Abstract
Purpose:
To compare the efficacy and safety between epirubicin-loaded DC Beads (DCB-TACE) and conventional TACE (cTACE) used in transarterial chemoembolization (TACE) for hepatocellular carcinoma (HCC).
Materials and Methods:
This retrospective study enrolled 64 patients (mean age, 73.3 years; 44 men, 20 women) who underwent initial DCB-TACE between 2014 and 2015, and 66 patients (mean age, 71.3 years; 38 men, 28 women) who underwent initial cTACE between 2011 and 2013 as historical controls. Treatment effects on the target lesions at 3 months after TACE, the period until re-treatment of the target lesion, and adverse events after TACE were compared between the groups. Univariate and multivariate analyses were also performed to estimate the factors influencing the treatment effects.
Results:
Based on the Response Evaluation Criteria in Cancer of the Liver version 2015, treatment response of the target lesions equivalent to a complete response and termed as TE4, was 51.0% (53/104) in the DCB-TACE group and 74.4% (64/86) in the cTACE group (p<0.001). Multivariate analysis revealed that the TACE procedure, Child-Pugh score, serum aspartate aminotransferase (AST) level, alpha fetoprotein level, and tumor size were independent significant predictors of TE4. The frequencies of elevated serum AST and alanine transaminase levels after TACE were significantly lower in patients in the DCB-TACE group (p<0.001 each). No significant difference in biliary/liver damage was evident between the groups.
Conclusion:
The local efficacy of cTACE was higher than that of DCB-TACE. Adverse events were milder after DCB-TACE than after cTACE.
Keywords: TACE, DC-Bead, lipiodol
Introduction
Transarterial chemoembolization (TACE) is widely performed for unresectable hepatocellular carcinoma (HCC) and is recommended as the first-line treatment for the intermediate stage of the Barcelona Clinic Liver Cancer (BCLC) classification, BCLC-B [1]. The conventional TACE (cTACE) procedure was developed in the 1980's [2]. A prospective study by the Japan and Korea Interventional Radiology in Oncology Study Group showed favorable local efficacy with reasonable survival data and tolerable adverse events [3].
On the other hand, TACE procedures using drug-eluting beads (DEB) have been reported since the 2000's [4], with good treatment effects and mild adverse events. Some randomized controlled trials comparing TACE using drug-eluting beads and TACE using lipiodol have been reported, and all such reports have indicated comparable treatment effects, with a lower frequency of adverse events in the drug-eluting bead group [5, 6].
Since 2014, two drug-eluting beads have been covered by insurance in Japan: DC Beads (Eisai, Tokyo, Japan) and Hepasphere (Nippon Kayaku, Tokyo, Japan). With increasing treatment options for TACE, the best use of lipiodol or drug-eluting beads should be considered for each clinical case.
The purpose of this study was to compare the local efficacy and safety between epirubicin-loaded DC Beads (DCB-TACE) and conventional TACE (cTACE) used in TACE for HCC.
Materials & Methods
Study design and participants
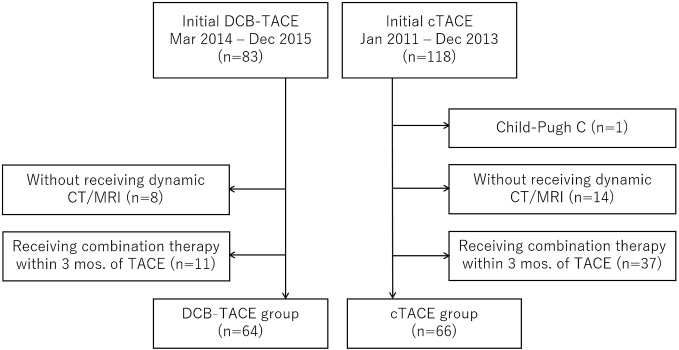
This retrospective study was conducted in a single institution. Eighty-three patients received initial DCB-TACE for HCC between March 2014 and December 2015 (DCB-TACE group). On the other hand, 118 patients received initial cTACE for HCC between January 2011 and December 2013 (cTACE group). A patient with Child-Pugh C (1 patient in the cTACE group), those who did not undergo dynamic computed tomography (CT) or magnetic resonance imaging (MRI) before and after TACE for any reasons (8 patients in the DCB-TACE group; 14 patients in the cTACE group), and those who received combination therapy with other treatments (e.g., radiofrequency ablation, operation, radiation therapy) within 3 months of TACE (11 patients in the DCB-TACE group; 37 patients in the cTACE group) were excluded. Finally, 64 patients in the DCB-TACE group and 66 patients in the cTACE group were enrolled in this study. A consort diagram is shown in Figure 1. The study protocol was approved (approval number 18008) by the institutional review board.
