Abstract
Objective
To identify the outcomes considered important to parents or caregivers of infants diagnosed with neonatal encephalopathy, hypoxic ischaemic encephalopathy or birth asphyxia in high-income and low- to middle-income countries (LMiCs), as part of the outcome-identification process in developing a core outcome set (COS) for the treatment of neonatal encephalopathy.
Design
A qualitative study involving 25 semistructured interviews with parents or other family members (caregivers) of infants who were diagnosed with, and treated for, neonatal encephalopathy, hypoxic ischaemic encephalopathy or birth asphyxia.
Setting
Interviews were conducted in high-income countries (HiCs) (n=11) by Zoom video conferencing software and in LMiCs (n=14) by phone or face to face.
Findings
Parents identified 54 outcomes overall, which mapped to 16 outcome domains. The domains identified were neurological outcomes, respiratory outcomes, gastrointestinal outcomes, cardiovascular outcomes, motor development, cognitive development, development (psychosocial), development (special senses), cognitive development, development (speech and social), other organ outcomes, survival/living outcomes, long-term disability, hospitalisation, parent-reported outcomes and adverse events.
Conclusions
This study provides insight into the outcomes that parents of infants diagnosed with neonatal encephalopathy have identified as the most important, to be considered in the process of developing a COS for the treatment of neonatal encephalopathy. We also provide description of the processes employed to ensure the inclusion of participants from LMiCs as well as HiCs.
Keywords: Neonatology, Neurology, Qualitative research
WHAT IS ALREADY KNOWN ON THIS TOPIC
Parents of newborn infants diagnosed and treated for neonatal encephalopathy, hypoxic ischaemic encephalopathy or birth asphyxia have extensive experience of caring for these infants.
Qualitative interviews are increasingly used in core outcome set (COS) development but little has been done to include participants from low- to middle-income countries (LMiCs).
WHAT THIS STUDY ADDS
This study highlights the outcomes that parents of infants diagnosed and treated for neonatal encephalopathy consider important to include in a COS.
Provides a transparent reporting of processes used to include parents from LMiCs in qualitative research contributing to COS development.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study was part of the process of developing a COS for use in trials and other studies for the treatment of neonatal encephalopathy.
Introduction
Neonatal encephalopathy is a condition of impaired neurological function that can occur in newborns. There can be many causes of neonatal encephalopathy including among others, infection, genetic or maternal risk factors, and a lack of oxygen supply to the brain of the infant.1 Neonatal encephalopathy is associated with impaired respiratory functioning, depressed reflexes and tone, reduced level of consciousness and the occurrence of seizures.2 The current leading treatment for neonatal encephalopathy is therapeutic hypothermia, which has been shown in randomised trials to reduce mortality and major disability in high-income countries (HiCs); however, mortality still occurred in some infants and only a borderline decrease in blindness and seizures was seen.3 Moreover, in a low-income to middle-income country (LMiC) setting, therapeutic hypothermia did not reduce the risk of death or disability, and instead increased the likelihood of death in these infants.4 This has resulted in studies of new treatments5 and the need to standardise the outcomes measured to allow for results to be compared and combined.
