Abstract
PFOS is one of the dominant PFAS detected in aquatic ecosystems. PFOS has been used in a wide range of industrial and consumer products for decades. The unique properties of PFOS, including its stability and resistance to degradation, have made it highly persistent in the aquatic environment. Due to its persistence, potential toxicity, and occurrence in aquatic ecosystems, interest in PFOS has increased in recent decades. Despite this interest, current information on the environmental distribution of PFOS in ambient surface waters of the United States (U.S) is fairly limited. This critical review summarizes currently available literature on PFOS occurrence in surface waters across the U.S. and highlights existing data gaps. Available data are largely from a handful of study areas with known PFAS manufacturing or industrial uses, with much of the data collected from freshwater systems in eastern states and the upper midwest. Measured PFOS concentrations in surface waters vary widely, over eight orders of magnitude, with the highest concentrations occurring downstream from manufacturing and industrial use plants, areas near aqueous film-forming foams (AFFF) use sites, and sites where PFOS precursors were used in textile treatment. Non-point source related occurrences are highest near urbanized areas with high population densities. Current data illustrates the occurrence of PFOS in surface waters across multiple U.S. states. Additional data are needed to better understand PFOS occurrence in U.S. aquatic ecosystems, particularly in estuarine and marine systems and where monitoring data are not available (e.g., southwestern, central, and western U.S.). Additional PFOS occurrence data would provide valuable information on potential spatial and temporal variability in surface waters, and possible risks posed to aquatic ecosystems.
Keywords: Perfluoroalkyl Substance (PFAS), Perfluorooctane Sulfonate (PFOS), Fate and Transport, Occurrence, Surface Waters
BACKGROUND
Perfluorooctane sulfonate (PFOS), and its salts, belong to the Per- and Polyfluoroalkyl Substances (PFAS) group of chemicals. PFAS are synthetic, organic compounds that consist of a carbon backbone and a unique functional group, such as sulfonate or carboxylic acid (CnF2n+1-R; Ahrens 2011; Buck et al. 2011; Lindstrom et al. 2011; Wang et al. 2017). Specifically, PFOS consists of an eight-carbon backbone and a sulfonate functional group (formula is C8F17 SO3−; CAS No. 45298–90-6 for anionic form). PFOS, and its salts, have been incorporated into a wide range of consumer and industrial products for decades (since the 1950s), including surface treatments for soil and stain resistance of textiles, paper, metals, pesticides, and are used in applications such as in aqueous film-forming foam (AFFF; Ahrens 2011; Buck et al. 2011; Lindstrom et al. 2011; Ahrens and Bundschuh 2014) and by 2002 the use of PFOS was phased with the exception of a few small applications (i.e., AFFF and hard chrome plating mist suppression) ( (Lindstrom et al. 2011; U.S. EPA 2000).
The manufacture of PFOS started in 1949 with the 3M Company (Paul et al 2009). Prior to 2000, the 3M Company was the major producer of perfluorooctanesulfonyl fluoride (PFOSF also known as POSF), the raw material that undergoes base-catalyzed hydrolysis in the Electrochemical Fluorination (ECF) process to make PFOS, with smaller producers in Europe and Asia (Paul et al. 2009; Lehmler 2005; Lindstrom et al. 2011). In 2000, the 3M Company manufactured approximately 78% of the estimated global PFOSF production (approximately 3,665 tons of 4,650 tons; OECD 2002). The estimated total cumulative production of PFOSF from the 3M Company and other western companies through 2002 is between 44,000 and 96,000 tons. Information on previous and current production of PFOSF from Asia and other production sources is limited (Prevedourous et al. 2006; Smithwick et al. 2006; Paul et al. 2009).
In May 2000, following negotiations between EPA and 3M, the 3M Company agreed to a voluntary phase out and to find substitutes for PFOS chemistry used to produce all but a few small applications (i.e., AFFF and hard chrome plating mist suppression) across their range of products by 2002 (Lindstrom et al. 2011; U.S. EPA 2000). Starting around the same time, a series of Significant New Use Rules (SNUR) were put into place by the EPA to restrict the production and use of chemicals that contain PFOS and its precursors in the U.S. (Lindstrom et al. 2011). Additionally, Canada phased out PFOS, its salts, and precursors (ECCC 2018). In 2009, PFOS and related compounds were listed under Annex B of the Stockholm Convention on Persistent Organic Pollutants; restricting global manufacturing and use of PFOS (OECD 2002; Ahrens 2011). Homologues, neutral precursor compounds, and new classes of PFAS continue to be produced; and therefore, are potential sources of PFOS (Ahrens 2011). The production of PFOS was estimated to be approximately 1,000 tons from 2002 and onward (Paul et al. 2009). However, while industrialized countries, like the U.S., phased-out the use of PFOS and its precursors, producers in other countries, such as China and Brazil, have scaled up their production to fill remaining demand (Wang et al. 2013). Despite the wide use in an array of industrial and consumer products globally, information on the sources, volumes, and emission of PFOS and its precursors are and have been limited (Paul et al. 2009; Zhang et al. 2016; Ankley et al. 2020).
PFOS is resistant to hydrolysis, photolysis, microbial degradation, and metabolism, making it persistent in aquatic environments (OECD 2002; Ahrens et al. 2011; Buck et al. 2011; Lindstrom et al. 2011). PFOS has been detected in tissues of aquatic organisms and both field and laboratory data show a propensity of PFOS to bioaccumulate and move through aquatic food webs (Houde et al. 2006 and 2011; Giesy et al. 2010). Current toxicity literature reports that PFOS exposures can have adverse effects on a diversity of aquatic organisms (Beach et al. 2006; Giesy et al. 2010). Despite the persistence, toxicity, and potential bioaccumulation of PFOS in the aquatic environment, current information on the environmental distribution of PFOS in surface waters of the U.S. is relatively limited (Ankley et al. 2020).
SOURCES OF PFOS TO AQUATIC ENVIRONMENTS
Aquatic environments and soil are thought to serve as a reservoir of PFOS, with 42,000 tons emitted to aquatic environments compared to 235 tons released globally into air between 1980 and 2002 (Paul et al. 2009; Rankin et al. 2016). Unlike other contaminants commonly found in aquatic ecosystems, such as metals, PFAS are synthetic compounds with no natural source. Thus, the occurrence of any PFAS compound in the environment is an indication of anthropogenic sources (Ahrens 2011). The occurrence of PFOS in aquatic environments can be attributed to both point and non-point sources, entering aquatic environments from industrial and consumer products during manufacturing, along supply chains, and during product use and/or disposal (Paul et al. 2009; Ahrens 2011; Kannan 2011; Ahrens and Bundschuh 2014). However, quantitative assessments of PFOS production, point and non-point source discharges, and environmental measurements are limited compared to other persistent bioaccumulative pollutants (Ahrens and Bundschuh 2014; Zhang et al. 2016)
Potential point sources of PFOS to the aquatic environment include both industrial facilities and municipal wastewater treatment plants (WWTPs). Additional point sources may include surface water runoff from industrial use sites such has metal plating facilities, areas that have received AFFF applications, landfills, and contaminated soils. Of these, industrial facilities, specifically those for fluorochemical manufacturing and other use facilities, are a primary source of PFOS to aquatic systems (Ahrens et al. 2011; Houtz et al. 2016; Sedlak et al. 2017). Estimated total global releases to water arising from discharge of PFOS during manufacturing from 1970 to 2002 ranged between 230 and 1,450 tons (Paul et al. 2009). Several studies have found increased concentrations of PFOS in municipal WWTP effluent compared to influent (Schultz et al. 2006; Sinclair and Kannan 2006). Schultz et al. (2006) observed an increase of PFOS concentrations following treatment of wastewater using standard technologies in a municipal WWTP. Similarly, Sinclair and Kannan (2006) observed a statistically significant increase (by 227 times ± 119%) of PFOS measured in effluent compared to influent from a municipal WWTP that received industrial inputs in New York. These studies indicate conventional municipal WWTP processes (i.e., primary and secondary treatment) may not be effective at removing PFOS and that the degradation of other PFAS may be contributing to the increased concentrations of PFOS in effluent (Schultz et al. 2006; Sinclair and Kannan 2006; Ahrens 2011).
Potential non-point PFOS sources to aquatic environments include: dry and wet atmospheric deposition, discharge of contaminated groundwater from manufacturing sites, runoff from impervious surfaces in urban environments, discharge of contaminated groundwater from use of AFFF, and land application of contaminated biosolids (OECD 2002; Paul et al. 2009; Ahrens et al. 2011; Kannan 2011). Identification of non-point PFOS sources and understanding their relative contribution to aquatic ecosystems can be difficult (Paul et al. 2009; Ahrens 2011). Overall, the presence of non-point PFOS sources and their relative contributions are dependent on the aquatic system, air, groundwater, and soil levels, and nearby land uses. For example, concentrations of PFAS, including PFOS, have been influenced by urban land use (Ahrens 2011; Zhang et al. 2016) and overall PFAS concentrations in the environment have been positively correlated with human population density. PFOS was detected in aquatic systems at elevated concentrations (ranging between 97 and 1,371 ng/L) in densely populated areas of the U.S. and Europe (Zhang et al. 2016 and Loos et al. 2009, respectively). Paul et al. (2009) estimated the total global PFOS emissions to air and water from 1970 to 2009 resulting from consumer use and disposal to be between 420 and 2,100 tons.
PFAS are still produced that can transform or degrade into compounds belonging to the perfluoroalkane sulfonic acids (PFSA) family of PFAS, including PFOS (Ahrens 2011). The metabolic transformation of PFAS precursors such as fluorotelomer sulfonates (FTSAs) and perfluoroalkyl sulfonamidoacetic acids (FASAAs), and the degradation of volatile PFAS such as perfluoroalkyl sulfonamidoethanols (FASEs), are known to degrade to PFOS. (Ahrens and Bundschuh 2014; Benskin et al. 2009; Boulanger et al. 2005; Buck et al. 2011; Lange 2000; Lui and Mejia Avendaño 2013; Plumlee et al. 2009; Rhoads et al. 2008; Wang et al. 2017). However, understanding of these transformation processes is limited, and additional work is needed to fully understand these processes and their role as a source of PFOS to aquatic environments (Lau et al. 2007; Buck et al. 2011; Lui and Mejia Avendaño 2013; Wang et al. 2017). The contribution of precursors to the presence of PFOS in the environment is unknown and difficult to quantify. However, these precursors can be a continuous source of PFOS. Particularly, the degradation of precursors may represent a potentially significant known source of PFOS to the aquatic environment, especially since PFOS production within the U.S. has not occurred since 2002 (Buck et al. 2011; Lui and Mejia Avendaño 2013). Nevertheless, PFOS-treated articles, such as fabrics, paper, and other treated materials, are still being imported in the U.S. and end up being released into the environment (Allred et al. 2015; Lang et al. 2016; Lui et al. 2014). The importation of PFOS treated articles is considered as production under the Toxic Substances Control Act (TSCA; U.S. EPA 2020).
PFOS can also be re-emitted to aquatic environments from PFOS sinks such as soil, groundwater, ice, and sediment. Sediment is an important sink of PFOS in the aquatic environment (Ahrens et al. 2011). Like other persistent organic pollutants, the movement of PFOS between groundwater, surface water, and sediment is complex and depends on the chemical properties of PFOS and site-specific physiochemical characteristics (including pH, temperature, organic carbon content, and salinity) of the aquatic environment. In general, PFOS may adsorb to sediments (with a Kd greater than 1 mL/g; Giesy et al. 2010). However, this sorption to sediments is limited and PFOS has a KOC of 2.57 indicating that PFOS is relatively mobile in water and the physicochemical characteristics of the sediment ultimately influence the sorption of PFOS (Ahrens et al. 2011; Higgins and Luthy 2006). While the release of PFOS from the transformation of other PFAS and the historical products still in use (e.g., consumer goods manufactured, imported and/or obtained before the PFOS discontinuation and regulations) will continue into the future, the re-emissions of PFOS from existing sinks are assumed to be slowly decreasing since the restrictions and regulations of PFOS have gone into place (Paul et al. 2009; Ahrens 2011; Ahrens and Bundschuh 2014, Washington and Jenkins 2005; Washington et al. 2015).
METHODS
An understanding of the occurrence of PFOS in ambient surface waters across the U.S. is needed to better identify the environmental relevance of concentrations reported in PFOS toxicity literature. In the present paper, PFOS occurrence data in U.S. ambient surface waters were obtained from publicly available literature, including peer-review journal articles, theses, and government and industry reports. Searches for such literature were conducted by developing a series of search terms related to the chemicals analyzed (e.g., perfluorooctane sulfonate or PFOS, and its salts), waterbody type sampled (e.g., ambient waterbodies such as rivers, streams, wetlands, or lakes), and location of sampled waterbody (which was specific to the U.S.). Databases searched were Science Direct, Google Scholar, and EPA’s ECOTOX Database (which includes ambient water concentrations for calculation of bioconcentration and bioaccumulation factors such as those in Burkard (2021); https://cfpub.epa.gov/ecotox/). Additionally, it should be noted that many states in the U.S. have conducted monitoring of PFOS. However, most of the state monitoring data for PFOS are currently not publicly available. Thus, after extensive literature searches (including searches by individual states and on state monitoring databases/websites) and reaching out to individual states, the data presented in this manuscript appear to be inclusive of all publicly available relevant PFOS data.
