Key Points
Adding idasanutlin to cytarabine to treat R/R AML improved overall remission rate (CR+CRi+CRp) but not overall survival (138).
Visual Abstract
Abstract
The phase 3 MIRROS (MDM2 antagonist Idasanutlin in Relapsed or Refractory acute myeloid leukemia [AML] for Overall Survival) trial (NCT02545283) evaluated the efficacy and safety of the small-molecule MDM2 antagonist idasanutlin plus cytarabine in patients with relapsed/refractory (R/R) AML. Adults (n = 447) with R/R AML whose disease relapsed or was refractory after ≤2 prior induction regimens as initial treatment or following salvage chemotherapy regimen, with Eastern Cooperative Oncology Group performance status ≤2 were enrolled regardless of TP53 mutation status and randomly assigned 2:1 to idasanutlin 300 mg or placebo orally twice daily plus cytarabine 1 g/m2 IV on days 1 to 5 of 28-day cycles. At primary analysis (cutoff, November 2019), 436 patients were enrolled, including 355 in the TP53 wild-type intention-to-treat (TP53WT-ITT) population. The primary endpoint, overall survival in the TP53WT-ITT population, was not met (median, 8.3 vs 9.1 months with idasanutlin-cytarabine vs placebo-cytarabine; stratified hazard ratio [HR], 1.08; 95% confidence interval [CI], 0.81-1.45; P = .58). The complete remission (CR) rate, a key secondary endpoint, was 20.3% vs 17.1% (odds ratio [OR], 1.23; 95% CI, 0.70-2.18). The overall response rate (ORR) was 38.8% vs 22.0% (OR, 2.25; 95% CI, 1.36-3.72). Common any-grade adverse events (≥10% incidence in any arm) were diarrhea (87.0% vs 32.9%), febrile neutropenia (52.8% vs 49.3%), and nausea (52.5% vs 31.5%). In summary, despite improved ORR, adding idasanutlin to cytarabine did not improve overall survival or CR rates in patients with R/R AML.
Introduction
Acute myeloid leukemia (AML) is a genetically heterogeneous hematologic disease characterized by uncontrolled clonal expansion of myeloid blast cells in the bone marrow, blood, and other tissues.1 Approximately 20% of patients with AML have primary refractory disease, and about half will experience relapse.2,3 In older patients, these rates are higher, with 30% having primary refractory disease and up to 74% experiencing relapse.4,5 There is no standard-of-care treatment for patients with relapsed or refractory (R/R) AML. Recommended salvage therapies include intensive cytarabine-based chemotherapy to achieve complete remission (CR), followed by allogeneic hematopoietic stem cell transplant (HSCT) in eligible patients, or best supportive care, low-intensity treatments, or clinical trials for unfit patients.6-8 Prognosis generally remains poor for patients with R/R AML, representing a high unmet need.2 In patients without HSCT after salvage therapy, the 5-year overall survival (OS) rate is approximately 12% to 16% after the second relapse, with a 4-year survival rate of 4% seen in refractory patients.9-11 In those who receive HSCT, the 5-year OS rate is approximately 42% to 48% in relapsed patients and 25% in refractory patients.11,12
The tumor suppressor TP53 induces the transcription of genes involved in cell-cycle control, apoptosis, DNA repair, differentiation, and senescence and is frequently mutated or deleted in human cancers, leading to dysfunctional p53. p53 can also be inactivated by its primary negative regulator, MDM2,13 an E3 ligase that marks p53 for degradation by the proteasome. MDM2 can be overexpressed in human tumors, including AML.14-16 Preclinical research indicates a correlation between MDM2 expression and resulting apoptosis from MDM2 antagonism.14,17 Approximately 80% of patients with R/R AML have TP53 wild-type (TP53WT) disease that may benefit from an MDM2-targeted therapy, stabilizing p53 and activating its tumor-suppressor function and promoting apoptosis.18 Furthermore, overexpression of MDM2/X in AML may result in a defective p53 pathway and has been associated with potential response to MDM2 inhibitors, although not all patients with high MDM2 levels respond equally well.19
Idasanutlin is a potent (Kd = 0.15 nM), selective small-molecule MDM2 antagonist that has demonstrated tolerable safety and encouraging clinical activity in an open-label, phase 1/1b trial in patients with AML, alone and in combination with cytarabine (composite CR rate, 35.6%).20 The study enrolled patients regardless of TP53 mutation status because some TP53 mutations can retain p53 function.21,22 Here, we report the results of the MIRROS (MDM2 antagonist Idasanutlin in Relapsed or Refractory AML for Overall Survival) trial, a multicenter, randomized, double-blind, phase 3 trial evaluating idasanutlin in combination with 1 g/m2 of cytarabine vs placebo in combination with cytarabine in patients with R/R AML.23
Patients and methods
Patients
MIRROS (NCT02545283) enrolled patients ≥18 years of age who had a confirmed AML per World Health Organization classification,24 both with R/R disease after ≤2 prior induction regimens (excluding HSCT) for first-line therapy, including cytarabine with an anthracycline or anthracenedione, and patients with R/R disease after a second chemotherapy regimen. Patients had an Eastern Cooperative Oncology Group performance status ≤2 and acceptable hepatic and renal function (serum total bilirubin ≤1.5 × upper limit of normal; aspartate aminotransferase or alanine aminotransferase ≤3 × upper limit of normal; serum creatinine within laboratory ranges, or creatinine clearance [by Cockcroft Gault formula] ≥50 mL/min). Patients were enrolled regardless of TP53 mutation status. Key exclusion criteria were a first CR duration of >1 year in first relapsed patients aged <60 years, >2 prior induction regimens as first-line therapy, documented refractory disease or relapse(s) within 90 days of receiving a cumulative dose of cytarabine ≥18 g/m2, and secondary AML.25
Trial design and procedures
Patients were randomly assigned in a 2:1 ratio to receive idasanutlin plus 1 g/m2 of cytarabine once daily (idasa-C) or cytarabine plus matching placebo (placebo-C). Stratification factors were age (<60 vs ≥60 years), cytogenetic or molecular risk per 2010 European LeukemiaNet recommendations25 (favorable or intermediate vs adverse), prior HSCT (yes or no), and prior response duration (refractory vs CR ≥90 days, and ≤1 year vs CR >1 year).
