Abstract
New treatment developments in ovarian cancer have led to a renewed interest in staging advanced ovarian cancer. The treatment of females with ovarian cancer patients has a strong multidisciplinary character with an essential role for the radiologist. This review aims to provide an overview of the current position of CT, positron emission tomography-CT, and MRI in ovarian cancer and how imaging can be used to guide multidisciplinary team discussions.
Introduction
Of all gynecological malignancies, ovarian cancer has the highest mortality rate. For a long time, no structural improvement of long-term survival for advanced-stage ovarian cancer patients had been reported.1 5-year overall survival ranges between 35 and 49%,1–6 and approximately 15% survive the 10-year mark.
A contributing factor to the generally poor survival of patients with ovarian cancer is that most females present with advanced-stage disease (75%), meaning that metastatic spread to the peritoneum has already occurred.7 One of the most important prognostic factors for these patients is whether all macroscopic tumor is removed during cytoreductive surgery (CRS).8 This extensive surgical procedure is performed either upfront (upfront or primary CRS) or after neoadjuvant chemotherapy (interval CRS). The role of neoadjuvant chemotherapy is to reduce the tumor burden and increase the likelihood of complete cytoreduction in more extensive cases.9,10 Recent developments have added new therapeutic options for gynecologic oncologists to consider in the first-line treatment of ovarian cancer. Anti-VEGF therapy (anti-vascular endothelial growth factor) showed improved progression-free survival (PFS) by 4 months in patients with International Federation of Gynecology and Obstetrics (FIGO) III ovarian cancer patients after incomplete CRS and FIGO IV ovarian cancer patients.11 PARP inhibitors (Poly ADP-ribose polymerase) showed a remarkable improvement in PFS in selected patients with BCRA-associated ovarian cancer after response to platinum-based therapy.
In addition, the OVHIPEC trial showed that patients undergoing interval CRS with Stage III ovarian cancer, can benefit from hyperthermic intraperitoneal chemotherapy (HIPEC) with a prolonged recurrence-free survival and overall survival than surgery alone.12 Currently, OVHIPEC-2 has started to evaluate the effect of HIPEC on overall survival in patients with FIGO Stage III epithelial ovarian cancer who are treated with primary CRS (with no or <2.5 mm residual disease after CRS).13 Together with advances in upper abdominal surgical procedures and radical surgery,9,14,15 these new therapeutic options will substantially improve the outcome of patients with advanced-stage ovarian cancer.
However, the recent developments demand a more stringent patient selection to provide a tailored treatment for each patient. Imaging can provide detailed information regarding the extent of the tumor and the location of peritoneal metastases. This is essential for the gynecologic oncologist to determine which therapeutic strategy is more appropriate. This review aims to provide an overview of the current role of imaging in advanced-stage ovarian cancer and how imaging can be used to guide multidisciplinary team (MDT) discussions.
Staging ovarian cancer
Of all malignant tumors originating from the ovaries, epithelial ovarian carcinoma (EOC) occurs most often (≈95%); other tumors include germ cell and sex-cord stromal cell cancer. EOC can be categorized into high grade serous, low grade serous, mucinous, endometrioid, clear cell, transitional, and epithelial-stromal subtypes. Of all subtypes of EOC, high-grade serous is the most common and accounts for the most deaths of all ovarian cancers (70–80%).16 The FIGO is a surgical staging system and provides the internationally recognized standard for staging ovarian cancer17 (Table 1). Advanced stage disease is defined as FIGO Stage IIb-IV, which means that cancer has spread on or into the adjacent pelvic peritoneal tissues, extended to the upper abdomen or outside the abdominal cavity.
Table 1.
2013 FIGO stage classification for ovarian cancer17
| FIGO stage | (TMN) | description |
|---|---|---|
| I | Tumor confined to the ovaries or fallopian tubes. | |
| - A | T1a N0 M0 | A: unilateral |
| - B | T1b N0 M0 | B: bilateral |
| - C | T1c N0 M0 | C: in one or both ovaries or fallopian tubes with (1) intraoperative surgical spill, (2) a ruptured capsule, or (3) malignant cells in the peritoneal washing or ascites |
| II | Primary peritoneal cancer or tumor in one or both ovaries or fallopian tubes with | |
| - A | T2a N0 M0 | A: Extension and/or implants on the uterus and/or fallopian tubes/ovaries |
| - B | T2b N0 M0 | B: Extension to other pelvic intraperitoneal tissues |
| III | Primary peritoneal cancer or tumor in one or both ovaries or fallopian tubes with | |
| - A | T3a NX M0 | A: (1) tumor positive retroperitoneal lymph nodes, (i |
| ) up to 10 mm in largest diameter or (ii) larger and/or (2) microscopic PM above the pelvic brim | ||
| - B | T3b NX M0 | B: macroscopic PM beyond the pelvis up to 2 cm in largest diameter |
| - C | T3c NX M0 | C: macroscopic PM beyond the pelvis more than 2 cm in largest diameter |
| IV | Distant metastases excluding PM | |
| - A | TX NX M1 | A: tumor positive pleural effusion |
| - B | TX NX M1 | B: Parenchymal metastases and metastases to extra abdominal organs (including inguinal lymph nodes and lymph nodes outside of the abdominal cavity) |
In addition to the FIGO and TNM classification, several scoring systems have been developed to predict the most important prognostic factor; whether complete removal of all visible disease during surgery is feasible. The main five scoring systems to predict the completeness of CRS in literature are based on the surgical exploration of the abdominal cavity; Peritoneal Cancer Index (PCI), Predictive Index Value (PIV), Eisenkop, Espada, and Kasper.18 The PCI and the PIV are used most frequently in literature and showed AUCs of 0.69–0.92 and 0.66–0.98 for predicting complete or optimal CRS.18
The Peritoneal Surface Oncology Group International has named the PCI the international standard for peritoneal surface malignancies19 The PCI divides the abdomen into 13 regions. According to the largest diameter of the PM, the PCI is scored in each region as; 0 (no tumor), 1 (tumor 0 > 0.5 cm), 2 (tumor 0.5–5 cm) and 3 (tumor > 5 cm). The PCI score ranges from 0 to 39. PCI is widely adopted in colorectal cancer to guide treatment decisions.
However, according to a survey of the European Network of Gynaecological Oncology Trial, surgical staging by laparoscopy is still only used in a minority of selected patients. In addition, only a minority of centers base their decision to proceed with CRS on a scoring system.19 This is because staging peritoneal disease with laparoscopy has several disadvantages; it is an invasive procedure with a small but significant risk of complications, and assessment of all regions of the abdominal cavity can be hindered by adhesions or tumor.