Figure 1.
Consort diagram
Treatment strategy for HCC
The treatment strategy for each HCC patient was determined through a multidisciplinary conference among hepatologists, surgeons, and radiologists. Selection of the TACE procedure was mainly determined by the interventional radiologists. The same TACE method was applied for recurrent or residual HCC at least twice; if the treatment effect was insufficient, the method was switched to the other.
TACE procedure
TACE was performed using the femoral approach under local anesthesia. Celiac and hepatic arteriograms as well as CT during arterial portography and CT during hepatic arteriography were performed to evaluate tumor localization and feeding arteries. The microcatheter was inserted into the feeding artery as selectively as possible, and embolic materials were infused under fluoroscopic guidance.
In the DCB-TACE group, a 2-mL vial of 100 μm -300 μm DC beads was loaded with 50 mg of epirubicin hydrochloride (Epirubicin hydrochloride “NK”; Nippon Kayaku, Tokyo, Japan) dissolved in 2 mL of distilled water. The solution was then diluted with 10 mL of 300 mg/mL nonionic contrast medium (Iomeron; Eisai, Tokyo, Japan) and 6 mL of normal saline, resulting in 20 mL of diluted DC bead solution (10× diluted solution). The DC bead solution was further diluted 100 times, according to the preference of the operator. The resulting DC bead solution was slowly injected until the tumor stain disappeared on digital subtraction angiography.
In the cTACE group, 5 mL of iodized oil (Lipiodol; Guerbet Japan, Tokyo, Japan) and 50 mg of epirubicin hydrochloride dissolved in 5 mL of non-ionic contrast media were mixed using a three-way stopcock. The total volume of the emulsion was adjusted according to the maximum tumor size. One-millimeter gelatin sponge particles (Gelpart; Nippon Kayaku, Tokyo, Japan) were subsequently injected until near stasis.
Study outcomes
The tumor response was evaluated using the arterial phase of dynamic CT or MRI before (median 34 days) and after treatment. The target lesions were defined as those with major axis >1 cm, up to the two largest lesions, selected based on CT or MRI before treatment. Treatment response of the target lesions (treatment effect [TE]) was evaluated using the Response Evaluation Criteria in Cancer of the Liver [7], based on CT or MRI performed approximately 3 months after TACE. The area of each target lesion before and after TACE was calculated by multiplying the length of the major axis by the maximum diameter crossing the major axis at a right angle. The necrotic (lipiodol accumulation) area of the target lesion after TACE was also calculated using the same method. After the size reduction rate and the necrotizing effect were calculated, the direct TE on each lesion was categorized into four categories: TE4 was equivalent to a complete response (CR) and was defined as 100% tumor-necrotizing effect or 100% tumor size reduction. TE3 was equivalent to partial response (PR) and defined as 50%-100% tumor necrotizing effect or 50%-100% tumor size reduction. TE2 was regarded as stable disease (SD) if the effect was neither PR nor progressive disease (PD). TE1 corresponded to PD with an increase in tumor size of 50% or more, excluding the area of treatment-induced necrosis. The period until re-treatment of the target lesion, adverse events after TACE, biliary injury, and liver damage were also evaluated. The period until re-treatment of target lesions was defined as the period from the day of treatment to the day that the recurrent or residual target lesion was judged as an indication for re-treatment based on follow-up images. Adverse events within 30 days after TACE were evaluated using the Common Terminology Criteria for Adverse Events version 4.0, and biliary injury (intrahepatic biliary duct dilatation and biloma) and liver damage (portal vein thrombosis and liver infarction) were evaluated using CT or MRI.
