ABSTRACT
Objective
To estimate the cost‐effectiveness of strategies to prevent spontaneous preterm delivery (PTD) in asymptomatic singleton pregnancies, using prevalence and healthcare cost data from the Swedish healthcare context.
Methods
We designed a decision analytic model based on the Swedish CERVIX study to estimate the cost‐effectiveness of strategies to prevent spontaneous PTD in asymptomatic women with a singleton pregnancy. The model was constructed as a combined decision‐tree model and Markov model with a time horizon of 100 years. Four preventive strategies, namely ‘Universal screening’, ‘High‐risk‐based screening’ (i.e. screening of high‐risk women only), ‘Low‐risk‐based screening’ (i.e. treatment of high‐risk population and screening of remaining women) and ‘Nullipara screening’ (i.e. treatment of high‐risk population and screening of nulliparous women only), included second‐trimester cervical length (CL) screening by transvaginal ultrasound followed by vaginal progesterone treatment in the case of a short cervix. A fifth preventive strategy involved vaginal progesterone treatment of women with previous spontaneous PTD or late miscarriage but no CL screening (‘No screening, treat high‐risk group’). For comparison, we used a sixth strategy implying no specific intervention to prevent spontaneous PTD, reflecting the current situation in Sweden (‘No screening’). Probabilities for a short cervix (CL ≤ 25 mm; base‐case) and for spontaneous PTD at < 33 + 0 weeks and at 33 + 0 to 36 + 6 weeks were derived from the CERVIX study, and probabilities for stillbirth, neonatal mortality and long‐term morbidity (cerebral palsy) from Swedish health data registers. Costs were based on Swedish data, except costs for cerebral palsy, which were based on Danish data. We assumed that vaginal progesterone reduces spontaneous PTD before 33 weeks by 30% and spontaneous PTD at 33–36 weeks by 10% (based on the literature). All analyses were from a societal perspective. We expressed the effectiveness of each strategy as gained quality‐adjusted life years (QALYs) and presented cost‐effectiveness as average (ACER; average cost per gained QALY compared with ‘No screening’) and incremental (ICER; difference in costs divided by the difference in QALYs for each of two strategies being compared) cost‐effectiveness ratios. We performed deterministic and probabilistic sensitivity analysis. The results of the latter are shown as cost‐effectiveness acceptability curves. Willingness‐to‐pay was set at a maximum of 500 000 Swedish krona (56 000 US dollars (USD)), as suggested by the Swedish National Board of Health and Welfare.
Results
All interventions had better health outcomes than did ‘No screening’, with fewer screening‐year deaths and more lifetime QALYs. The best strategy in terms of improved health outcomes was ‘Low‐risk‐based screening’, irrespective of whether screening was performed at 18 + 0 to 20 + 6 weeks (Cx1) or at 21 + 0 to 23 + 6 weeks (Cx2). ‘Low‐risk‐based screening’ at Cx1 was cost‐effective, while ‘Low‐risk‐based screening’ at Cx2 entailed high costs compared with other alternatives. The ACERs were 2200 USD for ‘Low‐risk‐based screening’ at Cx1 and 36 800 USD for ‘Low‐risk‐based screening’ at Cx2. Cost‐effectiveness was particularly sensitive to progesterone effectiveness and to productivity loss due to sick leave during pregnancy. The probability that ‘Low‐risk‐based screening’ at Cx1 is cost‐effective compared with ‘No screening’ was 71%.
Conclusion
Interventions to prevent spontaneous PTD in asymptomatic women with a singleton pregnancy, including CL screening with progesterone treatment of cases with a short cervix, may be cost‐effective in Sweden. © 2022 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: cost‐effectiveness analysis, preterm delivery, progesterone, screening, transvaginal ultrasound
CONTRIBUTION —
What are the novel findings of this work?
This is the first study to estimate the cost‐effectiveness of cervical length (CL) screening by transvaginal ultrasound and vaginal progesterone treatment in the case of a short cervix in a healthcare context different from that in the USA. All interventions to prevent spontaneous preterm delivery had better health outcomes compared with no screening.
What are the clinical implications of this work?
CL screening and vaginal progesterone treatment may be cost‐effective in the Swedish healthcare context. Ideally, each country should perform its own cost‐effectiveness analysis considering the conditions unique to their healthcare system and population.
INTRODUCTION
Preterm delivery (PTD) (before 37 weeks' gestation) increases the risk of perinatal mortality, severe neonatal morbidity and long‐term sequelae such as cerebral palsy (CP) and cognitive disability 1 , 2 , 3 , 4 . In 2014, the estimated global rate of PTD was 10.6%, ranging from 8.7% in Europe to 13.4% in North Africa 5 . In Sweden, the rate of PTD before 37 weeks in singletons was 4.4% in 2018, and the rate of PTD before 33 weeks was 0.9% 6 .
Common risk factors for PTD are multiple pregnancy, previous PTD, previous spontaneous PTD, previous late miscarriage and cervical conization 7 , 8 , 9 , 10 . Vaginal progesterone treatment may prevent spontaneous PTD in asymptomatic women with a singleton pregnancy and previous PTD 11 . A short cervix as measured by transvaginal sonography (TVS) in the second trimester is also a risk factor for spontaneous PTD in asymptomatic women with a singleton pregnancy 12 , 13 , and treating asymptomatic women with a singleton pregnancy and a short cervix with vaginal progesterone may reduce the risk of PTD and improve neonatal outcome 14 , 15 , 16 . Universal mid‐trimester sonographic cervical length (CL) screening of women with a singleton pregnancy has therefore been proposed 17 .
The cost of PTD for society is substantial, with an inverse relationship between gestational age at birth and cost 18 . Four studies reported CL screening of asymptomatic women with a singleton pregnancy followed by vaginal progesterone treatment if the cervix is short to be cost‐effective in the USA 19 , 20 , 21 , 22 . Only one study questioned the cost‐effectiveness of this strategy 23 . In the USA, the rate of PTD is higher (10.2% in 2019) 24 and healthcare expenditure makes up a higher percentage of the gross domestic product (GDP) in comparison with many European Union member states (18% of GDP in the USA in 2020 25 vs an average of 10% of GDP in Europe and 11% in Sweden in 2018 26 ).
The aim of this study was to estimate the cost‐effectiveness of various strategies for the prevention of spontaneous PTD in singleton pregnancies, using prevalence and healthcare cost data from a Swedish healthcare context.
METHODS
This study was reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 27 . We designed a decision analytic model to estimate the cost‐effectiveness of strategies aimed at preventing spontaneous PTD in asymptomatic women with a singleton pregnancy. Four preventive strategies (‘Universal screening’, ‘Low‐risk‐based screening’, ‘High‐risk‐based screening’ and ‘Nullipara screening’) included second‐trimester CL screening by TVS followed by vaginal progesterone treatment in the case of a short cervix. A fifth preventive strategy encompassed vaginal progesterone treatment of women with previous spontaneous PTD or late miscarriage but no CL screening (‘No screening, treat high‐risk group’). For comparison, we used a sixth strategy implying no specific intervention to prevent spontaneous PTD (‘No screening’), which reflects the current situation in Sweden (0.2% of all women with a singleton pregnancy redeemed their prescription for vaginal progesterone after 18 + 0 weeks during the years 2014 to 2017) 12 . The decision analytic model was based on the CERVIX study, a prospective blinded multicenter study that investigated the diagnostic performance of second‐trimester CL measurement for the prediction of spontaneous PTD in a Swedish population of asymptomatic singleton pregnancies 12 . The CERVIX study is briefly outlined below.
The CERVIX study
Women were recruited to the CERVIX study at their routine fetal ultrasound examination scheduled at 18 weeks' gestation. Those aged ≥ 18 years who had a live singleton pregnancy at 18 + 0 to 20 + 6 weeks were invited to participate. Exclusion criteria applied at the routine scan were fetal malformations, ruptured membranes, symptoms or findings suggesting ongoing miscarriage, current use of progesterone, cerclage in situ and difficulty understanding written or oral information about the study. Women with medical termination of the pregnancy after inclusion in the study or with missing information about pregnancy outcome were also excluded.
The study protocol included two measurements of CL: one between 18 + 0 and 20 + 6 weeks, performed on the day of the routine fetal ultrasound examination, and a second one between 21 + 0 and 23 + 6 weeks (optional) with at least 14 days between the two measurements. Participants and staff were blinded to the CL measurements. Gestational age was estimated based on ultrasound measurement of the fetal biparietal diameter 28 , 29 , or on the day of embryo transfer in case of in‐vitro fertilization, as recommended in Swedish guidelines 30 . PTD was defined as delivery before 37 + 0 weeks (259 days) and spontaneous PTD as delivery before 37 + 0 weeks (including late miscarriage occurring after inclusion in the study, i.e. spontaneous delivery at 18 + 0 to 21 + 6 weeks of a fetus showing no signs of life), either after spontaneous onset of labor or after preterm prelabor rupture of membranes, the latter regardless of whether labor was induced or not. To obtain reliable information on previous late miscarriages, we scrutinized the medical records of participants with self‐reported previous late miscarriage. We defined previous late miscarriage as: (1) spontaneous miscarriage at 16 + 0 to 21 + 6 weeks according to the last menstrual period, (2) missed abortion if fetal size measured with ultrasound corresponded to 16 + 0 to 21 + 6 weeks or (3) self‐reported miscarriage between 16 and 21 weeks if no information was found in the medical records 12 .
The CERVIX study, which was conducted between May 2014 and June 2017, included 11 072 women with delivery data and CL measurement at 18 + 0 to 20 + 6 weeks, 6288 women with delivery data and CL measurement at 21 + 0 to 23 + 6 weeks and 6179 women with delivery data and CL measurement at both 18 + 0 to 20 + 6 weeks and 21 + 0 to 23 + 6 weeks with at least 14 days between the two measurements. The results showed that CL measurements obtained at 21 + 0 to 23 + 6 weeks performed substantially better at predicting spontaneous PTD than measurements taken at the routine scan at 18 + 0 to 20 + 6 weeks 12 , 31 .
