ABSTRACT
Viral gastroenteritis has a global distribution and represents a high risk for vulnerable population and children under 5 years due to acute diarrhea, fever and dehydration. Human astroviruses (HAstV) have been identified as the third most important cause of viral gastroenteritis in pediatric and immunocompromised patients. Furthermore, HAstV has been reported in biopsies taken from patients with encephalitis, meningitis and acute respiratory infection, yet it is not clear how the virus reaches these organs. In this work we have tested the possibility that the released astrovirus particles could be associated with extracellular vesicles. Comparison between vesicles purified from HAstV Yuc8 infected and mock-infected cells showed that infection enhances production of vesicles larger than 150 nm. These vesicles contain CD63 and Alix, two markers of vesicular structures. Almost 70% of the extracellular virus present in clarified supernatant at 18 h postinfection was found associated with vesicular membranes, and this association facilitates cell infection in the absence of trypsin activation and protects virions from neutralizing antibodies. Our findings suggest a new pathway for HAstV spread and might represent an explanation for the extra-intestinal presence of some astrovirus strains.
IMPORTANCE Astroviruses are an important cause of diarrhea in vulnerable population, particularly children; recently some reports have found these viruses in extra-intestinal organs, including the central nervous system, causing unexpected clinical disease. In this work, we found that human astrovirus strain Yuc8 associates with extracellular vesicles, possibly during or after their cell egress. The association with vesicles doubled astrovirus infectivity in less susceptible cells and rendered virus particles insensitive to neutralization by antibodies. These data suggest that extracellular vesicles could represent a novel pathway for astrovirus to disseminate outside the gastrointestinal tract.
KEYWORDS: astrovirus, extracellular vesicles, vesicle mediated infection
INTRODUCTION
Astroviruses are considered the third most important cause of viral gastroenteritis in children, as well as in the young of many animal species (1, 2). Moreover, in some mammalian species astroviruses have been associated with different neurological disorders and have been found in biopsies of patients with encephalitis, meningitis or acute respiratory infections (1, 2). Given that mammalian astroviruses are considered intestinal viruses, the central question is: “How could astroviruses get into the central nervous system and respiratory tract?”.
From the structural point of view, astroviruses are small nonenveloped viruses, forming the family Astroviridae. They contain a single-stranded, positive sense RNA (ssRNA+) genome whose length ranges, in the case of mammalian astroviruses, from 6.1 to 6.8 kb. The astrovirus genome is organized into three open reading frames, named ORF1a, ORF1b, and ORF2, which encode nonstructural (ORF1a and ORF1b) and structural (ORF2) viral proteins (3, 4). Astrovirus cell entry is not completely understood, and the virus cell surface receptor is unknown, although the fact that the susceptibility of different cell lines to infection with astrovirus depends on the viral serotype (5–7), suggests that there could be more than one receptor. Astrovirus enters into cells by clathrin-mediated endocytosis, and it seems that entry process follows a classical route into late endosomes (3, 4). During maturation, the astrovirus particles are subjected to distinct proteolytic processes. First, the capsid protein VP90 of the newly assembled astrovirus particles is cleaved intracellularly by caspases to give immature virions composed by the viral protein VP70. This cleavage is associated with the release of the viral particles from the infected cell (8, 9). Then, once in the extracellular medium, the virion is processed by trypsin-like extracellular proteases to render infectious, mature virions, composed by the final protein products VP34 and VP27 (10, 11).
One of the less characterized phases of the astrovirus replication cycle, is cell egress. It has been proposed that astrovirus release is a nonlytic process, during which the extracellular virions appear to be associated with membranous structures (9, 12). In this regard, it is of interest that the cell exit of different viruses has been associated with extracellular vesicles (EV) (13–15). EV are a heterogeneous group of small vesicles with a lipidic bilayer, ranging from 50 nm to 1,000 nm of diameter (16). These vesicles are secreted by different types of cells and can be isolated from conditioned media of cultured cells, as well as from virtually any type of body fluid, including blood, urine, ascites, bronchoalveolar lavage, saliva and cerebrospinal (17). There are different types of EV, with exosomes being the better characterized, having a diameter of around 50 to 150 nm, and also well studied are microvesicles with diameter around 50 to 1,000 nm. Exosomes originate from the endosomal compartment by fusion of multivesicular bodies with the plasma membrane, while microvesicles originate from the plasma membrane by outward budding (18).
Viral infections affect cell physiology, as well as many cellular processes, including protein synthesis and degradation (19), intracellular trafficking and vesicle secretion (14, 20). In the last few years, the evidence regarding the interaction between EV and different types of viruses (21–23) has accumulated. Particularly, several positive-sense ssRNA viruses, like hepatitis C virus (HCV) and hepatitis E virus (HEV), have been found to associate with EV or to use the mechanism of EV biogenesis as an egress pathway (24–26). In addition, DNA viruses like HSV-1 (27) and JC polyomavirus (28) also have been observed interacting or being released with EV.
Given the possibility that EV could be involved in the human astrovirus (HAstV) cell egress, we tested the possibility that astrovirus particles could be released in association with this type of vesicles. To characterize the possible interaction between EV and the virus, Caco-2 cells infected with the Yuc8 strain of HAstV and EV were purified from the cell culture media by differential centrifugation coupled to polyethylene glycol 6000 (PEG) precipitation and affinity magnetic sorting. Our results suggest that astrovirus infection stimulates the secretion of EV and astrovirus particles seem to associate with EV. These vesicle-associated viruses acquire the ability to infect cells in the absence of trypsin activation. Also, viral particles associated with EV were refractory to the effect of neutralizing antibodies, suggesting that EV are able to protect the virions from this interaction.
RESULTS
Detergent treatment increases HAstV Yuc8 infectious titer in supernatant from 15 h postinfection.
It has been previously observed that a portion of the astrovirus particles produced in infected cells floats to low-density fractions when separated by density centrifugation, suggesting that they interact with membranous structures (12). To characterize the possible association of astrovirus particles with membranes in the cell culture media, we evaluated the kinetics of astrovirus release in Caco-2 cells. Media from astrovirus infected cells were collected at different time points after infection (from 12 to 24 hpi), and the virus titer was determined. The infectivity of the virus in the supernatant was activated with trypsin after the samples were treated or not with detergent (Triton X-100). Under these conditions, if astrovirus particles were associated with vesicles or membranes, the detergent treatment would release the virions, leading to an increase in viral titer compared to the titer of samples not treated with detergent. Triton X-100 treatment did not affect trypsin activity, nor it had any adverse effect on viral particles, as no decrease in viral titer was observed (results not shown). We observed the virus present in the supernatant starting at the first time point analyzed (12 hpi), without any significant change in the titer after Triton X-100 treatment (P > 0.05) (Fig. 1A), similar to previously published results (8). The titer of virus present in the media treated with detergent showed significant increase from 15 to 24 h postinfection, reaching above 180% compared to nontreated samples (Fig. 1A). No significant cellular damage was detected at the different time points, as determined by an LDH assay (Fig. 1B). In all the subsequent experiments shown here, media were harvested at 18 hpi.
FIG 1.
