Abstract
Background and Objetives
Hyperuricemia (HUA) and gout seriously influence patients’ quality of life. The current study was performed to investigate the prevalence of HUA and gout and the related risk factors in Chinese adults.
Methods
Data were collected from the National Survey of Thyroid Disorders and Diabetes (the Thyroid Disease, Iodine Status, and Diabetes National Epidemiological survey [TIDE]), a cross-sectional investigation conducted during 2015–2017. Using a random, multistage, and stratified sampling strategy, a representative sample (78,130 participants aged 18 years and above) was selected from the general population in 31 provinces of mainland China. The weighted prevalence rates of HUA and gout were calculated, and the related risk factors were analyzed.
Results
The weighted prevalence rates of HUA and gout in Chinese adults were 17.7% and 3.2%, respectively. The prevalence of HUA in males linearly decreased with age, while the prevalence in females showed the opposite trend (both P for trend < 0.01). The prevalence rate of gout exhibited a rising tendency with age in both genders (both P for trend < 0.05). The HUA and gout prevalence rates in males were the highest in Han and Tibetan nationalities, respectively. Logistic regression analysis showed that the morbidities of HUA and gout were differentially associated with age, residence location, nationality, smoking, and other complicating metabolic diseases in the two genders.
Conclusions
There are relatively high prevalence rates of gout and HUA in China, which is currently a developing country. Reducing their burden has become an urgent issue for Chinese people.
Key words: hyperuricemia, gout, prevalence, epidemiology, China
Introduction
Hyperuricemia (HUA) is a common biochemical abnormality caused by excessive production of urate and/or decreased renal uric acid excretion or their coexistence.[1] Gout is a crystal deposition disease caused by chronic elevation of uric acid level with main characteristic manifestations including recurrent acute arthritis, tophi, and uric acid kidney stones.[2] The prevalence rates of HUA and gout have been reported to vary greatly due to the differences in inhabiting regions, ethnicity, eating habits, etc. It has been found that HUA and gout are more common in the patients with hypertension, cardiovascular diseases, obesity, and diabetes.[3, 4, 5, 6]
The prevalence rates of HUA and gout have been found to be much higher in developed countries than in developing countries. The 2015–2016 National Health and Nutrition Survey (NHANES) showed that the prevalence of HUA in US adults was 20.1%, similar to that from the 2007–2008 survey.[7, 8] It was 16.6% in Australian adults.[9] The 2016 Korean NHANES (KNHANES) showed that the prevalence of HUA in Korean adults was 11.4% (17.0% for males and 5.9% for females).[10] The prevalence of gout is greater than 1% in most developed countries, including the USA (3.9%), Australia (5.2%), Canada (3.8%), Greece (4.75%), Germany (1.4%), and the UK (2.5%).[11, 12, 13, 14, 15] However, the prevalence of HUA in Thailand and Bangladesh was 10.6% and 9.3%, respectively.[16, 17] The prevalence of gout in developing countries, such as Bangladesh, India, and Pakistan, is basically under 0.5%.[18, 19, 20] As the largest developing country in the world, China’s geographic regions, economy, and ethnic groups are diversified. A large epidemiological data on HUA in Chinese adults came from the 2009–2010 National Chronic Kidney Disease Survey (NCKDS), which covered 13 provinces. That study estimated that the prevalence of HUA was 8.4%.[21] Another meta-analysis showed that the Chinese prevalence rates of HUA and gout during 2000–2014 were 13.3% and 1.1%, respectively.[22]
The Thyroid Disease, Iodine Status, and Diabetes National Epidemiological survey (TIDE) was a more representative national and cross-sectional survey conducted during 2015–2017, which covered 31 provinces of mainland China and five nationalities, including Han, Tibetan, Zhuang, Uyghur, and Hui. Based on the TIDE survey database, the epidemiological characteristics of HUA and gout in Chinese adults were analyzed, their prevalence rates were estimated, and the associated risk factors were further explored in the current study.
Methods
Study participants
In the TIDE survey, a representative sample from the general population in 31 provinces of mainland China was selected with a multistage, cluster-based, and stratified random sampling method.[23, 24] Two residential communities were randomly selected from each urban district or rural township. The latter was randomly selected from one city or village per province, and thus, a total of 124 communities were covered. The participants were included based on the following criteria: (1) ≥18 years old; (2) inhabiting in the selected community for up to 5 years; (3) no use of iodine-containing drugs or contrast agents during the last 3 months; and (4) females who were not pregnant. After 340 subjects without serum uric acid (SUA) measurement data were excluded, 78,130 participants were finally included in this study. All the subjects signed informed consent forms.
Data collection
All the questionnaires were administered by trained interviewers, and a database with a good-quality control system was established including all related information, such as smoking status, annual family income, and educational level.[23, 24] Physical examinations, including height, body weight, waist circumference, and blood pressure, were conducted at the medical examination center. The body mass index (BMI) was calculated as weight (kg)/height (m2). Fasting blood and spot urine samples were collected from each participant. The oral glucose tolerance test (OGTT) was administered to each subject without a personal history of diabetes.
Laboratory tests
As described in previous reports,[23, 24] blood glucose (BG) was measured with the hexokinase method. Triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were determined using Mindray’s kit and biochemical detector (BS-180; Mindray, Shenzhen, China). Glycated hemoglobin (HbA1c) was assayed with Bio-Rad reagents. SUA was measured using the uricase– peroxidase coupling method (ROCHE COBAS 8000).
Diagnostic criteria
HUA was defined as SUA above 420.0 μmol/L in males and 360.0 μmol/L in females.[25] Gout was self-reported through the following consultation as adopted by NHANES.[8] All the participants were asked, “Have you ever received a diagnosis of gout from a medical professional?” The diagnostic criteria for diabetes, impaired fasting glucose (IFG), impaired glucose tolerance (IGT), dyslipidemia, hypertension, and abdominal obesity were adopted as described in the previous study.[24]
Statistical analysis
To obtain the national prevalence estimates, all calculations were weighted, and standardized prevalence rates were acquired. The weighting coefficients were derived from the 2010 China Census data and the sampling strategy of this survey to represent the total Chinese adult population over the age of 18 years.[23] The chi-square test (or Fisher’s exact test) and the Cochran-Armitage test for trend were used to analyze categorical data expressed as percentages (95% confidence interval [CI]). Normally distributed data are shown as the mean ± standard deviation (SD), and comparisons were performed using independent sample t test or one-way analysis of variance (ANOVA). Multivariate logistic regression was used to calculate the corrected odds ratio (OR) and 95% CI to determine the independent correlations between HUA/gout and other variables. There was a statistically significant difference when P was less than 0.05 or the adjusted cut-off value due to multiple comparisons in the chi-square analysis. To identify the independent contribution of each ethnic group to the burden of HUA and gout, the population attribute risk percent (PAR%) was calculated for each ethnic subpopulation group. PAR% was calculated using the following formula: [PAR% = pd(RR - 1/RR)] according to the literature,[26] where pd referred to the proportion of cases exposed to the risk factor. However, the relative risk (RR) was substituted by OR in this retrospective study. The collinearity statistics was performed using the linear regression analysis to check the multicollinearity between the potential related factors with HUA and gout. Its existence was considered if the variance inflation factor (VIF) was >5. All the above statistical analyses were carried out with Statistical Package for the Social Sciences (SPSS) (version 21.0; IBM Corporation, Armonk, NY, USA) and SUDAAN (version 10.0; Research Triangle Institute, Raleigh, NC, USA) software.
