Abstract
Objectives:
To explore the predictive value of radiomics nomogram using pretreatment ultrasound for disease-free survival (DFS) after resection of triple negative breast cancer (TNBC).
Methods and materials:
A total of 486 TNBC patients from 3 different institutions were consecutively recruited for this study. They were categorized into the primary cohort (n = 216), as well as the internal validation cohort (n = 108) and external validation cohort (n = 162). In primary cohort, least absolute shrinkage and selection operator logistic regression algorithm was used to select recurrence-related radiomics features extracted from the breast tumor and peritumor regions, and a radiomics signature was constructed derived from the grayscale ultrasound images. A radiomic nomogram integrating independent clinicopathological variables and radiomic signature was established with uni- and multivariate cox regressions. The predictive nomogram was validated using an internal cohort and an independent external cohort regarding abilities of discrimination, calibration and clinical usefulness.
Results:
The patients with higher Rad-score had a worse prognostic outcome than those with lower Rad-score in primary cohort and two validation cohorts (All p < 0.05).The radiomics nomogram indicated more effective prognostic performance compared with the clinicopathological model and tumor node metastasis staging system (p < 0.01), with a training C-index of 0.75 (95% confidence interval (CI), 0.71–0.80), an internal validation C-index of 0.73 (95% CI, 0.69–0.78) and an external validation 0.71 (95% CI,0.66–0.76). Moreover, the calibration curves revealed a good consistency for survival prediction of the radiomics model.
Conclusions:
The ultrasound-based radiomics signature was a promising biomarker for risk stratification for TNBC patients. Furthermore, the proposed radiomics modal integrating the optimal radiomics features and clinical data provided individual relapse risk accurately.
Advances in knowledge:
The radiomics model integrating radiomic signature and independent clinicopathological variables could improve individual prognostic evaluation and facilitate therapeutic decision-making, which demonstrated the incremental value of the radiomics signature for prognostic prediction in TNBC.
Introduction
Triple negative breast cancer (TNBC) constitutes 15–20% of all invasive breast cancer, which lacks the expression of human epidermal growth factor receptor 2 and hormone receptors.1,2 Owing to its aggressive nature and lack of specific therapeutic targets, those TNBC patients who cannot totally benefit from neoadjuvant chemotherapy (NAC) have a worse prognosis compared with other non-TNBC patients.2–4 Additionally, TNBC is heterogeneous with large variations in clinical outcomes. Hence, efforts to establish a precise prognostic model for individualize management in TNBC are warranted and ongoing.
To date, in addition to the traditional tumor node metastasis (TNM) staging, molecular biomarkers are available for survival estimation used for risk classification due to improvements in biologic and genomic technologies. Some studies5,6 indicated that immune‐related genomic data provided reliable and practical prognostic information for breast cancer. Wang et al5 detected that high-proportioned activated NK cells and naive B cells stood for better prognostic indicators in TNBC patients. However, it is a limitation for these invasive means that none of them could provide comprehensive and accurate characterization about TNBC with large-scale analyses.
Imaging assessment of breast ultrasound before surgery has been applied to investigate the value of diagnosis and prognosis because of its effectivity and feasibility. Previous studies7–10 have found the correlation between the morphological features of ultrasound and prognostic factors in breast cancer. Ko et al8 showed that circumscribed margins and posterior acoustic enhancement have been correlated with the triple-negative subtype or aggressive biology. A recent study10 demonstrated that vertical direction of lesion on pre-operative ultrasound was indicated to be predictive for higher risk of axillary lymph node metastasis and unfavorable outcomes. However, manual evaluation of tumor tissue by radiologist mainly depends on semantic features but ignores many significant tumor heterogeneities derived from medical images.11
In comparison, a rapidly evolving topic called radiomics has been able to extract large amounts of quantitative features from digitally encrypted images, which facilitates improved clinical decision-making.11,12 Currently, although previous studies13,14 have suggested favorable predictive analyses in invasive breast cancer using intratumoral radiomics features from pre-operative MRI, most available studies ignored features of peritumoral region which may improve the accuracy of predicting recurrence. In order to get a comprehensive study design, we aimed to develop a radiomics signature based on intra- and peritumoral ultrasound features for risk stratification and to further construct and validate a radiomics nomogram for estimating the disease-free survival (DFS) in patients with TNBC.
Methods and materials
Patients
The retrospective analysis was approved by the ethics committee of all participating institutions, and written informed consent was waived. Between July 2012 and December 2017, a total of 486 female patients with TNBC were consecutively recruited. Among them, 324 patients who underwent curative-intent breast surgery from the First Affiliated Hospital of Nanjing Medical University. The cohort was randomly allocated into a primary data set (216 cases) and an internal validation data set (108 cases) at a 2:1 ratio. The independent external validation data set consisted of 162 patients at the remaining two institutions. Supplementary Material 1 and Figure S1 showed recruitment pathway of our study, along with the inclusion and exclusion criteria.
