Abstract
With recent advances in imaging technology, the frequency of detecting musculoskeletal lesions has also increased. Percutaneous image-guided needle biopsy is occasionally required for the diagnosis of such lesions. Moreover, in the era of personalized cancer care, chances in histopathological diagnosis and the importance of histopathological diagnosis by percutaneous needle biopsy are increasing. However, as percutaneous needle biopsy is not a common procedure for musculoskeletal lesions, careful planning and the application of adequate techniques such as hydrodissection and the trans-osseous approach are occasionally required. In this review, we have summarized the indications and techniques for percutaneous image-guided needle biopsy for musculoskeletal lesions, including lymphatic lesions.
Keywords: Biopsy, Image guidance, Osteoblastic lesion, Osteolytic lesion, Soft tissue
Introduction
With recent advances in imaging technologies such as computed tomography (CT), magnetic resonance imaging (MRI), and 18-fluorodeoxyglucose positron emission tomography (PET), the frequency of detecting musculoskeletal lesions, such as bone tumor, muscle metastases, and lymphatic lesions, has also increasing. Some lesions can be diagnosed by imaging features and clinical history, but non-specific lesions are often difficult to diagnose using imaging examinations alone, therefore, histopathological diagnosis is required in such cases.
For the histopathological diagnosis of bone lesions, surgical biopsy offers a high diagnostic accuracy of 93.3%-100% [1, 2], but it is invasive and imposes a substantial burden on patients. Percutaneous needle biopsy is often preferred as an initial procedure to obtain a histopathological diagnosis [3, 4]. For soft tissue tumors, surgical biopsy is thought to be the standard method with a high accuracy of 93.5%-100% [2, 3, 5-7]. Nevertheless, percutaneous needle biopsy is also performed because of its low invasiveness [9-13], and opportunities for percutaneous needle biopsy of musculoskeletal lesions are increasing as minimally invasive medicine becomes more prevalent. However, to accomplish safe and effective procedures, biopsies must be performed under adequate image guidance. Moreover, recent advances in molecularly targeted therapy have increased the need for biopsy, while simultaneously increasing the amount of specimen collected [12]. To obtain a sufficient amount of specimen, understanding the features of each target and the imaging modalities used for biopsy is also important.
The purpose of this review was to summarize the outcomes of image-guided percutaneous needle biopsy for bone and soft tissue tumors and provide some techniques for achieving safe and effective biopsy procedures.
Literature search
A literature search was performed using PubMed using the following key words: (bone, percutaneous, “needle biopsy”), (soft tissue, percutaneous, “needle biopsy”), and (lymphoma, percutaneous, “needle biopsy”). The titles and abstracts of the articles obtained from the search were scrutinized to identify relevant studies. In this review, we have focused on studies evaluating percutaneous needle biopsy techniques. Some publications considered important were added during the manuscript preparation and revision process (Fig. 1).
Fig. 1.
Flowchart of the result from literature
Indications and contraindications
Techniques for biopsy of tumor specimens include surgical and needle biopsies. Generally, surgical biopsy is invasive, but it can yield a greater volume of specimen; the converse is true for needle biopsy [13, 14]. The decision to perform surgical or needle biopsy should be determined based on the tumor location, condition of the disease, performance status of the patient, and the amount of specimen needed to meet the purpose of the procedure [15].
The general indications for image-guided percutaneous needle biopsy for bone lesions are as follows: a) determination of benign or malignant status is difficult from nonspecific imaging, b) confirmation of metastatic tumor and/or acquisition of a tumor specimen for genetic mutation analysis in a patient with known malignancy, c) exclusion of malignancy in a bone fracture, d) confirmation of a diagnosis of multiple myeloma, and e) investigation of infection [13, 14].
In contrast, indications for percutaneous needle biopsy of soft tissue tumors are as follows: a) histopathological diagnosis of malignant lymphoma, b) confirmation of metastatic tumor and/or acquisition of tumor tissue for genetic mutation analysis in a patient with known malignancy, and c) diagnosis of an enlarging soft tissue tumor [13, 14].
