Abstract
The gene (chi92) encoding the extracellular chitinase of Aeromonas hydrophila JP101 has been cloned and expressed in Escherichia coli. The mature form of Chi92 is an 842-amino-acid (89.830-kDa) modular enzyme comprised of a family 18 catalytic domain, an unknown-function region (the A region), and three chitin-binding domains (ChBDs; Chi92-N, ChBDCI, and ChBDCII). The C-terminally repeated ChBDs, ChBDCI and ChBDCII, were grouped into family V of cellulose-binding domains on the basis of sequence homology. Chitin binding and enzyme activity studies with C-terminally truncated Chi92 derivatives lacking ChBDs demonstrated that the ChBDs are responsible for its adhesion to unprocessed and colloidal chitins. Further adsorption experiments with glutathione S-transferase (GST) fusion proteins (GST-CI and GST-CICII) demonstrated that a single ChBD (ChBDCI) could promote efficient chitin and cellulose binding. In contrast to the two C-terminal ChBDs, the Chi92-N domain is similar to ChiN of Serratia marcescens ChiA, which has been proposed to participate in chitin binding. A truncated derivative of Chi92 that contained only a catalytic domain and Chi92-N still exhibited insoluble-chitin-binding and hydrolytic activities. Thus, it appears that Chi92 contains Chi92-N as the third ChBD in addition to two ChBDs (ChBDCI and ChBDCII).
Chitinases (EC 3.2.1.14) cleave the β-1,4-glycosidic bonds of chitin, a β-1,4-linked, unbranched polymer of N-acetylglucosamine (GlcNAc), which is a major component of insect exoskeletons, shells of crustaceans, and fungal cell walls. These enzymes have been detected in a variety of organisms, including organisms that do not contain chitin as a structural component, such as bacteria, plants, and animals. The production of chitinases by plants is thought to be involved in defense reactions against chitin-containing pathogens. Bacteria utilize chitinases for assimilation of chitin as a carbon and nitrogen source, and these enzymes play an important ecological role in the degradation of chitin.
Numerous chitinases have been characterized, and the corresponding genes have been analyzed. On the basis of the primary and secondary structures of the catalytic domains, chitinases are grouped into two distinct families (families 18 and 19) in the classification of glycosyl hydrolase (12, 13). The bacterial chitinases, except for Streptomyces griseus HUT 6037 ChiC (20), belong to glycosyl hydrolase family 18. In addition to the catalytic domain, many bacterial chitinases, like many polysaccharidases, such as cellulases and amylases, have a discrete binding domain that mediates adsorption to the substrate. In the past decade, studies on the molecular structure and function of substrate-binding domains have focused mainly on cellulose-binding domains (CBDs); however, relatively little is known about the chitin-binding domains (ChBDs). Most of the knowledge about bacterial ChBDs has been accumulated from studies on Bacillus circulans ChiA1 (11, 39), Clostridium paraputrificum ChiB (18), S. olivaceoviridis exo-ChiO1 (2), Alteromonas sp. strain O-7 ChiC (36), Serratia marcescens ChiC (31), and Pyrococcus kodakaraensis KOD1 ChiA (33). The above studies have shown that the chitinase lacking the ChBD lost much of its binding capacity and hydrolytic activity toward insoluble chitin. Thus, it has been suggested that the ChBD potentiates the catalytic activity against insoluble-chitin substrates.
Although several studies have revealed that deletion of the ChBD from chitinases reduces the capacity of the enzymes to bind and hydrolyze insoluble chitin, it is unclear by which mechanism the domain elicits its effect. In a previous study (6), the chitinase gene from Aeromonas hydrophila JP101 was cloned and expressed in Escherichia coli. In this paper, we describe the purification, biochemical properties, and primary structure of A. hydrophila JP101 Chi92. Furthermore, in order to investigate the molecular basis for the capacity of bacterial chitinases to bind chitin, we also carried out biochemical studies of C-terminally truncated derivatives and glutathione S-transferase (GST) fusion proteins of ChBDCI and ChBDCII.
MATERIALS AND METHODS
Bacterial strains, plasmids, and cultivation conditions.
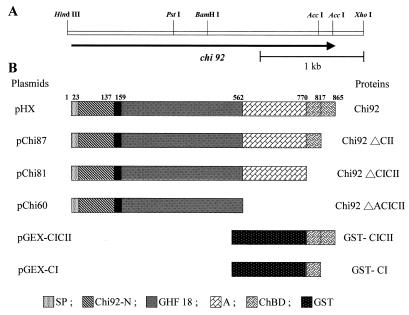
A. hydrophila JP101 was used in this study. It was initially isolated in our laboratory from shrimp shell-enriched soil (6). E. coli JM109 (41), JA221 (1), and XL1-Blue (Stratagene, La Jolla, Calif.) were used as hosts for recombinant plasmids. A. hydrophila JP101 was cultivated at 30°C in a medium containing 1% chitin, 0.2% glucose, 0.5% peptone, 0.5% yeast extract, 0.1% KH2PO4, and 0.3% NaCl. All E. coli strains were grown in Luria-Bertani (LB) medium (25) at 37°C. When necessary, the medium was supplemented with IPTG (isopropyl-β-d-thiogalactopyranoside) to a final concentration of 0.5 mM. Plasmid pBR322 was used in cloning experiments; plasmids pUC18, pGEM-7Zf (+) (Promega, Madison, Wis.), and pGEX-5X-3 (Amersham Pharmacia Biotech Inc., Uppsala, Sweden) were used in subcloning. A 3.0-kb HindIII-XhoI fragment was subcloned into the HindIII and XhoI sites of expression vector pGEM-7Zf (+) to produce pHX (Fig. 1A).
FIG. 1.