The identified citations were reviewed for reported ambient surface water concentrations of PFOS, including relevant summary statistics (e.g., minimum, maximum, mean, and median concentrations) reported by the individual study authors. Citations without PFOS data and/or with only drinking or ground water data were not included. Measured PFOS concentrations in ambient surface waters from the identified citations with appropriate information were extracted into a database (Supplemental Data Table S2). For the purposes of this overview and comparison, all concentrations reported here are in nanograms per liter (ng/L). Additional data extracted from the identified citations included: the location of the waterbody sampled (as both the location by state, waterbody name, and GPS coordinates), specific site name or description (if provided in the paper), identification of possible previous exposure to PFOS (as stated by the study authors of the individual papers), the date the sample was collected, the number of samples collected and/or analyzed, and analytical methods used to measure PFOS (including reported limits of quantification and limits of detection). Beyond the extraction of the aforementioned data, the identified citations were not further evaluated for data quality. Instead, all relevant PFOS occurrence data for ambient surface waters in the U.S. were captured in this present paper to provide an overview of PFOS occurrence in ambient surface waters across the U.S.
The extracted PFOS occurrence data were included in understanding the current distribution, frequency of detection, and summary statistics (specifically both arithmetic and geometric means, median, and overall range) of reported PFOS concentrations in ambient surface waters across the U.S. The PFOS occurrence data were also evaluated for potential spatial and/or temporal variability of PFOS in ambient surface waters. And lastly, the occurrence and concentrations of PFOS in ambient surface waters across the U.S. were generally compared to those reported globally.
RESULTS OF PFOS OCCURRENCE IN U.S. SURFACE WATERS
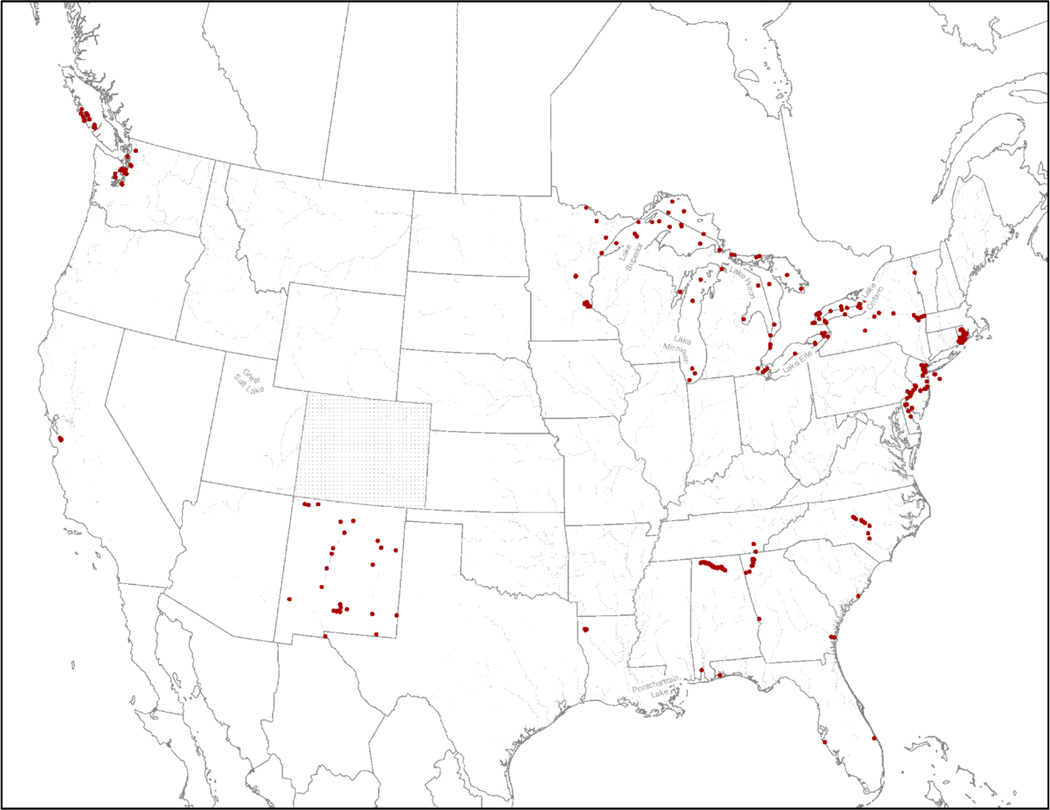
PFOS is one of the most commonly detected PFAS in aquatic ecosystems, along with PFOA (Ahrens 2011; Benskin et al. 2012; Zareitalabad et al. 2013; Dinglasan-Panlilio et al. 2014; Nakayama et al. 2017; Remucal 2019). Despite its wide use, and persistence in the aquatic environment, current information on the distribution of PFOS in ambient surface waters of the U.S. is relatively limited. Available data are largely collected from freshwater systems in eastern states, with most of the current, published PFOS occurrence data focused on a handful of study areas with known manufacturing or industrial uses of PFAS, such as the Mississippi River near a 3M facility, the Great Lakes, the Cape Fear Drainage Basin, and waterbodies near Decatur, Alabama and northern Georgia, along with areas of known AFFF use, such as fire-training areas on military bases (Anderson et al. 2016; Figure 1 and Table 1; Supplemental Data, Figure S1 and Table S1).
Figure 1.
Map indicating sampling locations for perfluorooctane sulfonate (PFOS) measured in surface waters across the United States (U.S.) based on data reported in the current, publicly available literature. Sampling locations for the Colorado data were not available and these data are represented by the dash marks to indicate measured PFOS surface water concentrations are available. Detailed information on sampling locations, including references, coordinates, and sampling site identification numbers and names, provided in Supplemental Data, Figure S1 and Table S1.
Table 1.
Current Publicly Available Measured Perfluorooctane Sulfonate (PFOS) Concentrations in Surface Waters Across the United States (U.S.). Additional details, including study specific sampling dates, number of measurements, and limits of detection and quantification, provided in Supplemental Data, Table S2.
| State | Waterbody1 | Arithmetic Mean PFOS Concentration (ng/L)2 | Median PFOS Concentration (ng/L)2 | Range of PFOS Concentration (ng/L) | Reference |
|---|---|---|---|---|---|
|
| |||||
| Lake Erie | 3.77 | 3 | 2.8 – 5.5 | Sinclair et al. (2006) | |
| 31.3 | 32.5 | 21.5 – 38.5 | Boulanger et al. (2004) | ||
| 2.84 | 2.63 | 2.49 – 3.41 | De Silva et al. (2011) | ||
| 4.5 | 4.2 | 4.0 – 5.3 | Furdui et al. (2008) | ||
| Lake Huron | 2.25 | 1.96 | 0.239 – 5.46 | De Silva et al.(2011) | |
| 1.73 | 1.5 | 1.2 – 2.7 | Furdui et al. (2007) | ||
| Lake Michigan | 2.03 | 2.03 | 0.93 – 3.13 | Simcik and Dorweiler (2005) | |
| 2.00 | 1.96 | 1.73 – 2.36 | De Silva et al. (2011) | ||
| not provided | 4.9 | 2.9 – 30 | (Sinclair et al.2006) | ||
| 55.4 | 59.8 | 16.5 – 85.5 | Boulanger et al. (2004) | ||
| Lake Ontario | 5.96 | 5.63 | 2.60 – 9.48 | De Silva et al. (2011) | |
| 8.69 | 6.6 | 3.6 – 37.6 | Furdui et al. (2008) | ||
| 2.20 | not provided | not provided | Houde et al. 2008 | ||
| 0.255 | 0.236 | 0.095 – 0.395 | De Silva et al. (2011) | ||
| Lake Superior | 0.233 | 0.3 | 0.1 – 0.3 | Furdui et al. (2008) | |
| 0.246 | 0.124 | 0.074 – 0.996 | Scott et al. (2010) | ||
| Alabama | Waterbody near Decatur | 58,016 | 41,027 | 9 – 150,000 | OECD (2002) |
| Waterbody in Decatur | 2.5 < x < 25 | 2.5 < x < 25 | 2.5 < x < 25 | 3MCompan y (2001) | |
| Pond in Decatur | 111 | 111 | 111 | ||
| Waterbody in Mobile | 30.3 | 35.5 | < 25 – 41.5 | 3MCompan y (2001) | |
| Pond in Mobile | 32.5 | 32.5 | 32.5 | ||
| Tennessee River (upstream of Baker’s Creek) | 30.85 | 29.80 | 16.0 – 52.6 | Hansen et al. (2002) | |
| Tennessee River (downstream of Baker’s Creek) | 103.9 | 107.0 | 30.3 – 144 | Hansen et al. (2002) | |
| California | Upper Silver Creek | not provided | not provided | 27 – 56 | Plumlee et al. (2008) |
| Coyote Creek | not provided | not provided | 4.8 – 25 | ||
| Animas River | < 0.48 | < 0.48 | < 0.48 | ||
| Arkansas River | 1.96 | 0.62 | 0.23 – 5.00 | ||
| Arvada Blunn Reservoir | 0.77 | 0.77 | 0.77 | ||
| Colorado | Barker Reservoir | < 0.49 | < 0.49 | < 0.49 | Colorado Department of Public Health and the Environment 2020 |
| Bessemer Ditch | 14.0 | 14.0 | 14.0 | ||
| Big Thompson River | 3.90 | 3.90 | 3.90 | ||
| Blue River | 1.20 | 1.20 | 1.20 | ||
| Boulder Feeder Canal | < 0.45 | < 0.45 | < 0.45 | ||
| Boyd Lake | 1.00 | 1.00 | 1.00 | ||
| Cache la Poudre River | 5.61 | 5.61 | < 0.45 – 11.0 | ||
| Clear Creek | 7.95 | 7.95 | 7.20 – 8.70 | ||
| Colorado River | 0.67 | 0.66 | 0.65 – 0.69 | ||
| Coon Creek | < 0.48 | < 0.48 | < 0.48 | ||
| Eagle River | 0.68 | 0.68 | 0.68 | ||
| East Plum Creek | < 0.43 | < 0.43 | < 0.43 | ||
| Erie Lake | 3.70 | 3.70 | 3.70 | ||
| Fairmount Reservoir | < 2.50 | < 2.50 | < 2.50 | ||
| Fountain Creek | 16.9 | 20.0 | 3.50 −24.0 | ||
| Fraser River | 1.00 | 1.00 | 1.00 | ||
| Gore Creek | 0.98 | 0.98 | 0.98 | ||
| Gunnison River | 0.71 | 0.71 | 0.71 | ||
| Horsetooth Reservoir | 0.51 | 0.51 | 0.51 | ||
| Jackson Creek | < 0.44 | < 0.44 | < 0.44 | ||
| Jerry Creek | < 0.485 | < 0.485 | < 0.48 - < 0.49 | ||
| Kannah Creek Flowline | < 0.49 | < 0.49 | < 0.49 | ||
| Lakewood Reservoir | < 0.45 | < 0.45 | < 0.45 | ||
| Little Fountain Creek | < 0.46 | < 0.46 | < 0.46 | ||
| Maple Grove Reservoir | 10.0 | 10.0 | 10.0 | ||
| Marstron Reservoir | 0.48 | 0.48 | 0.48 | ||
| McBroom Ditch | 4.90 | 4.90 | 4.90 | ||
| Mclellen Reservoir | 1.30 | 1.30 | 1.30 | ||
| Mesa Creek | < 0.49 | < 0.49 | < 0.49 | ||
| Michigan River | < 0.46 | < 0.46 | < 0.46 | ||
| Molina Power Plant Tail | < 0.50 | < 0.50 | < 0.50 | ||
| North Fork Gunnison River | < 0.47 | < 0.47 | < 0.47 | ||
| Purdy Mesa Flowline | < 0.49 | < 0.49 | < 0.49 | ||
| Purgatoire River | 0.47 | 0.47 | 0.47 | ||
| Ralston Reservoir | < 0.46 | < 0.46 | < 0.46 | ||
| Rio Grande | < 0.47 | < 0.47 | < 0.47 | ||
| Roaring Fork River | < 0.50 | < 0.50 | < 0.50 | ||
| San Juan River | < 0.44 | < 0.44 | < 0.44 | ||
| Sand Creek | 30.3 | 30.3 | 6.50 – 54.0 | ||
| Severy Creek | < 0.47 | < 0.47 | < 0.47 | ||
| Somerville Flowline | < 0.48 | < 0.48 | < 0.48 | ||
| South Boulder Creek | 0.50 | 0.50 | 0.50 | ||
| South Platte River | 10.5 | 11.5 | 3.80 – 16.0 | ||
| St. Vrain River | 3.90 | 3.90 | 3.90 | ||
| Strontia Springs | < 0.51 | < 0.51 | < 0.51 | ||
| Taylor River | < 0.45 | < 0.45 | < 0.45 | ||
| Uncompahgr e River (delta) | 0.54 | 0.54 | 0.54 | ||