Results
A total of 78,130 people were included in this study, consisting of 38,013 males and 40,117 females. The SUA level showed a normal distribution in both sexes, and there was a significant difference between them (Figure 1). As shown in Table 1, the overall standardized prevalence of HUA in Chinese adults was 17.7%, which corresponded to an estimated 185.13 million adults with HUA in China between 2015 and 2017. The prevalence of HUA in males (8741, 23.5%) was significantly higher than that of females (4391, 11.7%). Although the prevalence of HUA did not show a significant difference between urban and rural residents, the former’s SUA level was significantly higher than that of the latter (Table 2). Similarly, the SUA level of coastal residents was pronouncedly increased compared to that of inland inhabitants, although the HUA prevalence was not significantly different between them. Both males with higher education levels and females who smoked tended to develop HUA. Both the HUA prevalence and SUA level exhibited linear decreasing trends with increased age in males, while they showed the opposite alterations in females. In addition, they displayed a linearly upward trend with increase in BMI in both sexes. Interestingly, the changes in HUA prevalence exhibited a U-shaped curve with increased income in both genders (Table 1), while the SUA level showed a linear upward trend with the increase of income in males and a U-shaped curve trend in females (Table 2). Han nationality showed the highest SUA level among the five ethnic groups in both sexes, and Han males exhibited the highest HUA prevalence (Tables 1 and 2). Compared to Han nationality males, the prevalence of HUA was significantly decreased in Uyghur males (Table 1). In addition, the prevalence rates of HUA in males and females were compared among the 31 provinces in China. The highest male prevalence of HUA seemed to be in Liaoning (46.6%, range 43.5%–49.6%; Figure 2), while the highest female prevalence appeared to be in Guangdong (31.7%, range 17.2%–50.9%; Figure 2).
Figure 1.
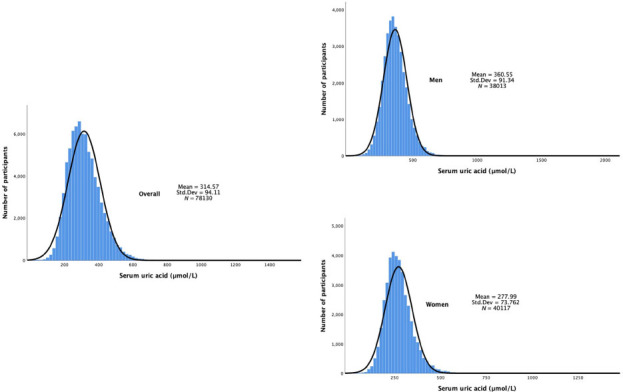
Distribution of serum uric acid levels in males and females. Std. Dev: standard deviation.
Table 1.
Age- and sex-standardized prevalence rates of hyperuricemia and gout in mainland China
| Prevalence of gout, % (95% CI) |
Prevalence of hyperuricemia, % (95 % CI) |
|||||
|---|---|---|---|---|---|---|
| Overall | Males | Females | Overall | Males | Females | |
| Total prevalence | 3.2 (2.8–3.7) | 4.4 (3.9–5.0) | 2.0 (1.6–2.5) | 17.7 (15.5–20.1) | 23.5 (21.2–26.0) | 11.7 (9.5–14.4) |
| Area of residence a | ||||||
| Urban | 3.7 (2.8–4.9) | 5.2 (4.2–6.5) | 2.2 (1.7–2.8) | 19.7 (14.9–25.6) | 25.8 (20.6–31.9) | 13.4 (8.9–19.7) |
| Rural | 2.7 (2.0–3.8) | 3.6 (2.7–4.9) | 1.8 (1.2–2.6) | 15.6 (12.7–19.0) | 21.1 (17.2–25.6) | 10.0 (7.9–12.5) |
| P for difference | 0.42 | 0.20 | 0.90 | 0.30 | 0.25 | 0.41 |
| Inland/coastal a | ||||||
| Inland | 3.4 (2.9–3.9) | 4.5 (3.9–5.3) | 1.7 (1.3–2.1) | 16.8 (15.3–18.3) | 22.8 (20.1–24.9) | 10.6 (9.4–11.8) |
| Coastal | 3.1 (2.4–3.9) | 4.3 (3.6–5.3) | 1.4 (0.9–2.2) | 19.0 (14.5–25.0) | 24.5 (19.8–29.9) | 13.4 (8.7–20.1) |
| P for difference | 0.46 | 0.62 | 0.37 | 0.39 | 0.54 | 0.30 |
| Education a | ||||||
| Below high school | 2.9 (2.6–3.4) | 3.8 (3.2–4.4) | 2.1 (1.7–2.5) | 16.1 (14.4–18.0) | 20.7 (18.6–23.0) | 11.4 (9.5–13.6) |
| High school and above | 3.5 (3.1–4.1) | 5.2 (4.6–5.9) | 1.9 (1.3–2.6) | 18.8 (15.7–22.3) | 25.0 (22.0–28.2) | 12.5 (9.1–17.0) |
| P for difference | 0.006 | 0.22 | <0.001 | 0.047 | 0.002 | 0.54 |
| Smoking a | ||||||
| No smoking | 3.3 (3.0–3.8) | 4.7 (4.2–5.2) | 2.0 (1.6–2.4) | 17.6 (15.5–20.0) | 23.6 (21.4–25.9) | 11.6 (9.3–14.3) |
| Smoking | 3.7 (2.9–4.7) | 4.2 (3.6–4.9) | 3.2 (2.1–4.9) | 22.4 (18.0–22.4) | 23.5 (20.9–26.4) | 16.6 (14.0–19.5) |
| P for difference | <0.001 | 0.82 | 0.03 | <0.001 | 0.35 | <0.001 |
| Ethnic groups a | ||||||
| Han | 3.2 (2.8–3.6) | 4.3 (3.8–4.9) | 2.0 (1.6–2.5) | 17.9 (15.6–20.5) | 23.9 (21.5–26.6) | 11.8 (9.5–14.7) |
| Tibetan | 11.8 (9.6–14.4 | ) 9.8 (9.1–10.7) | 13.8 (9.1–20.5) | 17.0 (15.5–18.6) | 22.9 (22.5–23.4) | 10.9 (8.4–13.9) |
| Zhuang | 5.2 (3.4–8.1)* | 8.8 (6.3– | 1.6 (0.5–4.6) | 16.4 (12.2–21.7) | 21.8 (18.5–25.4) | 10.9(6.1–18.6) |
| 12.2)* | ||||||
| Uyghur | 1.7 (1.1–2.6)* | 2.1 (1.9–2.2)* | 1.3 (0.5–3.5) | 4.6 (1.6–12.4)* | 4.0 (2.5–6.3)* | 5.3 (1.18–20.5) |
| Hui | 0.8 (0.6–1.0) | 0.6 (0.5–0.9) | 0.9 (0.7–1.2) | 4.0 (3.6–4.4) | 5.9 (5.9–6.0) | 2.0 (1.4–2.8) |
| P for difference | <0.001 | <0.001 | 0.50 | <0.001 | <0.001 | 0.23 |
| Age (years) b | ||||||
| 18–29 | 1.0 (0.7–1.3) | 1.1 (0.8–1.5) | 0.8 (0.5–1.3) | 20.0 (16.7–23.8) | 28.4 (24.5–32.6) | 11.5 (8.5–15.4) |
| 30–39 | 2.3 (1.8–2.9) | 3.4 (2.9–4.1) | 1.0 (0.6–1.8) | 17.4 (14.5–20.7) | 26.0 (23.2–29.1) | 8.3 (5.4–12.7) |
| 40–49 | 3.4 (2.9–3.9) | 5.0 (4.2–5.9) | 1.7(1.3–2.1) | 15.5 (13.5–17.6) | 22.3 (20.0–24.9) | 8.3 (6.2–11.1) |
| 50–59 | 5.2 (4.4–6.1) | 7.0 (5.9–8.3) | 3.2 (2.6–4.1) | 16.7 (15.1–18.5) | 19.2 (17.0–21.5) | 14.1 (12.3–16.2) |
| 60–69 | 5.9 (5.0–6.8) | 7.5 (6.5–8.8) | 4.1 (3.0–5.7) | 17.0 (14.8–19.3) | 18.3 (16.4–20.3) | 15.6 (12.8–18.9) |