This means that if imaging can accurately depict peritoneal metastases in a patient friendly and non-invasive manner, then it could play an important role in selecting patients in which complete CRS is possible.
Prediction models derived from CT findings have been extensively researched with limited success.20 Fortunately, several articles reported that MRI has a good diagnostic performance for detecting peritoneal metastases using surgical scoring systems.20–22 Currently, two large multicenter trials (MISSION and MROC) are ongoing to confirm whether MRI can accurately select patients for whom a complete/optimal CRS is feasible.23,24 If these trials show that MRI can accurately predict the extent of disease in the abdomen. In that case, current imaging guidelines will need to change to implement MRI as a non-invasive triaging tool in females with advanced oavarian cancer.
Treatment options for ovarian cancer
Early-stage ovarian carcinoma is treated with a staging procedure to establish the extent of the disease. During this staging procedure, the uterus, ovaries, fallopian tubes, greater omentum, and, if applicable enlarged pelvic and para-aortic lymph nodes are removed, and peritoneal biopsies are obtained from designated sites. Following the pathology result, adjuvant chemotherapy is indicated.
The cornerstone of advanced-stage ovarian cancer treatment is CRS. Macroscopic complete resection leads to the best survival benefit and should be the intent of surgery. A maximum effort of CRS resulting in a residual disease of no more than 1 cm across is typically referred to as “optimal CRS.” It is known to lead to a significant survival benefit in cases where complete cytoreduction cannot be achieved.8,25–27 Whether complete CRS is feasible is determined by the extent of peritoneal involvement and involvement of crucial structures. Surgery, therefore, starts with an open exploration of the peritoneal cavity to assess the extent of the disease. If cytoreduction is deemed feasible, surgery generally includes a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, resection of all peritoneal tumor sites including a peritonectomy, and if necessary (partial) resection of all affected organs and pelvic and para-aortic lymphadenectomy (only in the presence of suspicious lymph nodes28). Whether CRS is feasible depends on local surgical policies and surgical experience. However, the extent of peritoneal involvement can be described systematically by the PCI, which has shown to be highly predictive of surgical outcomes.29–31
Following upfront CRS, six cycles of platinum and taxane-based chemotherapy are advised in advanced ovarian cancer. Alternatively, neoadjuvant chemotherapy (three cycles) interrupted by interval CRS may be administered to reduce the tumor burden and increase the chance to preserve organs, and increase the chances of complete CRS in extensive cases.9 Also, patients at an older age and with a poor performance status may benefit from neoadjuvant chemotherapy to help reduce surgery-related morbidity and mortality.32 However, the role of neoadjuvant chemotherapy regarding its potential survival benefit is under debate as after four trials, the matter remains inconclusive.9,10,33,34 The much-anticipated TRUST trial may help resolve this issue.15
A recent randomized controlled trial reported an 11.8-month increase in overall survival after complete interval CRS with HIPEC compared to complete CRS alone in patients with advanced-stage ovarian cancer with FIGO Stage III.12 The role of HIPEC for patients undergoing primary CRS is currently under investigation.13,35 A meta-analysis of 37 studies revealed an improved overall survival of HIPEC in addition to CRS and chemotherapy for both primary and recurrent ovarian cancer.36 Developments for chemotherapy or targeted therapy in the first-line setting, like dose-dense chemotherapy, the addition of bevacizumab, and intraperitoneal therapy, have yet to show superior to the current standard but are considered acceptable alternatives in selected cases.37–39 For patients with a BRCA mutation, promising results were reported with olaparib which reduced the risk of disease progression or death by 70% compared to placebo at 3 years and has been implemented in current guidelines.40
Current role of imaging in advanced-stage ovarian cancer
Most females with ovarian cancer experience non-specific symptoms (e.g. abdominal pain or discomfort, urinary frequency, weight changes). Therefore, ovarian cancer is often diagnosed on CT while searching for a cause of non-specific symptoms or to evaluate the abdomen after worrisome ultrasound findings.
For the primary tumors, location, size, and invasion of nearby structures can influence treatment decisions. Presence of malignant nodes can also significantly impact the treatment strategy, especially malignant suprarenal lymph nodes, which could be a contraindication for surgery. The extent of peritoneal metastases can also be a contraindication for surgery. The ESMO-ESGO guidelines describe essential features that may contraindicate CRS. These are summarized in Table 2 ; involvement of small bowels, stomach, pelvic wall, and ureter, e.g. could result in a suboptimal CRS.41 The T.R.U.S.T. trial investigators set criteria that contraindicate primary CRS, which are also helpful as a reference for radiologists Table 3). For radiologists, a structured report can help to effectively communicate these important imaging findings, as shown by Chandramohan et al.42 This structured report incorporates findings relevant to estimating the FIGO stage and operability.
Table 2.
T.R.U.S.T. criteria indicating a contraindication for primary CRS and triage to neoadjuvant chemotherapy15
| - Lymph node enlargement above the renal hilum (larger than 10 mm short axis) |
|---|
| - Tumor involvement of the stomach or duodenum |
| - Tumor involvement of the pancreas |
| - Tumor involvement of the celiac trunk, hepatic artery, gastric artery |
| - Extensive tumor involvement of the mesentery and/or small bowel |
CRS, cytoreductive surgery.
Table 3.
ESGO 2017 recommendations for contraindications for CRS
| - Diffuse deep infiltration of the root of small bowel mesentery |
|---|
| - Diffuse carcinomatosis of the small bowel involving such large parts that resection would lead to a short bowel syndrome (remaining bowel < 1.5 m) |
| - Diffuse involvement/deep infiltration of: |
| - Stomach/duodenum |
| - Head or middle part of the pancreas |
| - Involvement of coeliac trunk, hepatic arteries, left gastric artery |
| - Central or multisegmental parenchymal liver metastases |
| - Multiple parenchymal lung metastases (preferably histologically proven) |
| - Non-resectable lymph nodes |
| - Brain metastases |
CRS, cytoreductive surgery.
Although CT is most commonly used to stage ovarian cancer patients, MRI and positron emission tomography (PET)-CT are increasingly used in specialized centers to stage advanced cases. These functional imaging modalities may provide additional information to determine whether a complete CRS is possible and guide further treatment, as mentioned below.