(A) Restriction endonuclease maps of a plasmid clone harboring the gene for chitinase (Chi92). (B) Domain structures of Chi92 and various derivative recombinant proteins used in this study. The numbers refer to the positions of amino acids. Abbreviations: SP, signal peptide; Chi92-N, all-β-strand N-terminal region; GHF 18, catalytic domains of glycosyl hydrolase family 18; A, unknown-function region. ChBD, chitin-binding domain; GST, glutathione S-transferase.
Nucleotide sequence analysis.
All sequences were determined by the dideoxy-chain termination method (26) with an automated laser fluorescence sequencer (model 377; ABI PRISM). Universal and reverse primers were used to obtain the initial sequences within the insert, and then specific primers for the sequences within the insert were generated. DNA was sequenced in both directions. The deduced amino acid sequence of Chi92 was analyzed by using BLAST searches of the databases at the National Center for Biotechnology Information. Multiple alignments of the amino acid sequences of the homologous proteins were carried out with the PC/GENE software package (Intelligenetics).
Purification of Chi92.
A. hydrophila JP101 was grown in chitin-supplemented medium at 30°C for 3 days, and the extracellular fraction was collected by centrifugation. The extracellular fraction was partially purified by affinity digestion in accordance with a previous report (6). The partially purified chitinase was applied to a HiTrap Q Sepharose column (5 ml; Amersham Pharmacia Biotech) equilibrated with 20 mM Tris-HCl buffer (pH 8.0). The enzyme was eluted with a linear gradient of 0 to 0.5 M NaCl in the same buffer. The eluted enzyme fraction was applied to a PD-10 column (Amersham Pharmacia Biotech) to remove salts. The purified chitinase was lyophilized and stored at −20°C. E. coli JM109 harboring plasmid pHX was grown to stationary phase in 1 liter of LB medium containing ampicillin at 100 μg/ml, and the periplasmic cell fraction was prepared by the osmotic-shock method (19). Meanwhile, the enzyme was purified by the method described above. Chitinases purified from E. coli(pHX) and A. hydrophila JP101 was separated by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS-PAGE) (17). Protein concentration was determined by the Bradford method (3).
Amino acid sequence analysis.
The purified Chi92 separated by SDS-PAGE was electroblotted onto polyvinylidene difluoride membrane. After visualization by Coomassie brilliant blue R-250 staining, the membrane was cut into pieces containing the 90-kDa protein. The membrane pieces were directly applied to a protein sequencer (model 477A; Applied Biosystems, Foster City, Calif.) for amino acid sequence analysis.
Construction of truncated chi92 derivatives and GST gene fusions.
The C-terminally truncated derivatives were constructed by PCR amplification of the 3′ region of Chi92 in plasmid pHX (Fig. 1B). On the basis of the nucleotide sequence of chi92, forward primer 5′-GAG TTC CTG CAG ACC TGG AAA TTC-3′ (the PstI site is underlined) and reverse primers 5′-TA GAC CAC TCG AGC ATC TCA ACC CAG-3′, 5′-T ACC TCG AGT TCA GGC CGG ATG GTT-3′, and 5′-C CGG CTC GAG CTG TCA CTC GCC GTG-3′ (the XhoI site is underlined, and the artificial stop codon is in boldface) were designed to amplify the sequence between positions 895 and 2477, 895 and 2329, and 895 and 1696. PCR was performed with a model PE2400 automatic thermocycler (Perkin-Elmer Cetus, Norwalk, Conn.). The reaction was carried out in a 100-μl volume containing 10 μl of 10× buffer (supplied with Pfu DNA polymerase), 50 pmol of each primer, 1 mM deoxynucleoside triphosphate, 2.5 U of Pfu DNA polymerase (Stratagene), and 25 ng of plasmid pHX. PCR conditions were 30 cycles at 94°C for 1.0 min, 52°C for 2.0 min, and 72°C for 2.0 min. The PCR products were digested with PstI and XhoI and ligated to PstI-XhoI-cut plasmid pHX. The recombinant plasmids were named pChi87, pChi81, and pChi60, respectively.
Plasmids pGEX-CICII and pGEX-CI, which encoded the protein fusions GST-CICII and GST-CI, respectively, were constructed by PCR amplification in plasmid pHX (Fig. 1B). The ChBD-encoding regions (positions 2278 to 2615 and 2278 to 2469) of chi92 were amplified by using forward primer 5′-TGG AAT TCC AGC GAT CCG GAT GCG-3′ (the EcoRI site is underlined) and reverse primers 5′-TCC GTC GAC CCA ACC CAG TTG CAC C-3′ and 5′ AT TGT CGA CTC GAG AGA TCA GTT GCA GC-3′ (the SalI site is underlined), which were fused in frame with the GST-encoding open reading frame (ORF) of pGEX-5X-3 (Amersham Pharmacia Biotech). These two PCR products were digested with EcoRI and SalI and cloned into pGEX-5X-3 cleaved with the same restriction enzymes. The nucleotide sequences of these constructs were checked by sequencing.
Purification of truncated derivatives of Chi92 and GST fusion proteins.
E. coli cells containing plasmids pChi87, pChi81, and pChi60 were collected, and the periplasmic proteins were prepared as described above. The periplasmic proteins were applied to a HiTrap Q Sepharose column (5 ml; Amersham Pharmacia Biotech) preequilibrated with 20 mM Tris-HCl buffer (pH 8.0). The protein fractions were eluted with a linear gradient of 0 to 0.4 M NaCl in the same buffer. The eluted protein fraction was concentrated on Centriprep-10 (Amicon) and then subjected to gel filtration on a Sephacryl S-200 column (2.6 by 60 cm; Amersham Pharmacia Biotech) preequilibrated with 50 mM Tris-HCl (pH 7.5). Fractions were eluted at a flow rate of 20 ml/h in the same buffer, and the active fractions were collected.