| ≥70 | 6.1 (5.1–7.3) | 8.2 (6.5–10.2) | 4.3 (3.2–5.7) | 19.9 (18.0–21.9) | 18.6 (16.6–20.8) | 21.0 (18.8–23.2) |
| P for trend | 0.01 | 0.01 | <0.001 | <0.001(U) | 0.002 | <0.001 |
| BMI (kg/m 2 ) b | ||||||
| <18.5 | 1.6 (1.0–2.5) | 1.5 (0.8–2.5) | 1.7 (0.9–3.4) | 9.0 (5.7–14.0) | 11.7 (8.2–16.3) | 6.3 (2.7–14.0) |
| 18.5–23.9 | 2.6 (2.2–3.1) | 3.4 (2.7–4.1) | 1.9 (1.5–2.3) | 13.0 (10.5–16.0) | 17.6 (14.8–20.7) | 8.4 (6.1–11.4) |
| 24.0–27.9 | 3.4 (3.0–4.0) | 4.8 (4.3–5.4) | 2.0 (1.5–2.7) | 20.4 (17.9–23.1) | 25.6 (22.7–28.8) | 15.0 (12.7–17.7) |
| ≥28.0 | 4.9 (4.1–5.8) | 6.9 (5.7–8.4) | 2.9 (2.2–3.8) | 31.4 (29.0–33.9) | 32.6 (34.4–38.8) | 26.1 (22.5–30.0) |
| P for trend | <0.001 | <0.001 | 0.32 | <0.001 | 0.01 | <0.001 |
| Income (CNY/year) b | ||||||
| <5000 | 4.2 (3.0–5.8) | 5.4 (3.9–7.4) | 2.9 (1.6–5.1) | 18.3 (14.6–22.7) | 22.7 (19.5–26.4) | 13.8 (9.0–20.6) |
| 5000–10,000 | 2.9 (2.2–3.8) | 4.0 (2.9–5.4) | 1.8 (1.3–2.5) | 17.7 (13.9–22.2) | 23.8 (19.1–29.3) | 11.4 (8.1–15.8) |
| 10,000–30,000 | 2.8 (2.4–3.2) | 3.6 (3.0–4.2) | 2.0 (1.5–2.5) | 16.4 (14.5–18.4) | 21.6 (19.4–23.9) | 11.0 (9.0–13.5) |
| 30,000–50,000 | 2.8 (2.4–3.4) | 3.6 (3.0–4.3) | 2.0 (1.5–2.8) | 17.0 (15.5–18.5) | 22.8 (21.1–24.6) | 11.0 (9.5–12.7) |
| 50,000–100,000 | 3.7 (3.2–4.2) | 5.5 (4.9–6.2) | 1.9 (1.3–2.6) | 18.0 (15.3–21.0) | 23.9 (21.0–27.1) | 11.9 (9.3–15.2) |
| >100,000 | 4.0 (3.5–4.6) | 6.2 (5.3–7.1) | 1.9 (1.4–2.4) | 21.4 (17.6–25.9) | 28.5 (23.9–33.6) | 14.3 (10.7–18.7) |
| P for trend | <0.001 (U) | <0.001 (U) | 0.32 | 0.01(U) | 0.01(U) | 0.04(U) |
BMI: body mass index; CNY: Chinese yuan (1 CNY = 0.1489 USD); (U): u-shaped curve trend; 95% CI: 95% confidence interval.
aThe prevalence rate within each subgroup was compared by the chi-square test.
bThe trend of prevalence rate within each subgroup was analyzed through Cochran-Armitage test for trend.
*Compared with Han people, the differences were statistically significant, in which P was less than the adjusted cutoff value (0.05/4 = 0.0125) due to multiple comparisons by the chi-square test.
Table 2.
Distribution of serum uric acid level analyzed by subgroups
| Serum uric acid level, mean ± SD (μmol/L) |
|||
|---|---|---|---|
| Overall | Males | Females | |
| Area of residence a | |||
| Urban | 319.76 ± 93.41 | 368.19 ± 88.33 | 274.86 ± 73.41 |
| Rural | 308.63 ± 94.56 | 352.02 ± 93.86 | 266.47 ± 73.92 |
| P for difference | <0.001 | <0.001 | <0.001 |
| Inland/coastal a | |||
| Inland | 312.62 ± 93.37 | 358.90 ± 90.77 | 269.26 ± 72.65 |
| Coastal | 319.32 ± 95.74 | 364.51 ± 92.57 | 275.31 ± 76.29 |
| P for difference | <0.001 | <0.001 | <0.001 |
| Education a | |||
| Below high school | 304.04 ± 91.92 | 346.17 ± 91.60 | 270.31 ± 77.07 |
| High school and above | 323.53 ± 95.05 | 371.05 ± 89.78 | 271.59 ± 70.24 |
| P for difference | <0.001 | <0.001 | 0.09 |
| Smoking a | |||
| No smoking | 299.27 ± 89.85 | 359.06 ± 91.40 | 270.48 ± 73.42 |
| Smoking | 357.54 ± 92.54 | 362.13 ± 91.29 | 286.61 ± 82.16 |
| P for difference | <0.001 | 0.94 | <0.001 |
| Age (years) b | |||
| 18–29 | 321.94 ± 96.46 | 373.36 ± 91.03 | 268.42 ± 68.92 |
| 30–39 | 311.64 ± 97.41 | 369.27 ± 90.12 | 257.68 ± 68.92 |
| 40–49 | 306.18 ± 93.91 | 356.79 ± 91.05 | 259.57 ± 69.09 |
| 50–59 | 314.11 ± 89.98 | 350.02 ± 90.39 | 282.02 ± 76.49 |
| 60–69 | 317.27 ± 89.17 | 348.17 ± 90.04 | 288.66 ± 78.15 |
| ≥70 | 320.70 ± 91.51 | 344.52 ± 92.33 | 298.70 ± 85.03 |
| P for trend | <0.001 (U) | <0.001 | <0.001 |
| BMI (kg/m 2 ) b | |||
| <18.5 | 280.04 ± 80.72 | 330.99 ± 80.80 | 251.22 ± 64.89 |
| 18.5–23.9 | 295.46 ± 86.49 | 343.68 ± 85.25 | 259.43 ± 67.86 |
| 24.0–27.9 | 329.34 ± 94.23 | 367.58 ± 90.37 | 282.81 ± 76.17 |
| ≥28.0 | 355.77 ± 102.01 | 393.08 ± 99.04 | 304.05 ± 81.42 |
| P for trend | <0.001 | <0.001 | <0.001 |
| Income (CNY/year) b | |||
| <5000 | 302.84 ± 92.26 | 347.54 ± 92.83 | 271.36 ± 77.72 |
| 5000–10,000 | 302.81 ± 93.60 | 346.72 ± 94.44 | 267.01 ± 76.03 |
| 10,000–30,000 | 306.10 ± 92.37 | 350.02 ± 92.17 | 268.97 ± 74.53 |
| 30,000–50,000 | 316.24 ± 93.24 | 361.39 ± 90.37 | 272.17 ± 72.65 |
| 50,000–100,000 | 324.06 ± 93.25 | 369.00 ± 86.96 | 272.76 ± 71.09 |
| >100,000 | 331.90 ± 98.72 | 380.59 ± 91.54 | 273.70 ± 71.82 |
| P for trend | <0.001 | <0.001 | <0.001(U) |
| Ethnic groups c | |||
| Han | 318.65 ± 93.64 | 364.56 ± 90.24 | 274.40 ± 73.34 |
| Tibetan | 298.24 ± 88.26* | 362.03 ± 79.93 | 261.58 ± 70.12* |
| Zhuang | 315.54 ± 91.60 | 363.68 ± 88.11 | 268.42 ± 67.22 |
| Uyghur | 243.22 ± 82.73* | 268.60 ± 88.42* | 221.28 ± 70.49* |
| Hui | 258.71 ± 80.46* | 301.64 ± 74.65* | 215.57 ± 60.57* |
| P for difference | <0.001 | <0.001 | <0.001 |
ANOVA: analysis of variance; BMI: body mass index; CNY: Chinese yuan (1 CNY = 0.1489 USD); SD: standard deviation; SUA: serum uric acid; (U): u-shaped curve trend.
aThe SUA level within each subgroup was compared by the independent sample t-test.
bThe trend test of SUA level within each subgroup was analyzed through one-way ANOVA.
cThe SUA level among the five ethnic groups was compared through one-way ANOVA followed by post hoc Bonferroni correction.