Statistical analysis
Differences between treatment groups were compared using the chi-square test for categorical variables and Welch's t-test or the Mann-Whitney U-test for continuous variables. The period until re-treatment was calculated using Kaplan-Meier curves, and differences between groups were analyzed using the log-rank test. To estimate the factors influencing the treatment effect, we performed univariate and multivariate analyses of the clinical variables. Univariate analysis was performed to compare the following variables between TE4 and non-TE4 groups using the chi-square test: sex, age, previous treatment, etiology, Child-Pugh class, BCLC stage, number and maximum size of HCC, blood test data (aspartate aminotransferase [AST], alanine transaminase [ALT], albumin, alpha-fetoprotein [AFP], des-gamma-carboxy prothrombin [DCP] ), and treatment group. With regard to continuous variables, the chi-square test was used with multiple cut-off levels, and the appropriate cut-off level was selected. Variables with values of p<0.1, were selected for multiple logistic regression analysis. All statistical tests were two-sided, and values of p<0.05 were regarded as statistically significant.
Results
Patient background
Baseline characteristics are shown in Table 1. The DCB-TACE group showed significantly greater frequencies of Child-Pugh A, BCLC-B, history of previous treatments, and multiple HCCs. Other variables showed no significant differences between the groups.
Table 1.
Baseline characteristics
| Variables | DCB-TACE
n=64 |
cTACE
n=66 |
P-value |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 44 (68.8) | 38 (57.6) | 0.187 |
| Female | 20 (31.2) | 28 (42.4) | |
| Age (y) | 73.3±9.2 | 71.3±13.3 | 0.316 |
| Previous treatments, n (%) | |||
| Yes | 57 (89.1) | 31 (47.0) | <0.001 |
| Resection | 26 (40.6) | 17 (25.8) | |
| RFA/PEIT | 31 (48.4) | 16 (24.2) | |
| cTACE | 49 (76.6) | 0 (0) | |
| Radiation therapy | 1 (1.8) | 0 (0) | |
| No | 7 (10.9) | 35 (53.0) | |
| Etiology, n (%) | |||
| HCV | 38 (59.4) | 43 (65.1) | 0.380 |
| HBV | 9 (14.0) | 7 (10.6) | |
| HCV+HBV | 1 (1.6) | 1 (1.5) | |
| Alcohol | 1 (1.6) | 5 (7.6) | |
| Other | 15 (23.4) | 10 (15.2) | |
| Child-Pugh class, n (%) | |||
| A | 53 (82.8) | 44 (66.7) | 0.034 |
| B | 11 (17.2) | 22 (33,3) | |
| BCLC, n (%) | |||
| 0 | 4 (6.3) | 6 (9.1) | 0.046 |
| A | 24 (37.5) | 39 (59.1) | |
| B | 31 (48.4) | 18 (27.3) | |
| C | 5 (7.8) | 3 (3.5) | |
| Maximum tumor size (mm) | 23.0±17.4 | 28.6±22.7 | 0.114 |
| Tumor number, n (%) | |||
| 1 | 12 (18.7) | 30 (45.4) | 0.002 |
| 2 | 11 (17.2) | 17 (25.8) | |
| 3 | 10 (15.6) | 6 (9.1) | |
| 4 | 3 (4.7) | 2 (3.0) | |
| 5 | 6 (9.4) | 4 (6.1) | |
| ≧6 | 22 (34.4) | 7 (10.6) | |
| AST (IU/L) | 51.9±27.1 | 53.5±25.2 | 0.739 |
| ALT (IU/L) | 36.0±18.1 | 43.7±26.3 | 0.054 |
| Total bilirubin (mg/dL) | 0.79±0.36 | 0.90±0.56 | 0.177 |
| Albumin (g/dL) | 3.63±0.48 | 3.55±0.54 | 0.348 |
| AFP (ng/mL) | 12 (2-14372) | 13.5 (2-30264) | 0.679 |
| DCP (mAU/mL) | 74.5 (10-44254) | 113.5 (11-50286) | 0.463 |
Note. RFA; radiofrequency ablation, PEIT; percutaneous ethanol injection therapy, DEB; drug-eluting beads, TAE: transarterial embolization, TAI; transarterial infusion, TKI; tyrosine kinase inhibitor, HCV; hepatitis C virus, HBV; hepatitis B virus, BCLC; Barcelona Clinic Liver Cancer, AST; aspartate aminotransferase, ALT; alanine transaminase, AFP; alpha fetoprotein, DCP; des-gamma-carboxy prothrombin.
