcystis as a coccal, unicellular cyanobacterium that grows as mucilaginous colonies of irregularly arranged cells (under natural conditions, while strain cultures usually grow as single cells). According to this tradition, morphological criteria such as size of the individual cells, colony morphology, and mucilage characteristics are used for species delimitation within Microcystis (i.e., morphospecies) (20, 21). Microcystin-producing strains as well as strains that do not synthesize microcystin have been reported for all species within the genus Microcystis. However, whereas most field samples and strains of Microcystis aeruginosa and Microcystis viridis studied to date were found to contain microcystins (17, 47, 49–51), strains of M. wesenbergii, M. novaceckii, and M. ichthyoblabe have only sporadically been reported to contain microcystins (34, 38, 49).
Beside microcystins, various other linear and cyclic oligopeptides such as aeruginosins, anabaenopeptilides, cyanopeptolins, anabaenopeptins, and microginins are found within the genus Microcystis (31). Similar to microcystins, these peptides possess unusual amino acids like 3-amino-6-hydroxy-2-piperidone (Ahp) in cyanopeptolins or 2-carboxy-6-hydroxyoctahydroindol (Choi) in aeruginosin-type molecules, and numerous structural variants also exist within these groups (14, 29, 31). These peptides show diverse bioactivities, frequently protease inhibition (31).
The presence of d-amino acids, unusual amino acids, as well as their small size suggests that the cyanobacterial oligopeptides mentioned above are synthesized nonribosomally by multifunctional enzyme complexes, generally termed peptide synthetases, a pathway studied intensively in other bacteria and fungi (1, 19). The nonribosomal synthesis of microcystins in the axenic strain Microcystis sp. strain PCC 7806 and of anabaenopeptilides in Anabaena sp. strain 90 was recently demonstrated by site-directed mutagenesis and sequencing (6, 42, 46). Nonribosomal peptide synthetase genes have so far been detected in all strains of the genus Microcystis, but genes encoding for the so-called microcystin synthetase are usually detected only in toxic (i.e., microcystin-containing) Microcystis spp. (7, 35). This corresponds to the observation of oligopeptides in all Microcystis strains investigated to date showing various combinations of microcystins and/or other oligopeptides such as aeruginosins, cyanopeptolins, or anabaenopeptins (8, 27, 31).
The cooccurrence of both microcystins and other oligopeptides such as anabaenopeptins and cyanopeptolins in natural Microcystis populations was recently demonstrated (10, 14, 36). It is well known that the species and genotype composition in natural Microcystis populations is heterogeneous, and both microcystin- and non-microcystin-containing strains have been isolated from the same sample (41, 48, 52). Rohrlack et al. (41) isolated 13 Microcystis strains from Lake Wannsee (Berlin, Germany) in 1995 which produced either microcystins or anabaenopeptins (T. Rohrlack, M. Erhard, and M. Henning, unpublished data). Furthermore, isolated strains may show both a different qualitative and quantitative microcystin pattern than the original population (41, 48). These results suggest a considerable diversity of genotypes with different oligopeptide patterns in natural Microcystis populations.
Our study aimed to investigate the inter- and intraspecific oligopeptide diversity in a natural population of the genus Microcystis. Since isolation of strains from natural populations is likely to be selective, we recorded the oligopeptide pattern directly in single Microcystis colonies selected from natural populations using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS).
MATERIALS AND METHODS
Sampling and isolation.
Microcystis spp. were harvested biweekly in hypertrophic Lake Wannsee, Berlin, Germany, from June to October 1999 with a plankton net (40-μm mesh size). The samples were stored in the cool and dark until isolation of Microcystis colonies the same day. The colonies were isolated by serial dilution with tap water and micromanipulation techniques using an inverted microscope at a magnification of ×160 to ×400. Isolated colonies were washed by transferring them to several drops of water until all other organisms were removed.
Epiphytic cyanobacteria and algae sticking in the mucilage of Microcystis aeruginosa could not be detached (Table 1). Cell size and morphological characteristics were recorded for each colony and species were determined according to Komárek and Anagnostidis (21) (Table 1). Additionally, aliquots of the concentrated net samples were taken and either frozen at −20°C and lyophilized or fixed with formaldehyde solution and stored in the dark for detailed cell size determination. The mean cell diameter of the species was determined by measuring the diameters of 50 cells (10 cells per colony) every sampling day.
TABLE 1.
Colony characteristics and cell size of Microcystis species isolated from Lake Wannsee in 1999
| Species | Colony characteristics | Mean cell diam (μm) ± SD | Reference |
|---|---|---|---|
| M. aeruginosa (Kützing) Kützing | More or less firm, elongated and lobate, usually with distinct holes, net-like clathrate; often more or less dense epiphytic populations of Pseudanabaena muscicola or Nitzschia spp. | 4.92 ± 0.13 | 21 |
| M. ichthyoblabe Kützing | Colonies more or less irregular-spherical, soft; distribution of cells in colonies is irregular or sponge like; margin of colony irregular | 3.39 ± 0.18 | 21 |
| M. wesenbergii (Komárek) Komárek in Kondrateva | Elongated, lobate cells in one layer, with visible margin of mucilage | 5.29 ± 0.24 | 21 |
Extraction and preparation of Microcystis colonies, lyophilized strains, and field samples for MALDI-TOF MS analysis.
Single colonies were directly transferred onto a stainless steel template, and immediately 1 μl of matrix (10 mg of 2,5-dihydroxybenzoic acid per ml in water-acetonitrile [1:1] with 0.03% trifluoroacetic acid) was added. The extraction of the oligopeptides from the cells was achieved by the solvent fraction of the matrix. An immediate change of the colony color from green to brownish yellow after addition of the matrix was observed due to the degradation of chlorophyll a by the acidic solution.
