Abstract
Radiomics is the extraction of a significant number of quantitative imaging features with the aim of detecting information in correlation with useful clinical outcomes. Features are extracted, after delineation of an area of interest, from a single or a combined set of imaging modalities (including X-ray, US, CT, PET/CT and MRI). Given the high dimensionality, the analytical process requires the use of artificial intelligence algorithms. Firstly developed for diagnostic performance in radiology, it has now been translated to radiation oncology mainly to predict tumor response and patient outcome but other applications have been developed such as dose painting, prediction of side-effects, and quality assurance. In gynecological cancers, most studies have focused on outcomes of cervical cancers after chemoradiation. This review highlights the role of this new tool for the radiation oncologists with particular focus on female GU oncology.
Introduction
Rapidly evolving, imaging is now the key diagnostic tool for many diseases and plays an important role in monitoring treatment response and predicting patient outcome.1 Radiation oncology is a pluri-disciplinary medical specialty combining clinical knowledge, medical physics, and imaging. In routine practice, radiation oncologists use different imaging modalities such as computed tomography (CT), positron emission tomography (PET-CT), or magnetic resonance imaging (MRI) to guide them at the different steps of patient treatment. New imaging sequences, modalities and analysis, have led to dramatic medical innovation, paralleled by improvements in computer hardware and software. For example, functional imaging such as diffusion-weighted imaging (DWI) has been widely used in radiation oncology for tumor segmentation and to predict tumor response.2–6 Recently, a new field of medical images analysis named radiomics has been proposed and tend to play an increasing role in personalized medicine.7 It consists in the extraction and analysis of multiple quantitative imaging features to create algorithm models correlated with clinical outcomes (mainly diagnosis, prediction of prognosis and treatment response).8 The analysis methods commonly use artificial intelligence (AI) tools such as machine or deep learning.9 The concept underlying radiomics is that solid cancers are heterogeneous (at cellular or genomic level, in terms of oxygenation, spatial organization, etc.), and this heterogeneity could be captured by radiomics analysis offering a more precise image evaluation by quantifying the internal conditions of the tumor invisible to a human observer.10
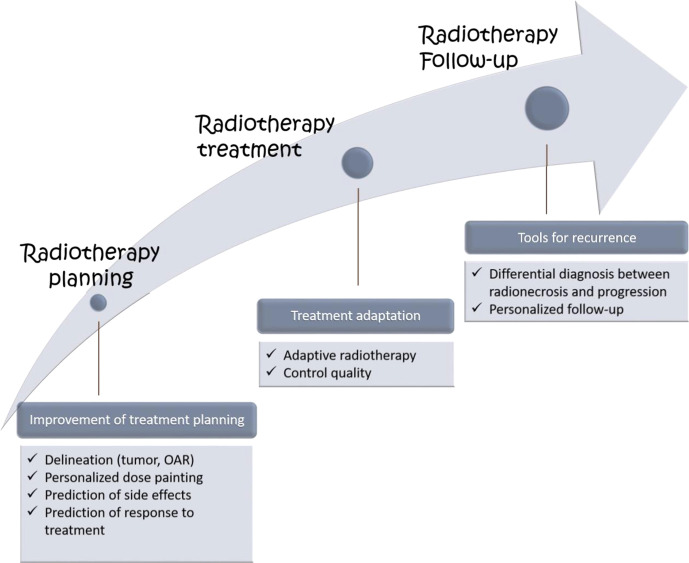
Combined with clinical data, radiomics may offer massive input in the scope of radiation oncology. The radiotherapy treatment workflow consists of different steps (radiotherapy planning, radiotherapy treatment, radiotherapy follow-up), and each of these steps could find applications in radiomics. Indeed, using radiomics in radiation oncology may help stratify patients according to their outcome (prediction of response to treatment or prediction of toxicity), improve tumor segmentation (tumor delineation), and finally optimize dose delivery (adaptive radiotherapy) and response assessment (follow-up, differential diagnosis between recurrence or radionecrosis)
The objective of this review is to highlight the recent studies useful for the radiation oncologists in the field of radiomics with particular focus on female GU oncology.
Workflow of radiomics-specifics of radiotherapy evaluation
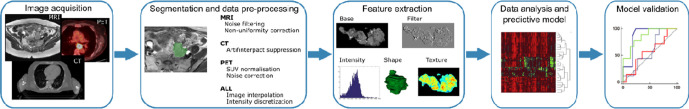
Different authors have described the usual workflow of radiomics procedure.8,10–12 Briefly, the method consists of five different steps (Figure 1). Please refer to article from Rizzo et al. from the same issue for a more detailed description (ref to be inserted).
Figure 1.
Work flow of radiomics process
Image acquisition: can be done on CT, MRI or PET/CT, PET/MRI
Image segmentation and data pre-processing
Segmentation is a time-consuming step during whicha physician has to delineate manually a region of interest (ROI). Currently,there are different tools trying to automatize this process.13,14 Historically, interest has been given to the visibletumor, but recently peritumoral environment has been shown to be informative aswell.15,16This may be of great interest in cervical cancer inevaluating parametrial invasion for example following radiotherapy. Despite the recent advances in the field ofcomputer vision and deep learning more specifically, fully automaticsegmentation remains a challenge, particularly when dealing with complexanatomy such as in the case of GU malignancies.17–19 Data pre-processing encompasses all the techniques,following image acquisition and preceding feature selection, aiming athomogenization of the samples. These include pixels resampling, normalizationand bin discretization.20
Feature extraction
Radiomics features are the translation of information contained within regions of interest (ROI) pixels and/or voxels. Four types of features are usually described:.21,22
Shape features are features describing the geometrical aspects of the tumor such as diameter, volume, sphericity etc..23
First-order statistics or histogram-based features are based on the distribution of pixel intensities throughout the entire ROI. They depict histogram characteristics.24 Their inconvenient is that they do not consider relationship between pixels/voxels.
Second-order statistics also known as textural features represent, as their ‘’texture’’ denomination indicates, spatial relationships between pixels/or voxels. The most famous is the grey level run-length matrix (GLRLM) described in 1975 by Galloway et al.25 It is the number of consecutive pixels having the same grey level intensity. We can also find the grey level co-occurrence matrix (GLCM)26 or neighborhood gray-level different matrix (NGLDM).
