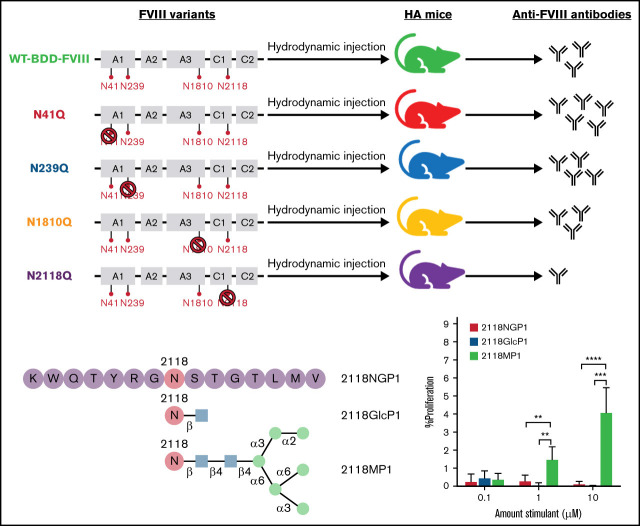
Key Points
Elimination of N-glycosylation at sites in the A and C domains of FVIII differentially affects its immunogenicity but not specific activity.
A potent mannosylated glycopeptide epitope at site N2118 was identified and characterized to activate T cell's.
Visual Abstract
Abstract
The most significant complication in hemophilia A treatment is the formation of inhibitors against factor VIII (FVIII) protein. Glycans and glycan-binding proteins are central to a properly functioning immune system. This study focuses on whether glycosylation of FVIII plays an important role in induction and regulation of anti-FVIII immune responses. We investigated the potential roles of 4 N-glycosylation sites, including N41 and N239 in the A1 domain, N1810 in the A3 domain, and N2118 in the C1 domain of FVIII, in moderating its immunogenicity. Glycomics analysis of plasma-derived FVIII revealed that sites N41, N239, and N1810 contain mostly sialylated complex glycoforms, while high mannose glycans dominate at site N2118. A missense variant that substitutes asparagine (N) to glutamine (Q) was introduced to eliminate glycosylation on each of these sites. Following gene transfer of plasmids encoding B domain deleted FVIII (BDD-FVIII) and each of these 4 FVIII variants, it was found that specific activity of FVIII in plasma remained similar among all treatment groups. Slightly increased or comparable immune responses in N41Q, N239Q, and N1810Q FVIII variant plasmid-treated mice and significantly decreased immune responses in N2118Q FVIII plasmid-treated mice were observed when compared with BDD-FVIII plasmid-treated mice. The reduction of inhibitor response by N2118Q FVIII variant was also demonstrated in AAV-mediated gene transfer experiments. Furthermore, a specific glycopeptide epitope surrounding the N2118 glycosylation site was identified and characterized to activate T cells in an FVIII-specific proliferation assay. These results indicate that N-glycosylation of FVIII can have significant impact on its immunogenicity.
Introduction
Inhibitor formation remains the major complication in treatment of hemophilia A (HA). Inhibitors, neutralizing antibodies specific to factor VIII (FVIII), form in approximately 30% of patients during FVIII replacement therapy.1,2 Many factors contribute to the induction of inhibitors3; however, much is still unknown about the key risk factors and the associated induction mechanism, including why inhibitor formation occurs in some patients, but not others. FVIII is a plasma glycoprotein. During processing in the endoplasmic reticulum and Golgi, sugar residues and oligosaccharide chains are covalently attached to the amino acids of its polypeptide chain. The differences in posttranslational modifications, such as glycosylation, may contribute to the differences in immune responses among patients.
In the last few years, multiple epidemiological studies have highlighted the differential immunogenicity of FVIII products used in the treatment of patients with HA.4,5 It was found that there is a 1.87-fold increase in inhibitor risk associated with recombinant FVIII (rFVIII) products compared with plasma-derived FVIII (pdFVIII) and a 1.6-fold increase in inhibitor risk associated with second-generation baby hamster kidney cell–derived rFVIII compared with third-generation Chinese hamster ovary cell–derived rFVIII.4 One hypothesis that may explain these differences is glycosylation as the glycan structures on each product differ based on the cell types from which they are derived.6,7 Further studies are needed to understand and elucidate the mechanisms of inhibitor development, particularly the effects of glycosylation on FVIII immunogenicity that could lead to different responses in patients.
Multiple studies have recently been performed to analyze the glycosylation of various pdFVIII and rFVIII products.8-10 Most N-linked glycosylation was detected in the B domain of FVIII, with only 4 sites identified outside of the B domain: N41 and N239 in the A1 domain, N1810 in the A3 domain, and N2118 in the C1 domain.8,10 Furthermore, while the locations of N-glycosylation sites remain the same in rFVIII and pdFVIII, the composition of glycoforms and occupancy at each site varies greatly from product to product.9,10
The development of inhibitors to FVIII is a T-cell–dependent process, and interactions between glycans and antigen-presenting cells (APCs) have been observed. Sialic acids are known to interact with sialic acid–binding immunoglobulin-type lectins (siglecs),11 including Siglec-5, an inhibitory receptor,12 as well as c-type lectin asialoglycoprotein receptor13 to protect FVIII from endocytosis. High mannose glycans on FVIII have been observed to interact with mannose-specific receptors on dendritic cells in vitro, potentially leading to FVIII endocytosis.14 Given the research suggesting the potential roles played by different types of glycans,11,13-15 we explored whether elimination of N-glycans at the 4 aforementioned sites in the A and C domains of B domain deleted FVIII (BDD-FVIII) would induce changes in the immune responses in a HA murine model when compared with wild-type (WT) BDD-FVIII in a gene therapy setting.
Methods
Mutagenesis of N-linked glycans
Mutagenesis of the BDD-FVIII cDNA in the liver-specific plasmid (pBS-HCRHPI-hFVIII16) was carried out using the Agilent QuikChange II XL Mutagenesis kit (Agilent Technologies, Inc.). A substitution was made swapping an asparagine (N) at the site of interest with a glutamine (Q) residue to eliminate N-glycosylation at the specified site. FVIII N to Q variants were created at sites N41, N239, N1810, and N2118 on the BDD-FVIII plasmid, respectively. Primers used are listed in Supplemental Figure 1.
Mice
All the experimental mice were housed in a specific pathogen-free facility at Seattle Children’s Research Institute according to the animal care guidelines of the National Institutes of Health and Seattle Children’s Research Institute. The experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee of Seattle Children’s Research Institute.
Delivery of plasmids carrying BDD-FVIII and its glycosylation variants genes into HA mice
Male HA mice with a FVIII exon 16 knockout in an Sv129/BL6 mixed background between the ages of 8 and 14 weeks were used in all experiments. Mice were injected with liver-specific plasmids encoding WT BDD-FVIII or one of the glycosylation variants (Figure 1) via hydrodynamic injection at a concentration of 25 µg/mL and a volume (mL) equal to 9% the body weight of the mouse (g). A second challenge of the same plasmids via hydrodynamic injection was performed on day 86 to elicit robust secondary immune responses. Blood was collected by retroorbital bleed in one-tenth volume of 3.8% sodium citrate periodically following plasmid injections.
