Abstract
Aim:
Clinicopathologic characterization of contemporary series of neuroendocrine (NE) differentiation in the setting of prostatic carcinoma (PCa).
Methods & Results:
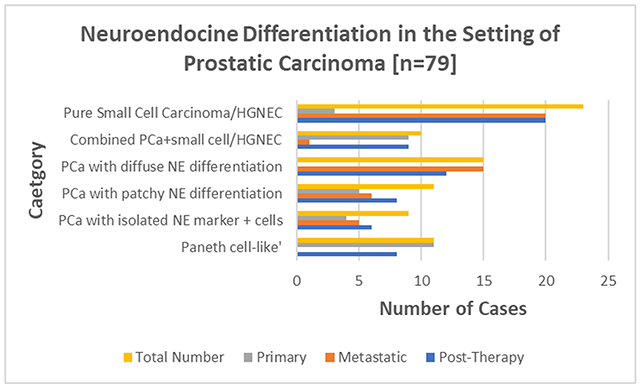
We reviewed institutional databases for in-house cases with history of PCa and histopathologic evidence of NE differentiation during the disease course. 79 cases identified: 32 primary and 47 metastases. Metastatic lesions were in liver [n=15], lymph node [n=9], bone [n=6], lung [n=3], brain [n=1], other sites [n=13]. 63 of 76 (82%) cases with NE differentiation and available history were post-therapy: 6 post-radiation therapy (RT), 24 post- androgen–deprivation therapy (ADT) and 33 post-RT+ADT. Morphologic assessment [n=79]: a. 23 pure small cell/high-grade NE carcinoma (HGNEC): 20/23 metastatic; b. 10 combined high-grade PCa and small cell/HGNEC: 9/10 primary; c. 15 PCa with diffuse NE immunohistochemistry (IHC) marker positivity/differentiation, associated with nested to sheet-like growth of cells with abundant cytoplasm and prominent nucleoli, yet diffuse positivity for at least one prostatic and one NE IHC marker: all metastatic; d. 11 PCa with patchy NE differentiation, displaying more than single cell positivity for NE IHC: 5 primary / 6 metastatic; e. 9 PCa with focal NE marker positive cells: 4 primary / 5 metastatic; f. 11 PCa with ‘Paneth cell-like’ change: all primary.
Conclusions:
In this contemporary series, the majority of NE differentiation in the setting of PCa was seen post-therapy. We highlight tendencies of small cell/HGNEC and PCa with diffuse NE differentiation by IHC to occur in metastatic settings, while morphologically combined high grade PCa+small cell/HGNEC and ‘Paneth cell-like’ change occur in primary disease.
Keywords: prostate, neuroendocrine, small cell
Graphical Abstract
Introduction
Widespread adoption of next generation androgen deprivation therapy (ADT) for the oncologic management of metastatic and more recently, localized high-risk prostatic carcinoma (PCa), has increased treatment efficacy but led to emergence of treatment-resistant phenotypes1–5. In metastatic castration resistant PCa, prolonged androgen receptor (AR) pathway inhibition can induce lineage switching or trans-differentiation1, resulting in histologic differentiation to neuroendocrine (NE) phenotypes2–3. This clinically termed, ‘treatment-emergent small cell NE prostate cancer’, ‘NE prostate cancer’ or ‘aggressive variant’ has been reported in approximately 15% of cases3–5, in contrast to a reported incidence of <1% for de-novo small cell carcinoma in the setting of usual PCa6–7. A recent multi-institutional prospective study of post-treatment metastatic castrate resistant PCa has reported wide variability in AR transcriptional activity/protein expression, outcomes, and histological features, with some tumors displaying morphology of usual high grade acinar PCa and others having a spectrum of features suggestive of NE differentiation3, 8.
In parallel with this body of evidence, there have been attempts to refine histopathologic classification of NE differentiation in the setting of PCa. The two best recognized proposals are those set forth by a 2013 Prostate Cancer Foundation working group9, and by the 2016 World Health Organization classification of genitourinary tumors, which have significant overlap10. Despite providing a framework, some manifestations continue to require elucidation, including tumors with indeterminate morphological features that do not fall neatly into binary categories of adenocarcinoma or small cell/NE carcinoma or that have variable NE marker expression, as well as PCa with so-called ‘Paneth cell-like neuroendocrine’ differentiation, a subset for which existing nomenclature may not best reflect the characteristics of the named cells. In this study, we report a consecutive case series of NE differentiation in the setting of PCa with detailed histopathologic characterization and annotation of therapeutic history.
Methods
We searched institutional databases (spanning a 5.5-year period) for patients with in-house tissue sampling, diagnosed with PCa and reported to have any form of NE differentiation at any time during their disease course. All available slides and charts were reviewed, including treatment history. Immunohistochemical (IHC) stains performed at the time of initial work-up were reviewed, when available, but no additional IHC staining was performed. This study was approved by our Institutional Review Board.
