Abstract
Objectives:
This cross-sectional study assessed the accuracy of emergency CT reports at presentation in acute aortic syndrome (AAS).
Methods:
Retrospective identification of cases of AAS presenting within a large health board with three acute hospitals receiving adult patients between January 2013 and December 2016. CT studies and reports at presentation were reviewed for discrepancies related to diagnosis, complications and classification by two cardiovascular radiologists. The specialist interest of the original reporters, clinically suspected diagnosis at referral for CT and technical adequacy of the scans were also assessed. False-positive diagnoses were identified and evaluated separately.
Results:
Among 88 consecutive confirmed cases of AAS at least one discrepancy was identified in 31% (n = 27), including failure to identify or misinterpretation of the AAS itself in 15% (n = 13), haemorrhage in 13% (n = 11), branch involvement in 9% (n = 8), and misclassification in 3% (n = 3). All discrepancies occurred among the 80% (n = 70) of cases reported by radiologists without specialist cardiovascular interest. 26% (n = 23/88) of AAS cases were not clinically suspected at referral for CT and although this was associated with suboptimal protocols, only 51% of CT scans among suspected cases were technically adequate. Seven false-positive diagnoses were identified, three of which related to motion artefact.
Conclusion:
Significant discrepancies are common in the emergency CT assessment of positive cases AAS and this study highlights important pitfalls in CT technique and interpretation. The absence of discrepancies among radiologists with specialist cardiovascular interest suggests both suspected and confirmed cases warrant urgent specialist review.
Advances in knowledge:
CT angiography is central to the diagnosis of AAS; however, significant radiology discrepancies are common among non-specialists. This study highlights important pitfalls in both CT technique as well as interpretation and supports routine specialist cardiovascular imaging input in the emergency assessment of AAS.
Introduction
Acute aortic syndrome (AAS) describes a spectrum of life-threatening conditions of the thoracic aorta, including aortic dissection (AD), intramural haematoma (IMH) and penetrating atherosclerotic ulcer (PAU).1 AD is the commonest form of AAS caused by a tear in the intima of the aortic wall, allowing blood to enter and dissect through the medial layer, creating a false channel separated from the true lumen by an intimomedial flap with variable extension along the aorta.2 IMH is defined by a haematoma within the medial layer of the aortic wall, often presumed due to rupture of the vasa vasorum in the absence of an intimal tear.3 PAU occurs with ulceration of an atherosclerotic plaque through the intima into the medial layer of the aortic wall, with outpouching beyond the normal outer aortic contour and can cause aneurysmal dilation, IMH and/or AD. The Stanford classification is the most widely used system to direct surgical intervention in AD and is also commonly applied to IMH where involvement of the ascending aorta has a similar mortality to type A AD.4,5 Type A involves the ascending aorta regardless of the site of the initial intimal tear and mandates emergency surgical repair.6 Type B describes all dissections not involving the ascending aorta, including dissections involving the arch and are managed medically in the absence of haemorrhagic or malperfusion complications requiring endovascular and/or surgical intervention.7 Haemorrhagic complications are common in AAS—the pericardium is the most frequently involved site and can rapidly cause cardiac tamponade, followed by haemorrhage into the mediastinum and pleural cavity.2 AD can also be complicated by branch involvement with various mechanisms of obstruction that can lead to end-organ malperfusion with a multitude of clinical and radiological manifestations.8
The urgency of diagnosis in AAS is emphasised by the historically cited untreated mortality rate of 1% per hour from presentation. The timely management of type A AD is particularly important with a significantly lower mortality rate of 18% in patients managed surgically vs 56% medically.5 However, AAS is a challenging diagnosis, with frequent clinical misdiagnosis due to overlapping features with other conditions, especially acute coronary syndrome, and inappropriate use of antiplatelet and anticoagulant medications increase the risk of major haemorrhage.9,10 Emergency imaging therefore plays a central role—CT angiography, ideally with motion-free imaging (typically achieved with ECG-gating), is recommended by national and international guidelines,6,11,12 favoured by wide availability, rapid acquisition and sensitivity approaching 100%.3 Furthermore, it allows detailed assessment of the entire thoracic aorta and accurate identification of potential complications.2 In light of the critical role of CT angiography, this study investigated the accuracy of emergency CT reports at presentation in AAS and the frequency of radiological discrepancies.
Methods and materials
Study population
Consecutive patients with acute AD or IMH and an emergency CT at presentation over a 4-year period were retrospectively identified across a health board in the United Kingdom with a population of 1.2 million and 3 acute hospitals receiving adult patients. Patients with uncomplicated PAU were not included. A keyword search was used to identify all CT reports from January 2013 to December 2016 containing the term ‘dissection’. Reports and scans were evaluated to identify cases of AAS in patients ≥18 years of age with an emergency CT performed at presentation within the relevant time period. Retrospective patient identification is detailed in Figure 1.
Figure 1.
Retrospective patient identification.AAS = acute aortic syndrome.
