Abstract
Computed tomography-guided lung biopsy is a well-established method for the histological diagnosis of pulmonary lesions. There is abundant literature regarding the diagnostic yield of and complications associated with computed tomography-guided lung biopsy. Many studies have investigated the risk factors influencing pneumothorax. Conversely, there are a limited number of reports detailing techniques for reducing the incidence of pneumothorax or other complications. This study reviews the indications, diagnostic accuracy, and complications of computed tomography-guided lung biopsy. In addition, techniques for reducing the incidence of these complications were reviewed.
Keywords: Computed tomography guided lung biopsy, lung cancer, pneumothorax
Introduction
Computed tomography (CT)-guided lung biopsy has gained wide acceptance as a method for the diagnosis of lung lesions. Its diagnostic yield is higher than that of transbronchial lung biopsy (TBLB). However, the complication rates of patients undergoing CT-guided biopsy are higher than that of those undergoing TBLB [1-5].
In this article, we review the current state of CT-guided lung biopsy, considering the indications, biopsy techniques, diagnostic yield, and complications.
Literature Search
A literature search was performed on PubMed in August 2020 using the following keywords: i) “CT-guided lung biopsy” and “complication” and ii) “CT-guided biopsy” and “transbronchial biopsy,” except meta-analysis, prospective, or retrospective clinical studies on systemic embolism. The search was limited to articles published in English in the past 25 years (between 1996 and 2020). In total, 913 articles were found in the initial search. The list of all articles was then examined to identify potentially relevant studies. Meta-analyses and prospective or retrospective clinical studies with more than 50 CT-guided biopsy procedures except for case reports of systemic air embolism were included. The following articles were excluded: i) those including a combination of CT and ultrasound (US) guidance and ii) those with less than 50 biopsy procedures. A few additional recent relevant papers were added during the manuscript revision process.
Biopsy Indications
In the past, flexible bronchoscopy and TBLB were commonly performed as an initial approach to arrive at a pathological diagnosis of lung lesions. For central lung cancer and endobronchial lesions, biopsy under flexible bronchoscopy had a sensitivity of 88% for diagnosing lung cancer. However, the diagnostic yield of bronchoscopy for peripheral lesions was lower, with a sensitivity of 34% [1]. Endobronchial US (EBUS)-guided transbronchial needle aspiration and radial EBUS (r-EBUS) are emerging technologies for the diagnosis of peripheral lung lesions. The diagnostic yield of r-EBUS for peripheral lung cancer was reportedly 73%-70.6% [1-4].
On the other hand, CT-guided biopsy is highly sensitive for diagnosing peripheral lung cancer, with higher sensitivity than EBUS-TBLB. Studies have reported that CT-guided biopsy for ground-glass nodules (GGNs) provided high diagnostic yields [6, 8-9, 11-12]. However, the complication rates reported for CT-guided biopsy were higher than those reported for EBUS-TBLB [5-7].
Therefore, CT-guided lung biopsy may be considered, particularly for lung lesions that have achieved diagnostic failure by transbronchial examination, are located in peripheral areas, or have GGNs [5-13]. Molecular testing of lung cancer requires an adequate amount of tissue samples. Therefore, CT-guided biopsy is likely to yield a better sample than conventional EBUS [14, 15].
Diagnostic Yield
Established diagnosis of pulmonary lesions
A previous study reported an overall diagnostic accuracy of 87.3% in 396 CT-guided pulmonary biopsy procedures, including 242 fine-needle aspiration biopsies (FNABs) and 154 cutting-needle core biopsies (CNCBs) [16]. Another study reported that a specific diagnosis of 530 lung lesion biopsies was 67% in 448 FNABs and 82% in 82 CNCBs [17].
Recently, some studies revealed that the diagnostic accuracy of FNAB was 82%-93% and that of CNCB was 85%-95% [18, 20, 21, 26, 30].
A meta-analysis of the diagnostic evaluation of peripheral lung lesions demonstrated that r-EBUS-guided TBLB had a point sensitivity of 69% for the diagnosis of peripheral lung cancer, which was lower than the sensitivity of CT-guided percutaneous transthoracic needle biopsy (94%) [5]. Another meta-analysis of the diagnostic evaluation of small lung lesions found that the pooled diagnostic yield was 75% using r-EBUS and 93% using CT-guided biopsy. For pulmonary lesions ≤2 cm in size, the percutaneous approach (pooled diagnostic yield: 92%) was superior to the bronchoscopic approach (66%) [7]. A meta-analysis reported that CT-guided biopsy for GGNs provided high diagnostic yields, with an estimated specificity of 94% and estimated sensitivity of 92% [9].
Selection of Needle Type
FNAB and cutting-needle biopsy techniques yield satisfactory results with acceptable complication rates; thus, the selection of needle type should be considered. In FNAB, cytological techniques are used for analysis, whereas tissue samples are used in CNCB. A tissue core biopsy using a cutting needle yielded a larger number of high-quality tissue samples suitable for histological evaluation and determination of biomarker profiles than FNAB [20].
Guimaraes et al. reported that the sensitivity, specificity, and accuracy of 362 FNABs were 82.6%, 81.3%, and 81.8% and those of 97 CNCBs were 93.8%, 97.3%, and 95.2%, respectively (all p < 0.05). The overall complication rate did not differ between FNAB (14.1%) and cutting-needle biopsy (12.4%) (p > 0.05); however, the incidence of pneumothorax was higher for FNAB and that of pulmonary hematoma was higher for cutting-needle biopsy (both p < 0.05) [20]. Hiraki et al. reported that the sensitivity, specificity, and accuracy of 1,000 CT fluoroscopy-guided CNCBs were 94.2%, 99.1%, and 95.2%, respectively [21]. These reports showed that CNCB yielded better results than FNAB. In conclusion, cutting-needle biopsy is recommended for CT-guided lung biopsy (Table 1).