Higher-order statistics features are obtained after applying filters on the original images to highlight or reveal a specific kind of information, such as increasing enhancing tumor conspicuity when using the square filter for instance.23
Predictive modeling and model validation
Predictive model construction is a two-step process. It requires features selection, then model training. Feature selection refers to the process by which, redundant features are removed, and relevant ones are selected as a potential signature for model training. This step helps reduce the overfitting risk, given the high dimensionality of radiomics features.23 A wide variety of algorithms are available to select appropriate features and no consensus has been achieved as to which one should be used for a specific dataset.
In oncology, model training is usually carried out in a supervised way, meaning that each sample is labeled before the training phase.27 In other areas, such as robotics, unsupervised learning could be an alternative if labeled data is lacking.
Algorithms for model training are based on conventional machine learning. Among these algorithms, neural networks offer promising results and seem to outperform the more traditional techniques.28 Model validation and testing is necessary to assess for model robustness.29 Two types of models exist: model validation performed throughout cross-validation and model testing with an independent cohort.
The quality metrics of a predictive model are usually reported with the area under the curve (AUC), the receiver operating characteristic curve (ROC), the sensitivity and the specificity among others.30
Application of radiomics in radiation oncology with specific focus on gynecological malignancies
In gynecological cancers, radiotherapy takes an important place in treatment of endometrial and cervical cancers. It is associated with chemotherapy, followed by brachytherapy, for cervical cancers staged FIGO stage IB-IVA31 or prescribed as an adjuvant treatment for endometrial cancers.32 As for other cancers, the first development in radiomics have focused primarily in diagnostic performances of radiomic methods.10,33,34
In cervical cancer for example, MRI radiomic, FDG-PET radiomic, and/or their combination have been linked with stage, histology, lymph nodes status, and/or lymphovascular space invasion (LVSI). For example, Yu et al evaluated MRI-based radiomics performance (including DWI sequences) on 153 patients with stage IB-IIA cervical cancers. The radiomics model (including clinical stage, MR-reported lymph node status and grey-level non-uniformity) showed better performance than usual clinical and radiological factors (AUC 0.870).35 Lymphovascular space invasion (LVSI) is another unfavorable prognostic factor. Li et al have investigated the relationship between LVSI and MRI radiomics features in 105 patients. In their study, radiomics model showed favorable discrimination between LVSI and non-LVSI groups with an AUC of 0.754 in the training cohort and 0.727 in the validation cohort using T1 post contrast sequence.35 More recently, radiomics model derived from the combination of T2, ADC, and T1 contrast images in a study of 168 patients was significantly associated with lymph nodes metastases and showed better predictive performance than signatures derived from either of them alone in both sets.36
Different authors have evaluated radiomics as a tool for improving pre-treatment staging in endometrial cancer. Yan et al published a multicenter study evaluating MRI-based radiomics to predict the pelvic lymph node metastasis in endometrial cancer. They included 622 patients divided in training and validation sets. The objective was to compare the diagnostic performance between radiologists alone and radiomics-aided radiologists. The AUC values were 0.623 and 0.643 for the radiologists of different centers versus 0.814 and 0.842 for radiomics-aided radiologists.37 The same question was asked by De Bernardi et al using 18F-FDG-PET/CT. They extracted imaging features from the primary tumor and created different univariate and multivariate models. They identified a unique heterogeneity feature able to predict lymph node metastasis with a sensitivity of 89% and a specificity of 80%, outperforming usual visual detection.38
Besides diagnosis performance, the direct applications of radiomics for the radiation oncologists are more recent and have focused mainly on predicting tumor response.27,39 However, additional benefits have been investigated with promising results discussed below and in Figure 2.
Figure 2.
Application of radiomics to the different steps of radiotheraphy treatment
Radiotherapy planning
Delineation is a crucial step in radiotherapy planning consisting of the determination of the target volume to treat, by drawing this volume on planning images (CT and MR scan). In radiation oncology, we use different volumes: GTV for gross tumor volume (macroscopic tumor), CTV for clinical target volume (microscopic extension around GTV) and PTV for planning target volume (volume treated). It has been shown that radiomics may help better volume delineation by visualizing more precisely tumor borders. The use in uterine cervical cancer yet need to be determined, since there are some evidences of utility in other cancers. Some authors already demonstrated the good accuracy of autosegmentation models, for example in prostate cancers delineation with model using deep decision forest algorithm.14 In prostate cancer, Shiradkar et al evaluated the potential use of radiomics-based on targeted radiotherapy planning with MRI. They demonstrated that using such detected tumor regions to generate treatment plans would result in significant reduced doses in organs at risk (rectum, bladder, penile bulb, femoral heads) and the possibility to deliver a boost dose to lesions up to 85.8 Gy with external beam radiotherapy.40 Nailon et al have proposed an automatic segmentation of the GTV and OAR in bladder cancer based on CT radiomics features. The approach significantly offered an accurate classification on axial, coronal and sagittal CT imaging planes of GTV using an unsupervised classification.41