Patients received 300 mg of idasanutlin or placebo, administered orally twice daily, and 1 g per square meter of body surface area of cytarabine, administered through IV once daily, on days 1 through 5 of induction treatment (supplemental Figure 1). The initial induction cycle was 28 days, and a maximum of 2 additional cycles of optional postremission therapy with 300 mg of idasanutlin or placebo administered orally once daily and cytarabine administered through IV (days 1-5) was allowed for patients who responded to treatment. Antibiotic (levofloxacin), antifungal (posaconazole), antidiarrheal (loperamide), and antiemetic (palonosetron, dexamethasone) prophylaxis during treatment cycles was mandated.
The primary endpoint was OS in the predefined intention-to-treat (ITT) population of patients with TP53WT status, including patients with TP53 missense mutations with retained functionality, as listed in the International Agency for Research on Cancer database.26 Key secondary endpoints evaluated in the TP53WT-ITT population were investigator-assessed CR rates during the induction cycle, overall response rate (CR, CR with incomplete platelet recovery [CRp], and CR with incomplete blood count recovery [CRi]), duration of remission following CR, rate of HSCT, and rate of minimal residual disease (MRD). Safety was evaluated according to the Common Terminology Criteria for Adverse Events version 4.03 of the National Cancer Institute. Further details on the study design, including the rationale for the selection of comparator and endpoints, have been previously published.23
Exploratory biomarker analysis
MRD was centrally evaluated for all patients on the basis of different from normal immunophenotypes by flow cytometry of bone marrow aspirates taken at screening and protocol-response assessment visits at the end of each treatment cycle and every 3 months during follow-up until relapse or HSCT. Bone marrow aspirate samples were mixed with heparin and shipped immediately at ambient temperature to the central laboratory for further processing. At least 100 000 CD45+ cells were counted for MRD analysis. MDM2 protein expression level was analyzed by flow cytometry on AML-gated blasts. MIC-1 was quantified using an enzyme-linked immunosorbent-based assay.
Interim analyses
An interim futility analysis based on both safety and efficacy criteria had been passed successfully, as described previously.23 An interim efficacy analysis of OS was conducted by the independent data monitoring committee after 80% (∼220) of death events had been observed in the TP53WT-ITT population. If the treatment effect was significant or futile at interim analysis, it would be considered the primary analysis.
Statistical analysis
The trial was designed to enroll 440 patients based on the assumption that 85% of patients will have TP53WT disease. The all-comer ITT population included all randomized patients who were TP53WT (including TP53 mutations known to retain full TP53 functionality), mutant, or unknown status; patients were analyzed according to the arm to which they were randomized, regardless of which treatments the patients received. The safety population, which included all patients who received any amount of the study drug, was analyzed according to the treatment received. Type I error was controlled at a 2-sided significance level (.05) using a fixed-sequence testing procedure to adjust for multiple statistical testing of the primary and key secondary efficacy endpoints. The trial had 82% power for the primary analysis of OS in patients in the TP53WT-ITT population. OS was compared between treatment groups using a stratified Cox proportional hazards regression to estimate an HR and a 95% confidence interval (CI). Additionally, due to the short survival times in this population, a reverse Kaplan-Meier analysis of potential follow-up time was performed that effectively measures survival time rather than time to death. In this method, alive and censoring data are treated as events, and death and event data are censored.27 Treatment response, including CR, was assessed at the end of each cycle and summarized by the study arm along with the 2-sided 95% CI by the Pearson-Clopper method. MRD was analyzed by descriptive statistics at the 0.1% cutoff. CD34+ and CD117+ cells were evaluated for MDM2 status by flow cell cytometry and correlated to treatment response at the end of induction.
Oversight
The trial sponsor, F. Hoffmann-La Roche/Genentech, provided idasanutlin and placebo and collaborated with an academic steering committee regarding the trial design and data collection, analysis, and interpretation. The trial was conducted according to the guidelines of Good Clinical Practice and the principles of the Declaration of Helsinki. All patients provided written informed consent. The trial was approved by multiple institutional review boards and ethics committees.
Roche data sharing
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, this request platform is Vivli (https://vivli.org/ourmember/roche/). For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than 1 data source external to Roche cannot, and should not, be linked due to a potential increase in the risk of patient reidentification.
Results
Patients and trial interventions
From 31 December 2015 through 13 January 2020, a total of 447 patients (ITT) were enrolled at 79 sites in 17 countries. The cutoff for the primary analysis, planned as an efficacy interim analysis, was in November 2019, at which time the ITT population had enrolled a total of 436 patients, with 342 patients (78%) in Europe, 27 (6%) in Australia and New Zealand, 19 (4%) each in South Korea and Israel, 14 (3%) in the United States and Canada, 12 (3%) in Russia, and 3 (1%) in Latin America. A total of 290 patients were randomized to receive idasanutlin plus cytarabine, and 146 were randomized to receive placebo plus cytarabine. The TP53WT-ITT population excluded patients with TP53 mutations that affect protein function as listed in the IARC database and those of unknown TP53 status. The TP53WT-ITT population included 355 patients (81%; 232 patients in the idasa-C group and 123 in the placebo-C group, including 3 and 1 patients, respectively, harboring TP53 mutations known to retain full TP53 functionality). Overall, the characteristics of the patients in the TP53WT-ITT groups at baseline were well balanced between the 2 treatment arms (Table 1). The numbers of patients who were still receiving the trial intervention at the time of analysis (unblinding: 10 January 2020) are shown in the Consolidated Standards of Reporting Trials diagram in Figure 1. The sponsor decided to close the study considering the outcomes of this analysis and the expected impact of the SARS-CoV-2/COVID-19 pandemic on further data collection. All participants discontinued the study by April 2020.