Computed tomography (CT)
On CT imaging, ovarian cancer typically presents as thick-walled cysts with septations. The CT report should address the location, size, and invasion of nearby structuresTable 4. For the nodal status, each lymph node’s size, shape, and border should be evaluated. For evaluating the abdominal nodal status lymph nodes, a short axis cut-off of 1 cm is often used to detect a malignant lymph node. However, CT has a disappointing diagnostic performance for detecting malignant abdominal lymph nodes with a sensitivity of 41% and specificity of 89%.43 This means that the decision to perform an infrarenal lymphadenectomy should not be solely based on CT imaging but also on pre-operative findings.43 Interestingly, for malignant cardiophrenic lymph nodes, size criteria seem to be more useful; a short-axis diameter of >7 mm had a positive predictive value of 86%.44 The presence of malignant abdominal lymph nodes is not the only feature that is being underestimated with CT. CT also structurally underestimates the presence of peritoneal metastases. In a recent meta-analysis, CT had a pooled sensitivity, specificity, and diagnostic odds ratio for the detection of PM for region-based studies of 68% (CI, 46–84%), 88% (CI, 81–93%), and 15.9 (CI, 4.4–58.0), respectively.45 Especially, the involvement of gastrointestinal organs and the mesentery can be difficult to recognize on CT. The presence of ascites can further impede an accurate overview of PM on CT images. However, CT seems to be relatively accurate in predicting diaphragm and omental involvement. Due to these shortcomings of CT, CT cannot accurately predict a (sub)optimal cytoreduction. Axtell et al demonstrated that CT has a sensitivity of 79% and specificity of 75% for predicting an optimal cytoreduction..46 Other studies showed similar disappointing results for the performance of CT for predicting surgical outcome.47,48 The PCI is structurally underestimated on CT images, and therefore it is not recommended to report the PCI in the CT report.49 CT results should be used cautiously when deciding between primary CRS and neoadjuvant chemotherapy.46 However, interpreting the literature on CT in ovarian cancer is challenging because the diagnostic performance of CT features varies significantly between studies, probably reflecting variations in surgical/radiological practice, experience, and technique.
In current clinical practice, determining response to chemotherapy is done by subjective measures on CT images because Response Evaluation Criteria in Solid Tumors v. 1.1 (RECIST 1.1) is of limited prognostic value for ovarian cancer as it is not associated with PFS nor overall survival.50,51 Therefore, RECIST is often supplemented by serum cancer antigen 125 (CA125) response. It should be noted that neither response assessment methods should determine eligibility for interval CRS as 73% of patients with RECIST stable disease and 49% of patients with no CA125 response received complete or optimal interval CRS.50
Positron emission tomography with 2-deoxy-2-fluorine-18-flu-D-glucose (18FDG-PET)
18FDG-PET imaging is not recommended for primary detection of ovarian cancer. In the ESGO/ISUOG/IOTA/ESGE Consensus Statement on pre-operative diagnosis of ovarian tumors, it stated that PET-CT cannot differentiate reliably between borderline and benign tumors. In addition, due to the low FDG uptake in clear cell and mucinous invasive subtypes PET-CT has a disappointing diagnostic performance for detecting the primary ovarian tumor. On the other hand the specificity of FDG-PET-CT for ovarian cancer is 78% due to false-positive results in endometriosis and hydrosalpinges.52 Another known pitfall is the FDG uptake in the late follicular to early luteal cysts in pre-menopausal females.53 There is also no clear cut-off value for maximum standardized uptake value for differentiation between benign and malignant ovarian tumors, although in general, there is higher FDG uptake in malignant lesions compared to benign or borderline lesions.54 Therefore, in ovarian FDG uptake in pre-menopausal females, the patient’s menstrual status, phase, ultrasound features, and tumor markers should be considered. Despite these drawbacks, PET-CT outperforms CT in detecting malignant lymph nodes, peritoneal metastases, and recurrent disease.55–57 Yuan et al reported similar results that FDG-PET-CT was more accurate than CT or MRI in detecting malignant lymph nodes. FDG-PET-CT had a sensitivity of 73% and a specificity of 97%, whereas those for CT were 43 and 95%, respectively, and those for MRI were 55 and 88%, respectively.58 In a recent meta-analysis, 18FDG-PET had a pooled sensitivity, specificity, and diagnostic odds ratio for the detection of PM for region-based studies of 80% (CI, 57–92%), 90% (CI, 80–96%), and 36.5 (CI, 6.7–199.5), respectively.45 Although this is better than CT, the problem with 18FDG-PET is its radiation exposure, higher cost, and limited depiction of small (peritoneal) tumor volumes. Especially, the limited depiction of small tumor volumes hinder accurate staging of (small) peritoneal disease. This means that PET-CT is accurate in predicting the presence of peritoneal disease but fails to detect small peritoneal metastases. This was also confirmed in a recent study of Lopez-Lopez et al, which suggested that the main utility of 18F-FDG-PET-CT is to evaluate a possible metastatic extraperitoneal spread of the disease in the work-up.59
Another indication for FDG-PET-CT seems to be to detect recurrent disease. A meta-analysis showed that PET-CT compared to CT and MRI is relatively accurate in detecting recurrent disease with a sensitivity and specificity of 91 and 88%, respectively.60 Lymph nodes were the most frequent site of relapse.61 Another advantage of PET-CT is that post-treatment changes, like fibrosis, do not show FDG uptake. This means that PET-CT may play a role in the follow up of females with the suspicion of recurrent disease.
In the future, PET/MRI may be an option for hybrid molecular anatomic imaging due to its high soft-tissue contrast and lower radiation dose. Also, other PET radiotracers, like such as 11C-methionine (MET) and 3′-deoxy-3′−18F-fluorothymidine (FLT),) might further improve the diagnostic performance of PET-CT.62 Although most data are from animal studies, new radiotracers can potentially improve the staging of ovarian cancer. However, these radiotracers are not yet used in clinics, and more research is needed to clarify their role in the diagnostic work-up of ovarian cancer patients.
Magnetic resonance imaging (MRI)
The characteristics of ovarian cancer on MR imaging are partly similar to CT; cystic lesions with septa and solid components. A septal or wall thickness of >3 mm, nodularity, papillary projections of >4 cm, and necrosis are features linked to ovarian cancer.63 Additional features of malignancy include involvement of pelvic organs or sidewall; peritoneal disease; ascites; and lymphadenopathy. A recent meta-analysis showed that MRI had a sensitivity of 91% and specificity of 85% for the diagnosis of ovarian cancer.64 This means that MRI outperforms CT and PET-CT for detecting ovarian cancer. MRI dynamic contrast-enhanced and diffusion-weighted MRI may therefore be used as a second-line tool after ultrasonography to further differentiate between benign, malignant, and borderline masses.65
However, like all imaging modalities, identifying abdominal malignant lymph nodes remains a problem for MRI with a sensitivity and specificity of 77 and 91%, respectively.
