ABSTRACT
African swine fever virus (ASFV) is the etiological agent of African swine fever (ASF), a devastating disease affecting domestic and wild swine and currently causing a global pandemic, severely affecting swine production. Here, we demonstrate that the deletion of the previously uncharacterized ASFV gene, H108R from the highly virulent ASFV-Georgia2007 (ASFV-G) genome strain, reduces virulence in domestic swine. ASFV-G-ΔH108R, a recombinant virus with the H108R gene deleted, was used to evaluate the involvement of the H108R gene for ASFV replication and virulence in swine. ASFV-G-ΔH108R showed a delayed replication in swine macrophage cultures. A group of five pigs, intramuscularly inoculated with 102 HAD50 of ASFV-G-ΔH108R, was observed over a 28-day period and compared with a similar group of animals inoculated with similar doses of the parental virulent virus. While all animals inoculated with ASFV-G developed an acute fatal disease, ASFV-G-ΔH108R inoculated animals, with the exception of one animal showing a protracted but fatal form of the disease, all survived the infection, remaining clinically healthy during the observational period. The surviving animals presented protracted viremias with lower virus titers compared with those of animals inoculated with the parental virus, and all of them developed a strong virus-specific antibody response. Importantly, all animals surviving ASFV-G-ΔAH108R infection were protected when challenged with the virulent parental strain, ASFV-G. This report constitutes the first evidence that the H108R gene is involved in ASFV virulence in swine and that the deletion of this gene may be used as a tool to increase the attenuation of currently experimental vaccines to improve their safety profiles.
IMPORTANCE Currently, there is no commercial vaccine available to prevent ASF. ASFV-Georgia2007 (ASFV-G) and its field isolate derivatives are producing a large pandemic which is drastically affecting pork production in Eurasia. We present here the discovery of a novel virus determinant of virulence, the H108R gene, which, when deleted from the ASFV-G genome, significantly reduces virus virulence in domestic swine. Additionally, animals that survive the inoculation with a recombinant virus harboring a deletion of the H108R gene, ASFV-G-ΔH108R, are protected against a challenge with the virulent parental virus. Although presenting residual virulence, ASFV-G-ΔH108R confers protection even at low doses (102 HAD50), demonstrating its potential to be used as an additional gene deletion to increase the safety profile of the preexisting vaccine candidate.
KEYWORDS: ASF, ASFV, African swine fever, African swine fever virus, H108R, pandemic, swine, virulence
INTRODUCTION
African swine fever (ASF) is a devastating disease of swine present in more than 20 sub-Saharan African countries and currently being considered a pandemic affecting countries in a large contiguous geographical area from Central Europe to East and Southeast Asia. In the last months, the disease was also detected in the Dominican Republic and Haiti after more than 50 years of being absent from the Western hemisphere. This situation is causing significant economic losses in the pig industry and a shortage in food availability in the affected countries. The causative agent of this epidemiological situation, African swine fever virus (ASFV) strain Georgia 2007 (ASFV-G), is a highly virulent strain that belongs to the ASFV genotype II group (1), which has remained genetically stable with only minor genome modifications since the initial outbreak in 2007 in the Republic of Georgia.
ASFV is a large and structurally complex virus, presenting a double envelope and harboring a large (180 to 190 kbp), double-stranded DNA genome (2), encoding approximately 150 to 160 genes. The roles of most of these genes remain unknown or have only been predicted by functional genomics. Actually, just a few virus proteins have been experimentally studied to determine their function (3–13).
Currently, there is no commercial vaccine available to prevent ASF, so control of the disease is based on the culling of the affected animals. Although a variety of experimental vaccines have been tested in past years (2), the use of live attenuated virus (LAV) strains is the more effective way to protect animals against challenges with virulent homologous field isolates (14–21). Attenuation of virulent viruses has been achieved by deleting the ASFV genes associated with virulence in swine through genetic manipulation. Using this approach, several experimental recombinant vaccine candidates have been developed which efficiently protect pigs against a challenge with the ASFV-G isolate or its field isolate derivatives (15–19, 22–26). Therefore, the discovery of virus genes important to the process of disease production is a critical first step in designing recombinant LAVs to prevent ASFV infection and disease.
Although the first-generation experimental ASF vaccines that use recombinant viruses appear to be promising tools, additional genetic deletions may be necessary to increase their safety profiles. Nevertheless, the combination of multiple gene deletions has been shown to usually lead to a diminished virus replication of the vaccine candidates when inoculated in pigs, along with a reduction of their protective efficacy (27–29). Interestingly, the deletion of even highly conserved virus genes has been shown to have different consequences in terms of virus virulence, depending on the virus strain considered (18, 22, 26, 30). All of these factors make the identification and characterization of novel genetic determinants of virulence an essential issue for the rational production of the next-generation live attenuated vaccine candidates to protect swine against the current pandemic strain of ASFV. This knowledge could be critical to increasing the safety profile of a current vaccine strain or to achieving attenuation in a novel, emerging ASFV isolate.
We present here the identification of a novel determinant of virulence, ASFV gene H108R. The protein encoded by the H108R gene has been shown to localize at the inner envelope of the virus particle (31), but nothing is known regarding its function. Deletion of the gene from the genome of a virulent ASFV-G isolate (producing ASFV-G-ΔH108R) drastically reduces its virulence when inoculated in swine. Animals surviving an ASFV-G-ΔH108R infection developed a strong ASFV-specific antibody response and are completely protected when challenged with the virulent parental ASFV-G.
RESULTS
Genetic diversity of the H108R gene of ASFV.
To evaluate the genetic diversity of the H108R gene, we used a set of nine ASFV isolates that represent the genetic diversity of this gene in nature. These isolates were chosen based on an initial screening produced by BLAST analysis using the ASFV strain Georgia 2007/1 (representative of the pandemic Eurasian lineage, genotype II) as a query. Final ASFV strains included: LIV 5 40 (genotype I), BA71V (genotype I), Pretoriuskop/96/4 (genotype XX), SPEC 57 (genotype III), RSA 2 2008 (genotype XXII), Malawi Lil-20/1 (genotype VIII), Ken06.Bus (genotype X), and Kenya 1950 (genotype X).
Our results, using pairwise analysis over the model p-distance and the bootstrap method to get a confidence interval of 95% in our calculations (32), indicated a high degree of conservation of H108R in nature. At the nucleotide and amino acid levels, the overall identity was calculated between 92.10% and 99.41% (~99.19%) and between 87.71% and 99.12% (~94.29%), respectively.