Table 1.
Baseline demographics and characteristics (TP53WT ITT population)
| Characteristic | Idasa-C | Placebo-C |
|---|---|---|
| n = 232 | n = 123 | |
| Age, median (range), y | 63.0 (21-79) | 62.0 (27-77) |
| Male, n (%) | 121 (52.2) | 67 (54.5) |
| White, n (%) | 169 (72.8) | 92 (74.8) |
| Age group, n (%) | ||
| <60 y | 95 (40.9) | 44 (35.8) |
| ≥60 y | 137 (59.1) | 79 (64.2) |
| n = 231 | n = 123 | |
| ECOG PS, n (%) | ||
| 0 | 107 (46.3) | 61 (49.6) |
| 1 | 116 (50.2) | 60 (48.8) |
| 2 | 8 (3.5) | 2 (1.6) |
| n = 228 | n = 121 | |
| ELN 2010 classification, n (%) | ||
| Adverse | 47 (20.6) | 25 (20.7) |
| Favorable or intermediate | 181 (79.4) | 96 (79.3) |
| n = 232 | n = 123 | |
| Duration of initial response, n (%) | ||
| CR >1 y | 55 (23.7) | 30 (24.4) |
| CR ≥90 d but ≤1 y | 94 (40.5) | 46 (37.4) |
| Refractory <90 d | 83 (35.8) | 47 (38.2) |
| Prior HSCT, n (%) | 48 (20.7) | 23 (18.7) |
| Time from initial diagnosis to randomization, median (range), mo | 10.1 (0.7-89.0) | 9.5 (1.1-133.4) |
| n = 231 | n = 123 | |
| AML disease status at screening, n (%) | ||
| Refractory to first induction | 68 (29.4) | 35 (28.5) |
| Refractory to second induction | 33 (14.3) | 15 (12.2) |
| First relapse | 115 (49.8) | 65 (52.8) |
| Second relapse | 15 (6.5) | 8 (6.5) |
| Highest prior cytarabine dose, n (%) * | ||
| High | 97 (42.0) | 51 (41.5) |
| Intermediate | 34 (14.7) | 15 (12.2) |
| Conventional | 97 (42.0) | 57 (46.3) |
| Low | 3 (1.3) | 0 |
ECOG PS, Eastern Cooperative Oncology Group performance status.
High dose = ≥2 g/m2; intermediate dose = 0.5 to <2.0 g/m2; conventional dose = 0.1 to <0.5 g/m2; and low dose = <0.1 g/m2.
Figure 1.
Consolidated Standards of Reporting Trials diagram showing the number of patients allocated to each treatment arm and the number of patients still receiving treatment.
Among safety-evaluable patients in the idasa-C group, the mean number of treatment cycles started (ie, ≥1 dose of study drug was administered in the cycle) was 1.2 (standard deviation [SD], 0.5). For patients in the placebo-C group, the mean number of treatment cycles initiated was 1.2 (SD, 0.6). The proportion of patients receiving an optional first postremission cycle was 17.3% (n = 49) in the idasa-C group and 14.4% (n = 21) in the placebo-C group. A second postremission cycle was administered to 5.3% (n = 15) and 9.6% (n = 14) of patients, respectively. The mean dose intensity of cytarabine over all treatment phases, including consolidation, was 99.3% (SD, 5.4%) in the idasa-C group and 99.6% (SD, 8.0%) in the placebo-C group. Additional exposure data are provided in supplemental Table 1 in the supplemental Appendix.
OS analysis
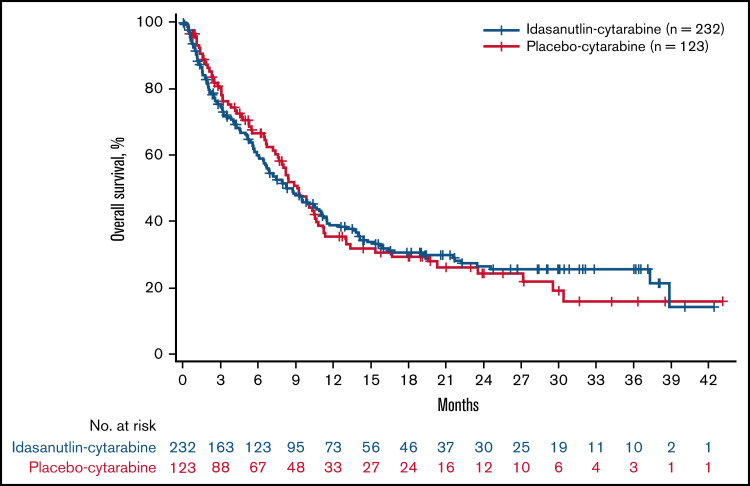
At the time of data cutoff, 230 OS events (84% of planned events) had occurred in the TP53WT-ITT population (152 events for idasa-C and 78 events for placebo-C). The median duration of follow-up was 6.7 months in both the idasa-C and placebo-C arms. The follow-up time in the TP53WT-ITT population for patients surviving at the time of OS analysis was calculated by reverse Kaplan-Meier analysis to be a median of 22.28 months. In the all-comer ITT population, a total of 197 (67.9%) patients in the idasa-C group and 99 (67.8%) in the placebo-C group had died. OS in the TP53WT-ITT population was similar between the idasa-C and placebo-C groups (median, 8.3 vs 9.1 months; stratified HR for death, 1.08; 95% CI, 0.81-1.45; P = .58) (Figure 2). The effects of treatment on OS in key subgroups are shown in supplemental Figure 2. No subgroup showed a different outcome for OS. The median OS in the all-comer ITT population was 6.8 months in the idasa-C group and 7.7 months in the placebo-C group (HR, 1.09; 95% CI, 0.84-1.41; P = .52). As the treatment effect was futile at this preplanned interim efficacy analysis, this became the primary analysis.