One of the advantages of MRI is the use of functional imaging techniques like diffusion-weighted (DWI) sequences to depict small peritoneal metastases (Figure 1). Several studies demonstrate that DWI-MRI is reliable to depict and quantify peritoneal metastases using the PCI.66,67 If the ongoing large multicenter studies (MISSION and MROC) confirm these promising results than this could lead to a paradigm shift in the diagnostic work-up of ovarian cancer patient23,24 (ref). MRI could become the standard imaging tool for advanced ovarian cancer to select those patients in which a completer CRS is possible. In order to detect all small peritoneal metastases on DWI images. it is essential to avoid the high signal in normal bowels and feces. Therefore, the use of pineapple juice prior to the examination is strongly advocated. Patients need to drink at least 1 liter pineapple juice 1 h prior to the examination to make sure that its manganese content results in a low signal on DWI images within the bowels. In this way, peritoneal metastases are more easily detected on DWI images. In addition to subjective evaluation of DW-MRI, a more quantitative approach using quantitative Kurtosis variables and apparent diffusion coefficient values could help in discriminating benign and malignant ovarian lesions.68 No concrete quantitative MR criteria are widely adopted in the daily clinic. Another advantage of MRI is that ascites does not hamper with visibility of nodular peritoneal metastases unlike CT. This might explain the promising results of MRI in staging the peritoneal disease. In the same meta-analysis which compared CT, PET-CT, and MRI for detecting peritoneal metastases, MRI had the highest pooled regionwise sensitivity and specificity of 92 and 84%, respectively.45 The additional value of MRI was also demonstrated in a study by Michielsen et al, which showed that MRI was superior to CT for primary tumor characterization and staging and the prediction of whether the extent of peritoneal metastases made a suboptimal resection feasible. In this study, MRI had a sensitivity of 94%, a specificity of 97.7%, and overall accuracy of 95% for predicting a suboptimal resection.69 Three other prospective cohort studies showed similar results; MRI was accurate in predicting surgical outcome, reporting area under the curves (AUCs) of 0.88–0.95 (Figure 2). Two multicenter studies are currently ongoing to define the role of MRI in females with advanced ovarian cancer.23,24 If these studies confirm the promising results, then a paradigm shift is imminent; females with advanced ovarian cancer could be individually stratified to their appropriate treatment according to their clinical and MR risk factors.
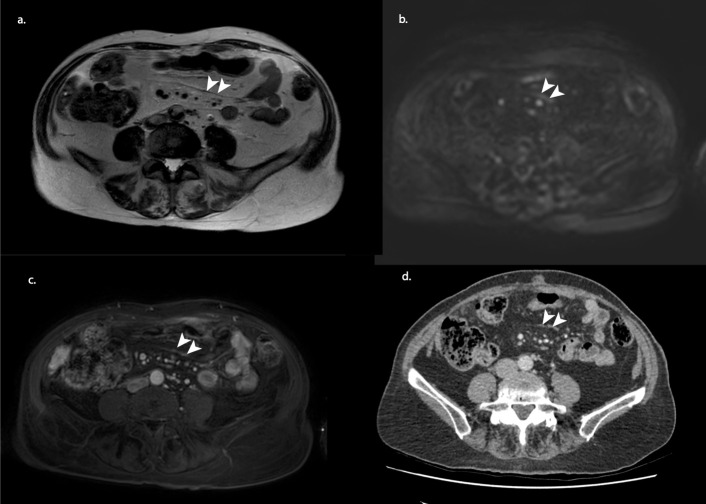
Figure 1.
MRI staging example of an 80-year-old female after three courses of neoadjuvant chemotherapy. T2 weighted (a) shows a suspicious dark thickening of the mesenteries (arrowheads), which showed diffusion restriction and contract enhancement on b1000 diffusion-weighted (b) and gadolinium-enhanced T1 weighted (c) imaging. At CRS, millimetric depositions were found on the mesenteric surface. A CT of 3 weeks earlier showed a similar pattern (arrowheads); however, with CT alone, no confident distinction can be made between ascites, fibrosis or PM. Therefore, the involvement of the mesenteries was not mentioned in the original CT report. CRS, cytoreductive surgery.
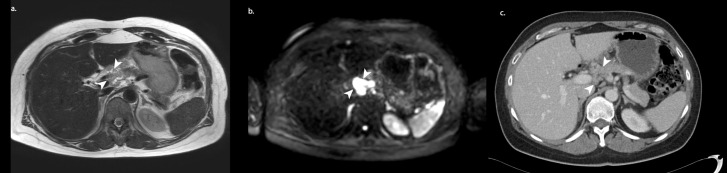
Figure 2.
MRI staging example of a 56-year-old female after three courses of neoadjuvant chemotherapy. On diffusion-weighted imaging (b), a conspicuous lesion (arrowheads) is found in the area of the hepatic hilum. On T2 weighted imaging, the lesion can be distinguished as malignant. With CT (2 weeks earlier) alone, this large lesion largely “fades“ into its surroundings, making it difficult to spot and was therefore not mentioned in the original CT report. This extensive disease in the liver hilum, among others, rendered the patient inoperable.
Future prospective
There are some promising studies that suggest that FDG-PET-MRI is superior to DW-MRI in estimating total spread of carcinomatosis in gynecological cancer.70 By combining the two functional imaging techniques, PET-MRI has the potential to be most accurate in peritoneal and lymph node metastases. In addition, it may help differentiate borderline from malignant ovarian tumors.71 However, cohort studies are small and PET-MRI is not widely available. More research is needed to clarify the role of MRI-PET in the work-up of ovarian cancer patients.
To further support the subjective image assessment by the radiologist, emerging advanced image analysis methods, like radiomics and artificial intelligence, may expose high-order imaging patterns that correlate with survival or genetic signatures. For example, Rizzo and colleagues demonstrated that from ovarian masses of 101 high-grade serous ovarian cancer patients, certain radiomic features from CT were associated with incomplete CRS and disease progression within 12 months.72 Similarly, an MRI radiomic model was associated with ovarian cancer stage and overall survival ; it could provide survival estimations with high accuracy. Vargas and colleagues showed that by segmenting all detectable sites of ovarian cancer, intersite heterogeneity could be characterized by radiomic features associated with overall survival and incomplete CRS.73 Other studies have also demonstrated associations between radiomics features and response to immunotherapy and histological subtypes.74,75 With complex feature selection techniques using deep-learning methods of CT imaging, a radiomic model was predictive of PFS and showed AUCs of 0.77–0.83 in the test and two validation cohorts.76 These studies show that it is possible to extract diagnostic and prognostic information from CT and MRI images which could help guide treatment strategies and provide an objective response to therapy for future clinical trials. This new field of radiology is rapidly evolving, and these new exciting ways of interpreting data could revolutionize the work-up of ovarian cancer patients in the future.
