Abstract
Gestational diabetes mellitus (GDM) during pregnancy is associated with health complications for both mother and infant, but patient numbers in the Waikato District Health Board region of New Zealand have not been well characterised. This study reviewed the full 2018 cohort of Waikato District Health Board hospital births (n = 4970) to report on GDM prevalence by ethnicity and age. The overall prevalence of GDM was 5.7% and is more likely to affect Asian, Pacific and Māori women as well as those of advanced maternal age.
Keywords: gestational diabetes, prevalence, maternal outcome
Gestational diabetes mellitus (GDM) is associated with significant complications for both mother and child, including miscarriage, preterm labour, caesarean section, macrosomia, neonatal hypoglycaemia and perinatal death. 1 , 2 Appropriate clinical assessment and/or intervention is required for women diagnosed with GDM to ensure optimal outcomes, 3 although workload planning and patient numbers depend on several factors including optimal pregnancy screening for GDM as well as immigration and population shifts. 4
Earlier studies from 2009 to 2013 report the prevalence of GDM in New Zealand (NZ) to be approximately 6% of all pregnancies. 5 , 6 , 7 , 8 , 9 , 10 However, overall prevalence is generally reported as a collective value for a particular geographical region or cohort, irrespective of the ethnic composition of that region and the fact that different ethnic groups appear to have different GDM risk profiles. 11 The Waikato District Health Board (WDHB) region has a distinct demographic profile, including a higher proportion of Māori (24%) and a lower proportion of Pacific (3%) and Asian (9%) compared with the national averages (16.6%, 6.7% and 33% respectively). 12 This potentially impacts on health system planning as all three ethnic groups have been shown to be more at risk than European women. 5 , 9
GDM prevalence is also determined by the level of diabetes in pregnancy (DiP) screening that occurs. With the exception of one small study, 10 all previous studies reported GDM prevalence when NZ DiP screening guidelines recommended that only ‘at‐risk’ women should be screened for GDM. 13 These guidelines have since been updated (in December 2014) to include screening for GDM in all pregnant women and not just those with known risk factors. 14 Currently, DiP screening in NZ involves the following: (i) a glycated haemoglobin (HbA1c) prior to 20 weeks gestation to test for probable undiagnosed diabetes or suggestive of impaired glucose tolerance; and (ii) a glucose challenge test to assess for GDM. For those at low risk of GDM (and/or normal HbA1c levels of ≤40 mmol/mol) a 50 g oral glucose challenge test (GCT) is recommended for all women at 24–28 weeks gestation followed by a 75 g oral glucose tolerance test (OGTT) for those whose polycose result is ≥7.8 mmol/L. 14 However, for women with suggested impaired glucose tolerance, an OGTT is directly recommended at 24–28 weeks. 14 Because the guidelines have been updated to recommend universal screening of GDM we suggest that the prevalence of GDM may have increased, particularly for those groups that have been significantly under‐screened in the past. Thus, the aim of the present study was to provide a report on the current prevalence of GDM in the Waikato region.
To undertake this study, retrospective electronically available data (National Health Index (NHI) number, age and ethnicity) were collected for a full annual cohort of women who birthed in the Waikato region between 1 January and 31 December 2018, including all deliveries at WDHB hospitals and regional tertiary birthing centres. Data were unavailable for women who birthed at home, and these were excluded from our study (total study n = 5019). A further 49 cases of mothers with type 1 and type 2 diabetes (either pre‐existing or diagnosed during pregnancy) were also excluded as screening for GDM is not required in this group of women, resulting in a final ‘GDM screening cohort’ of 4970.
Within this GDM screening cohort, 92.2% had completed an HbA1c screening test, with 83.1% of these occurring before the recommended 20 weeks gestation (Table 1). Further, 78.6% of women completed a GCT and/or OGTT test during pregnancy, although only 46.8% overall completed this screening test at the recommended time period of 24–28 weeks gestation (Table 1). Two hundred women had a HbA1c suggestive of impaired glucose tolerance (41–49 mmol/mol) and 18 of these women (9.0%) had their initial GCT/OGTT at 24–28 weeks gestation.
Table 1.
