Abstract
Radiation risks from diagnostic imaging have captured the attention of patients and medical practitioners alike, yet it remains unclear how these considerations can best be incorporated into clinical decision-making. This manuscript presents a framework to consider these issues in a potentially at-risk population, the so called “frequent flyer” patients undergoing a large amount of recurrent imaging over time. Radiation risks from the low-dose exposures of diagnostic imaging are briefly reviewed, as applied to recurrent exposures. Some scenarios are then explored in which it may be helpful to incorporate knowledge of a patient’s imaging history. There is no simple or uniformly applicable approach to these challenging and often nuanced clinical decisions. The complexity and variability of the underlying disease states and trajectories argues against alerting mechanisms based on a simple cumulative dose threshold. Awareness of imaging history may instead be beneficial in encouraging physicians and patients to take the long view, and to identify those populations of frequent flyers that might benefit from alternative imaging strategies.
A drop in the bucket
It is helpful to think about radiation exposure and radiation risk to patients through a simple analogy (Figure 1). Just as each drop in a slowly filling bucket contributes only a small amount to the volume within, the exposure and associated cancer risk from any one properly performed imaging exam is quite small. However, to put individual exams in context, it is instructive to consider the concept of filling a cumulative bucket of risk. The Surveillance, Epidemiology, and End Results (SEER) program data indicate that approximately 39% of the US population are expected to develop a cancer of one form or another during their lifetimes, making this bucket 39% full at baseline.1 Risk can be added to this bucket by a single large exposure, as has been observed in survivors of the atomic bomb blasts at Hiroshima and Nagasaki. Alternatively, as in the case of diagnostic imaging, risk may accrue in multiple small exposures over time. This simplified analogy certainly does not capture all the complexities of medical imaging. It considers only the accrual of potential cancer risks from radiation exposure, without incorporating other risks, and without inherently recognizing that these imaging exams are intended to impart clinical benefits that may in fact reduce other downstream risks to the patient. It should also be noted that probabilistic risk propagation is not strictly additive as in this analogy, though it closely approximates additive risk accumulation for very small individual exam risks: For example, 30 exams each imparting an excess cancer risk of 0.1% would be expected to diminish the probability of remaining cancer-free to (1–0.001)^30 = 0.9704, almost identical to the 3% risk one would estimate by adding the independent 0.1% risks over 30 exams. Simplifications aside, this way of thinking about risk accumulation nonetheless lends itself to certain ideas about interventions that might be employed to reduce cumulative radiation risks to patients over time, either by reducing the drip rate, or by reducing the drop size.
Figure 1.
A drop in the bucket. The bucket represents accumulated cancer risk to patients from ionizing radiation. Each droplet represents a discrete exposure. To the right are risks from one-time exposure, which may impart substantial cancer risk if large enough, as in the case of the atomic bomb survivors. To the left, recurrent small exposures describe the situation in diagnostic imaging. Before the scan, interventions to reduce drip rate include various measures to curb utilization of imaging that employs ionizing radiation. During the scan, interventions to reduce drop size include imaging techniques and advancing technologies to perform diagnostic quality scans with progressively lower radiation exposures.
Before the scan is where opportunities lie to reduce drip rate through measures to curb imaging utilization. These justification efforts should ideally rely on evidence-based guidance, where it exists, to define exam appropriateness for certain well-defined clinical scenarios. Further exam justification should emphasize a patient-specific weighing of anticipated benefit against exam risks, incorporating an understanding of the individual patient’s medical history and clinical presentation. In some situations, imaging algorithms may be designed to intentionally limit the use of ionizing radiation. Decision-support tools can be implemented that incorporate appropriateness and radiation risks of imaging.2 Duplicative imaging within short time frames is a natural target for intervention. Non-ionizing alternatives including ultrasound and MRI can be considered in place of CT, but only if those exams are the right ones to answer the clinical question at hand. These before-the-scan interventions are typically most successful when undertaken as collaborative efforts between radiology and leadership of the relevant referring services.
During the scan is where opportunities lie to reduce drop size, or to perform imaging exams with lower and lower doses. The objective is to dose-optimize protocols in order to find the lowest exposure appropriate for the clinical task, while still achieving robust diagnostic quality exams. These efforts rely on implementation of ever-improving imaging technologies and protocol optimization measures.3 Great strides have been made in recent years.4 These activities are largely in the purview of Radiology, and benefit from collaborative efforts between Radiologists, Medical Physicists, technologists, and equipment manufacturers.
