Key Points
Cladribine is regarded as the first treatment of choice for symptomatic hairy cell leukemia.
This large international study reports a complete response in 72% of cases and a continuous complete response in 20% of patients.
Introduction
Cladribine is universally regarded as the frontline treatment of choice for symptomatic hairy cell leukemia (HCL), as it has transformed an almost incurable disease into a well-controllable condition. Compared with the agents or procedures applied before it became widely available (namely, splenectomy and interferon-α), cladribine has been able to dramatically change the natural history of HCL, as most patients have demonstrated high rates of response to 1 course of treatment only.1,2 More specifically, cladribine-treated patients can achieve complete recovery of peripheral blood cytopenia and disappearance of marrow infiltration by hematoxylin and eosin staining in 80% to 90% of cases, as reported in the literature,3 albeit some complete responder patients still display residual marrow disease detectable by immunohistochemistry or molecular techniques.4,5 Besides that, a significant proportion of patients could maintain an efficient disease control over time with only 1 treatment course, possibly enjoying very long-lasting treatment-free intervals and having a chance of being cured.
The bulk of available data on the efficacy of cladribine and the correlated long-term follow-up of patients treated frontline with this agent comes from several retrospective series published since the late nineties.2 Most of the research, however, reports on patients treated either at disease onset or as second or later lines, therefore conveying little information on the efficacy of the drug applied upfront, especially as far as durable remissions after only 1 treatment course are concerned. The published international experience with frontline cladribine mainly consists of single-institution retrospective analyses, sometimes with a limited number of patients.6-9 Only a few multicentric studies deal with patients treated uniformly, but reports on multinational cooperation are lacking (Table 1).10-16
Table 1.
Recently published international experiences with frontline cladribine in the treatment of classic HCL
| Author | Year | Study Type | Site/Country | Patients, n | ORR | CR | Median DFS/TTF, yr | OS, % (yr) |
|---|---|---|---|---|---|---|---|---|
| Else6 | 2009 | Single center | London, UK | 45 | 100 | 76 | 11 | 100 (10) |
| Rosenberg7 | 2014 | Single center | La Jolla, CA | 83 | 100 | 88 | 5 | 80 (25) |
| Somasundaram8 | 2014 | Single center | New Delhi, India | 27 | 100 | 100 | — | — |
| Broccoli9 | 2021 | Single center | Bologna, Italy | 122 | 86 | 54 | 8 | 66 (25) |
| Ruiz-Delgado10 | 2012 | Multicenter | Mexico | 11 | 100 | 100 | — | 91 (11) |
| López Rubio11 | 2014 | Multicenter | Spain | 80 | 100 | 88 | 12 | — |
| Cornet12 | 2014 | Multicenter | France | 281 | 100 | 83 | 14 | 90 (20) |
| Hacioglu13 | 2015 | Multicenter | Turkey | 78 | 97 | 81 | 2 | 83 (19) |
| Criscuolo14 | 2019 | Multicenter | Italy | 557 | 92 | 65 | 12 | 82 (15) |
| Benz15 | 2020 | Multicenter | Switzerland | 221 | 88 | 49 | — | 67 (20) |
| Paillassa16 | 2020 | Multicenter | France | 159 | 99 | 83 | 14 | 60 (20) |
| This study | 2022 | Multicenter | Europe (4 sites) | 384 | 94 | 72 | 11 | 48 (28) |
ORR, overall response rate; TTF, time-to-treatment failure.
The aim of this research is to report the clinical experience with cladribine in a wide series of treatment-naïve HCL patients treated at 4 European centers, with the ambition of providing useful information on long-lasting responder patients due to the long available follow-up.
Methods
Disease-specific patient records have been reviewed at each center, and all patients requiring treatment who received frontline cladribine have been extrapolated. Responses have been classified according to the Consensus Resolution criteria published in 1987. A complete response (CR) was defined as the resolution of peripheral cytopenia (hemoglobin ≥12 g/dL, platelets >100 000 per mmc, and neutrophils >1500 per mmc), along with the disappearance of hairy cell marrow infiltrate with hematoxylin and eosin staining and no residual organomegaly. Bone marrow (BM) biopsy after treatment was necessary to confirm CR. BM aspiration was optional. Patients qualified for a partial response if peripheral cytopenia resolved and hepatosplenomegaly decreased ≥50%, although with persistent marrow infiltration (≥50% reduction). The main study objectives were long-term overall survival (OS), disease-free survival (DFS), and progression-free survival (PFS) rates. PFS calculation involved all study patients; DFS was determined only in patients with a CR. Determining events for DFS and PFS were disease progression (decline in hematologic parameters, the reappearance of marrow infiltration, and/or organomegaly), initiation of subsequent treatment, and death (due to HCL [for DFS] or for any cause [for PFS]). This retrospective study was approved by the institutional board of the coordinating center (IRCCS Azienda Ospedaliero-Universitaria di Bologna, id 1043/2021/OssAOUBo) and was conducted according to the Declaration of Helsinki. A shared database was used, and variables were strictly defined to avoid bias in reporting data.