Lyophilized Microcystis field samples, the axenic Microcystis strains PCC 7806 and PCC 7813, and the unialgal Microcystis strains HUB 5-2-4, HUB 5-3, and HUB 063 were extracted with acetonitrile-ethanol-water (1:1:1) with 0.03% trifluoroacetic acid. Then 1 μl of the extract was prepared for MALDI-TOF MS analysis as described for the single colonies.
MALDI-TOF MS analysis.
Positive ion mass spectra were recorded from each colony and the lyophilized field samples and strains using a MALDI-TOF mass spectrometer (Voyager DE-PRO; PerSeptive BioSystems, Framingham, Mass.) equipped with a reflectron. For desorption of the components, a nitrogen laser beam (λ = 337 nm) was focused on the template. The acceleration voltage was set at 20 kV. All measurements were carried out in the delayed extraction mode, allowing the determination of monoisotopic mass values (m/z; mass-to-charge ratio). Analyses were performed in the positive-ion mode, giving mainly singly protonated molecular ions ([M+H]+). Chlorophyll a degradation products phaeophytin a and pheophorbide a with mass values of m/z 871.57 and 593.27 [M+H]+, respectively, were used for internal calibration. A low mass gate of 500 Da improved measurement by filtering out the most intensive matrix ions.
After determination of monoisotopic mass values, post-source decay (PSD) measurements for recording fragment ions were performed directly from the same colony on the template. The precursor ions were selected with a time ion selector having a mass window of 10 mass units. The operating voltages of the reflectron were reduced stepwise to record 12 spectral segments sequentially.
PSD spectra of the most prominent peptides were recorded several times over the entire sampling period from single colonies and additionally from the lyophilized Microcystis samples.
RESULTS
Species determination.
The Microcystis population in Lake Wannsee in 1999 consisted of several species which were present during the entire investigation period. The majority of the isolated colonies belonged to one of three species with distinct colonial features and cell sizes (Table 1): M. aeruginosa (Kützing) Kützing, M. ichthyoblabe Kützing, and M. wesenbergii (Komárek) Komárek in Kondrateva. The other colonies isolated could not be unequivocally determined to species level and thus were grouped as Microcystis spp. About half of these colonies exhibited colonial characteristics similar to those of Microcystis ichthyoblabe but had larger cell sizes than the main phenotype isolated from this species. The other colonies showed diverse variations in cell size and colonial characteristics, often similar to features described for M. flos-aquae or M. novaceckii (Komárek) Compère. The sizes of the colonies isolated ranged between 0.2 and 4 mm, as measured at their longest dimension.
Oligopeptides identified by MALDI-TOF MS.
Positive-ion mass spectra were recorded from the Microcystis strains, from 258 Microcystis colonies, and from the entire Microcystis population each time colonies were isolated (Fig. 1 and 2). In the mass range of m/z 500 to 1,100 Da, numerous structural variants of microcystins, anabaenopeptins, microginins, aeruginosins, and one cyanopeptolin were identified by means of characteristic fragment ions obtained by PSD measurements (Table 2). Most of these components are well known, and data about their structures and PSD data have been published previously (9, 10, 12) (Table 2). As an example, the PSD spectrum of microcystin-LR with characteristic fragment ions leading to an unambiguous structure assignment is given in Fig. 3 (32). Fragment analysis assigned peptides with mass values of m/z 726, 728, 740, 742, 749, and 751 [M+H]+ as microginin variants previously identified by means of amino acid analysis and PSD measurements (Table 2) (U. Neumann, J. Weckesser, and M. Erhard, unpublished data). The compound with a molecular mass of m/z 603 [M+H]+ is probably a new variant of an aeruginosin-type peptide, as suggested by the fragment ion of m/z 140, indicating the presence of the unusual amino acid Choi, which is unique to aeruginosin-type molecules (8, 28, 29).
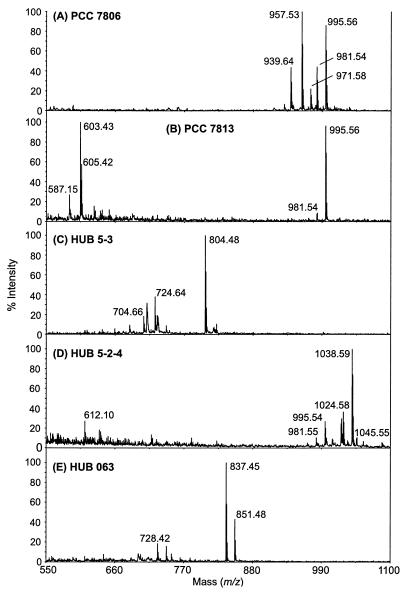
FIG. 1.
Positive-ion MALDI-TOF mass spectra (m/z range 550 to 1,100 Da) of Microcystis strains PCC 7806 (axenic) (A), PCC 7813 (axenic) (B), HUB 5-3 (unialgal) (C), HUB 5-2-4 (unialgal) (D), and HUB 063 (unialgal) (E). For structure assignments, see Table 2.