Risks of low-dose ionizing radiation
For this discussion, the focus will be purely on the stochastic risks of subsequent cancer development from ionizing radiation, rather than the deterministic tissue damaging effect following acute large exposures. Much of what is known about the risks of low dose ionizing radiation comes from longitudinal observations of the Life Span Study (LSS) cohort of survivors of the atomic bomb blasts at Hiroshima and Nagasaki.5 Statistically significant increases in excess relative risk (ERR) of solid cancers were found even in the lowest dose range from 0 to 100 mGy, with greater cancer rates observed in survivors receiving higher doses due to their location closer to the hypocenter of the bomb blast. The fundamental shape of the dose–response curve within this low-dose region cannot be definitively determined from the LSS analysis of all solid cancers combined, though the dose–response curves of multiple specific solid cancers have been found to be well described by linear models, including the lung, breast, colon, liver, and prostate.6–10
Radiation exposures from diagnostic imaging fall almost entirely below 100 mGy, the realm in which there is the most uncertainty about associated stochastic cancer risks. The most commonly used model to extend observed risks of higher radiation exposure to the low dose realm is that of a linear-no-threshold (LNT) dose–response curve, in which a doubling of the radiation exposure is assumed to impart a doubling of the associated cancer risk. The most commonly used risk model that incorporates this LNT assumption is the BEIR-VII (Biological Effects of Ionizing Radiation) model, which estimates cancer incidence and mortality for specific cancer sites.11 Overall risk for all cancer sites combined is shown in Figure 2 (used with permission from Kalra et al12).
Figure 2.
LAR of radiation-induced cancer from all organs combined, extracted from table 12D-1 of the BEIR-VII report.11 Figure reprinted in modified form, with permission, from Kalra et al.12 LAR represents an additive excess risk above baseline, and varies with patient gender, age at exposure, and the size of the radiation exposure. For the same exposure, females (red curve) generally have a higher cancer risk than males (blue curve), a finding borne out across multiple organs. Younger people have greater risks than older people, thought to be due in part to their more inherently radiation-sensitive organs, and to their longer remaining life expectancy in which to develop cancer from radiation-induced DNA damage. Where these curves cross the dotted horizontal line (for a 32-year-old female or 19-year-old male), there is an approximate excess risk of 1 in 1000 following a 10 mSv exposure. LAR, lifetime attributable risk.
There is certainly residual controversy around use of the LNT assumption, with alternative theories calling for non-linear dose–response curves.13,14 For example, the hormesis model asserts that low doses of radiation are protective, and reduce cancer risks. Other models incorporate a dose threshold below which DNA repair is presumed to be able to correct radiation-induced damage, which would thus eliminate cancer risk from radiation exposures below this threshold.
Another main controversy revolves around the question of how to translate observed risks from the one-time acute exposures of the atomic bombs to the situation in medical imaging in which patients may have many small exposures spread out over time. Multiple studies of cancer incidence following recurrent imaging exposures or protracted occupational radiation exposures have yielded varied ERRs of cancer risk, though most have demonstrated results that indicate risk accumulation from multiple exposures over time, and remain broadly compatible with an LNT model.15–18
Imaging frequent-flyers, and associated cumulative effective dose and risk estimates
In 2008, we began to notice a subset of so-called “frequent flyer” patients in our department who underwent a surprisingly large amount of recurrent CT imaging over time. We set out to quantify their rates of recurrent imaging, and to estimate their associated cumulative radiation doses and cancer risks.19,20 For the 31,462 patients who had an index diagnostic CT scan in calendar year 2007, Figure 3 displays the histogram of the number of CT scans each patient had undergone at our institution in the 22 years over which imaging records were available at that time. We found that a third of our patients had at least 5 CT scans in those 22 years, 5% had over 22 scans, and the top percentile had 38 scans or more, up to a maximum of 132 scans.
Figure 3.