This qualitative study is a part of a larger study (COHESION) to develop a core outcome set (COS) for the treatment of neonatal encephalopathy.6 A COS is a standardised list of outcomes considered to be the most important to all stakeholders (parents/caregivers, healthcare providers, researchers/academics), which should be measured as a minimum in all trials for a specific condition.7
The development of COSs involves a consensus process to prioritise outcomes for a particular condition. This process begins with identifying outcomes for the condition, usually by reviewing outcomes used in prior studies.7 8 More recently, COS developers also identify outcomes important to patients through qualitative research.9 Outcomes are then presented to stakeholders in an online Delphi survey to rate the importance of each outcome. The final COS is then agreed on at a consensus meeting.5 According to Keeley et al8 conducting qualitative research with patients, including parents of patients, to inform the list of outcomes should increase confidence that ‘all potentially relevant outcomes’ will be identified. Other benefits of incorporating qualitative methods for outcome identification in COS development include gaining a better understanding of ‘why’ outcomes are important to patients/parents of patients/caregivers. This context can strengthen outcomes and justify inclusion in the Delphi survey.8 Plain language explanations for outcomes presented in the Delphi survey can also be informed by the language used by participants of the interviews.8
In addition to including parents/caregivers of infants with neonatal encephalopathy, it is important that previously under-represented parents/caregivers from LMiCs are involved in developing this COS. A review by Karumbi et al in 202110 found that in the 370 published COSs reviewed, only 20% had included participants from LMiCs. Temporal, cultural and resource disparities have been suggested as barriers for inclusion of LMiCs.10 For a COS to have global relevance, more effort needs to be made to include the perspectives of patients from low-income and middle-income countries.
This study aims to identify the outcomes considered important to parents/caregivers of infants diagnosed with neonatal encephalopathy, hypoxic ischaemic encephalopathy or birth asphyxia in HiCs and LMiCs, as part of the outcome-identification process in developing a COS for interventions for the treatment of neonatal encephalopathy.
Previous qualitative studies exploring parents’ experiences of their child receiving therapeutic hypothermia treatment have been conducted in the USA and Sweden.11–14 However, the focus of COHESION is to develop a COS for all treatments of neonatal encephalopathy across all jurisdictions, for all treatments.6 This prompted the team to conduct primary qualitative research, with participants from both HiC and LMiCs, as opposed to a systematic review of previous qualitative research, as had been done in other COS development.15
Methods
This study is reported in line with reporting recommendations for qualitative research methods in COS development described by Jones et al.16 (online data supplemental file 1) and Consolidated criteria for reporting qualitative research guidelines (COREQ) (online data supplemental file 2).
bmjpo-2022-001550supp001.pdf (874KB, pdf)
Design
Using a qualitative design to underpin this inquiry allowed the research team to hear parents/caregivers speak to their experiences of an infant with neonatal encephalopathy and the outcomes they considered important to measure.
Participants
The eligibility criteria for inclusion in this study were that participants were over 18 years of age and a parent or other family member who care for, or have cared for, an infant that was diagnosed with, and received treatment for, neonatal encephalopathy, hypoxic-ischaemic encephalopathy or birth asphyxia (see standard operating procedure (SOP) online data supplemental file 3) and participant information leaflet (PIL) (online data supplemental file 4), these documents were developed by researchers and parent representatives on the COHESION Steering Group. The grade of brain injury was not included in the inclusion criteria. Participants’ infants were not required to have been part of a trial for treatment. On consultation with colleagues in Kenya, ‘birth asphyxia’ was included in the inclusion criteria, in addition to neonatal encephalopathy, to account for diagnosis criteria used locally.
Patient and Public Involvement
Parent representatives are members of the COHESION Study steering group and contributed to the design, conduct and reporting of this study. Parent representatives contributed to all documentation relating to the recruitment of parents/ caregivers to interviews and the conduct and reporting of the interviews.
Recruitment strategy
We planned to interview 8–12 parents/caregivers in HiCs and 4–6 parents/caregivers in each LMiC participating in the study (Kenya and Pakistan), informed by the numbers interviewed in previous qualitative studies.11–14 We were mindful that the concept of conceptual saturation17 (ie, when no new outcomes were identified as the interviews and analysis progressed) would guide the final interview numbers.