The length of the H108R protein varied between 108 and 111 amino acids. The largest versions of this protein are associated with the three amino acid insertions present in the isolates, Malawi Lil-20/1, Ken06.Bus, and Kenya 1950 (Fig. 1A). Also, an additional deletion at position 47 was recorded in the isolate, Kenya 1950. Overall, 100% of identity was observed among multiple isolates reported in the GenBank database as being associated with the epidemic Eurasian lineage, indicating the high conservation of this gene throughout this pandemic lineage. A unique feature in this lineage was the substitution S54F, observed in the Georgia 2007/1 isolate, a representative of the pandemic lineage (Fig. 1A).
FIG 1.
Evaluation of the H108R protein across ASFV isolates. (A) Amino acid alignment representing the diversity of the H108R protein of ASFV in the field. Residues in white spots represent changes between amino acids with different charges. Conservation plot scores reflect the nature of the change in specific sites, and high scores are associated with changes with similar biological properties. Alignment was produced using the Jalview version 2.11.1.4 software package. (B) Phylogenetic analysis conducted via the maximum likelihood method and the Tamura-3 parameter model, showing the diversity of the H108R gene of ASFV in the field. Based on the cluster distribution, isolates were categorized into three main groups. Numbers above internal branches represent bootstrap values (1,000 repetitions). (C) The graphic represents the dN (rate of evolution at nonsynonymous sites) to dS (rate of evolution at synonymous sites) ratio (dN-dS) at specific codon sites in the H108R gene of ASFV. Blue and red asterisks represent codon sites evolving under diversifying and purifying selection, respectively. Analyses were conducted using the evolutionary algorithms FEL and MEME, using cutoff values of P = 0.1.
Based on the genetic distance among the isolates, and supported by a phylogenetic analysis of the H10R gene, we predicted the existence of 3 main genetic groups being the Georgia 2007/1 isolate associated with the genetic group I (Fig. 1B).
No homology with other proteins was found when the H108R protein was compared with the other 19,175 protein families using the program Pfam (33). However, a transmembrane region between amino acids 6 and 23 was predicted in the N-terminal region of this protein. Interestingly, the essential role in the virulence of H108R reported herein is consistent with other small transmembrane proteins, such as I5L, present in poxviruses, where its function has been associated with the enhancement of viral replication and virulence (34).
To get more insights about the evolution of the H108R gene, we conducted a comprehensive evolutionary analysis. Overall, no evidence of recombination was found when the genetic algorithm for detecting recombination (GARD) (35) analyzed different representative isolates. We found overall dN-dS values equal to 0.434, indicating that purifying selection has been the main driver during the evolution of this gene. Consistent with this assumption, the algorithm fixed effects likelihood (FEL) identified 5 five codons evolving under purifying selection (Fig. 1C) (36). Interestingly, these codons, encoding amino acids 22, 23, 28, 41, and 45, are spanning at a highly conserved region of this protein (amino acids 1 to 46), indicating the potential relevance of these sites for the functionality of the H108R protein. Finally, only amino acid 50 was identified under positive selection by the algorithm and mixed effects model of evolution (MEME) (37). Interestingly, the substitution N50M was associated with the divergence of the isolates Malawi Lil-20/1, Ken06.Bus, and Kenya 1950 from the rest of the ASFV strains (Fig. 1C).
Transcriptional profile of the ASFV H108R gene.
To determine the transcriptional profile of the H108R gene during the replication cycle, a time course experiment was performed to analyze the kinetics of RNA transcription in primary swine macrophages infected with ASFV-G. Primary swine macrophage cultures were infected (MOI = 1) with ASFV-G, and samples of cell extracts were taken at 4, 6, 8, and 24 hpi. The presence of H108R RNA was detected by two-step RT-PCR, as described in the Materials and Methods section. Transcription of the H108R gene was detected at 4 hpi and remained stable until 24 hpi (Fig. 2). Two well-characterized ASFV proteins, the early protein p30 (CP204L) and the late protein p72 (B646L), were used as references of early and late transcription profiles, respectively. The expression of H108R overlaps the kinetics of the B646L gene; therefore, it should be considered a late gene. This suggests that the ASFV H108R gene encodes a protein that is expressed late during the virus replication cycle.
FIG 2.
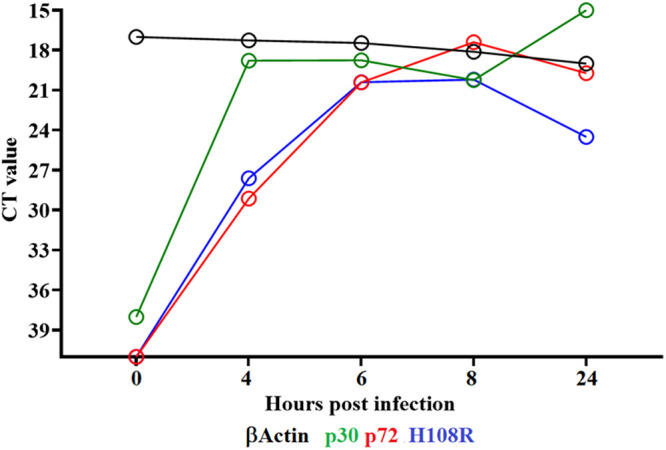
Expression profile of the H108R gene of ASFV during in vitro infection of porcine macrophages. Reverse transcription followed by qPCR was used to evaluate the expression profile of the H108R gene during in vitro infection at different time points, up to 24 h. As a reference for this analysis, we use qPCRs to specifically detect the expression of genes encoding ASFV proteins p30 (early expression) and p72 (late expression). Additionally, the β-actin gene was used as a control to evaluate the quality and levels of RNA during the infection at different time points.
Development of an H108R gene deletion mutant based on the ASFV strain Georgia.
The function of the protein encoded by the H108R gene is still unknown. To study the function of the H108R gene in virus replication in swine macrophages and in ASFV virulence in swine, a recombinant virus, which deleted the H108R gene, was developed (ASFV-G-ΔH108R). ASFV-G-ΔH108R was produced using the highly virulent ASFV strain, Georgia 2007 (ASFV-G), as the parental virus. The H108R gene was deleted following a homologous recombination procedure, as described in detail in the Materials and Methods section, substituting the H108R gene with a cassette containing a fluorescent reporter gene, mCherry, under the ASFV p72 promoter (Fig. 3). The stock of purified recombinant ASFV-G-ΔH108R was obtained after 16 limiting dilution purification steps based on the presence of fluorescent activity.
