Abstract
Pelvic exenteration (PE) is one of the most challenging gynecologic oncologic surgeries and is an overriding term for different procedures that entail radical en bloc resection of the female reproductive organs and removal of additional adjacent affected pelvic organs (bladder, rectum, anus, etc.) with concomitant surgical reconstruction to restore bodily functions. Multimodality cross-sectional imaging with MRI, PET/CT, and CT plays an integral part in treatment decision-making, not only for the appropriate patient selection but also for surveillance after surgery. The purpose of this review is to provide a brief background on pelvic exenteration in gynecologic cancers and to familiarize the reader with the critical radiological aspects in the evaluation of patients for this complex procedure. The focus of this review will be on how imaging can aid in treatment planning and guide management.
Introduction
Pelvic exenteration (PE) is one of the most challenging gynecologic oncologic surgeries. A high surgical skill level and precise knowledge of anatomy are vital prerequisites for a safe and successful outcome. PE is an overriding term for different procedures entailing radical en bloc resection of the female reproductive organs and removal of additional adjacent affected pelvic organs, with concomitant surgical reconstruction to restore bodily functions, body image, and sexual functioning.1–4 It is an uncommon procedure, with most institutions performing a few of these procedures annually.5
Indication for PE requires multidisciplinary expertise through formal interdisciplinary case presentation and discussion between gynecologic oncologists, colorectal surgeons, urologists, radiation oncologists, plastic reconstructive surgeons, and radiologists. Further, the procedure and its peri- and postoperative implications for quality of life need to be discussed thoroughly with patients.2,3,6
PE was first performed by the physician Alexander Brunschwig in 1948 as a palliative, radical surgical procedure for recurrent cervical carcinoma.7 From 1948 to the early 1950s, PE was limited to only a few centers and the rate of morbidity and mortality from PE was up to 35%. However, advancements in the medical field in the second half of the 20th century, such as those related to surgical technique, perioperative care, intensive care, and radiology, have significantly improved its outcomes; thus, it is now a viable option with acceptable morbidity in selected patients if they are treated at a specialized tertiary care center.2,3,8
In highly selected patients with non-metastatic gynecological cancers who present with recurrent or persistent disease after chemoradiotherapy, PE with curative intent has a 5-year survival rate of up to 50%,2,3 whereas in patients with recurrent gynecological cancers, the 2-year survival rate is only 25–32%.9,10 Survival benefit can only be derived if there is complete surgical clearance of cancerous tissue at surgery through achieving histologically tumor-free margins (i.e. R0 resection).11 Therefore, the critical question preoperatively is whether R0 resection is technically achievable; this is where radiology comes into play and allows for disease localization and evaluation of disease extent.1 Besides PE with curative intent, PE can also be performed with palliative intent.1–4
The most common indications for PE include central pelvic recurrence or persistent cervical cancer after chemoradiotherapy, recurrent vulvar cancer, and recurrent uterine cancer.2,4 The multidisciplinary decision to pursue PE is a balancing act between achieving beneficial outcomes against the risk of surgical complications affecting quality of life, in patients who have often already undergone several prior treatments.4 To date, post-surgical complications remains as high as 50%, as the previously irradiated surgical field is prone to wound disruption and superinfection after surgery.1–4,12 Apart from complications related to any major abdominopelvic surgery, common complications of PE include those of the urinary or bowel reconstruction and pelvic floor flaps; late post-operative complications may also occur, including anastomotic strictures, chronic fistula formations, and tumor recurrence.2,3
The purpose of this review is to provide a brief background on pelvic exenteration in gynecologic cancers and to familiarize the reader with the critical radiological aspects in the evaluation of patients for this complex procedure. The focus of this review will be on how imaging can aid in treatment planning and guide management. We will briefly describe the PE procedure, its variations, and common indications in gynecologic oncology. Subsequently, the relevant imaging aspects before and after PE will be discussed.
Types of pelvic exenteration
PE can be classified according to therapeutic intent, the structures removed, or the compartments removed.2,3
According to therapeutic intent
PE can be performed with either curative or palliative intent. Curative PE aims to prolong survival and the goal of the surgery is to remove all tumor tissue1–3 and achieve R0 resection. There must not be any evidence of distant metastases in order to consider curative PE.2,3 Several single-center studies reported 5-year survival rates from 45–50% in patients treated with curative PE.13,14 Occassionally, intraoperative local radiation therapy to the surgical bed can be administered.13,14 By contrast, palliative PE primarily aims at relieving refractory or intractable symptoms in patients who do not qualify for curative PE. The survival benefit and evidence of symptom palliation from case series or historical cohorts have shown variable results.15,16 Thus, the multidisciplinary decision to perform palliative PE implies extensive risk–benefit consideration.15,16
According to compartments involved
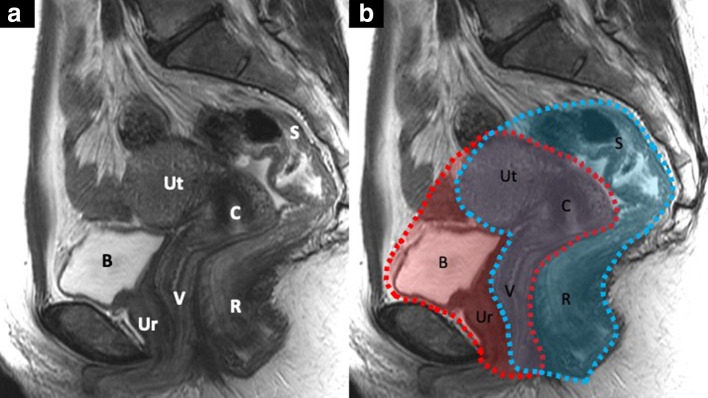
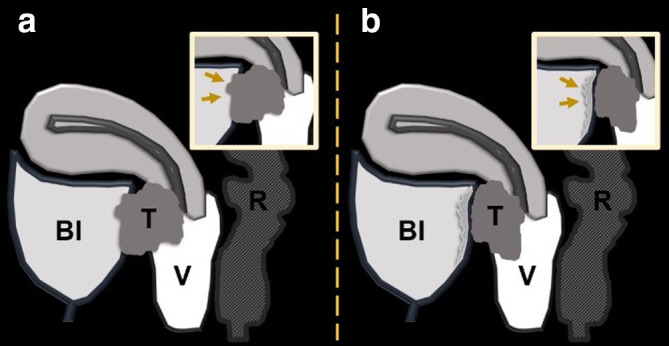
Any pelvic exenteration implies the removal of the female reproductive organs affected, i.e. “middle compartment” and adjacent affected organs. Anterior PE implies removing the bladder, pelvic ureters, and urethra; posterior PE involves removing the rectosigmoid colon, and total PE combines both (Figure 1). In cases of total PE, vaginectomy and vulvectomy may be required, depending on tumor location and extent.2–4,16
Figure 1.
Sagittal T2 weighted MR image with the different pelvic organs labeled with letters (A): B, bladder; C, cervix; R, rectum; S, sigmoid colon; Ut, uterus; Ur, urethra; V, vagina. Image (B) highlights the structures removed in an anterior, in red, and posterior exenteration, in blue. In a total pelvic exenteration, both of these outlined compartments are removed.