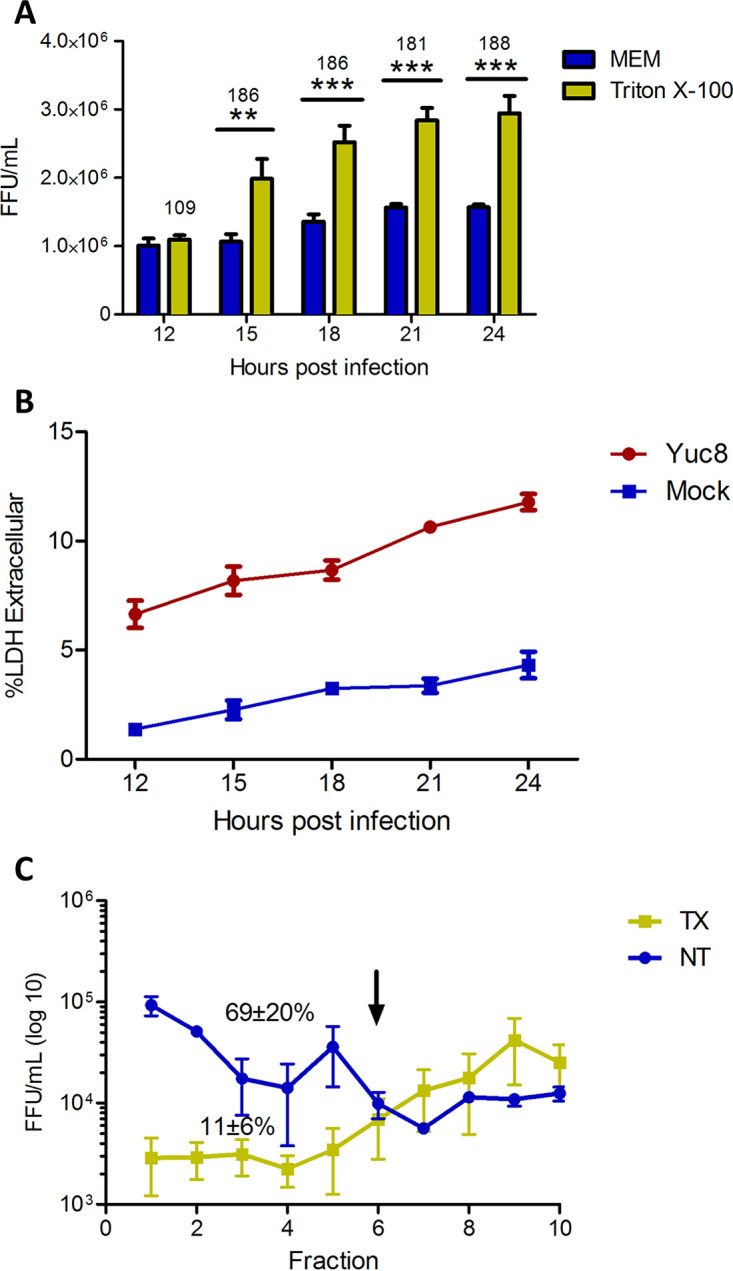
Kinetics of astrovirus release from infected Caco-2 cells. Caco-2 cells were infected with astrovirus Yuc8 at MOI of 5 and supernatants were collected at different time points postinfection (from 12 to 24 h). (A) Viral titer was determined with (yellow bars) and without (blue bars) 0.1% Triton X-100 treatment before trypsin activation. The results represent the mean focus forming units (FFU) per milliliter (mL) ± standard error of the mean of three independent experiments done in duplicate. The number above bars represent % of viral titer after Triton X-100 treatment taking as 100% infectivity of no treated virus. Statistical analysis was done with two-way ANOVA, P value **, P < 0.01; ***, P < 0.001. (B) The cell membrane integrity in each time point was confirmed by measuring lactic dehydrogenase (LDH) in media from infected (in red) or not infected (blue) cells, by In vitro toxicity assay kit, Lactic Dehydrogenase based (Sigma-Aldrich). (C) Supernatant was collected from infected cells; cell debris was removed, and content was precipitated. Pellet was resuspended, and one half was treated with Triton X-100 (yellow line), while second half was left untreated (blue line), before centrifugation. Samples were then separated on optiprep gradient, and 10 fractions were collected, from 1 (top), to 10 (bottom). Viral titers associated with fractions were obtained after virus activation with trypsin. Arrow divides gradient in top (light) fractions, and bottom (heavy) fractions. Number indicates % of virus present in top fraction in each condition (n3). One representative experiment of three is shown.
To test the amount of released virus associated with membrane structures (possibly extracellular vesicles), astrovirus–membrane complexes were separated by density centrifugation in optiprep gradient with or without previous detergent treatment. In samples without detergent treatment majority of astrovirus particles were floating in top portion of the gradient, and this presence was affected by detergent treatment, previous to centrifugation (Fig. 1C). As consequence, in top portion of the gradient (fractions 1–5), without detergent treatment there is 69% ± 20% (n3) of total infectious virus, while after detergent treatment this amount represents only 11% ± 6% (n3). These results suggest that astrovirus particles are released from infected cells before appreciable cell lysis, and that they could be associated with detergent soluble structures in the extracellular medium.
Astrovirus infection increases the secretion of extracellular vesicles from Caco-2 cells.
To characterize the effect of astrovirus infection on the production of EV in Caco-2 cells, supernatants from infected or mock-infected cells cultured in serum-free MEM were harvested at 18 hpi and processed by differential centrifugation. Initially, detached cells were pelleted at 500 g for 5 min, getting pellet 1 (P1). Pellet 2 (P2) was obtained by centrifugation of the remaining supernatant at 2,000 g for 30 min. We expected this fraction to contain very large vesicles and some cell debris and organelles. Pellet 3 was obtained by centrifugation of the remaining supernatant at 20,000 g for 1 h, to collect large extracellular vesicles (LEV), theoretically calculated to be over 122 nm (29). Finally, pellet 4 was obtained by overnight precipitation of remaining vesicles and particles in the remaining supernatant by 8% PEG 6000 and 0.5 M NaCl, followed by centrifugation at 10,000 g for 1 h, producing small extracellular vesicles fraction (SEV), calculated to be under 170 nm (29, 30). As consequence, we expect some size overlapping between LEV and SEV fractions.
An equal portion of each pellet fraction was analyzed after SDS-PAGE. By silver staining of the gel, it was clear that the amounts of total proteins present in each fraction was increased in astrovirus-infected cells (Fig. 2A). The presence of different cellular markers in the pelleted fractions was analyzed by immunoblotting; to identify both exosomes and microvesicles we have chosen EV specific markers CD63 (specific exosomal marker), ALIX (involved in both microvesicle and exosome biogenesis) (31, 32), and GM1 as general cell membrane marker, while endoplasmic reticulum associated PDI protein was used as non-EV associated protein control. In fractions P1 and P2, which probably contain cells, cell debris and large vesicles, all proteins markers were observed, and their presence has increased after astrovirus infection. Interestingly, the LEV and SEV fractions purified from astrovirus-infected Caco-2 cells showed a higher content of EV specific proteins (ALIX and CD63) compared to mock-infected cells (Fig. 2B), presumably representing larger amounts of EVs. (Fig. 2B). When analyzing PDI, it was present only in P1 and P2 fractions of mock-infected cells, while in infected cells low amount of PDI has been found even in LEV fraction, however it was absent in SEV fraction (Fig. 2B). It was reported previously that PDI could be found in some cases of extracellular vesicles (31, 32). It is possible that stimulation of secretion of EV after infection is responsible for PDI identification in LEV fraction.
FIG 2.
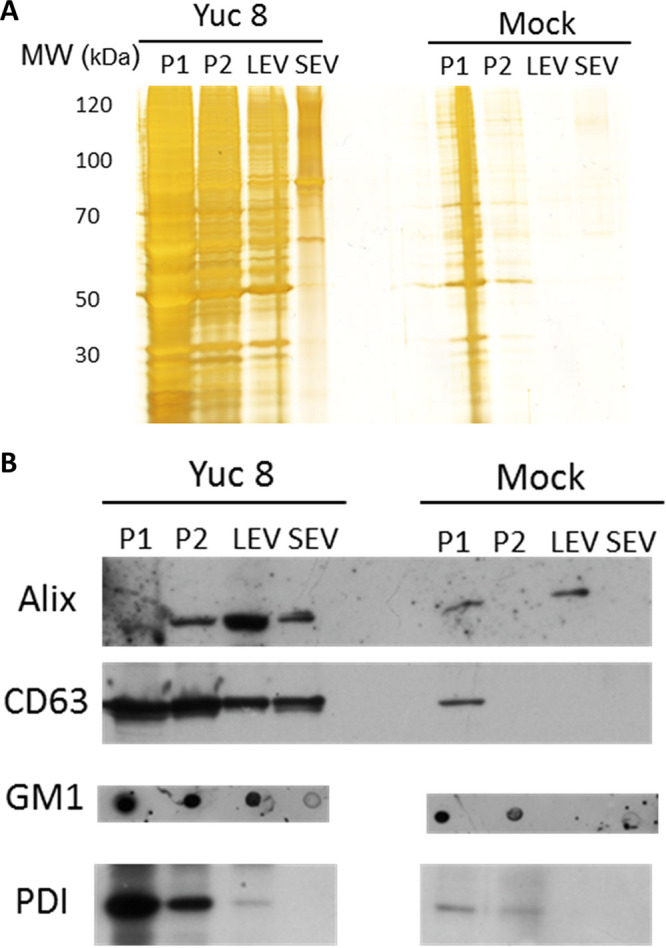
Identification of vesicular markers in vesicles purified from Caco-2 cells. Caco-2 cells were infected or mock-infected with astrovirus strain Yuc8 (MOI of 5) and 18 h postinfection the supernatant was collected. Different fractions were purified by differential centrifugation; at 500 g pellet 1 was obtained (P1), pellet 2 was obtained at 2,000 g (P2), large extracellular vesicles (LEV) were obtained after 20,000 g centrifugation and final small extracellular vesicles (SEV) fraction was obtained by precipitation with 8% polyethylene glycol 6000, 0.5M NaCl. (A) The same volume of each pelleted fraction was separated by SDS-PAGE and proteins were detected by silver staining. (B) Samples were resolved on SDS-PAGE and analyzed by Western blotting, using antibodies specific for CD63 and Alix as vesicle markers and protein disulfide isomerase (PDI) as endoplasmic reticulum protein to assess preparation contamination. To detect GM1 (general membrane marker) samples were directly blotted onto nitrocellulose membrane and processed. Immunoblots are representative of five independent experiments.