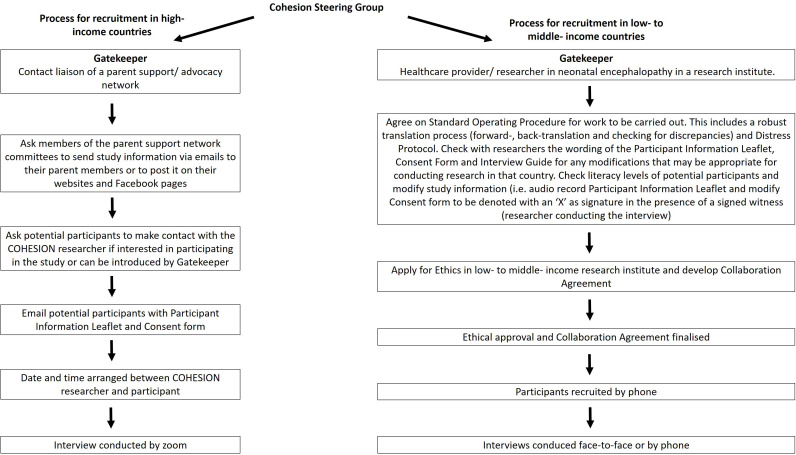
We developed a strategy for preparatory work and recruitment in both HiCs and LMiCs as follows (figure 1):
Figure 1.
Strategy for recruiting parents/ caregivers of infants diagnosed with neonatal encephalopathy in HiCs and LMiCs.
High-income countries
For the HiCs, we used purposeful sampling18 and developed a recruitment approach (figure 1) to recruit parents/caregivers. Gatekeepers were representatives of national and international parent support networks (see acknowledgements) to facilitate recruitment of parents/caregivers. Parents/caregivers were also recruited through the COHESION Study Facebook page where a recruitment video was uploaded and shared (https://www.facebook.com/100053069057849/videos/109673200811648/). The participant from India was also recruited in this way. Information (names and affiliations) on the researchers was provided in the recruitment of participants in all locations.
Low-income to middle-income countries
Gatekeepers were healthcare providers and/or researchers working in the area of neonatal encephalopathy. Gatekeepers in LMiCs used a purposeful and convenience sampling technique14 and invited parents/caregivers who had engaged previously with local healthcare or research teams about neonatal encephalopathy/birth asphyxia (see figure 1).
Data collection
A semistructured interview guide (online data supplemental file 5) was developed for data collection in HiCs and LMiCs. The guide was informed by those used for qualitative interviews conducted in previous COS development,19 20 and codeveloped with parent representatives and our colleagues in Kenya. Interviews were carried out between March 2020 and February 2021. Interviews were concept-elicitation interviews,21 which enabled participants to elicit spontaneous responses based on their experiences. A Distress Protocol document (online data supplemental file 6) adapted from Draucker22 was also available across all research sites. All interviews were conducted by experienced qualitative researchers. Colleagues involved in the interview process were academics and/or clinicians.
High-income countries
All interviews with participants from HiC countries (Ireland, Australia, the USA and the UK), and the interview with the participant from India, were conducted online by FQ and LB, in English, audio recorded and transcribed. Reflective notes were written during and after each interview (online supplemental table 1). Researchers in HiCs were not known to participants prior to recruitment.
bmjpo-2022-001550supp002.pdf (158.4KB, pdf)
Low-income to middle-income country
The SOP guided the processes of translating the PIL, consent form and interview guide to local dialects to ensure that participation was not restricted to English-speaking parents/caregivers. The translation process was guided by published principles of good practice23 24 and is described in figure 2. One interview was conducted in English with a participant from India who was fluent in English. In keeping with the country classification by the World Bank, we describe the data from this participant with the LMiC interviews. Interviews in LMiC countries were conducted face to face or by phone by interviewers in the local language using the interview guide, audio recorded and transcribed by COO, CB, VK and SK (Kenya) and SA, MN and FT (Pakistan). The process for transcription and translation of interview scripts is outlined in figure 3.
Figure 2.
Translation process for COHESION parent interviews in LMiCs.
Figure 3.
Translation processes for COHESION interviews conducted in LMiC.