Table 1.
Diagnostic Yield and Complications of CT-guided Lung Biopsies.
| Author | Year | No. of Patients | No. of Lesions | Needle Type | Conventional or Fluoroscopic | Diagnostic Accurasy | Complications | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pneumothorax | Bleeding | |||||||||||
| Fine Needle | Cutting Needle | Pneumothorax | Chest Tube Insertion | Intraparenchymal Bleeding | Hemoptysis | Hemothorax | ||||||
| Rubens et al, [17] | 2006 | 530 | 530 | 448 | 82 | Conventional | 70% | 84/628 (13.4%) | 24/628 (3.8%) | (-) | 3/628 (0.5%) | 2/628 (0.3%) |
| including thorax biopsy | including thorax biopsy | |||||||||||
| Laspas et al, [18] | 2008 | 409 | 409 | 409 | 0 | Conventional | 93.00% | 17 (4%) | 1 (0.2%) | 8 (2.0%) | 1 (0.2%) | 0 |
| Hiraki et al, [21] | 2009 | 901 | 1000 | 0 | 1000 | Fluoroscopic | 95.20% | 431/1000 (43.1%) | (-) | (-) | (-) | (-) |
| Loh et al. [19] | 2013 | 384 | 399 | 349 | 50 | (-) | 96.80% | 139 (34.8%) | 12 (3.0%) | (-) | 13 (3.3%) | 2 (0.5%) |
| Mendiratta-Lala et al, [30] | 2014 | 169 | 169 | 0 | 169 | Fluoroscopic | 85.20% | 69 (40.8%) | 10 (5.9%) | (-) | (-) | 3 (1.8%) |
| Filippo et al, [38] | 2014 | 538 | 538 | 538 | 0 | Conventional | (-) | 154 (28.6%) | 0 (0%) | 144 (26.8%) | 2 (0.4%) | (-) |
| Guimaraes et al, [20] | 2014 | 459 | 459 | 362 | 97 | Conventional | 81.8% (fine), 95.2% (cutting) | 40 (11.1%, fine), 3 (3.1%, cutting) | 11 (2.5%, fine), 0 (0%, cutting) | 4 (1.1%, fine), 7 (7.2%, cutting) | 7 (1.9%, fine), 2 (2.1%, cutting) | (-) |
| Vagn-Hansen et al, [26] | 2016 | 427 | 520 | 0 | 520 | Fluoroscopic | 85.40% | 154 (29.6%) | 79 (15%) | (-) | 12 (2.3%) | (-) |
CT Fluoroscopy-guided Biopsy versus Conventional CT-guided Biopsy
Recently, CT fluoroscopy guidance has been developed, and it is now widely used for CT-guided lung biopsies. CT fluoroscopy-guided biopsy allows the physician to continuously monitor the biopsy needle as it progresses toward the target lesion.
Several studies have shown that CT fluoroscopy is a safe, effective tool for guiding percutaneous interventional procedures [22, 23]. On the other hand, CT fluoroscopy guidance involves high amounts of radiation to which physicians and patients may be exposed. In particular, radiologists receive scattered exposure to the hands and body compared with that received in conventional CT guidance [22-24]. However, a comparison between CT fluoroscopy-guided and conventional CT-guided lung biopsies has not been performed. A prospective study evaluated the radiation doses, complication rates, and diagnostic accuracy of CT-guided FNAB procedures for pulmonary lesions performed with or without fluoroscopic guidance. A total of 142 patients were enrolled to undergo CT-guided FNAB with (group 1, n = 72) or without (group 2, n = 70) fluoroscopic guidance. The mean estimated effective radiation dose to patients (6.53 mSv in group 1 and 2.72 mSv in group 2, p < 0.001) and doctors (0.054 mSv in group 1 and 0.029 mSv in group 2, p < 0.001) were significantly different between the groups. Moreover, the complication rate was significantly different between the two groups (13.4% vs. 31.4%, p = 0.012). The sensitivity, specificity, and accuracy were 97.8%, 100%, and 98.4% in group 1 and 95.3%, 100%, and 89.5% in group 2, respectively (p > 0.05) [24].
Therefore, there is no certain recommendation for the selection of CT fluoroscopy guidance or conventional CT guidance.
Factors Affecting the Diagnostic Yield
Several reports have evaluated the factors affecting the diagnostic yield of CT-guided lung biopsy. They investigated the risk factors for diagnostic failure based on lesion size, lesion lobar location, pleural contact, and lesion depth [20, 21, 25, 26]. Guimaraes et al. reported the most optimal needle option from 362 FNABs and 97 CNCBs [20]. The success rates of both biopsy techniques were higher for larger-than-average (≥40 mm) than for smaller-than-average (<40 mm) lesions (p < 0.05) [20]. Hiraki et al. reported significant independent risk factors for diagnostic failure of 1,000 CT fluoroscopy-guided lung biopsies. The risk factors were lesion size (lesions ≤1.0 cm: 92.7%, p = 0.016; lesions 1.1-2.0 cm: 96.1%, lesions 2.1-3.0 cm: 97.7%, lesions ≥3.1 cm: 92.7%, p = 0.007), lobar location (upper and middle lobe: 96.7%, lower lobe: 92.9%, p = 0.003), and two or fewer specimens (≤2: 93.2%, ≥3: 96.7%, p = 0.007) [21]. Priola et al. reported a retrospective study of 612 consecutive procedures in which the lesion size affected diagnostic accuracy (lesions <1.5 cm: 68%, lesions 1.5-5.0 cm: 87%, lesions >5 cm: 78%, p < 0.05) [25]. Vagn-Hansen et al. assessed the risk of failed biopsy in approximately 520 CT-guided thorax biopsies. Only the lesion size (<1 cm: 60%, 1-4 cm: 84 %, >4 cm: 99%, p = 0.000001) had a significant influence on successful completion of the procedure [26].
