Abstract
Degradation of petroleum hydrocarbons was monitored in microcosms with diesel fuel-contaminated Arctic tundra soil incubated for 48 days at low temperatures (−5, 0, and 7°C). An additional treatment was incubation for alternating 24-h periods at 7 and −5°C. Hydrocarbons were biodegraded at or above 0°C, and freeze-thaw cycles may have actually stimulated hydrocarbon biodegradation. Total petroleum hydrocarbon (TPH) removal over 48 days in the 7, 0, and 7 and −5°C treatments, respectively, was 450, 300, and 600 μg/g of soil. No TPH removal was observed at −5°C. Total carbon dioxide production suggested that TPH removal was due to biological mineralization. Bacterial metabolic activity, indicated by RNA/DNA ratios, was higher in the middle of the experiment (day 21) than at the start, in agreement with measured hydrocarbon removal and carbon dioxide production activities. The total numbers of culturable heterotrophs and of hydrocarbon degraders did not change significantly over the 48 days of incubation in any of the treatments. At the end of the experiment, bacterial community structure, evaluated by ribosomal intergenic spacer length analysis, was very similar in all of the treatments but the alternating 7 and −5°C treatment.
Biological degradation and transformation of petroleum hydrocarbons in soil and water systems has gained increased interest and applications during the last decades (2). Biological treatment of contaminated soil is, when optimized properly, far more cost efficient than traditional methods such as incineration, storage, or concentration (4). When possible, contaminated soil is usually more efficiently treated and cleaned if the biological treatment can be done ex situ (2) since the addition of nutrients (nitrogen and phosphorus) and oxygen is then done more easily than in situ. Another important parameter to control is temperature. If possible, treatments of organic pollutants such as petroleum derivatives and aromatic hydrocarbons, are performed at moderate temperatures (20 to 37°C) in order to facilitate metabolic activity, diffusion, and mass transfer. A higher pollutant degradation rate is usually obtained at moderate than at lower temperatures (8, 24). It is generally believed that it is possible to use microorganisms to degrade and clean up soil contaminated with gasoline and diesel fuel hydrocarbons (mainly aliphatics) as long as there are no major limitations of bioavailability, oxygen, and temperature (2, 3, 8).
Numerous former and current Canadian military sites on the Arctic tundra have soil contaminated with Arctic diesel fuel. These sites are located in areas where the temperature rarely exceeds 10°C and the soil is frozen most of the year. In much of the Arctic, an active soil layer above the permafrost thaws for 1 to 2 months/year. Even during this summer period, the air temperature occasionally drops below 0°C. During the fall, when diurnal cycles begin to include periods of darkness, temperatures will fluctuate above and below freezing. These trends cause frequent freezing and thawing of surface soils. Most Arctic sites are remote, so shipping of contaminated soil for treatment in more temperate regions is not cost effective. Research shows that Arctic tundra soil has cold-adapted, hydrocarbon-degrading indigenous microorganisms (12, 19, 20). Similar findings have been reported for Antarctic and Alpine regions (10). Low-temperature bioremediation of petroleum hydrocarbon-contaminated polar and alpine tundra soil has been demonstrated in small-scale experiments (1, 10, 12). Permafrost microflora are metabolically active (incorporate acetate) at temperatures well below 0°C (15). However, temperatures below 15°C appear to be suboptimal for hydrocarbon biodegradation in Arctic tundra soils (12) and we are not aware of studies examining hydrocarbon biodegradation at 0°C or lower temperatures. Low-temperature limitation of hydrocarbon biodegradation may be due to the lower mass transfer rates and physical properties of the compounds at low temperatures rather than, or in addition to, direct temperature effects on the microorganisms involved. Understanding the effects of temperature is critical to efforts to bioremediate Arctic sites, since temperature clearly appears to limit that process. Most studies of temperature effects on microbial pollutant degradation rates and microbial communities have been done in the range of 5 to 30°C (9, 10). However, very little is known about how very low temperatures (0°C and below) and freeze-thaw cycles affect soil microbial pollutant degradation rates, overall metabolic activity, and population dynamics.
We performed a soil microcosm experiment, with biostimulation of indigenous microorganisms, to study the effect of temperatures of −5 to 7°C on the biological degradation of weathered diesel fuel in Arctic tundra soil. We also studied the effect of freeze-thaw cycles, which are likely common in Arctic surface soils. We monitored hydrocarbon removal, carbon dioxide production, populations of total heterotrophs and hydrocarbon degraders, RNA/DNA ratios as an indicator of bacterial metabolic activity, and community composition using ribosomal intergenic spacer (RIS) length analysis (RISA). To our knowledge, there are no previous reports on the effects of freezing and thawing on hydrocarbon degradation and community composition in hydrocarbon-contaminated Arctic soil.