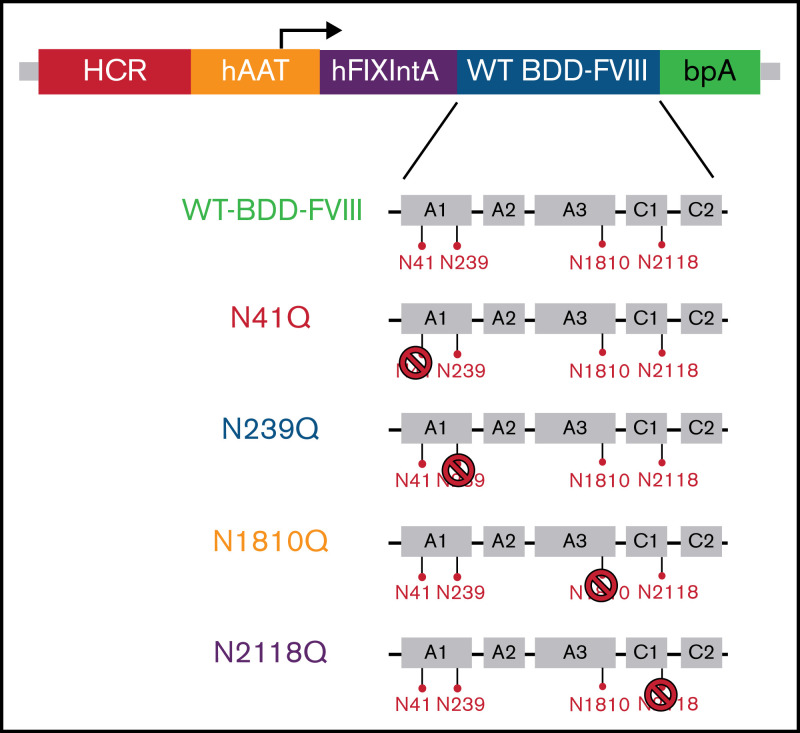
Figure 1.
WT BDD-FVIII plasmid used for N to Q mutagenesis and the resulting FVIII variant constructs used for in vivo gene therapy. The WT BDD-FVIII plasmid construct is shown at the top. The 4 N-glycosylation sites, N41, N239, N1810, and N2118, in the A1, A3, and C1 domains are separately represented by red pins. N to Q mutagenesis was performed on the WT BDD-FVIII backbone to eliminate glycosylation on each of the 4 sites represented by the stop symbol. BDD, B domain deleted; bpA, bovine growth hormone polyadenylation site; hAAT, human α1-antitrypsin promoter; HCR, hepatic control region; hFIXintA, human factor IX intron 1; WT, wild type.
Adeno-associated viral vector production, purification, and delivery
Adeno-associated viral (AAV) vector AAV-BDD-FVIII and AAV-N2118Q-FVIII were produced by the triple plasmid transfection system in HEK 293 cells. AAV production and determination of vector titers are described in the supplemental Methods. AAV vectors were administered into HA mice at a dosage of 1 × 1012 vg/mouse via tail vein injection.
Measurement of FVIII activity and antigen levels and anti-FVIII inhibitory antibodies
Posthydrodynamic injection of FVIII plasmids into mice, the coagulant activity of FVIII, FVIII:C, was measured by a 1-stage, activated partial thromboplastin time (aPTT)-based assay using a Stago Compact Max instrument.17,18 FVIII inhibitors were measured using Bethesda assay.17,18 Measurement of FVIII antigen levels (FVIII:Ag) and total anti-FVIII immunoglobulin G (IgG) were examined by enzyme-linked immunosorbent assay (ELISA) as described in the supplemental Methods.
CD4+ T-cell proliferation assay
Mice with high titer anti-FVIII inhibitors were generated as described in the supplemental Methods. Mouse splenocytes were isolated and stained with BD Horizon violet cell proliferation dye 450 (VPD450) (BD Biosciences). The labeled splenocytes were cultured and stimulated by adding FVIII as a positive proliferation control, Factor IX as a non-specific antigen control, or peptides, either glycosylated or non-glycosylated, corresponding to site N2118. Mannan (0.1, 1, and 10 µM, respectively) was added in the mannan inhibition experiments. After 96 hours, cells were stained with anti-mouse CD4 and Fixable Viability Stain (Fisher Scientific) and analyzed by flow cytometry (BD LSR II analyzer, Fisher Scientific). The enhancement of proliferation was calculated by subtracting the background levels in non-stimulated cells.
Synthesis of non-glycosylated and glycosylated peptides
Non-glycosylated peptides, 15 amino acids in length, corresponding to sequences including site N2118, were synthesized and provided by GenScript. Glycosylated peptides, including peptides with either a single N-acetyl-glucosamine (GlcNAc) attachment or a high mannose glycan (Man6GlcNAc2), were synthesized in house. Peptides with GlcNAc were synthesized by using wang-resin through the Fmoc-strategy. To prepare mannosylated N-glycopeptides, Man6GlcNAc oxazoline was prepared as previously described19 and coupled to GlNAc-attached peptides. The detailed synthetic methods of glycosylated peptides are described in the supplemental Methods.
Statistical analysis
All statistical analyses were carried out using GraphPad Prism 7 software. The data was compared using 1-way or 2-way analysis of variance or multiple t tests. P < .05 was considered statistically significant.
Results
Comparison of FVIII expression resulting from the WT and glycosylation variant genes
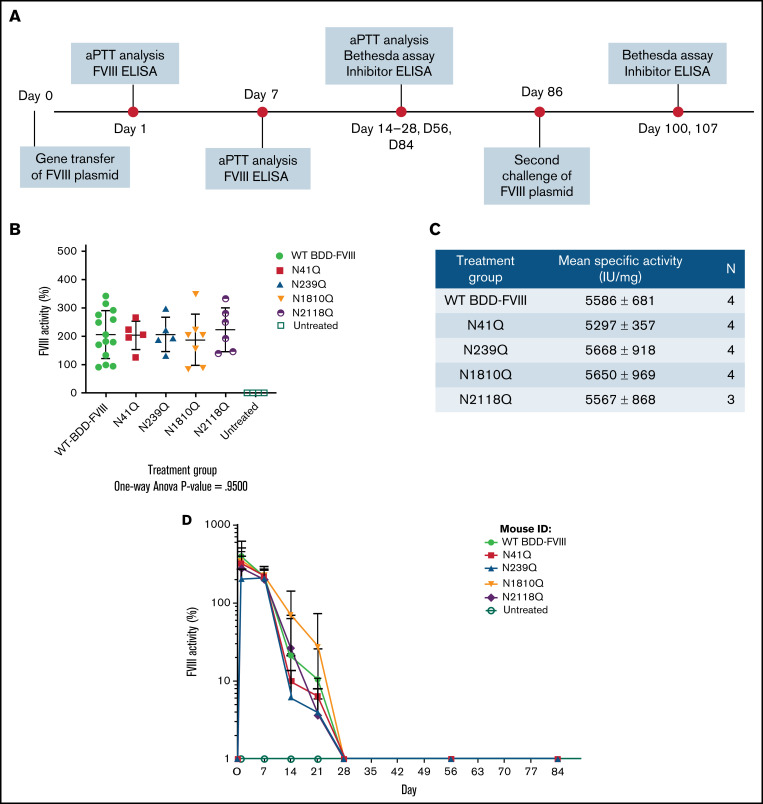
FVIII variants were created through site-directed mutagenesis of the cDNA of a WT BDD-FVIII plasmid at each of the 4 N-glycosylation sites in the A and C domains by substituting the N residue with a Q residue (pBS-HCRHPI-hFVIII)16 (Figure 1; supplemental Figure 1). These variants could effectively eliminate glycosylation at each site. The plasmids carrying the genes encoding BDD-FVIII and glycosylation variants were then hydrodynamically injected, respectively, into HA mice. FVIII activity levels in treated mouse plasma were evaluated 1 week after injection (Figure 2A). Mice injected with the WT BDD-FVIII plasmid had an average activity of 206 ± 84%, and mice injected with N41Q, N239Q, N1810Q, and N2118Q FVIII variant plasmids had average activities of 203 ± 50%, 206 ± 60%, 187 ± 91%, and 223 ± 77%, respectively (Figure 2B). No statistically significant differences in FVIII activities between the variants and WT BDD-FVIII were observed by a 1-way ANONVA (P close to 1). FVIII:Ag levels were determined by FVIII ELISA. The specific activities of FVIII produced in all treated groups also showed no statistical differences (Figure 2C). Taken together, these results indicated that mutation in each of these sites likely did not induce any significant conformational changes in the protein structure to affect FVIII activity and production in vivo. FVIII activities were monitored weekly for the duration of the experiment via aPTT analysis (Figure 2A). The % FVIII in circulation dropped to undetectable levels in all treatment groups by day 28 (Figure 2D).