| Welton Reservoir | 2.60 | 2.60 | 2.60 | ||
| White River | < 0.46 | < 0.46 | < 0.46 | ||
| Yampa River | < 0.47 | < 0.47 | < 0.47 | ||
| Delaware, New Jersey, Pennsylvani a | Delaware River | 3.98 | 3.5 | 0.97 – 6.92 | Pan et al. (2018) |
| Waterbody in Pensacola | 16.29 | 2.5 < x < 25 | <25 – 29 | ||
| Pond in Pensacola | 2.5 < x < 25 | 2.5 < x < 25 | 2.5 < x < 25 | ||
| Florida | Waterbody in Port St. | 50.83 | 2.5 < x < 25 | < 2.5 – 137.5 | 3MCompan y (2001) |
| Lucie Small pond in Port St. Lucie3 | 9,784 | 1,945 | 1,830 – 48,200 | ||
| Sarasota Bay | 0.90 | not provided | not provided | Houde et al. 2006 | |
| Waterbody in Columbus | 59.9 | 55 | 44.6 – 80 | 3Mcompan y (2001) | |
| Pond in Columbus | < 2.5 | < 2.5 | < 2.5 | ||
| Conasauga River | 162.1 | 192 | < 1.5 – 321 | ||
| Georgia | Altamaha River | 2.63 | 2.6 | 2.6 – 2.7 | Konwick et al. (2008) |
| Streams and ponds in Dalton | 70.36 | 70.73 | 10.5–119.5 | ||
| Oostanaula River | 150.3 | 151 | 148 – 152 | Lasier et al. (2011) | |
| Louisiana | Waterbodies (locations of concern) near Barksdale A.F.B. | 776.7 | 195.0 | < 10 – 7,070 | Cochran (2015); Lanza et al. (2017) |
| Reference waterbodies near Barksdale A.F.B. | < 10 | < 10 | < 10 | ||
| Raisin River | 3.5 | 3.5 | 3.5 | Kannan et al. (2005) | |
| Michigan | St Clair River | 2.6 | 2 | 1.9 – 3.9 | |
| Siskiwit Lake | 0.283 | 0.283 | 0.277 – 0.289 | Scott et al. (2010) | |
| Upper Mississippi River | 528l.9 | < 2 | < 2 – 18,200 | Newsted et al. (2017) | |
| Lake of the Isles | 2.47 | 2.47 | 2.47 | ||
| Lake Calhoun | 50.4 | 50.4 | 50.4 | ||
| Lake Harriet | 22.1 | 22.1 | 22.1 | ||
| Minnesota | Minnesota River | 9.21 | 9.21 | 9.21 |
Simcik and Dorweiler (2005)
|
| Lake Tettegouche | 0.23 | 0.23 | 0.23 | ||
| Lake Nipisiquit | < 0.27 | < 0.27 | < 0.27 | ||
| Lake Loiten | < 0.27 | < 0.27 | < 0.27 | ||
| Little Trout Lake | 1.2 | 1.2 | 1.2 | ||
| Echo Lake Reservoir | < 2 | < 2 | < 2 | ||
| New Jersey | Passaic River | 13.1 | 13.1 | 13.0 – 13.2 | NJDEP (2019) |
| Raritan River | 6.9 | 6.9 | 6.9 | ||
| Metedeconk River | 1.65 | 1.65 | < 2 – 2.8 | ||
| Pine Lake | 102 | 102 | 102 | ||
| Horicon Lake | 10 | 10 | 10 | ||
| Little Pine Lake | 100 | 100 | 100 | ||
| Mirror Lake | 72.9 | 72.9 | 72.9 | ||
| Woodbury Creek | 6.4 | 6.4 | 6.4 | ||
| Fenwick Creek | 3.1 | 3.1 | 3.1 | ||
| Cohansey River | < 2 | < 2 | < 2 | ||
| Harbortown Road | 1.93 | 1.93 | 1.93 | Zhang et al. (2016) | |
| Passaic River | 4.59 | 4.07 | 0.244 – 9.99 | ||
| Alamogordo Domestic Water Sys. | < 1 | < 1 | < 1 | ||
| Animas River | 0.799 | 0.625 | < 0.89 – 1.5 | ||
| Canadian River | 0.848 | 0.9 | < 0.89 – 1.2 | ||
| New Mexico | Cloud Country Estates WUA | < 0.93 | < 0.93 | < 0.93 | New Mexico Environment Department 2020–2021 |
| Gila River | < 0.93 | < 0.93 | < 0.93 | ||
| Holloman AFB Golf Course Pond 1 | 1,220 | 1,220 | 1,220 | ||
| Holloman AFB Golf Course Pond 2 | 878 | 878 | 878 | ||
| Holloman AFB Lagoon G | 310 | 310 | 310 | ||
| Holloman AFB Outfall | 951 | 951 | 951 | ||
| Holloman AFB Sewage Lagoon | 2,200 | 2,200 | 2,200 | ||
| Karr Canyon Estates | < 0.93 | < 0.93 | < 0.93 | ||
| La Luz MDWCA | < 1.3 | < 1.3 | < 1.3 | ||
| Lake Holloman | 4,033 | 4,500 | 1,700 – 5,900 | ||
| Mountain Orchard MDWCA | < 0.93 | < 0.93 | < 0.93 | ||
| Pecos River | 1.223 | 1.50 | <0.94 – 1.70 | ||
| Rio Chama | < 0.98 | < 0.98 | < 0.96 – < 1 | ||
| Rio Grande | 1.052 | 0.474 | < 0.465 – 2.90 | ||
| Rio Puerco | 4.35 | 4.35 | 3.10 – 5.60 | ||
| San Juan River | < 1.15 | < 1.15 | < 1.06 – < 1.24 | ||
| Tularosa Water System | 0.723 | 0.723 | < 0.89 – 1.0 | ||
| Washington Park Lake | 1.67 | 1.77 | < 0.25 – 2.88 | ||
| Rensselaer Lake | 7.11 | 6.58 | 5.85 – 9.3 | ||
| New York | Iroquois Lake | not provided | not provided | not provided | Kim and Kannan (2007) |
| Unnamed lake 1 outside Albany, NY | not provided | not provided | not provided | ||
| Unnamed lake 2 outside Albany, NY | not provided | not provided | not provided | ||
| Niagara River | 5.17 | 5.5 | 3.3 – 6.7 | ||
| Finger Lakes | not provided | 1.6 | 1.3 – 2.6 | ||
| Lake Onondaga | 681 | 756 | 198 – 1,090 | ||
| Lake Oneida | 3.5 | 3.5 | 3.5 | Sinclair et al. (2006) | |
| Erie Canal | 8.37 | 6.4 | 5.7 – 13 | ||
| Hudson River | not provided | 1.7 | 1.5 – 3.4 | ||
| Lake Champlain | not provided | 2.7 | 0.8 – 7.7 | ||
| Lower NY Harbor | 0.755 | 0.755 | 0.755 | ||
| Staten Island | 1.66 | 1.66 | 1.66 | Zhang et al.(2016) | |
| Hudson River | 1.81 | 1.81 | 0.79 – 2.84 | ||
| North Carolina | Cape Fear River | 31.2 | 28.9 | < 1 – 132 | Nakayama et al. (2007) |
| Narragansett Bay | 2.2 | 2.2 | 2.2 | Benskin et al. (2012) | |
| Allen Cove Inflow | 1.20 | 1.20 | 1.20 | ||
| Bristol Harbor | 0.508 | 0.46 | 0.437 – 0.626 | ||
| Brook at Mill Cove | 9.80 | 9.80 | 9.80 | ||
| Rhode Island | Buckeye Brook | 4.13 | 4.13 | 4.13 | Zhang et al.(2016) |
| Chickasheen Brook | < 0.05 | < 0.05 | < 0.05 | ||
| EG Town Dock | 0.735 | 0.735 | 0.735 | ||
| Fall River | 0.238 | 0.238 | 0.238 | ||
| Green Falls River | 0.291 | 0.291 | 0.29 – 0.292 | ||
| Hunt River | 1.48 | 1.48 | 1.48 | ||
| Mill Brook | 3.94 | 3.94 | 3.94 | ||
| Narrow River | 0.298 | 0.264 | 0.176 – 0.488 | ||
| Pawcatuck River | 0.561 | 0.561 | 0.509 – 0.612 | ||
| Pawtuxet River | 2.19 | 2.19 | 2.19 | ||
| Queens River | 0.334 | 0.334 | 0.334 | ||
| Sand Hill Brook | 1.82 | 1.82 | 1.82 | ||
| Secret Lake - Oak Hill Brook | < 0.05 | < 0.05 | < 0.05 | ||
| Slack’s Tributary | 0.777 | 0.777 | 0.777 | ||
| South Ferry Road Pier | 0.161 | 0.161 | 0.161 | ||
| Southern Creek | 3.74 | 3.74 | 3.74 | ||
| Woonasquat ucket River | 14.6 | 14.6 | 5.87 – 23.2 | ||
| South Carolina | Charleston Harbor | 12.0 | not provided | not provided | Houde et al. 2006 |
| Tennessee | Waterbody near Cleveland | 2.5 < x < 25 | 2.5 < x < 25 | < 2.5 – < 25 | 3MCompan y (2001) |
| Conasauga River | <0.0094 | <0.0094 | <0.0094 | Laiser et al.2011 | |
| Texas | Rio Grande | 4.17 | 4.1 | 2.0 – 6.5 | New Mexico Environment Department 2020 |
| Puget Sound | 2.3 | 1.45 | 0.2 – 5.9 | ||
| Washington | Clayoquot Sound | 0.32 | 0.3 | 0.25 – 0.4 | Dinglasan- Panlilio et al. (2014) |
| Barkley Sound | 0.7 | 0.7 | 0.7 | ||
| Multiple States (10 Air Force Bases across the continental U.S.) | Surface waters impacted by aqueous film forming foam use | not provided | 2,170 | 8,970,000 (maximum) | Anderson et al. (2016) |
Less than (<) values based on study specific LOD and LOQ values that the study authors reported, LOD = limit of detection and LOQ = limit of quantitation
Name of Waterbody Sampled for PFOS. Name or description of waterbody above is consistent with that provided in cited reference.
Calculation of arithmetic mean and median includes lower of ½ LOD or ½ LOQ, depending on information provided. See full occurrence table in Appendix N for waterbody-specific details.
. Study authors conducted additional sampling of this waterbody but were unable to detect the initial high PFOS concentrations in any of the additional samples.
. Reported as ng/g by the study authors.
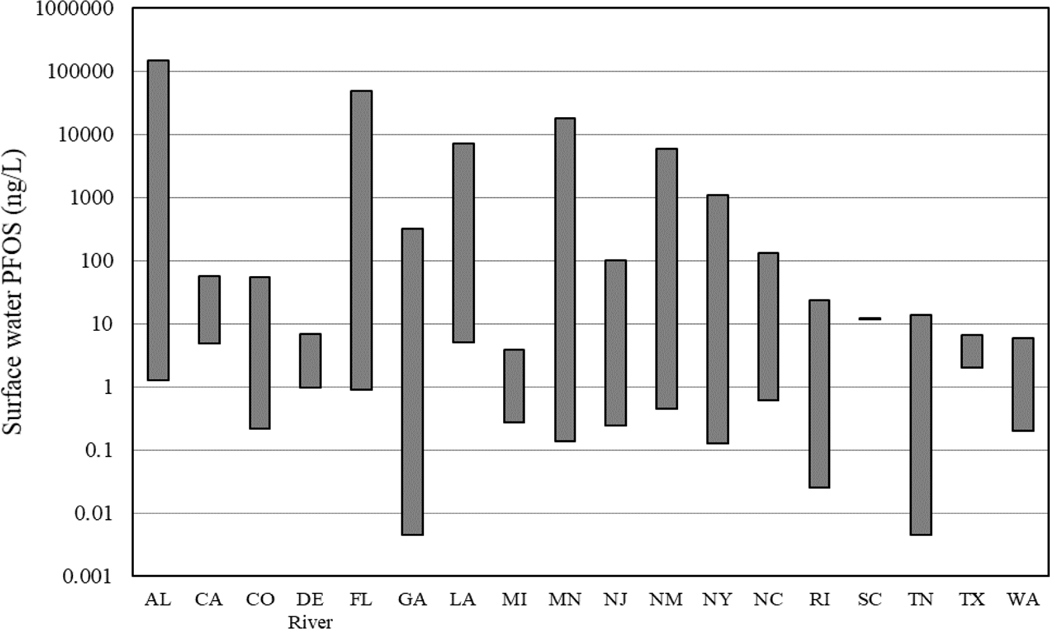
Concentrations of PFOS in surface waters vary widely, with observed concentrations ranging over eight orders of magnitude and detected generally between picogram and nanogram per liter. Some sites reported concentrations in the microgram and milligram per liter ranges.(Ahrens 2011; Zareitalabad et al. 2013). Measured surface water concentrations of PFOS in peer-reviewed journal articles and publicly available industry and government reports, range between 0.074 and 8,970,000 ng/L with an arithmetic mean concentration of 786.77 ng/L, a geometric mean concentration of 5.468 ng/L, and a median concentration of 3.6 ng/L. However, it should be noted that the mean and median concentrations reported here were calculated from the reported concentrations for individual samples. And therefore, these mean and median concentrations are not fully representative of all the measured PFOS concentrations in U.S. surface waters. In particular, should be noted that some of the papers (6 papers total without individual concentration data; see Supplemental Data Table S2) only reported summary information such as minimum and maximum concentrations and did not provide more detailed data (e.g., individual sample concentrations or sample site means) in the paper (Figure 2 and Table 1; Supplemental Data, Table S2).