Contraindications for biopsy for bone tumors and soft tissue tumors are as follows: a) uncorrected coagulopathy, b) inability to obtain a safe access route, and c) inability to obtain patient consent [13, 14]. In particular, to avoid bleeding complications, patients with uncorrected coagulopathy should not undergo percutaneous needle biopsy. Usually, Prothrombin Time-International Normalized Ratio <1.5 and platelet count >50,000 are recommended for safe coagulopathy at the time of interventional procedures [16, 17]. Anticoagulant or antiplatelet drugs may be discontinued before the procedure, as shown in several guidelines [18, 19]. The decision to perform bridging to short-acting parenteral agents such as heparin depends on the patients’ clinical status, the pharmacologic characteristics of the medication used, and procedural bleeding risks. For example, bridging should be considered for patients who are treated with warfarin for atrial fibrillation, mechanical heart valves, or drug-eluting stents following percutaneous coronary intervention [19]. However, when performing biopsy for tumors in superficial areas, the criteria might be loosened slightly, as the puncture site can be compressed [20].
Techniques
Bone biopsy
Image-guided biopsy is usually performed under local anesthesia [13]. Bone biopsy is performed using several modalities, including CT, fluoroscopy, ultrasonography (US), and MRI. CT is the most frequently used modality during bone biopsy due to its ability to clearly visualize bone structures and surrounding organs [21-24]. Generally, CT is the first-choice modality during bone lesion biopsy [13, 14]. Fluoroscopy is another option for image guidance, with clear visualization of bone structures [14, 22-24]. However, the needle should be carefully inserted as the depth is unclear on 2-dimensional images, and the surrounding organs are occasionally invisible. Cone-beam CT may help decrease the risk of complications when performing biopsies using fluoroscopy [25]. US is also used as image guidance for biopsy for several organs, offering the advantages of real-time image acquisition, high availability, and no radiation exposure. Biopsy for osteolytic lesions can be performed under US guidance [22, 23, 26], but osteoblastic lesions are difficult to detect on US. MRI- and PET-CT-guided biopsy have also been reported to offer clear visibility of the target tumor and high diagnostic accuracy, but the availability of these modalities is limited [27, 28].
The diagnostic accuracy of percutaneous biopsy for bone lesions based on previous studies is 77%-94% [23, 26, 29-32]. Percutaneous biopsy can be performed for both osteolytic and osteoblastic lesions. However, diagnostic accuracy is reportedly higher for osteolytic lesions because of the difficulty in detecting the target area containing tumor cells in radiologically visible sclerotic areas and maceration or degradation by crush artefacts during penetration of sclerotic bone tissue [30-33]. Moreover, samples with tumor cells comprising >20% are recommended for successful genetic mutation analysis [15]. The pre-analysis step of decalcification for specimens from osteoblastic bone lesions causes degeneration of nucleic acids and DNA, resulting in a lower success rate for molecular analysis [34, 35]. Biopsy should, thus, be performed for osteolytic parts when both osteolytic and osteoblastic lesions are accessible.
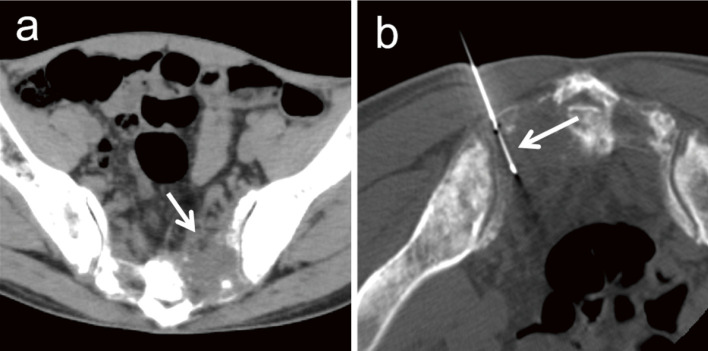
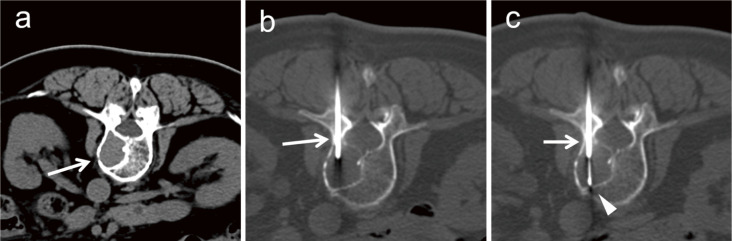
Osteolytic lesions can be biopsied with a commonly used 18- or 20-gauge biopsy needle (Fig. 2). Core-needle biopsy with aspiration has recently become available and reportedly allows obtaining greater amounts of specimens [36]. When osteolytic lesions exist in normal bone tissue, bone tissue can be passed through a 14-gauge bone biopsy needle. After placing the needle into the osteolytic lesion across the bone tissue, an 18-gauge biopsy needle can be inserted into the lesion through the bone biopsy needle (Fig. 3).