Population information for the gestational diabetes mellitus (GDM) 2018 annual GDM screening cohort
| Test | All women, n = 4970 | European, n = 2468 (49.7%) | Māori, n = 1517 (30.5%) | Pacific, n = 187 (3.8%) | Asian, n = 660 (13.3%) | MELAA, n = 114 (2.3%) | Other, n = 24 (0.5%) |
|---|---|---|---|---|---|---|---|
| HbA1c measured, n (%) | 4581 (92.2) | 2322 (94.1) | 1365 (90.0) | 165 (88.2) | 602 (91.2) | 107 (93.9) | 20 (83.3) |
| <20 weeks | 4131 (83.1) | 2203 (89.3) | 1123 (74.0) | 130 (69.5) | 564 (85.5) | 92 (80.7) | 19 (79.2) |
| ≥20 weeks | 450 (9.1) | 119 (4.8) | 242 (16.0) | 35 (18.7) | 38 (5.8) | 15 (13.2) | 1 (4.2) |
| HbA1c with further screening, n (%) | 3609 (72.6) | 1898 (81.7) | 906 (59.7) | 131 (70.1) | 561 (85.0) | 96 (84.2) | 17 (70.8) |
| HbA1c and no further screening, n (%) | 972 (19.6) | 424 (19.2) | 459 (30.3) | 34 (18.2) | 41 (6.2) | 11 (9.6) | 3 (12.5) |
| Median gestation of HbA1c test, (IQR) | 6.2 (4.5, 10.5) | 5.6 (4.3, 8.4) | 8.4 (5.4, 15.0) | 9.7 (5.8, 17.4) | 5.7 (4.3, 8.3) | 6.6 (4.3, 13.1) | 5.9 (4.1, 10.4) |
| GCT or GTT screening (any time point), n (%) | 3832 (77.1) | 1989 (80.6) | 972 (64.1) | 142 (75.9) | 608 (92.1) | 101 (88.6) | 20 (83.3) |
| Initial GCT/GTT <24 weeks | 396 (8.0) | 182 (7.4) | 86 (5.7) | 10 (5.3) | 103 (15.6) | 9 (7.9) | 6 (25.0) |
| Initial GCT/GTT 24–28 weeks | 2290 (46.1) | 1289 (52.2) | 472 (31.1) | 70 (37.4) | 377 (57.1) | 72 (63.2) | 10 (14.7) |
| Initial GCT/GTT >28 weeks | 1146 (23.1) | 518 (21.0) | 414 (27.3) | 62 (33.2) | 128 (19.4) | 20 (17.5) | 4 (16.7) |
| Median gestation of initial GCT/GTT test (IQR) | 27.0 (25.4, 28.4) | 27.0 (25.5, 28.1) | 27.4 (25.7, 29.1) | 27.7 (26.1, 30.0) | 26.4 (24.6, 27.9) | 26.3 (24.9, 27.5) | 25.0 (23.3, 27.0) |
| Women screened as per Ministry of Health guideline, 14 n (%) | 2071 (41.7) | 1201 (48.7) | 410 (27.0) | 58 (31.0) | 331 (50.2) | 63 (55.3) | 8 (33.3) |
GCT, glucose challenge test; GTT, glucose tolerance test; IQR, interquartile range; MELAA, Middle Eastern/Latin American/African.
Cases of GDM were identified from the DiP register at WDHB (April 2017–December 2018) and were matched to our full annual birthing cohort by NHI. GDM was defined as fasting glucose test result of ≥5.5 mmol/L and/or a 2‐h glucose result of ≥9.0 mmol/L following a 2‐h OGTT. 14 Patients who had an HbA1c suggestive of impaired glucose tolerance (41–49 mmol/mol) were not identified as GDM unless this was also supported with the OGTT result, as per the guideline recommendation. 14 Ethnicity was categorised based on WDHB recorded self‐identified ethnicity with prioritisation to manage multiple ethnicities.
The prevalence of GDM was calculated using 2018 GDM births as the numerator and the full 2018 GDM screening cohort data (n = 4970) as the denominator. Prevalence was calculated overall and by age groups and ethnicity (European, Māori, Pacific, Asian, MELAA (Middle Eastern/Latin American/African) and others). Ethics approval for this study was granted by the University of Waikato Health Ethics Committee (Ref.: HREC (Health) 2019#42).
A total of 285 patients with GDM was identified from the 2018 GDM screening cohort giving an overall prevalence of 5.7%. GDM prevalence was lowest in European (85/2468; 3.4%) and Māori (63/1517; 4.2%) and highest in Pacific (16/187; 8.6%), MELAA (14/114; 12.3%) and Asian women (107/660; 16.2%).