At the time of diagnosis, all cases were categorized based on morphology and available IHC markers into one of the following groups:
Small cell carcinoma/high grade neuroendocrine carcinoma (HGNEC): as classically described in other organs11–13 [Figure 1]
Combined high grade PCa + HGNEC/small cell carcinoma: in which there was typically an abrupt transition between the components [Figure 2]
PCa with diffuse neuroendocrine marker positivity/differentiation: nested to sheet-like growth of cells with abundant cytoplasm and prominent nucleoli, yet diffuse positivity for at least one prostatic and one NE IHC marker [Figure 3], distinguishing these carcinomas from typical small cell carcinoma. Cytoplasm ranged from pale to eosinophilic to amphophilic, with some cases displaying occasional mitoses or apoptotic debris. A few cases showed foci with vague microacinar or ‘rosette-like’ appearance in a background of predominant nested and/or sheet-like architecture.
PCa with patchy neuroendocrine differentiation: adenocarcinoma histology displaying more than scattered single cell positivity for NE IHC marker(s) [Figure 4]
-
PCa with isolated neuroendocrine marker positive cells: adenocarcinoma histology displaying scattered rare or isolated single cell positivity for NE IHC marker(s)
IHC in categories 3, 4, and 5 was performed due to architectural features +/− cytoplasmic alterations (amphophilic or basophilic, granular cytoplasm) suggestive of NE features or by clinician request due to unusual clinical features or suboptimal response to ADT.
PCa with prominent neuroendocrine granules (so-called ‘Paneth cell-like change’): adenocarcinoma with a variable number of cells displaying bland nuclei and coarse eosinophilic cytoplasmic granules present in usual acinar architecture or in cords / small clusters admixed with adenocarcinoma
Figure 1.
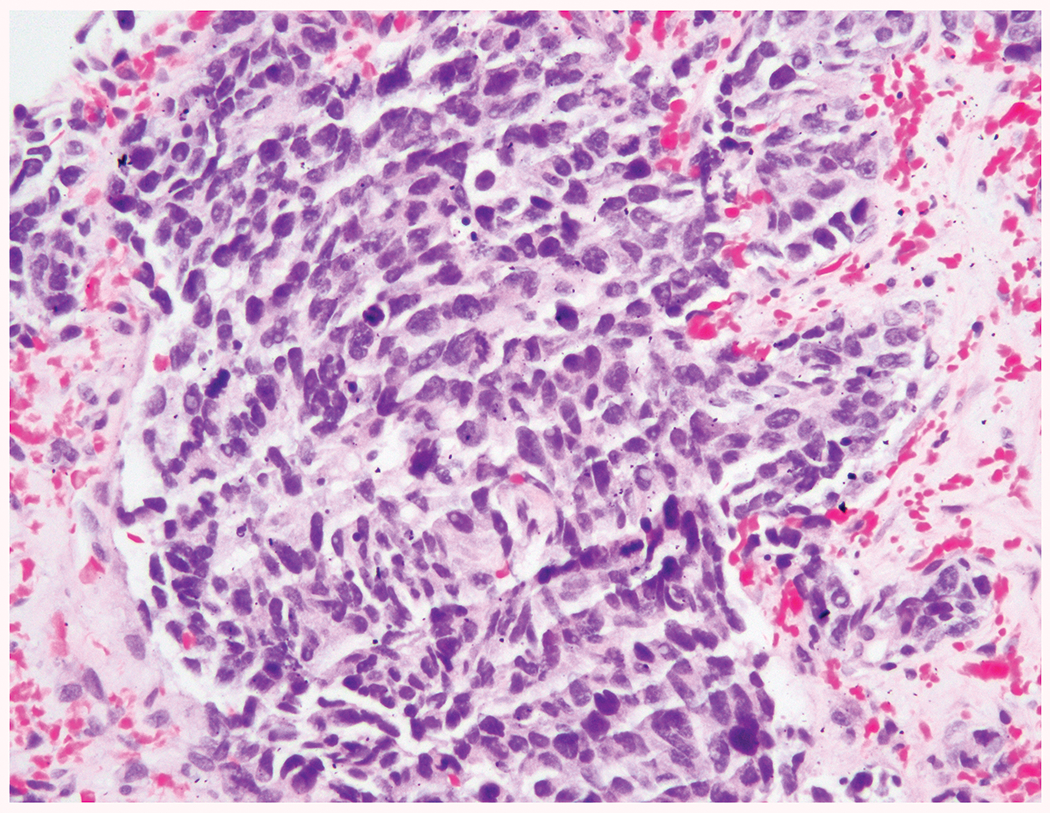
Small cell/ high grade neuroendocrine carcinoma at 20x magnification demonstrating high N-C ratio, hyperchromatic nuclei with nuclear molding and indistinct nucleoli and a high mitotic and apoptotic rate
Figure 2:
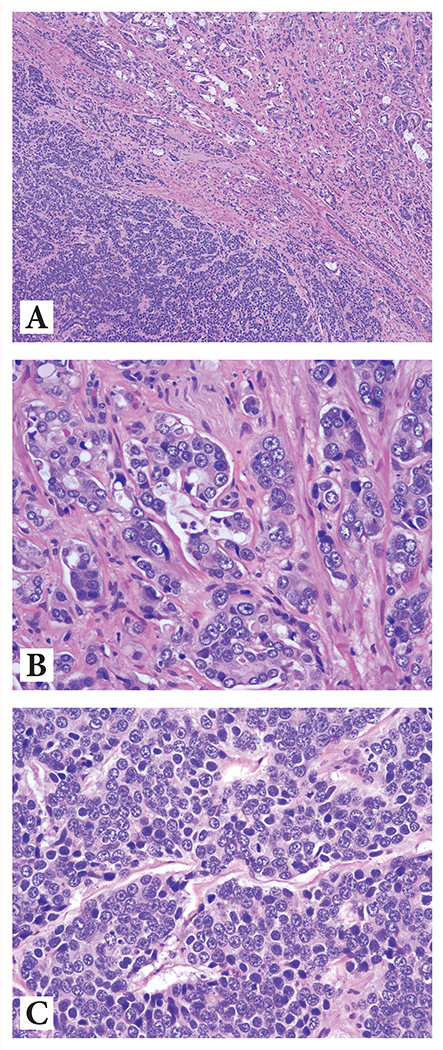
Combined (mixed) high grade prostatic adenocarcinoma and high-grade neuroendocrine carcinoma showing:
(A) Abrupt transition between the two components at 4x magnification
(B) Acinar adenocarcinoma component at 20x magnification highlighting intraluminal mucin, pale eosinophilic cytoplasm, and macronucleoli
(C) High-grade NE carcinoma component at 20x magnification highlighting basophilic cytoplasm, ‘salt and pepper’ nuclear chromatin, and 1-3 indistinct nucleoli
Figure 3:
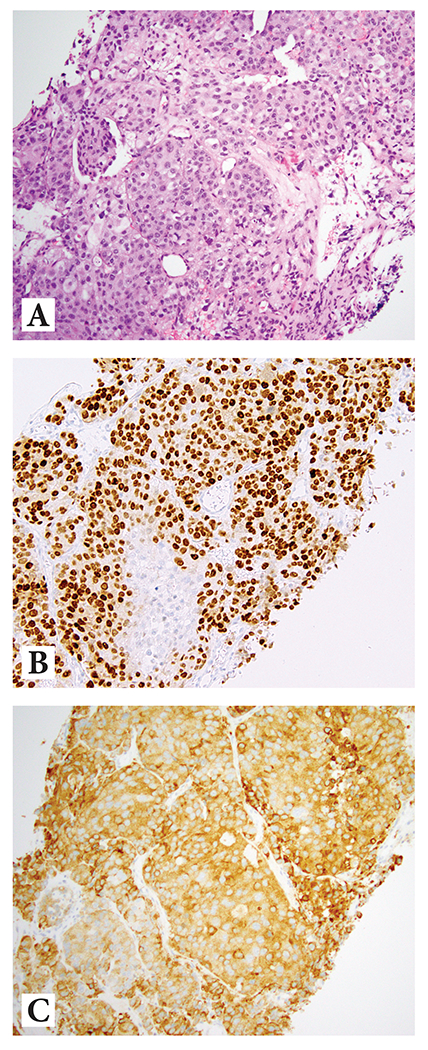
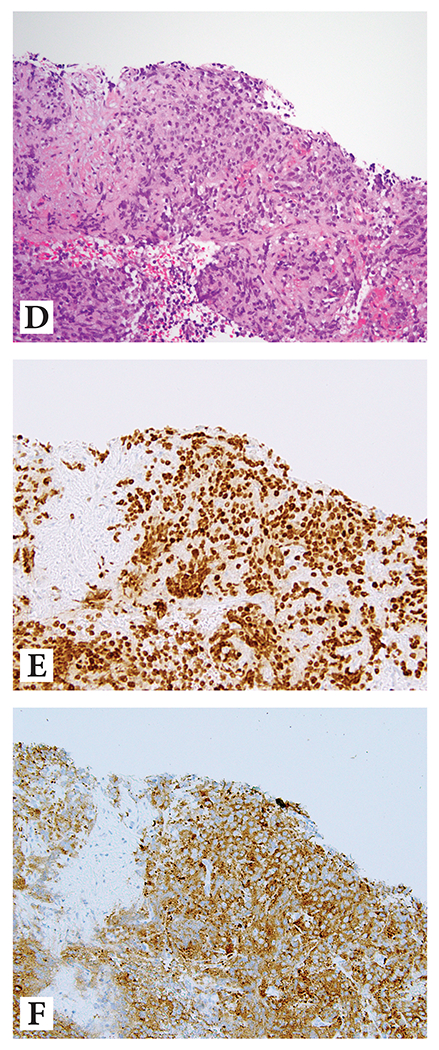
Prostate cancer with diffuse neuroendocrine differentiation
Example 1: (A) H&E at 10x magnification highlighting nested/compartmentalized arrangement of cells with abundant amphophilic cytoplasm; (B) diffuse reactivity with NKX3.1, and (C) diffuse reactivity with synaptophysin
Example 2: (D) H&E at 10x magnification showing sheet-like growth of cells with abundant amphophilic cytoplasm; (E) diffuse reactivity with NKX3.1, and (F) diffuse reactivity with synaptophysin
Figure 4:
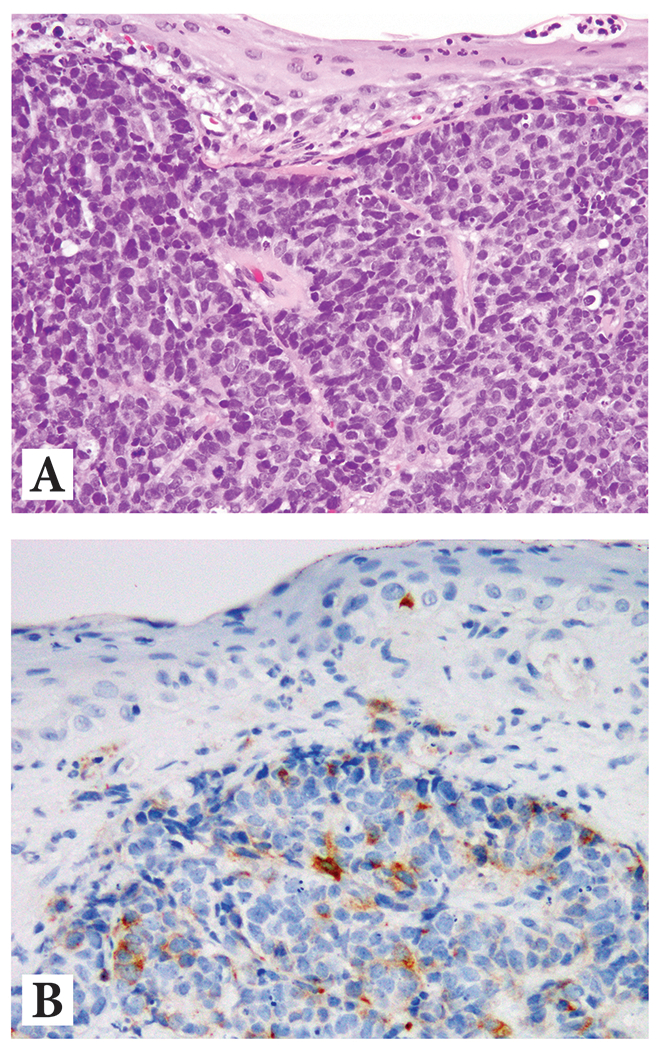
Example of metastatic prostate cancer with patchy neuroendocrine differentiation in a penile biopsy showing
(A) H&E at 10x magnification displaying high grade prostatic adenocarcinoma with
(B) More than isolated reactivity for synaptophysin
Cases of prostatic well-differentiated neuroendocrine tumor (carcinoid tumor) have been rarely described in the literature [14-16], but no such cases were identified in the current consecutive cohort.
Results
85 consecutive cases were reported to have evidence of some NE differentiation. A complete set of slides were available for 79 cases, which constituted the cohort, including 32 primary and 47 metastatic tumors. Of the primary tumors, 14 were in needle biopsies, 16 in radical prostatectomies and two in transurethral resections. Metastatic tumors were located in liver (n=15), lymph node (n=9), bone (n=6), lung (n=3), brain (n=1), and other, including various visceral and soft tissue sites (n=13).