MATERIALS AND METHODS
Chemicals.
Bushnell-Haas medium and tryptic soy broth were from Difco Laboratories (Detroit, Mich.), p-iodonitrotetrazolium violet, octane, dodecane, and hexadecane (all 99% pure or better) were from Sigma-Aldrich Canada Ltd. (Oakville, Ontario, Canada). Anhydrous sodium sulfate, hexane, and hexadecane (the latter two high-performance liquid chromatography grade) were from Fisher Scientific (Nepean, Ontario, Canada).
Soil microcosms.
Soil was from Canadian Forces Station Alert, located on the northeastern tip of Ellesmere Island, Nunavut, Canada (82o30′06" N, 62o19′47" W). The soil was transported and stored at 7°C and was used for this study approximately 6 months after collection. The soil contained approximately 1,000 μg of weathered Arctic diesel fuel (which is very similar to jet fuel) per g of dry soil. Microcosms were prepared in triplicate, each with 60 g of sieved soil (2-mm mesh) in 240-ml Teflon septum-sealed bottles (Supelco, Bellefonte, Pa.). The following were added per 60 g of soil: 6.2 mg of diammonium phosphate, 46.0 mg of urea, 4.0 ml of peat moss, 1.0 ml of sterile water, 0.33 ml of BioSolve surfactant (Westford Chemical Co., Westford, Mass.). For killed controls, 180 mg of sodium azide was added. The soil was thoroughly mixed with a spatula. There were five treatments, including microcosms incubated at 7, 0, and −5°C in darkness. An additional treatment was alternate incubation for 24-h periods at 7 and −5°C to simulate freezing and thawing effects. Killed controls were incubated at 7°C. There were additional triplicate sets of 7°C, 7 and −5°C, and killed treatments for headspace solid-phase microextraction (HS-SPME) analysis (see below).
At each sampling, two 0.5-g samples for DNA extraction were removed and stored at −70°C and one 3.0-g sample for petroleum hydrocarbon analysis was removed and stored at −20°C. All samples were taken at the end of a 7°C period of the 7 and −5°C treatment. The first samples were taken after 2 h of incubation on day 0.
Hydrocarbon extraction and analysis.
The 3.0-g soil samples for hydrocarbon analysis (12% water content) were dried with 3.0 g of anhydrous sodium sulfate. Dried samples were extracted with 5.0 ml of hexane in 20-ml vials with Teflon-lined screw caps by horizontal shaking at 150 oscillations per min in darkness for 24 h. Extracts were analyzed on a Hewlett-Packard GC 5890 Series II gas chromatograph with a flame ionization detector. The column was an HP-5 (length, 25 m; inside diameter, 0.32 mm; film thickness, 0.17 μm), and the carrier gas was H2 at a pressure of 7.5 lb/in2 and a flow rate of 1.8 ml/min. The temperature program was 40°C for 3 min, an increase of 30°C/min to 300°C, and holding for 10 min. The injector was set to 290°C, and the detector was set to 300°C. Volumes of 2.0 μl were injected in splitless mode for 1 min. The most abundant compounds were quantified by comparing the area under each individual peak in the chromatogram with that of a known amount of a standard in hexane. Total petroleum hydrocarbons (TPH) were quantified by comparing the total area under the chromatogram from 3 to 12 min with that of a known amount of Jet A-1 fuel in hexane.
Compounds were identified by with a Varian 3400Cx gas chromatograph with a Saturn 4D ion trap mass spectrum detector by comparing mass spectra with those in the National Institute of Standards and Technology library and by comparing retention times to those of standards. The column was a DB5-MS (length, 30 m; inside diameter, 0.25 mm; film thickness, 0.25 μm; J&W Scientific, Folsom, Calif.). The temperature program was 40°C for 5 min, an increase of 10°C/min to 220°C, and holding for 10 min. The carrier gas was helium at a pressure of 10 lb/in2. The Varian 1078 injector was operated at 230°C with splitless injection of 1.0 μl for 30 s on a 0.8-mm (inside diameter) liner. The ion trap was operated at 70 eV, and the scan range was m/z 50 to 400.
HS-SPME.