Figure 2.
In vivo experimental layout and comparison of activities of mutated FVIII variants and WT BDD-FVIII. Groups of HA mice were injected hydrodynamically with plasmids encoding WT BDD-FVIII and 4 N-glycosylation site mutated FVIII variants, respectively. (A) The experimental schedule of first and second challenges of FVIII plasmids via hydrodynamic injections and periodic blood collection for aPTT analysis, Bethesda assay, and inhibitor enzyme-linked immunosorbent assay (ELISA). (B) FVIII activities in plasma by aPTT at 1 week after plasmid injection. P = .95 calculated using 1-way analysis of variance among all plasmid treated groups. Untreated mice were used as negative controls. (C) Specific activities of each FVIII variant compared with WT BDD-FVIII. Antigen levels of FVIII were evaluated using a FVIII-specific ELISA. N indicates the number of animals per group. (D) FVIII activities evaluated over time. WT BDD-FVIII: N = 14; N41Q: N = 5; N239Q: N = 5; N1810Q: N = 7; N2118Q: N = 6; untreated: N = 4. Experiments for each group were repeated at least 3 times. Data are presented as averages from repeated experiments, with error bars indicating standard deviation.
Evaluation of the impact of N-glycosylation on the immunogenicity of FVIII following plasmid-mediated gene transfer
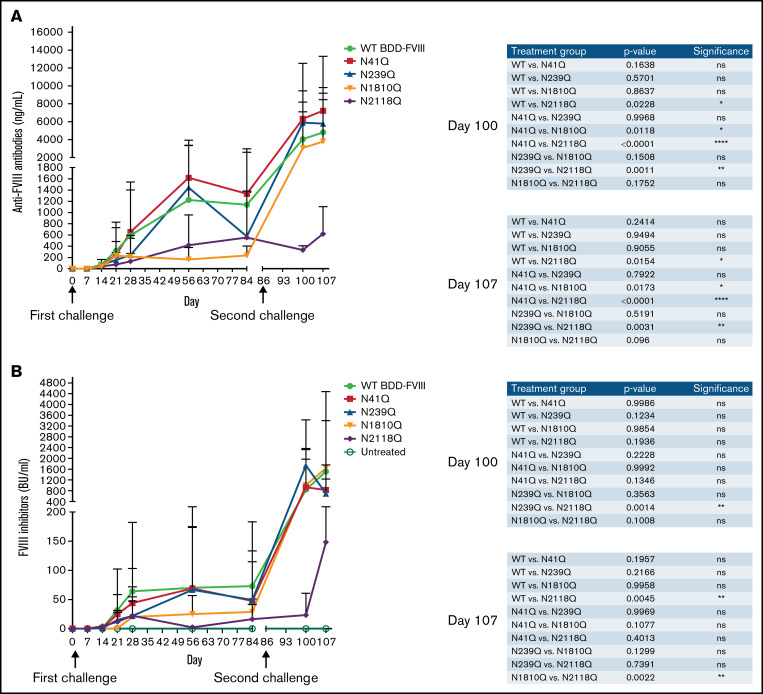
After confirming that our mutagenesis experiments to eliminate individual sites of glycosylation had not affected FVIII activity and stability in vivo and given the drop in FVIII activity by day 14, we explored the inhibitor responses in the treated mice. HA mice were injected hydrodynamically with WT BDD-FVIII plasmid or 1 of the 4 variant plasmids. Following plasmid transfer, anti-FVIII IgG, measured by ELISA, appeared at low levels (<100 ng/mL) in all injection groups by day 14 and then began to rise and peak around day 56, at which point some N1810Q and N2118Q groups had lower antibody levels compared with the other 3 groups. Subsequently, we performed a second plasmid challenge on day 84 via hydrodynamic injection. While all other groups experienced a drastic increase in anti-FVIII levels after the second challenge, N2118Q group did not (Figure 3A). Bethesda assays were also performed on the same samples and time points to examine the inhibitor responses (Figure 3B). These results parallels those of antibody responses, with N1810Q and N2118Q groups having lower inhibitor titers compared with other groups on day 56 and N2118Q group having significantly lower inhibitor titer after the second challenge. Taken together, these assays confirmed that when site N2118 is mutated to eliminate glycosylation, there is a decrease in inhibitor development compared with WT BDD-FVIII and the other N-glycosylation variants. It is also worth noting a trend that anti-FVIII antibody levels in N41Q and N239Q groups were higher than in the WT BDD-FVIII group.
Figure 3.
Inhibitor development in mice treated with plasmids carrying WT-BDD-FVIII and mutated BDD-FVIII variants. Groups of mice were injected hydrodynamically with plasmids encoding mutated BDD-FVIII variants and WT BDD-FVIII, respectively. Second challenges were performed via hydrodynamic injection in all groups on day 86. (A) FVIII-specific IgG levels were analyzed by ELISA. (B) Anti-FVIII inhibitor titers were measured using Bethesda assay. Arrows indicate the time of FVIII plasmid challenges. The comparison of inhibitor titers between different treatment groups at days 100 and 107 are shown in the right panel of each figure. WT BDD-FVIII: N = 16; N41Q: N = 7; N239Q: N = 6; N1810Q: N = 8; N2118Q: N = 6; untreated: N = 4. Experiments for each group were repeated at least 3 times. Data are presented as averages from repeated experiments, with error bars indicating standard deviation. The P values were calculated using 2-way analysis of variance among all plasmid treated groups. Untreated mice were used as negative controls.