Data analysis
Data analysis was carried out by FQ and LB using methods adopted by other COS developers,25 26 based on the approach by Corbin and Strauss.27 The transcripts were coded by (a) inductive coding, capturing definitive outcomes reported by the participants and (b) semantic coding, for references to outcomes inferred by the participants. This approach to coding was carried out for each interview independently, verified, and any differences were discussed before proceeding to the next stage. Similar codes (outcomes) were merged and deduplicated from interviews and grouped into higher-level thematic categories (outcome domains). The domain categorisation was largely based on domains used in previous similar COSs.15 28
The process of mapping direct quotes to outcomes and domains was not presented to participants but was presented to parent representatives and healthcare providers on the COHESION Steering Group for final verification and agreement, rather than presenting to participants.
Conceptual saturation17 was assessed iteratively during the analysis and was reached by the interview 11 in HiCs, by interview 5 in Pakistan and by interview 8 in Kenya.
Results
Interviews were carried out with 25 parents. The children of these parents ranged between 4 months at the time of interview and 17 years. Other parents initially showed interest but decided not to take part in the interviews due to the time, childcare and working from home commitments. The country breakdown is as follows: Ireland (n=8), Australia (n=1), the USA (n=1), the UK (n=1), India (n=1), Kenya (n=8) and Pakistan (n=5). The interviewees included twenty-one mothers and four fathers. Four of the infants of parents we interviewed had died. Fourteen children had received therapeutic hypothermia treatment and eleven received standard care. Twelve interviews were carried out by Zoom video conferencing; seven interviews face-to-face and six interviews by phone. Interviews took between 40 and 70 min.
Many of the outcomes mentioned and discussed by parents align with those previously measured in randomised trials of interventions for the treatment of neonatal encephalopathy.29–31 Fifty-four outcomes were identified overall by parents as important to measure in the treatment of neonatal encephalopathy. These outcomes mapped to sixteen outcome domains (online supplemental table 1). We have included whether parents mentioned the outcomes in HiCs alone, LMiCs alone or both. Four unique outcomes were identified by parents in LMiCs, and seven unique outcomes were identified by parents from HiCs. The outcomes, noted under the domain headings, are presented in the online supplemental table 1. Twenty-one of the outcomes are unique to this study and were not identified in our ongoing systematic review of randomised trials and systematic reviews of randomised trials for the treatment of neonatal encephalopathy, these outcomes are indicated with an Asterix in the online supplemental table 1. These unique outcomes were mapped across eight domains: neurological outcomes, respiratory outcomes, gastrointestinal outcomes, motor development, development (speech & social), survival/living outcomes, long-term disability and parent-reported outcomes. Extracts from the interviews providing illustrative examples of the parents/family caregivers narratives are reported in table 1. This table provides illustrative examples of the definitive (and inferred) outcomes considered important to parents/caregivers.
Table 1.
Examples of illustrative quotes mapped to outcomes
| Outcome identified | Illustrative quote | Participant details |
| Absence of neonatal reflexes | She told we cannot predict when he will be fine because when the pupillary reflex is not there, when other reflexes are not there. He’s not responding to any of the treatment | Interview H, mother, (LMiC) |
| Gag reflex (absent) | They did a test to see if he had a gag reflex and he had no gag reflex | Interview A, mother, (HiC) |
| Swallow (absent) | I remember them saying to me that’s a really good sign he can swallow | Interview E, mother, (HiC) |
| Sleep disorders | When you’re a mum and she’s sleeping four or four and a half hours between feeds you think it’s great, you don’t think or know that could be…a bad thing | Interview I, mother, (HiC) |
| Ability to breathe normally and unaided | He was able to breathe on his own he was kind of fighting it straight away. That was a good sign I suppose | Interview C, father, (HiC) |
| Need for neonatal resuscitation | He needed resuscitating while he was being warmed up twice while I was sitting there… that was terrifying | Interview E, mother, (HiC) |
| Meconium passage | Personally, I used to observe some problems on the baby. It got to a point that when he would pass stool, it had blood so for me I used to ask such questions because I thought it was related with the problem with the problem that he had | Interview S, mother, (LMiC) |
| Ability to undertake sport | Now he has no problems (motor development), he loves swimming, watching him in the water in the pool, watching him swimming around and having fun is fantastic | Interview E, mother, (HiC) |