FIG 3.
Diagram indicating the position of the H108R open reading frame in the ASFV-G genome. The donor plasmid with the homologous arms to ASFV-G and the mCherry under the control of the p72 promoter in the orientation are indicated. The final genomic changes introduced to develop ASFV-G-ΔH108R where the sequence of the donor plasmid mCherry reporter is introduced to replace the ORF of H108R are indicated. The nucleotide positions indicated refer to the nucleotide positions in the parental ASFV-G genome.
The accuracy of the genetic modifications during the development of ASFV-G-ΔH108R were evaluated by Next Generation Sequencing (NGS) along with an assessment of the genome integrity to confirm the purity of the recombinant virus stock. Comparative DNA sequence analyses between ASFV-G-ΔH108R and ASFV-G showed an 86 nucleotide deletion (from nucleotide positions 155,233 to 155,318) from the H108R gene, consistent with the designed modifications. The genome of ASFV-G-ΔH108R also showed an insertion of 1226 nucleotides which corresponds to the length of the p72mCherryΔH108R cassette replacing the deletion of the H108R gene. No undesired modifications were detected in the rest of the ASFV-G-ΔH108R genome.
Replication of ASFV-G-ΔH108R in primary swine macrophages.
To evaluate the effect of the H108R gene deletion in virus replication, in vitro cultures of primary swine macrophages, the natural target cell during ASFV infection in swine, were infected with ASFV-G-ΔH108R. The replication kinetics of ASFV-G-ΔH108R compared with those of the parental ASFV-G were assessed in multistep growth curves (Fig. 4). Swine macrophages were infected with an MOI of 0.01, and samples were harvested at 2, 24, 48, 72, and 96 h postinfection (hpi). Results demonstrated that, although at the final sample time ASFV-G-ΔH108R presented similar virus yields, its growth kinetic was significantly decreased (10 to 100 times) at 24, 48, and 72 hpi compared to parental ASFV-G. Therefore, the H108R gene is not essential for virus replication, but its absence slightly delayed the ability of ASFV-G to replicate in primary swine macrophage cell cultures.
FIG 4.
In vitro growth characteristics of ASFV-G-ΔH108R and parental ASFV-G. Primary swine macrophage cell cultures were infected (MOI = 0.01) with each of the viruses and virus yield titrated at the indicated times postinfection. Data represent means from three independent experiments. Sensitivity of virus detection: ≥1.8 log10 HAD50/mL. The unpaired t-test using the two-stage step-up (Benjamini, Krieger, and Yekutieli) method was used to assess statistical differences in viral yields between ASFV-G and ASFV-G-ΔH108R at different time points. The significance of these discoveries was evaluated by the false discovery rate method (FDR), with q values <0.05 (*) considered significant. Significant differences (*) in viral yields between both viruses at specific times points were determined using the Holm-Sidak method (α = 0.05), without assuming a consistent standard deviation. All calculations were conducted in the GraphPad Prism version 8 software package.
Evaluation of ASFV-G-ΔH108R virulence in swine.
To evaluate the effect of the deletion of the H108R gene in the process of disease development in swine, a group of domestic pigs were infected with ASFV-G-ΔH108R, and the virulent phenotype was compared with that of animals infected with parental virulent ASFV-G. Five animals (80 to 90 pounds) were intramuscularly (IM) inoculated with 102 HAD50 of ASFV-G-ΔH108R, and their clinical evolution was compared with that of a similar group of pigs inoculated with ASFV-G. All animals inoculated with parental ASFV-G showed an increase in body temperature (>104°F) by the fourth day postinfection (pi), with all animals presenting clinical signs associated with ASF (including anorexia, depression, purple skin discoloration, and occasional diarrhea). The disease progressively worsened, with all animals being euthanized by day 6 or 7 pi (Table 1 and Fig. 5). The animals inoculated with ASFV-G-ΔH108R showed different and heterogeneous disease kinetics. One of the animals presented a rise in body temperature by day 6 pi, followed by a protracted but fatal ASF clinical disease, and was euthanized by day 9 pi. The other four animals inoculated with ASFV-G-ΔH108R survived the whole 28 day observational period without showing any ASF-related signs except for the appearance of mild and transient increased body temperature in three of the animals (at day 8 pi in one animal, by days 8 to 9 in another, and between days 15 and 18 in a third) (Fig. 5). The fifth animal did not show any rise in body temperature during the 28 day observational period. Therefore, the deletion of the H108R gene produced a dramatic attenuation of the virulent ASFV-G strain.
TABLE 1.
Swine survival and fever response following infection with 102 HAD50 doses of ASFV-G-ΔH108R or parental ASFV-G
| Fever |
|||||
|---|---|---|---|---|---|
| Virus (102 HAD50) |
No. of survivors/total | Mean time to death (days ± SD) |
No. of days to onset (days ± SD) |
Duration No. of days (days ± SD) |
Maximum daily temp (°F ± SD) |
| ASFV-G | 0/5 | 6.25 (0.55)a | 4.4 (0.84) | 2 (1.1) | 104.97 (0.98) |
| ASFV-G-ΔH108R | 4/5 | 9b | 9.25 (11.5) | 2 (1.58) | 104.47 (0.99) |
All animals were euthanized due to humanitarian reasons following the corresponding IACUC protocol.
Only one animal was euthanized in this group.
FIG 5.
Kinetics of body temperature values (A) and mortality (B) in pigs IM inoculated with 102 HAD50 of either ASFV-G-ΔH108R or ASFV-G. In panel A, each curve represents an individual animal’s values in each of the groups.
Viremia kinetics in ASFV-G-infected animals presented, as expected, high titers (104.55 to 108.55 HAD50/mL) on day 4 pi. These titers increased (around 107.08 to 108.55 HAD50/mL) by day 7, when animals were euthanized due to the severity of the clinical disease (Fig. 6). Conversely, ASFV-G-ΔH108R showed a different pattern, with undetectable viremias in four of the animals and a mild titer in the fifth (105.3 HAD50/mL) at day 4 pi, reaching higher values (approximately 104.3 to 107.3 HAD50/mL, in particular, the animal euthanized by day 9 pi) by day 7 pi, peaking by day 11 pi (between 106.3 and 107.8 HAD50/mL), with titers steadily decreasing until day 28 pi in the remaining four animals. Therefore, the attenuation of ASFV-G-ΔH108R virulence is accompanied by a reduced but stable virus replication, presenting long viremias with relatively high titer values.