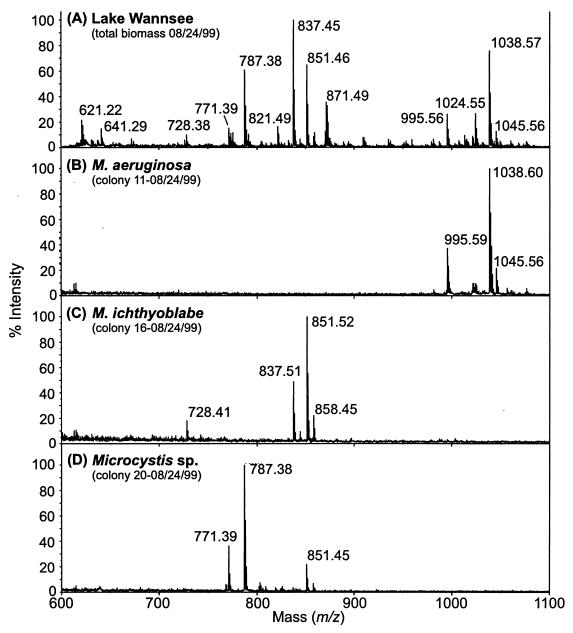
FIG. 2.
Positive-ion MALDI-TOF mass spectra (m/z range, 600 to 1,100 Da) of the entire Microcystis population (A) and of colonies of M. aeruginosa (B), M. ichthyoblabe (C), and Microcystis sp. (D) in Lake Wannsee on 24 August 1999. For structure assignments, see Table 2.
TABLE 2.
Oligopeptides detected in Microcystis colonies from Lake Wannsee in 1999 using MALDI-TOF MS (results from positive-ion mass spectra and PSD measurements)
| Substance class | [M+H]+ | Assignment | Reference(s) |
|---|---|---|---|
| Microcystin | 981 | [Dha7] MC-LR | 32, 12 |
| 995 | MC-LR | 32, 12 | |
| 1,024 | [Dha7] MC-RR | 18, 12 | |
| 1,038 | MC-RR | 32, 12 | |
| 1,045 | MC-YR | 32, 12 | |
| 1,049 | [H4] MC-YR | 33, 12 | |
| 1,068 | MC-WR | 32, 12 | |
| Anabaenopeptin | 821 | Anabaenopeptin I | 30 |
| 837 | Anabaenopeptin B | 13, 10 | |
| 844 | Anabaenopeptin A | 13 | |
| 851 | Anabaenopeptin F | 44, 9 | |
| 858 | Oscillamide Y | 43 | |
| Microginin | 726 | Microginin FR5 | PSD fragmentation; Neumann et al., unpublished data |
| 728 | Microginin FR3 | See microginin FR5 | |
| 740 | Microginin FR6 | See microginin FR5 | |
| 742 | Microginin FR2 | See microginin FR5 | |
| 749 | Microginin FR10 | See microginin FR5 | |
| 751 | Microginin FR9 | See microginin FR5 | |
| Aeruginosin | 561 | Aeruginosamide | 25 |
| 603 | Aeruginosin-type | PSD fragmentation | |
| 609 | Microcin SF608 | 2 | |
| 653a | Aeruginosin 102A | 28, 8 | |
| Cyanopeptolin | 846b/828c | Cyanopeptolin-S | 14, 8 |
| 957b/939c | Cyanopeptolin D | 27 |
[M − SO3 + H]+.
[M − SO3 + H]+.
[M − SO3 − H2O + H]+.
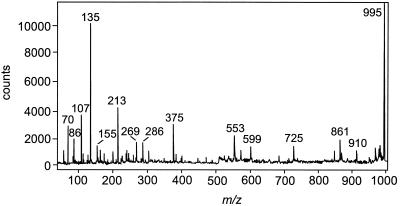
FIG. 3.
PSD spectrum of microcystin-LR: m/z 995 [M + H]+, 861 [M − 134 (Adda side chain) + H]+, 599 [Arg−Adda−Glu + H]+, 375 [C11H14O−Glu−Mdha]+, 286 [Arg−MeAsp + H]+, 213 [Glu−Mdha + H]+, 155 [Mdha−Ala + H]+, 135 [PhCH2CH(OCH3)]+, and 70 [Leu − CO + H]+.
In addition, many unknown substances were detected in the colonies, of which those with mass values of m/z 619, 635, 771, 787, 804, 846, 1,007, 1,009, 1,015, and 1,021 were the most abundant.
Inter- and intraspecific oligopeptide diversity.
Mass spectra from the whole Microcystis population in Lake Wannsee in 1999 showed a complex mixture of different microcystins, microginins, anabaenopeptins, and unknown components (Fig. 2A). In contrast, isolated Microcystis strains usually have a less diverse peptide pattern. As shown in Fig. 1, the axenic Microcystis strains PCC 7806 and PCC 7813 contain largely MC-LR and [Asp3] MC-LR and either cyanopeptolin D or the unknown peptide of m/z 603 [M+H]+ (Fig. 1A and B; Table 2) (27). Microcystins are the most abundant oligopeptides in the unialgal strain HUB 5-2-4, while in HUB 5-3 an unknown peptide of m/z 804 [M+H]+ and in HUB 063 anabaenopeptin B and F are found (Fig. 1C to E).
Similar to the clonal strains, the peptide patterns in the single Microcystis colonies were usually less complex (Fig. 2B to D). Comparison of the peptide composition in the total Microcystis population with that of single colonies suggests that the population is dominated by colonies with the specific oligopeptide patterns shown in Fig. 2B to D.