Strategies to prevent spontaneous PTD
Analyses were performed separately for CL screening at the routine second‐trimester scan at 18 + 0 to 20 + 6 weeks (Cx1) and for CL screening at a separate appointment at 21 + 0 to 23 + 6 weeks (Cx2). The cost‐effectiveness of the following strategies was investigated (Table 1; Figure 1): (1) ‘No screening’: no CL screening and no standardized treatment with vaginal progesterone (reflecting the current situation in Sweden); (2) ‘No screening, treat high‐risk group’: no CL screening but vaginal progesterone treatment of women with previous spontaneous PTD or late miscarriage (high‐risk women); (3) ‘Universal screening’: CL screening either at Cx1 or Cx2, with vaginal progesterone treatment of women with a short cervix (for definition of short cervix, see below and Table 1); (4) ‘High‐risk‐based screening’: CL screening at Cx2 of women with previous PTD, late miscarriage or cervical conization, with vaginal progesterone treatment of women with a short cervix; no screening and no standardized vaginal progesterone treatment of the remaining population; (5) ‘Low‐risk‐based screening’: vaginal progesterone treatment of women with a previous spontaneous PTD or late miscarriage (high‐risk) and CL screening of all other women (low‐risk) either at Cx1 or Cx2, followed by vaginal progesterone treatment of women with a short cervix; (6) ‘Nullipara screening’: CL screening of nulliparous women either at Cx1 or Cx2 followed by vaginal progesterone treatment of women with a short cervix; vaginal progesterone treatment of women with a previous spontaneous PTD or late miscarriage (high‐risk); no screening and no standardized vaginal progesterone treatment of the remaining population (i.e. parous women with only previous term deliveries or indicated PTD).
Table 1.
Strategies for prevention of spontaneous preterm delivery (sPTD) in asymptomatic women with a singleton pregnancy, based on sonographic cervical length (CL) screening and vaginal progesterone treatment
| Strategy | Population screened | Population not screened | Definition of short cervix | Treatment | |
|---|---|---|---|---|---|
| CL measurement at 18 + 0 to 20 + 6 weeks | CL measurement at 21 + 0 to 23 + 6 weeks | ||||
| No screening | NA | All women | NA | NA | No standardized treatment |
| No screening, treat high‐risk group | NA | All women | NA | NA | Women with previous sPTD or late miscarriage are treated with vaginal progesterone without screening |
| Universal screening | All women | NA | ≤ 25 mm (base‐case); ≤ 29 mm; ≤ 20 mm | ≤ 25 mm (base‐case); ≤ 27 mm; ≤ 20 mm | Screened women with a short cervix are treated with vaginal progesterone |
| High‐risk‐based screening | Women with previous PTD, previous late miscarriage or cervical conization | All women except those with previous PTD, previous late miscarriage or cervical conization | NA | ≤ 25 mm (base‐case); ≤ 27 mm; ≤ 20 mm | Screened women (previous PTD, late miscarriage or cervical conization) with a short cervix are treated with vaginal progesterone |
| Low‐risk‐based screening | All women except those with previous sPTD or previous late miscarriage | Women with previous sPTD or previous late miscarriage | ≤ 25 mm (base‐case); ≤ 29 mm; ≤ 20 mm | ≤ 25 mm (base‐case); ≤ 27 mm; ≤ 20 mm | Screened women with a short cervix are treated with vaginal progesterone; not‐screened women with a previous sPTD or late miscarriage are treated with vaginal progesterone |
| Nullipara screening | All nulliparous women (except those with a previous late miscarriage) | All women except screened nulliparous women | ≤ 25 mm (base‐case); ≤ 29 mm; ≤ 20 mm | ≤ 25 mm (base‐case); ≤ 27 mm; ≤ 20 mm | Screened women with a short cervix are treated with vaginal progesterone; not‐screened women with a previous sPTD or late miscarriage are treated with vaginal progesterone |
NA, not applicable.
Figure 1.
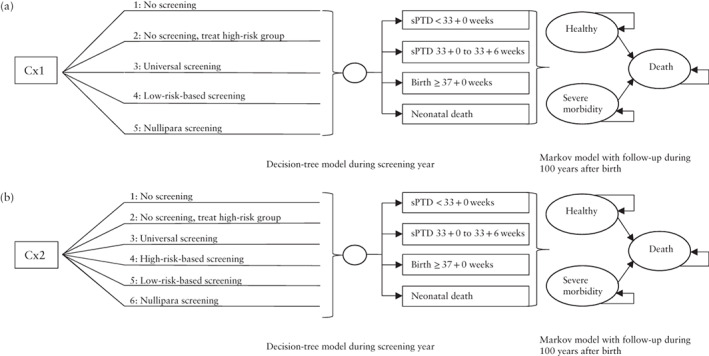
Overview of investigated strategies for prevention of spontaneous preterm delivery (sPTD) in asymptomatic women with a singleton pregnancy, based on sonographic cervical length (CL) screening and vaginal progesterone treatment, when CL is measured by transvaginal ultrasound at: (a) 18 + 0 to 20 + 6 weeks, on the day of the routine fetal scan (Cx1); and (b) 21 + 0 to 23 + 6 weeks, at an additional appointment (Cx2). The decision analytic model was constructed as a combined decision‐tree model (screening year) and a Markov model with three health states (healthy, long‐term morbidity or death) conducted with annual cycles for a time horizon of 100 years.
For screening of high‐risk women (‘High‐risk‐based screening’), we evaluated CL screening only at Cx2 for two reasons: first, the ability of sonographic CL at 21 + 0 to 23 + 6 weeks (Cx2) to correctly predict spontaneous PTD before 33 + 0 weeks is superior to that of CL measured at 18 + 0 to 20 + 6 weeks (Cx1) 12 , 31 , and second, an extra visit for CL measurement is justified in a high‐risk population.
In the base‐case scenario we defined a short cervix as one with a length of ≤ 25 mm (CL25) (Table 1) 14 . Other definitions were:
CL ≤ 20 mm (CL20) (used in other cost‐effectiveness analyses) 19 , 20 , 21 , 23 ;
CL ≤ 29 mm (CL29) at 18 + 0 to 20 + 6 weeks (best cut‐off, i.e. the cut‐off that yielded the largest number of correct classifications 32 , at Cx1 in the CERVIX study 12 );
CL ≤ 27 mm (CL27) at 21 + 0 to 23 + 6 weeks (best cut‐off 32 at Cx2 in the CERVIX study 12 ).
Probabilities
Probabilities for spontaneous PTD and indicated PTD before 33 + 0 weeks and at 33 + 0 to 36 + 6 weeks were derived from the study populations in the CERVIX study 12 , 31 . The distinction between PTD before 33 + 0 weeks and at 33 + 0 to 36 + 6 weeks was made because short‐term and long‐term complications are substantially more common in neonates born before 33 + 0 weeks than in those born at 33 + 0 to 36 + 6 weeks 3 , 33 . We assumed that vaginal progesterone reduces spontaneous PTD before 33 + 0 weeks by 30%, and spontaneous PTD at 33 + 0 to 36 + 6 weeks by 10% 14 , irrespective of which CL cut‐off (20, 25, 27 or 29 mm) was used. We assumed that vaginal progesterone also has this effect in women with previous spontaneous PTD or late miscarriage not undergoing CL screening. Probability estimates for preterm and term delivery for the strategies ‘No screening’, ‘No screening, treat high‐risk group’ and ‘Low‐risk‐based screening’ are shown in Table 2, and for all six strategies in Table S1.
Table 2.
Probability estimates for prevalence of preterm (< 33 + 0 weeks or at 33 + 0 to 36 + 6 weeks) and term (≥ 37 + 0 weeks) delivery for three strategies for prevention of spontaneous preterm delivery (sPTD) in asymptomatic women with a singleton pregnancy, at 18 + 0 to 20 + 6 weeks (Cx1) and at 21 + 0 to 23 + 6 weeks (Cx2)
| Strategy/variable | Probability at Cx1 (n = 11 072) | Probability at Cx2 (n = 6288) | ||||
|---|---|---|---|---|---|---|
| Absolute numbers | Point estimate | SE | Absolute numbers | Point estimate | SE | |
| No screening | ||||||
| sPTD < 33 + 0 weeks | 63/11 072 | 0.006 | 0.000001 | 26/6288 | 0.004 | 0.000001 |
| sPTD at 33 + 0 to 36 + 6 weeks | 354/11 072 | 0.032 | 0.000003 | 199/6288 | 0.032 | 0.000005 |
| iPTD < 33 + 0 weeks | 54/11 072 | 0.005 | 0.000001 | 27/6288 | 0.004 | 0.000001 |
| iPTD at 33 + 0 to 36 + 6 weeks | 114/11 072 | 0.010 | 0.000001 | 69/6288 | 0.011 | 0.000002 |
| Delivery ≥ 37 + 0 weeks | 10 487/11 072 | 0.947 | 0.000012 | 5967/6288 | 0.949 | 0.000020 |
| No screening, treat high‐risk group | ||||||
| No‐treatment population | ||||||
| sPTD < 33 + 0 weeks | 57/10 668 | 0.005 | 0.000001 | 23/6037 | 0.004 | 0.000001 |
| sPTD at 33 + 0 to 36 + 6 weeks | 308/10 668 | 0.029 | 0.000002 | 172/6037 | 0.028 | 0.000004 |
| iPTD < 33 + 0 weeks | 50/10 668 | 0.005 | 0.000001 | 25/6037 | 0.004 | 0.000001 |
| iPTD at 33 + 0 to 36 + 6 weeks | 106/10 668 | 0.010 | 0.000001 | 65/6037 | 0.011 | 0.000002 |
| Delivery ≥ 37 + 0 weeks | 10 147/10 668 | 0.951 | 0.000012 | 5752/6037 | 0.953 | 0.000021 |
| High‐risk women* treated | ||||||
| sPTD < 33 + 0 weeks | 6/404 | 0.015 | 0.006018 | 3/251 | 0.012 | 0.000046 |
| sPTD at 33 + 0 to 36 + 6 weeks | 46/404 | 0.114 | 0.015803 | 27/251 | 0.108 | 0.000314 |
| iPTD < 33 + 0 weeks | 4/404 | 0.010 | 0.004926 | 2/251 | 0.008 | 0.000031 |
| iPTD at 33 + 0 to 36 + 6 weeks | 8/404 | 0.020 | 0.006931 | 4/251 | 0.016 | 0.000060 |
| Delivery ≥ 37 + 0 weeks | 340/404 | 0.842 | 0.018166 | 215/251 | 0.857 | 0.000532 |
| Low‐risk‐based screening | ||||||
| All, except high‐risk women* | ||||||
| Prevalence of CL ≤ 25 mm | 419/10 668 | 0.039 | 0.001881 | 251/6037 | 0.042 | 0.002469 |
| If CL ≤ 25 mm: | ||||||
| sPTD < 33 + 0 weeks | 17/419 | 0.041 | 0.009639 | 8/251 | 0.032 | 0.011088 |
| sPTD at 33 + 0 to 36 + 6 weeks | 17/419 | 0.041 | 0.009639 | 21/251 | 0.084 | 0.017477 |
| iPTD < 33 + 0 weeks | 4/419 | 0.010 | 0.004750 | 2/251 | 0.008 | 0.005612 |
| iPTD at 33 + 0 to 36 + 6 weeks | 2/419 | 0.005 | 0.003367 | 5/251 | 0.020 | 0.008819 |
| Delivery ≥ 37 + 0 weeks | 379/419 | 0.905 | 0.014356 | 215/251 | 0.857 | 0.022124 |
| If CL > 25 mm: | ||||||
| sPTD < 33 + 0 weeks | 40/10 249 | 0.004 | 0.000616 | 15/5786 | 0.003 | 0.000669 |
| sPTD at 33 + 0 to 36 + 6 weeks | 291/10 249 | 0.028 | 0.000164 | 151/5786 | 0.026 | 0.002096 |
| iPTD < 33 + 0 weeks | 46/10 249 | 0.004 | 0.000660 | 23/5786 | 0.004 | 0.000827 |
| iPTD at 33 + 0 to 36 + 6 weeks | 104/10 249 | 0.010 | 0.000990 | 60/5786 | 0.010 | 0.001332 |
| Delivery ≥ 37 + 0 weeks | 9768/10 249 | 0.953 | 0.002089 | 5537/5786 | 0.957 | 0.002668 |
| High‐risk women* (all treated, none screened) | ||||||
| sPTD < 33 + 0 weeks | 6/404 | 0.015 | 0.006018 | 3/251 | 0.012 | 0.006859 |
| sPTD at 33 + 0 to 36 + 6 weeks | 46/404 | 0.114 | 0.015803 | 27/251 | 0.108 | 0.019557 |
| iPTD < 33 + 0 weeks | 4/404 | 0.010 | 0.004926 | 2/251 | 0.008 | 0.005612 |
| iPTD at 33 + 0 to 36 + 6 weeks | 8/404 | 0.020 | 0.006931 | 4/251 | 0.016 | 0.007904 |
| Delivery ≥ 37 + 0 weeks | 340/404 | 0.842 | 0.018166 | 215/251 | 0.857 | 0.022124 |
High‐risk women are those with previous sPTD or previous late miscarriage.