Evaluation of the impact of N-glycosylation on the immunogenicity of FVIII following AAV-mediated gene delivery
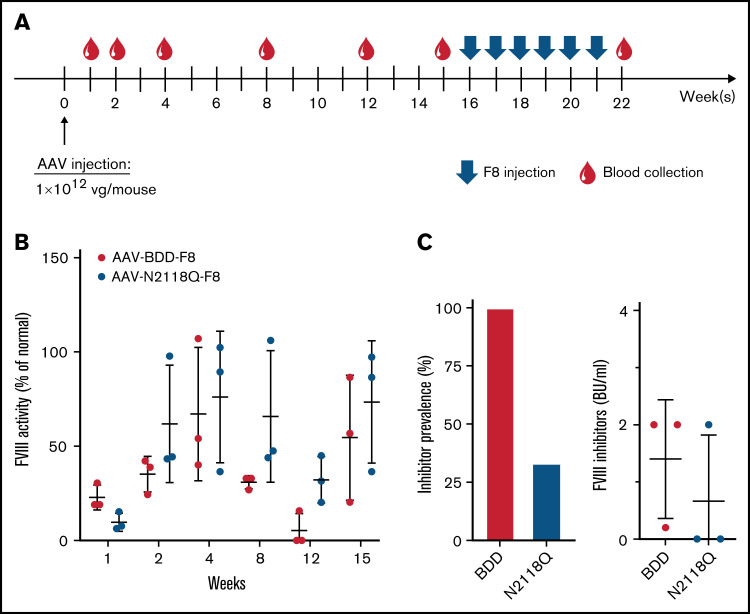
Groups of HA mice (n = 3/group) received AAV-BDD-FVIII and AAV-N2118Q-FVIII at a dosage of 1 × 1012 vg/mouse via intravenous injection (Figure 4A). FVIII activities were detected at week 1 and gradually increased to an average of ∼70% of normal plasma FVIII activity (100%) at week 4. In AAV-BDD-FVIII–treated mice, FVIII activities dropped to lower levels at week 8 and continued to decline to <10% (1 mouse) or undetectable levels (2 mice) at week 12 (Figure 4B). In the 2 mice with undetectable FVIII levels, we detected very low levels of anti-FVIII antibodies in ELISA but no inhibitor titers by Bethesda assay. In AAV-N2118Q-FVIII–treated mice, FVIII expression remained at similar levels at weeks 4 and 8 but moderately dropped to lower levels at week 12. Interestingly, both groups of mice had FVIII levels that reverted to higher levels at week 15, suggesting potential tolerance induction between weeks 12 and 15. When these experiments were repeated with a new batch of AAVs, the same trend was observed. Next, we challenged the groups of mice with 5 U of FVIII weekly for 6 weeks. One week after the final challenge, inhibitor titers were examined (Figure 4C). The prevalence of inhibitor development of AAV-BDD-FVIII– and AAV-N2118Q-FVIII–injected mice was 100% and 33%, respectively (Figure 4C), indicating that mice treated with AAV-N2118Q-FVIII were more tolerant to FVIII compared with mice treated with AAV-BDD-FVIII.
Figure 4.
Comparison of FVIII activity and inhibitor development following gene transfer of AAV carrying WT-BDD-FVIII or the mutated BDD-FVIII N2118Q variant. HA mice were intravenously injected with 1 × 1012 vg of AAV carrying WT-BDD-FVIII or the mutated FVIII N2118Q variant, respectively. (A) Schematic of the treatment and blood collection schedule. (B) The plasma was collected at marked timepoints for the detection of FVIII activity. Mice were subsequently challenged intravenously with 5 U of FVIII weekly for 6 weeks from weeks 16 to 21. (C) The prevalence of inhibitor development was calculated 1 week after final FVIII challenge. Mice with >0.6 BU inhibitor titer were considered inhibitor positive. The data are presented as means with standard deviation from 3 separate experiments.
Identification of a potential T-cell glycopeptide epitope surrounding site N2118 of FVIII
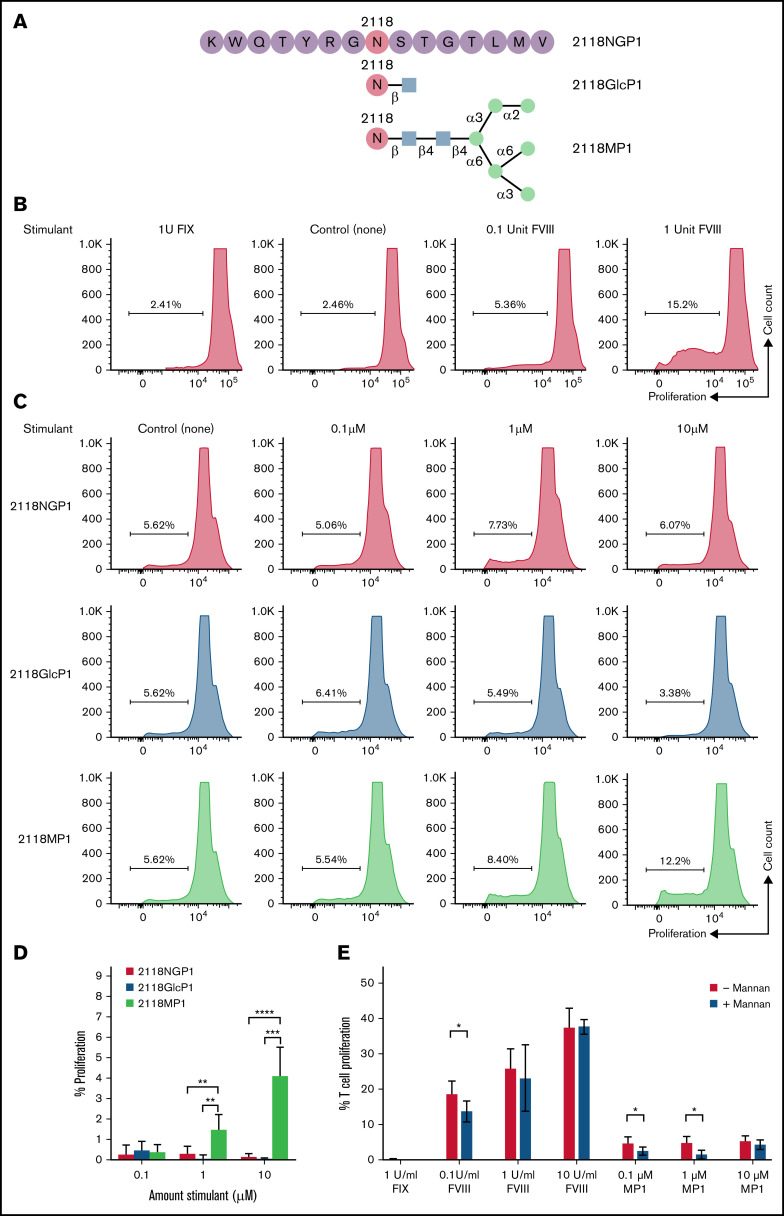
Given the reduced immune response in mice treated with the FVIII variant N2118Q seen in both the IgG ELISA and Bethesda assays, we wanted to explore the specific role of glycosylation at this site in interactions with the immune system. Because the development of inhibitors to FVIII is known to be a T-cell dependent process, we performed an in vitro proliferation experiment using a non-glycosylated peptide (NGP) (2118NGP1), a GlcNAc-attached peptide (2118GlcP1), and a mannosylated peptide (2118MP1) corresponding to the amino acid (aa) sequence around N2118 site of FVIII, with the asparagine located centrally within the 15-mer peptide (Peptide 1: K2111WQTYRGN2118STGTLMV2125), to investigate CD4+ T-cell responses (Figure 5A).
Figure 5.
CD4+ T-cell proliferation in response to FVIII glycosylated peptides. (A) A graphic representation of the synthetic 15 amino-acid peptides corresponding to N-glycosylation site 2118 was shown. A non-glycosylated peptide, 15 amino acids in length, centered around site N2118 (2118NGP1) was modified with the addition of either a single GlcNAc residue (2118GlcP1) or a high mannose glycan Man6GlcNAc2 (2118MP1). (B) CD4+ T-cell proliferation levels in response to 1 U of FIX as a non-specific control, no stimulant as the negative control, and increasing doses of rFVIII (0.1 and 1 U). (C) CD4+ T-cell proliferation rates measured after stimulation with 2118NGP1, 2118GlcP1, and 2118MP1 synthetic peptides, respectively. (D) Summary of the comparison of CD4+ T-cells proliferative rate after stimulation with different synthetic peptides from multiple experimental runs. The background was subtracted for each run. The data are presented as means with standard deviation from 3 separate experiments (**P < .01; ***P < .001; ****P < .0001). (E) CD4+ T-cell proliferation rates in response to FVIII and MP1 in the presence or absence of mannan, respectively (*P < .05).