Age, AST, ALT, Total bilirubin, albumin are expressed as mean ± standard deviation.
AFP, DCP are expressed as mean (range).
Treatment effect
The treatment effects after TACE in both groups are shown in Table 2. The TE4 rate of target lesions was 51.0% (53/104) in the DCB-TACE group and 74.4% (64/86) in the cTACE group (p<0.001). The TE4 + TE3 (partial response) rate of target lesions was 64.4% (68/104) in the DCB-TACE group and 87.2% (75/86) in the cTACE group (p<0.001).
Table 2.
Treatment effect on target lesions
| DCB-TACE
n=104 |
cTACE
n=86 |
P-value | |
|---|---|---|---|
| Treatment effect, n (%) | |||
| TE4 | 53 (51.0) | 64 (74.4) | |
| TE3 | 14 (13.5) | 11 (12.8) | |
| TE2 | 33 (31.7) | 9 (10.5) | |
| TE1 | 4 (3.8) | 2 (2.3) | |
| Complete response, n (%) | |||
| TE4 | 53 (51.0) | 64 (74.4) | <0.001 |
| TE1/2/3 | 51 (49.0) | 22 (25.6) | |
| Objective response, n (%) | |||
| TE3/4 | 67 (64.4) | 75 (87.2) | <0.001 |
| TE1/2 | 37 (35.6) | 11 (12.8) |
Note. RECICL (Response Evaluation Criteria in Cancer of the Liver) assessment of direct treatment effect on target lesions; TE4; tumor necrosis of 100% or 100% reduction in tumor size, TE3; tumor necrosis of 50-100% or 50-100% reduction in tumor size, TE2; effect other than TE3 or TE1, TE1; tumor enlargement of ≧50% (excluding the area of necrosis after treatment).
Univariate analysis indicated the following variables as significant predictors of complete response: TACE procedure (DCB-TACE vs. cTACE), etiology (hepatitis C vs. non-hepatitis C), AFP level (>100 ng/mL vs. ≤100 ng/mL), DCP level (>500 mAU/mL vs. ≤500 mAU/mL), Child-Pugh class (A vs. B), BCLC stage (0/A vs. B/C), serum AST level (>40 IU/L vs. ≤40 IU/L), serum albumin level (<3.6 g/dL vs. ≥3.6 g/dL), tumor number (1-3 vs. ≥4), and tumor size (>30 mm vs. ≤30 mm). Multivariate analysis revealed that the TACE procedure, Child-Pugh class, serum AST level, AFP level, and tumor size were independent significant predictors of complete response (TE4) (Table 3).
Table 3.