CL, cervical length; iPTD, indicated preterm delivery; SE, standard error.
Probability estimates for progesterone effectiveness, neonatal mortality and long‐term morbidity are shown in Table 3. We assumed 16 weeks' therapy for women treated with vaginal progesterone.
Table 3.
Estimates for progesterone effect and probability of neonatal mortality and long‐term morbidity used in decision analytic model estimating cost‐effectiveness of strategies to prevent spontaneous preterm delivery (sPTD) in asymptomatic women with a singleton pregnancy
| Variable | Absolute numbers | Point estimate | Range* | Reference |
|---|---|---|---|---|
| Estimated reduction of sPTD < 33 + 0 weeks with VP treatment | — | 0.300 | 0.050/0.550 | 14 |
| Estimated reduction of sPTD at 33 + 0 to 36 + 6 weeks with VP treatment | — | 0.100 | 0.0167/0.1833 | 14 |
| Stillbirth if: | ||||
| PTD < 33 + 0 weeks | 677/458 220 | 0.0014774 | ± 20% | MBR† |
| PTD at 33 + 0 to 36 + 6 weeks | 298/453 499 | 0.0006571 | ± 20% | MBR† |
| Delivery ≥ 37 + 0 weeks | 669/436 509 | 0.0014326 | ± 20% | MBR† |
| NND < 7 days after birth if: | ||||
| PTD < 33 + 0 weeks | 214/4044 | 0.0529179 | ± 20% | MBR† |
| PTD at 33 + 0 to 36 + 6 weeks | 67/16 702 | 0.0040138 | ± 20% | MBR† |
| Delivery ≥ 37 + 0 weeks | 158/435 840 | 0.0003625 | ± 20% | MBR† |
| NND at 7–27 days after birth if: | ||||
| PTD < 33 + 0 weeks | 82/4044 | 0.0202769 | ± 20% | MBR† |
| PTD at 33 + 0 to 36 + 6 weeks | 17/16 702 | 0.0010184 | ± 20% | MBR† |
| Delivery ≥ 37 + 0 weeks | 57/435 840 | 0.0001307 | ± 20% | MBR |
| Long‐term morbidity (CP) if: | ||||
| PTD < 33 + 0 weeks | 41.36/1000 | 0.04136 | ± 20% | 36‡ |
| PTD at 33 + 0 to 36 + 6 weeks | 5.63/1000 | 0.00563 | ± 20% | 36‡ |
| Delivery ≥ 37 + 0 weeks | 1.23/1000 | 0.00123 | ± 20% | 36‡ |
| Healthy newborn if: | ||||
| PTD < 33 + 0 weeks | 958.64/1000 | 0.9586 | ± 20% | 36‡ |
| PTD at 33 + 0 to 36 + 6 weeks | 994.37/1000 | 0.99437 | ± 20% | 36‡ |
| Delivery ≥ 37 + 0 weeks | 998.77/1000 | 0.99877 | ± 20% | 36‡ |
Range means values used in sensitivity analyses and is presented as min/max or as ± 20%.
Mortality rates from Swedish Medical Birth Register (MBR) for the years 2014, 2015, 2016 and 2017.
Personal communication with K. Himmelmann 36 .
CP, cerebral palsy; NND, neonatal death; PTD, preterm delivery; VP, vaginal progesterone.
We estimated the probability of stillbirth (at or after 22 + 0 weeks) and neonatal mortality (neonatal death before 7 days and neonatal death at 7 to 27 days) for each of the three gestational‐age categories at delivery (before 33 + 0 weeks, at 33 + 0 to 36 + 6 weeks and at or after 37 + 0 weeks) using data from the Swedish Medical Birth Register 34 including all singleton deliveries during the years 2014, 2015, 2016 and 2017. Long‐term morbidity was defined as CP, which comprises a group of permanent disorders involving movement and posture impairment due to a non‐progressive interference or lesion of the developing and immature brain 35 . Probabilities of CP for each gestational‐age category at delivery were derived from the CP register in western Sweden (all individuals with CP born between 1959 and 2002 and living in western Sweden) and personal communication with the first author of the study (K. Himmelmann) 36 .
Cost estimates
Cost estimates are shown in Table 4, presented in US dollars (USD) using an exchange rate of 1 USD = 9 Swedish krona (SEK) (22 November 2021). All costs are deflated to 2021 (July 2021) prices using a consumer price index 37 . The costs for treatment with vaginal progesterone were derived from Pharmaceutical specialties in Sweden 38 . The costs for CL screening include costs for teaching and training midwife sonographers to perform CL measurements as well as implementation of the screening program 39 , costs for maintenance of a quality program to ensure the quality of CL measurements 40 , 41 and costs for ultrasound examinations. Gross salary costs (including payroll taxes) for midwife sonographers and physicians and costs for ultrasound examinations are based on information from Skåne University Hospital, Sweden, in 2019. We estimated that 15 min needed to be added for CL measurement at the routine ultrasound examination at 18 + 0 to 20 + 6 weeks (Cx1) 39 , and we estimated the duration of an extra visit to measure CL at 21 + 0 to 23 + 6 weeks (Cx2) to be 30 min (including information, documentation and counseling). The costs for a quality program were approximated by using the model for the existing Swedish quality assurance program for prenatal screening for chromosomal abnormalities (combined ultrasound examination and blood test at 11–13 weeks). In the case of a short cervix, we assumed at least one visit to a physician for information and for prescription of progesterone. We did not account for any productivity loss for women considered at high risk for spontaneous PTD, i.e. those with short cervix, previous (spontaneous) PTD or late miscarriage or conization.
Table 4.
Unit cost estimates and quality‐adjusted life year (QALY) weights of decision analytic model estimating cost‐effectiveness of strategies to prevent spontaneous preterm delivery in asymptomatic women with a singleton pregnancy, based on sonographic cervical length (CL) screening and vaginal progesterone treatment
| Variable | Point estimate (US dollars) | Range* | Reference |
|---|---|---|---|
| Costs | |||
| Vaginal progesterone (16‐week course), per treated woman | 130 | ± 20% | 38 |
| Education of midwife sonographers and implementation of a screening program if: | 39; see Methods | ||
| Universal screening or screening of low‐risk women at 18 + 0 to 20 + 6 weeks | 3 224 222 | ± 20% | |
| Screening of nulliparous women at 18 + 0 to 20 + 6 weeks | 1 617 111 | ± 20% | |
| Universal screening or screening of low‐risk women at 21 + 0 to 23 + 6 weeks | 2 259 956 | ± 20% | |
| Screening of high‐risk women at 21 + 0 to 23 + 6 weeks | 813 556 | ± 20% | |
| Screening of nulliparous women at 21 + 0 to 23 + 6 weeks | 1 134 978 | ± 20% | |
| Quality control of screening program | 52 732 | ± 20% | See Methods |
| TVS at 18 + 0 to 20 + 6 weeks, per scan | 58 | ± 20% | See Methods |
| TVS at 21 + 0 to 23 + 6 weeks, per scan | 117 | ± 20% | See Methods |
| Visit to physician for check‐ups, per visit | 175 | ± 20% | See Methods |
| Cost per delivery < 33 + 0 weeks | 6431 | ± 20% | See Methods |
| Cost per delivery at 33 + 0 to 36 + 6 weeks | 5222 | ± 20% | See Methods |
| Cost per delivery ≥ 37 + 0 weeks | 4289 | ± 20% | See Methods |
| Productivity loss (parental leave) per baby born < 33 + 0 weeks | 10 833 | ± 20% | 43 |
| Neonatal care per delivery < 33 + 0 weeks | 69 586 | ± 20% | See Methods |
| Neonatal care per delivery at 33 + 0 to 36 + 6 weeks | 11 739 | ± 20% | See Methods |
| Cost of extra stay at postpartum ward per baby born at 35 + 0 to 36 + 6 weeks not admitted to NICU | 2100 | ± 20% | See Methods |
| Neonatal care per delivery ≥ 37 + 0 weeks | 8146 | ± 20% | See Methods |
| Long‐term disability: | |||
| Annual healthcare cost per individual 0–19 years | 6415 | ± 20% | 44,45 |
| Annual healthcare cost per individual 20–54 years | 2135 | ± 20% | 44,45 |
| Annual healthcare cost per individual > 54 years | 976 | ± 20% | 44,45 |
| Annual social cost per individual (pre‐school 1–6 years) | 76 511 | ± 20% | 44,45 |
| Annual social cost per individual (school 7–16 years) | 39 762 | ± 20% | 44,45 |
| Annual social cost for day‐care center per individual 0–18 years | 11 297 | ± 20% | 44,45 |
| Annual social cost for institutional residence per individual 0–18 years | 135 566 | ± 20% | 44,45 |
| Annual social cost for sheltered workshop per individual > 18 years | 16 796 | ± 20% | 44,45 |
| Annual social cost for day‐care center per individual > 18 years | 30 310 | ± 20% | 44,45 |
| Annual social cost for institutional residence per individual > 18 years | 119 929 | ± 20% | 44,45 |
| Annual social cost for temporary institutional residence per individual > 18 years | 9994 | ± 20% | 44,45 |
| Daily cost for absence from work per individual | 217 | ± 20% | 44,45 |
| QALY weights | |||
| Neonatal death | 0.00 | 46–48 | |
| Long‐term morbidity | 0.55 | 0.06 | 46–48 |
| Healthy neonate | 1.00 | 46–48 |
Range means values used in sensitivity analyses and is presented as standard error (SE) or as ± 20%.