These peptides were examined for their ability to induce FVIII-specific T-cell proliferation using splenocytes isolated from FVIII-primed HA mice with high titers of anti-FVIII IgGs. A dose-dependent increase was observed in response to increasing doses of FVIII in the FVIII-specific CD4+ T-cell proliferation assay, and no response was observed in the presence of factor IX (FIX) as a non-specific antigen control (Figure 5B). Having confirmed the FVIII specificity of cell proliferation, we then looked at CD4+ T-cell proliferation in response to stimulation with 2118NGP1, 2118GlcP1, or 2118MP1. Representative runs are shown in Figure 5C. Multiple runs were performed under the same conditions and the summary of data are shown in Figure 5D and supplemental Figure 2. In response to the increased doses of high mannose N2118 peptide (2118MP1) from 0.1 to 10 µM, CD4+ T cells showed increased levels of proliferation with 4.2 ± 1.3% at 10 µM, whereas 2118NGP1 and 2118GlcP1 had negligible effects on cell proliferation. These results indicated that the specific mannosylated peptide 2118MP1 may encompass a T-cell responsive glycopeptide epitope region of FVIII as opposed to the unglycosylated version.
Next, we performed mannan inhibition experiments to verify the effect of mannosylation of FVIII on induction of T-cell responses using the FVIII-specific T-cell proliferation assay. It was found that T-cell proliferation was only partially inhibited in response to 0.1 U/mL of FVIII, and no inhibition was observed at 1 or 10 U/mL of FVIII. However, mannan inhibited T-cell proliferation in response to all 3 concentrations of 2118MP1 (0.1, 1, and 1 µM) (Figure 5E). These data further confirmed that mannosylation at site 2118 is responsible for T-cell activation.
Screening of glycopeptide epitope surrounding N2118 using overlapping peptides
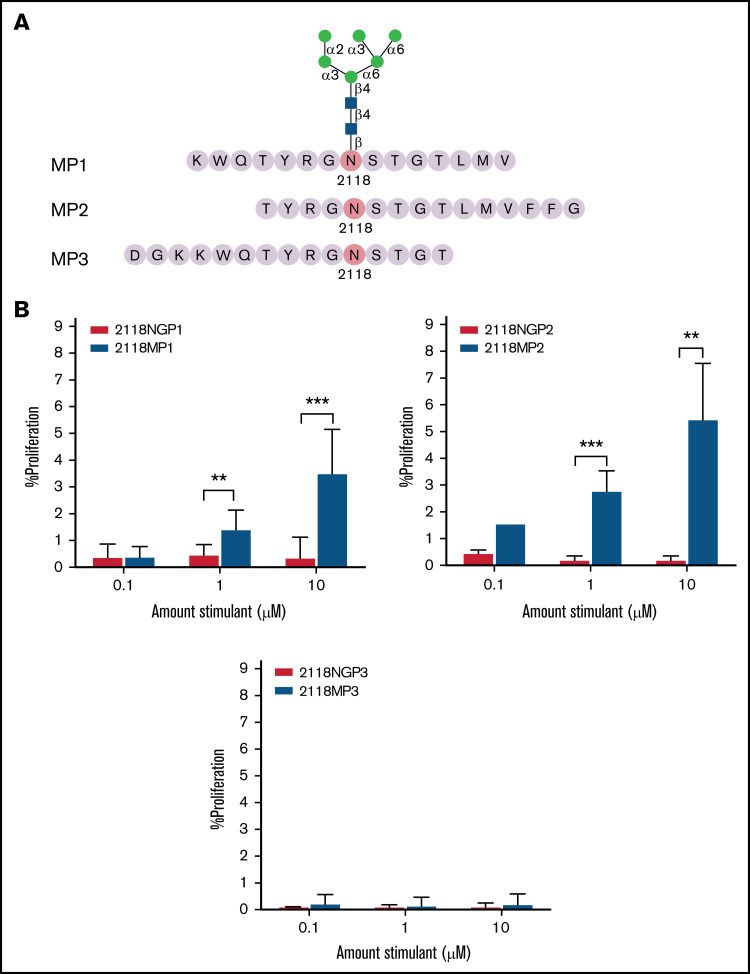
To further narrow down the region of FVIII responsible for eliciting an increased immune response in the presence of a mannosylated site N2118, we synthesized overlapping non-glycosylated and glycosylated (Man6GlcNAc2) peptides with site N2118 shifted toward either the N- or C-terminus of a 15-mer peptide (Peptide 2 (2118MP2, 2118NGP2): T2114YRGN2118 STGTLMVFFG2128; Peptide 3 (2118MP3, 2118NGP3): D2108 GKKWQTYRGN2118STGT2122). These peptides were analyzed to compare proliferation with mannosylated peptide 2118MP1 and non-glycosylated peptide 2118NGP1 (K2111WQTYRGN2118 STGTLMV2125), where the N-glycosylation site is centrally located (Figure 6A). Compared with 2118MP1 with enhanced proliferative CD4+ T-cell responses relative to its nonglycosylated counterpart 2118NGP1 (Figure 6B; supplemental Figure 3, top left panel), stimulation with 2118MP2 resulted in even higher levels of CD4+ T-cell proliferation than with 2118MP1, at 2.9 ± 0.6% at 1 µM and 5.5 ± 2.0% at 10 µM, and with significantly increased proliferation in comparison with its non-glycosylated counterpart 2118NGP2 (Figure 6B; supplemental Figure 3, top right panel). On the other hand, 2118MP3 showed little to no proliferation in comparison with the other mannosylated peptides and its non-glycosylated counterpart 2118NGP3 (Figure 6B; supplemental Figure 3, bottom panel). Proliferation of CD4+ T cells in the presence of all 3 NGPs was either at or near background levels. These data indicated that the essential sequence of the potential T-cell glycopeptide epitope region is located in the overlapping region of 2118MP1 and 2118MP2 (Figure 7).
Figure 6.
CD4+ T-cell proliferation in response to overlapping mannosylated peptides. (A) Peptides, 15 amino acids in length, overlapping with 2118MP1 were synthesized with high-mannose glycan Man6GlcNAc2 attachments. (B) CD4+ T-cell proliferation levels were measured in response to mannosylated peptides MP1 (top left panel), MP2 (top right panel), and MP3 (bottom panel) and their non-glycosylated counterparts (NGP1, NGP2, and NGP3). The data are presented as means with standard deviation from 3 separate experiments (**P < .01; ***P < .001).
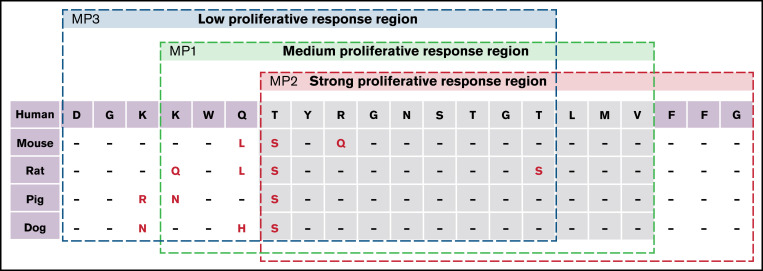
Figure 7.
Homology of FVIII peptides representative of site N2118. The amino acid sequences of 2118MP1, 2118MP2, and 2118MP3 were compared between the homologous sequences in human, mouse, rat, pig, and dog species.
Discussion
Posttranslational modifications of proteins, such as glycosylation, and the possible effects on the immune response in HA have increased in interest in recent years. Recent studies have determined the location of glycosylation sites and the composition of glycan structures in different FVIII products.8-10 Sialic acids as the terminal sugar may act as protective sugar moieties from mounting immune responses whereas high-mannose structures may act as immunogenic moieties.11,14,15 Given that the largest barrier to successful FVIII treatment remains inhibitor development in patients, we were prompted to investigate the roles of specific sites of N-glycosylation on FVIII immunogenicity through the removal and addition of glycans in an HA mouse model.