Clinical characteristics and follow-up
The clinical and histopathological variables, including age, tumor number, stage (T stage, N stage, clinical stage), histologic tumor grade, Ki-67 levels, lymphovascular invasion, surgical type and chemoradiotherapy, were evaluated. The end point of the study was DFS, defined as the period from the initial pathological results to tumor local recurrence, distant metastasis, last follow-up or death. According to the follow-up procedure adopted at three institutions, post-operative relapse was monitored by means of breast ultrasound examination every 3–6 months during the first 2 years and then at least annually. Tumor staging was generated on the basis of American Joint Committee on Cancer (AJCC) TNM Staging Manual, eighth Edition.
Ultrasound image acquisition, radiomics feature extraction
Ultrasound examinations were performed using MyLab Twice ultrasound machine (Esaote, Italy) or Siemens S3000 ultrasound device (Siemens Medical Solutions, USA), and two ultrasound instruments were equipped with a high-frequency probe (4–12 MHz). All the lesions were independently examined and assessed by two board-certified radiologists (with 8 and 10 years’ of breast oncologic imaging experience, respectively), who were blinded to the pathologic details and prognosis. Detailed diagnostic criteria are shown in Supplementary Material 1. The following descriptive ultrasound characteristics were further analyzed, including tumor size, shape, margin, orientation, posterior acoustic patterns. Any disagreements were settled by consensus. Intratumoral region of interest (ROI) was generated through drawing a line along the contour of primary tumor. Due to the pathophysiological changes in the surrounding tissues of the tumor, a 4-mm-wide peripheral ring was automatically created with dilation and shrinkage by 2 mm on previous boundary. Image pre-processing was described in Supplementary Material 1. Segmentation of tumor ROI was performed by means of the maximum cross-sectional area of the grayscale image with ITK-SNAP program (http://www.itksnap.org), and the radiomics features reflecting differences of phenotypic characteristics were automatically extracted using the open-source Pyradiomics package (https://github.com/Radiomics/pyradiomics). We extracted 96 quantified image features, consisting of 17 first-order statistical features, 11 shape features, and 68 textural features, which were normalized with z scores for reduce difference of a series of feature parameters. Each patient got 192 features due to biphasic radiomics with intra- and peritumoral imaging analysis.
The reliability was quantified by interclass correlation coefficients (ICCs). The interobserver agreement of feature extraction was verified with 100 randomly chosen images by 2 independent radiologists in a blinded fashion. To assess the intraobserver reliability, the first radiologist repeated the second extraction of feature according to the same procedure 1 month later. The ICC values greater than 0.80 representing almost perfect agreement were chosen for following analysis.
Radiomics feature selection and construction of signature
The minimum redundancy maximum relevance (MRMR) algorithm and least absolute shrinkage and selection operator (LASSO) was applied to choose the most significant prognostic features for avoiding the data overfitting. A radiomics signature incorporating the selected imaging features and their corresponding weights was created to calculate the ultimate radiomics score (Rad-score) for each patient. The optimum cut-off value of the Rad-score was identified according to time-dependent receiver operation characteristic (ROC) curve analysis, and patients were classified into high- or low-risk groups. Using Kaplan–Meier survival analysis, the relationship between the radiomics signature and prognosis was evaluated in the training and validation sets. The log-rank test was performed to compare significant differences in the survival curves between two groups.
Development and validation of the radiomics model
Predictors of recurrence with p values < 0.05 in the univariate analysis were incorporated in the multivariate regression analysis. Radiomics nomogram using the multivariate Cox proportional hazard model with the lowest Akaike information criteria score was developed to predict survival probability at the different point of time. Two other predictive models to evaluate prognosis were developed. One model was on the basis of the TNM staging system, while the other included only the significant clinicopathological characteristics. To demonstrate the incremental value of radiomics signature, the predictive accuracy and discriminative ability of our radiomics nomogram quantified by the concordance index (C-index) was compared with those of clinicopathological nomogram and TNM staging. Calibration curves were plotted in light of 1000 bootstrap resamples to assess the prognostic performance of the radiomics model. Validation of the radiomics nomogram was conducted by in internal and external validation cohorts respectively. In addition, decision curve analysis (DCA) was employed to determine clinical usefulness of radiomics model by quantitatively estimating the net benefits in both validation data sets.
Statistical analysis
The categorical variables were assessed by using the Pearson χ2 or Fisher’s exact test, and the Student’s t-test or non-parametric Mann–Whitney U test was applied to compare the difference for descriptive variables between primary and validation groups. A two-sided p < 0.05 was indicative of a statistical difference. Statistical analysis was performed with R software (v. 3.3.4; http://www.R-project.org) with R packages reported in the supplementary method.
Results
Patient characteristics and DFS
The clinicopathological and ultrasonic characteristics for primary, internal validation, and external cohorts were listed in Table 1, and the median follow-up time of these three datasets were 53 months (interquartile range [IQR], 36–62) ,47 months (IQR, 32–59) and 56 months (IQR, 37–65) respectively. Compared with the primary cohort, the histologic grade in external validation cohort was significantly lower, and other characteristics and the median follow-up duration were comparable between the training and two validation cohorts. Regarding the survival outcomes, a total of 63 of 216 patients (29.2%) in the primary cohort, 30 of 108 patients (27.8%) in the internal cohort, and 49 of 162 patients (30.2%) in the external cohort had experienced a confirmed disease relapse with the similar median DFS. Among all 142 relapsed patients, there were 62 (43.7%) locoregional recurrence, 56 (39.4%) distant metastasis, and 11 (7.7%) contralateral breast cancer, and the remaining 13 (9.2%) patents had both local regional and distant recurrence. Six patients relapsed within the initial 12 months of follow-up, and these may be caused by residual tissue.