To quantitate the concentration and size of the purified vesicles more precisely, the pellet 3 (LEV fraction) and the fraction purified after PEG 6000 precipitation (SEV fraction) were analyzed by nanoparticle tracking analysis (Fig. 3). There was a clear and significative increase in the vesicle number in the LEV fraction from Yuc8-infected cells (P < 0.05), compared to that of mock-infected cells (Fig. 3A and C). In the case of the SEV fraction obtained by PEG 6000 precipitation, there was only a small, not significant increase (P > 0.05) on the number of vesicles present in preparations obtained from either infected cells or mock-infected cells (Fig. 3B and C). These results suggest that astrovirus infection might stimulate the production of EV, particularly those present in the LEV fraction.
FIG 3.
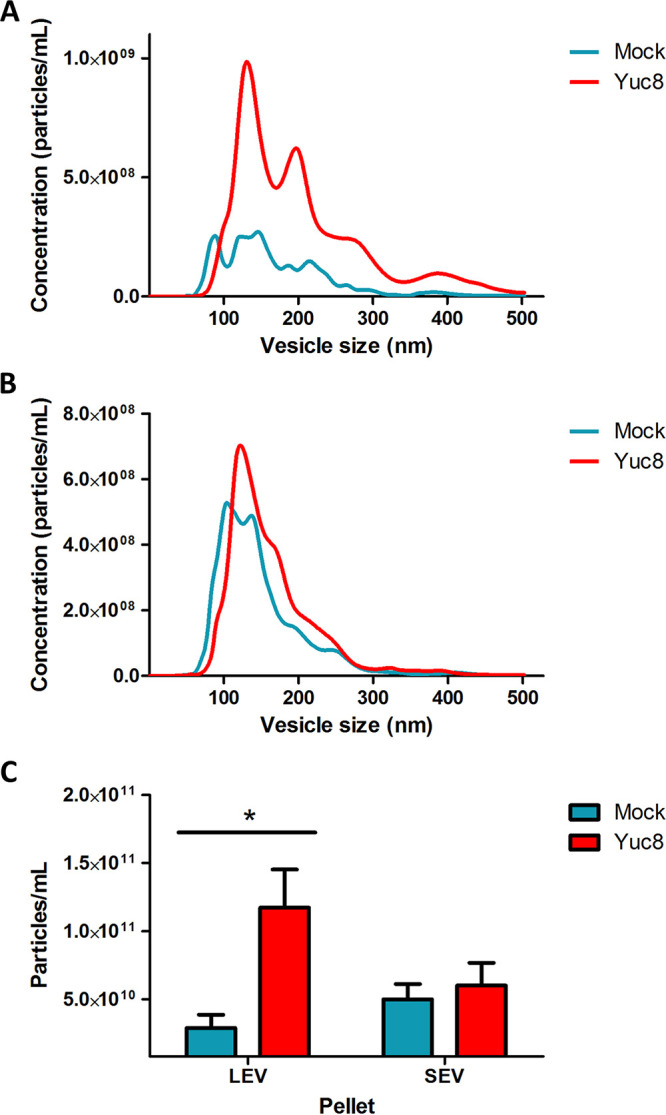
Astrovirus Yuc8 infection stimulates secretion of extracellular vesicles in Caco-2 cells. Fractions were purified from supernatants of Caco-2 cells infected with astrovirus Yuc8 (MOI of 5) or mock infected, 18 h postinfection and processed by differential centrifugation, after pelleting at 20,000 g (LEV) (A), and obtained after PEG 6000 precipitation (SEV) (B). Both fractions were resuspended in PBS and used for nanoparticle tracking analysis in the NanoSight NS300. In each experiment five videos were recorded and used for analysis. Distribution of particle-vesicle size (hydrodynamic diameter in nm) and concentration (particles/mL) from 3 to 5 independent experiments are shown. Vesicles purified from mock infected cells are represented by blue line, vesicles from Yuc8 infected cells are represented by red line. (C) Comparison of the mean number of particles present in LEV and SEV fractions shown in A and B. All results are expressed as the mean of the whole concentration of particles ± standard error of the mean of three independent experiments. Statistical analysis was done using two-way ANOVA *, P < 0.05.
Astrovirus particles seem to associate with extracellular vesicles.
Given the presence of vesicles with different size in the cell culture medium, we analyzed whether astrovirus particles were associated with a particular fraction and if an increased infectivity could be observed after treating the different fractions with Triton X-100 before activation of the virus with trypsin. We found that different amounts of infectious viral particles were present in fractions P2, LEV, and SEV; treatment with Triton X-100 before trypsin activation significantly increased virus titer in fractions P2 (P < 0.05) and LEV (P < 0.01), but not in fraction SEV (Fig. 4A). These observations suggest that a portion of the astrovirus particles could be present inside vesicles or, alternatively, that groups of viral particles could be associated with EV from the outside, and consequently membrane solubilization releases individual particles, increasing virus titer.
FIG 4.
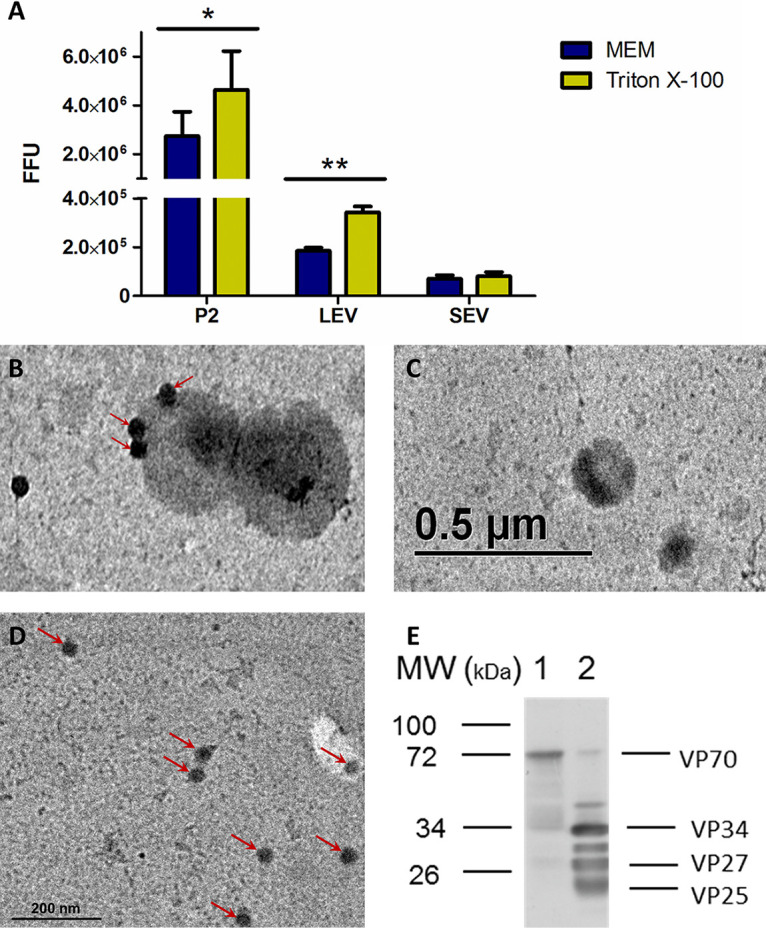
Astrovirus particles associate with large extracellular vesicles. Caco-2 cells were infected with astrovirus Yuc8 (MOI of 5) or mock infected, and supernatants were harvested 18 h postinfection and processed by differential centrifugation, fraction P2 at 2,000 g, fraction containing large extracellular vesicles (LEV) at 20,000 g and final small extracellular vesicles (SEV) fraction was obtained by precipitation with PEG 6000, and NaCl after centrifugation at 10,000 g. All fractions were resuspended in same volume (100 μL) of sterile PBS. (A) Viral titer was determined after activation with trypsin, with or without previous incubation with detergent. Viral content was expressed as total focus forming units. Yellow bars represent samples treated with 0.1% Triton X-100 prior trypsin activation; blue bars (MEM), represent samples activated with trypsin without Triton X-100 treatment. The mean of viral particles in each sample ± standard error of the mean of three independent experiments are shown. (B) Large extracellular vesicles purified from Yuc8 infected, or (C) non infected Caco-2 cells were further clarified by additional isolation using MagCapture Exosome isolation kit PS. One drop of sample was fixed onto carbon vaporized coper grids and negative stained with uranyl acetate. Samples were observed in EFTEM ZEISS Libra 120 electron microscope. Electro-dense particles of 30 nm, possibly viral particles, are pointed by arrows. (D) Purified Yuc8 virions were bound to carbon vaporized coper grids and stained as described in B. Electrodense particles, which resemble astrovirus particles are pointed by arrows. Size bars are shown. (E) Immunoblotting of LEV fraction. Sample used in A, purified from Caco-2 infected cells, was not-treated (lane 1) or treated (lane 2) with trypsin, and separated in SDS-PAGE, transferred to nitrocellulose membrane and viral proteins were detected using anti-astrovirus polyclonal antibody. Viral proteins are pointed on right hand side, while molecular weight in kilodaltons is shown on left hand side. Images are representative of three independent experiments yielding similar results. Statistical analysis was done with two-way ANOVA, P value *< 0.05; **, P < 0.01; ***, P < 0.001.