To account for changes in hydrocarbon bioavailability, HS-SPME was used. A manual 30-μm PDMS fiber (Supelco) was exposed to the headspace of individual bottles for 10 min at 7°C and injected within 1 min on the above-described gas chromatograph-flame ionization detector. Calibration was done by spiking uncontaminated soil from Alert with known amounts of Jet A-1 fuel or of individual compounds (octane, dodecane, and hexadecane). The uncontaminated soil had a composition and particle size distribution similar to that of the above-described contaminated soil. The uncontaminated soil was placed in bottles and amended as described above for the killed controls. These calibration standards were left to equilibrate for 24 h at 7°C before analysis by HS-SPME (5).
Carbon dioxide analysis.
Carbon dioxide was trapped in microcosms in a 10-ml vial containing 1.0 ml of 2.5 M NaOH placed on top of the soil. The vials were exchanged for new ones with fresh NaOH solution at each sampling time. The CO2 analysis was performed by sealing each vial with a septum, acidifying the solution with 1.0 ml of 3.0 M H2SO4, and withdrawing 10 μl of the headspace with a gas chromatography syringe for immediate analysis. Headspace samples were injected on a Shimadzu GC-8A equipped with a packed Haysep DB column (9-m length, 100–120 mesh, 3.125-mm inside diameter) and a thermal conductivity detector. The carrier gas was helium at a flow rate of 30 ml/min. The injector temperature was 140°C; the column was isothermal at 100°C; the detector temperature was 140°C, and the current was 140 mA. Calibration was done with known concentrations of CO2.
Enumeration of heterotrophs and hydrocarbon degraders.
An additional 1.0-g soil sample was removed at the last time point. Microorganisms were extracted by adding the soil to 9.0 ml of sterile 0.8% saline solution and vigorously shaking the mixture. For most-probable-number analysis, 10-fold dilutions of the extract in saline solution were used to inoculate triplicate wells in microtiter plates. Inocula of 20 μl were added to 180 μl of either tryptic soy broth for total culturable heterotrophs or to Bushnell-Haas medium with 1,000 ppm Jet A-1 fuel for hydrocarbon degraders. The microtiter plates were incubated for 30 days at 7°C in darkness. Growth of total heterotrophs was scored by visually checking for turbidity. The respiratory activity of hydrocarbon degraders was scored by adding to each well 50 μl of tetrazolium solution (3.0 g/liter), incubating them for an additional 24 h, and visually checking for the pink color from the reduction of tetrazolium by respiring cells (21).
RNA/DNA ratios.
DNA and RNA were simultaneously extracted from 0.5-g soil samples with a bead beater as described by Yu and Mohn (22). Separate DNA and RNA samples were obtained by treating aliquots of the nucleic acid extract with DNase-free RNase at 50°C for 30 min or with RNase-free DNase at 37°C for 1 h, respectively. The RNA/DNA ratio of the bacterial community was determined as described by Ka et al. (7). For this determination, competitive PCR assays, using universal bacterial primers, were used to quantify corresponding regions of 16S rRNA and 16S rRNA genes. Standard deviations of the ratios were calculated as the standard deviation of the mean of the ratios in triplicate. Statistical significance was tested by using an analysis-of-variance table (α = 0.1).
RISA.
Total DNA was extracted from 0.5 g of soil with a FastDNA Spin Kit (Bio 101, Quantum Technologies, La Jolla, Calif.). The bead beating time was extended to twice for 2.5 min at 5,000 oscillations per min. The obtained DNA was stored in Tris-EDTA buffer at −20°C. RISA was done as previously described by Yu and Mohn (23). Universal bacterial primers were used to amplify the intergenic spacer and flanking fragments of the 16S and 23S rRNA genes. The obtained PCR products (approximately 800 to 1,500 kb) were separated on a 2.0% agarose gel and stained with GelStar (BioWhittaker Molecular Applications, Rockland, Maine).
RESULTS
Hydrocarbon degradation.
The initial concentration of TPH in the soil was 907 μg/g of dry soil. The most abundant compounds from the extracted soil samples identified by gas chromatography-mass spectrometry were n-alkanes with starting concentrations (± the standard deviation, n = 5), per gram of dry soil, of 16 ± 0.4 μg of undecane, 32 ± 0.8 μg of dodecane, 60 ± 8.2 μg of tridecane, 51 ± 1.6 μg of tetradecane, and 20 ± 1.0 μg of pentadecane. The total amount of one- and two-ring aromatics was less than 10 μg/g of dry soil (data not shown).