Results and discussion
Three hundred and eighty-four patients affected by classic HCL (including 3 patients with HCL variant) were diagnosed and followed between 1969 and 2018. The median age at diagnosis was 56 years (range, 25-85 years). Male patients represented 86.5% of the sample. Splenomegaly was found in 53% of the cases. Median BM cellularity before treatment was 50%, with a median leukemic infiltration of 90%. All patients received cladribine as upfront systemic therapy (12 patients underwent previous splenectomy), either subcutaneously or by IV, according to era- and site-specific guidelines and experience. Most patients were treated in the decade 2000 to 2009 (54%), then between 2010 and 2019 (27%), and between 1990 and 1999 (18%). Before 1990, cladribine was considered experimental in Europe, and only 1% of the patients (5 in total) received it.
The overall response rate (sum of CR and partial response rates) was 94%, ranging between 86% and 100%, according to the experience of the different centers. A CR was obtained in 276 cases (72%), ranging from 54% to 85% (supplemental Table). Two hundred and ninety-three patients (76%) received no more therapy besides the initial course as they did not require further treatment for their disease. Among these patients, 208 (54%) displayed no further relapse or progression over time, within a range of follow-up of 1 to 22 years (median, 8.5 years). A continuous CR, which indicates a hematologic CR maintained for ≥5 years, was documented in 76 patients (20%), which corresponds to 28% of all complete responder subjects (supplemental Figure).
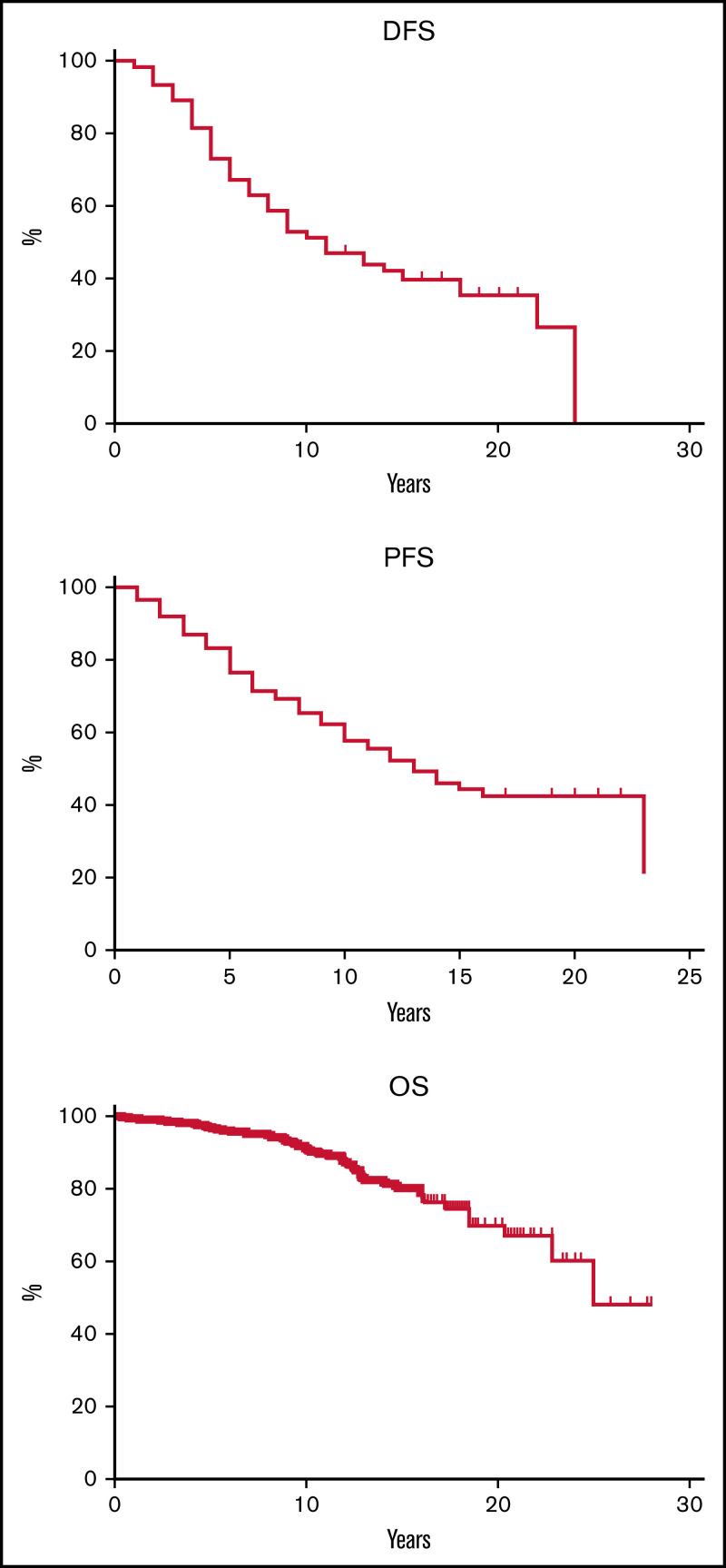
Median OS was reached at 25 years, with 48% of patients alive at 28 years. Death due to secondary neoplasms was registered in 4 cases (2 cases of lung adenocarcinoma, 1 acute myeloid leukemia, and 1 colon carcinoma). Median PFS was 13 years, with 43% of patients being free of progression at 22 years, whereas DFS was 26.5% at 22 years, and the median was reached at 11 years. These figures translate into 5-year, 10-year, and 20-year PFS and DFS of 77%, 58%, 43% and 73%, 51%, 35%, respectively (Figure 1).
Figure 1.
DFS, PFS, and OS curves for the whole study population.
Treatment with cladribine was early withdrawn in 18 cases because of adverse events: more specifically, hematologic toxicity (mostly neutropenia) and fever represented the cause of interruption in 5 cases each, followed by sepsis (3 cases), pneumonia, gastrointestinal bleeding, military tuberculosis, hepatic toxicity, and cutaneous rash (1 case each).