To determine if there is a direct association between virions and vesicles in the LEV fraction, we analyzed by transmission electron microscopy (TEM) this fraction purified from astrovirus infected Caco2 cells. The LEV fraction was chosen since the largest increase in virus infectivity when the trypsin activation was done after the Triton X-100 treatment was observed in this fraction. By TEM we found virus-like particles, associated with what appeared to be vesicles (Fig. 4B, pointed by arrows). The electro-dense virus-like particles observed in this micrograph, are similar in form and size (30 nm) to purified astrovirus particles (Fig. 4D), suggesting that they represent bona-fide virus particles associated with membranes. Such virus-like particles were not observed in vesicles present in LEV fraction purified from mock-infected cells (Fig. 4C). Since the infectivity of astroviruses requires activation by proteolytic processing of the VP70 protein precursor, we analyzed by Western blot the virus protein composition of the LEV-associated virions. We observed that the virus particles are mainly composed by the VP70 protein (70 kDa) with no evidence of neither VP90 precursor protein, nor any activated viral proteins of 34, 27, or 25 kDa proteins (Fig. 4E).
Vesicle-associated astrovirus particles are infectious without proteolytic treatment and are protected from antibody neutralization.
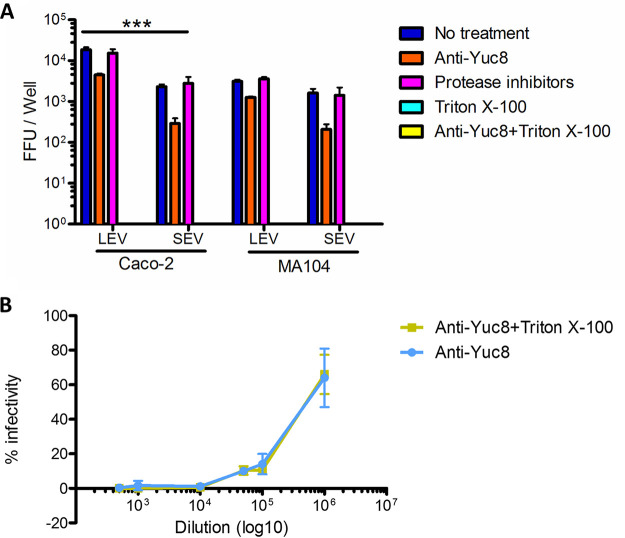
EVs have an intrinsic capacity to fuze with other cells, and thus to transfer proteins, genetic material, and even viral particles to recipient cells (14, 18, 33, 34). Using this mechanism, different types of viruses are able to infect otherwise refractory cells. Such is the case of human immunodeficiency virus 1 (HIV-1) (35, 36) and herpes simplex virus 1 (HSV-1) (27). The association with vesicles has also been shown to confer some viruses’ resistance to neutralization with specific antibodies [hepatitis A virus or HSV-1] (27, 37). To test whether vesicle-associated astrovirus strain Yuc8 is able to infect other cell lines, vesicles present in the LEV and SEV fractions purified from infected Caco-2 cells were added to Caco-2 and MA104 cells. Caco-2 cells were used as fully susceptible cell line, while MA104 cells are at least 100 times less susceptible to astrovirus Yuc8 infection (7, 38). Before adding to the cell monolayers, the samples were either not treated, or incubated with 0.1% Triton X-100 for 1 h to disrupt possible membranes; or incubated with polyclonal neutralizing polyclonal antibodies to Yuc8 to neutralize the infectivity of accessible viral particles; or incubated with detergent followed by neutralization with the neutralizing polyclonal antibodies in order to neutralize all viral particles present. Additionally, samples were treated with cocktail of protease inhibitors, to see if some cellular protease could activate astrovirus infectivity.
The results of these assays show that the LEV and SEV vesicle-associated astrovirus viral particles were able to infect both Caco-2 and MA104 cells (Fig. 5A), while viral infection was completely abolished after membrane solubilization with Triton X-100, suggesting that membranes or vesicles are indispensable for infection, since astrovirus particles in these assays were not proteolytically activated. Preincubation of both types of vesicles with anti-Yuc8 neutralizing polyclonal antibody left a fraction of virus particles infectious, suggesting that some of these viruses (10-20%) were protected from the neutralization by the antibodies, possibly by being inside the vesicles (Fig. 5A). Accordingly, pretreatment of the vesicle fractions with detergent, allowed complete antibody neutralization of the virus particles, supporting the hypothesis that vesicles in these fractions are important to allow viral infection and to shield viral particles from neutralizing antibodies. Dilution of anti-Yuc8 antibody used to neutralize virus-EVs complexes neutralizes >99% viral infectivity, and its activity is not affected by treatment with Triton X-100 (Fig. 5B). Pretreatment of samples with protease inhibitors did not have any effect on the infectivity, suggesting that cellular protease is not involved (Fig. 5A).
FIG 5.
Vesicle associated astrovirus is infective in Caco-2 and MA104 cells. (A) Large extracellular vesicles (LEV) and small extracellular vesicles (SEV) were purified by differential centrifugation and MagCapture exosome isolation kit PS from supernatants of Caco-2 cells infected with astrovirus (strain Yuc8). Samples were treated with MEM (blue); 0.1% Triton X-100 (cyan); anti-Yuc8 (orange); 0.1% TX-100 followed by anti-Yuc8 (yellow), or they were incubated with a mixture of protease inhibitors (aprotinin, leupeptin, and PMSF) (lila) before addition to Caco-2 or MA104 cells. Treated samples were let to adsorb for 2 h, after which time unbound vesicles were washed and infection was left to proceed for 18 h. Infected cells were detected by immuno-peroxidase staining. Focus forming units (FFU) of each sample were counted in 3 wells of three independent experiments. Bars represent the viral focus forming units (FFU) in each sample ± standard error of the mean. Samples treated with TX-100 and TX-100 plus anti-Yuc8 treatments did not show infected cells. Statistical analysis was done with two-way ANOVA, P value ***, P < 0.001. (B) Dilutions of anti-Yuc8 polyclonal antibody, treated or not with 0.1% TX-100, were incubated with fixed amount of Yuc8 for 1 h, after which time mixture was added to Caco-2 cells grown in 96-well plates. Results of minimum 3 experiments done in duplicate are shown. Infection is compared to infectivity of Yuc8 virus incubated in medium without antibody.
Protected viral particles were observed in both, LEV and SEV fractions, and they were able to infect a similar number of both Caco-2 and MA104 cells (Fig. 5A). When the infectivity was compared between Caco-2 and MA104 cells, Caco-2 cells showed more infected cells by LEV fraction than MA104 cells, while infection associated with SEV fraction was similar between both cell lines (Fig. 5A). In Caco-2 cells there were more infected cells after infection with vesicles from LEV fraction, compared to SEV fraction, while in MA104 cells infectivity of these two fractions was similar (Fig. 5A).
Association of nonactivated astrovirus Yuc8 with purified EVs enhances viral infectivity.
To further evaluate the possibility that the association of the virus with membranous structures promotes virus infectivity without the need of trypsin treatment, nonactivated purified Yuc8 particles were incubated for 1 h at 37°C with LEV and SEV fractions obtained from supernatants of mock-infected Caco-2 cells. After virus-EV incubation, the virus-vesicle mixture was left untreated, or subjected to the same treatments described in the previous experiment: neutralization with polyclonal anti-Yuc8 antibody, membrane solubilization by incubation with 0.1% Triton X-100, or detergent treatment followed by neutralization. After treatment, the samples were added to Caco-2 and MA104 monolayers and infection was left to proceed as described. As control, an equal amount of the purified not activated or trypsin activated astrovirus was added to cells without previous EV incubation.