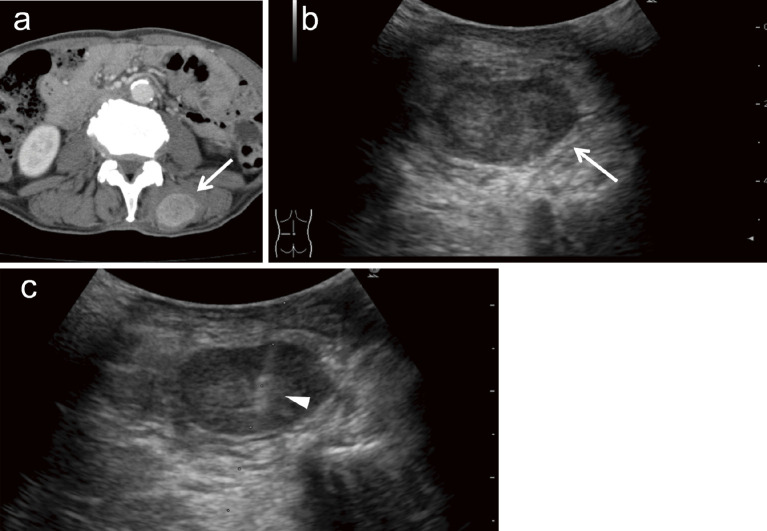
Fig. 2.
A 54-year-old man was referred to our hospital for investigation of a sacral tumor found after a consultation for lower back pain. (a) A 55-mm osteolytic tumor (arrow) has developed on the left side of the sacrum. (b) Biopsy is performed in a prone position under CT fluoroscopic guidance. The tumor is punctured by an 18-gauge semi-automatic biopsy needle (arrow) and biopsy is performed. The tumor is diagnosed as metastasis from lung adenocarcinoma.
Fig. 3.
An 80-year-old woman with a history of breast cancer treated surgically 10 years earlier and gastric cancer treated by endoscopic submucosal dissection 3 years earlier complained of back pain. (a) A 26-mm osteolytic tumor (arrow) has developed on the right side of the 11th thoracic vertebral body. (b) A 14-gauge bone biopsy needle (arrow) is inserted into the tumor through the bone tissue through the left pedicle of the vertebral arch. (c) An 18-gauge semi-automatic biopsy needle (arrowhead) is inserted through the bone biopsy needle (arrow) and biopsy is performed. The tumor is diagnosed as myeloma.
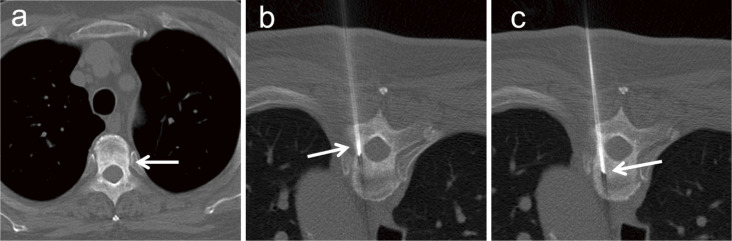
A bone biopsy needle is used at the time of biopsy for osteoblastic lesions. An 11-14-gauge bone biopsy needle is advanced into the lesion and retrieved while aspirating with a syringe. When the needle is advanced through a relatively hard tissue, the needle tends to deviate from the side opposite the needle bevel as the force is applied from the hard tissue [37]. Thus, when advancing the needle into the bone, the direction of the needle should be carefully checked and rotated as necessary (Fig. 4). A screw drill reportedly helps improve the diagnostic accuracy to 73%-92% for osteoblastic lesions [38, 39].
Fig. 4.