Biopsy Techniques
Pre-biopsy preparations
All patients must be informed about the purpose, adaptation, usefulness, and methodology of the biopsy procedure; possible complications such as pneumothorax, hemorrhage, and air embolism; and the treatment of these complications. Written consent for biopsy must be obtained from all patients.
CT-guided biopsy is considered a moderate-risk procedure. In patients who receive antiplatelet drugs such as aspirin, only aspirin can be continued and other antiplatelet drugs should be discontinued and resumed within 48 h after the procedure. In patients who receive anticoagulant drugs such as warfarin, treatment should be discontinued at least 5 days before the procedure [31].
Axial images at 1.0 mm collimation or high-resolution chest CT images should be acquired before performing the biopsy.
Pre-biopsy conferences should be held with radiologic technologists and nurses, and confirmation should be obtained before the biopsy is performed [32].
Biopsy procedure
Biopsy procedures are performed under local anesthesia with CT guidance. Before performing biopsy, it is important to select the patient's body position. The patient should be placed in the prone, supine, or lateral decubitus position, which allows for needle puncture of the lung to be performed safely using the shortest approach possible. In addition, the patient may be requested to perform breath-hold exercise before the biopsy.
Continuous cardiorespiratory monitoring is required for each institutional protocol. The puncture point of the needle is determined, and a biopsy needle path should be selected to avoid bullae, fissures, visible vessels, and bronchioles whenever possible. After reviewing the CT images, the needle entry point, direction, and length of approach for biopsy are planned. The patient's skin is disinfected. A 1% lidocaine solution for local anesthesia is administered subcutaneously to the pleural surface. The biopsy needle is introduced into the lesion under fluoroscopic or nonfluoroscopic guidance. Immediately after the biopsy, the needle is slowly withdrawn and gauzes are applied to the skin [29, 30].
Subsequently, an on-site cytotechnologist confirms whether the material is adequate for histologic diagnosis. The obtained tissue samples are immediately placed on glass slides and immersed in 10% formalin solution. Cell samples or FNAB samples are fixed in low-molecular-weight dextran-lactated Ringer's solution for staining.
Post-biopsy care
Whole-thorax CT is performed at the same position to detect complications such as pneumothorax, hemorrhage, or air bubbles in the systemic circulation. All symptoms, such as cough, hemosputum, chest discomfort, and changes in cardiorespiratory monitoring findings, must be evaluated [19, 35].
Complications
The common complications after CT-guided lung biopsy are pneumothorax and intrapulmonary hemorrhage.
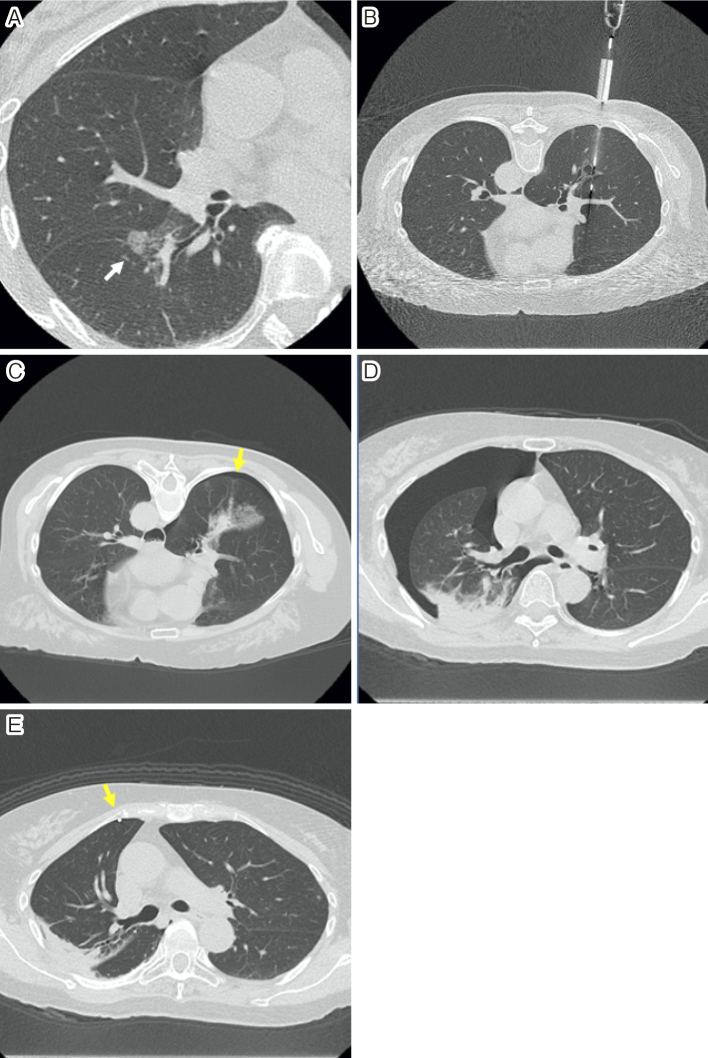
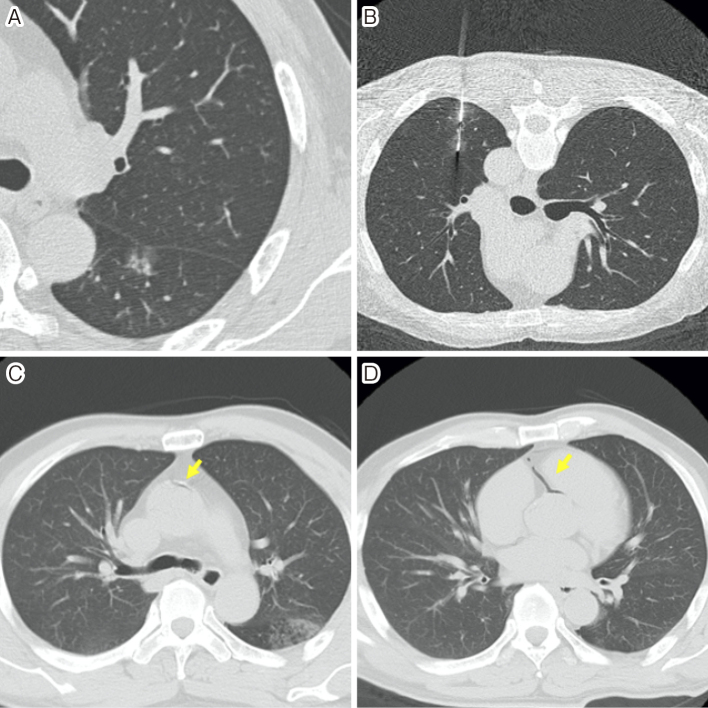
Tomiyama et al. conducted a survey of severe complications following CT-guided lung biopsy in Japan based on 9,783 biopsies. Tension pneumothorax (0.10%); hemothorax (0.092%); severe pulmonary hemorrhage or hemoptysis (0.061%); systemic air embolism (0.061%); tumor seeding at the site of the biopsy route (0.061%); and others, including cardiac arrest, shock, and respiratory arrest (0.26%) were reported. Further, 0.07% of the patients died (Fig. 1 and 2) [27].