FIG 6.

Viremia titers detected after infection in pigs IM inoculated with 102 HAD50 of either ASFV-G-ΔH108R (black curves) or ASFV-G (red curves). In addition, the figure also demonstrates viremia titers of the ASFV-G-ΔH108R inoculated animal after the IM challenge with 102 HAD50 of ASFV-G at 28 days postinfection (0 days postchallenge). Viremias of the control group, also IM challenged with 102 HAD50 of ASFV-G, are included (green curves). Each curve represents values from individual animals in each group. Sensitivity of virus detection: ≥1.8 log10 HAD50/mL.
The sentinel animal remained clinically normal until day 18 pi, when it started showing a sudden rise in body temperature with a quick progress of the clinical disease, eventually being euthanized due to the severity of the clinical signs by day 20 pi. Viremia titers remained undetectable until day 14 pi, presenting a titer of 105.55 HAD50/mL at the moment of the euthanasia.
Protective efficacy of ASFV-G-ΔH108R against a challenge with parental ASFV-G.
Usually, pigs which survive an ASFV infection develop a protective immunity against a challenge with the virulent homologous virus (14–21). To evaluate the ability of the ASFV-G-ΔH108R infected animals to be protected against a challenge with the highly virulent parental virus, ASFV-G, the four survivors of the ASFV-G-ΔH108R infection were IM challenged 28 days later with 102 HAD50 of the parental virus. An additional group (n = 4) of naive animals were also included as a control group and challenged under the same conditions.
The group of the control animals showed clinical signs of ASF by approximately the third to fifth day, postchallenge (dpc), followed by a quick increase in disease severity, with all animals euthanized between the third and seventh dpc (Table 2, Fig. 7). Conversely, the animals in the group infected with ASFV-G-ΔH108R remained clinically normal during the 21-day observation period, not even showing a transient rise in body temperature. Therefore, infection with ASFV-G-ΔH108R induced protection against clinical disease after animals were challenged with the highly virulent parental virus.
TABLE 2.
Swine survival and fever response in ASFV-G-ΔH108R-infected animals challenged with 102 HAD50 ASFV-G virus 28 days later
| Fever |
|||||
|---|---|---|---|---|---|
| Virus (102 HAD50) |
No. of survivors/total | Mean time to death (days ± SD) |
No. of days to onset (days ± SD) |
Duration No. of days (days ± SD) |
Maximum daily temp (°F ± SD) |
| Mock | 0/4 | 5 (1.83)a | 4 (1.15) | 1 (0.82) | 104.12 (1.16) |
| ASFV-G-ΔH108R | 4/4 | 102.5 (0.46) | |||
All animals were euthanized due to humanitarian reasons following the corresponding IACUC protocol.
FIG 7.
Kinetics of body temperature values (A) and mortality (B) in pigs IM inoculated with 102 HAD50 ASFV-G-ΔH108R and challenged 28 days later with 102 HAD50 of ASFV-G. A control group of animals was IM inoculated with 102 HAD50 of ASFV-G. In panel (A), each curve represents individual animal’s values in each of the groups.
As expected, viremia titers in the control animals challenged with ASFV-G were high (ranging between 107.3 and 108.3 HAD50/mL) on day 4 pi, remaining high until the animals were euthanized due to the severity of the clinical disease (Fig. 6). In general, after the challenge, the viremia values of the ASFV-G-ΔH108R infected animals progressively decreased until the end of the experimental period (21 days after challenge), where the present titers ranged from an undetectable ≤101.8 to 104.3 HAD50/mL.
Host antibody response in animals infected with ASFV-G-ΔH108R.
Although the immune mechanisms mediating protection against the infection with virulent ASFV strains in animals are still under discussion, in our experience, the presence of virus-specific antibodies is the only immunological parameter constantly associated with protection. We tried to relate the presence of anti-ASFV antibodies with protection against a challenge in the sera of animals surviving the infection with ASFV-G-ΔH108R. A strong ASFV-specific antibody response was detected in the sera of all animals surviving the ASFV-G-ΔH108R infection using an in-house developed, direct ELISA (Fig. 8). Antibody response, mediated by IgG isotypes, was detected in four of the animals by day 11 pi, and titers gradually increased until day 28 pi, when the animals were challenged. These results confirm our previous reports, in which we showed a close correlation between the presence of anti-ASFV antibodies at the moment of challenge and protection against the challenge (38).
FIG 8.
Anti-ASFV antibody titers detected by ELISA in pigs IM inoculated with 102 HAD50 of ASFV-G-ΔH108R. Each point represents a value from an individual animal.
DISCUSSION
The current ASF pandemic has energized research into the development of experimental vaccines to improve the epidemiological management of the disease. Among these experimental vaccines, those based on the use of attenuated strains constitute the most reliable approach to develop successful vaccines. Although approaches to develop attenuated ASFV strains have considered the use of naturally attenuated isolates (39, 40) and those obtained by adaptation to grow in cell cultures (41–43), vaccine candidates rationally designed and developed by genetic manipulation are the more promising ones (15–18, 23, 24, 26, 44–46). Thus, the specific removal of virus genes associated with virus virulence is currently the more reasonable approach by which to produce safe and efficacious vaccines. The critical first step in this approach is the discovery of those virus genes involved in disease production. Here, we described the discovery of an ASFV gene of previously unknown function, the H108R gene, which is involved in virus virulence in swine. Removal of the H108R gene reduced the virulence of the highly virulent ASFV-G when experimentally inoculated in swine. Importantly, animals surviving ASFV-G-ΔH108R infection are protected against a challenge with the virulent parental ASFV-G.
Discovering ASFV genes involved in the process of virulence has been a laborious and time-demanding process, based, essentially, on a systematic experimental approach. At the moment, only 13 ASFV genes have been identified as determinants of virulence, since their individual deletion produced a significant decrease in the virulence of the corresponding parental virulent strain. Nine of these determinants of virulence have been shown to produce attenuation in the ASFV-G or in any of its derivatives, and only five (9GL, I177L, I226R, A137R, and E184L) have been demonstrated to be useful in the development of an attenuated strain able to induce protection against a challenge with the field isolate responsible for the current pandemic affecting Eurasia (46). Therefore, the identification of a novel determinant of virulence in ASFV is of paramount importance, in particular, if that information can be used for the development of experimental vaccines.