There are other modifications that entail more radical resections in case of more extensive tumor involvement; their applicability and use depend on local surgical expertise. Laterally extended endopelvic resection (LEER) implies total infralevator PE along with the resection of the pelvic sidewall muscles, i.e. the obturator internus and piriformis muscles including the internal iliac vessels, pelvic ureters, and lateral pelvic lymph nodes, and may require other surgical specialties such as orthopedic surgery. Its indication remains controversial since R0 is achieved in fewer than 40% of cases.1–4,16 An extended or composite pelvic resection implies removing a portion of the bony pelvis involved by contiguous tumor extension.1–4,16
According to structures removed
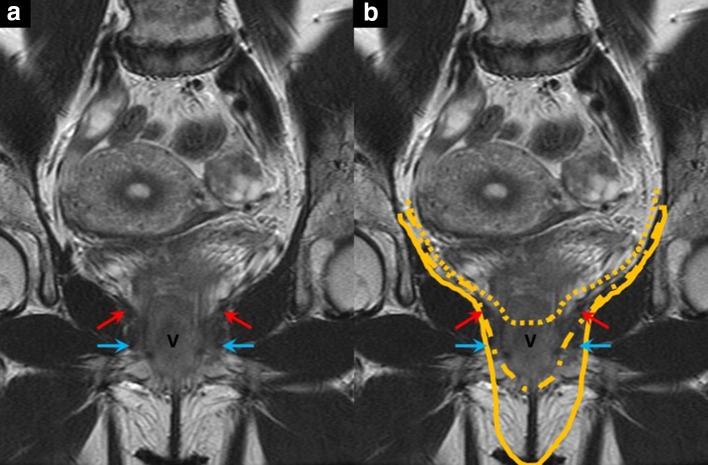
One relevant aspect is whether the pelvic floor musculature (levator ani, anal sphincter complex, and urogenital diaphragm) can be preserved. The levator ani is formed by the puborectalis, iliococcygeus, and pubococcygeus muscles. Based on the plane of the levator ani, a supralevator or infralevator PE can be distinguished. Infralevator PE involves the removal of the pelvic floor muscles and subsequent flap reconstruction in addition to the removal of the female reproductive organs (Figure 2).2–4,17 Its indication depends on the tumor location and extent. If the middle to lower third of the vagina is involved, then infralevator PE is considered.2–4
Figure 2.
Coronal T2 weighted MR image (A) with the arrows indicating the following anatomical landmarks: levator ani with red arrows, urogenital diaphragm with blue arrows. V = vagina. Image (B) shows the different resection planes of a supralevator and infralevator exenteration. The dotted line indicates a supralevator exenteration, the dash-dotted line an infralevator exenteration, and the solid line an infralevator exenteration with vulvectomy.
Indications and contraindications
Table 1 summarizes the indications and contraindications for both curative3,6,18 and palliative PE.2–4,6,18 Unilateral leg edema, obstructive uropathy, and sciatic nerve pain are considered tell-tale clinical signs for contraindication, implying venous, ureteral, and neural invasion. Of note, contraindications may also vary between institutions depending on the local surgical expertise and available technology, and radiologists should familiarize themselves with their local institutional practice, indications, and contraindications. Nevertheless, some generally applicable contraindications exist, and their knowledge is essential for clinically meaningful image interpretation.1–4 The most important contraindications for curative PE are distant metastases, peritoneal or serosal metastases, and pelvic nodal metastases. With regards to local pelvic extent of the tumoral mass, involvement of the sciatic foramen, external iliac vessels, S1/S2 nerve roots, and sacral nerve invasion above the S3 level would preclude surgery.
Table 1.
Summary of indications and contraindications for pelvic exenteration in gynecologic cancers
| Pelvic exenteration | |
|---|---|
| Indications | Contraindications |
Curative
|
Curative
|
Palliative*
|
Palliative
*
|
| * Please note that the role of palliative pelvic exenteration is controversial as it is associated with relatively high morbidity and mortality in exchange for moderate local symptom relief and only limited quality of life improvement.1 1 PelvEx Collaborative. Palliative pelvic exenteration: A systematic review of patient-centered outcomes15. | |
Radiological evaluation
A multimodality approach with different complementary imaging techniques is commonly used. MRI, positron emission tomography fused with CT (PET/CT), and CT are the most commonly used imaging modalities. Imaging provides relevant information about different tumor-related factors that are important for treatment planning.2–4
One critical task is the accurate localization and characterization of the disease in the pelvis, for which MRI is the modality of choice.2,4 The accurate description of tumor extent and of the involvement of essential anatomic structures allows the surgical team to predict the likelihood of achieving complete surgical resection, estimate the procedure complexity, and also help prepare for and inform the patient19,20 (Figure 3). Further, imaging helps exclude candidates who are unsuitable for a curative PE, particularly nodal and distant metastatic disease, for which PET/CT is the preferred modality1–4 (Figure 4).
Figure 3.
40-year-old female with recurrent cervical adenocarcinoma. Axial (A, B) T2 weighted MR images of the pelvis show a predominantly cystic central and right pelvic mass measuring 8.5 × 7.0 cm. The mass abuts the right external and internal iliac vessels (yellow arrows in A) and the right distal ureter (white arrow in B). Given the extent of disease and location, the patient subsequently underwent an anterior and supralevator exenteration. Since the posterior compartment was not involved, it could be spared.
Figure 4.
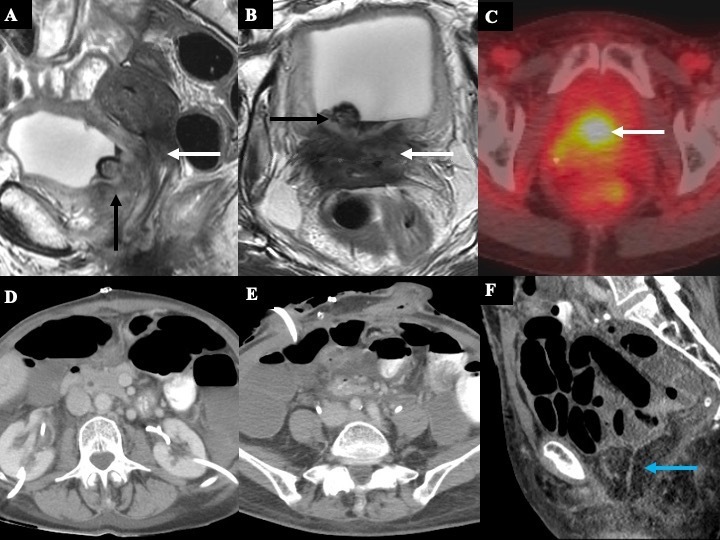
52-year-old female with recurrent dedifferentiated endometrial carcinoma who was symptomatic with left leg pain. Axial T2 weighted MR images (A-C) of the pelvis at different levels show a multilobulated solid mass at the left pelvic sidewall/obturator space, measuring 4.6 × 3.9 cm (white arrows in A-C, red arrow in D). The mass is encasing the left distal ureter (A-B), abutting the left internal iliac vessels (yellow arrows in A-C), and abutting the left acetabulum without frank osseous invasion (red arrow in D). This patient was not deemed a suitable candidate for pelvic exenteration. The multidisciplinary tumor board’s consensus decision was to perform a systemic therapy with immunotherapy due to her comorbidities, high perioperative risk, and high-risk histology.
Finally, imaging also plays an essential role in the post-operative setting for surveillance (Figure 5), recurrent disease diagnosis, and postoperative complications evaluation. CT is the most common first-line imaging modality for suspected complications due to its excellent diagnostic performance, acquisition speed, and availability.2,3
Figure 5.
49-year-old female with recurrent squamous cell cervical carcinoma complaining of chronic pain in the left lower extremity. Preoperative sagittal (A) and axial (B-C) contrast-enhanced CT images show a necrotic tumor centered in the cervix fistulizing to the mid/upper rectum (black arrow in A) and extending to the left pelvic sidewall and left sciatic foramen (green arrow in B). The left distal ureter is encased, and a ureteral stent has been placed (white arrow in C). The patient underwent a pelvic exenteration with palliative intent due to the chronic intractable pain and persistent, treatment-refractory fistula. A laterally extended and complete pelvic exenteration with an ileal conduit and end-colostomy were performed. Tumor dissection from the left lumbosacral plexus was necessary. Postoperative axial (D, F) and sagittal (E) contrast-enhanced CT images show the post-surgical appearance with left lower quadrant end-colostomy (D), and omental flap (yellow arrows in E and F, blue arrow in E). Note the haziness and infiltration within the omental flap, a normal finding that should not be mistaken for recurrent tumor or implants. The omentum contains normal vasculature and lymphatics and may show some haziness or fat infiltration.