A 66-year-old man with cancer of the duodenal papilla developed an osteoblastic lesion in the 4th thoracic vertebral body. (a) A 34-mm osteoblastic lesion is present on the dorsal side of the 4th thoracic vertebral body. (b) A 14-gauge bone biopsy needle (arrow) is inserted into the osteoblastic lesion. (c) The needle is advanced into the deep side of the lesion while rotating it (arrow). The obtained specimen is revealed to represent bone metastasis from a duodenal papilla cancer.
The sample number was reported to be 2-10 in previous reports [22, 26, 29, 30, 33, 35, 40-42]. However, needle size and sample number have not been shown to be significant factors for technical success in a meta-analysis [42]. In contrast, the radiologist operator was identified as a significant factor of technical success [42]. Careful planning with profound knowledge about images and considerable experience in interventional radiology procedures may contribute to good outcomes in biopsy.
The puncture route should be determined running shorter normal bone tissue, with avoiding major vessels or nerves [13, 14]. Although needle tract seeding during puncture procedures for malignant tumors is rare, with a reported incidence of less than 0.01% [43, 44], selecting the route in which later resection could be allowed is also important.
Soft tissue tumor biopsy
Soft tissue tumors can develop in various areas. As in biopsy for bone tumors, percutaneous biopsy for soft tissue tumors is also performed mainly under CT or US guidance under local anesthesia [7-11]. Biopsy is generally performed under US guidance for tumors in a superficial area and under CT guidance for tumors in a deep area [7, 13]. However, the modality should be determined by considering the location of each tumor and the surrounding organs.
The diagnostic yield of core-needle biopsy for soft tissue tumors, including lymphatic lesions, has been reported to be 76%-97% [7-10, 29, 45]. In particular, surgical biopsy for soft tissue tumors located in deep areas, such as the retroperitoneal or mediastinal regions, is invasive and places a substantial burden on the patient. Percutaneous needle biopsy is superior to surgical biopsy in terms of reduced invasiveness and earlier results from histopathological diagnosis; therefore, core-needle biopsy should be considered as the first choice for patients who require early diagnosis or with low performance status [7]. Several studies reported that no significant difference was found between diagnostic accuracy and needle size, although a larger number of specimens were obtained using a thick needle [46-48]. Nevertheless, the number of specimens obtained by core-needle biopsy is less than that obtained by surgical biopsy; therefore, the risk of false-negative results must also be considered. In particular, in the diagnosis of Hodgkin lymphoma, obtaining specimens containing many tumor cells is important, as Reed-Sternberg cells need to be confirmed for diagnosis. Thus, the biopsy technique should be determined according to the condition of the patient and the suspected disease type.
Image-guided needle biopsy for soft tissue tumors located in superficial areas can be performed under US guidance. Muscular metastasis is also an appropriate target for US-guided biopsy (Fig. 5).
Fig. 5.
A 66-year-old man who had been receiving chemotherapy for squamous cell lung carcinoma developed a tumor on the back. (a) A 37-mm tumor (arrow) showing enhancement by contrast media is detected in the left longissimus thoracis muscle. (b) The tumor (arrow) is evident on ultrasonography. (c) Biopsy is performed using an 18-gauge semi-automatic biopsy needle (arrowhead) under US guidance. Metastasis from squamous cell lung carcinoma is diagnosed, but genetic mutation analysis reveals no specific mutation.
Several techniques facilitate the biopsy of difficult lesions. When the tumor is located in the anterior mediastinum just behind the sternum, the tumor can be easily punctured using a trans-osseous approach (Fig. 6). The puncture route can also be secured by hydrodissection, which makes space by pushing organs in the way with lidocaine or saline injection while performing biopsy for mediastinal tumors, although lung parenchyma appears along the puncture route (Fig. 7). Hydrodissection is also helpful when performing biopsies for tumors located near the intestine (Fig. 8).
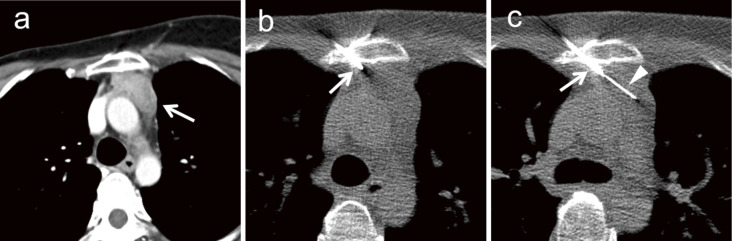
Fig. 6.