Histogram of cumulative CT scan counts for patients with an index scan in 2007, using institutional imaging records from the prior 22 years. The inset box expands the y-axis for the top percentile, to better display the right tail of the distribution. Reproduced with permission from Sodickson et al.19
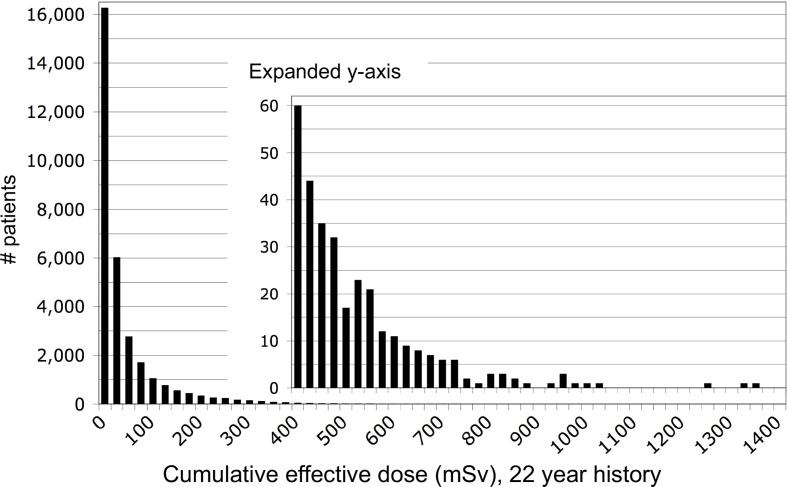
By assigning crude effective dose estimates for scans of each body region that were typical for the CT technology of the time, we calculated cumulative effective dose (CED) estimates as shown in the histogram of Figure 4. We found that 15% of our patients had accrued estimated CED of 100 mSv in their 22 year CT imaging histories, while 4% had CED estimates greater than 250 mSv, and the top percentile had CED estimates greater than 400 mSv, up to a maximum of 1375 mSv. Recent publications have identified similar groups of patients at numerous hospitals in the US and Europe who have accrued CEDs greater than 100 mSv from recurrent CT imaging within 1–5 year periods.21,22
Figure 4.
Cumulative effective dose estimates accrued by each patient over the prior 22 year institutional CT imaging history, calculated by adding typical effective doses for scans of each body region. Reproduced with permission from Sodickson et al.19
Finally, we applied the BEIR-VII risk model of Figure 2, incorporating patient gender, age at exposure, and size of exposure to calculate estimates of cancer incidence and mortality as shown in Figure 5. This chain of calculation admittedly incorporates multiple sources of potentially significant error, such as the dosimetry assigned to each scan, and most notably, the large uncertainties inherent in the BEIR-VII risk models, including extension of the atomic bomb risks to low dose and fractionated exposures, and uncertainty in the accuracy of the underlying LNT assumption.
Figure 5.
Cumulative estimates of lifetime attributable risk of cancer incidence and mortality, using the BEIR-VII risk model.11 Reproduced with permission from Sodickson et al.19
Despite the inherent limitations of our risk estimates, we were nonetheless largely reassured by our results, as we found that only 7% of our patients had estimated lifetime attributable risk (LAR) of 1% or more above baseline, with the top percentile having LAR estimates ranging from 2.6%, to 12%. While most of our patients thus had low estimated excess cancer risks from their prior CT imaging histories, these results did highlight the notion that certain patients undergoing a large amount of recurrent imaging over time might warrant special consideration.
Many frequent flyer patients already had a cancer history as the reason for their recurrent restaging or surveillance imaging. However, many of these patients were thought to have been cured or in remission, raising concerns about secondary cancer development related to their imaging exposures. We also found a sizeable minority of top-percentile patients with no cancer history, and with other chronic and complex medical conditions accounting for their recurrent imaging evaluations. This finding has been reproduced in recent study of other institutions.22,23
The most frequently imaged non-cancer patient in our cohort was a 44-year-old female with numerous ED visits for flank pain (clinical extract described in table 3 of Griffey and Sodickson20). She had a complex medical history including recurrent pyelonephritis, prior lithotripsy and stone extraction, and a remote history of ulcerative colitis with proctocolectomy. Her underlying conditions and persistent symptoms had stimulated a large amount of recurrent imaging over time, including 69 CT scans (all but one of the abdomen and pelvis), 3 nuclear medicine studies, 8 fluoroscopic exams or interventions, 122 other X-ray exams, and 62 ultrasounds. Of all her CT scans, one demonstrated an obstructing ureteral stone (after ultrasound had first revealed hydronephrosis), and one demonstrated CT findings of pyelonephritis. The remaining 66 abdominopelvic CT scans demonstrated no obstructive uropathy or other acute pathology, and only incremental changes in the size of her non-obstructing renal stones.