Deletion of the H108R gene from the genome of ASFV-G clearly produced an important decrease in virus virulence, with the exception of one animal presenting a slightly delayed fatal form of the disease. All other animals infected with ASFV-G-ΔH108R showed no clinical signs associated with ASF, with the exception of transient and mild peaks of elevation in body temperature. It is important to mention that NGS analysis of the virus obtained from the only animal presenting a fatal clinical disease as well as the sentinel animal shows the exclusive presence of ASFV-G-ΔH108R, eliminating the possibility that the disease of this particular animal was caused by the presence of residual parental ASFV-G in the ASFV-G-ΔH108R stock. It is possible that ASFV-G-ΔH108R retains some degree of residual virulence that may produce a delayed form of the disease in an animal that may present higher susceptibility than others. The presence of this potential residual virulence might also be expressed in other animals as a more chronic form of the disease if the observational period were to be notably extended. All of the animals surviving the infection with ASFV-G-ΔH108R showed relatively high viremia titers lasting until day 28 pi. Long viremia periods are a common event that occurs in animals infected with attenuated ASFV strains (16, 17, 19). In fact, in our experience, the presence of detectable viremias in animals infected with attenuated virus is strongly associated with protection against the challenge with virulent parental ASFV-G.
All animals surviving the ASFV-G-ΔH108R infection were protected against the challenge with the parental virulent ASFV-G at 28 dpi and did not develop any clinical signs associated with disease (not even a transient rise in body temperature), emphasizing the protective efficacy produced in those animals. The low dose (102 HAD50) of ASFV-G-ΔH108R needed for the induction of a protective immune response against a challenge with the virulent parental virus should be stressed.
As previously demonstrated in several reports presenting results on the development of experimental ASFV vaccine strains, a close association is seen in ASFV-G-ΔH108R inoculated animals between the levels of circulating virus-specific antibodies and protection against a challenge. Although the evidence presented here cannot establish a causative effect between the presence of virus-specific antibodies and protection during the challenge, it does corroborate the accumulative evidence that strongly associates these two factors (13–17), detecting high levels of ASFV-specific antibodies in all protected animals.
Taken together, this report demonstrated for the first time that the ASFV gene H108R is a novel ASFV determinant of virulence, since its deletion from the genome of the highly virulent ASFV-G isolate induced a significant attenuation. Also, a virus strain lacking the H108R gene, ASFV-G-ΔH108R, even when administered at a low dose, induced protection against the parental virus challenge, thus indicating that H108R gene deletion has promising potential in the development of novel vaccine candidates.
MATERIALS AND METHODS
Cell culture and viruses.
Primary swine macrophage cell cultures were prepared from heparin-treated swine blood as previously described in detail (47). Briefly, mononuclear leukocytes were separated over a Ficoll-Paque density gradient (Pharmacia, Piscataway, NJ) and the monocyte/macrophage cell fraction was cultured in plastic Primaria tissue culture flasks (Falcon; Becton, Dickinson Labware, Franklin Lakes, NJ) containing macrophage media composed of RPMI 1640 Medium (Life Technologies, Grand Island, NY) with 30% L929 supernatant and 20% fetal bovine serum (HI-FBS, Thermo Scientific, Waltham, MA) for 48 h at 37°C under 5% CO2. Adherent cells were detached from the plastic using 10 mM EDTA in phosphate-buffered saline (PBS) and were then reseeded into Primaria T25, 6- or 96-well dishes at a density of 5 × 106 cells per mL for use in assays 24 h later.
Growth curves comparing growth kinetics between the ASFV-G and ASFV-G-ΔH108R viruses were performed in primary swine macrophage cell cultures. Macrophage monolayers, prepared in 24-well plates, were infected at an MOI of 0.01. One hour later, the inoculum was removed, and the cells were rinsed twice with PBS, rinsed once with macrophage media, and incubated with 1 mL/well of macrophage media for 2, 24, 48, 72, and 96 h at 37°C under 5% CO2. At appropriate times postinfection, the cells were frozen at <−70°C, and the thawed lysates were used to determine virus titers in primary swine macrophage cell cultures. All samples were run simultaneously to avoid interassay variability.
Virus titration was performed on primary swine macrophage cell cultures in 96-well plates (15). Virus dilutions and cultures were performed by using macrophage medium. Presence of virus was assessed by hemadsorption (HA), and virus titers were calculated by the Reed and Muench method (48).
ASFV-Georgia (ASFV-G) was a field isolate kindly provided by Nino Vepkhvadze from the Laboratory of the Ministry of Agriculture (LMA) in Tbilisi, Republic of Georgia.
Construction of the recombinant ASFV-G-ΔH108R.
Recombinant ASFV-G-ΔH108R was generated by homologous recombination between the parental ASFV-G genome and recombination transfer vector p72mCherryΔH108R by infection and transfection procedures, using swine macrophage cell cultures as previously described in detail (38). The recombinant transfer vector p72mCherryΔH108R contained flanking genomic regions to the mapping, approximately 1kbp to the left and right from nucleotide positions 155,233 and 155,318 in ASFV-G. These are the first 85 nucleotides of the ORF for the H108R gene, along with the reporter gene cassette containing the mCherry gene with the ASFV p72 late gene promoter, p72mCherry. This construction created an 86 bp deletion at the beginning of the H108R ORF, resulting in the deletion of the first 29 amino acids of H108R (Fig. 3). This partial deletion was designed to not disrupt the promoter region of overlapping ASFV ORF H223R while still disrupting the H108R ORF. The recombinant transfer vector p72mCheryΔH108R was obtained via DNA synthesis (Epoch Life Sciences Missouri City, TX).
Next Generation Sequencing (NGS) of the ASFV-G-ΔH108R genome.
ASFV DNA was extracted from infected cells and quantified as described earlier. A full-length sequence of the virus genome was performed as described previously (49), using an Illumina NextSeq500 sequencer.
Animal experiments.
Animal experiments were performed under biosafety level 3AG conditions in the Plum Island Animal Disease Center (PIADC) animal facility, following protocols approved by the PIADC Institutional Animal Care and Use Committee of the US Departments of Agriculture and Homeland Security (protocol number 225.04-16-R, approved 09-06-19).