*Compared with Han people, the differences were statistically significant.
Figure 2.
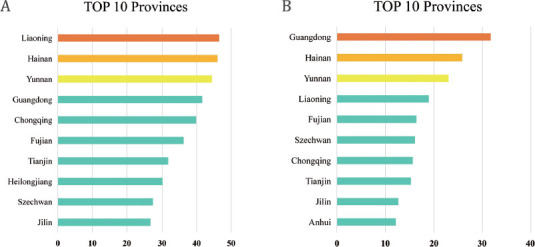
Regional distribution of the prevalence of HUA in mainland China: (A) prevalence in males (%); (B) prevalence in females (%). HUA: hyperuricemia.
The overall standardized prevalence of gout in all Chinese adults was 3.2% (Table 1), which corresponded to an estimated 25.56 million adults with gout in China during 2015–2017. The prevalence of gout in males (4.4%) was significantly higher than that in females (2.0%). No significant difference was found in the prevalence of gout between coastal and inland residents or between urban and rural inhabitants (Table 1). Females with a smoking history and those with low education levels tended to develop gout (Table 1). The gout prevalence exhibited a linear upward trend with increasing age in both sexes and with increase in BMI in males. It showed a U-shaped curve with the increase in income in males, which reached the lowest point at an annual income of 10,000–30,000 Chinese yuan. When compared to Han nationality, the prevalence of gout was markedly increased in Zhuang males, while it decreased in Uyghur males (Table 1). Although Tibetan males showed the highest prevalence of gout among the five ethnic groups studied, the difference was not statistically significant compared with Han males. It was inferred that this phenomenon could be due to the existence of some confounding factors (e.g., educational background and residency areas). Moreover, the prevalence rates of gout in males and females were analyzed among the 31 provinces in China. They were both highest in Tibet (9.9%, range 9.0%–10.8% in males and 13.8%, range 9.0%–20.5% in females; Figure 3).
Figure 3.
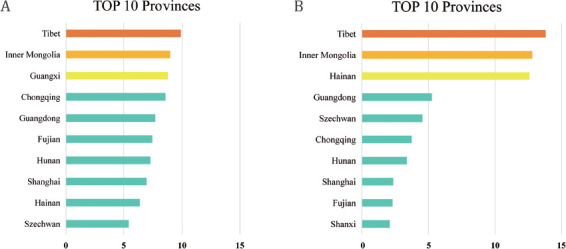
Regional distribution of the prevalence of gout in mainland China: (A) prevalence in males (%); (B) prevalence in females (%).
Dwelling in the urban and coastal areas, high educational backgrounds, and BMI ≥24 kg/m2 were all independent risk factors for HUA in males and females (Table 3). Moreover, IFG, IGT, hypertension, dyslipidemia, and abdominal obesity were all independently associated with the development of HUA in the two sexes. With increasing age, the risk of HUA was significantly reduced in males (Table 3). Female smokers were more likely to develop HUA. An annual income of 10,000–100,000 Chinese yuan was found to be a protective factor against HUA in females. Diabetes was found to be an independent risk factor for HUA in females and a protective factor against HUA in males. Han nationality was found as a risk factor for HUA in both genders (PAR%: 48.70% in males and 40.13% in females), whereas Zhuang and Uyghur were shown as its protective factors (PAR%: Zhuang: –3.27% in males and –2.83% in females, Uyghur: –1.72% in males and –1.46% in females; Tables 3 and 4).
Table 3.
Multiple logistic regression analysis of the associated factors with hyperuricemia and gout
| Risk factors | Hyperuricemia |
Gout |
||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||
|
| ||||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age per 10 years | 0.84 (0.82–0.85) | <0.01 | 1.01 (0.98–1.04) | 0.44 | 1.44 (1.38–1.50) | <0.01 | 1.37 (1.29–1.45) | <0.01 |
| High school and above | 1.16 (1.09–1.24) | <0.01 | 1.25 (1.15–1.36) | <0.01 | 1.28 (1.12–1.46) | <0.01 | 0.84 (0.69–1.01) | 0.07 |
| Current smoking | 0.99 (0.94–1.04) | 0.59 | 1.54 (1.30–1.82) | <0.01 | 1.04 (0.92–1.16) | 0.55 | 1.17 (0.81–1.69) | 0.40 |
| Urban residency | 1.13 (1.07–1.20) | <0.01 | 1.23 (1.14–1.33) | <0.01 | 1.30 (1.14–1.47) | <0.01 | 1.04 (0.88–1.22) | 0.69 |
| Coastal residency Ethnic groups | 1.09 (1.03–1.15) | <0.01 | 1.25 (1.16–1.34) | <0.01 | 1.05 (0.93–1.18) | 0.48 | 0.65 (0.54–0.78) | <0.01 |
| Hana | 2.07(1.85–2.32) | <0.01 | 1.76 (1.53–2.02) | <0.01 | 0.59 (0.49–0.72) | <0.01 | 0.56 (0.45–0.68) | <0.01 |
| Tibetanb | 1.11(0.91–1.36) | 0.32 | 1.01(0.81–1.26) | 0.93 | 3.60(2.73–4.76) | <0.01 | 4.89(3.85–6.21) | <0.01 |
| Zhuangc | 0.12(0.08–0.17) | <0.01 | 0.27(0.20-0.37) | <0.01 | 0.36(0.18–0.71) | <0.01 | 0.41(0.21–0.80) | 0.01 |
| Uyghurd | 0.25(0.19–0.34) | <0.01 | 0.21(0.13–0.35) | <0.01 | 0.22(0.10–0.49) | <0.01 | 0.31(0.14–0.66) | <0.01 |
| Huie | 0.99(0.83–1.18) | 0.87 | 1.03(0.82–1.30) | 0.80 | 3.08(2.31–4.12) | <0.01 | 1.11(0.62–1.99) (2.23-3.73) | 0.73 |
| Income (CNY/year) | ||||||||
| ≤5000 (reference) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 5000–10,000 | 1.03 (0.90–1.17) | 0.70 | 0.93 (0.81–1.07) | 0.33 | 0.81 (0.62–1.05) | 0.11 | 1.02 (0.80–1.31) | 0.86 |
| 10,000–30,000 | 0.93 (0.83–1.04) | 0.17 | 0.84 (0.74–0.94) | <0.01 | 0.80 (0.64–1.00) | 0.06 | 0.78 (0.62–0.99) | 0.04 |
| 30,000–50,000 | 0.94 (0.85–1.05) | 0.30 | 0.84 (0.75–0.96) | <0.01 | 0.79 (0.63–0.99) | 0.06 | 0.77 (0.57–1.00) | 0.04 |
| 50,000–100,000 | 0.97 (0.87–1.09) | 0.67 | 0.83 (0.73–0.95) | <0.01 | 1.06 (0.84–1.33) | 0.59 | 0.75 (0.57–1.00) | 0.05 |
| >100,000 | 1.08 (0.96–1.22) | 0.19 | 0.92 (0.79–1.07) | 0.29 | 1.39 (1.09–1.78) | <0.01 | 0.93 (0.66–1.30) | 0.66 |
| BMI (kg/m2) 18.5–23.9 (reference) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| <18.5 | 0.82 (0.69–0.97) | 0.02 | 0.85 (0.70–1.04) | 0.11 | 0.63 (0.38–1.05) | 0.08 | 0.94 (0.65–1.39) | 0.76 |
| 24.0–27.9 | 1.20 (1.12–1.29) | <0.01 | 1.47 (1.35–1.61) | <0.01 | 1.10 (0.94–1.28) | 0.24 | 0.97 (0.81–1.18) | 0.78 |
| ≥28.0 | 1.63 (1.48–1.78) | <0.01 | 2.12 (1.88–2.39) | <0.01 | 1.37 (1.12–1.66) | <0.01 | 1.09 (0.84–1.41) | 0.51 |