A 43-year-old woman who underwent breast cancer resection 8 years earlier developed mediastinal tumor. (a) CT reveals a 36-mm mediastinal tumor (arrow) posterior to the sternum. (b) A trans-osseous route is selected to avoid the penetration into the aortic arc by the needle. The sternum is penetrated using a 14-gauge bone biopsy needle (arrow). (c) An 18-gauge semi-automatic biopsy needle (arrowhead) is inserted thorough the bone biopsy needle (arrow) and safely inserted into the tumor. The tumor is diagnosed as breast cancer metastasis.
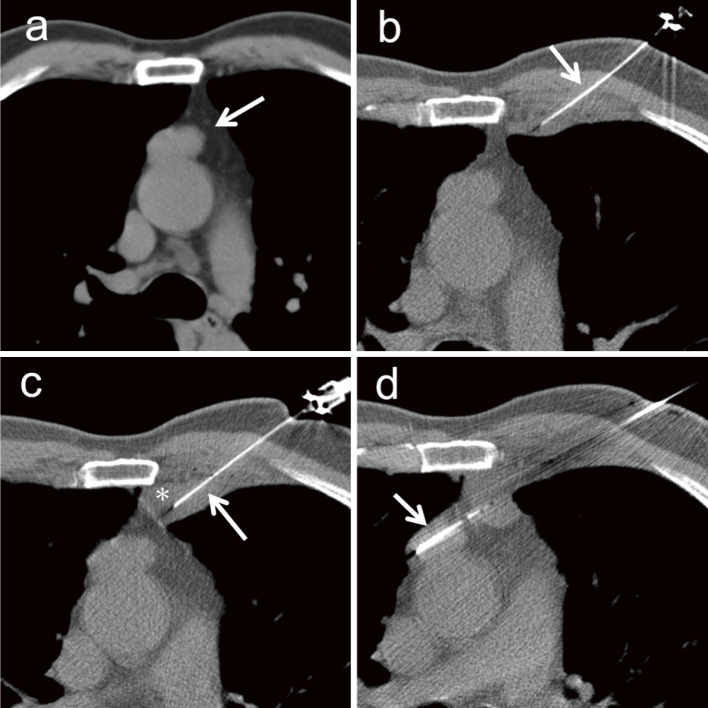
Fig. 7.
A 70-year-old woman was referred to our hospital for the investigation of an incidentally detected mediastinal tumor increasing in size. (a) A 27-mm mediastina tumor (arrow) anterior to the ascending aorta (b) To avoid puncturing, the needle is inserted towards the ascending aorta and passing the lung parenchyma, hydrodissection is intended. A 23-gauge needle (arrow) is inserted near the pleura, and saline is injected into the gap between the parietal and visceral pleura. (c) The needle (arrow) is inserted towards the mediastinum while injecting the saline (asterisk) under CT fluoroscopic guidance. (d) An 18-gauge semi-automatic biopsy needle (arrow) is inserted through the injected saline without puncturing the lung parenchyma. Biopsy is safely performed, and the tumor is diagnosed as thymoma.
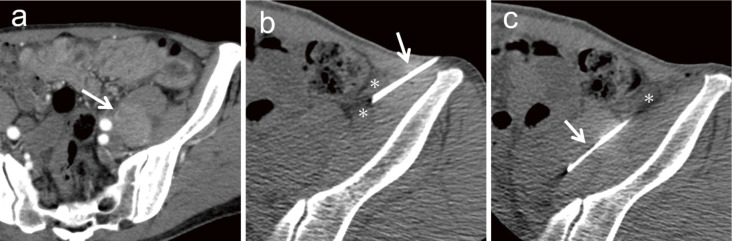
Fig. 8.
A 64-year-old man was referred to our hospital for investigation of an incidentally detected retroperitoneal tumor. (a) A 38-mm tumor (arrow) exists in the left psoas muscle, left external iliac artery, and vein. (b) A 23-gauge needle (arrow) is inserted into the left side of the descending colon and saline (asterisk) is injected. (c) Saline (asterisk) is used to push the descending colon to the right side. An 18-gauge semi-automatic biopsy needle (arrow) is inserted through the saline and biopsy is performed. The tumor is diagnosed as dedifferentiated liposarcoma.
