Abstract
Introduction
Recurrent tricuspid regurgitation (TR) is frequently observed after cardiac surgery; however, the correct approach remains controversial. We developed an algorithm for action on the tricuspid valve (TV) and conducted a 1-year follow-up study. The aim was to assess the efficacy of the algorithm to minimise residual TR after TV surgery. The hypothesis was that the TR rate at 1 year would be reduced by selecting the surgical approach in accordance with a set of preoperative clinical and echocardiographic variables.
Methods
A prospective, observational, single-centre study was performed in 76 consecutive patients with TV involvement. A protocol was designed for their inclusion, and data on their clinical and echocardiographic characteristics were gathered at 3 months and 1-year postsurgery. The treatment of patients depended on the degree of TR. Surgery was performed in all patients with severe or moderate-to-severe TR and in those with mild or moderate TR alongside the presence of certain clinical or echocardiographic factors. They underwent annuloplasty or extended valve repair when the TV was distorted. If repair techniques were not feasible, a prosthesis was implanted. Residual TR rates were compared with published reports, and predictors of early/late mortality and residual TR were evaluated.
Results
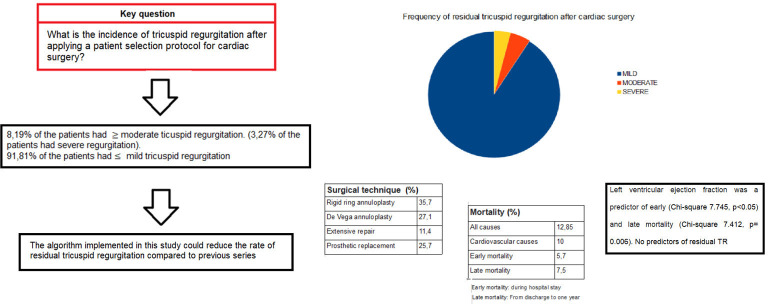
TR was functional in 69.9% of patients. Rigid ring annuloplasty was performed in 35.7% of patients, De Vega annuloplasty in 27.1%, extended repair in 11.4% and prosthetic replacement in 25.7%. TR was moderate or worse in 8.19% of patients (severe in 3.27%) at 1 year postintervention. No clinical, surgical or epidemiological variables were significantly associated with residual TR persistence, although annulus diameter showed a close-to-significant association. Total mortality was 12.85% for all causes and 10% for cardiovascular causes. In multivariate analysis, left ventricular ejection fraction was related to both early and late mortality.
Conclusions
Severe residual TR was significantly less frequent than reported in other series, being observed in less than 4% of patients at 1-year postsurgery.
Keywords: Cardiac Surgery, Tricuspid Valve Insufficiency, Heart Valve Diseases
What is already known on this topic
Recurrent tricuspid regurgitation (TR) is frequently observed after cardiac surgery, ranging between 10% and 30%.
TR after cardiac surgery continues to be frequent and associated with a poor prognosis.
What this study adds
We developed a new algorithm for action on the tricuspid valve and conducted a 1-year follow-up study to evaluate the recurrence or TR.
The hypothesis was that the TR rate at 1 year would be reduced. Secondary objectives were to determine the mortality and to evaluate predictors of TR at 1 year and predictors of early and late mortality.
How this study might affect research, practice or policy
Selecting the surgical approach according to a set of preoperative clinical and echocardiographic variables could reduce the recurrence of TR.
At 1 year, 8.19 % of the patients had ≥ moderate TR, including 3.27% with severe TR.
Introduction
The tricuspid valve (TV) was long known as the ‘forgotten valve’, because tricuspid regurgitation (TR) was considered resolved by left disease surgery. However, various researchers found that left heart valve treatment did not correct tricuspid annular dilatation or improve right ventricular (RV) function.1 Follow-up studies also observed TR persistence or progression in patients who had not undergone TV surgery and even in those who had. For instance, Shiran and Sagie found that only 33% of severe/moderate TR cases became mild after mitral valvuloplasty.2
TR is frequent in patients with concomitant heart disease, especially valve disease. The prevalence of TR is 15% overall and higher in patients with left disease, and it is the most frequent complication of mitral disease. In cases of heart failure (HF), TR grade >2/4 has been reported in 35% overall, in 30% of those with severe mitral regurgitation, and in 50% of those treated with mitral valve surgery.3
The surgical technique of choice remains under debate,4–7 although valve repair is the most common approach, especially rigid ring annuloplasty, with reports that 15% of the patients have residual TR in comparison to 20%–35% of those undergoing other procedures.1 Additional techniques applied in cases of marked valve deformity or ventricular remodelling include anterior leaflet extension, neochordae implantation, decalcification or commissuroplasty. Prosthetic replacement is a last resort.