Uni- and multivariate analyses
| Variables | Univariate
odds ratio (95%CI) |
P-value |
Multivariate
odds ratio (95%CI) |
P-value |
|---|---|---|---|---|
| Sex | ||||
| Male vs. Female | 1.395 (0.762-2.553) | 0.279 | ||
| Age | ||||
| ≧70 vs. <70 | 1.018 (0.546-1.899) | 0.955 | ||
| ≧80 vs. <80 | 1.648 (0.837-3.245) | 0.147 | ||
| Etiology | ||||
| HCV vs. non-HCV | 0.471 (0.249-0.893) | 0.020 | 0.436 (0.175-1.087) | 0.075 |
| HBV vs. non-HBV | 1.944 (0.778-4.858) | 0.150 | ||
| HBV/HCV vs. non-virus | 0.658 (0.323-1.342) | 0.248 | ||
| Previous treatment | ||||
| Yes vs. No | 1.197 (0.636-2.247) | 0.578 | ||
| Child Pugh | ||||
| A vs. B | 2.002 (1.026-3.905) | 0.040 | 3.727 (1.344-10.338) | 0.011 |
| BCLC | ||||
| 0/A vs. B/C | 2.799 (1.508-5.196) | <0.001 | 3.823 (0.949-15.402) | 0.059 |
| AST | ||||
| >40 vs. ≦40 | 0.506 (0.269-0.951) | 0.033 | 0.403 (0.169-0.962) | 0.041 |
| ALT | ||||
| >40 vs. ≦40 | 0.915 (0.502-1.666) | 0.770 | ||
| Alb | ||||
| <3.6 vs. ≧3.6 | 0.288 (0.155-0.534) | <0.001 | 0.713 (0.304-1.671) | 0.437 |
| Number | ||||
| 1 vs. ≧2 | 1.592 (0.768-3.300) | 0.209 | ||
| 1-3 vs. ≧4 | 1.676 (0.929-3.024) | 0.085 | 0.302 (0.073-1.256) | 0.100 |
| 1-5 vs. ≧6 | 1.702 (0.906-3.199) | 0.097 | ||
| Size | ||||
| >20 vs. ≦20 | 0.393 (0.214-0.721) | 0.002 | ||
| >30 vs. ≦30 | 0.240 (0.113-0.509) | <0.001 | 0.267 (0.098-0.729) | 0.010 |
| AFP | ||||
| ≧50 vs. <50 | 0.313 (0.160-0.613) | <0.001 | ||
| ≧100 vs. <100 | 0.248 (0.114-0.539) | <0.001 | 0.256 (0.101-0.649) | 0.004 |
| DCP | ||||
| ≧100 vs. <100 | 0.582 (0.323-1.051) | 0.071 | ||
| ≧500 vs. <500 | 0.294 (0.149-0.580) | <0.001 | 0.477 (0.201-1.131) | 0.093 |
| TACE procedure | ||||
| DCB-TACE vs. cTACE | 0.357 (0.192-0.663) | <0.001 | 0.128 (0.047-0.344) | <0.001 |
Note. HCV; hepatitis C virus, HBV; hepatitis B virus, BCLC; Barcelona Clinic Liver Cancer, AST; aspartate aminotransferase, ALT; alanine transaminase, Alb; albumin, AFP; alpha fetoprotein, DCP; des-gamma-carboxy prothrombin.
The Kaplan-Meier curve for the period until re-treatment is shown in Figure 2. The median period until re-treatment was 147 days in the DCB-TACE group and 453 days in the cTACE group. The period until re-treatment was significantly longer in the cTACE group (p<0.001).
Figure 2.
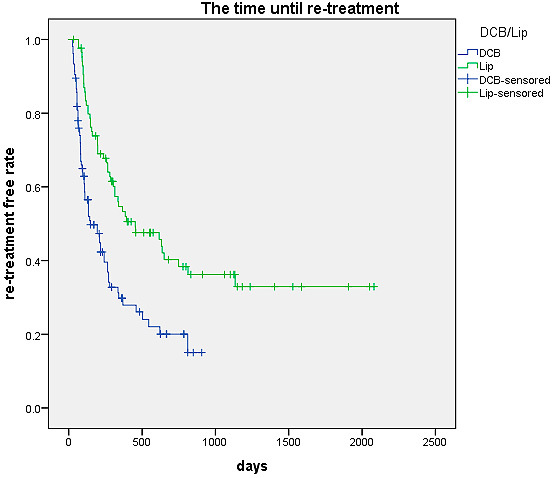
Kaplan-Meier curve of the time until re-treatment
Note. Kaplan-Meier curves for the time until re-treatment showed that the time until re-treatment in the cTACE group was significantly longer (P<0.001).