Besides better tumor margin delineation, radiomics may help distinguish intratumor heterogeneity which is known to be a cause of poor outcome and of progression after radiotherapy.42 For now, radiation therapy is usually applied assuming that the target tumor volume is homogeneous. However, it is well-known that tumor are heterogeneous. This heterogeneity and its link with radiomics has been particularly highlighted in ovarian cancer. For example, Vargas et al developed 12 quantitative metrics to capture spatial inter-site imaging heterogeneity in high-grade serous ovarian cancer. The authors demonstrated that radiomics features capturing the differences in texture similarities across sites were significantly associated with shorter overall survival (inter-site similarity entropy, similarity level cluster shade, and inter site similarity level cluster prominence) and incomplete surgical resection (similarity level cluster shade, inter site similarity level cluster prominence and inter site cluster variance).43 As such, the use of radiomics could offer the great advantage to describe more precisely this heterogeneity with the aim for example to deliver a boosted dose in more radioresistant/aggressive areas named as “dose painting”.44 Initial works using PET imaging have been recently proposed.45 Some authors used FDG-PET in cervix cancers as a tool to semi-automatically drawn the metabolic tumor volume and prescribe different doses with EBRT between this volume (central pelvis) and the latero-pelvis volume, in order to boost the tumor volume using brachytherapy.46 Other works have been also realized in other cancers. In prostate cancer, PET guided boost showed a higher biochemical recurrence-free survival (92% vs 85% without simultaneous integrated boost) with a significant trend.47 In pancreatic cancers, a correlation has been identified between baseline FDG-PET and residual metabolic activity after chemoradiotherapy, paving the way to escalated dose to this area.48,49
Predictive and prognostic value
Prediction of outcomes/prognosis after radiotherapy is one of the most interesting and studied field of radiomics. In radiation oncology, it plays a crucial role as the need of individual prognostic assessment is of great interest, allowing to intensify the treatment for patients with unfavorable prognostic or de-intensify for those with favorable prognostic (in order to prevent “unnecessary” toxicities). Exponential numbers of studies have been published during the last decade, focusing either on recurrence and/or survival. Radiomics as a noninvasive biomarker of response in uterine cervical cancer has particularly demonstrated increased interest. Studies on the value of radiomics in the prediction of survival and prediction of recurrence for patients with cervical cancer are outlined in Table 1. For example, Takada et al evaluated MRI-based radiomics for predicting prognosis of cervical cancers after definitive radiotherapy. On 107 patients, 25 presented a relapse. The radiomics AUC calculated for ADC MRI sequence with absolute rescaling was of 0.79 vs 0.52 for conventional factors such as tumor volume (p = 0.001).50 Lucia et al evaluated 18F-FDG-PET/CT and MRI-based radiomics features for prediction of outcomes in patients treated with chemotherapy for a locally advanced cervical cancer. They included 102 patients, with a median follow-up of 3.0 years. In multivariate analysis, they identified two radiomics features as independent prognostic factors: the Grey Level Non-Uniformity (GLRLM) in PET/CT and the entropy for apparent diffusion maps on DWI MRI. These two features largely outperformed usual clinical, biological and radiological factors, with accuracy of 94% (sensitivity 90% specificity 96%) for predicting recurrence and 100% for predicting locoregional control. At 3 years, the locoregional rate control was 98% for patients with high GLRLM versus 41% for patients with low GLRLM and 98% for patients with high entropy versus 45% for patients with low entropy.39 These results are consistent with other authors evaluating PET/CT-based features.51
Table 1.
Summary of the recent publications regarding the role of radiomics in cervical cancer
| Author | Year | Training /validation set |
Modality | Stage | Results |
|---|---|---|---|---|---|
| Studies Regarding LNM | |||||
| Shen et al.69 | 2017 | 85/85 | PET/CT | IB-IVA | Homogeneity derived from the GLCM was the sole feature in predicting LNM |
| Becker et al.70 | 2017 | 23 | T2-DWI | I-III | Skewness and Kurtosis were higher in patient with LNM |
| Kan et al.71 | 2018 | 100/43 | T2-T1 contrast | Ia2-IIb | Radiomics model including 10 features was able to differentiate LNM and non-LNM |
| Li et al.72 | 2018 | 64/30 | PETCT | IA-IIA | Skewness was able to predict LNM with AUC 0.803 in training cohort and 0.757 in validation cohort |
| Wang et al.73 | 2019 | 96 | T2 DWI | I-III | Radiomics model derived from joint T2WI and DWI yielded higher AUC compared to model derived from T2WI or DWI alone. |
| Wu et al74 | 2019 | 126/63 | T2-ADC | IB-IIB | A decision tree combining radiomics model of T2 (tumor +peritumoral area) and clinical LN status achieved best diagnostic performance, with AUC and sensitivity of 0.895 and 94.3%, 0.847 and 100% in the training and validation cohort, respectively. |
| Jin et al75 | 2020 | 100/32 | Ultrasound | I-II | Radiomics model was associated with able to predict LNM |
| Xiao et al76 | 2020 | 155/78 | T1-T2-DWI-ADC | IB-IIB | Radiomics model allowed the discrimination between the LNM and non-LNM groups, with a C-index of 0.856 in the primary cohort and 0.883 in the validation cohort. |
| Dong et al.77 | 2020 | 176/50 | CT | IA-IIB | A trained deep learning model had an area under curve (AUC) of 0.99 and an accuracy of 97.16% in the internal validation to predict LNM. |
| Hou et al.15 | 2020 | 168 | T2-ADC-T1 contrast | IB-IIA | Radiomics model derived from the combination of T2, ADC, and T1 contrast images, composed of 6 LN-status-related features, was significantly associated with LNM and showed better predictive performance than signatures derived from either of them alone in both sets. |
| Studies regarding overall and disease-free survival | |||||
| Ho et al.78 | 2017 | 44 | PETCT | IB2-IVA | Radiomics features were associated with overall survival |
| Ho et al.79 | 2017 | 69 | DWI-PETCT | IB1-IVB | Mean ADC value was the only features associated with DFS |
| Lucia et al.18 | 2017 | 69/33 | T1-T2-DWI-PETCT | IB1-IVA | Radiomics features associated with DFS |
| Shen et al.69 | 2018 | 77/65 | PETCT | IB-IIIB | Radiomics features associated with overall survival |
| Schernberg et al.80 | 2018 | 69/39 | PETCT | IB1-IVA | SUV peak combined with neutrophil count predictive of overall survival |
| Lucia et al. (81) | 2019 | 112/78 | T1-T2-DWI-PETCT | IB1-IVA | Externally validated radiomics features associated with DFS |
| Other evaluations | |||||
| Liu et al. (82) | 2019 | 160 | T2-ADC | IB-IV | Whole-tumor volumetric 3D radiomics analysis had a better performance than using the 2D center-slice of tumor in stratifying the histological grade of cervical cancer. |
| Wang et al. (83) | 2020 | 137 | T2-ADC | IB-IIA | Radiomics model was able to predict parametrial invasion. |
Prediction of prognosis was also evaluated in endometrial cancers (Table 2) Fasmer et al developed an MRI-based radiomics signature for the pre-treatment evaluation of aggressive endometrial cancers. The whole-tumor radiomic signatures predicted presence of high grade-endometrioid tumors or poor progression-free survival, more efficiently than tumor volume (HR 4.6–9.8; p < 0.05).52
Table 2.