Figure 2.
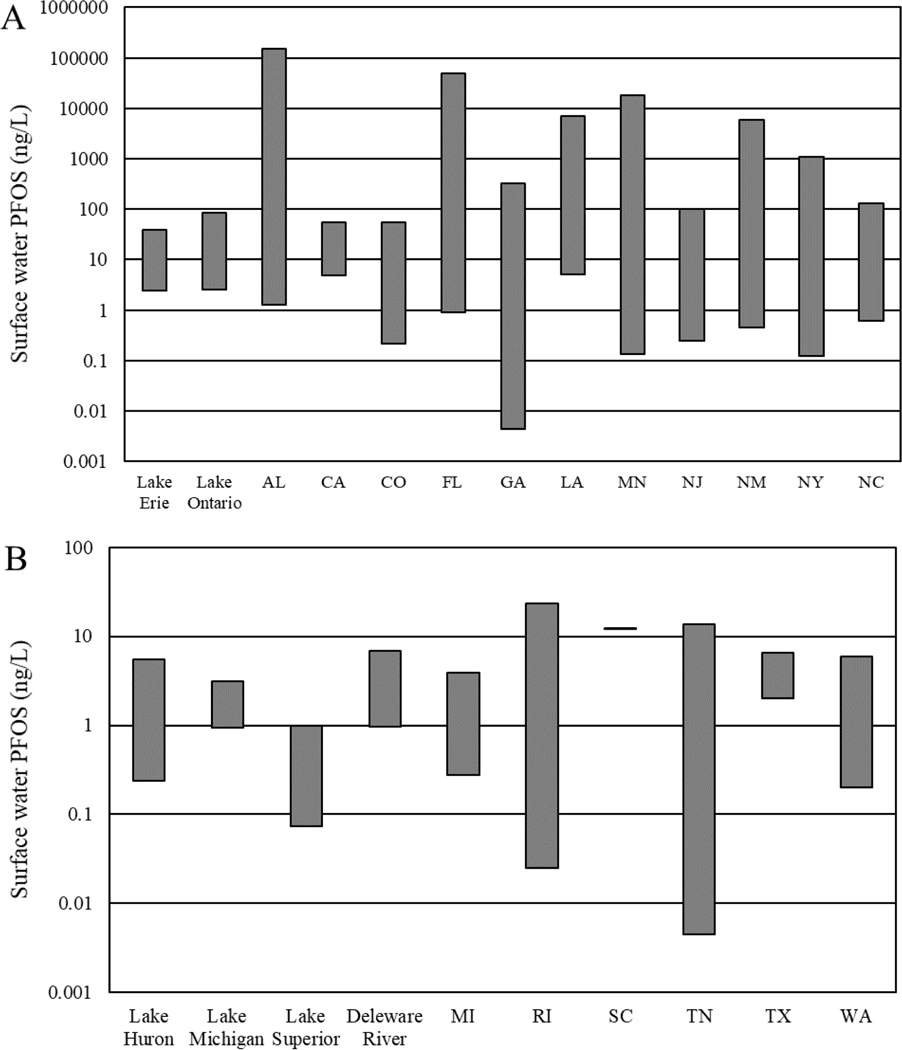
Distribution of the minimum and maximum concentrations (ng/L) of perfluorooctane sulfonate (PFOS) measured in surface waters for each state or waterbody (excluding the Great Lakes) with reported data in the current, publicly available literature and is not necessarily comprehensive of PFOS concentrations in surface waters across each state. The distribution is arranged alphabetically by state and waterbody.
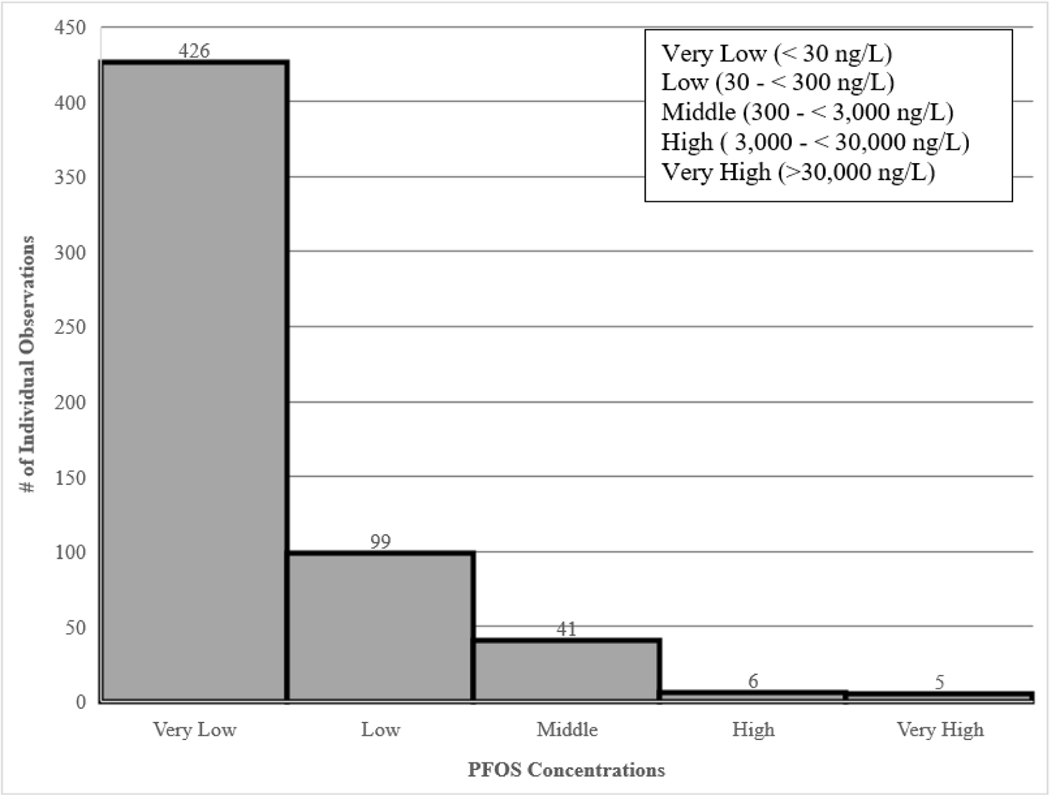
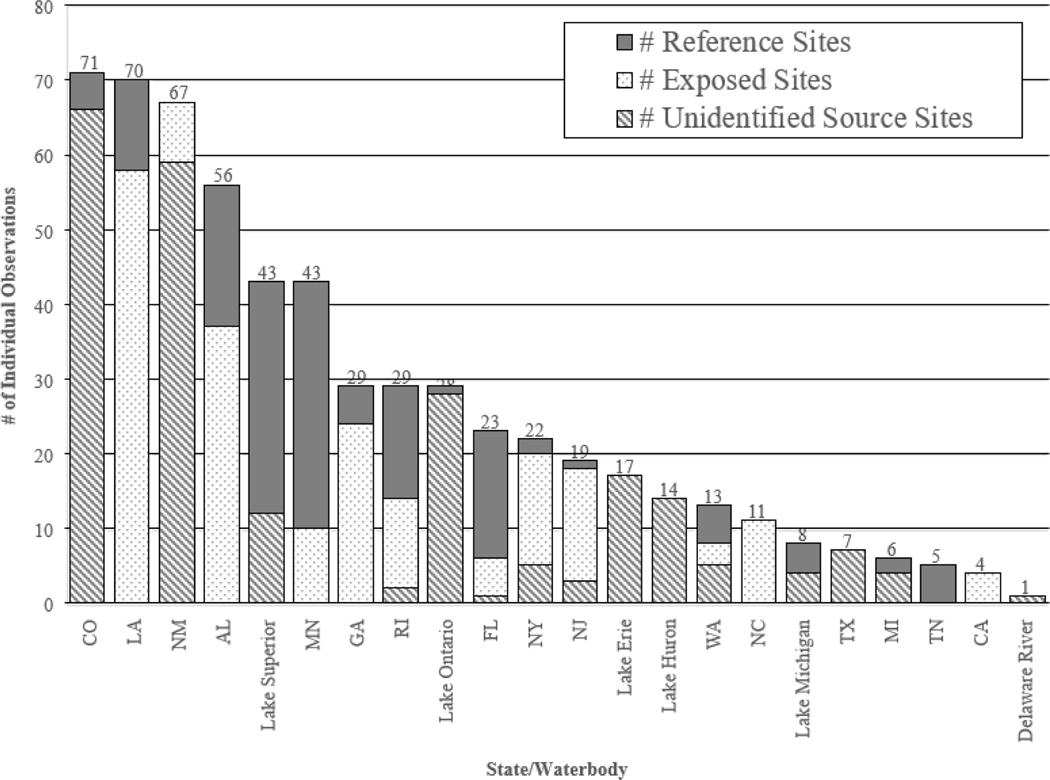
Consistent with the calculated median of 3.6 ng/L, a majority (90.99%) of measured PFOS concentrations in the current literature fall below 300 ng/L with fewer (9.01%) observed concentrations greater than 300 ng/L (Figure 3). As mentioned in the sources of PFOS section above, in contrast with other contaminants commonly found in aquatic ecosystems PFOS is a synthetic compound with no natural source. Thus, the occurrence of PFOS in surface water is an indication of anthropogenic sources, including consumer and industrial use, long-range transport, atmospheric deposition, surface water runoff, and general persistence in the environment (Ahrens 2011).The higher frequency of PFOS concentrations below 300 ng/L can likely be attributed to: (1) the increased tendency of study designs found in the current literature to include sites with no known previous exposures to PFAS in order to compare results to sites with known previous exposure to PFAS, which is depicted in Figure 4 and (2) it is likely that sites in which the study authors did not specify potential exposure to PFAS could be classified as relatively pristine sites with no known PFOS inputs since the reported measured concentrations indicate that the PFOS concentrations are generally similar to those observed in sites noted to have no known PFOS inputs (Table 2).
Figure 3.
Number of individual observations of measured perfluorooctane sulfonate (PFOS) concentrations that are very low (< 30 ng/L), low (30 – < 300 ng/L), middle (300 – < 3,000 ng/L), high (3,000 – < 30, 0000 ng/L), and very high (> 30,000 ng/L) in ambient surface waters across the United States. The bins of PFOS concentrations were determined from the currently available toxicity literature for PFOS.
Figure 4.
Distribution of individual observations among sites with no known previous exposure to PFASs (identified as Reference Sites by individual study authors, Exposed (sites with known previous exposure to PFASs and also identified as such by individual study authors), and Unidentified (sites in which the study authors did not specify sites as reference or potential exposure to PFASs) ambient surface water sites in the current, publicly available for PFOS occurrence data and is not necessarily comprehensive of PFOS concentrations in surface waters across each state.. The distribution is arranged by highest to lowest number of individual observations and grouped by state or waterbody.
Table 2.
Measured Perfluorooctane Sulfonate (PFOS) Concentrations in Reference, Exposed, and Unidentified Surface Waters Sites Across the United States (U.S.). Additional details, including site type based on classification provided in the individual paper, number of measurements, and limits of detection and quantification, provided in Supplemental Data, Table S2.
| Site Classification | Mean PFOS Concentration (ng/L) | Median PFOS Concentration (ng/L) | Range of PFOS Concentration (ng/L) |
|---|---|---|---|
|
| |||
| Exposed | 1,746 | 76 | < LOD – 8,970,000 |
| Reference | 776.94 (7.43)1 | 1 | < LOD – 51,100 (< LOD – 138)2 |
| Unidentified | 5.91 | 1.28 | < LOD – 121 |
LOD = limit of detection
Mean including concentrations for pond in Port St. Lucie, Florida, which was classified as a reference site by the study authors. The mean concentration excluding these concentrations (total of 13 individual observations excluded) presented in parentheses.
Range including concentrations for pond in Port St. Lucie, Florida, which as noted under footnote 1 above was classified as a reference site by the study authors. The range excluding these concentrations (total of 13 individual observations excluded presented in parentheses.