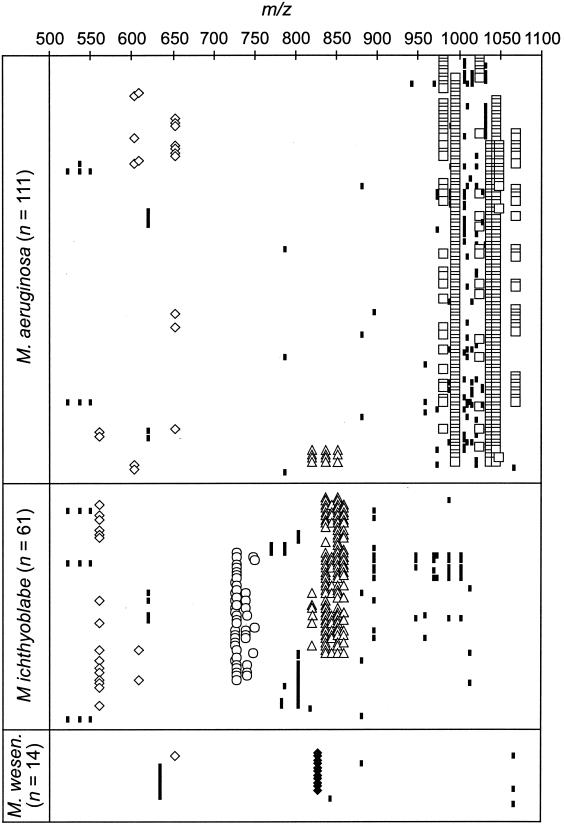
The oligopeptide patterns in colonies of M. aeruginosa, M. ichthyoblabe, and M. wesenbergii revealed pronounced differences (Fig. 2B to D and Fig. 4). In all but 2 of 111 M. aeruginosa colonies, microcystins were the chief oligopeptides detected (Fig. 2B, Fig. 4). The microcystin profiles within this species were rather homogeneous, with MC-RR, MC-YR, and MC-LR being codominant in most colonies. Similar microcystin compositions were found both by MALDI-TOF MS (Fig. 2A) and by high-pressure liquid chromatography (HPLC) analysis (data not shown) of the whole population in Lake Wannsee. MC-RR was detected in 79%, MC-YR in 89%, and MC-LR in 94% of all M. aeruginosa colonies. Minor microcystins, as indicated by low peak intensities and small amounts in HPLC analysis, found in M. aeruginosa were [Dha7] MC-RR, [Dha7] MC-LR, [H4] MC-YR, and MC-WR. They were detected in 13 to 37% of all colonies. In some colonies of M. aeruginosa, only [Dha7] MC-RR and [Dha7] MC-LR were found. In addition to microcystins, aeruginosins were present in some colonies, and in four colonies anabaenopeptins could be detected (Fig. 4). Unknown components were detected in many M. aeruginosa colonies, occasionally with molecular weights corresponding to those of known microcystin variants. However, signal intensity was too low to obtain reliable PSD spectra.
FIG. 4.
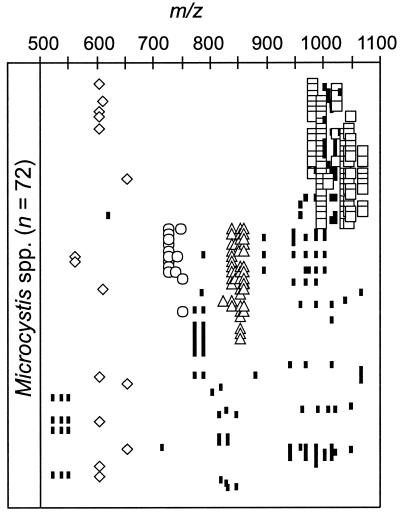
Molecular masses (m/z) oligopeptides and unknown components in the range of m/z 500 to 1,100 Da detected in colonies of M. aeruginosa, M. ichthyoblabe, and M. wesenbergii. Symbols for substance classes: open diamonds, aeruginosin; open circles, microginin; open triangles, anabaenopeptin; open squares, microcystin; solid diamonds, cyanopeptolin; small solid rectangles, unknown components. For structure assignments of compounds, see Table 2.
In order to control for the homogeneity of the colonies, eight large colonies of M. aeruginosa were divided into two to four parts, and each part was analyzed separately. No difference in the microcystin pattern was found between the single parts (data not shown). Furthermore, no relationship between the presence of epiphytic cyanobacteria and algae in the M. aeruginosa colonies and their peptide pattern was found.
By contrast, microcystins were never detected in colonies of M. ichthyoblabe (Fig. 2C, Fig. 4). The oligopeptide pattern within this group was more diverse: they contained either mainly anabaenopeptins or microginins or both microginins and anabaenopeptins or various unknown peptides. Anabaenopeptins B and F and oscillamide Y were the most prominent anabaenopeptins, while anabaenopeptins I and A were less abundant. Major microginins were FR5 and FR3, while all other variants of this substance class were less frequent. Aeruginosins were also detected in the colonies of M. ichthyoblabe (Fig. 4).
M. wesenbergii colonies also did not contain microcystins. The colonies of this species show very similar patterns, with 11 of 14 colonies containing cyanopeptolin-S together with an unknown peptide of m/z 635 [M+H]+ (Fig. 4).
Oligopeptide patterns of Microcystis colonies not identified to species level (Microcystis spp.) mainly fall into three clusters, with peptide patterns similar to those observed for M. aeruginosa and M. ichthyoblabe (Fig. 5). The colonies contained either microcystins, microginin and/or anabaenopeptin, or chiefly unknown peptides (Fig. 2D, Fig. 5). Aeruginosins were detected in all three clusters.
FIG. 5.
Molecular masses (m/z) of oligopeptides and unknown components in the range of m/z 500 to 1,100 Da detected in colonies of Microcystis spp. Symbols for substance classes: open diamonds, aeruginosin; open circles, microginin; open triangles, anabaenopeptin; open squares, microcystin; solid diamonds, cyanopeptolin; small solid rectangles, unknown components. For structure assignments of compounds, see Table 2.