The presence of residual TR and its grade are independent predictors of mortality, which is more likely with higher severity. The persistence of TR grade 3–4/4 after mitral valve replacement is associated with worse functional class and with higher mortality rates for HF and all causes.8
The frequency of residual TR after tricuspid surgery ranges between 10% and 30%9 according to the baseline characteristics of patients and the surgical approach, among other factors.10–12 In one follow-up study, only 32% of patients had no residual TR at 3 months postsurgery and only 22% at 5 months, while TR grade 3/4 was recorded at the same time points in 11% and 17% of patients, respectively.13 TR grade 3/4 was observed in 12% of patients after rigid ring annuloplasty, 16% after flexible prosthesis implantation, 24% after De Vega annuloplasty, 44% after periguard annuloplasty, and 19% after Kay’s annuloplasty.13
Annulus dilatation is known to be a preoperative predictor of residual TR. However, there is no consensus on other potential predictors, including the presence of right HF, pulmonary hypertension, increased atrial volume, atrial fibrillation (AFib), rheumatic mitral valve disease, marked RV remodelling/dysfunction or a history of ischaemic heart disease.14–16 There is wide agreement on the therapeutic response to severe TR, but the approach to lesser grades remains controversial. Thus, American clinical practice guidelines17 centre on severe or progressive TR, while European guidelines18 include recommendations for lower grades associated with certain predictors of residual TR. The evidence underlying European guidelines includes data on: the association of severe TR with congestive HF19; poor outcomes in isolated severe TR cases attributed to late patient management20; good survival rates after combined tricuspid and mitral valve surgery,21 and the predictive capacity of annulus size.1 22 Brescia et al23 followed European clinical practice guidelines in patients with functional TR and reported absent or only moderate TR in a large proportion of patients, with the persistence of good RV function. Other authors underscored the need for careful patient selection to improve outcomes.22 In contrast, one study found that survival was not improved by surgery in comparison to medical treatment.24 American guidelines cite multiple references related to TR of different grades/etiologies and associated predictive factors; however, almost all focus on the study of severe TR.
With the above background, we hypothesised that the TR rate at 1 year would be reduced by selecting the surgical approach in accordance with a set of preoperative clinical and echocardiographic variables. A corresponding algorithm was implemented in this study, following up patients during 1 year after TV surgery to evaluate TR recurrence. The primary objective of the study was to assess the efficacy of this novel algorithm for surgical intervention to TR, considering the residual TR as primary endpoint. Secondary objectives were to determine the mortality for all causes and for cardiovascular disease with implementation of the algorithm and to evaluate predictors of TR at 1 year and predictors of early and late mortality.
Methods
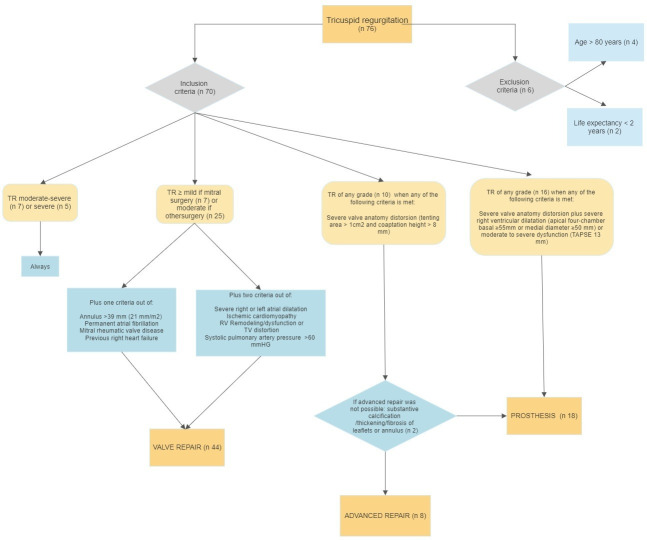
This prospective, observational, single-centre study included patients undergoing cardiac surgery. Surgery was indicated in patients with heart disease (eg, coronary or valve disease) who fulfilled the criteria for surgery in clinical practice guidelines. At the same time, patients underwent surgery on the TV according to the algorithm exhibited in table 1. The decision on TV valve intervention and the surgical technique was based on the degree of TR, aetiology, TV anatomy, RV function and other cardiological comorbidities (figure 1). Surgery was always performed in patients with moderate-to-severe or severe TR. Other factors were considered in patients with a lesser degree of TR. Thus, tricuspid surgery was performed when TR was mild (in mitral surgery cases) or moderate (in other cardiac surgery cases) when the patients had annulus >39 mm (21 mm/m2), permanent AFib, mitral rheumatic valve disease or previous right HF or if they met at least two of the following conditions: severe right or left atrial dilatation, ischaemic cardiomyopathy, RV remodelling/dysfunction, TV distortion or systolic pulmonary artery pressure (SPAP) >60 mm Hg.
Table 1.
Tricuspid valve surgery protocol
| 1. Surgical management of tricuspid valve regurgitation | |
| Moderate to severe or severe tricuspid regurgitation | Always Following indications of clinical guidelines: symptoms or progressive RV dilatation/declining RV function. |
| Mild tricuspid regurgitation if mitral surgery or moderate tricuspid regurgitation if other cardiac surgery | Plus one of the following criteria:
Plus two of the following criteria:
|
2. Criteria for extended repair
| |
3. Criteria for prosthesis implantation
| |
EF, ejection fraction; RV, right ventricle; SPAP, systolic pressure pulmonary artery; TAPSE, tricuspid annular plane systolic excursion; TV, tricuspid valve.
Figure 1.
Algorithm for surgical intervention of tricuspid regurgitation (TR). RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion; TV, tricuspid valve.
Valve repair with simple annuloplasty (ring annuloplasty or DeVega suture) was the technique of choice. Extended repair (neochordae implantation, enlargement of the tricuspid anterior leaflet or commissuroplasty) was performed in cases of severe valve distortion (tenting area >1.0 cm2 and coaptation >8 mm). A tricuspid prosthesis was implanted in patients with substantive calcification/thickening/fibrosis of leaflets or annulus and/or moderate to severe dilatation (apical four-chamber basal diameter ≥55 mm or medial diameter ≥50 mm) or severe dysfunction of the RV (tricuspid annular plane systolic excursion, TAPSE) <13 mm combined with depressed shortening fraction (<35%), depressed pulsed tissue Doppler (<9.5 cm/s), or depressed global longitudinal RV free wall strain (<20%).