ASFV-G-ΔH108R virulence was assessed in comparison to that of the virulent parental ASFV-G virus. using 80 to 90 pound commercial Yorkshire crossbreed swine. Groups of pigs (n = 5) were intramuscularly inoculated with 102 HAD50 of either recombinant ASFV-G-ΔH108R or parental ASFV-G virus. A sixth, noninoculated pig was incorporated into the group as a sentinel to detect the presence of virus shedding from the inoculated animals. The sentinel cohabitated with the inoculated group until day 28 pi, when it was removed from the room. The presence of clinical signs (anorexia, depression, fever, purple skin discoloration, staggering gait, diarrhea, and cough) and changes in rectal temperature were recorded daily throughout the experiment. In protection experiments, animals inoculated with ASFV-G-ΔH108R were IM challenged 28 days later with 102 HAD50 of parental virulent ASFV-G. The presence of clinical signs associated with the disease were recorded as described earlier (18).
Detection of H108R gene transcription.
The transcriptional profile of the H108R gene was evaluated using a real-time PCR assay (qPCR) during the infection of ASFV-G in swine macrophages cultures, using the expression of early CP204L (p30) and late B646L (p72) ASFV genes as reference genes. Briefly, cell cultures of porcine macrophages were infected with a stock of ASFV-G, using an MOI of 1. RNA extractions using an RNeasy Kit (Qiagen, Hilden, Germany) were conducted at 4, 6, 8, and 24 h postinfection. All extractions were treated with 2 units of DNase I (BioLabs, San Diego, CA), then purified using the Monarch RNA Cleanup Kit (New England BioLabs, Inc., Ipswich, MA). One μg of RNA was used to produce cDNA, using qScript cDNA SuperMix (Quanta bio, Beverly, MA), which was used for the qPCR.
The primers and probe for the detection of the H108R gene were designed using the ASFV Georgia 2007/1 strain (GenBank database NC_044959.2). Primer forward: 5′-CATATTCCCTACCAGTGGCTG-3′, reverse: 5′-AGGATCATAATTGCCCCACC-3′, and probe: 5′-FAM/ACTGAAGGAAGCTGAGAGC AAGTACTG/MGBNFQ-3′. All qPCRs were conducted using the TaqMan Universal PCR Master Mix (Applied Biosystems, Waltham MA), using the following amplification conditions: one step at 55°C for 2 min, followed by one denaturation step at 95°C for 10 min, then 40 cycles of denaturation at 95°C for 15 s and annealing/extension at 65°C for 1 min.
Detection of anti-ASFV antibodies.
ASFV antibody detection used an in-house ELISA performed as described previously (50). Briefly, ELISA antigen was prepared from ASFV-infected Vero cells. Maxisorb ELISA plates (Nunc, St. Louis, MO) were coated with 1 μg per well of infected or uninfected cell extract. The plates were blocked with phosphate-buffered saline containing 10% skim milk (Merck, Kenilworth, NJ) and 5% normal goat serum (Sigma, Saint Louis, MO). Each swine serum was tested at multiple dilutions against both infected and uninfected cell antigen. ASFV-specific antibodies in the swine sera were detected using an anti-swine IgG-horseradish peroxidase conjugate (KPL, Gaithersburg, MD) and SureBlue Reserve peroxidase substrate (KPL, Milford, MA). Plates were read at OD630 nm in an ELx808 plate reader (BioTek, Shoreline, WA). Sera titers were expressed as the log10 of the highest dilution in which the OD630 reading of the tested sera at least duplicated the reading of the mock-infected sera.
ACKNOWLEDGMENTS
We thank the Plum Island Animal Disease Center Animal Care Unit staff for excellent technical assistance. We wish to particularly thank Carmen V. Borca-Carrillo for editing the manuscript. This research was supported in part by an appointment to the Plum Island Animal Disease Center (PIADC) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the U.S. Department of Agriculture (USDA). ORISE is managed by ORAU under DOE contract number DE-SC0014664. All opinions expressed in this paper are the authors’ and do not necessarily reflect the policies and views of USDA, ARS, APHIS, DHS, DOE, or ORAU/ORISE.
This project was partially funded by the National Pork Board Project no. 21-137 and an interagency agreement with the Science and Technology Directorate of the U.S. Department of Homeland Security under Award Numbers 70RSAT19KPM000056 and 70RSAT18KPM000134.
The authors have no conflict of interest to declare.
Contributor Information
Douglas P. Gladue, Email: Douglas.Gladue@usda.gov.
Manuel V. Borca, Email: Manuel.Borca@usda.gov.