Conclusion
The importance of tissue sampling continues to increase in this era of personalized cancer care. Although biopsy for musculoskeletal and lymphatic lesions is not a common procedure, careful pre-procedural planning and using an adequate technique can contribute to the acquisition of adequate tissue samples with relatively low invasiveness for patients.
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
Yoshitaka Inaba is one of the Senior Editors of Interventional Radiology and on the journal's Editorial Board. Takaaki Hasegawa is one of the Associate Editors of Interventional Radiology and on the journal's Editorial Board. They were not involved in the editorial evaluation or decision to accept this article for publication at all.
References
- 1.Ieguchi M, Hoshi M, Takada J, Hidaka N, Nakamura H. Navigation-assisted surgery for bone and soft tissue tumors with bony extension. Clin Orthop Relat Res. 2012; 470: 275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pohlig F, Kirchhoff C, Lenze U, et al. Percutaneous core needle biopsy versus open biopsy in diagnostics of bone and soft tissue sarcoma: a retrospective study. Eur J Med Res. 2012; 17: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Layfield LJ, Schmidt RL, Sangle N, Crim JR. Diagnostic accuracy and clinical utility of biopsy in musculoskeletal lesions: a comparison of fine-needle aspiration, core, and open biopsy techniques. Diagn Cytopathol. 2014; 42: 476-486. [DOI] [PubMed] [Google Scholar]
- 4.Kasraeian S, Allison DC, Ahlmann ER, Fedenko AN, Menendez LR. A comparison of fine-needle aspiration, core biopsy, and surgical biopsy in the diagnosis of extremity soft tissue masses. Clin Orthop Relat Res. 2010; 468: 2992-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichenauer DA, Aleman BMP, André M, et al. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018; 29 Suppl 4: iv19-iv29. [DOI] [PubMed] [Google Scholar]
- 6.Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015; 26 Suppl 5: v116-125. [DOI] [PubMed] [Google Scholar]
- 7.Chatani S, Hasegawa T, Kato S, et al. Image-guided core needle biopsy in the diagnosis of malignant lymphoma: comparison with surgical excision biopsy. Eur J Radiol. 2020; 127: 108990. [DOI] [PubMed] [Google Scholar]
- 8.Qi D, Zhao M, Hu T, Zhang G. Diagnostic yield of percutaneous core needle biopsy in suspected soft tissue lesions of extremities. J Int Med Res. 2019; 47: 2598-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oldrini G, Leroux A, Vogin G, et al. Comparison of the histopathological results of the radioguided percutaneous microbiopsies and the operative specimens of soft tissue tumors of limbs, trunk and retroperitoneum. Presse Med. 2016; 45: e363-e368. [DOI] [PubMed] [Google Scholar]
- 10.Tomita K, Iguchi T, Hiraki T, et al. Computed tomography fluoroscopy-guided core needle biopsy of abdominal para-aortic lesions: a retrospective evaluation of the diagnostic yield and safety. Interventional Radiology. 2020; 5: 128-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kariya S, Nakatani M, Uemura Y, Yasuda K, Matsuda T, Tanigawa N. Contrast-enhanced CT-guided biopsy for IgG4-related disease of the ureter: a case report. Interventional Radiology. 2017; 2: 47-50 [Google Scholar]
- 12.Bubendorf L, Lantuejoul S, de Langen AJ, Thunnissen E. Nonsmall cell lung carcinoma: diagnostic difficulties in small biopsies and cytological specimens: number 2 in the series "Pathology for the clinician" edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev. 2017; 26: 170007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Exner GU, Kurrer MO, Mamisch-Saupe N, Cannon SR. The tactics and technique of musculoskeletal biopsy. EFORT Open Rev. 2017; 2: 51-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filippiadis DK, Charalampopoulos G, Mazioti A, Keramida K, Kelekis A. Bone and soft-tissue biopsies: what you need to know. Semin Intervent Radiol. 2018; 35: 215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smits AJ, Kummer JA, de Bruin PC, et al. The estimation of tumor cell percentage for molecular testing by pathologists is not accurate. Mod Pathol. 2014; 27: 168-174. [DOI] [PubMed] [Google Scholar]