| Heightened sensory sensitivity | They also told us that she could have a sensory condition where she’d be, where she mightn’t want to be touched | Interview K, mother, (HiC) |
| Suffering | He was very uncomfortable and crying shaking cause obviously they were cooling him down | Interview D, mother, (HiC) |
| Parental involvement in care | She let me hold him earlier than I was meant to… my instinct was this can’t be bad for him to be held by his mother | Interview D, mother, (HiC) |
| Parental attachment with their baby | I was in the post-surgery room and my son was in the Neonatal Intensive Care Unit. No-one was allowed to see my son except my husband | Interview H, mother, (LMiC) |
| Uncertainty for future well-being | I missed out on so much. I kept having to hug her and if she wasn’t there or gone to Montessori or something I would have to get a pillow or something just to hug | Interview E, mother, (HiC) |
| Parental psychological impact of Neonatal Intensive Care Unit experience | I went back to my room and I was in some kind of shock. I remember my mum and dad came in and there were conversations I had with doctors that had been there at his birth, a lot of them came to see me over those couple of days and I have no recollection of speaking to them | Interview F, mother, (HiC) |
| Impact of child’s condition on parents’ relationship | Probably nearly caused our divorce. There were some very, very hard times | Interview E, mother, (HiC) |
| Financial burden of healthcare costs of care for infant on parents | Like financially to take her to the hospital. It brings a lot of trouble | Interview P, mother, (LMiC) |
| Parental ability to work | You don’t go to work, you need to stay with the baby, to look after them. So it has effect | Interview Q, mother, (LMiC) |
| Impact of child’s condition and Neonatal Intensive Care Unit experience on wider family (stress, disappointment, sadness, grief etc) | As a family, we are not as before, everyone is very down. It has caused a big impact in our life | Interview H, mother, (LMiC) |
| Effective communication between parents and healthcare providers | What’s this mean? Numbers. What’s a good number for this? What’s a good range for that? And I just, I went deep. Every night I’d come home at ten o’clock, until two or three in the morning I’d be, numbers, what’s that mean? HIE studies, outcomes, grading, HIE one, two, three, long-term prognosis, everything like you know, cerebral palsy, what comes with this. So it broke it down over a few weeks and I learned as much as I could | Interview J, father, (HiC) |
HiC, high-income country; LMiC, low- to middle-income country.
Discussion
Outcomes considered important to parents of infants with neonatal encephalopathy are largely unreported in trials evaluating treatments.29–31 This qualitative study highlights the outcomes that these parents identified as most important in determining their child’s health following treatment. Twenty-one outcomes were identified by parents as important to measure in the treatment of neonatal encephalopathy that were not measured in randomised trials of interventions for the treatment of neonatal encephalopathy, as identified by our ongoing systematic review. This highlights the need for inclusion of parents’ perspectives in identifying important outcomes in the COS development process.
This study also highlights the need for qualitative data collection in COS development to be more inclusive of LMiCs. Although many outcomes were identified as important by parents from both LMiCs and HiCs, some were unique to either LMiC or HiC. While, the reasons for these differences in outcomes is beyond the scope of this work, all outcomes will be included in the next phase of the COS development.6 32
Using the processes we have reported to engage with gatekeepers in LMiCs, future COS developers have the opportunity to be more inclusive of the experiences and opinions of stakeholders on a given condition from both HiCs and LMiCs. In addition, using the translation processes described, language does not have to be a barrier for the inclusion of patients’ opinions of important outcomes in COS development.
Strengths and limitations
The findings of this work contribute to an under-researched area. We chose not to present parents with a list of outcomes from the literature, a method that has been used in other qualitative work informing COS development.20 This decision was pragmatic in that it was influenced by time frames of other work packages of this study. It also contributed to the iterative process underpinning the aim of this qualitative study that is, the focus was on the parents identifying outcomes they deemed important rather than offering comments on outcomes we presented to them. Parents will also be invited to participate in the next phase of this study, an online Delphi survey to prioritise outcomes and inform the final COS for use in future studies evaluating treatments for neonatal encephalopathy.