Conclusion
CT will remain the first step in the diagnostic work-up of ovarian cancer. However, due to new developments, especially in MRI, radiologists can now report imaging features that help the gynecologist to select patients where a complete cytoreduction can be achieved. To this end, MRI may shift from a problem-solving to a more central imaging tool for females with advanced ovarian cancer. MRI could help to individualize the treatment of females with ovarian cancer, and thus hopefully lead to a structural improvement of long-term survival for females with ovarian cancer. Currently, there is no universal definition of whether a CRS is feasible and it depends on patient features, local treatment policies and surgical experience of the surgical team. Therefore, MDT meetings will remain the cornerstone in treating females with ovarian cancer.
Contributor Information
Maurits Peter Engbersen, Email: m.engbersen@nki.nl.
Willemien Van Driel, Email: w.v.driel@nki.nl.
Doenja Lambregts, Email: d.lambregts@nki.nl.
Max Lahaye, Email: m.lahaye@nki.nl.
REFERENCES
- 1.Timmermans M, Sonke GS, Van de Vijver KK, van der Aa MA, Kruitwagen RFPM. No improvement in long-term survival for epithelial ovarian cancer patients: a population-based study between 1989 and 2014 in the Netherlands. Eur J Cancer 2018; 88: : 31–7p.. doi: 10.1016/j.ejca.2017.10.030 [DOI] [PubMed] [Google Scholar]
- 2.Sant M, Chirlaque Lopez MD, Agresti R, Sánchez Pérez MJ, Holleczek B, Bielska-Lasota M, et al. Survival of women with cancers of breast and genital organs in Europe 1999-2007: results of the EUROCARE-5 study. Eur J Cancer 2015; 51): : 2191–205p.. doi: 10.1016/j.ejca.2015.07.022 [DOI] [PubMed] [Google Scholar]
- 3.Trétarre B, Molinié F, Woronoff A-S, Bossard N, Bessaoud F, Marrer E, et al. Ovarian cancer in France: trends in incidence, mortality and survival, 1980-2012. Gynecol Oncol 2015; 139): : 324–9p.. doi: 10.1016/j.ygyno.2015.09.013 [DOI] [PubMed] [Google Scholar]
- 4.Chen T, Jansen L, Gondos A, Emrich K, Holleczek B, Katalinic A, et al. Survival of ovarian cancer patients in Germany in the early 21st century: a period analysis by age, histology, laterality, and stage. Eur J Cancer Prev 2013; 22): : 59–67p.. doi: 10.1097/CEJ.0b013e3283552e28 [DOI] [PubMed] [Google Scholar]
- 5.Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the International cancer benchmarking partnership): an analysis of population-based cancer registry data. Lancet 2011; 377): : 127–38p.. doi: 10.1016/S0140-6736(10)62231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Institute, N.C. SEER Cancer Stat Facts: Ovarian Cancer.. Available from: https://seer.cancer.gov/statfacts/html/ovary.html.
- 7.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol 2010; 177): : 1053–64p.. doi: 10.2353/ajpath.2010.100105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux pour les Etudes des cancers de l'Ovaire (GINECO. Cancer 2009; 115: : 1234p.. doi: 10.1002/cncr.24149 [DOI] [PubMed] [Google Scholar]
- 9.Fagotti A, Ferrandina MG, Vizzielli G, Pasciuto T, Fanfani F, Gallotta V, et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850. Int J Gynecol Cancer 2020; 30): : 1657–64p.. doi: 10.1136/ijgc-2020-001640 [DOI] [PubMed] [Google Scholar]
- 10.Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010; 363): : 943–53p.. doi: 10.1056/NEJMoa0908806 [DOI] [PubMed] [Google Scholar]
- 11.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011; 365): : 2473–83p.. doi: 10.1056/NEJMoa1104390 [DOI] [PubMed] [Google Scholar]
- 12.van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med 2018; 378): : 230–40p.. doi: 10.1056/NEJMoa1708618 [DOI] [PubMed] [Google Scholar]
- 13.Koole S, van Stein R, Sikorska K, Barton D, Perrin L, Brennan D, et al. Primary cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) for FIGO stage III epithelial ovarian cancer: OVHIPEC-2, a phase III randomized clinical trial. Int J Gynecol Cancer 2020; 30): : 888–92p.. doi: 10.1136/ijgc-2020-001231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope CR, Wilson TO, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol 2006; 107): : 77–85p.. doi: 10.1097/01.AOG.0000192407.04428.bb [DOI] [PubMed] [Google Scholar]
- 15.Reuss A, du Bois A, Harter P, Fotopoulou C, Sehouli J, Aletti G, et al. Trust: trial of radical upfront surgical therapy in advanced ovarian cancer (ENGOT ov33/AGO-OVAR OP7. Int J Gynecol Cancer 2019; 29): : 1327–31p.. doi: 10.1136/ijgc-2019-000682 [DOI] [PubMed] [Google Scholar]
- 16.Lisio M-A, Fu L, Goyeneche A, Gao Z-H, Telleria C. High-Grade serous ovarian cancer: basic sciences, clinical and therapeutic Standpoints. Int J Mol Sci 2019; 20): : 952p.. doi: 10.3390/ijms20040952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prat, J. and F. C.o.G. Oncology, Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 2014; 124): : 1–5p.. [DOI] [PubMed] [Google Scholar]
- 18.Engbersen MP, Lahaye MJ, Lok CAR, Koole SN, Sonke GS, Beets-Tan RGH, et al. Peroperative scoring systems for predicting the outcome of cytoreductive surgery in advanced-stage ovarian cancer - A systematic review. Eur J Surg Oncol 2021; 47: 1856-1861. doi: 10.1016/j.ejso.2021.03.233 [DOI] [PubMed] [Google Scholar]
- 19.Grotz TE, Fournier KF, Mansfield PF. Patient selection for cytoreductive surgery. Surg Oncol Clin N Am 2018; 27): : 443–62p.. doi: 10.1016/j.soc.2018.02.012 [DOI] [PubMed] [Google Scholar]
- 20.Engbersen M, et al. Whole-Body diffusion weighted MR-imaging (DW-MRI) to predict a complete resection in ovarian cancer patients; a pilot study. Pleura and Peritoneum 2018; 3: : sA36.p.. [Google Scholar]
- 21.Espada M, Garcia-Flores JR, Jimenez M, Alvarez-Moreno E, De Haro M, Gonzalez-Cortijo L, et al. Diffusion-Weighted magnetic resonance imaging evaluation of intra-abdominal sites of implants to predict likelihood of suboptimal cytoreductive surgery in patients with ovarian carcinoma. Eur Radiol 2013; 23): : 2636–42p.. doi: 10.1007/s00330-013-2837-7 [DOI] [PubMed] [Google Scholar]
- 22.Kasper SM, Dueholm M, Marinovskij E, Blaakær J. Imaging diagnostics in ovarian cancer: magnetic resonance imaging and a scoring system guiding choice of primary treatment. Eur J Obstet Gynecol Reprod Biol 2017; 210: : 83–9p.. doi: 10.1016/j.ejogrb.2016.10.034 [DOI] [PubMed] [Google Scholar]
- 23.The impact of multiparametric MRI on the staging and management of patients with suspected or confirmed ovarian cancer.2015 20/11/2018 Available from. Available from: http://www.isrctn.com/ISRCTN51246892..