GST fusion proteins GST-CICII and GST-CI were purified from E. coli XL1-Blue harboring either pGEX-CICII or pGEX-CI. The E. coli strains were grown at 37°C in 400 ml of LB medium with ampicillin to an optical density at 595 nm of 0.8 to 1.0, and then IPTG was added to 0.5 mM. After incubation for a further 4 h, the cells were harvested and resuspended with PBS buffer (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.3) and disrupted by sonication. After centrifugation, the crude extracts were applied to a glutathione Sepharose 4B column (Amersham Pharmacia Biotech). The GST fusion proteins were eluted with glutathione elution buffer (10 mM reduced glutathione in 50 mM Tris-HCl, pH 8.0). The eluted protein fractions were applied to a PD-10 column (Amersham Pharmacia Biotech) to remove salts, and the purified proteins were lyophilized and stored at −20°C.
Enzyme activity measurements.
Chitinase activity was assayed spectrophotometrically by using p-nitrophenyl-β-d-chitobioside (pNPC; Sigma) as a soluble substrate. The reaction was carried out at 37°C in 20 mM Tris-HCl (pH 7.5) containing 0.5 mM pNPC and the enzyme. One unit of chitinase activity was defined as the amount of activity that liberates 1 μmol of p-nitrophenol per min under the assay condition used. Alternatively, chitinase activity was determined by the dinitrosalicylic acid assay using colloidal chitin or unprocessed chitin from crab shells (Sigma C4666) as substrates. Colloidal chitin was prepared from unprocessed chitin by the method described by Shimahara and Takiguchi (27). One unit of activity was defined as the amount of enzyme that releases 1 μmol of reducing sugar per min.
Polysaccharide-binding studies.
For the binding assay, 50 μg of the enzyme was mixed with various amounts of insoluble polysaccharides (unprocessed chitin, colloidal chitin, cellulose, starch, and xylan) in a final volume of 1 ml of Tris-HCl buffer (pH 7.5). The mixture was kept under constant agitation at 4°C for 1 h and then centrifuged. The concentration of unbound enzyme was determined from the A280 and used to calculate the amount of enzyme bound to the polysaccharide.
Nucleotide sequence accession number.
The nucleotide sequence of chi92 has been deposited at the EMBL database under accession no. AF181852.
RESULTS
Nucleotide sequence of the chi92 gene.
Previous studies showed that pCH1001 contained a 4.5-kb HindIII fragment of A. hydrophila JP101 genomic DNA, which coded for active chitinase in E. coli JA221 (6). To determine the approximate location of the chi92 gene in the cloned DNA fragment, several deletion and restriction fragments were subcloned into pGEM-7Zf (+). For each subclone, chitinase activity was determined by measuring clearing zone formation on colloidal chitin-containing plates. The results suggested that the region required for maximal chitinase production is located in the 3.0-kb HindIII-XhoI fragment inserted into pHX (Fig. 1).
The nucleotide sequence of the 3.0-kb DNA fragment encompassing the chi92 gene was determined on both strands. Computer analysis of the nucleotide sequence revealed the existence of a chi92 ORF extending from an ATG start codon to a TGA stop codon at position 2598 that is sufficient to encode a protein of 865 amino acids. A putative ribosome-binding site (Shine-Dalgarno sequence), AGGAAG, was found 7 bp upstream from the ATG codon. At a point 63 bp downstream from the stop codon, there was a palindromic sequence between nucleotides 2661 and 2671. This structure would be expected to function as a transcriptional terminator. The G+C content of the coding region for chi92 was 63.17%.
Analysis of the Chi92 amino acid sequence.
Analysis of the deduced amino acid sequence revealed that the first 23 amino acids of the chi92 gene product have the characteristics of a bacterial signal peptide for the initiation of translocation across the cytoplasmic membrane. The predicted cleavage site was between Ala23 and Ala24, since amino acid residues 24 to 37 of the deduced gene product were identical to the N-terminal amino acid sequences determined for the purified native and cloned chitinases (see below). A search through the available protein sequence databases revealed that Chi92 has significant amino acid sequence similarity to bacterial chitinases. Using the program FASTA, it was found that Chi92 was 98.3% identical (only 15 amino acid substitutions in 865 residues) to ChiA of A. caviae (29). None of the 15 amino acid differences between the two proteins mapped to residues corresponding to the predicted catalytic site, which are shared with several chitinases. In comparison with the amino acid sequence of S. marcescens chitinase A, it has been suggested that the structure of mature A. caviae ChiA is that of a modular enzyme (29), consisting of three major domains: a 134-amino-acid all-β-strand N-terminal region; 331 amino acids in the middle region, similar to the major catalytic domain which inserts an α+β-fold domain (74 amino acid residues); and 101 amino acids in the C-terminal region. The C-terminal region consists of tandem repeats of about 40 residues with 51% identity, in which each repeat is similar to the ChBDs of bacterial chitinases and CBDs of cellulases and xylanase (Fig. 2). Since the deduced amino acid sequences of Chi92 and A. caviae ChiA are very similar, Chi92 is supposed to consist of three major domains. In addition to three domains, Chi92 also contains 208 amino acid residues, designated the A region, located between the catalytic domain and the C-terminal region. Although no linker sequence rich in proline and/or hydroxyamino acids, such as those present in a number of polysaccharidases, was detected between the individual domains of Chi92, a short, proline-rich region (PPVNKPP) located at the C-terminal border of the catalytic domain and the A region was found.
FIG. 2.
Optimal alignment of the putative ChBDs of A. hydrophila JP101 Chi92 with those of other proteins. The sequences listed are those of A. hydrophila JP101 chitinase 92, A. caviae chitinase A (29), S. coelicolor chitinase A (23), Vibrio harveyi chitinase A (32), S. griseus chitinase C (20), Aeromonas sp. ORF-1 to -4 (28), Aeromonas sp. chitinase II (37), J. lividum chitinase A (9), Alteromonas sp. strain O-7 chitinases 85 (35) and C (36), C. paraputrificum chitinase B (18), B. subtilis chitinase (accession no. AF069131), P. kodakaraensis KOD1 chitinase A (33), B. licheniformis chitinase (accession no. U71214), S. marcescens QMB1466 chitinase B (10), Cellulomonas pachnodae xylanase 10B (5), Bacillus sp. strain N-4 cellulases A and B (8), B. agaradhaerens cellulase 5A (7), and E. chrysanthemi endoglucanase Z (4). The amino acid numbers are listed on the right, and they are numbered from Met-1 of the proteins. The stop codon is indicated by an asterisk. The black background regions indicate highly conserved amino acid residues. Dashes represent gaps introduced during the alignment process.