Disease localization and characterization
MRI is the modality of choice for evaluation of the female pelvis due to its superior soft-tissue contrast. Technical considerations and imaging protocols of the female pelvis are beyond the scope of this article and are detailed elsewhere.21–23 MRI is used to diagnose tumor recurrence or persistence, establish tumor burden and extent, and provide a roadmap for surgical planning (Figure 6). Alternatively, PET/MRI is a promising modality for disease localization and characterization, combining morphological and metabolic information and thereby potentially increasing the diagnostic confidence.3,24,25 MRI provides information regarding tumor localization, size, morphology (solid, cystic, necrotic), and growth pattern (mass-like or infiltrative). Further, disease focality can be evaluated (unifocal or multifocal). The relationship of the tumor to the adjacent organs (e.g. bladder or rectum) and to the surrounding structures (e.g. pelvic floor musculature, Retzius or prevesical space, presacral space, or retro-rectal space) can be also evaluated.2,4,24–27
Figure 6.
48-year-old female with recurrent cervical squamous cell carcinoma. Contrast-enhanced MRI of the pelvis at baseline (A: sagittal T2 weighted; B: coronal T2 weighted; C: axial fat-suppressed contrast-enhanced T1 weighted) and T2 weighted MR images after chemotherapy (sagittal [D] and axial [E-F]). There is a solid mass (yellow short arrow in A-C) centered in the pelvis involving the sigmoid colon (black arrows in B) and posterior bladder wall (white arrows in A and C). After chemotherapy, there is a marked reduction in tumor size (white short arrow in D) with concomitantly decreased involvement of the sigmoid wall (black arrow in E) and the posterior bladder wall (white arrow in F). She subsequently underwent a complete infralevator exenteration with vaginectomy with an end colostomy, ileal conduit, and right-sided VRAM flap reconstruction.
Imaging signs to evaluate in a PE candidate
Table 2 lists the imaging signs to evaluate in a PE candidate. In every candidate, the evaluation begins with identifying the tumoral mass and its features, e.g. size, appearance, and localization. Surgical resectability is assessed by analyzing the presence and degree of contact between the tumor and the adjacent organ or structure under study. In this regard, it is important to evaluate whether there is a preserved fat plane between the tumor and the structure, which is best assessed with nonfat-suppressed MR images.2 If there is a loss of a separating fat plane, the next step is to assess the degree of tumoral contact with the adjacent structure as focal contact, broad-based contact, or frank invasion.
Table 2.
Checklist for imaging evaluation of candidates for pelvic exenteration.
| Task | Preferred imaging modality |
|---|---|
| Mass characterization (location, size, morphology, composition) | MRI |
| Assessment of surgical resectability (tumor extent, relation of tumor to adjacent organs, degree of adjacent structure involvement, see below in detail) | MRI, CT, PET/CT |
| Urinary bladder, distal ureters, and urethra | MRI |
| Vagina and vulva | MRI |
| Sigmoid colon, rectum, anal canal including sphincter complex | MRI |
| Pelvic side-walls, including the levator ani complex | MRI |
| Major pelvic vessels (internal iliac artery/vein, external iliac artery/vein) | CT +/−MRI |
| Lymph nodes | PET/CT, MRI, CT |
| Bony pelvis | CT +/−MRI |
| Assessment for contraindications | PET/CT, CT, MRI |
| Please note that surgical resectability assessment is not solely based on imaging; adjunct evaluations (e.g., clinical examination, endoscopy, cystoscopy) are commonly performed in the pre-surgical workup. | |
When evaluating tubular structures such as vessels or ureters, the degree of tumoral contact should be assessed semi-quantitatively in the radial and longitudinal dimension by describing whether the contact is merely focal, more broad-based and radially surrounding the structure for up to 180° (i.e. “abutment”), or radially surrounding the structure for more than 180° (i.e. “encasement”). Further, any associated deformity of the vessel contour, e.g. narrowing, irregularity, or occlusion, should be described. If the ureters are involved, the presence and degree of upstream dilation should be assessed.2,28
Relevant organs and structures that need to be evaluated and mentioned in the radiological report include the urinary bladder, distal ureters, urethra, vagina, sigmoid colon, rectum, anal canal with the sphincter complex, pelvic sidewalls (including the levator ani complex), major pelvic vessels, lymph nodes, and bony pelvis.2,4
Urinary bladder, urethra, rectum, and anal sphincter
The degree of involvement and depth of tumor invasion into the sigmoid colon, rectum, and urinary bladder can be evaluated in more detail on imaging.29 Abutment with loss of a fat plane between the tumor and the organ raises the suspicion for organ involvement. Interruption of the affected hollow organ’s low signal intensity muscular wall on MRI implies muscle invasion. Delineation of tumor nodules of similar signal intensity to the primary tumor in the mucosal layer of the affected organ suggests deeper invasion with mucosal involvement.30
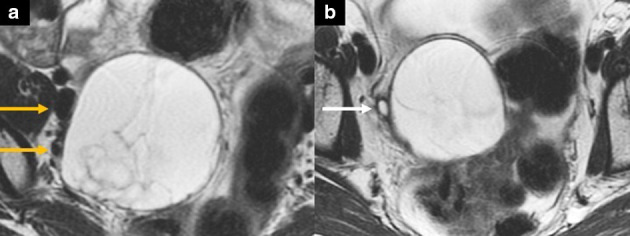
Advanced uterine, cervical, or vaginal tumors can infiltrate adjacent organs such as the bladder, more commonly the posterior bladder wall. On imaging, the most accurate sign of tumor invasion is nodularity or direct tumor extension with signal intensity similar to the primary tumor (Figure 7). A potential pitfall leading to overstaging on MRI is the presence of bullous edema, a bladder wall reactive edematous thickening due to inflammation but not due to direct tumor invasion. On MRI, it appears as a T2-hyperintense thickening, usually more hyperintense than the intermediate T2 signal of the tumor. Direct cystoscopic visualization can be performed in equivocal cases31,32 (Figure 8).
Figure 7.
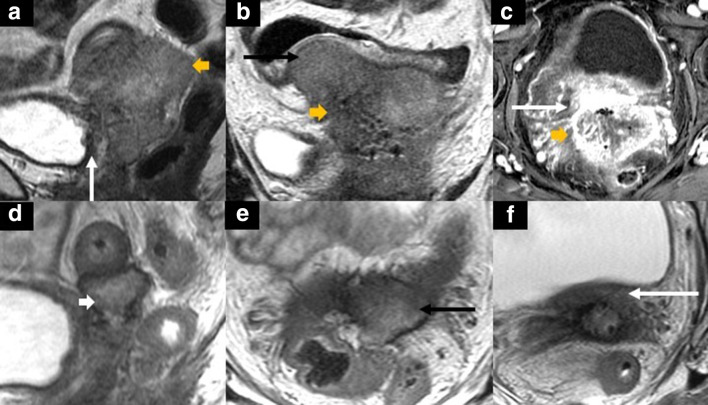
50-year-old female with recurrent cervical gastric-type adenocarcinoma after chemoradiotherapy. Sagittal (A) and axial (B) T2 weighted pelvic MR images show the recurrent mass at the anterior vaginal wall (white arrows in A-B) invading the posterior bladder wall with associated bladder hemorrhage (black arrows in A-B). The mass shows increased FDG uptake (SUVmax 5.3) on the fused axial 18FDG PET/CT (C). The patient underwent a complete pelvic exenteration, including bilateral salpingo-oophorectomy and radical hysterectomy and vaginectomy, cystectomy, and low anterior resection. Axial (D-E) and sagittal (F) contrast-enhanced CT images after the total pelvic exenteration with reconstructions including bilateral percutaneous nephrostomy (D), right lower quadrant ileal conduit (E), a left lower quadrant end-colostomy, and a VRAM flap (light blue arrow in F). PET, positron emission tomography.
Figure 8.
Schematic illustration of a sagittal image with a cervical tumor (T) extending to the posterior bladder wall (Bl) with frank invasion in A (arrows in the magnified image in A) and abutment of the posterior bladder wall in B with reactive or inflammatory bullous edema of the posterior bladder wall (arrows in the magnified image in B). Please note that the signal of bullous edema is different from the signal of the primary tumor. R = rectum, V = vagina.