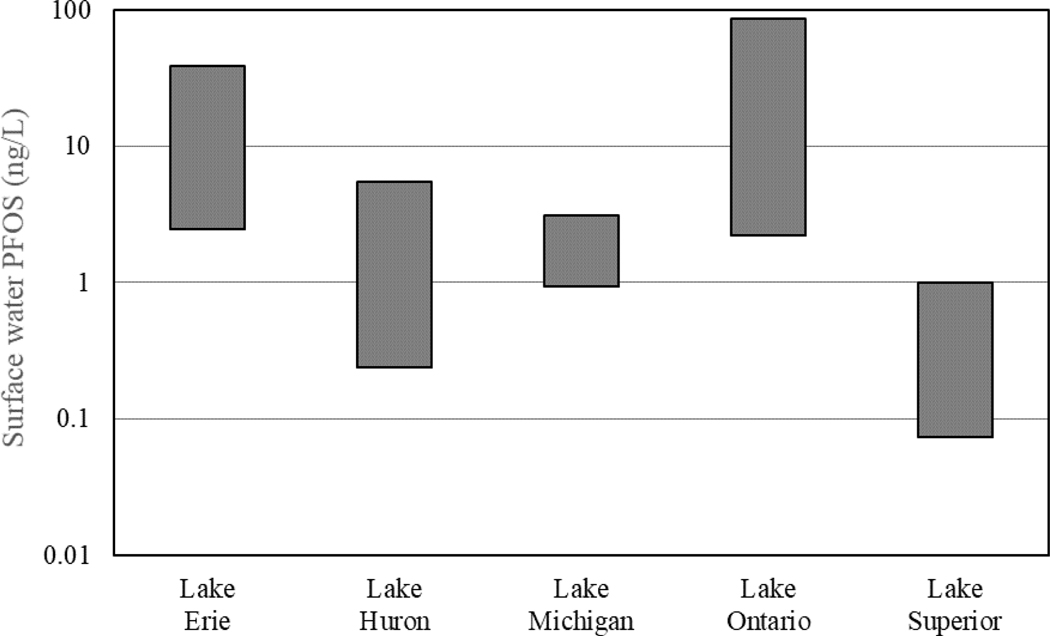
Numerous available studies report measured PFOS concentrations in surface waters across the U.S. (Figure 2 and Table 1), some of which are summarized below; however, more detailed information on PFOS occurrence in areas not previously sampled and spatial and temporal variability of PFOS remain limited. Prior to this review, there were few current analyses of spatial variability of PFOS concentrations in surface water across the U.S. (Remucal 2019). This review indicates that PFOS occurrence is widely reported in areas where sampling has been conducted. And that the presence and measured concentrations of PFOS in surface waters are similar between both lotic and lentic and freshwater and estuarine/marine systems, based on the limited data available (Supplemental Data Table S2). Higher PFOS concentrations in surface water tend to be dependent on the presence of a nearby source and generally increase with levels of urbanization. Across the Great Lakes region, PFOS concentrations were higher in the more southern lakes of Erie and Ontario compared to the upstream lakes of Superior, Michigan, and Huron (Table 1; Remucal 2019). Similarly, Zhang et al. (2016) observed that measured PFOS surface water concentrations in urban areas (with mean PFOS concentrations of 4.21 ng/L in urban sites within Rhode Island, New York, and New Jersey) were an order of magnitude higher than those at rural sites (with an mean PFOS concentration of 0.42 ng/L).
Currently, there are insufficient data to quantitatively evaluate temporal trends of PFOS in surface waters across the U.S. (Remucal 2019). However, recent studies have suggested that PFOS concentrations in surface waters with limited sampling sites in northeastern states appear to have generally decreased since the voluntary phase out of PFOS in 2002 (Zhang et al. 2016; Pan et al. 2018). While these more recent studies observed lower measured PFOS concentrations in surface waters compared to those reported in earlier reports (Hansen et al. 2002; Nakayama et al. 2007), few studies have measured PFOS concentrations from the same sampling locations over time. However, it appears that eight studies (six focused on the Great Lakes and two in New York on the Hudson River; Table 1) measured PFOS in the same waterbody over time (Supplemental Data, Table S2 and Figure S1). Thus, the observed lower concentrations reported in recent literature could be due to trends of PFOS concentrations decreasing since the 2002 PFOS phaseout, differences in sampling site locations, and/or advances in analytical methods for detecting PFOS that reduced detection limits. If the dataset is limited to Lake Ontario, which is one of the most well-studied waterbodies for PFOS occurrence in the U.S., data from 2002 to 2010 indicates an apparent decrease in PFOS concentrations over time. This and any future decreases would be likely due to the reduction in PFOS use in manufacturing. However, this downward trend of PFOS concentrations in Lake Ontario surface waters was not statistically significant (p > 0.05; Remucal 2019).
PFOS occurrence and concentrations in the Great Lakes region
The Great Lakes are among the most widely studied waterbodies in the U.S. for PFOS occurrence. However, occurrence data are still relatively limited for this system and were largely collected between 2003 and 2010. Comparisons across the Great Lake system indicate PFOS concentrations are higher in Lakes Erie and Ontario, ranging between 2.8 and 38.5 ng/L and 2.9 and 85.5 ng/L, respectively (Figure 5; Sinclair et al. 2006; Boulanger et al. 2004; De Silva et al. 2011; and Furdui et al. 2009), compared to the more northern Great Lakes. These northern Great Lakes have a maximum reported concentration of 5.46 ng/L in Lake Huron. However, current measured PFOS concentrations in Lakes Huron, Michigan, and Superior were not from sampling sites around urbanized areas (such as Chicago and Detroit) and may not be representative of the potential sources of PFOS related to these areas. The measured concentrations of PFOS in the surface waters of Lakes Huron and Michigan, range between 0.24 and 5.46 ng/L (Table 1; Remucal 2019; Furdui et al. 2008; De Silva et al. 2011) and 0.93 and 3.13 ng/L (De Silva et al. 2011; Simcik and Dorweiler 2013), respectively. In contrast measured PFOS concentrations observed in Lake Superior were considerably lower and range between 0.074 and 0.996 ng/L (Scott et al. 2010; De Silva et al. 2011; Furdui et al. 2011). The higher PFOS concentrations in Lakes Erie and Ontario are likely due to higher levels of industrial activities and urbanization around these lakes (Boulanger et al. 2004; Remucal 2019), and could also be associated with the sampling locations. A mass balance constructed for Lake Ontario by Boulanger et al. (2004) indicated wastewater effluent was the major source of PFOS to the lake. In contrast, inputs from Canadian tributaries and atmospheric deposition of PFOS, and other PFAS that may be transformed into PFOS, were the major contributing sources of PFOS to Lake Superior. Inputs from Canadian tributaries and atmospheric deposition were estimated to contribute 57 and 32% of PFOS inputs into Lake Superior respectively (Scott et al. 2010).
Figure 5.
Distribution of the minimum and maximum concentrations (ng/L) of perfluorooctane sulfonate (PFOS) measured in surface water samples collected from the Great Lakes as reported in the current, publicly available literature and is not necessarily comprehensive of PFOS concentrations in surface waters across each state.. This distribution is arranged alphabetically by waterbody.
PFOS occurrence and concentrations in the southeastern U.S.
Measured PFOS concentrations in southeastern U.S. surface waters were similar to those measured in Lakes Erie and Ontario, with the exception of some of the highest concentrations detected in waterbodies near areas with PFOS manufacturing (Figure 6 and Table 1). In 1999, the 3M Company conducted a multi-city study measuring PFOS concentrations across waterbodies with known manufacturing and/or industrial uses of PFOS (3M Company 2001). In the 3M Company’s 2001 report, PFOS concentrations from sites with known PFOS discharges were compared to PFOS concentrations measured in waterbodies with no known sources of any PFAS chemical (3M Company 2001). In this comparison study, cities with known PFOS exposure were Mobile and Decatur, Alabama; Columbus, Georgia; and Pensacola, Florida. Measured PFOS concentrations ranged from not detected (reported detection limit of 2.5 ng/L; 3M Company 2001) to 41.5 ng/L in the cities with known PFOS discharges (Table 1). These PFOS concentrations were compared to those measured in control cities. These study control cities were Cleveland, Tennessee and Port St. Lucie, Florida and PFOS concentrations ranged from not detected to 137.5 ng/L (3M Company 2001). The PFOS concentrations measured in Cleveland, Tennessee were below the limit of detection (2.5 ng/L1) and were lower than the PFOS concentrations observed in the cities with known PFOS exposure, as was expected in the report for the control cities. However, PFOS concentrations around Port St. Lucie, Florida, the other control city, were unexpectedly similar to and at times higher than the waterbodies with known PFOS discharges. The sources of PFOS near Port St. Lucie, Florida remain unknown; however, observed PFOS concentrations suggest the presence of a potential manufacturing/industrial source or the use of AFFF in this area (3M Company 2001).
Figure 6.
Comparison of relatively high (A; greater than 30 ng/L) and low (B; less than 30 ng/L) maximum perfluorooctane sulfonate (PFOS) concentrations (ng/L) measured in surface water samples collected across the United States (U.S.) as reported in the current, publicly available literature and is not necessarily comprehensive of PFOS concentrations in surface waters across each state.. The relatively high PFOS concentrations were associated with specific nearby consumer and/or industrial source. Both distributions are arranged alphabetically by waterbody or state.
Water samples were collected from ponds near all of the sampling sites except those in Cleveland, Tennesse. As reported in Table 1, PFOS concentrations in these additional pond sites were similar to those measured in Mobile, Alabama (ranging between 32 and 33 ng/L), lower than those observed in Columbus, Georgia (as PFOS was not detected with a detection limit of 2.5 ng/L), and higher than those measured in Decatur, Alabama (ranging between 108 and 111 ng/L) and in Port St. Lucie, Florida (ranging between 1,830 and 48,200 ng/L). Samples collected from the pond site near Port St. Lucie, Florida had some of the highest measured PFOS concentrations in publicly available literature with the maximum concentration of 48,200 ng/L. In the report, the 3M Company conducted additional sampling at the pond site in Port St. Lucie, Florida and determined that the measured PFOS concentrations at this site were more variable than the initial measurements alone indicated and were lower than the previous measurements, ranging between below detection (i.e., < 2.5 ng/L) and 2,340 ng/L. Aside from the samples collected in Port St. Lucie, Florida, this report demonstrated that measured PFOS concentrations in surface waters tend to be higher in areas with PFOS manufacturing and/or industrial use (3M Company 2001).
In separate studies, PFOS and PFOA concentrations were measured in surface waters by Hansen et al. (2002) near Decatur, Alabama and Konwick et al. (2008) in Georgia. Hansen et al. (2002) studied a stretch of the Tennessee River near Decatur, Alabama and Konwick et al. (2008) focused on the Conasauga River in Georgia, both areas with known PFOS discharge and use. In Hansen et al. (2002), discharge from a fluorochemical manufacturing facility entered the Tennessee River towards the middle of the study area. In contrast, Konwick et al. (2008) compared the PFOS concentrations measured in the Conasauga River with those from sites with no known exposure along the Altamaha River. In both studies, mean PFOS concentrations were higher in the study areas with PFOS sources. Specifically, Hansen et al. (2002) observed mean PFOS concentrations upstream of the fluorochemical manufacturing facility were 30.85 ng/L (ranging between 16.0 and 52.6 ng/L) and were 103.9 ng/L (ranging between 30.3 and 144 ng/L) downstream of the fluorochemical manufacturing facility. Similarly, Konwick et al. (2008) observed higher measured PFOS concentrations in the Conasauga River, which ranged from below limit of detection (with a limit of detection of 1.5 ng/L) to 321 ng/L, compared to those in the Altamaha River, ranging between 2.6 and 2.7 ng/L. Consistent with the report from the 3M Company summarized above, effluents from manufacturing facilities, WWTP, and carpet mill effluents were determined to be the source of increased PFOS concentrations in both the Tennessee and Conasauga Rivers (Hansen et al. 2002 and Konwick et al. 2008, respectively). These PFOS concentrations are relatively consistent with those measured in Alabama and Georgia as reported by the 3M Company (3M Company 2001).
Nakayama et al. (2007) and Cochran (2015) measured PFAS, including PFOS, in the Cape Fear Drainage Basin in North Carolina and waterbodies on Barksdale Air Force Base in Bossier City, Louisiana, respectively. PFOA and PFOS were found to be the dominant PFAS detected in both studies. Nakayama et al. (2007) detected PFOS in 97.5% of all samples above the limit of quantification of 1 ng/L. PFOS concentrations in the Cape Fear Drainage Basin ranged between < 1 (the lower limit of quantification) and 132 ng/L with a mean concentration of 31.2 ng/L. As in other studies summarized above, lower PFAS concentrations, including PFOS, were found in the upland tributaries and concentrations were highest in the middle reaches of the Cape Fear Drainage Basin, near expected sources. Municipal wastewater treatment plant effluents were identified as a source of PFAS to the study area. AFFF usage at the Department of Defense base in Fayetteville, North Carolina and the land application of biosolids likely contributed as well (Nakayama et al. 2007). Cochran (2015) detected PFOS in 79% of all water samples collected and concentrations ranged between below the limit of quantification (i.e., 10 ng/L) and 7,070 ng/L, with an average concentration of 776.7 ng/L. PFOS concentrations varied in samples collected in Barksdale Air Force Base based on proximity to fire training areas. Cochran (2015) attributed the evaluated PFOS concentrations to run off and ground infiltration of AFFF formally used on the base during firefighting and/or training.
PFOS occurrence and concentrations in the midwestern U.S.