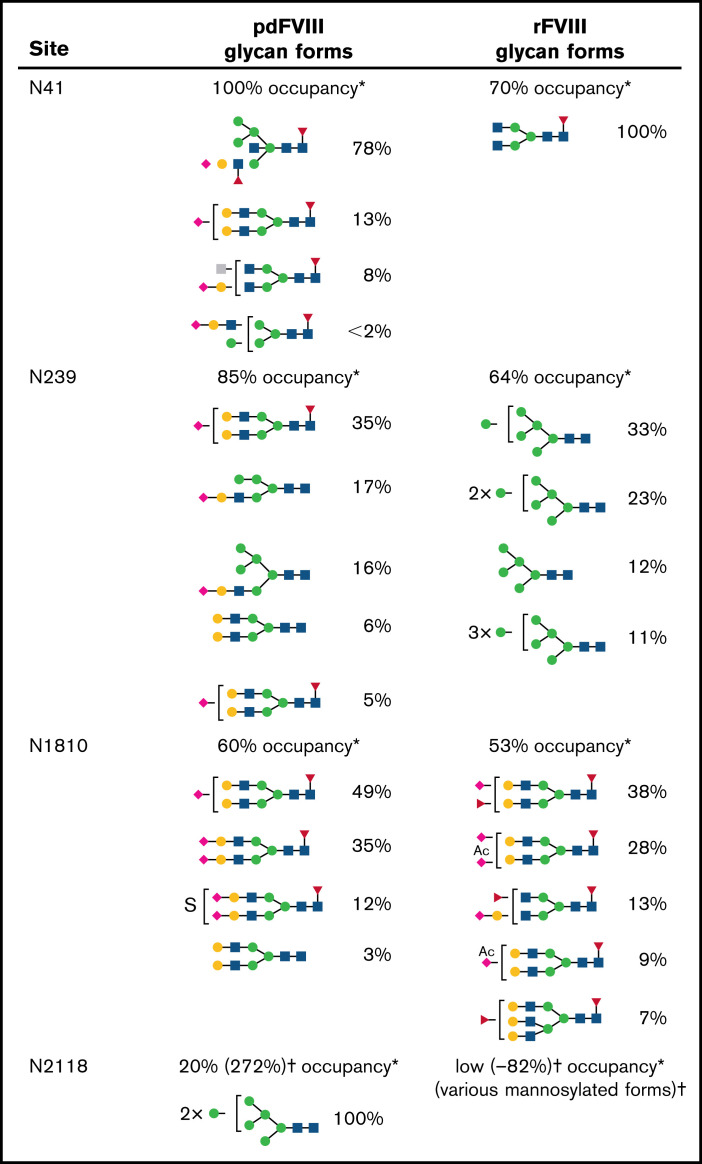
N-linked glycosylation occurs in the presence of an N-X-Serine (S)/Threonine (T) (where X is not proline) consensus sequence and is the most common form of glycosylation in human cells.20 There are 3 classes of N-linked glycans: complex type, high-mannose type, and hybrid type.20 Five consensus N-X-S/T sequences in FVIII have been identified outside of the B domain,8-10 including N41 and N239 in the A1 domain, N582 in the A2 domain, N1810 in the A3 domain, and N2118 in the C1 domain (Figure 1). However, no glycosylation was detected on N582 in either pdFVIII or rFVIII.8,10 Detailed analysis10 by our group illustrated a comparison of the percentages of various types of N-glycans on pdFVIII and rFVIII (Figure 8), revealing dramatic differences in the 4 N-glycosylation sites outside the B domain between pdFVIII and rFVIII. Most notably, in pdFVIII, N2118 was occupied by high-mannose glycans, whereas N41, N139, and N1810 sites were occupied mostly by sialylated complex- and hybrid-type glycoforms. On the other hand, in rFVIII, few high-mannose glycans were found in N2118 whereas in N239 only high-mannose glycans were found. It should also be noted that all 4 N-glycosylation sites are surface-exposed residues and thus able to interact with immune moieties in vivo.21,22
Figure 8.
Glycan form by percent occupancy. *The percentage listed next to each glycan form represents how often this glycan was detected at that site. Glycan forms at each glycosylation site were previously evaluated by mass spectrometry. Glycan forms for rFVIII were isolated from Kogenate FS (baby hamster kidney-cell derived) (Bayer AG) and pdFVIII glycan forms from FVIIIenriched cryoprecipitate obtained from Shanghai Lai Shi Blood Products Co., Ltd (Shanghai, China). The experiments and analyses were performed at Georgia State University.10 †For N2118 site, variable occupancies of mannosylation of this site have been reported.6,9,10
 N-acetylglucosamine (GlcNAc);
N-acetylglucosamine (GlcNAc);  N-acetylhexosamine (HexNAc);
N-acetylhexosamine (HexNAc);  Mannose (Man);
Mannose (Man);  Galactose (Gal);
Galactose (Gal);  N-acetylneuraminic acid (Neu5Ac);
N-acetylneuraminic acid (Neu5Ac);  L-Fucose; Ac, acetyl; S, sulfate.
L-Fucose; Ac, acetyl; S, sulfate.
Glycosylation of proteins can not only influence its interactions with immune cells but also contribute to the stability and function. We first examined if the mutagenesis of sites N41, N239, N1810, and N2118 from N to Q would affect FVIII activity and/or accelerate an immune response and FVIII clearance. The homologous Q residue is chosen because it is never glycosylated due to the requirement of the special N-X-S/T structure to form N/d-turn conformation to undergo glycosylation.23 It was reported that fully deglycosylated FVIII exhibited significantly decreased activity as well as decreased interactions with phosphatidylserine-containing membranes.24 Nevertheless, previous reports showed that a single mutation of these 4 N-glycosylation sites either decreased or maintained the same antigen levels and activity of FVIII.25-28 In our study, following delivery of plasmids carrying the WT BDD-FVIII and the 4 glycosylation variants, mice produced similar levels of FVIII expression and specific activities, based on aPTT and FVIII:Ag ELISA data. These results indicated that the removal of each of these 4 N-glycosylation sites, respectively, likely does not result in significant conformational changes that would affect protein activity/stability in these in vivo mouse models. These gene therapy–treated mice provided us the opportunity to focus on examining the impact of glycosylation on FVIII immunogenicity.
Given that anti-FVIII inhibitor development is the biggest obstacle standing in the way of successful FVIII treatment in patients, it is important to consider the impact that posttranslational modifications such as glycosylation may have upon its immunogenicity. As shown in the results, mice treated with plasmids carrying WT BDD-FVIII and the 4 glycosylation variants all developed anti-FVIII inhibitors. Following first plasmid challenge, groups of mice treated with N1810Q and N2118Q showed a trend of decreased inhibitor responses to FVIII compared with mice treated with WT BDD-FVIII plasmid, whereas no significant difference in inhibitor responses was observed among N41Q, N239Q, and WT BDD-FVIII plasmid-treated mice. Subsequently, we treated each group of mice with the same plasmid a second time on day 84. Mice treated with N2118Q showed statistically significant lower inhibitor responses compared with WT BDD-FVIII, whereas N41Q, N139Q, and N1810Q groups had slightly increased or comparable responses with the WT BDD-FVIII group. The reduction of inhibitor response by the N2118Q FVIII variant is also demonstrated in AAV-mediated gene transfer experiments. As shown in Figure 8, the glycoforms detected at site N2118 were all high-mannose–type glycans, whereas N41, N239, and N1810 sites were occupied 60% to 100% with complex- and hybrid-type glycans with mostly terminal sialic acids. It is striking that high-mannose glycans on site N2118 had such a significant impact on the immunogenicity of FVIII. It is also noteworthy that compared with pdFVIII, N41 lost the terminal sialic acid and N239 changed from complex- and hybrid-type sialylated glycans to high-mannose glycans in rFVIII (Figure 8). Whether these changes contribute to the higher immunogenicity of rFVIII warrants further investigation. Furthermore, the N2118Q variant could be used as a more tolerogenic FVIII compared with WT BDD-FVIII.