- 16.Veltri A, Bargellini I, Giorgi L, Almeida PAMS, Akhan O. CIRSE Guidelines on percutaneous needle biopsy (PNB). Cardiovasc Intervent Radiol. 2017; 40: 1501-1513. [DOI] [PubMed] [Google Scholar]
- 17.Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012; 23: 727-736. [DOI] [PubMed] [Google Scholar]
- 18.Davidson JC, Rahim S, Hanks SE, et al. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions-Part I: Review of Anticoagulation Agents and Clinical Considerations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019; 30: 1155-1167. [DOI] [PubMed] [Google Scholar]
- 19.Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions-Part II: Recommendations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019; 30: 1168-1184.e1. [DOI] [PubMed] [Google Scholar]
- 20.Mehta SD, Weber K, Fleisher L, et al. Assessing the need for preprocedural laboratory tests and stopping non-steroidal anti-inflammatory drugs/aspirin in patients undergoing percutaneous bone and soft tissue biopsies. Cardiovasc Intervent Radiol. 2019; 42: 1588-1596. [DOI] [PubMed] [Google Scholar]
- 21.Hillen TJ, Talbert RJ, Friedman MV, et al. Biopsy of CT-occult bone lesions using anatomic landmarks for CT guidance. Am J Roentgenol. 2017; 209: 214-221. [DOI] [PubMed] [Google Scholar]
- 22.Sung KS, Seo SW, Shon MS. The diagnostic value of needle biopsy for musculoskeletal lesions. Int Orthop. 2009; 33: 1701-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datir A, Pechon P, Saifuddin A. Imaging-guided percutaneous biopsy of pathologic fractures: a retrospective analysis of 129 cases. Am J Roentgenol. 2009; 193: 504-508. [DOI] [PubMed] [Google Scholar]
- 24.Cherdchukiatsakul S. The results of comparisons between CT-guided and fluoroscopic-guided spinal biopsy. J Med Assoc Thai. 2013; 96 Suppl 3: S59-63. [PubMed] [Google Scholar]
- 25.Liu JF, Jiao DC, Ren JZ, Zhang WG, Han XW. Percutaneous bone biopsy using a flat-panel cone beam computed tomography virtual navigation system. Saudi Med J. 2018; 39: 519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chira RI, Chira A, Calauz A, et al. Ultrasound-guided biopsy of osteolytic metastasis - could be less than three cores enough? Med Ultrason. 2018; 1: 50-56. [DOI] [PubMed] [Google Scholar]
- 27.Carrino JA, Khurana B, Ready JE, Silverman SG, Winalski CS. Magnetic resonance imaging-guided percutaneous biopsy of musculoskeletal lesions. J Bone Joint Surg Am. 2007; 89: 2179-2187. [DOI] [PubMed] [Google Scholar]
- 28.Govindarajan MJ, Nagaraj KR, Kallur KG, Sridhar PS. PET/CT guidance for percutaneous fine needle aspiration cytology/biopsy. Indian J Radiol Imaging. 2009; 19: 208-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Frassica FJ, Fayad L, Clark DP, Weber KL. Analysis of nondiagnostic results after image-guided needle biopsies of musculoskeletal lesions. Clin Orthop Relat Res. 2010; 468: 3103-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Du Y, Luo TY, et al. Factors influencing diagnostic yield of CT-guided percutaneous core needle biopsy for bone lesions. Clin Radiol. 2014; 69: e43-47. [DOI] [PubMed] [Google Scholar]
- 31.Rimondi E, Rossi G, Bartalena T, et al. Percutaneous CT-guided biopsy of the musculoskeletal system: results of 2027 cases. Eur J Radiol. 2011; 77: 34-42. [DOI] [PubMed] [Google Scholar]
- 32.Ní Mhuircheartaigh J, McMahon C, Lin YC, Wu J. Diagnostic yield of percutaneous biopsy for sclerotic bone lesions: Influence of mean Hounsfield units. Clin Imaging. 2017; 46: 53-56. [DOI] [PubMed] [Google Scholar]