Besides reporting data on a significant number of patients treated homogeneously at 4 centers of excellence with experience in the management of HCL, this work displays the first international effort in collecting data on symptomatic HCL patients treated with upfront cladribine since its introduction in Europe with a long-term follow-up. These results represent the everyday clinical practice with HCL patients at European sites, and outcomes reported for each participating center reflect the efficacy of cladribine as frontline treatment. This is underscored by the high percentages of good quality responses, with up to 72% of CR.
Recently published data by Chihara and coworkers have demonstrated the synergistic activity of rituximab with cladribine in the initial treatment of HCL.17 In a randomized trial, they showed that the concurrent administration of rituximab and cladribine yielded a higher chance of eradicating minimal residual disease (MRD) rather than cladribine given alone (or possibly followed by rituximab after 6 months or later), with 97% and 24% of patients in either arm being MRD-free at 6 months. Nevertheless, patients receiving concomitant rituximab and cladribine displayed the same overall response rate at 6 months as those receiving delayed rituximab (the latter qualifying as responders to cladribine only), and no statistically significant differences in CR rates were detected between treatment arms.17 Despite demonstrating its superiority in terms of MRD-free treatment interval, the true impact of rituximab + cladribine over cladribine alone on PFS, DFS, time-to-next treatment, and OS remains to be established, and longer follow-up is required.
MRD was not an endpoint of our study. Although it may be hypothesized that some patients have obtained MRD negativity,5 it is of note that deep disease control is achieved in a significant proportion of cases with only 1 course of treatment. More than 50% of treated patients, in fact, required no further therapy beyond upfront cladribine. Notably, high-quality responses have been maintained for >20 years in up to 35% of patients. For this reason, it is questionable whether MRD eradication in HCL affects long-term outcomes as it happens with other hematological malignancies. Even though it is plausible that relapse rates may be lower in those achieving an MRD-free status, persistent MRD detectability does not automatically translate into a rapid hematologic deterioration, nor to the immediate need for any subsequent treatment.2,6,9,14
In conclusion, data obtained from this large international cohort of patients integrate the results obtained from smaller single-center or national multicentric clinical experiences with purine analogs.6-16 We would like to underscore the concept that international multicenter collaborations are of great importance in gathering a high number of patients as far as rare diseases such as HCL are concerned.
Supplementary Material
The full-text version of this article contains a data supplement.