Figure 2.
OS in TP53WT ITT. Kaplan-Meier curves for OS were similar between the idasa-C and placebo-C groups.
The proportion of TP53WT patients receiving HSCT over the study period was 32.3% (n = 75) in the idasa-C group and 32.5% (n = 40) in the placebo-C group. The proportion of these patients who were in CR at the time of transplant was 42.7% (n = 32) in the idasa-C group and 40.0% (n = 16) in the placebo-C group; the proportion of patients in CRp or CRi at the time of transplant was 38.7% (n = 29) in the idasa-C group and 17.5% (n = 7) in the placebo-C group. The proportion of patients in treatment failure or relapse at the time of transplant was 16.0% (n = 12) for the idasa-C group and 40.0% (n = 16) in the placebo-C group.
HSCT provided a survival benefit in both patient groups. In patients who advanced to allogeneic HSCT at any time during the study (n = 115), OS following HSCT was numerically worse in the idasa-C group (n = 75), with a median of 23.5 months compared with 27.1 months in the placebo-C group (n = 40) (Figure 3A). When patients having received follow-up salvage therapies were excluded from the OS analysis, survival in the resulting subgroups was similar between the idasa-C and placebo-C groups (Figure 3B). A lower rate of use of salvage therapy (excluding conditioning) after study treatment was seen in the idasa-C arm compared with the placebo-C arm (57.6% vs 69.9%). Lower use rates were also seen with therapies that suggest the use of intensive follow-up regimens, such as cytarabine (16.2% vs 29.5%), fludarabine (6.9% vs 12.3%), and idarubicin (4.5% vs 10.3%) (supplemental Table 2).
Figure 3.
OS after allogeneic HSCT. The effect of HSCT on OS was explored by excluding the effect of follow-up salvage therapy. (A) In patients in the TP53WT ITT population who advanced to HSCT, OS was numerically worse in the idasa-C group than in the placebo-C group, and those who did not receive HSCT had worse OS. (B) In patients in the TP53WT ITT population, excluding those who received follow-up salvage therapies, survival was similar between treatment groups in both those who received HSCT and those who did not.
Response-rate outcomes
In the TP53WT-ITT population, the CR rate, as assessed by investigators, was 20.3% in the idasa-C group compared with 17.1% in the placebo-C group (odds ratio [OR], 1.23; 95% CI, 0.70-2.18; P = .408) (Table 2). The overall remission rate (CR + CRi + CRp) was 38.8% in the idasa-C group compared with 22.0% in the placebo-C group (OR, 2.25; 95% CI, 1.36-3.72; P = .008). Patients were analyzed according to response at the end of induction and grouped by CR, CRi or CRp, and no response. There was no significant difference seen in OS between patients achieving a CR vs those achieving a CRi or CRp (supplemental Figure 3).
Table 2.
Secondary endpoints (TP53WT ITT population)
| Idasa-C (n = 232) |
Placebo-C (n = 123) |
OR or HR (95% CI) |
p value (HR) | |
|---|---|---|---|---|
| ORR [CR, CRp, CRi], n (%) | 90 (38.8) | 27 (22.0) | 2.25 (1.36-3.72) |
.0008 |
| CR, n (%)* | 47 (20.3) | 21 (17.1) | 1.23 (0.70-2.18) |
.408 |
| CRp, n (%) | 23 (9.9) | 4 (3.3) | — | — |
| CRi, n (%) | 20 (8.6) | 2 (1.6) | — | — |
| Median EFS, wk† | 6.3 | 4.4 | 0.65 | .0005 |
| n = 28 | n = 10 | |||
| DOR, median (95% CI), mo‡ | 13.9 (6.4, 21.1) |
29.4 (8.2, NE) |
— | — |
EFS, event-free survival; NE, not estimable.
CR was defined at the end of induction according to ELN 2017 criteria: bone marrow blasts <5%; absence of blasts with Auer rods; absence of extramedullary disease; absolute neutrophil count ≥1000 per μL; platelets ≥100 000 per μL.
EFS was defined as the time from randomization to treatment failure (failure to achieve CR, set as the day of last assessment), relapse from CR, or death from any cause.
DOR was defined as the time from achieving CR until relapse from CR or death from any cause.
The median duration of CR was 13.9 months in the idasa-C group and 29.4 months in the placebo-C group (Table 2). In the idasa-C arm, events contributing to the duration of CR analysis for patients with a CR were 12 deaths (25.5%; 11 in remission and 1 in relapse) and 16 (34.0%) relapses, while 19 patients (40.4%) were still in remission at the time of analysis. In the placebo-C arm, 2 patients (9.5%) died in remission, 8 (38.1%) relapsed, and 11 (52.4%) were still in remission at the time of analysis (supplemental Table 3). Most patients who died before relapse from CR died after transplant and/or follow-up cancer therapy, and the latencies between the end of induction and death in the idasa-C group ranged from 27 days to 538 days (supplemental Figure 4).