NICU, neonatal intensive care unit; TVS, transvaginal sonography.
Costs for spontaneous PTD and indicated PTD were assumed to be equal. Costs for delivery, postpartum care of the mother and child and neonatal care (including intermediate and intensive neonatal care) differ depending on gestational age at birth (before 33 + 0 weeks, at 33 + 0 to 36 + 6 weeks and at or after 37 + 0 weeks), and delivery costs differ depending on delivery mode. We estimated the frequency of vaginal and Cesarean delivery in each gestational‐age category (< 33 + 0 weeks, at 33 + 0 to 36 + 6 weeks and ≥ 37 + 0 weeks) based on data from deliveries in the CERVIX study 12 , 31 and used this information to estimate the mean cost for delivery and postpartum ward care per patient in each of the three gestational‐age categories at delivery. We derived costs for delivery, including postpartum ward care of mother and child (cost per patient), from Sahlgrenska University Hospital, Gothenburg, Sweden, during the years 2018 and 2019, and costs for neonatal care (cost per neonate) from Sahlgrenska University Hospital during the years 2017, 2018 and 2019. The cost per baby born before 33 + 0 weeks was estimated by dividing the total cost for neonatal care of all babies born before 33 + 0 weeks by the total number of babies born before 33 + 0 weeks; the cost of neonatal care per baby born at 33 + 0 to 36 + 6 weeks or at or after 37 + 0 weeks was estimated in the same way. About 70% of neonates born between 35 + 0 and 36 + 6 weeks (included in the gestational‐age category 33 + 0 to 36 + 6 weeks) were not in need of intensive or intermediate neonatal care but needed prolonged postpartum ward care. For this subgroup, costs for 3.5 extra days of postpartum ward care were added per neonate based on data from the Sahlgrenska University Hospital during the years 2017, 2018 and 2019.
For parents with a baby born before 33 + 0 weeks, we estimated a temporary parental leave of 5 weeks for each parent. The indirect cost for absence from work was valued by the human capital approach assuming production loss to be valued at market price, i.e. gross salaries and payroll taxes 42 . We estimated this cost to be 1950 SEK, corresponding to 217 USD (including payroll taxes) per day 43 .
Costs for neonates surviving with long‐term morbidity (in this model defined as CP, as discussed above) were derived from the literature 44 , 45 and calculated as an average annual individual cost including both healthcare and social costs. Full‐time absence from work was estimated for the hypothetical population with long‐term morbidity according to Kruse et al. 44 , 45 , with an estimated daily cost of 217 USD (including payroll taxes) 43 .
Quality‐adjusted life years
The cost‐effectiveness analysis was performed based on gained quality‐adjusted life years (QALYs). A QALY weight of 0 equals ‘death’ and of 1 represents ‘perfect health’. QALY weights were derived from the literature 46 , 47 , 48 (Table 4). QALYs were calculated for a hypothetical population as QALY weight decrements from age‐specific health‐related quality of life based on the general population in Sweden 49 .
Decision analytic model
A decision analytic model was designed to estimate the effectiveness of each strategy expressed as gained QALYs. The model was constructed as a combined decision‐tree model and a Markov model. The decision‐tree model was conducted for the screening year, i.e. the period from sonographic screening until the baby's first birthday. The state‐transition Markov model with three health states (healthy, long‐term morbidity or death) was conducted with annual cycles for a time horizon of 100 years. Since there are (neonatal) mortality differences between the strategies, a lifelong perspective (set at maximum to 100 years in our model) is necessary to capture these differences. This is in line with recommendations on best practices for cost‐effectiveness analysis modeling 50 , 51 . We present the cost‐effectiveness of each analyzed strategy as average cost‐effectiveness ratio (ACER) and incremental cost‐effectiveness ratio (ICER). The ACER shows the difference in costs divided by the difference in QALYs for each strategy compared with ‘No screening’ (i.e. current situation in Sweden). The ICER shows the difference in costs divided by the difference in QALYs for two strategies being compared. If a strategy was both less expensive and resulted in more QALYs than another alternative, it was classified as ‘dominant’. The cost‐effectiveness analysis was based on a societal perspective, including both direct costs within the health and social‐care systems and productivity loss. An annual discount rate of 3% was applied for both costs and QALYs 52 . Maximum willingness to pay was set at 500 000 SEK (corresponding to approximately 56 000 USD) according to the definition of cost‐effectiveness suggested by the Swedish National Board of Health and Welfare 53 . The models were programmed in Microsoft Excel (Microsoft Corp., Redmond, WA, USA).
Sensitivity analyses
Deterministic and probabilistic sensitivity analyses were carried out for the base‐case (CL25) strategies to assess the effect on the cost‐effectiveness results of the uncertainty of assumed parameter values in the model. We performed deterministic sensitivity analyses to assess the impact of varying the model inputs one at a time, while keeping other variables fixed. The following deterministic sensitivity analyses were performed:
Costs for visit to a physician for follow‐up, either every 2 weeks or once a month from diagnosis until 34 + 0 weeks were added.
Costs for productivity loss (average 16 weeks) for either 50% or 100% of the women with a short cervix, previous spontaneous PTD or late miscarriage based on cost presented by Statistics Sweden 43 were added.
The effectiveness of progesterone treatment to reduce spontaneous PTD before 33 + 0 weeks was varied from 5% to 55% and that to reduce spontaneous PTD at 33 + 0 to 36 + 6 weeks from 1.7% to 18.3% 14 .
Costs for temporal parental leave were varied by ± 20%.
Costs for neonatal care per baby were varied by ± 20%.
Costs were calculated using only a healthcare perspective.
The discount rate of all costs was examined for 0% and for 5% 52 .
For the most cost‐effective base‐case strategies (CL25), a threshold analysis was performed to investigate the minimum required effect of progesterone for the strategy to remain cost‐effective.
A probabilistic sensitivity analysis varying all prevalence probabilities, effectiveness of progesterone (Tables 2 and 3), costs and QALY weights (Table 4) was undertaken using a Monte Carlo simulation 54 with 1000 simulations for the most cost‐effective base‐case (CL25) strategies. This was perfomed separately for the Cx1 and Cx2 strategies. In each simulation, the value for each probability, unit cost and QALY weights was sampled from its statistical distribution. The probabilities and QALY weights were modeled using beta distribution, since the probabilities and QALYs are bounded within 0 and 1 (0–100%). The remaining variables were simulated assuming a uniform distribution. We show the results of the simulations as cost‐effectiveness acceptability curves.
RESULTS
Base‐case strategies (CL25)
Table 5 shows the results in terms of differences in cost, screening‐year mortality and lifetime QALYs between the prevention strategies and no screening. For the Cx1 strategies (screening at 18 + 0 to 20 + 6 weeks), the intervention ‘No screening, treat high‐risk group’ reduced costs compared with ‘No screening’ both during the screening year and in the lifetime horizon. ‘Nullipara screening’ reduced costs compared with ‘No screening’ in the lifetime horizon. The intervention with the highest increase in total costs was ‘Universal screening’. All interventions had better health outcomes than did no intervention, with fewer screening‐year deaths and more lifetime QALYs. The Cx1 strategy with the best health outcomes was ‘Low‐risk‐based screening’, which reduced deaths during the screening year by six and increased lifetime QALYs by 206 per 100 000 women. For the Cx2 strategies (screening at 21 + 0 to 23 + 6 weeks), compared with ‘No screening’, the ‘No screening, treat high‐risk group’ strategy reduced costs both during the screening year and in the lifetime horizon, and the ‘High‐risk‐based screening’ strategy reduced costs in the lifetime horizon. Universal screening was the most expensive Cx2 strategy. All Cx2 strategies gave better health outcomes than did ‘No screening’. ‘Low‐risk‐based screening’ was the most beneficial Cx2 strategy in terms of improved health outcomes, but it was the second most expensive Cx2 strategy.
Table 5.
Differences in cost and health outcome per 100 000 women for the base‐case strategies (high risk of spontaneous preterm delivery (sPTD) indicated by sonographic cervical length (CL) ≤ 25 mm) in comparison with the ‘No screening’ strategy, according to CL screening at 18 + 0 to 20 + 6 weeks (Cx1) and at 21 + 0 to 23 + 6 weeks (Cx2)
| Strategy | Difference in cost compared with ‘No screening’ | Difference in health outcomes compared with ‘No screening’ | ||
|---|---|---|---|---|
| Screening year (USD) | Lifetime (USD) | Screening‐year mortality (n) | Lifetime QALYs (n) | |
| CL screening at Cx1 | ||||
| No screening, treat high‐risk group | –1 201 000 | –2 630 000 | –2.1 | + 71 |
| Universal screening | 5 894 000 | 3 327 000 | –4.0 | + 136 |
| Low‐risk‐based screening | 4 425 000 | 449 000 | –6.0 | + 206 |
| Nullipara screening | 1 426 000 | –1 454 000 | –4.3 | + 148 |
| CL screening at Cx2 | ||||
| No screening, treat high‐risk group | –832 000 | –2 124 000 | –1.8 | + 64 |
| Universal screening | 10 870 000 | 8 154 000 | –4.1 | + 141 |
| High‐risk‐based screening | 632 000 | –491 000 | –1.7 | + 58 |
| Low‐risk‐based screening | 10 234 000 | 6 663 000 | –5.3 | + 181 |
| Nullipara screening | 5 232 000 | 2 750 000 | –3.6 | + 124 |
QALYs, quality‐adjusted life years; USD, US dollar.