High-mannose glycans were postulated to facilitate the uptake of FVIII via the mannose receptors on dendritic cells and macrophages27,29; however, a controversial study showed conflicting data.30 We studied mannan inhibition in a T-cell proliferation assay. Two steps could be involved in mannan inhibition; the first step is the entry of FVIII with mannosylated residues into APCs involving mannose receptors, and the second step is the interaction of mannosylated peptides presented on APCs with the T-cell receptor. It was shown that mannan only partially inhibited the proliferation rate in response to FVIII at low FVIII concentrations but not at higher FVIII concentrations, indicating that another major pathway governs the uptake of FVIII by APCs in mice, consistent with previous studies.30,31 Whether this is the same in humans needs further investigation. While glycans can affect the immunogenicity of proteins by presenting as a glycoepitope,32 glycans can also shield other epitopes from T cells33 and antibodies,34 regulate presentation of neighboring epitopes,35 or be a part of a specific peptide epitope (glycopeptide epitope).36 Due to the significant impact of N2118Q mutation on FVIII immunogenicity in our gene therapy–treated HA mice, we considered the possibility of a potential glyco or glycopeptide epitope around the N2118 site. To test this hypothesis, three 15-mer peptides centered around N2118 (K2111WQTYRGN2118STGTLMV2125) were synthesized with or without GlcNAc or Man6GlcNAc2 attached, respectively. The highly mannosylated (Man6GlcNAc2) peptide 2118MP1 showed significant stimulatory effect of FVIII-specific T-cell responses, whereas the non-glycosylated 2118NGP1 and GlcNAc-attached 2118GlcP1 showed no stimulation. Next, we made additional overlapping peptides and found a strong stimulatory mannosylated peptide 2118MP2 (T2114YRGN2118STGTLMVFFG2128) with FVIII-specific T-cell responses and a non-stimulatory mannosylated peptide 2118MP3 (D2108GKKWQTYRGN2118STGT2122). Notably, none of the non-glycosylated versions of these peptides showed any stimulatory effect in the FVIII-specific proliferation assay. In addition, 2118MP2 encompasses the most conserved region among FVIII derived from different species, whereas 2118MP3 encompasses the least conserved region (Figure 7). These results together suggest a potent T-cell–specific glycopeptide epitope between K2111 and G2128 of FVIII. Thus, deletion of glycans at the N2118 site can significantly reduce the immunogenicity of FVIII due to the elimination of a potent glycopeptide epitope that can present antigens and interact with and activate T cells. These results also indicate that there is a specific glycopeptide epitope rather than a general glycoepitope surrounding the N2118 glycosylation site. Detailed investigation is currently ongoing to further characterize this specific glycopeptide epitope.
It would be helpful to analyze the FVIII glycoforms in gene therapy–treated HA mice because the glycosyltransferases present in mouse liver may be different from what is present in cells that produce FVIII in humans. However, this is limited by the difficulty to purify small quantities of mouse proteins. Furthermore, in addition to T-cell activation, several innate-like B-cell populations, including marginal zone macrophages and marginal zone B cells, have been shown to regulate early inhibitor development.37,38 Recent studies39-41 showed that antibodies may influence de novo antibody formation following exposure to distinct FVIII proteins. FVIII glycosylation may also influence the innate-like B-cell recognition of these glycan epitopes for the initiation of an antibody response,42 especially when considering that naturally occurring antibodies may facilitate inhibitor formation as was recently shown with xenoglycans.43 These studies, in addition to the present work, provide an understanding of how FVIII glycosylation may influence antibody formation against FVIII in a variety of contexts.
In conclusion, this study examined the impact of posttranslational modifications, specifically N-glycosylation, on the immunogenicity of FVIII synthesized in vivo following gene transfer of FVIII plasmids. Four N-glycosylation sites outside the B domain were examined. Three sites with predominantly sialylated complex-type or hybrid-type glycans did not significantly alter the immunogenicity of FVIII, whereas site N2118 which contains high-mannose glycans showed significant impact on FVIII immunogenicity. A potent T-cell–specific glycopeptide epitope surrounding site N2118 was identified and characterized in FVIII for the first time. These results can enhance the understanding of inhibitory antibody formation against FVIII and facilitate the development of a more tolerant FVIII molecule for replacement protein and gene therapy for treatment of HA.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (grant U54 HL142019) (W.X., L.L., and C.H.M.).
Authorship
Contribution: A.V.K. designed and performed experiments, analyzed results, and wrote the manuscript; S.W. synthesized the mannosylated glycopeptides; M.-N.F., A.C., J.Z., C.-Y.C., and X.C. performed experiments; W.X., B.A.K., and L.L. helped in the discussion and edited the manuscript; and C.H.M. conceived and supervised the project, analyzed results, and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carol H. Miao, Seattle Children’s Research Institute, 1900 9th Ave, Seattle, WA 98101; e-mail: carol.miao@seattlechildrens.org.