Figure 1.
•A woman in her 60s presented with cough.
•High-resolution computed tomography (CT) image (A) showing a ground-glass nodule in the right lower lobe.
•A biopsy was performed, with the patient in the prone position, using an 18-gauge cutting needle, under CT fluoroscopic guidance (B).
•Immediately after the biopsy, whole-body CT showed grade 1 pneumothorax (C, arrow).
•Chest radiograph and CT (D) performed 1 day after the biopsy revealed grade 2 pneumothorax.
•An 8-Fr. drainage catheter was inserted, and 1,200 ml of air was released manually (E, arrow).
Figure 2.
•A man in his 60s underwent computed tomography-guided lung biopsy.
•High-resolution CT image (A) showing a part-solid nodule in the left lower lobe.
•A biopsy was performed with the patient in the prone position, using an 18-gague cutting needle under CT fluoroscopic guidance (B).
•Immediately after the biopsy, he complained of oppressive chest pain and hemosputum occurred.
•The patient was then turned to the supine position, because of a suspected pneumothorax.
•Whole-thorax CT showing air within the ascending aorta (C) and coronary artery (D).
•A decrease in blood pressure to 75/47 mmHg and heart rate to 45/min were noted. The patient was placed in the Trendelenburg position, and 100% oxygen was administered. Brain CT showed no air within the cerebral artery.
•Brain magnetic resonance imaging revealed no abnormalities, and the patient left our hospital 1 day after biopsy with no symptoms.
Pneumothorax
The most common complication after CT-guided lung biopsy is pneumothorax. Pneumothorax has been reported to occur in 9%-43% of cases, and chest drainage is needed in 0%-15% of patients undergoing biopsy [18-20, 27-31, 33-37].
Several studies have evaluated the relationship between specific variables and pneumothorax [19, 20, 26, 34-36]. The risk factors of pneumothorax associated with needle biopsies should be considered for the classification of the factors of patients, radiologists, lesions, and procedure techniques. The patient factors include age, sex, and grade of emphysema. The radiologist factor is the number of years of experience. The lesion factors include lesion size, location, and pleural contact. The procedure factors include the needle type, needle size, patient position, length of needle passes, puncture angle, number of pleural punctures, number of punctures, and procedure time (Table 2).
Table 2.
The Significant Risk Factors of Pneumothorax
| Author | Year | No. of Procedure | Needle Type | Patient Factors | Lesion Factors | Procedure Factors | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fine Needle | Cutting Needle | sex | size | location | pleural contact | needle type | patient position | length of needle passes | puncture angle | number of pleural punctures | number of punctures | procedure time | |||
| Khan MF et al, [34] | 2008 | 135 | 0 | 135 | (-) | (-) | (-) | p < 0.05 | (-) | (-) | > 40mm, p < 0.05 | (-) | (-) | (-) | p < 0.05 |
| Kakizawa H et al, [35] | 2010 | 91 | (-) | (-) | (-) | < 20mm, p < 0.05 | lower, p < 0.05 | p < 0.05 | (-) | (-) | (-) | (-) | p < 0.05 | p < 0.05 | (-) |
| Hiraki T et al. [36] | 2010 | 1155 | (-) | 1155 | (-) | (-) | lower, p < 0.001 | (-) | (-) | (-) | p < 0.001 | < 45 degrees, p = 0.014 | (-) | (-) | (-) |
| Loh et al. [19] | 2013 | 399 | 349 | 50 | (-) | < 33mm, p < 0.05 | (-) | (-) | (-) | (-) | > 3mm, p < 0.05 | (-) | (-) | (-) | (-) |
| Guimaraes et al, [20] | 2014 | 459 | 362 | 97 | (-) | (-) | (-) | (-) | FNAB, p < 0.05 | (-) | (-) | (-) | (-) | (-) | (-) |
| Vagn-Hansen et al, [26] | 2016 | 520 | 0 | 520 | female, p = 0.02 | < 10mm, p = 0.00001 | upper, p = 0.00001 | (-) | (-) | lateral, p = 0.00001 | > 0mm, p = 0.00001 | (-) | (-) | (-) | (-) |
Details of Pneumothorax
Multivariate analysis of the risk factors for the severity of pneumothorax after CT-guided lung biopsy showed that lower locations, nonpleural contact lesions, increased number of pleural punctures, and increased number of harvested samples presented a higher severity of pneumothorax [35]. A study that evaluated the diagnostic yield and complication rates of 98 procedures reported that the rates of complications such as pneumothorax and pulmonary bleeding were correlated with the penetration depth of the lung; however, the difference was not statistically significant [33]. Another study evaluated the influence of various variables on the rate of pneumothorax and intrapulmonary hemorrhage associated with CT-guided lung biopsy for 135 procedures. The study found that intrapulmonary paths longer than 4 cm (p < 0.05) and longer puncture time (p < 0.05) were associated with significantly higher numbers of pneumothorax [34]. A retrospective analysis of 384 patients who underwent 399 CT-guided lung biopsies was performed to evaluate the diagnostic yield, accuracy, and complications. FNAB was performed in 349 cases, and cutting-needle biopsy was performed in 50 cases. The incidence of pneumothorax was significantly associated with the traversed lung length (>3 mm, p < 0.0001), lesion size (<33 mm, p = 0.0072), and lesion depth (>60 mm, p = 0.0442). However, there was no significant relationship between the incidence of pneumothorax and patient age, needle size, or number of passes [19]. A retrospective