DISCUSSION
MALDI-TOF MS analysis of the Microcystis populations from Lake Wannsee in 1995 to 1999 showed a complex and persisting mixture of microcystins, other oligopeptides, and unknown components (8, 10) (Fig. 2A). The cooccurrence of microcystins and cyanopeptolins in other Microcystis sp.-dominated field samples was reported previously (15, 36). By typing single Microcystis colonies, we could show for the first time that the actual peptide diversity in a natural population of this genus is substantially higher. Many of the substances detected belong to well-known groups of cyanobacterial peptides like microcystins, anabaenopeptins, microginins, cyanopeptolins, and aeruginosins, of which many have been discovered in Microcystis spp. (31). In addition, numerous unknown components have been detected in the colonies. However, the origin of these unknown components has yet to be investigated, since besides the observed epiphytic cyanobacteria and algae, heterotrophic bacteria are also known to be present in Microcystis colonies (4).
Usually more than one type of oligopeptide was detected in the Microcystis colonies from Lake Wannsee in 1999. With respect to known peptides, combinations of anabaenopeptins, microginins, and aeruginosins were observed, while microcystins were found along with aeruginosins. This correlates to the detection of aeruginosins as well as cyanopeptolins in both toxic and nontoxic Microcystis culture strains (Fig. 1) (6, 31). Anabaenopeptins and microginins were usually not detected together with microcystins with the exception of four colonies containing both microcystins and anabaenopeptins. Microcystins and anabaenopeptins were also never found simultaneously in more than 20 Microcystis strains investigated to date (M. Erhard, H. von Döhren, P. Jungblut, E. Dittmann, T. Börner, M. Henning, L. Rouhiainen, and K. Sivonen, Abstracts of the 4th European Workshop on the Molecular Biology of Cyanobacteria, p. 29, 1999). However, it must be considered that isolation of strains is selective and may pick up only those genotypes which are favored by the cultivation conditions. On the other hand, although one colony can be regarded as a clone and homogeneity was found for all of the divided colonies, a potential bias of conducting studies with selected colonies may be the contamination of a colony with cells of other clones.
Our data revealed a relationship between oligopeptide patterns and certain Microcystis taxa in Lake Wannsee. Microcystins were chiefly found in M. aeruginosa, while colonies of M. ichthyoblabe and M. wesenbergii did not contain microcystins but did contain anabaenopeptins, microginins, cyanopeptolins, or unknown peptides. Essentially the same results were found for the presence of the microcystin synthetase genes in single Microcystis colonies from Lake Wannsee in 2000 (24); microcystin synthetase genes were detected in 73% of M. aeruginosa colonies but in only 16% of colonies assigned to M. ichthyoblabe and in 0% of M. wesenbergii colonies. The low oligopeptide diversity within M. aeruginosa suggests that the species-related peptide patterns observed may have been caused by the dominance of only certain genotypes within the Microcystis species in Lake Wannsee in 1999. Restriction fragment length polymorphism of the mcyB gene indicates the presence of five different genotypes among M. aeruginosa colonies with similar microcystin profiles in Lake Wannsee in 2000 (24). The occurrence of various combinations of several types of oligopeptides in M. ichthyoblabe implies a higher genotype diversity within this species in Lake Wannsee in 1999.
M. aeruginosa is worldwide the species most often associated with toxic water blooms and microcystin-producing strains (38, 47, 50), while the majority of M. wesenbergii and M. ichthyoblabe strains reported in the literature did not produce microcystin, although microcystin-producing strains are occasionally described (34, 38, 49). Japanese strains of a M. aeruginosa S-type (i.e., small cell size), which were later classified as M. ichthyoblabe (51), also rarely contained microcystins (50). Rohrlack et al. (41) isolated 13 Microcystis strains from Lake Wannsee in 1995, of which the strains having larger cells contained microcystins, while those with smaller cells produced only anabaenopeptins (T. Rohrlack, M. Erhard, and M. Henning, unpublished data). Though these data support some relationship between morphospecies and microcystin production, we also detected the peptide patterns typical for M. aeruginosa and M. ichthyoblabe in colonies with colonial characteristics different from these species (these were grouped as Microcystis spp.).
However, colony morphology and cell size, traditionally used in taxonomic differentiation of the genus Microcystis (20, 21), may be questionable criteria for species distinction; both parameters have been found to be variable in laboratory cultures and in the field (22, 39, 40). In culture particularly some strains of M. aeruginosa, M. ichthyoblabe, and M. novaceckii have developed colony morphologies similar to each other (39). Recent studies investigating the taxonomy of the genus Microcystis using genetic criteria in comparison to morphological traits and microcystin production show contradictory results. 16S rRNA analysis revealed no differences between different toxic and nontoxic strains of M. aeruginosa, M. wesenbergii, and M. viridis (34). In contrast, sequence data for the 16S to 23S internally transcribed spacer lead to three clusters, of which cluster I contained both toxic and nontoxic strains of M. aeruginosa, M. novaceckii, and M. ichthyoblabe, cluster II only toxic strains of mainly M. viridis, and cluster III only nontoxic strains of mainly M. wesenbergii (38). Similarly, allozyme divergence studied by Kato et al. (16) characterized M. wesenbergii and M. viridis as well-established species. In contrast to Otsuka et al. (38), allozyme divergence (16) revealed a separation of Japanese M. aeruginosa L-type strains (equivalent to M. aeruginosa) from strains of M. aeruginosa type S (included in M. ichthyoblabe [51]), which corresponds to our observation of a different peptide pattern in these species. More data are needed to determine whether or not the oligopeptide pattern may be used as a chemotaxonomical feature to clarify the taxonomic uncertainties within the genus Microcystis. These should include systematic studies on the distribution of oligopeptides in combination with morphological and molecular characterization of more strains and original colonies from different regions.