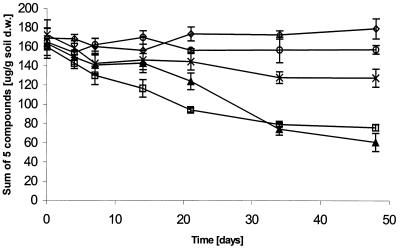
Temperature had a clear effect on the biodegradation of petroleum hydrocarbons. The killed control indicated that biodegradation was the main mechanism of hydrocarbon removal in the microcosms (Fig. 1; Table 1). At 7°C, biodegradation started without a lag phase and continued for 22 days. After that, there was no significant biodegradation during the last 28 days. In the 7 and −5°C treatment, there was a lag phase of 15 days before substantial biodegradation took place, but after 48 days, removal of the five most abundant hydrocarbons was slightly greater than in the 7°C treatment. The final TPH values were significantly different between all treatments at a 95% confidence level, except between the killed control and the 0°C treatment. TPH removal was greatest in the 7 and −5°C treatment (Table 1). Monitoring of the five most abundant hydrocarbons revealed a relatively small, but significant, amount of biodegradation in the 0°C treatment (Fig. 1). However, this biodegradation was not evident at 0°C when TPH was measured (Table 1). There was no significant hydrocarbon removal in the −5°C treatment. Comparison of the killed control and the −5°C treatment suggests that some abiotic losses, such as volatilization, did occur in treatments at temperatures above −5°C. At 7°C, abiotic losses appeared to account for less than 15% of the TPH.
FIG. 1.
Effect of temperature on petroleum hydrocarbon removal (sum of the five most abundant compounds, C11 to C15 n-alkanes) in Arctic soil microcosms. Symbols: □, 7°C; ▴, 7 and −5°C; ×, 0°C; ◊, −5°C; ○, killed control. Error bars show standard deviations (n = 3). d.w., dry weight.
TABLE 1.
Activity measurements after 48 days of treatment at different temperaturesa
| Treatment | Total petroleum hydrocarbon concn in soil (μg/g of soil [dry wt]) | Heterotrophs (MPN, 106)b | Hydrocarbon degraders (MPN, 106) |
|---|---|---|---|
| Start (day 0) | 907 (87)c | 23 (16) | 2.3 (1.2) |
| Killed control | 754 (40) | NDd | ND |
| −5°C | 974 (67) | 93 (40) | 1.5 (1.2) |
| 0°C | 701 (14) | 210 (190) | 4.3 (1.1) |
| 7°C | 426 (30) | 33 (16) | 3.6 (1.2) |
| 7 and −5°C | 290 (31) | 120 (40) | 9.3 (1.2) |
Values are means (n = 3) with standard deviations in parentheses.
MPN, most probable number.
n = 15.
ND, not determined.
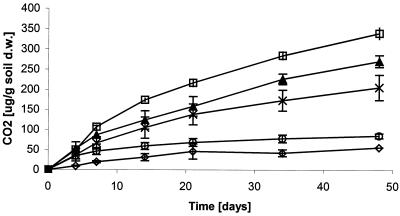
Generally, the three treatments with the most hydrocarbon biodegradation (Fig. 1; Table 1) had the most CO2 production (Fig. 2). However, the amount of CO2 production was higher in the 7°C treatment than in the 7 and −5°C treatment, despite slightly higher hydrocarbon biodegradation in the latter. Also, the amount of CO2 production in the 0°C treatment was relatively high while hydrocarbon biodegradation in that treatment was relatively low.
FIG. 2.
Effect of temperature on respiration (as CO2 production) in Arctic soil microcosms. Symbols: □, 7°C; ▴, 7 and −5°C; ×, 0°C; ◊, −5°C; ○, killed control. Error bars show standard deviations (n = 3). d.w., dry weight.
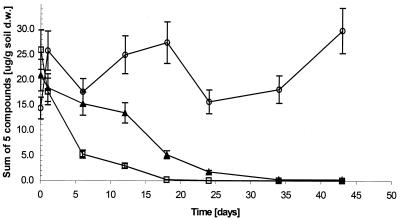
The five most abundant hydrocarbons disappeared from the headspace of the 7°C and 7 and −5°C treatments while remaining in the headspace of the killed control (Fig. 3). The kinetics of this disappearance corresponds well to the removal of these compounds from the soil, but substantial residual concentrations remained in the soil (Fig. 1). Complete disappearance of these compounds from the headspace occurred despite the fact that the headspace analysis is much more sensitive than analysis of extracted soil samples (14). Chromatograms from extracted samples and headspace indicated consistent mixtures of hydrocarbons and indicated that certain n-alkanes (not among the most abundant five) persisted. These persistent compounds were tentatively identified by gas chromatography-mass spectrometry as groups of branched alkanes with molecular weights similar to those of the n-alkanes that were removed, C11 to C15 (data not shown).