Pelvic sidewall
Pelvic sidewall involvement should be stated in the report to aid in LEER planning. Imaging-based criteria for pelvic sidewall invasion are tumor extension delineation within 3 mm of the pelvic sidewall muscles (internal obturator, levator ani, and piriformis muscles) and abutment with fat plane loss between the tumor and muscles on MRI.2,25
Iliac vessels
The distance to and relation of the tumor to the iliac vessels should be described, including whether or not the vessels are involved, which side is affected, and which vessels are affected (arteries, veins, or both). Potentially involved vessels in the pelvis include the common iliac veins and arteries, external iliac veins and arteries, and internal iliac veins and arteries. When evaluating the tumoral relationship with the vessels, again the preserved fat plane presence should be assessed. If there is a fat plane loss between the tumor and vessel, the degree of contact in the radial plane (best assessed on the imaging plane where the vessel luminal cross-section is seen orthogonally) should be quantified to differentiate between abutment and encasement, as described above. Other vessel involvement findings may include vessel contour deformity, including a teardrop deformity, change in caliber or focal narrowing, thrombosis, or vessel occlusion. Of note, involvement of the internal iliac vessels does not necessarily preclude a PE and the tumor can be deemed resectable with LEER (Figure 9). However, the involvement (especially encasement of the vessel) of the external iliac vessels is a contraindication due to the challenge of obtaining R0 and the expected associated morbidity33 due to risk of limb ischemia.1,3,34
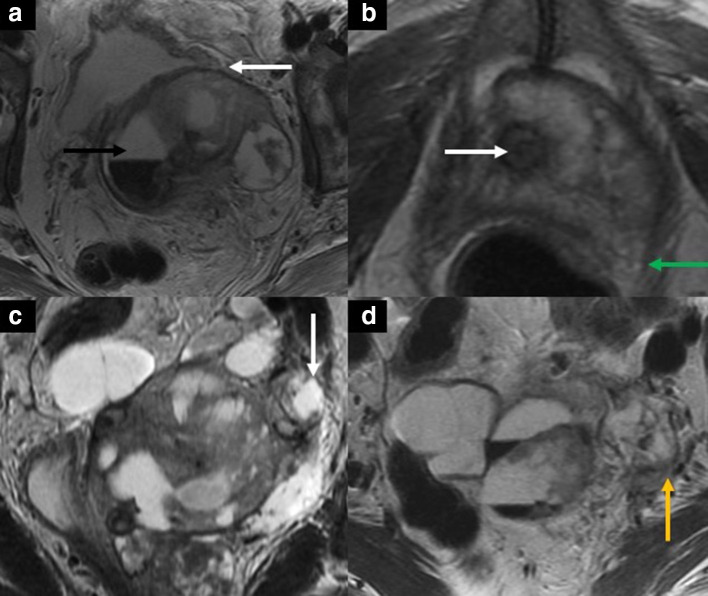
Figure 9.
40-year-old female with recurrent cervical squamous cell carcinoma. Axial (A, B, D) and coronal (C) T2 weighted images show a partially hemorrhagic solid-cystic mass in the left pelvis (black arrow in A), measuring 8.8 × 6.4 x 10.3 cm. The mass is inseparable from the posterior bladder wall and the bladder neck (white arrow in A) and encases the urethra (white arrow in B). There is the focal abutment of the left levator ani muscle (green arrow in B) and left distal ureteral encasement with mild upstream dilatation (white arrow in C). The mass also extends to the left pelvic sidewall with associated tumor thrombus in the left internal iliac vein (yellow arrow in D).
Bony pelvis
Several MRI features are suggestive of bony invasion. The sign with the highest negative predictive value for bone invasion is a separating fat plane between the tumor and the adjacent bone, best assessed on T1 weighted images without fat suppression. If there is loss of a fat plane and the tumor focally contacts the bony cortex, suspicion for superficial invasion of bone must be raised (Figure 4). Diagnostic confidence can be increased if there is irregularity or thinning of the low signal intensity cortex. Of note, CT is more sensitive for evaluating the bony cortex than MRI. Further, periosteal reaction, which may be better seen on CT or radiographs, may also suggest superficial bone invasion. In cases of deeper bony invasion, the normal hyperintense fatty marrow signal in T1 weighted images is replaced by a low signal intensity tumor. It is important to correlate any bone marrow edema signal seen on T2 weighted fat-suppressed images with that on non-fat suppressed T1 weighted images to differentiate frank tumor invasion from possibly reactive marrow edema and other benign mimickers such as insufficiency fracture.2,35–37
On PET/CT, early bone involvement may appear as focal areas with fludeoxyglucose (FDG) avidity with or without a corresponding correlate on CT or MRI. However, care should be taken not to misinterpret PET and CT/MRI misregistration and to ascertain that the bone involvement is contiguous with the tumor and not bone metastasis. The latter precludes curative PE.2,38
Sciatic nerve
MRI can help assess the likelihood of the involvement of neural structures. Specific anatomic landmarks should be carefully inspected. The normal signal intensity of peripheral nerves is isointense to muscle on T1- and T2 weighted images with symmetry in size and signal intensity to the contralateral side and a gradual decrease in size and caliber as they course distally.39 Larger nerves such as the sciatic nerve show a uniform fascicular pattern with little interspersed perifascicular fat and a halo of surrounding fat best assessed on non-fat-suppressed T1 weighted images.39 The nerves of the sacral plexus (L5–S2) should be evaluated along their course as the exit their neuroforamen. Further, the relationship of the tumoral mass to the greater sciatic foramen and the sciatic nerve should be assessed.
The sciatic nerve is formed from the L4–S3 nerve roots and courses through the greater sciatic foramen either inferior to (most commonly), superior to or through the piriformis muscle.39 The anatomy as well as normal and abnormal MRI appearance of pelvic nerves are detailed elsewhere.40 Signs suggesting invasion include loss of clear fat planes between the tumor and nerves, diameter of the nerve larger than the adjacent artery, and asymmetry of the affected nerve if compared with the contralateral side (the affected site will show increased signal intensity on T2 weighted images and asymmetrically increased contrast enhancement).2,41
Nodal disease
It is well-acknowledged that MRI and CT are limited in the detection of lymph node metastases, as they primarily rely on size thresholds and morphological criteria (i.e. rounded appearance, asymmetric thickening of the nodal cortex, fatty hilum loss).42 Nodal metastases can be subcentimeter and not meet these pre-defined size thresholds. Of note, the normal size of abdominopelvic nodes varies according to the anatomic region; some generally accepted threshold values for the short-axis diameter of nodes are 0.7 cm for the internal iliac region, 0.8 cm for the obturator region, 1.0 cm for the external iliac region, and 1.5 cm for inguinal nodes.42 One additional relevant feature is the location and knowledge of expected nodal drainage patterns, which can help evaluate the likelihood of observed nodes being metastatic. Notably, in pre-treated patients, classic nodal landing zones may be altered.42–44
PET/CT is superior to conventional anatomic imaging modalities for detecting nodal disease. In a recent meta-analysis, the pooled sensitivity and specificity of PET/CT for detection of pelvic and para-aortic lymph node metastases in patients with advanced cervical cancer were 88% and 93%, and 40% and 93%, respectively.45 The low specificity, specifically for para-aortic nodal metastases, elucidate the radiological shortcomings and the continued need for histopathological confirmation for accurate nodal staging.3,43,46
Diagnosing contraindications to PE
The main contraindications to curative intent PE are peritoneal carcinomatosis, abdominal nodal metastases, and distant metastases.2–4 A recent meta-analysis of CT, PET/CT, and MRI in patients with peritoneal carcinomatosis from gastrointestinal and ovarian cancers found lower diagnostic performance for CT (68% sensitivity and 88% specificity) and similar diagnostic performance for PET/CT and MRI (PET/CT: 80% sensitivity and 90% specificity; MRI: 92% sensitivity and 85% specificity).44 Another retrospective single-center study found that FDG PET/CT had 100% sensitivity and 73% specificity for detecting extra pelvic metastases in candidates for PE.47 The high sensitivity of PET allowed the detection and targeted biopsy of suspected sites of disease. Other groups had similar results.46,48,49
Imaging appearance after PE
The imaging appearance after PE depends on the type of exenteration and the reconstructive procedures performed (Figure 10). Therefore, knowledge of these factors as well as the timing of procedure (i.e., early or late postoperative phase) are essential for appropriate image interpretation and evaluation of potential complications.2,3
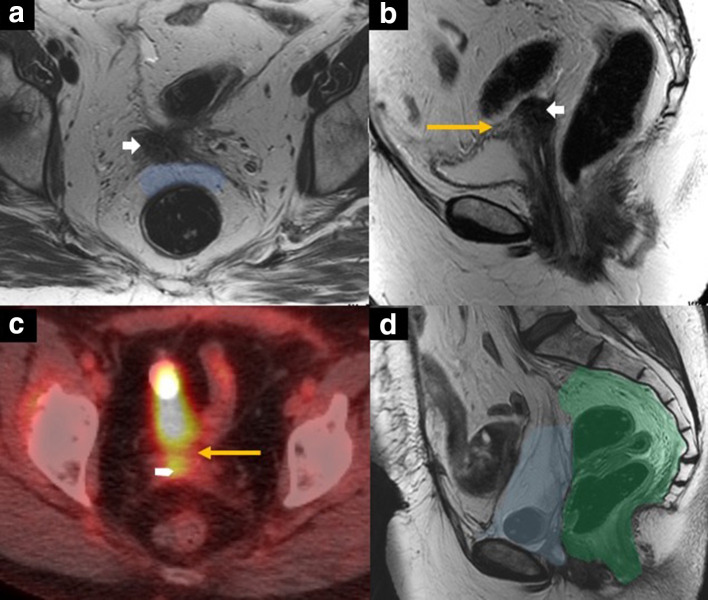
Figure 10.