Our study demonstrates the unequivocal identification of microcystins and other oligopeptides in single Microcystis colonies by employing MALDI-TOF MS. It is thus possible to directly identify the toxic species and genotypes in natural Microcystis populations without time-consuming and probably selective isolation procedures. The typing of single Microcystis colonies may be a valuable tool in early warning of toxic bloom formation, since it enables rapid detection of whether or not a population contains microcystin-producing genotypes in an early phase of population growth. Furthermore, the succession of toxic and nontoxic species may be followed and the influence of biotic and abiotic factors on genotype succession assessed. In Lake Wannsee in 1999, M. aeruginosa colonies showed a persisting composition of MC-RR, MC-YR, and MC-LR over the entire sampling period from June to October. Similar quantitative relations of microcystins were determined in the whole Microcystis population by both HPLC and MALDI-TOF MS during this time span. This indicates that this type of M. aeruginosa colony determined the overall microcystin pattern in 1999.
Hypotheses about possible functions of microcystins often focused on the toxicity of microcystins. Although microcystins have been shown to affect diverse aquatic organisms (for an overview, see reference 45), they do not necessarily seem to be produced as a defense mechanism against zooplankton grazing. Speculations about an inter- or intracellular function of microcystins raise the question about substances playing a similar role in genotypes without microcystins (37). This is supported by our observation of various oligopeptides in the Microcystis population investigated. The coexistence of genotypes producing either mainly microcystins or other oligopeptides throughout the investigation period suggests that a comprehensive understanding of their possible functions and ecological benefits requires studying oligopeptides as a group rather than focusing on microcystins.
ACKNOWLEDGMENTS
We thank Frank Grützner and Adam Antebi and his group (Max Plank Institute for Molecular Genetics, Berlin, Germany) for kindly providing micromanipulation facilities. Microcystis strains were kindly provided by Rosemarie Rippka (Institute Pasteur, Paris, France) and Manfred Henning (Humboldt University, Berlin, Germany).
This work was financially supported by funds from the EU (ENV4-CT98-802).
REFERENCES
- 1.Arment A R, Carmichael W W. Evidence that microcystin is a thiotemplate product. J Phycol. 1996;32:591–597. [Google Scholar]
- 2.Banker R, Carmeli S. Inhibitors of serine proteases from a waterbloom of the cyanobacterium Microcystis sp. Tetrahedron. 1999;55:10835–10844. [Google Scholar]
- 3.Bell S G, Codd G A. Cyanobacterial toxins and human health. Rev Med Microbiol. 1994;5:256–264. [Google Scholar]
- 4.Brunberg A K. Contribution of bacteria in the mucilage of Microcystis spp. (cyanobacteria) to benthic and pelagic bacterial production in a hypertrophic lake. FEMS Microbiol Lett. 1999;29:13–22. [Google Scholar]
- 5.Carmichael W W, Beasly V, Bunner D L, Eloff J N, Falconer I, Gorham P, Harada K I, Krishnamurty T, Min-Juan Y, Moore R E, Rinehart K, Runnegar M, Skulberg O M, Watanabe M. Naming cyclic heptapeptide toxins of cyanobacteria (blue-green algae) Toxicon. 1988;26:971–973. doi: 10.1016/0041-0101(88)90195-x. [DOI] [PubMed] [Google Scholar]
- 6.Dittmann E, Neilan B A, Erhard M, von Döhren H, Börner T. Insertional mutagenesis of a peptide synthetase gene that is responsible for hepatotoxin production in the cyanobacterium Microcystis aeruginosa PCC 7806. Mol Microbiol. 1997;26:779–787. doi: 10.1046/j.1365-2958.1997.6131982.x. [DOI] [PubMed] [Google Scholar]
- 7.Dittmann E, Christiansen G, Neilan B A, Fastner J, Rippka R, Börner T. Peptide synthetases genes occur in various species of cyanobacteria. In: Peschek G A, Loeffelhardt W, Schmetterer G, editors. The phototrophic prokaryotes. New York, N.Y: Kluwer Academic/Plenum Publishing Corp.; 1999. pp. 615–621. [Google Scholar]
- 8.Erhard M. Ph.D. thesis. Berlin, Germany: Technische Universität Berlin; 1999. [Google Scholar]
- 9.Erhard M, von Döhren H, Jungblut P. Rapid typing and elucidation of new secondary metabolites of intact cyanobacteria using MALDI-TOF mass spectrometry. Nat Biotechnol. 1997;15:906–909. doi: 10.1038/nbt0997-906. [DOI] [PubMed] [Google Scholar]
- 10.Erhard M, von Döhren H, Jungblut P. Rapid identification of the new anabaenopeptin G from Planktothrix agardhii HUB 011 using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 1999;13:337–343. doi: 10.1002/(SICI)1097-0231(19990315)13:5<337::AID-RCM488>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Falconer I R. Algal toxins and human health. In: Hrubec J, editor. The handbook of environmental chemistry, vol. 5, part C: quality and treatment of drinking water II. Berlin, Germany: Springer-Verlag; 1998. pp. 53–82. [Google Scholar]