The decision on the type of simple annuloplasty was taken by the surgeon. These patients were operated on by one of only three cardiovascular surgeons, one being an expert in mitral and TV repair and the other two having extensive experience in tricuspid ring annuloplasty and DeVega repair with very good long-term outcomes. DeVega annuloplasty was applied when there was little anatomical distortion of the valve.
Exclusion criteria were age >80 years or life expectancy <2 years. There were no cases of isolated TR in patients not requiring surgery for left valvular heart disease with severe systolic dysfunction or severe pulmonary hypertension. The primary outcome considered was the residual TR. Secondary outcomes were mortality for all causes, mortality for cardiovascular disease, predictors of TR at 1 year, and the early and late mortality.
The approach was selected in accordance with the algorithm exhibited in table 1. Cardiologists with expertise in echocardiography gathered data on sociodemographic, clinical, analytical and transthoracic echocardiographic (TTE) variables during the first year postsurgery. In a descriptive analysis, quantitative variables were expressed as means±SD when they were normally distributed (by Kolmogorov-Smirnov test) and medians±IQR when they were not, while qualitative variables were expressed as absolute numbers and percentages. The hypothesis was tested for a dichotomous qualitative sample (exact binomial test), defining group 1 as unfavourable outcome (≥moderate TR) and group 2 as favourable outcome ≤mild TR).
An exhaustive review of the literature over the past 30 years revealed that around 20% of patients had >moderate TR after TV surgery.8 9 We hypothesised that application of this algorithm would reduce TR recurrence after this surgery to 10%, that is, around half the rate previously recorded at our centre and generally reported in the literature.
Bivariate analyses were conducted on the relationship of variables with a favourable outcome, using the independent-samples Student’s t-test for numerical variables when normally distributed and the Mann-Whitney U test when not. Pearson’s or Fisher’s χ2 test was used for categorical variables. The predictive capacity of variables for residual TR was tested by binary logistic regression, entering variables that were statistically significant in bivariate analysis or considered clinically relevant. The model was constructed using a forward stepwise selection procedure. Predictors of hospital and 1-year postdischarge mortality were also evaluated. The level of significance was set at 0.05 with a CI of 95%.
Results
Seventy-six consecutive patients were enrolled in the study between 1 February 2018 and 1 February 2020, and six of these were excluded. The mean age of the 70 remaining patients was 65.47±10.3 years, and 75.7% were female. AFib was permanent in 79.5%, paroxysmal in 4.1%, and persistent in 1.4%; 46.4% had a history of right HF; 49.3% were in New York Heart Association functional class II, 41.1% in class III and 1.4% in IV. The mean SPAP was 52.45±15.96 mm Hg at baseline (presurgery) and 42.85 mm Hg ±11.02 mm Hg at 1 year (tables 2 and 3).
Table 2.
Baseline characteristics
| Baseline characteristics | |
| Age (years) | 65.47±10.3 |
| BMI (kg/m2) | 28.06±4.17 |
| Sex (female) (n/%) | 53/75.70 |
| Hypertension (n/%) | 38/56.20 |
| Diabetes (n/%) | 9/12.3 |
| Glomerular filtration rate (mL/min/m2) | 72.81/–18.45 |
| COPD (n/%) | 12/17.40 |
| Dyslipidaemia (n/%) | 30/43.80 |
| Cardiovascular history | |
| Left heart failure (n/%) | 30/42.80 |
| Right heart failure (n/%) | 32/46.40 |
| Preoperative atrial fibrillation (n/%) | 55/79.50 |
| Coronary heart disease (n/%) | 6/8.6 |
| Stroke (n/%) | 9/12.8 |
| SPAP (mm Hg) | 52.45±15.96 |
| LVEF (n/%) | |
| >52% in males or >54% in females | 56/80.0 |
| 41%–51/53% (males/females) | 10/14.2 |
| 30%–40% | 3/4.2 |
| <30% | 1/1.4 |
| RVEF (%) | |
| TAPSE >17 mm | 57/81.4 |
| 15–17 mm | 10/14.2 |
| <15 mm | 3/4.2 |
| Surgical variables | |
| Previous cardiac surgery (n/%) | 20/28.5 |
| Mean EuroSCORE (%) | 5±3.66 |
| Extracorporeal time (min) | 112±39.3 |
| Clamping time (min) | 83.42±46.71 |
| TR aetiology (n/%) | |
| Functional | 49/69.9 |
| Rheumatic | 14/20.5 |
| Myxomatous | 2/2.7 |
| Pacemaker mediated | 1/1.4 |
| Radiation mediated | 1/1.4 |
| Unspecified | 3/4.1 |
| Grade of TR (n/%) | |
| Mild | 7/10 |
| Moderate | 25/35.7 |
| Moderate-severe | 7/10 |
| Severe | 31/44.28 |
| Type of valve surgery (n/%) | |
| Tricuspid alone | 17/24.7 |
| Tricuspid+Mitral | 30/42.5 |
| Tricuspid+aortic | 2/2.7 |
| Trivalvular | 14/20.5 |
| Other type | 7/9.6 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; RVEF, right ventricular ejection fraction; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; TV, tricuspid valve.