Adverse events
Adverse events after TACE in both groups are shown in Table 4. Elevated serum AST and ALT levels (grade 3 or higher) were observed in 4 patients (6.25%) and 1 patient (1.56%) respectively, in the DCB-TACE group, and 37 patients (56.1%) and 21 patients (31.8%) respectively, in the cTACE group. These frequencies differed significantly between the groups (p<0.001 each). No significant differences in serum bilirubin or albumin levels were observed between the groups. The incidences of abdominal pain (p=0.003), fever (p=0.016), and fatigue (p=0.020) after TACE were significantly higher in the cTACE group, although these symptoms were all grade 2 or lower.
Table 4.
Adverse events after TACE
| DCB-TACE
n=64 |
cTACE
n=66 |
P-value | |
|---|---|---|---|
| Blood test (≧G3), n (%) | |||
| AST | 4 (6.3) | 37 (56.1) | <0.001 |
| ALT | 1 (1.6) | 21 (31.8) | <0.001 |
| T-bil | 0 (0) | 3 (4.5) | 0.084 |
| Alb | 1 (1.6) | 2 (3.0) | 0.577 |
| Clinical sign (≧G1), n (%) | |||
| Abdominal pain | 11 (17.2) | 27 (40.9) | 0.003 |
| Fever | 12 (18.8) | 25 (37.9) | 0.016 |
| Nausea | 18 (28.1) | 22 (33.3) | 0.520 |
| Vomiting | 8 (12.5) | 8 (12.1) | 0.948 |
| Fatigue | 14 (21.9) | 27 (40.9) | 0.020 |
Note. AST; aspartate aminotransferase, ALT; alanine transaminase, T-bil; total bilirubin, Alb; albumin.
The frequencies of biliary and liver damage after TACE in both the groups are shown in Table 5. Biliary damage (intrahepatic bile duct dilatation and/or biloma) was observed in 8 patients (12.5%) in the DCB-TACE group and 5 patients (7.6%) in the cTACE group. No significant differences were evident between the groups (p=0.349). Portal vein thrombosis was observed in 2 patients (3.0%) in the cTACE group. Liver infarction was observed in 2 patients (3.1%) in the DCB-TACE group and 4 patients (6.1%) in the cTACE group. No significant differences were evident between the two groups (p=0.160 and p=0.425, respectively).
Table 5.
Biliary and liver damage after TACE
| DCB-TACE
n=64 |
cTACE
n=66 |
P-value | |
|---|---|---|---|
| Biliary damage | |||
| Total | 8 (12.5) | 5 (7.6) | 0.349 |
| Biliary duct dilatation | 7 (10.9) | 5 (7.6) | 0.508 |
| Biloma | 3 (4.7) | 4 (6.1) | 0.729 |
| Liver damage | |||
| Portal vein thrombosis | 0 (0) | 2 (3.0) | 0.160 |
| Liver infarction | 2 (3.1) | 4 (6.1) | 0.425 |
Discussion
TACE is an effective treatment option for patients with unresectable HCC. However, some reports have demonstrated that good local treatment effects from TACE correlate with high survival rates [8, 9]. Treatment methods offering higher local treatment effects should thus be preferentially selected when TACE is performed for patients with HCC.