- 12.Fastner J, Erhard M, Carmichael W W, Sun F, Rinehart K L, Rönicke H, Chorus I. Characterization and diversity of microcystins in natural blooms and strains of the genera Microcystis and Planktothrix from German freshwaters. Arch Hydrobiol. 1999;145:147–163. [Google Scholar]
- 13.Harada K-I, Fujii K, Shimada T, Suzuki M, Sano H, Adachi K, Carmichael W W. Two cyclic peptides, anabaenopeptins, a third group of bioactive compounds from the cyanobacterium Anabaena flos-aquae NRC 525-167. Tetrahedron Lett. 1995;36:1511–1514. [Google Scholar]
- 14.Jacobi C, Oberer L, Quiquerez C, König W A, Weckesser J. Cyanopeptolin S, a sulfate containing depsipeptide from a water-bloom of Microcystis aeruginosa. FEMS Microbiol Lett. 1995;129:129–134. doi: 10.1111/j.1574-6968.1995.tb07569.x. [DOI] [PubMed] [Google Scholar]
- 15.Jacobi C, Rinehart K L, Codd G A, Carmienke I, Weckesser J. Occurrence of toxic water blooms containing microcystins in a German lake over a three year period. J Syst Appl Microbiol. 1996;19:249–254. [Google Scholar]
- 16.Kato T, Watanabe M F, Watanabe M. Allozyme divergence in Microcystis (Cyanophyceae) and its taxonomic inference. Algol Studies. 1991;64:129–140. [Google Scholar]
- 17.Kaya K, Watanabe M M. Microcystin composition of an axenic clonal strain of Microcystis viridis and Microcystis viridis-containing waterblooms in Japanese freshwaters. J Appl Phycol. 1990;2:173–178. [Google Scholar]
- 18.Kiviranta J, Namikoshi M, Sivonen K, Evans W R, Carmichael W W, Rinehart K L. Structure determination and toxicity of a new microcystin from Microcystis aeruginosa strain 205. Toxicon. 1992;30:1093–1098. doi: 10.1016/0041-0101(92)90054-9. [DOI] [PubMed] [Google Scholar]
- 19.Kleinkauf H, von Döhren H. A nonribosomal system of peptide biosynthesis. Eur J Biochem. 1996;236:335–351. doi: 10.1111/j.1432-1033.1996.00335.x. [DOI] [PubMed] [Google Scholar]
- 20.Komárek J. A review of water-bloom-forming Microcystis species, with regard to populations from Japan. Algol Studies. 1991;64:115–127. [Google Scholar]
- 21.Komárek J, Anagnostidis K. Cyanoprokaryota 1, Teil: Chroococcales. In: Ettl H, Gärtner G, Heynig H, Mollenhauer D, editors. Süßwasserflora von Mitteleuropa Band 19/1. Stuttgart, Germany: Gustav Fischer Verlag; 1999. [Google Scholar]
- 22.Krüger G H J, Eloff J N, Pretorius J A. Morphological changes in toxic and non-toxic Microcystis isolates at different irradiance levels. J Phycol. 1991;17:52–56. [Google Scholar]
- 23.Kuiper-Goodman T, Falconer I, Fitzgerald J. Human health aspects, p 113–153. In: Chorus I, Bartram J, editors. Toxic cyanobacteria in water. London, England: E & FN Spon; 1999. [Google Scholar]
- 24.Kurmayer, R., E. Dittmann, J. Fastner, and I. Chorus. Diversity of microcystin genes within a population of the toxic cyanobacterium Microcystis spp. in Lake Wannsee. Microb. Ecol., in press. [DOI] [PubMed]
- 25.Lawton L A, Morris L A, Jaspers M. A bioactive modified peptide, aeruginosamide, isolated from the cyanobacterium Microcystis aeruginosa. J Org Chem. 1999;64:5329–5332. doi: 10.1021/jo990247i. [DOI] [PubMed] [Google Scholar]
- 26.MacKintosh C, Beattie K A, Klump S, Cohen P, Codd G A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2a from both mammals and higher plants. FEBS Lett. 1990;264:187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- 27.Martin C, Oberer L, Ino T, König W A, Busch M, Weckesser J. Cyanopeptolins, new depsipeptides from the cyanobacterium Microcystis PCC 7806. J Antibiot. 1993;46:1550–1556. doi: 10.7164/antibiotics.46.1550. [DOI] [PubMed] [Google Scholar]
- 28.Matsuda H, Okino T, Murakami M, Yamaguchi K. Aeruginosins 102-A and B, new thrombin inhibitors from the cyanobacterium Microcystis viridis (NIES-102) Tetrahedron. 1997;52:14501–14506. [Google Scholar]
- 29.Murakami M, Ishida K, Okino T, Okita Y, Matsuda H, Yamaguchi K. Aeruginosin 98-A and-B, trypsin inhibitors from the blue-green alga Microcystis aeruginosa (NIES-98) Tetrahedron Lett. 1995;36:2758–2788. [Google Scholar]
- 30.Murakami M, Suzuki S, Itou Y, Kodani S, Ishida K. New anabaenopeptins, potent carboxypeptidase-A inhibitors from the cyanobacterium Aphanizomenon flos-aquae. J Nat Prod. 2000;63:1280–1282. doi: 10.1021/np000120k. [DOI] [PubMed] [Google Scholar]
- 31.Namikoshi M, Rinehart K L. Bioactive compounds produced by cyanobacteria. J Ind Microbiol. 1996;17:373–384. [Google Scholar]
- 32.Namikoshi M, Rinehart K L, Sakai R, Stotts R R, Dahlem A M, Beasley V R, Carmichael W W, Evans W R. Identification of 12 hepatotoxins from a Homer Lake bloom of the cyanobacteria Microcystis aeruginosa, Microcystis viridis, and Microcystis wesenbergii: nine new microcystins. J Org Chem. 1992;57:866–872. [Google Scholar]