analysis of 1,033 patients who underwent 1,155 CT fluoroscopy-guided lung biopsies evaluated the risk factors for pneumothorax. The significant independent risk factors were lesions in the lower lobe (p < 0.001), greater lesion depth (p < 0.001), and a needle trajectory angle of <45 degrees (p = 0.014); that for chest tube placement was greater lesion depth (p < 0.001) [36]. Another study reported the outcome of 520 CT fluoroscopy-guided biopsies using an 18-gauge cutting needle with respect to diagnostic accuracy and complications. The study reported a higher risk of pneumothorax in women (p = 0.02), smokers with more than 10 pack-years (p = 0.02), patient placed in a side lying lateral position (p = 0.0007), a distance of 5-10 cm from the skin surface to the lesion (p = 0.00001), and lesions of <1 cm in diameter (p = 0.00001) [26].
A study reviewed the CT images of 538 patients who underwent CT-guided FNAB to determine the correlation between pneumothorax and pulmonary hemorrhage. The study showed that high-grade (>6 mm in diameter) pulmonary hemorrhage is a protective factor for pneumothorax (p < 0.001) [38].
Furthermore, sealing of the biopsy track to reduce the incidence of pneumothorax has been proposed. The complications associated with pneumothorax results in an increased need for hospitalization and increased costs. Many sealing techniques using blood clots, collagen, fibrin, and liquid NaCl have been employed, with varying success rates.
The blood patch technique using autologous blood injection into the introducing needle was reported in 1988. A total 140 needle biopsies were divided into two groups: with the blood patch technique (52 biopsies, group A) and without the blood patch technique (88 biopsies, group B). Chest tube insertion was required in 7.7% of cases in group A and 9.1% of cases in group B. There was no statistically significant difference [39]. Instillation of NaCl solution was first reported in 2008. After biopsy with an 18-gauge coaxial system, 2-4 mL of 0.9 % NaCl solution was instilled into the puncture access route via the introduction of a cannula (16-gauge) in 70 patients (group A). In the other 70 patients, the coaxial sheath was withdrawn without instilling NaCl (group B). The incidence of pneumothorax was significantly lower in group A than in group B (8% vs. 34%; p < 0.001) [40]. A randomized controlled trial was performed to determine whether the use of autologous blood clot seal (ABCS) after biopsy of lung lesions can reduce or prevent the incidence of pneumothorax. One hundred patients were randomly assigned to one of two groups: those in whom the biopsy track was sealed with autologous blood clots (n = 50) and those who did not receive autologous blood clots (n = 50). Biopsy was performed with CT guidance and a 19-gauge coaxial system with 20-22-gauge aspiration biopsy needles. Pneumothorax occurred in 9% of those who had deep lesions and received autologous blood clots and in 47% in those who had deep lesions and did not receive autologous blood clots (p < 0.001). Therefore, the authors reported that plugging of biopsy tracks with ABCS significantly reduced the frequency of pneumothorax [41]. The study with the highest evidence level reported that the use of normal saline for sealing the needle track could reduce the incidence of pneumothorax and chest tube placement after CT-guided lung biopsy. A prospective, randomized, controlled trial enrolled 322 patients who were randomly assigned to one of two groups: those in whom the needle track was not sealed with normal saline (n = 161, group A) and those who received normal saline (n = 161, group B). CT-guided biopsy was performed using a coaxial system with a 19-gauge coaxial needle and a matching 20-gauge core cutting needle. The incidence of pneumothorax was 26.1% in group A and 6.2% in group B (p < 0.001). Nine patients in group A and one patient in group B required chest tube placement (p < 0.010). Furthermore, multiple logistic regression analysis showed that an increased risk of pneumothorax was associated with smaller lesion size, greater needle-pleural angle, longer lesion-pleural distance, presence of emphysema, and no sealing of the needle track with normal saline. The latter three factors were also associated with an increased risk of pneumothorax requiring chest tube placement [42].
A retrospective analysis of 236 CT-guided lung biopsies that referred to a more straightforward technique to reduce the incidence of pneumothorax was reported. With the standard technique, 41.6% had pneumothorax, with 18.0% requiring chest tube placement. With the puncture site-down positioning technique, 12.9% had pneumothorax, with 2.7% requiring chest tube placement [43].
Hemorrhage
A retrospective analysis of 384 patients who underwent 399 CT-guided lung biopsies reported that mild hemoptysis occurred in 3.2% of patients [19]. Another study reporting the outcome of 520 CT-guided core needle biopsies stated that hemoptysis occurred in 2.3% of cases [26]. Another retrospective study that reviewed data from 362 (71.6%) FNAB procedures and 97 (19.7%) cutting-needle procedures reported that the incidence of pulmonary hematoma was significantly higher for cutting-needle biopsies (7.2%) than for FNABs (1.1%, p < 0.05) [20].