FIG. 3.
HS-SPME analysis of the five most abundant compounds in the Alert soil (C11 to C15 n-alkanes) in the 7°C and 7 and −5°C treatments. Symbols: □, 7°C; ▴, 7 and −5°C; ○, killed control. Error bars show standard deviations (n = 3). d.w., dry weight.
Metabolic activity.
Midway through the incubation, on day 21, the 7°C treatment had the highest rate of TPH removal (Table 2). Simultaneously, the 7°C and 7 and −5°C treatments had similar rates of CO2 production and similar bacterial RNA/DNA ratios, which were higher than the values for the other treatments. The 0°C treatment also had rates of TPH removal and CO2 production that were significantly greater than those of the killed control, while the −5°C treatment did not. On day 21, the bacterial RNA/DNA ratios of the 7°C and 7 and −5°C treatments were significantly higher (P < 0.057, α = 0.1) than the initial ratio in the soil while the bacterial RNA/DNA ratio of the 0°C treatment was significantly lower (P < 0.050, α = 0.1) than the initial ratio.
TABLE 2.
Metabolic activities on day 21a
| Treatment | Hydrocarbon removalb (μg g of soil−1 day−1) | CO2 production (μg g of soil−1 day−1) | RNA/DNA ratio |
|---|---|---|---|
| Start | NDc | ND | 51 (12) |
| −5°C | −0.71 | 0.42 | ND |
| 0°C | 0.95 | 3.3 | 31 (3) |
| 7°C | 1.8 | 5.4 | 99 (29) |
| 7 and −5°C | 3.5 | 5.1 | 91 (20) |
| Killed (7°C) | 0.55 | 0.91 | ND |
Values are means (n = 3) with standard deviations in parentheses.
Based on the five most abundant hydrocarbons (C11 to C15 n-alkanes).
ND, not determined.
Heterotrophs and hydrocarbon degraders.
At the end of the incubation, on day 48, populations of total heterotrophs ranged from 3 × 107 to 21 × 107 cells per g of soil and populations of hydrocarbon degraders ranged from 1 × 106 to 9 × 106 cells per g of soil (Table 1). Given the large variability in the measured heterotroph populations, there was no significant difference between treatments or between the initial and final values. Populations of hydrocarbon degraders did increase significantly during incubation of the 0°C and 7 and −5°C treatments and were largest in the latter treatment at the end of the experiment.
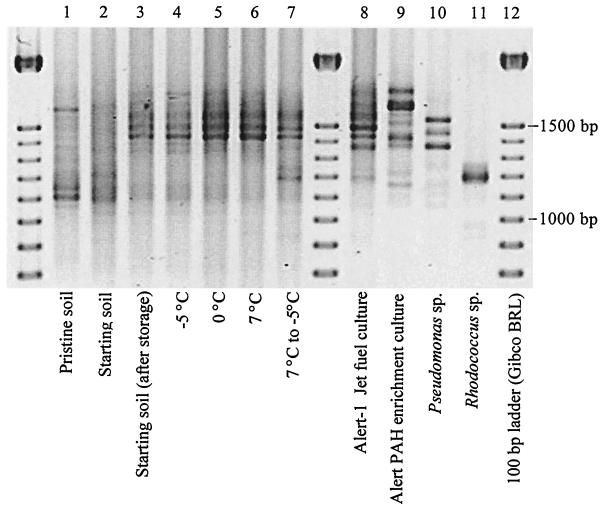
RISA.
At the end of the incubation, on day 48, all of the treatments had highly similar RIS length polymorphism banding patterns (Fig. 4). This pattern was also discernible in the starting soil for the experiment after storage at 7°C for 6 months but was not discernible in a subsample of the soil stored at −20°C. Several of the major bands in the pattern correspond to those from an enrichment culture that was inoculated with the same soil and selected by growth on jet fuel, followed by lyophilization. Of all of the treatments, only the 7 and −5°C treatment produced major bands that were not present initially. These bands correspond to those from a Rhodococcus sp. isolate taken from the same contaminated soil in a previous study (16).
FIG. 4.
RISA at day 48: Lanes: 1, pristine soil; 2, contaminated soil; 3, starting soil (after storage at 7°C); 4, −5°C; 5, 0°C; 6, 7°C; 7, 7 and −5°C; 8, enrichment culture on jet fuel (16); 9, enrichment culture grown on PAHs (unpublished data); 10, Pseudomonas sp. isolate from the soil (16); 11, Rhodococcus sp. isolate from the soil (16); 12, 100-bp ladder (Gibco BRL).