- 33.Namikoshi M, Choi B W, Sun F, Rinehart K L, Carmichael W W, Evans W R, Beasley V R. Seven more microcystins from Homer Lake cells: application of the general method structure assignment of peptides containing α,β-dehydroamino acid unit(s) J Org Chem. 1995;60:3671–3679. [Google Scholar]
- 34.Neilan B A, Jacobs D, del Dot T, Blackall L L, Hawkins P R, Cox P T, Goodman A E. rRNA sequences and evolutionary relationship among toxic and nontoxic cyanobacteria of the genus Microcystis. Int J Syst Bacteriol. 1997;47:693–697. doi: 10.1099/00207713-47-3-693. [DOI] [PubMed] [Google Scholar]
- 35.Neilan B A, Dittmann E, Rouhiainen L, Bass R A, Schaub V, Sivonen K, Börner T. Nonribosomal peptide synthesis and toxigenicity of cyanobacteria. J Bacteriol. 1999;181:4089–4097. doi: 10.1128/jb.181.13.4089-4097.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neumann U, Campos V, Cantarero S, Urrutia H, Heinze R, Weckesser J, Erhard M. Co-occurrence of non-toxic (cyanopeptolin) and toxic (microcystin) peptides in a bloom of Microcystis sp. from a Chilean lake. Syst Appl Microbiol. 2000;23:191–197. doi: 10.1016/S0723-2020(00)80004-1. [DOI] [PubMed] [Google Scholar]
- 37.Orr P T, Jones G J. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol Oceanogr. 1998;43:1604–1614. [Google Scholar]
- 38.Otsuka S, Suda S, Li R, Watanabe M, Oyaizu H, Matsumoto S, Watanabe M M. Phylogenetic relationships between toxic and non-toxic strains of the genus Microcystis based on 16S to 23S internal transcribed spacer sequences. FEMS Microbiol Lett. 1999;172:15–21. doi: 10.1111/j.1574-6968.1999.tb13443.x. [DOI] [PubMed] [Google Scholar]
- 39.Otsuka S, Suda S, Li R, Matsumoto S, Watanabe M M. Morphological variability of colonies of Microcystis morphospecies in culture. J Gen Appl Microbiol. 2000;46:39–50. doi: 10.2323/jgam.46.39. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds C S, Jaworski G H M, Cmiech H A, Leedale G F. On the annual cycle of the blue-green alga Microcystis aeruginosa Kütz. Emend Elenkin Phil Trans R Soc London B. 1981;293:419–477. [Google Scholar]
- 41.Rohrlack T, Henning M, Kohl J-G. Isolation and characterisation of colonyforming Microcystis aeruginosa strains. In: Chorus I, editor. Cyanotoxins—occurrence, causes, consequences. Heidelberg, Germany: Springer Verlag; 2001. pp. 152–158. [Google Scholar]
- 42.Rouhiainen L, Paulin L, Suomalainen S, Hyytiäinen H, Buikema W, Hasselkorn R, Sivonen K. Genes encoding syntheses of cyclic depsipeptides, anabaenopeptilides, in Anabaena strain 90. Mol Microbiol. 2000;37:156–167. doi: 10.1046/j.1365-2958.2000.01982.x. [DOI] [PubMed] [Google Scholar]
- 43.Sano T, Kaya K. Oscillamide Y, a chymotrypsin inhibitor from toxic Oscillatoria agardhii. Tetrahedron Lett. 1995;36:5933–5936. [Google Scholar]
- 44.Shin H J, Matsuda H, Murakami M, Yamaguchi K. Anabaenopeptins E and F, two new cyclic peptides from the cyanobacterium Oscillatoria agardhii (NIES-204) J Nat Prod. 1997;60:139–141. [Google Scholar]
- 45.Sivonen K, Jones G. Cyanobacterial toxins. In: Chorus I, Bartram J, editors. Toxic cyanobacteria in water. London, England: E & FN Spon; 1999. pp. 41–111. [Google Scholar]
- 46.Tillett D, Dittmann E, Erhard M, von Döhren H, Börner T, Neilan B A. Structural organisation of the microcystin biosynthesis in Microcystis aeruginosa PCC7806: an integrated peptide-polyketide synthetase system. Chem Biol. 2000;7:753–764. doi: 10.1016/s1074-5521(00)00021-1. [DOI] [PubMed] [Google Scholar]
- 47.Vasconcelos V M, Sivonen K, Evans W R, Carmichael W W, Namikoshi M. Isolation and characterization of microcystins (heptapeptide hepatotoxins) from Portuguese strains of Microcystis aeruginosa Kutz. emend Elekin. Arch Hydrobiol. 1995;134:295–305. [Google Scholar]
- 48.Vezie C, Brient L, Sivonen K, Bertru G, Lefeuvre J-C, SalkinojaSalonen M. Variation of microcystin content of cyanobacterial blooms and isolated strains in Lake Grand-Lieu (France) Microb Ecol. 1998;35:126–135. doi: 10.1007/s002489900067. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe M F, Oishi S, Harada K-I, Matsuura K, Kawai H, Suzuki M. Toxins contained in Microcystis species of cyanobacteria (blue-green algae) Toxicon. 1988;26:1017–1025. doi: 10.1016/0041-0101(88)90200-0. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe M F, Watanabe M, Kato T, Harada K-I, Suzuki M. Composition of cyclic peptide toxins among strains of Microcystis aeruginosa (blue-green algae, cyanobacteria) Bot Mag Tokyo. 1991;104:49–57. [Google Scholar]
- 51.Watanabe M. Isolation, cultivation and classification of bloom-forming Microcystis in Japan. In: Watanabe M F, Harada K-I, Carmichael W W, Fujiki H, editors. Toxic Microcystis. Boca Raton, Fla: CRC Press; 1996. pp. 13–35. [Google Scholar]
- 52.Welker M, Hoeg S, Steinberg C. Hepatotoxic cyanobacteria in the shallow lake Müggelsee. Hydrobiologia. 1999;408/409:263–268. [Google Scholar]