Table 3.
Complications
| Complications | % | N |
| Early | ||
| Kidney failure | 39.70 | 27 |
| Atrial fibrillation De novo | 8.2 | 6 |
| Acute myocardial infarction | 4.41 | 3 |
| Ischaemic stroke | 4.41 | 3 |
| Heart failure | 13.70 | 9 |
| Significant pericardial effusion | 7.6 | 7 |
| Bleeding requiring transfusion | 11 | 8 |
| Late | ||
| Kidney failure | 8.2 | 5 |
| De novo atrial fibrillation | 4 | 3 |
| Acute myocardial infarction | 1.4 | 1 |
| Significant pericardial effusion | 6.5 | 4 |
| Heart failure | 17.80 | 11 |
| Ischaemic stroke | 1.4 | 1 |
| Pacemaker implantation | 13.70 | 9 |
Left valve aetiology was rheumatic in 40% of patients, moderate and severe mitral stenosis in 20% and 9.5%, respectively, and moderate and severe mitral regurgitation in 17% and 24%, respectively (table 4). Baseline left ventricular function was >52% in males and >54% in females in 80% of patients, 41%–51%/53% in 14.28%, 30%–40% in 4.28% and <30% in 1.4%.
Table 4.
Prevalence of left valvular diseases
| No | Mild | Moderate | Severe | |
| Mitral stenosis (%/n) | 58.6/41 | 11.4/8 | 20/14 | 9.5/7 |
| Aortic stenosis (%/n) | 71.4/50 | 18.5/13 | 7.1/5 | 2.8/2 |
| Mitral regurgitation (%/n) | 25.7/18 | 32.8/23 | 17.0/12 | 24/17 |
| Aortic regurgitation (%/n) | 38.57/27 | 37.14/26 | 18.57/13 | 10.0/4 |
TR aetiology was functional in 69.9% of patients, rheumatic in 20.5%, myxomatous in 2.7%, pacemaker-mediated in 1.4%, radiation-mediated in 1.4% and unspecified in 4.1%. Baseline RV function showed TAPSE >17 mm in 81.4%, 15–17 mm in 14.2%, and <15 mm in 4.2%. Rigid ring annuloplasty was performed in 35.7% of patients, De Vega annuloplasty in 27.1%, extended repair in 11.4%, and prosthetic replacement in 25.7%. Valve surgery alone was conducted in 94.5% of patients and was combined with coronary or other type of surgery in the remainder. The type of valve surgery performed alongside tricuspid surgery was mitral in 42.5% of patients, aortic in 2.7%, trivalvular (aortic, mitral and tricuspid) in 20.5 %, and isolated tricuspid in 24.7%, while another type of surgery was performed in 9.6%.
At baseline, TR was mild in 10% of patients, moderate in 35.7%, moderate-to-severe in 10%, and severe in 44.28%. At 1 year, TR was mild in 45.2%, moderate in 4.8%, moderate-severe in 3.2%, severe in 1.6% and trivial or absent in 45.2%.
In comparison to patients undergoing repair, those receiving a prosthesis had lower SPAP, lower arterial hypertension, a tendency towards higher left ventricular ejection fraction (LVEF), and similar Euroscore at baseline. Around three-quarters of patients receiving prosthesis were female. Adverse factors in the prosthesis versus repair group included: worse RV ejection fraction (RVEF), worse TAPSE and lateral strain, more frequent AFib, rheumatic aetiology, more frequent pacemaker implantation during the follow-up, and a more marked RV modelling, with larger diameters. Semiquantitative and quantitative TR variables were all more severe. The two groups did not differ in the presence of residual TR at 1 year or in early or late mortality rates (table 5 and online supplemental material 1).
Table 5.
Comparison between repair and prosthesis groups
| Repair | Prosthesis | P value | |
| Age (years) | 64.36±11.7 | 64.87±6.7 | 0.86 |
| Body mass index (kg/m2) | 28.4861±4.78 | 27.49±4.34 | 0.75 |
| SPAP (mm Hg) | 55.35±16.81 | 39.58±10.51 | 0.02 |
| Glomerular filtration rate (mL/kg/min) |
74.25±18.38 | 73.68±19.03 | 0.92 |
| EuroSCORE (%) | 6.19±13.33 | 4.36±2.46 | 0.31 |
| Extracorporeal time (min) | 115.59±39.13 | 88.14±37.79 | 0.08 |
| Clamping time (min) | 96.89±36.22 | 37.55±48.69 | 0.01 |
| Sex (female) (%) | 75.8 | 80 | 0.49 |
| Arterial hypertension (%) | 54.8 | 26.7 | 0.02 |
| Previous stroke (%) | 12.7 | 6.7 | 0.37 |
| Ischaemic heart disease (%) | 9.7 | 6.7 | 0.12 |
| Atrial fibrillation (%) | 79 | 93.3 | 0.02 |
| NYHA functional class (%) | |||
| II III |
53.2 37.1 |
33.3 46.7 |
0.03 |
| Right heart failure (%) | 43.5 | 33.3 | 0.32 |
| LVEF (%) | |||
| Normal Mild Moderate |
85.5 12.9 1.6 |
93.3 6.7 |
0.67 |
| Death during hospital stay | 3.8 | 11 | 0.27 |
| Pacemaker implantation during first year of follow-up | 14.3 | 20 | 0.07 |
| Death during first year of follow-up | 6 | 12 | 0.47 |
| RVEF (%) | |||
| Normal Mild Moderate |
82.3 14.5 3.2 |
60 40 |
0.02 |
| TR aetiology (%) | |||
| Functional Rheumatic |
76.7 20 |
53.3 40 |
0.6 |
| Echocardiography | |||
| Basal diameter RV (mm) | 47.7±7.39 | 56.33±5.74 | 0.01 |
| Mid-diameter RV (mm) | 36.8±8.49 | 43.33±6.8 | 0.01 |
| Shortening fraction (%) | 39.71±13.67 | 39.42±16.06 | 0.94 |
| TAPSE (mm) | 19.64±5.02 | 18.07±2.64 | 0.15 |
| Longitudinal strain (%) | −20.39±5.25 | −17.52±2.87 | 0.05 |
| Vena contracta (mm) | 6.8±5.20 | 11.3 (1.92) | 0.09 |
| PISA radius (mm) | 8.57±2.64 | 8.25 (3.57) | 0.76 |
| Regurgitant orifice (cm2) | 0.4 (0.57) | 0.8 (0.23) | 0.01 |