Table 6 shows the ACERs and ICERs for the intervention strategies. The ACER shows the added costs per gained QALY compared with ‘No screening’. For the Cx1 strategies, ‘No screening, treat high‐risk group’ and ‘Nullipara screening’ had lower costs and better health outcomes than did ‘No screening’, i.e. they were dominant. The ICER shows the incremental cost for each strategy compared with the less expensive strategy when all dominated strategies are excluded. Since the strategy ‘No screening’ was dominated by ‘No screening, treat high‐risk group’, we used the latter strategy as the reference since it had the lowest costs. The ICER for ‘Nullipara screening’ compared with ‘No screening, treat high‐risk group’ was 15 300 USD per gained QALY, whereas the ICER for ‘Low‐risk‐based screening’ compared with ‘Nullipara screening’ was 32 800 USD per gained QALY.
Table 6.
Average and incremental cost‐effectiveness of base‐case strategies (high risk of spontaneous preterm delivery (sPTD) indicated by sonographic cervical length (CL) ≤ 25 mm) per 100 000 women in the lifetime horizon, according to CL screening at 18 + 0 to 20 + 6 weeks (Cx1) and at 21 + 0 to 23 + 6 weeks (Cx2)
| Strategy | Average cost per gained QALY (vs ‘No screening’) (USD) | Incremental cost per gained QALY (USD) |
|---|---|---|
| CL screening at Cx1 | ||
| No screening, treat high‐risk group | Dominant* | Reference |
| Nullipara screening | Dominant* | 15 300† |
| Low‐risk‐based screening | 2200 | 32 800‡ |
| Universal screening | 24 500 | Dominated§ |
| CL screening at Cx2 | ||
| No screening, treat high‐risk group | Dominant* | Reference |
| High‐risk‐based screening | Dominant* | Dominated¶ |
| Nullipara screening | 22 200 | Dominated** |
| Low‐risk‐based screening | 36 800 | 74 600†† |
| Universal screening | 58 000 | Dominated‡‡ |
Lower costs and better health outcomes compared with ‘No screening’.
Compared with ‘No screening, treat high‐risk group’ at Cx1.
Compared with ‘Nullipara screening’ at Cx1.
More expensive and worse health outcomes compared with ‘Low‐risk‐based screening’ at Cx1.
More expensive and worse health outcomes compared with ‘No screening, treat high‐risk group’ at Cx2.
Dominated (by extension) by ‘Low‐risk‐based screening’ and ‘No screening, treat high‐risk group’ at Cx2.
Compared with ‘No screening, treat high‐risk group’ at Cx2.
More expensive and worse health outcomes compared with ‘Low‐risk‐based screening’ at Cx2.
QALY, quality‐adjusted life year; USD, US dollar.
For the Cx2 strategies, ‘No screening, treat high‐risk group’ and ‘High‐risk‐based screening’ were both dominant compared with ‘No screening’ (ACER). The remaining Cx2 strategies were slightly more costly compared with ‘No screening’, but also gave substantially better health outcomes (Tables 5 and 6). The ACERs were 22 200 USD per gained QALY for ‘Nullipara screening’, 36 800 USD per gained QALY for ‘Low‐risk‐based screening’ and 58 000 USD per gained QALY for ‘Universal screening’. When considering the ICERs, the only strategies that were not dominated were ‘No screening, treat high‐risk group’ and ‘Low‐risk‐based screening’. The former strategy was the least costly and therefore served as the reference. The ICER for ‘Low‐risk‐based screening’ compared with ‘No screening, treat high‐risk group’ was 74 600 USD per gained QALY.
All strategies (CL20, CL25, CL27 or CL29)
Figure 2 and Table S2 show the results for all strategies. For the Cx1 strategies, the CL29 scenarios showed better health outcomes than did the CL25 (base‐case) and CL20 scenarios. The Cx1 strategies that were worth considering after excluding the dominated strategies were ‘Nullipara screening’ CL29 and ‘Low‐risk‐based screening’ CL29. The ICER for the latter vs the former strategy was 11 800 USD per gained QALY. For the Cx2 strategies, the CL27 scenarios produced better health outcomes than did the CL25 (base‐case) and CL20 scenarios (Figure 2b). The incremental cost‐effectiveness analysis showed that the viable strategies after excluding dominated strategies were ‘No screening, treat high‐risk group’, ‘Nullipara screening’ CL27 and ‘Low‐risk‐based screening’ CL27. The ICER for ‘Nullipara screening’ CL27 vs ‘No screening, treat high‐risk group’ was 37 500 USD per gained QALY, and the ICER for ‘Low‐risk‐based screening’ CL27 vs ‘Nullipara screening’ CL27 was 53 300 USD per gained QALY.
Figure 2.
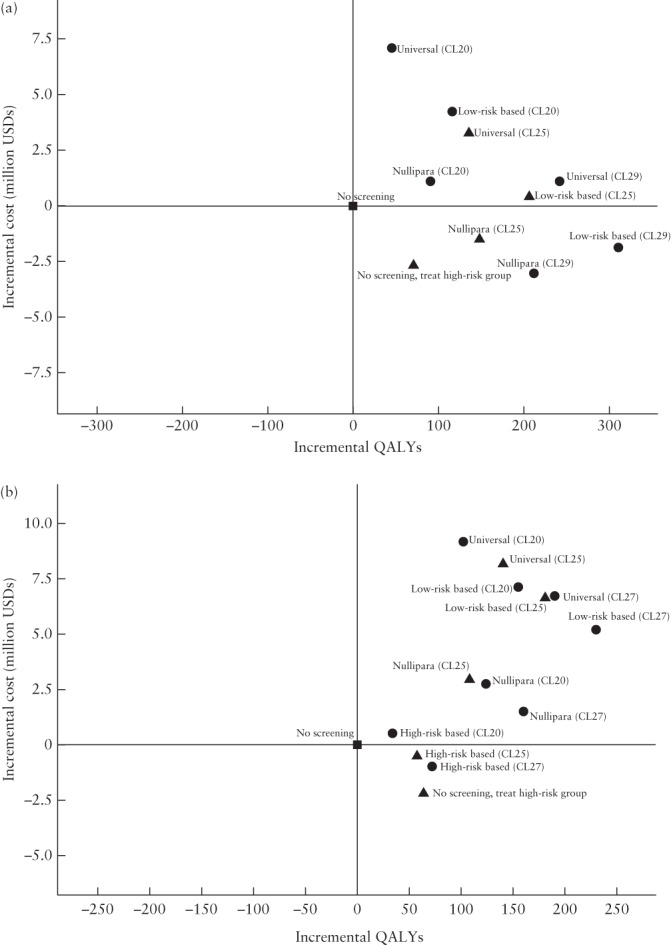
Cost‐effectiveness plane showing the incremental cost‐effectiveness ratio (ICER) per 100 000 women in the lifetime horizon for all screening strategies with cervical length (CL) measurement at 18 + 0 to 20 + 6 weeks (Cx1) (a) and at 21 + 0 to 23 + 6 weeks (Cx2) (b). ‘No screening’ strategy is used as reference. (a) At Cx1, ‘Nullipara screening’ CL29 dominates all other strategies. The ICER for ‘Low‐risk‐based screening’ CL29 vs ‘Nullipara screening’ CL29 is 11 800 US dollars (USD) per gained quality‐adjusted life year (QALY). (b) At Cx2, not dominated strategies are ‘No screening, treat high‐risk group’, ‘Nullipara screening’ CL27 and ‘Low‐risk‐based screening’ CL27. The ICER for ‘Nullipara screening’ CL27 vs ‘No screening, treat high‐risk group’ is 37 500 USD per gained QALY. The ICER for ‘Low‐risk‐based screening’ CL27 compared with ‘Nullipara screening’ CL27 is 53 300 USD per gained QALY.  , ‘No screening’ strategy;
, ‘No screening’ strategy;  , base‐case results (CL25);
, base‐case results (CL25);  , all other results (CL20, CL27, CL29). CL20, high risk of spontaneous preterm delivery (sPTD) indicated by CL ≤ 20 mm; CL25, high risk of sPTD indicated by CL ≤ 25 mm; CL27, high risk of sPTD indicated by CL ≤ 27 mm; CL29, high risk of sPTD indicated by CL ≤ 29 mm.
, all other results (CL20, CL27, CL29). CL20, high risk of spontaneous preterm delivery (sPTD) indicated by CL ≤ 20 mm; CL25, high risk of sPTD indicated by CL ≤ 25 mm; CL27, high risk of sPTD indicated by CL ≤ 27 mm; CL29, high risk of sPTD indicated by CL ≤ 29 mm.