63 of 76 cases (82%) with available history had received prior therapy; 6 received radiation therapy (RT) alone, 24 received androgen deprivation therapy (ADT) alone, and 33 received a combination of RT+ADT. Therapeutic history was not available for 3 patients. Patients who received ADT (with or without RT) received Lupron/Casodex (30 patients), Lupron/Casodex + Abiraterone or Enzalutamide (20 patients), or Abiraterone alone (3 patients). The type of ADT was not specified for four patients.
The incidence of each histologic category and breakdown of primary and metastatic location is summarized in Table 1. Notably, cases of pure small cell carcinoma/HGNEC (87%) and PCa with diffuse NE marker positivity/differentiation (100%) were overwhelmingly observed in the metastatic setting, while cases of combined PCa+small cell carcinoma/HGNEC (90%) and PCa with prominent NE granules (so-called ‘Paneth cell-like’ change) (100%) were overwhelmingly observed in the primary setting.
Table 1.
Breakdown of primary vs. metastatic cases for tumors with neuroendocrine differentiation in the setting of prostate cancer
| Category | Primary | Metastatic |
|---|---|---|
| Pure small cell carcinoma / HGNEC | 3/23 | 20/23 |
| Combined adenocarcinoma + small cell carcinoma / HGNEC | 9/10 | 1/10 |
| PCa with diffuse neuroendocrine differentiation | 0/15 | 15/15 |
| PCa with patchy neuroendocrine differentiation | 5/11 | 6/11 |
| PCa with isolated neuroendocrine marker positive cells | 4/9 | 5/9 |
| PCa with prominent neuroendocrine granules (‘Paneth cell-like’) | 11/11 | 0/11 |
| Total | 32 | 47 |
HGNEC: high-grade neuroendocrine carcinoma; PCa: prostate cancer
Among cases of PCa with diffuse NE marker positivity/differentiation, in addition to morphologic features, all cases showed diffuse positivity for at least one prostatic and one NE IHC marker. PSA, PSMA, and NKX3.1 (androgen receptor-related markers) showed diffuse labeling in 9, 6, and 6 cases, respectively, while synaptophysin and chromogranin (NE markers) showed diffuse labeling in 14 and 10 cases, respectively.
Table 2 highlights the therapeutic history of each morphologic group by tumor site (primary or metastatic). 19/20 small cell carcinoma/HGNEC and 12/15 PCa with diffuse NE marker positivity/differentiation seen in the metastatic setting were post-therapy, 90% including ADT. Likewise, among 11 cases of primary PCa with prominent NE granules, 8/11 were post-therapy.