Joanna L. Shisler, University of Illinois at Urbana Champaign
REFERENCES
- 1.Chapman DAG, Darby AC, Da Silva M, Upton C, Radford AD, Dixon LK. 2011. Genomic analysis of highly virulent Georgia 2007/1 isolate of African swine fever virus. Emerg Infect Dis 17:599–605. 10.3201/eid1704.101283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tulman ER, Delhon GA, Ku BK, Rock DL. African swine fever virus in lesser known large dsDNA viruses. 2009. Springer-Verlag; Berlin Heidelberg. P 43–87. [DOI] [PubMed] [Google Scholar]
- 3.Ramírez-Medina E, Vuono EA, Velazquez-Salinas L, Silva E, Rai A, Pruitt S, Berggren KA, Zhu J, Borca MV, Gladue DP. 2020. The MGF360-16R ORF of African swine fever virus strain Georgia encodes for a nonessential gene that interacts with host proteins SERTAD3 and SDCBP. Viruses 12:60. 10.3390/v12010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borca MV, O'Donnell V, Holinka LG, Ramírez-Medina E, Clark BA, Vuono EA, Berggren K, Alfano M, Carey LB, Richt JA, Risatti GR, Gladue DP. 2018. The L83L ORF of African swine fever virus strain Georgia encodes for a non-essential gene that interacts with the host protein IL-1beta. Virus Res 249:116–123. 10.1016/j.virusres.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Borca MV, O'Donnell V, Holinka LG, Rai DK, Sanford B, Alfano M, Carlson J, Azzinaro PA, Alonso C, Gladue DP. 2016. The Ep152R ORF of African swine fever virus strain Georgia encodes for an essential gene that interacts with host protein BAG6. Virus Res 223:181–189. 10.1016/j.virusres.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Nunez D, et al. 2015. CD2v interacts with adaptor protein AP-1 during African swine fever infection. PLoS One 10:e0123714. 10.1371/journal.pone.0123714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Chen X, Huang Q, Shao Z, Gao Y, Li Y, Yang C, Liu H, Li J, Wang Q, Ma J, Zhang Y-Z, Gu Y, Gan J. 2020. A unique DNA-binding mode of African swine fever virus AP endonuclease. Cell Discov 6:13. 10.1038/s41421-020-0146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrado-Gil L, Del Puerto A, Muñoz-Moreno R, Galindo I, Cuesta-Geijo MÁ, Urquiza J, Nistal-Villán E, Maluquer de Motes C, Alonso C. 2020. African swine fever virus ubiquitin-conjugating enzyme interacts with host translation machinery to regulate the host protein synthesis. Front Microbiol 11:622907. 10.3389/fmicb.2020.622907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen Z, Chen C, Yang Y, Xie Z, Ao Q, Lv L, Zhang S, Chen H, Hu R, Chen H, Peng G. 2021. A novel function of African swine fever virus pE66L in inhibition of host translation by the PKR/eIF2alpha pathway. J Virol 95 10.1128/JVI.01872-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuo Y, Guo Z, Ba T, Zhang C, He L, Zeng C, Dai H. 2021. African swine fever virus MGF360-12L inhibits type I interferon production by blocking the interaction of importin alpha and NF-kappaB signaling pathway. Virol Sin 36:176–186. 10.1007/s12250-020-00304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matamoros T, Alejo A, Rodríguez JM, Hernáez B, Guerra M, Fraile-Ramos A, Andrés G. 2020. African swine fever virus protein pE199L mediates virus entry by enabling membrane fusion and core penetration. mBio 11 10.1128/mBio.00789-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freitas FB, Frouco G, Martins C, Ferreira F. 2018. African swine fever virus encodes for an E2-ubiquitin conjugating enzyme that is mono- and di-ubiquitinated and required for viral replication cycle. Sci Rep 8:3471. 10.1038/s41598-018-21872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frouco G, Freitas FB, Coelho J, Leitão A, Martins C, Ferreira F. 2017. DNA-binding properties of African swine fever virus pA104R, a histone-like protein involved in viral replication and transcription. J Virol 91. 10.1128/JVI.02498-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Zhang Y, Chen T, Yang J, Yue H, Wang L, Zhou X, Qi Y, Han X, Ke J, Wang S, Yang J, Miao F, Zhang S, Zhang F, Wang Y, Li M, Hu R. 2021. Deletion of the L7L-L11L genes attenuates ASFV and induces protection against homologous challenge. Viruses 13:255. 10.3390/v13020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borca MV, Ramirez-Medina E, Silva E, Vuono E, Rai A, Pruitt S, Espinoza N, Velazquez-Salinas L, Gay CG, Gladue DP. 2021. ASFV-G-I177L as an effective oral nasal vaccine against the Eurasia strain of Africa swine fever. Viruses 13:765. 10.3390/v13050765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borca MV, Ramirez-Medina E, Silva E, Vuono E, Rai A, Pruitt S, Holinka LG, Velazquez-Salinas L, Zhu J, Gladue DP. 2020. Development of a highly effective African swine fever virus vaccine by deletion of the I177L gene results in sterile immunity against the current epidemic Eurasia strain. J Virol 94. 10.1128/JVI.02017-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Donnell V, Holinka LG, Gladue DP, Sanford B, Krug PW, Lu X, Arzt J, Reese B, Carrillo C, Risatti GR, Borca MV. 2015. African swine fever virus Georgia isolate harboring deletions of MGF360 and MGF505 genes is attenuated in swine and confers protection against challenge with virulent parental virus. J Virol 89:6048–6056. 10.1128/JVI.00554-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Donnell V, et al. 2015. African swine fever virus Georgia 2007 with a deletion of virulence-associated gene 9GL (B119L), when administered at low doses, leads to virus attenuation in swine and induces an effective protection against homologous challenge. J Virol 89:8556–8566. 10.1128/JVI.00969-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Donnell V, et al. 2017. Simultaneous deletion of the 9GL and UK genes from the African swine fever virus Georgia 2007 isolate offers increased safety and protection against homologous challenge. J Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li D, Yang W, Li L, Li P, Ma Z, Zhang J, Qi X, Ren J, Ru Y, Niu Q, Liu Z, Liu X, Zheng H. 2021. African swine fever virus MGF-505-7R negatively regulates cGAS-STING-mediated signaling pathway. J Immunol 206:1844–1857. 10.4049/jimmunol.2001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li D, Liu Y, Qi X, Wen Y, Li P, Ma Z, Liu Y, Zheng H, Liu Z. 2021. African swine fever virus MGF-110-9L-deficient mutant has attenuated virulence in pigs. Virol Sin 36:187–195. 10.1007/s12250-021-00350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis T, Zsak L, Burrage TG, Lu Z, Kutish GF, Neilan JG, Rock DL. 2000. An African swine fever virus ERV1-ALR homologue, 9GL, affects virion maturation and viral growth in macrophages and viral virulence in swine. J Virol 74:1275–1285. 10.1128/jvi.74.3.1275-1285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran XH, et al. 2021. African swine fever virus vaccine candidate ASFV-G-DeltaI177L efficiently protects European and native pig breeds against circulating Vietnamese field strain. Transbound Emerg Dis. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Zhao D, He X, Liu R, Wang Z, Zhang X, Li F, Shan D, Chen H, Zhang J, Wang L, Wen Z, Wang X, Guan Y, Liu J, Bu Z. 2020. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci China Life Sci 63:623–634. 10.1007/s11427-020-1657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teklue T, Wang T, Luo Y, Hu R, Sun Y, Qiu H-J. 2020. Generation and evaluation of an African swine fever virus mutant with deletion of the CD2v and UK genes. Vaccines (Basel 8:763. 10.3390/vaccines8040763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monteagudo PL, Lacasta A, López E, Bosch L, Collado J, Pina-Pedrero S, Correa-Fiz F, Accensi F, Navas MJ, Vidal E, Bustos MJ, Rodríguez JM, Gallei A, Nikolin V, Salas ML, Rodríguez F. 2017. BA71DeltaCD2: a new recombinant live attenuated African swine fever virus with cross-protective capabilities. J Virol 91 10.1128/JVI.01058-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez-Medina E, Vuono E, O’Donnell V, Holinka LG, Silva E, Rai A, Pruitt S, Carrillo C, Gladue DP, Borca MV. 2019. Differential effect of the deletion of African swine fever virus virulence-associated genes in the induction of attenuation of the highly virulent Georgia strain. Viruses 11:599. 10.3390/v11070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gladue DP, O’Donnell V, Ramirez-Medina E, Rai A, Pruitt S, Vuono EA, Silva E, Velazquez-Salinas L, Borca MV. 2020. Deletion of CD2-like (CD2v) and C-type lectin-like (EP153R) genes from African swine fever virus Georgia-9GL abrogates its effectiveness as an experimental vaccine. Viruses 12:1185. 10.3390/v12101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Donnell V, Holinka LG, Sanford B, Krug PW, Carlson J, Pacheco JM, Reese B, Risatti GR, Gladue DP, Borca MV. 2016. African swine fever virus Georgia isolate harboring deletions of 9GL and MGF360/505 genes is highly attenuated in swine but does not confer protection against parental virus challenge. Virus research 221:8–14. [DOI] [PubMed] [Google Scholar]
- 30.Borca MV, Carrillo C, Zsak L, Laegreid WW, Kutish GF, Neilan JG, Burrage TG, Rock DL. 1998. Deletion of a CD2-like gene, 8-DR, from African swine fever virus affects viral infection in domestic swine. J Virol 72:2881–2889. 10.1128/JVI.72.4.2881-2889.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alejo A, Matamoros T, Guerra M, Andrés G. 2018. A proteomic atlas of the African swine fever virus particle. J Virol 92 10.1128/JVI.01293-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mistry J, Chuguransky S, Williams L, Qureshi M, Salazar GA, Sonnhammer ELL, Tosatto SCE, Paladin L, Raj S, Richardson LJ, Finn RD, Bateman A. 2021. Pfam: the protein families database in 2021. Nucleic Acids Res 49:D412–D419. 10.1093/nar/gkaa913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiMaio D. 2014. Viral miniproteins. Annu Rev Microbiol 68:21–43. 10.1146/annurev-micro-091313-103727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosakovsky Pond SL, Posada D, Gravenor MB, Woelk CH, Frost SDW. 2006. GARD: a genetic algorithm for recombination detection. Bioinformatics 22:3096–3098. 10.1093/bioinformatics/btl474. [DOI] [PubMed] [Google Scholar]
- 36.Kosakovsky Pond SL, Frost SD. 2005. Not so different after all: a comparison of methods for detecting amino acid sites under selection. Mol Biol Evol 22:1208–1222. 10.1093/molbev/msi105. [DOI] [PubMed] [Google Scholar]
- 37.Murrell B, Wertheim JO, Moola S, Weighill T, Scheffler K, Kosakovsky Pond SL. 2012. Detecting individual sites subject to episodic diversifying selection. PLoS Genet 8:e1002764. 10.1371/journal.pgen.1002764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borca MV, O'Donnell V, Holinka LG, Sanford B, Azzinaro PA, Risatti GR, Gladue DP. 2017. Development of a fluorescent ASFV strain that retains the ability to cause disease in swine. Sci Rep 7:46747. 10.1038/srep46747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abrams CC, Goatley L, Fishbourne E, Chapman D, Cooke L, Oura CA, Netherton CL, Takamatsu H-H, Dixon LK. 2013. Deletion of virulence associated genes from attenuated African swine fever virus isolate OUR T88/3 decreases its ability to protect against challenge with virulent virus. Virology 443:99–105. 10.1016/j.virol.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallardo C, Soler A, Rodze I, Nieto R, Cano-Gómez C, Fernandez-Pinero J, Arias M. 2019. Attenuated and non-haemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transbound Emerg Dis 66:1399–1404. 10.1111/tbed.13132. [DOI] [PubMed] [Google Scholar]
- 41.Pires S, Ribeiro G, Costa JV. 1997. Sequence and organization of the left multigene family 110 region of the Vero-adapted L60V strain of African swine fever virus. Virus Genes 15:271–274. 10.1023/A:1007992806818. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez JM, et al. 2015. Genome sequence of African swine fever virus BA71, the virulent parental strain of the nonpathogenic and tissue-culture adapted BA71V. PLoS One 10:e0142889. 10.1371/journal.pone.0142889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krug PW, Holinka LG, O'Donnell V, Reese B, Sanford B, Fernandez-Sainz I, Gladue DP, Arzt J, Rodriguez L, Risatti GR, Borca MV. 2015. The progressive adaptation of a Georgian isolate of African swine fever virus to Vero cells leads to a gradual attenuation of virulence in swine corresponding to major modifications of the viral genome. J Virol 89:2324–2332. 10.1128/JVI.03250-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tran XH, Phuong LTT, Huy NQ, Thuy DT, Nguyen VD, Quang PH, Ngôn QV, Rai A, Gay CG, Gladue DP, Borca MV. 2022. Evaluation of the safety profile of the ASFV vaccine candidate ASFV-G-&Delta. I177L Viruses 14:896. 10.3390/v14050896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gladue DP, Ramirez-Medina E, Vuono E, Silva E, Rai A, Pruitt S, Espinoza N, Velazquez-Salinas L, Borca MV. 2021. Deletion of the A137R gene from the pandemic strain of African swine fever virus attenuates the strain and offers protection against the virulent pandemic virus. J Virol 95:e0113921. 10.1128/JVI.01139-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gladue DP, Borca MV. 2022. Recombinant ASF live attenuated virus strains as experimental vaccine candidates. Viruses 14:878. 10.3390/v14050878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borca MV, Berggren KA, Ramirez-Medina E, Vuono EA, Gladue DP. 2018. CRISPR/Cas gene editing of a large DNA virus: African swine fever virus. Bio-protocol 8 10.21769/BioProtoc.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. The American J Hygiene 27:493–497. 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 49.Borca MV, Holinka LG, Berggren KA, Gladue DP. 2018. CRISPR-Cas9, a tool to efficiently increase the development of recombinant African swine fever viruses. Sci Rep 8:3154. 10.1038/s41598-018-21575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carlson J, O’Donnell V, Alfano M, Velazquez Salinas L, Holinka L, Krug P, Gladue D, Higgs S, Borca M. 2016. Association of the host immune response with protection using a live attenuated African swine fever virus model. Viruses 8:291. 10.3390/v8100291. [DOI] [PMC free article] [PubMed] [Google Scholar]