Abstract
Objective:
Axillary lymph node status assessment has always been an important issue in clinical treatment of breast cancer. However, there has been no effective method to accurately predict the pathological complete response (pCR) of axillary lymph node after neoadjuvant chemotherapy (NAC). The objective of our study was to investigate whether conventional ultrasonography combined with contrast-enhanced ultrasonography (CEUS) can be used to evaluate axillary lymph node status of breast cancer patients after NAC.
Methods:
A total of 74 patients who underwent NAC were recruited for the present study. Prior to and after NAC, examinations of conventional ultrasonography and CEUS were performed. After evaluating the images of conventional ultrasonography, four characteristics were recorded: lymph node medulla boundary, cortex of lymph node, lymph node hilus, and lymph node aspect ratio. Two additional imaging characteristics of CEUS were analyzed: CEUS way and CEUS pattern. Receiver operating characteristiccurve analysis was applied to evaluate their diagnostic performance.
Results:
After 6~8 cycles of NAC, 46 (71.9%) patients had negative axillary lymph node, and 18 (28.1%) patients turned out non-pCR. According to statistical analysis, lymph node medulla, lymph node aspect ratio and CEUS way were independently associated with pCR of axillary lymph node after NAC. The area under the curve of the prediction model with three imaging characteristics was 0.882 (95% confidence interval: 0.608–0.958), and the accuracy to predict the patients’ lymph node status was 78.1% (p < 0.01).
Conclusions:
Conventional ultrasonography combined with CEUS technology can accurately predict axillary lymph nodes status of breast cancer patients after NAC.
Advances in knowledge:
The usefulness of CEUS technology in predicting pCR after neoadjuvant chemotherapy is highlighted.
Introduction
Breast cancer is one of the most threatening malignant tumours for females worldwide.1 Neoadjuvant therapy is an important way to treat early breast cancer. Based on the results of evidence-based medicine in the current literature, neoadjuvant therapy (NAC) can not only achieve similar benefits as conventional adjuvant therapy but can also enable some patients with high-risk factors (such as triple negative and human epidermal growth factor receptor 2-positive breast cancers) to undergo further intensive treatment and to benefit from it.2 Moreover, the long-term prognosis of patients who have obtained pathological complete response (pCR, with no residual infiltrating tumour in the breast and axilla) through neoadjuvant therapy is significantly better than that of patients with residual invasive tumour.3 Therefore, for these patients with good prognosis, with regard to the choice of surgical methods, breast-conserving surgery resulting in less trauma, faster recovery, and better quality of life is preferred. As lymphedema caused by axillary dissection is an important factor affecting the prognosis of breast cancer patients, studies have shown that sentinel lymph node biopsy (SLNB) could be sufficient for patients who are expected to achieve axillary pCR.4 However, according to The American College of Surgeons Oncology Group 1071, the overall false-negative rates (FNRs) were 13–14%, which didn't achieve necessary FNR <10% to be clinically acceptable.5 Therefore, an effective and accurate method to detect lymph node status after NAC is urgently required. In patients with axillary lymph node metastasis before neoadjuvant, after effective neoadjuvant treatment, the axillary lymph node metastasis disappears, allowing patients to avoid excessive dissection.4,6
Although NAC is effective for most breast cancer patients, some patients are still unresponsive to neoadjuvant therapy and cannot achieve pCR.7,8 Therefore, the prediction of neoadjuvant efficacy plays a critical role in the next clinical treatment strategy, especially the choice and method of surgery. For the evaluation of whether primary lesion achieves pCR, a combination of multiple imaging methods is always clinically adopted,9,10 especially with magnetic resonance technology,11–13 that can further improve the predictability of primary lesion pCR. However, to date, the most appropriate imaging detection method for predicting whether axillary lymph nodes achieve pCR has not been established.
Previous studies have reported that ultrasonography can effectively evaluate axillary lymph nodes.14–16 Contrast-enhanced ultrasonography (CEUS) is a new technology in the field of ultrasonography. It uses the nonlinear effect in a sound field of gas microbubbles in the blood and generated strong backscattering to obtain contrast-enhanced images. It has a good sensitivity for evaluating lymph node status.17,18 In this study, we aimed to use conventional ultrasonography combined with CEUS to evaluate the axillary lymph node status of breast cancer patients on neoadjuvant therapy and to identify whether it can predict the occurrence of axillary lymph node pCR in these patients.