| Diabetes | 0.85 (0.78–0.92) | <0.01 | 1.65 (1.50–1.81) | <0.01 | 1.25 (1.08–1.44) | <0.01 | 1.32 (1.08–1.61) | <0.01 |
| IFG | 1.11 (1.03–1.20) | 0.01 | 1.20 (1.07–1.34) | <0.01 | 1.08 (0.91–1.28) | 0.40 | 0.78 (0.59–1.03) | 0.07 |
| IGT | 1.42 (1.29–1.56) | <0.01 | 1.42 (1.27–1.59) | <0.01 | 1.35 (1.12–1.62) | <0.01 | 1.16 (0.91–1.48) | 0.23 |
| Hypertension | 1.22 (1.15–1.29) | <0.01 | 1.22 (1.13–1.33) | <0.01 | 1.16 (1.03–1.31) | 0.02 | 0.94 (0.79–1.12) | 0.49 |
| Dyslipidemia | ||||||||
| High TG | 1.77 (1.67–1.87) | <0.01 | 1.73 (1.60-1.87) | <0.01 | 1.62 (1.43–1.84) | <0.01 | 1.17 (0.98–1.40) | 0.08 |
| High TC | 1.35 (1.25–1.45) | <0.01 | 1.45 (1.32-1.60) | <0.01 | 1.23 (1.06–1.43) | <0.01 | 1.50 (1.23–1.80) | <0.01 |
| High LDL | 1.14 (1.06–1.23) | <0.01 | 1.20 (1.09-1.33) | <0.01 | 1.02 (0.88–1.19) | 0.78 | 0.82 (0.67–1.01) | 0.06 |
| Low HDL | 1.23 (1.15–1.32) | <0.01 | 1.40 (1.29-1.51) | <0.01 | 1.27 (1.10–1.50) | <0.01 | 0.90 (0.76–1.08) | 0.26 |
| Abdominal obesity | 1.30 (1.21–1.39) | <0.01 | 1.32 (1.20-1.44) | <0.01 | 1.23 (1.06–1.42) | <0.01 | 1.16 (0.96–1.41) | 0.13 |
OR: odds ratio; 95% CI: 95% confidence interval; BMI: body mass index; CNY: Chinese yuan (1 CNY = 0.1489 USD); IFG: impaired fasting glucose; IGT: impaired glucose tolerance; TG: triglyceride; TC: total cholesterol; LDL: low-density lipoprotein; HDL: high-density lipoprotein.
aAs compared with other ethnic groups including Tibetan, Zhuang, Uyghur, and Hui.
bAs compared with other ethnic groups including Han, Zhuang, Uyghur, and Hui.
cAs compared with other ethnic groups including Han, Tibetan, Uyghur, and Hui.
dAs compared with other ethnic groups including Han, Tibetan, Zhuang, and Hui.
eAs compared with other ethnic groups including Han, Tibetan, Zhuang, and Uyghur.
Table 4.
PAR% of each ethnic group for the prevalence of hyperuricemia and gout
| Ethnic groups | Hyperuricemia |
Gout |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||||||
|
| ||||||||||||
| pd (%) | OR | PAR% (%) | pd (%) | OR | PAR% (%) | pd (%) | OR | PAR% (%) | pd (%) | OR | PAR% (%) | |
| Han | 94.22 | 2.07 | 48.70 | 92.94 | 1.76 | 40.13 | 86.88 | 0.59 | -60.37 | 80.33 | 0.56 | -63.12 |
| Tibetan | - | - | - | - | - | - | 6.77 | 3.60 | 4.89 | 15.85 | 4.89 | 12.61 |
| Zhuang | 0.45 | 0.12 | -3.27 | 1.05 | 0.27 | -2.83 | 0.63 | 0.36 | -1.12 | 1.07 | 0.41 | -1.54 |
| Uyghur | 0.57 | 0.25 | -1.72 | 0.39 | 0.21 | -1.46 | 0.42 | 0.22 | -1.48 | 0.95 | 0.31 | -2.12 |
| Hui | - | - | - | - | 5.30 | 3.08 | 3.58 | - | - | - | ||
pd: proportion of cases exposed to risk factor; OR: odds ratio; PAR%: population attribute risk percent; which was calculated using the following formula: [PAR% = pd(RR - 1/RR)] according to the literature[26]; -: not an independent risk or a protective factor in multiple logistic regression analysis; RR: relative risk.
As shown in Table 3, increase in age, diabetes and high serum TC were all independently associated with the occurrence of gout in both male and female. Living in urban areas, high educational backgrounds, BMI ≥28 kg/ m2, and an annual income over 100,000 Chinese yuan were all independent risk factors for gout in males. Besides, hypertension, high serum TG level, low HDL-C level, and abdominal obesity were independently associated with the development of gout in males only. However, both living in coastal areas and annual income of 10,000–30,000 Chinese yuan were protective factors against gout in females. They showed sex-differential actions on the development of gout. Han, Zhuang, and Uyghur were all found as protective factors against gout in both genders (PAR%: Han: –60.37% in males and –63.12% in females, Zhuang: –1.12% in males and –1.54% in females, Uyghur: –1.48% in males and –2.12% in females), while Tibetan was found to be a risk factor for gout in both genders (PAR%: 4.89% in males and 12.61% in females) and Hui was a risk factor only in males (PAR%: 3.58%; Tables 3 and 4).
Since there were several influencing factors related to the occurrence of HUA and gout as found in this study, the multicollinearity between these potential risk factors had been checked. However, all their VIF values were <5, indicating no multicollinearity between them (Table 5).
Table 5.
Collinearity statistics analysis of the potential related factors with hyperuricemia and gout
| Risk factors | Hyperuricemia |
Gout |
||
|---|---|---|---|---|
| Male | Female | Male | Female | |
|
| ||||
| VIF | VIF | VIF | VIF | |
| Age | 1.393 | 1.679 | 1.400 | 1.699 |
| High school and above | 1.370 | 1.495 | 1.377 | 1.516 |
| Current smoking | 1.032 | 1.006 | 1.033 | 1.005 |
| Urban residency | 1.169 | 1.218 | 1.181 | 1.232 |
| Coastal residency | 1.024 | 1.029 | 1.028 | 1.036 |
| Ethnicities | 1.065 | 1.060 | 1.079 | 1.077 |
| Income (CNY/year) | 1.232 | 1.235 | 1.239 | 1.253 |
| BMI (kg/m2) | 1.814 | 1.671 | 1.809 | 1.667 |
| Diabetes | 1.146 | 1.166 | 1.149 | 1.164 |
| IFG | 1.053 | 1.039 | 1.056 | 1.041 |
| IGT | 1.051 | 1.051 | 1.051 | 1.050 |
| Hypertension | 1.155 | 1.235 | 1.158 | 1.233 |
| High TG | 1.279 | 1.248 | 1.280 | 1.252 |
| High TC | 1.828 | 1.818 | 1.839 | 1.840 |
| High LDL | 1.741 | 1.174 | 1.753 | 1.740 |
| Low HDL | 1.133 | 1.156 | 1.138 | 1.152 |
| Abdominal obesity | 1.754 | 1.681 | 1.750 | 1.674 |
IFG: impaired fasting glucose; IGT: impaired glucose tolerance; TG: triglyceride; TC: total cholesterol; LDL: low-density lipoprotein; HDL: high-density lipoprotein; VIF: variance inflation factor.