Among patients achieving CR after the first cycle, 37 patients in the idasa-C group and 17 patients in the placebo-C group were evaluable for MRD. CRMRD− at the <0.1% threshold was infrequent, occurring in 1 patient (3%) in the idasa-C group and no patients in the placebo-C group. The median MRD at CR was 0.49% (range, 0.05% to 3.25%) in the idasa-C group and 0.58% (range, 0.14% to 5.17%) in the placebo-C group. As only 1 patient was found to be CRMRD− the prognostic impact on OS was not analyzed.
MDM2 expression was evaluated on CD34+ and CD117+ cells by comparing responders (CR, CRp, or CRi at the end of induction; n = 36) with nonresponders (treatment failure or relapse; n = 63). There was no significant difference in MDM2 expression between the response groups (supplemental Figure 5).
MIC-1, a transcriptional target of TP53, was induced. Median levels of MIC-1 increased from baseline to cycle 1, day 5, in the idasa-C group compared with the placebo-C group in patients with TP53WT (n = 143) and mutant (n = 28) disease, demonstrating the expected target engagement. The increases in MIC-1 seen in the idasa-C groups were similar in the TP53WT and mutant groups (supplemental Figure 6).
Safety
Among patients in the safety population, adverse events (AEs), regardless of attribution, occurred in 100% of 284 patients in the idasa-C group and 99.3% of 146 patients in the placebo-C group (supplemental Table 4). The most common any-grade AEs occurring in the idasa-C vs placebo-C groups were diarrhea (87.0% vs 32.9%), febrile neutropenia (52.8% vs 49.3%), and nausea (52.5% vs 31.5%) (supplemental Table 5). In addition to febrile neutropenia, other myelosuppression AEs were common, such as thrombocytopenia (41.2% vs 47.9%) and neutropenia (12.7% vs 8.9%). Sepsis occurred in 12.0% vs 4.8%. The most common any-grade treatment-related AEs (≥5% incidence in either arm) occurring in the idasa-C vs placebo-C group were diarrhea (79.6% vs 16.4%), nausea (44.0% vs 15.1%), febrile neutropenia (32.0% vs 30.1%), and thrombocytopenia (29.2% vs 27.4%) (Table 3). Treatment-related sepsis occurred in 6.0% vs 2.1%.
Table 3.
Any-grade treatment-related AEs and serious AEs (safety population)
| AE, n (%) | Idasa-C (n = 284) |
Placebo-C (n = 146) |
|---|---|---|
| Any-grade treatment-related AEs (≥5% incidence in any arm) | ||
| Diarrhea | 226 (79.6) | 24 (16.4) |
| Nausea | 125 (44.0) | 22 (15.1) |
| Febrile neutropenia | 91 (32.0) | 44 (30.1) |
| Thrombocytopenia | 83 (29.2) | 40 (27.4) |
| Vomiting | 70 (24.6) | 9 (6.2) |
| Anemia | 57 (20.1) | 28 (19.2) |
| Pyrexia | 44 (15.5) | 15 (10.3) |
| Hypokalemia | 41 (14.4) | 9 (6.2) |
| Decreased appetite | 34 (12.0) | 8 (5.5) |
| Hyperbilirubinemia | 34 (12.0) | 5 (3.4) |
| Mucosal inflammation | 31 (10.9) | 7 (4.8) |
| Rash | 31 (10.9) | 15 (10.3) |
| Neutropenia | 30 (10.6) | 12 (8.2) |
| Erythema | 25 (8.8) | 3 (2.1) |
| Abdominal pain | 23 (8.1) | 4 (2.7) |
| Asthenia | 23 (8.1) | 6 (4.1) |
| Fatigue | 18 (6.3) | 5 (3.4) |
| Sepsis | 17 (6.0) | 3 (2.1) |
| Headache | 17 (6.0) | 8 (5.5) |
| Stomatitis | 15 (5.3) | 3 (2.1) |
| Pneumonia | 13 (4.6) | 9 (6.2) |
| Constipation | 8 (2.8) | 10 (6.8) |
| Epistaxis | 8 (2.8) | 11 (7.5) |
| Serious AEs (>2% in system organ class or >5% in preferred term) | ||
| Infections and infestations | 97 (34.2) | 33 (22.6) |
| Sepsis (PT) | 32 (11.3) | 7 (4.8) |
| Pneumonia (PT) | 18 (6.3) | 13 (8.9) |
| Septic shock (PT) | 9 (3.2) | 8 (5.5) |
| Blood and lymphatic system disorders | 37 (13.0) | 14 (9.6) |
| Febrile neutropenia (PT) | 27 (9.5) | 13 (8.9) |
| General disorders and administration site conditions | 18 (6.3) | 5 (3.4) |
| Gastrointestinal disorders | 20 (7.0) | 2 (1.4) |
| Nervous system disorders | 16 (5.6) | 4 (2.7) |
| Hepatobiliary disorders | 12 (4.2) | 3 (2.1) |
| Respiratory, thoracic, and mediastinal disorders | 10 (3.5) | 5 (3.4) |
| Immune system disorders | 10 (3.5) | 4 (2.7) |
| Cardiac disorders | 7 (2.5) | 4 (2.7) |
| Vascular disorders | 7 (2.5) | 1 (0.7) |
| Renal and urinary disorders | 6 (2.1) | 1 (0.7) |
| Injury, poisoning, and procedural complications | 3 (1.1) | 3 (2.1) |
PT, preferred term.