Methods and materials
Patients
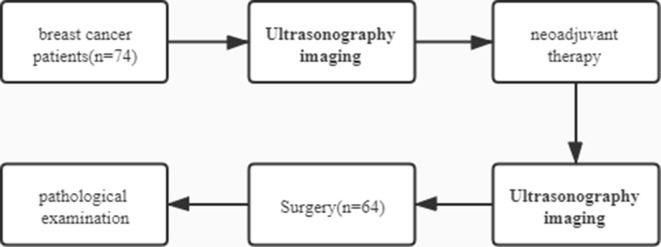
Patients with early breast cancer who underwent neoadjuvant therapy at Harbin Medical University Cancer Hospital from 2018 to 2020 were included, and axillary lymph nodes of patients were examined by conventional ultrasonography combined with CEUS before and after neoadjuvant therapy. The inclusion criteria were as follows: (1) pathological diagnosis of invasive breast cancer ; (2) patients that met the neoadjuvant therapy population criteria according to the National Comprehensive Cancer Network (NCCN) guidelines; (3) fully informed and agreed to CEUS examination; (4) in good health, without severe heart, liver, or kidney function insufficiency, and could tolerate neoadjuvant therapy; (5) upper limbs were sound without vessel-related diseases; and (6) without history of surgical trauma to the axilla and breast. The exclusion criteria were as follows:(1) presence of severe organic diseases and inability to tolerate neoadjuvant therapy, (2) a history of severe allergies, (3) unsound upper limbs, (4) a history of surgical trauma to the axilla and breast, (5) ultrasonography imaging was not performed, and (6) refusal of CEUS(Figure 1).
Figure 1.
The flowchart of this article.
Experimental methods
True-cut biopsy with 16G needle was performed from the primary breast lesion and swollen lymph nodes to determine the pathological type and immunohistochemical type.
According to NCCN guidelines, NAC or combined neoadjuvant endocrine therapy was performed on patients with different subtypes of breast cancer until the end of the prescribed therapy cycle. Surgery was then performed. We defined positive lymph node before NAC as: (1) True-cut biopsy confirmed positive lymph node before chemotherapy; (2) Lymph node before chemotherapy was negative, but post-operative pathological results confirmed the presence of cancer cells or reactions after chemotherapy. Positive lymph node after NAC was defined as: residual axillary lymph node metastasis confirmed by pathology.
In this study, the Canon Aplio i900 ultrasound diagnostic instrument (Canon Medical Systems USA, Inc., Tustin, CA) equipped with an i18L × 5 ultrasonography probe (Canon Medical Systems USA, Inc.) of 6 MHz was used for conventional ultrasonography. SonoVue (Bracco, Milan, Italy) as a contrast agent was used in CEUS examination. To avoid rupture of SonoVue microbubbles under high-frequency ultrasound irradiation, the frequency of the probe used for CEUS was 5.5 MHz. A total of 4 ml SonoVue suspension was injected through the patient’ s elbow vein, followed by 5 ml of saline to flush the tube. The dynamic image was stored in real time for 60 s, and the contrast observation continued until lesion enhancement disappeared.
Conventional ultrasonography was used to find and locate enlarged lymph nodes under the armpit. We observed and recorded the aspect ratio of lymph nodes, cortical thickness, lymphatic hilum status, and lymph node boundary conditions and classified them. CEUS was performed to assess the axillary lymph nodes. The enhancement entrance and mode were classified in combination with conventional ultrasonography as follows:
Lymph node medulla boundary: A, clear; B, unclear
Cortex of lymph node: A, ≤3 mm; B, ≥3 mm
Lymph node hilus: A, clear; B, unclear
Lymph node aspect ratio: A, ≥2; B, <2
CEUS way: A, hilus; B, mix/around
CEUS pattern: A, even-enhanced; B, uneven-enhanced
Imaging examinations were performed twice for every patient, both before and after NAC. The accuracy of the methods in evaluating judge lymph node metastasis was compared(Figure 2).