The summary of the recent publications regarding the role of radiomics in endometrial cancer
| Author | Year | Training /validation set |
Modality | Stage | Results |
|---|---|---|---|---|---|
| Studies Regarding LNM | |||||
| De Bernardi et al.17 | 2018 | 86/29 | PETCT | I-IV | Imaging features from primary tumor increase nodal staging sensitivity |
| Xu et al.73 | 2019 | 140/60 | T2-3D LAVA | I-IV | Four models show predictive ability to predict LNM (AUC = 0.883) |
| Yan et al.74 | 2020 | 351/271 | T1-T2-DWI-ADC | I-IV | Radiomics-aided radiologists are better than radiologists alone |
| Crivellaro et al. (84) | 2020 | 167 | PETCT | I-IV | Volume density is the most predictive feature for LNM |
| Studies regarding overall and disease-free survival | |||||
| Fasmer et al.30 | 2020 | 108/30 | T1 VIBE – DIXON VIBE | I-IV | Whole-tumor radiomic signature help to predict PFS and aggressive disease |
| Other evaluations | |||||
| Stanzione et al. (85) | 2020 | 43/11 | T2-ADC | I-IV | Radiomics can predict deep myometrial infiltration with accuracy of 91% |
| Luo et al. (86) | 2020 | 101/43 | T1-T2-DWI | I-IV | Radiomic-based machine-learning model can predict LVSI with AUC of 0.820 |
Usually, the gross tumor volume (GTV) is used as the region of interest (ROI) for the development of prognostic radiomics-based models, but we currently know that peritumoral environment is an important factor for treatment resistance and metastatic spread. Hao et al analyzed this peritumoral environment on PET/CT images of cervical cancers developing a radiomic feature named “shell feature”, which corresponds to the periphery of the tumor at the interface between tumor and peri-tumoral environment. They demonstrated that this shell feature could predict more efficiently distant failure than other usual radiomics GTV-based features (AUC 0.83, sensitivity 0.81, specificity 0.80, accuracy 0.80, p < 0.005).53 Those results are particularly interesting highlighting the prognostic role of the peritumoral environment.
The predictive value of radiomics is also well evaluated in other cancers, such as rectal cancers. Using radiotherapy treatment planning CT, Wang et al demonstrated that radiomics features can improve the prediction of overall survival from 0.672 with clinical features to 0.730 with clinical and radiomics features.54 Good results were also obtained in hematologic oncology, where a radiomic feature has been identified as an independent prognostic predictor of PFS and OS on pre-treatment 18F-FDG PET in patients treated for Hodgkin lymphoma55 or diffuse large B-cell lymphoma.56
Prediction of side effects
In radiation oncology, two categories of side-effects exist: acute and late side-effects. They are consecutive to irradiation of the normal tissue surrounding the tumor. Acute side-effects (during and within 3 months after treatment) are transient and usually well supported by appropriate symptomatic treatments. On the contrary, late side-effects (more than 3 to 6 months after treatment) may be permanent and can be life threatening. They are mainly related to treatment and/or patient characteristics. Identifying such radiomics-based characteristics can lead to modify the prescribed dose or the target volume offering a more personalized medicine. The use in radiotherapy for cervical cancers needs to be determined, for example to predict recto-proctitis and cystitis after radiotherapy.57 In lung cancers, a model has been developed on planning CT images to predict radiation pneumonitis after SBRT, with a 0.75 AUC.58 Cunliffe et al identified 12 features changing significantly between, pre- and post-radiotherapy CT scan. These features can discriminate patients with and without radiation pneumonitis by identifying lung tissue reaction after radiotherapy.59 Other authors also evaluated the ability of radiomic features to predict radiation pneumonitis after SBRT and found a dose-response relationship.60 A similar work evaluated radiomics for urinary and gastro-intestinal toxicities after prostate cancer radiotherapy with a 0.71 AUC.57 Such results could lead to change the dose prescription for patients with high risk of toxicities. Radiation-induced xerostomia is the other side-effect of interest for radiomic studies. Sheikh et al demonstrated that using baseline CT and MR images-extracted features, we can stratify xerostomia risk by evaluation of salivary gland function.61 This is concordant with other studies on xerostomia risk prediction using CT where models can predict acute xerostomia with a precision of 0.9220 with a sensitivity of 100%,62 or PET/CT scan where intensity and textural features of parotid glands extracted from pre-treatment PET/CT were associated with the risk of developing xerostomia 12 months after radiotherapy.63 These concerns are really helpful for the radiation oncologists because xerostomia can be partly avoided by greater protection of salivary glands. In prostate radiotherapy, models of late toxicity prediction were significantly improved by addition of rectal and bladder texture analysis (example for rectal bleeding, AUC from 0.58 to 0.73).64
Differential diagnosis between radionecrosis and tumor progression
Such tool could be useful for cervical cancers, where the difference between radiotherapy effect (radionecrosis or fibrosis) and recurrence or persistence of tumor is sometimes hard to distinguish after radiotherapy, but to our knowledge there is no work existing on this topic, contrary to other cancers as brain or lung tumors.
This question is frequent for brain metastases after SRS (Stereotactic RadioSurgery) where radionecrosis is hard to distinguish from true progression, despite advances of imaging modalities (multimodality MRI, PET scan). The diagnostic certainty is only obtained by pathologic confirmation for symptomatic patients requiring a resection. Peng et al identified 82 resected lesions (from 66 patients). With a predictive model from MR-based radiomic features, they obtained a sensitivity of 66%, a specificity of 87%, with an area under the curve of 0.81.65 Compared to two expert neuroradiologists, a textural feature with support vector machine classifier identified more frequently radionecrosis from recurrent brain tumors (12/15 cases correctly identified vs 7/15 and 8/15). Other authors tend to similar results.66
In lung cancer, Mattonen et al found a radiomic signature consisting of 5 features which demonstrated an excellent discrimination (AUC 0.85) between early recurrence and post treatment changes.67
Radiation quality assurance
The quality assurance of a radiotherapy is usually based on a γ comparison between irradiated measurement device with the treatment plan and the expected dose on the treatment planning system. Different authors showed an advantage to use radiomics and machine learning-based methods to identify unexpected treatment delivery errors from patient-specific γ images. Using 3 sets of planar doses (error-free case, random multileaf collimator (MLC) error case or systematic MLC error case) exported from 23 IMRT plans and delivered to an electronic portale device, Nyflot et al compared planned and and measured doses to obtain γ images using radiomics approaches to extract images features The highest accuracy to detect presence or absence of error was achieved using deep learning-based methods, and both methods were better than usual approaches without radiomics.68,69 Similar results were obtained by other teams with artificial intelligence-based methods of quality assurance.69 In gynecological cancers, as for other cancers, the potential benefit is to increase the quality assurance of irradiation, minimizing the risk of difference between prescribed dose and delivered dose.