Table 1.
Clinical characteristics of the primary cohort and two validation cohorts
| Characteristic | Primary cohort (n = 216) | Internal validation cohort (n = 108) | P value | External validation cohort (n = 162) | P value |
|---|---|---|---|---|---|
| Age | 0.812 | 0.530 | |||
| <50 | 123 (56.9) | 63 (58.3) | 87 (53.7) | ||
| ≥50 | 93 (43.1) | 45 (41.7) | 75 (46.3) | ||
| aMedian | 46(37-54) | 48(38-59) | 0.762 | 45(36-53) | 0.658 |
| T stage | 0.406 | 0.502 | |||
| T1(≤2 cm) | 120 (55.6) | 64 (59.3) | 91 (56.2) | ||
| T2(2–5 cm) | 89 (41.2) | 38 (35.2) | 62 (38.3) | ||
| T3(≥5 cm) | 7 (3.2) | 6 (5.5) | 9 (5.5) | ||
| N stage | 0.590 | 0.442 | |||
| N0 | 140 (64.8) | 76 (70.4) | 115 (71.0) | ||
| N1 | 48 (22.2) | 21 (19.4) | 29 (17.9) | ||
| N2 + N3 | 28 (13.0) | 11 (10.2) | 18 (11.1) | ||
| Stage | 0.490 | 0.175 | |||
| I | 93 (43.1) | 54 (50.0) | 78 (48.1) | ||
| II | 97 (44.9) | 42 (38.9) | 58 (35.8) | ||
| III | 26 (12.0) | 12 (11.1) | 26 (16.1) | ||
| Grade | 0.365 | 0.004 | |||
| I | 17 (7.9) | 4 (3.7) | 18 (11.1) | ||
| II | 37 (17.1) | 19 (17.6) | 48 (29.6) | ||
| III | 162 (75.0) | 85 (78.7) | 96 (59.3) | ||
| Tumor shape | 0.627 | 0.330 | |||
| Regular | 80 (37.0) | 43 (39.8) | 68 (42.0) | ||
| Irregular | 136 (63.0) | 65 (60.2) | 94 (58.0) | ||
| Tumor margin | 0.722 | 0.290 | |||
| Smooth | 56 (25.9) | 30 (27.8) | 50 (30.9) | ||
| Non-smooth | 160 (74.1) | 78 (72.2) | 112 (69.1) | ||
| Tumor orientation | 0.708 | 0.273 | |||
| Parallel | 191 (88.4) | 97 (89.8) | 137 (84.6) | ||
| Not Parallel | 25 (11.6) | 11 (10.2) | 25 (15.4) | ||
| Posterior acoustic pattern | 0.460 | 0.123 | |||
| No change | 110 (50.9) | 51 (47.2) | 93 (57.4) | ||
| Enhancement | 70 (32.4) | 31 (28.7) | 35 (21.6) | ||
| Shadowing | 28 (13.0) | 20 (18.5) | 28 (17.3) | ||
| Combined pattern | 8 (3.7) | 6 (5.6) | 6 (3.7) | ||
| Ki16 index | 0.578 | 0.356 | |||
| <30% | 95 (44.0) | 44 (40.7) | 79 (48.8) | ||
| ≥30% | 121 (56.0) | 64 (59.3) | 83 (51.2) | ||
| Lymphovascular invasion | 0.510 | 0.426 | |||
| Present | 78 (36.1) | 35 (32.4) | 65 (40.1) | ||
| Absent | 138 (63.9) | 73 (67.6) | 97 (59.9) | ||
| Surgery type | 0.655 | 0.057 | |||
| Mastectomy | 161 (74.5) | 78 (72.2) | 134 (82.7) | ||
| Lumpectomy | 55 (25.5) | 30 (27.8) | 28 (17.3) | ||
| Adjuvant chemotherapy | 0.396 | 0.494 | |||
| Yes | 178 (82.4) | 93 (86.1) | 129 (79.6) | ||
| No | 38 (17.6) | 15 (13.9) | 33 (20.4) | ||
| Adjuvant radiotherapy | 0.520 | 0.119 | |||
| Yes | 134 (62.0) | 63 (58.3) | 114 (70.4) | ||
| No | 82 (38.0) | 45 (41.7) | 48 (29.6) |
Data are number of patients; data in parentheses are percentage.
Data are medians, with interquartile range in parentheses.