- 24.Clinical Impact of Dedicated MR Staging of Ovarian Cancer.. Available from: https://ClinicalTrials.gov/show/NCT03399344.
- 25.Rutten MJ, Sonke GS, Westermann AM, van Driel WJ, Trum JW, Kenter GG, et al. Prognostic value of residual disease after interval debulking surgery for FIGO stage IIIC and IV epithelial ovarian cancer. Obstet Gynecol Int 2015;. . 2015: 4641232015: p. doi: 10.1155/2015/464123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timmermans M, van der Hel O, Sonke GS, Van de Vijver KK, van der Aa MA, Kruitwagen RF. The prognostic value of residual disease after neoadjuvant chemotherapy in advanced ovarian cancer; a systematic review. Gynecol Oncol 2019; 153): : 445–51p.. doi: 10.1016/j.ygyno.2019.02.019 [DOI] [PubMed] [Google Scholar]
- 27.Polterauer S, Vergote I, Concin N, Braicu I, Chekerov R, Mahner S, et al. Prognostic value of residual tumor size in patients with epithelial ovarian cancer FIGO stages IIA-IV: analysis of the OVCAD data. Int J Gynecol Cancer 2012; 22): : 380–5p.. doi: 10.1097/IGC.0b013e31823de6ae [DOI] [PubMed] [Google Scholar]
- 28.Harter P, Sehouli J, Lorusso D, Reuss A, Vergote I, Marth C, et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N Engl J Med 2019; 380): : 822–32p.. doi: 10.1056/NEJMoa1808424 [DOI] [PubMed] [Google Scholar]
- 29.Jonsdottir B, et al. The peritoneal cancer index is a strong predictor of incomplete cytoreductive surgery in ovarian cancer. Ann Surg Oncol 2020;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosendahl M, Harter P, Bjørn SF, Høgdall C. Specific regions, rather than the entire peritoneal Carcinosis index, are predictive of complete resection and survival in advanced epithelial ovarian cancer. Int J Gynecol Cancer 2018; 28): : 316–22p.. doi: 10.1097/IGC.0000000000001173 [DOI] [PubMed] [Google Scholar]
- 31.Llueca A, Serra A, Rivadulla I, Gomez L, Escrig J, .MUAPOS working group (Multidisciplinary Unit of Abdominal Pelvic Oncology Surgery) . Prediction of suboptimal cytoreductive surgery in patients with advanced ovarian cancer based on preoperative and intraoperative determination of the peritoneal carcinomatosis index. World J Surg Oncol 2018; 16: 37p.. doi: 10.1186/s12957-018-1339-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aletti GD, Santillan A, Eisenhauer EL, Hu J, Aletti G, Podratz KC, et al. A new frontier for quality of care in gynecologic oncology surgery: multi-institutional assessment of short-term outcomes for ovarian cancer using a risk-adjusted model. Gynecol Oncol 2007; 107): : 99–106p.. doi: 10.1016/j.ygyno.2007.05.032 [DOI] [PubMed] [Google Scholar]
- 33.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (chorus): an open-label, randomised, controlled, non-inferiority trial. Lancet 2015; 386): : 249–57p.. doi: 10.1016/S0140-6736(14)62223-6 [DOI] [PubMed] [Google Scholar]
- 34.Onda T, Satoh T, Ogawa G, Saito T, Kasamatsu T, Nakanishi T, et al. Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomised trial. Eur J Cancer 2020; 130: : 114–25p.. doi: 10.1016/j.ejca.2020.02.020 [DOI] [PubMed] [Google Scholar]
- 35.Lim MC, Chang S-J, Yoo HJ, Nam B-H, Bristow R, Park S-Y. Randomized trial of hyperthermic intraperitoneal chemotherapy (HIPEC) in women with primary advanced peritoneal, ovarian, and tubal cancer. Journal of Clinical Oncology 2017; 35(15_suppl): 5520. doi: 10.1200/JCO.2017.35.15_suppl.5520 [DOI] [Google Scholar]
- 36.Huo YR, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) in ovarian cancer: a systematic review and meta-analysis. European Journal of Surgical Oncology 2015; 41): : 1578–89p.. [DOI] [PubMed] [Google Scholar]
- 37.Chan JK, Brady MF, Penson RT, Huang H, Birrer MJ, Walker JL, et al. Weekly vs. Every-3-Week paclitaxel and carboplatin for ovarian cancer. N Engl J Med 2016; 374): : 738–48p.. doi: 10.1056/NEJMoa1505067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-Dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 2009; 374): : 1331–8p.. doi: 10.1016/S0140-6736(09)61157-0 [DOI] [PubMed] [Google Scholar]
- 39.Walker JL, Brady MF, Wenzel L, Fleming GF, Huang HQ, DiSilvestro PA, et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: an NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol 2019; 37): : 1380–90p.. doi: 10.1200/JCO.18.01568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore K, Colombo N, Scambia G, Kim B-G, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018; 379): : 2495–505p.. doi: 10.1056/NEJMoa1810858 [DOI] [PubMed] [Google Scholar]
- 41.Bristow RE, Duska LR, Lambrou NC, Fishman EK, O'Neill MJ, Trimble EL, et al. A model for predicting surgical outcome in patients with advanced ovarian carcinoma using computed tomography. Cancer 2000; 89): : 1532–40p.. doi: [DOI] [PubMed] [Google Scholar]
- 42.Chandramohan A, et al. Management driven structured reporting in ovarian cancer. Journal of Gastrointestinal and Abdominal Radiology 2020; 03): : 153–62p.. [Google Scholar]