Multicollinearity was considered to exist if VIF >5.
Discussion
To the best of our knowledge, this study is currently the largest epidemiological survey of HUA and gout in China and is very helpful for better understanding the impacts of demographic, economic, and geographic factors on the prevalence of HUA and gout. In this nationwide survey of the general Chinese adult population, we observed that the weighted prevalence rates of HUA and gout in China were 17.7% (23.5% for males, 11.7% for females) and 3.2% (4.4% for males, 2.0% for females), corresponding to an estimated 185.31 million adults with HUA and 25.56 million adults with gout, respectively, in our country during 2015–2017. The prevalence of HUA estimated in the present study is obviously higher than that reported by previous studies. The NCKDS performed in China during 2009–2010 showed that the prevalence of HUA in Chinese adults was 8.4% (9.9% for males and 7.0% for females).[21] Another survey of the elderly population from 28 provinces in China showed that the prevalence of HUA was estimated to be 6.4% (7.9% for males and 4.9% for females).[27] The Chinese Physiological Constant and Health Condition survey was carried out from 2007 to 2011. It was found that 13.0% of Chinese adults had HUA (18.5% for males and 8.0% for females).[28] These studies are summarized in Supplementary Tables 1 and 2. Moreover, the prevalence of HUA in males in China found in our study (23.52%) was even overtly higher than that found in some developed countries such as the USA (20.2%) and Australia (16.6%).[7, 9] The prevalence of gout estimated in China by our study (3.23%) was not only markedly higher than that reported by a previous study (1.1%),[22] but also close to or even higher than that found in some developed countries such as the USA (3.9%), Canada (3.8%), Sweden (1.8%), France (0.9%), the UK (2.49%), Denmark (0.68%), and Italy (0.91%).[12,29, 30, 31] The lower prevalence of HUA and gout in China reported by previous studies may be due to insufficient sample sizes, incomplete geographic coverage, or/and rapid development of China’s economy. We speculate that the increased prevalence rates of HUA and gout in China may be related to the rapid development of China’s economy in recent years and the westernization of Chinese dietary habits.[32, 33, 34]
Previous studies reported that the prevalence of HUA in Chinese urban residents was higher than that in rural residents (14.9% vs. 6.6%).[21] In the present study, there was no significant difference in the prevalence of HUA or gout between urban and rural residents. We inferred that the rapid development of society and the economy, the progress of urbanization, industrialization, and the improvement of rural medical care may contribute to the gradually shrinking difference between urban and rural areas. In addition, we analyzed whether the prevalence rates of HUA and gout were related to geographic location. Although they did not show significant differences between inland and coastal residents, the SUA level of the former was significantly lower than that of the latter, which might be related to the seafood preferences of the coastal people.[35] In the current study, although living in a coastal area was found to be a risk factor for HUA in both males and females, gout was found to be more common in inland areas of China, especially in Tibet, and living in a coastal area was suggested to be a protective factor against gout in females. Such opposite phenomena may be due to the preference of iodine-rich foods by the people living in coastal regions. Another study derived from the TIDE project by Lu et al. revealed that urinary iodine concentration (UIC) was negatively correlated with the prevalence rates of HUA and gout.[36] However, as shown in Table 1, there was no statistically significant difference in the prevalence rates of gout between the females living in coastal areas and the females inhabiting inlands. It also suggests that living in coastal areas may be considered as a protective factor against gout in females only when the other risk factors, such as diabetes, advanced age, and high TC, are avoided at the same time. On the other hand, the onset of gout is closely related to temperature, air pressure, and air humidity. Tibet is a high-altitude region with high air pressure and low air density. Under these conditions, urate in the joint cavity of HUA patients is more likely to form deposits.[37] Additionally, Tibetans mostly lead a nomadic lifestyle with a preference for beef, barley, and buttered tea. Close to 50% of the Tibetan adults are alcohol consumers, far exceeding the national average level.[38] Finally, estrogens can increase renal urate clearance and inhibit renal reabsorption,[39] which can contribute to sex differences in the development of the two diseases. All these results indicate that there are a few external and internal factors affecting the deposition of urate. They may further influence the development of HUA into gout and cause inconsistency between the occurrence of HUA and gout. Besides, it was interesting to find that the logistic regression analysis and PAR% indicated Zhuang nationality as a protective factor against HUA and gout in both males and females and Hui nationality as an independent risk factor for gout in males. They were inconsistent with the tendencies in the standardized prevalence rates of HUA and gout among these ethnic groups. We infer there may be other unknown confounding factors related to both age and the occurrence of gout and HUA, which could finally affect their standardized prevalence rates. We admit that the potential influencing factors analyzed in this study are limited and some others are needed for investigation in the future work.
Our results have further demonstrated the sex differences in the prevalence of HUA and gout, which was markedly higher in males than in females. Such sex differences may be related to the protective actions of estrogens, as stated above.[39] Additionally, in the current study, the HUA prevalence in males decreased with age and exhibited an opposite trend in females, which was consistent with the findings of previous Chinese studies.[40, 41] NHANES I also suggested that SUA levels in females were positively related to age.[24] Such sex differences may be due to the drinking habits or lack of exercise in young men and changes in estrogenic levels in postmenopausal women.[39, 42] Our data showed that the HUA prevalence was significantly higher in females over 50 years of age (age 50–59 years: 14.1%; age 60–69 years: 15.6%; age ≥70 years: 21.0%) than in those under 50 years of age (age 18–29 years: 11.5%; age 30–39 years: 8.3%; age 40–49 years: 8.3%). The significant decrease in serum estrogen levels of postmenopausal women may reduce the clearance of uric acid by the kidneys and eventually make them more susceptible to HUA.
In addition, we found that smoking was a risk factor for HUA in females. The 2016 KNHANES VII-1 also suggested that smoking was closely related to SUA levels in females and increased the risk of HUA in them.[43] Previous research has shown that long-term exposure to smoke may exert adverse influences on renal function.[44, 45] The levels of total protein and glutathione S-transferase in the urine of smokers were significantly increased as compared with those of nonsmokers.[46] Glomerular mesangial cells showed proliferative and degenerative changes in rats exposed to cigarette smoke for a long period of time, which eventually led to renal dysfunction.[47]
In this study, we also analyzed the relationship between HUA and other metabolic diseases. We found that diabetes was associated with an increased risk of female HUA, consistent with previous studies.[27, 41] However, it was a protective factor against HUA in males, which was consistent with the results of another study published in 2019.[48] Elevated urine glucose level could inhibit uric acid reabsorption in proximal tubules, which may explain the above phenomenon in males.[49] However, the exact mechanism of the sex difference in the relationship between diabetes and HUA remains to be elucidated. This study also suggests that IFG, IGT, hypertension, BMI ≥24 kg/m2, abdominal obesity, and dyslipidemia are all risk factors for HUA in both genders. Previous studies have demonstrated the close associations between HUA, insulin resistance, and metabolic diseases.[50]
Our study has several strengths. First, our study is currently the largest epidemiological survey to assess the impact of demographic, economic, and geographic factors on the prevalence of HUA and gout in China. Second, the multivariable logistic model adjusted for a number of potentially influencing covariables, including age, sex, education, annual income, smoking, BMI, regional differences, and ethnicity, which increased the reliability of the results. These potential risk factors were selected for this study based on the previous reports[8, 9, 10] and the variable availability in our current database. Moreover, the sample size of this study could provide sufficient statistical power, so they were all included in the regression models. Their multicollinearity has been checked in the linear regression analysis; no multicollinearity between them was indicated in this study. However, the study has several limitations also. First, this was a cross-sectional study, and the results could have been influenced by some unmeasured confounding factors and reverse causation. Thus, subsequent longitudinal studies are needed to further confirm our findings. Second, the present study analyzed only limited factors. The impacts of extra factors on the prevalence rates of HUA and gout need also be considered, such as serum creatinine level, food intake, physical activity, alcohol consumption, use of urate-lowering drugs, and accessibility to health care. Finally, the gout diagnosis methodology in this survey was simply a self-reported physician diagnosis, which may affect its accuracy.