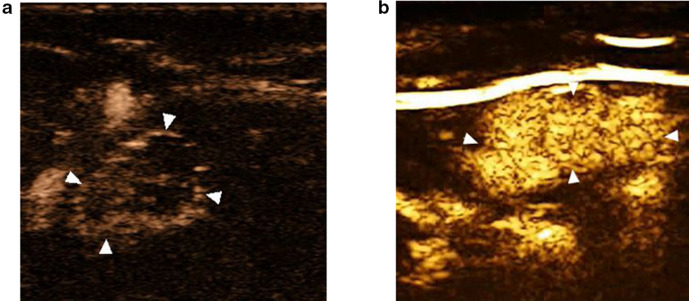
Figure 2.
(A) The imaging example of positive lymph node, which showed enhancement around the lymph node with uneven enhancement pattern; (B) the imaging example of negative lymph node, which showed enhancement originated from lymphatic hilum with even enhancement pattern.
Statistical analysis
Statistical Package for Social Sciences v. 19.0 (IBM Corp., Armonk, NY) was used to analyse the data of all patients. Logistics regression analysis was performed, and a receiver operating characteristic (ROC) curve was drawn to analyse patient data before neoadjuvant therapy and evaluate their sensitivity and specificity. After NAC, the combination method was used to reanalyse the patient data. For the multivariable analysis, we selected those covariates with p values less than 0.3 in the univariable analysis. An odds ratio greater than 1 meant higher odds of residual axillary lymph node metastasis. We evaluated its predictive ability and compared the accuracy differences. The subgroup analysis included patients with positive lymph nodes before NAC.
Results
Patients
74 patients who underwent pre-neoadjuvant conventional ultrasonography combined with CEUS were included in this study, of whom 20 had negative nodes before NAC (27.0%), and 54 (73.0%) had positive nodes. 64 people completed all 2 examinations and underwent surgeries, of whom 52 (70.3%) underwent axillary lymph node dissection, and another 12 (16.2%) underwent sentinel lymph node biopsy (Table 1).
Table 1.
Patient and tumour characteristics
| Before NAC (N = 74) | After NAC (N = 64) | |
|---|---|---|
| Age,years | 50.8 ± 8.35 | 52.66 ± 8.95 |
| Tumour size | ||
| T1 | 2 (2.7%) | 46 (71.9%) |
| T2 | 36 (48.6%) | 14 (21.9%) |
| T3 | 31 (41.9%) | 4 (6.3%) |
| T4 | 5 (6.8%) | 0 (0%) |
| Lymph node | ||
| pN0 | 20 (27.0%) | 46 (71.9%) |
| pN1 | 42 (56.8%) | 16 (25.0%) |
| pN2-3 | 12 (16.2%) | 2 (3.1%) |
| Subtype (IHC) | ||
| TNBC | 20 (27.0%) | 16 (25.0%) |
| HER2+ | 34 (45.9%) | 32 (50.0%) |
| HR+, HER2− | 20 (27.0%) | 16 (25.0%) |
| Regimens of NAC | ||
| TCb | 20 (27.0%) | 16 (25.0%) |
| THP | 34 (45.9%) | 32 (50.0%) |
| AC-T | 20 (27.0%) | 16 (50.0) |
| Axillary surgery type | ||
| SLND | 12 (16.2%) | 12 (18.8%) |
| ALND | 52 (70.2%) | 52 (71.2%) |
| Unknown | 10 (13.8%) | 0 (0%) |
| Total | 74 (100%) | 64 (100%) |
AC-T,adriamycin with cyclophosphamide plus docetaxel;ALND, axillary lymph node dissection; HER2, Human epidermal growth factor receptor; HR, Hormone receptor;IHC, Immunohistochemical; NAC, Neoadjuvant chemotherapy;SLNB, sentinel lymph node biopsy; TCb,Docetaxel with carboplatin;THP,Docetaxel with Trastuzumab andPertuzumab; TNBC, Triple negative breast cancer.