Discrepancies
Cases were reviewed for radiological discrepancies related to AAS in the presenting CT report by two cardiovascular radiologists (JD and GR with 6 years and 15 years of experience in ECG-gated CT, respectively). JD undertook the initial unblinded evaluation of reports and scans as part of the retrospective case identification. GR reviewed the scans blinded to the clinical report which was treated as the gold-standard. Discrepancies were categorised as failure to identify, misinterpretation or inadequate characterisation of the AAS, acute haemorrhage, branch involvement, and misclassification. False-positive AAS diagnoses were evaluated separately. All discrepancies were referred to local Radiology Events And Learning Meetings (REALMs)13 and cases with clinical implications were communicated urgently to clinical teams.
AAS diagnosis
AD was defined by the presence of a false channel separated from the true lumen by an intimomedial flap demonstrated at CT angiography,2 and IMH by crescentic high attenuation within the aortic wall with lack of intravenous contrast enhancement.3
Haemorrhage
Haemorrhagic complications were defined by high attenuation collections at CT within the pericardial, mediastinal, or pleural spaces.2
Branch involvement
Multiple mechanisms of branch involvement that can lead to end-organ malperfusion and are readily demonstrated by CT angiography were assessed,8 including: direct extension of the intimomedial flap into a branch with associated luminal stenosis (‘static’ obstruction); tight compression of the true lumen by a high-pressure false lumen leading to direct pressure of the broad side of the intimomedial flap against branch ostia and obstruction (‘dynamic’ obstruction); avulsion of branch ostia intima by the intimomedial flap with precarious false lumen-dependent perfusion (‘ostial disconnection’); and systemic arterial thromboembolism due to altered flow dynamics in the dissected aorta or branches.
Classification
The Stanford classification was used to classify both AD and IMH.4
Reporting radiologists
Original consultant radiologist reporters were categorised as to whether or not they had a specialist cardiovascular interest. Provisional reports by radiologists in training were assessed separately and compared to the subsequent verified reports by the supervising consultants.
Clinically suspected diagnosis
Clinical information provided in the CT referral for each case was evaluated as to whether or not AAS was suspected.
CT technique
The technical adequacy of CT examinations was compared to national and international guidelines; specifically, an unenhanced CT of the thoracic aorta, CT angiography of the entire aorta, arterial contrast enhancement ≥250 HU (Hounsfield units), and ECG-gated motion-free imaging of the aortic root.6,11,12
Statistical analysis
Statistical analysis was performed using SPSS (v. 21, IBM, Armonk, NY). Non-normally distributed variables (Shapiro-Wilk test) are presented as median and interquartile range (IQR). Categorical variables are expressed as percentages and interrogated with χ2 tests (two-sided). Univariate logistic regression identified predictors of radiological discrepancy related to AAS and significant univariate variables were tested in a multivariate model to determine independent associations. Significance was set at p < 0.05.
Results
Study population
A total of 88 patients met the inclusion criteria from the 6445 reports identified from the keyword search (retrospective patient identification detailed in Figure 1), with an annual incidence of 1.8 per 100,000 population within the health board covered. 50% of patients were male with a median age of 71 years (IQR 61, 78 years). AD accounted for 81% (n = 71/88) and IMH 19% (n = 17/88) of cases, 60% (n = 53/88) type A and 40% (n = 35/88) type B. Demographics and AAS types are detailed in Table 1.
Table 1.
Demographics, AAS types, complications and discrepancies
| AAS | Aortic dissection | Intramural haematoma | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Type A | Type B | All | Type A | Type B | All | Type A | Type B | |
| 88 (100%) | 53 (60%) | 35 (40%) | 71 (81%) | 48 (68%) | 23 (32%) | 17 (19%) | 5 (29%) | 12 (71%) | |
| Age | 71 (61, 78) | 70 (61, 78) | 71 (60, 79) | 67 (58, 78) | 67 (61, 76) | 69 (54, 79) | 75 (67, 80) | 84 (70, 88) | 74 (66, 76) |
| Male | 44 (50%) | 26 (59%) | 18 (49%) | 37 (52%) | 24 (50%) | 13 (57%) | 7 (41%) | 2 (40%) | 5 (42%) |
|
Overall discrepancy rate (false positives not included) |
27 (31%) | 18 (34%) | 9 (26%) | 21 (30%) | 17 (35%) | 4 (17%) | 6 (35%) | 1 (20%) | 5 (42%) |
| AAS diagnosis discrepancy | 13 (15%) | 8 (15%) | 5 (14%) | 7 (10%) | 7 (15%) | 0 (0%) | 6 (35%) | 1 (20%) | 5 (42%) |
| Haemorrhage | 31 (35%) | 26 (49%) | 5 (14%) | 24 (34%) | 21 (44%) | 3 (13%) | 7 (41%) | 5 (100%) | 2 (17%) |
| Discrepancy rate (overall) | 11 (13%) | 10 (19%) | 1 (3%) | 10 (14%) | 9 (19%) | 1 (4%) | 1 (6%) | 1 (20%) | 0 (0%) |
| Discrepancy rate (when present) | 11 (35%) | 10 (38%) | 1 (20%) | 10 (42%) | 9 (43%) | 1 (33%) | 1 (14%) | 1 (20%) | 0 (0%) |
| Branch Involvement (AD only) | 34 (48%) | 26 (54%) | 8 (35%) | 34 (48%) | 26 (54%) | 8 (35%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Discrepancy rate (overall) | 8 (9%) | 7 (13%) | 1 (3%) | 8 (11%) | 7 (15%) | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Discrepancy rate (when present) | 8 (24%) | 7 (27%) | 1 (13%) | 8 (24%) | 7 (27%) | 1 (13%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Classification discrepancy | 3 (3%) | 1 (2%) | 2 (6%) | 3 (4%) | 0 (0%) | 3 (13%) | 0 (0%) | 0 (0%) | 0 (0%) |
AAS, acute aortic syndrome; AD, aortic dissection; IMH, intramural haematoma.