- 43.Widschwendter P, Blersch A, Friedl TWP, Janni W, Kloth C, de Gregorio A, et al. CT Scan in the Prediction of Lymph Node Involvement in Ovarian Cancer - a Retrospective Analysis of a Tertiary Gyneco-Oncological Unit. Geburtshilfe Frauenheilkd 2020; 80): : 518–25p.. doi: 10.1055/a-1079-5158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim T-H, Lim MC, Kim SI, Seo S-S, Kim SH, Park S-Y. Preoperative prediction of cardiophrenic lymph node metastasis in advanced ovarian cancer using computed tomography. Ann Surg Oncol 2016; 23): : 1302–8p.. doi: 10.1245/s10434-015-5015-0 [DOI] [PubMed] [Google Scholar]
- 45.van 't Sant I, Engbersen MP, Bhairosing PA, Lambregts DMJ, Beets-Tan RGH, van Driel WJ, et al. Diagnostic performance of imaging for the detection of peritoneal metastases: a meta-analysis. Eur Radiol 2020; 30: 3101–12p.. doi: 10.1007/s00330-019-06524-x [DOI] [PubMed] [Google Scholar]
- 46.Axtell AE, Lee MH, Bristow RE, Dowdy SC, Cliby WA, Raman S, et al. Multi-Institutional reciprocal validation study of computed tomography predictors of suboptimal primary cytoreduction in patients with advanced ovarian cancer. J Clin Oncol 2007; 25): : 384–9p.. doi: 10.1200/JCO.2006.07.7800 [DOI] [PubMed] [Google Scholar]
- 47.Rutten MJ, van de Vrie R, Bruining A, Spijkerboer AM, Mol BW, Kenter GG, et al. Predicting surgical outcome in patients with international Federation of gynecology and obstetrics stage III or IV ovarian cancer using computed tomography: a systematic review of prediction models. Int J Gynecol Cancer 2015; 25): : 407–15p.. doi: 10.1097/IGC.0000000000000368 [DOI] [PubMed] [Google Scholar]
- 48.Rutten IJG, van de Laar R, Kruitwagen RFPM, Bakers FCH, Ploegmakers MJM, Pappot TWF, et al. Prediction of incomplete primary debulking surgery in patients with advanced ovarian cancer: an external validation study of three models using computed tomography. Gynecol Oncol 2016; 140): : 22–8p.. doi: 10.1016/j.ygyno.2015.11.022 [DOI] [PubMed] [Google Scholar]
- 49.van 't Sant I, van Eden WJ, Engbersen MP, Kok NFM, Woensdregt K, Lambregts DMJ, et al. Diffusion-Weighted MRI assessment of the peritoneal cancer index before cytoreductive surgery. Br J Surg 2019; 106: 491–8p.. doi: 10.1002/bjs.10989 [DOI] [PubMed] [Google Scholar]
- 50.Morgan RD, McNeish IA, Cook AD, James EC, Lord R, Dark G, et al. Objective responses to first-line neoadjuvant carboplatin-paclitaxel regimens for ovarian, fallopian tube, or primary peritoneal carcinoma (ICON8): post-hoc exploratory analysis of a randomised, phase 3 trial. Lancet Oncol 2021; 22): : 277–88p.. doi: 10.1016/S1470-2045(20)30591-X [DOI] [PubMed] [Google Scholar]
- 51.Bogani G, Matteucci L, Tamberi S, Ditto A, Sabatucci I, Murgia F, et al. RECIST 1.1 criteria predict recurrence-free survival in advanced ovarian cancer submitted to neoadjuvant chemotherapy. Eur J Obstet Gynecol Reprod Biol 2019; 237: : 93–9p.. doi: 10.1016/j.ejogrb.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 52.Rieber A, Nüssle K, Stöhr I, Grab D, Fenchel S, Kreienberg R, et al. Preoperative diagnosis of ovarian tumors with MR imaging: comparison with transvaginal sonography, positron emission tomography, and histologic findings. AJR Am J Roentgenol 2001; 177: : 123p.. doi: 10.2214/ajr.177.1.1770123 [DOI] [PubMed] [Google Scholar]
- 53.Nishizawa S, Inubushi M, Okada H. Physiological 18F-FDG uptake in the ovaries and uterus of healthy female volunteers. Eur J Nucl Med Mol Imaging 2005; 32): : 549–56p.. doi: 10.1007/s00259-004-1703-x [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto Y, Oguri H, Yamada R, Maeda N, Kohsaki S, Fukaya T. Preoperative evaluation of pelvic masses with combined 18F-fluorodeoxyglucose positron emission tomography and computed tomography. Int J Gynaecol Obstet 2008; 102): : 124–7p.. doi: 10.1016/j.ijgo.2008.02.019 [DOI] [PubMed] [Google Scholar]
- 55.Kitajima K, Murakami K, Yamasaki E, Kaji Y, Fukasawa I, Inaba N, et al. Diagnostic accuracy of integrated FDG-PET/contrast-enhanced CT in staging ovarian cancer: comparison with enhanced CT. Eur J Nucl Med Mol Imaging 2008; 35): : 1912–20p.. doi: 10.1007/s00259-008-0890-2 [DOI] [PubMed] [Google Scholar]
- 56.Kitajima K, Murakami K, Yamasaki E, Domeki Y, Kaji Y, Fukasawa I, et al. Performance of integrated FDG-PET/contrast-enhanced CT in the diagnosis of recurrent ovarian cancer: comparison with integrated FDG-PET/non-contrast-enhanced CT and enhanced CT. Eur J Nucl Med Mol Imaging 2008; 35): : 1439–48p.. doi: 10.1007/s00259-008-0776-3 [DOI] [PubMed] [Google Scholar]
- 57.Tawakol A, Abdelhafez YG, Osama A, Hamada E, El Refaei S. Diagnostic performance of 18F-FDG PET/contrast-enhanced CT versus contrast-enhanced CT alone for post-treatment detection of ovarian malignancy. Nucl Med Commun 2016; 37): : 453–60p.. doi: 10.1097/MNM.0000000000000477 [DOI] [PubMed] [Google Scholar]
- 58.Yuan Y, Gu Z-X, Tao X-F, Liu S-Y. Computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with ovarian cancer: a meta-analysis. Eur J Radiol 2012; 81): : 1002–6p.. doi: 10.1016/j.ejrad.2011.01.112 [DOI] [PubMed] [Google Scholar]