When faced with a scenario like this, one can’t help but wonder whether this is a valuable imaging approach for this patient, or whether we should be doing something differently. As demonstrated in Figure 6, if our underlying dose estimates and the BEIR-VII risk model are reasonably accurate, then we estimate her excess cancer risk to be nearly 10% above baseline, attributable solely to her past CT imaging at our institution.
Figure 6.
A) Estimated CED and LAR for a 44-year-old female undergoing recurrent imaging. Each data point corresponds to the estimated effective dose (red circles, left y-axis) and incremental LAR (blue triangles, right axis) for each of the 69 CT scans in her history. CED, cumulative effective dose; LAR, lifetime attributable risk.
Can cumulative risk estimates be incorporated into clinical decision-making?
The ability to estimate cancer risk, however crudely, naturally gives rise to efforts to incorporate these risks into risk/benefit decision-making for our patients.
For better or worse, much of our decision-making in medicine is episodic, particularly in urgent or emergent situations, with a physician meeting the patient for the first time without the benefit of internalizing their long history. In these situations, it is typically most relevant to balance incremental risk against incremental benefit. The incremental risk of the 50th CT scan is quite similar to that of the first CT scan. In this framing, the small incremental risk of imaging must be outweighed by the benefits of a clinically justified scan in order to proceed to imaging. One of the challenges in making this determination is that the benefits of CT imaging, while well documented, can rarely be numerically quantified, and certainly not in the same units as the counterbalancing radiation risks.24–26 Imaging appropriateness is not binary but falls on a continuum of shades of grey between clearly appropriate and clearly lacking in clinical value. That said, the radiation risks of any individual scan are so low that any substantial clinical benefit would typically outweigh the small associated radiation risk.
How then can cumulative dose or cumulative risk be incorporated into clinical decision-making? When weighing risk against benefit in a patient with large CED, it is often tempting to balance cumulative risk against incremental benefit, which is certainly not appropriate.27,28 This inherently biases against a decision to reimage the patient, which can lead to faulty and potentially harmful clinical decisions if the patient is truly in need of diagnostic clarity. There is certainly no abrupt threshold of cumulative dose or risk that can be used to offset the need for critical or otherwise clinically important imaging evaluations. On the other hand, dismissing the incremental risk of each new scan entirely would allow the risk bucket to fill unchecked. It is worth noting that some of the key technological and methodological advances in radiation dose reduction in recent years were motivated by prevalent desires to minimize radiation risks.
Alternatively, in scenarios such as the patient vignette presented above, a physician able to take the long view of a patient’s needs (such as a primary care physician or treating oncologist) might be able to reframe from a relative weighing of incremental risk against incremental benefit to that of cumulative risk vs cumulative benefit. This requires an inherently gestalt assessment, since imaging benefit is not easily quantifiable, and radiation risk estimates are inherently error prone and reliant on a number of unproven assumptions. Nonetheless, a physician using this mindset might be better able to recognize when the patient’s presentation is substantively different from her multiple other episodes in which recurrent imaging did not uncover a cause for her symptoms. In this particular vignette, the primary intellectual leap is the recognition that this patient’s long history of imaging has proven the incremental clinical benefit of the 50th scan to be far smaller than that of the first, tipping the balance in favor of avoiding repeat imaging. While cumulative dose and risk estimates play little role in this decision, it may nonetheless be helpful to reframe from the narrowly focused episodic view to a higher-level assessment of the patient’s care over time.
One of the core principles of effective decision-support tools is that advice must be actionable, or easily mapped to an appropriate action, in order to impact ordering decisions.29 About a decade ago, we had embarked on an effort to develop informatics tools to automatically extract more accurate cumulative radiation exposures and associated risks from CT and nuclear medicine exams.30–32 One of our early intentions for doing this was to embed these data in the electronic medical record, and to create decision-support tools that would present a patient’s imaging history, associated CED and cancer risk at the time of order entry, and query the provider if they still wished to proceed with imaging. We soon realized that this approach would inherently (and inappropriately) balance cumulative risk against incremental benefit. We also found in focus group sessions with care providers that they had no notion of how to incorporate this information into their decision-making process, rendering our theorized decision support intervention completely non-actionable. Instead, we refocused our decision-support efforts into identifying requests for exams that were identical to others recently performed in those patients, and querying the provider about whether an interval change in the patient’s clinical status would justify the repeat scan.33,34
Taking the long view
Returning to the patient vignette, if we do not make any changes in our collective approach to imaging this patient, we would expect to continue accumulating effective dose and LAR in her future care, as shown in Figure 7, which projects a nearly 25% lifetime excess cancer risk. The good news is that there are many measures available to help us flatten these curves, either by reducing the drip rate or reducing the drop size, as described earlier.