Deterministic sensitivity analysis (CL25)
Table 7 shows the results of the deterministic sensitivity analyses for the base‐case strategies (CL25). We show the preferred strategy, based on ICER, assuming the maximum acceptable cost per gained QALY to be 56 000 USD 53 . For Cx1, the preferred strategies from a cost‐effectiveness perspective varied mainly between ‘Low‐risk‐based screening’ and ‘Nullipara screening’. However, if we assumed that high‐risk women (those with previous spontaneous PTD or late miscarriage and those with a short cervix) would be on sick leave during pregnancy (average 16 weeks production loss for 50% or 100% of the women), then none of the screening strategies would have an ICER below 56 000 USD, and instead ‘No screening’ would become the preferred strategy. This is partly explained by the cost of one single day of productivity loss being much higher than the total cost of progesterone treatment for one woman. The ability of progesterone to reduce the number of spontaneous PTDs (progesterone effectiveness) is also important from a cost‐effectiveness perspective. For the Cx1 strategies, the ‘Low‐risk‐based screening’ strategy was the preferred strategy as long as progesterone reduced spontaneous PTD before 33 + 0 weeks by at least 21% and spontaneous PTD at 33 + 0 to 36 + 6 weeks by at least 7%. For Cx2, the preferred strategies varied between ‘No screening, treat high‐risk group’, ‘Low‐risk‐based screening’ and ‘No screening’, depending on how we changed the model assumptions. If we assumed low effectiveness of progesterone or productivity loss because of sick leave during pregnancy, then ‘No screening’ would be the preferred strategy from a cost‐effectiveness perspective. If we assumed a high effectiveness of progesterone, then ‘Low‐risk‐based screening’ would become the most cost‐effective strategy. For ‘Low‐risk‐based screening’ at Cx2 to be the preferred strategy, progesterone treatment would need to reduce the number of spontaneous PTDs before 33 + 0 weeks by at least 36% and the number of spontaneous PTDs at 33 + 0 to 33 + 6 weeks by at least 12%.
Table 7.
Results of deterministic sensitivity analysis for the base‐case strategies (high risk of spontaneous preterm delivery (sPTD) indicated by sonographic cervical length (CL) ≤ 25 mm), according to CL screening at 18 + 0 to 20 + 6 weeks (Cx1) and at 21 + 0 to 23 + 6 weeks (Cx2)
| Sensitivity analysis | Preferred strategy at Cx1* | Preferred strategy at Cx2* |
|---|---|---|
| Physician visits | ||
| Every 2 weeks until 34 + 0 weeks | Nullipara screening | No screening, treat high‐risk group |
| Once a month until 34 + 0 weeks | Low‐risk‐based screening | No screening, treat high‐risk group |
| Productivity loss owing to sick leave during pregnancy | ||
| 50% of women | No screening | No screening |
| 100% of women | No screening | No screening |
| Progesterone effectiveness† | ||
| 5% and 1.7%‡ | No screening, treat high‐risk group | No screening |
| 15% and 5%‡ | No screening, treat high‐risk group | No screening, treat high‐risk group |
| 45% and 15%‡ | Low‐risk‐based screening | Low‐risk‐based screening |
| 55% and 18.3%‡ | Low‐risk‐based screening | Low‐risk‐based screening |
| Productivity loss owing to parental leave | ||
| Low (−20%) | Low‐risk‐based screening | No screening, treat high‐risk group |
| High (+ 20%) | Low‐risk‐based screening | No screening, treat high‐risk group |
| Cost of neonatal care | ||
| Low (−20%) | Low‐risk‐based screening | No screening, treat high‐risk group |
| High (+ 20%) | Low‐risk‐based screening | No screening, treat high‐risk group |
| Type of perspective | ||
| Healthcare perspective (does not include societal costs) | Low‐risk‐based screening | No screening, treat high‐risk group |
| Discount rate | ||
| Low (0%) | Low‐risk‐based screening | Low‐risk‐based screening |
| High (5%) | Nullipara screening | No screening, treat high‐risk group |
The preferred strategy is based on the maximum acceptable cost per gained quality‐adjusted life year being 500 000 Swedish krona (corresponding to 56 000 US dollars) according to the Swedish National Board of Health and Welfare 53 .
The spectrum of effectiveness of progesterone for prevention of sPTD between 33 + 0 and 36 + 6 weeks was modulated in the same proportion as the effect for prevention of sPTD < 33 + 0 weeks.
The first number indicates the estimated effectiveness of progesterone to reduce sPTD < 33 weeks and the second number indicates the estimated effectiveness of progesterone to reduce sPTD at 33 + 0 to 36 + 6 weeks.
Probabilistic sensitivity analysis (CL25)
The results of our probabilistic sensitivity analyses are shown as cost‐effectiveness acceptability curves in Figure 3. At Cx1, the probability of ‘Low‐risk‐based screening’ being cost‐effective compared with ‘No screening’ is 71% if willingness to pay is at most 56 000 USD per gained QALY. The likelihood of the strategy ‘No screening, treat high‐risk group’ to be cost‐effective compared with ‘No screening’ is approximately 95%, explained by this strategy being cost‐saving compared with ‘No screening’ in most of the simulations. The probability of ‘Low‐risk‐based screening’ being cost‐effective compared with ‘No screening, treat high‐risk group’ is 58% (Figure 3a).
Figure 3.
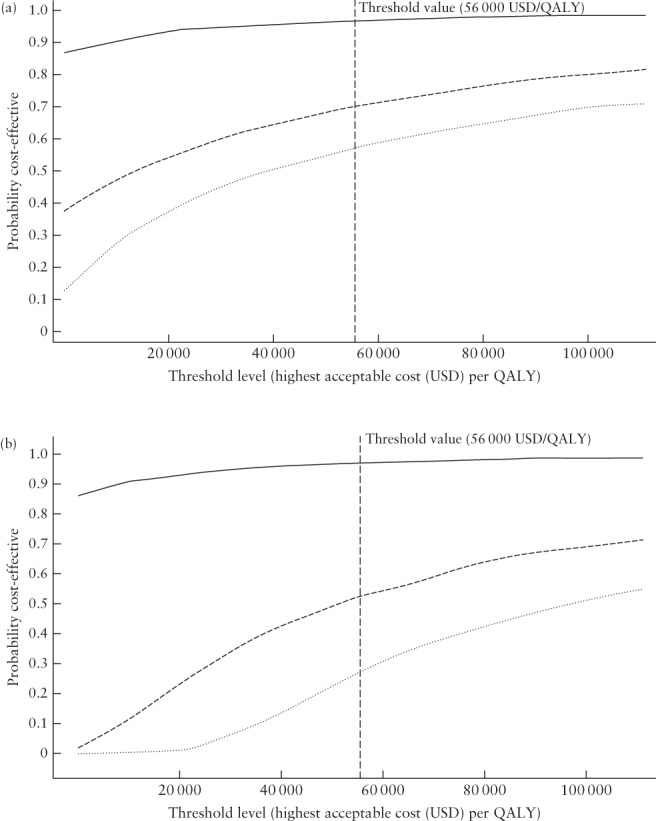
Cost‐effectiveness acceptability curves for cervical length (CL) screening at 18 + 0 to 20 + 6 weeks (Cx1) (a) and at 21 + 0 to 23 + 6 weeks (Cx2) (b) when CL ≤ 25 mm is used to indicate high risk of spontaneous preterm delivery (base‐case), showing the likelihood of the strategies ‘No screening, treat high‐risk group’ and ‘Low‐risk‐based screening’ CL25 being cost‐effective compared with each other, as well as in comparison with ‘No screening’. Willingness to pay (in US dollars (USD)) is shown on the x‐axis, and the likelihood of the strategy being cost‐effective is shown on the y‐axis. (a) At Cx1, ‘No screening, treat high‐risk group’ is likely to be cost‐effective at a low threshold level for willingness to pay per gained quality‐adjusted life year (QALY) compared with ‘No screening’ (approximately 95%) ( ). The probability of ‘Low‐risk‐based screening’ CL25 being cost‐effective if the willingness to pay is at most 500 000 Swedish krona (SEK) (56 000 USD) per gained QALY
53
is 71% compared with ‘No screening’ (
). The probability of ‘Low‐risk‐based screening’ CL25 being cost‐effective if the willingness to pay is at most 500 000 Swedish krona (SEK) (56 000 USD) per gained QALY
53
is 71% compared with ‘No screening’ ( ) and 58% compared with ‘No screening, treat high‐risk group’ (
) and 58% compared with ‘No screening, treat high‐risk group’ ( ). (b) At Cx2, the strategy ‘No screening, treat high‐risk group’ is likely to be cost‐effective at a low threshold level for willingness to pay per gained QALY compared with ‘No screening’ (approximately 90%) (
). (b) At Cx2, the strategy ‘No screening, treat high‐risk group’ is likely to be cost‐effective at a low threshold level for willingness to pay per gained QALY compared with ‘No screening’ (approximately 90%) ( ). The probability of ‘Low‐risk‐based screening’ CL25 being cost‐effective if the willingness to pay is at most 500 000 SEK (56 000 USD) per gained QALY
53
is 53% compared with ‘No screening’ (
). The probability of ‘Low‐risk‐based screening’ CL25 being cost‐effective if the willingness to pay is at most 500 000 SEK (56 000 USD) per gained QALY
53
is 53% compared with ‘No screening’ ( ) and 28% compared with ‘No screening, treat high‐risk group’ (
) and 28% compared with ‘No screening, treat high‐risk group’ ( ).
).
At Cx2, the likelihood that ‘No screening, treat high‐risk group’ is cost‐effective compared with ‘No screening’ is at least 90% even at a low threshold level of willingness to pay, explained by this strategy being cost‐saving compared with ‘No screening’ in most of the simulations. Whether ‘Low‐risk‐based screening’ at Cx2 is cost‐effective compared with ‘No screening’ or compared with ‘No screening, treat high‐risk group’ is uncertain. If willingness to pay is at most 56 000 USD per gained QALY, the likelihood that it is cost‐effective compared with ‘No screening’ is 53% and the likelihood that it is cost‐effective compared with ‘No screening, treat high‐risk group’ is 28% (Figure 3b).
DISCUSSION
All interventions to prevent spontaneous PTD in asymptomatic singleton pregnancies gave better health outcomes than did ‘No screening’. The best strategy in terms of improved health outcomes when using the 25‐mm CL cut‐off (base‐case) was ‘Low‐risk‐based screening’, irrespective of whether screening was performed at 18 + 0 to 20 + 6 weeks at the time of the routine ultrasound scan (Cx1) or at 21 + 0 to 23 + 6 weeks at an extra appointment (Cx2). ‘Low‐risk‐based screening’ at Cx1 was cost‐effective, while ‘Low‐risk‐based screening’ at Cx2 may be questioned because of its high costs compared with the other alternatives. Cost‐effectiveness was particularly sensitive to the ability of progesterone to reduce the number of spontaneous PTDs and to productivity loss due to sick leave during pregnancy.