Construction and evaluation of the radiomics signature
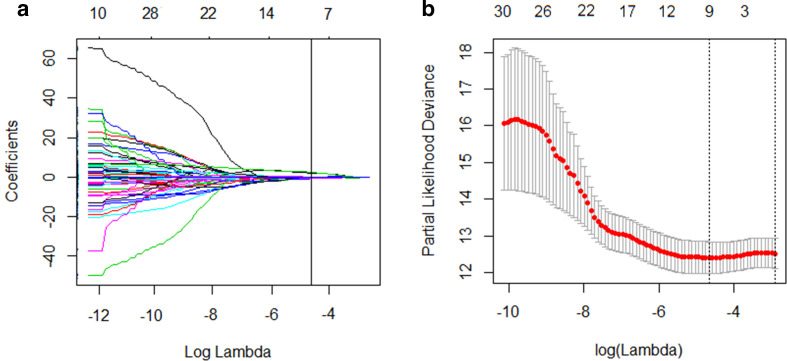
Among 192 radiomics parameters, a total of 167 high reproducible features with ICC values of more than 0.80 in the intra- and interobserver analysis were chosen for next evaluation. Then, the top 30 candidate variables with highest ranking were considered using the MRMR algorithm. In term of the LASSO Cox regression, nine most robust potential predictors with a non-zero coefficient were identified in the training data set to build a prognostic classifier (Figure 1). A radiomics signature established by a calculation formula generated an intuitive parameter score, which was presented in the supplementary data. The radiomics signature yielded a C-index of 0.71 [95% confidence interval (CI): 0.66, 0.74] in the training set, 0.70 (95% CI: 0.64, 0.74) in the internal validation cohort, and 0.69 (95% CI: 0.63, 0.72) in the external validation set, showing moderate predictive performance for DFS.
Figure 1.
Feature selection using the LASSO cox regression model. (A) A vertical line was drawn at the optimal λ value in the LASSO coefficient profiles, which resulted in nine features with nonzero coefficients. LASSO, least absolute shrinkage and selection operator.(B) Selection of the tuning parameter (λ) in the LASSO model via 10-fold cross-validation was based on minimum criteria. Two vertical lines were drawn at the optimal value by using the minimum criteria and the one standard error of the minimum criteria. The optimal λ value of 2.761*10−5 with log (λ) = −4.559 was chosen.
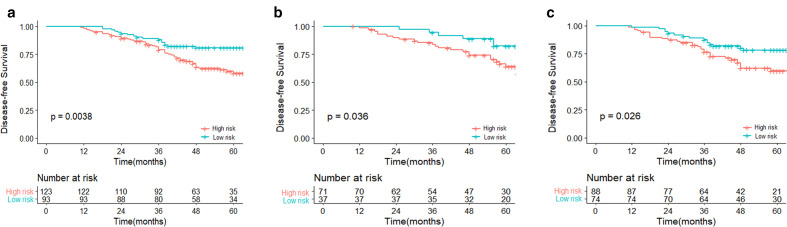
The optimum cut-off point of the Rad-score (2.213) was generated based on the maximum Youden index by the time-dependent ROC curve for survival prediction(Figure S2) , and it was then applied to classify patients into a low-risk group (Rad-score ≤2.213) and a high-risk group (Rad-score >2.213). In the training data set, patients in the low-risk group showed a statistically superior prognostic performance than that in the high-risk group (p = 0.0038), and this result was confirmed in both test datasets (p = 0.036 and 0.026) (Figure 2).
Figure 2.
Kaplan–Meier survival analyses according to the best cut-off value of the Rad-score for TNBC patients in the primary cohort (A), internal validation cohort (B) and external validation cohort (C). TNBC, triple negative breast cancer.
Development, performance and validation of prediction models
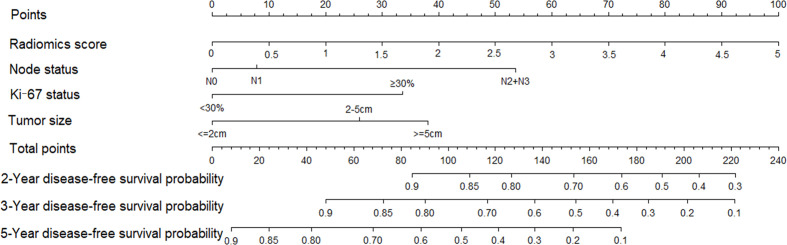
Univariate cox regression analysis presented in Supplementary Material 1 revealed the possible association between the selected predictors and DFS. Results of multivariate cox regression with backward stepwise selection by eliminating non-significant variables are listed in Table 2. A clinicopathological nomogram was constructed on the basis of three predictive factors (tumor size, axillary lymph node status and Ki-67 index). A radiomics model incorporating radiomics signature and three prognostic variables was established and displayed as a form of nomogram (Figure 3).
Table 2.
Multivariable analysis of variables to predict disease-specific survival in TNBC
| Variable | Clinical-pathologic Model | Radiomics Model | ||
|---|---|---|---|---|
| Hazard Ratio(95% CI) | P value | Hazard Ratio(95% CI) | P value | |
| T stage | ||||
| T1(≤2 cm) | Reference | Reference | ||
| T2(2–5 cm) | 2.26 (1.12–3.56) | 0.013 | 2.06 (1.05–2.73) | 0.029 |
| T3(≥5 cm) | 2.34 (1.26–6.25) | 0.026 | 1.86 (0.69–3.17) | 0.27 |
| N stage | ||||
| N0 | Reference | Reference | ||
| N1 | 1.67 (0.76–2.45) | 0.584 | 1.36 (0.48–2.34) | 0.803 |
| N2 + N3 | 5.22 (2.96–8.10) | <0.001 | 2.76 (1.36–4.81) | 0.009 |
| Ki-67 index | ||||
| <30% | Reference | Reference | ||
| ≥30% | 2.23 (1.15–3.91) | 0.035 | 2.62 (1.45–4.17) | 0.022 |
| Radiomics score | NA | NA | 5.42 (2.65–8.21) | <0.001 |
TNBC, triple negative breast cancer.