Purification and characterization of Chi92.
Native Chi92 and recombinant Chi92 were purified from the culture supernatant of A. hydrophila JP101 and from the periplasmic fraction of E. coli JM109(pHX), respectively, by affinity digestion and Sephacryl S-200 column chromatography. After examination of both purified protein preparations by SDS-PAGE, a single band with a molecular mass of 90 kDa was observed (data not shown), which is in good agreement with the value estimated from the deduced amino acid sequence of the mature form of Chi92. A single active chitinase band was presented in the crude enzyme preparations from culture supernatant of A. hydrophila JP101 grown on chitin, indicating that Chi92 is a major extracellular chitinolytic enzyme produced by this strain whose mature form has a molecular mass of 90 kDa (data not shown).
Thin-layer chromatographic analysis revealed that the products of colloidal chitin hydrolysis by purified native or recombinant Chi92 were mainly (GlcNAc)2 with some GlcNAc (data not shown). When chitooligosaccharides from the dimer to the hexamer were used as substrates, native or recombinant Chi92 hydrolyzed (GlcNAc)3–6 to give (GlcNAc)2 and GlcNAc as the main products but they did not digest (GlcNAc)2. Purified recombinant Chi92 had the same maximum activity within a pH range of 6.5 to 7.0 and at 42°C as that of native Chi92 when colloidal chitin was used as the substrate. The N-terminal 14-amino-acid sequences of the two enzymes were identical and determined to be AAPGKPTIGSGPTK, which was completely identical to the deduced amino acid sequence of Chi92 at amino acid positions 24 to 37.
Polysaccharide-binding capacity of Chi92 and C-terminally truncated Chi92 derivatives.
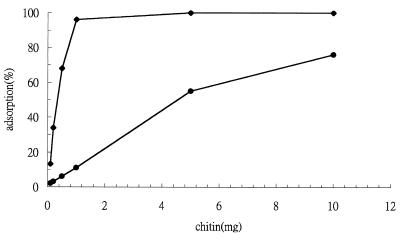
To investigate the binding capacity and specificity of Chi92, we measured the adsorption of Chi92 to various amounts of colloidal chitin, unprocessed chitin, cellulose, xylan, and starch. The results indicated that Chi92 had an approximately 10-fold higher affinity for colloidal chitin than that of unprocessed chitin (Fig. 3). In addition to chitin binding, Chi92 showed low but detectable cellulose-binding capacity but it did not bind xylan or starch (data not shown). Furthermore, a time course experiment showed that more than 90% of the enzyme was bound to the chitin preparation within 2 min (data not shown). This suggests that Chi92 bound rapidly and specifically to chitin.
FIG. 3.
Chitin substrate-binding capacity of Chi92. The binding of Chi92 to various amounts of chitin was measured as described in Materials and Methods. The concentration of Chi92 was 50 μg/ml; the concentrations of colloidal and unprocessed chitin were in the range 0.1 to 10 mg/ml. Symbols: ♦, colloidal chitin; ●, unprocessed chitin.
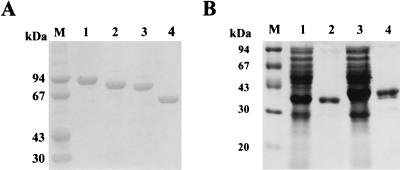
As the C-terminal repeats of Chi92 (designated ChBDCI and ChBDCII) were assumed to be the ChBD, various C-terminally truncated derivatives of Chi92 were constructed via PCR to verify this hypothesis. Moreover, their chitin substrate-binding capacities were evaluated. The truncated derivatives were obtained from the periplasmic fractions of cells after expression in E. coli JM109 recombinants and purified by Q Sepharose and Sephacryl S-200 column chromatography. The purified proteins showed a single band on SDS-PAGE, and their molecular sizes were 87, 81, and 60 kDa. These were in agreement with the expected values for Chi92ΔCII, Chi92ΔCICII, and Chi92ΔACICII, respectively (Fig. 4A). The abilities of Chi92ΔCII, Chi92ΔCICII, and Chi92ΔACICII to bind chitin substrates were measured and compared with that of Chi92. As shown in Table 1, the levels of binding of Chi92ΔCII and Chi92ΔCICII to unprocessed chitin were 50 and 30% of that of Chi92, respectively, and 30 and 20% of the levels of binding to colloidal chitin, respectively. These results indicated that the C-terminal portion of Chi92 is important for the insoluble-chitin binding of Chi92 and that both the ChBDCI and ChBDCII domains are necessary for maximal binding to insoluble chitins. Chi92ΔACICII exhibited significant binding to chitin substrates due to the control protein (bovine serum albumin) adhered negligibly to the same substrate. Also, its binding capacity was nearly equal to that observed with Chi92ΔCICII. The results indicated that the N-terminal two-thirds of Chi92 has some affinity for the insoluble chitins. It also showed that deletion of the A region did not affect insoluble-chitin binding.
FIG. 4.