- 33.Wu JS, Goldsmith JD, Horwich PJ, Shetty SK, Hochman MG. Bone and soft-tissue lesions: what factors affect diagnostic yield of image-guided core-needle biopsy? Radiology. 2008; 248: 962-970. [DOI] [PubMed] [Google Scholar]
- 34.Singh VM, Salunga RC, Huang VJ, et al. Analysis of the effect of various decalcification agents on the quantity and quality of nucleic acid (DNA and RNA) recovered from bone biopsies. Ann Diagn Pathol. 2013; 17: 322-326. [DOI] [PubMed] [Google Scholar]
- 35.Toffart AC, Asfari S, Mc Leer A, et al. Percutaneous CT-guided biopsy of lytic bone lesions in patients clinically suspected of lung cancer: diagnostic performances for pathological diagnosis and molecular testing. Lung Cancer. 2020; 140: 93-98. [DOI] [PubMed] [Google Scholar]
- 36.Iguchi T, Hiraki T, Matsui Y, et al. Difference in specimen weights with semi-automatic cutting biopsy needles. Jpn J Radiol. 2020; 38: 579-586. [DOI] [PubMed] [Google Scholar]
- 37.Khadem M, Rossa C, Sloboda RS, Usmani N, Tavakoli M. Ultrasound-guided model predictive control of needle steering in biological tissue. Journal of Medical Robotics Research. 2016; 1: 1640007. [Google Scholar]
- 38.Cohen MG, McMahon CJ, Kung JW, Wu JS. Comparison of battery-powered and manual bone biopsy systems for core needle biopsy of sclerotic bone lesions. Am J Roentgenol. 2016; 206: W83-86. [DOI] [PubMed] [Google Scholar]
- 39.Schnapauff D, Marnitz T, Freyhardt P, et al. CT guided bone biopsy using a battery powered intraosseous device. Cardiovasc Intervent Radiol. 2013; 36: 1405-1410. [DOI] [PubMed] [Google Scholar]
- 40.Huang AJ, Halpern EF, Rosenthal DI. Incidence of delayed complications following percutaneous CT-guided biopsy of bone and soft tissue lesions of the spine and extremities: a 2-year prospective study and analysis of risk factors. Skeletal Radiol. 2013; 42: 61-68. [DOI] [PubMed] [Google Scholar]
- 41.Kiatisevi P, Thanakit V, Sukunthanak B, Boonthatip M, Bumrungchart S, Witoonchart K. Computed tomography-guided core needle biopsy versus incisional biopsy in diagnosing musculoskeletal lesions. J Orthop Surg (Hong Kong). 2013; 21: 204-208. [DOI] [PubMed] [Google Scholar]
- 42.Kubo T, Furuta T, Johan MP, Sakuda T, Ochi M, Adachi N. A meta-analysis supports core needle biopsy by radiologists for better histological diagnosis in soft tissue and bone sarcomas. Medicine (Baltimore). 2018; 97: e11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Exner GU, Kurrer MO, Mamisch-Saupe N, Cannon SR. The tactics and technique of musculoskeletal biopsy. EFORT Open Rev. 2017; 2: 51-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iemsawatdikul K, Gooding CA, Twomey EL, et al. Seeding of osteosarcoma in the biopsy tract of a patient with multifocal osteosarcoma. Pediatr Radiol. 2005; 35: 717-721. [DOI] [PubMed] [Google Scholar]
- 45.Yao L, Nelson SD, Seeger LL, Eckardt JJ, Eilber FR. Primary musculoskeletal neoplasms: effectiveness of core-needle biopsy. Radiology. 1999; 212: 682-686. [DOI] [PubMed] [Google Scholar]
- 46.Groneck L, Quaas A, Hallek M, Zander T, Weihrauch MR. Ultrasound-guided core needle biopsies for workup of lymphadenopathy and lymphoma. Eur J Haematol. 2016; 97: 379-386. [DOI] [PubMed] [Google Scholar]
- 47.Han F, Xu M, Xie T, et al. Efficacy of ultrasound-guided core needle biopsy in cervical lymphadenopathy: A retrospective study of 6,695 cases. Eur Radiol. 2018; 28: 1809-1817. [DOI] [PubMed] [Google Scholar]
- 48.Silverman SG, Lee BY, Mueller PR, Cibas ES, Seltzer SE. Impact of positive findings at image-guided biopsy of lymphoma on patient care: evaluation of clinical history, needle size, and pathologic findings on biopsy performance. Radiology. 1994; 190: 759-764. [DOI] [PubMed] [Google Scholar]