Table 2.
Therapy history for each morphologic group by tumor site – primary/metastatic disease
| Cancer Location | Number of Cases (N=) | Therapy | ||||
|---|---|---|---|---|---|---|
| RT | ADT | RT+ADT | NONE | |||
| Small cell carcinoma / HGNEC | Total | 23 | 3 | 5 | 12 | 3 |
| Primary | 3 | 1 | 0 | 0 | 2 | |
| Metastatic | 20 | 2 | 5 | 12 | 1 | |
| Combined adenocarcinoma + small cell carcinoma / HGNEC | Total | 10 | 0 | 6 | 3 | 1 |
| Primary | 9 | 0 | 6 | 2 | 1 | |
| Metastatic | 1 | 0 | 0 | 1 | 0 | |
| PCa with diffuse NE marker positivity / differentiation | Total | 15 | 1 | 3 | 8 | 3 |
| Primary | 0 | 0 | 0 | 0 | 0 | |
| Metastatic | 15 | 1 | 3 | 8 | 3 | |
| PCa with patchy NE differentiation | Total | 11* | 0 | 3 | 5 | 2 |
| Primary | 5 | 0 | 2 | 1 | 2 | |
| Metastatic | 6 | 0 | 1 | 4 | 0 | |
| PCa with isolated NE marker positive cells | Total | 9* | 0 | 4 | 2 | 1 |
| Primary | 4 | 0 | 2 | 1 | 1 | |
| Metastatic | 5 | 0 | 2 | 1 | 0 | |
| PCa with prominent NE granules (‘Paneth cell-like’) | Total | 11 | 2 | 3 | 3 | 3 |
| Primary | 11 | 2 | 3 | 3 | 3 | |
| Metastatic | 0 | 0 | 0 | 0 | 0 | |
One case of PCa with patchy NE differentiation and 2 cases of PCa with isolated NE marker positive cells had no therapy history available
HGNEC: high-grade neuroendocrine carcinoma; PCa: prostate cancer; NE: neuroendocrine; RT: radiation therapy; ADT: androgen deprivation therapy
Discussion
Although NE manifestations in the setting of PCa are considered rare, the prostate gland represents one of the most frequent sites for carcinoma with NE differentiation outside the lung17. These phenomena have garnered increasing interest in the last decade due to implementation of next generation AR pathway inhibitors which have been associated with increased survival rates in advanced PCa18–19. It has been postulated that sustained blockade of the AR pathway leads to a switch of the lineage identity of tumor cells – ‘phenotypic plasticity’ or ‘trans-differentiation’ –associated with treatment resistance3, 20–21. The resulting advanced disease, referred to as treatment emergent PCa, has been characterized by a constellation of findings: low/absent AR expression, increased incidence of small cell carcinoma/HGNEC morphology, and clinically by bulky tumor masses, and visceral or lytic (rather than typical blastic) bone metastases4.
This study represents the first consecutive series of cancers with NE features in the setting of PCa. Herein, we have detailed the incidence of various well-defined, as well as less well recognized manifestations, shown that the majority of cases – regardless of morphology – are associated with a therapeutic (RT +/− ADT) history, and noted the presentation of specific morphologies at either the primary or metastatic sites.
Small cell carcinoma/HGNEC comprised 30% of all NE differentiation in the setting of PCa in our study, 87% (20/23) in metastatic sites, with only 3 of 23 cases in primary tumors. These carcinomas histologically resembled the spectrum of HGNEC described at other sites11–12, 22, with more common features including diffuse sheets of round blue hyperchromatic cells with nuclear molding, scant cytoplasm and inconspicuous nucleoli, and high apoptotic and mitotic rates, to occasional tumors with large nested, trabecular, or palisaded architecture, large cells with abundant cytoplasm and prominent nucleoli, and rare geographic necrosis. The latter descriptor, which may fit criteria for large cell NE carcinoma, are exceedingly rare in the setting of PCa, with one small series showing strong association with palliative transurethral resection specimens in patients with prior ADT23. These tumors were classified as ‘small cell carcinoma/HGNEC’ in our series, given known associations between this range of histological features and aggressive biological behavior6, 23–25.
Prior studies have noted that up to one half of HGNEC of the prostate may exhibit an abrupt transition between the conventional acinar and the small cell carcinoma/HGNEC components24 and have been referred to as “mixed” NE carcinoma-acinar adenocarcinoma9. These have been shown to share loss of tumor suppressor genes TP53 and Rb1, despite exhibiting immunohistochemical differences corresponding to different lineages, including differences in AR expression26. Recent studies27 have found that loss-of-function of TP53 and Rb1 may also confer lineage plasticity on AR dependent PCa, that may later gain multiple phenotypes, including small cell carcinoma/HGNEC.