Another study that reviewed CT images of 538 patients who underwent CT-guided FNAB of pulmonary nodules reported that intrapulmonary parenchymal hemorrhage occurred in 26.8% of patients [38].
Systemic Air Embolism
Ibukuro et al. presented the details and incidence of systemic air embolism and needle track implantation in patients who underwent 1,400 percutaneous CT-guided thoracic biopsies at a single institution, and three cases (0.21%) of systemic air embolism were reported [44]. Hiraki et al. reported 4 cases of systemic air embolism complicated by CT-guided transthoracic needle biopsy among 1,010 procedures performed at a single institution [45]. Hirasawa et al. reported one case of systemic air embolism in which CT fluoroscopy images revealed air entry during the procedure [46]. Tomabechi et al. also reported a case of systemic air embolism treated with hyperbaric oxygen therapy [47]. These cases were nonfatal, but systemic air embolism was potentially fatal [49, 50].
The mechanism of systemic air embolism was thought to be a communication between the airway and the pulmonary vein during needle biopsy [46-48]. Hirasawa et al. suspected two possible mechanisms by which systemic air embolism could have occurred: (a) when the needle tip was positioned within a pulmonary vein, communication between the atmosphere and pulmonary vein was established; (b) when the needle penetrated the pulmonary vein and a bronchus or the air-filled portion of a cavity lesion or a cyst, communication between the airway and pulmonary vein may have been established. Coughing or a Valsalva maneuver may increase airway pressure, facilitating aspiration of air into the pulmonary vein [46, 47]. A case-control study that evaluated data from 2,216 CT-guided lung biopsy procedures revealed that systemic air embolism occurred in 10 cases (0.5%). Univariate and multivariate analyses showed that lesions in the lower lobe (p = 0.025), occurrence of parenchymal hemorrhage (p = 0.019), and the use of a larger biopsy needle (p = 0.014) were significant risk factors [51].
Ramaswamy et al. proposed that if arterial embolism is suspected, the patient should be placed in the right lateral decubitus position to prevent air from entering the systemic circulation by trapping air in the left ventricle [48]. However, further discussion is necessary for an appropriate position to prevent systemic air embolism. The treatment of systemic air embolism consists of administering 100% oxygen, providing routine supportive measures such as steroid and anticonvulsant therapy, and using hyperbaric oxygen chambers.
Tumor Seeding
A retrospective analysis of 4,365 patients who underwent percutaneous FNAB at the same institution reported that eight patients developed implantation metastasis related to the procedure [52]. On the other hand, a study of 171 patients who underwent CT-guided coaxial biopsy before surgery reported no significant association between the biopsy and intraoperative pleural lavage cytology results. Therefore, percutaneous CT-guided lung biopsy with a coaxial needle does not seem to cause pleural dissemination [53].
Conclusions
CT-guided lung biopsy is a minimally invasive and established method for the diagnosis of pulmonary lesions. This procedure may be considered for lung lesions with diagnostic failure by transbronchial examination and are present in peripheral areas or present with GGNs. However, there is a higher incidence of complications associated with CT-guided lung biopsy than with transbronchial examinations, including pneumothorax, intrapulmonary hemorrhage, systemic air embolism, or tumor seeding.
Conflict of Interest
None
References
- 1.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer 3rd ed. American college of chest physicians evidence-based clinical practice guidelines. Chest 2013; 143: e142S-e165S. [DOI] [PubMed] [Google Scholar]
- 2.Mondoni M, Sotgiu G, Bonifazi M, et al. Transbronchial needle aspiration in peripheral pulmonary lesions: a systematic review and meta-analysis. Eur Respir J 2016; 48: 196-204. [DOI] [PubMed] [Google Scholar]
- 3.Steinfort DP, Khor YH, Manser RL, Irving LB. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011; 37: 902-910. [DOI] [PubMed] [Google Scholar]
- 4.Ali MS, Trick W, Mba BL, Mohananey D, Setni J, Musani A. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Respirology 2017; 22: 443-453. [DOI] [PubMed] [Google Scholar]
- 5.Zhan P, Zhu QQ, Miu YY, et al. Comparison between endobronchial ultrasound-guided transbronchial biopsy and CT-guided transthoracic lung biopsy for the diagnosis of peripheral lung cancer: A systematic review and meta-analysis. Transl Lung Cancer Res 2017; 6: 23-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fielding DI, Chia C, Nguyen P, et al. Prospective randomized trial of endobronchial ultrasound-guide sheath versus computed tomography-guided percutaneous core biopsies for peripheral lung lesions. Intern Med J 2012; 42: 894-900. [DOI] [PubMed] [Google Scholar]