DISCUSSION
Although TPH values are normally used to measure hydrocarbon biodegradation and are normally the criteria used for assessment of contaminated sites, measurement of other values may be more informative. TPH measurements will not indicate whether particular hydrocarbons are degraded or persist (11). Further, TPH measurements may include soil organic matter that does not originate from hydrocarbon contamination. In this study, the removal of TPH (Table 1) was generally consistent with removal of the five most abundant n-alkanes (Fig. 1) when treatments were compared. Thus, TPH was a meaningful basis on which to compare treatments.
Substantial rates of hydrocarbon biodegradation occurred at low temperatures. This activity in the 7°C treatment is consistent with previous reports. The amount of TPH removed during 48 days at 7°C agrees with reported hydrocarbon degradation rates on the order of 1 to 2 μg of TPH g of soil−1 day−1 (10, 24). A higher initial rate of approximately 30 to 50 μg of TPH g of soil−1 day−1 during the first 30 days is typical for starting concentrations higher than 2,500 μg of TPH per g of soil (10, 16), which is likely due to high bioavailability of hydrocarbons.
In the treatments with the greatest extent of hydrocarbon removal, biodegradation activity stopped at the same time that hydrocarbons disappeared from the headspace (Fig. 1 and 3). This correspondence indicates that bioavailability was probably the limiting factor and likely accounts for persistent residual levels of degradable hydrocarbons in the soil. Results of this study agree very well with the hypothesis that there exist distinct available and residual fractions of soil contaminants (2, 13, 18). The latter fraction is biodegraded only very slowly and is likely not detectable by headspace analysis under the conditions used (5). The soil used in this study was also used in others (16), and the residual level of TPH was consistently 300 to 500 μg/g of soil.
RISA suggests that a few predominant populations developed in the soil during storage at 7°C prior to the experiment (Fig. 4). Addition of nutrients and further incubation at 7°C did not alter the RIS pattern, although the predominant bands became more intense, suggesting that these populations increased further in relative abundance. While band density cannot be used to quantify populations, a qualitative inference about relative population sizes is reasonable in this case, where very similar samples were analyzed in parallel. It is important to bear in mind that RISA probably did not detect populations of low relative abundance and may have missed predominant populations if they lacked sequence similarity to the PCR primers used. A significant increase in the culturable hydrocarbon degrader population was not detected in the 7°C treatment (Table 1). However, this population may have increased during the period of hydrocarbon biodegradation, after which RISA was performed, and then decreased during the latter part of the incubation, when starvation may have occurred, after which the culturable population was measured. The bacterial RNA/DNA ratios (Table 2) suggest that in the 7°C treatment, the metabolic activity of the bacterial community increased as a result of nutrient addition. We have previously observed this response to nutrient addition (J.-O. Ka et al., unpublished data).
Hydrocarbon biodegradation at 0°C was slow but significant (Fig. 1; Table 1). On the other hand, there was no evidence of hydrocarbon biodegradation or general metabolic activity at −5°C. Presumably, abiotic hydrocarbon losses were lower at 0°C than at 7°C. Therefore, comparison of the 0°C treatment to the killed control incubated at 7°C provides a conservative estimate of biological hydrocarbon removal at 0°C. The kinetics of both hydrocarbon biodegradation and respiration, measured as CO2 production, at 0°C suggest that these activities were slowing by day 48. Therefore, the lower temperature appears to limit the potential extent of hydrocarbon removal, as well as the rate of removal. After 48 days, hydrocarbon removal at 0°C was 38% of that at 7°C (Table 1). In close agreement, after 48 days, respiration at 0°C was 38% of that at 7°C (Fig. 2). The negative effect of the lower temperature on metabolic activity is also reflected in the low bacterial RNA/DNA ratio at 0°C relative to both the initial ratio (before lowering of the soil temperature to 0°C) and the corresponding ratio of the 7°C treatment (Table 2). RISA suggests that the 0°C incubation selected for the same predominant populations as the 7°C incubation (Fig. 4). The increase in intensity of the RIS bands during incubation at 0°C again suggests an increase in the predominant populations and is consistent with the metabolic activity observed.