Figure 3.
A radiomics nomogram was developed with axillary lymph node status, Ki-67 index, tumor size and radiomics signature for estimation of disease-free survival for TNBC patients. TNBC, triple negative breast cancer.
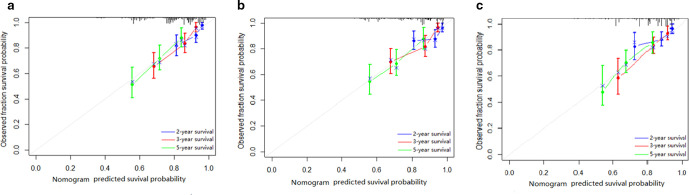
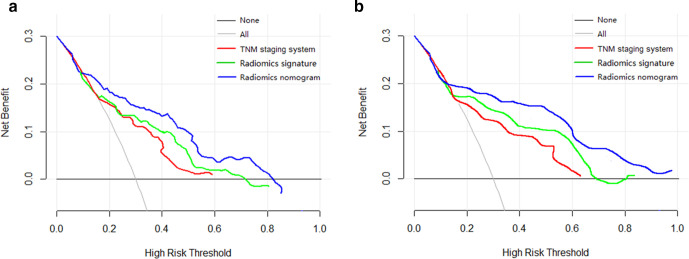
The radiomics nomogram yielded a favorable discrimination capability for predicting DFS in the training dataset (0.75; 95% CI: 0.71, 0.80) and in the internal validation set (0.73; 95% CI: 0.69, 0.78), and it also presented a similar C-index (0.71, 95% CI: 0.66, 0.76) in the external validation set. The prognostic performance of the radiomics model was better than (All p < 0.05) that of radiomics signature, clinicopathological model and TNM system (Table 3). The calibration curves for the probability of DFS at second, third, and fifth year after treatment were showed in Figure 4 with a favorable consistency between the estimation by radiomics nomogram and actual observation. The DCA graphically revealed that the radiomics nomogram supplied better clinical net benefit across the majority range of reasonable threshold probabilities than either the clinicopathological model or the present TNM stage in two validation cohorts (Figure 5).
Table 3.
Prognostic performance of models
| Model | Primary cohort | Internal Validation cohort | External Validation cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C index (95% CI) | P value | AIC | C index (95% CI) | P value | AIC | C index (95% CI) | P value | AIC | |
| Radiomics model | 0.75 (0.71–0.80) | Reference | 575 | 0.73 (0.69–0.78) | Reference | 602 | 0.71 (0.66–0.76) | Reference | 665 |
| Clinical-pathologic model | 0.72 (0.67–0.76) | <0.01* | 612 | 0.71 (0.67–0.73) | <0.01* | 645 | 0.70 (0.65–0.74) | <0.05* | 689 |
| AJCC TNM (eighth edition) | 0.65 (0.60–0.70) | <0.001* | 683 | 0.64 (0.59–0.68) | <0.001* | 712 | 0.63 (0.58–0.66) | <0.001* | 745 |
| Radiomics signature | 0.71 (0.66–0.74) | <0.01* | 655 | 0.70 (0.64–0.74) | <0.01* | 684 | 0.69 (0.63–0.72) | <0.01* | 703 |
AIC, Akaike information criteria; TNM, tumor node metastasis.
Figure 4.
Calibration curves of the radiomics nomogram in the primary cohort (A), internal validation cohort (B) and external validation cohort (C) in terms of the agreement between the estimated and the observed 2-, 3-, and 5 year outcomes.
Figure 5.
Decision curve analysis for each model in predicting disease-free survival in patients with TNBC in internal validation cohort (A) and external validation cohort (B). The y-axis represents the net benefit, which was calculated by summing the benefits (true-positive results) and subtracting the harms (false-positive results), weighting by the relative harm of forgoing treatment compared with the harm of an unnecessary treatment. According to the threshold probabilities obtained, the radiomics nomogram (blue curve) had the greatest net benefit compared with the other models and simple strategies such as follow-up of all patients (gray line) or no patients (horizontal black line) across the full range of threshold probabilities at which a patient would choose to undergo imaging follow-up. TNBC, triple negative breast cancer.
Discussion
In present study, we developed and validated a recurrence risk model based on radiomics features from ultrasound for TNBC patients. A radiomics signature consisting of nine robust features successfully categorized patients into high- and low-risk groups with statistically different prognosis. Furthermore, in this multi-institutional study, the radiomics nomogram incorporated multi-feature-based radiomics signature and clinicopathological risk factors achieved significantly better prognosis value for individualized DFS than clinical model and traditional TNM staging, which suggested the favorable value of the radiomics signature served as an effective complementary to clinical features for prognostic prediction in TNBC.