60-year-old female with central pelvic recurrence of cervical cancer after radical hysterectomy and pelvic radiation therapy. Axial T2 weighted MR image (A) showing nodular mass (white arrow in A and B) at the vaginal cuff. There is a well-defined preserved plane of mesorectal fat between the mass and the rectum (blue-shaded area in A). Sagittal T2 weighted MR image (B) showing broad-based abutment and tethering of the posterior wall (yellow arrow in B) by the mass (white short arrow in B). On 18FDG PET/CT (C), the mass is hypermetabolic (white short arrow in C), and the posterior bladder focal abutment is again seen (yellow arrow in C). Sagittal T2 weighted MR image (D) after anterior pelvic exenteration with ileal conduit urinary diversion showing the postoperative situs with bowel loops and mesentery filling the former vesical space (blue-shaded area in D). The posterior compartment with the rectum and the anal sphincter complex (green shaded area in D) were spared.
After removing the bladder, urinary diversion is created which can be either a non-continent ostomy (i.e., ileal conduit) or continent (through the creation of a reservoir, such as a continent pouch or orthotopic neobladder).2,3 After colorectal resection (with or without resection of the anal sphincter complex), bowel reconstruction can include either a permanent colostomy (if an infralevator PE was performed) or a low-rectal anastomosis in cases of a preserved anal sphincter complex (in a supralevator PE).2,3
As primary closure of the complex wound cavity after PE is associated with high morbidity and infection rates of up to 66%, different surgical flap reconstructions are commonly performed to reduce dead space and provide structural support.50 Generally, there are two types of flaps: myocutaneous and non-myocutaneous. One of the most commonly used myocutaneous flaps is the vertical rectus abdominis myocutaneous flap (VRAM), a composite flap containing skin, subcutaneous fat and muscle supplied by a vascular pedicle fed by the deep inferior epigastric artery.51 The VRAM flap is harvested by dissecting it from the rectus sheath and tunneling it through an intraperitoneal route into the pelvis.51 On imaging, the abdominal wall of the affected the donor site will be thinner and lacking the rectus muscle belly. Another commonly used myocutaneous flap for vaginal reconstruction is the gracilis flap, containing the gracilis muscle released from the medial thigh adductors and advanced to the pelvis through a subcutaneous tunnel.52 A commonly used non-myocutaneous flap is the omental flap, which is often used in conjunction with the VRAM flap. It helps obliterate post-surgical dead space, promotes wound healing, and may introduce beneficial locoregional angiogenic and immunologic effects to the recipient site.2,3,50,53,54
Complications
Table 3 lists complications from PE. Since PE is a radical surgical procedure, major complications such as anastomotic leaks, fistulas, and small bowel or ureteral obstructions may occur in up to 50% of procedures. Minor complications can occur in up to 80–90% of cases.55,56 Recent reports from our institution focused exclusively on anterior pelvic exenteration, revealing a rate of 36% for major complication rates involving pelvic abscess and urosepsis.1 Apart from general complications associated with any major surgery (i.e. postoperative fever, pneumonia, deep vein thrombosis with or without pulmonary embolism), specific complications associated with PE are dependent on the surgical type and post-surgical timing.2,3,54
Table 3.
Complications after pelvic exenteration.
| Types | Complication | Preferred imaging modality | |
|---|---|---|---|
| Related to primary surgical resection and anastomoses/diversions | Early | Urinary tract infections Surgical site infections Anastomotic insufficiency or leak Seroma, hematoma Lymphocele Abscess Fistula formation |
CT |
| Late | Anastomotic stricture | CT | |
| Chronic fistula formation | CT, MRI | ||
| Tumor recurrence | MRI, PET/CT, CT | ||
| Related to the reconstructive flaps | Post-surgical fluid collections and infections (at the donor site, at recipient site) | ||
| Flap ischemia and necrosis | |||
| Abdominal wall hernias (from abdominal wall donor sites) | |||
| Fat necrosis (e.g. omental flap) | |||
Early post-operative period complications include urinary tract infections, surgical site infections (i.e., wound infection), anastomotic insufficiency or leak, seroma, hematoma, lymphoceles, abscesses, and fistula formation. Many post-surgical complications are identified clinically, and imaging is not necessarily required.3 Nevertheless, some common complications, e.g. collections, ileus, and anastomotic leaks, require imaging for the diagnosis and to guide interventional procedures (Figures 11 and 12).2 In suspected bowel obstruction, CT aids in differentiating bowel obstruction with transition points from postoperative ileus. Further, CT can depict the extent and location of abscess collections and help in the planning of interventions such as fluid aspiration or percutaneous drainage.2,3,54 Moreover, CT can be used to delineate postoperative leaks and fistula formation (in these cases, the protocol should ideally include oral and rectal contrast, if applicable).2,3,54 A CT urography, including late excretory phase images, should be performed if urinary leak or urinoma is suspected after urinary diversion procedures.2,3,54
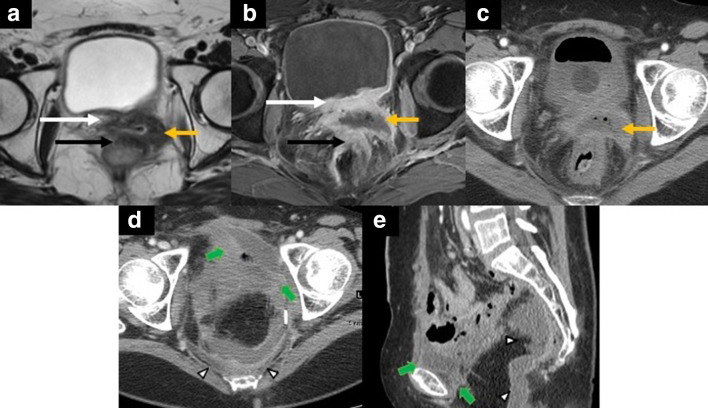
Figure 11.