The nonactivated astrovirus particles that were incubated with both types of purified vesicles (LEV and SEV fractions) acquired the capacity to infect both Caco-2 and MA104 cells (Fig. 6). Detergent treatment of the samples before addition to the cells abolished the infectivity in both cell lines, again confirming the contribution of membrane vesicles to viral infectivity of particles nonactivated by trypsin (results not shown). Pre-incubation with neutralizing antibodies abolished infectivity, as did the combined treatment of detergent and neutralizing antibodies. Of note, no infection was detected when either cell line was incubated with the same amount of nonactivated virus, in the absence of vesicles, unless the virus was activated by treatment with trypsin (Fig. 6). These results suggest that free viral particles could associate with vesicles, and this interaction facilitates their cell entry and infectivity, even if the virus is not activated. When the infectivity of the vesicle-associated nonactivated astrovirus particles was compared in Caco-2 and MA104 cells, both LEV and SEV fractions showed similar capacity to promote infection in both cell lines (Fig. 6). Of interest, nonactivated astrovirus particles incubated with purified EV, infected MA104 cells more efficiently (>200%) than the same amount of free virus activated with trypsin (Fig. 6), while in Caco-2 cells (astrovirus fully permissive cell line), the vesicle-associated particles had a 17% average infectivity of the free, trypsin activated virus (Fig. 6).
FIG 6.
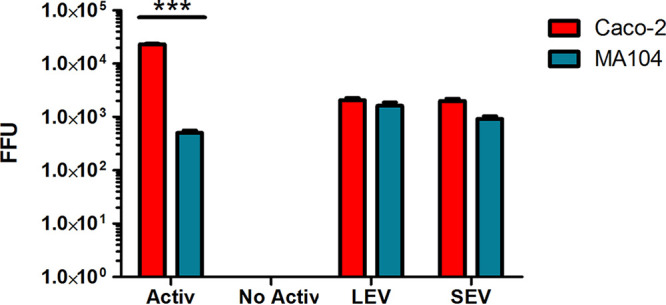
The interaction of nonactivated astrovirus Yuc8 with vesicles enhances their infectivity. Large extracellular vesicles (LEV) and small extracellular vesicles (SEV) were purified by differential centrifugation and MagCapture exosome isolation kit PS from supernatants of noninfected Caco-2 cells. Purified vesicles were incubated with purified nonactivated astrovirus Yuc8 particles for 1 h at 37°C. After incubation fractions were let to adsorb to Caco-2 (red bars) or MA104 (blue bars) cells for 2 h, after which time the unbound vesicles and viral particles were washed out. Infected cells observed were counted in three wells of three independent experiments and compared with the same amount of trypsin activated (Activ) and nonactivated (No activ) astrovirus without preincubation with EV in Caco-2 and MA104 cells. Graphic shows the number of infected cells in each sample expressed as focus forming units (FFU). Bars represent the mean FFU ± standard error of the mean. Statistical analysis was done with two-way ANOVA, P value ***, P < 0.001.
DISCUSSION
Astrovirus cell release has been reported to be a nonlytic process promoted by caspase processing of the viral capsid-precursor protein VP90 to VP70 (8, 9). It is a gradual process in which the majority of the new particles (about 90% of the total progeny) remain inside infected cells, and only 10% are released to the extracellular media (8). In this study, we found that a portion of the new progeny was present in the cell supernatant as early as 12 hpi and the amount of released virus increased with time. Interestingly, starting at 15 hpi, a significant portion of the released virus particles were not susceptible to trypsin treatment, and required to be solubilized from their association with membranous structures by detergent treatment, to become accessible to the protease. The amount of virus protected from trypsin increased with time. This observation suggests that there could be more potentially infectious virus particles in the extracellular media than originally thought (8), most probably explained by the association of viruses with EVs. Viral particles associated to EV could behave as no infectious, but after release become normal infectious particles, while no infectious viral particles, which are generated during replication of RNA viruses would remain no infectious independently of treatment realized.
Using differential centrifugation, we purified several fractions of EV from the media of astrovirus Yuc8 infected Caco-2 cells, and two of these fractions were characterized in more detail: the LEV fraction, obtained after cellular debris depletion and by centrifugation for 1 h at 20,000 g; and the SEV fraction, purified after PEG 6000 based precipitation and centrifugation at 10,000 g (29, 39). Both fractions contained markers of extracellular vesicles, CD63 and Alix. Since CD63 is an specific marker of exosomes (32, 40), and ALIX has been reported to be involved in both microvesicle and exosome biogenesis (34), it is possible that both of these vesicles, which overlap in size, are present in both fractions. Analysis of the concentration and size of the vesicles in LEV and SEV fractions by nanoparticle tracking showed partial overlap in size, however the LEV fractions showed a significative increase in the vesicle number when infected versus mock-infected conditions were compared. A similar increase in EV secretion after infection was also reported with other viruses, like HIV-1 (41, 42), HSV-1 (27, 43), rotavirus (22) and tick-borne Langat virus (44).
Different viruses have been found to be able to interact directly with EV. Among these, hepatitis A virus (HAV) (37), HCV (24), HSV-1 (27), and JC polyomavirus (28). Analysis of the LEV fraction, purified from astrovirus-infected cells, showed electro-dense astrovirus-like particles associated with vesicles of about 200 nm, resembling the appearance of extracellular vesicles (45). The membranous structures observed by TEM seem to associate with more than one viral particle. This observation opens up the possibility that during astrovirus infection, EV could participate both as virion carriers, protecting the virions, as well as a form of concentrating viral particles, forming the so called collective infectious units (CIU), capable to gather together several infectious particles (46). Similar observations have been made recently for rotavirus and norovirus, where several viral particles were reported to be associated with vesicles (22, 47).
It has been described previously that the association with vesicles could protect some viruses from neutralization by antibodies, for example hepatitis A, B, and C viruses (24, 37, 48), and HSV-1 (27), among others. In this work we observed that a portion of the astrovirus particles present in LEV and SEV fractions remained infectious even after incubation with an anti-Yuc8 neutralizing antibody, suggesting that a portion of the isolated astrovirus particles were inaccessible to the neutralizing antibodies. The presence of vesicles was crucial for the infectivity of these nonactivated viral particles, since the solubilization of membranes with detergent abrogated all infectivity. To confirm that vesicles are important in nonactivated astrovirus infectivity, LEV and SEV vesicles were purified from noninfected Caco-2 cells, and then incubated with nontrypsin activated purified astrovirus particles. Our results showed that the purified virus, was able to interact with these vesicles, and acquired the capacity to enter and infect the cells without protease activation. It is not clear if these interactions between astrovirus particle and EVs interaction are specific or not, but the virus particles in this mix acquired the capacity to infect even low susceptibility cells like MA104. The infectivity was abolished by solubilization of the vesicles with detergent, or by incubation with neutralizing antibodies, suggesting that the interaction between viral particles and EV somehow facilitates the interaction between the virus and the cell surface.
Since extracellular vesicles could facilitate the internalization of the virus, apparently through a viral receptor independent pathway, the viral particles associated with vesicles could be internalized by a mechanism triggered by vesicles themselves (49). The incubation of nonactivated purified astrovirus with LEV or SEV fractions leads to similar level of infection in both Caco-2 and MA104 cells, while nonactivated purified astrovirus particles were not able to infect these cell lines. These results suggest that the astrovirus proteolytic processing by trypsin (activation) is important for virus-cell adhesion and/or entry but probably not for the uncoating process. The ability of astrovirus particles associated with vesicles to infect not only susceptible Caco-2 cells, but also the poorly susceptible MA104 cell line (Fig. 6), suggest that the vesicle-associated virus particles could bypass certain blocks in astrovirus tropism, probably the specific virus-receptor interaction, potentially increasing their pathogenicity. This observation also suggest that extracellular vesicles could help astrovirus to disseminate outside the gastrointestinal tract like it has been reported before for HAstV serotype 4 and the novel astroviruses strains MLB and VA (2), possibly by allowing astroviruses to avoid the immune response and cellular barriers until they get into permissive cells far away from their common environment (gastrointestinal tract).
Our observations suggest the possibility that EV could be acting as platforms to create collective infectious units, rendering virus particles insensitive to neutralization with antibodies and promoting their internalization in a nonreceptor dependent manner. The mechanisms by which EV promote viral internalization in new cells remain unclear, as well as the contribution of EV to the whole astrovirus infectivity.
MATERIALS AND METHODS
Cell lines, virus, reagents, and antibodies.