Currently, radiomics has been utilized to explore in survival estimation of several kinds of cancers by previous research, including hepatocellular carcinoma,15 non-small cell lung cancer,16 and pancreatic ductal adenocarcinoma.17 Ultrasound-based radiomics analysis have also demonstrated promising ability in diagnostic and predictive analyses.18–20 In our study, we extracted radiomics features based on intratumor and peripheral ring. A radiomics signature concluded by the best nine radiomics features was considered to be an independent prognostic indicator in primary set and two validation sets (all p < 0.05). Among nine radiomics features, five GLCM-related features, which was calculated from the interaction between neighboring image pixels, obtained high weights in our equation in consistence with previous studies.13,21 The study of Koh et al21 demonstrated that GLCM quantifying tumor texture and heterogeneity had more value for predicting system recurrence in TNBC. Regarding the relationship between entropy and prognosis, entropy has been emphasized particularly in many prior studies22,23 as a well-known radiomics feature. Kim23 et al reported that entropy, which reflects internal pixel distribution patterns, was a robust significant predictor of recurrence-free survival among primary breast cancer patients. In addition, the radiomics signature also contained three important peritumoral features. Evidences have demonstrated that quantitative radiomic analysis of the tumor itself and the adjacent peritumoral tissue can provide a valuable insight into outcome-associated information. In study by Braman et al,24 it was suggested that quantitative imaging features of the tumor and its surroundings in breast MRI could identify molecular subtypes of HER2+ breast cancers and evaluate likelihood of immune response to targeted therapy. Another recent study25 showed that quantitative ultrasound (QUS) features of intratumoral and peritumoral regions achieved better performance in indicating breast cancer response to NAC and predicting 5-year recurrence-free survival. Accordingly, radiomics approaches provide important complementary data on tumor biological character, as well as tumor microenvironment, which are difficult to be recognized by the naked eye.
We constructed a nomogram combining radiomics signature with tumor size, axillary lymph node status and Ki-67 value to achieve superior prognostic performance. The number of axillary lymph node metastases has been confirmed as one of the best-established prognostic factors,9 and the high level of Ki-67 value which correlates strongly with tenacious proliferation potential was also considered to attribute to the early relapse of TNBC, especially during the first 3-years follow-up period.26 Nevertheless, the uncertainty regarding the Ki-67 cut-off value to define highly proliferative tumors in TNBC has been acknowledged, ranging from 10 to 40%.26–29 The optimal cut-off value of Ki-67 in our study for further prognostic classification in TNBC patients was 30%, which was in line with the study of Zhu et al.29 In the study, their findings suggested the Ki-67 cut-off at 30% could decrease interobserver deviation and be considered as a clinically applicable biologic marker on the prognostic assessment of TNBC.
In addition, our radiomics models may facilitate individualized treatment decision and surveillance policy. In the CREATE-X trial,30 the addition of capecitabine therapy showed effectiveness as an adjuvant option in prolonging DFS in TNBC patients with positive residual tumor tissue after standard NAC. Therefore, if TNBC patients presented a high risk recurrence, neoadjuvant treatment should be taken into significant consideration and capecitabine could be administered in combination with chemotherapy, which could improve long-term prognostic outcomes for high-risk subsets and identify the patients most likely to benefit from chemotherapy.
Some limitations have to be recognized in our research. First, this was a retrospective study with the relatively small sample size which may result in inherent biases. Thus, getting more confirmatory evidence from prospective collaborations is required to further verify our model before clinical application. Second, although our radiomics nomogram provided stable prognostic ability, repeatability and variability of radiomics analysis based on conventional B-mode ultrasound image can be influenced by different radiologists and machine parameters. In addition, QUS retains raw radiofrequency signal generated by ultrasonic backscatter, which reveals more valuable information associated to tumoral heterogeneity. Furthermore, QUS-based radiomics have been confirmed to promote the classification performances in predicting response to NAC.31,32 Further texture analysis within QUS parametric images will be used to obtain more accurate prediction models. Third, genomic features of TNBC were not available in our study. Further studies will focus on assessing relationship between genomics and sonographic radiomics.
Conclusions
Our finding indicated a radiomics signature based on ultrasound images could be considered as a potential biomarker to stratify patients into distinct prognostic groups in patients with TNBC. Moreover, the radiomics nomogram, which offer notable advantages over clinicopathological model and traditional TNM staging system, may improve individualized prognosis evaluation and facilitate therapeutic decision-making.
Footnotes
Competing interests: The authors declare they have no competing financial interests.
Funding: The present study was supported by grants from the National Key Research & Development Plan of Ministry of Science and Technology of the People’s Republic of China (No.2018YFC1314900, 2018YFC1314901), the 2017 projects of Jiangsu Provincial, Department of Finance (No.2150510), the 2018 project of Jiangsu Committee of Health (No. H2018071).
Ethics approval and consent to participate: The retrospective analysis was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University, and written informed consent was waived.