| Regurgitating volume (mL) | 40.43±22.04 | 58.33 (34.8) | 0.03 |
| Tenting area (cm2) | 1.41±1.21 | 3.5 (3.57) | 0.06 |
| Coaptation distance (mm) | 6.9±3.3 | 16.1 (21.4) | 0.01 |
LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PISA, proximal isovelocity surface area; RV, right ventricle; RVEF, right ventricular ejection fraction; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
openhrt-2022-002011supp001.pdf (109.2KB, pdf)
Total mortality was 12.85% for all causes and 10% for cardiovascular disease. Cardiovascular causes were responsible for 100% of the early mortality and 60% of the late mortality. Globally, the early mortality rate was 5.7% and the late mortality rate 7.5% (figure 2).
Figure 2.
Figure summary of the study. Data measured 1 year after surgery. TR, tricuspid regurgitation.
At 1 year, 8.19% of the patients had ≥moderate TR, including 3.27% with severe TR. Binomial exact test results evidenced a significant reduction (χ2 of 4.129, p=0.024) in the percentage of present patients with residual TR in comparison to the percentage generally reported in the literature (20%). A recent meta-analysis25 reported moderate-to-severe TR in 9% of patients vs 3.27% of the present series. At our centre, the percentage of patients with residual TR was significantly lower (p=0.02) after (8.19%) than before (21%) implementation of the algorithm.
The capacity of preoperative variables to predict residual TR and early and late mortality was examined using binary logistic regression analysis. Given the scant presence of TR during the follow-up, no association was observed between an unfavourable TR outcome and any study variable, as shown in online supplemental tables 2 and 3. Another relevant finding was that the percentage of patients with TR did not significantly differ between 3 months and 1-year postsurgery (p=0.823).
A 1% increase in LVEF was associated with a 1.24-fold decrease in early mortality (χ2 7.745, p<0.05). The tested model explained 36.4% of the variance of the dependent variable (Nagelkerke R2 of 0.364), and 96.8% of predictions based on the model were correct (online supplemental table 4). Death during the first year of follow-up (late mortality) was 40-fold more likely in patients with LVEF below vs above 40% (χ2 7.412, p=0.006). The model explained 25.7% of the variance of the dependent variable (Nagelkerke R2 of 0.257), and 93.8% of predictions based on the model were correct (online supplemental table 5).
No predictors of residual TR emerged in the model, likely because only five patients had an unfavourable outcome and only two of these had severe TR (online supplemental table 6). Furthermore, the clinical relevance of moderate residual TR (observed in the other three patients) is not clear.
Discussion
Inadequate evidence is available on TV surgical indications and the relevant preoperative factors, leading to discrepant recommendations and a variability in protocols among centres and even among surgeons in the same centre. Moreover, repair techniques other than annuloplasty are much less widely applied in TV than in mitral valve surgery and are more complex, besides the lesser experience accrued in centres. A consensus has generally been reached on the management of severe TR but not on the approach to lower grades of TR, for which divergent recommendations have even been published.
American guidelines17 prescribe surgery when TR is severe and in the presence of certain other factors, including symptoms, left surgery, right HF and RV dilatation or progressive dysfunction. They do not expressly refer to lower TR grades except for ‘progressive TR with annulus dilatation or history of HF and concomitant left surgery’, based on evidence level IIA and with no definition of progressive TR. The same surgical indications for severe TR are included in European guidelines18; however, they also make recommendations for moderate TR with concomitant left surgery and for mild or moderate TR with annulus dilatation and concomitant left surgery. Hence, unlike American guidelines, they support surgery for annulus dilatation even in patients with mild TR grades.
Following clinical practice guidelines (symptoms, progressive dilatation or RV dysfunction), all patients with severe or moderate-to-severe TR underwent TV intervention. For those with a lesser degree of TR, an exhaustive bibliographic search8 14 26–38 was carried out, selecting variables referred to in the literature associated with the persistence of TR after surgery and its magnitude. In this way, preoperative clinical and echocardiographic variables were selected for the decision to intervene or not intervene on the TV in patients with mild or moderate TR (table 1).
When there were anatomical factors (table 1) that hindered or could predict valve repair failure with simple annuloplasty, an extended repair was chosen as the technique of first choice.