The incidence of grade 3 to 5 AEs, regardless of attribution, was 94.7% in the idasa-C group and 95.2% in the placebo-C group (supplemental Table 6). The most common grade 3 to 5 events in these groups (≥20%) were febrile neutropenia (52.5% vs 49.3%), thrombocytopenia (40.8% vs 47.9%), and anemia (23.2% vs 28.1%) (supplemental Table 5). The incidence of grade 3 to 5 diarrhea was 16.9% vs 6.2%, respectively (supplemental Table 5). Serious AEs were reported in 58.5% of patients in the idasa-C group and 46.6% of patients in the placebo-C group. The most common events according to system organ class were infections and infestations, which occurred in 34.2% of participants in the idasa-C group (including sepsis in 11.3%) vs 22.6% in the placebo-C group (including sepsis in 4.8%), blood and lymphatic system disorders (13.0% vs 9.6%), and gastrointestinal disorders (7.0% vs 1.4%) (Table 3). Regardless of attribution, grade 5 AEs with >2% incidence in the idasa-C group vs the placebo-C group included sepsis (5.3% [n = 15] vs 1.4% [n = 2]) and pneumonia (2.1% [n = 6] vs 2.1% [n = 3]) (supplemental Table 7). The 30-day mortality rates were 7.9% vs 5.5%, respectively (supplemental Table 4).
In TP53WT-ITT patients, neutrophil counts at baseline were slightly lower in the idasa-C group than in the placebo-C group (0.54 vs 0.59 103/mm3) and were persistently lower in the idasa-C group through cycle 1, day 56 (Figure 4). The time to recovery of neutrophils in patients achieving CR or CRp was longer in the idasa-C arm than in the placebo–chemotherapy arm and was not improved by treatment with granulocyte-colony stimulating factor (GCSF). Without GCSF administration, recovery of neutrophils to above 1.0 × 109/L occurred at a median of 37.5 days from day 1 of treatment in the idasanutlin–chemotherapy arm and at a median of 33.5 days in the placebo-C arm. With GCSF administration, median neutrophil recovery time was 36 days in the idasa-C arm vs 22 days in the placebo-C arm.
Figure 4.
Median (minimum–maximum) change from baseline in neutrophils (TP53WT ITT). Neutrophil counts were persistently lower in the idasa-C group than in the placebo-C group through cycle 1, day 56.
Discussion
As observed with several other investigational agents,28-30 the addition of idasanutlin to intermediate-dose cytarabine failed to improve the median OS in patients with TP53WT R/R AML. Similar to results from studies with laromustine30 and clofarabine28 in R/R AML, this failure to improve OS was observed after a near doubling of the ORR in patients randomized to idasa-C (driven by CRp/CRi rates for idasa-C, 18.5%; vs 4.9% for placebo-C). In addition, no differences in OS were detected in the ITT population when the TP53-mutant cohort, which comprised approximately 15% of the all-comer population, was analyzed with the TP53WT cohort.
Improved survival was seen primarily in patients receiving allogeneic HSCT. As the strong antileukemic effect of HSCT is essential for long-term remission in R/R AML,7 the similar proportion of patients who received transplantation may explain the lack of survival difference between the study arms. Another contributing element may have been very low CRMRD– rates in both arms. Factors contributing to the lack of improved OS in the idasa-C cohort may have been increased toxicity observed in the idasa-C arm, the higher incidence of intensive salvage therapies (70% vs 58%), and more frequent use of consolidation therapy in the placebo-C arm. Patients achieving ORR were eligible for optional consolidation, which was more frequently used in the placebo-C arm (21 of 27 patients) than in the idasa-C arm (40 of 90 patients). Higher rates of AEs such as gastrointestinal toxicities might have prevented patients in the idasa-C arm from receiving more intensive therapies or, conversely, conferred a higher risk of infection and death in patients who did receive intensive chemotherapy. MDM2 overexpression failed to identify patients more likely to benefit from idasa-C.
In the current study, the median OS in the cytarabine control arm of the entire patient cohort (independent of TP53-mutation status) was slightly longer than had been previously reported in the VALOR29 and CLASSIC I28 trials (7.7 months in MIRROS placebo-C vs 6.1 months in VALOR and 6.3 mo in CLASSIC I). These differences may reflect an improvement in the supportive care of patients with R/R AML or an increase in transplant rate, but they may also be explained by reported differences between these study populations. Although comparisons between these studies may be limited by the differences in trial design and the eligibility criteria, such as the exclusion of patients who were R/R to ≥1 treatment, the rate of transplant in the current study was higher than that observed in VALOR (29.5% in MIRROS vs 19.0% in VALOR) and might reflect a general trend toward transplant now being more often performed among patients with R/R AML.1 Nevertheless, the effect of subsequent HSCT has also been observed in those trials, indicating a general challenge in drug development in the R/R AML fit-patient setting, for which HSCT must remain a posttreatment option.
There were no new or unexpected safety findings observed, in line with prior experience with idasanutlin and with AEs considered class effects among MDM2 inhibitors.31 Idasanutlin exposure in the MIRROS study was above that of the previous phase 1/1b study, in which clinical efficacy was seen, highlighting that the observed gastrointestinal side effects did not prevent the achievement of therapeutic levels despite the oral route of administration of idasanutlin.32
At the dosing regimen selected for this study, higher rates of treatment-related AEs, serious AEs, and fatal AEs were seen in the idasa-C arm. Idasa-C was associated more frequently with diarrhea as a serious AE (2.8% vs 0.7%). The myelosuppressive effect was also more pronounced in the idasa-C arm, as evidenced by the longer duration of severe neutropenia that did not appear to be responsive to growth factor support (data not shown). In addition, higher rates of fatal infections appeared to contribute to shorter OS in the idasa-C arm, but the proportion of patients who died during the treatment-emergent period was similar between arms. Duration of response was limited in the idasa-C arm by more deaths occurring in this arm. Grade 5 AEs occurring after CR at the end of induction were not considered related to study treatment because of the long latencies between the end of induction and the occurrence of death, as well as the effect of subsequent HSCT with its associated transplant-related mortality for most patients.