Discrepancies
At least one discrepancy was identified in 31% (n = 27/88) of cases of AAS, detailed in Table 1. The study identified discrepancies de novo in 41% (n = 11/27) of cases, with 1 case requiring urgent communication to the clinical team and all referred to relevant REALMs. Of the remaining discrepancy cases, 30% (n = 8/27) had already been identified following ad hoc specialist review, 19% (n = 5/27) on follow-up scans and 11% (n = 3/27) on consultant review of cases provisionally reported by trainees. The influence of demographics, clinically suspected diagnosis, and technical parameters were evaluated as predictors of radiological discrepancy related to AAS with logistic regression analysis, detailed in Table 2. The odds of any AAS-related discrepancy were 3.9-fold higher in cases where AAS was suspected clinically, in both uni- and multivariate models.
Table 2.
Logistic regression analysis to identify predictors of radiological discrepancy
| Variable | Univariate OR [95% CI] | p-value | Multivariate OR (95% CI) | p-value |
|---|---|---|---|---|
| Age | 0.99 [0.97–1.03] | 0.98 | … | … |
| Male | 1.11 [0.45–2.76] | 0.82 | … | … |
| AAS diagnosis suspected | 3.90 [1.05–14.52] | 0.04 | 3.92 [1.04–14.8] | 0.04 |
| Unenhanced CT thoracic aorta | 0.65 [0.26–1.63} | 0.36 | … | … |
| CT angiogram | 0.64 [0.16–2.53] | 0.52 | … | … |
| Aortic enhancement ≥250 HU | 0.79 [0.23–2.75] | 0.79 | … | … |
| Coverage of whole aorta | 0.64 [0.19–2.19] | 0.48 | … | … |
| ECG-gating capable scanner | 6.37 [0.78–51.72] | 0.08 | 6.40 [0.77–53.00] | 0.09 |
AAS, acute aortic syndrome; CI, confidence interval; CT, computed tomography; HU, Hounsfield unit; OR, odds ratio.
AAS diagnosis
Failure to diagnose the AAS itself was the most common discrepancy, observed in 15% (n = 13/88) of all cases and most frequent among IMH cases with 35% (n = 6/17) false-negative diagnosis at presentation. Example discrepancies in the diagnosis of AAS are included in Figure 2.-
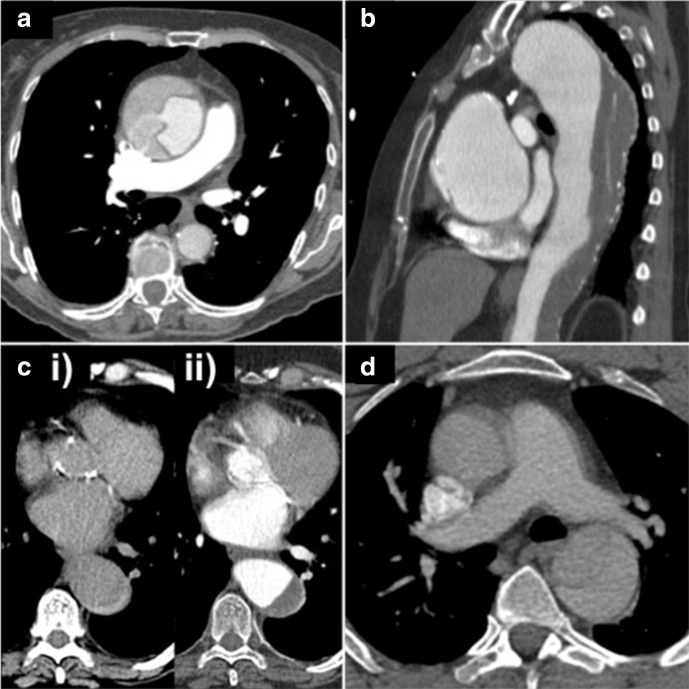
Figure 2.
AAS diagnosis and classification – example discrepancies. (a) CT pulmonary angiogram demonstrating an acute type A AD with aneurysmal dilation of the ascending aorta, not identified in the initial report. (b) Short segment acute type A AD in an aneurysmal ascending aorta not identified at presentation and chronic thrombosed type B AD in the diffusely aneurysmal descending thoracic aorta misinterpreted as atheromatous plaque. (c) Acute type B IMH misinterpreted as atheroma. (i) Unenhanced CT demonstrating crescentic high attenuation. (ii) CT angiogram. (d) Acute type B AD correctly diagnosed, however, motion artefact in the ascending aorta misinterpreted as extension of the dissection flap and incorrectly classified as type A. AAS, acute aortic syndrome; AD, aortic dissection; IMH, intramural haematoma.