Having rigorous procedures in place, including developing an SOP and Distress Protocol helped ensure that similar processes were followed for all parents regardless of what country they were participating from.
A strength of this study was that fathers of infants with neonatal encephalopathy/birth asphyxia were also interviewed and contributed their unique experience of outcomes they considered to be important. A lack of inclusion of fathers was listed as a limitation of interviews carried out by Duffy et al.25
We must acknowledge the limitations of this study. The age range of participants children was broad (children ranged from 4 months to 17 years) at the time of these interviews. The eldest ‘child’ had taken part in a trial for therapeutic hypothermia and so this informed the higher age threshold. We are aware that the age range of the children may have modified parents experiences and observations. However, for the purposes of identifying outcomes as part of a COS development process, the diversity of outcomes at different age milestones is an important factor and ensures all relevant outcomes (short term and long term) are considered in deciding the final COS. We did not seek in-depth demographic information of the parents taking part in the interviews (eg, level of education, socioeconomic status etc). We acknowledged the parents by the World Bank classification of their country of residence in terms of income status (see https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html). However, we suggest that further exploration of demographic data would be interesting.
This study was conducted during the COVID-19 pandemic. The multiple methods of recruitment reported were used as the recruitment took longer than planned, perhaps this could be aligned to the pandemic. Due to public health guidance and social distancing, some of the interviews were conducted via phone and not face to face as we had originally planned.
Including parents in this stage of COS development ensures that the outcomes presented in the next rounds of the COS development (ie, the Delphi survey) will present outcomes that are important to parents from HiC and LMiCs. Including parents in all stages of the COS development ensures the COS will be relevant to those in HiCs and LMiCs. This is particularly important as different treatments are being investigated and disparities have been shown in the effectiveness of treatments between HiC and LMiC populations.3 4
Conclusion
Our findings present outcomes identified by parents as important to measure and report in future trials for interventions for the treatment of neonatal encephalopathy. In addition to physiological/clinical outcomes measured by healthcare providers and researchers in studies, parents also highlighted outcomes of parental and familial involvement in the care of the infant and their overall well-being. This study also offers processes for the inclusion of participants from LMiCs in COS development. The next phase of COHESION Study will combine these outcomes with those from a systematic review of studies for the treatment of neonatal encephalopathy. Unique outcomes will be scored by stakeholders in an online consensus process called a Delphi survey. The final outcomes to include in the COS will be decided through discussion with stakeholders in online consensus meetings.6
Supplementary Material
Acknowledgments
On behalf of the COHESION Study team, we would sincerely like to thank all parents who gave their time and took part in our interviews. We would also like to thank the Irish Neonatal Health Alliance, Hope for HIE, Miracle Babies Foundation and Life’s Little Treasures Foundation for sharing our study information and playing a vital part in recruitment to this study. Dr Malcolm Battin would like to acknowledge the receipt of his research fellowship (Health Research Council of New Zealand grant number 20/030).
Footnotes
Twitter: @Fiona_Quirke_, @shabina
Contributors: FQ, FHB, MD, DD, DMH, PH, TH, JJK, SM, EM, ENB, KW and LB conceived and designed this qualitative study. FQ, LB, PH, DD, MD, ENB, CB and COO contributed to the study design of the interview guide and SOP. FQ, LB, PH, DD, MD and ENB contributed to the study design of all other supplementary material including the Participant Information Leaflet and Distress Protocol. SA, FT, MN, CB, VK, COO and SK conducted the recruitment, interviews, transcription and translation in LMiCs. FQ and LB conducted the recruitment, interviews and transcription in HiCs. FQ and LB analysed the data. DD, PH and MB verified the data analysis. FQ and LB wrote the paper. FQ is the corresponding author and guarantor of this manuscript. All authors critically revised the manuscript. All authors reviewed and approved the final manuscript.