SDS-PAGE analysis of truncated Chi92 derivatives and GST fusion proteins. (A) Chi92ΔCII, Chi92ΔCICII, and Chi92ΔACICII purified by Q Sepharose and Sephacryl S-200 chromatography as described in Materials and Methods. Lanes: 1, Chi92 purified from E. coli JM109(pHX); 2, Chi92ΔCII purified from E. coli JM109(pChi87); 3, Chi92ΔCICII purified from E. coli JM109(pChi81); 4, Chi92ΔACICII purified from E. coli JM109(pChi60). (B) GST-CICII and GST-CI purified by glutathione Sepharose 4B chromatography. Lanes: 1, crude cell extract from E. coli XL1-Blue(pGEX-CI); 2, purified GST-CI; 3, crude cell extract from E. coli XL1-Blue(pGEX-CICII); 4, purified GST-CICII. The proteins were analyzed by SDS-PAGE, and the gel was stained with Coomassie brilliant blue R-250. Molecular mass standards were run in lanes M.
TABLE 1.
Binding capacity of Chi92 and its truncated derivatives for insoluble polysaccharidesa
| Insoluble polysaccharide | Amt of protein associated with polysaccharide
|
|||||
|---|---|---|---|---|---|---|
| Chi92 | Chi92ΔCII | Chi92ΔCICII | Chi92ΔACICII | GST-CICII | GST-CI | |
| Unprocessed chitin | 37∗ | 18† | 11‡ | 10‡ | 39∗ | 19† |
| Colloidal chitin | 47∗ | 14† | 9‡ | 10‡ | 50∗ | 13† |
| Cellulose | 8∗ | 3† | ND | ND | 10∗ | 4† |
Duncan's multiple-range test was employed for the statistical analysis. Different symbols (∗, †, and ‡) in the same column indicate significant (P < 0.05) differences. One milligram of colloidal chitin or 10 mg of insoluble polysaccharides was incubated with 50 μg of purified enzyme for 1 h at 4°C, and the concentration of bound enzyme was determined from the A280. ND, not detectable.
Polysaccharide-binding capacity of GST fusion proteins.
To evaluate whether chitin binding by the ChBDs was dependent on the presence of other components of Chi92 and whether ChBDCI and ChBDCII could function independently of each other, GST gene fusions were constructed. The GST gene fusions were expressed in E. coli XL1-Blue, and the fusion proteins were purified to homogeneity by affinity chromatography. Figure 4B shows that the fusion proteins had apparent masses of 41 and 35 kDa, which corresponds well to the expected sizes of GST-CICII and GST-CI, respectively. The adsorption of GST fusion proteins to the two forms of chitin and cellulose is shown in Table 1. In contrast to the adsorption of GST-CI and GST-CICII to chitin substrates, there was no apparent adsorption of GST alone to either chitins or cellulose (data not shown). GST-CICII also displayed the greatest binding activity toward colloidal chitin. GST-CI exhibited significant binding to colloidal and unprocessed chitin, suggesting that a single ChBD can function independently. Furthermore, the abilities of GST-CI to bind unprocessed chitin and colloidal chitin were about twofold and fourfold lower than those of GST-CICII.
Catalytic activities of Chi92 and C-terminally truncated Chi92 derivatives.
To evaluate the effect of the C-terminal domains on the catalytic activity of Chi92, the specific activities of full-length Chi92, Chi92ΔCII, Chi92ΔCICII, and Chi92ΔACICII on soluble and insoluble substrates were determined. The substrates tested included pNPC as a soluble substrate and colloidal and unprocessed chitin as insoluble substrates. The data presented in Table 2 show that full-length Chi92 and Chi92ΔCII and Chi92ΔCICII displayed similar activities against pNPC, whereas Chi92ΔACICII had approximately twofold lower catalytic activities than full-length Chi92. These data indicated that deletion of the A region decreased the hydrolytic activity of Chi92 on a soluble substrate. When colloidal chitin was used as the substrate, Chi92ΔCII, Chi92ΔCICII, and Chi92ΔACICII retained 45, 34, and 32% of their catalytic activities, respectively. Chi92ΔCII, Chi92ΔCICII, and Chi92ΔACICII retained 73, 33, and 30% of their specific activities on unprocessed chitin, respectively.
TABLE 2.
Catalytic activity of Chi92 and its truncated derivatives against various chitinase substratesa
| Substrate | Catalytic activity (U/μmol of enzyme)
|
|||
|---|---|---|---|---|
| Chi92 | Chi92ΔCII | Chi92ΔCICII | Chi92ΔACICII | |
| pNPC | 98.0∗ | 98.6∗ | 99.2∗ | 45.7† |
| Colloidal chitin | 86.4∗ | 39.2† | 29.5‡ | 27.6‡ |
| Unprocessed chitin | 3.3∗ | 2.4† | 1.1‡ | 1.0‡ |
Duncan's multiple-range test was employed for the statistical analysis. Different symbols (∗, †, and ‡) in the same column indicate significant (P < 0.05) differences.
DISCUSSION
The data presented in this report describe the characterization of an extracellular chitinase, designated Chi92, from A. hydrophila JP101. Chi92 is the major chitinase secreted by this bacterium when it is induced with chitin, and it is encoded by an ORF of 2,598 bp coding for 865 amino acids. The deduced molecular mass of the mature protein, 89.830 kDa, correlates well with the value determined by SDS-PAGE of purified Chi92 from A. hydrophila JP101. There were no apparent differences between the enzymatic characteristics of the purified chitinase from A. hydrophila JP101 and those that were obtained from E. coli containing the cloned chitinase gene. The deduced amino acid sequence located in the middle region of Chi92 showed remarkable similarity to the catalytic domains of S. marcescens ChiA (15), which were also found in a wide range of other bacterial chitinases belonging to glycosyl hydrolase family 18. According to the crystal structure of S. marcescens ChiA (21) and site-directed mutagenesis studies of B. circulans chitinase (38), Glu315 and Asp391 are the most important catalytic residues; Phe191, Trp275, Phe316, Met388, Try444, and Arg446 are likely to be involved in the catalytic mechanism. Sequence analysis revealed that all of the residues corresponding to these catalytic residues are clearly present in A. hydrophila JP101 Chi92.