Experimental results
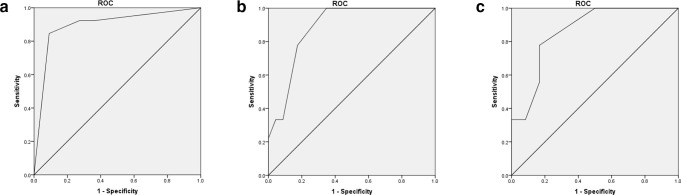
Lymph node metastasis was diagnosed in 74 people who underwent conventional ultrasonography combined with CEUS for axillary lymph node assessment before NAC. According to the multivariate analysis, “Cortex of lymph node” [OR: 9.7 (1.9–49.4)] and “Contrast-enhanced ultrasonography way” [OR: 7.4 (1.5–36.2)] showed statistical significance, and accordingly, a logistics regression model was built (Table 2). A ROC curve was plotted, with an area under the ROC curve (AUC) of 0.895 (0.808–0.982) (Table 3, Figure 3), sensitivity of 63.6%, specificity of 92.3%, and accuracy of 83.8% (p < 0.0001) (Table 3).
Table 2.
Multivariate analysis using the backward elimination method of conventional ultrasonography combined with contrast-enhanced ultrasonography before and after NAC according to the axillary lymph nodes
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR | p-value | OR | p- value | |
| Before NAC | ||||
| Lymph node medulla boundary: | - | - | - | - |
| Cortex of lymph node: | 32.0 (8.0–128.0) | p < 0.0001 | 9.7 (1.9–49.4) | p = 0.006 |
| Lymph node hilus: | - | - | - | - |
| Lymph node aspect ratio: | 9.6 (3.0–30.4) | p < 0.0001 | - | - |
| Contrast-enhanced ultrasonography way: | 24.8 (6.6–92.6) | p < 0.0001 | 7.4 (1.5–36.2) | p = 0.013 |
| Contrast-enhanced ultrasonography pattern: | - | - | - | - |
| After NAC | ||||
| Lymph node medulla boundary | 8.4 (2.1–33.5) | p = 0.003 | 11.7 (2.1–63.9) | p = 0.005 |
| Cortex of lymph node: | - | - | - | - |
| Lymph node hilus: | - | - | - | - |
| Lymph node aspect ratio: | 13.1 (3.3–52.4) | p < 0.0001 | 15.4 (3.0–77.0) | p = 0.001 |
| Contrast-enhanced ultrasonography way: | 3.5 (1.1–11.1) | p = 0.03 | 2.4 (1.1–5.3) | p = 0.231 |
| Contrast-enhanced ultrasonography pattern: | - | - | - | - |
NAC,neoadjuvant chemotherapy;OR,odds ratio.
Table 3.
The ROC of conventional ultrasonography combined with contrast-enhanced ultrasonography to diagnose axillary lymph nodes
| ROC | AUC | NPV | PPV | Sensitivity | Specificity | Accuracy | Sig. |
|---|---|---|---|---|---|---|---|
| Before NAC | 0.895 (0.808–0.982) |
85.7% | 77.8% | 63.6% | 92.3% | 83.8% | p < 0.0001 |
| After NAC | 0.882 (0.802–0.962) |
62.5% | 83.3% | 87.0% | 55.6% | 78.1% | p < 0.0001 |
| Positive lymph node |
0.861 (0.751–0.971) |
77.8% | 83.3% | 83.3% | 77.8% | 81.0% | p < 0.0001 |
ROC,Receiver operating characteristic curve;AUC,Area under ROC;PPV,Positive predictive value; NPV, Negative predictive value; Sig., Significance value.
Figure 3.
(A) The ROC of contrast-enhanced ultrasonography combined model before NAC (AUC = 0.895, p < 0.0001); (B) the ROC of contrast-enhanced ultrasonography combined model after NAC (AUC = 0.882, p < 0.0001); (C) The ROC of contrast-enhanced ultrasonography combined model in lymph node-positive patients (AUC = 0.861, p < 0.0001). AUC, area under the curve; NAC, neoadjuvant chemotherapy; ROC, receiver operating characteristic.