52-year-old female with central pelvic recurrence of cervical cancer after radical hysterectomy and adjuvant chemoradiation therapy. Axial T2 weighted MR image (A) showing an intermediate signal irregular mass at the vaginal cuff (yellow arrow in A) with abutment and tethering of the posterior bladder wall (white arrow in A) and abutment of the anterior rectal wall without preserved fat planes (black arrow in A). Axial fat-suppressed post-contrast T1 weighted MR image (B) depicts the centrally necrotic mass (yellow arrow in B). Note the thickened posterior bladder wall (white arrow in B) and the anterior rectal wall (black arrow in B), suggesting invasion. Axial contrast-enhanced CT image (C) depicts the rim-enhancing, centrally necrotic mass with central foci of gas (yellow arrow in C). The patient underwent a total pelvic exenteration since the tumor involved the anterior and posterior compartments. Axial (D) and sagittal (E) contrast-enhanced CT images showing the postoperative situs complicated by rim-enhancing fluid-collections in the retropubic (short green arrows in D, E) and presacral space (white arrowheads in D, E) consistent with abscess collections.
Figure 12.
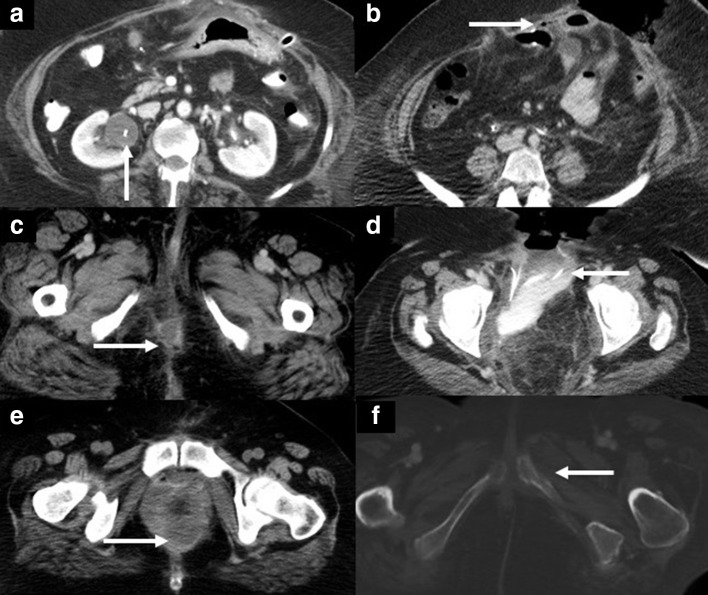
60-year-old female with recurrent vaginal cancer after chemoradiotherapy. A complete pelvic exenteration with ileostomy and end-colostomy were performed. The patient experienced several post-surgical complications (depicted by arrows in axial contrast-enhanced CT images, (A-F): right hydroureteronephrosis due to reflux from the ileal conduit (A), dehiscence of the anterior abdominal wall with protrusion of small bowel loops (B), perineal fluid collection (C), bowel anastomosis dehiscence with contrast extravasation into a pelvic collection (D), pelvic hematoma (E), and chronic osteomyelitis of the left pubic bone (F).
In the late post-operative setting, complications include anastomotic stricture, chronic fistula formation, and tumor recurrence. Most tumor recurrences occur within 2 years of the surgery, manifesting as a recurrent pelvic mass or adenopathy.2 Any new asymmetric soft tissue in the post-surgical bed should be carefully evaluated for possible recurrent tumor and differentiated from expected post-surgical findings or complications by carefully scrutinizing prior available imaging studies for comparison.2,3,54 Although some useful radiological signs such as restricted diffusion and avid enhancement on MRI and increased metabolic activity on PET/CT help raise suspicion, the final diagnosis commonly relies on the combination of clinical findings, imaging studies, and ultimately, tissue sampling.2,3,54
The most common complications related to the reconstructive flaps are post-surgical fluid collections, wound infections, flap ischemia, and necrosis. The gracilis flap has the highest risk of flap ischemia and necrosis due to the scarcity of perforating vessels.50,57 Another complication associated with VRAM is abdominal wall hernias at the donor site due to the donor site abdominal wall weakening, for which reinforcement with mesh placement is sometimes performed.58 The omental flap contains normal lymphatic tissue and can undergo infarction with development of fat necrosis that may present as fat infiltration and nodularity within the flap; such findings should not be mistaken for a recurrent tumor (Figure 5).2,50,53,54
Conclusion
Pelvic exenteration is a relatively uncommon and complex procedure requiring a high level of surgical expertise and diligent selection of appropriate patients, given its radicality and implications. The ultimate goal of curative intent PE is to achieve R0 resection, which is the most important factor affecting prognosis. The radiologist plays an essential role in this multidisciplinary setting and selection process, by providing the most accurate possible information on local disease extent and by identifying findings which may complicate a R0 resection or even represent a contraindication. Radiologists should be aware of essential aspects in reporting imaging studies and be knowledgeable of the post-treatment imaging appearance to differentiate normal post-surgical findings from tumor recurrence. Furthermore, imaging in the postoperative setting can help diagnose and guide the management of post-operative complications.
Source of funding
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Acknowledgment: The authors would like to thank Joanne Chin for her editorial support.
Contributor Information
Pamela Ines Causa Andrieu, Email: causapamela@gmail.com, CausaAnP@mskcc.org.
Sungmin Woo, Email: woos@mskcc.org.
Eric Rios-Doria, Email: riosdore@mskcc.org.
Yukio Sonoda, Email: sonoday@mskcc.org.
Soleen Ghafoor, Email: soleen.ghafoor@usz.ch.
REFERENCES
- 1.Andikyan V, Khoury-Collado F, Sonoda Y, Gerst SR, Alektiar KM, Sandhu JS, et al. Extended pelvic resections for recurrent or persistent uterine and cervical malignancies: an update on out of the box surgery. Gynecol Oncol 2012; 125: 404–8. doi: 10.1016/j.ygyno.2012.01.031 [DOI] [PubMed] [Google Scholar]
- 2.Lakhman Y, Nougaret S, Miccò M, Scelzo C, Vargas HA, Sosa RE, et al. Role of Mr imaging and FDG PET/CT in selection and follow-up of patients treated with pelvic exenteration for gynecologic malignancies. Radiographics 2015; 35: 1295–313. doi: 10.1148/rg.2015140313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambrou NC, Pearson JM, Averette HE. Pelvic exenteration of gynecologic malignancy: indications, and technical and reconstructive considerations. Surg Oncol Clin N Am 2005; 14: 289–300. doi: 10.1016/j.soc.2004.11.011 [DOI] [PubMed] [Google Scholar]
- 4.Sagebiel TL, Viswanathan C, Patnana M, Devine CE, Frumovitz M, Bhosale PR. Overview of the role of imaging in pelvic exenteration. Radiographics 2015; 35: 1286–94. doi: 10.1148/rg.2015140127 [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee S, Chen L, Jones N, Tergas AI, Burke WM, Hou JY, et al. National trends in total pelvic exenteration for gynecologic malignancies. Am J Obstet Gynecol 2016; 215: 395–6. doi: 10.1016/j.ajog.2016.06.031 [DOI] [PubMed] [Google Scholar]
- 6.Matsuo K, Mandelbaum RS, Adams CL, Roman LD, Wright JD. Performance and outcome of pelvic exenteration for gynecologic malignancies: a population-based study. Gynecol Oncol 2019; 153: 368–75. doi: 10.1016/j.ygyno.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunschwig A. Complete excision of pelvic viscera for advanced carcinoma; a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer 1948; 1: 177–83. doi: [DOI] [PubMed] [Google Scholar]
- 8.Brown KGM, Solomon MJ, Koh CE. Pelvic exenteration surgery: the evolution of radical surgical techniques for advanced and recurrent pelvic malignancy. Dis Colon Rectum 2017; 60: 745–54. doi: 10.1097/DCR.0000000000000839 [DOI] [PubMed] [Google Scholar]