Like many extracellular hydrolases that hydrolyze insoluble polysaccharides, Chi92 is also a multidomain protein, having a signal peptide, an all-β-strand N-terminal region (Chi92-N), an unknown-function region (the A region), and C-terminal repeat domains (ChBDs), in addition to a catalytic domain. Chitin-binding and enzyme activity studies with C-terminally truncated Chi92 derivatives lacking ChBDs demonstrated that the ChBDs are responsible for its adhesion to colloidal chitin and unprocessed chitin. This is accompanied by a significant decrease in the enzyme activities of Chi92 on colloidal and unprocessed chitin but not on a soluble chitin substrate. In addition, the truncated derivatives without two ChBDs (Chi92ΔCICII) exhibited lower affinities and catalytic activities on colloidal and unprocessed chitin than did those without a single ChBD (Chi92ΔCII). Further adsorption experiments with GST fusion proteins revealed that a single domain, ChBDCI, preferentially bound to colloidal chitin, followed by unprocessed chitin, and exhibited weak but significant binding to cellulose. Thus, the two C-terminal repeats of Chi92 apparently represent two ChBDs that function independently of each other, can bind specifically to insoluble chitin, and have some affinity for cellulose. Furthermore, in comparison with GST-CI, GST-CICII has about twofold higher affinity for unprocessed chitin and cellulose and about fourfold higher affinity toward colloidal chitin. Thus, the results suggested that the binding capacities of two ChBDs (ChBDCI and ChBDCII) have additive effects on unprocessed chitin and a synergistic effect on colloidal chitin.
The N-terminal 563 residues of Chi92 exhibited high homology (74.6%) to S. marcescens full-length ChiA, but Chi92 is longer by 302 amino acid residues at the C terminus, which corresponds to the A region and ChBDCICII domains of Chi92. Although both of the C-terminally truncated derivatives, Chi92ΔCICII and Chi92ΔACICII, have similar levels of binding and hydrolytic activity toward colloidal and unprocessed chitin, the hydrolytic activity of Chi92ΔACICII was lower than that of Chi92ΔCICII on a soluble substrate. Thus, our findings suggest that the A region does not facilitate the hydrolytic activity toward colloidal and unprocessed chitin but may likewise play a role in enhancing the enzymatic activity of Chi92 during the catalysis of a soluble-chitin substrate by Chi92. Chi92ΔACICII, which corresponds to the N-terminal 563 residues of Chi92, has significant affinity and enzymatic activity toward insoluble chitins, although its binding activities toward colloidal and unprocessed chitin are slightly lower than those of S. marcescens ChiA (data not shown). The results indicate that Chi92 without the A region and the two C-terminal ChBDs still retained binding and hydrolytic activities toward insoluble and soluble chitin substrates. Alignment of S. marcescens ChiA and Chi92ΔACICII revealed that the sequence of the all-β-strand domain (114-residue domain) located in the N terminus of Chi92 is 75% identical and 83% similar (identical residues plus conservative changes) to the N-terminal domain (ChiN, residues Ala24 to His137) of S. marcescens ChiA, and thus, the all-β-strand domain in Chi92 was named the Chi92-N domain. ChiN is a fibronectin III-like fold consisting of 11 β-strands that has been proposed to participate in chitin binding (22, 30). Since Chi92-N exhibited high homology to ChiN, it seems likely that Chi92-N also serves as the ChBD in Chi92, as is the case with ChiN in S. marcescens ChiA. Thus, it appears that Chi92 contains Chi92-N as the third ChBD, in addition to ChBDCI and ChBDCII. Tanaka et al. recently reported that a chitinase obtained from P. kodakaraensis KOD1 possesses three ChBDs (33).
More than 200 different CBDs from β-1,4-glucanases have been investigated and can be classified into 13 different families on the basis of amino acid similarities. The three-dimensional structures of five CBDs that specifically bind both crystalline and amorphous cellulose have been determined (4, 16, 24, 34, 40). Among them, Brun et al. proposed that CBDEGZ and some ChBDs might form a new family (family V) (4). They also pointed out that the ChBDs of B. circulans ChiA1 and D1 are presumably not included in family V because they do not exhibit the conserved AKWWTQG motif, which corresponds to Ala41, Asn42, Trp43, Tyr44, Thr45, and Ala46 in CBDEGZ. Thus, the ChBDs that share significant similarity to CBDEGZ have been divided into two groups, (i) those that do not have the AKWWTQG motif, like the S. marcescens 2170 chitinase CI, Aeromonas sp. strain 10S chitinase II, Janthinobacterium lividum chitinase 69a, and B. circulans ChiD1 and ChiA1 (11), and (ii) those that contain the AKWWTQG motif, as summarized in Fig. 2. The structural analysis of CBDEGZ has shown that it exhibits a ski boot shape and that its binding to the cellulose surface may occur through three exposed aromatic residues, Trp18, Trp43, and Tyr44 (where Trp43 and Tyr44 correspond to the two aromatic residues in the AKWWTQG motif), which are localized on the surface of the protein molecule. On the other hand, the ChBDs in the first group, including ChBDChiA, probably have substrate-binding surfaces that differ from those of the ChBDs and CBDs, including CBDEGZ in the second group (family V). Recently, a structural analysis by Ikegami et al. revealed that ChBDChiA does not have a planar array of aromatic residues on its surface (14), further supporting the idea that the ChBDs in the first group form a new family (family V-1) that is distinct from family V. As shown in Fig. 2, both ChBDCI and ChBDCII contain the AKWWTQG motif and are highly similar to the ChBDs of family V, indicating that both domains belong to family V. Our data indicate that ChBDCI preferentially binds to colloidal chitin, followed by unprocessed chitin, and exhibits weak but significant binding to cellulose, as mentioned above. Morimoto et al. (18) also reported that ChiB of C. paraputrificum, which belongs to family V, exhibits affinity for chitin and cellulose. Similarly, binding to cellulose, in addition to colloidal and unprocessed chitins, has been demonstrated with a GST fusion with ChBD from Alteromonas sp. strain O-7 chitinase C (36). In contrast to family V CBDs, ChBDChiA bound specifically to insoluble chitin but lacks the ability to bind cellulose. Although the binding specificities of the various ChBDs in families V and V-1 and their substrate affinities require further study, it seems reasonable to suggest that family V CBDs might have binding properties similar to those of ChBDCI. On the other hand, family V-1 CBDs might have binding properties similar to those of ChBDChiA.