- 7.Han Y, Kim HJ, Kong KA, et al. diagnosis of small pulmonary lesions by transbronchial lung biopsy with radial endobronchial ultrasound and virtual bronchoscopic navigation versus CT-guided transthoracic needle biopsy: A systematic review and meta-analysis. PLoS One 2018; 13: e0191590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hur J, Lee HJ, Nam JE, et al. Diagnostic accuracy of CT fluoroscopy-guided needle aspiration biopsy of ground-glass opacity pulmonary lesions. Am J Roentgenol 2009; 192: 629-634. [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi Y, Izumi Y, Nakatsuka S, et al. Diagnostic performance of percutaneous core needle lung biopsy under multi-CT fluoroscopic guidance for ground-glass opacity pulmonary lesions. Eur J Radiol 2011; 79: e85-e89. [DOI] [PubMed] [Google Scholar]
- 10.Inoue D, Gobara H, Hiraki T, et al. CT fluoroscopy-guided cutting needle biopsy of focal pure ground-glass opacity lung lesions: Diagnostic yield in 83 lesions. Eur J Radiol 2012; 81: 354-359. [DOI] [PubMed] [Google Scholar]
- 11.Yamagami T, Yoshimatsu R, Miura H, et al. Diagnostic performance of percutaneous lung biopsy using automated biopsy needles under CT-fluoroscopic guidance for ground-glass opacity lesions. Br J Radiol 2013; 86: 20120447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang JS, Liu YM, Mao YM, et al. Meta-analysis of CT-guided transthoracic needle biopsy for the evaluation of the ground-glass opacity pulmonary lesions. Br J Radiol 2014; 87: 20140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsui Y, Hiraki T, Mimura H, et al. Role of computed tomography fluoroscopy-guided cutting needle biopsy of lung lesions after transbronchial examination resulting in negative diagnosis. Clin Lung Cancer 2011; 12: 51-55. [DOI] [PubMed] [Google Scholar]
- 14.Gill RR, Murphy DJ, Kravets S, Sholl LM, Janne PA, Johnson BE. Success of genomic profiling of non-small cell lung cancer biopsies obtained by trans-thoracic percutaneous needle biopsy. J Surg Oncol 2018; 118: 1170-1177. [DOI] [PubMed] [Google Scholar]
- 15.Porrello C, Gullo R, Gagliardo CM, et al. CT-guided transthoracic needle biopsy: advantages in histopathological and molecular tests. Future Oncol 2020; 16: 27-32. [DOI] [PubMed] [Google Scholar]
- 16.Ohno Y, Hatabu H, Takenaka D, Imai M, Ohbayashi C, Sugimura K. Transthoracic CT-guided biopsy with multiplanar reconstruction image improves diagnostic accuracy of solitary pulmonary nodules. Eur J Radiol 2004; 51: 160-168. [DOI] [PubMed] [Google Scholar]
- 17.Rubens C, Rony KI, Luciana MV, Yu LS, Aita AA, Soares EA. Computed tomography guided needle biopsy: experience from 1,300 procedures. Sao Paulo Med J 2006; 124: 10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laspas F, Roussakis A, Efthimiadou R, Papaioannou D, Papadopoulos S, Andreou J. Percutaneous CT-guided fine-needle aspiration of pulmonary lesions: results and complications in 409 patients. J Med Imaging Radiat Oncol 2008; 52: 458-462. [DOI] [PubMed] [Google Scholar]
- 19.Loh SEK, Wu DDF, Venkatesh SK, et al. CT-guided thoracic biopsy: evaluating diagnostic yield and complications. Ann Acad Med Singap 2013; 42: 285-290. [PubMed] [Google Scholar]
- 20.Guimaraes MD, Marchiori E, Hochhegger B, Rubens C. CT-guided biopsy of lung lesions: defining the best needle option for a specific diagnosis. Clinics (Sao Paulo) 2014; 69: 335-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiraki T, Mimura H, Gobara H, et al. CT fluoroscopy-guided biopsy of 1,000 pulmonary lesions performed with 20-gauge coaxial cutting needles. Chest 2009; 136: 1612-1617. [DOI] [PubMed] [Google Scholar]
- 22.Paulson EK, Sheator DH, Enterline DS, McAdamus HP, Yoshizumi TT. CT fluoroscopy-guided interventional procedures: techniques and radiation dose to radiologists. Radiology 2001; 220: 161-167. [DOI] [PubMed] [Google Scholar]
- 23.Heck SL, Blom P, Berstad A. Accuracy and complications in computed tomography fluoroscopy-guided needle biopsies of lung masses. Eur Radiol 2006; 16: 1387-1392. [DOI] [PubMed] [Google Scholar]
- 24.Kim GR, Hur J, Lee SM, et al. CT fluoroscopy-guided lung biopsy versus conventional CT-guided lung biopsy: a prospective controlled study to assess radiation doses and diagnostic performance. Eur Radiol 2011; 21: 232-239. [DOI] [PubMed] [Google Scholar]
- 25.Priola AM, Priola SM, Cataldi A, et al. Accuracy of CT-guided transthoracic needle biopsy of lung lesions: factors affecting diagnostic yield. Radiol Med 2007; 112: 1142-1159. [DOI] [PubMed] [Google Scholar]
- 26.Vagn-Hansen C, Pedersen MR, Rafaeisen SR. Diagnostic yield and complications of transthoracic computed tomography-guided biopsies. Dan Med J 2016; 63: A5239. [PubMed] [Google Scholar]
- 27.Tomiyama N, Yasuhara Y, Nakajima Y, et al. CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol 2006; 59: 60-64. [DOI] [PubMed] [Google Scholar]
- 28.Geraghty PR, Kee ST, McFariane G, Razavi MK, Sze DY, Dake MD. CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. Radiology 2003; 229: 475-481. [DOI] [PubMed] [Google Scholar]
- 29.Yamagami T, Iida S, Kato T, et al. Usefulness of new automated cutting needle for tissue-core biopsy of lung nodules under CT fluoroscopic guidance. Chest 2003; 124: 147-154. [DOI] [PubMed] [Google Scholar]
- 30.Mendiratta-Lata M, Sheiman R, Brook OR, Gourtsoyianni S, Mahadevan A, Siewert B. CT-guided core biopsy and percutaneous fiducial seed placement in the lung: can these procedures be combined without an increase in complication rate or decrease in technical success? Eur J Radiol 2014; 83: 720-725. [DOI] [PubMed] [Google Scholar]