Funding: This study was conducted as part of a PhD project funded by the Health Research Board (HRB) as part of the Neonatal Encephalopathy PhD Training Network (NEPTuNE).
Competing interests: No, there are no competing interests.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; internally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Public data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Received
Ethics approval
This study involves human participants and was approved by the National University of Ireland, Galway, Research Ethics Committee (REC) (Reference 19 April 2014). The Moi University/Moi Teaching and Referral Hospital Institutional Ethics Review Committee (IREC, reference IREC/2016/243, approval number: 0001874). Aga Khan University Ethics Research Committee (ERC) (Reference 2020-5263-14425). Participants gave informed consent to participate in the study before taking part.
References
- 1.Aslam S, Strickland T, Molloy EJ. Neonatal encephalopathy: need for recognition of multiple etiologies for optimal management. Front Pediatr 2019;7:142. 10.3389/fped.2019.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Vries LS, Jongmans MJ. Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 2010;95:F220–4. 10.1136/adc.2008.148205 [DOI] [PubMed] [Google Scholar]
- 3.Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2013;69:CD003311. 10.1002/14651858.CD003311.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thayyil S, Pant S, Montaldo P, et al. Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (helix): a randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob Health 2021;9:e1273–85. 10.1016/S2214-109X(21)00264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAdams RM, Berube MW. Emerging therapies and management for neonatal encephalopathy-controversies and current approaches. J Perinatol 2021;41:661–74. 10.1038/s41372-021-01022-9 [DOI] [PubMed] [Google Scholar]
- 6.Quirke FA, Healy P, Bhraonáin EN, et al. Cohesion: core outcomes in neonatal encephalopathy (protocol). Trials 2021;22:125. 10.1186/s13063-021-05030-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson PR, Altman DG, Bagley H, et al. The comet Handbook: version 1.0. Trials 2017;18:280. 10.1186/s13063-017-1978-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keeley T, Williamson P, Callery P, et al. The use of qualitative methods to inform Delphi surveys in core outcome set development. Trials 2016;17:230. 10.1186/s13063-016-1356-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorst SL, Gargon E, Clarke M, et al. Choosing important health outcomes for comparative effectiveness research: an updated review and user survey. PLoS One 2016;11:e0146444. 10.1371/journal.pone.0146444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karumbi J, Gorst SL, Gathara D, et al. Inclusion of participants from low-income and middle-income countries in core outcome sets development: a systematic review. BMJ Open 2021;11:e049981. 10.1136/bmjopen-2021-049981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemmon ME, Donohue PK, Parkinson C, et al. Parent experience of neonatal encephalopathy. J Child Neurol 2017;32:286–92. 10.1177/0883073816680747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nassef SK, Blennow M, Jirwe M. Parental viewpoints and experiences of therapeutic hypothermia in a neonatal intensive care unit implemented with Family-Centred care. J Clin Nurs 2020;29:4194–202. 10.1111/jocn.15448 [DOI] [PubMed] [Google Scholar]
- 13.Craig AK, James C, Bainter J, et al. Parental perceptions of neonatal therapeutic hypothermia; emotional and healing experiences. J Matern Fetal Neonatal Med 2020;33:2889–96. 10.1080/14767058.2018.1563592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemmon ME, Donohue PK, Parkinson C, et al. Communication challenges in neonatal encephalopathy. Pediatrics 2016;138:e20161234. 10.1542/peds.2016-1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webbe J, Brunton G, Ali S, et al. Parent, patient and clinician perceptions of outcomes during and following neonatal care: a systematic review of qualitative research. BMJ Paediatr Open 2018;2:e000343. 10.1136/bmjpo-2018-000343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones JE, Jones LL, Keeley TJH, et al. A review of patient and carer participation and the use of qualitative research in the development of core outcome sets. PLoS One 2017;12:e0172937. 10.1371/journal.pone.0172937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis JJ, Johnston M, Robertson C, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health 2010;25:1229–45. 10.1080/08870440903194015 [DOI] [PubMed] [Google Scholar]