In this study, we have shown that a major extracellular chitinase, Chi92, containing three binding domains in addition to a catalytic domain and an A region is produced by A. hydrophila JP101. We suppose that this aquatic bacterium utilizes chitin as a source of carbon and nitrogen, mainly through Chi92. Since it effectively binds to chitin-containing substrates, it is especially important in an aquatic environment that ChBDs could confer a selective advantage on chitinase by promoting intimate enzyme-substrate contact. The presence of multiple ChBDs in Chi92 may be due to this selective superiority. Further studies are therefore required to elucidate whether Chi92-N can play a role identical to that of ChBDCI and ChBDCII and how the binding capacity of two ChBDs (ChBDCI and ChBDCII) has an additive effect on unprocessed chitin and a synergistic effect on colloidal chitin.
REFERENCES
- 1.Beggs J D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978;275:104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- 2.Blaak H, Schrempf H. Binding and Substrate specificities of a Streptomyces olivaceoviridis chitinase in comparison with its proteolytically processed form. Eur J Biochem. 1995;229:132–139. doi: 10.1111/j.1432-1033.1995.tb20447.x. [DOI] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Brun E, Moriaud F, Gans P, Blackledge M J, Barras F, Marion D. Solution structure of the cellulose-binding domain of the endoglucanase Z secreted by Erwinia chrysanthemi. Biochemistry. 1997;36:16074–16086. doi: 10.1021/bi9718494. [DOI] [PubMed] [Google Scholar]
- 5.Cazemier A E, Verdoes J C, van Ooyen A J, Op den Camp H J M. Molecular and biochemical characterization of two xylanase-encoding genes from Cellulomonas pachnodae. Appl Environ Microbiol. 1999;65:4099–4107. doi: 10.1128/aem.65.9.4099-4107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J P, Nagayama F, Chang M C. Cloning and expression of a chitinase gene from Aeromonas hydrophila in Escherichia coli. Appl Environ Microbiol. 1991;57:2426–2428. doi: 10.1128/aem.57.8.2426-2428.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies G J, Dauter M, Brzozowski A M, Bjornvad M E, Andersen K V, Schulein M. Structure of the Bacillus agaradherans family 5 endoglucanase at 1.6 Å and its cellobiose complex at 2.0 Å resolution. Biochemistry. 1998;37:1926–1932. doi: 10.1021/bi972162m. [DOI] [PubMed] [Google Scholar]
- 8.Fukumori F, Sashihara N, Kudo T, Horikoshi K. Nucleotide sequence of two cellulase genes from alkalophilic Bacillus sp. strain N-4 and their strong homology. J Bacteriol. 1986;168:479–485. doi: 10.1128/jb.168.2.479-485.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gleave A P, Taylor R K, Morris B A, Greenwood D R. Cloning and sequencing of a gene encoding the 69-kDa extracellular chitinase of Janthinobacterium lividum. FEMS Microbiol Lett. 1995;131:279–288. doi: 10.1111/j.1574-6968.1995.tb07788.x. [DOI] [PubMed] [Google Scholar]
- 10.Harpster M H, Dunsmuir P. Nucleotide sequence of the chitinase B gene of Serratia marcescens QMB1466. Nucleic Acids Res. 1989;17:5395. doi: 10.1093/nar/17.13.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto M, Ikegami T, Seino S, Ohuchi N, Fukada H, Sugiyama J, Shirakawa M, Watanabe T. Expression and characterization of the chitin-binding domain of chitinase A1 from Bacillus circulans WL-12. J Bacteriol. 2000;182:3045–3054. doi: 10.1128/jb.182.11.3045-3054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henrissat B. A classification of glycosyl hydrolase based on amino acid sequence similarities. Biochem J. 1991;280:309–316. doi: 10.1042/bj2800309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henrissat B, Bairoch A. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J. 1993;293:781–788. doi: 10.1042/bj2930781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikegami T, Okada T, Hashimoto M, Seino S, Watanabe T, Shirakawa M. Solution structure of the chitin-binding domain of Bacillus circulans WL-12 chitinase A1. J Biol Chem. 2000;275:13654–13661. doi: 10.1074/jbc.275.18.13654. [DOI] [PubMed] [Google Scholar]
- 15.Jones J D G, Grady K L, Suslow T V, Bedbrook J R. Isolation and characterization of genes encoding two chitinase enzymes from Serratia marcescens. EMBO J. 1986;5:467–473. doi: 10.1002/j.1460-2075.1986.tb04235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraulis P J, Clore G M, Nilges M, Jones T A, Pettersson G, Knowles J, Gronenborn A M. Determination of the three-dimensional solution structure of the C-terminal domain of cellobiohydrolase I from Trichoderma reesei. A study using nuclear magnetic resonance and hybrid distance geometry-dynamical simulated annealing. Biochemistry. 1989;28:7241–7257. doi: 10.1021/bi00444a016. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Morimoto K, Karita S, Kimura T, Sakka K, Ohmiya K. Cloning, sequencing, and expression of the gene encoding Clostridium paraputrificum chitinase ChiB and analysis of the functions of novel cadherin-like domains and a chitin-binding domain. J Bacteriol. 1997;179:7306–7314. doi: 10.1128/jb.179.23.7306-7314.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neu H C, Heppel L A. The release of enzyme from Escherichia coli by osmotic shock during the formation of spheroplasts. J Biol Chem. 1965;240:3685–3692. [PubMed] [Google Scholar]