Our study showed that the complete response rate of cTACE was higher than that of DCB-TACE. Treatment methods (cTACE or DCB-TACE) were also independently associated with a complete response. Moreover, the period until re-treatment was longer in the cTACE group than in the DCB-TACE group. However, no previous reports have described local treatment effects from cTACE to be higher than those from DCB-TACE. One report recently described that selective cTACE appeared to have greater efficacy for local tumor control, than selective DCB-TACE [10]. One important factor for recurrence after TACE is the portal venous supply to the recurrent tumor [11, 12]. Embolization of both the feeding artery and drainage portal vein would enhance local treatment effects. Iodized oil injected into the feeding artery would have flowed into the portal vein through the peribiliary plexus, vasa vasorum of the portal vein, or the HCC itself [13]. Miyayama et al. reported that ultra-selective TACE enabled embolization of both the feeding artery and drainage portal vein, and sufficient infusion of iodized oil into the portal vein during a procedure correlated significantly with lower local recurrence rates [2]. On the other hand, in most cases of DCB-TACE, each bead reached a distal feeding artery that a single bead could occlude [14]. Therefore, each bead could not reach the drainage portal vein. Although both embolization and antitumor effects of anticancer drugs are important for local treatment effects, cTACE may be more effective than DCB-TACE in achieving high local embolization effects.
Our study revealed some other predictive factors for complete response: Child-Pugh class, serum AST level, AFP level, and tumor size. Some previous reports have described AST to platelet ratio index, AFP level, and tumor size as predictive factors for complete response [15-17], similar to our study. Although no previous report has described Child-Pugh class as a predictive factor for complete response, Child-Pugh class has been shown to be a predictive factor for overall survival after TACE [18].
Adverse events were less frequent after DCB-TACE than after cTACE, as reported earlier [5, 6]. This advantage is particularly useful for elderly patients and those with poor liver function.
Our study showed that the frequency of liver/biliary damage after TACE did not differ significantly between the groups. Previous reports have described the frequency of such damage as significantly higher after DEB-TACE than after cTACE [19, 20]. The frequency of these injuries after DCB-TACE was low in our study (12.5%) compared with that in previous reports (30.4-36.8%). Our endpoint for DCB-TACE was the disappearance of tumor staining, and not near stasis of the feeding arteries. Further dilution of the DC bead solution was performed to avoid over-embolization. The resulting preservation of feeding arteries may have reduced the incidence of liver/biliary damage.
Our study has several limitations. First, this study was retrospective in design. Second, some background characteristics of patients differed significantly between the groups: previous treatment, Child-Pugh class, BCLC stage, and number of HCC. In particular, most patients (76.6%) in the DCB-TACE group had undergone cTACE. Some recurrent tumors in these patients may have become TACE-refractory because of changes in the tumor biology and damage to the hepatic artery as well as the development of collateral circulation. This may be a disadvantage for the DCB-TACE group. Multivariate analysis was therefore performed, revealing that the TACE procedure was an independent factor associated with complete response. Third, an iodized oil emulsion with a 1:1 ratio was used in our study. This was an oil-in-water emulsion, not a water-in-oil emulsion; therefore, its embolic effects on tumor vessels may be weaker than that of an oil-in-water emulsion.
In conclusion, our study showed that local efficacy was higher in the cTACE group than in the DCB-TACE group. Adverse events were milder after DCB-TACE than after cTACE. The frequency of liver/biliary damage after TACE did not differ significantly between the groups. Our study suggests that cTACE should be performed preferentially, with higher local control and tolerable adverse events.
Conflict of interest
The authors declare that they have no conflicts of interest to report.
This study has been presented at JSIR 2017, JSIR & ISIR 2018.