When valve distortion or RV remodelling was so marked that even advanced repair was inadequate, prosthesis implantation was the last surgical technique of choice. Regarding selection of the type of prosthesis, 72% of the implanted prostheses were biological. Only five mechanical prostheses were implanted, by decision of the patients after receiving detailed information on their advantages and disadvantages.
The TR recurrence rate was lower in the patients undergoing rigid ring or De Vega annuloplasty than has been reported in the literature. This may be attributable to the strict criteria applied for the candidate’s clinical characteristics, TR severity and RV remodelling or valvular distortion. Among the five recurrences observed, two were after rigid ring annuloplasty, one after De Vega annuloplasty, one after extended repair and one after prosthetic replacement. The degree of TR was moderate in three of the five patients and severe in the other two. Importantly, although moderate residual TR was considered an unfavourable outcome, its impact on survival has not been fully elucidated. Some authors have suggested that progression and a moderate or worse degree of TR are associated with clinical events.39
There are no recommendations on the timing of TR measurements in the literature, and the variations in their timing among studies generates a potential bias. The first measurement was conducted at 3 months in this study, providing data on the short-term effects of surgery, and the second was at 1 year to evaluate its progression. In fact, there was no difference in residual TR progression between measurements at 3 months and at 1 year, so that the result obtained at 3 months predicted the degree of residual TR for up to 1-year postsurgery (Z score −0.223, p=0.823).
Repair has conventionally been preferred over replacement, on the grounds that it delivers a superior outcome and ventricular function is better preserved. However, there have been few comparative studies, and replacement is usually performed in patients with more advanced disease or with a more dilated or dysfunctional RV. In this way, patients who received a prosthesis in this study had a worse RVEF, more marked RV modelling, and higher TR grade. However, the repair and prosthesis groups did not differ in the presence of residual TR or in early or late mortality rates, which may be attributable to the application of our algorithm to select the most appropriate surgical approach for each patient.
The mortality rate was lower in the present series than generally reported in the literature, indicating that implementation of the algorithm to select the surgical approach did not have a negative impact on survival. We highlight that extended repair failed in one patient who died in hospital and in another who died postdischarge (2.8% of the series), who both required prolonged surgery for prosthetic replacement after the repair attempt. This suggests that extended repair, which requires greater experience and longer surgery, may pose a greater risk to the patient than direct prosthesis implantation, especially in patients with unfavourable anatomical features. Severe TR was observed in a very small number of patients during the follow-up, supporting the usefulness of the management algorithm to select treatment (prosthesis, extended repair or annuloplasty) as a function of RV characteristics and TV distortion.
Tricuspid annulus dilatation was not found to be a predictor of residual TR because annular dilatation was a criterion for the ordering of corrective annuloplasty, even with a mild degree of TR. In addition, it is difficult to find statistically significant predictors in a multivariate analysis when there are only five events (ie, cases of moderate or worse TR). Nevertheless, annular dilatation showed a tendency towards statistical significance despite the few events, although this association has also not been found in other studies with larger numbers of patients.39
According to the present findings, treatment decision making could be improved by taking into account the TV characteristics and other preoperative clinical and echocardiographic variables considered in this study. However, further studies with larger samples and greater statistical power are needed to provide solid evidence on the association of preoperative clinical and imaging variables with the mid-term and long-term outcomes of different surgical approaches.
Limitations
Comparison of these findings with published results is hampered by the wide variability in methodologies adopted by previous investigations. The study was also limited by its non-randomised design, although differences among clinical practice guidelines and the lack of a consensus in the literature (see above) prevented the formation of a group treated according to a standard protocol for comparative purposes. It was also not possible to precisely control the decision to conduct valve repair or prosthesis implantation or switch to another approach during repair, given the largely subjective nature of these choices by the surgical team. Hence, no specific conclusions could be drawn from these comparisons.
MRI is the technique of choice for RV assessment but was not used in this study. This is because both MRI and CT have limitations in the anatomical assessment of the TV and can be affected by image artefacts in these patients, who often have metallic prostheses and pacemaker leads. TTE echocardiography was used as the reference technique. A three-dimensional TTE was also routinely performed; however, it was not included in subsequent analyses because it was considered insufficiently precise for anatomical assessment of the valve in a substantial number of patients. The reduced number of patients and events probably explains the failure to identify statistically significant predictors of mortality. Likewise, the very few patients with severe TR during the 1-year follow-up would account for the absence of significant predictors of residual TR. In addition, the possibility of a higher frequency/grade of residual TR beyond 1-year postdischarge cannot be ruled out, and longer-term studies are needed.
Conclusions
TR has major clinical repercussions, affecting functional class, exercise capacity and survival. Persistent TR after cardiac surgery continues to be frequent and associated with a poor prognosis. Clinical practice guidelines offer discordant recommendations based on inadequate evidence. The algorithm implemented in this study could reduce the rate of residual TR in comparison to previous series. Further long-term research is warranted to verify these findings.
Acknowledgments
To all the authors for their contribution, especially to Dr. García-Orta.
Footnotes
Contributors: DRT and RGO did the planning, conducting and reporting of the work. LTQ, DSR, JMGJ, MEM, FGM and EME did the planning of the work. DRT is responsible for the overall content as guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: The authors certify that they have no affiliations with or involvement in any organisation or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership or other equity interest; and expert testimony or patent-licensing arrangements), or non-fi nancial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Ethics and Research Committee of the Hospital Virgen de las Nieves. Participants gave informed consent to participate in the study before taking part.