- 59.Lopez-Lopez V, Cascales-Campos PA, Gil J, Frutos L, Andrade RJ, Fuster-Quiñonero M, et al. Use of (18)F-FDG PET/CT in the preoperative evaluation of patients diagnosed with peritoneal carcinomatosis of ovarian origin, candidates to cytoreduction and hipec. A pending issue. Eur J Radiol 2016; 85): : 1824–8p.. doi: 10.1016/j.ejrad.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 60.Gu P, Pan L-L, Wu S-Q, Sun L, Huang G. Ca 125, PET alone, PET-CT, CT and MRI in diagnosing recurrent ovarian carcinoma: a systematic review and meta-analysis. Eur J Radiol 2009; 71): : 164–74p.. doi: 10.1016/j.ejrad.2008.02.019 [DOI] [PubMed] [Google Scholar]
- 61.Dragosavac S, Derchain S, Caserta NMG, DE Souza G. Staging recurrent ovarian cancer with (18)FDG PET/CT. Oncol Lett 2013; 5): : 593–7p.. doi: 10.3892/ol.2012.1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aide N, Kinross K, Cullinane C, Roselt P, Waldeck K, Neels O, et al. 18F-FLT PET as a surrogate marker of drug efficacy during mTOR inhibition by everolimus in a preclinical cisplatin-resistant ovarian tumor model. J Nucl Med 2010; 51): : 1559–64p.. doi: 10.2967/jnumed.109.073288 [DOI] [PubMed] [Google Scholar]
- 63.Tsili AC, Tsampoulas C, Charisiadi A, Kalef-Ezra J, Dousias V, Paraskevaidis E, et al. Adnexal masses: accuracy of detection and differentiation with multidetector computed tomography. Gynecol Oncol 2008; 110: : 22p.. doi: 10.1016/j.ygyno.2008.03.022 [DOI] [PubMed] [Google Scholar]
- 64.Dai G, et al. A meta-analysis on the diagnostic value of diffusion-weighted imaging on ovarian cancer. Journal of BU on. Official Journal of the Balkan Union of Oncology 2019; 24): : 2333–40p.. [PubMed] [Google Scholar]
- 65.Timmerman D, Planchamp F, Bourne T, Landolfo C, du Bois A, Chiva L, et al. ESGO/ISUOG/IOTA/ESGE consensus statement on pre-operative diagnosis of ovarian tumors. Int J Gynecol Cancer 2021; 31: 961-982.p. ijgc-2021-002565. doi: 10.1136/ijgc-2021-002565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia Prado J, González Hernando C, Varillas Delgado D, Saiz Martínez R, Bhosale P, Blazquez Sanchez J, et al. Diffusion-Weighted magnetic resonance imaging in peritoneal carcinomatosis from suspected ovarian cancer: diagnostic performance in correlation with surgical findings. Eur J Radiol 2019; 121: : 108696p.. doi: 10.1016/j.ejrad.2019.108696 [DOI] [PubMed] [Google Scholar]
- 67.Engbersen MP, Van' T Sant I, Lok C, Lambregts DMJ, Sonke GS, Beets-Tan RGH, et al. Mri with diffusion-weighted imaging to predict feasibility of complete cytoreduction with the peritoneal cancer index (PCI) in advanced stage ovarian cancer patients. Eur J Radiol 2019; 114: : 146–51p.. doi: 10.1016/j.ejrad.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 68.Mokry T, Mlynarska-Bujny A, Kuder TA, Hasse FC, Hog R, Wallwiener M, et al. Ultra-High-b-Value Kurtosis Imaging for Noninvasive Tissue Characterization of Ovarian Lesions. Radiology 2020; 296): : 358–69p.. doi: 10.1148/radiol.2020191700 [DOI] [PubMed] [Google Scholar]
- 69.Michielsen K, Dresen R, Vanslembrouck R, De Keyzer F, Amant F, Mussen E, et al. Diagnostic value of whole body diffusion-weighted MRI compared to computed tomography for pre-operative assessment of patients suspected for ovarian cancer. Eur J Cancer 2017; 83: : 88–98p.. doi: 10.1016/j.ejca.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 70.Jónsdóttir B, Ripoll MA, Bergman A, Silins I, Poromaa IS, Ahlström H, et al. Validation of 18F-FDG PET/MRI and diffusion-weighted MRI for estimating the extent of peritoneal carcinomatosis in ovarian and endometrial cancer -a pilot study. Cancer Imaging 2021; 21): : 34.p.. doi: 10.1186/s40644-021-00399-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jung DC, Choi HJ, Ju W, Kim SC, Choi K-G. Discordant MRI/FDG-PET imaging for the diagnosis of borderline ovarian tumors. Int J Gynecol Cancer 2008; 18): : 637–41p.. doi: 10.1111/j.1525-1438.2007.01116.x [DOI] [PubMed] [Google Scholar]
- 72.Rizzo S, Botta F, Raimondi S, Origgi D, Buscarino V, Colarieti A, et al. Radiomics of high-grade serous ovarian cancer: association between quantitative CT features, residual tumour and disease progression within 12 months. Eur Radiol 2018; 28): : 4849–59p.. doi: 10.1007/s00330-018-5389-z [DOI] [PubMed] [Google Scholar]
- 73.Vargas HA, Veeraraghavan H, Micco M, Nougaret S, Lakhman Y, Meier AA, et al. A novel representation of inter-site tumour heterogeneity from pre-treatment computed tomography textures classifies ovarian cancers by clinical outcome. Eur Radiol 2017; 27): : 3991–4001p.. doi: 10.1007/s00330-017-4779-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Himoto Y, et al. Computed Tomography–Derived radiomic metrics can identify responders to immunotherapy in ovarian cancer. JCO Precision Oncology 2019; 3): : 1–13p.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu H, Ai Y, Zhang J, Zhang J, Jin J, Xie C, et al. Preoperative nomogram for differentiation of histological subtypes in ovarian cancer based on computer tomography Radiomics. Front Oncol 2021; 11: 642892. doi: 10.3389/fonc.2021.642892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang S, Liu Z, Rong Y, Zhou B, Bai Y, Wei W, et al. Deep learning provides a new computed tomography-based prognostic biomarker for recurrence prediction in high-grade serous ovarian cancer. Radiother Oncol 2019; 132: : 171–7p.. doi: 10.1016/j.radonc.2018.10.019 [DOI] [PubMed] [Google Scholar]