References
- 1.Darby SC, Keeling DM, Spooner RJ, et al. ; UK Haemophilia Centre Doctors’ Organisation . The incidence of factor VIII and factor IX inhibitors in the hemophilia population of the UK and their effect on subsequent mortality, 1977-99. J Thromb Haemost. 2004;2(7):1047-1054. [DOI] [PubMed] [Google Scholar]
- 2.Hoyer LW, Scandella D. Factor VIII inhibitors: structure and function in autoantibody and hemophilia A patients. Semin Hematol. 1994;31(2 suppl 4):1-5. [PubMed] [Google Scholar]
- 3.Lacroix-Desmazes S, Voorberg J, Lillicrap D, Scott DW, Pratt KP. Tolerating factor VIII: recent progress. Front Immunol. 2020;10:2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai J, Hough C, Tarrant J, Lillicrap D. Biological considerations of plasma-derived and recombinant factor VIII immunogenicity. Blood. 2017;129(24):3147-3154. [DOI] [PubMed] [Google Scholar]
- 5.Peyvandi F, Cannavò A, Garagiola I, Palla R, Mannucci PM, Rosendaal FR; SIPPET Study Group . Timing and severity of inhibitor development in recombinant versus plasma-derived factor VIII concentrates: a SIPPET analysis. J Thromb Haemost. 2018;16(1):39-43. [DOI] [PubMed] [Google Scholar]
- 6.Lai JD, Swystun LL, Cartier D, et al. N-linked glycosylation modulates the immunogenicity of recombinant human factor VIII in hemophilia A mice. Haematologica. 2018;103(11):1925-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnamoorthy S, Liu T, Drager D, et al. Recombinant factor VIII Fc (rFVIIIFc) fusion protein reduces immunogenicity and induces tolerance in hemophilia A mice. Cell Immunol. 2016;301:30-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannicht C, Ramström M, Kohla G, et al. Characterisation of the post-translational modifications of a novel, human cell line-derived recombinant human factor VIII. Thromb Res. 2013;131(1):78-88. [DOI] [PubMed] [Google Scholar]
- 9.Canis K, Anzengruber J, Garenaux E, et al. In-depth comparison of N-glycosylation of human plasma-derived factor VIII and different recombinant products: from structure to clinical implications. J Thromb Haemost. 2018;16(8):1592-1603. [DOI] [PubMed] [Google Scholar]
- 10.Qu J, Ma C, Xu XQ, et al. Comparative glycosylation mapping of plasma-derived and recombinant human factor VIII. PLoS One. 2020;15(5):e0233576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perdicchio M, Ilarregui JM, Verstege MI, et al. Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells. Proc Natl Acad Sci USA. 2016;113(12):3329-3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pegon JN, Kurdi M, Casari C, et al. Factor VIII and von Willebrand factor are ligands for the carbohydrate-receptor Siglec-5. Haematologica. 2012;97(12):1855-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bovenschen N, Rijken DC, Havekes LM, van Vlijmen BJ, Mertens K. The B domain of coagulation factor VIII interacts with the asialoglycoprotein receptor. J Thromb Haemost. 2005;3(6):1257-1265. [DOI] [PubMed] [Google Scholar]
- 14.Dasgupta S, Navarrete AM, Bayry J, et al. A role for exposed mannosylations in presentation of human therapeutic self-proteins to CD4+ T lymphocytes. Proc Natl Acad Sci USA. 2007;104(21):8965-8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira MS, Alves I, Vicente M, et al. Glycans as key checkpoints of T cell activity and function. Front Immunol. 2018;9:2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miao CH. A novel gene expression system: non-viral gene transfer for hemophilia as model systems. Adv Genet. 2005;54:143-177. [DOI] [PubMed] [Google Scholar]
- 17.Chen AC, Cai X, Li C, Khoryati L, Gavin MA, Miao CH. A Treg-selective IL-2 mutein prevents the formation of factor VIII inhibitors in hemophilia mice treated with factor VIII gene therapy. Front Immunol. 2020;11:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CY, Tran DM, Cavedon A, et al. Treatment of hemophilia A using factor VIII messenger RNA lipid nanoparticles. Mol Ther Nucleic Acids. 2020;20:534-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umekawa M, Huang W, Li B, et al. Mutants of Mucor hiemalis endo-beta-N-acetylglucosaminidase show enhanced transglycosylation and glycosynthase-like activities. J Biol Chem. 2008;283(8):4469-4479. [DOI] [PubMed] [Google Scholar]
- 20.Lyons JJ, Milner JD, Rosenzweig SD. Glycans Instructing immunity: the emerging role of altered glycosylation in clinical immunology. Front Pediatr. 2015;3:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen BW, Spiegel PC, Chang CH, et al. The tertiary structure and domain organization of coagulation factor VIII. Blood. 2008;111(3):1240-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoilova-McPhie S, Villoutreix BO, Mertens K, Kemball-Cook G, Holzenburg A. 3-Dimensional structure of membrane-bound coagulation factor VIII: modeling of the factor VIII heterodimer within a 3-dimensional density map derived by electron crystallography. Blood. 2002;99(4):1215-1223. [DOI] [PubMed] [Google Scholar]
- 23.Imperiali B, O’Connor SE. Effect of N-linked glycosylation on glycopeptide and glycoprotein structure. Curr Opin Chem Biol. 1999;3(6):643-649. [DOI] [PubMed] [Google Scholar]
- 24.Kosloski MP, Miclea RD, Balu-Iyer SV. Role of glycosylation in conformational stability, activity, macromolecular interaction and immunogenicity of recombinant human factor VIII. AAPS J. 2009;11(3):424-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei W, Misra S, Cannon MV, et al. Molecular mechanisms of missense mutations that generate ectopic N-glycosylation sites in coagulation factor VIII. Biochem J. 2018;475(5):873-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selvaraj SR, Miao H, Pipe S. Elucidation of the roles of individual asparagine-linked glycans outside of the B domain on factor VIII secretion. Blood. 2011;118(21):2238. [Google Scholar]
- 27.Delignat S, Rayes J, Dasgupta S, et al. Removal of mannose-ending glycan at Asn2118 abrogates FVIII presentation by human monocyte-derived dendritic cells. Front Immunol. 2020;11:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito J, Baldwin WH, Cox C, et al. Removal of single-site N-linked glycans on factor VIII alters binding of domain-specific monoclonal antibodies. J Thromb Haemost. 2022;20(3):574-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Repessé Y, Dasgupta S, Navarrete AM, Delignat S, Kaveri SV, Lacroix-Desmazes S. Mannose-sensitive receptors mediate the uptake of factor VIII therapeutics by human dendritic cells. J Allergy Clin Immunol. 2012;129(4):1172-1175. [DOI] [PubMed] [Google Scholar]
- 30.Herczenik E, van Haren SD, Wroblewska A, et al. . Uptake of blood coagulation factor VIII by dendritic cells is mediated via its C1 domain. J Allergy Clin Immunol. 2012;129(2):501-509. [DOI] [PubMed] [Google Scholar]
- 31.Delignat S, Repessé Y, Navarrete AM, et al. Immunoprotective effect of von Willebrand factor towards therapeutic factor VIII in experimental haemophilia A. Haemophilia. 2012;18(2):248-254. [DOI] [PubMed] [Google Scholar]
- 32.Wang D. Glyco-epitope diversity: an evolving area of glycomics research and biomarker discovery. J Proteomics Bioinform. 2014;7(2):23539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gram AM, Oosenbrug T, Lindenbergh MF, et al. The Epstein-Barr virus glycoprotein gp150 forms an immune-evasive glycan shield at the surface of infected cells. PLoS Pathog. 2016;12(4):e1005550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavie M, Hanoulle X, Dubuisson J. Glycan shielding and modulation of hepatitis C virus neutralizing antibodies. Front Immunol. 2018;9:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Xu CF, Blais S, et al. Proximal glycans outside of the epitopes regulate the presentation of HIV-1 envelope gp120 helper epitopes. J Immunol. 2009;182(10):6369-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun L, Paschall AV, Middleton DR, et al. Glycopeptide epitope facilitates HIV-1 envelope specific humoral immune responses by eliciting T cell help. Nat Commun. 2020;11(1):2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navarrete A, Dasgupta S, Delignat S, et al. Splenic marginal zone antigen-presenting cells are critical for the primary allo-immune response to therapeutic factor VIII in hemophilia A. J Thromb Haemost. 2009;7(11):1816-1823. [DOI] [PubMed] [Google Scholar]
- 38.Zerra PE, Cox C, Baldwin WH, et al. Marginal zone B cells are critical to factor VIII inhibitor formation in mice with hemophilia A. Blood. 2017;130(23):2559-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batsuli G, Ito J, Mercer R, et al. Anti-C1 domain antibodies that accelerate factor VIII clearance contribute to antibody pathogenicity in a murine hemophilia A model. J Thromb Haemost. 2018;16(9):1779-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartholt RB, Wroblewska A, Herczenik E, et al. Enhanced uptake of blood coagulation factor VIII containing immune complexes by antigen presenting cells. J Thromb Haemost. 2017;15(2):329-340. [DOI] [PubMed] [Google Scholar]
- 41.Vollack N, Friese J, Bergmann S, Cragg MS, Tiede A, Werwitzke S. Anti-FcγRIIB (CD32) antibodies differentially modulate murine FVIII-specific recall response in vitro. Scand J Immunol. 2017;86(2):91-99. [DOI] [PubMed] [Google Scholar]
- 42.Zerra PE, Arthur CM, Chonat S, et al. Fc gamma receptors and complement component 3 facilitate anti-fVIII antibody formation. Front Immunol. 2020;11:905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arthur CM, Zerra PE, Shin S, et al. Nonhuman glycans can regulate anti-factor VIII antibody formation in mice. Blood. 2022;139(9):1312-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.