R/R AML is heterogenous, and mechanisms of resistance to chemotherapy in R/R AML are not well understood. The myelosuppressive effect of idasanutlin appears to have limited treatment activity, leading to increased response rates, but it is plagued by prolonged neutropenia. It remains unanswered whether testing MDM2 inhibitors with different treatment regimens or dosages could reduce myelosuppression and permit the full benefit of MDM2 inhibition. Also, further research is needed to clarify whether MDM2 inhibitors such as idasanutlin may still have a potential role in earlier-stage AML or other cancer settings.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
Third-party writing assistance for this manuscript was provided by Christopher Lum of Health Interactions, Inc.
Funding support for this article was provided by F. Hoffmann-La Roche Ltd.
Authorship
Contribution: All authors verify that the trial was conducted according to the protocol and vouch for the accuracy and completeness of the data. All drafts of the manuscript were prepared by the authors, with editorial assistance from professional medical writers funded by the sponsor.
N.V., O.O., M.O., B.M.B., K.M., P.F., and A.H.W. conceptualized and designed the study; M.Y.K., C.R., D.D., J.K., P.M., J.A.S., O.O., D.T., S.M., B.M.B., O.C., M.G., K.M., C.J., and P.F. collected and assembled data; M.Y.K., C.R., D.D., L.G., J.K., P.M., J.A.S., O.O., M.P., A.R., D.T., N.V., S.-S.Y., P.F., and A.H.W. provided the provision of study material or patients; and all authors contributed to data analysis and interpretation and approved the final manuscript.
Conflict-of-interest disclosure: All authors received grants and nonfinancial support from F. Hoffmann-La Roche during the conduct of the study. Editorial support, funded by the sponsor, was provided by an independent medical writer under the guidance of the authors. M.Y.K. has received grants from AbbVie, Genentech, Stemline Therapeutics, Forty-Seven, Kisoji, Eli Lilly, Cellectis, Calithera, Ablynx, Agios, Ascentage, AstraZeneca, Rafael Pharmaceutical, and Sanofi; has consulted for Stemline Therapeutics, Amgen, Forty-Seven, and Kisoji; and holds stock options/royalties related to a patent for Reata Pharmaceutical. In addition, M.Y.K. has a patent (US 7 795 305 B2) for CDDO-compounds and combination therapies with royalties paid to Reata Pharmaceutical, has a patent for combination therapy with a mutant IDH1 inhibitor and a BCL-2 licensed to Eli Lilly, and has a patent (62/993 166) for the combination of an MCL-1 inhibitor and midostaurin, uses and pharmaceutical compositions thereof pending to Novartis. C.R. has consulted for AbbVie, Amgen, Astellas, Bayer, BMS, Celgene, Janssen, Jazz, Novartis, Pfizer, and Roche; and has received grants from AbbVie, Novartis, Pfizer, and Roche. L.G. is employed by the Federal State Budgetary Institution “Almazov National Medical Research Centre” of the Ministry of Health of the Russian Federation. G.M. has received personal fees from Amgen, Incyte, Pfizer, Celgene, Janssen, Jazz Pharmaceuticals, AbbVie, Novartis, Daiichi Sankyo, and Amgen. A.R. has served in advisory roles for Pfizer, Italfarmaco, Sanofi Aventis, Astellas, Roche, Jazz Pharmaceuticals, Omeros, and AbbVie. C.R. has received grants from AbbVie, Amgen, Astellas, BMS/Celgene, Jazz Pharmaceuticals, Agios, Daiichi-Sankyo, MaaT Pharma, and Novartis; and has received personal fees from AbbVie, Amgen, Astellas, BMS/Celgene, Jazz Pharmaceuticals, Agios, Daiichi-Sankyo, Incyte, Magrogenics, Janssen, Novartis, and Otsuka. R.R.-V. has participated in speakers’ bureaus for Jazz Pharmaceuticals and Astellas. N.V. has received personal fees from BMS/Celgene, Amgen Novartis, and Jazz Pharmaceuticals. M.O., S.M., O.C., M.G., K.M., and C.J. are employees of F. Hoffmann-La Roche. B.M.B. is an employee of F. Hoffmann-La Roche and owns stock for F. Hoffmann-La Roche and Novartis. P.F. reports grants, advisory roles, and honorarium from Celgene, Roche, AbbVie, and Jazz Pharmaceuticals. A.H.W. reports grants from Novartis, AbbVie, Servier, Celgene/BMS, Amgen, and AstraZeneca; honoraria and advisory roles with Novartis, Astellas, Pfizer, Macrogenics, AbbVie, Genentech, Servier, Celgene/BMS, Amgen, AstraZeneca, and Janssen; participation in speakers’ bureaus for Novartis, AbbVie, and Celgene/BMS; and consulting roles with Servier. Additionally, A.H.W. is a former employee of the Walter and Eliza Hall Institute and receives a fraction of its royalty stream related to venetoclax. J.C., D.D., P.M., J.A.S., O.O., M.P., J.K., A.P., D.T., and S.-S.Y. have nothing further to disclose.
Correspondence: Marina Y. Konopleva, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1400 Holcombe Blvd, FC3.3048, Houston, TX 77030; e-mail: mkonople@mdanderson.org.