In conclusion, this study has shown that the prevalence rates of HUA and gout among Chinese adults during 2015–2017 were much higher than those reported in previous studies of the Chinese population and even higher than those found in some developed countries. It further suggests that the morbidities of HUA and gout are differentially influenced by a few factors, including sex, age, residence location, nationality, smoking, and other complicating metabolic diseases. The high prevalence rates of HUA and gout would lead to increased consumption of public health resources in China. Reducing this burden has become an urgent issue for Chinese people.
Footnotes
Supplementary Materials
Supplementary materials mentioned in this article are online available at the journal's official site only.
Conflict of Interest
The authors declare that they have no conflict of interest.
Source of Funding
This work was supported by the Clinical Research Fund of the Chinese Medical Association (No. 15010010589). The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report. The authors thank all the participants in this study.
Authors’ Contributions
Teng W, Li J, and Shan Z conceived and designed the study. The Thyroid Disorders, Iodine Status, and Diabetes Epidemiological Survey Group conducted the epidemiological survey for data acquisition. Song J and Jin C performed the data analysis and statistical analysis. Song J, Jin C, and Li J drafted the manuscript.
Ethics Approval and Consent to Participate
This survey was approved by the Ethics Committee of China Medical University (2014-103-2). All the participants had signed the informed consents.
Availability of Data and Materials
The data underlying this article would be shared on making reasonable request to the corresponding author, after getting approval from the TIDE Survey Group.
Supplementary Material.
References
- 1.Bardin T, Richette P. Definition of hyperuricemia and gouty conditions. Curr Opin Rheumatol. 2014;26:186–91. doi: 10.1097/BOR.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 2.Luk AJ, Simkin PA. Epidemiology of hyperuricemia and gout. Am J Manag Care. 2005;11:S435–442. quiz S465–38. [PubMed] [Google Scholar]
- 3.Gibson TJ. Hypertension, its treatment, hyperuricaemia and gout. Curr Opin Rheumatol. 2013;25:217–22. doi: 10.1097/BOR.0b013e32835cedd4. [DOI] [PubMed] [Google Scholar]
- 4.Lin WY, Liu CS, Li TC, Lin T, Chen W, Chen CC. In addition to insulin resistance and obesity, hyperuricemia is strongly associated with metabolic syndrome using different definitions in Chinese populations: a population-based study (Taichung Community Health Study) Ann Rheum Dis. 2008;67:432–3. doi: 10.1136/ard.2007.073601. et al. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Zhang N, Sun G, Guo X, Yu S, Yang H. Metabolically healthy obesity also has risk for hyperuricemia among Chinese general population: A cross-sectional study. Obes Res Clin Pract. 2016;10:S84–S95. doi: 10.1016/j.orcp.2016.03.008. et al. [DOI] [PubMed] [Google Scholar]
- 6.Choi BG, Kim DJ, Baek MJ, Ryu YG, Kim SW, Lee MW. Hyperuricaemia and development of type 2 diabetes mellitus in Asian population. Clin Exp Pharmacol Physiol. 2018;45:499–506. doi: 10.1111/1440-1681.12911. et al. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136–41. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 8.Singh G, Lingala B, Mithal A. Gout and hyperuricaemia in the USA: prevalence and trends. Rheumatology (Oxford) 2019;58:2177–80. doi: 10.1093/rheumatology/kez196. [DOI] [PubMed] [Google Scholar]
- 9.Ting K, Gill TK, Keen H, Tucker GR, Hill CL. Prevalence and associations of gout and hyperuricaemia: results from an Australian population-based study. Intern Med J. 2016;46:566–73. doi: 10.1111/imj.13006. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y, Kang J, Kim GT. Prevalence of hyperuricemia and its associated factors in the general Korean population: an analysis of a population-based nationally representative sample. Clin Rheumatol. 2018;37:2529. doi: 10.1007/s10067-018-4130-2. –. [DOI] [PubMed] [Google Scholar]
- 11.Anagnostopoulos I, Zinzaras E, Alexiou I, Papathanasiou AA, Davas E, Koutroumpas A. The prevalence of rheumatic diseases in central Greece: a population survey. BMC Musculoskelet Disord. 2010;11:98. doi: 10.1186/1471-2474-11-98. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 2015;74:661–7. doi: 10.1136/annrheumdis-2013-204463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Annemans L, Spaepen E, Gaskin M, Bonnemaire M, Malier V, Gilbert T. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000-2005. Ann Rheum Dis. 2008;67:960–6. doi: 10.1136/ard.2007.076232. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 20072016. Arthritis Rheumatol. 2019;71:991–9. doi: 10.1002/art.40807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rai SK, Avina-Zubieta JA, McCormick N, De Vera MA, Shojania K, Sayre EC, et al. The rising prevalence and incidence of gout in British Columbia, Canada: Population-based trends from 2000 to 2012. Semin Arthritis Rheum. 2017;46:451–6. doi: 10.1016/j.semarthrit.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lohsoonthorn V, Dhanamun B, Williams MA. Prevalence of hyperuricemia and its relationship with metabolic syndrome in Thai adults receiving annual health exams. Arch Med Res. 2006;37:883–9. doi: 10.1016/j.arcmed.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Ali N, Perveen R, Rahman S, Mahmood S, Rahman S, Islam S. Prevalence of hyperuricemia and the relationship between serum uric acid and obesity: A study on Bangladeshi adults. PLoS One. 2018;13:e0206850. doi: 10.1371/journal.pone.0206850. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haq SA, Darmawan J, Islam MN, Uddin MZ, Das BB, Rahman F. Prevalence of rheumatic diseases and associated outcomes in rural and urban communities in Bangladesh: a COPCORD study. J Rheumatol. 2005;32:348–53. et al. [PubMed] [Google Scholar]
- 19.Chopra A, Patil J, Billempelly V, Relwani J, Tandle HS; WHO-ILAR COPCORD Study. WHO International League of Associations from Rheumatology Community Oriented Program from Control of Rheumatic Diseases. Prevalence of rheumatic diseases in a rural population in western India: a WHO-ILAR COPCORD Study. J Assoc Physicians India. 2001;49:240–6. [PubMed] [Google Scholar]
- 20.Farooqi A, Gibson T. Prevalence of the major rheumatic disorders in the adult population of north Pakistan. Br J Rheumatol. 1998;37:491–5. doi: 10.1093/rheumatology/37.5.491. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Zhang XM, Wang YL, Liu BC. Prevalence of hyperuricemia among Chinese adults: a national cross-sectional survey using multistage, stratified sampling. J Nephrol. 2014;27:653–8. doi: 10.1007/s40620-014-0082-z. [DOI] [PubMed] [Google Scholar]