Human colon adenocarcinoma cells (Caco-2), and rhesus monkey epithelial cells (MA104), were obtained from American type culture collection (ATCC, Manassas, VA). Dulbecco modified Eagle medium–high glucose (DMEM) was purchased from Sigma-Aldrich (San Luis, MI, USA), while Advanced-DMEM (A-DMEM), fetal bovine serum (FBS), and trypsin were obtained from Gibco (Thermo Fisher Scientific, USA). Triton X-100 was acquired from Boehringer Mannheim, (Germany), whereas Polyethylene glycol 6000, soybean trypsin inhibitor and Minimum Essential Medium (MEM) were acquired from Sigma-Aldrich (San Luis, MI, USA). Formaldehyde was obtained from J.T. Baker (USA), and MagCapture exosome isolation kit PS was from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Human astrovirus serotype 8, strain Yuc8 was originally isolated in our laboratory (50). Polyclonal rabbit antibody specific for Yuc8 virus (anti-Yuc8) was prepared in our laboratory (9). Rabbit polyclonal antibodies specific for anti-CD63 and anti-Alix were acquired from Santa Cruz (Santa Cruz Biotechnology, CA, USA), and Aviva Systems Biology (Aviva Systems Biology, CA, USA) respectively, while monoclonal antibody specific to anti- protein disulfide isomerase (PDI, clone 1D3) was obtained from Enzo Life Sciences, Inc (C. Mexico, Mexico). Anti-rabbit peroxidase conjugated antibody was from KPL (MD USA), and protein A, peroxidase conjugate and cholera B subunit, conjugated with biotin were from Sigma-Aldrich, and streptavidin peroxidase was from Zymed (Zymed, Thermo Fisher scientific, Rockford, IL, USA).
Cell culture and viral propagation.
Caco-2 cells were cultured in DMEM supplemented with nonessential amino acids and 15% heat-inactivated FBS, in a 10% CO2 atmosphere at 37°C (12). MA104 cells were grown in A-DMEM, supplemented with 5% FBS, at 37°C in a 5% CO2 atmosphere.
A working stock of human astrovirus serotype 8, strain Yuc8 (50), was prepared as previously described (51). The virus was activated just prior to infection with 200 μg/mL of trypsin (Sigma-Aldrich), for 1 h at 37°C, followed by inactivation with 200 μg/mL of soybean trypsin inhibitor (Sigma-Aldrich). Before infection, Caco-2 cell monolayers were washed with MEM and incubated with activated virus for 1 h at 37°C. Then, cell monolayers were washed twice with MEM, to remove nonadsorbed virus. Finally, MEM without trypsin or FBS was added to the cells, and infection was left to proceed for 48 h at 37°C.
Astrovirus particles were purified essentially as described previously. Briefly, Caco-2 cells were infected with HAstV serotype 8 (Yuc8) at an MOI of 5 as described above and the infection was left to proceed for 48 h. After this time cells were detached, and frozen and thawed three times. Then, cellular lysate was clarified by centrifugation at 2,000 g for 10 min and then passed through a 0.45-μm filter (Milipore). Filtered supernatants were pelleted at 60,000 g for 16 h at 4°C in a SW28 Ti rotor (Beckman), and the resulting pellet was resuspended in TNE buffer (50 mM Tris-HCl [pH 7.4], 0.1 M NaCl, 10 mM EDTA). This suspension was adjusted to 0.5% vol/vol with octyl glucoside in TNE buffer and incubated for 30 min on ice. Finally, virus was pelleted through a 30% wt/vol sucrose cushion in TNE buffer for 2 h at 200,000 g in a SW55 Ti Beckman rotor. The pelleted viral particles were resuspended in TNE buffer.
Viral infectivity assay.
Viral titers were determined by immuno-peroxidase staining to detect infectious focus forming units (FFU) as described previously (9). In brief, Caco-2 cells were cultured to confluence in 96 wells plates and washed with serum-free MEM before infection. Viral samples were activated with trypsin (200 μg/mL), for 1 h at 37°C, soybean trypsin inhibitor was added (200 μg/mL), and serial 2-fold dilutions of activated viral samples were performed. Diluted samples were added to each well and let to adsorb for 1 h at 37°C. After adsorption period, the virus inoculum in each well was removed, cells were washed twice with MEM and infection was left to proceed in fresh MEM for 18 h at 37°C. Cells were fixed for 20 min with 2% formaldehyde in phosphate-buffered saline (PBS), then they were washed three times with PBS and permeabilized by a 15 min incubation with 0.2% Triton X-100 solution in PBS. Finally, cells were washed again three times with PBS and incubated with a polyclonal rabbit anti-Yuc8 overnight at 4°C. Next day cells were washed out three times with PBS and incubated with peroxidase conjugated protein A for 2 h at 37°C. After washing protein A, infected cells were revealed by carbazole precipitation and FFU were counted. In each experiment, three dilutions done in duplicate were counted.
To test neutralization capacity of polyclonal anti-Yuc8 antibody, dilutions of antibody were incubated with constant amount of virus for 1 h at 37°C. After incubation, antibody-virus mixture was transferred to Caco-2 cells grown in 96-well plates for 1 h. After this time cells were washed, and infection and staining were done as described above. When desired, antibody was incubated with 0.1% Triton X-100 before dilutions.
Kinetics of viral release.
Caco-2 cells were grown to confluence in 24 wells plates. Cell monolayers were washed twice with MEM and infected with activated HAstV Yuc8 strain (at an MOI of 5). No trypsin or FBS were added to MEM. Supernatants were harvested at 3 h intervals starting at 12 h postinfection (hpi) until 24 hpi and centrifuged for 5 min at 500 g to separate cellular debris. At the same time, MEM was added to cellular monolayers and cells were lysed by two cycles of freeze-thaw. Infectious viral particles associated to cells and present in supernatants were determined by an immune-peroxidase assay as described above. Before trypsin activation, supernatants were incubated for 30 min at 37°C with MEM or with 0.1% Triton X-100 diluted in MEM. The cell membrane integrity in each time point was confirmed by measuring lactic dehydrogenase (LDH) in media by in vitro toxicity assay kit, Lactic Dehydrogenase based (Sigma-Aldrich), as described by the manufacturer.
Separation of extracellular vesicles on density gradient.
Caco-2 cells were grown in 150cm2 flasks until confluence, washed twice with MEM without FBS and antibiotics and infected with Yuc8 (MOI of 1), for 1 h at 37°C. After this time, cells were washed twice, and the infection was left to proceed for 18 h in serum free MEM. Supernatants were collected, possible floating cells and cell debris were removed by centrifugation at (2, 000 g), and the remaining content was mixed with an equal volume of a solution of 16% polyethylene glycol 6000 (PEG), 1 M sodium chloride and left overnight at 4°C. The mixture was then centrifuged at 10,000 g for 1 h. Resulting pellet was resuspended in TNC (10 mM Tris pH 8.0, 140 mM NaCl, 10 mM CaCl2) and loaded in bottom of a centrifugation tube (SW55, Beckman) in 40% iodixanol/250 mM sucrose. To analyze possible association of virus particles with membrane vesicles, one half of the sample was treated with 1% Triton X-100 at 37°C for 1 h before addition of iodixanol/sucrose mixture and centrifugation. Samples were overlaid with iodixanol (35%, 30%, 25%, and 15% in TNC/250 mM sucrose) with pure TNC being put on the top. Samples were centrifuged for 4 h at 200,000 g, and 10 fractions were collected from the top of the tubes.
Purification of extracellular vesicles.
Caco-2 cells, grown to confluence in 150 cm2 flasks, were washed twice with MEM and infected with trypsin activated HAstV Yuc8 at an MOI of 5. As a control, cells were mock infected using an identical protocol without virus. Supernatants were harvested at 18 hpi and processed by differential centrifugation essentially as described before (30, 39). Briefly, supernatants were centrifuged at 500 g for 5 min to obtain P1, and the supernatant was again centrifuged at 2,000 g for 30 min, obtaining P2. The remaining supernatant was centrifuged at 20,000 g for 1 h, producing pellet 3. Finally, the last supernatant was mixed with an equal volume of a solution of 16% polyethylene glycol 6000 (PEG), 1 M sodium chloride and left overnight at 4°C. The mixture was then centrifuged at 10,000 g for 1 h, yielding pellet 4. As proposed by a theoretical analysis of sedimentation (29), the purified fraction in pellet 3 was considered to contain LEV, while the fraction of pellet 4 contains SEV. All centrifugations were performed at 4°C and all pellets were resuspended in sterile PBS. Virus titer in purified fractions was determined by immune-peroxidase assay, with and without TX-100 treatment as described above for supernatants in the assays of viral release kinetics.
For some experiments, in order to remove possible contaminants (i.e., free contaminating virions or protein aggregates) from purified vesicles in LEV or SEV fractions (pellets 3 and 4, respectively), the vesicle fractions were additionally purified using the MagCapture exosome isolation kit PS (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), according to the manufacturer protocol.