Consent for publication: We confirm that the manuscript has been read and approved for the publication in the journal by all named authors.
Author Contributions: Feihong Yu performed imaging acquisition, data analysis and wrote the manuscript. Jing Hang and Bin Yang contributed to manuscript preparation. Jianxiang Wang contributed to statistical analysis. Jing Deng and Xinhua Ye designed the research and performed the manuscript editing. Yun Liu provided fund and technical support. All the authors read and approved the final manuscript.
Contributor Information
Feihong Yu, Email: yu551437@126.com.
Jing Hang, Email: hang_jing2021@126.com.
Jing Deng, Email: docterdengjing@126.com.
Bin Yang, Email: yangbin12yb@126.com.
Jianxiang Wang, Email: wangjx_1976@126.com.
Xinhua Ye, Email: ye_xinhua2021@126.com.
Yun Liu, Email: liuyun_nmu@126.com.
REFERENCES
- 1.Foulkes WD, Smith IE, Reis-Filho JS. Triple-Negative breast cancer. N Engl J Med 2010; 363: 1938–48. doi: 10.1056/NEJMra1001389 [DOI] [PubMed] [Google Scholar]
- 2.Boyle P. Triple-Negative breast cancer: epidemiological considerations and recommendations. Ann Oncol 2012; 23 Suppl 6: vi7–12. doi: 10.1093/annonc/mds187 [DOI] [PubMed] [Google Scholar]
- 3.Sharma P. Biology and management of patients with triple-negative breast cancer. Oncologist 2016; 21: 1050–62. doi: 10.1634/theoncologist.2016-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-Negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 2016; 13: 674–9010.1038 /nrclinonc.2016.66. doi: 10.1038/nrclinonc.2016.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K, Li H-L, Xiong Y-F, Shi Y, Li Z-Y, Li J, HL L, , et al. Development and validation of nomograms integrating immune-related genomic signatures with clinicopathologic features to improve prognosis and predictive value of triple-negative breast cancer: a gene expression-based retrospective study. Cancer Med 2019; 8: 686–700. doi: 10.1002/cam4.1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. Cd4⁺ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013; 123: 2873‐–9210.1172 / JCI67428. doi: 10.1172/JCI67428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Au-Yong ITH, Evans AJ, Taneja S, Rakha EA, Green AR, Paish C, et al. Sonographic correlations with the new molecular classification of invasive breast cancer. Eur Radiol 2009; 19: 2342–8. doi: 10.1007/s00330-009-1418-2 [DOI] [PubMed] [Google Scholar]
- 8.Ko ES, Lee BH, Kim H-A, Noh W-C, Kim MS, Lee S-A. Triple-Negative breast cancer: correlation between imaging and pathological findings. Eur Radiol 2010; 20: 1111–7. doi: 10.1007/s00330-009-1656-3 [DOI] [PubMed] [Google Scholar]
- 9.Li J-W, Li N, Jiang Y-Z, Liu Y-R, Shi Z-T, Chang C, et al. Ultrasonographic appearance of triple-negative invasive breast carcinoma is associated with novel molecular subtypes based on transcriptomic analysis. Ann Transl Med 2020; 8: 435. doi: 10.21037/atm.2020.03.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Zhan W, Chen W, Li Y, Chen X, Shen K. Sonography with vertical orientation feature predicts worse disease outcome in triple negative breast cancer. Breast 2020; 49: 33–4010.1016 /j. breast.2019.10.006. doi: 10.1016/j.breast.2019.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bi WL, Hosny A, Schabath MB, Giger ML, Birkbak NJ, Mehrtash A, et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin 2019; 69: 127–5710. 3322/caac.21552. doi: 10.3322/caac.21552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017; 14: 749–62. doi: 10.1038/nrclinonc.2017.141 [DOI] [PubMed] [Google Scholar]
- 13.Park H, Lim Y, Ko ES, Cho H-H, Lee JE, Han B-K, et al. Radiomics signature on magnetic resonance imaging: association with disease-free survival in patients with invasive breast cancer. Clin Cancer Res 2018; 24: 4705–14CCR-17-3783. doi: 10.1158/1078-0432.CCR-17-3783 [DOI] [PubMed] [Google Scholar]
- 14.Kim J-H, Ko ES, Lim Y, Lee KS, Han B-K, Ko EY, ES K, EY K, et al. Breast cancer heterogeneity: MR imaging texture analysis and survival outcomes. Radiology 2017; 282: 665–7510.1148 /radiol. 2016160261. doi: 10.1148/radiol.2016160261 [DOI] [PubMed] [Google Scholar]
- 15.Ji G-W, Zhu F-P, Xu Q, Wang K, Wu M-Y, Tang W-W, GW J, , et al. Radiomic features at contrast-enhanced CT predict recurrence in early stage hepatocellular carcinoma: a multi-institutional study. Radiology 2020; 294: 568‐–79. doi: 10.1148/radiol.2020191470 [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, Liu Z, He L, Chen X, Pan D, Ma Z, et al. Radiomics signature: a potential biomarker for the prediction of disease-free survival in early-stage (I or II) non-small cell lung cancer. Radiology 2016; 281: 947‐–57. doi: 10.1148/radiol.2016152234 [DOI] [PubMed] [Google Scholar]