Figure 7.
Projected CED accumulation and associated LAR if imaging continues beyond the historical scans of Figure 6 (in inset box, bottom left). If the rate of recurrent imaging continues unabated, dose and risk accumulation continue to high levels (top dotted red and blue lines, respectively), reaching LAR of nearly 25% above baseline. Reducing the “drip rate” of CT utilization to 1/2 or 1/4 of historical imaging (or alternatively, reducing “drop size” by incorporating newer CT technologies and optimized imaging techniques) can substantially flatten these curves (downward blue arrows between blue LAR curves). CED, cumulative effective dose; LAR, lifetime attributable risk.
While it is challenging to formally incorporate imaging history, dose and risk into decision-making, awareness of recurrent imaging may be beneficial to identify frequent flyer patients who might benefit from special consideration. Depending on the clinical scenario, it might be appropriate to employ longer watch-and-wait observation periods before reimaging, or to use non-ionizing alternatives if they have adequate diagnostic accuracy for the question at hand, even if they are less commonly considered in the general patient population due to issues of access, convenience, speed, or cost.
It can be instructive to explore various scenarios that commonly lead to frequent recurrent imaging, and to consider whether a patient’s history of recurrent imaging and high cumulative dose should modify the approach to justification of subsequent imaging. One such approach is to focus on patient cohorts that tend to undergo a lot of recurrent imaging by virtue of their underlying chronic medical conditions, and to design imaging alternatives or algorithms specifically targeted to these at-risk groups. Examples include the use of MRI rather than CT for patients with Crohn’s disease,35 or the use of rapid tailored MRI protocols for young patients needing recurrent imaging for hydrocephalus or other conditions.36 When considering whether alternative strategies are warranted, it is also important to consider anticipated life expectancy, because for patients with little remaining life expectancy due to significant acute or chronic illnesses, their radiation exposures will likely have very little impact on their remaining lifespan.
In the case of a cancer patient undergoing repetitive restaging or surveillance imaging, the question is primarily one of optimal interval between CT scans. This must be primarily determined by the underlying biology and typical growth rates of the specific cancer type, as the risk to the patient from delayed identification of recurrent disease is typically far greater than any later radiation-induced cancer risk. However, in select circumstances, alternative surveillance strategies using MRI might be possible.
It may be difficult to avoid CT imaging in patients with recurrent chest pain and a history of pulmonary embolus or deep venous thrombosis. If the patient has already committed to a course of anticoagulation, the key question becomes that of recurrent thromboembolism despite anticoagulation. In this scenario, it is reasonable to begin with lower extremity venous ultrasound to determine if an IVC filter is needed. If clot is present in the legs, repeat pulmonary embolus CT only changes management if there is clinical concern for a significant pulmonary embolus clot burden warranting thrombolysis or thrombectomy. However, this approach requires provider comfort to manage the patient without direct knowledge of the presence or extent of pulmonary embolism.
The patient described in the vignette above had numerous repeated CT scans of the abdomen and pelvis to assess for obstructive uropathy or other causes of abdominal pain. Her recurrent low-yield imaging presents a situation of diminishing returns, warranting consideration of alternative strategies. In this scenario, there are viable alternatives that do not require ionizing radiation. Ultrasound can be used to identify hydronephrosis, and may occasionally identify the offending stone, although much of the ureter is not well assessed by ultrasound. The usual problem with this approach is that this initial ultrasound is often followed by CT, either to explore alternative causes for symptoms when there is no hydronephrosis by ultrasound, or to demonstrate size and location of the offending stone when ultrasound reveals hydronephrosis without directly identifying the ureteral stone. Avoidance of CT using this strategy requires treating physicians to be comfortable managing these patients conservatively without directly visualizing the ureteral stone. Alternatively, a further reduced-dose CT strategy could be used in order to characterize the ureteral stone following ultrasound-detected hydronephrosis.