In our cohort, all except one of the combined adenocarcinoma+small cell carcinoma/HGNEC were seen in the primary setting, and typically showed an abrupt transition between the two components. In uncommon cases reported in the literature, merging of the two components is observed, with a continuum of morphology including sheet-like architecture and scant cytoplasm, yet visible nucleoli, a phenomenon that has been referred to as ‘prostate carcinoma with overlapping features of small cell carcinoma and acinar adenocarcinoma’9. Finding combined adenocarcinoma+ small cell carcinoma/HGNEC mostly in primary PCa may be partly attributed to the ability to evaluate the entire tumor in radical prostatectomy specimens, as opposed to sampling bias encountered in metastatic biopsy material. Likewise, difficulty performing metastatic biopsies from bone and visceral sites28 may contribute to detection of this combined phenotype primarily in the prostate gland.
We have previously used the term prostatic adenocarcinoma with diffuse neuroendocrine marker positivity/differentiation to describe a subset of tumors do not fit neatly into contemporary classification schema29. It denotes tumors with nested to sheet-like growth of cells with abundant, often variegated, cytoplasm and prominent nucleoli, yet diffuse positivity for at least one prostatic and one NE IHC marker (often more than one of each). As noted, NE IHC was triggered in these cases due to architectural and/or cytoplasmic features. Prior studies have utilized other terminologies for tumors in this spectrum, including ‘amphicrine carcinoma’30 and ‘hybrid PCa with mixed luminal and NE phenotype’31, alluding to the histologic pliability of these tumors. Beyond nomenclature, the limited mention of this specific subset of tumors in the literature raises uncertainty about clinical characteristics/outcome, which may translate to a dilemma in selecting therapeutic options. Specifically, in these tumors in which the AR axis appears to be active, consonant with labeling for prostatic markers such as PSA, PSMA, and NKX3.1, the diffuse NE marker expression in the absence of small cell carcinoma/HGNEC morphology, raises the question of whether ADT alone or in combination with platinum-based chemotherapy is appropriate.
While a small number of presumed de novo cases of PCa with diffuse neuroendocrine marker positivity/differentiation have recently been described32–33, all 15 such cases in the current cohort were metastatic lesions; 12 were post-therapy, 11 of which received ADT. Possibly reflecting the dual marker positive nature of this lesions, metastases were noted in both sites common for PCa (lymph nodes, bone), as well as in visceral sites (liver, lung) in which NE manifestations are more typically seen4. Although outcome data is beyond the scope of this study, the overlapping morphologic and IHC features incorporating acinar and NE phenotypes, as well as the prevalence of this entity in metastatic sites and overwhelmingly in the post ADT setting, suggest that this represents a transient/intermediate state in the lineage plasticity continuum1, 20. For these reasons, we have adopted the clinical practice of appending the verbiage of ‘not small cell carcinoma/HGNEC’ as a note to the diagnosis when reporting this group of tumors. Thus, we aim to differentiate this state(s) from small cell carcinoma/HGNEC, which represents an extreme end of the continuum and for which the current standard is platinum-based therapy. We also emphasize the non-focal/non-isolated cell nature of the NE marker positivity by using the term ‘diffuse’, further separating this from an entity with potentially less significant clinical ramifications for therapy.
In contrast to the diffuse expression of NE markers in the histologic subtypes described above, our series included eleven cases with typical morphology of adenocarcinoma yet displaying more than single cell positivity for NE markers. These were labelled as having ‘patchy’ NE differentiation. Nine additional cases showed NE marker positivity in isolated tumor cells of adenocarcinoma. These morphologic groups were nearly equally seen in primary and metastatic lesions.
Prior studies34–37 have demonstrated that scattered NE cells are present in 10-100% of prostatic adenocarcinoma, varying with the number of NE markers performed, and the extent of tumor evaluated. When tumor grade is accounted for, there is no definitive data showing that extent of NE marker positive cells has an independent effect on survival34, 38–43, and therefore routine staining of all PCa for NE markers is not recommended. In metastatic sites, older studies suggest that the specific ADT utilized influences the presence of NE marker-positive cells, but no correlation with extent of disease and disease specific survival is seen42. In pathology reports from such cases with more limited – patchy or isolated cell – NE IHC marker positivity, it is crucial to distinguish from small cell carcinoma/HGNEC manifestations, as there is no current indication for platinum-based therapy in the former.
There is a subset of PCa with cells displaying prominent NE granules on routine H&E stains, that has been termed ‘Paneth cell-like (neuroendocrine)’44. Such cells have been reported across the spectrum of grades of prostate cancer. When present as cords, nests, or single cells they may recapitulate high grade PCa; however, in reported series, pathologic and clinical outcomes are dependent on grading, quantitation, and staging features of conventional adenocarcinoma areas45–47. PCa with prominent NE granules comprised ~ 15% of all cases in this series, exclusively in primary disease, and with over 70% (8/11) seen in the post-therapy setting. Since these cells more closely resemble intestinal NE cells (NE markers positive and lack of lysozyme) rather than true Paneth cells (which are NE marker negative and contain lysozyme)45, and may express AR48, an alternative nomenclature, such as “PCa with prominent NE granules”, may be warranted, which also distinguishes this NE manifestation from small cell carcinoma/HGNEC.