References
- 1.Dreyfus GD, Corbi PJ, Chan KMJ, et al. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg 2005;79:127–32. 10.1016/j.athoracsur.2004.06.057 [DOI] [PubMed] [Google Scholar]
- 2.Shiran A, Sagie A. Tricuspid regurgitation in mitral valve disease incidence, prognostic implications, mechanism, and management. J Am Coll Cardiol 2009;53:401–8. 10.1016/j.jacc.2008.09.048 [DOI] [PubMed] [Google Scholar]
- 3.Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham heart study). Am J Cardiol 1999;83:897–902. 10.1016/S0002-9149(98)01064-9 [DOI] [PubMed] [Google Scholar]
- 4.Kundi H, Popma JJ, Cohen DJ, et al. Prevalence and outcomes of isolated tricuspid valve surgery among Medicare beneficiaries. Am J Cardiol 2019;123:132–8. 10.1016/j.amjcard.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 5.Hamandi M, Smith RL, Ryan WH, et al. Outcomes of isolated tricuspid valve surgery have improved in the modern era. Ann Thorac Surg 2019;108:11–15. 10.1016/j.athoracsur.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 6.Mangoni AA, DiSalvo TG, Vlahakes GJ, et al. Outcome following isolated tricuspid valve replacement. Eur J Cardiothorac Surg 2001;19:68–73. 10.1016/S1010-7940(00)00598-4 [DOI] [PubMed] [Google Scholar]
- 7.Zack CJ, Fender EA, Chandrashekar P, et al. National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol 2017;70:2953–60. 10.1016/j.jacc.2017.10.039 [DOI] [PubMed] [Google Scholar]
- 8.Katsi V, Raftopoulos L, Aggeli C, et al. Tricuspid regurgitation after successful mitral valve surgery. Interact Cardiovasc Thorac Surg 2012;15:102–8. 10.1093/icvts/ivs107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy PM, Bhudia SK, Rajeswaran J, et al. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg 2004;127:674–85. 10.1016/j.jtcvs.2003.11.019 [DOI] [PubMed] [Google Scholar]
- 10.Kwon D-A, Park J-S, Chang H-J, et al. Prediction of outcome in patients undergoing surgery for severe tricuspid regurgitation following mitral valve surgery and role of tricuspid annular systolic velocity. Am J Cardiol 2006;98:659–61. 10.1016/j.amjcard.2006.03.047 [DOI] [PubMed] [Google Scholar]
- 11.Mahesh B, Wells F, Nashef S, et al. Role of concomitant tricuspid surgery in moderate functional tricuspid regurgitation in patients undergoing left heart valve surgery. Eur J Cardiothorac Surg 2013;43:2–8. 10.1093/ejcts/ezs441 [DOI] [PubMed] [Google Scholar]
- 12.Chikwe J, Anyanwu AC. Surgical strategies for functional tricuspid regurgitation. Semin Thorac Cardiovasc Surg 2010;22:90–6. 10.1053/j.semtcvs.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 13.Navia JL, Nowicki ER, Blackstone EH, et al. Surgical management of secondary tricuspid valve regurgitation: annulus, commissure, or leaflet procedure? J Thorac Cardiovasc Surg 2010;139:1473–82. 10.1016/j.jtcvs.2010.02.046 [DOI] [PubMed] [Google Scholar]
- 14.Song H, Kim M-J, Chung CH, et al. Factors associated with development of late significant tricuspid regurgitation after successful left-sided valve surgery. Heart 2009;95:931–6. 10.1136/hrt.2008.152793 [DOI] [PubMed] [Google Scholar]
- 15.Topilsky Y, Khanna AD, Oh JK, et al. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation 2011;123:1929–39. 10.1161/CIRCULATIONAHA.110.991018 [DOI] [PubMed] [Google Scholar]
- 16.Fender EA, Petrescu I, Ionescu F, et al. Prognostic importance and predictors of survival in isolated tricuspid regurgitation: a growing problem. Mayo Clin Proc 2019;94:2032–9. 10.1016/j.mayocp.2019.04.036 [DOI] [PubMed] [Google Scholar]
- 17.Otto CM, Nishimura RA, Bonow RO. ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American heart association joint Committee on clinical practice guidelines. Circulation 2020;2021:e72–227. [DOI] [PubMed] [Google Scholar]
- 18.Vahanian A, Beyersdorf F, Praz F. ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 2021;2021:727–800. [DOI] [PubMed] [Google Scholar]
- 19.Kadri AN, Menon V, Sammour YM, et al. Outcomes of patients with severe tricuspid regurgitation and congestive heart failure. Heart 2019;105:1813–7. 10.1136/heartjnl-2019-315004 [DOI] [PubMed] [Google Scholar]
- 20.Dreyfus J, Ghalem N, Garbarz E. Timing of referral of patients with severe isolated tricuspid valve regurgitation to surgeon. Am J Cardiol 2018;122:323326. [DOI] [PubMed] [Google Scholar]
- 21.Van de Veire NR, Braun J, Delgado V, et al. Tricuspid annuloplasty prevents right ventricular dilatation and progression of tricuspid regurgitation in patients with tricuspid annular dilatation undergoing mitral valve repair. J Thorac Cardiovasc Surg 2011;141:1431–9. 10.1016/j.jtcvs.2010.05.050 [DOI] [PubMed] [Google Scholar]