Challenges
As an emergent post-processing tool, radiomic face some challenges such as the lack of standardization in the methods used for data extraction or analysis.56–59
In radiation oncology, a frequent pitfall shared with radiomics workflow is the variability of segmentation. It can first be related to the thresholds selected for delineation on the different imaging methods.70,71 Then it is also related to inter and intraobserver variability. The recent development of auto-segmentation methods should solve this issue.17 The method already demonstrated superiority to manual segmentation with better feature quantification reproducibility and robustness.72 However, we need more studies for the generalization of such procedures.
Repeatability and reproducibility are frequent pitfalls of radiomics-based models. Fiset et al evaluated the stability of T2W MRI-based features in cervical cancers. For that, they used a retrospective cohort of patients who underwent chemoradiation for cervical cancer. This cohort was separated in 3 groups of comparison: comparison test – retest (on the same images), comparison between diagnostic MRI and simulation MRI, and comparison between different observers. They found that the most reproducible features were those between different observers but on the same images (95.2% with an intraclass correlation coefficient (ICC) ≥0.75). Conversely, the worst reproducible features were those between diagnostic MRI and simulation MRI, highlighting the lack of reproducibility between different acquisition protocols. The most stable features were shape features.73 The inter observer variability of radiomic features, and the ways to improve it, was also evaluated by Traverso et al. Eighty-one ADC maps derived from pre-treatment cervical cancers DWI-MRI were delineated by two observers. They found an approach (urine-normalized approach) improving reproducibility (78% of features with ICC ≥0.75, vs 63% without normalization).74
Despite promising results, the “dose painting” struggles to become a standard of care. Previous studies often focused to boosted dose of hypoxic areas identified with different imaging modalities. It is now well known that oxygenation is probably not the only factor of intra-tumoral heterogeneity. We need to better understand this heterogeneity underlying by radiomics to adapt the different steps of planning and treatments.75
The ability of radiomics to predict side effects is of great interest in our daily practice. The benefits/risks balance is the main question raised by the radiation oncologist. To date, the predictive performances are not sufficient for changing the radiotherapy prescription plan. Before being integrated in clinical practice, the methods need to be evaluated in randomized clinical trials, as other predictive biomarkers.76
Conclusion
Radiomics is an interesting and emergent tool of development for the radiation oncology practice. It can be used at different steps of treatment preparation or treatment evaluation. A few researches have been performed in the female GU oncology field, requiring more data for the generalization of radiomics in routine practice (mainly due to the lack of reproducibility). Overcoming these challenges, we can imagine that “radiotherapy-radiomics” could be incorporated into routine practice as decision-support or computer-aided diagnosis tools to predict tumor aggressiveness and response to therapy.
Contributor Information
Morgan Michalet, Email: morgan.michalet@icm.unicancer.fr.
David Azria, Email: david.azria@icm.unicancer.fr.
Marion Tardieu, Email: marion.tardieu@icm.unicancer.fr.
Hichem Tibermacine, Email: hichem.tiber@gmail.com.
Stéphanie Nougaret, Email: stephanie.nougaret@icm.unicancer.fr.
REFERENCES
- 1.Giardino A, Gupta S, Olson E, Sepulveda K, Lenchik L, Ivanidze J, et al. Role of imaging in the era of precision medicine. Acad Radiol 2017; 24: 639–49. doi: 10.1016/j.acra.2016.11.021 [DOI] [PubMed] [Google Scholar]
- 2.Gui B, Miccò M, Valentini AL, Cambi F, Pasciuto T, Testa A, et al. Prospective multimodal imaging assessment of locally advanced cervical cancer patients administered by chemoradiation followed by radical surgery-the "PRICE" study 2: role of conventional and DW-MRI. Eur Radiol 2019; 29: 2045–57. doi: 10.1007/s00330-018-5768-5 [DOI] [PubMed] [Google Scholar]
- 3.Thomeer MG, Vandecaveye V, Braun L, Mayer F, Franckena-Schouten M, de Boer P, et al. Evaluation of T2-W MR imaging and diffusion-weighted imaging for the early post-treatment local response assessment of patients treated conservatively for cervical cancer: a multicentre study. Eur Radiol 2019; 29: 309–18. doi: 10.1007/s00330-018-5510-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jalaguier-Coudray A, Villard-Mahjoub R, Delouche A, Delarbre B, Lambaudie E, Houvenaeghel G, et al. Value of dynamic contrast-enhanced and diffusion-weighted MR imaging in the detection of pathologic complete response in cervical cancer after neoadjuvant therapy: a retrospective observational study. Radiology 2017; 284: 432–42. doi: 10.1148/radiol.2017161299 [DOI] [PubMed] [Google Scholar]
- 5.Levy A, Caramella C, Chargari C, Medjhoul A, Rey A, Zareski E, et al. Accuracy of diffusion-weighted echo-planar MR imaging and ADC mapping in the evaluation of residual cervical carcinoma after radiation therapy. Gynecol Oncol 2011; 123: 110–5. doi: 10.1016/j.ygyno.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 6.Schreuder SM, Lensing R, Stoker J, Bipat S. Monitoring treatment response in patients undergoing chemoradiotherapy for locally advanced uterine cervical cancer by additional diffusion-weighted imaging: a systematic review. J Magn Reson Imaging 2015; 42: 572–94. doi: 10.1002/jmri.24784 [DOI] [PubMed] [Google Scholar]