Haemorrhage
Acute haemorrhage was observed in 35% (n = 31/88) of patients with a related discrepancy in 13% (n = 11/88) of all cases and a discrepancy rate of 35% (n = 11/31) among cases where haemorrhage was actually present. Example discrepancies in haemorrhagic complications are included in Figure 3.
Figure 3.
Haemorrhagic complications – example discrepancies. (a) Acute type A AD correctly diagnosed. Moderate haemopericardium not identified. (b) Acute type A AD of the aneurysmal ascending aorta correctly diagnosed. Pseudoaneurysm arising from the posterior wall of the ascending aorta not identified. (c) Acute type A AD correctly diagnosed. Moderate haemomediastinum not identified. (d) Acute type B AD and periaortic haematoma correctly diagnosed. Large acute haemothorax misinterpreted as a pleural-based mass. AD, aortic dissection.
Branch involvement
Involvement of the aortic branches, with or without evidence of end-organ malperfusion, was present in 48% (n = 34/71) of AD cases. No branch involvement was observed among IMH cases. Discrepancies related to branch involvement occurred in 11% (n = 8/71) of all AD cases and 24% (n = 8/34) of cases where branch involvement was present. Example discrepancies in branch involvement are included in Figure 4.
Figure 4.

Branch involvement and malperfusion complications – example discrepancies. (a) i) CT at presentation: acute type B AD with intimal flap extension into the SMA correctly diagnosed but tight compression of the true lumen and ‘dynamic’ obstruction of the coeliac axis and SMA not recognised. (ii) CT 2 days from presentation: persistent dynamic obstruction and subsequent small bowel infarction. (b) Type B AD correctly diagnosed. Severe renal artery stenosis supplying the single functioning right kidney not identified; the patient developed refractory hypertension. (c) Acute type A AD with extension into right CCA and right SA extension correctly identified, but both erroneously described as extending into the ‘left’ CCA and SA with potential implications for antegrade cerebral perfusion at surgery. AD, aortic dissection; CCA, common carotid artery; SA, subclavian artery; SMA, superior mesenteric artery.
Classification
Discrepancies related to AAS classification occurred in 3% (n = 3/88) of all cases, exclusively in type B AD. In two cases, arch involvement without extension into the ascending aorta was incorrectly classified as type A, and in one case motion artefact in the ascending aorta was misinterpreted as a dissection flap and incorrectly classified as type A. No classification discrepancies were observed among IMH cases, but this is limited to the 65% (n = 6/17) that were actually identified at presentation. An example misclassification discrepancy is included in Figure 2.
Overdiagnosis
Seven cases of false-positive AAS diagnosis were identified. In three cases, motion artefact in the aortic root was misinterpreted as AD, two of which were clarified by a subsequent ECG-gated scan. In one case, a bioprosthetic aortic valve suture line was misinterpreted as a short dissection flap. Findings misinterpreted as a PAU in the remaining three cases included a prominent ductus arteriosus, a surgical pledget on the ascending aorta, and the stump of an occluded coronary bypass graft. Example overdiagnosis discrepancies are included in Figure 5.
Figure 5.

False positive AAS diagnosis – example discrepancies. (a) i) CT performed to rule out AAS – acutely occluded CABG graft misinterpreted as an ulcerated plaque in the anterior ascending aorta consistent with an AAS. (ii) Cardiac-gated CT performed 2 months earlier demonstrating a previously patent saphenous vein to obtuse marginal coronary artery graft. (b) Non-diagnostic quality images of the aortic root due to motion artefact on a non-gated CT interpreted as an acute type A AD, clarified on a subsequent cardiac-gated scan. (c) Equivocal appearances at the aortic root on another non-gated CT also interpreted as a type A AD but again clarified following a repeat cardiac-gated scan. AAS, acute aortic syndrome; AD, aortic dissection; CABG, coronary artery bypass graft; .
Reporters
No discrepancies occurred among the 20% (n = 18/88) of cases verified by consultants with specialist cardiovascular interest. Among the 80% (n = 70/88) of cases verified by 46 consultants without specialist cardiovascular interest, there was a discrepancy rate of 39% (n = 27/70), involving 46% (n = 21/46) of the consultants. In total, 116 consultant radiologists were involved in emergency CT reporting across the 3 acute hospitals. The 38% (n = 33/88) of cases provisionally reported by radiologists in training observed a discrepancy rate of 27% (n = 9/33) involving 33% (n = 8/24) of trainees, with discrepancies corrected in only 3 cases by the verifying consultant. Discrepancies are compared between cardiovascular specialists and other consultants in Table 3. All false-positive AAS diagnoses occurred among consultants without specialist cardiovascular interest.
Table 3.