Freeze-thaw cycles had a temporary inhibitory effect on hydrocarbon biodegradation in soil, affecting the removal of the five most abundant n-alkanes from both the soil (Fig. 1) and the headspace (Fig. 3), as well as total respiration (Fig. 2). However, the rate and extent of hydrocarbon biodegradation in the 7 and −5°C treatment ultimately exceeded those in the 7°C treatment (Fig. 1; Tables 1 and 2). This latter effect is substantial if one assumes that the former treatment had little degradation activity during the time it was incubated at −5°C. Two causes may contribute to the eventual stimulatory effect of freeze-thaw cycles. First, freezing will affect the physical properties of the soil and may have increased the bioavailability of hydrocarbons. Such an effect was previously studied for polycyclic aromatic hydrocarbons (PAHs), with the conclusion that periodic freezing and thawing did not enhance the degradation of weathered, nonextractable PAHs in soil (6). However, diesel range alkanes are, in general, more volatile than PAHs and therefore it may be possible to get a positive effect on degradation by the physical changes in the soil during freezing and thawing. Our data can neither confirm nor disprove this effect. Second, cells may have died during the freezing period, providing nutrients for the survivors, with the net result that nutrients were cycled faster and both the death and growth rates of hydrocarbon degraders increased. The data do not support the second possible cause. The similar RNA/DNA ratios in the 7 and −5°C and 7°C treatments (Table 2) suggest that the bacterial populations undergoing those treatments had similar specific growth rates on day 21. Also, the greater total respiration in the 7°C treatment (Fig. 2; Table 2) is not consistent with faster nutrient cycling in the 7 and −5°C treatment.
The freeze-thaw cycles also affected the composition of the microbial community. The 7 and −5°C treatment is the only one that resulted in a unique RIS pattern (Fig. 4). It appears that the temperature regimen selected for at least one additional population. Such a change in community composition is consistent with the low initial, and subsequently high, rate of hydrocarbon removal observed in the 7 and −5°C treatment (above). It is noteworthy that the 7 and −5°C treatment and the jet fuel enrichment culture have similar RIS patterns and had two similar selective pressures, hydrocarbons as the primary substrate, and freezing (the enrichment culture was frozen and thawed twice). It appears likely that the Rhodococcus sp. isolated from the enrichment culture, and shown to be abundant in that culture (16), also became a predominant member of the soil community under the freeze-thaw regimen. This organism appears to be well adapted to survival during freezing. Wardell (17) previously found that gram-positive bacteria showed a high resistance to temperatures below 0°C and may be well adapted to hydrocarbon biodegradation in soil in polar regions with low temperatures.
This study confirms that bioremediation of hydrocarbons in soil is feasible at low temperatures. The limitations to the rate and, perhaps more importantly, the extent of hydrocarbon removal at 0°C may make this temperature inadequate for bioremediation applications. There is no evidence that significant hydrocarbon biodegradation will occur at temperatures below 0°C. Freeze-thaw activity does not necessarily inhibit hydrocarbon biodegradation and may even increase the bioavailability of hydrocarbons. However, hydrocarbon-degrading communities must adapt to freeze-thaw conditions, in part through selection of certain populations.
ACKNOWLEDGMENT
This work was supported by a Strategic Project Grant from the Natural Science and Engineering Research Council of Canada.
REFERENCES
- 1.Aislabie J, Mcleod M, Fraser R. Potential for biodegradation of hydrocarbons in soil from the Ross Dependency, Antarctica. Appl Microbiol Biotechnol. 1998;49:210–214. [Google Scholar]
- 2.Alexander M. Biodegradation and bioremediation. 2nd ed. San Diego, Calif: Academic Press; 1999. [Google Scholar]
- 3.Atlas R M. Microbial degradation of petroleum hydrocarbons: an environmental perspective. Microbiol Rev. 1981;45:180–209. doi: 10.1128/mr.45.1.180-209.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cookson J T., Jr . Bioremediation engineering design and application. New York, N.Y: McGraw-Hill Inc.; 1995. pp. 1–25. [Google Scholar]