- 7.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016; 278: 563–77. doi: 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB, et al. Radiomics: the process and the challenges. Magn Reson Imaging 2012; 30: 1234–48. doi: 10.1016/j.mri.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parmar C, Grossmann P, Bussink J, Lambin P, Aerts HJWL. Machine learning methods for quantitative radiomic biomarkers. Sci Rep 2015; 5: 13087. cited 2020 Nov 5. doi: 10.1038/srep13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RGPM, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012; 48: 441–6. doi: 10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, Tha KK, Xing L, Li R. Radiomics and radiogenomics for precision radiotherapy. J Radiat Res 2018; 59(suppl_1): i25–31. doi: 10.1093/jrr/rrx102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bibault J-E, Xing L, Giraud P, El Ayachy R, Giraud N, Decazes P, et al. Radiomics: a primer for the radiation oncologist. Cancer Radiother 2020; 24: 403–10. doi: 10.1016/j.canrad.2020.01.011 [DOI] [PubMed] [Google Scholar]
- 13.Anders LC, Stieler F, Siebenlist K, Schäfer J, Lohr F, Wenz F. Performance of an atlas-based autosegmentation software for delineation of target volumes for radiotherapy of breast and anorectal cancer. Radiother Oncol 2012; 102: 68–73. doi: 10.1016/j.radonc.2011.08.043 [DOI] [PubMed] [Google Scholar]
- 14.Macomber MW, Phillips M, Tarapov I, Jena R, Nori A, Carter D, et al. Autosegmentation of prostate anatomy for radiation treatment planning using deep decision forests of radiomic features. Phys Med Biol 2018; 63: 235002. doi: 10.1088/1361-6560/aaeaa4 [DOI] [PubMed] [Google Scholar]
- 15.Braman NM, Etesami M, Prasanna P, Dubchuk C, Gilmore H, Tiwari P, et al. Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res 2017; ; 19: 57.18. doi: 10.1186/s13058-017-0846-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dou TH, Coroller TP, van Griethuysen JJM, Mak RH, Aerts HJWL. Peritumoral radiomics features predict distant metastasis in locally advanced NSCLC. PLoS One 2018; 13: e0206108. doi: 10.1371/journal.pone.0206108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardenas CE, Yang J, Anderson BM, Court LE, Brock KB. Advances in Auto-Segmentation. Semin Radiat Oncol 2019; ; 29: 185–97Jul. doi: 10.1016/j.semradonc.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Lu J, Qin G, Shen L, Sun Y, Ying H, et al. Technical note: a deep learning-based autosegmentation of rectal tumors in Mr images. Med Phys 2018; 45: 2560–4. doi: 10.1002/mp.12918 [DOI] [PubMed] [Google Scholar]
- 19.Ibragimov B, Xing L. Segmentation of organs-at-risks in head and neck CT images using convolutional neural networks. Med Phys 2017; 44: 547–57. doi: 10.1002/mp.12045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray JM, Kaissis G, Braren R, Kleesiek J. A primer on radiomics. Radiol 2020; 60: 32–41. [DOI] [PubMed] [Google Scholar]
- 21.Lohmann P, Bousabarah K, Hoevels M, Treuer H. Radiomics in radiation oncology-basics, methods, and limitations. Strahlenther Onkol 2020; 196: 848–55. doi: 10.1007/s00066-020-01663-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larue RTHM, Defraene G, De Ruysscher D, Lambin P, van Elmpt W. Quantitative radiomics studies for tissue characterization: a review of technology and methodological procedures. Br J Radiol 2017; 90: 20160665. doi: 10.1259/bjr.20160665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizzo S, Botta F, Raimondi S, Origgi D, Fanciullo C, Morganti AG, et al. Radiomics: the facts and the challenges of image analysis. Eur Radiol Exp 2018; 2: 36. doi: 10.1186/s41747-018-0068-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quantitative histogram analysis of images. Comput Phys Commun 2006; 175: 620–3. [Google Scholar]
- 25.Galloway MM. Texture analysis using gray level run lengths. Comput Graph Image Process 1975; 4: 172–9. [Google Scholar]
- 26.Haralick RM, Shanmugam K, Dinstein I. Textural features for image classification. IEEE Trans Syst Man Cybern 1973; 3):: 610–21Nov;SMC-. [Google Scholar]
- 27.Zhang Y, Oikonomou A, Wong A, Haider MA, Khalvati F. Radiomics-based prognosis analysis for non-small cell lung cancer. Sci Rep 2017; 7: 46349. doi: 10.1038/srep46349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosny A, Parmar C, Coroller TP, Grossmann P, Zeleznik R, Kumar A, et al. Deep learning for lung cancer prognostication: a retrospective multi-cohort radiomics study. PLoS Med 2018; 15: e1002711. doi: 10.1371/journal.pmed.1002711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y-Q, Liang C-H, He L, Tian J, Liang C-S, Chen X, et al. Development and validation of a Radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. J Clin Oncol 2016; 34: 2157–64. doi: 10.1200/JCO.2015.65.9128 [DOI] [PubMed] [Google Scholar]
- 30.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010; 21: 128–38. doi: 10.1097/EDE.0b013e3181c30fb2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhatla N, Berek JS, Cuello Fredes M, Denny LA, Grenman S, Karunaratne K. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet 2019; 145: 129–35. [DOI] [PubMed] [Google Scholar]
- 32.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol 2016; 27: 16–41. [DOI] [PubMed] [Google Scholar]
- 33.Avanzo M, Stancanello J, El Naqa I, Naqa E I. Beyond imaging: the promise of radiomics. Phys Med 2017; 38: 122–39. doi: 10.1016/j.ejmp.2017.05.071 [DOI] [PubMed] [Google Scholar]
- 34.Tagliafico AS, Piana M, Schenone D, Lai R, Massone AM, Houssami N. Overview of radiomics in breast cancer diagnosis and prognostication. Breast 2020; 49: 74–80. doi: 10.1016/j.breast.2019.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu YY, Zhang R, Dong RT, Hu QY, Yu T, Liu F, et al. Feasibility of an ADC-based radiomics model for predicting pelvic lymph node metastases in patients with stage IB-IIA cervical squamous cell carcinoma. Br J Radiol 2019; 92: 20180986. doi: 10.1259/bjr.20180986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou L, Zhou W, Ren J, Du X, Xin L, Zhao X, et al. Radiomics analysis of multiparametric MRI for the preoperative prediction of lymph node metastasis in cervical cancer. Front Oncol 2020; 10: 1393. doi: 10.3389/fonc.2020.01393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan BC. Radiologists with MRI-based radiomics AIDS to predict the pelvic lymph node metastasis in endometrial cancer: a multicenter study. Eur Radiol 12. [DOI] [PubMed] [Google Scholar]