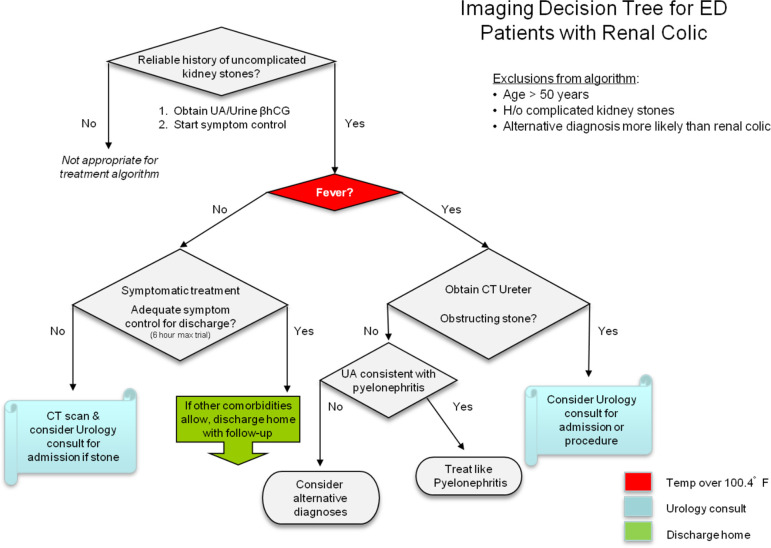
For other patients with recurrent imaging for renal colic, we designed an algorithm in collaboration between Radiology, Emergency Medicine, and Urology (Figure 8), in which imaging is avoided entirely in favor of a trial of symptomatic treatment, except in patients with signs of urinary infection and concern for infection above an obstructing ureteral stone.37
Figure 8.
Imaging decision tree for emergency department patients with a history of uncomplicated kidney stones (reproduced with permission from Raja et al.37). Afebrile patients with suspected renal colic undergo a trial of symptom control before undergoing repeat imaging.
Few practices have access to real-time CED or risk estimates during clinical decision-making, and even if they were available, their numerical values do not translate to actionable guidance on how best to proceed. Instead, frequency of imaging is a reasonable and commonly used surrogate to identify frequent flyer patients in order to trigger targeted interventions such as the examples described above.
Conclusion
Awareness of patients’ histories of recurrent imaging can be helpful to identify frequent flyer patients who have undergone a high rate of recurrent imaging over time and are thus at potentially elevated risk of subsequent radiation-induced cancer development. It is critical to avoid the common impulse to weigh perceived cumulative risk against incremental benefit when deciding whether to image the patient again. However, there are many potential interventions to limit the rate of further dose and risk accumulation over time. Some of these occur “during the scan” in the form of adoption of dose-reduction tools and optimization techniques that allow the acquisition of high-quality scans at progressively lower doses. Others occur “before the scan” in the form of justification measures to avert unneeded radiation exposures through implementation of decision support tools or imaging algorithms appropriately tailored to relevant populations such as those frequently imaged for well-defined clinical scenarios. The available remediation measures must be tailored to the clinical scenario, and caution is needed to avoid placing too much weight in the decision-making process on inherently non-actionable estimates of cumulative dose and associated radiation-induced cancer risk.
REFERENCES
- 1.SEER Cancer of Any Site - Cancer Stat Facts [Internet].. Available from: https://seer.cancer.gov/statfacts/html/all.html [30 Apr 2021].
- 2.American College of Radiology ACR Appropriateness Criteria [Internet].. Available from: http://www. acr.org/secondarymainmenucategories/quality_ safety/app_criteria.aspx [08 Jun 2011].