Similar PFOS concentrations were reported in the publicly available literature for waterbodies in urban areas across the midwestern U.S. Lower PFOS concentrations were reported in areas with no previous PFAS exposure (identified as remote areas by the individual study authors) in the same states (Simcik and Dorweiler 2005; Newsted et al. 2017). In Minnesota, Simcik and Dorweiler (2005) observed PFOS concentrations ranging between 2.4 and 50.4 ng/L in urban areas near Minneapolis and concentrations ranging between less than the limit of quantification of 0.29 ng/L and 1.2 ng/L were observed in remote areas in northern Minnesota (Table 1). Additionally, Newsted et al. (2017) reported an average PFOS concentration of 528.9 ng/L (ranging between below limit of quantification and 18,200 ng/L; limit of quantification not provided) in surface waters collected from the Upper Mississippi River near the Minneapolis/St. Paul, Minnesota metropolitan area with a maximum concentration of 18,200 ng/L. The occurrence of PFOS at these urban sites was attributed to the presence of manufacturing source, runoff, and wastewater discharge (Simcik and Dorweiler 2005 and Newsted et al. 2017).
PFOS occurrence and concentrations in the northeastern U.S.
Several studies measured PFOS concentrations in surface waters in the northeastern U.S that are comparable to those reported in Minnesota (Sinclair et al. 2006; NJ DEP 2019). Sinclair et al. (2006) measured PFOS in various waterbodies across New York state and observed a median concentration of 756 ng/L in surface waters collected from a Superfund site at Lake Onondaga (ranging between 198 and 1,090 ng/L; Table 1) and attributed these elevated concentrations to several industries located along Lake Onondaga. All other observed concentrations of PFOS in New York, including sites along the Niagara River, the Finger Lakes, Lakes Oneida and Champlain, the Erie Canal, and Hudson River, had lower median PFOS concentrations ranging between 0.8 and 13 ng/L (Table 1; Sinclair et al. 2006).
New Jersey Department of Environmental Protection (NJ DEP) measured PFOS in surface water samples collected from 14 different sites across New Jersey. PFOS concentrations ranged from below the detection limit of 1.0 ng/L to 102 ng/L (NJ DEP 2019). Individual samples collected along Pine, Little Pine, and Mirror Lakes had measured PFOS concentrations of 102, 100, and 72.9 ng/L, respectively. All other observed concentrations of PFOS in New Jersey freshwaters were below 15 ng/L (Table 1). NJ DEP attributed the elevated concentrations of PFOS observed at Pine, Little Pine, and Mirror Lakes to the use of AFFF in training and/or firefighting on the Department of Defense (DoD) Joint Base McGuire-Dix-Lakehurst (NJ DEP 2019).
PFOS occurrence and concentrations in the western U.S.
PFOS concentrations in surface waters of western U.S. states are generally consistent with the lower-end concentrations (less than 100 ng/L) measured in eastern states; however, the monitoring data for PFOS was limited in the western U.S. Plumlee et al. (2008) measured PFOS and PFOA in Coyote Creek and a tributary of Upper Silver Creek in San Jose, California and determined PFOS concentrations in both Coyote and Upper Silver Creeks to be similar to those measured in eastern states, which are summarized above (Figure 6). Concentrations of PFOS in Coyote Creek ranged from 4.8 to 25 ng/L and concentrations in Upper Silver Creek ranged from 27 to 56 ng/L. The source of PFOS to these aquatic systems was unknown, however, Plumlee et al. (2008) stated that a combination of atmospheric deposition of volatile precursors and surface runoff were likely sources of PFOS to both Coyote and Upper Silver Creeks.
State level data are currently available for Colorado and New Mexico. In particular Colorado Department of Public Health and the Environment (2020) measured PFOS in surface water samples collected from 71 different sampling locations across Colorado. PFOS concentrations ranged from below the detection limit (which varied between 0.42 and 2.50 ng/L across sites) to 54 ng/L. New Mexico Environment Department (2021) also measured PFOS concentrations collected from 67 surface water sampling sites across the state. These PFOS concentrations ranged from below detection limit (which varied between 0.86 and 1.9 ng/L across sites with some limits not reported) to 5,900 ng/L. The elevated concentrations of PFOS were observed in sampling locations near Holloman Air Force Base (New Mexico Environment Department 2021).
Lastly, Dinglasan-Panlilio et al. (2014) measured PFOS concentrations in surface waters along the Puget Sound in Washington, as well as Clayoquot and Barkley Sounds in British Columbia, Canada. PFOS concentrations measured by Dinglasan-Panlilio et al. (2014) were lower than those observed from sites in eastern states (such as those summarized above for Alabama, Florida, and North Carolina with known manufacturing and/or industrial use of PFOS (Table 1) Concentrations ranging from 0.2 to 5.9 ng/L in Puget Sound and 0.25 to 0.7 ng/L in Clayoquot and Barkley Sound, British Columbia. These concentrations are consistent with those reported in the publicly available literature for areas identified as remote by individual study authors, such as in Minnesota (Simcik and Dorweiler 2005) and in New York (Sinclair et al. 2006), as summarized above. The study authors indicated specific regional sources and atmospheric deposition were likely PFOS sources to these areas with no previous PFAS exposure (Dinglasan-Panlilio et al. 2014).
Summary of PFOS occurrence and concentrations across the U.S.
Despite the wide use and persistence of PFOS in aquatic ecosystems and unlike the sampling of PFOS in drinking water sources2, groundwater, and fish tissue monitoring3, current information on the environmental distribution of PFOS in ambient surface waters across the U.S. remains very limited. Additionally, sampling efforts in other media corroborate that PFOS occurrence in aquatic ecosystems is relatively widespread, particularly in urban areas with detection frequencies of 73% in fish tissue samples from urban rivers and 100% in fish tissue samples from the Great Lakes (Stahl et al. 2014). Similar conclusions were reached in a sampling effort across Canada by Gewurtz et al. (2013), which demonstrated that distribution of PFOS detected in multiple media types (i.e., air, water, sediment, and fish and bird tissue) generally related to urbanization with PFOS concentrations reported in surface water for 23 out of 31 sampling locations.
Present surface water occurrence data are largely collected from freshwater systems in eastern states and in the upper midwest and focused on a handful of study areas with known manufacturing or industrial uses of PFAS or use of AFFF. Current data indicate that PFOS concentrations measured in U.S. surface waters vary widely, across eight orders of magnitude (Table 1). PFOS concentrations in areas with little to no PFAS manufacturing and/or industrial use) range between 0.074 to 23.23 ng/L (Figure 4 and Table 1). This contrasts with PFOS concentrations measured in areas with known PFAS manufacturing, industrial use, and/or application of AFFF, which vary widely and reach up the maximum observed concentration of 8,970,000 ng/L at a site impacted by AFFF (Figure 6 and Table 1). While current PFAS occurrence data illustrate the prevalence and quantify concentrations of PFOS in ambient surface waters across the U.S., additional data, particularly in central, southwestern, and western freshwaters as well as saltwater systems, is needed to better understand PFOS occurrence in aquatic ecosystems across the U.S.
COMPARISON OF PFOS OCCURRENCE IN THE U.S. TO GLOBAL AMBIENT SURFACE WATERS
Similar surface waters in the U.S., generally PFOS and PFOA were the most commonly detected PFAS in surface waters around the world (Ahrens 2011). However, it should be noted that the frequency of PFOS and PFOA detection may be an artifact resulting from regulations of these compounds and the lack of available standardized analytical methods for other PFAS. Generally, on a global scale PFOS concentrations in surface waters generally range between picogram/liter and nanogram/liter with some concentrations in the milligram/liter range. PFOS concentrations in the U.S. were comparable to those reported in studies with sampling sites in other countries. Global surface water PFOS concentrations reported in the public literature ranged between not detected and 2,100,000 ng/L. These global surface water concentrations are summarized below to provide a comparison with those observed in the U.S.
In Canada elevated PFOS concentrations in surface waters generally occurred in urbanized areas, suggesting urban areas with high population densities contributed to the elevated PFOS concentrations, similar to indications from U.S. data (Gewurtz et al. 2013; Scott et al. 2009). And PFOS was monitored and assessed for locations across Canada from 2006 through 2011, and it was concluded that PFOS posed a risk to the aquatic environment (ECCC 2018). PFOS concentrations measured by Gewurtz et al. (2013) ranged between not detected (with a detection limit of 2 ng/L) and 10 ng/L in surface waters across Canada. PFOS was rarely detected in surface water samples collected from non-urban areas (Gewurtz et al. 2013). In a systematic, cross-Canada study of PFAS in surface waters, Scott et al. (2009) observed PFOS and PFOA as the dominant PFAS detected and that generally PFOS concentrations were higher, overall ranging between < 0.02 and 34.6 ng/L, than PFOA concentrations, which ranged between 0.044 and 9.9 ng/L. These studies indicated PFOS concentrations in Canadian surface waters were lower than those in the U.S., Europe and Asia (Scott et al. 2009). However, PFOS ranged from not detected (with a detection limit of 4 ng/L) to 2,100,000 ng/L in Etobicoke Creek, a tributary to Lake Ontario, after an accidental spill of a fire-retardant foam containing perfluorinated surfactants was released at L.B. Pearson International Airport in Toronto, Ontario in June 2000 (Moody et al. 2002). The elevated concentrations of PFOS measured by Moody et al. (2002) were higher than those measured in U.S. surface waters and are consistent with the presence of elevated concentrations in surface waters near a source of PFOS (Ahrens 2011).
PFOS concentrations measured in surface waters across Europe were similar to those observed in the U.S. Specifically, in a European Union (EU)-wide study of polar organic persistent pollutants, Loos et al. (2009) observed a median PFOS concentration of 6 ng/L in surface waters sampled across a wide range of sampling sites (including contaminated and pristine rivers and streams of various sizes). However, relatively high median PFOS concentrations between 32 (from the Rhine River in Germany) and 1,371 ng/L (from Krka River in Slovenia) were also observed. Mean PFOS concentrations observed by Pan et al. (2018) were similar to those reported in Loos et al. (2009) and across the U.S., with mean surface water concentrations from waterbodies across western Europe, specifically the Thames River, Mälaren Lake, and Rhine River, ranging between 3.15 and 13.8 ng/L and a maximum concentration of 18.8 ng/L measured in the Thames River. Kwadijk et al. (2010) reported PFOS concentrations between 4.7 and 32 ng/L in surface water samples collected from 20 sampling locations across the Netherlands. Lastly, similar and some slightly higher PFOS concentrations, with averages ranging between 16 and 449 ng/L, were observed by Huset et al. (2008) in the Glatt Valley Watershed in Switzerland. Like in the U.S. and Canada, concentrations of PFOS in surface waters across Europe were higher in urbanized areas and sources have been attributed to municipal waste water treatment plant effluent, AFFF spills, and fluorochemical manufacturing facilities (Loos et al. 2007 and 2009; Huset et al. 2008; Kwadijk et al. 2010; Ahrens 2011; Pan et al. 2018).
Lastly, like PFOS concentrations observed in Canada and Europe, PFOS occurrence in surface waters across Asia were generally similar to those reported in the U.S., with lower reported maximum concentrations being observed in Asia compared to the U.S. (Xu et al. 2013). In Japan, Saito et al. (2003) observed PFOS concentrations ranging between 0.3 and 157 ng/L with a median of 1.68 ng/L in 142 surface water samples collected from various locations. Similarly, Nguyen et al. (2011) reported PFOS concentrations ranging between 1 and 156 ng/L collected from an urbanized section of the Marina catchment in Singapore. However, PFOS concentrations in more recently collected surface water samples reported by Pan et al. (2018) were lower than those previously reported in Asia from publicly available literature. Median surface water PFOS concentrations from samples collected from the Yangtze (sample size (n) = 35), Yellow (n = 15), Pearl (n = 13), Liao (n = 6), Han (n = 6), and Huai (n = 9) Rivers and Chao (n = 13) and Tai (n = 15) Lakes ranged between 1.41 and 8.56 ng/L with the an overall maximum PFOS concentration of 29.7 ng/L in Choa Lake (Pan et al. 2018). Overall, the PFOS concentrations observed in Asia were similar to the lower end of those reported in the U.S.
Overall, these studies show the widespread distribution and variability of PFOS concentrations in surface waters around the world and demonstrate that surrounding land use has a large influence on PFOS concentrations in ambient surface waters. Urbanized areas with high population densities tended to have elevated PFOS concentrations in surface waters (Loos et al. 2007 and 2009; Scott et al. 2009; Ahrens 2011; Gewurts et al. 2013). Like in the U.S., PFOS concentrations in surface waters around the world vary widely and current information on the environmental distribution of PFOS in ambient surface waters around the world is relatively limited.
CONCLUSIONS
Currently PFOS is one of the most commonly observed PFAS detected in surface waters (Ahrens 2011; Benskin et al. 2012; Zareitalabad et al. 2013; Dinglasan-Panlilio et al. 2014; Nakayama et al. 2017; Remucal 2019). As demonstrated in this review, PFOS has been detected in a number of ambient surface waters across the U.S. and concentrations of PFOS vary widely (over eight orders of magnitude). The occurrence of PFOS in surface waters indicates the presence of an anthropogenic source, such as consumer and/or industrial use and/or atmospheric deposition, surface water runoff or groundwater discharge, and results from the general persistence and mobility of these chemicals in the environment.(Ahrens 2011). This review indicates that elevated PFOS concentrations are generally associated with a nearby source or urbanization (Table 2). PFOS concentrations measured in areas with known PFAS sources varied widely with a maximum observed concentration of 8,970,000 ng/L (Table 1 and Figure 6A) in comparison to detected PFOS concentrations measured in areas with little or no PFAS sources, which ranged between 0.074 and 23.23 ng/L (Figure 6B). Additionally, some ambient surface water concentrations in the U.S. are within the range of observed toxicity values reported in current literature (with effect concentrations ranging between 28 and 5000,000,000 ng/L; https://cfpub.epa.gov/ecotox/).