Immunodetection of cellular and viral proteins.
The fractions purified by differential centrifugation from supernatants of infected and mock-infected Caco-2 cells were mixed with Laemmli sample buffer (50 mM Tris, pH 7.5, 2% SDS, 2% β-mercaptoethanol, 10 mM EDTA, and 0.1% bromophenol blue), boiled for 5 min and the proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to a nitrocellulose membrane (Millipore, Bedford, MA). Membranes were blocked with 5% nonfat dried milk in PBS. The proteins of interest were detected with specific primary antibodies followed by incubation with secondary peroxidase-conjugated reagents. Primary antibodies were incubated with membranes overnight at 4°C, washed three times with PBS 0.1% Tween (PBS-T) and incubation continued with peroxidase conjugated secondary antibody or protein A for 90 min, at room temperature. After these incubations the membranes were washed again with PBS-T and proteins were visualized by Western Lightning Chemiluminescence Reagent Plus (Perkin Elmer). To detect ganglioside GM1, 3 μL of samples were directly blotted onto nitrocellulose membrane and dried. Detection was done using Cholera B subunit, conjugated with biotin, followed by incubation with peroxidase conjugated streptavidin.
Infectivity associated to the extracellular vesicles.
Fractions were purified from supernatant of astrovirus infected Caco-2 cells by differential centrifugation coupled with isolation with magnetic beads (MagCapture exosome isolation kit PS), as described above. Vesicle containing fractions were diluted in MEM and added to Caco-2 and MA104 cells, grown to confluence in 96 wells plates, washed twice with MEM before addition. The fractions were submitted to the following treatments before adsorption: with 0.1% Triton X-100 for 30 min at 37°C; or were preincubated with an anti-Yuc8 neutralizing antibody (final dilution 1:1500) for 1 h at 37°C; or by an incubation with 0.1% Triton X-100 for 30 min at 37°C, followed by neutralization with anti-Yuc8 antibody (1 h at 37°C), or they were incubated with a mixture of protease inhibitors (20 μg/mL aprotinin, 20 μg/mL leupeptin, and 1 mM PMSF). Experimental, nontreated samples were incubated under the same conditions but using an equivalent volume of FBS-free MEM instead of Triton X-100 and/or anti-Yuc8 antibody. Vesicles were left to adsorb to cells during 2 h at 37°C, then cells were washed, fresh medium was added, and the infection was left to proceed for 18 h.
To test the capacity of purified EV to promote infection with externally bound virus particles, LEV and SEV vesicles were purified from noninfected Caco-2 cells as described above, including the isolation step with the MagCapture exosome isolation kit PS. These vesicles were then incubated for 1 h at 37°C with a known amount of nonactivated purified HAstV Yuc8 (1100 FFU per well). After incubation, the vesicles were treated as described above (0.1% Triton X-100 for 30 min at 37°C, anti-Yuc8 neutralizing antibody for 1 h at 37°C or 0.1% Triton X-100 followed by neutralization with anti-Yuc8 antibody). After the treatments, vesicles with virus were added to Caco-2 or MA104 cells grown in 96 wells, and incubated during 2 h at 37°C. After this time cells were washed, and infection was left to proceed for 18 h. As control, an equal amount of the identical purified trypsin activated or nonactivated astrovirus was used under the same conditions without EV incubation. Infected cells were counted in selected area, of two wells per sample using 20X lens. Images were acquired with 10X lens in a Nikon Diaphot 300 microscope.
Transmission electron microscopy.
LEV vesicular fraction was purified from infected Caco-2 cells as described above, using differential centrifugation coupled to isolation with the MagCapture exosome isolation kit PS. Purified fraction and nonactivated purified virus particles were bound on carbon vaporized copper grids covered with Formvar and negatively stained with 3% uranyl acetate. Images were acquired using a Zeiss Libra 120 electron microscope operating at 80 KV coupled with a GATAN Multiscan 600HP 794 CCD camera.
Nanoparticle tracking analysis.
Nanoparticle tracking analysis, was conducted using a NanoSight NS300 (Malvern Instruments Ltd., Worcestershire, UK) to assess the hydrodynamic diameter of nonactivated virus particles and vesicles purified by differential centrifugation from infected and noninfected cells supernatants. Purified fractions were analyzed after diluting the samples in sterile and microfiltered PBS (1:100-200 in the case of vesicles and 1:1,000 in case of purified virus particles). For each condition 5 videos of 1-min length each, were recorded sizing 20–40 particles/frame and analyzed using the NanoSight NTA 3.1 software (52). This technique uses dynamic light scattering to measure the diffusion coefficient of particles moving under Brownian motion and converts it to hydrodynamic diameter using the Stokes-Einstein equation (53). A blank of sterile filtered PBS was used for particle calculations in every measurement, and after each measurement the flushing lines were thoroughly washed three times to prevent contamination.
Statistical analysis.
Statistical analysis of the obtained results was performed using the GraphPad prism 5.0 software (GraphPad Software, Inc.), with an interval of confidence of 95%.
ACKNOWLEDGMENTS
This work was supported by grants 254608 and 302965 from CONACyT (Mexico). We thank to Dra. Guadalupe Zavala for her assistance in transmission electron microscopy. Carlos Baez-N was the recipient of a scholarship from CONACyT (Mexico), No. 464741 for MS degree, and had support from the postgraduate studies program (PAEP) of the National Autonomous University of Mexico (UNAM).
Contributor Information
Pavel Iša, Email: pavel.isa@ibt.unam.mx.
Rebecca Ellis Dutch, University of Kentucky College of Medicine.
REFERENCES
- 1.Meliopoulos V, Schultz-Cherry S. 2012. Astrovirus pathogenesis, p 65–75. In Schultz-Cherry S (ed), Astrovirus research. Springer, New York, NY. [Google Scholar]
- 2.Vu DL, Bosch A, Pinto RM, Guix S. 2017. Epidemiology of classic and novel human astrovirus: gastroenteritis and beyond. Viruses 9:33. 10.3390/v9020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosch A, Pinto RM, Guix S. 2014. Human astroviruses. Clin Microbiol Rev 27:1048–1074. 10.1128/CMR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendez E, Arias CF. 2013. Astroviruses, p 1347–1401. In Knipe DM (ed), Fields vVirology. Lippincot Williams and Wilkins, Philadephia. [Google Scholar]
- 5.Aguilar-Hernandez N, Meyer L, Lopez S, DuBois RM, Arias CF. 2020. Protein disulfide isomerase A4 is involved in genome uncoating during human astrovirus cell entry. Viruses 13:53. 10.3390/v13010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arias CF, DuBois RM. 2017. The astrovirus capsid: a review. Viruses 9:15. 10.3390/v9010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinker JP, Blacklow NR, Herrmann JE. 2000. Human astrovirus isolation and propagation in multiple cell lines. Arch Virol 145:1847–1856. 10.1007/s007050070060. [DOI] [PubMed] [Google Scholar]
- 8.Banos-Lara M.dR, Méndez E. 2010. Role of individual caspases induced by astrovirus on the processing of its structural protein and its release from the cell through a non-lytic mechanism. Virology 401:322–332. 10.1016/j.virol.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 9.Mendez E, Salas-Ocampo E, Arias CF. 2004. Caspases mediate processing of the capsid precursor and cell release of human astroviruses. J Virol 78:8601–8608. 10.1128/JVI.78.16.8601-8608.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguilar-Hernandez N, Lopez S, Arias CF. 2018. Minimal capsid composition of infectious human astrovirus. Virology 521:58–61. 10.1016/j.virol.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Mendez E, Fernandez-Luna T, Lopez S, Mendez-Toss M, Arias CF. 2002. Proteolytic processing of a serotype 8 human astrovirus ORF2 polyprotein. J Virol 76:7996–8002. 10.1128/jvi.76.16.7996-8002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendez E, Aguirre-Crespo G, Zavala G, Arias CF. 2007. Association of the astrovirus structural protein VP90 with membranes plays a role in virus morphogenesis. J Virol 81:10649–10658. 10.1128/JVI.00785-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altan-Bonnet N, Chen YH. 2015. Intercellular transmission of viral populations with vesicles. J Virol 89:12242–12244. 10.1128/JVI.01452-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barteneva NS, Maltsev N, Vorobjev IA. 2013. Microvesicles and intercellular communication in the context of parasitism. Front Cell Infect Microbiol 3:49. 10.3389/fcimb.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schorey JS, Cheng Y, Singh PP, Smith VL. 2015. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep 16:24–43. 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raposo G, Stoorvogel W. 2013. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383. 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach J-M, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, et al. 2018. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7:1535750. 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thery C, Ostrowski M, Segura E. 2009. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9:581–593. 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 19.Karpe YA, Meng XJ. 2012. Hepatitis E virus replication requires an active ubiquitin-proteasome system. J Virol 86:5948–5952. 10.1128/JVI.07039-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai FW, Lichty BD, Bowdish DM. 2015. Microvesicles: ubiquitous contributors to infection and immunity. J Leukoc Biol 97:237–245. 10.1189/jlb.3RU0513-292RR. [DOI] [PubMed] [Google Scholar]