- 3.Sodickson A. Strategies for reducing radiation exposure in multi-detector row CT. Radiol Clin North Am 2012; 50: 1–14. doi: 10.1016/j.rcl.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 4.McCollough CH. Computed tomography Technology-and Dose-in the 21st century. Health Phys 2019; 116: 157–62. doi: 10.1097/HP.0000000000000997 [DOI] [PubMed] [Google Scholar]
- 5.Grant EJ, Brenner A, Sugiyama H, Sakata R, Sadakane A, Utada M, et al. Solid cancer incidence among the life span study of atomic bomb survivors: 1958-2009. Radiat Res 2017; 187: 513–37. doi: 10.1667/RR14492.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahoon EK, Preston DL, Pierce DA, Grant E, Brenner AV, Mabuchi K, et al. Lung, laryngeal and other respiratory cancer incidence among Japanese atomic bomb survivors: an updated analysis from 1958 through 2009. Radiat Res 2017; 187: 538. doi: 10.1667/RR14583.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner AV, Preston DL, Sakata R, Sugiyama H, de Gonzalez AB, French B, et al. Incidence of breast cancer in the life span study of atomic bomb survivors: 1958-2009. Radiat Res 2018; 190: 433. doi: 10.1667/RR15015.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugiyama H, Misumi M, Brenner A, Grant EJ, Sakata R, Sadakane A, et al. Radiation risk of incident colorectal cancer by anatomical site among atomic bomb survivors: 1958-2009. Int J Cancer 2020; 146: 635–45. doi: 10.1002/ijc.32275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadakane A, French B, Brenner AV, Preston DL, Sugiyama H, Grant EJ, et al. Radiation and risk of liver, biliary tract, and pancreatic cancers among atomic bomb survivors in Hiroshima and Nagasaki: 1958-2009. Radiat Res 2019; 192: 299. doi: 10.1667/RR15341.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mabuchi K, Preston DL, Brenner AV, Sugiyama H, Utada M, Sakata R, et al. Risk of prostate cancer incidence among atomic bomb survivors: 1958–2009. Radiat Res 2020; 195: 66–76. doi: 10.1667/RR15481.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Research Council (U.S.) Committee to assess health risks from exposure to low level of ionizing radiation. health risks from exposure to low levels of ionizing radiation: BEIR VII, phase 2. 2006;. [PubMed]
- 12.Kalra MK, Sodickson AD, Mayo-Smith WW. Ct radiation: key concepts for gentle and wise use. Radiographics 2015; 35: 1706–21. doi: 10.1148/rg.2015150118 [DOI] [PubMed] [Google Scholar]
- 13.Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci U S A 2003; 100: 13761–6. doi: 10.1073/pnas.2235592100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardarelli JJ, Ulsh BA. It is time to move beyond the linear No-Threshold theory for low-dose radiation protection. Dose Response 2018; 16: 155932581877965. doi: 10.1177/1559325818779651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012; 380: 499–505. doi: 10.1016/S0140-6736(12)60815-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 2013; 346(may21 1f2360. doi: 10.1136/bmj.f2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sodickson A. Ct radiation risks coming into clearer focus. BMJ 2013; 346(may21 1f3102. doi: 10.1136/bmj.f3102 [DOI] [PubMed] [Google Scholar]
- 18.Leuraud K, Richardson DB, Cardis E, Daniels RD, Gillies M, O'Hagan JA, et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol 2015; 2: e276–81. doi: 10.1016/S2352-3026(15)00094-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sodickson A, Baeyens PF, Andriole KP, Prevedello LM, Nawfel RD, Hanson R, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology 2009; 251: 175–84. doi: 10.1148/radiol.2511081296 [DOI] [PubMed] [Google Scholar]
- 20.Griffey RT, Sodickson A. Cumulative radiation exposure and cancer risk estimates in emergency department patients undergoing repeat or multiple CT. AJR Am J Roentgenol 2009; 192: 887–92. doi: 10.2214/AJR.08.1351 [DOI] [PubMed] [Google Scholar]
- 21.Rehani MM, Yang K, Melick ER, Heil J, alátŠalát D, Sensakovic WF, et al. Patients undergoing recurrent CT scans: assessing the magnitude. Eur Radiol 2020; 30: 1828–36. doi: 10.1007/s00330-019-06523-y [DOI] [PubMed] [Google Scholar]