- 17.Xie T, Wang X, Li M, Tong T, Yu X, Zhou Z. Pancreatic ductal adenocarcinoma: a radiomics nomogram outperforms clinical model and TNM staging for survival estimation after curative resection. Eur Radiol 2020; 30: 2513‐–24. doi: 10.1007/s00330-019-06600-2 [DOI] [PubMed] [Google Scholar]
- 18.Wang K, Lu X, Zhou H, Gao Y, Zheng J, Tong M, et al. Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut 2019; 68: 729–41. doi: 10.1136/gutjnl-2018-316204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu F-H, Wang J-X, Ye X-H, Deng J, Hang J, Yang B. Ultrasound-based radiomics nomogram: a potential biomarker to predict axillary lymph node metastasis in early-stage invasive breast cancer. Eur J Radiol 2019; 119: 108658. doi: 10.1016/j.ejrad.2019.108658 [DOI] [PubMed] [Google Scholar]
- 20.Zheng X, Yao Z, Huang Y, Yu Y, Wang Y, Liu Y, et al. Deep learning radiomics can predict axillary lymph node status in early-stage breast cancer. Nat Commun 2020; 11: 1236. doi: 10.1038/s41467-020-15027-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koh J, Lee E, Han K, Kim S, Kim D-K, Kwak JY, et al. Three-Dimensional radiomics of triple-negative breast cancer: prediction of systemic recurrence. Sci Rep 2020; 10: 297610.1038 /s41598 -020-59923-2. doi: 10.1038/s41598-020-59923-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Win T, Miles KA, Janes SM, Ganeshan B, Shastry M, Endozo R, et al. Tumor heterogeneity and permeability as measured on the CT component of PET/CT predict survival in patients with non-small cell lung cancer. Clin Cancer Res 2013; 19: 3591‐–9. doi: 10.1158/1078-0432.CCR-12-1307 [DOI] [PubMed] [Google Scholar]
- 23.Kim J-H, Ko ES, Lim Y, Lee KS, Han B-K, Ko EY, ES K, EY K, et al. Breast cancer heterogeneity: MR imaging texture analysis and survival outcomes. Radiology 2017; 282: 665–7510.1148 /radiol. 2016160261. doi: 10.1148/radiol.2016160261 [DOI] [PubMed] [Google Scholar]
- 24.Braman N, Prasanna P, Whitney J, Singh S, Beig N, Etesami M, et al. Association of peritumoral radiomics with tumor biology and pathologic response to preoperative targeted therapy for HER2 (ERBB2)-positive breast cancer. JAMA Netw Open 2019; 2: e192561: e19256110.1001 /jamanetwork open.2019.2561. doi: 10.1001/jamanetworkopen.2019.2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tadayyon H, Sannachi L, Gangeh MJ, Kim C, Ghandi S, Trudeau M, et al. A priori prediction of neoadjuvant chemotherapy response and survival in breast cancer patients using quantitative ultrasound. Sci Rep 2017; 7: 45733. doi: 10.1038/srep45733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keam B, Im S-A, Lee K-H, Han S-W, Oh D-Y, Kim JH, SA I, , et al. Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res 2011; 13: R22. doi: 10.1186/bcr2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munzone E, Botteri E, Sciandivasci A, Curigliano G, Nolè F, Mastropasqua M, et al. Prognostic value of Ki-67 labeling index in patients with node-negative, triple-negative breast cancer. Breast Cancer Res Treat 2012; 134: 277‐–82. doi: 10.1007/s10549-012-2040-6 [DOI] [PubMed] [Google Scholar]
- 28.Wu Q, Ma G, Deng Y, Luo W, Zhao Y, Li W, et al. Prognostic value of Ki-67 in patients with resected triple-negative breast cancer: a meta-analysis. Front Oncol 2019; 9: 1068. doi: 10.3389/fonc.2019.01068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu X, Chen L, Huang B, Wang Y, Ji L, Wu J, et al. The prognostic and predictive potential of Ki-67 in triple-negative breast cancer. Sci Rep 2020; 10: 225. doi: 10.1038/s41598-019-57094-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masuda N, Lee S-J, Ohtani S, Im Y-H, Lee E-S, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017; 376: 2147–5910.1056/ NEJMoa1 612645. doi: 10.1056/NEJMoa1612645 [DOI] [PubMed] [Google Scholar]
- 31.Sannachi L, Gangeh M, Tadayyon H, Sadeghi-Naini A, Gandhi S, Wright FC, et al. Response monitoring of breast cancer patients receiving neoadjuvant chemotherapy using quantitative ultrasound, texture, and molecular features. PLoS One 2018; 13: e018963410.1371/journal. pone.0189634. doi: 10.1371/journal.pone.0189634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiCenzo D, Quiaoit K, Fatima K, Bhardwaj D, Sannachi L, Gangeh M, et al. Quantitative ultrasound radiomics in predicting response to neoadjuvant chemotherapy in patients with locally advanced breast cancer: results from multi-institutional study. Cancer Med 2020; 9: 5798–806. doi: 10.1002/cam4.3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.