Our study is the first to estimate the cost‐effectiveness of interventions to prevent PTD in a healthcare context different from that in the USA (the search strategy is detailed in Appendix S1). The prevalences of a short cervix and of spontaneous PTD are based on the results of our blinded Swedish multicenter study 12 , 31 . It is a strength of the study that we distinguish between spontaneous and indicated PTD when classifying pregnancy outcome and when defining the high‐risk group of women with previous PTD. In addition, we meticulously estimated the costs of delivery and postpartum and neonatal care based on Swedish data, differentiating costs for babies born before 33 weeks, at 33 to 36 weeks and at or after 37 weeks. We also carefully estimated the costs for long‐term morbidity, taking into account that the risk of CP depends on gestational age at birth and including a variety of healthcare and societal costs.
It is a limitation of the study that we did not include costs for productivity loss due to prolonged examination time at the 18–20‐week scan or for the additional visit at 21–23 weeks to measure CL. Moreover, we did not include costs for possible hospitalization owing to a short cervix or for possible productivity loss in a lifetime perspective for parents with a child with long‐term disability or for informal caregivers. It was impossible to estimate the costs for the latter two items, and we did not expect women to be hospitalized because of a short cervix 55 . Another limitation is that we assumed the same progesterone effect for CL ≤ 20 mm, ≤ 25 mm, ≤ 27 mm and ≤ 29 mm, despite the progesterone effect being insufficiently known if the cervix measures 26 to 30 mm 16 , and that progesterone might have no effect if the CL is ≤ 9 mm 14 . Moreover, we assumed the same effectiveness of progesterone in women with previous spontaneous PTD or late miscarriage as in women with a short cervix, despite the magnitude of the progesterone effect in the former group being uncertain 11 , 16 . For the strategy ‘High‐risk‐based screening’, we assumed that progesterone reduces spontaneous PTD in women with a short cervix after conization, despite this not necessarily being the case 31 . The uncertainties of all our assumptions are dealt with in the probabilistic sensitivity analyses. Finally, another limitation of the study is that the CERVIX‐study population includes a slightly higher proportion of pregnancies at increased risk of PTD than the Swedish background population of singleton pregnancies 12 .
Published studies estimating the cost‐effectiveness of CL screening with progesterone treatment if the cervix is short discuss cost‐effectiveness from a USA perspective 19 , 20 , 21 , 22 , 23 . In most studies, costs were calculated based on costs in the USA, willingness to pay per gained QALY was set at 100 000 USD at most, while information on the prevalence of a short cervix and its effect on the risk of PTD was obtained from sources not necessarily representative of the situation in the USA. No previous study has distinguished between spontaneous and indicated PTD. Four studies found CL screening with progesterone treatment in case of a short cervix to be cost‐effective 19 , 20 , 21 , 22 . The only study that did not find it cost‐effective assumed that progesterone reduces PTD by only 11% based on reanalysis of trial data specifically for US women 23 . The other studies assumed that progesterone reduces PTD by 39% to 45% 19 , 20 , 21 , 22 .
In agreement with previous studies, our cost‐effectiveness results were sensitive to the ability of progesterone to reduce the number of PTDs. Others found that for CL screening to be cost‐effective, progesterone effectiveness needed to be at least 19% 19 , 20 , 21 or 36% 23 . In our study, for the strategy ‘Low‐risk‐based screening’ at Cx1 to be cost‐effective, progesterone effectiveness needed to be at least 21% to prevent spontaneous PTD before 33 weeks and 7% to prevent spontaneous PTD at 33 + 0 to 36 + 6 weeks, and for it to be cost‐effective at Cx2 progesterone effectiveness needed to be at least 36% and 12%, respectively. Jain et al. 23 emphasized the need for future progesterone efficacy and effectiveness studies before recommending CL screening. In our study, productivity loss due to sick leave during pregnancy also substantially affected cost‐effectiveness. This cost was not taken into account in other studies 19 , 20 , 21 , 22 , 23 . Sick leave (bed‐rest) is often prescribed for women judged to be at high risk of spontaneous PTD, but has no proven benefit 56 , 57 .
CL screening at 21–23 weeks may be preferable to screening at 18–20 weeks because the ability of CL at 21–23 weeks to correctly predict spontaneous PTD is superior to that at the earlier timepoint 12 , 31 , 58 . Postponing the routine second‐trimester scan to 21–23 weeks is not an option in Sweden because termination of pregnancy (e.g. because of fetal malformation) is not allowed after 21 + 6 weeks 59 . Therefore, in Sweden, CL screening at 21–23 weeks would require an extra visit. It is not possible to compare the cost‐effectiveness of CL screening at 18 weeks with that at 21 weeks, because screening at 18 weeks includes late miscarriage as a pregnancy outcome. In a clinical setting, threatening periviable births must be dealt with.
In our base‐case scenarios we used a CL cut‐off of 25 mm to indicate increased risk of spontaneous PTD. However, the CL29 strategies at Cx1 and the CL27 strategies at Cx2 were the most cost‐effective when all strategies were compared. It would be advantageous to use the 27‐mm and 29‐mm cut‐offs, provided that progesterone effectiveness is the same for CL ≤ 27 mm and ≤ 29 mm as for CL ≤ 25 mm. There is some evidence to support this, but it is not strong 16 .
In conclusion, interventions to prevent spontaneous PTD, including CL screening with progesterone treatment of women with a short cervix, may be cost‐effective in Sweden.
Supporting information
Appendix S1 Search strategy for studies estimating the cost‐effectiveness of second‐trimester cervical length screening by transvaginal ultrasound followed by progesterone treatment in case of short cervix to prevent preterm delivery
Table S1 Probability estimates for preterm and term delivery for all strategies for prevention of spontaneous preterm delivery in asymptomatic women with a singleton pregnancy
Table S2 Results per 100 000 women for all strategies considered and costs presented in US dollars
ACKNOWLEDGMENTS
We acknowledge the contribution of Karin Sävman, MD, PhD, who provided us with information on costs for neonatal care, Helena Fadl, MD, associate professor, local principal investigator and responsible for the data acquisition to the CERVIX‐study from Örebro University hospital, Örebro, Sweden, and Jan Wesström MD, PhD, local principal investigator and responsible for the data acquisition to the CERVIX‐study from Falu Hospital, Falun, Sweden.
The CERVIX study was funded by The Swedish Research Council (Dnr 2014‐06998), Forskning och Utbildning (FoU) Södra Älvsborg, by the Swedish state under the agreement between the Swedish government and the county councils, the ALF‐agreement (ALFGBG‐136431, ALFGBG‐426411, ALFGBG‐71859), and The Swedish National Patient Insurance Company (LÖF). The funders had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the work for publication. The researchers were independent of the funders.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the article or in the supplementary material of this article
REFERENCES
- 1. Ward RM, Beachy JC. Neonatal complications following preterm birth. BJOG 2003; 110: 8–16. [DOI] [PubMed] [Google Scholar]
- 2. Moster D, Lie RT, Markestad T. Long‐term medical and social consequences of preterm birth. N Engl J Med 2008; 359: 262–273. [DOI] [PubMed] [Google Scholar]
- 3. Swamy GK, Ostbye T, Skjaerven R. Association of preterm birth with long‐term survival, reproduction, and next‐generation preterm birth. JAMA 2008; 299: 1429–1436. [DOI] [PubMed] [Google Scholar]
- 4. Crump C, Sundquist K, Sundquist J, Winkleby MA. Gestational age at birth and mortality in young adulthood. JAMA 2011; 306: 1233–1240. [DOI] [PubMed] [Google Scholar]
- 5. Chawanpaiboon S, Vogel JP, Moller A‐B, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, Lewis C, Rattanakanokchai S, Teng DN, Thinkhamrop J, Watananirun K, Zhang J, Zhou W, Gülmezoglu AM. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019; 7: e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Socialstyrelsen.se :. https://www.socialstyrelsen.se/globalassets/sharepoint‐dokument/artikelkatalog/statistik/2020‐2‐6622‐tabeller.xls [Accessed 23 May 2021].
- 7. Menzies R, Li ALK, Melamed N, Shah PS, Horn D, Barrett J, Murphy KE. Risk of singleton preterm birth after prior twin preterm birth: a systematic review and meta‐analysis. Am J Obstet Gynecol 2020; 223: 204.e1–204.e8. [DOI] [PubMed] [Google Scholar]
- 8. Laughon SK, Albert PS, Leishear K, Mendola P. The NICHD Consecutive Pregnancies Study: recurrent preterm delivery by subtype. Am J Obstet Gynecol 2014; 210: 131.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Celik E, To M, Gajewska K, Smith GC, Nicolaides KH. Cervical length and obstetric history predict spontaneous preterm birth: development and validation of a model to provide individualized risk assessment. Ultrasound Obstet Gynecol 2008; 31: 549–554. [DOI] [PubMed] [Google Scholar]
- 10. Poon LC, Savvas M, Zamblera D, Skyfta E, Nicolaides KH. Large loop excision of transformation zone and cervical length in the prediction of spontaneous preterm delivery. BJOG 2012; 119: 692–708. [DOI] [PubMed] [Google Scholar]
- 11. Jarde A, Lutsiv O, Beyene J, McDonald SD. Vaginal progesterone, oral progesterone, 17‐OHPC, cerclage, and pessary for preventing preterm birth in at‐risk singleton pregnancies: an updated systematic review and network meta‐analysis. BJOG 2019; 126: 556–567. [DOI] [PubMed] [Google Scholar]
- 12. Kuusela P, Jacobsson B, Hagberg H, Fadl H, Lindgren P, Wesström J, Wennerholm UB, Valentin L. Second trimester transvaginal ultrasound measurement of cervical length for prediction of preterm birth: a blinded prospective multicentre diagnostic accuracy study. BJOG 2021; 128: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, Thom E, McNellis D, Copper RL, Johnson F, Roberts JM. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med 1996; 334: 567–572. [DOI] [PubMed] [Google Scholar]
- 14. Romero R, Conde‐Agudelo A, Da Fonseca E, O'Brien JM, Cetingoz E, Creasy GW, Hassan SS, Nicolaides KH. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta‐analysis of individual patient data. Am J Obstet Gynecol 2018; 218: 161–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dodd JM, Jones L, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database Syst Rev 2013; 7: CD004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. EPPPIC Group . Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta‐analysis of individual participant data from randomised controlled trials. Lancet 2021; 397: 1183–1194. [DOI] [PubMed] [Google Scholar]
- 17. Campbell S. Prevention of spontaneous preterm birth: universal cervical length assessment and vaginal progesterone in women with a short cervix: time for action! Am J Obstet Gynecol 2018; 218: 151–158. [DOI] [PubMed] [Google Scholar]
- 18. Mangham LJ, Petrou S, Doyle LW, Draper ES, Marlow N. The cost of preterm birth throughout childhood in England and Wales. Pediatrics 2009; 123: e312–327. [DOI] [PubMed] [Google Scholar]
- 19. Werner EF, Han CS, Pettker CM, Buhimschi CS, Copel JA, Funai EF, Thung SF. Universal cervical‐length screening to prevent preterm birth: a cost‐effectiveness analysis. Ultrasound Obstet Gynecol 2011; 38: 32–37. [DOI] [PubMed] [Google Scholar]
- 20. Werner EF, Hamel MS, Orzechowski K, Berghella V, Thung SF. Cost‐effectiveness of transvaginal ultrasound cervical length screening in singletons without a prior preterm birth: an update. Am J Obstet Gynecol 2015; 213: 554.e1–6. [DOI] [PubMed] [Google Scholar]
- 21. Einerson BD, Grobman WA, Miller ES. Cost‐effectiveness of risk‐based screening for cervical length to prevent preterm birth. Am J Obstet Gynecol 2016; 215: 100.e1–7. [DOI] [PubMed] [Google Scholar]
- 22. Cahill AG, Odibo AO, Caughey AB, Stamilio DM, Hassan SS, Macones GA, Romero R. Universal cervical length screening and treatment with vaginal progesterone to prevent preterm birth: a decision and economic analysis. Am J Obstet Gynecol 2010; 202: 548.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jain S, Kilgore M, Edwards RK, Owen J. Revisiting the cost‐effectiveness of universal cervical length screening: importance of progesterone efficacy. Am J Obstet Gynecol 2016; 215: 101.e1–7. [DOI] [PubMed] [Google Scholar]
- 24. Cdc.gov :. https://www.cdc.gov/nchs/products/databriefs/db418.htm#section_4 [Accessed 23 May 2021].