Conclusions
In this contemporary case series of NE differentiation in the setting of prostate cancer, we found that most NE manifestations, not only small cell carcinoma/HGNEC, are seen after therapy. We highlight 15 cases of PCa with diffuse NE marker positivity/differentiation in metastatic sites, an under-recognized phenotype which may well fall along the lineage plasticity spectrum. We further demonstrate a propensity for detection of small cell carcinoma/HGNEC and PCa with diffuse NE differentiation in metastatic settings, and conversely, detection of combined high grade PCa+small cell carcinoma/HGNEC and PCa with prominent NE granules (‘Paneth cell-like’) in primary disease. These findings expand current knowledge regarding NE differentiation in PCa and await molecular and further clinical outcome correlation.
Funding
This study was supported in part through NIH/NCI Prostate Cancer SPORE Award P50CA092629 (MSKCC) and NIH/NCI Cancer Center Support grant P30CA008748
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
Data Availability Statement
Data available on request from authors.
References
- 1.Beltran H, Hruszkewycz A, Scher HI, et al. The Role of Lineage Plasticity in Prostate Cancer Therapy Resistance. Clin Cancer Res 2019; 25(23):6916–6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abida W, Cyrta J, Heller G, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci U S A 2019;116(23):11428–11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal R, Huang J, Alumkal JJ, et al. Clinical and Genomic Characterization of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer: A Multi-institutional Prospective Study. J Clin Oncol 2018;36(24):2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aparicio AM, Harzstark AL, Corn PG, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res 2013. Jul;19(13):3621–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aparicio AM, Shen L, Tapia EL, et al. Combined Tumor Suppressor Defects Characterize Clinically Defined Aggressive Variant Prostate Cancers. Clin Cancer Res 2016;22(6):1520–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tetu B, Ro JY, Ayala AG, et al. Small cell carcinoma of the prostate. I. A clinicopathologic study of 20 cases. Cancer 1987;59(10):1803–1809. [DOI] [PubMed] [Google Scholar]
- 7.Abbas F, Civantos F, Benedetto P, et al. Small cell carcinoma of the bladder and prostate. Urology 1995;46(5):617–630. [DOI] [PubMed] [Google Scholar]
- 8.Shah RB, Mehra R, Chinnaiyan AM, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res 2004;64:9209–9216. [DOI] [PubMed] [Google Scholar]
- 9.Epstein JI, Amin MB, Beltran H, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol 2014;38:756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moch H, Humphrey PA, Ulbright TM, et al. WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th ed. Lyon: IARC; 2016. [DOI] [PubMed] [Google Scholar]
- 11.Rekhtman N Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med 2020;134:1628–1638. [DOI] [PubMed] [Google Scholar]
- 12.Travis WD. Pathology and diagnosis of neuroendocrine tumors: lung neuroendocrine. Thorac Surg Clin 2014;24:257–266. [DOI] [PubMed] [Google Scholar]
- 13.Wenk RE, Bhagavan BS, Levy R, et al. Ectopic ACTH, prostatic oat cell carcinoma, and marked hypernatremia. Cancer 1977;40:773–778. [DOI] [PubMed] [Google Scholar]
- 14.Almagro UA. Argyrophilic prostatic carcinoma: case report with literature review on prostatic carcinoid and “carcinoid-like” prostatic carcinoma. Cancer 1985;55:608–614. [DOI] [PubMed] [Google Scholar]
- 15.Azumi N, Shibuya H, Ishikura M. Primary prostatic carcinoid tumor with intracytoplasmic prostatic acid phosphatase and prostate specific antigen. Am J Surg Pathol 1984;8:545–550. [DOI] [PubMed] [Google Scholar]
- 16.Ghannoum JE, DeLellis RA, Shin SJ. Primary carcinoid tumor of the prostate with concurrent adenocarcinoma: a case report. Int J Surg Pathol 2004;12:167–170. [DOI] [PubMed] [Google Scholar]
- 17.Galanis E, Frytak S, Lloyd RV. Extrapulmonary small cell carcinoma. Cancer 1997;79:1729–1736. [DOI] [PubMed] [Google Scholar]
- 18.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187–1197. [DOI] [PubMed] [Google Scholar]
- 20.Davies AH, Beltran H, Zoubeidi A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat Rev Urol 2018;15(5):271–286. [DOI] [PubMed] [Google Scholar]
- 21.Wang HT, Yao YH, Li BG, et al. Neuroendocrine Prostate Cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis-a systematic review and pooled analysis. J Clin Oncol 2014;32(30):3383–3390. [DOI] [PubMed] [Google Scholar]
- 22.Klimstra DS, Beltran H, Lilenbaum R, et al. The spectrum of neuroendocrine tumors: histologic classification, unique features and areas of overlap. Am Soc Clin Oncol Educ Book 2015:92–103. [DOI] [PubMed] [Google Scholar]
- 23.Evans AJ, Humphrey PA, Belani J, et al. Large cell neuroendocrine carcinoma of the prostate: a clinicopathologic summary of 7 cases of a rare manifestation of advanced prostate cancer. Am J Surg Pathol 2006;30:684–693. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Epstein JI. Small cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol 2008;32:65–71. [DOI] [PubMed] [Google Scholar]