- 9.Seebacher V, Sturdza A, Bergmeister B, Polterauer S, Grimm C, Reinthaller A, et al. Factors associated with post-relapse survival in patients with recurrent cervical cancer: the value of the inflammation-based Glasgow prognostic score. Arch Gynecol Obstet 2019; 299: 1055–62. doi: 10.1007/s00404-018-4993-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida K, Kajiyama H, Utsumi F, Niimi K, Sakata J, Suzuki S, et al. A post-recurrence survival-predicting indicator for cervical cancer from the analysis of 165 patients who developed recurrence. Mol Clin Oncol 2018; 8: 281–5. doi: 10.3892/mco.2017.1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolomainen DF, Barton DPJ. Pelvic exenteration for recurrent gynaecological cancer after radiotherapy. Obstet Gynecol 2017; 19: 109–18. doi: 10.1111/tog.12383 [DOI] [Google Scholar]
- 12.Höckel M, Dornhöfer N. Pelvic exenteration for gynaecological tumours: achievements and unanswered questions. Lancet Oncol 2006; 7: 837–47. doi: 10.1016/S1470-2045(06)70903-2 [DOI] [PubMed] [Google Scholar]
- 13.Berek JS, Howe C, Lagasse LD, Hacker NF. Pelvic exenteration for recurrent gynecologic malignancy: survival and morbidity analysis of the 45-year experience at UCLA. Gynecol Oncol 2005; 99: 153–9. doi: 10.1016/j.ygyno.2005.05.034 [DOI] [PubMed] [Google Scholar]
- 14.Morris M, Alvarez RD, Kinney WK, Wilson TO. Treatment of recurrent adenocarcinoma of the endometrium with pelvic exenteration. Gynecol Oncol 1996; 60: 288–91. doi: 10.1006/gyno.1996.0040 [DOI] [PubMed] [Google Scholar]
- 15.PelvEx Collaborative . Palliative pelvic exenteration: a systematic review of patient-centered outcomes. Eur J Surg Oncol 2019; 45: 1787–95. doi: 10.1016/j.ejso.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 16.Schmidt A-M, Imesch P, Fink D, Egger H. Indications and long-term clinical outcomes in 282 patients with pelvic exenteration for advanced or recurrent cervical cancer. Gynecol Oncol 2012; 125: 604–9. doi: 10.1016/j.ygyno.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 17.Loubeyre P, Copercini M, Petignat P, Dubuisson JB. Levator ani muscle complex: anatomic findings in nulliparous patients at thin-section MR imaging with double opacification. Radiology 2012; 262: 538–43. doi: 10.1148/radiol.11111014 [DOI] [PubMed] [Google Scholar]
- 18.Benn T, Brooks RA, Zhang Q, Powell MA, Thaker PH, Mutch DG, et al. Pelvic exenteration in gynecologic oncology: a single institution study over 20 years. Gynecol Oncol 2011; 122: 14–18. doi: 10.1016/j.ygyno.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rausa E, Kelly ME, Bonavina L, O'Connell PR, Winter DC. A systematic review examining quality of life following pelvic exenteration for locally advanced and recurrent rectal cancer. Colorectal Dis 2017; 19: 430–6. doi: 10.1111/codi.13647 [DOI] [PubMed] [Google Scholar]
- 20.Rezk YA, Hurley KE, Carter J, Dao F, Bochner BH, Aubey JJ, et al. A prospective study of quality of life in patients undergoing pelvic exenteration: interim results. Gynecol Oncol 2013; 128: 191–7. doi: 10.1016/j.ygyno.2012.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laifer-Narin SL, Genestine WF, Okechukwu NC, Hecht EM, Newhouse JH. The role of computed tomography and magnetic resonance imaging in gynecologic oncology. PET Clin 2018; 13: 127–41. doi: 10.1016/j.cpet.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 22.Nougaret S, Horta M, Sala E, Lakhman Y, Thomassin-Naggara I, Kido A, et al. Endometrial cancer MRI staging: updated guidelines of the European Society of urogenital radiology. Eur Radiol 2019; 29: 792–805. doi: 10.1007/s00330-018-5515-y [DOI] [PubMed] [Google Scholar]
- 23.Sala E, Rockall AG, Freeman SJ, Mitchell DG, Reinhold C. The added role of Mr imaging in treatment stratification of patients with gynecologic malignancies: what the radiologist needs to know. Radiology 2013; 266: 717–40. doi: 10.1148/radiol.12120315 [DOI] [PubMed] [Google Scholar]
- 24.Beiderwellen K, Grueneisen J, Ruhlmann V, Buderath P, Aktas B, Heusch P, et al. 18)F]FDG PET/MRI vs. PET/CT for whole-body staging in patients with recurrent malignancies of the female pelvis: initial results. Eur J Nucl Med Mol Imaging 2015; 42: 56–65. doi: 10.1007/s00259-014-2902-8 [DOI] [PubMed] [Google Scholar]
- 25.Vargas HA, Burger IA, Donati OF, Andikyan V, Lakhman Y, Goldman DA, et al. Magnetic resonance imaging/positron emission tomography provides a roadmap for surgical planning and serves as a predictive biomarker in patients with recurrent gynecological cancers undergoing pelvic exenteration. Int J Gynecol Cancer 2013; 23: 1512–9. doi: 10.1097/IGC.0b013e3182a41e61 [DOI] [PubMed] [Google Scholar]
- 26.Brown WE, Koh CE, Badgery-Parker T, Solomon MJ. Validation of MRI and surgical decision making to predict a complete resection in pelvic exenteration for recurrent rectal cancer. Dis Colon Rectum 2017; 60: 144–51. doi: 10.1097/DCR.0000000000000766 [DOI] [PubMed] [Google Scholar]
- 27.Chew M-H, Brown WE, Masya L, Harrison JD, Myers E, . Clinical, MRI, and PET-CT criteria used by surgeons to determine suitability for pelvic exenteration surgery for recurrent rectal cancers: a Delphi study. Dis Colon Rectum 2013; 56: 717–25. doi: 10.1097/DCR.0b013e3182812bec [DOI] [PubMed] [Google Scholar]
- 28.Colombo P-E, Quenet F, Alric P, Mourregot A, Neron M, Portales F, et al. Distal pancreatectomy with celiac axis resection (modified Appleby procedure) and arterial reconstruction for locally advanced pancreatic adenocarcinoma after Folfirinox chemotherapy and chemoradiation therapy. Ann Surg Oncol 2021; 28: 1106–8. doi: 10.1245/s10434-020-08740-y [DOI] [PubMed] [Google Scholar]
- 29.Nougaret S, Nikolovski I, Paroder V, Vargas HA, Sala E, Carrere S, et al. Mri of tumors and tumor mimics in the female pelvis: anatomic pelvic Space-based approach. Radiographics 2019; 39: 1205–29. doi: 10.1148/rg.2019180173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rockall AG, Ghosh S, Alexander-Sefre F, Babar S, Younis MTS, Naz S, et al. Can MRI rule out bladder and rectal invasion in cervical cancer to help select patients for limited EUA? Gynecol Oncol 2006; 101: 244–9. doi: 10.1016/j.ygyno.2005.10.012 [DOI] [PubMed] [Google Scholar]
- 31.Kim SH, Han MC. Invasion of the urinary bladder by uterine cervical carcinoma: evaluation with MR imaging. AJR Am J Roentgenol 1997; 168: 393–7. doi: 10.2214/ajr.168.2.9016214 [DOI] [PubMed] [Google Scholar]