- 22.Liu R, Han C, Wu D, Xia X, Gu J, Guan H. Prevalence of Hyperuricemia and Gout in Mainland China from 2000 to 2014: A Systematic Review and Meta-Analysis. Biomed Res Int. 2015;2015:762820. doi: 10.1155/2015/762820. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. doi: 10.1136/bmj.m997. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Gao Y, Li Y, Teng D, Xue Y, Yan L. The Presence of Serum TgAb Suggests Lower Risks for Glucose and Lipid Metabolic Disorders in Euthyroid General Population From a National Survey. Front Endocrinol (Lausanne) 2020;11:139. doi: 10.3389/fendo.2020.00139. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–10. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 26.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–9. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song P, Wang H, Xia W, Chang X, Wang M, An L. Prevalence and correlates of hyperuricemia in the middle-aged and older adults in China. Sci Rep. 2018;8:4314. doi: 10.1038/s41598-018-22570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J, Qiu L, Cheng XQ, Xu T, Wu W, Zeng XJ. Hyperuricemia and clustering of cardiovascular risk factors in the Chinese adult population. Sci Rep. 2017;7:5456. doi: 10.1038/s41598-017-05751-w. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zobbe K, Prieto-Alhambra D, Cordtz R, Hojgaard P, Hindrup JS, Kristensen LE. Secular trends in the incidence and prevalence of gout in Denmark from 1995 to 2015: a nationwide register-based study. Rheumatology (Oxford) 2019;58:836–9. doi: 10.1093/rheumatology/key390. et al. [DOI] [PubMed] [Google Scholar]
- 30.Bardin T, Bouée S, Clerson P, Chalès G, Flipo RM, Lioté F, et al. Prevalence of Gout in the Adult Population of France. Arthritis Care Res (Hoboken) 2016;68:261–6. doi: 10.1002/acr.22660. [DOI] [PubMed] [Google Scholar]
- 31.Dehlin M, Drivelegka P, Sigurdardottir V, Svard A, Jacobsson LT. Incidence and prevalence of gout in Western Sweden. Arthritis Res Ther. 2016;18:164. doi: 10.1186/s13075-016-1062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Hu XM, Chen TJ, Bai MJ. Rural-Urban Differences of Dietary Patterns, Overweight, and Bone Mineral Status in Chinese Students. Nutrients. 2016;8 doi: 10.3390/nu8090537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson RJ, Titte S, Cade JR, Rideout BA, Oliver WJ. Uric acid, evolution and primitive cultures. Semin Nephrol. 2005;25:3–8. doi: 10.1016/j.semnephrol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Lee YH, Wang Z, Chiang TC, Liu CT. Beverage Intake, Smoking Behavior, and Alcohol Consumption in Contemporary China-A Cross-Sectional Analysis from the 2011 China Health and Nutrition Survey. Int J Environ Res Public Health. 2017;14 doi: 10.3390/ijerph14050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li R, Yu K, Li C. Dietary factors and risk of gout and hyperuricemia: a meta-analysis and systematic review. Asia Pac J Clin Nutr. 2018;27:1344. doi: 10.6133/apjcn.201811_27(6).0022. –. [DOI] [PubMed] [Google Scholar]
- 36.Lu X, Shi X, Li Y, Chi H, Liao E, Liu C. A negative association between urinary iodine concentration and the prevalence of hyperuricemia and gout: a cross-sectional and population-based study in Mainland China. Eur J Nutr. 2020;59:3659–68. doi: 10.1007/s00394-020-02199-z. et al. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Ruiz F, Herrero-Beites AM. Crystal arthritis: Environment and genetics in gout: a maze for clinicians? Nat Rev Rheumatol. 2014;10:8–9. doi: 10.1038/nrrheum.2013.173. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Q, Gong H, Lin C, Liu Q, Baima Y, Wang Y. The prevalence of gout and hyperuricemia in middle-aged and elderly people in Tibet Autonomous Region, China: A preliminary study. Medicine (Baltimore) 2020;99:e18542. doi: 10.1097/MD.0000000000018542. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anton FM, Garcia Puig J, Ramos T, Gonzalez P, Ordas J. Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2) on the renal handling of urate. Metabolism. 1986;35:343–8. doi: 10.1016/0026-0495(86)90152-6. [DOI] [PubMed] [Google Scholar]
- 40.Liu L, Lou S, Xu K, Meng Z, Zhang Q, Song K. Relationship between lifestyle choices and hyperuricemia in Chinese men and women. Clin Rheumatol. 2013;32:233–9. doi: 10.1007/s10067-012-2108-z. [DOI] [PubMed] [Google Scholar]
- 41.Yu S, Yang H, Guo X, Zhang X, Zhou Y, Ou Q. Prevalence of hyperuricemia and its correlates in rural Northeast Chinese population: from lifestyle risk factors to metabolic comorbidities. Clin Rheumatol. 2016;35:1207–15. doi: 10.1007/s10067-015-3051-6. et al. [DOI] [PubMed] [Google Scholar]
- 42.Puig JG, Mateos FA, Miranda ME, Torres RJ, de Miguel E, Perez de Ayala C, et al. Purine metabolism in women with primary gout. Am J Med. 1994;97:332–8. doi: 10.1016/0002-9343(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 43.Kim SK, Choe JY. Association between smoking and serum uric acid in Korean population: Data from the seventh Korea national health and nutrition examination survey 2016. Medicine (Baltimore) 2019;98:e14507. doi: 10.1097/MD.0000000000014507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hussain T, Al-Attas OS, Alrokayan SA, Ahmed M, Al-Daghri NM, Al-Ameri S. Deleterious effects of incense smoke exposure on kidney function and architecture in male albino rats. Inhal Toxicol. 2016;28:364–73. doi: 10.1080/08958378.2016.1179372. et al. [DOI] [PubMed] [Google Scholar]
- 45.Baggio B, Budakovic A, Dalla Vestra M, Saller A, Bruseghin M, Fioretto P. Effects of cigarette smoking on glomerular structure and function in type 2 diabetic patients. J Am Soc Nephrol. 2002;13:2730–6. doi: 10.1097/01.asn.0000032422.81130.68. [DOI] [PubMed] [Google Scholar]
- 46.IA EL-S, Gadallah M, Shouman AE, Nessim DE. DE Subclinical nephrotoxicity caused by smoking and occupational silica exposure among Egyptian industrial workers. Arch Med Res. 2003;34:415–21. doi: 10.1016/S0188-4409(03)00077-8. [DOI] [PubMed] [Google Scholar]
- 47.Pekmez H, Ogeturk M, Ozyurt H, Sonmez MF, Colakoglu N, Kus I. Ameliorative effect of caffeic acid phenethyl ester on histopathological and biochemical changes induced by cigarette smoke in rat kidney. Toxicol Ind Health. 2010;26:175–82. doi: 10.1177/0748233710362380. [DOI] [PubMed] [Google Scholar]
- 48.Shen Y, Wang Y, Chang C, Li S, Li W, Ni B. Prevalence and risk factors associated with hyperuricemia among working population at high altitudes: a cross-sectional study in Western China. Clin Rheumatol. 2019;38:1375–84. doi: 10.1007/s10067-018-4391-9. [DOI] [PubMed] [Google Scholar]
- 49.Nan H, Dong Y, Gao W, Tuomilehto J, Qiao Q. Diabetes associated with a low serum uric acid level in a general Chinese population. Diabetes Res Clin Pract. 2007;76:68–74. doi: 10.1016/j.diabres.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 50.Sattui SE, Singh JA, Gaffo AL. Comorbidities in patients with crystal diseases and hyperuricemia. Rheum Dis Clin North Am. 2014;40:251–78. doi: 10.1016/j.rdc.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