- 25.Yao JL, Madeb R, Bourne P, et al. Small cell carcinoma of the prostate: an immunohistochemical study. Am J Surg Pathol 2006;30:705–712. [DOI] [PubMed] [Google Scholar]
- 26.Hansel DE, Nakayama M, Luo J, et al. Shared TP53 gene mutation in morphologically and phenotypically distinct concurrent primary small cell neuroendocrine carcinoma and adenocarcinoma of the prostate. Prostate 2009;69:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ku SY, Rosario S, Wang Y, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 2017;355(6320):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beltran H, Tomlins S, Aparicio A, et al. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res 2014;20:2846–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fine SW. Neuroendocrine tumor of the prostate. Mod Pathol 2018;31:S122–S132. [DOI] [PubMed] [Google Scholar]
- 30.Prendeville S, Al-Bozom I, Comperat E, et al. Prostate carcinoma with amphicrine features: further refining the spectrum of neuroendocrine differentiation in tumours of primary prostatic origin? Histopathology 2017;71:926–933. [DOI] [PubMed] [Google Scholar]
- 31.Wu C, Wyatt AW, Lapuk AV, et al. Integrated genome and transcriptome sequencing identifies a novel form of hybrid and aggressive prostate cancer. J Pathol 2021;227:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdulfatah E, Reichert ZR, Davenport MS, et al. De novo neuroendocrine transdifferentiation in primary prostate cancer-a phenotype associated with advanced clinicopathologic features and aggressive outcome. Med Oncol. 2021;38(3):26. [DOI] [PubMed] [Google Scholar]
- 33.Galea LA, Mow C, Fine SW, et al. Primary Prostatic Carcinoma With De Novo Diffuse Neuroendocrine Differentiation. Int J Surg Pathol. 2022;30(2):232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma. The Prostate 1999;39:135–148. [DOI] [PubMed] [Google Scholar]
- 35.Mucci NR, Akdas G, Manely S, et al. Neuroendocrine expression in metastatic prostate cancer: evaluation of high throughput tissue microarrays to detect heterogeneous protein expression. Hum Pathol 2000;31:406–414. [DOI] [PubMed] [Google Scholar]
- 36.Huang J, Yao JL, di Sant’Agnese, Yang Q, Bourne PA, Na Y. Immunohistochemical characterization of neuroendocrine cells in prostate cancer. The prostate 2006;66:1399–1406. [DOI] [PubMed] [Google Scholar]
- 37.Komiya A, Suzuki H, Imamoto T, et al. Neuroendocrine differentiation in the progression of prostate cancer. Int J Urol 2009;16:37–44. [DOI] [PubMed] [Google Scholar]
- 38.Weinstein MH, Partin AW, Veltri RW, et al. Neuroendocrine differentiation in prostate cancer: enhanced prediction of progression after radical prostatectomy. Hum Pathol 1996;27:683–687. [DOI] [PubMed] [Google Scholar]
- 39.Noordzij MA, van der Kwast TH, van Steenbrugge GJ, et al. The prognostic influence of neuroendocrine cells in prostate cancer: results of a long-term follow-up study of patients treated by radical prostatectomy. Int J Cancer 1995;62:252–258. [DOI] [PubMed] [Google Scholar]
- 40.Abrahamsson PA, Cockett ATK, di Sant’Agnese PA. Prognostic significance of neuroendocrine differentiation in clinically localized prostatic carcinoma. The Prostate [Suppl] 1998;8:37–42. [PubMed] [Google Scholar]
- 41.Aprikian AG, Cordon-Cardo C, Fair WR, et al. Characterization of neuroendocrine differentiation in human benign prostate and prostatic adenocarcinoma. Cancer 1993;71:3952–3965. [DOI] [PubMed] [Google Scholar]
- 42.Aprikian AG, Cordon-Cardo C, Fair WR, et al. Neuroendocrine differentiation in metastatic prostatic adenocarcinoma. J Urol 1994;151:914–919. [DOI] [PubMed] [Google Scholar]
- 43.McWilliam LJ, Manson C, George NJR. Neuroendocrine differentiation and prognosis in prostatic adenocarcinomas. Br J Urol 1997;80:287–290. [DOI] [PubMed] [Google Scholar]
- 44.Weaver MG, Abdul-Karim FW, Srigley J, et al. Paneth cell-like change of the prostate gland: a histological, immunohistochemical, and electron microscopic study. Am J Surg Pathol 1992;16:62–68. [DOI] [PubMed] [Google Scholar]
- 45.Tamas EF, Epstein JI. Prognostic significance of Paneth cell-like neuroendocrine differentiation in adenocarcinoma of the prostate. Am J Surg Pathol 2006;30:980–985. [DOI] [PubMed] [Google Scholar]
- 46.So JS, Gordetsky J, Epstein JI. Variant of prostatic adenocarcinoma with Paneth cell-like neuroendocrine differentiation readily misdiagnosed as Gleason pattern 5. Hum Pathol 2014;45:2388–2393. [DOI] [PubMed] [Google Scholar]
- 47.Salles DC, Mata DA, Epstein JI. Significance of Paneth cell-like differentiation in prostatic adenocarcinoma: a retrospective cohort study of 80 cases. Hum Pathol 2020;102:7–12. [DOI] [PubMed] [Google Scholar]
- 48.Kaur H, Samarska I, Lu J, et al. Neuroendocrine differentiation in usual-type prostatic adenocarcinoma: Molecular characterization and clinical significance. Prostate 2020. Sep;80(12):1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from authors.