- 32.Nam H, Huh SJ, Park W, Bae DS, Kim BG, Lee JH, et al. Prognostic significance of MRI-detected bladder muscle and/or serosal invasion in patients with cervical cancer treated with radiotherapy. Br J Radiol 2010; 83: 868–73. doi: 10.1259/bjr/6646798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romeo A, Gonzalez MI, Jaunarena J, Zubieta ME, Favre G, Tejerizo JC. Pelvic exenteration for gynecologic malignancies: postoperative complications and oncologic outcomes. Actas Urol Esp 2018; 42: 121–5. doi: 10.1016/j.acuro.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 34.Zaky AM, Wolfgang CL, Weiss MJ, Javed AA, Fishman EK, Zaheer A. Tumor-Vessel relationships in pancreatic ductal adenocarcinoma at multidetector CT: different classification systems and their influence on treatment planning. Radiographics 2017; 37: 93–112. doi: 10.1148/rg.2017160054 [DOI] [PubMed] [Google Scholar]
- 35.Elias DA, White LM, Simpson DJ, Kandel RA, Tomlinson G, Bell RS, et al. Osseous invasion by soft-tissue sarcoma: assessment with MR imaging. Radiology 2003; 229: 145–52. doi: 10.1148/radiol.2291020377 [DOI] [PubMed] [Google Scholar]
- 36.Kaur H, Silverman PM, Iyer RB, Verschraegen CF, Eifel PJ, Charnsangavej C, Diagnosis CC. Diagnosis, staging, and surveillance of cervical carcinoma. AJR Am J Roentgenol 2003; 180: 1621–31. doi: 10.2214/ajr.180.6.1801621 [DOI] [PubMed] [Google Scholar]
- 37.Sakaguchi M, Maebayashi T, Aizawa T, Ishibashi N. Risk factors for sacral insufficiency fractures in cervical cancer after whole pelvic radiation therapy. Anticancer Res 2019; 39: 361–7. doi: 10.21873/anticanres.13120 [DOI] [PubMed] [Google Scholar]
- 38.Ulaner G. Fundamentals of oncologic PET/CT. 1st Edition. : Elselveier,Inc 2019;. [Google Scholar]
- 39.Weissman E, Boothe E, Wadhwa V, Scott K, Chhabra A. Magnetic resonance neurography of the pelvic nerves. Semin Ultrasound CT MR 2017; 38: 269–78. doi: 10.1053/j.sult.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 40.Garwood ER, Duarte A, Bencardino JT. Mr imaging of entrapment neuropathies of the lower extremity. Radiol Clin North Am 2018; 56: 997–1012. doi: 10.1016/j.rcl.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Li M, Guan J, Wang H, Li S, Guo Y, et al. Evaluation of the sacral nerve plexus in pelvic endometriosis by three-dimensional Mr neurography. J Magn Reson Imaging 2017; 45: 1225–31. doi: 10.1002/jmri.25435 [DOI] [PubMed] [Google Scholar]
- 42.McMahon CJ, Rofsky NM, Pedrosa I. Lymphatic metastases from pelvic tumors: anatomic classification, characterization, and staging. Radiology 2010; 254: 31–46. doi: 10.1148/radiol.2541090361 [DOI] [PubMed] [Google Scholar]
- 43.Adam JA, van Diepen PR, Mom CH, Stoker J, van Eck-Smit BLF, Bipat S. 18F]FDG-PET or PET/CT in the evaluation of pelvic and para-aortic lymph nodes in patients with locally advanced cervical cancer: A systematic review of the literature. Gynecol Oncol 2020; 159: 588–96. doi: 10.1016/j.ygyno.2020.08.021 [DOI] [PubMed] [Google Scholar]
- 44.van 't Sant I, Engbersen MP, Bhairosing PA, Lambregts DMJ, Beets-Tan RGH, van Driel WJ, et al. Diagnostic performance of imaging for the detection of peritoneal metastases: a meta-analysis. Eur Radiol 2020; 30: 3101–12. doi: 10.1007/s00330-019-06524-x [DOI] [PubMed] [Google Scholar]
- 45.Burger IA, Vargas HA, Donati OF, Andikyan V, Sala E, Gonen M, et al. The value of 18F-FDG PET/CT in recurrent gynecologic malignancies prior to pelvic exenteration. Gynecol Oncol 2013; 129: 586–92. doi: 10.1016/j.ygyno.2013.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yen T-C, Ng K-K, Ma S-Y, Chou H-H, Tsai C-S, Hsueh S, et al. Value of Dual-phase 2-fluoro-2-deoxy-D-glucose positron emission tomography in cervical cancer. J Clin Oncol 2003; 21: 3651–8. doi: 10.1200/JCO.2003.01.102 [DOI] [PubMed] [Google Scholar]
- 47.Husain A, Akhurst T, Larson S, Alektiar K, Barakat RR, Chi DS. A prospective study of the accuracy of 18fluorodeoxyglucose positron emission tomography (18FDG PET) in identifying sites of metastasis prior to pelvic exenteration. Gynecol Oncol 2007; 106: 177–80. doi: 10.1016/j.ygyno.2007.03.018 [DOI] [PubMed] [Google Scholar]
- 48.Lai C-H, Yen T-C, Chang T-C. Positron emission tomography imaging for gynecologic malignancy. Curr Opin Obstet Gynecol 2007; 19: 37–41. doi: 10.1097/GCO.0b013e32801195c9 [DOI] [PubMed] [Google Scholar]
- 49.Chu Y, Zheng A, Wang F, Lin W, Yang X, Han L, et al. Diagnostic value of 18F-FDG-PET or PET-CT in recurrent cervical cancer: a systematic review and meta-analysis. Nucl Med Commun 2014; 35: 144–50. doi: 10.1097/MNM.0000000000000026 [DOI] [PubMed] [Google Scholar]
- 50.Bura V, Visrodia P, Bhosale P, Faria SC, Pintican RM, Sharma S, et al. Mri of surgical flaps in pelvic reconstructive surgery: a pictorial review of normal and abnormal findings. Abdom Radiol 2020; 45: 3307–20. doi: 10.1007/s00261-019-02211-z [DOI] [PubMed] [Google Scholar]
- 51.Sagebiel TL, Faria SC, , Sacks JM, You YN, Bhosale PR. Pelvic reconstruction with omental and VRAM flaps: anatomy, surgical technique, normal postoperative findings, and complications. Radiographics 2011; 31: 2005–19. doi: 10.1148/rg.317115112 [DOI] [PubMed] [Google Scholar]
- 52.Sagebiel TL, Faria SC, Balachandran A, Butler CE, Garvey PB, Bhosale PR. Pelvic reconstruction with pedicled thigh flaps: indications, surgical techniques, and postoperative imaging. AJR Am J Roentgenol 2014; 202: 593–601. doi: 10.2214/AJR.13.11394 [DOI] [PubMed] [Google Scholar]
- 53.Kukar M, Platz TA, Schaffner TJ, Elmarzouky R, Groman A, Kumar S, et al. The use of modified four-dimensional computed tomography in patients with primary hyperparathyroidism: an argument for the abandonment of routine sestamibi single-positron emission computed tomography (SPECT. Ann Surg Oncol 2015; 22: 139–45. doi: 10.1245/s10434-014-3940-y [DOI] [PubMed] [Google Scholar]
- 54.Paspulati RM, Dalal TA. Imaging of complications following gynecologic surgery. Radiographics 2010; 30: 625–42. doi: 10.1148/rg.303095129 [DOI] [PubMed] [Google Scholar]
- 55.Diver EJ, Rauh-Hain JA, Del Carmen MG. Total pelvic exenteration for gynecologic malignancies. Int J Surg Oncol 2012; 2012: 1–9. doi: 10.1155/2012/693535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westin SN, Rallapalli V, Fellman B, Urbauer DL, Pal N, Frumovitz MM, et al. Overall survival after pelvic exenteration for gynecologic malignancy. Gynecol Oncol 2014; 134: 546–51. doi: 10.1016/j.ygyno.2014.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lall C, Tirkes TA, Patel AA, Lamba R, Verma S, Jennings SG, et al. Flaps, slings, and other things: CT after reconstructive surgery--expected changes and detection of complications. AJR Am J Roentgenol 2012; 198: W521–33. doi: 10.2214/AJR.11.7552 [DOI] [PubMed] [Google Scholar]
- 58.Zoucas E, Bjarnevik C..editors. Primary abdominal wall reinforcement with synthetic mesh following harvesting of vertical rectus abdominis myocutaneous flaps in multivisceral. Pelvic Resections 2015;. [Google Scholar]