- 25. statista.com :. https://www.statista.com/statistics/184968/us‐health‐expenditure‐as‐percent‐of‐gdp‐since‐1960/ [Accessed 18 December 2021].
- 26. ec.europa.eu :. https://ec.europa.eu/eurostat/en/web/products‐eurostat‐news/‐/ddn‐20201202‐1 [Accessed 18 December 2021].
- 27. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E, CHEERS Task Force . Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013; 346: f1049. [DOI] [PubMed] [Google Scholar]
- 28. Selbing A, Kjessler B. Conceptual dating by ultrasonic measurement of the fetal biparietal diameter in early pregnancy. Acta Obstet Gynecol Scand 1985; 64: 593–607. [DOI] [PubMed] [Google Scholar]
- 29. Saltvedt S, Almstrom H, Kublickas M, Reilly M, Valentin L, Grunewald C. Ultrasound dating at 12–14 or 15–20 weeks of gestation? A prospective cross‐validation of established dating formulae in a population of in‐vitro fertilized pregnancies randomized to early or late dating scan. Ultrasound Obstet Gynecol 2004; 24: 42–50. [DOI] [PubMed] [Google Scholar]
- 30. https://www.sfog.se/media/336451/fetometri.pdf.
- 31. Wikström T, Hagberg H, Jacobsson B, Kuusela P, Wesström J, Lindgren P, Fadl H, Wennerholm UB, Valentin L. Effect of second‐trimester sonographic cervical length on the risk of spontaneous preterm delivery in different risk groups: A prospective observational multicenter study. Acta Obstet Gynecol Scand 2021; 100: 1644–1655. [DOI] [PubMed] [Google Scholar]
- 32. Habibzadeh F, Habibzadeh P, Yadollahie M. On determining the most appropriate test cut‐off value: the case of tests with continuous results. Biochem Med (Zagreb) 2016; 26: 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bilsteen JF, Taylor‐Robinson D, Børch K, Strandberg‐Larsen K, Nybo Andersen A‐M. Gestational Age and Socioeconomic Achievements in Young Adulthood: A Danish Population‐Based Study. JAMA Netw Open 2018; 1: e186085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. https://www.socialstyrelsen.se/en/statistics‐and‐data/registers/national‐medical‐birth‐register/.
- 35. Surveillance of Cerebral Palsy in Europe . Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE). Dev Med Child Neurol 2000; 42: 816–824. [DOI] [PubMed] [Google Scholar]
- 36. Himmelmann K, Sundh V. Survival with cerebral palsy over five decades in western Sweden. Dev Med Child Neurol 2015; 57: 762–777. [DOI] [PubMed] [Google Scholar]
- 37. scb.se :. https://www.scb.se/hitta‐statistik/statistik‐efter‐amne/priser‐och‐konsumtion/konsumentprisindex/konsumentprisindex‐kpi/pong/tabell‐och‐diagram/konsumentprisindex‐kpi/kpi‐faststallda‐tal‐1980100/ [Accessed 23 May 2021].
- 38. https://www.fass.se/LIF/product?userType=0&nplId=20181017000017#packages‐prices.
- 39. Romosan G, Lindberg C, Banos N, Valentin L. Resources needed to teach midwife sonographers to measure cervical length with transvaginal ultrasound in the second trimester. Acta Obstet Gynecol Scand 2020; 99: 1568–1569. [DOI] [PubMed] [Google Scholar]
- 40. Kuusela P, Wennerholm UB, Fadl H, Wesström J, Lindgren P, Hagberg H, Jacobsson B, Valentin L. Second trimester cervical length measurements with transvaginal ultrasound: A prospective observational agreement and reliability study. Acta Obstet Gynecol Scand 2020; 99: 1476–1485. [DOI] [PubMed] [Google Scholar]
- 41. Boelig RC, Feltovich H, Spitz JL, Toland G, Berghella V, Iams JD. Assessment of Transvaginal Ultrasound Cervical Length Image Quality. Obstet Gynecol 2017; 129: 536–541. [DOI] [PubMed] [Google Scholar]
- 42. Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes (4th edn). Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- 43. scb.se :. https://www.scb.se/hitta‐statistik/statistik‐efter‐amne/arbetsmarknad/loner‐och‐arbetskostnader/lonestrukturstatistik‐hela‐ekonomin/pong/tabell‐och‐diagram/genomsnittlig‐manadslon‐efter‐sektor/ [Accessed 23 May 2021].
- 44. Kruse M, Michelsen SI, Flachs EM, Brønnum‐Hansen H, Madsen M, Uldall P. Lifetime costs of cerebral palsy. Dev Med Child Neurol 2009; 51: 622–628. [DOI] [PubMed] [Google Scholar]
- 45. Kruse M, Michelsen SI, Flachs EM. Livstidsomkostninger ved cerebral parese. [Lifetime costs of cerebral palsy]. Research report. National Institute of Public Health, University of Southern Denmark: Copenhagen, Denmark, 2006. [Google Scholar]
- 46. Vandenbussche FP, De Jong‐Potjer LC, Stiggelbout AM, Le Cessie S, Keirse MJ. Differences in the valuation of birth outcomes among pregnant women, mothers, and obstetricians. Birth 1999; 26: 178–183. [DOI] [PubMed] [Google Scholar]
- 47. Pham C, Crowther C. Birth outcomes: utility values that postnatal women, midwives and medical staff express. BJOG 2003; 110: 121–127. [PubMed] [Google Scholar]
- 48. Tengs TO, Wallace A. One thousand health‐related quality‐of‐life estimates. Med Care 2000; 38: 583–637. [DOI] [PubMed] [Google Scholar]
- 49. Burström K, Johannesson M, Diderichsen F. Swedish population health‐related quality of life results using the EQ‐5D. Qual Life Res 2001; 10: 621–635. [DOI] [PubMed] [Google Scholar]
- 50. O'Mahony JF, Newall AT, van Rosmalen J. Dealing with Time in Health Economic Evaluation: Methodological Issues and Recommendations for Practice. Pharmacoeconomics 2015; 33: 1255–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weinstein MC, O'Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, Luce BR, ISPOR Task Force on Good Research Practices – Modeling Studies . Principles of good practice for decision analytic modeling in health‐care evaluation: report of the ISPOR Task Force on Good Research Practices – Modeling Studies. Value Health 2003; 6: 9–17. [DOI] [PubMed] [Google Scholar]
- 52. TLV.se :.https://www.tlv.se/download/18467926b615d084471ac3396a/1510316400272/LAG‐lfnar‐2003‐2pdf [Accessed 18 December 2021].
- 53. Socialstyrelsen.se :. https://www.sbu.se/globalassets/ebm/metodbok/sbushandbok_kapitel11.pdf.
- 54. Briggs AH, Gray AM. Handling uncertainty in economic evaluations of healthcare interventions. BMJ 1999; 319: 635–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shainker SA, Modest AM, Hacker MR, Ralston SJ. The Effect of a Universal Cervical Length Screening Program on Antepartum Management and Birth Outcomes. AJP Rep 2016; 6: e206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Walsh CA. Maternal activity restriction to reduce preterm birth: Time to put this fallacy to bed. Aust N Z J Obstet Gynaecol 2020; 60: 813–815. [DOI] [PubMed] [Google Scholar]
- 57. Matenchuk B, Khurana R, Cai C, Boulé NG, Slater L, Davenport MH. Prenatal bed rest in developed and developing regions: a systematic review and meta‐analysis. CMAJ Open 2019; 7: E435–E445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dilek TU, Yazici G, Gurbuz A, Tasdelen B, Gulhan S, Dilek B, Dilek S. Progressive cervical length changes versus single cervical length measurement by transvaginal ultrasound for prediction of preterm delivery. Gynecol Obstet Invest 2007; 64: 175–179. [DOI] [PubMed] [Google Scholar]
- 59. Socialstyrelsen.se :. https://www.socialstyrelsen.se/regler‐och‐riktlinjer/foreskrifter‐och‐allmanna‐rad/konsoliderade‐foreskrifter/200915‐om‐abort2/ [Accessed 11 December 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Search strategy for studies estimating the cost‐effectiveness of second‐trimester cervical length screening by transvaginal ultrasound followed by progesterone treatment in case of short cervix to prevent preterm delivery
Table S1 Probability estimates for preterm and term delivery for all strategies for prevention of spontaneous preterm delivery in asymptomatic women with a singleton pregnancy
Table S2 Results per 100 000 women for all strategies considered and costs presented in US dollars
Data Availability Statement
The data that supports the findings of this study are available in the article or in the supplementary material of this article