- 20.Ohno T, Armand S, Hata T, Nikaidou N, Henrissat B, Mitsutomi M, Watanabe T. A modular family 19 chitinases found in the prokaryotic organism Streptomyces griseus HUT 6037. J Bacteriol. 1996;178:5065–5070. doi: 10.1128/jb.178.17.5065-5070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrakis A, Tews I, Dauter Z, Oppenheim A B, Chet I, Wilson K S, Vorgias C E. Crystal structure of a bacterial chitinase at 2.3 Å resolution. Structure. 1994;2:1169–1180. doi: 10.1016/s0969-2126(94)00119-7. [DOI] [PubMed] [Google Scholar]
- 22.Perrakis A, Ouzounis C, Wilson K S. Evolution of immunoglobulin-like modules in chitinases: their structural flexibility and functional implications. Fold Des. 1997;2:291–294. doi: 10.1016/S1359-0278(97)00040-0. [DOI] [PubMed] [Google Scholar]
- 23.Redenbach M, Kieser H M, Denapaite D, Eichner A, Cullum J, Kinashi H, Hopwood D A. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolor A3 (2) chromosome. Mol Microbiol. 1996;21:77–96. doi: 10.1046/j.1365-2958.1996.6191336.x. [DOI] [PubMed] [Google Scholar]
- 24.Sakon J, Irwin D, Wilson D B, Karplus P A. Structure and mechanism of endo/exocellulase E4 from Thermomonospora fusca. Nat Struct Biol. 1997;4:810–818. doi: 10.1038/nsb1097-810. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimahara K, Takiguchi Y. Preparation of crustacean chitin. Methods Enzymol. 1988;161:417–423. [Google Scholar]
- 28.Shiro M, Ueda M, Kawaguchi T, Arai M. Cloning of a cluster of chitinase genes from Aeromonas sp. no. 10S-24. Biochim Biophys Acta. 1996;1305:44–48. doi: 10.1016/0167-4781(95)00213-8. [DOI] [PubMed] [Google Scholar]
- 29.Sitrit Y, Vorgias C E, Chet I, Oppenheim A B. Cloning and primary structure of the chiA gene from Aeromonas caviae. J Bacteriol. 1995;177:4187–4189. doi: 10.1128/jb.177.14.4187-4189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki K, Suzuki M, Taiyoji M, Nikaidou N, Watanabe T. Chitin binding protein (CBP21) in the culture supernatant of Serratia marcescens 2170. Biosci Biotechnol Biochem. 1998;62:128–135. doi: 10.1271/bbb.62.128. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki K, Taiyoji M, Sugawara N, Nikaidou N, Henrissat B, Watanabe T. The third chitinase gene (chiC) of Serratia marcescens 2170 and the relationship of its product to other bacterial chitinases. Biochem J. 1999;343:587–596. [PMC free article] [PubMed] [Google Scholar]
- 32.Svitil A L, Kirchman D L. A chitin-binding domain in a marine bacterial chitinase and other microbial chitinases: implications for the ecology and evolution of 1,4-β-glycanases. Microbiology. 1998;144:1299–1308. doi: 10.1099/00221287-144-5-1299. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka T, Fujiwara S, Nishikori S, Fukui T, Takagi M, Imanaka T. A unique chitinase with dual active sites and triple substrate binding sites from the hyperthermophilic archaeon Pyrococcus kodakaraensis KOD1. Appl Environ Microbiol. 1999;65:5338–5344. doi: 10.1128/aem.65.12.5338-5344.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tormo J, Lamed R, Chirino A J, Morag E, Bayer E A, Shoham Y, Steitz T A. Crystal structure of a bacterial family-III cellulose-binding domain: a general mechanism for attachment to cellulose. EMBO J. 1996;15:5739–5751. [PMC free article] [PubMed] [Google Scholar]
- 35.Tsujibo H, Orikoshi H, Tanno H, Fujimoto K, Miyamoto K, Imada C, Okami Y, Inamori Y. Cloning, sequence, and expression of a chitinase gene from a marine bacterium. Alteromonas sp. strain O-7. J Bacteriol. 1993;175:176–181. doi: 10.1128/jb.175.1.176-181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsujibo H, Orikoshi H, Shiotani K, Hayashi M, Umeda J, Miyamoto K, Imada C, Okami Y, Inamori Y. Characterization of chitinase C from a marine bacterium. Alteromonas sp. strain O-7 and its corresponding gene and domain structure. Appl Environ Microbiol. 1998;64:472–478. doi: 10.1128/aem.64.2.472-478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueda M, Kawaguchi T, Arai M. Molecular cloning and nucleotide sequence of the gene encoding chitinase II from Aeromonas sp. no. 10S-24. J Ferment Bioeng. 1994;78:205–211. [Google Scholar]
- 38.Watanabe T, Kobori K, Miyashita K, Fujii T, Sakai H, Uchida M, Tanaka H. Identification of glutamic acid 204 and aspartic acid 200 in chitinase A1 of Bacillus circulans WL-12 as essential residues for chitinase activity. J Biol Chem. 1993;268:18567–18572. [PubMed] [Google Scholar]
- 39.Watanabe T, Ito Y, Yamada T, Hashimoto M, Sekine S, Tanaka H. The roles of the C-terminal domain and type III domains of chitinase A1 from Bacillus circulans WL-12 in chitin degradation. J Bacteriol. 1994;176:4465–4472. doi: 10.1128/jb.176.15.4465-4472.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu G Y, Ong E, Gilkes N R, Kilbern D G, Muhandiram D R, Harris-Brandts M, Carver J P, Kay L E, Harvey T S. Solution structure of a cellulose-binding domain from Cellulomonas fimi by nuclear magnetic resonance spectroscopy. Biochemistry. 1995;34:6993–7009. [PubMed] [Google Scholar]
- 41.Yanisch P C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]