- 5.Eriksson M, Fäldt J, Dalhammar G, Borg-Karlson A-K. Determination of hydrocarbons in old creosote contaminated soil using headspace solid phase microextraction and GC-MS. Chemosphere. 2001;44:1641–1644. doi: 10.1016/s0045-6535(00)00371-4. [DOI] [PubMed] [Google Scholar]
- 6.Eschenbach A, Wienberg R, Mahro B. Fate and stability of nonextractable residues of [14C]PAH in contaminated soils under environmental stress conditions. Environ Sci Technol. 1998;32:2585–2590. [Google Scholar]
- 7.Ka, J.-O., Yu, Z., and W. W. Mohn. Monitoring the size and metabolic activity of the bacterial community during biostimulation of fuel-contaminated soil using competitive PCR and RT-PCR. Microb. Ecol., in press. [DOI] [PubMed]
- 8.Leahy J G, Colwell R R. Microbial degradation of hydrocarbons in the environment. Microbiol Rev. 1990;54:305–315. doi: 10.1128/mr.54.3.305-315.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacNaughton S J, Stephen J R, Venosa A D, Davis G A, Chang Y-J, White D C. Microbial population changes during bioremediation of an experimental oil spill. Appl Environ Microbiol. 1999;65:3566–3574. doi: 10.1128/aem.65.8.3566-3574.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margesin R, Shinner F. Biological decontamination of oil spills in cold environments. J Chem Technol Biotechnol. 1999;74:381–389. [Google Scholar]
- 11.McKenna E A, Youngren S H, Baker S R, Schroeder J R, Piccin T B, Weisman W H, Long C. Evaluation of the total petroleum hydrocarbon (TPH) standard for JP-4 jet fuel. J Soil Contam. 1995;4:355–406. [Google Scholar]
- 12.Mohn W W, Stewart G R. Limiting factors for hydrocarbon biodegradation at low temperature in Arctic soils. Soil Biol Biochem. 2000;32:1161–1172. [Google Scholar]
- 13.Nocentini M, Pinelli D, Fava F. Bioremediation of a soil contaminated by hydrocarbon mixtures: the residual concentration problem. Chemosphere. 2000;41:1115–1123. doi: 10.1016/s0045-6535(00)00057-6. [DOI] [PubMed] [Google Scholar]
- 14.Pawliszyn J. Solid phase microextraction theory and practise. New York, N.Y: Wiley-VCH; 1997. [Google Scholar]
- 15.Rivkina E M, Friedman E I, Mckay C P, Gilichinsky D A. Metabolic activity of permafrost bacteria below the freezing point. Appl Environ Microbiol. 2000;66:3230–3233. doi: 10.1128/aem.66.8.3230-3233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomassin-Lacroix E J M. Fate and effects of hydrocarbon-degrading strains used to inoculate soil for on-site bioremediation in the Arctic. M.S. thesis. 2000. Faculty of the Royal Military College of Canada, Kingston, Ontario, Canada. [Google Scholar]
- 17.Wardell L J. Potential for bioremediation of fuel-contaminated soil in Antarctica. J Soil Contam. 1995;4:111–121. [Google Scholar]
- 18.Weissenfels W D, Klewer H J, Langhoff J. Adsorption of polycyclic aromatic hydrocarbons (PAHs) by soil particles: influence on biodegradability and biotoxicity. Appl Microbiol Biotechnol. 1992;36:689–696. doi: 10.1007/BF00183251. [DOI] [PubMed] [Google Scholar]
- 19.Whyte L G, Slagman S J, Pietrantonio F, Bourbonnière L, Koval S F, Lawrence J R, Innis W E, Greer C W. Physiological adaptations involved in alkane assimilation at a low temperature by Rhodococcus sp. strain Q15. Appl Environ Microbiol. 1999;65:2961–2968. doi: 10.1128/aem.65.7.2961-2968.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whyte L G, Greer C W, Innis W E. Assessment of the biodegradation potential of psychrotrophic microorganisms. Can J Microbiol. 1995;42:99–106. doi: 10.1139/m96-016. [DOI] [PubMed] [Google Scholar]
- 21.Wrenn B A, Venosa A D. Selective enumeration of aromatic and aliphatic hydrocarbon degrading bacteria by a most-probable-number procedure. Can J Microbiol. 1996;42:252–258. doi: 10.1139/m96-037. [DOI] [PubMed] [Google Scholar]
- 22.Yu Z, Mohn W W. Killing two birds with one stone: simultaneous extraction of DNA and RNA from activated sludge biomass. Can J Microbiol. 1999;45:269–272. [Google Scholar]
- 23.Yu Z, Mohn W W. Bacterial diversity and community structure in an aerated lagoon revealed by ribosomal intergenic spacer analyses and 16S ribosomal DNA sequencing. Appl Environ Microbiol. 2000;67:1565–1574. doi: 10.1128/AEM.67.4.1565-1574.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou E, Crawford R L. Effects of oxygen, nitrogen and temperature on gasoline biodegradation in soil. Biodegradation. 1995;6:127–140. doi: 10.1007/BF00695343. [DOI] [PubMed] [Google Scholar]