Discrepancies compared between specialist and non-specialist reporters
| All AAS cases | Specialist report | Non-specialist report | p-value | |
|---|---|---|---|---|
| 88 (100%) | 18 (20%) | 70 (80%) | ||
|
Overall discrepancy rate (false positives not included) |
27 (31%) | 0 (0%) | 27 (39%) | 0.001 |
| AAS diagnosis discrepancy | 13 (15%) | 0 (0%) | 13 (19%) | 0.062 |
| Haemorrhage discrepancy | 11 (13%) | 0 (0%) | 11 (16%) | 0.111 |
| Branch involvement discrepancy | 8 (9%) | 0 (0%) | 8 (11%) | 0.336 |
| Classification discrepancy | 3 (3%) | 0 (0%) | 3 (4%) | 0.999 |
AAS, acute aortic syndrome; AD, aortic dissection.
Clinically suspected diagnosis
A total of 1002 emergency/presenting CT scans were performed in 991 patients over the 4-year period for suspected AAS, with 6.5% (n = 65/1002) of cases positive for AAS. 26% (n = 23/88) of AAS cases were not clinically suspected at referral for CT, with pulmonary embolism accounting for 52% (n = 12/23) of unsuspected cases and 14% (n = 12/88) of positive cases overall. Other clinically suspected diagnoses included malignancy (n = 6/23), acute abdomen (n = 3/23), and chest sepsis (n = 2/23). The overall discrepancy rate was significantly higher among clinically suspected cases, where there were also higher rates of type A AAS, haemorrhage and branch involvement. Clinical suspicion of AAS is compared with discrepancy rates, AAS types, complications and specialist reporters in Table 4.
Table 4.
Clinical suspicion of AAS at referral for CT compared with discrepancy rates, AAS type, complications and specialist reporters
| All cases | AAS clinically suspected | Non-AAS diagnosis suspected | p-value | |
|---|---|---|---|---|
| 88 (100%) | 65 (74%) | 23 (26%) | ||
|
Discrepancy rate (false positives not included) |
27 (31%) | 24 (37%) | 3 (13%) | 0.038 |
| Type A AAS | 53 (60%) | 43 (66%) | 10 (43%) | 0.082 |
| AD | 71 (81%) | 53 (82%) | 18 (78%) | 0.763 |
| IMH | 17 (19%) | 12 (71%) | 5 (29%) | 0.763 |
| Haemorrhage | 31 (35%) | 28 (43%) | 3 (13%) | 0.011 |
| Branch involvement | 34 (48%) | 26 (40%) | 8 (11%) | 0.790 |
| Specialist reporter | 18 (20%) | 14 (22%) | 4 (17%) | 0.772 |
AAS, acute aortic syndrome; AD, aortic dissection; IMH, intramural haematoma.
CT technique
Technically adequate CT angiography was achieved in the vast majority of positive suspected AAS cases, but only 51% (n = 33/65) included an unenhanced CT of the thoracic aorta. 15% (n = 13/88) of examinations were performed on CT scanners with ECG-gating capability, but even on these scanners ECG-gating was employed in only 31% (n = 4/13). The technical adequacy of CT examinations is compared with clinical suspicion of AAS in Table 5.
Table 5.
Technical adequacy of CT examinations compared with clinical suspicion of AAS
| All cases | AAS clinically suspected | Non-AAS diagnosis suspected | p-value | |
|---|---|---|---|---|
| 88 (100%) | 65 (74%) | 23 (26%) | ||
| Unenhanced CT thoracic aorta | 36 (41%) | 33 (51%) | 3 (13%) | 0.001 |
| CT angiogram | 75 (85%) | 63 (97%) | 12 (52%) | <0.0001 |
| Aortic enhancement ≥250 HU | 73 (83%) | 63 (97%) | 10 (43%) | <0.0001 |
| Coverage of whole aorta | 71 (81%) | 59 (91%) | 12 (52%) | <0.0001 |
| ECG-gating capable scanner | 13 (15%) | 9 (14%) | 4 (17%) | 0.736 |
| ECG-gated CT performed | 4 (5%) | 3 (5%) | 1 (4%) | 0.999 |
| ECG-gating capable scanner and ECG-gated CT performed | 4 (31%) | 3 (33%) | 1 (25%) | 0.999 |
| All technical parameters met (except ECG-gated imaging) | 35 (40%) | 32 (49%) | 3 (13%) | 0.003 |
AAS, acute aortic syndrome; HU, Hounsfield units.
Discussion
Radiological discrepancies occurred in nearly one-third of CT reports at presentation in AAS, most commonly related to diagnosis of the AAS itself, but also haemorrhagic and malperfusion complications, as well as misclassification. These discrepancies are concerning given the central role of CT in the diagnosis of AAS and frequently cited sensitivity of 100% depended upon clinically for safely excluding the condition.3 Inaccurate reports risk further jeopardising outcomes when considered in the context of high reported rates of clinical misdiagnosis of 25–39%.9,10
Interobserver variability and discrepancies in radiology are well documented, the causes of which can be individual-specific (errors of perception, interpretation and communication) and system-related (technical factors, inadequate clinical information, workload etc.).14–16 Although there are no objective standards for acceptable levels of discrepancies, formal review of cases is recommended as part of clinical governance, quality improvement and patient safety.17 Previous studies have investigated radiological discrepancies and outcomes in other settings including missed lung cancer at chest radiography, acute abdominal CT reports, and CT in intracranial haemorrhage.18–20 False-positive diagnosis of AAS has previously been investigated,21,22 but to the authors' knowledge this is the first study of CT reports in AAS with regards to false-negative diagnosis.