- 21.Altan-Bonnet N. 2016. Extracellular vesicles are the Trojan horses of viral infection. Curr Opin Microbiol 32:77–81. 10.1016/j.mib.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isa P, Perez-Delgado A, Quevedo IR, Lopez S, Arias CF. 2020. Rotaviruses associate with distinct types of extracellular vesicles. Viruses 12:763. 10.3390/v12070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meckes DG, Jr. 2015. Exosomal communication goes viral. J Virol 89:5200–5203. 10.1128/JVI.02470-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z, Zhang X, Yu Q, He JJ. 2014. Exosome-associated hepatitis C virus in cell cultures and patient plasma. Biochem Biophys Res Commun 455:218–222. 10.1016/j.bbrc.2014.10.146. [DOI] [PubMed] [Google Scholar]
- 25.Nagashima S, Takahashi M, Kobayashi T, Nishizawa T, Nishiyama T, Primadharsini PP, Okamoto H, Tanggis . 2017. Characterization of the quasi-enveloped hepatitis E virus particles released by the cellular exosomal pathway. J Virol 91. 10.1128/JVI.00822-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamai K, Shiina M, Tanaka N, Nakano T, Yamamoto A, Kondo Y, Kakazu E, Inoue J, Fukushima K, Sano K, Ueno Y, Shimosegawa T, Sugamura K. 2012. Regulation of hepatitis C virus secretion by the Hrs-dependent exosomal pathway. Virology 422:377–385. 10.1016/j.virol.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Bello-Morales R, Praena B, de la Nuez C, Rejas MT, Guerra M, Galan-Ganga M, Izquierdo M, Calvo V, Krummenacher C, Lopez-Guerrero JA. 2018. Role of microvesicles in the spread of herpes simplex virus 1 in oligodendrocytic cells. J Virol 92. 10.1128/JVI.00088-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris-Love J, Gee GV, O’Hara BA, Assetta B, Atkinson AL, Dugan AS, Haley SA, Atwood WJ. 2019. JC polyomavirus uses extracellular vesicles to infect target cells. mBio 10. 10.1128/mBio.00379-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livshits MA, Livshts MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE, Generozov EV, Govorun VM. 2015. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci Rep 5:17319. 10.1038/srep17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thery C, Amigorena S, Raposo G, Clayton A. 2006. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol [DOI] [PubMed] [Google Scholar]
- 31.Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, Gangoda L, Mathivanan S. 2016. ExoCarta: A web-based compendium of exosomal cargo. J Mol Biol 428:688–692. 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, Bond VC, Borràs FE, Breakefield X, Budnik V, Buzas E, Camussi G, Clayton A, Cocucci E, Falcon-Perez JM, Gabrielsson S, Gho YS, Gupta D, Harsha HC, Hendrix A, Hill AF, Inal JM, Jenster G, Krämer-Albers E-M, Lim SK, Llorente A, Lötvall J, Marcilla A, Mincheva-Nilsson L, Nazarenko I, Nieuwland R, Nolte-'t Hoen ENM, Pandey A, Patel T, Piper MG, Pluchino S, Prasad TSK, Rajendran L, Raposo G, Record M, Reid GE, Sánchez-Madrid F, Schiffelers RM, Siljander P, Stensballe A, Stoorvogel W, Taylor D, Thery C, Valadi H, van Balkom BWM, et al. 2012. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol 10:e1001450. 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kourembanas S. 2015. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol 77:13–27. 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 34.van Niel G, D'Angelo G, Raposo G. 2018. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19:213–228. 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 35.Mack M, Kleinschmidt A, Bruhl H, Klier C, Nelson PJ, Cihak J, Plachy J, Stangassinger M, Erfle V, Schlondorff D. 2000. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med 6:769–775. 10.1038/77498. [DOI] [PubMed] [Google Scholar]
- 36.Rozmyslowicz T, Majka M, Kijowski J, Murphy SL, Conover DO, Poncz M, Ratajczak J, Gaulton GN, Ratajczak MZ. 2003. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS 17:33–42. 10.1097/00002030-200301030-00006. [DOI] [PubMed] [Google Scholar]
- 37.Feng Z, Hensley L, McKnight KL, Hu F, Madden V, Ping L, Jeong SH, Walker C, Lanford RE, Lemon SM. 2013. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 496:367–371. 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aguilar-Hernandez N. 2018. Caracterizacion de las interacciones tempranas de astrovirus de humano con celulas permisivas y resistentes a la infeccion. master. Universidad Nacional Autonoma de Mexico. [Google Scholar]
- 39.Rider MA, Hurwitz SN, Meckes DG, Jr, 2016. ExtraPEG: a polyethylene glycol-based method for enrichment of extracellular vesicles. Sci Rep 6:23978. 10.1038/srep23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C. 2016. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA 113:E968–77. 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arenaccio C, Chiozzini C, Columba-Cabezas S, Manfredi F, Affabris E, Baur A, Federico M. 2014. Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4+ T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism. J Virol 88:11529–11539. 10.1128/JVI.01712-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kulkarni R, Prasad A. 2017. Exosomes derived from HIV-1 infected DCs mediate viral trans-infection via fibronectin and galectin-3. Sci Rep 7:14787. 10.1038/s41598-017-14817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deschamps T, Kalamvoki M. 2018. Extracellular vesicles released by herpes simplex virus 1-infected cells block virus replication in recipient cells in a STING-dependent manner. J Virol 92. 10.1128/JVI.01102-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou W, Woodson M, Neupane B, Bai F, Sherman MB, Choi KH, Neelakanta G, Sultana H. 2018. Exosomes serve as novel modes of tick-borne flavivirus transmission from arthropod to human cells and facilitates dissemination of viral RNA and proteins to the vertebrate neuronal cells. PLoS Pathog 14:e1006764. 10.1371/journal.ppat.1006764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rikkert LG, Nieuwland R, Terstappen L, Coumans FAW. 2019. Quality of extracellular vesicle images by transmission electron microscopy is operator and protocol dependent. J Extracell Vesicles 8:1555419. 10.1080/20013078.2018.1555419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanjuan R. 2017. Collective infectious units in viruses. Trends Microbiol 25:402–412. 10.1016/j.tim.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santiana M, Ghosh S, Ho BA, Rajasekaran V, Du WL, Mutsafi Y, De Jesus-Diaz DA, Sosnovtsev SV, Levenson EA, Parra GI, Takvorian PM, Cali A, Bleck C, Vlasova AN, Saif LJ, Patton JT, Lopalco P, Corcelli A, Green KY, Altan-Bonnet N. 2018. Vesicle-cloaked virus clusters are optimal units for inter-organismal viral transmission. Cell Host Microbe 24:208–220 e8. 10.1016/j.chom.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y, Han Q, Hou Z, Zhang C, Tian Z, Zhang J. 2017. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell Mol Immunol 14:465–475. 10.1038/cmi.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA. 2015. Exosomes: mechanisms of uptake. J Circ Biomark 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mendez-Toss M, Romero-Guido P, Munguia ME, Mendez E, Arias CF. 2000. Molecular analysis of a serotype 8 human astrovirus genome. J Gen Virol 81:2891–2897. 10.1099/0022-1317-81-12-2891. [DOI] [PubMed] [Google Scholar]
- 51.Mendez E, Salas-Ocampo MP, Munguia ME, Arias CF. 2003. Protein products of the open reading frames encoding nonstructural proteins of human astrovirus serotype 8. J Virol 77:11378–11384. 10.1128/jvi.77.21.11378-11384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quevedo IR, Olsson ALJ, Clark RJ, Veinot JGC, Tufenkji N. 2014. Interpreting deposition behavior of polydisperse surface-modified nanoparticles using QCM-D and sand-packed columns. Environmental Engineering Science 31:326–337. 10.1089/ees.2013.0302. [DOI] [Google Scholar]
- 53.Hassellow M, Kaegi R. 2009. Analysis and characterization of manufactured nanoparticles in aquatic environments., p 211–266. In Lead JR, Smith E (ed), Environmental and human health impacts of nanotechnology. Wiley, Chichester, West Sussex, UK. [Google Scholar]