As restrictions of PFOS have gone into place, concentrations in ambient surface waters are expected to decrease. Several studies have suggested that PFOS concentrations in U.S. surface waters have decreased since 2002 (Zhang et al. 2016; Pan et al. 2018). Although these studies observed lower measured PFOS concentrations in surface waters compared to those reported in earlier literature (Hansen et al. 2002; Nakayama et al. 2007), to the authors’ knowledge, there has not been a systematic sampling effort to measure PFOS concentrations in sites previously sampled or a comparison between analytical methods to confirm the decrease in PFOS concentrations. Recent studies have reported a shift in the PFAS compounds reported in the aquatic environment. Concentrations of shorter-chained PFAS, particularly PFSAs and perfluoroalkyl carboxylic acids (PFCAs), have increased compared to those of PFOS and PFOA (Möller et al. 2009; Pan et al. 2018) as use has switched to shorter chain PFAS. While the shift in PFAS use and manufacturing may result in a decrease in PFOS concentrations entering aquatic environments it will likely take decades or longer for existing sources of PFOS to be reduced to the point that they do not impact water quality in aquatic systems.
Despite the historical wide use, high persistence, and increased public interest in PFAS generally (including PFOS, which is one of the most commonly detected PFAS in surface waters) current information on the distribution of PFOS in aquatic environments across the U.S. is fairly limited and is largely based on studies that have targeted sites where PFAS were known to have been used. However, it should be noted that many states in the U.S. have conducted monitoring of PFOS and most of the state monitoring data for PFOS are currently not publicly available. Thus, the current dataset contains sampling location and study design bias (Figures 1, 3, and 4). Currently, PFOS occurrence data are limited in western states across the U.S., with the existing dataset only including data for California and Washington (Figure 1). Additionally, PFOS occurrence data are limited in marine and estuarine environments. Therefore, both of these areas continue to be PFOS occurrence data gaps in the U.S. Future PFOS sampling efforts should include consideration of filling these data gaps. Additionally, many of the current PFOS occurrence studies in the public literature include numerous sites with no known PFAS exposure in order to compare PFOS concentrations to exposed sites. This general study design may result in a higher frequency of measured PFOS concentrations below 300 ng/L compared to middle, high, and very high concentrations. This tendency may skew the median and mean concentrations of PFOS in ambient surface waters across the U.S. to the lower end of the concentrations. However, based on the great difference between the arithmetic mean concentration of 786.77 ng/L, a geometric mean concentration of 5.468 ng/L, and the median concentration of 3.6 ng/L for measured PFOS concentrations across the U.S. in the currently available public literature, the measured PFOS concentrations in ambient surface waters occur over a wide range. Also, the increased frequency of measured PFOS concentrations at sites with no known previous PFAS exposure or sites that were not identified by the individual study authors as either sites with or without previous exposure (Table 2 and Figure 4), may skew the measured PFOS concentrations in U.S. ambient surface waters toward the lower end of the wide range of measured concentrations. To better understand the occurrence of PFOS in ambient surface waters across the U.S, additional data from previously exposed sites and in urban areas, particularly those in areas where little or no data are available, are needed. Specifically, a systematic study focused on measuring PFAS (including PFOS, PFOA and their precursors and shorter chain PFAS) in ambient surface waters across the U.S. is needed to eliminate potential bias from differences in analytical methods, sample collection, and/or location, and to fill in existing data gaps. Filling these data gaps would provide a more robust understanding of the spatial and temporal variability of PFOS occurrence in U.S. ambient surface waters. Given the widespread use of PFAS and the persistence of PFOS in the aquatic environment, a thorough understanding of the total environmental distribution of PFAS in surface waters (particularly of PFOS itself and the volatile compounds that can transform PFSA) is needed to fully understand the occurrence of PFOS in the environment and any potential risks it may pose in aquatic ecosystems.
Supplementary Material
Acknowledgment—
We thank Tyler Linton, Keith Taulbee, and Dale White from Great Lakes Environmental Center. We also thank the following reviewers from EPA: Laurence Libelo (Office of Land and Emergency Management), Mark Strynar (Office of Research and Development), and Christine Bergeron (Office of Water) for their technical review and input.
Footnotes
Disclaimer—The views expressed in this manuscript are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
Supplemental Data—The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.xxxx.
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi:10.1002/etc.5147.
Limits of detection and quantification differ across the PFOS occurrence literature and is dependent on the specific lab and the analytical methods used in a particular study. Specific limits of detection and quantification provided in the subsequent text for each individual study.
EPA’s database for the Unregulated Contaminant Monitoring Rule (UCMR) that includes data for treated surface waters, (https://www.epa.gov/dwucmr)
EPA’s National Rivers and Streams Assessment (NRSA; https://www.epa.gov/national-aquatic-resourcesurveys/ncca) and the Great Lakes Human Health Fish Tissue Study component of the EPA National Coastal Condition Assessment (NCCA/GL)
Data availability statement—
Data, associated metadata, and calculation tools are available from the corresponding author (jarvis.amanda@epa.gov).
REFERENCES
- 3M Environmental Laboratory. 2011. Environmental monitoring – multi-city study water, sludge, sediment, POTW effluent and landfill leachate samples. Executive Summary. https://static.ewg.org/files/MultiCity_execsum.pdf [Google Scholar]
- Ahrens L 2011. Polyfluoroalkyl compounds in the aquatic environment: a review of their occurrence and fate. J. Environ. Monit 13:20–31. [DOI] [PubMed] [Google Scholar]
- Ahrens L Yeung LWY, Taniyasu S, Lam PKS, Yamashita N 2011. Partitioning of perfluorooctanoate (PFOA), perfluorooctane sulfonate (PFOS) and perfluorooctane sulfonamide (PFOSA) between water and sediment. Chemosphere. 85:731–737. [DOI] [PubMed] [Google Scholar]
- Ahrens L and Bundschuh M. 2014. Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: a review. Environ. Toxicol. Chem 33:1921–1929. [DOI] [PubMed] [Google Scholar]
- Allred BM, Lang JR, Barlaz MA, Field JA 2015. Physical and biological release of poly- and perfluoroalkyl substances (PFASs) from municipal solid waste in anaerobic model landfill reactors. Environ Sci Technol. 49:768–7656. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Long GC, Porter RC, Anderson JK. 2016. Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foams release sites other than fire-training areas: field-validation of critical fate and transport properties. Chemosphere. 150: 678–685. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Cureton P, Hoke RA, Houde M, Kumar A, Kurias J, Lanno R, McCarthy C, Newsted J, Salice CJ, Sample BE, Sepúlveda Steevens J, Valsecchi S 2020. Assessing the ecological risks of Per- and Polyfluoroalkyl substances: current state-of-the science and a proposed path forward. Environ. Toxicol. Chem 40: 564–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SA, Newsted JL, Coady K, Giesy JP. 2006. Ecotoxicological evaluation of perfluorooctanesultonate (PFOS). Rev Environ Contam Toxicol. 186:133–174. [DOI] [PubMed] [Google Scholar]
- Benskin PJ, Mui DCG, Scott BF, Spencer C, De Silva AO, Kylin H, Martin JW, Morris A, Lohmann R, Tomy G, Rosenberg B, Taniyasu S, Yamashita N. 2012. Perfluoroalkyl acids in the Atlantic and Canadian Arctic oceans. Environ Sci Technol. 46:5815–5823. [DOI] [PubMed] [Google Scholar]
- Benskin JP. Holt A, Martin JW 2009. Isomer-specific biotransformation rates of a perfluoruooctane sulfonate (PFOS)-precursor by cytochrome P450 isozymes and human liver miscrosomes. Environ Sci Technol 43: 8566–8572. [DOI] [PubMed] [Google Scholar]
- Boulanger B, Vargo JD, Schnoor JL, Hornbuckle KI. 2005. Evaluation of perfluorooctane surfactants in wastewater treatment system and in commercial surface protection product. Environ Sci Technol. 39: 5524–5530. [DOI] [PubMed] [Google Scholar]
- Boulanger B, Vargo J, Schnoor JL, Hornbuckle KC 2004. Detection of perfluorooctane surfactants in Great Lakes Water. Enrion Sci Technol 38:4064–4070. [DOI] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, De Voogt P, Jensen AA, Kannan K, Mabury SA, Van Leeuwen SPJ. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr Environ Assess Manag. 7:513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhard LP. 2021. Evaluation of published bioconcentration factor (BCF) and bioaccumulation factor (BAF) data for Per- and Polyfluoroalkyl substances across aquatic species. Environ. Toxicol. Chem 40: 1530–1543. [DOI] [PubMed] [Google Scholar]
- Cochran RS. 2015. Evaluation of perfluorinated compounds in sediment, water, and passive samplers collected from the Barksdale Air Force Base. MS Thesis. Texas Tech University, Lubbock, Texas United States. [Google Scholar]
- Colorado Department of Public Health and the Environment. 2020. PFAS sampling project results: surface water. https://cohealthviz.dphe.state.co.us/t/EnvironmentalEpidemiologyPublic/views/PFAS_results_DRAFT/Samplelocationsdash?%3AshowAppBanner=false&%3Adisplay_count=n&%3AshowVizHome=n&%3Aorigin=viz_share_link&%3AisGuestRedirectFromVizportal=y&%3Aembed=y
- De Silva AO, Spencer C, Scott BF, Backus S, Muir DCG. 2011. Detection of a cyclic perfluorinated acid, perfluoroethylcyclohexane sulfonate, in the Great Lakes of North America. Environ Sci Technol. 45:8060–8066. [DOI] [PubMed] [Google Scholar]
- Dinglasan-Panlilio M, Prakash SS, Baker JE 2014. Perfluorinated compounds in the surface waters of Puget Sound, Washington and Clayoquot and Barkley Sounds, British Columbia. Marine Pollut Bullet. 78:173–180. [DOI] [PubMed] [Google Scholar]
- Environment and Climate Change Canada (ECCC). 2018. Federal Environmental Quality Guidelines Perfluorooctance Sulfonate (PFOS). https://www.canada.ca/en/environment-climate-change/services/evaluatingexisting-substances/federal-environmental-quality-guidelines-perfluorooctanesulfonate.html
- Furdui VI, Crozier PW, Reiner EJ, Mabury SA. 2008. Trace level determination of perfluorinated compounds in water by direct injection. Chemosphere. 73:524–530. [DOI] [PubMed] [Google Scholar]
- Gewurtz SB, Backus SM, De Silva AO, Ahrens L, Armellin A, Evans M, Fraser S, Gledhill M, Guerra P, Harner T, Helm PA, Hung H, Khera N, Kim MG, King M, Lee SC, Letcher RJ, Martin P, Marvin C, McGoldrick DJ, Myers AL, Pelletier M, Pomeroy J, Reiner EJ, Rondeau M, Sauve MC, Sekela M, Shoeib M, Smith DW, Smyth SA, Struger J, Spry D, Syrgiannis J, Waltho J. 2013. Perfluoroalkyl acids in the Canadian environment: Multi-media assessment of current status and trends. Environ Int 59:183–200. [DOI] [PubMed] [Google Scholar]
- Giesy JP, Naile JE, Khim JS, Jones PD, Newsted JL. 2010. Aquatic toxicology of perfluorinated chemicals. Rev Environ Contam Toxicol 202:1–52. [DOI] [PubMed] [Google Scholar]
- Hansen KJ, Johnson HO, Eldridge JS, Butenhoff JL, Dick LA. 2002. Quantitative characterization of trace levels of PFOS and PFOA in the Tennessee River. Environ Sci Technol. 36:1681–1685. [DOI] [PubMed] [Google Scholar]
- Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DCG. 2006. Biological monitoring of polyfluoroalkyl substances: A review. Environ Sci Technol 40:3463–3473. [DOI] [PubMed] [Google Scholar]
- Houde M, De Silva AO, Muir DCG, Letcher RJ. 2011. Monitoring or perfluorinated compounds in aquatic biota: an updated review. Environ Sci Technol. 45:7962–7973. [DOI] [PubMed] [Google Scholar]