AAS diagnosis
Failure to identify or misinterpretation of AAS was the most common discrepancy, particularly among IMH cases where 35% (n = 6/17) were not correctly diagnosed at presentation. Misinterpretations of typical CT appearances of IMH included atheroma and aortitis, with similar errors of interpretation observed among AD cases with partially thrombosed/poorly perfused false lumens. Short dissection flaps limited to the aortic root/ascending aorta were also found among the small number of missed AD cases. Although it was beyond the scope of this study to investigate the clinical outcomes in discrepancy cases, the potential consequences of undiagnosed AAS are emphasised by the high mortality rate among untreated cases and dangers of antithrombotic treatments that may be administered following false exclusion of AAS.5,9,10
Haemorrhage
All but one discrepancy related to haemorrhagic complications occurred in cases of type A AAS and although emergency surgery may be warranted by classification alone, the identification of haemorrhage can be of value in emergency surgical planning.23 The discrepancies observed emphasise the importance of careful inspection of pericardial and mediastinal spaces for haematoma and extravasation.
Branch involvement
The majority of discrepancies relating to branch involvement occurred in type A AD, with only some cases relieved by surgical repair of the ascending aorta at the site of the primary tear and decompression of the false lumen. However, surgical repair of the ascending aorta does not guarantee relief of distal branch obstruction not identified at imaging. Dynamic obstruction of renal and mesenteric branches arising from the true lumen, both associated with high mortality rates,5 were frequently not identified at presentation, often due to a lack of familiarity with this unusual mechanism of obstruction outside of the context of AD.
Classification
Misclassifications were uncommon but stress important pitfalls. Firstly, traditional classification systems fail to account for the small proportion of dissections involving the aortic arch but not the ascending aorta, which favour medical therapy in the absence of complications and should be classified as type B.7 The second pitfall is motion artefact in the ascending aorta, misinterpreted in one case as extension of a type B dissection flap leading to incorrect classification as type A, emphasising the importance of guideline-recommended ECG-gated motion-free imaging.12 No cases of retrograde type A AD (i.e. AD originating from a primary intimal tear in the descending aorta propagating retrograde into the ascending aorta) were identified in this cohort; however, this well described entity, which the authors suspect is also vulnerable to misinterpretation or inadequate characterisation at CT, has important implications for surgical and/or interventional strategies.24
Overdiagnosis
Misinterpretation of motion artefact and other findings leading to false positive diagnosis of AAS occurred in a small, but nonetheless concerning, number of patients compared to the total number of CT scans performed for suspected AAS (0.7%, n = 7/1002). None of the false positive cases identified in this study underwent inappropriate surgery or intervention, but this is a recognised risk with potentially grave consequences21,22 and regarded as a ‘Never Event’ in the NHS, emphasising the importance of both ECG-gated motion-free imaging and specialist review. Reports frequently cited motion artefact as a technical limitation and this arguably applied to the overwhelming majority of cases.
Reporters
Discrepancies were common (39%, n = 27/70) among the majority of cases reported by consultants without a specialist cardiovascular interest and nearly half (46%, n = 21/46) of these reporters had at least one discrepancy in their reports. Similar discrepancy rates occurred among cases reported provisionally by trainees with only a fraction corrected by the supervising consultant. Although AAS is the most common emergency of the aorta,2 it is nonetheless relatively rare with the annual incidence in this cohort of 1.8 per 100,000 population broadly similar to previous studies.25,26 Unfamiliarity with potentially complex appearances of this infrequently encountered condition is probably the most significant factor in the high discrepancy rate amongst non-cardiovascular specialists. Furthermore non-emergent follow-up imaging of AAS cases is typically allocated to cardiovascular specialists, further limiting exposure among other consultants. The absence of discrepancies among cardiovascular specialists suggests both suspected and confirmed cases routinely warrant urgent specialist review. An ‘imaging checklist’ summarising the key findings that should be included in CT reports in AAS is detailed in Figure 6.
Figure 6.
Imaging checklist in acute aortic syndrome. AAS, acute aortic syndrome; HU, Hounsfield units; IMH, intramural haematoma; PAU, penetrating atherosclerotic ulcer.