64 people who underwent conventional ultrasonography combined with CEUS for axillary lymph node assessment completed NAC and underwent surgeries. According to the multivariate analysis, “Lymph node medulla boundary” [OR: 11.7 (2.1–63.9)], “Lymph node aspect ratio” [OR: 11.7 (2.1–63.9)], and “Contrast-enhanced ultrasonography way” [OR: 2.4 (1.1–5.3)] showed statistical significance, and accordingly, a logistics regression model was built (Table 2). The ROC curve was plotted, with an AUC of 0.882 (0.802–0.962) (Table 3, Figure 3), sensitivity of 87.0%, specificity of 55.6%, and accuracy of 78.1% (p < 0.0001) (Table 3).
In addition, we used these three imaging factors to analyse the imaging data of patients with positive lymph nodes before NAC. The ROC curve showed that the AUC was 0.861 (0.751–0.971), the sensitivity was 83.3%, the specificity was 77.8%, and the accuracy was 81.0% (p < 0.0001) (Table 3)(Figure 3) It was significantly higher than conventional ultrasonography.
Discussion
In this study, CEUS has obvious advantages in evaluating the patients’ axillary lymph nodes during neoadjuvant therapy. It greatly contributes to accurate diagnosis, with an accuracy of more than 80% in clinical practice. The results can accurately predict the efficacy of NAC through imaging methods, and help clinicians to plan surgery, such as choosing less traumatic breast-conserving surgery or avoiding axillary dissection and selecting sentinel lymph node biopsy instead, which is consistent with previous research.19. Li etc. conducted an animal model to demonstrate that CEUS allows for accurate visualisation of lymph nodes, and helps to choose correct surgical method clinically.20 In this experiment, CEUS also played an excellent role in locating sentinel lymph nodes. Previous studies have shown that CEUS is more accurate than conventional ultrasonography for detecting tumour lymph node metastasis without chemotherapy.18 Agliata etc. demonstrated that CEUS is an inspection method with overall high sensitivity for evaluation of axillary lymph nodes in breast cancer, which is close to the reference-standard sentinel lymph node biopsy.17 In our centre, CEUS has good clinical application value, especially for predicting the curative effect of neoadjuvant therapy. For patients with initial positive lymph nodes, the status of lymph nodes after NAC is an important factor affecting their prognosis. At the same time, for patients with axillary lymph node pCR, axillary lymph node dissection can be avoided, which can greatly reduce the incidence of lymphedema and improve the patients’ quality of life. This study innovatively used CEUS to evaluate the outcome of axillary lymph nodes after neoadjuvant therapy and obtained a good prediction result (AUC = 0.882). In retrospective samples of our centre, the accuracy of using conventional ultrasonography alone to diagnose axillary lymph nodes was less than 70% for neoadjuvant patients. As a non-invasive examination, CEUS can accurately predict pCR of axillary lymph nodes, with an accuracy rate of approximately 80% in lymph node-positive patients. This can better avoid unnecessary axillary dissections, improve the quality of life, and reduce the cost of diagnosis and treatment for patients.
In some previous articles, researchers often pay attention to the quantitative indicators of lymph node angiography and believe that data such as time to peak and mean time to enhance of node can predict the degree of remission of the lesion.21–23 However, in this study, the data for lymph nodes did not show an obvious correlation. This may be related to the individual differences in lymph node blood supply and differences in haemodynamics between different individuals, and it may also be related to differences in computer software analysis. In addition, these type of data require post-processing, which is not conducive to clinical use. Therefore, we only selected some qualitative indicators, which makes the evaluation by ultrasound physicians in clinical practice more convenient. Due to the different number of people and different factors included, we cannot directly compare the accuracy of this imaging technique before and after NAC. However, we can still see that this simple and convenient evaluation method has a good application prospect for patients after NAC.
This study has some limitations. Due to the small sample size, we could not establish a more accurate prediction model, resulting in a lack of extensive standards in clinical applications. However, this study has already given good preliminary prediction results. We believe that subsequent expansion of the sample size and further upgradation of statistical methods can establish more accurate prediction models for clinical practice.