Overall, GDM prevalence increased from 2.4% in those aged 15–20 years to 13.5% in those aged 41–45 years, although increased prevalence with age was most pronounced in Asian, Pacific and Māori women. Although the proportion of women aged 41–45 years at the time of delivery was low (1.7% of all deliveries), GDM prevalence was highest in this group: 40% for Asian (four of 10 women), 33% for Pacific (two of six women) and 17.4% for Māori (four of 23 women). In contrast, prevalence remained relatively stable (below 5%) in European women across all age groups. The prevalence of GDM by age and ethnicity is shown in Figure 1. Pacific and MELAA are not shown due to the small number (≤15) of women overall in each ethnic group.
Figure 1.
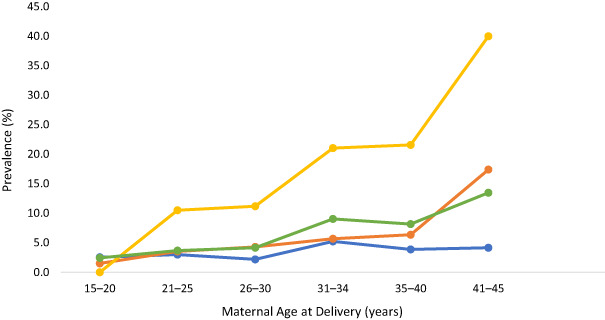
Prevalence of gestational diabetes mellitus (GDM) by age and ethnicity for women (European, Māori and Asian) who gave birth during 2018 in the Waikato region (total, n = 4970). Numbers in brackets in the legend represent the number of women with GDM. Other ethnicities are not presented due to small sample sizes. ( ), European (n = 83); (
), European (n = 83); ( ), Māori (n = 64); (
), Māori (n = 64); ( ), Asian (n = 107); (
), Asian (n = 107); ( ), total (n = 285).
), total (n = 285).
Discussion
This analysis of all births from 2018 showed a GDM prevalence in the Waikato region of 5.7%, This is consistent with previously reported NZ data, 6 , 8 , 9 , 10 despite the fact that universal screening for GDM recommendations were implemented in late 2014. 14 However, as discussed earlier, overall prevalence is a partly a reflection of the level of DiP screening that occurs in the region. Waikato data from 2017 10 and 2018 (Table 1) show that while universal GDM screening is recommended, at least one‐quarter of pregnant women did not undertake any screening test for GDM during the study periods. GDM screening was also reported to be lower in Māori than in non‐Māori women, 10 suggesting that several cases of GDM likely went undiagnosed. Assuming a similar GDM risk in our current cohort as in that described in the 2017 study, 10 this would equate to approximately 65 additional GDM cases (13 Māori and 52 non‐Māori), and a predicted overall prevalence of 7.0%. This agrees with the prevalence of GDM reported in an earlier screened population from the same region, 10 and clearly there is a need to optimise GDM screening in this region to ensure that all pregnant women can access appropriate and timely care for GDM as required. In particular, approximately one‐third of Māori women in our screening cohort did not undertake a GCT and/or OGTT and a missed diagnosis could have implications for both mother and child. Regardless, our data do suggest that the GDM prevalence for several ethnic groups may have increased in recent years. Compared to data reported from Counties Manukau DHB in 2011/2012, 9 the prevalence of GDM has almost doubled for Māori (4.2% vs 2.3%), Pacific (8.6% vs 5.58%) and Asian (16.2% vs 8.7%) women, while European remained constant at 3.4% in both data sets. Overall, this represents a substantial potential increase in clinical workload, though whether these changes are due to regional differences, changes in screening guidelines and/or other factors need be explored further. We also note that our study excluded women who delivered via home birth and these should also be included in further studies.
Importantly, our data also agree with previous literature that certain ethnic groups (e.g. Māori, Asian and Pacific) are more at risk of developing GDM. Some have suggested that this may be due to differences in maternal obesity, particularly as body mass index (BMI) thresholds for GDM risk differ by racial/ethnic groups. 15 However, we would suggest that ethnic groups with lower BMI (e.g. Asian) may have a higher prevalence of GDM because OGTT are not adjusted for maternal weight. This should also be explored in future studies as it could dramatically impact on the number of patients requiring clinical intervention.