Authorship
Contribution: A.B. and P.L.Z. conceived the study; A.B., L.A., and P.L.Z. wrote the manuscript; A.B., M.C., A.J., E.M., X.T., T.R., C.D., M.E., and D.C. provided study data; L.A. analyzed the data; and all authors read and approved the final version of the manuscript after revising it critically. All authors have access to the final database.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pier Luigi Zinzani, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Istituto di Ematologia “Seràgnoli”; Dipartimento di Medicina Specialistica, Diagnostica e Sperimentale, Università di Bologna. Via Massarenti, 9 – 40138 Bologna, Italy; e-mail: pierluigi.zinzani@unibo.it.
References
- 1.Troussard X, Maître E, Cornet E. Hairy cell leukemia 2022: update on diagnosis, risk-stratification, and treatment. Am J Hematol. 2022;97(2):226-236. [DOI] [PubMed] [Google Scholar]
- 2.Goodman GR, Burian C, Koziol JA, Saven A. Extended follow-up of patients with hairy cell leukemia after treatment with cladribine. J Clin Oncol. 2003;21(5):891-896. [DOI] [PubMed] [Google Scholar]
- 3.Andrasiak I, Rybka J, Wrobel T. Response to therapy in hairy cell leukemia: systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk. 2018;18(6):392-399.e3. [DOI] [PubMed] [Google Scholar]
- 4.Noel P. Definition of remission, minimal residual disease, and relapse in hairy cell leukemia bone marrow biopsy histology and immunohistology specimens. Leuk Lymphoma. 2011;52(sup2 Suppl 2):62-64. [DOI] [PubMed] [Google Scholar]
- 5.Broccoli A, Terragna C, Nanni L, et al. Droplet digital polymerase chain reaction for the assessment of disease burden in hairy cell leukemia. Hematol Oncol. 2022;40(1):57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Else M, Dearden CE, Matutes E, et al. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br J Haematol. 2009;145(6):733-740. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg JD, Burian C, Waalen J, Saven A. Clinical characteristics and long-term outcome of young hairy cell leukemia patients treated with cladribine: a single-institution series. Blood. 2014;123(2):177-183. [DOI] [PubMed] [Google Scholar]
- 8.Somasundaram V, Purohit A, Aggarwal M, et al. Hairy cell leukemia: a decade long experience of North Indian Hematology Center. Indian J Med Paediatr Oncol. 2014;35(4):271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broccoli A, Argnani L, Nanni L, et al. The treatment of hairy cell leukemia with a focus on long lasting responses to cladribine: a 30-year experience. Am J Hematol. 2021;96(10):1204-1210. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Delgado GJ, Tarín-Arzaga LC, Alarcón-Urdaneta C, Calderón-García J, Gómez-Almaguer D, Ruiz-Argüelles GJ. Treatment of hairy cell leukemia: long-term results in a developing country. Hematology. 2012;17(3):140-143. [DOI] [PubMed] [Google Scholar]
- 11.López Rubio M, Da Silva C, Loscertales J, et al. Hairy cell leukemia treated initially with purine analogs: a retrospective study of 107 patients from the Spanish Cooperative Group on Chronic Lymphocytic Leukemia (GELLC). Leuk Lymphoma. 2014;55(5):1007-1012. [DOI] [PubMed] [Google Scholar]
- 12.Cornet E, Tomowiak C, Tanguy-Schmidt A, et al. ; Société Française d’Hématologie . Long-term follow-up and second malignancies in 487 patients with hairy cell leukaemia. Br J Haematol. 2014;166(3):390-400. [DOI] [PubMed] [Google Scholar]
- 13.Hacioglu S, Bilen Y, Eser A, et al. Multicenter retrospective analysis regarding the clinical manifestations and treatment results in patients with hairy cell leukemia: twenty-four year Turkish experience in cladribine therapy. Hematol Oncol. 2015;33(4):192-198. [DOI] [PubMed] [Google Scholar]
- 14.Criscuolo M, Broccoli A, Galli E, et al. Multicenter long term follow-up in hairy cell leukemia patients treated with cladribine: a thirty-year experience. Blood. 2020;136(Supplement 1):32-33. [ASH annual meeting abstracts] [Google Scholar]
- 15.Benz R, Arn K, Andres M, et al. Prospective long-term follow-up after first-line subcutaneous cladribine in hairy cell leukemia: a SAKK trial. Blood Adv. 2020;4(15):3699-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paillassa J, Cornet E, Noel S, et al. Analysis of a cohort of 279 patients with hairy-cell leukemia (HCL): 10 years of follow-up. Blood Cancer J. 2020;10(5):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chihara D, Arons E, Stetler-Stevenson M, et al. Randomized phase II study of first-line cladribine with concurrent or delayed rituximab in patients with hairy cell leukemia. J Clin Oncol. 2020;38(14):1527-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.