- 38.Bernardi ED. Radiomics of the primary tumour as a tool to improve 18F-FDG-PET sensitivity in detecting nodal metastases in endometrial cancer. 2018; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucia F, Visvikis D, Desseroit M-C, Miranda O, Malhaire J-P, Robin P, et al. Prediction of outcome using pretreatment 18F-FDG PET/CT and MRI radiomics in locally advanced cervical cancer treated with chemoradiotherapy. Eur J Nucl Med Mol Imaging 2018; 45: 768–86. doi: 10.1007/s00259-017-3898-7 [DOI] [PubMed] [Google Scholar]
- 40.Shiradkar R. Radiomics based targeted radiotherapy planning (Rad-TRaP): a computational framework for prostate cancer treatment planning with MRI. 2016; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nailon WH, Redpath AT, McLaren DB. Characterisation of radiotherapy planning volumes using textural analysis. Acta Oncol 2008; 47: 1303–8. doi: 10.1080/02841860802256467 [DOI] [PubMed] [Google Scholar]
- 42.Glowa C, Karger CP, Brons S, Zhao D, Mason RP, Huber PE, et al. Carbon ion radiotherapy decreases the impact of tumor heterogeneity on radiation response in experimental prostate tumors. Cancer Lett 2016; 378):: 97–10310;. doi: 10.1016/j.canlet.2016.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vargas HA, Veeraraghavan H, Micco M, Nougaret S, Lakhman Y, Meier AA, et al. A novel representation of inter-site tumour heterogeneity from pre-treatment computed tomography textures classifies ovarian cancers by clinical outcome. Eur Radiol 2017; 27: 3991–4001. doi: 10.1007/s00330-017-4779-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nioche C, Orlhac F, Boughdad S, Reuzé S, Goya-Outi J, Robert C, et al. LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res 2018; 78):: 4786–915;. doi: 10.1158/0008-5472.CAN-18-0125 [DOI] [PubMed] [Google Scholar]
- 45.Reuzé S, Schernberg A, Orlhac F, Sun R, Chargari C, Dercle L, et al. Radiomics in nuclear medicine applied to radiation therapy: methods, pitfalls, and challenges. Int J Radiat Oncol Biol Phys 2018; 102: 1117–42. doi: 10.1016/j.ijrobp.2018.05.022 [DOI] [PubMed] [Google Scholar]
- 46.Lin AJ, Dehdashti F, Grigsby PW. Molecular imaging for radiotherapy planning and response assessment for cervical cancer. Semin Nucl Med 2019; 49: 493–500. doi: 10.1053/j.semnuclmed.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 47.Schlenter M, Berneking V, Krenkel B, Mottaghy FM, Vögeli T-A, Eble MJ. Intensity-modulated radiotherapy of prostate cancer with simultaneous integrated boost after molecular imaging with 18F-choline-PET/CT : Clinical results and quality of life. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al 2018; 194: 638–45. [DOI] [PubMed] [Google Scholar]
- 48.Wilson JM, Mukherjee S, Chu K-Y, Brunner TB, Partridge M, Hawkins M. Challenges in using 18F-fluorodeoxyglucose-PET-CT to define a biological radiotherapy boost volume in locally advanced pancreatic cancer. Radiat Oncol Lond Engl 2014; 9: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu T-T, Lam S-K, To L-H, Tse K-Y, Cheng N-Y, Fan Y-N, et al. Pretreatment prediction of adaptive radiation therapy eligibility using MRI-based Radiomics for advanced nasopharyngeal carcinoma patients. Front Oncol 2019; 9: 1050. doi: 10.3389/fonc.2019.01050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takada A, Yokota H, Watanabe M, Horikoshi T, Uno T. OC-0509 MRI radiomics analysis for predicting prognosis of cervical cancer after definitive radiotherapy. Radiother Oncol 2019; 133: S264–5. [Google Scholar]
- 51.Reuzé S, Orlhac F, Chargari C, Nioche C, Limkin E, Riet F, et al. Prediction of cervical cancer recurrence using textural features extracted from 18F-FDG PET images acquired with different scanners. Oncotarget 2017; 8: 43169–79. doi: 10.18632/oncotarget.17856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fasmer KE, Hodneland E, Dybvik JA, Wagner‐Larsen K, Trovik J, Ø S. Whole‐Volume tumor MRI Radiomics for prognostic modeling in endometrial cancer. J Magn Reson Imaging 2020; 27444Nov 16;jmri.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hao H, Zhou Z, Li S, Maquilan G, Folkert MR, Iyengar P, et al. Shell feature: a new radiomics descriptor for predicting distant failure after radiotherapy in non-small cell lung cancer and cervix cancer. Phys Med Biol 2018; 63: 095007. doi: 10.1088/1361-6560/aabb5e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Shen L, Zhong H, Zhou Z, Hu P, Gan J, et al. Radiomics features on radiotherapy treatment planning CT can predict patient survival in locally advanced rectal cancer patients. Sci Rep 2019; 9: 15346. doi: 10.1038/s41598-019-51629-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lue K-H, Wu Y-F, Liu S-H, Hsieh T-C, Chuang K-S, Lin H-H, et al. Prognostic value of pretreatment radiomic features of 18F-FDG PET in patients with Hodgkin lymphoma. Clin Nucl Med 2019; 44: e559. doi: 10.1097/RLU.0000000000002732 [DOI] [PubMed] [Google Scholar]
- 56.Lue K-H, Wu Y-F, Lin H-H, Hsieh T-C, Liu S-H, Chan S-C, et al. Prognostic Value of Baseline Radiomic Features of 18F-FDG PET in Patients with Diffuse Large B-Cell Lymphoma. Diagnostics 2020; 11: E3628 12 2020. doi: 10.3390/diagnostics11010036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mostafaei S, Abdollahi H, Kazempour Dehkordi S, Shiri I, Razzaghdoust A, Zoljalali Moghaddam SH, et al. Ct imaging markers to improve radiation toxicity prediction in prostate cancer radiotherapy by stacking regression algorithm. Radiol Med 2020; 125: 87–97. doi: 10.1007/s11547-019-01082-0 [DOI] [PubMed] [Google Scholar]
- 58.Hirose T-A, Arimura H, Ninomiya K, Yoshitake T, Fukunaga J-I, Shioyama Y. Radiomic prediction of radiation pneumonitis on pretreatment planning computed tomography images prior to lung cancer stereotactic body radiation therapy. Sci Rep 2020; 10: 20424. doi: 10.1038/s41598-020-77552-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cunliffe A, Armato SG, Castillo R, Pham N, Guerrero T, Al-Hallaq HA. Lung texture in serial thoracic computed tomography scans: correlation of radiomics-based features with radiation therapy dose and radiation pneumonitis development. Int J Radiat Oncol Biol Phys 2015; 91: 1048–56. doi: 10.1016/j.ijrobp.2014.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moran A, Daly ME, Yip SSF, Yamamoto T. Radiomics-based assessment of radiation-induced lung injury after stereotactic body radiotherapy. Clin Lung Cancer 2017; 18: e425–31. doi: 10.1016/j.cllc.2017.05.014 [DOI] [PubMed] [Google Scholar]