- 22.Badhwar V, Rankin JS, He M, et al. Performing concomitant tricuspid valve repair at the time of mitral valve operations is not associated with increased operative mortality. Ann Thorac Surg 2017;103:587–93. 10.1016/j.athoracsur.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 23.Brescia AA, Ward ST, Watt TMF, et al. Outcomes of guideline-directed concomitant annuloplasty for functional tricuspid regurgitation. Ann Thorac Surg 2020;109:1227–32. 10.1016/j.athoracsur.2019.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Axtell AL, Bhambhani V, Moonsamy P, et al. Surgery Does Not Improve Survival in Patients With Isolated Severe Tricuspid Regurgitation. J Am Coll Cardiol 2019;74:715–25. 10.1016/j.jacc.2019.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veen KM, Etnel JRG, Quanjel TJM, et al. Outcomes after surgery for functional tricuspid regurgitation: a systematic review and meta-analysis. Eur Heart J Qual Care Clin Outcomes 2020;6:10–18. 10.1093/ehjqcco/qcz032 [DOI] [PubMed] [Google Scholar]
- 26.Matsuyama K, Matsumoto M, Sugita T, et al. Predictors of residual tricuspid regurgitation after mitral valve surgery. Ann Thorac Surg 2003;75:1826–8. 10.1016/S0003-4975(03)00028-6 [DOI] [PubMed] [Google Scholar]
- 27.Wang G, Sun Z, Xia J, et al. Predictors of secondary tricuspid regurgitation after left-sided valve replacement. Surg Today 2008;38:778–83. 10.1007/s00595-007-3721-3 [DOI] [PubMed] [Google Scholar]
- 28.Yoshida J, Ikenaga H, Hayashi A, et al. Predictors and outcomes of persistent tricuspid regurgitation after transcatheter aortic valve implantation. Am J Cardiol 2019;124:772–80. 10.1016/j.amjcard.2019.05.066 [DOI] [PubMed] [Google Scholar]
- 29.Dumont C, Galli E, Oger E, et al. Pre- and postoperative tricuspid regurgitation in patients with severe symptomatic aortic stenosis: importance of pre-operative tricuspid annulus diameter. Eur Heart J Cardiovasc Imaging 2018;19:319–28. 10.1093/ehjci/jex031 [DOI] [PubMed] [Google Scholar]
- 30.Bianchi G, Solinas M, Bevilacqua S, et al. Which patient undergoing mitral valve surgery should also have the tricuspid repair? Interact Cardiovasc Thorac Surg 2009;9:1009–20. 10.1510/icvts.2009.217570 [DOI] [PubMed] [Google Scholar]
- 31.García Fuster R, Vázquez A, Peláez AG, et al. Factors for development of late significant tricuspid regurgitation after mitral valve replacement: the impact of subvalvular preservation. Eur J Cardiothorac Surg 2011;39:866–74. 10.1016/j.ejcts.2010.11.014 [DOI] [PubMed] [Google Scholar]
- 32.Zhu T-Y, Min X-P, Zhang H-B, et al. Preoperative risk factors for residual tricuspid regurgitation after isolated left-sided valve surgery: a systematic review and meta-analysis. Cardiology 2014;129:242–9. 10.1159/000367589 [DOI] [PubMed] [Google Scholar]
- 33.Izumi C, Miyake M, Takahashi S, et al. Progression of isolated tricuspid regurgitation late after left-sided valve surgery. clinical features and mechanisms. Circ J 2011;75:2902–7. 10.1253/circj.CJ-11-0718 [DOI] [PubMed] [Google Scholar]
- 34.Matsunaga A, Duran CMG. Progression of tricuspid regurgitation after repaired functional ischemic mitral regurgitation. Circulation 2005;112:I453–7. 10.1161/CIRCULATIONAHA.104.524421 [DOI] [PubMed] [Google Scholar]
- 35.Kim H-K, Kim Y-J, Kim K-I, et al. Impact of the maze operation combined with left-sided valve surgery on the change in tricuspid regurgitation over time. Circulation 2005;112:I14–19. 10.1161/CIRCULATIONAHA.104.524496 [DOI] [PubMed] [Google Scholar]
- 36.Fukuda S, Song J-M, Gillinov AM, et al. Tricuspid valve tethering predicts residual tricuspid regurgitation after tricuspid annuloplasty. Circulation 2005;111:975–9. 10.1161/01.CIR.0000156449.49998.51 [DOI] [PubMed] [Google Scholar]
- 37.De Bonis M, Lapenna E, Sorrentino F, et al. Evolution of tricuspid regurgitation after mitral valve repair for functional mitral regurgitation in dilated cardiomyopathy. Eur J Cardiothorac Surg 2008;33:600–6. 10.1016/j.ejcts.2008.01.011 [DOI] [PubMed] [Google Scholar]
- 38.Yilmaz O, Suri RM, Dearani JA, et al. Functional tricuspid regurgitation at the time of mitral valve repair for degenerative leaflet prolapse: the case for a selective approach. J Thorac Cardiovasc Surg 2011;142:608–13. 10.1016/j.jtcvs.2010.10.042 [DOI] [PubMed] [Google Scholar]
- 39.Bertrand PB, Overbey JR, Zeng X, et al. Progression of tricuspid regurgitation after surgery for ischemic mitral regurgitation. J Am Coll Cardiol 2021;77:713–24. 10.1016/j.jacc.2020.11.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2022-002011supp001.pdf (109.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.