Clinically suspected diagnosis
The 6.5% (n = 65/1002) of clinically suspected cases positive for AAS is similar to rates of 2.7–13.0% observed in previous studies.27,28 A sizeable minority of AAS cases (26%) were not suspected at CT referral. Pulmonary embolism was the most common clinical misdiagnosis, particularly concerning given the potential for empirical administration of antithrombotic treatments and delayed definitive diagnosis with CT. However, the significantly higher rate of haemorrhage observed among clinically suspected cases contradicts previous studies,9,10 probably because this study did not account for other potential initial clinical misdiagnoses warranting antithrombotic treatment that may have preceded the CT referral, particularly acute coronary syndrome. Interestingly, the radiological discrepancy rate was significantly higher among suspected cases (37% vs 13%), despite similar proportions of specialist reporters, and the logistic regression analysis revealed that cases with clinical suspicion of AAS were significantly and independently more likely to have a radiological discrepancy in a multivariate model. This unusual finding is likely accounted for by higher rates of type A AAS, haemorrhage and branch involvement among suspected cases, suggesting more complex imaging manifestations that may increase the likelihood of error.
CT technique
Only a minority of examinations were technically adequate with suboptimal protocols more common among unsuspected cases, but even when scrutinising cases suspected clinically and making an exception for lack of ECG-gating, only 49% (n = 32/65) were adequate when compared against national and international guidelines, primarily due to the absence of an unenhanced scan. CT with ECG-gating was employed in only a few suspected cases, even at centres with a capable scanner due to lack of radiographic expertise out-of-hours. This is despite the recognised risks of false positive and negative diagnosis from motion artifacts.12,21,22 The limited number of cardiac-capable CT scanners and accredited workforce across the UK clearly presents a challenge to the widespread use of ECG-gated imaging in suspected AAS.29 The absence of an unenhanced scan in nearly half of suspected cases is particularly concerning given the 35% (n = 6/17) discrepancy rate in the diagnosis of IMH. Routine availability of technically robust CT protocols for the emergency assessment of AAS without delay, with particular emphasis on ECG-gated motion-free imaging, must be considered mandatory for any emergency radiology service in the UK.
Limitations
Retrospective identification of cases was limited to CT reports, and therefore AAS cases missed at presentation but not identified on review of initial imaging or follow-up could not be included. Uncomplicated PAUs were excluded due to small numbers. It was beyond the scope of this study to fully investigate the clinical consequences of discrepancies and compare outcomes due to the small numbers and considering the diversity of complications and comorbidities needing to be accounted for to perform meaningful comparisons.
Conclusion
Potentially life-threatening radiological discrepancies are common in the emergency CT assessment of AAS and both suspected and confirmed cases warrant urgent review by a consultant with cardiovascular imaging expertise.
Footnotes
Ethics approval: This study was compliant with the Health Research Authority requirements for retrospective research in the National Health Service of the UK.
Contributor Information
John G Dreisbach, Email: john.dreisbach3@nhs.scot.
Jonathan CL Rodrigues, Email: j.rodrigues1@nhs.net.
Giles Roditi, Email: Giles.Roditi@glasgow.ac.uk.
REFERENCES
- 1.Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA 2000; 283: 897–903. doi: 10.1001/jama.283.7.897 [DOI] [PubMed] [Google Scholar]
- 2.McMahon MA, Squirrell CA, a MM, a SC. Multidetector CT of aortic dissection: a pictorial review. Radiographics 2010; 30: 445–60. doi: 10.1148/rg.302095104 [DOI] [PubMed] [Google Scholar]
- 3.Chiu KWH, Lakshminarayan R, Ettles DF. Acute aortic syndrome: CT findings. Clin Radiol 2013; 68: 741–8. doi: 10.1016/j.crad.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 4.Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg 1970; 10: 237–47. doi: 10.1016/S0003-4975(10)65594-4 [DOI] [PubMed] [Google Scholar]
- 5.Arturo E, IE M, Eduardo B, GT G, EM D, Udo S. Insights from the International registry of acute aortic dissection. Circulation 2018; 137: 1846–60. [DOI] [PubMed] [Google Scholar]
- 6.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE. ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of cardiology Foundation/American heart association Task force on practice guidelines, a. Circulation 2010; 121: e266–e369. [DOI] [PubMed] [Google Scholar]
- 7.Lempel JK, Frazier AA, Jeudy J, Kligerman SJ, Schultz R, Ninalowo HA, et al. Aortic arch dissection: a controversy of classification. Radiology 2014; 271: 848–55. doi: 10.1148/radiol.14131457 [DOI] [PubMed] [Google Scholar]
- 8.Valente T, Rossi G, Lassandro F, Rea G, Marino M, Muto M, et al. Mdct evaluation of acute aortic syndrome (aas. Br J Radiol 2016; 89: 201508252016/04/01. doi: 10.1259/bjr.20150825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol 2007; 99: 852–6. doi: 10.1016/j.amjcard.2006.10.055 [DOI] [PubMed] [Google Scholar]
- 10.Pourafkari L, Tajlil A, Ghaffari S, Parvizi R, Chavoshi M, Kolahdouzan K, et al. The frequency of initial misdiagnosis of acute aortic dissection in the emergency department and its impact on outcome. Intern Emerg Med 2017; 12: 1185–95. doi: 10.1007/s11739-016-1530-7 [DOI] [PubMed] [Google Scholar]
- 11.Erbel R, Aboyans V, Boileau C, Bossone E, Di Bartolomeo R, Eggebrecht H. Esc guidelines on the diagnosis and treatment of aortic diseases. European Heart Journal 2014; 35: 2873–926. [DOI] [PubMed] [Google Scholar]
- 12.Vardhanabhuti V, Nicol E, Morgan-Hughes G, Roobottom CA, Roditi G, Hamilton MCK, et al. Recommendations for accurate CT diagnosis of suspected acute aortic syndrome (AAS)--on behalf of the British Society of Cardiovascular Imaging (BSCI)/British Society of Cardiovascular CT (BSCCT. Br J Radiol 2016; 89: 20150705. doi: 10.1259/bjr.20150705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The Royal College of Radiologists. Radiology Events and Learning (REAL) [Internet. RCR 2019;. [Google Scholar]
- 14.Fitzgerald R. Error in radiology. Clin Radiol 2001; 56: 938–46. doi: 10.1053/crad.2001.0858 [DOI] [PubMed] [Google Scholar]
- 15.Brady A, Laoide Risteárd Ó, McCarthy P, McDermott R. Discrepancy and error in radiology: concepts, causes and consequences. Ulster Med J 2012; 81: 3–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Brady AP. Error and discrepancy in radiology: inevitable or avoidable? Insights Imaging 2017; 8: 171–82. doi: 10.1007/s13244-016-0534-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Royal College of Radiologists Standards for learning from discrepancies meetings. 2014;.