- 61.Sheikh K, Lee SH, Cheng Z, Lakshminarayanan P, Peng L, Han P, et al. Predicting acute radiation induced xerostomia in head and neck cancer using Mr and CT Radiomics of parotid and submandibular glands. Radiat Oncol 2019; 14: 131. doi: 10.1186/s13014-019-1339-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, Shi H, Huang S, Chen X, Zhou H, Chang H, et al. Early prediction of acute xerostomia during radiation therapy for nasopharyngeal cancer based on delta radiomics from CT images. Quant Imaging Med Surg 2019; 9: 1288–302. doi: 10.21037/qims.2019.07.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Dijk LV, Noordzij W, Brouwer CL, Boellaard R, Burgerhof JGM, Langendijk JA, et al. 18F-FDG PET image biomarkers improve prediction of late radiation-induced xerostomia. Radiother Oncol 2018; 126: 89–95. doi: 10.1016/j.radonc.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 64.Rossi L, Bijman R, Schillemans W, Aluwini S, Cavedon C, Witte M, et al. Texture analysis of 3D dose distributions for predictive modelling of toxicity rates in radiotherapy. Radiother Oncol 2018; 129: 548–53. doi: 10.1016/j.radonc.2018.07.027 [DOI] [PubMed] [Google Scholar]
- 65.Peng L, Parekh V, Huang P, Lin DD, Sheikh K, Baker B, et al. Distinguishing true progression from radionecrosis after stereotactic radiation therapy for brain metastases with machine learning and Radiomics. Int J Radiat Oncol Biol Phys 2018; ; 102: 1236–4315. doi: 10.1016/j.ijrobp.2018.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lohmann P, Kocher M, Ceccon G, Bauer EK, Stoffels G, Viswanathan S, et al. Combined FET PET/MRI radiomics differentiates radiation injury from recurrent brain metastasis. Neuroimage Clin 2018; 20: 537–42. doi: 10.1016/j.nicl.2018.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mattonen SA, Palma DA, Johnson C, Louie AV, Landis M, Rodrigues G, et al. Detection of local cancer recurrence after stereotactic ablative radiation therapy for lung cancer: physician performance versus radiomic assessment. Int J Radiat Oncol Biol Phys 2016; 94: 1121–8. doi: 10.1016/j.ijrobp.2015.12.369 [DOI] [PubMed] [Google Scholar]
- 68.Nyflot MJ, Thammasorn P, Wootton LS, Ford EC, Chaovalitwongse WA. Deep learning for patient-specific quality assurance: identifying errors in radiotherapy delivery by radiomic analysis of gamma images with convolutional neural networks. Med Phys 2019; 46: 456–64. doi: 10.1002/mp.13338 [DOI] [PubMed] [Google Scholar]
- 69.Ma C, Wang R, Zhou S, Wang M, Yue H, Zhang Y, et al. The structural similarity index for IMRT quality assurance: radiomics-based error classification. Med Phys 2021; 48: 80-93. doi: 10.1002/mp.14559 [DOI] [PubMed] [Google Scholar]
- 70.Lee JA. Segmentation of positron emission tomography images: some recommendations for target delineation in radiation oncology. Radiother Oncol 2010; 96: 302–7. doi: 10.1016/j.radonc.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 71.He L, Huang Y, Ma Z, Liang C, Liang C, Liu Z. Effects of contrast-enhancement, reconstruction slice thickness and convolution kernel on the diagnostic performance of radiomics signature in solitary pulmonary nodule. Sci Rep 2016; 6: 34921. doi: 10.1038/srep34921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.P C, E RV RL. M J, S C, Rh M, et al. Robust Radiomics feature quantification using semiautomatic volumetric segmentation [Internet. Vol. 9, PloS one. PLoS One 2014;Available from. cited 2020 Dec 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fiset S, Welch ML, Weiss J, Pintilie M, Conway JL, Milosevic M, et al. Repeatability and reproducibility of MRI-based radiomic features in cervical cancer. Radiother Oncol 2019; 135: 107–14. doi: 10.1016/j.radonc.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 74.Traverso A, Kazmierski M, Welch ML, Weiss J, Fiset S, Foltz WD, et al. Sensitivity of radiomic features to inter-observer variability and image pre-processing in apparent diffusion coefficient (ADC) maps of cervix cancer patients. Radiother Oncol 2020; 143: 88–94. doi: 10.1016/j.radonc.2019.08.008 [DOI] [PubMed] [Google Scholar]
- 75.Li YQ, Tan JSH, Wee JTS, Chua MLK, YQ L, , JTS W. Adaptive radiotherapy for head and neck cancers: fact or fallacy to improve therapeutic ratio? Cancer Radiother 2018; 22: 287–95. doi: 10.1016/j.canrad.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 76.Azria D, Riou O, Castan F, Nguyen TD, Peignaux K, Lemanski C, et al. Radiation-Induced CD8 T-lymphocyte apoptosis as a predictor of breast fibrosis after radiotherapy: results of the prospective multicenter French trial. EBioMedicine 2015; 2: 1965–73. doi: 10.1016/j.ebiom.2015.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Limkin EJ, Sun R, Dercle L, Zacharaki EI, Robert C, Reuzé S. Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann Oncol Off J Eur Soc Med Oncol 2017; 28: 1191–206. [DOI] [PubMed] [Google Scholar]
- 78.Elhalawani H, Lin TA, Volpe S, Mohamed ASR, White AL, Zafereo J, et al. Machine learning applications in head and neck radiation oncology: lessons from open-source Radiomics challenges. Front Oncol 2018; 8: 294. doi: 10.3389/fonc.2018.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lubner MG, Smith AD, Sandrasegaran K, Sahani DV, Pickhardt PJ. Ct texture analysis: definitions, applications, biologic correlates, and challenges. Radiographics 2017; 37: 1483–503. doi: 10.1148/rg.2017170056 [DOI] [PubMed] [Google Scholar]
- 80.Zwanenburg A, Vallières M, Abdalah MA, Aerts HJWL, Andrearczyk V, Apte A, et al. The image biomarker standardization initiative: standardized quantitative Radiomics for high-throughput image-based phenotyping. Radiology 2020; 295: 328–38. doi: 10.1148/radiol.2020191145 [DOI] [PMC free article] [PubMed] [Google Scholar]