Lastly, while we have described the prevalence of GDM here in the Waikato region of NZ, this has been restricted to the use of local screening and diagnosis criteria. 14 Other countries follow different criteria which, in turn, impact on the clinical workload. The use of stricter cut‐offs is debatable in NZ, though future studies are also warranted to evaluate the potential prevalence of GDM using international guidance.
In conclusion, the Waikato region appears to have a similar overall prevalence of GDM compared to other regions of NZ, though rates of GDM for Māori, Asian and Pacific are higher in our study than reported elsewhere. Further work is required to understand what factors are contributing to the prevalence of GDM and the increasing rate seen in different ethnic groups.
Acknowledgements
The authors thank the Waikato Medical Research Foundation for providing funding for this study. Open access publishing facilitated by University of Waikato, as part of the Wiley ‐ University of Waikato agreement via the Council of Australian University Librarians.
Funding: This study was funded by the Waikato Medical Research Foundation.
Conflict of interest: None.
References
- 1. Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA et al. Gestational diabetes and pregnancy outcomes – a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth 2012; 12: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Billionnet C, Mitanchez D, Weill A, Nizard J, Alla F, Hartemann A et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 2017; 60: 636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vääräsmäki MJD. Is it worth treating gestational diabetes: if so, when and how? Diabetologia 2016; 59: 1391–5. [DOI] [PubMed] [Google Scholar]
- 4. Yuen L, Wong VW, Simmons D. Ethnic disparities in gestational diabetes. Curr Diab Rep 2018; 18: 1–2. [DOI] [PubMed] [Google Scholar]
- 5. Yapa M, Simmons D. Screening for gestational diabetes mellitus in a multiethnic population in New Zealand. Diabetes Res Clin Pract 2000; 48: 217–23. [DOI] [PubMed] [Google Scholar]
- 6. Lawrence RL, Wall CR, Bloomfield FH. Prevalence of gestational diabetes according to commonly used data sources: an observational study. BMC Pregnancy Childbirth 2019; 19: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ekeroma AJ, Chandran GS, McCowan L, Ansell D, Eagleton C, Kenealy T. Impact of using the international association of diabetes and pregnancy study groups criteria in South Auckland: prevalence, interventions and outcomes. Aust N Z J Obstet Gynaecol 2015; 55: 34–41. [DOI] [PubMed] [Google Scholar]
- 8. McGrath NM, Baker C, Simkins A. Increased detection of gestational diabetes mellitus by using HbA1c screening in the first antenatal blood tests. Diabet Med 2014; 31: 1277. [DOI] [PubMed] [Google Scholar]
- 9. Winnard DAP, MacLennan L, Okesene‐Gafa K. Diabetes in pregnancy in Counties Manukau District Health Board. Trends over time, a 2011 snapshot and service implications. 2013. [cited 2021 Nov]. Available from URL: https://countiesmanukau.health.nz/assets/About-CMH/Reports-and-planning/Diabetes/86012bec3e/2011-Diabetes-in-Pregnancy-Trends-2011-snapshot.pdf
- 10. Chepulis LPR, Lewis‐Hills E, Ratnaweera M, McLean N, Wolmarans L, Tamatea J. Ethnic inequities in screening for diabetes in pregnancy in New Zealand – adherence to national guidelines. N Z Med J 2020; 133: 106–13. [PubMed] [Google Scholar]
- 11. Wong VW. Gestational diabetes mellitus in five ethnic groups: a comparison of their clinical characteristics. Diabet Med 2012; 29: 366–71. [DOI] [PubMed] [Google Scholar]
- 12. Ministry of Health . Population of Waikato DHB. 2021. [cited 2022 Feb]. Available from URL: https://www.health.govt.nz/new-zealand-health-system/my-dhb/waikato-dhb/population-waikato-dhb
- 13. New Zealand College of Midwives . NZCOM consensus statement gestational diabetes 1996. 2020. Available from URL: www.midwife.org.nz
- 14. Ministry of Health . Screening, Diagnosis and Management of Gestational Diabetes in New Zealand: A Clinical Practice Guideline. Wellington: Ministry of Health; 2014. [Google Scholar]
- 15. Hedderson M, Ehrlich S, Sridhar S, Darbinian J, Moore S, Ferrara A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care 2012; 35: 1492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