Conclusions
In conclusion, conventional ultrasonography combined with CEUS has a broad prospective application for predicting the efficacy of neoadjuvant therapy for breast cancer patients. In the future, we will further expand the sample size and utilise more imaging methods to establish a more accurate and efficient prediction model.
Footnotes
Acknowledgement: We would like to thank Editage for English editing.
Authors’ contributions: Guarantor of integrity of the entire study – Guoqiang Zhang
Study concepts and design – Shiyang Jin, Jinxing Zhang, Zhenfeng Huang
Literature search – Shiyang Jin, Ming Shan
Clinical studies – Xue Han, Yue Yang, Guoqiang Zhang
Experimental studies/data analysis – Xue Han, Ming Shan, Huajing Yang, Yue Yang, Guoqiang Zhang
Statistical analysis – Chuan He, Hongyan Guo, Jiguang Han
Manuscript preparation – Shiyang Jin, Ming Shan, Huajing Yang, Guoqiang Zhang
Manuscript editing – Shiyang Jin, Ming Shan
Funding: This study was supported by the Hei Longjiang Postdoctoral Foundation (LBH-Z16120) and the Hai Yan Science Foundation of Harbin Medical University Cancer Hospital (JJZD2019-05).
Ethical considerations: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The authors Ming Shan and Guoqiang Zhang contributed equally to the work.
Data statement: The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Code availability: The codes are available from the corresponding author on reasonable request.
Contributor Information
Xue Han, Email: 282268570@qq.com.
Shiyang Jin, Email: 784224552@qq.com.
Huajing Yang, Email: yanghuajing0416@outlook.com.
Jinxing Zhang, Email: 1843562148@qq.com.
Zhenfeng Huang, Email: 2445861891@qq.com.
Jiguang Han, Email: han_jg112@163.com.
Chuan He, Email: he-chuan@sohu.com.
Hongyan Guo, Email: Guohongyan5564@sohu.com.
Yue Yang, Email: yuelianghaiyang@126.com.
Ming Shan, Email: shanming@ems.hrbmu.edu.cn.
Guoqiang Zhang, Email: zhangguoqiang@hrbmu.edu.cn.
REFERENCES
- 1.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA Cancer J Clin 2019; 69: 438–51. doi: 10.3322/caac.21583 [DOI] [PubMed] [Google Scholar]
- 2. Early breast cancer Trialists' Collaborative Group (EBCTCG). long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 2018; 19: 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384: 164–72. doi: 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 4.Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013; 14: 609–18. doi: 10.1016/S1470-2045(13)70166-9 [DOI] [PubMed] [Google Scholar]
- 5.Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013; 310: 1455–61. doi: 10.1001/jama.2013.278932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubio IT. Sentinel lymph node biopsy after neoadjuvant treatment in breast cancer: work in progress. Eur J Surg Oncol 2016; 42: 326–32. doi: 10.1016/j.ejso.2015.11.018 [DOI] [PubMed] [Google Scholar]
- 7.Masuda N, Lee S-J, Ohtani S, Im Y-H, Lee E-S, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017; 376: 2147–59. doi: 10.1056/NEJMoa1612645 [DOI] [PubMed] [Google Scholar]
- 8.von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 2019; 380: 617–28. doi: 10.1056/NEJMoa1814017 [DOI] [PubMed] [Google Scholar]
- 9.Kim Y, Kim SH, Song BJ, Kang BJ, Yim K-I, Lee A, et al. Early prediction of response to neoadjuvant chemotherapy using dynamic contrast-enhanced MRI and ultrasound in breast cancer. Korean J Radiol 2018; 19: 682–91. doi: 10.3348/kjr.2018.19.4.682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones EF, Hathi DK, Freimanis R, Mukhtar RA, Chien AJ, Esserman LJ, et al. Current landscape of breast cancer imaging and potential quantitative imaging markers of response in ER-positive breast cancers treated with neoadjuvant therapy. Cancers 2020; 12: 151109 06 2020. doi: 10.3390/cancers12061511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hylton NM, Blume