References
- 1.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908-943. [DOI] [PubMed] [Google Scholar]
- 2.Miyayama S, Matsui O, Yamashiro M, Ryu Y, Kaito K, Ozaki K, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol 2007; 18: 365-376. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda M, Arai Y, Park SJ, Takeuchi Y, Anai H, Kim JK et al. Prospective study of transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: an Asian cooperative study between Japan and Korea. J Vasc Interv Radiol 2013; 24: 490-500. [DOI] [PubMed] [Google Scholar]
- 4.Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, Fan ST. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol 2007; 5: 1100-1108. [DOI] [PubMed] [Google Scholar]
- 5.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the Precision V study. Cardiovasc Interv Radiol 2010; 33: 41-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer 2014; 111: 255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kudo M, Ueshima K, Kubo S, Sakamoto M, Tanaka M, Ikai I, et al. Response Evaluation Criteria in Cancer of the Liver (RECICL) (2015 revised version). Hepatol Res 2016; 46: 3-9. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002; 359: 1734-1739. [DOI] [PubMed] [Google Scholar]
- 9.Kalva SP, Pectasides M, Yeddula K, Ganguli S, Blaszkowsky LS, Zhu AX. Factors affecting survival following chemoembolization with doxorubicin-eluting microspheres for inoperable hepatocellular carcinoma. J Vasc Interv Radiol 2013. 24: 257-265. [DOI] [PubMed] [Google Scholar]
- 10.Bannangkoon K, Hongsakul K, Tubtawee T, McNeil E, Sriplung H, Chongsuvivatwong V. Rate and Predictive factors for sustained complete response after selective transarterial chemoembolization (TACE) in patients with hepatocellular carcinoma. Asian Pac J Cancer Prev 2018; 19: 3545-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekelund L, Lin G, Jeppsson B. Blood supply of experimental liver tumors after intraarterial embolization with gelfoam powder and absolute ethanol. Cardiovasc Interv Radiol 1984; 7: 234-239. [DOI] [PubMed] [Google Scholar]
- 12.Goseki N, Nosaka T, Endo M, Koike M. Nourishment of hepatocellular carcinoma cells through the portal blood flow with and without transcatheter arterial embolization. Cancer 1995; 76: 736-742. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura H, Hashimoto T, Oi H, Sawada S. Iodized oil in the portal vein after arterial embolization. Radiology 1988; 167: 415-417. [DOI] [PubMed] [Google Scholar]
- 14.Namur J, Citron SJ, Sellers MT, Dupuis MH, Wassef M, Manfait M, Laurent A. Embolization of hepatocellular carcinoma with drug-eluting beads: doxorubicin tissue concentration and distribution in patient liver explants. J Hepatol 2011; 55: 1332-1338. [DOI] [PubMed] [Google Scholar]
- 15.Jeong SO, Kim EB, Jeong SW, Jang JY, Lee SH, Kim SG, et al. Predictive factors for complete response and recurrence after transarterial chemoembolization in hepatocellular carcinoma. Gut Liver 2017; 11: 409-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda M, Inaba Y, Tanaka T, Sugawara S, Kodama Y, Aramaki T, et al. A prospective randomized controlled trial of selective transarterial chemoembolization using drug-eluting beads loaded with epirubicin versus selective conventional transarterial chemoembolization using epirubicin-lipiodol for hepatocellular carcinoma: The JIVROSG-1302 PRESIDENT study. J Clin Oncol 2020. 38(15_suppl) (suppl; abstr 4518) [Google Scholar]
- 17.Zhu GQ, Wang K, Wang B, Zhou YJ, Yang Y, Chen EB, et al. Aspartate aminotransferase-to-platelet ratio index predicts prognosis of hepatocellular carcinoma after postoperative adjuvant transarterial chemoembolization. Cancer Manag Res 2019; 11: 63-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamakado K, Miyayama S, Hirota S, Mizunuma K, Nakamura K, Inaba Y, et al. Subgrouping of intermediate-stage (BCLC stage B) hepatocellular carcinoma based on tumor number and size and Child-Pugh grade correlated with prognosis after transarterial chemoembolization. Jpn J Radiol 2014; 32: 260-265. [DOI] [PubMed] [Google Scholar]
- 19.Guiu B, Deschamps F, Aho S, Munck F, Dromain C, Boige V, et al. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol vs. drug-eluting beads. J Hepatol 2012; 56: 609-617. [DOI] [PubMed] [Google Scholar]
- 20.Monier A, Guiu B, Duran R, Aho S, Bize P, Deltenre P, et al. Liver and biliary damages following transarterial chemoembolization of hepatocellular carcinoma: comparison between drug-eluting beads and lipiodol emulsion. Eur Radiol 2017; 27: 1431-1439. [DOI] [PubMed] [Google Scholar]