- 31.Aktas AR, Goziek E, Yilmaz O, et al. CT-guided transthoracic biopsy: histopathologic results and complication rates. Diagn Interv Radiol 2015; 21: 67-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee MJ, Fanelli F, Haage P, Hausegger K, Van Lienden KP. Patient safety interventional radiology: a CIRSE IR checklist. Cardiovasc Intervent Radiol 2012; 35: 244-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muehistaedt M, Bruening R, Diebold J, Mueller A, Helmberger T, Reiser M. CT/fluoroscopy-guided transthoracic needle biopsy: sensitivity and complication rate in 98 procedures. J Comput Assist Tomogr 2002; 26: 191-196. [DOI] [PubMed] [Google Scholar]
- 34.Khan MF, Straub R, Moghaddam SR, et al. Variables affecting the risk of pneumothorax and intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur Radiol 2008; 18: 1356-1363. [DOI] [PubMed] [Google Scholar]
- 35.Kakizawa H, Toyota N, Hieda M, et al. Risk factors for severity of pneumothorax after CT-guided percutaneous lung biopsy using the single-needle method. Hiroshima J Med Sci 2010; 59: 43-50. [PubMed] [Google Scholar]
- 36.Hiraki T, Mimura H, Gobara H, et al. Incidence of and risk factors for pneumothorax and tube placement after CT fluoroscopy-guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9-year period. Am J Roentgenol 2010; 194: 809-814. [DOI] [PubMed] [Google Scholar]
- 37.Nakatani M, Tanigawa N, Kariya S, Komemushi A, Yagi R, Sawada S. Analysis of factors influencing accuracy and complications in CT-guided lung biopsy. Minim Invasive Ther 2012; 21: 415-422. [DOI] [PubMed] [Google Scholar]
- 38.De Filippo M, Saba L, Silva M, et al. CT-guided biopsy of pulmonary nodules: is pulmonary hemorrhage a complication or an advantage? Diagn Interv Radiol 2014; 20: 421-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bourgouin PM, Shepard JA, McLoud TC, Spizarny DL, Dedrick CG. Transthoracic needle aspiration biopsy: evaluation of the blood patch technique. Radiology 1988; 166: 93-95. [DOI] [PubMed] [Google Scholar]
- 40.Billich C, Muche R, Brenner G, et al. CT-guided lung biopsy: incidence of pneumothorax after instillation of NaCl into the biopsy track. Eur Radiol 2008; 18: 1146-1152. [DOI] [PubMed] [Google Scholar]
- 41.Lang EK, Ghavami R, Schreiner VC, Autologous blood clot seal to prevent pneumothorax at CT-guided lung biopsy. Radiology 2000; 216: 93-96. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Du Y, Luo TY, et al. Usefulness of normal saline for sealing the needle track after CT-guided lung biopsy. Clin Radiol 2015; 70: 1192-1197. [DOI] [PubMed] [Google Scholar]
- 43.Kinoshita F, Kato T, Sugiura K, et al. CT-guided transthoracic needle biopsy using a puncture site-down positioning technique. Am J Roentgenol 2006; 187: 926-932. [DOI] [PubMed] [Google Scholar]
- 44.Ibukuro K, Tanaka R, Takeguchi T, et al. Air embolism and needle track implantation complicating CT-guided percutaneous thoracic biopsy: single-institution experience. Am J Roentgenol 2009; 193: W430-W436. [DOI] [PubMed] [Google Scholar]
- 45.Hiraki T, Fujiwara H, Sakurai J, et al. Nonfatal systemic air embolism complicating percutaneous CT-guided transthoracic needle biopsy: four cases from a single institution. Chest 2007; 132: 684-690. [DOI] [PubMed] [Google Scholar]
- 46.Hirasawa S, Hirasawa H, Taketomi-Takahashi A, et al. Air embolism detected computed tomography fluoroscopically guided transthoracic needle biopsy. Cardiovasc Intervent Radiol 2007; 31: 219-221. [DOI] [PubMed] [Google Scholar]
- 47.Tomabechi M, Kato K, Sone M, et al. Cerebral air embolism treated with hyperbaric oxygen therapy following percutaneous transthoracic computed tomography-guided needle biopsy of the lung. Radiat Med 2008; 26: 379-383. [DOI] [PubMed] [Google Scholar]
- 48.Ramaswamy R, Narsinh KH, Tuan A, Kinney TB. Systemic air embolism following percutaneous lung biopsy. Semin Intervent Radiol 2014; 31: 375-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wescott JL. Air embolism complicating percutaneous needle biopsy of the lung. Chest 1973; 63: 108-110. [DOI] [PubMed] [Google Scholar]
- 50.Sinner WN. Complications of percutaneous transthoracic needle aspiration biopsy. Acta Radiol Diagn 1976; 17: 813-828. [DOI] [PubMed] [Google Scholar]
- 51.Ishii H, Hiraki T, Gobara H, et al. Risk factors for systemic air embolism as a complication of percutaneous CT-guided lung biopsy: multicenter case-control study. Cardiovasc Intervent Radiol 2014; 37: 1312-1320. [DOI] [PubMed] [Google Scholar]
- 52.Kim JH, Kim YT, Lim HK, Kim YH, Sung SW. Management for chest wall implantation of non-small cell lung cancer after fine-needle aspiration biopsy. Eur J Cardiothorac Surg 2003; 23: 828-832. [DOI] [PubMed] [Google Scholar]
- 53.Sano Y, Date H, Toyooka T, et al. Percutaneous computed tomography-guided lung biopsy and pleural dissemination. Cancer 2009; 5526-5533. [DOI] [PubMed] [Google Scholar]
- 54.Robertson EG, Baxter G. Tumour seeding following percutaneous needle biopsy: the real story! Clin Radiol 2011; 66: 1007-1014. [DOI] [PubMed] [Google Scholar]
- 55.Wu CC, Maher MM, Shepard J-AO. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. Am J Roentgenol 2011; 196: W678-W682. [DOI] [PubMed] [Google Scholar]
- 56.Wang T, Luo L, Zhou Q. Risk of pleural recurrence in early stage lung cancer patients after percutaneous transthoracic needle biopsy: A meta-analysis. Sci Rep 2017; 7: 42762. [DOI] [PMC free article] [PubMed] [Google Scholar]