- 22.Frija G, Damilakis J, Paulo G, Loose R, Vano E, .European Society of Radiology (ESR) . Cumulative effective dose from recurrent CT examinations in Europe: proposal for clinical guidance based on an ESR EuroSafe imaging survey. Eur Radiol 2021; 31: 5514–23. doi: 10.1007/s00330-021-07696-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rehani MM, Melick ER, Alvi RM, Doda Khera R, Batool-Anwar S, Neilan TG, et al. Patients undergoing recurrent CT exams: assessment of patients with non-malignant diseases, reasons for imaging and imaging appropriateness. Eur Radiol 2020; 30: 1839–46. doi: 10.1007/s00330-019-06551-8 [DOI] [PubMed] [Google Scholar]
- 24.Pandharipande PV, Reisner AT, Binder WD, Zaheer A, Gunn ML, Linnau KF, et al. Ct in the emergency department: a real-time study of changes in physician decision making. Radiology 2016; 278: 812–21. doi: 10.1148/radiol.2015150473 [DOI] [PubMed] [Google Scholar]
- 25.Pandharipande PV, Alabre CI, Coy DL, Zaheer A, Miller CM, Herring MS, et al. Changes in physician decision making after CT: a prospective multicenter study in primary care settings. Radiology 2016; 281: 835–46. doi: 10.1148/radiol.2016152887 [DOI] [PubMed] [Google Scholar]
- 26.McCollough CH. To scan or not to scan: consideration of medical benefit in the Justification of CT scanning. Health Phys 2016; 110: 287–90. doi: 10.1097/HP.0000000000000391 [DOI] [PubMed] [Google Scholar]
- 27.Eisenberg JD, Lewin SO, Pandharipande PV. The fisherman's cards: how to address past and future radiation exposures in clinical decision making. AJR Am J Roentgenol 2014; 202: 362–7. doi: 10.2214/AJR.13.10896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durand DJ, Dixon RL, Morin RL. Utilization strategies for cumulative dose estimates: a review and rational assessment. J Am Coll Radiol 2012; 9: 480–5. doi: 10.1016/j.jacr.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 29.Ip IK, Lacson R, Hentel K, Malhotra S, Darer J, Langlotz C, et al. Journal Club: predictors of provider response to clinical decision support: lessons learned from the Medicare imaging demonstration. AJR Am J Roentgenol 2017; 208: 351–7. doi: 10.2214/AJR.16.16373 [DOI] [PubMed] [Google Scholar]
- 30.Sodickson A, Warden GI, Farkas CE, Ikuta I, Prevedello LM, Andriole KP, et al. Exposing exposure: automated anatomy-specific CT radiation exposure extraction for quality assurance and radiation monitoring. Radiology 2012; 264: 397–405. doi: 10.1148/radiol.12111822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikuta I, Sodickson A, Wasser EJ, Warden GI, Gerbaudo VH, Khorasani R. Exposing exposure: enhancing patient safety through automated data mining of nuclear medicine reports for quality assurance and organ dose monitoring. Radiology 2012; 264: 406–13. doi: 10.1148/radiol.12111823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikuta I, Warden GI, Andriole KP, Khorasani R, Sodickson A. Estimating patient dose from X-ray tube output metrics: automated measurement of patient size from CT images enables large-scale size-specific dose estimates. Radiology 2014; 270: 472–80. doi: 10.1148/radiol.13122727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wasser EJ, Prevedello LM, Sodickson A, Mar W, Khorasani R. Impact of a real-time computerized duplicate alert system on the utilization of computed tomography. JAMA Intern Med 2013; 173: 1024–6. doi: 10.1001/jamainternmed.2013.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connor SD, Sodickson AD, Ip IK, Raja AS, Healey MJ, Schneider LI, et al. Journal Club: requiring clinical Justification to override repeat imaging decision support: impact on CT use. AJR Am J Roentgenol 2014; 203: W482–90. doi: 10.2214/AJR.14.13017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gee MS, Harisinghani MG. Mri in patients with inflammatory bowel disease. J Magn Reson Imaging 2011; 33: 527–34. doi: 10.1002/jmri.22504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tekes A, Senglaub SS, Ahn ES, Huisman TAGM, Jackson EM. Ultrafast brain MRI can be used for indications beyond shunted hydrocephalus in pediatric patients. AJNR Am J Neuroradiol 2018; 39: 1515–8. doi: 10.3174/ajnr.A5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raja AS, Pourjabbar S, Ip IK, Baugh CW, Sodickson AD, O'Leary M, et al. Impact of a health information Technology-Enabled appropriate use criterion on utilization of emergency department CT for renal colic. AJR Am J Roentgenol 2019; 212: 142–5. doi: 10.2214/AJR.18.19966 [DOI] [PubMed] [Google Scholar]