JD, Bernreuter WK, Pisano ED, Rosen MA, Morris EA, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy--results from ACRIN 6657/I-SPY TRIAL. Radiology 2012; 263: 663–72. doi: 10.1148/radiol.12110748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheel JR, Kim E, Partridge SC, Lehman CD, Rosen MA, Bernreuter WK, et al. Mri, clinical examination, and mammography for preoperative assessment of residual disease and pathologic complete response after neoadjuvant chemotherapy for breast cancer: ACRIN 6657 trial. AJR Am J Roentgenol 2018; 210: 1376–85. doi: 10.2214/AJR.17.18323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton EJ, Onishi N, Fehr DA, Dashevsky BZ, Sadinski M, Pinker K, et al. A machine learning model that classifies breast cancer pathologic complete response on MRI post-neoadjuvant chemotherapy. Breast Cancer Res 2020; 22: 57. doi: 10.1186/s13058-020-01291-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim R, Chang JM, Lee H-B, Lee SH, Kim S-Y, Kim ES, et al. Predicting axillary response to neoadjuvant chemotherapy: breast MRI and US in patients with node-positive breast cancer. Radiology 2019; 293: 49–57. doi: 10.1148/radiol.2019190014 [DOI] [PubMed] [Google Scholar]
- 15.Hotton J, Salleron J, Henrot P, Buhler J, Leufflen L, Rauch P, et al. Pre-Operative axillary ultrasound with fine-needle aspiration cytology performance and predictive factors of false negatives in axillary lymph node involvement in early breast cancer. Breast Cancer Res Treat 2020; 183: 639–47. doi: 10.1007/s10549-020-05830-z [DOI] [PubMed] [Google Scholar]
- 16.Eun NL, Son EJ, Gweon HM, Kim J-A, Youk JH. Prediction of axillary response by monitoring with ultrasound and MRI during and after neoadjuvant chemotherapy in breast cancer patients. Eur Radiol 2020; 30: 1460–9. doi: 10.1007/s00330-019-06539-4 [DOI] [PubMed] [Google Scholar]
- 17.Agliata G, Valeri G, Argalia G, Tarabelli E, Giuseppetti GM. Role of contrast-enhanced sonography in the evaluation of axillary lymph nodes in breast carcinoma: a monocentric study. J Ultrasound Med 2017; 36: 505–11. doi: 10.7863/ultra.16.04012 [DOI] [PubMed] [Google Scholar]
- 18.Cui X-W, Jenssen C, Saftoiu A, Ignee A, Dietrich CF. New ultrasound techniques for lymph node evaluation. World J Gastroenterol 2013; 19: 4850–60. doi: 10.3748/wjg.v19.i30.4850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boca Bene I, Dudea SM, Ciurea AI. Contrast-Enhanced ultrasonography in the diagnosis and treatment modulation of breast cancer. J Pers Med 2021; 11: 8130 01 2021. doi: 10.3390/jpm11020081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Mori S, Kodama M, Sakamoto M, Takahashi S, Kodama T. Enhanced sonographic imaging to diagnose lymph node metastasis: importance of blood vessel volume and density. Cancer Res 2013; 73: 2082–92. doi: 10.1158/0008-5472.CAN-12-4200 [DOI] [PubMed] [Google Scholar]
- 21.Wan C-F, Liu X-S, Wang L, Zhang J, Lu J-S, Li F-H, , FH L. Quantitative contrast-enhanced ultrasound evaluation of pathological complete response in patients with locally advanced breast cancer receiving neoadjuvant chemotherapy. Eur J Radiol 2018; 103: 118–23. doi: 10.1016/j.ejrad.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Yuan C, Dai W, Tang L, Shi J, Li Z, et al. Evaluating pathologic response of breast cancer to neoadjuvant chemotherapy with computer-extracted features from contrast-enhanced ultrasound videos. Phys Med 2017; 39: 156–63. doi: 10.1016/j.ejmp.2017.06.023 [DOI] [PubMed] [Google Scholar]
- 23.Lee YJ, Kim SH, Kang BJ, Kim YJ. Contrast-Enhanced ultrasound for early prediction of response of breast cancer to neoadjuvant chemotherapy. Ultraschall Med 2019; 40: 194–204. doi: 10.1055/a-0637-1601 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data statement: The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.