- 18.Quekel LG, Kessels AG, Goei R, van Engelshoven JM. Miss rate of lung cancer on the chest radiograph in clinical practice. Chest 1999; 115: 720–4. doi: 10.1378/chest.115.3.720 [DOI] [PubMed] [Google Scholar]
- 19.Howlett DC, Drinkwater K, Frost C, Higginson A, Ball C, Maskell G. The accuracy of interpretation of emergency abdominal CT in adult patients who present with non-traumatic abdominal pain: results of a UK national audit. Clinical Radiology 2017; 72: 1–13. [DOI] [PubMed] [Google Scholar]
- 20.Strub WM, Leach JL, Tomsick T, Vagal A. Overnight preliminary head CT interpretations provided by residents: locations of misidentified intracranial hemorrhage. AJNR Am J Neuroradiol 2007; 28: 1679–82. doi: 10.3174/ajnr.A0653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raymond CE, Aggarwal B, Schoenhagen P, Kralovic DM, Kormos K, Holloway D. Prevalence and factors associated with false positive suspicion of acute aortic syndrome: experience in a patient population transferred to a specialized aortic treatment center. cardiovascular diagnosis and therapy; Vol 3, no 4 (December 2013. Cardiovascular Diagnosis and Therapy 2013;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karras R, Ricci M, Salerno TA, Gologorsky E. Motion artifact resulting in a false positive CT angiogram for a presumed aortic dissection. J Card Surg 2011; 26: 223–4. doi: 10.1111/j.1540-8191.2011.01200.x [DOI] [PubMed] [Google Scholar]
- 23.Galvin SD, Perera NK, Matalanis G. Surgical management of acute type A aortic dissection: branch-first arch replacement with total aortic repair. Ann Cardiothorac Surg 2016; 5: 236–44. doi: 10.21037/acs.2016.05.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiMusto PD, Rademacher BL, Philip JL, Akhter SA, Goodavish CB, De Oliveira NC, et al. Acute retrograde type A aortic dissection: morphologic analysis and clinical implications. J Surg Res 2017; 213: 39–45. doi: 10.1016/j.jss.2017.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melvinsdottir IH, Lund SH, Agnarsson BA, Sigvaldason K, Gudbjartsson T, Geirsson A. The incidence and mortality of acute thoracic aortic dissection: results from a whole nation study. Eur J Cardiothorac Surg 2016; 50: 1111–7. doi: 10.1093/ejcts/ezw235 [DOI] [PubMed] [Google Scholar]
- 26.Howard DPJ, Sideso E, Handa A, Rothwell PM, Incidence RPM. Incidence, risk factors, outcome and projected future burden of acute aortic dissection. Ann Cardiothorac Surg 2014; 3: 278–84. doi: 10.3978/j.issn.2225-319X.2014.05.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovy AJ, Bellin E, Levsky JM, Esses D, Haramati LB. Preliminary development of a clinical decision rule for acute aortic syndromes. Am J Emerg Med 2013; 31: 1546–50. doi: 10.1016/j.ajem.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nazerian P, Mueller C, Soeiro AdeM, Leidel BA, Salvadeo SAT, Giachino F, et al. Diagnostic accuracy of the aortic dissection detection risk score plus D-dimer for acute aortic syndromes: the advised prospective multicenter study. Circulation 2018; 137: 250–8. doi: 10.1161/CIRCULATIONAHA.117.029457 [DOI] [PubMed] [Google Scholar]
- 29.Dreisbach JG, Nicol ED, Roobottom CA, Padley S, Roditi G. Challenges in delivering computed tomography coronary angiography as the first-line test for stable chest pain. Heart 2018; 104: 921–927. doi: 10.1136/heartjnl-2017-311846 [DOI] [PMC free article] [PubMed] [Google Scholar]