References
- 1.Bohl SR, Bullinger L, Rücker FG. New targeted agents in acute myeloid leukemia: new hope on the rise. Int J Mol Sci. 2019;20(8):1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Megías-Vericat JE, Martínez-Cuadrón D, Sanz MA, Montesinos P. Salvage regimens using conventional chemotherapy agents for relapsed/refractory adult AML patients: a systematic literature review. Ann Hematol. 2018;97(7):1115-1153. [DOI] [PubMed] [Google Scholar]
- 3.Walter RB, Othus M, Burnett AK, et al. Resistance prediction in AML: analysis of 4601 patients from MRC/NCRI, HOVON/SAKK, SWOG and MD Anderson Cancer Center. Leukemia. 2015;29(2):312-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnett AK, Russell NH, Hills RK, et al. A comparison of clofarabine with ara-C, each in combination with daunorubicin as induction treatment in older patients with acute myeloid leukaemia. Leukemia. 2017;31(2):310-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnett AK, Russell NH, Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol. 2012;30(32):3924-3931. [DOI] [PubMed] [Google Scholar]
- 6.Thol F, Schlenk RF, Heuser M, Ganser A. How I treat refractory and early relapsed acute myeloid leukemia. Blood. 2015;126(3):319-327. [DOI] [PubMed] [Google Scholar]
- 7.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Acute Myeloid Leukemia. V3.2020
- 9.Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23(9):1969-1978. [DOI] [PubMed] [Google Scholar]
- 10.Burnett A, Wetzler M, Löwenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29(5):487-494. [DOI] [PubMed] [Google Scholar]
- 11.Othus M, Appelbaum FR, Petersdorf SH, et al. Fate of patients with newly diagnosed acute myeloid leukemia who fail primary induction therapy. Biol Blood Marrow Transplant. 2015;21(3):559-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wattad M, Weber D, Döhner K, et al. Impact of salvage regimens on response and overall survival in acute myeloid leukemia with induction failure. Leukemia. 2017;31(6):1306-1313. [DOI] [PubMed] [Google Scholar]
- 13.Moll UM, Petrenko O. The MDM2-p53 interaction. Mol Cancer Res. 2003;1(14):1001-1008. [PubMed] [Google Scholar]
- 14.Kojima K, Konopleva M, Samudio IJ, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106(9):3150-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Momand J, Jung D, Wilczynski S, Niland J. The MDM2 gene amplification database. Nucleic Acids Res. 1998;26(15):3453-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seliger B, Papadileris S, Vogel D, et al. Analysis of the p53 and MDM-2 gene in acute myeloid leukemia. Eur J Haematol. 1996;57(3):230-240. [DOI] [PubMed] [Google Scholar]
- 17.Arva NC, Talbott KE, Okoro DR, Brekman A, Qiu WG, Bargonetti J. Disruption of the p53-Mdm2 complex by Nutlin-3 reveals different cancer cell phenotypes. Ethn Dis. 2008;18(2 suppl 2):S2-1-8. [PMC free article] [PubMed] [Google Scholar]
- 18.Panuzzo C, Signorino E, Calabrese C, et al. Landscape of tumor suppressor mutations in acute myeloid leukemia. J Clin Med. 2020;9(3):802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanz G, Singh M, Peuget S, Selivanova G. Inhibition of p53 inhibitors: progress, challenges and perspectives. J Mol Cell Biol. 2019;11(7): 586-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding Q, Zhang Z, Liu JJ, et al. Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J Med Chem. 2013; 56(14):5979-5983. [DOI] [PubMed] [Google Scholar]
- 21.Yee K, Martinelli G, Vey N, et al. Phase 1/1b study of RG7388, a potent MDM2 antagonist, in acute myelogenous leukemia (AML) patients (Pts). Blood. 2014;124(21):116. [Google Scholar]
- 22.Martinelli G, Pappayannidis C, Yee K, et al. Phase 1B results of idasanutlin + cytarabine (Ara-C) in acute myelogenous leukemia (AML) patients (Pts). Haematologica. 2016; S504. Presented at the 21st Congress of the European Hematology Association, Copenhagen, Denmark; 9-12 June 2016. [Google Scholar]
- 23.Montesinos P, Beckermann BM, Catalani O, et al. MIRROS: a randomized, placebo-controlled, Phase III trial of cytarabine ± idasanutlin in relapsed or refractory acute myeloid leukemia. Future Oncol. 2020;16(13):807-815. [DOI] [PubMed] [Google Scholar]
- 24.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. [DOI] [PubMed] [Google Scholar]
- 25.Döhner H, Estey EH, Amadori S, et al. ; European LeukemiaNet . Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453-474. [DOI] [PubMed] [Google Scholar]
- 26.International Agency for Research on Cancer. IARC TP53 Database. Vol. 2020. https://p53.iarc.fr/. Accessed 17 December 2020.
- 27.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343-346. [DOI] [PubMed] [Google Scholar]
- 28.Faderl S, Wetzler M, Rizzieri D, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. J Clin Oncol. 2012;30(20):2492-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravandi F, Ritchie EK, Sayar H, et al. Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): a randomised, controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2015;16(9):1025-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giles F, Vey N, DeAngelo D, et al. Phase 3 randomized, placebo-controlled, double-blind study of high-dose continuous infusion cytarabine alone or with laromustine (VNP40101M) in patients with acute myeloid leukemia in first relapse. Blood. 2009;114(19):4027-4033. [DOI] [PubMed] [Google Scholar]
- 31.Pi L, Rooprai J, Allan DS, et al. Evaluating dose-limiting toxicities of MDM2 inhibitors in patients with solid organ and hematologic malignancies: a systematic review of the literature. Leuk Res. 2019;86:106222. [DOI] [PubMed] [Google Scholar]
- 32.Yee K, Papayannidis C, Vey N, et al. Murine double minute 2 inhibition alone or with cytarabine in acute myeloid leukemia: results from an idasanutlin phase 1/1b study. Leuk Res. 2021;100:106489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.