- Houtz EF, Sutton R, Park J, Sedlak M. 2016. Poly- and perfluoroalkyl substances in wastewater: significance of unknown precursors, manufacturing shifts, and likely AFFF impacts. Water Res. 95: 142–149. [DOI] [PubMed] [Google Scholar]
- Huset CA, Chiaia AC, Barfosky DF, Jonkers N, Kohler H- PE, Ort C, Giger W, Field JA. 2008. Occurrence and mass flows of fluorochemicals in the Glatt Valley watershed, Switzerland. Environ Sci Technol 42:6369–6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan K, Tao L, Sinclair E, Pastva SD, Jude DJ, Giesy JP. 2005. Perfluorinated compounds in aquatic organisms at various trophic levels in a Great Lakes food chain. Arch Environ Contam Toxicol. 48: 559–566. [DOI] [PubMed] [Google Scholar]
- Kannan K 2011. Perfluoroalkyl and polyfluoroalkyl substances: current and future perspectives. Environ Chem. 8:333–338. [Google Scholar]
- Kim S and Kannan K. 2007. Perfluorinated acids in air, rain, snow, surface runoff, and lakes: relative importance of pathways to contamination of urban lakes. Environ Sci Technol. 41:8328–8334. [DOI] [PubMed] [Google Scholar]
- Konwick BJ, Tomy GT, Ismail N, Peterson JT, Fauver RJ, Higginbotham D, Fisk AT. 2008. Concentrations and patterns of perfluoroalkyl acids in Georgia, USA surface waters near and distant to a major use source. Environ Toxicol Chem. 27:2011–2018. [DOI] [PubMed] [Google Scholar]
- Kwadijk CJAF, Korytar P, Koelmans AA. 2010. Distribution of perfluorinated compounds in aquatic systems in The Netherlands. Environ Sci Technol 44:3746–3751. [DOI] [PubMed] [Google Scholar]
- Lang JR, Allred BM, Peaslee GF, Field JA, Barlaz MA. 2016. Release of per- and polyfluoroalkyl substances (PFASs) from carpet and clothing in model anaerobic landfill reactors. Envrion Sci Technol 50: 5024–5032. [DOI] [PubMed] [Google Scholar]
- Lange CC. 2000. The aerobic biodegradation of N-EtFOSE alcohol by the microbial activity present in municipal wastewater treatment sludge. The 3M Company. St. Paul, MN. [Google Scholar]
- Lasier PJ, Wahsington JW, Hassan SM, Jenkins TM. 2011. Perfluorinated chemicals in surface water and sediments from northwest Georgia, USA, and their bioaccumulation Lumbriculus variegatus. Environ Toxicol Chem. 20(10): 2194–2201. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. 2007. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 99:366–394. [DOI] [PubMed] [Google Scholar]
- Lehmler H 2005. Synthesis of environmentally relevant fluorinated surfactants – a review. Chemosphere. 58:1471–1496. [DOI] [PubMed] [Google Scholar]
- Lindstrom A, Strynar M, Libelo EL. 2011. Polyfluorinated compounds: past, present, and future. Environ. Sci. Technol 45:7954–7961. [DOI] [PubMed] [Google Scholar]
- Liu X, Guo Z, Krebs KA, Pope RH, Roache NF. 2014. Concentrations and trends of perfluorinated chemicals in potential indoor sources from 2007 through 2011 in the US. Chemosphere 98: 51–57. [DOI] [PubMed] [Google Scholar]
- Liu J and Avendaño SM 2013. Microbial degradation of polyfluoroalkyl chemicals in the environment: a review. Environ Intern 61:98–114. [DOI] [PubMed] [Google Scholar]
- Loos R, Wollgast J, Huber T, Hanke G 2007. Polar herbicides, pharmaceutical products, perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and nonylphenol and its carboxylates and ethoxylates in surface and tap waters around Lake Maggiore in northern Italy. Annul Bioanal Chem. 387:1469–1478. [DOI] [PubMed] [Google Scholar]
- Loos R, Gawlik BM, Locoro G, Rimaviciute E, Contini S, Bidoglio G. 2009. EU-wide survey of polar organic persistent pollutants in European river waters. Environ Pollut 157:561–568. [DOI] [PubMed] [Google Scholar]
- Möller A, Ahren L, Sturm R, Ebinghaus R. 2009. Identification of point sources of polyfluoroalkyl compounds (PFCs) along the River Rhine watershed and their transportation into the North Sea. Coastline Reports. 13–143–154. [Google Scholar]
- Moody CA, Martin JW, Kwan WC, Muir DCG, Mabury SA. 2002. Monitoring perfluorinated surfactants in biota and surface water samples following an accidental release of fire-fighting foam into Etobicoke Creek. Environ Sci Technol 36:545–551. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Strynar MJ, Helfant L, Egeghy P, Ye X, Lindstrom AB. 2007. Perfluorinated compounds in the Cape Fear Drainage Basin in North Carolina. Environ Sci Technol. 41:5271–5276. [DOI] [PubMed] [Google Scholar]
- New Jersey Department of Environmental Protection Divison of Science, Research, and Environmental Health. 2019. Investigation of levels of perfluorinated compounds in New Jersey fish, surface water, and sediment. SR15–010. https://www.nj.gov/dep/dsr/publications/Investigation%20of%20Levels%20of%20Perfluorinated%20Compounds%20in%20New%20Jersey%20Fish,%20Surface%20Water,%20and%20Sediment.pdf
- New Mexico Environment Department. 2020–2021. https://www.env.nm.gov/pfas/data/
- Newsted JL, Holem R, Hohenstein G, Lange C, Ellefson M, Reagen W, Wolf S. 2017. Spatial and temporal trends of poly- and perfluoroalkyl substances in fish fillets and water collected from pool 2 of the upper Mississippi River. Environ Toxicol Chem. 36:3138–3147. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Reinhard M, Karina GY. 2011. Occurrence and source characterization of perfluorochemicals in urban watershed. Chemosphere. 82: 1277–1285. [DOI] [PubMed] [Google Scholar]
- [OECD] Organisation for Economic Co-operation and Development. 2002. Hazard assessment of perfluorooctane sulfonate (PFOS) and its salts. Report ENV/JM/RD(2002)17/FINAL. [Google Scholar]
- Oliaei F Kriens D Weber R, Watson A 2013. PFOS and PFC releases and associated pollution from a PFC production plant in Minnesota (USA). Environ Sci Pollut Res. 20: 1977–1992. [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhang H, Cui Q, Sheng N, Yeung LWY, Sun Y, Guo Y, Dai J. 2018. Worldwide distribution of novel perfluoroether carboxylic and sulfonic acids in surface water. Environ Sci Technol. 52:7621–7629. [DOI] [PubMed] [Google Scholar]
- Paul AG, Jones KC, Sweetman AJ. 2009. A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ Sci Technol 43:386–392. [DOI] [PubMed] [Google Scholar]
- Plumlee MH, McNeil K, Reinhard M. 2009. Indirect photolysis of perfluorochemicals: hydroxyl radical-initiated oxidation of N-ethyl perfluorooctane sulfonamido acetate (N-EtFOSAA) and other perfluoroalkanesulfonamides. Environ Sci Technol. 43: 3662–3668. [DOI] [PubMed] [Google Scholar]
- Plumlee MH, Larabee J, Reinhard M. 2008. Perfluorochemicals in water reuse. Chemosphere. 72:1541–1547. [DOI] [PubMed] [Google Scholar]
- Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. 2006. Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol 40:32–44. [DOI] [PubMed] [Google Scholar]
- Remucal CK. 2019. Spatial and temporal variability of perfluoroalkyl substances in the Laurentian Great Lakes. Environ Sci Processes Impacts. 11:1816–1834. [DOI] [PubMed] [Google Scholar]
- Rankin K, Mabury SA, Jenkins TM, Washington JW. 2016. A North American and global survey of perfluoroalkyl substances in surface soils: distribution patterns and mode of occurrence. 161: 331–341. [DOI] [PubMed] [Google Scholar]
- Rhoads KR, Janssen EML, Luthy RG, Criddle CS. 2008. Aerobic biotransformation and fate of N-ethyl perfluorooctane sulfonamidoethanol (N-EtFOS) in activated sludge. Environ Sci Technol. 42: 2873–2878. [DOI] [PubMed] [Google Scholar]
- Saito N, Sasaki K, Nakatome K, Harada K, Yoshinaga T, Koizumi A. 2003. Perfluorooctane sulfonate concentrations in surface water in Japan. Arch Environ Contam Toxicol. 45:149–158. [DOI] [PubMed] [Google Scholar]
- Scott BF, Spencer C, Lopez E, Muir DCG. 2009. Perfluorinated alkyl acid concentrations in Canadian rivers and creeks. Water Qual Res J Can. 44:263–277. [Google Scholar]
- Scott BF, De Silva AO, Spencer C, Lopez E, Backus SM, Muir DCG. 2010. Perfluoroalkyl acids in Lake Superior water: trends and sources. J. Great Lake Res 36:277–284. [Google Scholar]
- Schultz MM, Higgins CP, Huset CA, Luthy RG, Barofsky DF, Field JA 2006. Fluorochemical mass flows in a municipal wastewater treatment facility. Envion Sci Technol. 40–7350–7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak MD. Benskin JP, Wong A, Grace R, Greig DJ 2017. Per- and polyfluoroalkyl substances (PFASs) in San Francisco Bay wildlife: temporal trends, exposure pathways, and notal presence of precursor compounds. Chemosphere. 185:1217–1226. [DOI] [PubMed] [Google Scholar]
- Simcik MF and Dorweiler KJ. 2005. Ratio of perfluorochemical concentrations as a tracer of atmospheric deposition to surface water. Environ Sci Technol. 39:8678–8683. [DOI] [PubMed] [Google Scholar]
- Sinclair E and Kannan K. 2006. Mass loading and fate of perfluoroalkyl surfactants in wastewater treatment plans. Environ Sci Technol. 40:1408–1414 [DOI] [PubMed] [Google Scholar]
- Sinclair E, Mayack DT, Roblee K, Yamashita N, Kannan K. 2006. Occurrence of perfluoroalkyl surfactants in water, fish, and birds from New York State. Arch Environ Contam Toxciol. 50:398–410. [DOI] [PubMed] [Google Scholar]
- Smithwick M, Norstrom RJ, Mabury SA, Solomon K, Evans TJ, Stirling I, Taylor MK, Muir DCG. 2006. Temporal trends of perfluoroalkyl contaminants in polar bears Ursus maritimus from two locations in the North American arctic. Environ. Sci. Technol 40:1139–1143. [DOI] [PubMed] [Google Scholar]
- Stahl LL. Snyder BD, Olsen AR, Kincaid TM, Wathen JB, McCarty HB 2014. Perfluorinated compounds in fish from U.S. urban rivers and the Great Lakes. Sci Total Environ. 499: 185–195. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. National Coastal Condition Assessment. https://www.epa.gov/nationalaquatic-resource-surveys/ncca
- U.S. EPA. Monitoring the occurrence of unregulated drinking water contaminants. https://www.epa.gov/dwucmr.
- U.S. EPA. ECOTOX Knowledgebase. https://cfpub.epa.gov/ecotox/ [Google Scholar]
- U.S. EPA. 2020. Long-Chain Perfluoroalkyl Carboxylate and Perfluoroalkyl Sulfonate Chemical Substances; Significant New Use Rule. Office of Pollution Prevention and Toxics. EPA-HQ-OPPT-2013–0225; FRL-10010–44. https://www.epa.gov/sites/production/files/2020-06/documents/10010-44_prepubcopy_fr_doc_epa_admin_esign_2020-06-22final.pdf [Google Scholar]
- Wang Z, DeWitt JC, Higgins CP, Cousins IT. 2017. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ. Sci. Technol 51:2508–2518. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Buck RC, Hungerbühler K. 2014. Global emission inventories C4 – C14 perfluoroaklyl carboxylic acid (PFCA) homologues from 1951 to 2030, part I: production and emission from quantifiable sources. Environ Internat 60: 62–75. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Buck RC, Hungerbühler K. 2014. Global emission inventories for C4 – C14 perfluoroaklyl carboxylic acid (PFCA) homologues from 1951 to 2030, part II: the remaining pieces of the puzzle. Environ Internat 60:166–176. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Hungerbühler K 2013. Fluorinated alternatives to long-chain perfluoroalkyla carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential percursors. Environ Internat. 60: 242–248. [DOI] [PubMed] [Google Scholar]
- Washington JW, and Jenkins TM. 2015. Abiotic hydrolysis of fluorotelomer polymers as a source of perfluorocarboxylates at the global scale. Environ Sci Technol 49:14129–14135. [DOI] [PubMed] [Google Scholar]
- Washington JW, Jenkins TM, Rankin K, Naile JE. 2015. Decades-Scale Degradation of Commercial, Side-Chain, Fluorotelomer-based Polymers in Soils & Water. Environ Sci Tech 49(2):915–923. [DOI] [PubMed] [Google Scholar]
- Xu X Xu H, Zhang D, Shen X, Tong G, Lu Y, Wang W 2013. Occurrence of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in aquatic systems from Asia. Appl Mechan and Materials. 295–298:513–519. [Google Scholar]
- Zareitalabad P, Siemens J, Hamer M, Amelung W. 2013. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater – a review on concentrations and distribution coefficients. Chemosphere. 91:725–732. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lohmann R, Dassuncao C, Hu XC, Weber AK, Vecitis CD, Sunderland EM. 2016. Source attribution of poly- and perfluoroalkyl substances (PFASs) in surface waters from Rhode Island and the New York Metropolitan Area. Environ. Sci. Technol. Lett 3:316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data, associated metadata, and calculation tools are available from the corresponding author (jarvis.amanda@epa.gov).