- 18.Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks, CA: SAGE publications, 2002. [Google Scholar]
- 19.Fish R, Sanders C, Williamson PR, et al. Core outcome research measures in anal cancer (CORMAC): protocol for systematic review, qualitative interviews and Delphi survey to develop a core outcome set in anal cancer. BMJ Open 2017;7:e018726. 10.1136/bmjopen-2017-018726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dadouch R, Faheim M, Juando-Prats C, et al. Development of a core outcome set for studies on obesity in pregnant patients (COSSOPP): a study protocol. Trials 2018;19:655. 10.1186/s13063-018-3029-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nabbout R, Auvin S, Chiron C, et al. Development and content validation of a preliminary core set of patient- and caregiver-relevant outcomes for inclusion in a potential composite endpoint for Dravet syndrome. Epilepsy Behav 2018;78:232–42. 10.1016/j.yebeh.2017.08.029 [DOI] [PubMed] [Google Scholar]
- 22.Draucker CB, Martsolf DS, Poole C. Developing distress protocols for research on sensitive topics. Arch Psychiatr Nurs 2009;23:343–50. 10.1016/j.apnu.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 23.Brelsford KM, Ruiz E, Beskow L. Developing informed consent materials for non-English-speaking participants: an analysis of four professional firm translations from English to Spanish. Clin Trials 2018;15:557–66. 10.1177/1740774518801591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wild D, Grove A, Martin M, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (pro) measures: report of the ISPOR Task force for translation and cultural adaptation. Value Health 2005;8:94–104. 10.1111/j.1524-4733.2005.04054.x [DOI] [PubMed] [Google Scholar]
- 25.Duffy J, Thompson T, Hinton L, et al. What outcomes should researchers select, collect and report in pre-eclampsia research? A qualitative study exploring the views of women with lived experience of pre-eclampsia. BJOG 2019;126:637–46. 10.1111/1471-0528.15616 [DOI] [PubMed] [Google Scholar]
- 26.Gonçalves A-C, Marques A, Samuel D, et al. Outcomes of physical activity for people living with dementia: qualitative study to inform a core outcome set. Physiotherapy 2020;108:129–39. 10.1016/j.physio.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 27.Corbin JM, Strauss A. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol 1990;13:3–21. 10.1007/BF00988593 [DOI] [Google Scholar]
- 28.Healy P, Gordijn SJ, Ganzevoort W, et al. A core outcome set for the prevention and treatment of fetal growth restriction: deVeloping endpoints: the COSGROVE study. Am J Obstet Gynecol 2019;221:339.e1–339.e10. 10.1016/j.ajog.2019.05.039 [DOI] [PubMed] [Google Scholar]
- 29.Azzopardi D, Brocklehurst P, Edwards D, et al. The TOBY study. whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr 2008;8:17. 10.1186/1471-2431-8-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs SE, Morley CJ, Inder TE, et al. Whole-Body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med 2011;165:692–700. 10.1001/archpediatrics.2011.43 [DOI] [PubMed] [Google Scholar]
- 31.Laptook AR, Shankaran S, Tyson JE, et al. Effect of therapeutic hypothermia initiated after 6 hours of age on death or disability among newborns with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA 2017;318:1550–60. 10.1001/jama.2017.14972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quirke FA, Healy P, Bhraonáin EN, et al. Multi-round compared to real-time Delphi for consensus in core outcome set (COS) development: a randomised trial. Trials 2021;22:142. 10.1186/s13063-021-05074-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjpo-2022-001550supp001.pdf (874KB, pdf)
bmjpo-2022-001550supp002.pdf (158.4KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Public data relevant to the study are included in the article or uploaded as supplementary information.